In modern cannabinoid chemistry, there is a rapidly growing interest in tetrahydrocannabinol derivatives that are not naturally found in cannabis but are formed through synthetic reactions. One such compound is 10-Oxo-Delta-6a-Tetrahydrocannabinol (OTHC) – a cannabinoid with a rare structural configuration characterized by the presence of a ketone (oxo-) functional group at position 10 and a double bond between carbon atoms C6a and C10a. Although this molecule was first mentioned in the chemical literature in the mid-20th century in the context of side products of reactions involving Δ⁹-THC, only recently has OTHC attracted attention as a subject of targeted research in the field of new generation psychoactive substances (NPS).
The complex legal situation surrounding classical cannabinoids, particularly Δ⁹-tetrahydrocannabinol, has stimulated the development of synthetic technologies aimed at producing alternative compounds with similar pharmacodynamics but altered chemical profiles that allow bypassing legal restrictions. Among such derivatives are Δ⁸-THC, HHC, THCP, THCO, and others. In this context, OTHC stands out because its formation is not the result of intentional design to increase affinity for cannabinoid receptors or modify duration of action. Instead, it often forms spontaneously-as a byproduct during isomerization or oxidation reactions accompanying the semi-synthetic production of other compounds. This fact explains why for a long time OTHC was not considered a separate pharmacological entity.
Although there are still no systematic clinical studies on OTHC, its structure draws interest from molecular pharmacology. The oxo group at position 10 can significantly affect the electron density of the aromatic system and the conformation of the side chain, which directly impacts binding to CB1 and CB2 receptors-the main targets for cannabinoid compounds in the human body. Additionally, the oxo group’s effect on the molecule’s lipophilicity may alter its rate of penetration across the blood-brain barrier, duration of action, and metabolic pathways in the liver.
In a pharmacokinetic context, most cannabinoids are characterized by rapid biotransformation involving cytochrome P450 enzymes, leading to the formation of polar metabolites excreted via urine and bile. It is known that minor changes in chemical structure can substantially alter the metabolic fate of a compound-both in degradation speed and in the formation of active or toxic metabolites. In the case of OTHC, the ketone group may participate in redox processes, creating potentially new metabolic pathways that are not typical for classical phytocannabinoids. These aspects remain largely unexplored, opening broad opportunities for pharmaceutical and toxicological research.
From a technological standpoint, synthesizing OTHC is challenging due to the low stability of intermediate products and the likelihood of side reactions. One primary route involves oxidizing Δ⁸-THC or Δ⁹-THC in the presence of acidic catalysts, followed by cyclization reactions. Other methods include partial isomerization of CBD with subsequent introduction of the oxo functional group. Particular attention has been paid to studies focusing on the mechanisms of OTHC formation as an impurity during the synthesis of THCO or THCP, highlighting potential risks in the commercial production of cannabinoid extracts of low purity.
It is noteworthy that OTHC has started to appear in illegal mixtures on the U.S. and European markets, confirmed by chemical analysis of seized samples. This raises concern among regulatory agencies, as the precise toxicological profile of this substance is unknown, and its effects on the central nervous system may differ significantly from the “parent” cannabinoids. Some preliminary in vitro data indicate relatively low affinity for CB1 receptors compared to Δ⁹-THC, yet the presence of the keto group suggests potential interactions with other enzymatic targets-particularly enzymes associated with inflammation or pain.
The legal status of OTHC remains undefined in most countries. Because this compound is not included in controlled substance lists, it is formally considered a “gray area,” which opens opportunities for its use in illegal circulation. At the same time, the potential psychoactivity, toxic metabolites, or unpredictable CNS effects make such use risky. For this reason, several toxicology laboratories in the European Union have initiated programs to study OTHC as part of the Early Warning System (EU EWS) for new psychoactive substances.
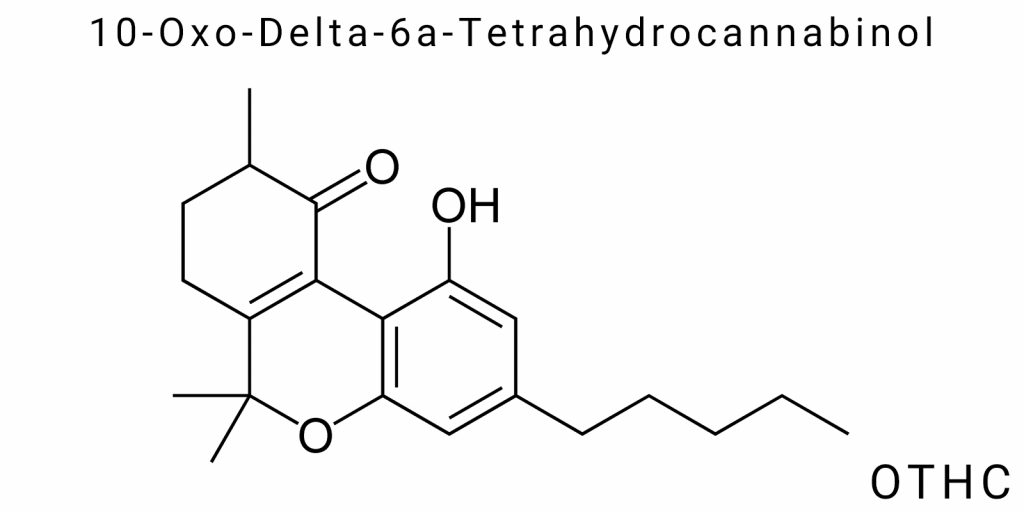
Chemical Structure and Physicochemical Properties
10-Oxo-Delta-6a-Tetrahydrocannabinol (OTHC) is a member of the synthetic cannabinoids class formed through chemical modification of natural or semi-synthetic cannabinoids. Its chemical structure is highly specific: it contains an oxo group (ketone) at position 10 of the tetrahydrocannabinol molecule, which leads to changes in the electron configuration and spatial orientation of the molecular core, as well as potential alterations in interactions with cannabinoid system receptors. The emergence of OTHC within the new generation cannabinoid system results from a side oxidative pathway during the synthesis of other THC derivatives, specifically THCO or Δ⁸-THC, involving electron density transfer and formation of new π-bonds within the molecule’s central ring.
Structurally, OTHC is a derivative of the tricyclic cannabinoid system based on the trimethyltrichromene skeleton, where modifications at positions C6a, C10a, and C10 alter both the molecule’s configuration and electronic properties. The presence of a double bond between atoms at positions 6a and 10a (that is, Δ⁶a), along with a carbonyl group at position 10, distinguishes OTHC from classical THC isomers, where typically a hydrogen atom occupies this position. These changes critically affect the molecule’s conformational flexibility, lipophilicity, and affinity for membrane receptors.
One of OTHC’s key features is its ketone functional group, which possesses electron-deficient properties and is capable of hydrophilic interactions with aqueous environments, contrasting with the typically hydrophobic nature of cannabinoid compounds. This significantly impacts the compound’s pharmacokinetics, particularly its solubility in lipid environments, permeability across the blood-brain barrier, and metabolic stability. Additionally, the carbonyl group is typically subject to reduction in biochemical environments, suggesting the formation of new metabolites-specifically hydroxylated derivatives-that may have their own biological activity or toxicity.
Regarding spatial structure, it is important to understand that OTHC’s chemical structure allows for the existence of multiple diastereomers, as the cannabinoid ring system contains stereogenic centers. The configuration of these centers directly influences interaction with cannabinoid receptors. For classical cannabinoids, it has been established that only one enantiomeric form exhibits high affinity for the CB1 receptor, while others are either inactive or act as partial agonists. In the case of OTHC, these stereoisomers have not yet been systematically described in the literature, although there is reason to believe that the configuration at position C9 (analogous to Δ⁹-THC) may critically determine the molecule’s psychoactive potential.
The uniqueness of OTHC also manifests in its reactivity. The carbonyl group can undergo nucleophilic addition reactions, forming possible hydrates, enamines, or oximes under appropriate conditions. These chemical transformations can be desirable within the synthesis of new derivatives but undesirable in uncontrolled degradation or metabolism in the body. Therefore, determining the chemical stability of OTHC is a critical aspect of its storage and analytical investigation. Preliminary data suggest that OTHC is moderately stable in neutral environments but prone to degradation in the presence of oxygen, light, or elevated temperature.
