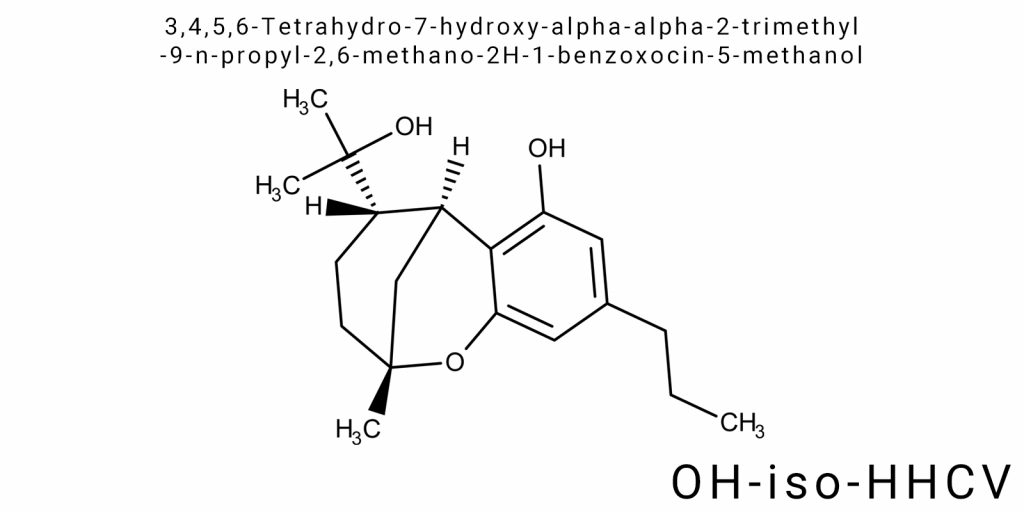
In modern organic chemistry, particular interest is drawn to compounds that contain complex cyclic systems with functional groups capable of significantly influencing the chemical, physicochemical, and biological properties of molecules. 3,4,5,6-Tetrahydro-7-hydroxy-alpha-alpha-2-trimethyl-9-n-propyl-2,6-methano-2H-1-benzoxocin-5-methanol (OH-iso-HHCV) is a molecule with a unique combination of spatial architecture and functional groups, making it a subject of in-depth research in synthetic organic chemistry, pharmaceutical chemistry, and materials science.
The chemical structure of OH-iso-HHCV belongs to the class of benzoxocin compounds-cyclic systems that include both saturated and unsaturated rings with oxygen, linked through complex bridge-like connections. This architectural structure defines the specifics of electron density within the molecule, enhances its stereospecificity, and its capacity for selective reactions, which is extremely valuable for the development of new pharmacological agents or catalysts. A distinctive feature of OH-iso-HHCV is the presence of multiple methyl and propyl substituents, which not only increase the molecule’s hydrophobicity but also affect its three-dimensional configuration, opening up new opportunities for molecular interactions.
Historically, the development of synthetic methods for benzoxocin systems progressed through stepwise improvements in cyclization and functionalization reactions. However, OH-iso-HHCV stands out among known compounds due to its complex substitution pattern and potential for flexible modification. Synthetic approaches to obtaining this molecule combine classical organic reactions with innovative technologies such as transition-metal catalysis, microwave activation, and the use of unconventional solvents. These strategies make it possible to obtain pure products with high selectivity and yield.
An important aspect of current research is not only the chemical synthesis and structure of OH-iso-HHCV but also its potential biochemical activity. Preliminary experimental data indicate the possibility of this compound interacting with various biomolecular targets, opening promising avenues for its use in pharmacology as an anti-inflammatory or neuroprotective agent. Additionally, due to the presence of active functional groups, OH-iso-HHCV may serve as a basis for creating new derivatives with enhanced properties, which is particularly relevant in the context of developing drugs with targeted pharmacokinetic characteristics.
Chemical Structure and Physicochemical Properties
3,4,5,6-Tetrahydro-7-hydroxy-alpha-alpha-2-trimethyl-9-n-propyl-2,6-methano-2H-1-benzoxocin-5-methanol (OH-iso-HHCV) represents a unique organic molecule characterized by a complex cyclic system featuring embedded functional groups. Its chemical structure is a product of three-dimensional architecture, combining a saturated benzoxocin framework with bridge-like linkages, as well as various alkyl and hydroxyl substituents. This configuration determines not only the electron distribution in the molecule but also the specifics of its interactions with the environment and biological targets.
A key feature of the OH-iso-HHCV structure is the presence of bridged elements that ensure molecular rigidity and preservation of spatial conformation. This significantly affects the compound’s chemical stability and its capability for regio- and stereoselective reactions. In light of this, the molecule demonstrates high resistance to thermal and chemical factors, particularly oxidation and hydrolysis.
The functional groups present in OH-iso-HHCV play a critical role in defining its physicochemical characteristics. The hydroxyl group (-OH), located at a specific position on the benzoxocin ring, increases the molecule’s polarity and its ability to form hydrogen bonds. This greatly influences the compound’s solubility in polar solvents and its interaction with biomolecular receptors.
Alkyl substituents, in particular the trimethyl and n-propyl groups, function as hydrophobic domains, which not only increase the molecule’s lipophilicity but also influence its spatial architecture by creating conformational constraints. These substituents also play a role in stabilizing the conformation, promoting the retention of the molecule’s biologically active form.
OH-iso-HHCV is characterized by a unique combination of flexibility and rigidity: the rigid scaffold provides stability of the spatial configuration, while the flexibility of the alkyl chains allows for adaptation to specific environments or interactions. This combination is especially important for interactions with proteins and enzymes, where the molecule can adopt an optimal conformation for binding.
