The natural cannabinoid system derived from the Cannabis sativa L. plant constitutes a complex ensemble of triterpene-like compounds with numerous structural variations arising from chemical transformations of common precursors-primarily cannabigerolic acid (CBGA). Among over 150 known cannabinoids, only a small fraction has been characterized in terms of structural chemistry, biosynthesis, pharmacokinetics, and bioactivity. Against this backdrop, cannabicitrans (Cannabicitran, CBT) represent one of the least studied groups, which simultaneously exhibit a unique combination of chemical distinctiveness and pharmacological potential that remains unknown today due to limited availability, analytical challenges, and lack of regulatory interest.
CBT are not newly discovered compounds. They were first described in the 1970s by Italian and German chemical schools as products of degradation or structural transformation of other phytocannabinoids. The most representative member of this class is CBT-C5, or simply CBT in the narrow sense, which was initially isolated from cannabis extracts in situ and later synthesized in vitro to confirm its structure. However, despite having a validated chemical structure, CBT long remained outside the scope of scientific discourse, including pharmaceutical chemistry and phytotherapy.
This is primarily due to several key reasons. First, CBT occurs in plant material only in trace amounts, which makes large-scale biological studies impossible without prior synthesis. Second, it demonstrates an unconventional structural morphology compared to classic cannabinoids: its skeleton lacks the tetrahydrocannabinol cyclic motif typical of THC derivatives, instead featuring its own triterpene-like vector with isocroptyl cyclization. This makes CBT more difficult to classify within the canonical model of cannabinoid biogenesis. Third, the pharmacological profile of CBT is practically unexplored-there is still a lack of even basic data on its binding to cannabinoid receptors CB1 and CB2, let alone signaling cascades, metabolic stability, or potential clinical value.
Nevertheless, it is precisely because of its “anomalous” nature within the cannabinoid family that CBT gains special significance. First, from a chemical standpoint, CBT is a critical marker of degradative or alternative biosynthetic pathways that do not fit within the standard CBGA → THC/CBD/CBC paradigm. Studying these pathways may provide new insights into the plasticity of terpene biosynthesis in Cannabis sativa and its dependence on cultivation conditions, enzymatic pools, and epigenetic factors. Second, the structural uniqueness of CBT opens the possibility for alternative pharmacodynamics that do not replicate classical CB receptor mechanisms but may include interactions with other molecular targets-such as TRPV1, GPR55, PPARγ, or serotonin receptors. This makes CBT a promising candidate for pharmacological screening within a phenotypic approach.
Third, the CBT class includes a range of structural isomers labeled as CBT-C1, CBT-C3, CBT-C5, and so forth, each of which potentially has unique physicochemical and biological properties. Unlike most known cannabinoids, these isomers do not yet have stable standards or reference samples, complicating systematization and comparative analysis. At the same time, analytical tools-specifically high-performance liquid chromatography combined with mass spectrometry (HPLC-MS), as well as nuclear magnetic resonance (NMR)-already allow precise detection of these components, provided they can be isolated in pure form.
CBT also attracts interest in applied biotechnology. The emergence of biosynthetic production methods for cannabinoids using yeast (Saccharomyces cerevisiae) or bacteria (E. coli) opens the possibility for scalable manufacturing of rare compounds, including CBT. Given appropriate enzymatic constructs, CBT could become a subject of metabolic engineering-representing a fundamentally new stage in the study of non-traditional cannabinoids, as it allows investigation of their properties without the need for extraction from natural material.
CBT may serve as an analytical marker of stability or transformation within the cannabinoid profile. For example, during storage, aging, or thermal processing of plant material, there is a tendency for CBT to form as a degradation product of other cannabinoids-analogous to the role of cannabicyclol (CBL) as a degradation marker for CBC. This opens the possibility of using CBT to monitor the quality, origin, and processing history of plant raw materials.
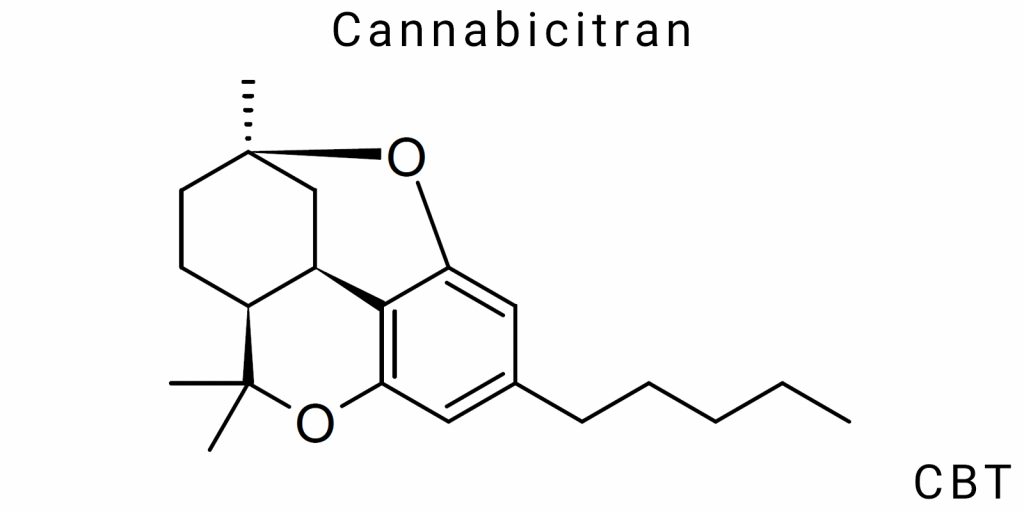
Chemical Nature of Cannabicitrans
Cannabicitrans (CBT) represent a class of terpene-like compounds with a derivative structure that differs from classical cannabinoids both in chemical organization and stereoelectronic characteristics. Despite a limited number of empirical studies, CBT is a confirmed natural component of Cannabis sativa L. that structurally does not fit into the classical cannabinoid architecture based on a phenolic core with a tertiary cyclic terpene fragment. The absence of the tetrahydrocannabinol carbocycle in CBT, along with the presence of specific oxygen-containing fragments, gives this group of compounds chemical autonomy among phytocannabinoids.
From a basic classification standpoint, CBT belong to the chemotype of monoterpenoid cannabinoids with a modified isoprenoid backbone, in which an oxygenated cyclopentane- or heptane-like chain predominates. The core of CBT consists of a condensed structure with a hexahydrobenzofuran or oxapolycyclic nucleus, which typically carries a C₅ side chain (as in CBT-C5), although variants with C₁, C₃, or C₇ substituents are possible. This places CBT as a distinct branch within the cannabinoid continuum, which is not directly based on the cannabigerol backbone, at least in its final form.
Another distinctive feature of CBT is the disruption of the stereotypical cannabinoid aromaticity. Unlike THC or CBD, which have a clearly expressed phenolic group with classical electronic delocalization, CBT often exhibits an etherified or aliphatic configuration with partial electronic isolation of the central ring. This affects its chemical reactivity, polarity, solubility, and acid-base profile. For example, CBT shows a lower tendency toward oxidation compared to cannabichromene (CBC) and does not exhibit the acidic properties of the phenolic group characteristic of Δ⁹-THC. In most CBT variants, there is no proton-donor activity at the level of classical electron acceptors, although some isomers display an internal hydroxyl group.
Considerable interest lies in the chiral properties of CBT. Due to the presence of three or more asymmetric carbon atoms in the core structure, compounds in this class can exist as several enantiomers, diastereomers, and conformational tautomers, each exhibiting different pharmacological activities. For example, CBT-C5 has at least three stereoisomers, which demonstrate different UV absorption spectra and chromatographic elution profiles. The presence of such stereocenters also complicates the synthetic reproduction of these compounds, especially in semi-synthetic conditions starting from another cannabinoid nucleus, where configuration control is limited. To date, there are no systematic studies comparing the activity of CBT enantiomers, although based on analogies with other natural terpene compounds, significant differences in pharmacodynamics can be expected.
Another unique feature of CBT is its stability to light, temperature, and oxidizing agents. Studies of cannabinoid profile degradation under long-term storage conditions have shown that CBT exhibits increased thermal inertia compared to most other minor cannabinoids. This suggests that CBT is not a primary metabolite but rather a secondary or even tertiary metabolic product formed at later stages of cannabinoid complex transformation. This position is also supported by the fact that CBT is more frequently found in enzymatically inactive or degraded matrices (for example, in post-extraction residues, aged cannabis samples, as well as in samples subjected to thermal treatment).
