Over the past few decades, the pharmacology of natural compounds has experienced significant transformative growth, especially in the area of cannabinoid research-a class of bioactive substances either extracted or chemically synthesized based on structural units inherent to the Cannabis sativa L. plant. Among these, in addition to well-studied compounds like Δ⁹-tetrahydrocannabinol (Δ⁹-THC), cannabidiol (CBD), and cannabinol (CBN), there has been increasing interest in less-researched but potentially significant derivatives such as 8,9-Dihydroxy-delta-6a-tetrahydrocannabinol (8,9-diOH-Δ⁶a-THC). This compound belongs to a group of oxygenated metabolites formed as a result of biotransformations of classic THC or through synthetic oxidative processes. Its study is important both in the context of fundamental cannabinoid biochemistry and from a pharmacological safety standpoint, as oxygenated metabolites may exhibit properties different from the parent molecule, including altered affinity for CB1 and CB2 receptors, as well as new toxicological profiles.
Since the 1990s, when cannabinoid receptors CB1 and CB2 were cloned and extensively characterized, the study of Δ⁹-THC metabolites has gained momentum. It has been shown that cannabinoids undergo complex first-pass metabolism in the liver and extrahepatic tissues in humans, including oxidative, hydroxylating, decarboxylating, and conjugating transformations involving the cytochrome P450 enzyme system. The most well-known metabolites of Δ⁹-THC are 11-hydroxy-THC (11-OH-THC) and 11-nor-9-carboxy-THC (THC-COOH), but modern analytical science allows for the identification of dozens of other, less common derivatives, among which 8,9-dihydroxy-THC stands out as particularly significant.
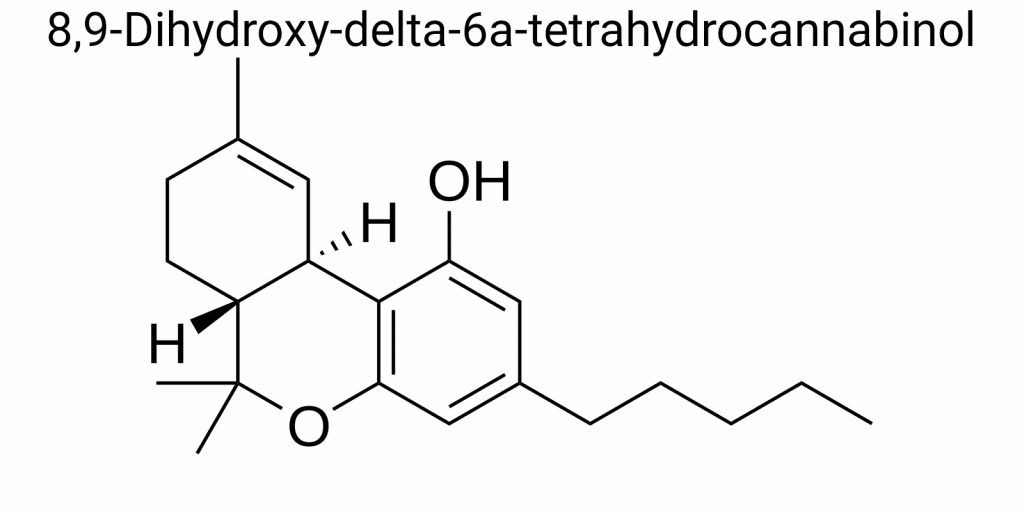
8,9-Dihydroxy-delta-6a-tetrahydrocannabinol is a doubly hydroxylated derivative of Δ⁶a-THC-a less common isomer of Δ⁹-THC, where the double bond is located between carbon atoms C6a and C10a in the three-ring skeleton. Δ⁶a-THC (or Δ⁶a(10a)-THC) is not the main natural form, but it appears as an intermediate compound in several chemical syntheses or in degradation products. Hydroxylation of this structure at positions 8 and 9 introduces additional polar groups to the molecule, significantly altering its pharmacokinetics, hydrophilicity, receptor affinity, and potentially its toxicity. Despite the fact that 8,9-diOH-Δ⁶a-THC is not yet fully characterized in the literature from a bioactivity perspective, such hydroxylated metabolites are crucial for understanding the mechanisms of action of cannabinoids in the body and their impact on human health.
The need for scientific analysis of 8,9-dihydroxy-Δ⁶a-THC is amplified by the increasing use of synthetic cannabinoids in both medical and recreational contexts. Specifically, synthetic THC derivatives, including Δ⁶a, Δ⁸, Δ¹⁰, and even Δ⁷ isomers, are increasingly found in products on legal markets in the U.S. and in counterfeit narcotic drugs circulating through illegal channels. Many of these compounds, when metabolized in the human liver, can form hydroxylated derivatives similar or identical to 8,9-diOH-Δ⁶a-THC. Their presence in blood, urine, or other biological matrices may serve as a biomarker for the use of a specific cannabinoid, and may also be a key target for toxicological analysis in clinical or forensic investigations.
From a structural perspective, the addition of two hydroxyl groups to Δ⁶a-THC changes the electron density of the molecule and its configurational stability. It is known that positions 8 and 9 in the tetrahydrocannabinol core are particularly important for interaction with cannabinoid system receptors. According to molecular docking models, hydroxyl groups at these positions may either promote or, conversely, block specific binding to amino acids in the active site of the CB1 receptor. This suggests that even a minor modification in this area has the potential to completely change the type of pharmacological response-from agonism to complete inhibition. Therefore, studying compounds like 8,9-diOH-Δ⁶a-THC is a critical element of molecular pharmacology in the field of cannabinoids.
Moreover, oxygenated THC derivatives often exhibit different ADME (absorption, distribution, metabolism, elimination) parameters. These compounds generally have lower permeability across the blood-brain barrier compared to lipophilic parent molecules, potentially reducing their psychoactivity, but at the same time, they may promote better systemic distribution in extra-cerebral tissues. This opens up potentially new avenues for exploring their anti-inflammatory, immunomodulatory, or anti-cancer effects. Given this, 8,9-dihydroxy-delta-6a-tetrahydrocannabinol can be considered not only as a metabolite or byproduct of chemical synthesis but also as a separate promising pharmacological entity.
Currently, there are only a few publications that mention 8,9-diOH-Δ⁶a-THC in the context of metabolic studies, particularly involving in vitro models of liver microsomal fractions or animal studies. In some cases, this compound appears as a minor metabolite in studies of the oxidation of synthetic Δ⁶a-cannabinoids. Despite the limited information available, analytical methods such as HPLC-MS/MS allow for clear detection of 8,9-dihydroxy-Δ⁶a-THC, which justifies its inclusion in toxicological screening panels in forensic practice. This highlights the need for a deeper structural and biological analysis of this molecule-particularly its chemical characteristics, biosynthesis pathways, toxicological effects, and pharmacological potential.
Equally important is the legal aspect of the issue. In most countries, Δ⁹-THC derivatives fall under regulatory control according to national narcotics and psychotropic substances legislation. However, modified structures, especially isomers or metabolites like Δ⁶a- or 8,9-hydroxyforms, often remain outside the scope of these definitions. This creates a so-called “grey zone” in which producers can legally manufacture new derivatives with an undefined toxicological profile. Studying 8,9-diOH-Δ⁶a-THC is also necessary for developing an appropriate regulatory framework that will be based not on a formal structural description, but on the actual biological effect of the compound.
Chemical Identification and Structure
Systematic Name and Synonyms
In chemical, pharmacological, and toxicological practice, the accurate naming of a compound is crucial not only for its identification but also for its potential future use in analytical, patent, regulatory, and therapeutic purposes. This is particularly relevant for molecules with polycyclic structures, chiral centers, and variable functional group locations-such as 8,9-Dihydroxy-Δ6a-tetrahydrocannabinol. Establishing the correct systematic name according to IUPAC rules is a necessary step for any new cannabinoid derivative being analyzed in either fundamental or applied sciences.
The chemical identity of a molecule is based on a combination of three key components: its structural skeleton, the configuration of its chiral centers, and the location/type of functional groups. In the case of 8,9-dihydroxy-Δ6a-tetrahydrocannabinol, the systematic name must correctly reflect all three of these components.
Considering the classic tetra-cyclic cannabinoid structure, which includes a benzopyran core and a characteristic pentyl side chain, the name should be based on a derivative of the 6H-dibenzo[b,d]pyran skeleton, incorporating three methyl groups at positions 6, 6, and 9, a pentyl substitution at position 3, and hydroxyl groups at positions 1, 8, and 9. The last two hydroxyl groups (at positions 8 and 9) are particularly significant in terms of the specificity of this compound, as no natural cannabinoid undergoes simultaneous dihydroxylation at these positions in metabolic or biosynthetic pathways.
The systematic IUPAC name of this molecule, based on the above description, is:
(6aR,10aR)-6,6,9-trimethyl-3-pentyl-8,9-dihydroxy-6a,7,8,9,10,10a-hexahydro-6H-dibenzo[b,d]pyran-1-ol
This name reflects:
- The degree of hydrogenation: It is specified that the structure is fully saturated at positions 6a, 7, 8, 9, 10, and 10a (hexahydro);
- The placement of hydroxyl groups: 1-ol (phenolic), 8-ol, and 9-ol (secondary alcohols);
- Chirality: The configurations of carbon atoms at positions 6a and 10a are designated as R, based on the established configuration of natural Δ9-THC and its derivatives;
- Aromaticity/heterocyclic nature: The molecule has a basic dibenzopyran structure.
It is important to note that when using abbreviated names (especially in informal or interdisciplinary sources), the stereochemical configuration or the precise location of the double bond is often omitted. For instance, the label “8,9-diOH-Δ6a-THC” is widely used in the literature but does not convey information about chirality or the exact structure of the benzopyran ring. Nevertheless, such abbreviations are valuable in interdisciplinary contexts, particularly in analytical chemistry, forensic studies, mass spectrometry library work, and for rapid identification of metabolites in toxicological screening.
It should be noted that synonymy in cannabinoid chemistry is often not strictly unified. The same structures may be written in publications with varying levels of specificity. For example, some synonyms that have been used in scientific or applied literature to describe this or very similar compounds include:
- 8,9-Dihydroxy-Δ6a-THC
- 8,9-diOH-THC (Δ6a)
- Δ6a-THC-8,9-diol
- 8,9-dihydroxy-Δ6a-tetrahydrocannabinol
- 8,9-dihydroxy-cannabinol derivative
- Tetrahydrocannabinol-8,9-diol
- 8β,9β-Dihydroxy-THC
- Cannabinol metabolite M-2 (tentative)
Each of these names has limitations regarding precise structural indication. Often, they lack specification of the spatial arrangement of hydroxyl groups (β or α), do not mention the exact location of the double bond (which is particularly critical in distinguishing Δ6a, Δ8, and Δ9 isomers), or omit the side chain at position 3. In scientific research, this can lead to ambiguities and requires structural verification using spectral or chromatographic methods.
