Over the past few decades, cannabinoids, once subjects of criminalization, have gradually transformed into a focus of intense scientific interest. Amid the limitations of classical pharmacotherapeutic approaches, molecules derived from Cannabis sativa L. have begun to be explored as promising agents for targeted treatment of complex, multi-etiological conditions, such as pharmacoresistant epilepsy, autism spectrum disorders (ASD), neurodegenerative diseases, and various forms of chronic pain. However, despite the success of CBD (cannabidiol) as the first officially recognized therapeutic agent, most other cannabinoids remain on the periphery of scientific development.
The new focus of research is shifting from the study of major cannabinoids (Δ9-THC and CBD) to minor, less explored molecules, such as cannabidivarin (CBDV), cannabidivarinic acid (CBDVA), and isomers like Cannabielsoic Acid B (CBDA-B). These compounds, distinct in their structure and mechanisms of action, are promising to expand the existing pharmacological toolkit by interacting with alternative biological targets, such as transient receptor potential (TRP) channels, PPAR receptors, and the serotoninergic system. Such interactions open new therapeutic possibilities, particularly for modulating neuroregenerative processes, regulating inflammatory cascades, and providing neuroprotection.
A key challenge remains the biotechnological production of rare cannabinoids in sufficient quantities for research and clinical application. Traditional extraction methods require large amounts of raw materials and carry the risk of degradation of the acidic forms of molecules. Innovative approaches, such as the genetic modification of Saccharomyces cerevisiae for cannabinoid biosynthesis, already offer alternatives to agricultural production, potentially shifting the balance of availability for these substances.
At the same time, there is a growing need to revise the methodology for cannabinoid preparation: purity of substances, determination of stereochemistry, and stability control in pharmaceutical forms. Without these changes, it will be impossible to advance lesser-known cannabinoids to the level of real pharmaceutical drugs.
Chemical Structure and Biogenesis of CBDV
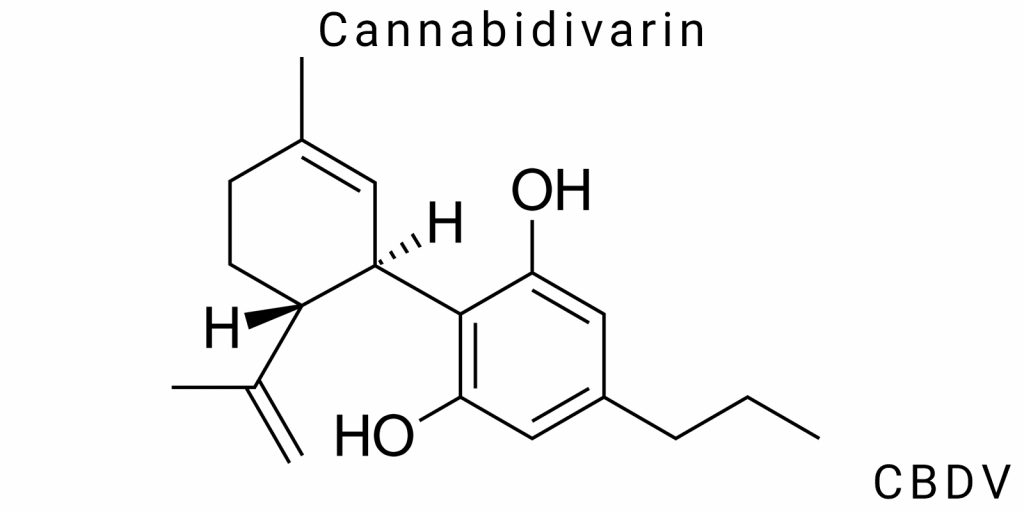
Chemical Structure of Cannabidivarin
Cannabidivarin (CBDV) is a natural bioactive compound from the group of phytocannabinoids, with its chemical identity determined by a key modification in the alkyl chain: the presence of a three-carbon (propyl) radical instead of the typical five-carbon (pentyl) group found in cannabidiol (CBD). This substitution creates a distinct chemical microenvironment within the molecule and defines its unique properties in terms of interaction with biological targets.