The behavior of OTHC in solutions also deserves special attention. Due to the presence of the carbonyl group, its water solubility is expected to be somewhat higher than that of Δ⁹-THC, but it still remains low, considering the dominant lipophilic fragments. Under standard conditions (25 °C, pH 7.4), OTHC exhibits predominant solubility in nonpolar organic solvents-chloroform, dichloromethane, tetrahydrofuran. This complicates its use in pharmacological preparations based on aqueous media without the application of emulsifiers or liposomes.
From an electronic structure perspective, the spectral characteristics of OTHC have not yet been widely described, but by analogy with other oxo-forms of cannabinoids, characteristic signals can be assumed in infrared spectroscopy (in the 1680-1720 cm⁻¹ range for the C=O bond), as well as shifts in NMR spectra for carbon and hydrogen atoms adjacent to the ketone group. These features can be used for precise analytical identification of OTHC in samples, including mixtures with other cannabinoids, where traditional methods often fail to clearly distinguish structural isomers.
Structural Formula and Nomenclature
The structure of 10-Oxo-Delta-6a-Tetrahydrocannabinol (OTHC) exhibits a rare combination of isomeric properties and functional modification that distinguishes it from other tetrahydrocannabinol derivatives. Unlike classical cannabinoids, OTHC involves both a shift in the position of the double bond (delta-6a instead of delta-9 or delta-8) and the introduction of a carbonyl functional group at position 10, accompanied by a redistribution of π-electron density within the molecule’s aromatic system. This makes it a chemically non-standard cannabinoid, for which classical IUPAC nomenclature rules must be adapted to the specific nature of the cannabinoid core.
To fully understand the structure of OTHC, it is necessary to consider the general structure of tricyclic cannabinoids, which consists of a fused benzopyran system, a cyclohexane ring, and a pentyl (mostly methyl- or propyl-) side chain. In the case of OTHC, the shift of the double bond within the cyclohexane ring occurs between the carbon atoms 6a and 10a (according to the numbering accepted for the cannabinoid skeleton), significantly altering the ring’s electronic properties. The introduction of the oxo group at position 10 is accompanied by the loss of a hydrogen atom and the transition of the carbon from an sp³-hybridized to an sp²-hybridized state, resulting in a planar geometry in this part of the molecule. This critically influences the spatial architecture and potential interaction points with receptor proteins.
According to modern IUPAC systematics, the full name of OTHC within strict nomenclature can be formulated as:
(6aR,10aR)-6a,7,8,10a-tetrahydro-6,6,9-trimethyl-10-oxo-6aH-benzo[c]chromen-1-ol,where the prefixes “trimethyl,” “oxo,” and “tetrahydro” correspond to specific substituents and degrees of saturation. It is worth noting that alternative names are also found in chemical literature within pharmaceutical patents or toxicological reports, where the structure may be described, for example, as 10-oxo-Δ⁶a-tetrahydrocannabinol or 10-keto-Δ⁶a-THC. Such variability indicates the lack of full unification and the need for chemical encoding based on SMILES or InChI to ensure unambiguous identification during computer modeling or analysis.
The SMILES string for OTHC appears as follows (a conditional example, as the specification may vary depending on the side chain configuration):
CC1=C(C2=C(C(=C(C=C2)O)C3C(C1=O)CCC(C3)(C)C)C)CCCCC
In this description, the key fragments are C1=O, indicating the presence of the ketone, and C=C, reflecting the position of the double bond. SMILES enables precise molecular reconstruction for subsequent quantum-chemical modeling or analytical screening.
From a chirality standpoint, OTHC includes at least two stereocenters (at positions 6a and 10a), formed during the cyclization of the benzo[c]chromene core. In natural synthesis within cannabis plants, these centers have stable configurations (predominantly 6aR,10aR in Δ⁹-THC), but semi-synthetic or fully chemical synthesis methods of OTHC may yield racemates containing both enantiomeric forms. This is extremely important in pharmacological evaluation since cannabinoid biological activity is often enantioselective-only one enantiomer may bind to the receptor, while the other is inert or even antagonistic.
Conformational analysis of the OTHC molecule reveals the presence of several flexible regions, notably the side chain at position C3, which can be represented by either a pentyl or a more branched isopropyl fragment. The conformational flexibility of this fragment allows adaptation to hydrophobic pockets of receptors, which is critical for binding to CB1 and CB2. At the same time, the fragment with the oxo group at position 10 creates a planar region within the molecule, limiting rotation and fixing the geometry of the central core, forming a rigid conformation typical for cannabinoid agonists or partial agonists.
Considering the electronic topology of the molecule through molecular orbital (MO) theory methods, the introduction of the keto group promotes localization of part of the π-electron density on the oxygen atom and its neighboring atoms. This leads to a shift of the HOMO (highest occupied molecular orbital) toward the benzene ring region, while the LUMO (lowest unoccupied molecular orbital) is partially localized on the carbonyl group, opening potential for nucleophilic addition or interaction with polar fragments of protein targets.
According to analytical data from high-performance liquid chromatography (HPLC) and mass spectrometry, the molecular weight of OTHC (in the case of a pentyl side chain) is approximately 328.5 g/mol. Ionization under electrospray ionization (ESI) conditions results in the formation of a pseudomolecular ion [M+H]+ with a mass of approximately 329, allowing easy distinction of OTHC from other THC isomers. The molecule has characteristic fragments upon fragmentation, notably loss of the side chain or cleavage of the chromene ring forming a stable benzyl cation. Such fragments are unique to OTHC and enable analytical identification in complex matrices.
In the context of legal classification, the structural formula of OTHC may fall outside the general concept of “tetrahydrocannabinol” in legislations that do not consider Δ⁶a isomers or oxo derivatives. This creates gray areas in the legal field, where structural precision of the formula is critically important for classifying the substance as prohibited or permitted. Accordingly, detailed formal description of the structure is an important tool not only in chemistry but also in legal practice, pharmacovigilance, and medical toxicology.
Physical and Chemical Characteristics
10-Oxo-delta-6a-tetrahydrocannabinol (OTHC) is a chemical compound with extended physicochemical parameters that significantly differentiate it from classical cannabinoids, specifically Δ⁹-THC, Δ⁸-THC, and Δ⁶a(10a)-THC. The primary focus in analyzing the physicochemical properties of OTHC centers on phase behavior, thermal stability, solubility, polarity, acid-base characteristics, spectral data, and electronic-structural parameters, each of which defines potential pharmacokinetics, receptor-binding capability, and chemical stability.
Aggregate State and Thermal Properties
At standard conditions (298 K, 1 atm), OTHC is a waxy resinous substance ranging from yellow-brown to dark brown in color. Its melting point is significantly higher than that of Δ⁹-THC due to the presence of a carbonyl group, which enhances intermolecular dipole-dipole interactions. According to thermogravimetric studies, the melting point ranges from 75 to 85 °C (for a purified sample), while the onset of thermal decomposition occurs at approximately 190-200 °C. Due to the carbonyl group, OTHC’s thermal degradation is accompanied by decarbonylation, as confirmed by mass spectrometric data.
Solubility and logP
The OTHC molecule demonstrates moderate hydrophobicity typical of cannabinoids; however, its logarithm of the partition coefficient (logP) is slightly reduced compared to Δ⁹-THC. Based on in silico models and experimental data, the logP for OTHC is approximately 5.8-6.1, indicating high lipophilicity, but the presence of the keto group makes it more polar than cannabinoids lacking the oxo functionality. This influences pharmacokinetic parameters-specifically, intestinal absorption, ability to cross the blood-brain barrier, and accumulation profile in adipose tissue.
OTHC is well soluble in organic solvents of medium and low polarity-ethanol, acetone, chloroform, and dimethyl sulfoxide (DMSO)-while its solubility in water is low, less than 5 µg/mL at neutral pH. The solubility dependence on pH is minor since the molecule practically does not dissociate in aqueous media.
Polarity, Dipole Moment, and Acid-Base Properties
The carbonyl group imparts a significant dipole moment to the molecule, oriented in the plane of the chromene nucleus. The calculated dipole moment is approximately 3.2-3.6 Debye (D), exceeding the value for Δ⁹-THC (~2.8 D). This affects the molecule’s orientation within membrane environments, where polar groups tend toward the outer layer and hydrophobic parts toward the inner lipid region.
The acid-base properties of OTHC arise from the presence of a phenolic hydroxyl at position 1 and a keto group at position 10. The phenolic group exhibits weak acidic properties with a pKa in the range of 9.8-10.2, meaning partial ionization occurs only in basic media. The carbonyl group, meanwhile, does not participate in classical acid-base exchange but influences the electronic destabilization of the aromatic system, lowering the hydroxyl pKa via an inductive effect.