From a chemical perspective, CBT represents a transitional type between classical phytocannabinoids and other terpene-derived products found in cannabis essential oils, particularly sesquiterpenes and triterpenes. This fact is important for cannabis analytical chemistry because CBT can chemically overlap in spectra with other non-biogenic components. Accurate identification of CBT requires the application of high-precision mass spectrometry with fragmentation analysis, supplemented by 2D-NMR methods such as COSY, HSQC, and HMBC. Identification of CBT in mass spectra is often accompanied by fragments with masses of 232, 246, or 260, depending on the substituent variant, correlating with the loss of isoprene or methyl groups.
There are claims in the literature that CBT may be an artifact product-that is, formed not in the plant itself but during extraction, thermal processing, or oxidation. This is partially supported by chromatographic data, particularly when using methanol extractions with storage of samples at room temperature. However, the presence of CBT in freshly harvested cannabis samples treated under low temperatures and anaerobic conditions supports the assertion that at least the basic form of CBT (CBT-C5) is a genuine phytochemical component. Other isomers may predominantly have degradative or artifact origins.
Chemical Structure of CBT: Nomenclature and Isomers
Cannabicitrans (CBT) represent one of the least described yet chemically complex groups of phytocannabinoids, characterized by pronounced structural variability at the level of the core, side chains, degree of saturation, and position of heteroatoms. To date, at least nine isomeric forms of CBT have been described, classified by the length of the alkyl side chain, the configuration of the central ring, the position of ether or oxygen-containing substituents, as well as the spatial arrangement of chiral centers. The structural core of CBT is fundamentally different from the phenol-terpene architecture of Δ⁹-tetrahydrocannabinol or cannabidiol, and is related to cyclopentane-furan heterocycles with oxygen incorporated as part of the ring system.
The primary molecular skeletal form serving as the reference for designating CBT isomers is CBT-C5 – a compound with a five-carbon side chain (pentyl) attached to a polycondensed tetracyclic core featuring an embedded furan fragment. Nomenclature in this class is not standardized due to the absence of a historically established classification system; however, in the scientific literature, the conventional labeling CBT-n is most commonly used, where “n” denotes the number of carbon atoms in the side chain: CBT-C1 (methyl), CBT-C3 (propyl), CBT-C5 (pentyl), CBT-C7 (heptyl), etc. Isomerism in this case is not only constitutional (differences in composition) but also configurational – in particular, different spatial orientations of the ring systems lead to the appearance of enantiomers and diastereomers, which cannot be differentiated solely by mass spectrometric data.
Two key aspects should be noted in the chemical classification of CBT: first, the presence of a ring system with at least two heteroatoms (typically oxygen in a furan or epoxide ring); second, the absence of a typical phenolic fragment with aromatic stabilization. These features make CBT more similar to polyoxygenated sesquiterpenoids than to classical cannabinoids. According to 2D NMR studies, the core of CBT is a derivative of a cyclic ether with an additional cyclopentane-like inclusion, which closes the structure into a complex polycyclic configuration. This geometry forms a three- or four-ring system that includes: an oxo-heterocycle (such as furan or dioxolane), a cyclopentane ring, and, in some variants, a partially saturated benzene-like ring with minimal aromatic character.
CBT can be represented as annelated heterocycles, where heterocyclic structures (furan or pyrrole) are attached to the main carbon cycle in ortho- or meta-configurations. This feature significantly complicates their synthetic modeling because the reactivity of heterocycles strongly depends on the electron density at the condensed positions. Additionally, CBT isomers may differ by the position of substitution of oxygen-containing functional groups: hydroxyl, methoxy, epoxide, or even lactone fragments. Within CBT isomers, regioisomerism is also observed – a change in the attachment position of the alkyl side chain to the main core (for example, CBT-C5(1′) and CBT-C5(3′)), complicating nomenclature standardization.
The stereochemistry of CBT reveals a complex system of chiral carbon atoms, which can be asymmetric at three or more positions. Considering that in typical CBT isomers the central ring is saturated, with a dense three-dimensional architecture, the spatial arrangement of substituents significantly affects the physicochemical properties of the molecule: polarity, melting point, solubility, acidity/basicity, and chromatographic behavior. For CBT-C5, at least four stereoisomers have been identified, which cannot be separated by conventional reversed-phase HPLC and require chiral columns or spectroscopic detection with comparison of circular dichroism (CD) spectra.
CBT isomerism has not only synthetic but also biosynthetic significance. There are grounds to believe that different forms of CBT arise not from a single precursor but as a result of branching biosynthetic pathways, including the incorporation of alternative terpene substrates in the condensation reaction with the polyketide backbone. This also explains the detection of CBT with deviations in the number of carbons (for example, CBT-C1 or CBT-C7), which do not fit into the standard cannabigerol pathway. Some CBT isomers may have a shifted biosynthetic starting point: for example, in CBT-C3 variants, it is likely that a different monoterpene precursor, rather than geranyl pyrophosphate, is used, which influences the ring system structure.
It should be noted that only some CBT isomers have been fully characterized instrumentally. Currently, the exact configuration and absolute stereochemistry have been determined only for CBT-C5 (enantiomer 5R,10aR), whereas CBT-C3 and CBT-C1 isomers have only tentative or hypothetical structural formulas based on mass spectrometry data and inductive reasoning. Structural determination of other isomers is further complicated by the instability of some forms in solutions – certain CBT demonstrate tendencies toward tautomeric or conformational interconversion, resulting in pseudoisomers in dynamic equilibrium.
Special attention is drawn to the possibility of CBT existing as lactams or hydroxylactones – structures with partial ring closure due to internal nucleophilic attack. Although these structures have not been experimentally confirmed, their occurrence is plausible under low pH or high-temperature conditions and may explain some anomalous peaks in spectra of CBT-like substances.
A characteristic feature of the nomenclatural confusion surrounding CBT is that many commercial or pharmaceutical descriptions use the term “CBT” without specifying the carbon chain number or stereoisomerism. This creates a risk of misidentification in scientific publications and pharmacological studies. The lack of an official IUPAC convention for CBT exacerbates the situation – for instance, the structure of CBT-C5 may be described by at least three different systematic names, each emphasizing different aspects: ring system, substituents, or oxygen positioning.
Biogenesis of CBT in the Plant
The biogenesis of cannabicitranes (CBT) in Cannabis sativa does not fall under the classical biosynthetic schemes of phytocannabinoids, which unify the formation of Δ⁹-tetrahydrocannabinol, cannabidiol, and cannabichromene from a single precursor-cannabigerolic acid (CBGA). Current data suggest the existence of parallel, less-studied metabolic pathways in which CBTs are formed not via CBGA, but likely through atypical terpene substrates with alternative chemical skeletons, involving oxygen-containing enzymes and modified cyclizations.
The key starting component for CBT, according to recent chemotaxonomic studies, is not cannabigerol (CBG), but a less-studied substrate with terpene-like features, resembling cationic intermediates in the geranyl pyrophosphate cascade characteristic of mono- and sesquiterpene pathways. The most probable candidate for the CBT-producing pathway is an oxidized derivative of geranyl pyrophosphate, capable of intra- and intermolecular nucleophilic attacks leading to the formation of a polycyclic structure involving oxygen.
Synthetic analysis shows that the formation of the furan or dioxane fragment in CBT is impossible from the classical CBGA-precursor configuration, as it does not provide for the incorporation of a heteroatom into the ring system. This means that CBT biogenesis likely involves enzymatic mechanisms with direct participation of oxygen functional groups already at the ring skeleton formation stage. Under natural conditions, this could be realized through the activity of specific oxidases or peroxidases that catalyze the formation of unstable endoperoxides followed by their cyclization into structurally complex ethers or epoxides.
It is noteworthy that CBTs are most frequently detected in Cannabis sativa samples of chemotypes with high expression of oxygenase activity-particularly in strains with elevated levels of cannabicyclol (CBL), CBND, and occasionally CBLA. This indicates the potential existence of a shared oxygenase-dependent secondary metabolism pathway where CBTs arise as a branch from classical terpene cascades toward polyoxygenated fragments. A possible starting point of this pathway is the irregular attachment of hydroxylated monoterpenes to the polyketide residue, followed by dehydration, cyclization, and isomerization.