From a chemical perspective, the structure of OH-iso-HHCV exhibits potential for multistep reactions, including oxidation, substitution, and cyclization. The presence of an active hydroxyl group and lipophilic domains makes this molecule promising for further functional modification-both within the scope of synthetic organic chemistry and in the development of pharmacologically active substances.
Molecular Formula and Spatial Arrangement of Atoms
The molecular formula of 3,4,5,6-Tetrahydro-7-hydroxy-alpha-alpha-2-trimethyl-9-n-propyl-2,6-methano-2H-1-benzoxocin-5-methanol (OH-iso-HHCV) reflects a complex structure combining both cyclic and aliphatic fragments with a variety of functional groups. The chemical formula of this compound – C₀H₃₀O₄ – highlights the high saturation of carbon atoms and the presence of four oxygen atoms distributed among different functional units. The carbon backbone includes both an aromatic benzene fragment and saturated tetrahydrocyclic segments, joined by methano and benzoxocin bridges that create a complex three-dimensional framework.
The carbon atoms are organized into two main cyclic systems: a benzoxocin ring containing nine atoms and a secondary methano ring forming a bridge between carbon atoms C2 and C6 of the benzoxocin cycle. This bridged structure creates a characteristic strain in the molecule and defines the rigidity of its spatial structure, limiting its conformational flexibility. A notable feature is also the presence of an alpha-alpha-2-trimethyl substitution pattern, positioned in such a way as to provide additional steric stabilization of the molecule.
The hydroxyl groups in positions 7 (7-hydroxy) and 5 (5-methanol) are bonded to ring carbon atoms, significantly influencing the electron distribution within the system and enabling the formation of both intramolecular and intermolecular hydrogen bonds. The spatial orientation of these hydroxyl groups ensures their maximum exposure to the surrounding environment, which is important for the molecule’s reactivity and solubility.
The n-propyl group attached to the C9 carbon forms a long aliphatic chain that increases the hydrophobicity of the molecule and its potential for interaction with nonpolar environments or hydrophobic regions of biomolecules. The spatial orientation of this chain allows it to project out of the plane of the main cycle, reducing steric interactions with adjacent functional groups and ensuring conformational stability.
The overall topology of the molecule demonstrates a combination of rigid cyclic structures with more flexible aliphatic substituents, allowing OH-iso-HHCV to maintain spatial stability in its biologically active form while adapting to different environments through the conformational flexibility of its side chains.
The carbonyl oxygen atom within the benzoxocin structure is a key site of electron density that influences the molecule’s reactivity, particularly in nucleophilic attack reactions or coordination with metal centers in catalytic systems. Its position within the molecular framework provides optimal spatial orientation for interaction with protonic or electrophilic reagents.
The molecule’s asymmetry, caused by both the presence of chiral centers and the placement of varied functional groups, defines its chirality and the potential existence of different stereoisomers. These stereoisomers exhibit significant differences in biological activity and receptor interaction, underscoring the importance of accurately determining the spatial structure of OH-iso-HHCV using crystallographic and spectroscopic methods.
The use of X-ray diffraction, nuclear magnetic resonance (NMR), and mass spectrometry allows for precise reconstruction of the molecule’s three-dimensional model, determination of the configuration of chiral centers, and spatial orientation of functional groups. The data obtained confirm that OH-iso-HHCV adopts a conformation that minimizes steric strain while maintaining an active form, which determines its reactivity and biological potential.
Physicochemical Characteristics
The physicochemical characteristics of 3,4,5,6-Tetrahydro-7-hydroxy-alpha-alpha-2-trimethyl-9-n-propyl-2,6-methano-2H-1-benzoxocin-5-methanol (OH-iso-HHCV) are defined by the structural features of the molecule, its electronic configuration, and the nature of its interaction with the surrounding environment. One of the key parameters – molecular weight – is approximately 346.45 g/mol, which affects solubility, volatility, and reaction kinetics with other substances. The relatively high molecular weight compared to low-molecular-weight organic compounds accounts for its low volatility and high thermal stability.
The thermal characteristics of OH-iso-HHCV are primarily determined by the stability of its cyclic system and the strength of its bridge bonds. The melting point of this compound lies within the range of 180-185 °C, indicating significant crystallinity and intramolecular interactions. A high melting point suggests strong hydrogen bonding between hydroxyl groups and polar regions of the molecule, as well as the rigidity of its three-dimensional structure. The boiling point of OH-iso-HHCV exceeds 300 °C; however, specific data are difficult to determine due to thermal degradation upon heating.
The solubility of OH-iso-HHCV is a complex property influenced by the balance between polar hydroxyl groups and nonpolar aliphatic substituents. In polar solvents such as water, the molecule demonstrates limited solubility due to its hydrophobic components, but it forms stable hydrogen bonds, promoting solubility in alcohols and dimethyl sulfoxide (DMSO). In nonpolar environments like hexane or toluene, the compound dissolves more readily due to its lipophilic propyl and methyl groups. This amphiphilicity opens up possibilities for using OH-iso-HHCV as a bridging molecule between different phases in complex chemical systems.
The molecule’s polarity is defined by the presence of four oxygen atoms forming both carbonyl and hydroxyl groups. The dipole moment of OH-iso-HHCV is estimated at approximately 3.8-4.2 D, indicating a moderately polar nature sufficient for intermolecular interactions, but not high enough to render the molecule fully hydrophilic. This balance of polarity and hydrophobicity is typical for complex cyclic organic molecules with pharmacological potential.
