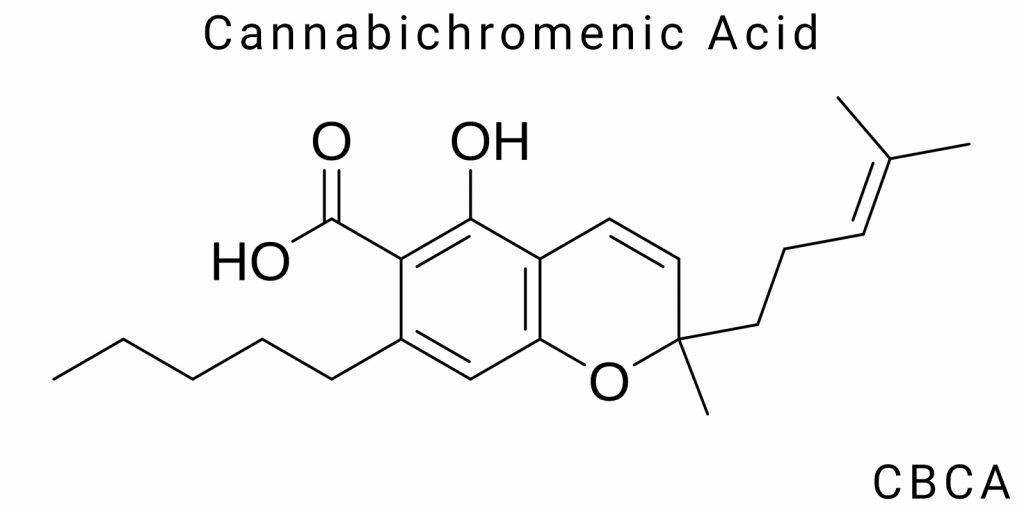
Cannabinoids are chemical compounds found in the Cannabis sativa plant, which has a long history of medicinal use. They belong to the group of secondary metabolites produced by the plant to protect against stressors such as ultraviolet radiation and pests. The major cannabinoids include Δ9-tetrahydrocannabinol (THC), cannabidiol (CBD), and their derivatives. However, recent research has also focused on lesser-known compounds, such as cannabichromenic acid (CBCA).
Overview of Cannabinoids as Secondary Metabolites of Cannabis sativa
Like other plant secondary metabolites, cannabinoids do not play a role in the primary biological processes such as the growth or development of the plant. However, their role in protecting against harmful environmental influences is crucial. Cannabinoids, in particular, can interact with various receptors in organisms, including humans, which underlies their biological activity. These compounds are synthesized in Cannabis sativa in their acidic forms, which then undergo decarboxylation to form neutral cannabinoids like THC and CBD.
Position of CBCA among the Acidic Forms of Cannabinoids
Cannabichromenic acid (CBCA) is one of the primary acidic precursors of cannabichromene (CBC), a cannabinoid that has gained attention due to its potential medical properties, particularly its anti-inflammatory and analgesic effects. CBCA is the precursor compound that converts into CBC after the loss of the carboxyl group. However, it is important to note that CBCA has its own unique properties, which may be beneficial for medicine, although research on this acid is ongoing.
Defining CBCA as a Biochemical Precursor to Cannabichromene (CBC) and a Promising Standalone Compound
While CBCA is a key precursor to cannabichromene (CBC), the acid itself holds certain biological potential. Studies suggest that CBCA may influence some physiological processes, notably through its ability to interact with cannabinoid receptors, although its activity in this regard requires further investigation. Given this, CBCA has potential as an independent compound, especially in the context of its potential use in medical or therapeutic applications.
Considering all of this, research into CBCA as a standalone compound may lead to new discoveries in cannabinoid pharmacology and contribute to the development of novel cannabinoid-based treatments
Biogenesis and Chemical Characteristics
Cannabichromenic acid (CBCA) is a component of the secondary metabolism of Cannabis sativa, which produces dozens of bioactive compounds with pharmacological potential. The biogenesis of CBCA begins with cannabigerolic acid (CBGA), a key precursor to almost all major cannabinoids. This metabolite is formed through the condensation of olivetolic acid with geranyl pyrophosphate in the presence of a specific synthase enzyme. Subsequently, CBCA synthase, a flavoprotein enzyme, catalyzes the cyclization of CBGA, forming the three-ringed structure of CBCA. The uniqueness of this pathway lies in the fact that CBCA synthase only produces CBCA, highlighting its enzyme specificity (Taura et al., 2009, NIH.gov).
