Within the chemical diversity of Cannabis sativa L., which includes over 150 identified phytocannabinoids, a significant portion of compounds remains either uncharacterized or poorly studied. One such structurally unique, yet scientifically underexplored compound is Cannabicyclol A (CBLA). Despite its name similarity to Cannabicyclol (CBL), CBLA is not its full structural analog, but rather considered an isomeric or derivative form that is formed through the involvement of other cannabis metabolites, likely cannabichromenic acid (CBCA), under conditions of natural or induced transformation.
From a scientific perspective, CBLA is potentially a stereoisomer or cyclic derivative containing characteristic cannabinoid skeleton fragments but with a different spatial configuration or linkage type between the rings. Its structural instability or tendency to undergo transformations under the influence of light, temperature, or pH may explain the lack of clear identification of this molecule for decades. Earlier literature references CBLA as a “potential degradation artifact,” though this approach is now considered outdated, as modern analytical chemistry methods allow for the verification of its presence in small but reproducible quantities in specific cannabis fractions.
The relevance of studying CBLA stems from a broader trend in contemporary pharmacognosy: the shift from well-known primary cannabinoids to secondary and micro-components that may exhibit atypical or selective bioactivity. In the context of searching for new molecular targets, especially beyond the classical endocannabinoid system (CB1, CB2), CBLA could be considered a promising candidate-specifically for research on interactions with GPCR receptors, TRP channels, or signaling pathways related to oxidative stress modulation.
Despite the fact that CBLA has never been the subject of clinical studies, its structural similarity to several natural cyclic cannabinoids suggests the possibility of pharmacodynamic activity distinct from classic CB receptor agonists. From a scientific perspective, this opens the opportunity to consider CBLA not only as a subject of fundamental chemical interest but also as a chemical platform for the creation of synthetic analogs with predicted properties.
At the same time, the historical “analytical invisibility” of CBLA in botanical and chemical studies of Cannabis sativa can be attributed to several factors. First, early analytical methods, such as thin-layer chromatography (TLC) and basic spectrophotometric approaches, lacked the sensitivity needed to identify small quantities of isomers with a similar polar profile. Second, the presence of CBLA may have gone unnoticed due to its overlap with peaks of other cannabinoids, such as CBCA, when using methods with insufficient resolution. Third, in mid-20th-century phytochemical research, the dominant interest was in psychoactive components, particularly Δ9-THC, while secondary compounds were considered irrelevant to pharmacology.
Today, with the development of advanced analytical techniques-such as LC-QTOF, high-resolution NMR, and ion mobility spectrometry-the detection of CBLA has become more feasible. Moreover, the application of mathematical modeling (in silico), proteomics, and chemoinformatics allows for the evaluation of the bio-potential of compounds even before their full experimental validation.
Thus, Cannabicyclol A is not merely a derivative structure of the well-known CBL but a distinct molecular entity with potential biological activity that has remained beyond the focus of academic interest until now. Its study could not only expand our understanding of cannabinoid chemistry but also open new horizons in the pharmacological modeling of natural compounds.