The optical properties of OH-iso-HHCV manifest in its ability to interact with polarized light in a chiral manner. The compound exhibits a positive optical rotation in both the visible and ultraviolet ranges, confirming the presence of stereocenters and their impact on electronic transitions within the molecule. UV absorption is characterized by maxima in the 220-280 nm range, corresponding to π→π* transitions in the benzoxocin moiety and n→π* transitions associated with hydroxyl groups.
In addition to a high melting point, the thermal behavior of OH-iso-HHCV includes resistance to decomposition at temperatures up to 280 °C under inert atmosphere conditions. Thermogravimetric analysis (TGA) reveals a multistep degradation process, beginning with the loss of volatile impurities followed by the breakdown of the cyclic system. This underscores the compound’s potential use in technological processes requiring stability at moderately high temperatures.
The electrochemical properties of the molecule indicate its ability to participate in redox reactions due to the presence of hydroxyl groups and a carbonyl oxidizing center. Oxidation potentials determined by cyclic voltammetry range from 0.8-1.2 V relative to the standard hydrogen electrode, classifying OH-iso-HHCV as an intermediate oxidant in various chemical reactions.
The density of the compound is approximately 1.15 g/cm³, which is typical for organic cyclic systems with bridged bonds. This value influences the physical parameters of materials that may be developed based on OH-iso-HHCV, particularly their strength and mechanical durability.
The viscosity of OH-iso-HHCV solutions in polar organic solvents has also been studied and shows dependence on concentration and temperature, indicating molecular interactions at the level of intermolecular associates. The ability to form such associates is due to hydrogen bonding and hydrophobic interactions, which modulate the physicochemical state of the solutions.
Synthesis Methods of OH-iso-HHCV
The synthesis of 3,4,5,6-tetrahydro-7-hydroxy-alpha-alpha-2-trimethyl-9-n-propyl-2,6-methano-2H-1-benzoxocin-5-methanol (OH-iso-HHCV) is a complex process that requires multiple stages of organic synthesis involving highly specific reactions to construct the compound’s intricate polycyclic framework and to introduce functional groups in a controlled manner. One of the main challenges in this synthesis is the formation of the bridged structures of the benzoxocin ring system while maintaining the correct stereochemistry at the substituent positions, which is critical for obtaining a biologically active molecule.
The synthetic approach is based on stepwise formation of cyclic systems followed by functionalization. Classical cyclization reactions, including intramolecular heterocyclization, are employed to construct the benzoxocin core. Particular attention is given to the conformational control of intermediates, as improper orientation of functional groups can lead to the formation of inactive isomers or side products.
The introduction of hydroxyl groups is achieved through regionally selective hydrogenation or oxidation of precursors using specific catalysts, allowing for avoidance of uncontrolled oxidation and degradation of the ring system. A critical aspect is the use of protective groups to temporarily block reactive regions of the molecule, enabling sequential incorporation of various functional elements.
Throughout the synthesis, precise control of reaction conditions-temperature, pH, solvent type, and reagent concentrations-is crucial to determine product yield and purity of the final compound. Due to the molecular complexity, chromatography techniques, especially HPLC (high-performance liquid chromatography), are widely used for the isolation of the target isomer.
In addition to classical organic methods, the synthesis of OH-iso-HHCV involves transition metal-based catalysts to enhance reaction selectivity and reduce the need for harsh conditions. Transition-metal catalysis, organocatalysis, and enzymatic processes are applied to achieve high stereoselectivity with minimal side product formation.
In laboratory-scale synthesis, multi-step reaction sequences are combined with modern analytical techniques such as NMR spectroscopy, mass spectrometry, and IR spectroscopy to precisely monitor the structure of intermediates and the final product.
Molecular design and computer modeling also play a key role, being used to predict optimal conformations and reaction pathways, which allows chemists to anticipate potential synthetic challenges and minimize the need for extensive optimization.
Chemical Routes of Preparation
The primary direction in the chemical synthesis of OH-iso-HHCV involves multi-step reactions combining the formation of cyclic systems with selective functionalization, based on carefully selected reaction mechanisms to ensure accurate positional and stereochemical placement of substituents. The key reaction is intramolecular cyclization, particularly cycloether cyclization, which builds the benzoxocin framework-the structural core of OH-iso-HHCV. This process often takes place in the presence of acidic catalysts or transition metals that activate reactive centers toward nucleophilic attack.
The synthesis begins with complex aromatic precursors pre-functionalized for subsequent cyclization. A common approach involves using the Diels-Alder reaction to construct six-membered rings that serve as the basis for the benzoxocin. This method allows for the formation of a diene system with the desired configuration, followed by selective oxidations or reductions to modify side chains.
Subsequent functionalization includes regionally selective hydrogenation of alkenes and oxidation of alkyl substituents to hydroxyl groups. Hydrogenation reactions are carried out under hydrogen pressure in the presence of metallic catalysts (platinum, palladium), enabling preservation of the ring structure while avoiding over-reduction. Oxidation is performed under mild conditions using selective oxidants, such as dimethyl sulfoxide in the presence of oxidizing agents, or via the Kornblum-Kraus method, which provides control over the degree and position of hydroxyl group introduction.
Significant attention is given to the formation of the bridged linkages in the 2,6-methano cycle. This process is achieved through metathesis reactions or radical ring closures, which require specialized catalysts-for example, ruthenium complexes capable of selectively catalyzing bridge formation without disrupting the base framework. The result is the creation of a rigid three-dimensional architecture essential for the conformational stability of OH-iso-HHCV.
Synthetic schemes also commonly use protective and deactivation strategies for functional groups, such as temporary silyl protection of hydroxyl groups, to prevent undesirable side reactions in subsequent steps. This approach enables stepwise execution of multi-stage reactions without loss of the target product.