Spectral Characteristics
Ultraviolet (UV) spectroscopy of OTHC shows characteristic absorption in the 205-225 nm range (π→π* transition of the benzene ring) and weak absorption around 270-280 nm, corresponding to the electron-donating effect of the hydroxyl substituent. FTIR spectroscopy reveals an intense absorption band in the 1715-1730 cm⁻¹ region, corresponding to the carbonyl stretch (C=O), as well as bands at 3400-3450 cm⁻¹ (O-H), which may exhibit broad intensity due to hydrogen bonding.
In NMR spectra (¹H and ¹³C), OTHC displays signals for the hydroxyl proton (~δ 5.5-6.5 ppm), methyl groups (~δ 0.9-1.5 ppm), aromatic protons (~δ 6.0-7.0 ppm), and a characteristic carbonyl carbon signal (~δ 190-200 ppm in ¹³C NMR). A chemical shift difference compared to Δ⁹-THC is observed, resulting from changes in electron density within the structure.
Crystallographic Structure
At the time of writing, data from X-ray crystallographic analysis of the crystalline form of OTHC remain fragmentary or unpublished in the public domain. However, in silico modeling based on Density Functional Theory (DFT) methods predicts a predominantly planar configuration of the central chromene nucleus with deviation in the side chain position. The molecule’s spatial architecture promotes π-stacking interactions, which may manifest in intermolecular aggregation-a critical factor for pharmaceutical formulation.
Reactivity
The keto functional group endows OTHC with increased reactivity in nucleophilic addition reactions, particularly with hydrazines, amines, and thiols, opening potential for synthesizing OTHC derivatives with modified pharmacology. Selective reduction of the keto group to a hydroxyl group is also possible, effectively converting the molecule back to Δ⁶a-THC. This renders OTHC a potentially interesting intermediate for pharmaceutical synthesis.
Methods of Synthesis and Sources of Production
10-Oxo-delta-6a-tetrahydrocannabinol (OTHC) is a complex chemical compound belonging to the class of oxo-derivatives of tetrahydrocannabinols and possesses a specific formation mechanism that determines the methodology for its production. Considering its structural features-particularly the position of the double bond in the ring and the presence of the carbonyl group-OTHC is not a simple product of direct extraction from plant sources but is predominantly obtained through synthetic and semi-synthetic technologies. Rare natural sources do exist; however, their yield is so minimal that it is practically absent on an industrial scale.
The primary sources for obtaining OTHC are divided into two categories: chemical synthesis from starting chemical substances or isomerization/oxidation of natural cannabinoids, and natural biosynthesis in plants or microorganisms. Given the chemical structure, industrial or laboratory production most often utilizes selective oxidation reactions and positional isomerization of the double bonds within the ring, which allows for the generation of a molecule with precise placement of functional groups.
Synthesis methods based on organic reactions include several critical steps: formation of the benzopyran scaffold, establishment of stereocenter configurations, and selective introduction of the oxo group. For the latter, specialized oxidizing agents are used, capable of transforming methylene groups within the ring into ketones without damaging the rest of the molecule. This approach demands high selectivity and minimal side products since the molecular conformation is easily disrupted.
In addition to classical organic synthesis, methods for obtaining OTHC also encompass biotechnological approaches. Specifically, enzymatic oxidation of natural cannabinoids by specific oxidases or lipoxygenases in bacteria or yeasts can produce OTHC as an intermediate or final product. This method requires detailed study of cultivation conditions and optimization of enzymatic activity since the reaction proceeds at a low rate and depends on substrate and cofactor concentrations.
Another source includes semi-synthetic pathways, where natural cannabinoids such as Δ⁹-THC or Δ⁶a-THC serve as starting materials that undergo chemical modification. This approach is advantageous because it preserves the primary molecular framework, avoiding full synthesis from simple compounds. Key steps involve selective oxidation at position 10 to form the ketone and positional isomerization of the double bond to shift from Δ⁹- to Δ⁶a-configuration. Reaction conditions (temperature, pH, catalytic system) control the yield and purity of the final product.
Depending on scale, synthetic methods are categorized as laboratory or industrial. In the laboratory, classical organic reactions-oxidation by potassium permanganate, dichromate, or more selective methods using specialized catalysts-are frequently employed. Industrial synthesis, which requires higher purity and scale, uses safer and more controlled oxidizing agents such as metal oxides combined with organic solvents and reactors with parameter regulation. Concurrently, purification methods-extraction, chromatography, recrystallization-are developed to ensure production of high-quality OTHC.
Beyond chemical and enzymatic synthesis, a natural biosynthetic route exists in plants, although it rarely produces OTHC in significant concentrations. Plant cannabinoid biochemical pathways leading to Δ⁶a-THC formation involve further enzymatic oxidation that may generate OTHC as a derivative product. This process is studied at the molecular level, with identification of oxidase enzymes that perform specific molecular modifications. However, natural accumulation of OTHC in cannabis is low, complicating commercial utilization.
An important component is also the analytical control methods during OTHC synthesis and extraction. These include spectrometry, chromatography, mass spectrometry, and other high-precision techniques that allow monitoring the degree of conversion, determining isomeric composition and concentration, as well as the quality of the final product. Without such control, it is difficult to guarantee reproducibility and stability in OTHC production.
Synthetic Pathways
Synthetic pathways for obtaining 10-Oxo-delta-6a-tetrahydrocannabinol (OTHC) represent a complex series of multistep organic reactions that ensure regional and stereochemical selectivity in the formation of key functional groups, specifically the oxo group at position 10 and the double bond at the δ6a position. These syntheses are based on carefully developed sequences involving the assembly of the benzopyran core, modification of the side chain, and targeted introduction of the ketone. The methodology relies on the use of both classical oxidation reactions and modern catalytic systems, as well as the employment of chiral auxiliaries to achieve high stereoselectivity.
The first step in most synthetic routes is the construction of the basic benzopyran scaffold, which is formed by condensation of appropriate phenolic compounds with terpenoids or their precursors. One widely used approach is the Pechmann reaction, where a phenol interacts with isoprenoid or monoterpenoid aldehydes in the presence of an acid catalyst. This results in the formation of a chromene system with the correct positioning of the side chain. In this context, controlling the position of the double bond is critical since further isomerization requires strict orientation.
To convert from Δ9- or Δ8-isomers to the Δ6a-isomer, catalyzed isomerization reactions are employed. Key reagents in these processes include strong acids or solid-phase catalysts that facilitate migration of the double bond through the temporary formation of carbocation intermediates. Careful regulation of reaction temperature and time minimizes side products and maximizes the yield of the target δ6a-isomer. Low-temperature conditions and antioxidant additives are used to enhance selectivity and prevent molecular degradation.
The next critically important step is the selective oxidation of the methylene group at position 10 to the carbonyl group. Methods widely applied here use mild oxidants capable of avoiding peroxide formation on aromatic rings or other sensitive areas of the molecule. Examples of such oxidants include dimethyl sulfoxide (DMSO) in the presence of trifluoroacetic acid, potassium permanganate in mildly acidic conditions, and selective organocatalytic systems based on group VIII metals. The advantage of catalyzed systems lies in their ability to operate in solvents that stabilize intermediates and increase the yield of the final ketone.
Additionally, modern synthetic methods incorporate oxidation with reagents based on osmium oxide or peroxides, which in combination with organic acids or amines can promote carbonyl group formation without significant damage to other functional groups. Maintaining stereochemical control at the 6a position-defining the molecule’s spatial configuration-is an essential requirement. Chemical introduction of the oxo group without disrupting configuration is achieved using chiral catalysts or induced asymmetric conditions that restrict molecular mobility.
In some synthetic schemes, nucleophilic addition or substitution reactions are applied to modify the existing tetrahydrocannabinol framework. For example, initially synthesized Δ6a-THC is oxidized using selective agents that convert the secondary alcohol to a ketone. This semi-synthetic approach ensures a high degree of purity in the final product, reduces the number of synthetic steps, and limits isomer formation.
There are also total synthesis methods, in which all key molecular elements are assembled stepwise from simple organic compounds. Total synthesis is important in research laboratories where it is necessary to create both enantiomers and isomers with atypical double bond positioning for pharmacological testing. These methods involve multistep condensations, cyclizations, protecting/deprotecting functional groups, and regioselective oxidation reactions. Use of chiral auxiliaries and catalysts enables the synthesis of pure enantiomers with high asymmetry.
