Cannabichromanon (CBCF) is one of the lesser-studied members of the cannabinoid class – natural phenolic compounds produced by the Cannabis sativa plant. Despite significant progress in researching major cannabinoids such as Δ9-tetrahydrocannabinol (THC) and cannabidiol (CBD), knowledge about the biochemical and pharmacological properties of less common cannabinoids, including CBCF, remains fragmentary. This creates a scientific gap that complicates a full understanding of the biochemistry and potential medical applications of this group of compounds.
CBCF belongs to the class of cannabichromanons – cannabinoids characterized by the presence of a chromane core with a ketone functional group. The chromane skeleton is a structural core that provides the compound with unique chemical properties, including increased chemical stability compared to other cannabinoids. The molecular peculiarity of CBCF lies in the combination of the phenolic part typical for cannabinoids with the chromane ring, which modifies the molecule’s electronic structure and potentially affects its bioactivity. At the same time, this class of compounds remains insufficiently studied from a chemical and pharmacological perspective.
From a scientific point of view, it is important to distinguish CBCF from more common cannabinoids, as structural and functional small modifications in molecules can lead to significantly different interactions with biological targets. In particular, changes in the chromane core can substantially influence affinity for cannabinoid receptors CB1 and CB2, as well as potential interactions with other proteins involved in regulating inflammatory processes, pain sensitivity, or neuromodulation. However, precise data on CBCF’s pharmacodynamics is currently limited due to the lack of systematic studies and the difficulty in obtaining the pure substance in large quantities.
Another important component in the study of CBCF is understanding its biogenetic origin. Cannabinoid biosynthesis in Cannabis sativa is a complex system involving enzymatic transformations of precursors such as olivetolic acid and germacrene B, leading to the formation of main cannabinoids, including cannabinoid acids (e.g., CBGA) and their subsequent conversion into active forms. Resolving the question of the pathway for CBCF formation requires detailed study of enzymatic mechanisms and possible oxidative processes that may cause the emergence of the chromanone structure. At present, this remains a subject of hypotheses and indirect experimental evidence based on modern analytical methods such as liquid chromatography-mass spectrometry (LC-MS) and nuclear magnetic resonance (NMR).
Beyond biogenesis, significant attention is given to methods of synthesizing CBCF, which are divided into semi-synthetic, fully synthetic, and biotechnological approaches. Semi-synthetic methods involve modifying natural cannabinoids or their acidic forms using various chemical reagents and catalysts. Full laboratory synthesis, although less common, opens the way for detailed control over the molecular structure and the possibility of creating analogs with improved properties. Biotechnological approaches, including the use of genetically modified microorganisms, are gaining popularity as environmentally friendly and promising for large-scale production.
Research into the biological potential of CBCF is at an early stage. Preliminary data suggest a possible impact of the compound on cannabinoid and other receptor systems, which could serve as a basis for developing new pharmacological agents with anti-inflammatory, neuroprotective, or analgesic effects. However, the lack of comprehensive toxicological and pharmacokinetic studies limits the clinical application of CBCF and its derivatives.
Given the above, scientific interest in CBCF follows several directions: first, detailed chemical analysis of its structure, including studying stereochemistry and physicochemical properties; second, establishing the biogenetic pathways of its formation in the plant; third, optimizing methods for obtaining CBCF for research and potentially industrial purposes; fourth, assessing biological activity and pharmacological profile with subsequent validation in preclinical models.
The absence of comprehensive studies on CBCF creates an urgent need for systematization and in-depth investigation of this compound. Modern analytical techniques, including high-resolution mass spectrometry and nuclear magnetic resonance, enable identification of CBCF even at low concentrations, opening opportunities for its study as a potential biomarker of specific Cannabis sativa chemotypes. This is important not only for cannabinoid chemistry but also for the development of new tools for personalized medicine and biotechnological platforms.
Chemical Identity of CBCF
Cannabichromanon (CBCF) belongs to the class of cannabinoids characterized by the presence of a chromane ring with a ketone functional group, which distinguishes it from classical cannabinoids with a tetrahydrocannabinol backbone. This structural feature gives CBCF unique chemical properties that define its physicochemical profile and potential biological functions. The chemical identity of CBCF includes not only its molecular formula and structure but also its spatial configuration, electronic organization, as well as the range of reactions it can undergo under various conditions.
The characterization of CBCF is based on determining its molecular composition and structure, which serve as the foundation for further understanding of its chemical reactivity and biological activity. This compound belongs to the group of phenolic chromanones – natural or semi-synthetic substances that have a cyclic structure with substituted benzene rings and simultaneously contain a ketone functional group within the chromane ring. The presence of the ketone in the molecular framework is a defining factor that modifies the electron distribution and provides particular chemical stability.
It is important to note that CBCF differs from other cannabinoids, such as cannabichromene (CBC), which has only the chromane backbone without the carbonyl group. The introduction of the ketone fragment leads to the formation of the chromanone core, making CBCF unique among phytocannabinoids. This is highly significant for the chemical properties of the molecule, particularly its reactivity, spectral characteristics, and stability.
Considering modern analytical methods, CBCF identification is carried out using high-precision analytical instruments – nuclear magnetic resonance (NMR) spectroscopy, high-resolution liquid chromatography-mass spectrometry (LC-HRMS), and infrared (IR) spectroscopy. The compound, either synthesized or extracted from plant materials, is studied for compliance with structural parameters critical to establishing its chemical identity. In this context, the high resolution of spectroscopic methods allows confirmation of the presence of the chromanone ring and ketone group, as well as determination of the spatial configuration.
CBCF has the potential to serve as a marker in comprehensive chemotyping of Cannabis sativa, which is an important component in studying the plant’s cannabinoid profile. Unlike more common cannabinoids, CBCF is found in relatively low concentrations, which complicates its analytical detection but also makes it valuable for researchers aiming to reveal finer aspects of metabolic diversity. The detection of CBCF may indicate specific enzymatic pathways or oxidative processes occurring in the plant and thus serve as an indicator of the biochemical characteristics of particular strains.
From a chemical standpoint, CBCF is a promising object of study due to its ability to participate in various reactions, which may include redox processes, nucleophilic attacks, or conjugate formation with biomolecules. The presence of the ketone group opens possibilities for forming different derivatives, expanding the potential for chemical modification and the creation of synthetic analogs with desired properties. This is especially relevant in pharmaceutical chemistry, where small changes in cannabinoid structures can significantly affect their biological activity.
Studying the reactivity of CBCF is also important for determining its stability in biological environments. Generally, cannabinoids are lipophilic compounds, which affects their solubility and bioavailability, as well as their metabolic pathways in the body. CBCF, due to its specific chromanone structure, may have different solubility and stability characteristics compared to more traditional cannabinoids. It is known that ketone compounds can be more resistant to hydrolysis or enzymatic degradation, potentially influencing the duration of their action and the way they interact with cellular targets.
Molecular Structure and Nomenclature
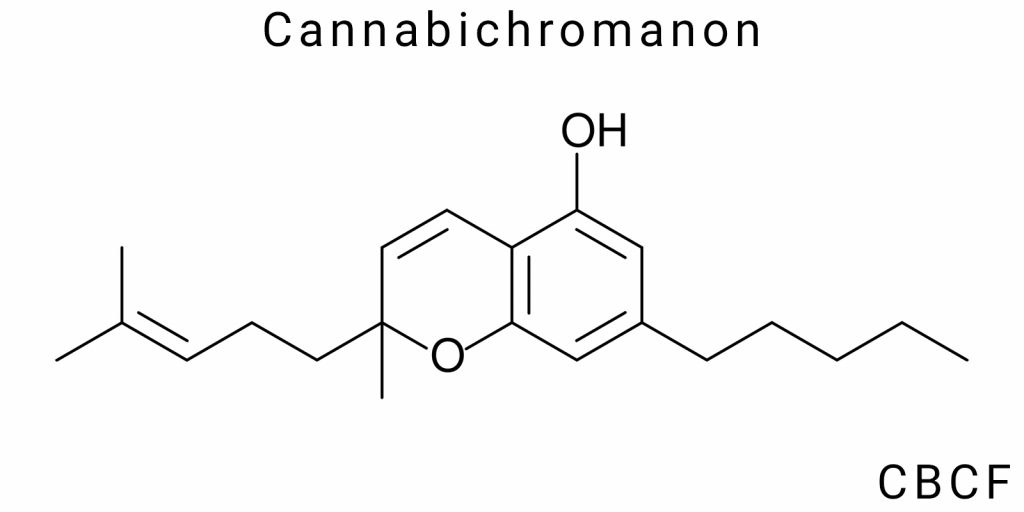
Cannabichromanon (CBCF) belongs to the class of chromanones with substituted phenolic groups, and its molecular architecture is formed by the combination of three key fragments: a partially hydrogenated benzopyran core (chromanone), an alkyl side chain at position 2, and a phenolic benzene ring at position 8. The central element is the chromanone system-a fused ring consisting of a benzene and tetrahydropyran fragment with a carbonyl group at position 4, which formally classifies the structure as a 4-chromanone.