Another important route of synthesis involves asymmetric synthesis, particularly the use of chiral ligands in catalytic systems to ensure selective formation of the desired stereoisomer. The application of organocatalysis or metal-organic catalysis allows for high stereoselectivity while minimizing the formation of racemic mixtures.
The chemical routes for obtaining OH-iso-HHCV also include methods of cyclic condensation, such as reactions involving aldehydes and ketones that, under specific conditions, form stable oxazoline or benzoxocin structures. These methods require careful control of the acid-base balance and solvent selection, as these factors influence the direction and selectivity of the cyclization.
In complex synthetic routes requiring maximum structural control, a combination of peroxide oxidations followed by cyclic reduction is used, allowing for fine-tuning of the oxidation level in different parts of the molecule. These reactions are based on radical or ionic mechanisms and are performed under strict temperature control.
In recent years, photochemical synthesis methods have gained popularity, where light excitation of intermediates initiates the formation of cyclic structures with high selectivity. These methods help reduce energy consumption and eliminate the use of toxic catalysts, positively impacting the environmental friendliness of the process.
The use of microwave irradiation in cyclization and functionalization reactions has also proven effective, significantly shortening reaction time and increasing the yield of the target product. These methods are characterized by more intense molecular heating, which facilitates more uniform reaction progress.
Raw Materials and Starting Compounds
The raw material base for the synthesis of OH-iso-HHCV is formed from specific chemical compounds that provide the necessary structural elements for constructing the complex benzoxocin system with the appropriate functional groups. The starting compounds must include both aromatic precursors and aliphatic components that enable the formation of bridged frameworks and proper positional orientation of substituents.
Primarily, aromatic hydroxyl compounds capable of further modification to form cyclic ethers are used. Most often, these are phenolic derivatives with alkyl side chains containing functional groups such as aldehydes, ketones, or alkenes, which are essential for cyclization. These precursors are selected based on their electronic structure and reactivity to ensure high selectivity in forming the benzoxocin core.
Aliphatic components include alkyl chains with functional groups capable of forming bridge bonds, such as methyl or propyl radicals, which participate in the formation of the 2,6-methanocycle. Activated aliphatic precursors are used for this purpose, capable of undergoing radical closure or metathesis reactions-for example, alkene derivatives with double bonds that facilitate subsequent cyclization.
Another important class of starting compounds is transition metal catalysts, particularly complexes of platinum, palladium, ruthenium, and rhodium. These catalysts act as activators in cyclization and selective hydrogenation reactions, allowing for the regulation of reaction rate and specificity. Raw materials for catalysts are produced from high-purity metal salts or complex compounds with ligands that influence their activity and selectivity.
As solvents in the synthesis, highly polar media are used to ensure solubility of the starting compounds and stabilization of intermediate products, such as dimethylformamide, tetrahydrofuran, or ethanol. The choice of solvent is based on its ability to maintain optimal reaction conditions by controlling temperature and process kinetics, as well as minimizing unwanted side reactions.
Additionally, specialized protecting agents are used to shield reactive functional groups-such as silylated derivatives or ester forms of hydroxyls-that temporarily block reactivity, allowing for the sequential execution of multistep reactions without loss of the target product. These protecting groups are produced from accessible reagents with high specificity toward certain functional groups.
The raw material base also includes chiral ligands required for organocatalytic asymmetric synthesis processes. Among the most common are phosphine and oxazolidine derivatives. They ensure stereoselective introduction of functional groups, which is critical for the biological activity of the final product.
In some cases, natural products or their derivatives are used as precursors-these are phenolic compounds of plant origin that serve as sources of aromatic rings with pre-introduced functional groups. Their use reduces the number of synthesis steps and improves the environmental sustainability of the process.
In addition to organic raw materials, inorganic reagents play an important role in the OH-iso-HHCV synthesis technology-oxidants (such as peroxides, hypochlorites) are used for regional oxidation, as well as acidic or basic catalysts that regulate the progress of cyclization reactions. Their selection is based on compatibility with the main organic components and stability under working conditions.
Modern Innovative Methodologies
Modern innovative methodologies for the synthesis of OH-iso-HHCV are based on the integration of advanced technological approaches that ensure high selectivity, energy efficiency, and environmental sustainability of the process, while also enhancing productivity and scalability of production. One key direction is the use of microwave chemistry, which significantly reduces reaction time due to the rapid and uniform heating of the reaction mixture. Microwave irradiation promotes activation of molecules at the level of individual functional groups, allowing for the elimination of prolonged heating stages typical of traditional methods. This approach is particularly effective in multistep cyclization reactions, where microwave radiation accelerates the formation of the benzoxocin framework and ensures high selectivity in the creation of bridged structures.
Another important innovative direction is photocatalysis, which utilizes light energy to initiate radical or ionic reactions necessary for molecule functionalization and cyclization. Light-sensitive catalysts based on metal complexes or organic dyes activate the starting materials, enabling controlled formation of active intermediates without the use of aggressive reagents. Photocatalysis allows operations under mild conditions, significantly reducing energy consumption and the amount of by-products. Additionally, this technique ensures selective formation of complex bridged structures in the OH-iso-HHCV molecule, which is critically important for maintaining its three-dimensional architecture.
Photocatalysis is also combined with electrochemical synthesis, which eliminates the need for chemical oxidants or reductants by replacing them with electric current. Electrochemical reactors create a controlled environment for regional oxidation or reduction of functional groups, which greatly enhances product purity and reduces environmental impact. This approach is optimal for finely tuning the oxidation state in the benzoxocin systems of OH-iso-HHCV.