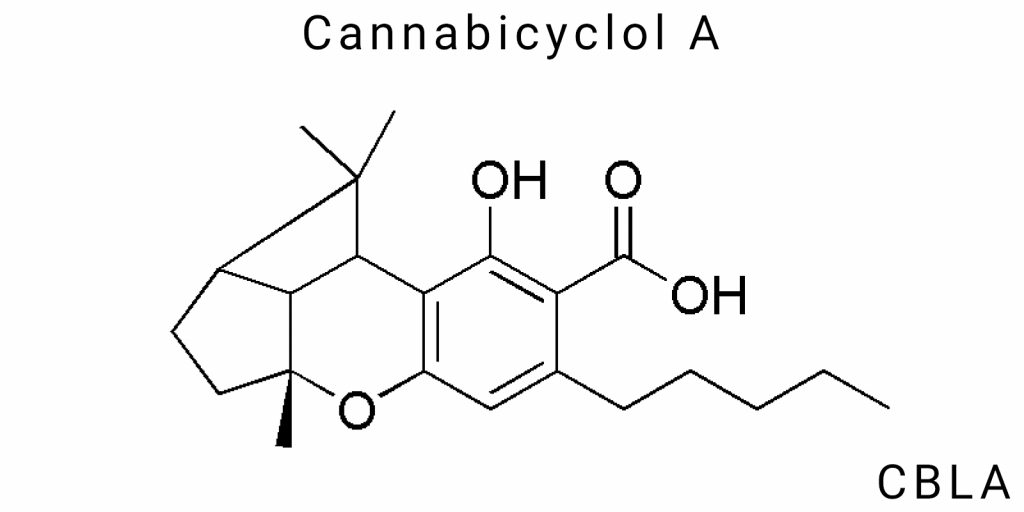
Molecular Structure and Chemical Identity of CBLA
Cannabicyclol A (CBLA) is a lesser-studied but promising chemical compound that differs from its isomer, cannabicyclol (CBL). It has a unique molecular structure that could potentially open new mechanisms of interaction with biological systems, distinct from the more widely known cannabinoids. However, this compound still requires further investigation to accurately determine its structure and mechanism of action.
Chemical Structure of CBLA: Properties and Predicted Functional Group Placement
CBLA shares similarities with CBL but primarily differs in the arrangement of double bonds and potential shifts in functional groups. The difficulty in determining the exact chemical composition of CBLA lies in its origin as a metabolic transformation product, which is often observed in cannabinoid studies. This means its structure can vary depending on environmental conditions, such as temperature, humidity, and exposure to ultraviolet light, which alter the interactions between molecular components.
One of the key features of the CBLA molecule is its ability to form different stereoisomers due to the mobility of double bonds in the structure. This allows CBLA to have several different configurations, which could significantly affect its biological activity and pharmacological properties. Depending on the spatial organization of functional groups and the number of double bonds, CBLA may interact differently with various receptor types, including cannabinoid receptors CB1 and CB2, as well as other molecular targets.
Mechanism of CBLA Formation: Chemical Reactions and Photochemical Processes
CBLA is likely a product of photoisomerization, a process where the CBCA molecule undergoes restructuring under the influence of ultraviolet light. As a result, the spatial arrangement of double bonds in CBCA changes, leading to the formation of new isomers, one of which could be CBLA. This complicates the identification and measurement of CBLA concentrations in natural cannabis samples.
In addition to photoisomerization, the formation of CBLA may also involve oxidation mechanisms, where the CBCA molecule reacts with molecular oxygen or peroxide compounds. This can occur during the storage or processing of cannabinoid extracts, adding complexity to the detection of CBLA in natural conditions. Oxidation may affect the molecular structure, creating new functional groups like ketones or aldehydes, which can significantly alter the physicochemical properties of the molecule.
Another potential pathway for CBLA formation involves acid- or base-catalyzed cleavage of CBCA. Through the use of acidic or basic catalysts, the CBCA molecule may undergo an electrophilic attack, leading to the formation of stable structures with new functional groups.
Relationship Between CBLA and CBCA: Structural Differences
Although CBLA and CBCA share a common origin, their structures and properties are significantly different. Cannabicyclol A (CBLA) may result from further transformations, which include not just simple changes to double bonds but also the restructuring of the entire molecular skeleton. CBLA likely lacks the structural elements found in classic CBL, such as certain methyl or hydroxyl groups, which define the stability and activity of the latter. However, CBLA possesses properties that could make it significant in a pharmacological context, particularly due to its ability to bind to other molecular targets.
It is important to note that the chemical and structural changes in the CBLA molecule may lead to new physiological effects. For example, the shift in spatial groups and the stability of the molecule may significantly impact CBLA’s ability to cross the blood-brain barrier, which opens up possibilities for using this compound in neurobiological research and therapy for neurological disorders.