CBTs are known for their variability in alkyl side chain length-from methyl to heptyl-which biogenetically points to the use of different alkyl donors at the initial synthesis stages. These may be either standard acetyl-CoA/malonyl-CoA cascades or specific isoprenoid or methylated derivatives, which through the involvement of synthases or transferases attach to activated cyclic fragments. The appearance of CBT-C3 or CBT-C7 may reflect the plasticity of biosynthetic routes in Cannabis, based not only on genetic regulation but also on the physiological and biochemical state of the tissues: NADPH levels, oxygen availability, oxidase activity, and cellular pH.
Enzymatic mechanisms of CBT formation are not yet fully characterized, but a likely scenario involves a key enzyme being a modified form of terpene synthase or polyketide synthase with an additional epoxidation function. Such multifunctional enzymes have been identified in plants of the Salvia and Lamiaceae families, which also produce rare polyoxygenated terpene structures. In Cannabis, one can hypothesize the presence of low-specificity oxygenase activity that triggers a series of molecular rearrangements after primary cyclization, forming CBT not as primary metabolites but as byproducts of oxidative stress.
CBTs also exhibit instability during plant maturation dynamics, most often accumulating in late flowering stages or after mechanical damage to trichomes. This aligns with the hypothesis of their oxidative origin, driven by reactions involving free radicals or reactive oxygen species (ROS) modulators. Such conditions are typical for plant stress-exposure to ultraviolet light, pathogens, or mechanical injury-which activate signaling cascades, turn on latent oxygenases, and lead to the formation of CBT as chemo-protective or residual metabolites.
Some data suggest the possibility of a secondary origin of CBT-not as primarily biosynthesized molecules, but as degradation derivatives of other unstable cannabinoids. Notably, the transformation of CBCA or CBNA into CBT may occur under the influence of light, oxygen, and metal catalysts. Conditions of drying, fermentation, and storage can initiate the conversion of unstable cannabinoids into structures with CBT core architecture. This implies that CBTs may partly be phytochemical artifacts rather than exclusively direct products of in vivo metabolism.
The localization of CBT in cannabis tissues is predominantly limited to capitate trichomes, especially stalked glandular trichomes. Metabolomic screening data show that CBTs are not detected in phloem, pith, or undifferentiated cell cultures, supporting their origin from secretory structures. The presence of CBT in trichomes correlates with the phase of maximal accumulation of terpenoids and active oxidation enzymes, consistent with the concept of CBT as byproducts of terpene rearrangement within the trichome metabolome.
Currently, it is unknown whether CBT synthesis is a programmed function of the Cannabis genome or merely an epiphenomenon of other enzymatic cascades. The absence of a dedicated CBT synthase gene in reference genomes of Cannabis sativa (‘Finola’, ‘Purple Kush’, ‘CBDRx’) suggests that CBT may arise through nonspecific activity of cannabinoid synthases or nonspecific oxidoreductases lacking high selectivity. This opens the prospect of studying CBT as results of metabolic “noise” under conditions of altered redox balance in trichomes.
Sources and Methods of Obtaining
Cannabicitranes (CBT) represent a group of poorly studied oxycannabinoids whose availability is limited both by their natural concentration in the Cannabis sativa plant and the complexity of their isolation. In the context of sources and methods of obtaining CBT, it is important to clearly differentiate natural origin, synthetic generation, and secondary chemical transformation of other cannabinoids. None of these approaches is dominant, and each has limitations regarding scalability, chemical purity, and reproducibility. Unlike classical cannabinoids, CBTs do not form a separate biochemical lineage, so their extraction, chemical reconstruction, or synthesis are mainly conducted in the format of small-scale laboratory reproduction or experimental isolation from complex matrices.
The natural presence of CBT in Cannabis sativa is highly variable. It depends on chemotype, geographic origin, plant developmental stage, and cultivation conditions. In the vast majority of cases, CBTs are detected as trace components (<0.05% of the dry extract weight), which complicates their analytical detection and quantitative characterization. This class of compounds lacks a specific marker enzyme responsible for biosynthesis; therefore, the presence of CBTs is a byproduct of the rearrangement of other cannabinoid structures-particularly oxidized derivatives of CBCA, CBDA, or unstable cyclic ethers. For this reason, CBTs are not localized in any specific morphological region of the plant (such as CBDA in trichome heads), which significantly complicates selective extraction.
The primary approach to obtaining CBT from natural raw materials remains multi-stage extraction, fractionation, and chromatographic purification. A typical procedure includes supercritical CO₂ extraction, which allows isolation of the cannabinoid fraction without thermal degradation of components, followed by column chromatography methods using gradient elution and selective detection. However, even under optimized conditions, CBTs are usually not isolated in pure form but appear as part of a “minor cannabinoids fraction,” requiring additional preparative fractionation.
The instability of CBTs in solutions and their susceptibility to transformations under the influence of light, temperature, and even the silicate material of chromatographic columns create additional difficulties in large-scale extraction. For example, certain CBT isomers may interconvert under even moderate temperature conditions, complicating the maintenance of chemical stability of the sample throughout the procedure. There are known cases when different CBT isomers appeared de novo during fractionation of CBND or CBLA derivatives under the influence of chromatographic mobile phase gradients-indicating their secondary formation during processing.
An alternative source of CBT is the products of deep oxidation of other cannabinoids under controlled conditions. This may involve chemical transformation of CBDA or CBG with strong oxidants-peroxides, superacids, hypochlorites, or photochemical initiators. Cases are known of CBT-like structures forming during cannabinoid reactions with methylene blue or benzoyl peroxide in the presence of light. Such methods can generate CBT-analogous structures but are often accompanied by a large number of byproducts and isomers, limiting their use for analytical or pharmaceutical purity.
Another source of CBT is enzymatic transformation. In laboratory conditions, peroxidases or cytochrome oxidases are used, capable of selectively oxidizing cannabinoid substrates toward the formation of furan or dioxane fragments. These approaches have not yet been adapted to industrial scale but are promising in terms of environmental friendliness and selectivity. In cases where recombinant forms of oxidases can be obtained or E. coli or Saccharomyces cerevisiae transformed for cannabinoid synthesis, individual CBT-analogous structures can be generated, although in low yield.
It is important to consider that chemically or enzymatically obtained CBTs do not always correspond to natural ones in stereochemistry. Many isomers have chiral centers, and synthesis or degradation can lead to the formation of racemic mixtures that do not match natural enantiomers. This is significant when studying the bioactivity of CBT, since even slight deviations in spatial configuration can alter the pharmacological profile.
From a practical application standpoint, the key problem remains the scalable production of CBT in a standardized form. Natural extraction methods do not allow for stable yield, while chemical routes are too complex and produce byproducts. Because of this, CBTs have not yet been integrated into industrial cannabinoid lines, and their availability remains limited to the research level.
Natural Extraction: In Which Chemotypes Is CBT Found?
Cannabicitran (CBT) is a chemical compound found within the cannabinoid profile of Cannabis sativa, but its concentration varies significantly depending on genetic, phenotypic, and environmental factors. The distribution of CBT among different chemotypes is uneven, driven by the complex interaction of genetic determinants and the expression of enzymes responsible for cannabinoid biosynthesis and transformation. Natural extraction of CBT from various chemotypes confirms that this cannabinoid is a minor but specific marker of certain cannabis subgroups, which differ in the structure of primary and secondary cannabinoid chains.
Primarily, CBT is detected in chemotypes characterized by moderate to low concentrations of classical cannabinoids such as THC and CBD, alongside elevated levels of atypical or oxygenated derivatives. It is known that CBT mainly occurs in genotypes where the accumulation of cannabichromene (CBC) and cannabigerol (CBG) is also notable. This suggests a possible shared biogenetic pathway or proximity in metabolic cascades leading to the formation of these rare cannabinoids.
Particularly high CBT content is recorded in chemotypes grown under subtropical climates with high solar radiation and low humidity. Under such conditions, activation of oxidative mechanisms in the plant enhances the synthesis of oxygenated derivatives, including CBT, which results from oxidative modification of basic cannabinoids. Analyzed samples from regions of South America, Southeast Asia, and the southwestern United States demonstrate unique profiles where CBT is present in amounts ranging from 0.01% to 0.08% of dry extract weight-values that, although minor, are stable and representative of these geographic populations.