The molecular formula of cannabidivarin is C₁₉H₂₆O₂, with a molecular mass of 286.41 g/mol. The core structure consists of an aromatic benzene ring, where hydroxyl groups (–OH) are located at positions 1 and 3, providing the molecule with polar characteristics and the ability to form hydrogen bonds in biological environments.
The cyclic part of the molecule features a modified monoterpene fragment (a cyclohexene backbone), which is linked to the benzene ring through a conjugated system of double bonds. Particular attention is given to the isoprene portion, which includes a double bond in the prop-1-en-2-yl group, oriented in such a way as to minimize steric hindrance and optimize interaction with receptor structures.
X-ray crystallographic analysis of CBDV crystals has shown stable satellite packing of molecules through intermolecular hydrogen bonds between the hydroxyl groups of neighboring molecules. This architecture may explain the relatively high stability of CBDV in its solid state compared to some other cannabinoids. Additionally, it has been observed that at physiological temperatures, the molecule maintains a specific semi-flat conformation, potentially enhancing its ability for passive diffusion across cell membranes.
The presence of a shorter propyl chain affects the electron density distribution along the molecule: spectroscopic studies (NMR, FT-IR) showed shifts in characteristic peaks compared to CBD, particularly in the C–C and C–O bond regions, indicating altered polarization of the molecule and its potential to participate in non-covalent interactions.
Electronic models (using density functional theory, DFT) confirm that the propyl version has a lowered energy barrier for rotation around certain σ-bonds, making CBDV more flexible in dynamic biological environments (plasma, intercellular fluid) compared to the more rigid CBD structure.
It is also important to note that CBDV, having a smaller molecular polar surface area (PSA) compared to CBD, demonstrates improved pharmacokinetic behavior, particularly in terms of absorption through the intestinal epithelium.
Biosynthetic Origin of Cannabidivarin
Formation of Major Precursors
The biogenesis of cannabidivarin (CBDV) is based on an alternative metabolic branch of the classic cannabinoid pathway in Cannabis sativa. The primary source for cannabinoid synthesis is polyketide compounds, specifically olivetolic acid and its varin form. The classical pathway uses hexanoyl-CoA, which leads to the formation of pentyl derivatives. In contrast, the key starting substrate for the varin series is butyryl-CoA. This difference results in the formation of a shorter, propyl side chain in the structure of the final cannabinoids.
The condensation of butyryl-CoA with three malonyl-CoA molecules, catalyzed by a specific polyketide synthase, leads to the formation of varin olivetolic acid. Further prenylation with geranyl pyrophosphate, mediated by a variant of CBG synthase, forms cannabigerovarinic acid (CBGVA), which is a universal precursor for the formation of both cannabidivarinic acid (CBDVA) and tetrahydrocannabidivarinic acid (THCVA).
Synthetase Specificity and Enzymatic Features
The conversion of CBGVA into CBDVA is catalyzed by enzymes that are mutant or variant versions of the classic CBDA synthase. These synthases exhibit a changed spatial organization of the active site, providing higher affinity for short-chain substrates. Structural biological studies have shown that the substitution of only a few amino acids in the active site can radically change the enzyme’s selectivity in favor of the varin series.
There are also data suggesting the activity of hybrid synthases capable of producing both pentyl and propyl products depending on substrate availability within plant tissues. This enzymatic plasticity allows breeding programs to create high-CBDV content strains without the need for genetic engineering.
Decarboxylation and Stability of CBDVA
CBDVA, formed in plant tissues, is an unstable molecule and undergoes decarboxylation under the influence of temperature, light, or prolonged storage. Decarboxylation is a chemical reaction that eliminates the carboxyl group in the form of CO₂, converting CBDVA into the active CBDV.