Unlike more well-known acidic forms of cannabinoids, such as THCA or CBDA, CBCA is less studied; however, its biochemical nature indicates an important functional role. CBCA contains a carboxyl group, which not only determines its acidity but also influences its physicochemical properties – particularly its ionization ability, solubility in polar environments, and chemical stability. The molecular structure of CBCA features a tricyclic core, which results in spatial rigidity and likely dictates its interaction with proteins or receptors, distinguishing it from more flexible, linear cannabinoid forms.
Particular attention should be given to the thermal lability of CBCA. When stored at room temperature, and especially when heated, CBCA gradually undergoes decarboxylation, releasing carbon dioxide and converting into its neutral form – cannabichromene (CBC). This process is typical of many acidic cannabinoids; however, in the case of CBCA, it holds additional scientific value because it allows the production of CBC free from the contamination of other cannabinoids. This is especially important in laboratory settings, where reaction purity impacts the validity of pharmacological studies (Morimoto et al., 1998, PubMed).
It should be noted that even in its natural environment, CBCA may undergo spontaneous conversion into CBC, particularly under conditions of elevated temperature or humidity. This fact has practical implications for the storage of Cannabis sativa raw material, as well as for determining the optimal conditions for cannabinoid extraction for research or medical purposes.
Interest in CBCA is also growing in the context of finding new biomarkers for cannabis chemotyping. A high level of CBCA in certain strains may indicate specific activity of CBCA synthase, which could be valuable for breeding programs and pharmacological screening. Furthermore, the chemical uniqueness of this compound – particularly its distinct stereochemistry – could lead to interactions with atypical molecular targets, expanding the scope of its medical applications.
Pharmacological Properties Research
Unlike well-known phytocannabinoids such as Δ9-tetrahydrocannabinol (THC) or cannabidiol (CBD), cannabi chromenic acid (CBCA) has long been considered a secondary metabolite in the biosynthesis of cannabichromene (CBC). Initially regarded as a mere precursor, recent years have brought new data that allow for a reassessment of CBCA’s potential as a bioactive compound. Below, we outline the main areas of research that are shaping the understanding of its pharmacological properties.
Anti-inflammatory Activity
Inhibition of Pro-inflammatory Factors: CBCA is capable of influencing the processes underlying inflammation. At the cellular level, it can inhibit the expression of enzymes that catalyze the production of pro-inflammatory molecules. Specifically, CBCA has been shown to reduce the activity of cyclooxygenase-2 (COX-2), a key enzyme in the biosynthesis of prostaglandins—compounds that initiate swelling, pain, and redness during inflammatory responses.
Immunomodulation: Certain in vitro studies have demonstrated that CBCA reduces the release of pro-inflammatory cytokines such as interleukin-6 (IL-6) and tumor necrosis factor alpha (TNF-α). This suggests that CBCA may function as a modulator of the immune response, softening excessive activation commonly observed in chronic inflammatory conditions, without fully suppressing it.
Clinical Application Potential: This anti-inflammatory activity is particularly significant in the search for alternatives to non-steroidal anti-inflammatory drugs (NSAIDs), which often cause adverse gastrointestinal effects. Given that CBCA shows no toxicity at physiologically relevant doses, it warrants further preclinical investigation as a mild immunomodulator or as an adjunct in anti-inflammatory therapy.
Antibacterial Properties
Action on Pathogenic Microorganisms: Similar to other acidic cannabinoids, CBCA demonstrates antibacterial activity against a range of Gram-positive bacteria. Its effect against Staphylococcus aureus, including antibiotic-resistant strains, is of particular interest. In laboratory conditions, it disrupts the bacterial cell wall structure, leading to cell death.