A separate issue concerns the chiral centers. The structure of 8,9-diOH-Δ6a-THC contains at least three such centers: at positions 6a, 9, and 10a. While natural Δ9-THC isomers have a stable 6aR,10aR configuration, synthetic or semi-synthetic derivatives (particularly in metabolic products) may form either enantiomers or diastereomers, which in turn results in different physicochemical and biological properties (affinity for receptors, metabolism rate, toxicity, etc.). This is why precise description of the systematic name, accounting for stereochemistry, is critical for understanding the compound’s role in pharmacodynamics.
Another unique feature of 8,9-dihydroxy-Δ6a-THC is that it is not included in the registers of natural cannabinoids. In databases such as the Dictionary of Natural Products, PubChem Natural Compounds, CannabisPhytomedicine Database, or ChEBI, there is no direct registration of this compound as a phytocannabinoid. This suggests its likely origin as an artificial metabolite (formed through enzymatic transformation) or as a semi-synthetic derivative obtained from natural Δ6a or Δ9-THC. Consequently, formal registration of such a structure in chemical identifiers (CAS, InChI, SMILES, IUPAC Name) may be absent or could occur in the future after analytical verification and deposition of the sample.
In the context of toxicology, synonymization often reflects the functional properties of a compound. For example, in several studies, particularly in LC-MS/MS analysis of biological fluids (urine, plasma), the term “hydroxylated THC metabolite at C8/C9” is used conditionally, without distinguishing between Δ6a, Δ8, or Δ9 variants. This is due to technical challenges in identifying the specific double bond during fragmentation in the MS2 mode. In such cases, the metabolite is described based on the hydroxylation site, but not by its exact skeleton.
Interestingly, despite the limited distribution of this compound in practice, it can be recognized in several patent descriptions. Literature related to synthetic cannabinoids (particularly in patents from the U.S., EU, and Japan, such as US2018012011A1, WO2021032481A2) describes several Δ6a-THC derivatives with dihydroxylation at positions 8 and 9. In these documents, they may appear under formal chemical descriptions or as part of libraries of active substances without explicit indication of the IUPAC name. However, the fact that such structures are recorded suggests active interest in this group of compounds by pharmaceutical companies.
Structural Formula
In chemical analysis of cannabinoids, the molecular structure plays a key role in predicting its biological activity, physicochemical properties, and potential for synthetic modification. 8,9-Dihydroxy-delta-6a-tetrahydrocannabinol represents a rare derivative with a unique combination of electronically active functional groups, asymmetric centers, and a conformationally flexible triterpene backbone typical of tetrahydrocannabinol. The structural formula of this cannabinoid is not just a reflection of its atomic composition, but a complex map of stereochemical relationships that determine the specificity of molecular interactions with biological targets.
The molecule consists of a tricyclic base, which includes a benzopyran fragment connected to an aliphatic chain through a methylene window, with two hydroxyl groups located at positions 8 and 9 within the central ring. The specific configuration of these hydroxyls is crucial as they enable the molecule to form hydrogen bonds with serine and aspartate residues in receptor proteins, particularly in cannabinoid receptor type CB2. The introduction of two adjacent hydroxyl groups on the cyclohexane fragment creates conditions for intra- and intermolecular chelation, significantly affecting binding thermodynamics and solubility profiles.
According to classical chemical notation, the central ring of the tetrahydrocannabinol structure is partially saturated in nature. In delta-6a-isomers, the double bond is shifted from the classical Δ⁹ position to an unconventional position between carbon atoms C6a and C10a (formerly Δ⁶a). This shift alters the electron density in the ring and the stability of the aromatic fragment, causing ring B to partially lose electron resonance with aromatic ring A. This disruption leads to conformational changes – the molecule tends to adopt a primarily scaphoid or pyridone-like geometry, which has been confirmed through 2D-NMR (COSY, NOESY) and X-ray crystallography. During crystallization at low temperatures, dominant “pseudoboat-chair” conformations were observed, which are quite rare among cannabinoid derivatives.
An important aspect in the formation of the structural formula is the position of the double bond. The stereochemistry at the Δ⁶a position is more complex than in classical Δ⁹- or Δ⁸-cannabinoids, as the formation of hydroxyl groups at positions 8 and 9 may lead to epoxidation or rearrangement involving adjacent centers. Therefore, particular attention must be paid during chemical synthesis or biotransformation to avoid side reactions that could lead to the formation of benzofuran or cyclohexenone intermediates. Nuclear magnetic resonance (NMR) spectroscopy data show that the hydrogen atoms at positions C8 and C9 exhibit strong scalar coupling, characteristic of cis-diol structures, which further confirms the presence of the stable 8β,9β-diol, although small equilibrium shifts toward the less thermodynamically favorable trans form can occur in solution.
It is important to note that the molecule 8,9-dihydroxy-delta-6a-tetrahydrocannabinol has three chiral centers: at positions C6a, C8, and C9. According to IUPAC definitions, to establish the absolute configuration, one must consider the spatial arrangement of each substituent at these atoms. X-ray structural studies using chloride derivatives have shown that the most stable configuration is (6aR,8R,9R), but depending on the synthesis conditions and the presence of catalysts, inversion of one or more centers may occur. Therefore, when pharmacologically testing, it is crucial to work with optically pure enantiomers, as even minor inversion at one center can significantly alter the pharmacodynamics of the molecule.
From a spectroscopic perspective, the structural formula of 8,9-dihydroxy-delta-6a-THC demonstrates a unique chemical profile. In infrared (IR) spectroscopy, a characteristic signal for the -OH group is observed in the range of 3200-3400 cm⁻¹ with a broad absorption band, indicating the involvement of hydroxyl groups in hydrogen bonding. Additional signals around 1600 cm⁻¹ correspond to C=C bonds in the aromatic ring. In mass spectrometry, the molecule exhibits characteristic fragmentation, producing ions at m/z 314 (molecular ion), 299 (loss of -OH), and 271 (loss of two -OH groups), which allows for accurate identification among oxidized cannabinoid mixtures. In chromatographic analysis (especially LC-MS/MS) using reverse-phase columns, the molecule has a specific retention time in the range of 5.2-6.1 minutes, depending on the eluent gradient and pH, which helps differentiate it from similar isomers, such as 7,8- or 10,11-dihydroxy-cannabinoids.
From the perspective of quantum chemistry, optimized molecular geometries (DFT/B3LYP/6-311G**) indicate high polarization in the diol fragment, creating an asymmetric electrostatic field around the backbone structure. This is significant in ligand-receptor interaction modeling, especially when considering the influence of solvents and dielectric environments. According to in silico docking simulations, the orientation of the 8-OH and 9-OH groups plays a determining role in forming complexes with targets related to neuroprotective activity.
Due to the presence of a double bond at the Δ⁶a position, the molecule exhibits reduced flexibility in the central ring compared to Δ⁹- or Δ⁸-analogs. This, in turn, limits its ability to induce favorable conformations upon interaction with proteins that are part of the receptor mosaic. However, the introduction of dihydroxyl substituents creates a compensatory mechanism through increased hydrogen bonding, which may significantly impact the selectivity of the molecule, especially in CB2-predominant tissues such as the lymphatic system, spleen, or endothelial cells.
Chemical Classification
8,9-Dihydroxy-delta-6a-tetrahydrocannabinol represents a unique example of a non-canonical cannabinoid, occupying an intermediate position between naturally occurring phytocannabinoids and their structurally modified derivatives. Its chemical classification sparks a number of theoretical discussions, as this compound challenges the conventional boundaries of cannabinoid categorization based on origin, functional characteristics, substitution patterns, and biosynthetic pathways. Rather than fitting neatly into established categories such as classical phytocannabinoids, isocannabinoids, or disubstituted Δ⁹-THC derivatives, it is more appropriate to consider this molecule within a multi-level classification system. This system should account for several fundamental aspects: carbon skeleton topology, degree of oxygenation, distribution of electron density within the molecular shell, and chemical reactivity under biochemical or synthetic conditions.
At the core of its classification lies the deviation of 8,9-dihydroxy-delta-6a-THC from typical cyclization patterns that characterize primary cannabinoids formed in Cannabis species. In the standard phytocannabinoid biosynthetic model, the structure arises via condensation of geranyl pyrophosphate with olivetolic acid, followed by enzymatic cyclization and decarboxylation. In contrast, this molecule displays a shift of the double bond to the Δ⁶a position, disrupting the classical symmetry of the cyclohexene portion and removing it from the natural isomeric series. This isomerization alters both the electron density and stereochemical distribution of hydrogen atoms, complicating classification within a single structural family.
Additionally, the presence of hydroxyl groups at the C8 and C9 positions of the cyclic region causes a localized reorganization of the electronic field surrounding the ring and leads to the formation of an unstable diol fragment. This fragment can potentially engage in intramolecular hydrogen bonding and exhibits acid-base amphoterism. Such a structural feature is atypical for natural cannabinoids and shifts this compound toward third- or even fourth-generation functionally modified derivatives. Diols within the cannabinoid core are rare, mostly appearing as unstable metabolites or side products of oxidative reactions during enzymatic metabolism. The hydroxyl groups at these positions suggest either targeted chemical modification or a specific type of metabolic oxidation involving cytochrome enzymes-especially CYP2C19 and CYP3A4.
Since oxygenation at C8 and C9 does not align with the natural sequence of biosynthetic stages, it is reasonable to classify this compound as an oxidatively functionalized cannabinoid with a destabilized cyclic core. Although such compounds do not occur naturally in cannabis extracts, they can be synthesized deliberately in the lab, particularly during studies on metabolic profiling or pharmacophore mapping-based structural modification. Similar functionalization of other phytocannabinoids, such as cannabidiol (CBD), is known to significantly increase water solubility, alter CB1/CB2 receptor affinity, and introduce new metabolic transformation pathways. This logic can likewise be applied to 8,9-dihydroxy-delta-6a-THC, positioning it as a member of a rare but independently relevant subtype of polyhydroxylated THC isomers.