The main backbone structure of CBCF includes a 2-allyl-8-hydroxy-4H-chromen-4-one system with characteristic substitutions at side positions. At the carbon position 2 (according to IUPAC numbering of the chromanone ring), a propyl or butyl chain is attached (in various isomeric forms-such as 1-methylpropyl or n-pentyl), which results from natural biosynthetic interaction with olivetol or similar precursors. In most chemically characterized variants of CBCF, the presence of an n-pentyl side group is observed, which is typical for a range of phytocannabinoids.
Another key structural fragment is the phenolic group at position 8, which enables the molecule’s participation in electron exchange and hydrogen bonding interactions. Its presence is not only structural but also a functionally active part of the molecule, particularly due to its ability for electron delocalization within the aromatic core. The position of the hydroxyl group on the phenolic ring is precisely determined by spectroscopic analysis, especially ^1H NMR, where its characteristic signal serves as a diagnostic marker to differentiate CBCF from related chromanones.
The generalized chemical name for CBCF according to IUPAC (for the most stable and common isomer) can be formulated as: 2-pentyl-8-hydroxy-4H-chromen-4-one, or more precisely accounting for alkylation of the aromatic ring-2-(n-pentyl)-8-hydroxy-1-benzopyran-4-one. The use of the trivial name “cannabichromanon” is justified within cannabinoid chemistry but is not a formal systematic nomenclature. The name “CBCF” is derived by analogy to cannabichromene (CBC), with the addition of “F” as an abbreviation for “flavonoid” or “phenone” structure, although this classification does not conform to strict chemical terminology rules.
The spatial configuration of CBCF is predominantly planar within the aromatic core but includes conformational flexibility in the chain at C2 and the tetrahydropyran ring region. This results in various tautomeric and conformational states observable in spectral analysis. The CBCF structure allows the existence of both keto and hydroxy-enol forms, with a potential shift in equilibrium depending on pH and environment. The keto form is more stable in nonaqueous systems; however, in aqueous environments, particularly at physiological pH, partial enolization may occur.
Chemical stability of CBCF is attributed to resonance stabilization of the carbonyl group within the chromanone structure, as well as intramolecular hydrogen bonding between the hydroxyl at position 8 and the oxygen of the ketone group. This interaction modifies the electron density around the oxygen atoms, reducing the molecule’s reactivity toward nucleophilic attack. This can influence the metabolic fate of the compound in biological systems, slowing enzymatic oxidation or hydrolysis.
It is also worth noting the possibility of isomeric forms of CBCF due to variations in the alkyl side chain, hydroxyl position, or the degree of saturation of the chromanone ring. According to theoretical modeling, the most thermodynamically stable isomer is the one with an n-pentyl substitution at C2, whereas branched chains may exhibit higher reactivity but lower stability. These structural differences are directly relevant to chemical synthesis and further applications of CBCF in pharmacological research.
The stereochemical component of CBCF is predominantly achiral in the main backbone; however, in cases of further chemical modifications or in a biogenetic context, stereocenters may appear, particularly upon hydroxylation of the side chain or hydrogenation of double bonds in the ring. In such cases, chiral chromatography or chiral-sensitive spectroscopic analysis is required to determine the absolute configuration.
Identification of CBCF as a distinct chemical entity requires not only structural analysis but also classification within current chemical nomenclature systems. In databases (including PubChem, ChEMBL, ChemSpider), CBCF may be recorded as a specific chromanone cannabinoid with an assigned CAS number, provided sufficient validation. However, to date, standardized registration of CBCF as an individual compound remains limited due to the lack of large-scale isolation or synthesis. Thus, its chemical identity remains partly provisional and depends on the accuracy of structural description in scientific literature.
Physicochemical Characteristics
The physicochemical characteristics of cannabichromanon (CBCF) are determined by its molecular architecture, electronic configuration, and functional groups, which influence thermodynamic parameters, spectral properties, phase behavior, solubility, reactivity, and stability in various environments. These characteristics are critical for its chemical behavior, pharmacokinetics, and analytical monitoring capabilities. A comprehensive physicochemical profiling of CBCF encompasses both macroscopic properties (melting and boiling points, density, color) and microscopic parameters (dipole moment, polarizability, molecular orbital energy levels).
Under standard conditions, CBCF is a solid with a yellowish-brown color, attributable to the presence of an aromatic system with delocalized π-electrons that absorb in the visible range. Its typical form is amorphous or microcrystalline with no pronounced polymorphism. In cases where CBCF crystallizes, X-ray structural analysis reveals orthorhombic or triclinic crystal systems, depending on purification methods and precipitation conditions. The melting point ranges approximately from 130 to 145 °C, showing minor dependence on the length of the alkyl side chain. This range indicates the presence of intermolecular hydrogen bonding and π-π stacking interactions between aromatic fragments.
CBCF is thermally stable up to 200-220 °C, after which decomposition begins with the formation of volatile products, including phenolic fragments, saturated hydrocarbons, and ketones. Thermogravimetric analysis (TGA) shows a single-stage mass loss during degradation, indicating its chemical homogeneity. Differential scanning calorimetry (DSC) records a clearly defined endothermic phase transition prior to decomposition, without intermediate glassy or mesophase states.
CBCF’s solubility reflects its amphiphilic nature: it is highly soluble in nonpolar organic solvents (chloroform, dichloromethane, ethyl acetate, acetone) and poorly soluble in water, less than 5 μg/mL at pH 7.0. The polar hydroxyl group at position 8 contributes to solubility in alcohols (ethanol, methanol, propanol), but the presence of a hydrophobic alkyl side chain counterbalances this effect in aqueous media. CBCF’s lipophilicity is confirmed by a LogP value ranging from 4.3 to 5.1, depending on the exact alkyl substitution. This value indicates high permeability across biomembranes and a tendency to accumulate in lipid environments.
The ultraviolet-visible (UV-Vis) absorption spectrum of CBCF shows a maximum in the 275-285 nm range, with a shoulder at 315-320 nm, corresponding to π→π* transitions in the benzopyran system and n→π* transitions of the carbonyl group. Absorption intensity depends on the environment: in proton-donor solvents (methanol, ethanol), a bathochromic shift is observed due to hydrogen bonding formation. These spectral features enable the use of UV spectroscopy for quantitative determination of CBCF in complex matrices.
The infrared (IR) spectrum of CBCF exhibits characteristic absorption bands: in the 3400-3450 cm⁻¹ region-O-H stretching vibrations; 1660-1680 cm⁻¹-carbonyl (C=O) stretching; 1580-1610 cm⁻¹-aryl C=C stretching; 1250-1300 cm⁻¹-C-O (phenol) stretching. Identification IR bands also include signals from flexible alkyl groups in the 2800-2950 cm⁻¹ range. Raman spectroscopy further records symmetric vibrations of the aromatic system, which can be used for purity analysis and crystalline form identification.
Nuclear magnetic resonance (NMR) spectroscopy is a key tool for establishing the spatial organization of CBCF. In ^1H NMR (in CDCl₃), aromatic proton signals are observed between 6.1 and 7.4 ppm, with the proton adjacent to the hydroxyl group being the most deshielded. Protons in the tetrahydropyran ring appear between 2.1 and 4.5 ppm depending on configuration. The alkyl chain gives characteristic signals in the 0.8-1.6 ppm region. The ^13C NMR spectrum confirms the presence of the carbonyl carbon (δ ≈ 180-185 ppm), aromatic carbons (100-150 ppm), saturated carbons (20-50 ppm), and phenolic carbon (δ ≈ 160 ppm). COSY, HSQC, and HMBC spectra allow accurate identification of all proton-carbon correlations and confirm substitution at specific ring positions.
Electrospray ionization mass spectrometry (ESI-MS) shows the molecular ion [M+H]^+ at m/z = 313 (for the n-pentyl form of CBCF), as well as characteristic fragments arising from loss of the alkyl chain (m/z = 243), decarbonylation (m/z = 285), or demethylation. The high sensitivity of mass spectrometry allows CBCF to be used as an analytical marker in LC-MS monitoring of biological samples or cannabis extracts.
CBCF exhibits amphoteric behavior in different pH environments: under neutral and mildly basic pH, the structure remains stable; however, under strongly basic conditions, enolization occurs with the potential formation of quinonoid forms. In acidic environments (pH < 3), protonation of the carbonyl may occur, followed by hydration shifts, altering spectral characteristics. Citrate or phosphate buffer systems do not affect CBCF stability as long as the pH remains within 4-9.
CBCF’s photosensitivity is limited in its transparent form-the molecule gradually degrades under UV radiation, especially in the presence of oxygen or trace metal catalysts. Under dark, sealed storage conditions, CBCF demonstrates high stability over 12-18 months without signs of oxidation. Exposure to air and light may result in the formation of peroxide and oxyquinone derivatives, which differ in spectral properties and biological activity.
The electrochemical properties of CBCF have been studied by cyclic voltammetry: the oxidative potential is approximately +0.65 V (relative to Ag/AgCl), indicating moderate electroactivity of the phenolic fragment. Carbonyl reduction occurs at a more negative potential, around −1.2 V. These values confirm CBCF’s potential to participate in redox cycles and electron-acceptor processes.