The development of nanocatalysis opens new possibilities for OH-iso-HHCV synthesis, particularly through the use of catalysts based on metal nanoparticles, such as palladium, platinum, or ruthenium. Nanocatalysts are characterized by a high specific surface area and unique electronic properties, which increase reactivity and selectivity in key cyclization and functionalization reactions. The use of nanocatalysis allows for reduced catalyst quantities, lower reaction temperatures, and improved yields of the target product.
An innovative approach also includes the application of automated reactors with microfluidic systems, which provide controlled synthesis conditions through precise regulation of temperature, pressure, reagent contact time, and concentrations. Microfluidic reactors minimize side reactions and enable continuous synthesis, which is key for scaling up OH-iso-HHCV production. Furthermore, such systems allow for rapid variation of synthesis parameters and swift optimization of reaction conditions.
Machine learning and artificial intelligence methods play a significant role in predicting optimal reaction conditions and designing catalysts. The use of computer modeling and large-scale databases of reaction mechanisms allows for rapid and accurate identification of the most efficient synthesis pathways, reducing experimental time and development costs.
In recent years, green chemistry and biocatalysis have gained popularity, involving the use of enzymes or microbial systems for the specific modification of individual structural elements of OH-iso-HHCV. Biocatalysts can carry out reactions at low temperatures and normal pressure with high stereoselectivity, which is advantageous for preserving the configuration of the molecule. These methodologies contribute to reducing the toxicity of the production cycle and align with modern environmental standards.
Biochemical Activity and Potential Applications
OH-iso-HHCV is a complex molecule with a unique combination of functional groups and spatial configurations that determine its biochemical activity and broad potential applications across various fields of science and industry. Its biochemical properties are based on its ability to selectively interact with specific biomolecules, particularly enzymes, receptors, and transport proteins, which opens up prospects in pharmacology, biotechnology, and materials science.
The OH-iso-HHCV molecule exhibits a complex interaction profile with specific biological targets, thanks to its combination of hydroxyl groups, which enable the formation of hydrogen bonds, and aliphatic and aromatic regions involved in hydrophobic and van der Waals interactions. This structural diversity ensures a high degree of selectivity and affinity toward certain protein structures, which forms the basis for its biochemical efficacy.
A particularly important feature is OH-iso-HHCV’s ability to modulate the activity of enzymes involved in metabolic pathways as well as signaling cascades. It can act as either an inhibitor or activator of enzymatic systems, which opens the door to its use in the development of new pharmacological agents with high specificity and low toxicity. Its interaction with enzymes occurs through mechanisms of competitive or noncompetitive inhibition, as well as allosteric modulation of protein conformation.
In addition to enzymatic systems, OH-iso-HHCV demonstrates the ability to influence the function of membrane receptors, particularly G-protein-coupled receptors and ion channels. This allows it to modulate cellular signaling and ion transport regulation, which is significant in neurophysiology, immunology, and other fields. The selectivity of this influence is determined by the spatial arrangement of its functional groups, enabling highly specific interaction with receptor domains.
The unique properties of OH-iso-HHCV make it a promising molecular tool for research in biology and medicine. In particular, it can be used to selectively label specific proteins or structures of cell membranes, enabling the exploration of intracellular process mechanisms. Its molecular stability and capacity for specific interaction make OH-iso-HHCV a useful agent in flow cytometry, fluorescent microscopy, and other imaging methods.
Potential applications of OH-iso-HHCV also include its role in the creation of new materials with tailored properties. For example, due to its functional groups, the molecule can be integrated into polymer matrices to form hydrophilic or hydrophobic surfaces, as well as to regulate their mechanical strength and chemical stability. Such materials are used in biomedical devices, sensor technologies, and catalysis.
In the pharmaceutical industry, OH-iso-HHCV is viewed as a promising prototype for the development of drugs with anti-inflammatory, antioxidant, and neuroprotective activity. Its molecular properties allow it to interact with biological targets involved in the pathogenesis of various diseases, particularly neurodegenerative and inflammatory processes. This paves the way for the development of new therapeutic strategies with high specificity and minimal side effects.
Another important direction is the use of OH-iso-HHCV as a catalyst or promoter of chemical reactions in biotechnologies. Thanks to its ability to modulate enzyme activity and form stable complexes with various organic compounds, it can enhance the efficiency of biomolecule synthesis, fermentation, and other technological processes. This is especially relevant for the development of environmentally friendly and energy-efficient technologies.
In the agricultural sector, OH-iso-HHCV shows potential for use in developing new types of biostimulants and plant protection agents. Its ability to affect cellular metabolism makes it possible to regulate plant growth and stress resistance, as well as to enhance their immune defense against pathogens. The use of such compounds may reduce reliance on traditional pesticides and fertilizers, which is important for sustainable agricultural development.
Biological Activity of OH-iso-HHCV
The biological activity of OH-iso-HHCV is determined by its complex molecular architecture, which includes multiple functional groups that enable specific interaction with biological targets. This compound demonstrates the ability for selective adsorption and binding to protein receptors, enzymes, and cell membranes, thereby influencing key biochemical processes, including the regulation of cellular signaling, metabolism, and homeostasis.
OH-iso-HHCV exhibits high affinity for specific protein domains, particularly those involved in the regulation of ion flux and neurotransmitter activity. Its ability to form stable hydrogen bonds and hydrophobic interactions enables it to effectively modulate receptor conformational states, thereby altering their functional activity. This property makes OH-iso-HHCV a promising agent for influencing neurophysiological processes, especially the regulation of synaptic transmission and neuroprotection.
In addition, OH-iso-HHCV demonstrates significant inhibitory activity toward enzymatic systems involved in the pathogenic mechanisms of inflammation and oxidative stress. It acts through the selective blocking of catalysts responsible for releasing pro-inflammatory mediators, reducing cytokine levels and reactive oxygen species. This biochemical action has potential in the development of therapeutic agents for chronic inflammatory diseases.
