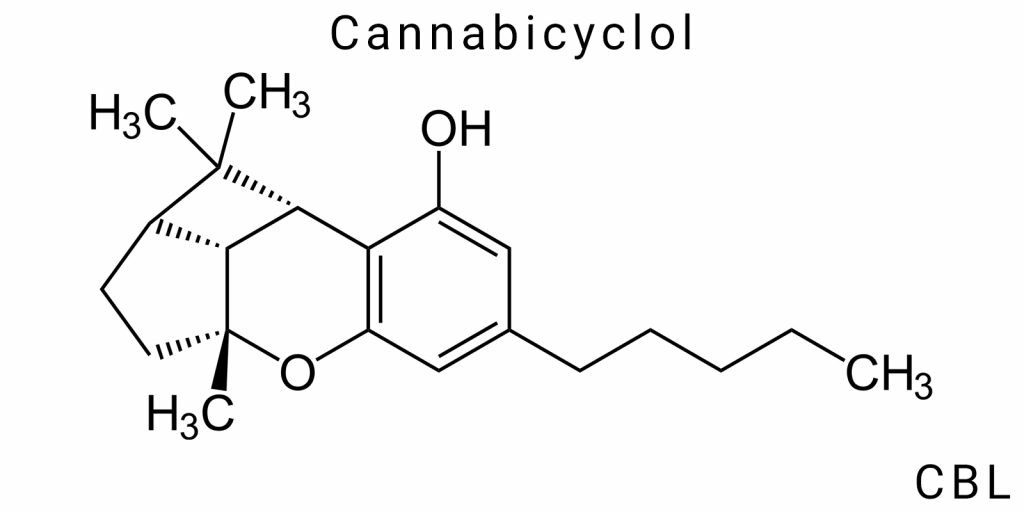
Cannabinoids are a large group of naturally occurring compounds synthesized by the Cannabis sativa L. plant. Their biological activity, pharmacological potential, and effects on the human body have been studied for over half a century. However, scientific focus has long been centered primarily on the most well-known cannabinoids-Δ9-tetrahydrocannabinol (THC) and cannabidiol (CBD). Meanwhile, dozens of other cannabinoids present in trace amounts remain largely unexplored, despite their potential to possess equally important properties.
One such lesser-known cannabinoid is cannabicyclol (CBL). This compound is not a major metabolite of cannabis but rather forms as a result of natural photochemical reactions that occur in plant material exposed to ultraviolet (UV) light. Due to its stability, unique chemical structure, and low concentration in the plant, CBL has long gone under the radar of researchers. However, recent trends in cannabinoid science indicate a growing interest in rare and under-studied molecules like this one.
The scientific value of such compounds lies in their potential to reveal new mechanisms of action, novel therapeutic targets, and opportunities to develop medications that do not act through the classic cannabinoid receptors. In the ongoing search for alternatives to current treatments for chronic pain, inflammation.
Chemical Nature and Origin of Cannabicyclol (CBL)
Cannabicyclol (CBL) is one of over 120 known phytocannabinoids. Unlike more widely recognized compounds such as Δ9-tetrahydrocannabinol (THC) and cannabidiol (CBD), CBL is not a primary product of the biosynthetic pathways in Cannabis sativa. Its chemical characteristics, method of formation, and potential role within the cannabinoid profile set it apart, making it a promising subject for future research.
Structural Characteristics: CBL as a Non-Biosynthetic Cannabinoid
CBL is a tricyclic, non-psychoactive compound with the molecular formula C₂₁H₃₀O₂. Its structure features a fully saturated carbocyclic skeleton, distinguishing it from most cannabinoids by the absence of double bonds in both the side chain and aromatic ring. As a result, it is considered a non-aromatic analog of cannabichromene (CBC)-the compound from which it is typically derived through external transformation.
CBL is classified as a photolytic product of CBC, meaning it forms through a chemical reaction triggered by ultraviolet (UV) light exposure. This transformation occurs via a process known as photoisomerization, in which UV photons induce a rearrangement of double bonds within the CBC molecule, resulting in the formation of a new condensed ring structure. Notably, this process is irreversible-CBL remains stable and does not revert back to CBC once UV exposure ends, unlike many other cannabinoids.