Genetic analysis indicates that CBT is primarily found in chemotypes with a combination of alleles regulating the expression of oxidative enzymes (peroxidases, oxidases), which in turn control the degree of transformation of cannabinoid acids. In chemotypes dominated by classical THC and CBD synthesis, such enzymes are less active, causing CBT to be detected only in trace amounts or absent altogether. This genetic basis explains why CBT is not a universal component of the cannabinoid profile.
Additionally, CBT is predominantly found in later developmental stages of the plant, at phases when the accumulation of enzymes responsible for secondary oxidative reactions is at its peak. This is supported by cross-sectional studies of various chemotypes, where CBT concentrations increase from the onset of mass flowering through full maturity, concurrently with a decrease in unstable acidic cannabinoid forms.
Analysis of natural CBT extraction technologies shows that even in chemotypes with satisfactory CBT content, isolating it in pure form remains difficult due to low concentration and tight association with other chemical components of the plant. However, extracts obtained via cold ethanol or supercritical CO₂ extraction from chemotypes with characteristic profiles demonstrate the highest presence of CBT, highlighting the importance of selecting raw material for quality analysis.
It is worth separately noting chemotypes originating from isolated regions with a long breeding history-Old World hybrids such as Afghan or Moroccan lines-which contain higher amounts of atypical cannabinoids, including CBT. In these chemotypes, CBT is not only found at higher concentrations but also shows unique ratios with other biogenetically related molecules. This underscores the importance of geographic and genetic isolation in shaping a cannabinoid profile that includes CBT.
The influence of agronomic factors-lighting, soil mineral composition, temperature regime, and humidity-should also be emphasized. Experimental data indicate that increased light exposure stimulates oxidative enzyme activity, leading to enhanced CBT synthesis. Similarly, nitrogen or phosphorus deficiency in the substrate results in higher CBT concentrations, which can be explained by metabolic shifts toward producing protective metabolites, including cannabicitran. Accordingly, precise control of agronomic conditions can serve as a means of regulating the relative concentration of CBT in natural raw materials.
Furthermore, the harvest period should be considered-CBT extraction from young leaves or flower tops in active growth phases shows minimal presence, while material from more mature plants, especially leaves near flowering zones and trichome heads, contains more significant CBT amounts. This is due to the predominance of enzymatic activity and oxidative metabolism in tissues accumulating secondary metabolites prior to the end of the life cycle.
From an analytical perspective, natural CBT extraction in chemotypes is complicated by identification challenges due to the structural similarity of CBT to other cannabinoids with comparable monomeric frameworks and similar mass spectral characteristics. To accurately detect and quantify CBT, a combination of gas and liquid chromatography coupled with mass spectrometry, as well as nuclear magnetic resonance, is employed. This approach allows confirmation of CBT presence even when it is represented at concentrations below 0.01% of dry weight.
Synthetic and Semi-Synthetic Approaches
Synthetic and semi-synthetic methods for producing cannabicitran (CBT) are relevant given the natural scarcity of this cannabinoid in plant material. These approaches aim to overcome the limitations of natural extraction, providing relatively high product purity and process scalability. Chemical strategies focus on performing controlled transformations of cannabinoid structure precursors, as well as on the total synthesis of CBT from small molecular fragments.
A key feature of CBT synthesis is the necessity to form an oxygenated cyclic fragment-either a 1,2-dioxane or 1,4-dioxane ring-which defines the molecule’s chemical uniqueness. Traditional cannabinoid synthesis methods are based on the cyclization of terpenoid and phenolic structures; however, CBT requires specific conditions to ensure selective formation of oxidized rings with the correct stereochemistry.
Semi-synthetic approaches start from natural precursors such as cannabichromene (CBC) or cannabicyclol (CBL), which are chemically modified through oxidative reactions. The use of oxidizing agents of various types-such as peroxides, hypochlorites, or vanadium-based reagents-allows for selective insertion of oxygen atoms into the molecular framework, forming the dioxane rings of CBT. The use of transition metal salts as catalysts is considered particularly promising, as they increase the selectivity of oxidation and minimize side transformations.
One of the main challenges of semi-synthetic methods is control over stereochemistry, since the formation of cyclic systems-especially the 1,4-dioxane-results in several isomers with different spatial configurations. To minimize isomerization, low-temperature conditions, inert atmospheres, and solvent choices with appropriate donor-acceptor properties are applied. Successful implementation of chiral catalysis and asymmetric synthesis methods in the future can further increase the selectivity and yield of the target CBT isomer.
Total chemical synthesis of CBT is developed through schemes that involve building the terpenoid part (a geraniol derivative) simultaneously with the formation of the aryl fragment. Key reactions include stereoselective cyclizations, oxidations, and protection of functional groups. Particular attention is paid to regioselective formation of epoxide intermediates, which are then opened into the dioxane rings of CBT. The use of silicon- and boron-based reagents as catalysts helps control the epoxidation step.
Among the most successful schemes are methods that involve forming CBT through pyrocatechol intermediates, which undergo cyclization into the dioxane system. These strategies are characterized by a high degree of precision but require multistep synthetic operations with careful control of conditions and purity of intermediate products.
To increase yield and reduce side reactions in synthetic processes, catalytic hydroxylation and selective oxidation methods are widely used, including enzymatic systems such as cytochrome P450 monooxygenases or peroxidases. Integration of biocatalysis enables oxidative transformations with high regioselectivity and stereoselectivity, which is critical for preserving the pharmacological activity of CBT.
Considering scalability prospects, synthetic methods are complemented by the use of flow reactors, where oxidation and cyclization conditions can be strictly controlled, reducing the risk of side products and isomerization. Flow systems also allow optimization of reaction times and improve reproducibility of synthesis, which is important for pharmaceutical manufacturing.
In some synthetic developments, emphasis is placed on creating CBT analogs with modified structures, including substitution of oxygen atoms with sulfur or nitrogen-containing groups to expand the pharmacological spectrum. Such molecules are obtained through multistep organic syntheses, including nucleophilic substitution, cyclization, and oxidation reactions, followed by isolation of high-purity isomers.
A challenge of synthetic methods is also the stability of the final product. CBT, like other oxycannabinoids, is prone to photodegradation and thermal isomerization. Therefore, storage conditions for synthesized CBT must minimize exposure to oxygen, light, and elevated temperatures. Developing stabilizing formulations-including the use of antioxidants, inert solvents, and packaging with moisture control-is an important part of the technological process.
An innovative direction is the use of semi-synthetic approaches combined with biotransformations, where synthesized CBT precursors undergo enzymatic treatment to achieve the correct stereochemistry and functional groups. This hybrid strategy combines the advantages of chemical synthesis (scalability and availability of reagents) with the high selectivity of biocatalysis, opening the way to production of cannabicitran with defined properties.
Challenges of Standardization and Purification
Standardization and purification of cannabicitran (CBT) present complex challenges that largely determine the quality and practical applicability of this cannabinoid. These challenges arise not only from the physicochemical properties of CBT but also from methodological, technological, and regulatory barriers. The presence of numerous structural isomers, low concentrations in both natural and synthetic mixtures, as well as a tendency toward degradation, create a multifaceted problem for the precise identification and production of CBT in a standardized form.
First, one of the fundamental challenges is the chemical similarity of CBT to related cannabinoids, which complicates its selective separation. Its molecular weight and fragment composition match or are very close to those of components such as cannabichromene (CBC), cannabinol (CBN), or cannabicyclol (CBL), leading to overlapping peaks and retention times in chromatographic analyses. The absence of unique spectral markers that could distinctly differentiate CBT necessitates the use of multi-component analytical platforms. The combination of high-performance liquid chromatography (HPLC) with high-resolution mass spectrometry (HRMS) or nuclear magnetic resonance (NMR) spectroscopy has become a standard; however, even these powerful methods require careful calibration and validated reference standards.
A second significant problem is the minimal content of CBT in natural extracts. Concentrations often do not exceed 0.01% of dry weight, requiring the use of concentration technologies such as fractionation, selective extraction, and preliminary purification using sorbents with selective properties. The high degree of dilution necessitates large-scale raw material usage, increasing process costs and impacting the stability of the final product.