The natural decarboxylation time depends on environmental humidity, temperature, and the degree of biomass grinding: the higher the surface area in contact with air, the faster the reaction occurs. According to laboratory data, heating plant material to 110–120°C results in decarboxylation of CBDVA into CBDV with over 90% efficiency within 30 minutes.
Genetic and Environmental Factors
The genetic basis for CBDV production is linked to allelic variations in the genes encoding CBDA synthase and CBG synthase. Plants with high CBDV content possess specific mutations that facilitate more efficient prenylation of short-chain precursors. Certain wild populations of cannabis from Asia (especially from northern Thailand and southern China regions) demonstrate natural selection favoring the varin biosynthetic pathway.
Climatic conditions significantly influence the ratio of pentyl to propyl cannabinoids. Studies have shown that increased ultraviolet (UV) radiation, particularly UV-B, stimulates the activity of the polyketide complex, increasing the concentration of varin compounds. Furthermore, nitrogen deficiency in the soil correlates with an increased proportion of short-chain products among cannabinoids.
Biotechnological Prospects
A deep understanding of the biogenesis of CBDV has laid the foundation for the development of biotechnological strategies to optimize its production. Genetically edited Cannabis sativa lines, created using CRISPR-Cas9 technology, enable the induction of overexpression of varin-specific synthases. An alternative approach is the synthesis of CBDV in heterologous systems, such as Saccharomyces cerevisiae or Escherichia coli, through the integration of genetic constructs encoding the enzymes of the complete biosynthetic cascade.
Industry prospects include the creation of cannabis strains that produce high levels of CBDV through modification of enzymatic cascades or by optimizing agronomic conditions for growing to stimulate the varin metabolic branch.
Natural Sources: The Role of Genetic Mutations in Cannabis
The Impact of Genetic Mutations on the Cannabinoid Profile of Cannabis
Genetic mutations play a crucial role in shaping the cannabinoid profile of Cannabis sativa. Studies of the cannabis genome and the molecular mechanisms of cannabinoid biosynthesis demonstrate that mutations in genes encoding cannabinoid synthases determine not only species diversity but also the plant’s ability to synthesize different classes of cannabinoids, including varin-type compounds such as CBDV, which is the primary focus of this topic.
Genetic Modifications and Variations in Cannabinoid Synthases
Natural mutations, particularly single nucleotide polymorphisms (SNPs), can alter the structure of the active sites of the enzymes responsible for cannabinoid synthesis. In the cannabidivarin series, mutations in the genes encoding CBDA synthase and THCA synthase are especially important, enabling plants to produce cannabinoid variants with a short propyl side chain instead of the standard pentyl chain. Differences in these genes result in the formation of distinct classes of cannabinoids, which may possess significantly different biological activities.
Research conducted on various cannabis strains has shown that mutations in the active site of CBDA synthase alter its efficiency in interacting with cannabinoid precursors. In cannabis producing high levels of CBDV, specific mutations have been identified in the genes responsible for the synthesis of cannabidivarinic acid (CBDVA). This indicates the presence of specific alleles favoring the formation of varin-type cannabinoids over the traditional pentyl forms such as CBD or THC.
Genetic Mutations in Natural Cannabis Populations
Genetic mutations leading to an increased CBDV content are often found in natural populations of cannabis growing in isolated or extreme environments. These plants are characterized by the accumulation of mutations within gene pools where there is limited crossbreeding with other populations. This allows such mutations to become stable under local conditions. For instance, in regions of East Asia and the southwestern United States, wild cannabis specimens exhibiting high levels of CBDV have been documented, resulting from local mutations and adaptations to climatic conditions.
Such plants typically preserve variations in cannabinoid synthase genes that are transmitted to subsequent generations. These variations may result from natural selection, promoting survival under conditions of climatic stress or pathogen pressure. For example, in mountainous regions with high solar radiation intensity, cannabis plants with elevated levels of varin-type cannabinoids may exhibit greater resilience to ultraviolet exposure and drought.