Key Differences Between CBLA and Other Cannabinoids
One of the primary aspects that differentiate CBLA from more extensively studied cannabinoids, such as CBL, lies in its structural differences and potential molecular properties. As mentioned, CBLA is not simply an isomer of CBL, as it may exist in other isomeric forms due to shifts in double bonds. This gives CBLA unique pharmacological properties that warrant separate study to understand its effects on the body.
Analytical Methods for Detection and Confirmation of CBLA
Detecting cannabicyclol A (CBLA) is a challenging task due to its low concentration in natural sources and the chemical transformations that can occur during the storage or processing of plant material. Despite its significance for pharmacology and molecular biology, CBLA remains a poorly studied cannabinoid, and scientific confirmation of its existence requires advanced analytical methods capable of detecting even trace amounts of this compound among numerous other cannabinoids.
History of Scientific Efforts to Confirm CBLA as a Distinct Cannabinoid
Early attempts to identify CBLA were based on its similarity to the more well-known cannabicyclol (CBL), but at the time, many aspects of its molecular structure and pharmacological properties remained unclear. The lack of sufficient raw material and the difficulty of isolating CBLA from cannabis biomass hindered its reliable identification. One of the primary challenges was that CBLA might be present at significantly lower concentrations than other cannabinoids like THC or CBD, making it difficult to detect using standard analytical techniques.
A turning point in the study of CBLA came with the use of more modern chromatography and spectroscopy methods, which allow for more precise analysis of cannabinoids. Current research is also focused on developing standard procedures for analyzing CBLA and other underexplored cannabinoids in the context of growing interest in their pharmacological properties.
Modern Analytical Techniques
One of the primary methods for detecting CBLA is liquid chromatography-mass spectrometry (LC-MS/MS). This technique enables researchers to separate complex cannabinoid mixtures through liquid chromatography and then identify them using mass spectrometry. Due to its high sensitivity, LC-MS/MS can detect even trace amounts of CBLA, making it indispensable when the concentration of this compound in samples is extremely low. A key feature of this method is its ability to provide detailed analysis of molecules, including the determination of molecular mass and structural fragments, allowing for the confirmation of CBLA presence based on spectral data.
Another important method is nuclear magnetic resonance (NMR) spectroscopy, which provides precise information about the atoms and their bonds within the molecule. For CBLA, this technique allows researchers to obtain a detailed picture of the molecule’s spatial organization, determine the placement of functional groups, and even detect potential stereoisomers. While NMR is less sensitive than LC-MS/MS, it is highly valuable for structural analysis and confirming the chemical identity of a compound, as demonstrated in studies of other cannabinoids.
Spectroscopy in the visible and UV ranges is also used for identifying CBLA. Due to its optical properties, cannabinoids, including CBLA, can be detected through absorption or fluorescence spectroscopy in various light ranges. Detection of specific absorption peaks in the UV range confirms the presence of compounds with a certain chemical structure, which is an essential step toward understanding CBLA’s properties.
Challenges of Low Concentration and Possible CBLA Degradation
The primary challenge in detecting CBLA is its low concentration in natural cannabis samples. In many cases, especially in plants not specifically cultivated for research, the amount of CBLA may be at trace levels, making it difficult to detect even with highly sensitive methods. Furthermore, during the storage and processing of cannabis biomass, CBLA may undergo degradation due to factors such as high temperatures, light, or oxygen exposure. This can lead to the breakdown or transformation of CBLA into other molecules that may lack the same biological activity or be difficult to distinguish using standard analytical methods.
Particular attention should be paid to the conditions under which samples are stored, as the loss of CBLA molecules due to degradation can significantly complicate the research results, leading to inaccuracies in their interpretation. One potential solution to this problem could be the development of new methods for stabilizing cannabinoids, which would preserve their properties even during long-term storage. In this context, standardizing procedures for harvesting, storing, and processing biomass for research purposes becomes crucial, as it significantly enhances the reliability of the results obtained.