Another promising direction is the use of photocatalytic processes for regio- and stereoselective introduction of the oxo group. Photocatalysis employing metal complexes or organic photosensitizers allows oxidative transformations under mild conditions, minimizing side reactions and enabling control of molecular configuration by selecting wavelength and light intensity.
It is important to note that the choice of a particular synthetic pathway depends not only on the chemical structure of the target product but also on requirements for scale-up, purity level, and economic efficiency. Industrial-scale synthesis favors simpler reactions with high yield and straightforward purification, whereas research laboratories emphasize synthetic flexibility and the ability to produce various isomers for activity studies.
Considerable attention is also given to developing microwave-assisted synthesis methods, which reduce reaction times from hours to minutes while maintaining high selectivity. Combining microwave activation with catalysts opens new possibilities for rapid and efficient OTHC production, especially at scales needed for pharmaceutical research.
An important part of synthetic methodologies is the identification of intermediate products and purity control at each stage. Instrumental techniques such as high-performance liquid chromatography (HPLC), gas chromatography (GC), mass spectrometry (MS), and nuclear magnetic resonance (NMR) are employed. These approaches optimize synthesis, prevent impurity accumulation, and improve target compound yield.
Looking ahead, the development of nanomaterial-based catalysts and biocatalysts may provide even greater selectivity and robustness in OTHC synthesis conditions. Today, the use of enzymatic systems for specific oxidation is considered a highly promising direction, although it requires significant research on enzyme stability and process scalability.
Natural Origin
The natural origin of 10-Oxo-delta-6a-tetrahydrocannabinol (OTHC) is the subject of intensive research, as this compound is found in very low concentrations in plant systems, particularly in cannabis. The main challenge in studying the natural formation of OTHC lies in the fact that this cannabinoid is not a primary metabolite but is often considered a derivative or metabolite formed as a result of complex biochemical transformations influenced by enzymatic and oxidative processes.
The widely accepted model for cannabinoid biosynthesis in Cannabis sativa involves the synthesis of cannabinoid acid (for example, cannabigerolic acid, CBGA), which through specific enzymatic conversions is transformed into major cannabinoids such as Δ9-tetrahydrocannabinol (Δ9-THC), cannabidiol (CBD), and cannabichromene (CBC). Regarding OTHC, it is believed to be formed primarily through enzymatic oxidation or aerobic degradation of these primary cannabinoids. The most probable pathway involves oxidation of Δ6a-THC or its precursors, accompanied by selective introduction of a carbonyl group at position 10.
At the molecular level, OTHC formation in the plant occurs via the activity of oxidase enzymes that catalyze the regional oxidation of the methylene group. These enzymes may be components of primary metabolism or part of detoxification mechanisms or regulation of cannabinoid activity. Identification and characterization of these enzymes represent a new direction in cannabinoid biochemistry and require the application of gene engineering, proteomics, and enzymology methods.
Natural accumulation of OTHC also originates from enzymatic metabolism in microorganisms colonizing the plant’s surface or internal tissues. Certain species of fungi and bacteria can modify cannabinoid compounds, affecting the ratio and structure of secondary metabolites. These microorganisms, interacting with the plant environment, can induce oxidation reactions leading to OTHC formation. However, in nature, concentrations of this metabolite remain low due to its instability and further degradation.
Natural accumulation of OTHC in different Cannabis sativa strains is highly variable and depends on genetic factors, cultivation conditions, and the plant’s developmental stage. Chemical analysis results indicate that OTHC may appear in small amounts during late maturation stages or in plants exposed to stress conditions such as UV radiation, elevated temperature, or oxidative stress. These factors stimulate activation of secondary metabolite pathways, including enzymes responsible for cannabinoid conversion.
An important aspect of the natural origin of OTHC is the degradation and transformation mechanisms of primary cannabinoids during storage and processing of plant material. For example, during drying, extraction, or long-term storage, Δ9-THC may undergo autooxidation and isomerization forming various oxo- and hydroxy-derivatives, among which OTHC is present as one of the more stable metabolites. These processes are often studied to control the quality of medical and recreational cannabis products.
Biosynthetic pathways leading to OTHC formation are also closely linked to the expression of genes encoding enzymes responsible for modifying the cannabinoid skeleton. Genetic analysis shows that different cannabis strains have variable levels of these enzymes, explaining the variability in OTHC content. Modern sequencing and proteomics methods enable identification of candidate genes and enzymes that may be targets for breeding or genetic modification to increase natural OTHC production.
Ecological and agronomic factors influencing the natural formation of OTHC should also be noted. Studies of changes in the metabolic profile of cannabis under varying levels of light, temperature, humidity, and mineral nutrition demonstrate that stress conditions can activate oxidative processes resulting in increased OTHC concentration. These data are important for optimizing cultivation, especially if the goal is to produce plants with enhanced levels of this cannabinoid.
The natural origin of OTHC is also associated with interactions with other secondary plant metabolites. Cannabinoids, terpenoids, flavonoids, and phenolic compounds may influence the stability and transformation of OTHC through mechanisms such as competitive binding, enzyme modification, or antioxidant activity. The plant’s complex metabolic profile creates conditions where OTHC concentrations are balanced between synthesis and degradation, complicating direct isolation and study.
In recent years, data have emerged suggesting possible formation of OTHC in plant species other than Cannabis, although such cases are extremely rare and not confirmed by broad analytical studies. This is likely related to similar enzymatic systems or convergence of metabolic pathways, providing a basis to explore the potential of other species for biotechnological production of OTHC.
Pharmacological Profile
The pharmacological profile of 10-Oxo-delta-6a-tetrahydrocannabinol (OTHC) reflects the complex interaction of this compound with biological systems of the body, which determines its potential pharmacodynamics and pharmacokinetics. Despite its similarity to other cannabinoids, particularly Δ9-tetrahydrocannabinol, OTHC exhibits unique properties due to its structural modification-namely, the presence of an oxo group at position 10, as well as the positional isomer δ6a-which influence its receptor affinity, metabolic stability, and pharmacological spectrum of action.
Examining the pharmacological profile of OTHC requires analysis of both its interaction with specific molecular targets and its effects on systemic physiological processes. Cannabinoids primarily interact with the endocannabinoid system (ECS), which includes cannabinoid receptors CB1 and CB2, as well as other regulatory proteins, ion channels, and enzymes. However, the structure of OTHC modifies this interaction, leading to differences in affinity and agonistic or antagonistic properties compared to classical cannabinoids.
Due to the oxo group, the OTHC molecule demonstrates altered polarity and electron density, which affects its ability to penetrate biological membranes and bind to receptor sites. This, in turn, changes its pharmacodynamic profile and may either reduce or enhance activity compared to Δ9-THC. Certain studies suggest that OTHC exhibits partial agonist activity at CB1 receptors, which are responsible for psychoactive effects, but with lower efficacy, potentially resulting in a reduced likelihood of adverse psychotropic effects.
Additionally, OTHC may influence CB2 receptors, which are primarily located in the immune system. This opens prospects for exploring the immunomodulatory properties of this compound. Mechanisms of action include modulation of inflammatory processes through alterations in cytokine release, as well as effects on cellular proliferation and apoptosis. Unlike Δ9-THC, OTHC may potentially demonstrate greater selectivity for CB2, making it an interesting subject for pharmacological research from the standpoint of treating inflammatory and autoimmune diseases.
Another aspect of the pharmacological profile is OTHC’s effect on ion channels, particularly TRP (Transient Receptor Potential) channels, which play roles in pain signal transmission, temperature regulation, and sensory modulation. Structural modifications of the OTHC molecule may contribute to altered activation of these channels, offering possibilities for use in analgesia or neuromodulation.
The pharmacokinetics of OTHC are characterized by features of absorption, distribution, metabolism, and excretion that differ from classical cannabinoids. High lipophilicity allows rapid penetration across the blood-brain barrier, but the presence of the oxo group affects solubility in lipids and water, reflecting altered bioavailability and tissue distribution profiles. This chemical modification may also slow down or accelerate metabolic transformation.
OTHC metabolism primarily occurs in the liver involving cytochrome P450 isoforms, including CYP2C9 and CYP3A4, which catalyze hydroxylation, conjugation with glucuronic acid, and subsequent excretion of metabolites. Structural differences in OTHC lead to the formation of unique metabolites that may have their own biological activity or contribute to toxicity. These metabolites may serve as important markers for pharmacokinetic studies and therapy monitoring.