Natural Formation Mechanism: CBC Photoisomerization
CBL does not form within the plant itself. Instead, it emerges after harvesting, most often during drying, storage, or improper light exposure. Its immediate precursor, CBC, is biosynthesized in the plant via CBC synthase from cannabigerolic acid (CBGA), much like other primary cannabinoids (e.g., THCA, CBDA, CBCA).
When CBC is exposed to ultraviolet light-particularly in the UV-B range (280–320 nm)-a reaction known as cyclodehydration occurs. This process involves a shift in one of the double bonds, leading to the closure of a new ring and resulting in CBL’s tricyclic structure. This reaction has been confirmed through various analytical techniques including chromatography, nuclear magnetic resonance (NMR), gas chromatography–mass spectrometry (GC-MS), and UV spectroscopy, all of which show a direct correlation between light exposure and CBL levels in plant samples.
Environmental Factors Affecting CBL Formation
The formation of CBL is highly dependent on several physical and chemical conditions:
- Type of light exposure: UV-B radiation is most effective at initiating isomerization. However, long-term storage under fluorescent lighting can also slowly convert CBC into CBL.
- Storage temperature: Higher temperatures accelerate photodegradation. Studies show that temperatures above 95°F (35°C) can double the rate of this reaction.
- Humidity: Elevated humidity may catalyze oxidative processes that either complement CBL formation or contribute to overall cannabinoid breakdown.
- Storage duration: Plant material stored for over six months with exposure to light shows significantly higher CBL levels compared to fresh or cryogenically preserved samples.
Thus, CBL is considered a typical degradation product in the cannabinoid profile, often formed due to improper storage or prolonged processing.
CBL as a Chemical Marker
Because of its stability and origin, CBL has potential use as a marker of cannabis product “age” or as an indicator of environmental exposure in forensic and chemical analysis. This is especially relevant in the context of medical cannabis standardization, where cannabinoid profiling is a key component of quality assurance protocols.
neurodegenerative disorders, or infectious diseases, rare cannabinoids like CBL could become a source of meaningful innovation.
Detection and Isolation Methods for Cannabicyclol (CBL)
The study of cannabicyclol (CBL) requires specialized methods due to its low natural abundance and its unique biosynthetic origin. CBL is formed as a result of the photoisomerization of cannabichromene (CBC), necessitating techniques capable of detecting and analyzing trace amounts of this cannabinoid in plant material.
Detection Methods for CBL
Since CBL is not a major component of cannabis, its detection demands highly sensitive analytical approaches. One of the primary techniques used is gas chromatography coupled with mass spectrometry (GC-MS). This method allows for precise analysis of decarboxylated cannabinoids, including CBL, through identification via characteristic mass spectra. However, one limitation of GC-MS is the potential for thermal degradation of some compounds during analysis, which can hinder the detection of certain cannabinoid derivatives.
To address this, high-performance liquid chromatography (HPLC)-especially when coupled with mass spectrometry (LC-MS)-is used as an alternative. HPLC avoids thermal breakdown, preserving the molecular structure and enabling more accurate identification of thermolabile compounds like CBL.
For definitive structural identification, researchers often rely on nuclear magnetic resonance (NMR) spectroscopy. NMR provides detailed information about the chemical environment of atoms within the molecule, allowing scientists to confirm the tricyclic structure of CBL and its interactions with other compounds in cannabis extracts. Together, GC-MS, HPLC, and NMR enable the detection of CBL even at very low concentrations-an essential requirement for further pharmacological investigation.
Extraction and Isolation of CBL
Extracting CBL from plant material poses significant challenges due to its low natural concentration. One of the most common techniques is supercritical CO₂ extraction, which produces high-quality extracts without the use of harsh chemical solvents. However, the efficiency of this method is highly dependent on extraction parameters.
Another widely used approach is solvent-based extraction using organic solvents such as ethanol or hexane. Following initial extraction, further purification is performed using flash chromatography or preparative HPLC, which helps separate CBL from other cannabinoids like CBC or CBG-compounds with similar chemical structures.
