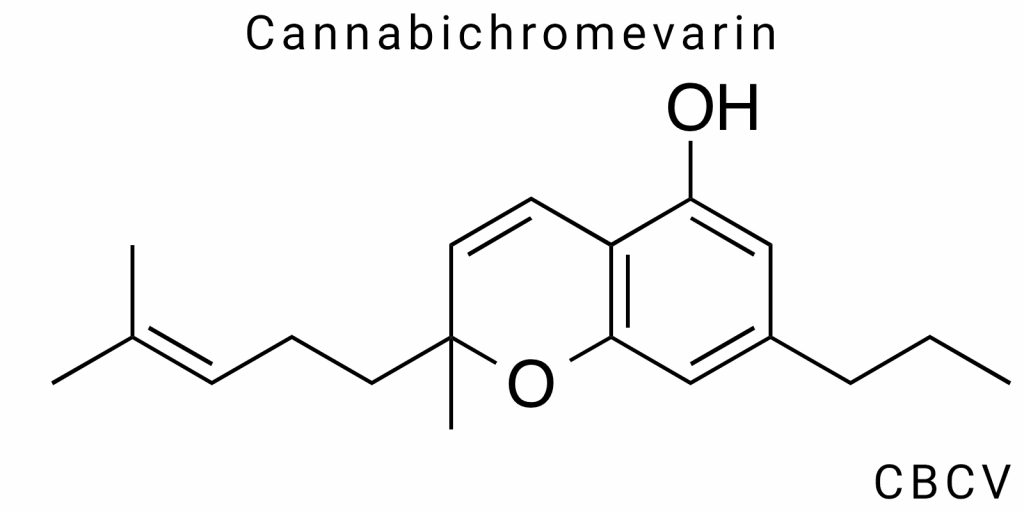
Cannabichromavarin (CBCV) is one of the lesser studied phytocannabinoids, also called cannabivarichromene, and occurs naturally in plants of the genus Cannabis. It is also a variation of the better-known cannabichromene (CBC), but has a shorter side alkyl chain, which distinguishes its pharmacological profile.CBCV belongs to a group of cannabinoids known as “propyl cannabinoids,” which have garnered attention from researchers due to their potential therapeutic properties and unique interactions with the endocannabinoid system.
Although CBCV is present in cannabis in only trace amounts, modern analytical techniques, such as HPLC (High-Performance Liquid Chromatography), now allow for precise detection and study of this cannabinoid. The first mentions of CBCV appeared in scientific literature in the 1970s, but interest in minor cannabinoids, particularly CBCV, has surged over the last decade due to advancements in extraction and purification technologies, as well as a growing interest in personalized medicine based on natural compounds.
Current research on CBCV is still in its early stages; however, preliminary data suggests it may play a role in regulating inflammation, pain, and neuroprotection. This review aims to systematically compile existing scientific knowledge about CBCV, exploring its chemical structure, biosynthesis, methods of extraction, potential mechanisms of action, and its prospects for medical applications.
Origin and Biogenetic Pathway of Сannabivarichromene
CBCV as a Member of Propyl Cannabinoids
Cannabichromevarin (CBCV) is part of the lesser-studied group of propyl (varin) cannabinoids, which are compounds characterized by a three-carbon alkyl side chain (propyl group), in contrast to the more common cannabinoids (THC, CBD, CBC) that feature a five-carbon pentyl side chain. Although this structural difference may seem minimal at first glance, it leads to significant changes in the biochemistry, pharmacokinetics, and potential biological effects of the compound.
While CBCV is chemically similar to cannabichromene (CBC), it arises from an entirely different genetic and biosynthetic pathway, unique to the varin chemotype of Cannabis plants.
Varin Chemotype: A Rare Genetic Trait
In natural populations of Cannabis sativa, several chemotypes (biochemical phenotypes) exist, defined by the predominance of certain cannabinoids. Among the most well-known are the THC-dominant (Type I), CBD-dominant (Type II), and mixed (Type III) chemotypes. A lesser-known but highly interesting chemotype is the varin chemotype (Type IV), which primarily synthesizes propyl derivatives such as tetrahydrocannabivarin (THCV), cannabidivarin (CBDV), and, to a lesser extent, CBCV.