Pharmacological and Biochemical Hypothesis: CBLA as a Molecular Candidate
Assumptions about the Bioactivity of CBLA Based on Its Chemical Structure
Cannabicyclol A (CBLA), like other cannabinoids, features a cyclic structure that allows it to interact with the body’s endocannabinoid system. However, unlike classical cannabinoids, CBLA has certain structural characteristics that may influence the specificity of its biological activity. Based on its structure, it is hypothesized that CBLA could interact with cannabinoid receptors CB1 and CB2. However, its binding to these receptors may differ in strength or nature compared to well-known cannabinoids like THC or CBD.
In addition to interacting with the endocannabinoid system, CBLA could also exhibit activity on receptors not directly associated with this system. For instance, its molecular structure suggests the potential to bind to vanilloid receptors (TRPV1), which are involved in processes related to pain and temperature sensitivity. Moreover, potential interactions with receptors like GPR55 and other non-cannabinoid molecular targets could open new possibilities in treating a wide range of diseases, from inflammatory conditions to neurological disorders.
Modeling CBLA Interaction with Cellular Receptors
The assumptions about CBLA’s interaction with various molecular targets are based on predictions regarding its structural features and functional groups. CBLA may bind to CB1 and CB2 receptors, which mediate most cannabinoid effects in the body. However, its more complex molecular structure could indicate other types of interactions. Given its cyclic nature, CBLA might interact with receptors that were not previously considered in the context of cannabinoids, such as TRPV1 receptors. These receptors play a crucial role in pain, inflammation, and thermoregulation, making them potentially important targets for cannabinoids like CBLA.
Another promising direction is the interaction of CBLA with GPR55, a receptor often associated with neuroplasticity regulation and metabolism. Although this receptor remains poorly studied, its potential in treating neurodegenerative diseases and inflammatory processes could be significant. Research into how CBLA may modulate GPR55 could uncover new pathways for therapeutic applications.
Data from Databases and Availability of Pharmacokinetic Studies
According to available data from PubChem and NCBI, CBLA is not yet a widely studied cannabinoid. However, its chemical structure and potential bioactivity have piqued interest among researchers. Nevertheless, mentions of CBLA in these databases are limited, primarily concerning theoretical aspects that require further validation through more specific experiments.
As for the pharmacokinetic properties of CBLA, there is currently no concrete information regarding its metabolism, bioavailability, or distribution in the body. This presents a significant barrier to further research and the practical application of this cannabinoid. Therefore, it is crucial to focus on specialized studies that provide data on its pharmacokinetics, including absorption, metabolism, and excretion processes.
Prospects for Medical Use of CBLA
Given the potential of CBLA to modulate receptors related to pain, inflammation, neuroprotection, and other biological processes, this cannabinoid could become an important molecular candidate for the treatment of chronic conditions. Its therapeutic potential is likely to increase as it is more thoroughly studied in the context of specific diseases such as chronic pain, inflammatory disorders, and neurological disturbances.
To gain more attention from researchers and medical professionals, comprehensive clinical trials are needed to determine CBLA’s efficacy, safety, and mechanisms of action. Additionally, detailed studies on the pharmacokinetics and pharmacodynamics of CBLA will help better understand its role in the potential treatment of numerous diseases and identify any risks associated with its use.
Challenges in the Experimental Study of CBLA
Reasons Why CBLA Has Not Become a Focus of Systematic Biomedical Research
Cannabicyclol A (CBLA) remains largely overlooked in the scientific community, especially when compared to other more widely studied cannabinoids such as THC and CBD. Several key factors contribute to this, with the foremost being technical challenges related to its detection in natural samples, its low concentration in plants, and the overall lack of scientific awareness about this cannabinoid.
Since CBLA is present in minute quantities, its identification and analysis require highly sensitive and sophisticated analytical methods. These difficulties often prevent researchers from obtaining sufficient material for further studies. Moreover, due to the lack of data regarding its structure and chemical activity, there is considerable uncertainty about the potential bioactivity of CBLA within the scientific community.
Thus, mainly due to technical limitations and a lack of data on its mechanisms of action, CBLA has not yet become a fully-fledged object of pharmacological and clinical research. Systematic studies in this area are only beginning to gain momentum, but they have not yet developed to a large scale.