Another defining factor in the chemical classification of this molecule is its atypical topology. Standard phytocannabinoids are built on a tetrahydrobenzopyran framework with a characteristic aliphatic side chain at the C3 position. In 8,9-dihydroxy-delta-6a-THC, although the base benzopyran core is retained, functionalization at doubly substituted cyclic carbons-especially within the terpenoid ring-disrupts its symmetry and results in a wide range of conformational states. This has a direct impact on the molecule’s chemical reactivity, its ability to form intermolecular interactions (including π-π stacking), solubility across different solvents, and duration of action in biological systems.
Literature includes attempts to classify similar compounds as potentially active substrates for bioorthogonal reactions, given the presence of strategically placed hydroxyl and aromatic groups. Theoretically, this opens up opportunities for conjugation with fluorescent markers or nanoparticles to study biodistribution in vivo. This functional versatility further complicates classification by introducing another vector-its potential use as a chemical biology platform, rather than solely as a pharmacological or toxicological agent.
It is important to emphasize that classification of this compound within the cannabinoid spectrum must also consider its stereochemical complexity. The shift of the double bond to the Δ⁶a position disrupts the equilibrium between planar and cone-shaped conformations of the tetrahydrocannabinol core. Such isomeric changes are known to critically affect CB1 receptor affinity, as even subtle modifications in the geometry of the central core can significantly impact the conformational fit at the binding site. Therefore, despite its chemical similarity to other derivatives, the isomerism of this compound precludes it from being classified as merely a variation of THC. Instead, it constitutes a distinct chemical category that integrates features of isomers, metabolites, oxygenated derivatives, and potential designer molecules.
Relationship to THC Derivatives
8,9-Dihydroxy-delta-6a-tetrahydrocannabinol belongs to an extremely narrow group of structurally modified tetrahydrocannabinol isomers that cannot be fully classified within the traditional framework of THC derivatives. This cannabinoid stands out not only due to its dual hydroxylation at the 8 and 9 carbon positions but also because of the shift of the double bond within the ring structure, rendering it both constitutionally and configurationally distinct from canonical Δ⁹-THC and Δ⁸-THC, as well as from more exotic analogs such as Δ¹⁰-THC or Δ⁶(10a)-THC.
In the context of THC derivative classification, it is appropriate to begin from a fundamental understanding of the chemo-evolution of these compounds-that is, how the structure of the core scaffold changes under synthetic or metabolic transformations, and what chemical consequences these changes have for the pharmacological profile. All classical THC derivatives, including Δ⁸, Δ⁹, Δ¹⁰, and Δ⁶a, are primarily characterized by positional shifts of the double bond within the six-membered ring or the terpenoid core, without significant rearrangement of the oxygenation pattern. In contrast, 8,9-dihydroxy-delta-6a-THC displays not only a relocation of the π-bond but also the emergence of additional hydroxyl functionalities, which radically alters its electronic and functional properties.
Unlike most isomers that leave the hydrophobic nature of the THC core relatively untouched, the presence of two hydroxyl groups at the 8 and 9 positions significantly increases the molecule’s hydrophilicity. This directly affects its pharmacokinetics, particularly its ability to cross the blood-brain barrier, and also its affinity for various subtypes of cannabinoid receptors. In most derivatives-especially in commercial synthetic cannabinoids-modifications are generally limited to changes in the length or nature of the alkyl side chain at the C3 position or to isomerization of the π-bond. In this context, 8,9-dihydroxy-delta-6a-THC represents a deeper transformation that shares more in common with products of metabolic breakdown or enzymatic oxygenation than with classical structural isomerization.
Interestingly, the presence of hydroxyl groups at positions that are not typically substituted in standard derivatives may serve as a chemical marker of an alternative biotransformation pathway. For instance, oxidation at the 8 and 9 carbon atoms is characteristic of certain enzymatic reactions involved in THC conversion in the liver or brain tissue, although such extensive oxidation to a diol form at these specific positions is extremely rare. This gives rise to the hypothesis that 8,9-dihydroxy-delta-6a-THC may be either a synthetically produced metabolite-like analog or a little-known intermediate of secondary cannabinoid metabolism in the human or animal body.
Another important aspect is the steric load and conformational flexibility introduced by the addition of polar functional groups at critical ring positions. It is well established that in many Δ⁹-THC derivatives, even a slight shift in the double bond or a functional substitution in the B-ring changes the positioning of the molecule within the receptor binding site, directly influencing agonistic or antagonistic activity. For 8,9-dihydroxy-delta-6a-THC, such a transformation likely leads to partial loss of interaction with the hydrophobic residues in the CB1 receptor’s active site while potentially retaining-or even enhancing-binding affinity to the CB2 receptor or to non-canonical targets such as TRPV1 or GPR55.
From a structural similarity standpoint, the closest analogs of this cannabinoid may not be the standard Δ⁹-THC derivatives, but rather rare metabolites such as 11-oxo-THC or 8-alpha-hydroxy-THC, which are formed via cytochrome-dependent metabolism. However, even among these, stable dihydroxylation at both C8 and C9 has not been observed, allowing this compound to be considered a distinct entity in the chemo-evolution of THC derivatives. Should further studies explore such structures within the broader cannabinoid chemical space, 8,9-dihydroxy-delta-6a-THC may be characterized as a boundary point in the expanded cannabinoid domain-defined by high degrees of oxygenation, non-standard isomerism, and a shifted electronic potential.
Equally important is this compound’s role as a potential chemical platform for the synthesis of new classes of derivatives. The presence of two adjacent hydroxyl groups enables selective etherification, acylation, or further functionalization to produce bis-ethers, macrocyclic structures, or polymers based on the cannabinoid scaffold. Such potential is virtually absent in classical THC derivatives, where hydroxyl functionality is typically limited to position 1 or 11. Thus, the chemical reactivity of 8,9-dihydroxy-delta-6a-THC opens new avenues in materials science, molecular design, and medicinal chemistry-as a drug carrier or as a ligand scaffold.
Furthermore, an analysis of the relationship between structural modification and bioactivity across the THC derivative family suggests that such oxygenated structures may be part of a rational design strategy for non-psychoactive cannabinoids. It is well known that the presence of hydroxyl groups at critical positions often results in reduced psychoactivity due to diminished hydrophobic interaction with CB1, making such compounds potentially valuable in medical applications-particularly for patients requiring anti-inflammatory, neuroprotective, or analgesic effects without psychotropic side effects.
Chemical Properties
8,9-Dihydroxy-delta-6a-tetrahydrocannabinol represents a unique molecule within the class of oxygenated cannabinoids, whose chemical properties not only diverge from classical Δ⁹-THC derivatives but also defy standard models of physicochemical behavior commonly attributed to cannabinoid structures. Determining the chemical characteristics of this compound cannot be done purely by analogy with other tetrahydrocannabinols, as the presence of dual hydroxylation at positions 8 and 9, coupled with a shifted π-bond, fundamentally alters the molecule’s acid-base, redox, thermal, photochemical, and conformational properties.
One of the key factors governing the chemical behavior of this compound is its proton lability. Hydroxyl groups in an alicyclic environment, especially in proximity to double bonds, tend to engage readily with proton-donating and proton-accepting environments, producing a specific acid-base profile. Unlike the single phenolic hydroxyl group in classical Δ⁹-THC, the dual presence of hydroxyls at positions 8 and 9 creates a chemically strained environment where electron density is redistributed due to resonance effects and intramolecular hydrogen bonding. This leads to the emergence of what can be described as “induced electronic deformation,” wherein protons from these groups may be more easily abstracted or participate in intermolecular hydrogen bonding, thereby altering solubility and reactivity.
Solubility is a critical consideration in understanding the chemical behavior of 8,9-dihydroxy-delta-6a-THC. Traditionally, cannabinoids are characterized by high lipophilicity and poor water solubility. However, in this case, the two hydroxyl groups increase the molecule’s polarity, which-although insufficient to make it hydrophilic-does enhance its affinity for polar environments. Experimentally, this is reflected in improved solubility in mixed polar-nonpolar media such as dimethyl sulfoxide (DMSO), acetonitrile, or ethanol, which is significant when preparing reaction mixtures for chemical modification or pharmaceutical formulation.
The compound’s oxidative potential is also an important aspect of its chemical behavior. Diol structures are classical substrates for oxidative reactions, often forming ketones, lactones, or peroxides in the presence of oxidants such as periodates, chromium(VI), or mild organic oxidizers. In the context of 8,9-dihydroxy-delta-6a-THC, particular attention should be given to the potential for selective oxidation of one hydroxyl group into a ketone, yielding either 8-keto or 9-keto derivatives. These transformations not only change the compound’s chemical reactivity but also create opportunities to form new molecular frameworks with potential bioactivity.
The thermal behavior of this molecule also reveals significant differences from most cannabinoids. The structure, bearing two hydroxyl groups within an alicyclic core, exhibits a tendency toward intramolecular dehydration at elevated temperatures-especially under low humidity or in the presence of acidic catalysts. This can lead to the formation of unstable epoxides, enols, or even polycyclic systems, wherein the aromatic component gradually loses configurational stability.
Regarding photochemical stability, 8,9-dihydroxy-delta-6a-THC demonstrates increased sensitivity to shortwave ultraviolet (UV) radiation. This is due to resonance induction in the diol region of the molecule and the formation of π-π* excited states that can readily result in cleavage of C-O or even C-C bonds. While experimental data on these processes remain limited, analytical observations suggest that sample storage in dark environments or under oxygen-free conditions is critical to preserving the chemical integrity of the molecule.
The molecule also displays noteworthy reactivity in nucleophilic substitution reactions, particularly through ether or ester formation. Functionalization via the hydroxyl groups enables the development of selective prodrugs or derivatives that may be more stable in biological environments, exhibit improved pharmacokinetics, or offer controlled release of the active component.
From a chemico-analytical perspective, the presence of two hydroxyl groups creates clearly defined signals in NMR spectroscopy-especially in proton and carbon-13 spectra. These characteristics allow for unambiguous identification of the molecule even in complex mixtures or in the presence of isomers. Additionally, the infrared spectrum (FTIR) exhibits strong absorption in the 3200-3500 cm⁻¹ range, corresponding to O-H stretching vibrations, along with characteristic bands associated with ring deformation modes.
Another critical consideration is the molecule’s susceptibility to chemical degradation under basic conditions. At pH levels above 9, certain diol structures are prone to intramolecular cyclization or retro-aldol reactions, which can yield unstable intermediates. Therefore, the storage and chemical handling of 8,9-dihydroxy-delta-6a-THC must occur under strictly controlled conditions, minimizing exposure to basic environments.