The basis of this phenomenon lies in a mutation or variation in the genes that code for specific enzymes responsible for the first step in cannabinoid synthesis-converting fatty acid derivatives into CBG-based compounds.
Instead of the pentyl precursor (oleic acid), plants with the varin chemotype accumulate divarinic acid, a source of short-chain cannabinoids. This compound is subsequently combined with geranylgeranyl pyrophosphate (GPP) to form cannabigerovarinic acid (CBGVA), a precursor to all varin cannabinoids.
Biogenetic Pathway of CBCV
The biosynthesis of CBCV begins with cannabichromevarin acid (CBGVA), a derivative of cannabigerolic acid (CBGA), in which the side chain is propyl rather than pentyl. In the next step, through the action of the specific enzyme CBCV synthase, CBGVA is converted into cannabichromevarinic acid (CBCA-V). Like other acidic cannabinoids, CBCA-V is not the active form. It only becomes the active neutral compound, CBCV, after exposure to heat, light, or time-through natural or thermal decarboxylation.
The pathway can be summarized as follows:
- CBGVA (Cannabichromevarinic Acid)
- CBCA-V (Cannabichromevarinic Acid)
- CBCV (Cannabichromene)
This biosynthetic route follows the general logic of cannabinoid biosynthesis, but the propyl variant remains insufficiently studied. This is due to both technical challenges in isolating CBCV and the limited distribution of varin genotypes in cultivated cannabis.
Genetic Features of CBCV-Producing Plants
In plants with the varin chemotype, specific alleles of enzymes are active, determining the presence of propyl forms. In particular, the enzyme CBCA synthase, which typically synthesizes CBCA from CBGA in “regular” plants, has a propyl variant known as CBCA-V synthase, which works with CBGVA. The presence of such genetic variations is the result of mutations in DNA sequences that encode the respective synthases. This means that CBCV-producing plants possess a unique combination of genes that activate the synthesis of propyl cannabinoids, and this trait can be stably passed to offspring.
From a breeding perspective, this opens the possibility of developing high-specificity cannabis strains focused on the content of minor cannabinoids like CBCV through targeted breeding or genomic editing. However, in practice, such selection requires precise genetic screening and enzymatic activity control, which is still in the active research phase.
Chemical Properties of CBCV: A Variation that Alters Pharmacology
cannabichromevarin (CBCV) as a Homolog of Cannabichromene (CBC)
Cannabichromevarin (CBCV) is the propyl homologue of cannabichromene (CBC), characterized by the presence of a three-carbon (propyl) side chain in place of the five-carbon (pentyl) side chain typical of most naturally occurring cannabinoids. Its molecular formula is C₁₉H₂₆O₂, and although the difference in the side chain may seem minor, it fundamentally alters both the physicochemical properties and pharmacological profile of the compound.
Impact of the Side Chain on Lipophilicity
Lipophilicity plays a critical role in the pharmacokinetic behavior of a molecule: it affects solubility in cell membranes, bioavailability, the ability to cross the blood-brain barrier (BBB), and accumulation in adipose tissue. The propyl side chain of CBCV, with fewer carbon atoms, reduces the overall hydrophobicity of the molecule, as evidenced by a lower calculated logP value. For CBC, this value is approximately 6.3, whereas for CBCV, it is around 5.2, indicating a reduced tendency to accumulate in lipophilic compartments.
Research on membrane transport modeling has shown that even a one-unit reduction in logP can lead to significant changes in pharmacokinetics, such as reduced ability to cross the BBB, but at the same time, it can increase circulation in the blood and availability in peripheral tissues.
Membrane Permeability and Cellular Availability
The smaller size and lower lipophilicity of CBCV change its ability to diffuse across biological membranes. On one hand, the propyl derivative is more easily transported through less dense epithelial barriers, which may be relevant for enteral or dermal administration. On the other hand, permeability across the BBB, which heavily depends on lipid interactions, may be reduced. This potentially limits the central activity of CBCV, making its effects more peripheral, a critical consideration when evaluating its therapeutic applications.