Impact of Legal Restrictions on Cannabinoid Research
Another significant barrier to the study of CBLA is the legal status of cannabis in many countries. Due to the legal status of cannabis, many research organizations and universities face substantial difficulties in accessing the plant material required for experiments. In countries where cannabis is banned or highly regulated, obtaining permission to collect, process, and analyze plant material becomes a challenging task. This greatly slows the development of scientific research that could open new avenues for studying cannabinoids like CBLA.
Overall, the lack of a unified regulatory framework for cannabinoid research significantly hinders the study of lesser-known compounds. Legal restrictions may even apply to laboratory use of cannabis in experiments, further complicating scientific inquiry. In more legally permissive jurisdictions, researchers may face a lack of clear and standardized methods for processing plant material, which reduces the scientific precision of results.
Lack of Standardized Reference Samples
One of the primary issues in CBLA research is the absence of standardized reference samples of this compound. For effective experimentation, especially for accurately determining the concentration of CBLA in plant material, well-validated and standardized samples are essential. The absence of such reference samples severely limits the ability of scientific laboratories to conduct highly precise studies.
Existing chemical analysis methods can only detect trace amounts of CBLA, which restricts the scale of research. Due to the lack of standardized samples, interpreting the results becomes challenging, and comparisons between different laboratories are problematic. Furthermore, the lack of a standardized approach to sample preparation can lead to significant variations in results, creating obstacles to the replication and verification of studies.
Presence of Only Single Analytical Signals of CBLA in Plant-Derived Samples
Another serious issue is that CBLA is found in very low concentrations in natural samples. Most cannabis samples used in scientific research contain only trace amounts of CBLA, making it difficult to detect using standard methods. It’s also important to note that various external factors, such as temperature, humidity, lighting, and other storage conditions, can affect the stability of Cannabicyclol A, further complicating its identification.
According to numerous studies, even when highly accurate analysis methods like liquid chromatography-mass spectrometry (LC-MS) or ultraviolet spectroscopy are used, signals indicating the presence of CBLA may be so weak that they are difficult to interpret. This creates a significant problem for the reproducibility of results in other laboratories and may lead to ambiguous conclusions.
Theoretical Models of Pharmaceutical Application of CBLA
CBLA as a Potential Precursor for Creating New Classes of Semi-Synthetic Cannabinoids
CBLA, with its unique molecular structure and potential for modification, represents an interesting candidate for the development of new classes of semi-synthetic cannabinoids. Similar to other cannabinoids with cyclic structures, CBLA can be used as a precursor for synthesizing new compounds with various properties. Semi-synthetic cannabinoids, typically derived from natural cannabinoids but modified chemically, may exhibit improved pharmacological properties such as enhanced bioavailability, greater specificity for certain receptors, or reduced toxicity compared to their natural counterparts.
For example, modifying groups in the CBLA molecule through methyl, ethyl, or other alkyl substitutions can alter their affinity for specific cannabinoid receptors like CB1 and CB2. These modifications may pave the way for the creation of drugs targeting chronic diseases, including neurological, inflammatory, and oncological conditions.
Furthermore, due to its structural similarities with other cannabinoids, CBLA could be utilized to create drugs combining multiple cannabinoid effects, potentially improving therapeutic outcomes. For instance, blending CBLA with other cannabinoids or terpenes may lead to the development of medications with enhanced effects due to the synergistic interaction of various components.
CBLA’s Role in the “Entourage Effect” and Possible Synergistic Interaction with Terpenes or Other Cannabinoids
The “entourage effect” refers to the interaction of various components of the cannabis plant (cannabinoids, terpenes, and other bioactive compounds) that enhance or modulate each other’s effects. This concept is crucial for understanding CBLA’s potential as part of this system. The entourage effect is particularly relevant in therapeutic cannabinoid use because the interaction between different active compounds may result in a better therapeutic outcome than the use of individual substances.