Mechanism of Evolution of the Cannabinoid Pathway
The evolution of the cannabinoid metabolic pathway in cannabis plants is the result of a prolonged adaptation process to changing environmental conditions. One adaptation mechanism involves the activation of alternative cannabinoid biosynthetic pathways. For instance, plants growing under extreme conditions may activate genetic variants that lead to the preferential synthesis of varin-type cannabinoids (such as CBDV), thus enhancing stress resistance.
Studies of the phytocannabinoid system in cannabis, conducted through phylogenetic methods, have shown that such adaptive mutations are the outcome of long-term environmental pressures. For example, in areas with high ultraviolet radiation activity, cannabis rich in varin cannabinoids tends to suffer less UV-B damage, likely because varin cannabinoids possess stronger antioxidant properties.
Breeding and Modification of Cannabinoid Pathways
Breeding programs aimed at developing cannabis varieties with high CBDV content utilize both natural mutations and genetic engineering methods. By introducing point mutations into the genes encoding cannabinoid synthases, it is possible to create plants with a desired cannabinoid profile without the use of chemical reagents or synthetic processes. However, such technologies require careful monitoring and study of potential ecological and evolutionary consequences.
One promising direction is the development of genetically modified cannabis plants utilizing specific genetic variants of cannabinoid synthases to enhance the concentration of varin-type cannabinoids. Laboratory trials of such plants have demonstrated a significant increase in CBDV production efficiency through optimization of enzymatic pathways. Additionally, these strains have potential for cultivation in regions with challenging agro-climatic conditions.
Thus, cannabis breeding aimed at increasing CBDV content represents an innovative strategy based on genetic modifications, with implications for both the pharmaceutical and agricultural industries. Considering the regulatory features of metabolic pathways in plants, such genetic approaches could be applied to the development of new cannabis varieties with improved properties and enhanced stress resistance.
Methods of Obtaining Cannabidivarin: From Classical to Biotechnological Approaches
The production of cannabidivarin (CBDV) and other cannabinoids can be accomplished through various methods, including classical extraction techniques from plant materials and more innovative biotechnological approaches. The choice of method depends on many factors: the targeted outcome, variations in cannabinoid composition, economic feasibility, and regulatory restrictions. Let us examine the main approaches to obtaining CBDV, starting with traditional methods and moving toward modern biotechnological solutions that promise significantly more efficient results in the future.
Classical Methods of CBDV Extraction
Traditional methods of obtaining CBDV largely rely on the extraction of cannabinoids from plant materials. Since CBDV is present in low concentrations in cannabis plants, extraction often includes several stages of purification and concentration.
Solvent Extraction
One of the most common methods is extraction using organic solvents such as ethanol, butane, or CO₂ (in its liquid state). The method utilizing organic solvents is simple and accessible but requires additional purification steps to remove traces of solvents. In some cases, this can lead to the formation of toxic residues, making it crucial to employ purification methods that ensure the safety of the product.
During ethanol extraction, plants are ground and immersed in an ethanol solution, after which the mixture is heated to evaporate the solvent, leaving behind a concentrate of cannabinoids, including CBDV. This method allows for the isolation of samples with a high concentration of cannabinoids but requires further steps to remove unwanted substances such as chlorophyll and waxes.
Supercritical CO₂ Extraction
Supercritical CO₂ extraction is a more complex yet more efficient method for obtaining pure cannabinoids, including CBDV. This process utilizes supercritical conditions (temperature and pressure) that allow CO₂ to act as a solvent, leaving no toxic residues. This is particularly important because the method enables the production of highly pure cannabinoids with minimal loss of active components while preserving their biological activity.
This method is widely used in the pharmaceutical and cosmetic industries to obtain high-quality extracts. However, its high cost and complexity make it less accessible for small producers.