Interaction with Cellular Receptors: TRP, PPAR, GPR55
TRP channels (transient receptor potential) are a family of ion channels sensitive to temperature, ion concentration, and chemical signals. CBC is known to be an agonist of TRPA1 and an antagonist of TRPM8. Receptor sites on TRP channels are highly sensitive to the steric properties of ligands, such as flexibility, chain length, and charge distribution.
For CBCV, the presence of a propyl side chain, as opposed to a pentyl one, could result in reduced hydrophobic interaction with the internal domains of TRP receptors, potentially leading to a decrease in affinity or even a change in the type of effector response (from agonism to partial antagonism). These effects are currently the subject of hypothetical modeling and require verification through in vitro studies.
PPAR receptors (peroxisome proliferator-activated receptors), particularly the γ isoform (PPARγ), regulate lipid homeostasis, inflammatory cascades, and cell differentiation. CBC has established activity toward PPARγ, while CBCV, due to its shorter side chain, is expected to have reduced affinity. However, activation of other isoforms, particularly PPARα, involved in the control of β-oxidation of fatty acids in the liver, cannot be ruled out.
GPR55, the so-called “orphan” cannabinoid receptor, is intensively studied as a potential target for oncological conditions, hypertension, and inflammatory diseases. CBC acts as an antagonist of GPR55, inhibiting cellular proliferation. Structural modification in CBCV, specifically a smaller contact area, could reduce its affinity, but at the same time increase its selectivity for certain tissue-specific variants of GPR55.
Pharmacological Potential of CBCV: Hypotheses and Preliminary Data
Although cannabichromevarin (CBCV) is one of the lesser-studied phytocannabinoids, its pharmacological potential has sparked interest due to its unique chemical structure and functional similarities with more widely researched cannabis compounds. At present, CBCV research is mainly limited to cell line experiments, biochemical modeling, and extrapolation of data from closely related cannabinoids. However, even these initial findings provide the foundation for several hypotheses that warrant further validation.
Interactions of CBCV with Non-Classical CB1/CB2 Receptor Systems
Although CBCV, like CBC, does not exhibit significant affinity for CB1 and CB2 receptors, this does not imply an absence of pharmacological activity. On the contrary, modern cannabinoid research is gradually moving away from a narrow focus on CB1/CB2, acknowledging the existence of a broader system, often referred to as the endocannabinoid signaling network. This network includes targets such as TRP receptor family members, PPAR, GPR55, and enzymes responsible for the breakdown of endocannabinoids (FAAH, MAGL).
According to studies involving other varin cannabinoids, CBCV likely has the ability to influence signal transduction through non-canonical receptors, especially TRP channels. This activity does not require direct binding with cannabinoid receptors but may indirectly modulate neurotransmitter release, inflammatory mediators, or neuronal excitability.
TRPV1, TRPA1, TRPM8: Potential Targets for Pain and Inflammation Modulation
TRP receptors, particularly TRPV1 (vanilloid receptor) and TRPA1 (ankyrin-sensitive receptor), are located on sensory neurons and immune cells. Activation or inhibition of these receptors directly influences pain signaling, temperature sensitivity, oxidative stress, and inflammatory processes.
Given the structural features of CBCV-its propyl side chain and chromene core-there are grounds to believe that this molecule may modulate TRP receptors in a manner similar to CBDV and THCV. Although these effects have not yet been confirmed in vivo for CBCV, in vitro studies with cannabinoids containing shorter side chains have demonstrated more selective and less psychoactive activity. This selectivity is advantageous for the development of pharmacological agents.
The potential mechanism of action may be allosteric, meaning that the substance alters the receptor’s sensitivity to natural ligands rather than replacing them in the binding pocket. Such a mechanism would allow for subtle modulation of signaling cascades without disrupting homeostasis.
Potential Role in Cell Proliferation Regulation
Although direct data on the antiproliferative activity of CBCV is lacking, the question of its involvement in cell cycle regulation, particularly in epithelial and oncologically transformed cells, remains relevant. It is known that cannabinoids can inhibit signaling pathways related to PI3K/Akt, MAPK/ERK, and other key proliferation cascades. Based on structural similarities with CBC and THCV, it is plausible to hypothesize that CBCV could indirectly influence the expression of genes responsible for apoptosis and cell division.