Solvent-based extraction allows for the recovery of cannabinoids without damaging sensitive molecules, but the process requires careful control of temperature and storage conditions to prevent oxidation or photodegradation. To ensure the stability of CBL during extraction, it is crucial to shield it from direct UV light exposure, which could trigger further isomerization.
Laboratory Synthesis of CBL
Due to the difficulty of obtaining sufficient quantities of CBL from natural sources, researchers have developed methods for laboratory synthesis. The most promising approach involves photoisomerization of CBC, its direct precursor. Under controlled UV light exposure (typically at 300–320 nm) in solvents such as chloroform or methanol, CBC is converted into CBL. Achieving a high-quality end product requires precise control over the exposure time and light intensity, as excessive irradiation can lead to the formation of unwanted isomers or degradation products.
Other synthetic strategies are also under investigation, including Lewis acid-catalyzed isomerization and photosensitized reactions, both of which aim to improve the efficiency of the conversion process. However, these methods remain largely experimental and require further refinement. Given the technical challenges and relatively low yields, synthetic production of CBL remains confined to research laboratories and demands continued investment in chemical development.
Outlook and Challenges
Despite the challenges associated with extraction and synthesis, CBL holds promise for future pharmacological research and applications. Its unique structure and potential to form specific bioactive molecules open new doors for drug discovery, particularly for targeting novel biological pathways. However, to fully harness CBL’s potential, researchers must overcome current methodological and technical barriers, including low natural yields and difficulties in scalable synthesis.
Pharmacological Profile of Cannabicyclol (CBL): Current Knowledge
Cannabicyclol (CBL) is a cannabinoid attracting growing scientific interest due to its potential pharmacological properties. Although CBL is present in cannabis in significantly lower concentrations compared to more well-known cannabinoids, its biological activity-including anti-inflammatory, antimicrobial, and neuroprotective effects-has generated increasing attention. As a product of the photoisomerization of cannabichromene (CBC), CBL’s rarity in plant material adds to the intrigue surrounding its therapeutic potential. While research is still in its early stages, preliminary findings suggest CBL may hold considerable value in medical applications.
Anti-Inflammatory Properties
One of the primary focuses in the pharmacological research of CBL is its anti-inflammatory activity. Inflammatory processes are central to the development of many chronic diseases, including arthritis, cardiovascular disorders, gastrointestinal diseases, and autoimmune conditions. Many cannabinoids exert anti-inflammatory effects through interactions with cannabinoid receptors CB1 and CB2, as well as by modulating other immune-regulatory molecules.
Studies have demonstrated that CBL can reduce levels of key pro-inflammatory cytokines such as interleukin-1 (IL-1), interleukin-6 (IL-6), and tumor necrosis factor-alpha (TNF-α). These molecules are central to the regulation of inflammation, and their suppression may significantly mitigate inflammatory responses in the body. In several animal models, administration of CBL led to marked reductions in tissue inflammation, indicating its potential as a therapeutic agent for inflammatory disorders. Importantly, unlike traditional anti-inflammatory drugs such as NSAIDs, CBL may offer a milder profile with fewer adverse side effects, making it a promising candidate for long-term use in chronic conditions.
Antimicrobial Properties
Given the escalating problem of antibiotic resistance, the antimicrobial potential of cannabinoids-including CBL-has gained particular significance. CBL has been shown to exhibit antimicrobial activity against a range of pathogenic microorganisms, including both Gram-positive and Gram-negative bacteria, as well as certain fungi. As cannabinoid-based therapies are explored for combating drug-resistant bacterial infections, the antimicrobial profile of CBL presents a compelling area for further research.
Specifically, researchers have observed that CBL effectively inhibits the growth of Staphylococcus aureus, including methicillin-resistant strains (MRSA), as well as Escherichia coli. Other studies suggest it also exhibits antifungal activity against Candida albicans. While CBL’s antiviral properties are less studied, some preliminary data suggest potential activity against certain viruses, though these findings require further verification in the absence of large-scale clinical trials.
Neuroprotective Properties
A particularly promising area of CBL research involves its neuroprotective potential. The endocannabinoid system plays a crucial role in neuroprotection, especially in the context of neurodegenerative diseases such as Alzheimer’s and Parkinson’s, as well as in cases of stroke and traumatic brain injury. Cannabinoids, including CBL, may help counteract oxidative stress, which is a key driver of neuronal damage in these disorders.