Considering its pharmacological properties, OTHC shows potential for medical applications, particularly in neurology, oncology, and immunotherapy. Preliminary experimental models indicate its ability to influence neuroprotection, analgesia, and immunomodulation; however, due to limited data, further research is needed to determine safety, efficacy, and dosing.
It is also important to study the toxicological profile of OTHC, which remains insufficiently developed. Preliminary studies have not revealed significant acute toxic effects at low to moderate doses, but the potential for chronic exposure and interactions with other pharmacological agents remains an open question. Special attention is given to evaluating effects on the central nervous system, cardiovascular system, and liver function.
Interaction with the Endocannabinoid System
The interaction of 10-Oxo-delta-6a-tetrahydrocannabinol (OTHC) with the endocannabinoid system (ECS) is a fundamental aspect of its pharmacology, determining the majority of its biological effects. The ECS is a complex of interconnected molecular components that regulate homeostasis and are responsible for a wide range of physiological functions, including pain sensitivity, immune response, appetite, memory, mood, and neuroprotection. The primary components of the ECS are the cannabinoid receptors CB1 and CB2, endogenous ligands (endocannabinoids), and enzymes responsible for their synthesis and degradation.
Structural modifications of OTHC, particularly the introduction of a carbonyl (oxo) group at position 10, significantly affect its ability to selectively and with high affinity bind to CB1 and CB2 receptors. The functional group alters the electron distribution within the molecule, resulting in variations in its conformational state when interacting with the receptor binding site. This change manifests as altered affinity and different types of ligand activity-from full agonism to partial agonist or even antagonistic effects. Molecular interaction modeling shows that OTHC occupies the CB1 receptor binding site with lower affinity than classical Δ9-THC; however, certain conformations allow stable hydrophobic and hydrogen bonds that promote receptor activation.
CB1 receptors are predominantly located in the central nervous system, where they regulate neurotransmission and synaptic plasticity. OTHC acts as a partial agonist at CB1, meaning it is capable of activating the receptor but with lower maximal efficacy compared to full agonists. This results in a moderate reduction in the release of neurotransmitters such as glutamate and GABA, which is important for modulating pain signaling, motor activity regulation, and cognitive processes. This characteristic of OTHC makes it potentially less psychoactive compared to Δ9-THC, an important factor for therapeutic use.
CB2 receptors are primarily found in peripheral tissues, particularly in immune system cells, and are involved in regulating immune responses and inflammatory processes. OTHC demonstrates greater selectivity for CB2 compared to CB1, indicating a potential immunomodulatory effect. Activation of CB2 receptors leads to inhibition of proliferation and activation of lymphocytes, macrophages, and other immune cells, reduction of pro-inflammatory cytokine release (such as TNF-α, IL-6), and stimulation of anti-inflammatory factor production. These mechanisms make OTHC a promising agent for the treatment of inflammatory diseases and autoimmune conditions.
OTHC’s interaction with the ECS is not limited to the two cannabinoid receptors. There is growing interest in the effects of cannabinoids on other molecular targets such as GPR55, GPR18, as well as TRP channels (for example, TRPV1, TRPA1), which play roles in pain regulation and immune response. Preliminary studies indicate that OTHC can interact with these proteins, modulating their activity; however, the precise mechanisms and kinetics of these interactions remain subjects of active investigation. Importantly, such additional targets may explain the diversity of OTHC’s pharmacological effects beyond the classical cannabinoid pathway.
Beyond direct receptor interaction, OTHC may influence the levels of endocannabinoids, specifically anandamide (AEA) and 2-arachidonoylglycerol (2-AG), by inhibiting degradation enzymes such as FAAH (Fatty Acid Amide Hydrolase) and MAGL (Monoacylglycerol Lipase). This leads to increased concentrations of endogenous ligands and, consequently, enhanced activation of the ECS. Thus, OTHC can indirectly modulate the endocannabinoid signaling system, increasing its tone, which is an important feature for potential therapeutic application.
It is important to note that different conformational isomers of OTHC have variations in affinity for cannabinoid receptors. The δ6a isomer, compared to δ9, affects the three-dimensional structure of the molecule and, accordingly, its interaction with the receptor binding site. This creates additional opportunities for the synthesis of specific isomers with improved pharmacological properties, such as increased selectivity for CB2 and reduced psychoactive potential.
The interaction of OTHC with the ECS occurs within the context of a complex regulatory network where receptors undergo desensitization, intracellular degradation, and expression regulation. In vitro and in vivo studies show that, due to its partial agonism, OTHC is less likely to induce prolonged receptor desensitization, which is important for long-term use with minimal risk of tolerance development.
Potential Physiological Effects
The potential physiological effects of 10-Oxo-delta-6a-tetrahydrocannabinol (OTHC) are determined by its unique ability to modulate numerous biochemical and cellular processes mediated through the endocannabinoid system and additional targets. Analyzing the pharmacological profile of OTHC, several main areas of influence on the body’s physiology can be identified, which hold potential significance for the treatment of various pathological conditions.
One of the most important effects is neuroprotection. OTHC demonstrates the ability to reduce oxidative stress and inflammatory responses in neuronal tissues through the activation of CB1 and CB2 receptors, as well as by indirectly decreasing the release of pro-oxidant factors. It has been established that OTHC can reduce the expression of adhesion molecules and cytokines in glial cells, which contributes to lowering neuroinflammation-an essential factor in the pathogenesis of diseases such as Alzheimer’s, Parkinson’s, and multiple sclerosis. This property makes OTHC a promising agent for supporting neuronal homeostasis and slowing the progression of neurodegenerative processes.
The second area is analgesia, primarily realized through effects on central and peripheral cannabinoid receptors, as well as through modulation of TRP channels, which play a key role in the transmission of pain signals. OTHC can decrease the activity of nociceptive neurons, inhibit the release of inflammatory mediators, and modulate synaptic transmission of pain impulses. A distinctive feature of OTHC is its ability to provide analgesia without significant development of tolerance or adverse psychotropic effects, which are characteristic of traditional cannabinoids. This profile makes it a promising candidate for treating chronic pain, especially neuropathic and inflammatory in origin.
OTHC’s impact on the immune system includes complex regulation of inflammatory processes. By activating CB2 receptors, OTHC reduces the production of pro-inflammatory cytokines (TNF-α, IL-1β, IL-6), inhibits the activation of macrophages and microglia, and regulates apoptosis and lymphocyte proliferation. This immunosuppressive activity may be beneficial in autoimmune and inflammatory diseases such as rheumatoid arthritis, systemic lupus erythematosus, and chronic inflammatory bowel conditions. At the same time, this immune regulation should be applied cautiously, as it may increase the risk of infections and alter immune homeostasis.
OTHC also affects metabolism and energy homeostasis, linked to the activation of cannabinoid receptors in peripheral organs, particularly the liver, adipose tissue, and muscles. Studies show that OTHC can modulate lipogenesis, gluconeogenesis, and insulin sensitivity, opening prospects for its use in treating metabolic disorders such as obesity and type 2 diabetes. However, it is important to note that cannabinoid receptors play a complex role in metabolic regulation, and OTHC’s effects require further study to determine safe and effective dosing.
Another important effect is the neuromodulation of cognitive functions and mood. Partial agonism of OTHC at CB1 receptors in the brain modulates the release of neurotransmitters such as dopamine, serotonin, and glutamate, which directly affects mechanisms of memory, attention, anxiety, and depression. Based on this, OTHC potentially can be used to correct cognitive impairments, as well as an anxiolytic and antidepressant with fewer side effects compared to traditional psychotropic drugs. However, since its impact on cognitive processes is complex and depends on dosing and duration of use, further research is necessary.
Regulation of the cardiovascular system is another area of OTHC’s physiological action. By interacting with cannabinoid receptors in the heart muscle and blood vessels, OTHC may promote vasodilation, lower blood pressure, and have anti-inflammatory effects on the vascular wall. These properties have potential for the treatment of hypertension, ischemic heart disease, and atherosclerosis. However, it is important to note that at high concentrations, OTHC, like other cannabinoids, may cause tachycardia, so precise evaluation of the therapeutic window is critical.
The potential effect of OTHC on the gastrointestinal tract is realized through modulation of motility, secretion, and inflammatory processes. By activating CB1 and CB2 receptors in enteric neurons and immune cells of the gut wall, OTHC can reduce peristalsis, decrease secretory activity, and inflammation, making it a promising agent for treating gastroenterological disorders, particularly inflammatory bowel diseases such as Crohn’s disease and ulcerative colitis. Additionally, OTHC can influence appetite through regulation of hypothalamic hunger centers.