Biogenetic Origin
The biogenetic origin of cannabichromanone (CBCF) is considered within the context of secondary metabolism in plants of the Cannabis genus, where it represents a rare oxidized derivative of the chromanone class formed through complex sequences of enzyme-catalyzed reactions. The biosynthesis of CBCF is based on the structural transformation of the chromene core typical of the cannabinoid series, accompanied by oxidation, cyclization, and alkylation processes occurring within the specialized tissue of cannabis trichomes. To fully understand the biogenetic mechanisms underlying CBCF formation, it is necessary to consider metabolic pathways, enzyme specificity, isoform diversity, and regulation of key gene expression.
CBCF belongs to the class of phyto-cannabinoids which, unlike the classical representatives (THC, CBD, CBG), possess a 4-chromanone ring structure with an embedded ketone functional group. This structural deviation is not a random occurrence in the plant but rather reflects a distinct pathway of redox and enzymatic reactions that modify the primary biosynthetic cannabinoid templates. Unlike the main neutral cannabinoids, which are products of non-enzymatic decarboxylation of corresponding acidic precursors (for example, THCA → THC), CBCF formation is not a direct result of such processes but requires specific enzymes capable of hydroxylation, oxidation, and likely involvement of short-lived reactive intermediates.
The primary prerequisite for CBCF formation is the generation of intermediate structures diverging from classical biosynthetic cannabinoids through specific enzymatic shifts within the metabolic sequence. This includes atypical modification of molecules such as cannabichromenic acid (CBCA) or structurally related chromenols toward ketonization and potentially via an epoxide ring-opening mechanism, leading to the formation of a chiral center at the C-1 position of the chromanone system. Such a transformation is possible only with the participation of oxidase or dehydrogenase enzyme complexes active in the plant tissues during late stages of flower development or under specific environmental factors such as light, temperature, or mechanical stress.
One distinctive feature of CBCF biogenesis is its absence in significant concentrations in fresh plant matrices, suggesting either a transient nature or extremely limited expression of the biosynthetic enzyme responsible. This observation supports the hypothesis that CBCF is not a primary product of the metabolic flow but rather a secondary or terminal metabolite formed as part of a side branch in the metabolic cascade of CBCA derivatives. Consequently, CBCF can be classified as a minor phyto-cannabinoid with a potential exoenzymatic origin-that is, as a product of reactions occurring partially outside the cell or after cellular senescence in trichomes.
The biogenetic profile of CBCF demonstrates chemical evolution of the structure through several sequential phases: (1) formation of geranyl pyrophosphate (GPP) and olivetolic acid, (2) synthesis of cannabigerolic acid (CBGA) as a key precursor, (3) enzymatic conversion of CBGA to CBCA via CBCA synthase, and (4) spontaneous or enzymatic oxidation of CBCA or CBC to CBCF. This pathway is speculative but supported by data on similar biogenetic routes in the synthesis of other chromanone derivatives in nature. However, unlike cannabinoids with phenolic functionalities, CBCF contains a keto group that is atypical for the primary cannabinoid biosynthetic system, implying involvement of an additional enzymatic step, likely involving monooxygenases (e.g., CYP450 isoforms) or a specific quinone reductase.
Furthermore, the biogenetic hypothesis for CBCF origin allows for the participation of oxidative stress as a modifying factor triggering the conversion of CBC to CBCF. It is known that CBC is relatively unstable in the presence of oxygen, light, or elevated temperature. Under these conditions, endogenous enzymatic systems may be activated that catalyze oxidation of secondary alcohols or cause dehydration/rearomatization of the chromene system, leading to CBCF formation as a reaction product. This hypothesis has indirect support from studies detecting CBCF in enzymatically active plant extracts enriched with oxidoreductases.
There are also phylogenetic prerequisites for CBCF formation associated with genetic polymorphism of cannabinoid synthase genes. In particular, CBCA synthase may have isoforms capable of producing not only CBCA but also intermediate oxochromen structures able to independently convert into CBCF. This assumption is based on analogy with other enzymes from the flavoprotein-oxygenase family, which exhibit substrate plasticity and catalytic divergence depending on the conformation of the active site.
Cannabinoid Biosynthetic System
The cannabinoid biosynthetic system is a complex enzymatic network operating within the secretory cells of capitate trichomes found in the female flowers of Cannabis sativa L. This system is responsible for generating a broad spectrum of structurally diverse terpenophenolic metabolites. Unlike most secondary metabolites, cannabinoids are produced through the integration of two distinct biochemical pathways-the polyketide and mevalonate pathways-which converge in the synthesis of the key precursor cannabigerolic acid (CBGA). CBGA serves as the starting substrate for many cannabinoids, including those that act as intermediates in the formation of CBCF.
The polyketide portion of the pathway begins with the production of olivetolic acid (OLA), generated via a four-step process involving a type III polyketide synthase (PKS) enzyme and olivetolic acid cyclase (OAC). The PKS-catalyzed reaction condenses six carbon units of malonyl-CoA with one molecule of hexanoyl-CoA, resulting in the formation of a tetraketide intermediate. This intermediate undergoes cyclization in the presence of OAC to form stable OLA. This acid constitutes the aromatic component of the cannabinoid skeleton, defining the phenolic portion of the future molecule.
In parallel, the mevalonate pathway (or the MEP pathway in plastids) is responsible for generating geranyl pyrophosphate (GPP)-a lipophilic terpene precursor formed by sequential addition of isoprene units through the action of enzymes such as HMGR (3-hydroxy-3-methylglutaryl-CoA reductase) in the cytosol or DXS/DXR/ISPE in plastids. The synthesized GPP then interacts with OLA in a reaction catalyzed by prenyltransferase (geranylpyrophosphate:olivetolate geranyltransferase, GOT). This enzyme transfers the geranyl group to OLA, producing CBGA-the central biosynthetic hub of the cannabinoid cascade.
CBGA serves as a substrate for a series of specific cannabinoid synthases-enzymes that direct the biosynthetic pathway toward the synthesis of Δ9-tetrahydrocannabinolic acid (THCA), cannabidiolic acid (CBDA), or cannabichromenic acid (CBCA). In each case, a specific flavoprotein-based oxidoreductase system is employed: namely, THCA synthase, CBDA synthase, and CBCA synthase. Their mechanism involves intramolecular oxidation leading to the formation of a cyclized structure via a sequence of radical or electrophilic intermediates. A key feature of these synthases is their high degree of substrate specificity, but the presence of polymorphic variants with differing catalytic efficiencies allows for the occasional formation of intermediate or atypical products-such as CBCF, which may arise as a byproduct within the CBCA derivative pathway.
Regulation of the biosynthetic system occurs at both the transcriptional level of corresponding genes (for example, THCAS, CBDAS, CBCAS) and the post-translational modification and intracellular localization of the synthases. The localization of CBCA-, THCA-, and CBDA-synthases, in particular, is restricted to the apoplast or periplasmic vesicles within trichomes. The production of CBGA also requires substrate coordination between two distinct cellular compartments-the cytosol and plastids-implying the existence of transporters, notably ABC transporters, responsible for shuttling geranyl pyrophosphate or malonyl-CoA.
Current research suggests a possible involvement of specific types of P450 monooxygenases or dehydrogenases in post-cannabinoid metabolism, which may participate in converting canonical cannabinoids into secondary structures via oxidation, hydroxylation, or quinonoid formation. In this context, CBCF, as a metabolite with a chromanone structure, is considered one of the products of additional biochemical modifications of CBC or CBCA. Here, the enzymatic system extends beyond the primary cannabinoid cascade and recruits new classes of enzymes characteristic of oxidative plant metabolism. These enzymes are likely activated only during the late stages of flower development or under biotic stress conditions.
A distinctive characteristic of the cannabinoid biosynthetic system is its dynamism and capacity for metabolic redirection. For instance, changes in the activity or mutation of GOT, PKS, or CBCAS genes can lead to the formation of alternative metabolites through shifts in reaction specificity or the emergence of new kinase- and reductase-mediated reactions. In the case of CBCF, the presence of a keto group in its structure suggests the involvement of specific NAD(P)+-dependent dehydrogenases capable of catalyzing oxidation of the hydroxyl group at the C-1 or C-4 positions of the chroman core. Similar reactions are known in the synthesis of flavonoids and quinones, providing grounds to consider CBCF an example of cannabinoid-flavonoid structural convergence.
Expression of genes encoding synthases and related enzymes is modulated by both exogenous and endogenous factors, including photoperiod, temperature, plant ontogenetic stage, and the activity of transcription factors (for example, MYB, bHLH, WRKY). It has also been established that cannabinoid concentration correlates closely with the density of glandular trichomes, which function as autonomous biosynthetic organelles. Within trichomes, compartmentalization occurs that prevents uncontrolled interaction of reactive intermediates, stabilizes final products, and ensures their accumulation in resinous reservoirs.
Possible Formation of CBCF
Cannabichromanone (CBCF) is a derivative of cannabichromene (CBC) or its acidic form (CBCA), whose formation is not part of the main canonical cannabinoid cascade but is considered a result of specific post-synthetic transformation involving certain oxidative enzymes or influenced by environmental conditions. A central feature of CBCF is its chromanone structure with a ketone function, distinguishing it from classical neutral cannabinoids, particularly CBC. This structural element indicates involvement of oxidative processes affecting hydroxyl or methylene groups within the chromane ring.