CBL appears to reduce levels of free radicals, thereby mitigating oxidative stress. Animal studies have shown that CBL administration improves brain function under neurotoxic conditions, which are common in many neurodegenerative diseases. Furthermore, CBL may reduce neuroinflammation-another major contributor to the pathogenesis of these conditions. The prospect of using CBL as a neuroprotective agent opens new avenues for developing therapies for neurological diseases that currently lack effective treatments.
Receptor Interactions
While the interaction of CBL with cannabinoid receptors remains underexplored, initial evidence suggests it may modulate the activity of CB1 and CB2 receptors. CB1 receptors are predominantly located in the central nervous system and are involved in regulating pain, mood, and memory. CB2 receptors are mainly found in peripheral tissues and play a role in immune modulation and inflammation.
Similar to other cannabinoids, CBL may interact with these receptors to exert anti-inflammatory and neuroprotective effects. However, the precise molecular mechanisms of these interactions are not yet fully understood, and further research is required to elucidate the pathways through which CBL exerts its biological effects.
Preliminary Laboratory Findings
Important insights into the pharmacological properties of CBL have been published in scientific journals such as the Journal of Natural Products and indexed databases like PubMed. For example, one study in the Journal of Natural Products reported that CBL reduced levels of pro-inflammatory molecules in animal models, highlighting its potential for treating inflammatory conditions. Other studies referenced in PubMed indicate that CBL shows promise in combating infectious diseases due to its antimicrobial activity against drug-resistant pathogens.
These early findings underscore the therapeutic potential of CBL but also emphasize the need for further clinical trials and molecular studies to confirm its safety and efficacy.
Biomedical Significance and Scientific Hypotheses: The Potential of CBL in Pharmacology, Toxicology, and Neurobiology
Cannabicyclol (CBL) is one of the lesser-studied compounds in the cannabinoid family that holds significant potential for biomedical research. While this cannabinoid has not attracted as much attention as its more well-known relatives, such as Δ9-THC or CBD, its unique physiological properties could form the basis for new pharmacological strategies. Despite the fact that research on CBL is still in its early stages, its relevance to fields such as pharmacology, toxicology, neurobiology, and even psychiatry can already be identified. The uniqueness of this molecule lies not only in its chemical structure but also in its ability to interact with various biological systems, opening up opportunities for the development of new therapeutic agents.
CBL in Pharmacology: New Therapeutic Opportunities
The study of CBL’s pharmacological properties is gaining increasing popularity due to its ability to interact with numerous molecular targets in the body, particularly with cannabinoid receptors type 1 (CB1) and 2 (CB2), as well as other biological structures. These receptors are typically involved in regulating various physiological processes such as pain, inflammation, mood, appetite, and many others. However, unlike Δ9-THC, CBL does not exhibit strong psychoactive effects, making it a promising candidate for the development of therapeutic drugs without the undesirable side effects typically associated with psychoactive cannabinoids.
CBL has already demonstrated its effectiveness in several experimental models as a potent antioxidant and anti-inflammatory agent. This suggests that it could be developed into new therapeutic treatments for chronic inflammatory diseases such as arthritis, Crohn’s disease, or even certain types of cancer. Given that CBL can inhibit certain enzymes responsible for inflammation, this compound may be valuable in developing drugs for treating inflammatory processes without negatively affecting other systems in the body.
CBL in Toxicology: Protection from Harmful Effects
From a toxicological perspective, CBL is also an intriguing molecule. Its antioxidant and anti-inflammatory properties could provide reliable protection from oxidative stress, which is one of the main causes of many diseases. Oxidative stress is caused by the excessive activity of free radicals, leading to cellular and tissue damage. Cannabicyclol, through its properties, may reduce the levels of these harmful molecules, improving the overall condition of the body and lowering the risk of developing chronic diseases associated with oxidative stress.
In the context of toxicology, it is also worth noting that CBL could play a role in detoxification. To understand this, it is important to explore the mechanisms through which CBL supports the clearance of harmful metabolites from cells, such as heavy metals or toxic compounds that accumulate due to environmental exposure or intoxications. Research into CBL in this area could lead to the development of new drugs for detoxification or for supporting the normal functioning of the liver-an organ responsible for neutralizing and eliminating toxins from the body.