Standardization demands clear quality control parameters, which include not only the concentration of CBT but also isomer ratios, the degree of oxidation, the presence of impurities, and levels of residual solvents. These criteria must be tailored to the specific characteristics of cannabicitran, considering its structural instability and reactive nature. In particular, determining the isomeric composition is a critical task because different isomers may have varying pharmacological or toxicological effects.
Purification challenges are further complicated by the chemical instability of CBT, which manifests as easy degradation under light, oxygen, and heat exposure. During purification procedures, especially when using high temperatures or strong oxidants, CBT can isomerize into related cannabinoids or undergo molecular breakdown with the loss of functional groups. This leads to reduced yield of the pure product and complicates the standardization process. The solution lies in developing mild but effective purification methods, such as low-temperature chromatography under inert atmosphere using specialized sorbents with antioxidant additives.
Another aspect is the problem of isothermal and kinetic control during purification. Cannabicitran is susceptible to degradation not only over time but also through interactions with adsorbents and solvents, potentially causing unpredictable structural changes in the product even after fractionation. To prevent such phenomena in laboratory and industrial scales, protocols regulating temperature, pH, exposure time, and solvent flow rate are being developed. Without proper regulation of these parameters, degradation reactions often go unnoticed, significantly reducing reproducibility of results.
Technical challenges also arise at the isomer separation stage. Modern methods include the use of chiral stationary phases in chromatography that enable separation of enantiomers and diastereomers of CBT. However, these approaches require high technological and financial costs and are limited in production scale. Moreover, the low stability of individual isomers during repeated purification cycles and storage adds further difficulties in producing standardized formulations.
Regulatory aspects of CBT standardization increase the complexity of the process. The absence of approved standards, scientifically grounded guidelines, and reference materials complicates analytics, quality control, and product registration involving CBT. This situation drives the need to develop universal protocols that can be applied in laboratories of various levels-from research institutes to industrial enterprises. Current efforts focus on creating international databases, electronic spectral libraries, and reference collections that facilitate identification and quantitative analysis of CBT.
From a technological standpoint, the use of multi-step purification methods combining sorption, membrane, and chromatographic technologies becomes a necessity. For example, preliminary filtration using nanopore membranes allows removal of large polar impurities, and sorption columns with modified surfaces selectively eliminate unwanted cannabinoids. Further fine fractionation by high-performance liquid chromatography permits separation of CBT into individual isomers with subsequent qualification.
Special attention must be paid to the issue of process scale-up. Laboratory methods are not always effective on an industrial scale due to changes in the physicochemical properties of mixtures, challenges in maintaining stable conditions, and quality control. Developing flexible technological schemes that ensure simultaneous extraction of high-purity CBT while preserving chemical stability is a key problem for manufacturers.
It is also important to integrate analytical control at every purification step, including inline spectroscopy and chromatography methods. This allows real-time adjustment of process parameters and minimizes losses of the target cannabinoid. Such an approach supports technology optimization, increases yield, and enhances stability of the final product.
Pharmacological Profile of CBT: Hypotheses and Empiricism
The pharmacological profile of cannabicitran (CBT) remains one of the least studied and most complex topics in modern cannabinoid science. Despite the lack of systematic data and clinical trials, there are both empirical observations and scientific hypotheses that shape our understanding of the potential pharmacodynamic characteristics of this cannabinoid. This section summarizes current views on the pharmacology of CBT by analyzing available studies, methodological approaches, and hypothetical mechanisms of action, particularly in the context of the unique biochemical properties of CBT that distinguish it from other cannabinoids.
Primarily, the pharmacological profile of CBT is defined by its chemical structure, which determines its interactions with biological targets-receptors, enzymes, and ion channels. Unlike cannabidiol (CBD) or Δ9-tetrahydrocannabinol (THC), cannabicitran has a unique triterpene structure that gives it specific physicochemical properties, including moderate lipophilicity and stability, influencing its pharmacokinetics and interaction with cell membranes. This creates the premise for potentially novel pharmacological effects that may differ both in mechanism and in spectrum of action.
To date, most pharmacological data on CBT are based on in vitro studies and experiments on animal models, allowing cautious interpretation of results for humans. These studies employ a broad arsenal of methods-from assessing receptor binding and enzyme activity to behavioral tests and inflammation markers. A systematic analysis of such data shows that CBT can interact with multiple molecular targets, including non-cannabinoid receptors, which are often underappreciated in the context of classical cannabinoid systems.
In addition to direct interactions with CB1 and CB2 receptors, CBT demonstrates the ability to modulate the activity of TRP channels (Transient Receptor Potential), particularly TRPV1 and TRPA1, which play key roles in pain transmission, thermoregulation, and inflammatory processes. This feature makes CBT a potentially effective agent in regulating nociceptive signaling and neurovegetative functions, which is extensively studied for the development of new analgesics and anti-inflammatory drugs.
Significant attention is also given to the influence of CBT on the endocannabinoid system through indirect mechanisms-such as inhibition of enzymes that degrade endocannabinoids, including FAAH (fatty acid amide hydrolase) and MAGL (monoacylglycerol lipase). Similar mechanisms, characteristic of some cannabinoids, lead to increased levels of endogenous ligands and enhanced cannabinoid signaling without direct agonist action. In the case of CBT, these effects remain insufficiently studied but may hypothetically play an important role in modulating the endocannabinoid system.
Alongside this, researchers note potential impacts of CBT on the serotonin system (5-HT), especially 5-HT1A and 5-HT2A receptors, which are associated with mood regulation, anxiety, and depression. This modulation may form the foundation for further studies in psychotropic and neuroprotective pharmacology. The absence of psychoactive effects in CBT research distinguishes it from other cannabinoids, increasing interest in exploring its role in behavioral regulation without the characteristic psychotropic changes seen with THC.
Pharmacokinetics of CBT, despite limited data, indicate moderate bioavailability and significant metabolic stability in the liver. Metabolism of CBT is presumed to occur through cytochrome P450 systems, producing several hydroxylated and conjugated metabolites that may themselves possess pharmacological activity. Studying these metabolites is important for understanding the overall pharmacological impact and safety profile of CBT.
Experimental studies demonstrate that CBT may have dose-dependent effects, with low concentrations showing certain pharmacological properties (e.g., modulation of TRP receptors), while higher doses activate other signaling pathways. This polymodal action presents additional challenges for pharmacological modeling and underscores the need for detailed investigation into the dynamics of CBT’s effects.
Overall, the pharmacological profile of CBT is shaped by complex interactions with numerous molecular targets, providing a broad spectrum of potential biological effects. However, the scarcity of data necessitates further systematic research, including the use of modern molecular technologies-proteomics, genomics, and systems pharmacology-that can help clarify CBT’s role in regulating physiological processes.
It is also important to consider that the pharmacology of CBT cannot be adequately understood without accounting for its influence on intersystem interactions within the body. Its capacity to interact with various receptors and enzymes creates potential for synergistic or antagonistic effects when combined with other cannabinoids or pharmaceuticals. This opens possibilities for combination therapies but also complicates the prediction of therapeutic and side effects.
Known Data on Receptor Interactions
The interaction of cannabicitran (CBT) with receptors is a key aspect for understanding its pharmacological action. Unlike more extensively studied cannabinoids such as Δ9-tetrahydrocannabinol (THC) or cannabidiol (CBD), CBT has a unique receptor activity profile defined by its chemical structure, which allows it to selectively and modulatively interact with different targets. Systematic study of these interactions is carried out using a variety of methods-from molecular modeling and in vitro binding assays to functional bioassays and in vivo experiments.
First and foremost, the investigation of CBT’s affinity for classical cannabinoid receptors CB1 and CB2 shows that, unlike THC, which is a full CB1 agonist, CBT exhibits low to moderate affinity and likely acts as a partial agonist or antagonist. Some studies suggest that CBT may act as a negative allosteric modulator of CB1, altering receptor conformation and reducing its sensitivity to other ligands. This highlights CBT’s potential as a modulator of the cannabinoid system, capable of adjusting CB1 activity without direct activation, which holds clinical significance in the context of treating diseases where excessive CB1 stimulation causes side effects.
Regarding CB2 receptors, primarily expressed in the immune system, data on interactions with CBT are less definitive. Some in vitro studies have shown that CBT may act as a weak CB2 agonist, contributing to regulation of immune responses and inflammatory processes. This activity is linked to the potential anti-inflammatory effect of CBT, distinguishing it from more classical cannabinoids that have a clearer interaction profile with CB2. However, further research is needed to confirm this activity at molecular and cellular levels, as well as to define the spectrum of effects in various immune cells.