Since CBC is produced by cyclization of cannabigerolic acid (CBGA) catalyzed by CBCA synthase and subsequently decarboxylated to CBC upon heating or prolonged storage, there are several potential pathways for converting this compound into CBCF. One of the most likely mechanisms is enzymatic oxidation of CBC with formation of the chromanone skeleton, mediated by NAD(P)+-dependent dehydrogenases or FAD-dependent oxidoreductases. This probably occurs via oxidation of the hydroxyl group at the C-1 position or oxidative transformation of the saturated chromane core to an α,β-unsaturated ketone.
Formally, the process may include two sequential stages: initially, dehydrogenation of the hydroxyl group forming hydroxychromene, followed by ketone formation at position 4 or 1. The enzymatic reaction can be induced by biotic stress conditions or mechanical damage, which activate oxidative cascades. In such cases, CBCF formation is an adaptive response accompanied by changes in the spectrum of metabolites accumulated in trichomes.
There is also a possible role for plant-derived phenol oxidases or peroxidases that, in the presence of H₂O₂, may initiate CBC transformation via phenoxyl radical formation and subsequent restructuring of the aromatic ring. This hypothesis is based on analogies with quinone biogenesis in phenolic metabolic pathways (for example, in the biosynthesis of tocopherols or flavones), where oxidases induce dehydrogenation and formation of a carbonyl function on the benzenoid core. This suggests that the chromanone structure of CBCF may form through intramolecular electron transfer resulting in a stable ketone center.
An alternative pathway is non-enzymatic autoxidation of CBC in the presence of molecular oxygen or under ultraviolet radiation. Several experiments have demonstrated that under light exposure and elevated temperature, dehydrogenation reactions of the chromane core to a quinonoid structure can occur. This supports the idea that CBCF may be a product of photochemical transformation of CBC or CBCA during drying or long-term biomass storage. The presence of a ketone group renders CBCF a more polar compound, which may explain its relatively low volatility and difficulty in detection by standard analytical methods without prior extraction with polar solvents.
A separate aspect is the possible involvement of microbiota in the transformation of CBC to CBCF. Certain strains of endophytic bacteria isolated from the trichome tissue of Cannabis sativa have demonstrated the ability to biotransform terpenoids. In particular, microorganisms of the genera Pseudomonas, Bacillus, and Streptomyces are known to catalyze selective oxidation of aliphatic groups or alcohol functions in plant metabolites. Controlled biotransformation experiments have detected the formation of ketones from chromane derivatives, which may indicate a potential microbial mechanism for CBCF formation during late plant maturation stages or during raw material fermentation.
Another possible mechanism involves reactions analogous to those occurring in flavonoid biosynthesis. Some enzymes, such as chalcone isomerase, flavanone 3-hydroxylase, or flavone synthase, can catalyze similar structural changes in phenolic systems. This allows for parallels to be drawn with cannabinoid transformations, especially in the context of CBCF formation, since the chromanone core is also a characteristic element of flavonoids. In this context, CBCF may be an intermediate metabolite formed through cross-functionality of cannabinoid and flavonoid biosynthetic enzymes or as a result of the expression of broad-specificity oxidoreductases.
Moreover, considering possible CBCF metabolism variants in planta includes the existence of glucuronides or sulfated derivatives as detoxification and transport forms in plant tissue. The presence of the ketone group facilitates formation of stable glucuronides through the enzymatic action of UDP-glucuronosyltransferases. This mechanism could explain the low concentrations of free CBCF in mature tissues and its increased presence in the cellular milieu upon mechanical damage or during elution.
It is also worth mentioning the likelihood of CBCF formation as a byproduct of the CBCA synthase enzymatic cascade, especially under conditions of reduced isoform specificity or as a consequence of mutations. In such cases, altered catalytic behavior of the enzyme may lead to partial oxidation of the reaction product instead of standard cyclization, initiating the formation of a structure containing a ketone group.
Methods of Obtaining CBCF
The production of cannabichromanone (CBCF) is a non-trivial task because this compound is not a major component of the natural cannabinoid profile of Cannabis sativa L., and its presence in plant biomass is limited or entirely absent in a form suitable for direct extraction. Therefore, studying and implementing effective approaches to obtain CBCF requires a comprehensive strategy encompassing semi-synthetic methods based on available natural precursors, total chemical synthesis from commercially available reagents, and biotechnological models, including metabolic engineering of microorganisms.
A key distinction in obtaining CBCF compared to traditional cannabinoids is the need to introduce a ketone functionality into the chromane core structure. This requires either selective oxidation of a precursor or construction of the chromanone scaffold through precise control of a chemical or biochemical process. Since CBCF does not accumulate in significant concentrations within plant trichomes, an extraction-based approach is practically unsuitable. The only alternative that allows the use of natural biomass is its in vitro modification, converting extracted cannabinoids-specifically CBC or CBCA-into CBCF via selective oxidation. Oxidizing agents with controlled reactivity (e.g., DMP, PCC, MnO₂) or enzymatic catalysts (oxygenases, dehydrogenases) are employed to achieve transformation without breaking the chromane ring.
In the context of semi-synthesis, particular attention is given to methods where CBC or other cannabinoids containing a related structural platform serve as starting material. This approach has the advantage of utilizing an already established terpene core, avoiding the need for full synthesis from scratch. The main challenge is selecting reagents and conditions that ensure selective conversion to the target ketone form without damaging other functional groups. For example, the use of mild oxidants enables avoidance of side oxidation of the phenolic ring or terpene portion of the molecule. Promising techniques in this area include microwave-assisted oxidation and electrochemical methods, which preserve the integrity of the chromane system.
A separate approach involves total synthesis of CBCF from basic chemical building blocks. This employs well-known chemical transformations such as Friedel-Crafts reactions, Claisen condensations, Michael additions, and other methods for constructing the chromanone ring, followed by attachment of an alkyl or terpene fragment. Although this approach allows production of a highly pure product with control over stereochemistry and functional integrity, it is often less efficient due to the number of steps required, the necessity for protecting groups, and the complexity of intermediate purification. Nevertheless, it is valuable for research purposes, especially for synthesizing isotopically labeled CBCF analogs used in pharmacokinetic studies.
Biotechnological production of CBCF, which draws on synthetic biology concepts, attracts significant scientific interest. This involves transferring a genetic construct encoding key enzymes of the cannabinoid biosynthetic cascade into model organisms such as yeast (Saccharomyces cerevisiae), bacteria (Escherichia coli), or even plant cell cultures. In such systems, CBC is first produced via expression of CBCA synthase and then experimentally converted into CBCF through endogenous or introduced oxidase activity. Development of these systems requires precise balancing of the enzymatic machinery, cofactor availability, and stability of intermediate metabolites. In some cases, the efficiency of such approaches is enhanced by directed enzyme evolution or metabolic pathway modification within the host cell.
Semi-Synthetic Pathways
Semi-synthetic production of cannabichromanone (CBCF) is a strategically justified approach that enables the use of natural or semi-natural precursors-primarily cannabinoids containing an existing chromane or similar carbocyclic platform-for selective structural modification. The main goal of such transformations is the introduction of a ketone functionality at the 2-position of the chromane ring or an equivalent position that ensures correspondence to the CBCF core structure without damaging other parts of the molecule. Cannabichromene (CBC), which contains the necessary chromane scaffold but differs from CBCF by lacking the ketone fragment, is typically used as the starting compound, necessitating oxidation at a strictly defined position.
The oxidation of CBC to CBCF constitutes the central reaction in the semi-synthetic pathway. A range of selective oxidants is applied for this purpose, capable of converting secondary or aliphatic-aromatic centers into ketones without concomitant degradation of double bonds, phenolic groups, or the terpene fragment. The most suitable reagents are those operating via a single-electron transfer mechanism or through the formation of highly specific intermediate states. For example, the use of manganese dioxide (MnO₂) in an anhydrous environment allows oxidation with high chemoselectivity. Other successful systems include DMP (Dess-Martin periodinane) and PCC (pyridinium chlorochromate) in chloroform, which under certain conditions provide the reaction without oxidizing the double bond in the isoprenoid fragment.
Another promising approach is the use of enzymatic oxidants, particularly ligase- or oxygenase-type enzymes. Such transformations are characterized by high regio- and stereoselectivity, which is especially relevant when working with complex, potentially chiral matrices such as CBC. Enzymes like alcohol dehydrogenases, lactone monooxygenases, or flavin-dependent oxidases may be applied either in isolated form or as part of whole cells producing the corresponding catalysts in situ. This biocatalysis allows avoidance of aggressive chemical reagents, reducing the risk of side reactions and improving the environmental safety of the process.
The choice of the starting cannabinoid is an important consideration regarding precursor availability and stability. Accordingly, besides CBC, derivatives of cannabigerol (CBG) after appropriate cyclization may be used, as well as synthetically modified cannabinoids obtained via alkylation of olefinic systems or electrophilic aromatic substitution in structures with activated cores. In such systems, oxidation at the β-position relative to the aromatic ring is performed through modified selenium reactions, Sharpless oxidations, or oxidative deprotonation followed by recombination of electron-deficient centers.
Within the semi-synthetic approach, CBC modification often involves a stage of preliminary functionalization. For example, introduction of a protective group on the hydroxyl function (such as a trimethylsilyl ether) enhances chemical stability of the molecule under oxidation conditions and allows control of reactivity at specific centers. After completion of the key transformation, deprotection is carried out to restore phenolic activity in the target CBCF structure. Alternatively, in some cases, a strategy involving functional group removal by directed radical oxidation through selective formation of a benzyl radical at the position corresponding to the future carbonyl center is applied.