CBL in Neurobiology: Neuroprotective Properties
One of the most promising areas of CBL research is its application in neurobiology. CBL’s neuroprotective properties could prove useful in treating neurodegenerative diseases, such as Alzheimer’s disease, Parkinson’s disease, as well as strokes and brain injuries. Understanding the mechanisms through which CBL affects the nervous system is crucial for developing new therapeutic strategies aimed at protecting neurons from damage.
These properties of CBL may be due to its ability to reduce inflammation in nervous tissue and activate protective mechanisms in brain cells. Since inflammation is one of the main factors in neurodegeneration, cannabicyclol could be significant in preventing diseases such as Alzheimer’s, which are characterized by memory and cognitive dysfunction. Furthermore, CBL can interact with neuropeptides and neurotransmitters, which may help maintain the normal function of the nervous system.
The Potential of CBL as an Underexplored Compound: New Hypotheses and Scientific Discoveries
The uniqueness of CBL lies not only in its biological properties but also in the fact that it remains largely unexplored, opening up opportunities for new scientific discoveries. While many other cannabinoids, such as THC and CBD, have been studied for decades, CBL remains relatively uncharted. This allows researchers to explore new mechanisms of action and potential applications of this compound in biomedicine without the constraints imposed by more widely studied cannabinoids.
Underexplored compounds like CBL have immense potential, as they can lead to the discovery of new molecular pathways that were previously unavailable to other, more studied cannabinoids. Investigating CBL could shed light on the mechanisms behind a range of diseases, such as inflammation, neurodegeneration, autoimmune disorders, or even certain types of cancer.
Specifically, research on such underexplored compounds could contribute to the development of new therapeutic strategies that take into account individual patient characteristics, including genetic variations, thus paving the way for personalized medicine. Furthermore, these compounds could form the basis for the creation of new drugs to treat chronic and severe diseases, where traditional treatment methods are insufficient.
Regulatory and Practical Barriers to Studying Cannabicyclol: Systemic Challenges and Potential Solutions
Despite the growing interest in lesser-studied cannabinoids like cannabicyclol (CBL), the field of their research faces numerous constraints, both external-legal and economic-and internal-methodological. All of these barriers create a rather complex context in which scientists are forced to work. While the general global trend is toward increasing the role of cannabinoids in medical research, CBL as a subject of fundamental science remains on the periphery of both academic and applied attention.
Legal Environment: Between Regulatory Uncertainty and Limited Access
The first and perhaps most significant barrier is the legal regulation of cannabis, particularly its use for scientific purposes. Many countries, including Ukraine, continue to regulate cannabis as a narcotic substance, requiring special permits for any use, even for research. In most cases, CBL is found in trace amounts in aged, degraded cannabis samples or as a result of photoisomerization of other cannabinoids, such as CBC. As a result, access to quality raw materials containing traces of CBL often becomes a problem for laboratories. Consequently, scientists face not only restrictions on the importation or cultivation of cannabis but also a lack of standardized samples.
In many jurisdictions, the procedure for obtaining research permits for cannabinoids is cumbersome and expensive. This is especially true for small or university-based laboratories that do not have the resources to navigate lengthy regulatory processes. Specific initiatives-such as the Cannabis Research License Program in the U.S., managed by the FDA and DEA-create mechanisms for legal access to cannabis but remain difficult for researchers from other countries to access.
Financial Motivation: Lack of Commercial Driver
Another important limiting factor is the commercial unavailability of CBL. Unlike well-studied cannabinoids like THC or CBD, CBL does not demonstrate pronounced psychoactivity or immediate pharmacological effects that could attract the attention of investors or pharmaceutical companies. As a result, research into this compound often remains purely academic, without support from the private sector.
It is known that most pharmaceutical developments are driven not only by scientific potential but also by the economic value of the molecule. CBL, which currently has no registered pharmaceutical forms, clinical applications, or significant consumer popularity, remains outside the realm of economic interest. Furthermore, even from a purely chemical perspective, isolating CBL is a resource-intensive process due to its microconcentration in natural sources-only nanograms or micrograms of this compound can be isolated from tens of grams of dry plant material.