CBLA, as part of the cannabinoid profile, can influence the bioactivity of other cannabinoids through its effects on CB1 and CB2 receptors. This opens new possibilities for developing cannabinoid combinations. For example, when combined with well-known cannabinoids like CBD or CBG, CBLA could help enhance their therapeutic efficacy due to the synergistic influence.
Moreover, terpenes, compounds found in cannabis, can modulate CBLA’s action. Terpenes, in particular, may improve the penetration of cannabinoids through the blood-brain barrier or alter their metabolism in the body, thereby promoting better absorption or prolonging their effects. This synergy between cannabinoids and terpenes can expand the therapeutic potential of these compounds.
Exploring CBLA in Non-Cannabinoid Mechanisms: Antioxidant, Antibacterial, and Membrane-Stabilizing Effects
In addition to studying CBLA as a traditional cannabinoid, it is important to explore its possible effects through non-cannabinoid mechanisms. One such avenue is the investigation of CBLA’s antioxidant activity. Like many other natural compounds, cannabinoids may possess the ability to neutralize free radicals and reduce oxidative stress, which is a critical factor in treating diseases such as neurodegenerative disorders, cardiovascular diseases, and cancer.
Some cannabinoids have already demonstrated antioxidant activity, and CBLA, due to its chemical structure, may exhibit similar properties. This opens up prospects for using CBLA in therapies related to diseases that arise from elevated oxidative stress, such as chronic inflammatory processes, and for protecting cells from damage caused by oxidative reactions.
Another potential research area is the exploration of CBLA’s antibacterial properties. Some cannabinoids have already shown promise as natural antibacterial agents. Specifically, CBLA could possess the ability to inhibit the growth of various pathogens, such as bacteria or fungi, by interacting with their membranes or other molecules critical for their survival.
Membrane-stabilizing properties are also worth investigating. Studies suggest that some cannabinoids can stabilize cell membranes, preventing damage under stress or inflammation. CBLA may have a similar effect, which could be beneficial for treating diseases where maintaining cell membrane integrity is crucial, such as neurodegenerative diseases or trauma-related conditions.
Perspectives and Open Questions
Why CBLA Deserves Investment in Scientific Research: Arguments Based on Biochemical Potential
Cannabicyclol A (CBLA), as a less-studied isomer of cannabicyclol (CBL), opens new opportunities in pharmaceutical science due to its unique molecular structure and potential biochemical impact. Given its structural similarity to other cannabinoids, yet with specific modifications, CBLA has the capacity to exhibit biological effects that are not characteristic of more widely studied compounds such as THC or CBD. Its unique properties make CBLA an important candidate for further research in therapeutic applications, including treatments for inflammatory diseases, neurodegenerative disorders, and therapies that involve antioxidant and antibacterial activities.
CBLA’s biochemical potential is a key factor to explore. There is evidence suggesting that CBLA may influence the endocannabinoid system, modulating the functions of CB1 and CB2 receptors, as well as other mechanisms, such as vanilloid receptors (TRPV1) and GPR55. Studying these effects could lead to the discovery of new pathways for treating diseases related to chronic pain, neuropathies, or autoimmune disorders. Given the complexity of chemical and biochemical interactions that occur with cannabinoids, gaining a deeper understanding of CBLA’s properties could significantly contribute to the development of new therapeutic strategies.
The potential for modification and synthesis of new molecules from CBLA, along with the possibility of combining it with other cannabinoids or terpenes, increases its value for investors in the pharmaceutical sector. This not only offers the chance to explore CBLA’s therapeutic applications but also to incorporate it into more complex treatment formulations that could offer higher bioavailability and specificity for treating particular conditions.
Experimental Models to Start With: Cell Lines, In Silico Modeling, Metabolic Mapping
To advance CBLA research, it is crucial to employ modern experimental models that assess not only the physicochemical properties of the molecule but also its biological activity. One such model is the use of cell lines, which enable the study of how CBLA interacts with cellular receptors and other molecular targets. For example, utilizing cell lines expressing CB1, CB2, or TRPV1 receptors can help evaluate the specificity of CBLA to these receptors and reveal how it modulates their function.