This hypothesis is further supported by data regarding the activity of varin cannabinoids (CBDV, THCV), which in in vitro experiments demonstrated a reduction in cancer cell viability without toxic effects on healthy cells. If CBCV exhibits similar selectivity, it would open avenues for further research in oncoprevention or adjunctive treatment.
Obstacles in Research and Strategic Directions
One reason why CBCV remains relatively unstudied is the difficulty in its extraction and quantification. This cannabinoid is synthesized in low concentrations, predominantly in varin-type cannabis chemotypes, while most commercially cultivated cannabis strains are oriented toward high THC or CBD content. As a result, even for basic in vitro experiments, CBCV must be synthetically or semi-synthetically obtained, which limits the scope of research.
Moreover, there are currently no established in vivo protocols for testing CBCV on laboratory models. This requires the development of methods for administration, metabolite analysis, and toxicity profiling. However, with the growing interest in varin cannabinoids, particularly in Europe (e.g., Italy, Germany, Israel), it is expected that the first preclinical models may emerge soon.
CBCV in the Spectrum of Varin Cannabinoids: What Sets It Apart
Varin cannabinoids, such as cannabichromevarin (CBCV), represent a distinct, underexplored subgroup within the class of phytocannabinoids. These molecules feature a propyl chain substitution instead of the more typical butyl group (as found in THC or CBD). This structural feature gives them unique physicochemical properties and the potential for specific pharmacological activities. The study of varin cannabinoids has opened new horizons for therapeutic cannabis applications, particularly due to their possible effects on various biological systems without significant psychoactive effects. Comparing CBCV with other varin cannabinoids, such as THCV and CBDV, provides a better understanding of the potential of this group of molecules in medical practice.
Comparison with THCV and CBDV
Psychoactivity:
One of the key characteristics that distinguishes CBCV from other varin cannabinoids is its lack of psychoactive effects. Unlike THCV, which, in higher doses, can exhibit weak psychoactive effects (though much less pronounced than THC), CBCV does not induce changes in mental state or perception. This makes CBCV particularly attractive for medical use, where minimizing cognitive and emotional changes is necessary, as well as allowing for combinations with other cannabinoids without the risk of psychoactive influence.
Medical Effects:
- THCV: By blocking CB1 receptors, THCV has potential for appetite reduction and may be used as a treatment for metabolic disorders such as obesity or type 2 diabetes. However, its psychoactive properties limit its use in certain patient populations.
- CBDV: Like CBD, CBDV is being studied for its anticonvulsant properties and its potential impact on psychiatric conditions and autism spectrum disorders. CBDV lacks psychoactive effects and can influence neuroplasticity while reducing hyperactivity in neural networks.
- CBCV: Not only is CBCV non-psychoactive, but it may also play an adjuvant role when combined with other cannabinoids. Due to its ability to modulate various biological pathways (e.g., through TRP or PPAR receptors), CBCV can enhance the effects of cannabinoids like CBDV or THCV while reducing potential side effects.
Varin Cannabinoids: A New Direction in Pharmaceuticals
Pharmaceutical Properties of Varin Cannabinoids:
The most significant aspect of varin cannabinoids, including CBCV, is their potential for developing new pharmaceutical agents. Due to their molecular structure, varin cannabinoids show potential in several key pharmacological areas:
- Anticonvulsant Properties: Like CBDV, varin-type cannabinoids have demonstrated efficacy in epilepsy treatment. They do not produce psychoactive effects and can be used as adjunctive therapy to reduce the frequency of seizures in patients who do not respond to traditional anticonvulsant drugs.
- Metabolic Effects: Cannabinoids have the ability to influence metabolism, particularly through anti-insulin activity or possible modulation of lipid metabolism. This makes them interesting for treating metabolic syndrome, type 2 diabetes, and obesity prevention, as they can regulate appetite and reduce the risk of insulin resistance.