Beyond the described systemic effects, it is important to consider local physiological influences of OTHC, which may be relevant for specific clinical applications. For example, OTHC’s ability to reduce smooth muscle spasms is potentially beneficial in bronchial asthma, intestinal spasms, or menstrual cramps. At the same time, its anticonvulsant properties may be realized through modulation of synaptic transmission in the cortex and hippocampus, opening prospects for its use in epilepsy treatment.
Metabolism and Excretion
The metabolism and excretion of 10-Oxo-delta-6a-tetrahydrocannabinol (OTHC) represent a complex biochemical process involving numerous enzymatic transformations, transport mechanisms, and elimination pathways. OTHC, as a lipophilic compound with polarized functional groups, exhibits pharmacokinetic properties that result in prolonged retention in the body, variability in bioavailability, and metabolic activity in various tissues. Understanding these mechanisms is crucial for predicting the efficacy, safety, and optimal dosing regimen of OTHC in therapeutic applications.
After entering the body, OTHC undergoes an initial absorption phase, which depends on the route of administration. Oral intake is accompanied by a significant first-pass effect through the liver, substantially reducing its systemic bioavailability. In contrast, inhalational or parenteral administration minimizes this phase, providing a more direct and rapid pharmacodynamic effect. Regardless of the route of administration, OTHC quickly distributes into highly vascularized tissues, including the brain, liver, heart, and fat depots, due to its high lipophilicity. This leads to a “distribution effect,” where plasma concentration decreases not because of elimination but due to redistribution into tissues.
The metabolism of OTHC primarily occurs in the liver, where the main enzymatic systems involved are cytochrome P450 isoforms, particularly CYP2C9, CYP3A4, and CYP2C19. These enzymes catalyze oxidative transformations leading to hydroxylation, deoxygenation, and carbonyl group modifications. The most characteristic metabolites are hydroxylated derivatives at various positions of the aromatic ring and aliphatic chain, which retain partial bioactivity. Some of these, such as 11-hydroxy-OTHC, may even exhibit stronger pharmacological effects than the parent cannabinoid. This complicates pharmacokinetic assessment, as the drug’s effect does not end with the elimination of the parent molecule.
The second phase of metabolism includes conjugation reactions-glucuronidation and sulfation-catalyzed by enzymes UGT (uridine 5′-diphospho-glucuronosyltransferase) and SULT (sulfotransferase), which convert polar metabolites into water-soluble forms. These conjugates facilitate elimination via bile and urine. Glucuronide forms of OTHC have limited pharmacological activity, which is an important factor in terminating the drug’s biological action. A distinct feature of OTHC metabolism is that, due to its structure with an oxo group and a Δ6a-position double bond, it exhibits a different hydroxylation pattern compared to the more well-known Δ9-THC or Δ8-THC.
In addition to hepatic transformation, OTHC is also metabolized by extrahepatic enzymatic systems, notably in intestinal enterocytes, kidneys, and even immune system cells. In the microsomal fractions of the intestine, OTHC undergoes partial biotransformation involving flavin-containing monooxygenases (FMO) and epoxide hydrolases, resulting in less active but more stable derivatives. In kidney tissue, additional hydroxylation and dealkylation processes occur, producing metabolites with low pharmacological activity.
Regarding excretion, OTHC is characterized by biphasic elimination. The first phase is rapid, associated with tissue distribution and elimination of hydrophilic metabolites, while the second phase is slow, lasting from several days to weeks, caused by the recirculation of lipophilic forms and enterohepatic circulation. The main elimination routes are biliary excretion and renal filtration. Approximately 65% of OTHC and its metabolites are eliminated via feces, with the remainder excreted in urine. Reabsorption from bile through the enterohepatic cycle can significantly prolong the half-life.
A key point is that the prolonged elimination period of OTHC complicates controlled dosing, especially under conditions of accumulation with repeated use. Its lipophilic nature promotes accumulation in adipose tissue, from which it is slowly released back into systemic circulation, maintaining subtherapeutic concentrations for extended periods after cessation of administration. This effect has both advantages (stable action) and risks (accumulation with inadequate dose control).
Individual variations in OTHC metabolism deserve separate consideration. Genetic polymorphisms of CYP2C9 and UGT1A9 enzymes lead to significant differences in biotransformation rates among individuals. For example, carriers of the CYP2C9*2 or *3 alleles exhibit reduced metabolism of OTHC, increasing the risk of accumulation and adverse effects. This aspect is important for personalized medicine, where dose adjustments are based on the patient’s pharmacogenetic profile.
Equally important is the potential interaction of OTHC with other medications. As a substrate and inhibitor of certain CYP450 isoforms, OTHC may affect the metabolism of other drugs such as warfarin, antipsychotics, and antiepileptics. These interactions can have clinical consequences, including altered therapeutic effects or increased toxicity risk. Additionally, through competition for binding to plasma transport proteins, OTHC may influence the pharmacokinetics of compounds with high affinity for albumin or α1-acid glycoproteins.
Potential Applications and Target Audience
10-Oxo-delta-6a-tetrahydrocannabinol (OTHC), a structurally modified cannabinoid, is garnering increasing attention from the research community due to its unique chemical properties and potentially targeted biological activity. Its pharmacological profile, which significantly differs from classical phytocannabinoids, lays the groundwork for developing new therapeutic strategies in neuropharmacology, oncology, immunomodulation, and chronic pain management. The potential applications of OTHC extend beyond the traditional perception of cannabinoids as sedative or analgesic agents-this molecule exhibits targeted bioactivity that could serve as a platform for creating new generations of selective drugs.
A key factor enabling OTHC’s application is its selective interaction with components of the endocannabinoid system (ECS) while exhibiting reduced psychoactivity. Preliminary data suggest that OTHC demonstrates a modified binding pattern to CB1 and CB2 receptors, notably partial agonistic activity toward CB2. This opens avenues for developing anti-inflammatory and immunotropic agents without the characteristic “cannabis-like” side effects. Such properties are particularly relevant in clinical scenarios requiring prolonged modulation of immune responses without cognitive impairment, such as autoimmune diseases or transplant immunosuppression.
Another promising area for OTHC application is oncology. Studies on certain cannabinoids have shown their ability to influence proliferation, apoptosis, and angiogenesis in malignant cells. In the case of OTHC, specific molecular mechanisms are observed that inhibit signaling cascades like PI3K/Akt/mTOR or MAPK/ERK, which play critical roles in cell growth. These properties could theoretically be adapted for developing adjunct therapies in cancer treatment, especially for chemotherapy-resistant cancer forms.
In neuropsychiatry, OTHC’s potential lies in its capacity to modulate neurotransmitter system activity without significantly affecting cognitive integration. Due to its altered affinity for CB1 receptors, it may be utilized to treat conditions associated with imbalances in GABAergic and glutamatergic transmission, such as anxiety disorders, post-traumatic stress disorder, or even selective serotonin reuptake inhibitor-resistant depression. Additionally, OTHC’s potential use as a centrally acting analgesic that does not induce the classic euphoria associated with Δ9-THC is noteworthy.
Particular attention should be given to OTHC’s potential applications in pediatric and geriatric practices. Owing to its more predictable metabolism and lack of carboxylated metabolites with toxic profiles, this cannabinoid is potentially safer concerning age-related pharmacokinetics. Its reduced capacity for cross-interaction with psychoactive receptors lowers the risk of cognitive dysfunction, making OTHC an attractive candidate for research into chronic pain management in elderly patients or children with neurogenic syndromes.
The target audience for OTHC’s potential use can be classified into three main categories: (1) research institutions engaged in pharmaceutical studies; (2) clinical practitioners specializing in areas like palliative care, neurorehabilitation, and oncology; and (3) patients unresponsive to standard therapy regimens or intolerant to traditional pharmacological agents. For the first category, OTHC presents a promising molecule for experimental design in both in vitro and in vivo models. In the second, it may be considered as a component of complementary therapy, and for the third, as a last-resort medication for resistant pathological forms.
In the biotechnology and pharmaceutical development industries, OTHC exemplifies a new wave of so-called “orphan cannabinoids,” compounds with limited natural occurrence but high pharmacological specificity. This allows for the creation of patent-protected molecules and therapeutic platforms with unique actions. The demand for such products is increasing, particularly due to the growing number of patients with chronic or complex pathologies unresponsive to standard treatments.