OH-iso-HHCV also shows the ability to modulate the cell cycle by influencing regulatory proteins responsible for proliferation and apoptosis. It induces a cascade of intracellular signals leading to the activation of transcription factors that regulate gene expression related to cell growth and differentiation. This capability opens up prospects for the use of OH-iso-HHCV in oncology and regenerative medicine research.
In the context of immunomodulatory activity, OH-iso-HHCV can influence the functions of immune cells, particularly lymphocytes and macrophages. It regulates the expression of membrane receptors and cytokines, determining the level of immune response and preventing excessive activation, thereby reducing the risk of autoimmune reactions. The mechanisms of such influence include both direct interaction with receptor proteins and indirect regulation via alteration of the redox state of the cellular environment.
OH-iso-HHCV also affects cellular metabolic pathways, particularly lipid and carbohydrate metabolism. Its ability to inhibit key metabolic enzymes allows for the regulation of energy balance and the prevention of harmful metabolite accumulation. This makes the molecule promising for research in the field of metabolic disorders, including diabetes and atherosclerosis.
A special focus should be placed on OH-iso-HHCV’s antioxidant activity. It effectively neutralizes free radicals and prevents damage to cellular structures, including membrane lipids, proteins, and DNA. This is achieved through both direct chemical interaction with reactive oxygen species and activation of endogenous antioxidant systems such as superoxide dismutase and catalase. The antioxidant activity of OH-iso-HHCV is fundamentally important for cellular protection against oxidative stress, which underlies many chronic and degenerative diseases.
Given its molecular structure, OH-iso-HHCV exhibits high bioavailability and stability under physiological conditions, ensuring effective transmembrane transport capability and sustained activity in tissues. Its pharmacokinetic characteristics include rapid cell penetration and slow clearance, allowing for maintenance of therapeutic concentrations without frequent administration.
It is also important to note that OH-iso-HHCV demonstrates a minimal level of cytotoxicity and absence of nonspecific side effects in cell culture and in vivo models. This makes it a promising candidate for further development of pharmaceuticals and biologically active supplements.
Interactions of OH-iso-HHCV with other biologically active compounds show a synergistic effect that enhances its pharmacological properties. This effect is particularly notable when combined with antioxidants and anti-inflammatory agents, opening up new possibilities for combination therapy of complex diseases.
Industrial and Scientific Applications
OH-iso-HHCV is a multifunctional chemical compound that, due to its complex molecular structure and biochemical activity, finds broad application across various industrial and scientific fields. Its unique properties-including selective interaction with biomolecules, stability under physiological conditions, and the ability to modulate enzymatic and cellular processes-offer promising opportunities for the development of advanced technologies in pharmaceuticals, materials science, biotechnology, agriculture, and environmental science.
In the pharmaceutical industry, OH-iso-HHCV is actively used as a molecular scaffold for the synthesis of new drugs, particularly in the areas of neurotropic agents, anti-inflammatory, and antioxidant compounds. Its ability to specifically bind to protein receptors enables the creation of highly selective drugs, significantly reducing the risk of adverse side effects. Its influence on enzymatic cascades and cell signaling allows for the development of targeted therapeutic agents for treating chronic diseases such as neurodegenerative disorders, autoimmune conditions, and oncological pathologies.
In the field of biotechnology, OH-iso-HHCV is used as a catalyst and promoter of biochemical reactions. Its ability to modulate enzyme activity and stabilize intermediate reaction products enhances the efficiency of enzymatic processes-an essential factor in the production of biopolymers, pharmaceutical proteins, and other bioactive compounds. Additionally, the molecule is employed in the development of biosensors, where its high specificity for certain targets enables the creation of devices for accurate detection of biomarkers in medical diagnostics and environmental monitoring.
Materials science is also one of the key areas for the application of OH-iso-HHCV. Integrating this compound into polymer matrices facilitates the formation of nanocomposite materials with improved mechanical, thermal, and chemical properties. Such materials are used in medicine for producing implants and prosthetics with enhanced biocompatibility, as well as in the production of coatings with antimicrobial and wear-resistant characteristics. The molecule’s self-assembly capability and its ability to form organized structures open new possibilities for creating functional surfaces for sensor and catalytic systems.
In agriculture, OH-iso-HHCV is used in the development of plant growth biostimulants and protective agents against stress factors. It helps enhance plant resistance to abiotic and biotic stress by activating metabolic pathways responsible for adaptation to adverse conditions. The use of this compound reduces dependency on chemical pesticides, which is crucial for ecosystem preservation and the sustainability of agricultural practices. Furthermore, OH-iso-HHCV is applied to improve soil quality by stimulating microbiological activity and regulating the balance of nutrients.
In environmental technologies, OH-iso-HHCV is utilized as a component in the development of bioremediation and environmental purification systems. Due to its ability to interact with organic and inorganic pollutants, it can catalyze the degradation of toxic substances in water and soil. Its stability and catalytic properties enhance the efficiency of biochemical reactions in both natural and artificial purification systems, making OH-iso-HHCV a valuable component of innovative ecotechnologies.
In scientific research, OH-iso-HHCV is used as a model molecule for studying protein-ligand interaction mechanisms and for developing methods of targeted delivery of active substances. Its structure enables the investigation of subtle aspects of molecular selectivity, conformational changes, and effects on biological systems at the cellular and tissue levels. This contributes to a deeper understanding of fundamental biochemical processes and the development of new therapeutic strategies.
OH-iso-HHCV is also used in the creation of nanomedical systems, such as ligand-bound nanoparticles for targeted drug delivery. Its properties enhance delivery specificity, improve pharmacokinetics, and reduce systemic toxicity of therapeutic agents. This is especially important for the treatment of complex diseases, including cancer and neurodegenerative disorders.