Impact on the Microbiome:
Recent studies suggest that cannabinoids, including varins, may influence the gut microbiome, particularly the microbiota involved in fatty acid metabolism. The concept of the gut microbiota as an integral part of the endocannabinoid system is new, but scientists speculate that cannabinoids, especially varins, may modulate bacterial balance to promote better fat processing and anti-inflammatory activity.
Interaction with Other Phytocannabinoids: Enhancing Effects
One of the key aspects of varin cannabinoids’ application is their adjuvant role when combined with other cannabinoids. Like CBDV, CBCV can enhance the anti-inflammatory, antiproliferative, and neuroprotective activity of other cannabinoids. Such effects are possible due to their interaction at receptor or signaling pathway levels.
Furthermore, varin cannabinoids help minimize the psychoactive effects of THC while enabling a more targeted impact on specific biological pathways. This allows for the development of balanced medications that meet the specific medical needs of patients without the risk of adverse psychoactive side effects.
Current Research on CBCV:
Despite the promising potential of varin cannabinoids like cannabichromevarin (CBCV), the current state of research still leaves much uncertainty. To date, there are no registered clinical trials evaluating CBCV as a standalone compound. This lack of clinical studies limits our understanding of CBCV’s pharmacokinetics, safety, dosage, and efficacy in different clinical contexts.
However, this does not mean that interest in this molecule is lacking. An increasing number of studies are focusing on understanding its potential pharmacological effects and therapeutic applications. With the development of extraction methods and the popularity of full-spectrum cannabinoid extracts, CBCV is becoming available as part of extracts containing various cannabinoids, although in most cases, its concentration is not controlled, making it difficult to ensure the accuracy and reproducibility of results.
Lack of Clinical Trials for CBCV as a Standalone Compound
Currently, CBCV does not have officially registered clinical trials where it is applied as a standalone compound for treating specific diseases. All studies so far are limited to in vitro research or studies using full-spectrum extracts containing cannabinoids, including CBCV. In these extracts, CBCV is often found in small amounts, and the lack of control over its concentration complicates precise study of its pharmacological properties and potential medical uses.
Nevertheless, this does not reduce CBCV’s potential, as its effects may still be significant in the context of improving the properties of cannabinoid extracts or as an adjunct in combination therapies with other cannabinoids. However, clinical trials are necessary to establish clear pharmacokinetic profiles and dosing standards.
Potential Applications of CBCV in Various Clinical Areas
Neurology: Antispasmodic Effects
One of the main areas where CBCV’s potential could be realized is neurology, particularly in the treatment of neurological disorders associated with spasms. There is a hypothesis that CBCV may exhibit antispasmodic effects similar to other cannabinoids like CBD and CBDV, which have already shown efficacy in epilepsy, peripheral spasms, and neurological disorders.
Through interactions with TRPV1 and TRPA1 receptors and potential modifications of the endocannabinoid system, CBCV may help reduce neural excitability and lower pain levels caused by nerve impulses. This makes CBCV potentially useful for diseases like multiple sclerosis, Parkinson’s disease, or muscle spasms, where spasms are a primary symptom.
Dermatology: Potential in Skin Disorders
Another promising field for CBCV is dermatology. Some cannabinoids, such as CBD and THCV, have already demonstrated their effectiveness in treating inflammatory skin disorders like psoriasis or eczema. Given CBCV’s anti-inflammatory activity (especially through activation of PPAR receptors), there is reason to believe that this molecule may have a similar effect, contributing to reducing inflammation and promoting skin wound healing.
The use of cannabinoids in dermatology opens new horizons for treating skin diseases, and CBCV may prove to be a useful tool in this regard, particularly for treating chronic skin inflammatory processes.
Enterology: Inflammatory Processes in the Digestive System
Additionally, CBCV could show effectiveness in enterology, particularly in inflammatory bowel diseases such as Crohn’s disease or ulcerative colitis. Cannabinoids have already demonstrated their ability to influence inflammatory processes in the digestive system through interactions with TRPV1 and TRPA1 receptors, which regulate pain sensations and inflammatory reactions in the gut.