Mechanism of Action: Membrane Dysfunction: Unlike classic antibiotics, CBCA does not interfere with protein or DNA synthesis in bacteria. Instead, it likely destabilizes the lipid membrane, creating pores through which the bacterium loses its osmotic balance. This unique mechanism is important as it potentially avoids the traditional pathways of resistance development.
Synergy Potential: Another area of exploration is the possibility of combining CBCA with other antimicrobial agents. Some observations suggest that CBCA may enhance the effect of compounds like cannabigerolic acid (CBGA) or even traditional antibiotics. This opens up the potential for new strategies to combat multidrug-resistant infections.
Interaction with TRP Channels
Effect on Sensory Receptors: CBCA has shown activity with members of the Transient Receptor Potential (TRP) family of receptors, specifically TRPA1 and TRPV1. These ion channels are involved in the perception of temperature, chemical irritants, and pain. In experimental settings, CBCA is able to activate these channels, which may explain its potential in reducing pain or altering thermoregulatory responses.
Therapeutic Implications: Activation of TRP channels is associated with the relief of chronic pain, reduction of neuropathic symptoms, and even involvement in controlling inflammation in nerve tissue. This positions CBCA as a potential component in the development of new treatments for conditions associated with sensory disturbances, such as migraines, fibromyalgia, or thermo neuropathies.
Need for Further Study
Pharmacokinetic Aspects: Currently, there is limited data on the absorption, distribution, metabolism, and excretion of CBCA in humans or animals. Due to its acidic nature and potential instability in neutral environments, studying its pharmacokinetics is critical for future therapeutic applications.
Importance as an Independent Compound: CBCA should not be regarded solely as a precursor in the path to CBC. Its own bioactivity, absence of psychoactive effects, and broad action on various biological systems position it as a promising compound for pharmacology, especially when a gentle modulation of physiological processes is needed without overt interference.
Scientific and Clinical Appeal: The use of CBCA in medicine could be attractive both as a monotherapy and in combination with other natural compounds. Modern synthesis and purification methods provide opportunities for standardization, which is a necessary condition for the initiation of preclinical and clinical studies.
Scientific Perspectives and Challenges
Despite the growing interest in rare cannabinoids, cannabic hromenic acid (CBCA) remains underexplored compared to compounds like Δ9-tetrahydrocannabinol (THC) or cannabidiol (CBD). Determining its place in modern biomedicine requires a systematic approach—from basic research to clinical application—which currently faces a number of interdisciplinary challenges.
Lack of Large-Scale Clinical Studies
Currently, none of the major pharmacological or clinical studies published in databases like PubMed, ClinicalTrials.gov, or NIH.gov focus exclusively on CBCA. Available information about its biological effects is primarily based on previous laboratory studies (in vitro) or animal models (in vivo). This creates a significant gap between preclinical results and potential clinical application.
For example, promising data on CBCA’s anti-inflammatory and antibacterial activities cannot be automatically translated to human physiology without clinical confirmation. An additional issue is the low bioavailability of acidic cannabinoid forms, which complicates the assessment of their effectiveness in oral or parenteral administration. Moreover, existing international clinical research protocols do not account for the specific features of rare phytocannabinoids, making their registration in research initiatives more difficult.
Entomedicine Context: The Need for a Systematic Approach
Like other acidic cannabinoids, CBCA may exhibit pharmacological activity not only in isolation but also in combination with other components of cannabis. This is why it should be studied within the framework of entomedicine—a concept suggesting that the synergistic action of cannabinoids, terpenoids, and flavonoids provides a complex biological response (the entourage effect).
At this stage of scientific development, there is a lack of studies evaluating CBCA as part of the chemical ensemble. Specifically, it remains unclear how CBCA affects the activity of CB1/CB2 receptors in the presence of other acidic forms, whether it can modulate the effects of CBDA or THCA, or whether there is synergy with terpenoids affecting the central or peripheral nervous system.
This opens the prospect of creating standardized phytocomplexes, in which CBCA would play a role not only as a precursor to CBC but also as a modulator of the body’s systemic response.