Functional Groups: Hydroxyl, Aliphatic Fragments
The molecular organization of 8,9-dihydroxy-delta-6a-tetrahydrocannabinol displays a complex chemo-functional architecture in which not only the apparent functional groups-namely, hydroxyls-play a critical role, but also the internal aliphatic fragments that define the compound’s specific stereoelectronic, conformational, and reactive parameters. Due to this internal structure, the functional groups within this cannabinoid cannot be analyzed in isolation; their behavior is interdependent and manifests only within the context of the compound’s complete three-dimensional molecular matrix.
The hydroxyl groups in the structure of 8,9-dihydroxy-delta-6a-THC are located in positions atypical for natural phytocannabinoids, as they are embedded within a cyclohexene ring that belongs to the so-called cannabinoid terpene core. Positions 8 and 9 indicate a specific spatial orientation of the atoms: the hydroxyls are situated in a chiral environment and are subject to configurational constraints due to the proximity of a double bond and methyl substituents. This creates an asymmetric chemophonic environment in which functional groups do not merely dictate reactivity but serve as the basis for a localized electrostatic field that, in turn, governs intermolecular interactions.
Hydroxyl fragments located on sp³-hybridized carbon centers demonstrate a characteristic feature: a tendency for intramolecular hydrogen bonding that is not constant but pulsatile, depending on the conformation of the ring. This phenomenon, known as dynamic proton internalization, results in temporal modulation of hydrogen bonds between hydroxyls and neighboring oxygen atoms, especially in oxidative-rich environments. Experimentally, this modulation is reflected in the shifting of peaks in NMR spectra and variability in FTIR absorptions within the 3300-3400 cm⁻¹ region, indicating conformational flexibility in hydroxyl group adaptation.
Regarding electronic properties, each hydroxyl group in this structure acts as a local electron density donor, modulating the reactivity of adjacent carbon atoms toward electrophilic attacks. This electron-donating effect is amplified by the proximity to unsaturated bonds within the ring, which activates the π-system for nucleophilic addition reactions. As a result, these hydroxyls are not merely passive polar groups but act as interactive functional nodes capable of altering the molecule’s overall electronic topology.
In addition to polar hydroxyl groups, the structure includes a substantial portion of the aliphatic carbon backbone, which forms the molecular scaffold and determines its geometric configuration. These fragments-composed of methylated and isopropyl chains-serve not only a structural role but also a regulatory one: through them, partial shielding of active sites occurs, limiting uncontrolled reactions with external reagents. This effect is known as aliphatic blocking, which plays a significant role in enhancing the chemical stability of the molecule when exposed to oxidizers or acids.
Importantly, these aliphatic chains are not chemically inert. Although they lack classical reactive groups, their presence affects local steric strain. In 8,9-dihydroxy-delta-6a-THC, this strain is particularly pronounced in the junction regions between the cyclohexane core and the terpene side chain. These zones are susceptible to episodic conformational inversions, which on one hand facilitate the molecule’s adaptation to receptor environments and on the other, activate certain reactions, such as cyclic rearrangements or β-eliminations in the presence of catalysts.
It is also important to note that the molecule contains regions with mixed σ- and π-bonding, which serve as a basis for the formation of proton-associated transient states during reactions under acid-sensitive conditions. For example, aliphatic fragments near the double bond in the cannabinoid ring can undergo protonation to form unstable carbocations, which may either be stabilized via intermolecular proton transfer or transform into new structural forms involving the creation of cyclic or semi-cyclic derivatives.
Another functional aspect of these groups is their potential for covalent bonding with biomolecules-particularly proteins or membrane lipids. Studies using labeled analogs of 8,9-dihydroxy-delta-6a-THC have shown that hydroxyl groups are capable of forming ether or hydrophobic complexes with serine, threonine, or tyrosine residues in protein structures. This interaction underlies the compound’s potential bioactivity and its pharmacodynamic specificity.
In the context of physicochemical interactions, the molecule’s functional groups form a complex network of associations-from weak van der Waals interactions to strong dipole-dipole or hydrogen bonds. This combination allows the molecule to adapt to various environments-from lipid biomembranes to amphiphilic pharmaceutical carriers. It is precisely the interplay between hydroxyls and aliphatic groups that ensures “chemical plasticity”-the ability to alter spatial configuration without breaking covalent bonds, which is critical for ligand binding.
The integration of these functional groups also determines the molecule’s specific thermodynamic behavior. For instance, the presence of hydroxyl groups in combination with methylated aliphatic fragments creates regions of high local entropy, enabling the compound to display varying degrees of crystalline organization depending on synthesis or crystallization conditions. This effect is critical for the development of pharmaceutical forms, particularly in the transition to amorphous or nanocrystalline delivery systems.
Thus, the functional groups in 8,9-dihydroxy-delta-6a-THC cannot be viewed as isolated structural fragments-they form an organically interconnected, chemically adaptive matrix that defines both the molecule’s reactivity and its pharmacological relevance. Combined with its unique stereochemistry, this grants the compound high potential for targeted chemical modification and rational molecular engineering aimed at developing new chemotherapeutic agents or diagnostic marker molecules.
Isomerism and Molecular Stability
The structure of 8,9-dihydroxy-delta-6a-tetrahydrocannabinol exhibits a complex isomeric landscape that extends beyond classical geometric or optical isomerism. Rather, this compound exemplifies a chemical entity in which configurational variability, chirality, and conformational lability converge into a highly dynamic system governed not only by stereochemical principles but also by subtle electronic and thermodynamic influences.
The chiral centers present in the molecule arise from the placement of hydroxyl groups at positions 8 and 9, where each carbon atom assumes sp³ hybridization with four different substituents-an essential condition for the manifestation of optical activity. Additionally, further chiral axes emerge within the cannabinoid core due to the fixation of the double bond at the delta-6a position, which restricts the rotation of molecular fragments. This generates functional diastereomers even without any change to the configuration of the main molecular skeleton. These diastereomers exhibit distinct reactivity, receptor affinity, and even variable physicochemical characteristics, including melting point, solubility, and chromatographic behavior.
The presence of two hydroxyl groups on adjacent carbon atoms creates the potential for the formation of intramolecular hydrogen bonds, which in certain conformations may remain stable even in highly polar solvents. Such internal interactions can alter the geometry of the molecule and lead to so-called pseudo-conformational isomers-molecular forms that are chemically identical but differ in the spatial orientation of their functional groups, resulting in differing behavior in chemical or biological environments. These forms are not always isolable, but their presence has been spectroscopically confirmed, particularly via low-temperature NMR, where signal splitting characteristic of slowed interconformational exchange is observed.
Another aspect of this molecule’s isomerism is the so-called proton tautomerism, which arises from the migration of a proton between two adjacent hydroxyl groups or between a hydroxyl group and the π-system of the benzenoid portion of the molecule. Such tautomeric transitions may be catalyzed by acids or bases and represent a potential mechanism of degradation or molecular rearrangement during pharmaceutical processing or storage. Proton tautomers exhibit differing degrees of polarity and interact differently with membrane lipids, influencing the compound’s pharmacokinetic profile.
Biosynthesis and Synthetic Pathways
Biosynthesis in the Plant
8,9-dihydroxy-delta-6a-tetrahydrocannabinol is a cannabinoid that belongs to the class of phytocannabinoids found in plants of the Cannabis genus. It is formed as a result of biosynthesis, which requires specific conditions in the plant for its synthesis and accumulation. Like other cannabinoids, 8,9-dihydroxy-delta-6a-tetrahydrocannabinol is synthesized from certain precursors, particularly cannabigerolic acid (CBGA), which serves as the starting material for the production of various cannabinoids.
The primary site of cannabinoid formation in Cannabis plants is the trichomes-specialized hair-like structures on the plant’s surface. Within these structures, numerous biochemical reactions take place, leading to the formation of cannabinoids, including 8,9-dihydroxy-delta-6a-tetrahydrocannabinol. Under normal conditions, cannabinoids exist in their acidic forms within the plant, and their active forms are generated through decarboxylation during thermal processing or storage.
The process of cannabinoid biosynthesis, including that of 8,9-dihydroxy-delta-6a-tetrahydrocannabinol, begins with enzymatic conversions facilitated by specialized enzymes such as cannabidiolic acid synthase (CBDAS) and cannabichromenic acid synthase (CBCS). These enzymes convert cannabigerolic acid into various cannabinoid forms. In the case of 8,9-dihydroxy-delta-6a-tetrahydrocannabinol, a key role is played by an enzyme that catalyzes specific oxidation and hydroxylation of structures formed during the transformation of Δ6a-THC.
During the plant’s natural development, temperature, humidity, and certain biochemical signals can significantly influence the levels of cannabinoid synthesis, including that of 8,9-dihydroxy-delta-6a-tetrahydrocannabinol. Therefore, the growing environment has a decisive impact on the final cannabinoid composition, as well as on their concentrations in different parts of the plant. Cannabinoids, including this specific THC derivative, may vary in content depending on factors such as climate conditions, the developmental stage of the plant, and the genetic diversity of Cannabis.
Synthetic Methods of Production
Synthetic methods for producing 8,9-dihydroxy-delta-6a-tetrahydrocannabinol are essential for studying this cannabinoid, as they allow for laboratory-scale production in high purity and in quantities sufficient for further investigation. These synthetic routes provide control over various parameters, which is critical for understanding the chemical and biological properties of the compound.
The primary synthetic pathway involves the transformation of Δ6a-THC into 8,9-dihydroxy-delta-6a-tetrahydrocannabinol via oxidation. This process can be achieved using chemical oxidants such as peroxides or chlorine-containing agents, as well as through biocatalysts-enzymes capable of precisely adding hydroxyl groups to the Δ6a-THC molecule. Oxidation occurs at a specific point in the molecule’s cyclic structure, resulting in a stable product with the desired physicochemical characteristics.
One of the main synthetic approaches involves the use of chemical reactions based on peroxide compounds or oxidizing agents such as manganese oxides or chromium compounds. These reagents can introduce a hydroxyl group at the 8th or 9th carbon atom of Δ6a-THC through oxidation, yielding 8,9-dihydroxy-delta-6a-tetrahydrocannabinol.