CBCV could help regulate inflammatory cytokines and reduce oxidative stress, which is an important aspect in treating chronic inflammatory gastrointestinal diseases. While specific studies on CBCV are still lacking, analogies with other cannabinoids suggest significant potential for this molecule in this area.
Technologies for Isolating CBCV:
The isolation of cannabichromevarin (CBCV) presents significant challenges due to its low concentrations in cannabis plants, which are the primary source of this cannabinoid. This necessitates the use of highly precise analytical methods, such as high-performance liquid chromatography (HPLC) and gas chromatography coupled with mass spectrometry (GC-MS), for accurate measurement and identification of CBCV.
Fractional Extraction and Chromatographic Separation
One of the main methods for isolating CBCV is fractional extraction, which allows for the separation of plant material components into different fractions based on their solubility in specific solvents. This method is often used for initial purification and concentration of cannabinoids before more detailed chromatographic methods are applied. The choice of solvent is crucial, as it must selectively extract cannabinoids, including rare molecules like CBCV, from the plant material.
After initial purification, chromatographic separation techniques are used to achieve high accuracy and purity in the analysis. By using HPLC or GC-MS, CBCV can be separated from other cannabinoids, allowing for precise measurement of its quantity and structural identification. A unique feature of CBCV is its ability to be chromatographically separated based on the length of its carbon chain. The CBCV molecule contains a propyl chain, which distinguishes it from other cannabinoids like THCV or CBDV that have ethylamine or other functional groups. Methods that account for these physicochemical properties are particularly effective for separating varin cannabinoids.
Analytical Challenges: Isomerism and Instability
One of the biggest challenges in analyzing CBCV is its isomerism. Like many other cannabinoids, CBCV can exist in different stereochemical forms that may have similar physicochemical properties but different biological effects. This complicates the accurate determination and quantitative assessment of each isomer, especially when they are present in low concentrations. Analysts need to account for this issue by using specialized mass spectrometry techniques to precisely recognize and separate isomeric forms.
Moreover, the instability of CBCV without stabilizers is another critical factor that complicates its isolation and storage. Like many cannabinoids, CBCV is prone to oxidation or degradation when exposed to light, temperature, or oxygen, which requires careful control of conditions to prevent loss of the compound during extraction and analysis. To ensure CBCV’s stability in samples and prevent degradation, antioxidants or low-temperature storage are often used.
Use of High-Precision Analytical Methods
For accurate isolation and identification of CBCV, high-performance liquid chromatography (HPLC) methods with detection via ultraviolet (UV) or fluorescent detectors are often employed. These methods provide high sensitivity and accuracy even at low concentrations of CBCV, which may be present in cannabis plants or extracts.
For more detailed analysis and determination of structural features of cannabinoids like CBCV, gas chromatography coupled with mass spectrometry (GC-MS) is actively used. This method not only detects the presence of CBCV but also structurally identifies the molecule by revealing characteristic fragmentation ions in its mass spectrometric analysis. The combination of GC-MS with fractional extraction allows for precise studies of the composition of cannabinoid extracts, including those containing varin cannabinoids such as CBCV.
Prospects for Developing Analytical Methods
Despite the existing challenges, modern technologies are constantly improving. These advancements enable better precision and accessibility of analytics for rare cannabinoids like CBCV. New approaches in chromatography and mass spectrometry are enhancing the sensitivity and selectivity of the analysis of varin cannabinoids, even at minuscule concentrations in plant samples.
Further advancements in extraction methods and equipment are essential for more efficiently isolating and identifying CBCV and similar molecules with minimal time and resource costs. This will enable scientists and pharmacists to obtain more precise data for the development of potential therapeutic agents based on varin cannabinoids.
Legal Status and Prospects for the Use of CBCV
Legal Status of CBCV
As of 2024, cannabichromevarin (CBCV) is not listed as a psychoactive substance in most countries, including Ukraine and the United States. This means that CBCV is not subject to the strict regulatory restrictions that apply to cannabinoids such as THC. However, it falls into what is known as the “grey zone,” where its use is not clearly regulated but is also not explicitly banned. This status allows for scientific and clinical research into the properties and potential of CBCV, but it does not permit the free commercial use of this cannabinoid in products without proper regulatory oversight.