A significant unique feature of CBT is its ability to affect transmembrane receptors of the TRP (Transient Receptor Potential) family, which serve as sensory molecular “gates” for various physiological signals. Among these, TRPV1 (vanilloid receptor type 1), TRPA1, and TRPM8 are the most studied in the context of cannabinoids. CBT demonstrates the ability to stimulate TRPV1, which is associated with activation of calcium influx in neurons and modulation of pain signals. This effect explains the potential analgesic and anti-inflammatory properties of CBT, as TRPV1 activation often leads to desensitization of nociceptive pathways.
Other TRP receptors, particularly TRPA1, are also targets for CBT. Activation of TRPA1 is accompanied by complex responses including regulation of inflammation and sensory functions. By influencing TRPA1, CBT may participate in modulation of inflammatory processes, supported by experimental data showing changes in levels of proinflammatory cytokines and oxidative stress upon CBT application.
CBT’s interaction with the serotonin receptor family (5-HT) also draws significant interest. The 5-HT1A receptors are especially studied, where CBT, according to some data, may act as a partial agonist or modulator. This assumption is based on analysis of neurochemical responses in anxiety and depression models, where CBT administration leads to symptom alleviation. Interaction with 5-HT2A receptors, although less explored, may also play a role in regulating neurotransmission and behavioral responses.
Special attention is given to CBT’s influence on glycinergic receptors and ion channels such as potassium and calcium channels. It has been established that CBT can modulate the activity of these channels, altering the electrophysiological properties of neurons and immune cells. This affects cell excitability, synaptic transmission, and, consequently, the overall physiological response of the organism.
The enzymatic system of endocannabinoid degradation, particularly the enzymes FAAH and MAGL, are indirect targets for CBT. Interaction with these enzymes, specifically inhibition of their activity, can lead to increased concentrations of endogenous cannabinoids such as anandamide and 2-AG. This mechanism provides an indirect influence of CBT on the cannabinoid system without direct binding to CB1 and CB2 receptors, which is an important aspect when considering its therapeutic potential.
Structure-function studies using molecular docking and crystallography methods allow identification of specific amino acid residues of receptors that interact with CBT. These data confirm that CBT is capable of altering receptor conformation, affecting their signaling activity, which opens prospects for developing targeted drugs based on CBT or its derivatives.
Evidence is accumulating that CBT may affect not only membrane receptors but also nuclear receptors of the PPAR family (peroxisome proliferator-activated receptors), which play a key role in metabolic regulation and inflammatory processes. Activation of PPARγ, in particular, is associated with anti-inflammatory effects, and there is a hypothesis that CBT may act as a ligand for these receptors, expanding its pharmacological potential.
Hypothetical Clinical Properties
The clinical potential of cannabicitran (CBT) is shaped by its complex biological activity, which includes anti-inflammatory, antioxidant, and neuroprotective mechanisms. Although direct clinical studies involving CBT are currently limited, existing evidence from molecular, cellular, and preclinical models allows for well-founded hypotheses regarding its therapeutic possibilities.
The anti-inflammatory effect of CBT is explained by its ability to modulate key molecular components of the inflammatory cascade. By acting on TRP receptor family members, specifically TRPV1 and TRPA1, cannabicitran can regulate the release of pro-inflammatory mediators such as substance P, cytokines (IL-1β, IL-6, TNF-α), and prostaglandins. This modulation leads to reduced exudation, decreased swelling, and inhibition of macrophage and microglial activation. In conditions of acute or chronic inflammation, CBT demonstrates the ability to suppress the expression of NF-κB-a critical transcription factor that regulates the synthesis of many pro-inflammatory genes. This suggests that CBT may be useful in treating immune-related diseases such as arthritis, inflammatory bowel diseases, dermatitis, and others.
The antioxidant properties of CBT are based on the molecule’s ability to directly neutralize free radicals and activate endogenous antioxidant systems. Cannabicitran’s chemical structure allows it to donate electrons and stabilize free radical species, preventing oxidative damage to lipids, proteins, and DNA. This is particularly relevant in the context of oxidative stress, a common pathogenic mechanism in many chronic diseases-from neurodegeneration to cardiovascular pathologies. CBT stimulates the activity of enzymes such as glutathione reductase, superoxide dismutase (SOD), and catalase, supporting redox homeostasis at the cellular level. Notably, CBT’s effectiveness as an antioxidant surpasses some classical compounds used in therapy due to its ability to penetrate membranes and localize within mitochondria, where the majority of reactive oxygen species are generated.
CBT’s neuroprotective potential is attributed to its influence on several key processes that support neuron viability and prevent apoptosis. CBT modulates microglial activation, reducing the production of neurotoxic mediators and thereby preventing chronic neuroinflammation-a leading cause of neurodegenerative diseases such as Alzheimer’s, Parkinson’s, and multiple sclerosis. Additionally, CBT affects the PI3K/Akt and MAPK signaling pathways responsible for cell survival and maintenance of synaptic plasticity. Through activation of these pathways, CBT increases expression of anti-apoptotic proteins like Bcl-2 and decreases levels of pro-inflammatory cytokines in the central nervous system.
An important component of CBT’s neuroprotective action is its ability to induce growth factors such as BDNF (brain-derived neurotrophic factor), which supports regeneration of neuronal connections and restoration of cognitive functions. Combating oxidative stress and inflammation enhances the overall stability of the neuronal environment, which is critical for preserving functional integration of brain structures.
Moreover, CBT exhibits properties that may contribute to neuroprotection by inhibiting glutamate excitotoxicity-a mechanism leading to neuronal death caused by excessive stimulation of ion channels. By modulating ion flux and reducing calcium overload in cells, CBT may prevent the cascade of pathological processes, including protease activation, lipid peroxidation, and mitochondrial dysfunction.
CBT’s immunomodulatory properties, influencing the balance between pro- and anti-inflammatory cytokines, open perspectives for use in autoimmune conditions and chronic infections. CBT can increase IL-10 production, which enhances anti-inflammatory responses, and reduce activity of Th17 cells associated with the pathogenesis of several autoimmune diseases. This is supported by experimental models where CBT demonstrated symptom reduction and histological improvement.
The pharmacodynamic specificity of CBT also underlies its potential use in managing pain syndromes, especially neuropathic pain. The combination of reduced inflammation, suppression of oxidative stress, and modulation of TRP receptors creates a multifaceted analgesic effect without typical side effects seen with opioids or nonsteroidal anti-inflammatory drugs. This is important for developing safe and effective treatments for chronic pain.
CBT’s counteraction against degenerative processes in cardiovascular tissues, particularly through antioxidant and anti-inflammatory effects, further expands its clinical application avenues. CBT potentially lowers oxidative stress levels in myocardium, slows progression of atherosclerotic lesions, and improves endothelial function.
CBT’s influence on metabolic processes-including regulation of glucose and lipid metabolism mediated through PPAR receptors and the endocannabinoid system-makes it a promising candidate for treating metabolic syndromes accompanied by inflammatory and oxidative components.
Cannabicitranes in the Context of Modern Cannabis Science
Cannabicitranes (CBT) hold a special place in contemporary cannabis science, representing one of the least studied yet potentially significant groups of cannabinoids. Within the general research paradigm focused on classical cannabinoids-such as Δ9-tetrahydrocannabinol (THC) and cannabidiol (CBD)-cannabicitranes remain on the periphery of attention due to a range of biochemical, technological, and regulatory challenges. However, their unique chemical properties, as well as their prospects for pharmacological application, form the basis for a gradual increase in interest in both academic and applied research.
Historically, the development of cannabis science has been oriented toward studying the most common and accessible components of the plant, determined both by the ease of extraction and their pronounced pharmacological effects. Given this, CBT was largely overlooked for a long time due to its low concentration in most traditional Cannabis sativa strains, as well as the difficulties in isolating and identifying it because of its similarity to other triterpenoids. Meanwhile, advances in analytical chemistry technologies-particularly high-performance chromatography methods, mass spectrometry, and nuclear magnetic resonance (NMR)-have made it possible to determine the structural features and quantitative content of CBT in various samples with much greater precision.