Emerging Pharmaceutical Applications
Another promising direction is the inclusion of CBCA in pharmaceutical formulations. However, several challenges must be overcome before it can be implemented in clinical practice:
Chemical Instability: CBCA is prone to rapid decarboxylation even at moderate temperatures or during prolonged storage. This process converts it into CBC, altering both its chemical and biological properties. To ensure stability, special storage and delivery conditions need to be developed, such as microencapsulation or antioxidant environments.
Methods of Administration: CBCA’s acidic nature can complicate its absorption in the gastrointestinal tract. It requires further investigation into its transport across biological membranes, the effect of pH environments, and enzymatic breakdown. Promising forms of delivery include sublingual drops, transdermal patches, or inhalers.
Need for Pharmacopoeial Standardization: None of the leading pharmacopoeias—such as USP, EMA, or JP—currently contain standards for CBCA, making its official use in medical practice impossible. To remedy this, methods for quantitative analysis, impurity profiling, and stability testing need to be established, as has already been done for CBD.
Interdisciplinary Barriers: Ethics, Law, and Technology
Ethical Aspects: In many jurisdictions, cannabinoids are still classified as controlled substances, regardless of whether they have psychoactive properties. This means researchers must undergo the same regulatory procedures as when working with THC, even when studying CBCA—an acid with no known psychoactive effects. Overcoming these barriers requires changes in regulatory frameworks and educational programs for ethical committees.
Legal Obstacles: CBCA does not have a separate legal status in most countries. Its presence in cannabis extracts automatically places it under general restrictions, even if the product contains it only in microdoses. This complicates patenting, cross-border transportation, licensing for production, and participation in international collaborative studies.
Technological Difficulties: The low natural concentration of CBCA in cannabis plants makes its economic extraction difficult using traditional methods. Moreover, during standard raw material processing (such as drying, heating, or ethanol/CO₂ extraction), the compound degrades or converts into CBC. Biotechnology methods, such as producing CBCA via genetically modified yeast or cannabis cell cultures, hold promise for creating standardized raw materials for research and pharmaceutical use.
Conclusions
Cannabichromenic acid (CBCA) is increasingly gaining attention as a distinct target for scientific research, rather than just as a chemical precursor to cannabichromene (CBC). From a structural perspective, it is a relatively stable compound within the acidic forms of cannabinoids, which opens up possibilities for its storage, standardization, and potential therapeutic application without the immediate need for decarboxylation.
The presence of the carboxyl group imparts unique properties to CBCA, including its influence on cell membrane permeability, potential for conjugation with other biomolecules, and distinct pharmacokinetics compared to decarboxylated analogs. Although its biological activity is not fully understood, it suggests potential for regulating immune responses, antimicrobial action, and interactions with TRP receptors—areas that deserve further experimental investigation.
CBCA should be considered not only in the context of its natural origin or role in the secondary metabolism of Cannabis sativa, but also as a promising pharmacological entity. Further research into its properties requires a multidisciplinary approach, from natural product chemistry to pharmacology and biotechnology. It is also crucial to establish legal, technological, and ethical frameworks for incorporating CBCA into future clinical programs.
References Used:
- Shoyama, Y., et al. (2008). Biosynthesis and function of cannabinoid synthases. Journal of Natural Medicines, https://doi.org/10.1007/s11418-008-0251-5
- Gülck, T., & Møller, B. L. (2020). Phytocannabinoids: Origins and biosynthesis. Trends in Plant Science, https://doi.org/10.1016/j.tplants.2020.06.001
- Appendino, G., et al. (2008). Antibacterial cannabinoids from Cannabis sativa: A structure–activity study. Journal of Natural Products, https://doi.org/10.1021/np8002673
- Russo, E. B. (2011). Taming THC: Potential cannabis synergy and phytocannabinoid-terpenoid entourage effects. British Journal of Pharmacology, https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3165946
- U.S. National Library of Medicine, PubChem Database. Cannabichromenic Acid (CBCA), https://pubchem.ncbi.nlm.nih.gov/compound/CBCA
- National Institutes of Health (NIH). Cannabinoid Research,https://cancer.gov
- European Monitoring Centre for Drugs and Drug Addiction (EMCDDA). Cannabis and cannabinoids: pharmacology and toxicology, https://www.emcdda.europa.eu