Additionally, in silico modeling is an important component of research. Computational methods can predict the potential biological effects of CBLA, its affinity for various receptors, and even interactions with other molecules. Molecular docking simulations can help foresee how CBLA might interact with different biological molecules, potentially guiding the creation of new drugs. Such studies are rapid and cost-effective, providing crucial insights that can expedite experimental testing.
Metabolic mapping is also a vital step. Investigating CBLA’s metabolism, its transformations in the body, and the formation of active metabolites can enhance our understanding of how this compound exerts its therapeutic effects. Since cannabinoids are typically metabolized in the liver, it is important to determine if CBLA undergoes metabolic changes that might reduce its effectiveness or cause unwanted side effects.
Could CBLA Become a Marker of Chemical Stability for Cannabis sativa-Based Products?
An intriguing possibility is the use of CBLA as a marker for the chemical stability of Cannabis sativa-based products. Due to the instability and degradation of certain cannabinoids during storage, CBLA could serve as an indicator for monitoring the stability of other cannabinoids in pharmaceutical formulations. As a molecule with a unique chemical structure, CBLA may remain stable under various conditions, making it an ideal control marker for pharmaceutical research.
Given that CBLA results from certain chemical transformations, its presence or absence in a product could indicate the stability of other components or the potential degradation of cannabinoids due to external factors such as temperature, humidity, or light exposure. Assessing the stability of CBLA in combination with other cannabinoids could assist in developing new methods for storing and transporting cannabis-based products and help identify the optimal conditions for preserving their activity.
As CBLA has potential as a stability marker, further research should focus on its chemical stability in various pharmaceutical forms, such as oils, tinctures, or capsules. Understanding these aspects will not only advance new manufacturing technologies but also ensure high quality and safety for cannabinoid products on the market.
Conclusions
Cannabicyclol A (CBLA) represents a molecule with a high degree of scientific uncertainty, yet its potential in pharmacology and biochemistry is significant for the further expansion of the cannabinoid pharmacopoeia. Although there are still substantial gaps in the research on this cannabinoid, its unique structure and probable biological activity make CBLA an interesting subject for scientific efforts. Investigating this molecule could be a crucial step towards developing new therapeutic strategies for treating a variety of diseases, such as neuropathies, chronic pain, autoimmune disorders, or other conditions that require innovative approaches.
Modern instrumental methods, including chromatography, spectroscopy, nuclear magnetic resonance (NMR), and mass spectrometry, enable scientists to move from theoretical hypotheses to real-world verification of the molecule. These technologies open up new possibilities for accurately determining the structure of CBLA, as well as for studying its interaction with biological targets. With these tools, researchers can not only confirm the presence of CBLA in plant material but also begin to explore its pharmacokinetic and pharmacodynamic characteristics in greater depth.
However, to fully understand the biological profile of CBLA, further academic, interdisciplinary research is necessary. Collaboration among chemists, biologists, pharmacologists, and toxicologists is critical for building a well-founded knowledge base about this molecule. Only through a comprehensive approach to study-combining theoretical and experimental methods-can the potential of CBLA in medical and pharmaceutical applications be uncovered.
As a result, CBLA could become a key tool in expanding the existing pharmaceutical arsenal, particularly in the context of treating complex pathologies where traditional approaches may not yield the desired results.
References:
- PubMed – A database of scientific articles containing numerous publications on cannabinoids and their pharmacological properties.
https://pubmed.ncbi.nlm.nih.gov/ - National Center for Biotechnology Information (NCBI) – A resource for scientific research containing articles on biochemistry, molecular biology, and pharmacology.
https://www.ncbi.nlm.nih.gov/ - ScienceDirect – An electronic platform for accessing scientific articles across various disciplines, including chemistry and pharmacology of cannabinoids.
https://www.sciencedirect.com/ - SpringerLink – A platform for accessing scientific articles and books on chemistry, pharmacology, and toxicology of cannabinoids.
https://link.springer.com/