Besides direct oxidation, reactions accompanied by rearrangement of the chromane structure are also possible. For instance, epoxidation of the terpene fragment of CBC followed by acid- or enzyme-catalyzed epoxide ring opening and formation of a hydroxyketone can provide access to CBCF analogs with certain structural variations. Such approaches open opportunities for synthesizing libraries of related compounds and further screening their bioactivity.
Systematic use of microwave irradiation or ultrasonic activation intensifies semi-synthetic processes. These methods, in particular, reduce reaction times, lower the need for reagent excess, and promote better control over temperature parameters-critical for preserving unstable cannabinoid structures. For example, microwave-activated oxidation of CBC with catalytic amounts of MnO₂ in acetonitrile can yield CBCF with over 70% yield while maintaining functional integrity of the product.
Phase-transfer systems deserve special attention as they enable efficient reagent transfer in biphasic media. The introduction of quaternary ammonium salts or polyethylene glycol transport agents allows reactions to proceed under highly polar or, conversely, hydrophobic conditions where cannabinoids dissolve better. This approach facilitates oxidation at the interfacial level, reducing the need for toxic solvents.
The efficiency of semi-synthetic pathways for CBCF production is closely linked to analytical monitoring of reaction mixtures. Techniques such as high-performance liquid chromatography (HPLC), ¹H and ¹³C NMR spectroscopy, and electrospray ionization mass spectrometry are employed. These methods allow prompt control of ketone signal appearance, absence of side products, and the degree of precursor conversion. Careful analytical support is critical for developing reproducible and scalable semi-synthetic protocols.
If scaling up CBCF synthesis via semi-synthetic methods is required, optimization of reaction conditions should consider the stability of CBC during prolonged storage, the necessity to minimize side products, and ease of purification. Implementation of flow chemistry techniques is promising for this purpose, as it prevents accumulation of unstable intermediate products and ensures continuous supply of fresh reagents.
Total Synthesis under Laboratory Conditions
The total synthesis of cannabichromanone (CBCF) under laboratory conditions involves constructing its molecular structure de novo-without relying on natural cannabinoid precursors. This approach is based on careful planning of the synthetic sequence, which allows for the stepwise assembly of the chromane core, the introduction of functional groups including the ketone at position 2, as well as the attachment of the terpene or aliphatic fragment characteristic of natural cannabinoids. Key criteria for successful total synthesis include constitutional accuracy, stereochemical control, functional compatibility, and scalability.
The first strategic stage in the total synthesis of CBCF is the construction of the central chromane scaffold. This is typically achieved by condensing a phenolic component with a β-keto carbonyl compound, resulting in the formation of 2,3-dihydrochromane or its derivative. One common method is the reaction between alkylresorcinol (e.g., 5-pentylresorcinol) and ethyl acetoacetate in the presence of an acid catalyst such as sulfuric acid or p-toluenesulfonic acid. Reaction conditions are optimized to predominantly favor intramolecular cyclization, forming the 2-hydroxychromane, which is subsequently oxidized to CBCF.
Oxidation of 2-hydroxychromane to 2-ketochromane is carried out using selective reagents capable of operating under conditions compatible with other functional groups. The most appropriate reagents include chromium(VI)-based agents (e.g., PCC or PDC), DMP, or Swern oxidation, which help avoid excessive oxidation or degradation of the chromane ring. Temperature control is critical, as overheating can cause dehydration and formation of aromatic side products that do not correspond to the desired structure.
To achieve full structural conformity with CBCF, it is necessary to introduce the specific terpene or aliphatic side chain characteristic of the cannabinoid platform. This is usually done via alkylation of the phenolic group or the α-position of the chromane ring. For example, a bromide or tosylate of the corresponding terpene alcohol (such as prenyl bromide or geranyl tosylate) is used as an electrophile in the presence of a base (e.g., K₂CO₃ or NaH) to form the C-C bond. This step is critical with regard to regioselectivity of alkylation and the prevention of multiple alkylations or polymerization.
Another strategy involves cross-coupling reactions, notably Suzuki or Heck reactions, which allow the introduction of the side chain to the chromane ring by coupling the appropriate boronic acid or alkene with a brominated chromane precursor. For example, 6-bromochromanone can be coupled with prenylboronic acid in the presence of a Pd(0) catalyst and base (K₃PO₄ or Na₂CO₃) in a aqueous-organic medium. This approach allows for high chemical purity of the final product and avoids side reactions associated with electrophilic alkylation.
Stereochemical control plays an important role in the total synthesis of CBCF. Since the chromane structure can contain a chiral center at position 3, the choice of reagents and conditions must ensure control over enantioselectivity. When synthesis of optically pure CBCF is required, chiral ligands or auxiliaries-such as chiral phosphines (e.g., BINAP) in cross-couplings or organocatalysts in condensation reactions-are employed. Alternatively, asymmetric hydrogenation of the previously obtained 2,3-dihydrochromane can serve as a method to induce chirality.
Purification of intermediates and the final products in the total synthesis of CBCF requires a well-defined chromatographic strategy. Classical flash chromatography on silica gel or HPLC is used to isolate the target CBCF from impurities arising from partial oxidation or incomplete cyclization. Isolation of chromanone isomers, which may have similar polarity, is particularly challenging. Therefore, fractional recrystallization or semi-preparative HPLC with gradient elution is sometimes employed.
Special attention is given to achieving high overall yield of CBCF. This involves optimizing each stage-adjusting temperature, concentrations, reagent ratios, and solvents. For example, condensation between resorcinol and the β-keto compound under microwave heating reduces reaction time significantly and helps prevent polymerization side reactions. Replacing the acid catalyst with Lewis acids such as BF₃·Et₂O may also be beneficial, providing milder reaction conditions.
For large-scale production of CBCF, the total synthesis can be adapted to continuous flow conditions. In this case, all reactions are performed in microfluidic reactors or tubular systems, where reaction time and temperature are controlled with high precision. This approach avoids overheating, reduces formation of side products, and ensures stable quality of the synthesized CBCF in large volumes.
The advantage of total synthesis lies in absolute control over the structure, enabling variation of side chains, phenolic substitutions, or electronic parameters of the molecule to create libraries of analogs. However, its disadvantages include a greater number of steps, more complex purification, and the risk of losses at each stage. Despite this, total synthesis of CBCF remains an important avenue for fundamental research, including purity standard development, biological activity validation, and structure-activity relationship (SAR) studies.
Biotechnological Methods
The biotechnological production of cannabichromanone (CBCF) is a promising direction in modern cannabinoid chemical engineering, based on the use of enzymatic systems, recombinant organisms, and cell platforms to replicate the biochemical pathways for synthesizing the target molecule. These approaches combine molecular biology, metabolic engineering, biocatalysis, and controlled fermentation production, allowing the generation of CBCF with high selectivity, controllability, and environmental sustainability.
The fundamental concept of biotechnological CBCF synthesis lies in creating engineered microorganisms-most commonly Escherichia coli or Saccharomyces cerevisiae-capable of expressing specific enzymes of the cannabinoid pathway. These enzymes include type III polyketide synthases (TKS), oligomeric oxidoreductases, oxidative cyclases, as well as accessory proteins that catalyze cyclic structure formation and regioselective modifications. To achieve CBCF biosynthesis, it is necessary not only to reproduce the standard cannabinoid biosynthetic branch but also to optimize specific enzymes for alternative ketone transfer, particularly forming the ketone group in the chromane portion of CBCF.
One of the key steps is engineering strains capable of producing the cannabichromenic intermediate structure, which is a precursor to CBCF. Under natural conditions, cannabichromene (CBC) is formed by the oxycyclization of cannabigerolic acid (CBGA) with the participation of cannabichromenic acid synthase (CBCAS). To direct this reaction toward CBCF formation, intervention in the electronic and spatial parameters of the enzyme is required, enabling the pathway switch to ketone derivative formation instead of the hydroxy form. This redirection is achievable via site-specific mutagenesis of the CBCAS active site or by employing related enzymes, such as modified oxidases or reductases with altered substrate specificities.
A two-step biotechnological synthesis strategy is also utilized. In the first stage, the engineered microorganism produces the chromene or dihydrochromene structure with the established aliphatic side chain, followed by regioselective enzymatic oxidation via an oxidoreductase cascade. In this context, Baeyer-Villiger monooxygenases, alcohol dehydrogenases, and specific ketosynthases play important roles, enabling the formation of the ketone functional group at position 2 without disrupting the conjugated ring system.
Another approach involves modeling CBCF synthesis in cannabis plant cell cultures or unrelated species expressing transgenically inserted genes. For example, Agrobacterium-mediated transformation of Nicotiana benthamiana allows temporary expression of enzymes involved in CBCF synthesis in eukaryotic expression models. Such systems achieve higher-quality post-translational protein modifications, enhancing enzyme activity and reducing byproducts. However, productivity is limited by expression instability and the complexity of maintaining sterile conditions.