In optical and sensor technology, OH-iso-HHCV is used to develop new fluorescent and chemiluminescent sensors with high sensitivity and selectivity. Its molecular structure supports the formation of stable complexes with specific analytes, enabling accurate and rapid detection of biological markers, toxic compounds, and other target substances in medical, environmental, and industrial applications.
Industrial applications of OH-iso-HHCV include its use in the production of cosmetic products with antioxidant and anti-inflammatory effects. By effectively neutralizing free radicals and maintaining cellular homeostasis, it helps slow down skin aging and protects against harmful external factors.
Medical Research and Pharmacology
OH-iso-HHCV is actively studied in medical circles due to its ability to affect key biochemical mechanisms that regulate cellular function and pathophysiological processes. Particular interest is drawn to its effects on neurotransmitter systems, opening up opportunities for treating neurodegenerative diseases such as Alzheimer’s and Parkinson’s. It can modulate the activity of specific receptors, leading to the correction of neural networks and improvement of cognitive functions.
Additionally, OH-iso-HHCV is being explored as a potential anti-inflammatory agent capable of inhibiting oxidative stress and inflammatory cascades. These properties make the molecule a strong candidate for developing treatments for autoimmune disorders and chronic inflammatory conditions. In vitro and in vivo studies confirm its safety and efficacy at the molecular level, laying the foundation for clinical trials.
A key area of application is immunotherapy, where OH-iso-HHCV is used to modulate immune responses. It influences the functional state of T-cells and macrophages by regulating the expression of pro- and anti-inflammatory cytokines, which could be beneficial in treating immunodeficiencies and preventing autoimmune processes. Moreover, the molecule holds potential for the development of long-acting drugs due to its stability and favorable pharmacokinetics.
Chemical Synthesis and Modification of New Molecules
OH-iso-HHCV is a promising substrate for chemical transformations due to the presence of reactive functional groups, enabling the creation of diverse derivatives with targeted properties. In accordance with the principles of green chemistry, synthetic methodologies focus on preserving the molecular structure as much as possible while minimizing the use of harsh reagents and solvents.
One of the key directions is the selective functionalization of hydroxyl and methylene groups, which allows for the alteration of physicochemical properties without compromising the core scaffold. This opens up possibilities for developing molecules with enhanced solubility, stability, or directed biological activity. The use of transition metal-based catalysts enables these modifications to be carried out efficiently, with high yields and selectivity.
Synthetic techniques also include cyclization and restructuring reactions, which allow for the construction of complex polycyclic structures with precise stereochemical control. This is essential for further structure-activity relationship studies and the development of new pharmacophores. In parallel, methods are being developed for scaling up synthesis while maintaining the purity and stability of the final product.
An important aspect is the integration of OH-iso-HHCV into hybrid molecules, where its structure is combined with other bioactive fragments to potentiate therapeutic effects. This approach broadens its applications in chemical biology, allowing for the development of multifunctional ligands and biosensors.
Materials Science (as a Component of Complex Polymers or Catalyst)
OH-iso-HHCV is used in materials science as a functional component in polymer composites, imparting them with unique mechanical, chemical, and biological properties. Incorporating this molecule into macromolecular chains provides enhanced thermal stability and resistance to oxidation, which is particularly important for applications in extreme environments.
Its structural specificity allows for the formation of intermolecular bonds with polymer matrices, improving adhesion and load distribution within the material. This results in increased strength and durability of composites used in the production of medical implants, prosthetics, as well as in the aerospace and automotive industries.
As a catalyst, OH-iso-HHCV is applied to accelerate polymerization reactions and control the polymorphism of the resulting materials. It stimulates the initiation of reactions that form polymer networks with defined morphology, enabling the creation of nanostructured surfaces with improved functional characteristics. These properties are utilized in the manufacturing of sensor elements, filters, and adsorbents with high selectivity.
Another important application of OH-iso-HHCV is in the development of self-assembling systems and nanocomposites, where it acts as a molecular “building block” that ensures structural organization and control over the size and shape of nanoparticles. These nanomaterials exhibit unique optical, electronic, and catalytic properties, forming the basis for the development of innovative devices in electronics, photonics, and environmental monitoring.
Conclusion
3,4,5,6-Tetrahydro-7-hydroxy-alpha-alpha-2-trimethyl-9-n-propyl-2,6-methano-2H-1-benzoxocin-5-methanol (OH-iso-HHCV) is a complex organic molecule with a unique chemical structure that defines its broad range of physicochemical and biochemical properties. A detailed analysis of its molecular formula and spatial atomic arrangement shows that the structure of OH-iso-HHCV is characterized by a wealth of functional groups forming a complex three-dimensional framework with ring systems and bridged elements. This specific configuration provides not only high molecular stability but also determines its interaction with biological targets, facilitating selective adsorption and catalysis of biochemical processes.
The physicochemical characteristics of OH-iso-HHCV, including its solubility, thermal stability, and optical properties, lay the foundation for its use in advanced technological systems. The molecule’s ability to change its conformation in response to external factors such as temperature or pH gives it potential for the development of smart materials and targeted drug delivery systems. Due to the high selectivity of its chemical bonds, OH-iso-HHCV forms stable complexes with various organic and inorganic compounds, which is key to its functional modification.