Insufficient Interdisciplinary Collaboration and Weak Government Support
Another aspect is the limited support for research from government institutions. In countries where science is funded on a residual basis, research into lesser-known cannabinoids rarely makes it to the priority list. The lack of systematic grant programs at the national level prevents laboratories from initiating long-term projects aimed at exploring the biological potential of such substances.
Nevertheless, there are positive examples. For instance, the University of Mississippi has had official approval from the DEA to cultivate cannabis for research purposes since 1968. Thanks to such state-supported projects, scientists gain legal access to plant material and the opportunity to conduct experiments with lesser-known cannabinoids, including CBL.
Conclusions
Cannabicyclol (CBL) is one of those cannabinoids that remain in the shadow of their more well-known “relatives,” such as Δ⁹-tetrahydrocannabinol (THC) and cannabidiol (CBD). However, its scientific potential cannot be ignored. As a product of the natural photoisomerization of cannabichromene (CBC), CBL is a unique example of molecular transformation that occurs due to external influences-ultraviolet radiation, time, and temperature. This formation pathway grants it the status of not only a rare but also potentially bioactive compound that deserves special attention in pharmacology, biochemistry, and neuro-sciences.
At present, knowledge of the pharmacological profile of CBL remains limited. Initial studies suggest possible antimicrobial, anti-inflammatory, and neuroprotective properties, but these hypotheses require extensive experimental verification. Another challenge is its low concentration in natural cannabis samples, which complicates both analytical detection and isolation. Despite this, progress in chromatography, spectroscopy, and semi-synthetic methods opens new possibilities for laboratory studies of CBL, including in the pharmaceutical context.
Another key challenge is the limited legal and economic space for researching lesser-known cannabinoids. Due to the legal status of cannabis and the lack of commercial interest, many laboratories cannot access the necessary raw materials or funding. However, it is academic science, supported by government backing and interdisciplinary collaboration, that has the potential to change this situation.
References:
- ElSohly, M. A., & Slade, D. (2005). Chemical constituents of marijuana: The complex mixture of natural cannabinoids. Life Sciences, 78(5), 539–548. https://doi.org/10.1016/j.lfs.2005.09.011
- Turner, C. E., Hadley, K. W., & Fetterman, P. S. (1975). Constituents of Cannabis sativa L. XVII. A Review of the Natural Constituents. Journal of Natural Products, 38(2), 105–110. https://doi.org/10.1021/np50038a001
- U.S. National Library of Medicine. PubChem Database. Cannabicyclol. Retrieved from:https://pubchem.ncbi.nlm.nih.gov/compound/Cannabicyclol
- U.S. Department of Health and Human Services, National Institutes of Health. National Center for Biotechnology Information (NCBI). Cannabicyclol – Compound Summary.https://www.ncbi.nlm.nih.gov
- Appendino, G., Chianese, G., & Taglialatela-Scafati, O. (2011). Cannabinoids: Occurrence and Medicinal Chemistry. Current Medicinal Chemistry, 18(7), 1085–1099. https://doi.org/10.2174/092986711795029660
- Andre, C. M., Hausman, J. F., & Guerriero, G. (2016). Cannabis sativa: The Plant of the Thousand and One Molecules. Frontiers in Plant Science, 7, 19. https://doi.org/10.3389/fpls.2016.00019
- Russo, E. B. (2011). Taming THC: potential cannabis synergy and phytocannabinoid-terpenoid entourage effects. British Journal of Pharmacology, 163(7), 1344–1364. https://doi.org/10.1111/j.1476-5381.2011.01238.x
- Senneff, M. (2023). The List of Cannabis Cannabinoids. CannabidiolCBD.org. Retrieved from:https://cannabidiolcbd.org/the-list-of-cannabis-cannabinoids/
- U.S. Food & Drug Administration (FDA). (2023). Scientific Data and Information about Products Containing Cannabis or Cannabis-Derived Compounds. Retrieved from:https://www.fda.gov
- University of Mississippi – National Center for Natural Products Research (NCNPR). Cannabis Research. Retrieved from: https://pharmacy.olemiss.edu/ncnpr/