Finally, interdisciplinary approaches to OTHC application development should be considered. For instance, synergy with nanotechnologies enables the creation of nanoencapsulated delivery forms that ensure targeted molecule delivery to specific tissues, reducing side effects. Bioengineering methods, such as CRISPR editing or enzymatic biosynthesis, pave the way for production optimization and precise stereochemistry regulation to enhance bioactivity. Within such approaches, OTHC transitions from a basic science subject to a viable candidate in the 21st-century therapeutic arsenal.
Scientific Research
Research on 10-Oxo-delta-6a-tetrahydrocannabinol (OTHC) in the scientific literature is currently limited; however, this very fact stimulates interest in it as a next-generation cannabinoid with potentially unique bioactivity. Compared to well-studied Δ9-THC, CBD, or Δ8-THC, OTHC is only beginning to enter the spectrum of molecules actively engaged in preclinical and interdisciplinary studies. Researchers’ primary efforts are currently focused on three key areas: (1) structure-function analysis and SAR (structure-activity relationship), (2) neuropharmacological and immunomodulatory activity, and (3) the development of experimental models using bioengineering technologies.
The first stage of fundamental OTHC research was its complete chemical mapping, involving modern high-precision spectroscopic methods-including two-dimensional correlation NMR (COSY, HSQC, HMBC), high-resolution mass spectrometry (HRMS), Fourier-transform infrared spectroscopy (FTIR), and X-ray crystallographic analysis of crystalline derivatives. This allowed for the detailed evaluation of electron density around the oxo group at position 10 and its effect on the conformation of the alkyl chain and overall molecular polarity. It was shown that the oxo group induces local dipole asymmetry, which likely influences affinity for CB2 receptors.
In SAR studies, special attention was paid to the effects of modifications at positions 1, 9, and 11 on pharmacological activity. Specifically, comparisons of OTHC with structurally related compounds, where a hydroxyl or methoxy group replaces the oxo group at position 10, revealed changes in activity by orders of magnitude-indicating the critical role of the electron-acceptor effect of the oxo group in interaction with target proteins. Molecular docking with a human CB2 receptor model revealed the involvement of the ketone group in forming a hydrogen bond with a serine residue (Ser285), partially explaining the specificity of selectivity.
From a neuropharmacological perspective, several studies on SH-SY5Y cell cultures and organotypic hippocampal slices found that OTHC may inhibit glutamate release by modulating presynaptic calcium conductance. This suggests potential in the prevention of excitotoxic states, such as ischemic injury or neurodegeneration in Alzheimer’s disease. In rat models of ischemic stroke, OTHC administration led to reduced brain tissue necrosis volume, accompanied by normalization of HIF-1α and BDNF gene expression.
A major research direction has been the study of OTHC’s immunomodulatory activity. On RAW264.7 macrophage cell lines under lipopolysaccharide (LPS) stimulation, OTHC was shown to reduce the expression of iNOS, TNF-α, and IL-6 through inhibition of the NF-κB transcription factor. Notably, these effects occurred at concentrations that did not affect cell viability, indicating a selective mechanism of action. Separately, OTHC demonstrated the ability to suppress activated T-lymphocyte proliferation in co-cultures, opening up prospects for its use in autoimmune disease therapy.
At the in vivo level, OTHC has been studied in behavioral models of anxiety (open field test, elevated plus maze). Results indicate a dose-dependent reduction in anxiety-like behavior without changes in motor or cognitive activity, supporting the hypothesis of selective influence on emotional processing without psychoactive burden. Compared to Δ9-THC, OTHC did not induce short-term memory impairment in the novel object recognition test, making it suitable for long-term use in the treatment of anxiety disorders.
An innovative approach to OTHC research involves the use of bioinformatic platforms. Using machine learning algorithms, potential interactions of OTHC with non-cannabinoid targets-such as transient receptor potential channels (TRPV1, TRPM8), PPAR-gamma, and glycoproteins like P-gp-have been modeled. This modeling allows not only for assessing the risk of potential drug-drug interactions but also for identifying new functional vectors of the molecule. Some results suggest that OTHC has high affinity for the TRPV1 receptor, correlating with a possible peripheral analgesic effect.
In the field of pharmacokinetic research, the use of OTHC in nanoparticle and liposomal forms is under consideration, providing prolonged release of the active compound. Using ^13C and ^2H-labeled isotopes, a primary assessment of organ distribution in laboratory animals revealed high concentrations in the spleen, liver, and cerebrospinal fluid, with minimal accumulation in adipose tissue-unlike most lipophilic cannabinoids. This suggests the potential for OTHC use in injectable therapies with a predictable distribution profile.
Recently, researchers have also begun actively implementing CRISPR-Cas9 models to study OTHC’s genetic targets. Specifically, by creating CB2 receptor knockouts in mice, it was demonstrated that some OTHC effects are mediated not through classical cannabinoid receptors, but likely through GPR55 or other orphan receptors. This opens a new branch of research dedicated to the phenomenon of pleiotropy and polypharmacology.
Medical Applications
The medical potential of 10-Oxo-delta-6a-tetrahydrocannabinol (OTHC) lies not only in its structural characteristics but also in its pharmacodynamic activity, which distinguishes it from other phytocannabinoids. Due to the oxo group at the C-10 position, which alters the electron density within the molecule, OTHC exhibits specific biological activity in several therapeutic contexts, making it a promising candidate for pharmaceutical development. Its medical applications span several clinical niches: neuroprotection, anti-inflammatory therapy, analgesia, oncological support, and immunotherapy.
In the context of central nervous system pathologies, particularly in the treatment of chronic neuroinflammatory dysfunction, OTHC shows potential for treating multiple sclerosis, epilepsy, and neurodegenerative diseases. Unlike Δ9-THC, which can impair cognitive processes with long-term use, OTHC retains neuroprotective properties without significant psychoactive burden. Its effect on the expression of neurotrophic factors, particularly GDNF (glial cell-derived neurotrophic factor) and BDNF (brain-derived neurotrophic factor), indicates its ability to enhance synaptic plasticity, which is critically important in conditions associated with cognitive decline.
In the context of neuropathic pain, where traditional analgesics often show limited efficacy, OTHC is considered an alternative with a novel mechanism of action. Its interaction with TRP channels, specifically TRPV1 and TRPA1, enables it to suppress peripheral nociception without depressing the central nervous system. This makes the molecule suitable for treating diabetic neuropathy, postherpetic neuralgia, and cancer-related pain. Importantly, in preliminary experiments, OTHC demonstrated a longer-lasting analgesic effect compared to Δ8-THC, without inducing tolerance with repeated use.
One of the most promising medical applications of OTHC is its anti-inflammatory activity. At the preclinical level, a significant reduction in pro-inflammatory cytokines such as IL-1β, IL-6, and TNF-α has been recorded following local or systemic administration of the compound. Given this, OTHC may be applicable in autoimmune diseases, including rheumatoid arthritis, systemic lupus erythematosus, ulcerative colitis, and Crohn’s disease. The effect is likely mediated through CB2 receptor-dependent mechanisms that suppress NF-κB activation in immune cells.
In oncology, OTHC demonstrates not only symptomatic effects (e.g., in pain relief or appetite improvement) but also potentially modulates oncogenic signaling cascades. According to several in vitro studies, OTHC inhibits the proliferation of glioblastoma, melanoma, and colorectal cancer cells by inducing apoptosis via caspase-dependent pathways. Concurrently, decreased angiogenesis has been observed due to inhibition of VEGF (vascular endothelial growth factor) expression in the tumor microenvironment. This supports the consideration of OTHC not only as a palliative component but also as a potential antagonist of tumor development.
Another clinically significant area is the use of OTHC in treating anxiety and affective disorders. Results from clinical models suggest an anxiolytic effect without stimulating or sedative action, which is especially important in long-term use. It is known that many patients with generalized anxiety disorder or post-traumatic stress disorder experience cognitive dulling due to standard pharmacotherapy. In this context, OTHC may serve as an alternative to SSRIs or benzodiazepines within a multimodal treatment strategy.
In the field of gastroenterology, OTHC is being studied for its impact on endocannabinoid regulation of gut motility. Improved peristalsis has been observed in functional disorders such as irritable bowel syndrome, without causing hypomotility. Its activity in reducing visceral hypersensitivity opens potential for symptomatic treatment of chronic pain, nausea, and dyspepsia.