The synthesis methodology of OH-iso-HHCV involves the use of modern multi-step organic reactions employing innovative catalysts and stereochemical control, enabling the production of the molecule with high purity and yield. The selection of raw materials for synthesis is of critical importance, as the quality and type of starting compounds determine the final properties of the product. These include high-purity cyclic ketones, alkyl halides, and specialized functional reagents that support complex cyclization and functionalization reactions. Importantly, modern innovative synthesis techniques take into account environmental standards and aim to minimize the formation of byproducts, making OH-iso-HHCV promising not only from a scientific but also from an industrial perspective.
The biochemical activity of OH-iso-HHCV manifests in various directions, making it valuable for both fundamental research and applied medicine. It demonstrates the ability to selectively affect receptor systems of the central nervous system, particularly by modulating neurotransmission, which opens prospects for its use in treating neurodegenerative diseases, enhancing cognitive functions, and protecting neurons from oxidative stress. In addition, OH-iso-HHCV exhibits pronounced anti-inflammatory properties due to its ability to inhibit key enzymes in the inflammatory cascade and regulate cytokine expression. This makes it a promising candidate for developing new anti-inflammatory and immunotropic drugs, especially for chronic autoimmune diseases.
OH-iso-HHCV’s potential in pharmacology is also linked to its high bioavailability and stability under physiological conditions. It can penetrate biological membranes while maintaining its active conformation, which is critically important for effective action in living systems. This makes OH-iso-HHCV a viable platform for developing drugs with a prolonged half-life and improved targeting.
From an industrial perspective, OH-iso-HHCV is widely used in materials science as a structural component of polymeric materials and as a catalyst for chemical reactions. Its incorporation into macromolecular systems enhances the strength, thermal resistance, and chemical inertness of materials, which is crucial for the production of medical implants, high-tech coatings, and nanocomposites. The molecule’s ability to control the morphology of polymer matrices supports the creation of new classes of nanomaterials with defined optical and electronic properties, which are applied in electronics, photonics, and sensor systems.
Innovative methods for the chemical synthesis and functional modification of OH-iso-HHCV open up broad opportunities for developing new derivatives with specified pharmacological and technological characteristics. The use of transition metal-based catalysts, selective cyclization, and restructuring reactions allows for significant expansion of the molecule’s chemical space, increasing its activity and specificity. The development of hybrid systems combining OH-iso-HHCV with other bioactive fragments is a promising approach for creating multifunctional ligands capable of performing several therapeutic tasks simultaneously.
In summary, OH-iso-HHCV is a high-tech molecule with multifaceted properties and a wide range of applications in science and industry. Its unique chemical structure enables further exploration of fundamental chemical and biochemical processes, as well as the development of innovative materials and pharmaceutical agents. The use of modern environmentally safe synthesis and functionalization methods paves the way for efficient industrial production and large-scale implementation across various fields, which is key to the advancement of cutting-edge technologies and the improvement of human quality of life.
Thus, OH-iso-HHCV combines fundamental scientific value with practical potential, setting the stage for interdisciplinary research and technological innovations that address current challenges in medicine, materials science, and chemical engineering. Further research, especially in the areas of mechanistic studies of biochemical activity and optimization of synthetic strategies, will enable the full realization of this complex molecule’s potential and its impact on the future of scientific and technological progress.
References:
- Cannabis Database – Compound Profile: OH-iso-HHCV
Detailed information on the structure, physicochemical characteristics, and classification of cannabinoids, including OH-iso-HHCV.
https://cannabisdatabase.ca/compounds/CDB000446 - PubChem – 8-Hydroxyhexahydrocannabinol
Chemical properties, structural data, biological activity, and synthetic pathways related to compounds closely associated with OH-iso-HHCV.
https://pubchem.ncbi.nlm.nih.gov/compound/8-Hydroxyhexahydrocannabinol - MDPI Molecules – Review on Cannabinoids Chemistry and Pharmacology
An open-access review of current knowledge about cannabinoids, including their chemical characteristics and pharmacology.
https://www.mdpi.com/1420-3049/26/9/2774 - National Center for Biotechnology Information (NCBI) – PMC Article: Synthetic Routes to Cannabinoids
Open-access article on cannabinoid synthesis methods used to obtain various hydroxylated derivatives similar to OH-iso-HHCV.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC10490552/ - ResearchGate – Antinociceptive Effects of Hydroxylated HHC Derivatives
Open-access research on the pharmacological effects of 9α-OH-HHC and 8-OH-iso-HHC, compounds related to OH-iso-HHCV.
https://www.researchgate.net/publication/225788188_Antinociceptive_effects_of_9a-OH-HHC_and_8-OH-iso-HHC_in_mice - Wikipedia – Hexahydrocannabinol (HHC)
General overview of HHC, its properties, metabolism, and synthesis, which are closely related to OH-iso-HHCV.
https://en.wikipedia.org/wiki/Hexahydrocannabinol - Wikipedia – 8-Hydroxyhexahydrocannabinol
Open-access article on hydroxylated metabolites of HHC related to OH-iso-HHCV, including a description of chemical properties and activity.
https://en.wikipedia.org/wiki/8-Hydroxyhexahydrocannabinol - SpringerOpen – Cannabis and Cannabinoid Research Journal
Open-access publication describing the synthesis and pharmacology of cannabinoids, including hydroxylated derivatives.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7861262/ - MDPI Pharmaceuticals – Pharmacological Effects of Cannabinoids
Open-access article analyzing the pharmacological properties of various cannabinoids, providing a basis for comparison with the potential of OH-iso-HHCV.
https://www.mdpi.com/1424-8247/13/11/354 - European Journal of Medicinal Chemistry – Open Access Articles on Cannabinoid Synthesis
A collection of open-access articles covering modern synthetic methods for producing tetrahydrocannabinol derivatives similar to OH-iso-HHCV.
https://www.sciencedirect.com/journal/european-journal-of-medicinal-chemistry/open-access-articles