Since CBCV is non-psychoactive and lacks toxic properties, it could potentially be considered for therapeutic use. However, due to the lack of a clear regulatory framework, scientists and manufacturers have limited access to its widespread application.
Prospects for the Use of CBCV
Prescription Products (Rx-Oriented Medicines)
One of the main prospects for CBCV is its potential in creating prescription medical products. Similar to other varin cannabinoids, CBCV could be useful for developing pharmaceutical drugs targeting specific conditions, such as neurological diseases (e.g., epilepsy or multiple sclerosis), inflammatory processes, and skin disorders. When combined with other cannabinoids, such as CBDV or THCV, CBCV may enhance the effectiveness of treatment, reducing side effects and improving therapeutic outcomes.
Although clinical studies directly confirming the medical efficacy of CBCV are still lacking, its potential use in prescription medications could be an important direction, especially considering its potential adjuvant role in cannabinoid therapy combinations.
Topical Products for Targeted Application
Another promising area is the development of topical products based on CBCV, such as creams, ointments, and balms. Cannabinoids, particularly varin types, show anti-inflammatory and analgesic properties, making them potentially useful for treating skin diseases (e.g., acne, psoriasis, eczema) and localized inflammatory conditions. Topical forms may be more appealing to patients, as they provide targeted application with a lower risk of side effects compared to oral forms.
Due to its non-psychoactive nature, CBCV can be used in such topical products without the risk of psychotropic effects, making it an attractive option for developing safe and effective topical agents.
Combination with CBDV/THCV
A direction for the development of CBCV-based products is its combination with other cannabinoids, such as CBDV (cannabidivarin) and THCV (tetrahydrocannabivarin). Combining these cannabinoids may enhance or modulatively alter their effects, creating a synergistic effect that could be more effective in treating various conditions, such as metabolic disorders, neurological diseases, and mental health issues.
To improve therapeutic strategies, varin cannabinoids like CBCV could be combined with other cannabinoids that have similar properties. This approach could help address important clinical issues related to therapeutic dosing and side effects of cannabinoids in general, enhancing the safety and effectiveness of treatment.
The legal status of CBCV and its therapeutic potential suggest that, with continued research and clearer regulatory frameworks, this cannabinoid could play a significant role in future medical applications, particularly in the areas of neurology, dermatology, and inflammatory diseases.
Conclusions:
Сannabivarichromene (CBCV) is one of the lesser-studied cannabinoids, yet its chemical and pharmacological properties open up new possibilities for medical and scientific research. While there are currently no clinical studies specifically focused on CBCV, preliminary experimental data suggest its potential use in treating a variety of conditions, particularly in neurology, dermatological disorders, and inflammatory processes. The ability of CBCV to interact with receptors such as TRPV1, TRPA1, and others points to its potential in developing new therapeutic strategies for managing pain, inflammation, and even cancer-related processes.
Despite its limited exploration, CBCV remains an important subject for further scientific investigation. It not only holds promise for clinical applications but also could contribute to a deeper understanding of cannabinoid pharmacology and how these compounds impact the body. However, to fully explore and integrate CBCV into medical practice, additional studies are needed, including clinical trials, as well as advancements in extraction methods and standardization of this cannabinoid.
References:
- PubMed (https://pubmed.ncbi.nlm.nih.gov/) – a database of scientific articles containing numerous publications on cannabinoids, their properties, and pharmacological potential.
- Google Scholar (https://scholar.google.com) – for searching scientific articles and publications on specific cannabinoids, particularly CBCV.
- National Institute on Drug Abuse (NIDA) (https://www.drugabuse.gov) – the institute provides numerous studies on the pharmacology of cannabinoids and their effects on the body.
- Journal of Natural Products (https://pubs.acs.org/action/doSearch?AllField=Cannabichromevarin) – publications on the chemistry of natural compounds, including cannabinoids.
- Cannabis and Cannabinoid Research (https://www.liebertpub.com/can) – a specialized journal dedicated to cannabis and cannabinoids, featuring studies and reviews of the latest developments in the field.