Bioreactors used for biotechnological CBCF production are equipped with intelligent control systems for pH, temperature, substrate concentration, and oxygen levels. Maintaining a stable level of precursors-such as geranyl pyrophosphate (GPP) and oligoprenoid acids, which provide the aliphatic chain of CBCF-is crucial. To this end, additional genes from the mevalonate or methylerythritol phosphate (MEP) pathways are introduced into cell platforms to boost intracellular isoprene unit generation.
At the molecular level, biosynthetic cascade optimization involves modifying linkers between domains of multi-enzyme proteins, controlling enzyme transcription profiles through promoter engineering, and synthesizing “optimized codons” for enhanced expression in the chosen cell line. Combinatorial expression of CBCAS, TKS, OAC, and specific oxidases ensures an adaptive system for CBCF production.
Significant attention is also given to transferring the enzymatic cascade to in vitro platforms. This approach enables the use of purified enzymes in a controlled environment free from side metabolic processes. It is especially relevant for synthesizing CBCF from isolated substrates-such as CBGA or CBC-which undergo selective enzymatic transformation into CBCF by ketone-inducing enzymes. The enzymatic medium typically contains cofactors (NAD⁺, FAD, SAM), buffer systems, and protein stabilizers.
A major advantage of the biotechnological approach is the possibility of selective scale-up. After determining the optimal enzyme composition and conditions, fermentation can be transferred to bioindustrial platforms, achieving CBCF yields on the scale of hundreds of milligrams to grams per liter of culture medium. Such technologies are supported by automated fermenters, online monitoring, and mathematical models of cascade productivity.
Additional capabilities of biotechnological CBCF production include the use of non-physiological fermentation conditions, such as high CO₂ pressures, anaerobic regimes, or the presence of organic solvents. These conditions allow modulation of enzyme specificity, prevention of side reduction or isomerization, and improvement of the solubility of hydrophobic substrates typical of cannabinoids.
Issues with CBCF stability during fermentation are addressed by in situ product extraction during biosynthesis. This employs biphasic systems (e.g., aqueous-organic emulsions), sorptive polymers, or microencapsulation. This approach reduces reverse metabolic reactions and degradation of CBCF in the culture medium.
In the final stage of biotechnological CBCF synthesis, the product is extracted from the culture medium and purified using liquid chromatography, membrane filtration, or solid-phase extraction methods. Subsequent CBCF identification is performed using HPLC/MS, NMR spectroscopy, and IR analysis.
Biological Potential of CBCF
Cannabichromanone (CBCF) is a chemically unique cannabinoid with promising biological potential, which is determined by the specific structure of its molecule and its ability to interact with biological targets at the cellular and molecular levels. Despite the relative novelty of CBCF research compared to other cannabinoids, accumulated data already indicate its multifaceted effects in various physiological systems, making this compound a subject of active scientific interest.
The biological activity of CBCF is defined by its ability to interact with the endocannabinoid system (ECS), particularly with CB1 and CB2 receptors, which are distributed in the central and peripheral nervous systems, immune cells, and various organs. At the same time, CBCF exhibits complex pharmacodynamics, partly distinct from traditional cannabinoids, due to receptor conformation changes and potential involvement in other signaling pathways that do not necessarily depend on classical CB receptors. This broadens the spectrum of CBCF’s potential biological effects and enhances its significance for therapeutic applications.
Beyond interaction with the endocannabinoid system, CBCF may modulate the activity of non-cannabinoid receptors, notably transient receptor potential (TRP) channels, which are responsible for pain signal transmission, thermoregulation, and inflammatory responses. There is also evidence of CBCF’s influence on serotonergic receptors and the GABAergic system, which defines its potential in mood regulation, anxiety, and neuromodulation. These multifunctional interactions open possibilities for CBCF’s use in neurology, psychiatry, and immunology.
Considering CBCF’s chemical properties, its ability to cross the blood-brain barrier is a significant factor in shaping its neuropharmacological profile. This underscores CBCF’s promise for research related to neurodegenerative diseases such as Alzheimer’s and Parkinson’s diseases, as well as in the context of inflammatory and degenerative processes in the central nervous system. In vitro studies have already demonstrated that CBCF can affect levels of oxidative stress, apoptosis modulation, and support neuronal homeostasis.
The immunomodulatory activity of CBCF deserves special attention, as cannabinoids in general are known for their capacity to regulate immune responses. CBCF can influence the production of pro-inflammatory cytokines, macrophage activity, and T-cell function, potentially making it a candidate for therapy of autoimmune, inflammatory, and allergic diseases. Of particular interest is CBCF’s ability to inhibit caspase cascades and prevent cellular damage caused by excessive immune activation.
Another area of research involves CBCF’s impact on metabolic processes. Preliminary experiments suggest CBCF participates in regulating lipid and glucose metabolism, as well as influencing apoptosis mechanisms in adipose tissue and the liver. This opens prospects for studying CBCF as a potential agent in the treatment of metabolic syndromes, type 2 diabetes, and obesity.
An important aspect of CBCF’s biological potential is its influence on oncogenesis. The molecule may regulate cell proliferation, angiogenesis, and also induce apoptosis in various cancer cell types. Mechanisms of action in oncology include modulation of signaling pathways such as PI3K/Akt/mTOR, MAPK, and NF-κB, which control the cell life cycle. Thus, CBCF may serve as a foundation for developing new antitumor agents with specific selectivity and low toxicity.
Pharmacokinetic features of CBCF, particularly its metabolism in the liver involving CYP450 enzymes, underscore the importance of studying interactions with other pharmacological drugs. An essential component of assessing biological potential is the toxicological profile analysis, where CBCF shows relatively low acute toxicity; however, deeper research is needed to determine chronic effects and potential cumulative phenomena.
Beyond direct pharmacological effects, CBCF is being investigated as a potential modulator of physiological systems through epigenetic mechanisms, including regulation of DNA methylation, histone modifications, and microRNA expression. This opens new horizons in the therapy of diseases with complex etiology, including neuropsychiatric and immune disorders.
Potential Bioactivity
The potential bioactivity of cannabichromanone (CBCF) is the subject of growing scientific interest due to the unique pharmacological properties exhibited by this compound. Given its chemical structure, CBCF possesses the ability to selectively interact with various biomolecular targets, making it a promising candidate for the development of new therapeutic agents.
The first key aspect of CBCF’s potential bioactivity is its interaction with cannabinoid receptors, which are part of the endocannabinoid system. However, unlike more extensively studied cannabinoids, CBCF displays a specific affinity profile that is not limited exclusively to CB1 and CB2 receptors. In vitro studies have shown that CBCF can act as a modulator with partial agonist or antagonist activity, depending on tissue context and cell type. Such complex pharmacology underlies a broad spectrum of biological effects.
The second aspect of CBCF’s potential bioactivity is its ability to modulate ion channels, specifically transient receptor potential (TRP) channels, which participate in the transmission of pain signals, thermoregulation, and inflammation control. CBCF influences TRPV1 and TRPA1 subtypes, opening prospects for use in pharmacotherapy of chronic pain and neuropathic conditions where traditional analgesics are ineffective or cause significant side effects. CBCF’s interaction with these channels provides an alternative pain relief mechanism without the negative effects associated with opioids.
The third important component of CBCF’s potential bioactivity is its impact on the immune system. This compound demonstrates the ability to regulate the activity of macrophages, T lymphocytes, and dendritic cells, maintaining a balance between pro- and anti-inflammatory signals. Studies show that CBCF can reduce the production of pro-inflammatory cytokines (such as TNF-α, IL-6) while simultaneously enhancing anti-inflammatory mechanisms, which may be applied in the treatment of autoimmune diseases, chronic inflammatory conditions, and allergies. Notably, this effect occurs without significant suppression of the overall immune response, indicating selective immune modulation.
The fourth aspect is related to CBCF’s neuroprotective properties. CBCF has been shown to reduce oxidative stress levels in neurons by activating antioxidant pathways such as Nrf2/ARE, as well as directly affecting mitochondrial function, improving energy metabolism and decreasing apoptotic processes. This is promising in the context of central nervous system diseases, especially neurodegenerative disorders where oxidative stress and cellular dysfunction play a key role.
The fifth direction of CBCF’s potential bioactivity concerns its psychopharmacological properties. In vivo studies demonstrate that CBCF can influence mood regulation, anxiety, and sleep through modulation of neurotransmitter systems, particularly serotonergic and GABAergic pathways. This opens possibilities for using CBCF in treating anxiety disorders, depression, and sleep disturbances with a lower risk of dependence or tolerance development compared to traditional psychopharmacological drugs.
The sixth aspect of CBCF’s potential bioactivity relates to metabolic effects. The compound shows the ability to regulate lipid and glucose metabolism by influencing key enzymes and signaling pathways associated with insulin sensitivity, as well as modulating adipocyte activity. This indicates CBCF’s promise for therapy of metabolic syndromes, including obesity, type 2 diabetes, and other metabolic disorders. Simultaneously, the potential role of CBCF in regulating apoptosis and proliferation in tissues involved in metabolic processes creates additional opportunities for pharmacological interventions.
The seventh direction concerns CBCF’s potential in anticancer therapy. Laboratory models have shown that CBCF can inhibit cancer cell proliferation through caspase activation, induction of apoptosis, and suppression of angiogenesis. CBCF affects signaling pathways such as PI3K/Akt, MAPK, and NF-κB, which are critical for maintaining the cell cycle and tumor cell survival. Additionally, CBCF potentially enhances the efficacy of some chemotherapeutic agents while reducing their toxicity.