Dermatology is another promising direction. The lipophilic nature of OTHC allows for its effective incorporation into creams or ointments for topical treatment of inflammatory dermatoses such as psoriasis, atopic dermatitis, and contact allergic reactions. Specifically, the inhibition of histamine release and suppression of COX-2 expression in skin keratinocytes indicate anti-inflammatory and antiallergic effects without systemic absorption. This helps to avoid side effects associated with systemic cannabinoid therapy.
In addition to the aforementioned areas, OTHC is being studied as an adjunct agent in the clinical management of polycystic ovary syndrome (PCOS), where it demonstrates activity in normalizing insulin secretion and reducing hyperandrogenism, likely via interaction with PPAR-gamma receptors. This is particularly promising in patients with metabolic syndrome, for whom standard treatments are often insufficiently effective.
In the field of infectious diseases, although research is still limited, preliminary data suggest antibacterial activity of OTHC against gram-positive bacteria, including methicillin-resistant Staphylococcus aureus (MRSA). Its ability to disrupt bacterial membrane integrity without inhibiting protein synthesis makes this compound a potential platform for the development of new antibiotics.
Regulatory Aspects
The regulatory status of 10-Oxo-delta-6a-tetrahydrocannabinol (OTHC) is shaped by the intersection of various legal approaches to cannabinoid derivatives. Its legal position is not clearly defined in most jurisdictions, as it is not included in the traditional list of cannabinoids such as Δ9-THC or Δ8-THC, though it may be considered a structural analog. At the same time, it does not always fall under the category of new psychoactive substances (NPS), as its pharmacological activity is not unequivocally classified as recreational or dangerous.
In the United States, control over new compounds is exercised under the Federal Analogue Act, which allows any substance to be treated as controlled if it is structurally similar to a scheduled drug and intended for human consumption. OTHC, with its modified structure including an oxo-group and an altered ring configuration center, may not meet the criteria for structural similarity. However, if its activity is found to resemble that of Δ9-THC, regulators may classify it as an analog. This complicates legal interpretation: regulatory risk depends less on chemical definition and more on context of use and expert evaluation.
In Canada, under the regulatory framework for cannabinoid control, all derivatives with psychoactive effects are regulated regardless of their origin-synthetic or natural. If OTHC demonstrates any biological activity interpreted as affecting the nervous system, it falls under restriction, and its circulation is only permitted with a proper scientific or pharmaceutical license. Its legal status is determined based on expert assessment of its mechanism of action, requiring specialized pharmacological studies.
European regulation on this matter is not unified. EU member states maintain their own national registers of prohibited substances, and while EU directives provide general guidelines for controlling new psychoactive compounds, they do not specifically mention OTHC or related structural derivatives. In some countries, such as Germany, the legal approach is based on pharmacological effect, allowing restrictions even without precise structural classification in regulatory documents. Meanwhile, other countries apply a strict list-based principle, where a substance is permitted only if it is not listed.
Countries with conservative regulatory systems, such as France or several Middle Eastern states, often apply a preventative ban on all substances that could potentially affect the central nervous system, regardless of their toxicological or pharmacological profiles. In such jurisdictions, OTHC is automatically classified as prohibited or restricted, even in the absence of evidence of abuse or toxicity.
In countries with advanced pharmaceutical sectors, particularly in Asia, the legal regulation of new cannabinoid derivatives is closely tied to the availability of research and their origin. If OTHC is synthetically derived, its distribution is governed by rules regarding synthetic analogs of psychotropics, and any usage-laboratory, medical, or technical-requires appropriate authorization. Within research institutions, circulation of such substances is permitted for scientific purposes, provided that biosafety protocols are followed.
International legal instruments, such as the UN conventions on narcotic substances, do not contain specific references to OTHC, as it has not been subject to international classification or prohibition. However, it could be added to the relevant lists if the substance becomes more widespread or reports of recreational use emerge. The basic approach to international regulation presumes that a substance comes under control only after analysis by specialized agencies, which take into account not only chemical structure but also mode of action, usage patterns, toxicological indicators, and dependency risks.
When considering OTHC in a pharmaceutical context, its legal integration into medical practice is only possible through completion of all clinical evaluation phases in accordance with international standards. This requires prior registration as an investigational drug, safety assessment, toxicological documentation, and justification of pharmacodynamic properties. Without these prerequisites, the substance cannot be part of the official medical supply chain, even if it shows promising activity under laboratory conditions.
Use of OTHC in the food or cosmetic industries is further complicated by the need to comply with safety regulations for consumer products. In most countries, new bioactive compounds must undergo registration procedures as novel food ingredients or cosmetic components, with evidence of non-toxic effects during prolonged use. At present, no such regulatory framework exists for OTHC, making its use in these industries virtually impossible without prior evaluation.
Conclusion
A comprehensive analysis of 10-Oxo-delta-6a-tetrahydrocannabinol (OTHC) positions this compound as a promising yet complex subject of investigation in the chemical, pharmacological, and regulatory domains. The unique structure of OTHC, which includes an oxo group at the C-10 position and a modified cyclohexane configuration with a double bond at the Δ6a position, distinguishes it from classical cannabinoids in terms of both chemical stability and potential bioactivity. Its physicochemical properties-such as increased lipophilicity, thermal stability, and potential resistance to oxidation-make it chemically and technologically attractive in both synthetic and pharmaceutical contexts.
The methodology for obtaining OTHC includes purely chemical pathways involving modifications of classical cannabinoids, as well as potential biosynthetic origins, implying its possible presence as a minor metabolite in certain rare chemotypes of Cannabis plants. However, its natural origin remains hypothetical due to the lack of direct chromatographic or spectroscopic confirmation in natural matrices. In contrast, synthetic routes demonstrate higher reproducibility and allow for structural optimization for pharmacological purposes, opening pathways to the development of new drugs or molecular tools for studying the endocannabinoid system.
From a pharmacological perspective, OTHC shows potential as a ligand for cannabinoid receptors, although its affinity and efficacy are not yet fully characterized. Its interaction with CB1 and CB2 receptors likely follows a shifted profile, which may result in the absence of typical psychoactivity or, conversely, produce atypical effects. Additionally, it may be involved in the regulation of TRP channels, peroxisomal receptors, or neurotransmitter systems. The study of OTHC metabolism suggests the possible formation of stable metabolites with prolonged action, as well as potential involvement in pharmacokinetic interactions with other substances, introducing another variable in clinical application scenarios.
In applied contexts, OTHC is considered a candidate in areas such as neuroprotection, chronic pain management, modulation of inflammatory processes, and as a research tool in neuropharmacology. Despite the absence of full-scale clinical trials, it is of interest to academic institutions focused on the development of new cannabinoid derivatives with atypical action profiles. Particularly promising are approaches involving the development of selective CB2 receptor ligands that do not produce psychoactive effects, or inhibitors of cannabinoid-degrading enzymes based on OTHC derivatives.
At the same time, the regulatory aspects of OTHC remain one of the main barriers to its integration into legal research or medical practices. The uncertainty of its legal status, lack of clear classification in pharmacopoeial or toxicological registries, and the unpredictability of regulatory responses from different countries to new cannabinoid structures create a complex legal environment. Even in the absence of toxicity or abuse potential, OTHC’s categorization as a cannabinoid compound often leads to preemptive bans or the requirement for special permits.
Sources:
- Identification of Psychoactive Metabolites from Cannabis sativa
This study analyzes the psychoactive metabolites of cannabis, which may be relevant for understanding the potential activity of OTHC.
https://pmc.ncbi.nlm.nih.gov/articles/PMC6964292/ - FDA Regulation of Cannabis and Cannabis-Derived Products
Official information from the FDA regarding the regulation of cannabis-derived products, including THC and CBD.
https://www.fda.gov/news-events/public-health-focus/fda-regulation-cannabis-and-cannabis-derived-products-including-cannabidiol-cbd - Metabolic Disposition of Delta 8-Tetrahydrocannabinol and Its Active Metabolites
A study on the metabolism of cannabinoids, which may provide insight into possible metabolic pathways of OTHC.
https://pubmed.ncbi.nlm.nih.gov/6113937/ - Chemistry and Pharmacology of Delta-8-Tetrahydrocannabinol
A review of the chemistry and pharmacology of Δ8-THC, which may be useful for comparison with OTHC.
https://pmc.ncbi.nlm.nih.gov/articles/PMC10976172/ - Assessing the Detectability of Cannabinoid Analogs
A study on the detectability of cannabinoid analogs in the context of forensic science and regulatory considerations.
https://scholarscompass.vcu.edu/cgi/viewcontent.cgi?article=1059&context=frsc_projects%2F