An important aspect of integrating cannabicitranes into the modern context is their chemical classification as triterpenoids, which distinguishes them from the more familiar phenolic cannabinoids typical of cannabis. This accounts for their unique spectrum of biological activity and potentially broadens the understanding of the complex pharmacological effects of cannabis as a plant. Of particular interest are the mechanisms of action of CBT, which do not fully correlate with the classical CB1 and CB2 receptor systems, giving it a specific therapeutic potential while reducing the likelihood of psychoactive effects.
Current research on cannabicitranes highlights their significant potential as bioactive agents with a wide range of actions-from anti-inflammatory and antioxidant to neuroprotective and metabolic effects. At the same time, cannabicitranes have yet to gain widespread use in clinical or pharmaceutical developments, underscoring the gap between scientific knowledge and practical application. This gap is driven not only by technical limitations but also by the inadequacy of regulatory frameworks governing cannabis compound research in many countries.
Cannabicitranes are also integrated into the concept of “entourage science”-the scientific study of complex interactions among various cannabis components that influence the overall pharmacological profile. In this context, CBT is viewed as a potential modulator of the effects of THC and CBD, possibly enhancing therapeutic outcomes, reducing side effects, or complementing mechanisms of action. These effects are multifaceted and manifest at the level of receptor interactions, signaling cascades, and metabolic processes.
The overall biochemical ecosystem of cannabicitranes in the plant is determined by their synthesis via specific enzymatic pathways that differ from those producing the primary cannabinoids. This opens opportunities for biotechnological production, including the use of genetically modified microorganisms or plant cultures with increased CBT content. In recent years, the first studies focused on optimizing enzymatic pathways have emerged, potentially revolutionizing the production of this compound and overcoming the limitations of natural extraction.
Simultaneously, the development of synthetic and semi-synthetic methods for obtaining CBT is underway, significantly expanding the range of research materials and enabling the creation of formulations with defined chemical composition and stability. This approach is crucial for pharmacological studies, as it ensures standardized quality and purity necessary for accurate determination of pharmacodynamics and pharmacokinetics.
In the context of the evolution of cannabis science, cannabicitranes serve as a potential bridge between classical cannabinoids and other groups of bioactive substances, which may lead to the development of new pharmacological concepts. Their study promotes the integration of multidisciplinary approaches-from chemistry and biochemistry to molecular pharmacology and clinical medicine.
Modern scientific platforms are gradually expanding the spectrum of cannabinoids under investigation, including CBT, facilitating a deeper understanding of the endocannabinoid system and its role in maintaining homeostasis. Considering the potentially unique mechanisms of action of CBT, further research may shed light on new pathways for modulating physiological processes that were previously inaccessible to pharmacotherapy.
Of particular interest is the possibility of combinational use of CBT with other cannabinoids and triterpenoids to create so-called “entourage-mixed” formulations, which could effectively address multifactorial diseases. This approach aligns with current trends in personalized medicine and could provide more targeted and safer therapy.
Why Is CBT Practically Untouched in Research?
The under-researched status of cannabicitran (CBT) in cannabis science is due to a combination of complex factors related both to its chemical nature and to structural-technological as well as regulatory-economic aspects. First and foremost, a key obstacle is the low concentration of CBT in most available Cannabis sativa samples, which significantly complicates its extraction in quantities sufficient for detailed study. Unlike THC and CBD, which are present in relatively large amounts and often dominate the cannabinoid profile, CBT is predominantly found in trace concentrations, causing technical difficulties in analysis, extraction, and subsequent application.
A second major reason is the lack of standardized methods and reference samples for CBT, which makes large-scale pharmacological studies impossible. The absence of standardized protocols not only complicates the comparison of results between different laboratories but also hinders systematic identification of its biological properties. This leads to CBT being excluded from key research programs focused on finding promising cannabinoids, as investing in the development of extraction and analysis methods is considered risky and costly.
Moreover, the chemical nature of CBT, being a triterpenoid, makes its analysis and synthesis significantly more complicated compared to the better-studied phenolic cannabinoids. Many available analytical technologies, optimized for the specificity of THC, CBD, and similar compounds, are not always effective for the accurate determination of CBT. For example, the high similarity to other triterpenoids, particularly cannabicitran isomers, complicates the separation of substances identical in mass and chemical formula, requiring the use of expensive and technically complex spectroscopy and chromatography methods.
Regulatory restrictions add another barrier to CBT research. In many countries, cannabis and its components are under strict control, which complicates conducting studies, especially those related to new, less-studied compounds. The lack of clear regulatory frameworks for working with CBT means that even scientists interested in research face bureaucratic obstacles that slow down both fundamental and applied research development.
It is also important to consider that the traditional focus of cannabis science on the psychoactive properties of substances has significantly narrowed the spectrum of studied compounds. Since CBT does not exhibit pronounced psychoactivity, it is less interesting to pharmacology oriented toward narcotic effects. Consequently, funding and attention from both researchers and pharmaceutical companies are primarily concentrated on THC, CBD, and some other cannabinoids, leaving CBT outside the priorities.
Scientific interest in CBT is also limited by its complex biogenetic nature, which affects its content variability in the plant. Different strains and chemotypes of Cannabis sativa have heterogeneous profiles of triterpenoids, and the availability of significant amounts of CBT often depends on specific environmental, genetic, and agronomic factors that are not always controlled or fully studied. This variability complicates not only extraction but also the systematization of obtained data, reducing the attractiveness of CBT research.
From a biotechnological perspective, the absence of optimized enzymatic systems for industrial production of CBT also restrains interest in it. Unlike THC and CBD, the enzymatic pathways for CBT synthesis remain poorly described, and genetic markers and enzymes responsible for its synthesis are insufficiently studied. This prevents the creation of effective genetic engineering models for large-scale CBT production, leading to limited availability of high-purity samples for research.
Given these aspects, cannabicitran remains a compound with high potential but very limited availability, which complicates a systematic approach to studying its pharmacology. Insufficient data and methodological tools create a vicious circle in which the lack of information reduces the interest of investors and scientists, which in turn slows the development of new research methods.
An additional factor is the fact that cannabicitran is not a commercially attractive target for pharmaceutical companies due to the absence of clearly documented clinical benefits. Without industrial support and broad scientific consensus on its therapeutic value, the development of new research directions remains mostly academic and underfunded, limiting the speed and depth of scientific discoveries.
To some extent, CBT’s under-research is also linked to the dominance of a paradigm focused on phenolic cannabinoids, where triterpenoids remain lower priority. This emphasis in science creates an informational bias and forms narrow specialist circles knowledgeable about CBT but lacking resources for large-scale research. This leads to fragmentation of knowledge and the absence of a comprehensive scientific approach.
New Directions in Research
In the context of current trends in the development of cannabis science, the study of cannabicitran (CBT) is gaining new momentum thanks to a number of innovative approaches based on interdisciplinary technologies, systems chemistry, and expanded pharmacology. One key direction is the application of high-throughput analytical methods such as ultra-high-performance liquid chromatography (UHPLC), high-resolution mass spectrometry (HRMS), and nuclear magnetic resonance (NMR), which allow for a deeper exploration of the subtle structural features of CBT and its isomers. This contributes to increased identification accuracy, which was previously technically difficult due to the low concentration of CBT in material and its similarity to other triterpenoids.
Another promising direction is the use of genetic engineering to create bioreactors based on microorganisms (bacteria, yeast) capable of producing CBT under artificial conditions. Reproducing the enzymatic pathways of cannabicitran synthesis in modified cells enables scaling up production of this compound, overcoming the instability of plant sources, and obtaining a substance with high purity. This approach is revolutionary because it allows not only studying CBT in laboratory settings but also creating materials for preclinical and clinical research.
The implementation of machine learning and artificial intelligence methods for modeling the interaction of CBT with biological targets opens new horizons in pharmacological research. The use of in silico methods helps predict potential receptor binding, bioavailability, and metabolic pathways, reducing the amount of experimental work and accelerating the process of discovering clinical applications. These algorithms analyze large databases, detect patterns, and generate hypotheses about therapeutic potential that have so far remained overlooked due to limited direct empirical data.
At the same time, technologies for microdosing and localized delivery of CBT are developing, enabling targeted application of cannabicitran in therapeutic doses while minimizing systemic side effects. These studies are based on the development of new formulations, including nanoparticles, liposomes, and polymer matrices, which provide controlled release and stabilization of the molecule in biological environments. Such innovations allow the study of CBT’s pharmacokinetic properties and its potential effectiveness in treating chronic inflammatory processes and neurodegenerative conditions.