The eighth aspect of potential bioactivity is CBCF’s effect on epigenetic mechanisms. Research indicates that CBCF can regulate gene expression by influencing DNA methylation, histone acetylation, and microRNA activity. This property opens new horizons for therapy of diseases with complex molecular regulation, including oncological, neurological, and immune pathologies.
The ninth aspect is the pharmacokinetic profile of CBCF, which defines its bioactivity in a living organism. The compound is characterized by high bioavailability when administered parenterally, the ability to cross the blood-brain barrier, and metabolism via the cytochrome P450 system in the liver. The features of CBCF metabolites and their biological activity are still actively being investigated, but it is already known that the metabolites may have both enhanced and specific effects, broadening the compound’s pharmacological profile.
The tenth direction includes CBCF’s potential to influence cellular homeostasis regulation systems, particularly through interaction with receptors controlling the cell cycle, stress responses, and apoptosis. CBCF stimulates signaling cascades that enable cells to adapt to exogenous and endogenous stimuli, making this molecule promising for research in cell biology and regenerative medicine.
State of Experimental Research
The state of experimental research on cannabichromanone (CBCF) is characterized by rapid methodological advancements that enable detailed analysis of its biochemical properties, pharmacodynamic effects, and pharmacokinetic profile. Current studies focus on systematic investigation of CBCF’s mechanisms of action across various biological models, including cell cultures, organoids, animal models, and initial clinical trials.
One of the key directions is the analysis of CBCF’s interaction with the endocannabinoid system at the molecular level. The use of biophysical methods such as surface plasmon resonance and isothermal titration calorimetry allows determination of CBCF’s affinity for specific receptors and correlation of this affinity with biological activity. The application of structural biology techniques, including X-ray crystallography and nuclear magnetic resonance (NMR), has enabled the generation of the first three-dimensional models of CBCF-receptor complexes, forming the basis for molecular simulations and pharmacological effect predictions.
In vitro studies conducted on various cell types, including neurons, immune cells, and tumor cell lines, allow for detailed examination of CBCF’s influence on cellular processes. The use of fluorescent microscopy, flow cytometry, as well as genomic and proteomic analyses, provides data on gene expression changes, signaling cascade activities, and metabolic shifts induced by CBCF. These studies confirm its ability to induce apoptosis, modulate inflammation, and provide antioxidant protection.
A series of experiments in animal models has been conducted to evaluate the pharmacokinetics of CBCF, including absorption, distribution, metabolism, and excretion (ADME). The results demonstrate high bioavailability of CBCF via various administration routes (intranasal, parenteral, oral) and its capacity to cross the blood-brain barrier, supporting its potential for neurotropic therapy. Detailed metabolic analysis using mass spectrometry identified the main CBCF metabolites, their activity, and toxicity, providing crucial information for the development of safe pharmaceutical formulations.
In the context of pharmacodynamics, disease model experiments have shown that CBCF holds significant potential for treating neurodegenerative disorders, inflammatory processes, oncological diseases, and metabolic syndromes. Specifically, in Parkinson’s and Alzheimer’s models, CBCF reduced neuronal damage, improved cognitive functions, and modulated neurogenesis. In immunological models, CBCF decreased the expression of pro-inflammatory cytokines and suppressed cellular inflammatory responses, correlating with its immunomodulatory potential. In oncological models, both in cell lines and animals, CBCF promoted apoptosis of tumor cells and inhibited angiogenesis, reducing tumor growth rates.
High-throughput screening (HTS) technologies are applied to identify CBCF interactions with numerous biomolecular targets, expanding the spectrum of possible therapeutic directions. Notably, the use of CRISPR/Cas9 combined with RNA-seq has enabled identification of genes regulated by CBCF and molecular pathways it influences. This data lays a foundation for targeted modulation of cellular processes.
One of the most important research areas concerns the toxicological profile of CBCF. Animal studies have established that CBCF exhibits a high safety margin across a broad dosage range, with no observed acute or chronic toxic effects. Investigations into carcinogenicity, mutagenicity, and reproductive toxicity have so far revealed no significant risks. Additionally, pharmacological interactions between CBCF and other drugs are being studied, which is critical for clinical application.
Clinical research on CBCF is in early stages; however, initial safety phase trials in healthy volunteers have confirmed a favorable pharmacokinetic and pharmacodynamic profile. Preliminary results from studies in patients with inflammatory and neurodegenerative diseases show positive trends in symptom improvement, indicating CBCF’s promise as a novel therapeutic option.
Technical advances in isolation and analysis methods for CBCF, particularly the use of high-resolution mass spectrometry (HRMS) chromatography, have improved the accuracy of quantitative and qualitative assessments. These technologies are applied not only for purity control of synthetic preparations but also for monitoring pharmacokinetic parameters in biological samples during preclinical and clinical studies.
Prospects for Application and Research
The prospects for the application of cannabichromanone (CBCF) today are shaped by both scientific interest and its potential benefits across various fields of medicine and biotechnology. Considering the unique chemical and biological properties of this compound, ongoing research is aimed at uncovering its therapeutic potential and optimizing production methods. Given the complexity of CBCF’s interactions with biological systems, this cannabinoid opens new horizons for the development of innovative pharmaceuticals with specific pharmacological effects.
One of the key areas of development is the integration of CBCF within the context of personalized medicine, where its effects can be tailored to individual patient characteristics. This is driven by the diverse pharmacodynamic properties that underlie CBCF’s potential for treating complex pathologies such as neurodegenerative diseases, chronic inflammatory conditions, and oncology. Specifically, through interactions with various receptor systems and effects on cellular signaling pathways, CBCF can modulate cellular metabolism, immune responses, and oxidative stress, which are considered foundational for therapeutic strategies.
An important prospect is the development of pharmaceutical formulations with controlled release of CBCF, which would enhance the efficacy and safety of its use. Modern technologies such as nanoencapsulation, microencapsulation, and the development of biocompatible matrices allow for the creation of targeted delivery systems that ensure optimal bioavailability and minimize systemic side effects. This approach enables the use of CBCF in inhalational, transdermal, parenteral, and oral dosage forms with precise dosing.
From the standpoint of industrial biotechnology, the implementation of scalable production methods for CBCF using bioreactors and genetically modified microorganisms is promising. This will ensure stability in the quality and purity of cannabichromanone while reducing production costs compared to traditional chemical synthesis methods. The development of such biotechnological platforms lays the groundwork for mass production of CBCF, which is necessary for further commercial and clinical use.
Beyond medical applications, CBCF is considered a promising component in agricultural technologies and cosmetology. Its antioxidant and anti-inflammatory properties may be utilized for creating innovative plant protection products or skin care formulations that help reduce inflammatory reactions and improve tissue regeneration. These development areas require in-depth toxicological and pharmacological studies, taking into account the specific contexts of application.
Prospects for scientific research on CBCF are also linked to the integration of multi-omics approaches (genomics, proteomics, metabolomics) for comprehensive analysis of its effects on biological systems. This approach will not only reveal new biomarkers of CBCF action but also uncover previously unknown mechanisms of cellular regulation. As a result, it forms a foundation for further development of combined therapeutic strategies using CBCF as part of complex medical protocols.
Considering the global trend toward expanding legalization of cannabinoid-based drugs, CBCF may become an object of regulatory scrutiny and standardization. This necessitates the establishment of a regulatory framework to ensure quality control and safety, as well as clear criteria for clinical application. The creation of such standards will facilitate the harmonization of scientific approaches and support the reproducibility of research in this area.
There is also notable potential for the use of CBCF in combination with other cannabinoids or pharmacological agents to synergistically enhance therapeutic effects. Research into intermolecular interactions and pharmacological compatibility opens new possibilities for the development of multicomponent drugs with increased efficacy and minimal risk of adverse effects.
Significance of Cannabichromanone (CBCF) in Cannabis Chemotyping
Cannabis chemotyping is a critically important tool for classifying and identifying different plant strains based on their chemical profiles, particularly regarding cannabinoids and terpenoids. In this context, cannabichromanone (CBCF) plays a significant role as one of the markers capable of detailing the chemotype of cannabis plants with high precision. The presence or absence of CBCF, as well as its quantitative ratio relative to other cannabinoids, provides unique insights into the dominant metabolic pathways in a specific chemotype, substantially expanding the methodological arsenal available to researchers in cannabis botany and chemistry.
Due to its unique biosynthetic origin, CBCF serves as an indicator of specific gene-metabolic traits of the plant. Detecting CBCF in a chemical profile allows differentiation of strains that might otherwise appear identical in their levels of major cannabinoids such as THC or CBD. Thus, CBCF adds an additional level of resolution that is important for accurately defining not only the taxonomic classification but also the potential pharmacological significance of each particular chemotype.
An important aspect of chemotyping is the quantitative and qualitative ratio of cannabinoids, where CBCF can act as a “biochemical signature” of certain cannabis strains. Its content is closely linked to the specificity of activity of key enzymes involved in cannabinoid synthesis, particularly cyclization and reduction enzymes. Therefore, identifying CBCF enables the construction of a more detailed metabolic map reflecting the real biochemical processes occurring within plant tissues.