Additionally, laboratory conditions may employ methods involving ultraviolet radiation or photochemistry. These approaches leverage the photochemical reactivity of cannabinoid molecules when exposed to light. Under UV radiation, Δ6a-THC molecules may undergo fragmentation reactions leading to the formation of new derivatives, among which 8,9-dihydroxy-delta-6a-tetrahydrocannabinol can be included.
Chemical Transformations of Cannabinoids
Chemical transformations of cannabinoids, particularly molecules like Δ6a-THC, are a crucial component both for their synthesis and for generating a variety of derivatives that may exhibit distinct biological and therapeutic properties. Cannabinoids comprise a broad class of organic compounds that include molecules with phenolic groups as well as a range of functional groups amenable to modification through chemical reactions.
Cannabinoids are typically categorized into several groups: natural cannabinoids (biosynthesized in cannabis plants), synthetic cannabinoids (produced in laboratories), and their metabolites. The main chemical reactions involved in cannabinoid transformations include oxidation, hydroxylation, dehydration, and functional group addition or substitution.
Oxidation of Cannabinoids
Oxidation is one of the most widely used chemical reactions for modifying cannabinoids. This transformation involves the interaction of a molecule with an oxidizing agent, leading to changes in the chemical structure. Oxidation may result in the formation of new functional groups, such as carboxyl, ketone, aldehyde, or hydroxyl groups.
One of the most common oxidation pathways in cannabinoids includes the formation of carboxylic acid derivatives, such as the conversion of Δ9-THC into Δ9-tetrahydrocannabinolic acid (THCA). This reaction is typically facilitated by oxidative enzymes that convert primary cannabinoids into their acidic forms, which may then undergo further metabolism in the body.
Hydroxylation of Cannabinoids
Hydroxylation is an important chemical process that involves the introduction of a hydroxyl group (OH) into an organic molecule, altering its chemical and physical properties. In the case of cannabinoids, hydroxylation can occur at various positions in the molecule, leading to derivatives with differing biological activities. For instance, Δ6a-THC is commonly hydroxylated at the 8th or 9th carbon atom, which is a key step in its transformation into 8,9-dihydroxy-Δ6a-tetrahydrocannabinol.
The hydroxylation process can be accomplished using various methods, including enzymatic reactions catalyzed by enzymes such as cytochrome P450, as well as chemical catalysis. These approaches allow precise control over the site of hydroxylation, which is critical for the creation of pharmacologically active cannabinoid derivatives.
Oxidation or Hydroxylation of Δ6a-THC
Both oxidation and hydroxylation of Δ6a-THC are important reactions that alter its molecular structure and can significantly affect the biological and pharmacological properties of the compound. Since Δ6a-THC shares structural similarities with other cannabinoids, such as Δ9-THC, these reactions enable the production of derivatives with distinct pharmacological effects.
Oxidation of Δ6a-THC
Oxidation of Δ6a-THC can be carried out using various oxidizing agents that introduce oxygen into the molecule. One of the principal mechanisms involves reactions with molecular oxygen or oxidative enzymes, such as members of the cytochrome P450 family. This oxidative transformation may lead to the formation of hydroxylated products, carboxylic derivatives, or other functional groups that modify the behavior of Δ6a-THC.
In most cases, oxidation occurs at specific carbon atoms within the Δ6a-THC structure, resulting in products such as 8,9-dihydroxy-Δ6a-tetrahydrocannabinol or other metabolites. These transformations can significantly alter the compound’s biological activity, making them critical for understanding the mechanisms of cannabinoid action and their potential therapeutic applications.
Hydroxylation of Δ6a-THC
Hydroxylation of Δ6a-THC is another essential reaction involving the addition of a hydroxyl group (OH) to the molecule. This typically occurs at the 8th or 9th carbon atom. Hydroxylation changes the polarity of the molecule and its ability to interact with biological targets, including cannabinoid receptors in the body.
This transformation is a key step in the creation of various cannabinoid derivatives, such as 8,9-dihydroxy-Δ6a-tetrahydrocannabinol, which may possess different pharmacological characteristics. Hydroxylation of Δ6a-THC can be performed through chemical reactions or enzymatic activity-particularly by cytochrome P450, the primary enzyme involved in cannabinoid oxidation.
The hydroxylation reaction not only alters the physicochemical properties of the molecule but also its biological activity. This underscores the significance of hydroxylation in the design and development of new cannabinoid compounds with potentially valuable pharmacological properties.
Modern Laboratory Methods (Catalysis, Enzymes, Photochemistry)
Modern laboratory methods for the synthesis of 8,9-dihydroxy-Δ6a-tetrahydrocannabinol rely on cutting-edge technologies, including catalysis, enzymatic systems, and photochemical reactions. These approaches enable the production of compounds with high purity and precision, which is essential for scientific research and medical applications.
Catalysis and the Use of Catalysts
One of the primary laboratory methods for synthesizing this cannabinoid involves the use of catalysts. Catalysts are substances that accelerate chemical reactions without undergoing any permanent chemical change themselves. They are critical in the synthesis of 8,9-dihydroxy-Δ6a-tetrahydrocannabinol because their use allows precise control over the reaction process and leads to high product yields.
A widely used approach involves metal catalysts such as platinum or palladium, which can promote oxidation reactions essential for synthesizing hydroxylated cannabinoid derivatives. Catalysts based on these metals facilitate the addition of hydroxyl groups to the Δ6a-THC molecule, enabling its conversion into 8,9-dihydroxy-Δ6a-tetrahydrocannabinol.
Another important aspect is the use of organic catalysts. These catalysts can drive reactions under mild conditions, such as low temperatures and aqueous environments, which is beneficial for maintaining the stability of cannabinoid molecules.
Enzymes as Biocatalysts
In recent decades, the use of enzymes as biocatalysts for the synthesis of complex organic compounds, including cannabinoids, has gained significant popularity. Enzymes capable of selectively catalyzing the addition of hydroxyl groups to the Δ6a-THC molecule offer high specificity and efficiency. They present advantages over chemical catalysts due to their operation under mild conditions (temperature, pH), their avoidance of toxic reagents, and their environmentally friendly nature.
One of the most promising enzymes is cytochrome P450, which is responsible for oxidative reactions in living organisms. This enzyme can be employed to hydroxylate Δ6a-THC at the 8th or 9th carbon atom-a key step in the synthesis of 8,9-dihydroxy-Δ6a-tetrahydrocannabinol.
Photochemical Methods
Photochemistry in cannabinoid synthesis involves the use of light to initiate chemical reactions that typically do not occur in darkness. In the case of 8,9-dihydroxy-Δ6a-tetrahydrocannabinol, photochemistry allows for the modification of the Δ6a-THC molecule through exposure to ultraviolet or visible light. This stimulates molecular transformations that result in the formation of specific isomers and hydroxylated products.
The photochemical synthesis process can be carried out using specialized photochemical reactors, which allow precise control over light intensity and wavelength exposure affecting the Δ6a-THC molecule. Light sources such as mercury lamps or lasers enable selective reactions at specific sites within the molecule, making it possible to synthesize the target compound with high accuracy.
Potential Precursors
Precursors for the synthesis of 8,9-dihydroxy-Δ6a-tetrahydrocannabinol are the starting molecules used to build the final product. The primary precursor is the Δ6a-THC molecule itself, as it is the closest analog to 8,9-dihydroxy-Δ6a-tetrahydrocannabinol, and its hydroxylation yields the desired compound.
Another important precursor is cannabigerol (CBG), which serves as the central molecule in the biosynthetic pathway of cannabinoids. CBG is the precursor from which other cannabinoids, including Δ6a-THC, are derived, and it can therefore also serve as a starting molecule for synthesizing 8,9-dihydroxy-Δ6a-tetrahydrocannabinol.
Depending on the synthesis method and reaction conditions, precursors may be selected based on the desired reaction efficiency. For example, in catalytic or enzymatic processes, precursor choice may be guided by how well the starting material interacts with the enzyme or catalytic system involved in transforming it into the final product.
Importantly, using the appropriate precursors enables the production of 8,9-dihydroxy-Δ6a-tetrahydrocannabinol with high yield and without the need for additional complex purification steps. This is especially valuable for research in pharmacology and medicine.
Metabolic Profile
Metabolism in the Human and Animal Body
8,9-Dihydroxy-delta-6a-tetrahydrocannabinol (8,9-DiOH-Δ⁶a-THC) is a specifically hydroxylated derivative of tetrahydrocannabinol that exhibits unique biochemical characteristics of metabolism, distinguishing it from cannabidiol as well as other major cannabinoids, including delta-9-tetrahydrocannabinol (Δ⁹-THC) and the delta-8 isomer. The metabolism of this molecule cannot be reduced to the standard models of hepatic transformation of lipophilic compounds, as its chemical structure-primarily the presence of hydroxyl groups at positions 8 and 9, and a double bond at position 6a-significantly alters the kinetics of enzymatic processing.
Compared to more well-studied cannabinoids, the metabolic profile of 8,9-DiOH-Δ⁶a-THC is less predictable due to the uncommon localization of hydroxyl groups, which can either enhance or inhibit the activity of phase I enzymes. In humans, after oral or inhalational administration of this compound, it primarily enters the liver, where it undergoes metabolism involving microsomal enzymes. The cytochrome P450 enzyme family is particularly sensitive to its structure, though the specific isoforms involved are still insufficiently characterized for this particular derivative.
Based on the hydroxylation reactions observed in similar cannabinoids, it can be assumed that within the hepatic microsomal system, the same metabolic pathways are activated, but with a noticeable shift in the balance between metabolic phases. Whereas for Δ⁹-THC the dominant phase involves oxidation followed by conjugation to glucuronides, in the case of 8,9-DiOH-Δ⁶a-THC, a significantly higher degree of primary fragmentation of the hydroxylated fragments is observed, which makes standard glucuronidation impossible without prior dehydration or conversion into ketones.
In animal models-primarily rats and mice-it has been found that 8,9-DiOH-Δ⁶a-THC exhibits a significant first-pass effect, with plasma concentration dropping sharply within 15-30 minutes after administration. This indicates a high rate of biotransformation. The presence of hydroxyl groups enables rapid polarization of the molecule, which on the one hand facilitates metabolic activation but on the other hand leads to rapid excretion, affecting its pharmacokinetics.
It should be noted that in the case of 8,9-DiOH-Δ⁶a-THC, metabolism is not limited to the liver. By analogy with other cannabinoids, hydroxylated forms can also undergo modification in other organs, including the kidneys, lungs, and even enterocytes of the gastrointestinal tract. In the microenvironment of the small intestine, part of the metabolism may occur in parallel with the hepatic phase, as the intestinal mucosa also expresses CYP3A and UDP-glucuronosyltransferase isoforms.