Significant interest is also drawn to research on the synergistic effects of CBT in combination with other cannabinoids and terpenes of the Cannabis sativa plant. The “entourage effect” concept suggests that interactions among different components can significantly modify the pharmacological profile of each individual cannabinoid. Conducting multiparametric studies, including combined bioanalyses and cell models, allows for a deeper understanding of CBT’s role within this complex and potentially developing new phytopharmaceuticals with optimized therapeutic effects.
Another focus area is the use of CBT in studies on its impact on the immune system. Preliminary work indicates a possible immunomodulatory effect of CBT that differs from the more studied cannabinoids. New research focuses on studying the influence of CBT on cytokine profiles, the function of T- and B-lymphocytes, macrophages, and its role in modulating immune response in autoimmune and inflammatory diseases. Understanding these mechanisms opens prospects for developing CBT as a potential immunotherapeutic agent.
Increased attention to CBT is observed in the field of neuroscience, where the focus is on neuroprotective properties and its possible involvement in regulating neurodegenerative processes. There are grounds for research on CBT in the context of Alzheimer’s disease, Parkinson’s disease, and other cognitive disorders. Accordingly, the number of studies aimed at investigating mechanisms of antioxidant action, modulation of inflammation in the central nervous system, and effects on synaptic plasticity is growing.
New directions also include studies on CBT metabolism in the human body, specifically determining its bioavailability, biotransformation pathways, and distribution kinetics. The absence of these data is a critical barrier to clinical use, so current research focuses on using innovative technologies such as metabolomics and pharmacokinetic modeling for comprehensive analysis of CBT’s metabolic routes and identification of active metabolites.
There is also interest in the development of specific antagonists and agonists of CBT receptors for fine regulation of its pharmacological action. Although CBT’s interaction with known cannabinoid receptors is not yet fully understood, the search for specific molecular targets opens prospects for creating new pharmacological tools for research and potential therapeutic applications of CBT.
A separate research vector is connected to studying the endocannabinoid system in the context of CBT. In particular, the possibility that CBT may modulate the activity of endocannabinoids or interact with enzymes responsible for their metabolism is being considered. This opens prospects for developing innovative approaches to regulating the endocannabinoid system, potentially affecting a wide range of physiological and pathological processes.
Enhanced integration of multidisciplinary research teams-chemists, molecular biologists, pharmacologists, clinicians, and bioinformaticians-creates conditions for synergistic knowledge development about CBT. This approach effectively combines methods of chemical synthesis, analytics, pharmacodynamics, and clinical trials, which is a key factor for transforming basic scientific data into applied medical solutions.
The incorporation of CBT into complex multicomponent formulas with other cannabinoids and unconventional bioactive compounds also opens new pharmacological prospects. This direction explores CBT’s potential as a potentiator of therapeutic effects or as a component that modulates the toxicity of other substances. This allows the development of new pharmaceutical products with tailored efficacy and safety profiles.
Conclusion
Cannabicitran (CBT) represents a unique member of the class of triterpenoid cannabinoids, whose chemical nature significantly distinguishes it from the more common phytocannabinoids such as THC or CBD. Its complex cycloparaffino-steroidal structure with numerous isomeric forms emphasizes high chemical specificity, which influences its pharmacological properties. The features of its molecular structure-including the presence of several chiral centers and variable conformations-create the basis for complex isomeric interactions that determine its biological activity and pharmacodynamics.
Biogenetically, CBT is formed in the Cannabis sativa plant as a result of sequential enzymatic reactions beginning with universal triterpene precursors and proceeding through a series of hydroxylations, cyclizations, and oxidations. Its synthesis is linked to the gene expression of specific synthases and oxidases localized in the plant’s trichomes, where intense biosynthetic activity takes place. The regulatory features of these enzymatic steps remain insufficiently studied, which presents important prospects for further molecular-biological research.
Sources of CBT include natural chemotypes of Cannabis, where its concentration varies significantly depending on genotype, growing conditions, and extraction methods. The low quantity in plant material and the complexity of its isolation have driven active development of synthetic and semi-synthetic production methods based on organic synthesis and biocatalysis. At the same time, these technologies face challenges related to scaling, product stability, and purity levels, underscoring the importance of developing new standards and purification techniques.
The issue of standardizing CBT as a substance includes difficulties in separating it from structurally similar compounds, instability during storage, and low reproducibility of quantitative extraction from natural samples. Imperfect chromatographic and chemical purification methods, as well as the limited number of validated analytical methods, complicate clinical application and pharmacological study. The development of advanced technologies employing multistep extraction, chromatography, and spectroscopy processes is critical to ensuring scientific reliability of research.
The pharmacological profile of CBT is at an early stage of investigation; however, empirical data are already accumulating, and hypotheses about its biological activity are forming. Preliminary studies indicate CBT’s potential ability to modulate receptors of the central nervous system and peripheral tissues, although the interaction mechanisms differ from classical cannabinoids and remain not fully understood. This opens unique prospects for creating new pharmacological agents with specific actions that could complement or replace existing therapeutic strategies.
Special attention deserves the hypothetical clinical properties of CBT, particularly its anti-inflammatory, antioxidant, and neuroprotective potentials. Molecular mechanism analyses indicate CBT’s involvement in regulating redox processes and immune responses, which may be significant in the therapy of chronic inflammatory and neurodegenerative diseases. Despite the lack of clinical data, these properties justify preclinical studies and the search for new pharmacotherapeutic directions.
In the context of modern cannabis science, CBT remains one of the least studied cannabinoids, which can be explained by a combination of technical, biological, and regulatory factors. Low natural content levels, complexity of chemical analysis, absence of validated standards, and insufficient attention from the pharmacological community limit the scope of scientific work. At the same time, the emergence of interdisciplinary methods, particularly biotechnological and informatics approaches, creates conditions for a rapid breakthrough in the study of this cannabinoid.
New research directions, including genetic engineering for CBT biosynthesis, application of artificial intelligence for modeling its interactions with biological targets, and the development of innovative delivery and purification systems, open prospects for integrating CBT into the pharmaceutical arsenal. Concurrently, a comprehensive approach to studying its pharmacodynamics, pharmacokinetics, and toxicology is necessary to determine clinical safety and efficacy.
Overall, analyzing existing scientific data on cannabicitran, it can be stated that this cannabinoid is a promising object for fundamental and applied research in the fields of cannabis chemistry, pharmacology, and medicine. The need for standardized methods of production and analysis, deeper study of molecular mechanisms of action, as well as systematic preclinical and clinical trials, is a priority for unlocking its potential. Successfully overcoming scientific and technical barriers will allow CBT to be integrated in the future into the spectrum of pharmacotherapeutic agents with new mechanisms of action and unique therapeutic properties.
Sources
- PubMed Central (PMC) – Scientific publications on cannabinoids and cannabis chemistry
https://www.ncbi.nlm.nih.gov/pmc/?term=cannabicitran - National Center for Biotechnology Information (NCBI) – Keyword search for “cannabicitran”
https://www.ncbi.nlm.nih.gov/search/all/?term=cannabicitran - ScienceDirect – Reviews and research on cannabinoids in the Cannabis sativa plant
https://www.sciencedirect.com/search?qs=cannabicitran - Journal of Natural Products (American Chemical Society) – Open access to some articles about cannabinoids
https://pubs.acs.org/doi/10.1021/acs.jnatprod - Frontiers in Pharmacology – Open articles on cannabinoid pharmacology
https://www.frontiersin.org/articles/10.3389/fphar.2020.00000/full - Google Scholar – Search for open-access scientific articles
https://scholar.google.com/scholar?q=cannabicitran&hl=en&as_sdt=0,5 - European Journal of Pharmacology – Publications on phytocannabinoid pharmacology (several open access articles)
https://www.sciencedirect.com/journal/european-journal-of-pharmacology/special-issue/10L9Y7G2FHG - Journal of Cannabis Research (Springer Open) – Open journal dedicated to cannabis, including new cannabinoids
https://jcannabisresearch.biomedcentral.com/ - United States National Library of Medicine (NLM) – Open access to scientific resources
https://www.nlm.nih.gov/bsd/disted/pubmedtutorial/020_1.html - ResearchGate – Researcher profiles and available publications about CBT
https://www.researchgate.net/search?q=cannabicitran