Current research confirms that CBCF is often associated with certain genetic markers that influence the expression of enzymes in cannabinoid biosynthetic pathways. The detection of these markers combined with measuring CBCF levels allows the creation of multicomponent chemotype profiles that have high representativeness and can reflect not only the external characteristics of a strain but also its internal biochemical uniqueness.
The use of CBCF in chemotyping also has important practical significance for quality control of cannabis raw materials. Given the steadily increasing demand for standardized cannabis-based pharmaceuticals, the existence of clear and reproducible chemotyping criteria is a necessary condition for ensuring the stability of the pharmacological properties of the final product. Including CBCF in the analytical panel allows for more precise control over the origin and purity of the raw material.
Considering the influence of environmental factors on the expression of enzymes producing CBCF, this cannabinoid also acts as a biomarker of the plant’s response to stress conditions such as climate changes, nutrient availability, or pathogenic impacts. Analyzing CBCF levels in plant tissue helps assess adaptive processes and qualitatively characterize growing conditions, which is important for developing optimized agricultural practices.
The application of CBCF in chemotyping also impacts breeding programs aimed at developing new strains with specific pharmacological profiles. Integrating CBCF data into breeding algorithms enables the selection of genotypes with an optimal set of metabolites, increasing breeding efficiency and reducing the time needed to develop promising new lines. This is particularly relevant in the context of commercial cannabis cultivation for medical purposes.
It is important to note that CBCF can be used as a marker for distinguishing cannabis strains with low or absent psychoactive effects, which is significant for the pharmaceutical industry. The presence or absence of this cannabinoid in a chemotype allows evaluation of a strain’s potential in terms of safety and specificity of therapeutic action.
Given the rapid advancement of analytical technologies, such as high-performance liquid chromatography (HPLC) combined with mass spectrometry, quantitative determination of CBCF within cannabinoid profiles has become significantly more accessible and accurate. This expands the capabilities for applying CBCF in chemotyping systems and allows for deep analysis of complex samples with high resolution.
Directions for Future Research
The prospects for further study of cannabichromanone (CBCF) involve a comprehensive interdisciplinary approach that includes expanding fundamental knowledge of its molecular biosynthesis mechanisms, pharmacological activity, as well as technological aspects of its production and application. One key area is the detailed investigation of enzymatic processes responsible for CBCF formation in cannabis plants. Despite existing data, understanding the specifics of enzymatic activity-including enzyme kinetics and regulation-remains incomplete. Future efforts should focus on the isolation, crystallographic study, and mutagenesis of key enzymes that control the cyclic conversion of precursors into CBCF. These studies will lay the foundation for genetic modification of plants aimed at increasing yield or controlling cannabinoid profiles.
A separate issue is the study of molecular interactions between CBCF and receptors in living organisms, particularly cannabinoid receptors CB1 and CB2, as well as other potential molecular targets. Systematic mapping of CBCF affinity to various receptors using modern molecular docking techniques, biophysical methods, and cellular models will help establish its specificity of action and potential signaling pathways. Additionally, research on CBCF metabolism in biological systems-including its bioavailability, pharmacokinetics, and metabolic transformations affecting pharmacodynamics-is necessary.
Another important direction is the development and improvement of analytical methods for quantitative determination of CBCF in complex biological and plant matrices. Advancements in chromatography coupled with mass spectrometry, as well as the implementation of nuclear magnetic resonance (NMR) and infrared spectroscopy methods, will enhance sensitivity and selectivity, lower detection limits, and expand capabilities for simultaneous analysis of complex cannabinoid mixtures. These technological improvements will be critical for large-scale investigation of the natural variation of CBCF across different chemotypes.
Research should also focus on uncovering ecological and agronomic factors that influence CBCF synthesis in cannabis plants. Studying the effects of cultivation conditions-including soil type, lighting, temperature, water regime, and fertilizer use-will enable development of optimal cultivation strategies to maximize CBCF yield. The creation of such methods has direct practical significance for the pharmaceutical industry and agriculture, especially in the context of raw material standardization and improving the quality of medicinal products.
Special attention should be given to investigating CBCF’s potential within complex bioactive compositions. Experimental studies of the combinatorial effects of CBCF with other cannabinoids, terpenoids, and flavonoids will help reveal mechanisms of synergy or antagonism that may be critical for therapeutic applications. Further evaluation of these interactions at molecular, cellular, and organismal levels will pave the way for developing more effective, targeted, and safer multi-component pharmaceuticals.
In biotechnology, an important focus will be engineering microorganisms capable of synthesizing CBCF at commercially viable scales. Developing recombinant systems based on bacteria, yeast, or plant cells with high productivity and synthesis stability requires optimizing genetic constructs, cultivation conditions, and scaling processes. The use of CRISPR/Cas technologies to edit the genomes of natural cannabinoid producers could significantly increase CBCF yields and improve the quality of the final product.
Further research should encompass systemic analysis of metabolic networks involving CBCF using metabolomics, proteomics, and transcriptomics methods. Integrating these data will enable the creation of comprehensive models of cannabinoid metabolism regulation, opening new possibilities for precise influence on CBCF biosynthesis and regulation.
Another crucial direction is the development of preclinical and clinical studies to assess the pharmacological efficacy and safety of CBCF. Considering its potential, standardized testing protocols need to be established, including models in cell cultures, target organs, and animal disease models. This will provide comprehensive data on therapeutic potential, toxicity, side effects, and optimal dosages required for further medical application.
Moreover, significant attention must be paid to social, legal, and ethical aspects related to CBCF use. Given the complexity of regulatory requirements in different countries, research on the impact of legal frameworks on the development of scientific and commercial projects is important for forming strategies to implement new technologies in production and medical practice.
Conclusion
Cannabichromanone (CBCF) is a chemical compound with unique structural and physicochemical properties belonging to the cannabinoid group-a class of natural phenolic metabolites characteristic of plants in the Cannabis genus. Its molecular structure features a distinctive cyclic conformation that determines its specific chemical reactivity and biological activity. Careful study of the physicochemical characteristics of CBCF enables the identification of parameters that define its stability, solubility, and interactions with biomolecules, forming the basis for further scientific and technological applications.
The biogenetic origin of CBCF is closely linked to the cannabinoid biosynthetic system, in which a complex interplay of enzymes and precursors leads to the formation of this molecule. The specifics of enzymatic mechanisms responsible for cyclic reactions and the particular set of functional groups remain subjects of active research. Revealing the precise biosynthetic pathways of CBCF is fundamentally important for controlling and regulating its production in natural sources.
Methods of obtaining CBCF include semi-synthetic approaches, total chemical synthesis, and biotechnological techniques. Each of these methods has its advantages and challenges: semi-synthetic routes allow modification of natural substances, laboratory synthesis provides the possibility of producing pure isomers, and biotechnological methods-particularly the use of recombinant microorganisms-offer prospects for large-scale and environmentally safe production. This comprehensive approach creates a foundation for a wide range of CBCF applications in pharmaceuticals and industry.
The biological potential of CBCF is characterized by significant promise in terms of potential bioactivity. Despite limited available experimental data, there are grounds to believe that CBCF can interact with various molecular targets in the body, demonstrating diverse pharmacological effects. Scientific investigation of this aspect requires further preclinical and clinical testing to confirm safety and efficacy.
The role of CBCF in cannabis chemotyping opens new horizons for classification and standardization of plant strains based on detailed cannabinoid profiles. This facilitates the development of more precise and controlled cultivation methods and allows for the creation of products with specified pharmacological properties. Thus, CBCF becomes an important marker that complements traditional cannabis classification systems.
Future research directions are aimed at a deeper understanding of the biochemical, molecular, and pharmacological mechanisms of CBCF action, development of new synthesis methods, improvement of analytical technologies, and the design of clinically relevant applications. Integration of interdisciplinary studies will not only expand fundamental knowledge but also effectively implement CBCF in medicine, pharmaceuticals, and other fields that require innovative bioactive compounds.
Therefore, CBCF represents a promising subject of scientific research with considerable potential for practical use. Its comprehensive study opens new opportunities for developing innovative therapeutic agents and technological solutions, emphasizing the importance of continued systematic work in this area.
References
- Structure-Activity Relationship of Cannabis Derived Compounds for Cannabinoid Receptors.
https://pmc.ncbi.nlm.nih.gov/articles/PMC6099582/ - Cannabichromene (Wikipedia).
https://en.wikipedia.org/wiki/Cannabichromene - Phytocannabinoids: A Unified Critical Inventory.
https://www.nature.com/articles/c6np00074f - Evolution of the Cannabinoid and Terpene Content during the Growth of Cannabis sativa Plants from Different Chemotypes.
https://pubs.acs.org/doi/abs/10.1021/acs.jnatprod.5b00949 - Antitumor Activity of Plant Cannabinoids with Emphasis on the Effect of Cannabidiol on Human Breast Carcinoma.
https://pubmed.ncbi.nlm.nih.gov/16798734/ - Cannabichromene, Related Phytocannabinoids, and 5-Fluoro-cannabichromene Have Anticonvulsant Properties in a Mouse Model of Dravet Syndrome.
https://pubs.acs.org/doi/abs/10.1021/acschemneuro.0c00556