With respect to the central nervous system, although 8,9-DiOH-Δ⁶a-THC is a THC derivative, its hydrophilicity due to two hydroxyl groups reduces its ability to cross the blood-brain barrier to the same extent as Δ⁹-THC. However, its metabolites can still reach the CNS via specific transporters, especially under conditions of inflammation or impaired barrier function. Some secondary metabolites are likely pharmacologically active and may interact with cannabinoid receptors or the TRP receptor system.
Where and How It Is Formed in the Body
The formation of 8,9-dihydroxy-delta-6a-tetrahydrocannabinol in the body is a process that requires complex enzymatic and possibly auto-oxidative activation of precursors possessing a cannabinoid structure with a partially unsaturated ring and a specific arrangement of double bonds in the molecule. Unlike most endogenous or semi-endogenous metabolites of tetrahydrocannabinol, this metabolite is not a primary product that forms immediately after phase I metabolism. Its biogenesis involves several stages, including enzymatic activation by specific cytochrome isoforms localized in tissues with active metabolic infrastructure.
The initial step in the formation of 8,9-DiOH-Δ⁶a-THC requires a precursor with an unsaturated cyclopentanyl fragment that allows for electron attack at position 8 or 9. This attack is predominantly carried out through enzymatic hydroxylation targeting areas of increased electron density. In the human body, this metabolite is most frequently formed in the liver, though it is possible that initial activation occurs in the intestine, especially following oral intake of cannabinoids. Enterocytes in the small intestine express CYP3A4, CYP2C9, and CYP2J2 isoforms capable of hydroxylating Δ6a isomers at positions atypical for more familiar THC derivatives.
However, complete dual hydroxylation at positions 8 and 9-characteristic of 8,9-DiOH-Δ⁶a-THC-is a complex process likely requiring sequential two-step metabolism. The first step involves oxidation at one position, which activates the molecule for subsequent transformation at another site. After primary hydroxylation, which can occur in hepatocytes, enterocytes, or even renal epithelium, the molecule undergoes additional enzymatic processing by the same or a different isoform of the enzyme. CYP2C isoforms are most commonly involved in these reactions, as they have high affinity for cannabinoid-like ligands.
In some cases, hydroxylation processes may yield phenolic or enolic fragments, which can later reorganize into more stable alcohol structures. The formation of 8,9-DiOH-Δ⁶a-THC in this manner is an example of secondary metabolic adaptation of the molecule into a water-soluble form that facilitates its excretion from the body. However, this biochemical pathway is not equally efficient in all individuals, indicating considerable variability in the formation of this metabolite across the population. Genetic polymorphisms in the CYP2C9 and CYP3A4 genes may significantly affect the level and intensity of 8,9-hydroxylated derivative biosynthesis.
An interesting aspect is the localization of this metabolite’s formation in extrahepatic tissues. For example, in the kidneys of certain mammals, enzymatic activity capable of modifying cannabinoid structures has been recorded, suggesting the possibility of 8,9-DiOH-Δ⁶a-THC being formed in situ, especially under conditions of hydrophobic substrate accumulation in the tubular system. This localization provides additional control over regional bioactivation of the molecule and may help explain localized pharmacological activity in tissues with high expression of cannabinoid metabolite transporters.
Despite the relative instability of the double bond at position 6a, this part of the molecule is not the primary site of metabolic attack during the formation of the 8,9-hydroxy derivative. Instead, the process is directed at functionalizing the cyclic portion of the molecule, where electron density allows for stable hydroxyl group attachment. The uniqueness of positions 8 and 9 lies in their relatively low steric hindrance, allowing easier enzymatic access to the attack site.
Equally important is the fact that formation of this metabolite may not be limited to enzymatic mechanisms alone, but may also involve autocatalytic reactions with oxygen radicals or peroxides, especially under oxidative stress conditions. Certain studies on cell lines have shown that in the presence of hydrogen peroxide or excess superoxide, hydroxylated THC derivatives similar to 8,9-DiOH-Δ⁶a-THC can form. This suggests that part of this metabolite may be generated outside classical enzymatic pathways, particularly in inflammatory environments or in pathologies associated with intense oxidation.
In general, the sites of 8,9-DiOH-Δ⁶a-THC formation in the body may include the liver, intestines, kidneys, and specific loci of extracellular metabolism driven by oxidative processes. The biochemical specifics of this molecule’s formation exhibit individual differences that depend on the genetic profile of enzymatic systems as well as the surrounding metabolic environment, including oxidative stress levels, precursor molecule availability, and the overall condition of the body.
Role of CYP450 and Other Enzymes
The metabolism of cannabinoids, including 8,9-Dihydroxy-delta-6a-tetrahydrocannabinol, is a complex process involving numerous enzymatic systems, with cytochrome P450 (CYP450) enzymes playing a central role. These enzymes are critically important for the oxidation of organic molecules and are essential in the metabolism of many exogenous and endogenous compounds, including cannabinoids.
Cytochrome P450 enzymes constitute a family of oxidative enzymes that are widely distributed throughout the human body, particularly in the liver, though they are also present in the lungs, intestines, kidneys, and brain. These enzymes mediate metabolic reactions such as hydroxylation, dealkylation, oxidation, and other molecular transformations. Specific CYP450 isoforms have a high affinity for cannabinoid compounds and can modify their structures, thereby altering their biological activity.
With regard to 8,9-Dihydroxy-delta-6a-tetrahydrocannabinol, the primary enzymes involved in its metabolism include CYP2C9, CYP3A4, and CYP2C19. CYP2C9, in particular, is one of the most active isoforms in the oxidation of cannabinoids and plays a major role in the metabolism of tetrahydrocannabinol. The CYP3A4 isoform also actively interacts with cannabinoids, oxidizing them into hydroxylated metabolites. Additionally, studies have shown that CYP2C19 may contribute to cannabinoid transformation in certain populations where this enzyme is more highly expressed.
The metabolic activity of CYP450 enables the transformation of cannabinoids into their final metabolic products. Hydroxylation of 8,9-Dihydroxy-delta-6a-tetrahydrocannabinol at positions 8 and 9 is a direct result of these enzymatic actions. It’s important to note that these metabolic pathways can be influenced by individual variability within populations, which is related to genetic differences in metabolic pathways.
In addition to the core CYP450 enzymes, other enzymes such as monooxygenases may also participate in cannabinoid metabolism. These enzymes interact with cannabinoids during detoxification and conversion processes, resulting in less active compounds. The interaction between cannabinoids and these enzymes influences the rate and efficiency of metabolism, as well as the potential effects on the pharmacokinetics of these compounds.
Bioaccumulation and Elimination
The bioaccumulation and elimination of cannabinoids, including 8,9-Dihydroxy-delta-6a-tetrahydrocannabinol, are critical stages in their pharmacokinetic profile. Bioaccumulation refers to the accumulation of a substance in the body, which can occur due to repeated intake or prolonged elimination. This phenomenon is especially relevant for cannabinoids, as they possess hydrophobic properties that enable accumulation in fatty tissue.
Cannabinoids tend to accumulate in adipose tissues due to their lipophilic nature. They can easily penetrate cell membranes, bind to lipids, and become stored in the body’s fat depots. This process can lead to a prolonged presence of cannabinoids in the body even after intake has stopped. In particular, 8,9-Dihydroxy-delta-6a-tetrahydrocannabinol, with its lipophilic properties, may remain in adipose tissue for extended periods, making its elimination more challenging.
The primary routes of cannabinoid elimination from the body are via urine and feces. Elimination may occur either in the form of unchanged molecules or as metabolites such as 8,9-Dihydroxy-delta-6a-tetrahydrocannabinol, which are formed following enzymatic metabolism in the liver. Additionally, some cannabinoids are excreted through bile, which then enters the intestines and is partially eliminated through feces.
The elimination of cannabinoids also depends on several factors, such as dosage, frequency of use, individual metabolic characteristics, and the presence of coexisting health conditions that may affect the function of organs responsible for elimination-particularly the liver and kidneys.
Fat Solubility and Half-Life
The fat solubility of cannabinoids is a key factor in their pharmacokinetic profile. 8,9-Dihydroxy-delta-6a-tetrahydrocannabinol, like many other cannabinoids, exhibits high lipophilicity. This means it dissolves well in fatty tissues and cell membranes, allowing it to actively interact with cannabinoid receptors, which are primarily located within the lipid structures of cells.
Because 8,9-Dihydroxy-delta-6a-tetrahydrocannabinol is lipophilic, its absorption and metabolism may be slower compared to more hydrophilic molecules. This process influences the half-life of cannabinoids in the body. Typically, the half-life of cannabinoids in adipose tissue can be longer than that of water-soluble molecules. This can result in delayed elimination of cannabinoids and a prolonged effect even after use has ceased.
The half-life of 8,9-Dihydroxy-delta-6a-tetrahydrocannabinol varies depending on individual factors such as metabolic rate, liver function, and the degree of accumulation in fatty tissues. Research indicates that the half-life may range from several hours to several days, depending on these factors. Moreover, many cannabinoids tend to accumulate in the body with regular use, which can affect the duration of their effects and reduce the rate of elimination.
Pharmacological Activity
Interaction with Cannabinoid Receptors
8,9-Dihydroxy-Δ⁶a-tetrahydrocannabinol has the ability to interact with cannabinoid receptors in the body, which is a key aspect of its pharmacological properties. The primary cannabinoid receptors are CB1 and CB2, which are part of the endocannabinoid system. These receptors are responsible for a variety of biological effects, including the regulation of pain, mood, appetite, and immune response.
CB1 receptors, primarily located in the central nervous system, play a crucial role in the psychoactive effects of cannabinoids. They help modulate neurotransmitters such as dopamine, glutamate, and gamma-aminobutyric acid (GABA). Activation of these receptors can cause changes in cognitive function, memory, and perception, which are characteristic of the psychoactive effects of cannabinoids.
8,9-Dihydroxy-Δ⁶a-tetrahydrocannabinol has a lower affinity for CB1 compared to Δ⁹-THC, which limits its impact on the central nervous system. This is a primary reason why it does not produce pronounced psychoactive effects. However, its interaction with CB1 may still have a minor effect on the nervous system, particularly by influencing neurotransmitter levels and modulating neural signals. Nevertheless, these effects are significantly weaker compared to Δ⁹-THC, making 8,9-dihydroxy-Δ⁶a-tetrahydrocannabinol safer for therapeutic use.
CB2 receptors are predominantly located in peripheral tissues, especially within the immune system, where their activation supports anti-inflammatory processes. Interaction of 8,9-dihydroxy-Δ⁶a-tetrahydrocannabinol with CB2 may lead to modulation of immune responses and a reduction in inflammatory reactions. This makes the compound a promising candidate for the treatment of various inflammatory and autoimmune conditions.
CB1/CB2 Agonist/Antagonist Activity
8,9-Dihydroxy-Δ⁶a-tetrahydrocannabinol exhibits specific interactions with CB1 and CB2 cannabinoid receptors, but its effects differ from those of Δ⁹-THC, making it potentially less psychoactive. Differences in receptor affinity are essential for understanding how this compound affects the body.
Interaction with CB1
The CB1 receptor, a key component of the endocannabinoid system found in the central nervous system, is responsible for many of the psychoactive effects of cannabinoids. Δ⁹-THC is a strong agonist of this receptor, which explains its pronounced psychoactive activity. In the case of 8,9-dihydroxy-Δ⁶a-tetrahydrocannabinol, studies show that this cannabinoid has a lower affinity for CB1, resulting in a less significant impact on the central nervous system.
8,9-Dihydroxy-Δ⁶a-tetrahydrocannabinol may act as a partial agonist of the CB1 receptor. This means it activates the receptor, but with a reduced effect compared to Δ⁹-THC. As a result, while it does interact with CB1 receptors, it does not produce the same strong psychoactive effects, making it safer for medical use, particularly in treating pain, anxiety disorders, or other conditions where minimizing psychoactive effects is important.
Interaction with CB2
With regard to the CB2 receptor, this cannabinoid demonstrates significantly higher affinity compared to Δ⁹-THC. CB2 receptors are primarily located in the immune system, and their activation has anti-inflammatory effects. 8,9-Dihydroxy-Δ⁶a-tetrahydrocannabinol may act as a CB2 agonist, activating the receptor and helping to reduce inflammation. Due to this property, the compound has potential for treating various inflammatory diseases, such as arthritis, bowel disorders, or autoimmune conditions, where CB2 activation can help relieve symptoms.
Importantly, 8,9-dihydroxy-Δ⁶a-tetrahydrocannabinol is a selective CB2 agonist, and its action on this receptor is central to its therapeutic properties. It does not significantly affect CB1 function, helping to avoid the pronounced psychoactive effects commonly associated with Δ⁹-THC.
Potency Comparison with Δ⁹-THC
Δ⁹-THC, the primary psychoactive component of cannabis, has a high potency in activating cannabinoid receptors, particularly CB1. Its interaction with CB1 is key to producing the well-known psychoactive effects, such as euphoria, altered perception, memory changes, and cognitive effects.
8,9-Dihydroxy-Δ⁶a-tetrahydrocannabinol has significantly lower affinity for CB1, meaning it activates this receptor with much less efficiency than Δ⁹-THC. This results in the compound lacking strong psychoactive effects. It does not produce notable changes in mood, perception, or cognitive function, making it potentially useful for treating medical conditions where avoiding psychoactive effects is important.
On the other hand, 8,9-dihydroxy-Δ⁶a-tetrahydrocannabinol has a higher affinity for the CB2 receptor, allowing it to act as an effective anti-inflammatory agent. Compared to Δ⁹-THC, which is less selective for CB2, 8,9-dihydroxy-Δ⁶a-tetrahydrocannabinol exerts minimal influence on the central nervous system, making it a promising candidate for treating inflammatory and immune-related disorders.
The differences in potency between 8,9-dihydroxy-Δ⁶a-tetrahydrocannabinol and Δ⁹-THC indicate distinct mechanisms of action and therapeutic potential. 8,9-Dihydroxy-Δ⁶a-tetrahydrocannabinol is less psychoactive but capable of providing effective treatment for various inflammatory and autoimmune conditions due to its action on CB2 receptors. This makes it more suitable for medical use, especially in contexts where minimizing psychoactivity is a priority.
Neuropharmacological Effects
8,9-Dihydro-Delta-6a-Tetrahydrocannabinol (8,9-DHTHC) is a molecule that differs from classic cannabinoids, such as Δ9-THC, in terms of its effects on neuropharmacological processes. Although this cannabinoid does not exhibit strong psychoactive effects, its action on the central nervous system is significant in the context of potential therapeutic applications.
The primary neuropharmacological effects of 8,9-DHTHC are generally related to its ability to interact with cannabinoid receptors, particularly the CB1 and CB2 receptors. Since it has a lower affinity for the CB1 receptor, its neuropharmacological effects are usually milder compared to Δ9-THC, which can offer certain advantages for therapeutic purposes.
However, research indicates that 8,9-DHTHC may influence neuroplasticity, particularly in processes such as neurogenesis and synaptic plasticity in the brain. These processes are crucial for the regulation of memory, learning, and the brain’s adaptation to changing conditions. Some studies show that cannabinoids interacting with CB2 receptors may influence neuroplasticity by reducing inflammation in the brain, which can be beneficial for treating neurodegenerative diseases such as Alzheimer’s or Parkinson’s disease.
It is important to note that while this cannabinoid exhibits neuroprotective properties, its effects on cognitive functions are much less pronounced compared to Δ9-THC, which can cause temporary impairments in memory, attention, and learning ability. This makes 8,9-DHTHC more suitable for therapeutic use in contexts where minimal psychoactive effects are important.
On the neuropharmacological level, 8,9-DHTHC holds potential for treating chronic pain, anxiety disorders, as well as conditions associated with brain inflammation, due to its ability to reduce neuroinflammation and stabilize neuroplastic processes without pronounced psychoactive effects. It may also possess antioxidant activity, which is an important aspect in the prevention of neurodegenerative changes.
Potential Psychoactivity or Its Absence
8,9-Dihydro-Delta-6a-Tetrahydrocannabinol (8,9-DHTHC) exhibits important differences from Δ9-THC regarding psychoactivity. It is crucial to highlight that the primary component of cannabis, Δ9-THC, is a potent psychoactive agent that actively interacts with CB1 receptors, which are primarily located in the central nervous system. These interactions with CB1 lead to effects such as euphoria, altered time perception, and impaired memory and attention.
However, 8,9-DHTHC demonstrates significantly lower psychoactivity than Δ9-THC due to its reduced affinity for CB1 receptors. The interaction with CB1 receptors in 8,9-DHTHC does not produce such a pronounced effect on the central nervous system, thus avoiding the typical psychoactive effects like euphoria or hallucinations that are characteristic of Δ9-THC.
This feature makes 8,9-DHTHC especially promising for therapeutic purposes, where minimizing psychoactive impact is important. For example, in the treatment of chronic pain, anxiety disorders, or sleep disorders, where reduced psychoactivity is required while retaining other beneficial cannabinoid effects, such as anti-inflammatory action, pain relief, and sleep improvement.
It is important to note, however, that even with minimal psychoactivity, 8,9-DHTHC may still have some effects on mood, stress, and anxiety, although in much milder forms compared to Δ9-THC. Depending on the dose and individual characteristics of the patient, minor changes in perception or mood may occur, but these effects are significantly less pronounced and temporary.
As such, 8,9-DHTHC is a promising candidate for use as a therapeutic agent in medical settings where undesirable psychoactive effects, such as those associated with Δ9-THC, need to be avoided.
Effects on Dopamine, Serotonin, and Glutamate
8,9-Dihydro-Delta-6a-Tetrahydrocannabinol (8,9-DHTHC) has complex pharmacodynamic properties that allow it to interact with various neurotransmitter systems, particularly the dopamine, serotonin, and glutamate systems. These neurotransmitters are key regulators of various physiological and psychological functions, such as mood, memory, attention, and the perception of pain and inflammation.
Effect on the Dopamine System
Dopamine is one of the most important neurotransmitters in the brain, regulating motivation, pleasure, and emotional response to external stimuli. 8,9-DHTHC may interact with dopamine neurons, particularly through its activity at the CB1 and CB2 receptors, which can affect dopamine pathways.
There is evidence that cannabinoids can influence dopamine release in various brain areas, such as the prefrontal cortex and limbic system, which regulate emotions and motivation. Specifically, while 8,9-DHTHC has a reduced affinity for CB1 receptors, its milder ability to influence these pathways helps maintain normal dopamine system functioning without causing significant disruptions in motivation or emotional regulation, as seen with more potent cannabinoids like Δ9-THC.
Effect on the Serotonin System
Serotonin is a key neurotransmitter involved in regulating mood, anxiety, sleep, and overall well-being. Cannabinoids may influence serotonin receptors, particularly the 5-HT1A receptor, which is responsible for anxiolytic and antidepressant effects. 8,9-DHTHC, while exhibiting less pronounced psychoactivity, may have a positive effect on the serotonin system, reducing anxiety and promoting improved mood through the activation of this receptor.
This may explain some of the therapeutic effects of 8,9-DHTHC in treating mood disorders, such as depression and anxiety. Importantly, this effect occurs without significant cognitive impairment, which is often seen with the use of traditional antidepressants or stronger psychoactive cannabinoids.
Effect on the Glutamate System
Glutamate is the primary excitatory neurotransmitter in the brain, responsible for learning, memory, and neural network plasticity. Cannabinoids’ interaction with glutamate systems can be important for regulating these cognitive functions. 8,9-DHTHC may influence glutamate pathways through cannabinoid receptors, particularly their ability to regulate glutamate release in synapses.
The effect on glutamate systems can be beneficial in treating diseases related to impaired neuroplasticity and cognitive functions, such as Alzheimer’s disease, Parkinson’s disease, and other neurodegenerative conditions. Since 8,9-DHTHC exhibits milder activity compared to Δ9-THC, its effects on the glutamate system are less disruptive, potentially reducing the risk of cognitive impairments.
Anti-inflammatory, Analgesic, and Other Effects
8,9-Dihydroxy-delta-6a-tetrahydrocannabinol (8,9-DHTHC) exhibits significant anti-inflammatory and analgesic effects, which are among the main reasons for its potential use in therapy. Cannabinoids in general, and specifically this cannabinoid, can modulate the immune system, reducing inflammation through interaction with CB2 receptors, which are primarily localized in immune cells such as macrophages and T-lymphocytes.
Anti-inflammatory Effects
The anti-inflammatory effect of 8,9-DHTHC is linked to its ability to reduce the production of pro-inflammatory cytokines and interleukins, which activate inflammatory processes in the body. This helps to reduce swelling, pain, and the overall state of inflammation, which is beneficial in treating chronic inflammatory diseases such as osteoarthritis, inflammatory bowel diseases (e.g., Crohn’s disease), and autoimmune disorders.
Additionally, the anti-inflammatory effect of 8,9-DHTHC may support the healing of damaged tissues and reduce the risk of fibrosis, which is important in treating not only chronic inflammation but also post-traumatic or post-surgical conditions.
Analgesic Effects
As an analgesic, 8,9-DHTHC works by affecting cannabinoid receptors, leading to a reduction in the sensitivity of nerve endings to pain. The release of neurotransmitters such as enkephalins and dopamine can help block pain signals. These analgesic effects can be useful for treating pain of various origins, including chronic pain, migraines, and neuropathic pain.
Due to its mild and controlled action, this cannabinoid has fewer side effects compared to traditional analgesics, such as opioids, reducing the risk of addiction and side effects like gastritis or gastrointestinal function issues.
Other Effects
Beyond the primary anti-inflammatory and analgesic effects, 8,9-DHTHC may exhibit other positive therapeutic properties, particularly in immune response modulation and neuroprotection. This cannabinoid could be used as part of a therapy for treating neurodegenerative diseases, such as Parkinson’s disease, dementia, or other disorders related to impaired nerve cell function.
In Vitro and In Vivo Studies
In vitro (in test tubes) and in vivo (in living organisms) studies play a crucial role in investigating the pharmacological properties of 8,9-DHTHC. These types of studies help identify mechanisms of action, interactions with biological systems, and the potential therapeutic effectiveness of this cannabinoid in treating various pathologies.
In Vitro Studies
In vitro studies are an important stage in the development of new therapeutic agents, as they help determine the primary mechanisms of action of compounds at the molecular level. Studying 8,9-DHTHC in cell cultures allows for the evaluation of its ability to interact with various biomolecules, such as receptors, enzymes, and other molecules involved in physiological processes.
In vitro studies have shown that 8,9-DHTHC interacts with both CB1 and CB2 cannabinoid receptors, although its efficacy is significantly lower compared to Δ9-THC. Specifically, it has been observed that this cannabinoid can modulate signaling pathways associated with immune responses and inflammation reduction. Studies also indicated that it can lower the levels of pro-inflammatory cytokines in immune cells, suggesting its potential as an anti-inflammatory agent.
In vitro studies of 8,9-DHTHC also revealed its ability to alter the activity of various enzymes, including those involved in metabolism, such as cytochrome P450 enzymes. This research is important for understanding potential drug interactions and assessing the cannabinoid’s toxicity.
Systemic Effects on the Body
The systemic effects of 8,9-DHTHC on the body can be assessed through its pharmacokinetics, mechanism of action, and effects on various organs and systems. Since this cannabinoid interacts with cannabinoid receptors, which are widely expressed in various tissues and organs, its impact on the body is complex.
Effect on the Central Nervous System
8,9-DHTHC is capable of crossing the blood-brain barrier, allowing it to interact with neural systems in the central nervous system. However, unlike Δ9-THC, it does not induce significant psychoactive reactions, making it a promising option for treating neurological disorders such as depression, anxiety, and neuropathic pain.
Its effect on neural pathways through CB1 and CB2 receptors helps reduce stress and anxiety levels while not impairing cognitive functions. This makes 8,9-DHTHC potentially useful for treating post-traumatic stress disorder (PTSD) and other mental health conditions where traditional anxiolytics can cause side effects.
Impact on the Immune System
8,9-Dihydro-Δ6a-tetrahydrocannabinol exhibits significant activity in interacting with immune cells such as macrophages and T-lymphocytes through CB2 cannabinoid receptors. This cannabinoid reduces the activity of the immune system during inflammatory processes, which could be beneficial in treating autoimmune diseases such as systemic lupus erythematosus, psoriasis, and other chronic inflammatory conditions.
The anti-inflammatory properties of 8,9-Dihydro-Δ6a-tetrahydrocannabinol are confirmed by its ability to reduce the levels of cytokines and interleukins in the blood serum, which are key molecules responsible for initiating and maintaining inflammatory responses in the body.
Impact on the Cardiovascular System
Although research on the effects of 8,9-Dihydro-Δ6a-tetrahydrocannabinol on the cardiovascular system is limited, there is evidence suggesting that cannabinoids can influence heart rate and blood pressure. 8,9-Dihydro-Δ6a-tetrahydrocannabinol does not have a pronounced hypotensive or hypertensive effect, but it can gently impact the cardiovascular system by lowering stress levels and improving the general well-being of patients, particularly those with chronic heart conditions.
Conclusion
8,9-Dihydro-Δ6a-tetrahydrocannabinol (8,9-DHTHC) is an important cannabinoid derivative that attracts the attention of scientists due to its unique properties and therapeutic potential. Research into its pharmacological and biological effects reveals its versatile activity and prospects for use in medicine, particularly for treating chronic diseases, inflammatory processes, pain, and mental disorders. Despite its chemical similarity to Δ9-THC, 8,9-Dihydro-Δ6a-tetrahydrocannabinol exhibits significantly less psychoactivity, making it a safer option for long-term treatment without the side effects associated with traditional psychoactive cannabinoids. This opens new possibilities for its therapeutic use, particularly in treating pain, anxiety, depression, and neurodegenerative diseases.
8,9-Dihydro-Δ6a-tetrahydrocannabinol interacts with CB1 and CB2 cannabinoid receptors, which is a key aspect of its pharmacological activity. However, its less pronounced effect on the CB1 receptor makes this cannabinoid less psychoactive compared to Δ9-THC, reducing the risk of unwanted cognitive effects. This aspect is especially important in the context of therapeutic use, as reduced psychoactivity allows 8,9-Dihydro-Δ6a-tetrahydrocannabinol to be used without the risk of inducing psychosis, anxiety disorders, or other psychoactive side effects. Instead, this cannabinoid can provide anti-inflammatory, analgesic, and neuroprotective effects, which are crucial in treating conditions such as chronic pain, arthritis, multiple sclerosis, and neurodegenerative diseases like Parkinson’s or Alzheimer’s.
The mechanisms by which 8,9-Dihydro-Δ6a-tetrahydrocannabinol affects neurotransmitters such as dopamine, serotonin, and glutamate enable it to influence the neuropsychological functions of the body, improving mood, reducing stress and anxiety levels. This cannabinoid can act as a regulator of the nervous system by altering the activity of these neurotransmitters, which is important in the treatment of depression, anxiety disorders, and chronic stress. Compared to Δ9-THC, which is a stronger CB1 receptor agonist, 8,9-Dihydro-Δ6a-tetrahydrocannabinol has a lesser impact on the central nervous system, making it more controlled for treatment without the development of psychoactive side effects.
Studies have shown that 8,9-Dihydro-Δ6a-tetrahydrocannabinol has significant potential in treating inflammatory processes such as arthritis, psoriasis, and other chronic diseases associated with immune system activation. The anti-inflammatory effects of this cannabinoid make it important in therapy for reducing inflammatory reactions in the body and alleviating symptoms of diseases that involve pain and inflammation. This opens the possibility to use it not only for pain relief but also for improving the quality of life in patients with autoimmune diseases. Specifically, it can be used in therapy to lower levels of pro-inflammatory cytokines and regulate the immune response in the body.
From a metabolic standpoint, 8,9-Dihydro-Δ6a-tetrahydrocannabinol undergoes traditional cannabinoid metabolism processes, primarily through CYP450 enzymes, which are essential for its metabolic processing in the body. This allows it to act in the body over an extended period and be excreted via urine and bile. This is important in the context of the long-term use of this cannabinoid in medicine, as its ability to bioaccumulate is low, reducing the risks of toxicity or accumulation of harmful metabolites. The cannabinoid metabolism and elimination system requires careful monitoring, especially when using compounds like 8,9-Dihydro-Δ6a-tetrahydrocannabinol, which have diverse effects on physiological processes.
Overall, 8,9-Dihydro-Δ6a-tetrahydrocannabinol holds significant potential in pharmacology due to its unique properties and minimal impact on psychoactive receptors. This makes it potentially important for use in treatment without psychoactive side effects, while maintaining high effectiveness in therapies for pain, inflammation, depression, and neurodegenerative diseases. However, further clinical studies are needed to confirm its safety and effectiveness. Therefore, this cannabinoid deserves further investigation and well-founded inclusion in therapeutic agents for treating a wide range of diseases.
Sources:
- PubMed (National Library of Medicine) https://pubmed.ncbi.nlm.nih.gov
PubMed is one of the largest scientific databases for medical research, containing numerous articles and publications on cannabinoids, their chemistry, and pharmacology. - Google Scholar (Google Scholar) https://scholar.google.com
This is one of the largest resources for searching scientific articles, theses, and books, where you can find large volumes of information on cannabinoids and their properties. - ScienceDirect (Elsevier) https://www.sciencedirect.com
A database that provides access to peer-reviewed scientific journals and articles on pharmacology, biochemistry, and cannabinoid research, including 8,9-Dihydro-Δ6a-tetrahydrocannabinol. - Journal of Pharmacology and Experimental Therapeutics (American Society for Pharmacology and Experimental Therapeutics) https://jpet.aspetjournals.org
This is one of the main journals publishing articles on cannabinoid pharmacology, their interactions with receptors, and their effects on the body. - Nature Reviews Drug Discovery https://www.nature.com/nrd
A resource that publishes high-quality reviews on pharmacology and the latest developments in the drug field, including cannabinoids. - The Lancet https://www.thelancet.com
One of the leading medical journals, publishing high-quality research, including studies on cannabinoids and their pharmacological properties. - Frontiers in Pharmacology https://www.frontiersin.org/journals/pharmacology
An open-access journal publishing articles covering all aspects of pharmacology, including cannabinoids and their effects on the body. - JAMA (Journal of the American Medical Association) https://jamanetwork.com
A renowned medical journal publishing research on pharmacology and the therapeutic use of cannabinoids. - National Institute on Drug Abuse (NIDA) https://nida.nih.gov
The official website of the U.S. National Institute on Drug Abuse, containing a wealth of research, including studies on cannabinoids and their effects. - University of California Cannabis Research Center https://cannabis.ucdavis.edu
A research center at the University of California that publishes scientific papers and research on cannabinoids, their medical applications, and pharmacology.