Steam Distillation
Steam distillation is an older and well-known method traditionally used for the purification of essential oils. In the context of cannabis, it allows for the isolation of cannabinoids by heating plant material to a temperature that promotes the evaporation of active components. Vacuum distillation may be employed to lower the evaporation temperature and prevent the degradation of thermolabile substances such as CBDV. Although this method is fairly effective, it requires precise temperature control and optimal conditions to obtain a high-quality end product.
Solid Phase Extraction (SPE)
The solid-phase extraction (SPE) method reduces the amount of organic solvents required for extraction. Using this method, cannabinoids can be concentrated on a solid phase and then extracted using appropriate eluents. This method is mainly used for the separation and purification of cannabinoids following primary extraction.
Biotechnological Methods of CBDV Production
Thanks to advances in biotechnology, new methods for obtaining CBDV have emerged, significantly reducing costs and improving the quality of the final product.
Genetic Engineering and Microbiological Approaches
One of the most promising ways to produce CBDV is the use of genetic engineering to introduce cannabinoid synthase genes into microorganisms. The application of bacteria and yeast for cannabinoid synthesis is actively being researched within the framework of synthetic biology. Researchers introduce genes encoding cannabinoid synthases (CBDV synthase, CBGVA synthase) into the genomes of yeasts or bacteria. As a result, these microorganisms are capable of efficiently synthesizing CBDV under laboratory conditions.
This approach offers significant advantages: reduced production costs, the ability to control synthesis conditions, and the minimization of environmental impact. Modern developments also foresee the possibility of scaling this method, opening opportunities for mass production of CBDV for pharmaceutical purposes.
Cell Culture Fermentation
Another approach is the use of cannabis plant cell cultures to obtain CBDV. In this case, cell cultures are used, which can be stimulated to produce specific cannabinoids. It is known that cannabis cells can synthesize CBDV when treated with specific enzymes or growth conditions that stimulate the varin cannabinoid biosynthesis pathway.
This method allows for the efficient cultivation of cannabinoids under controlled conditions without the need to grow entire plants, making it more economically viable for large-scale producers. Moreover, this method significantly reduces the levels of toxic components that could otherwise enter the final product.
Synthetic Biosynthesis
One of the most revolutionary approaches to obtaining CBDV is fully synthetic biosynthesis through the use of complex biochemical reactions. In this case, artificial conditions are created for the conversion of cannabinoid precursors into CBDV through the use of specialized enzymes that catalyze these reactions. Synthetic biosynthesis technologies are becoming increasingly popular due to the ability to produce cannabinoids in pure form without the need for plant extraction.
Synthetic methods allow for complete control over the composition of the cannabinoids produced while reducing negative environmental impacts, since it eliminates the need for large-scale cannabis cultivation. However, this method also requires significant investments in technology development and process optimization.
Pharmacological Mechanisms of CBDV Action via TRP Channels
Cannabidivarin (CBDV) belongs to the class of cannabinoids that exhibit a wide range of pharmacological activities, particularly through interactions with transient receptor potential (TRP) channels. TRP channels constitute a group of ion channels that play a crucial role in a number of the body’s physiological functions, including sensory perception of pain, temperature, mechanical stimuli, as well as inflammatory and thermoregulatory processes. Studying the interaction between CBDV and TRP channels allows for a deeper understanding of its mechanism of action and provides insight into how this cannabinoid may be used for the treatment of a variety of diseases associated with pain, inflammation, and temperature dysregulation.
TRP channels demonstrate tremendous diversity in terms of their functional characteristics. They can open or close in response to external or internal stimuli, such as temperature, mechanical pressure, chemical substances, or changes in the electrical potential of the cell membrane. These channels form the basis of sensory experiences such as pain, temperature, and touch, and they play an important role in sensory and reflex functions. They are also involved in the regulation of ionic balance within cells and organs and thus are critical for maintaining overall homeostasis. Importantly, certain TRP channel subtypes are associated with different pathogenic mechanisms, including pain and inflammation.
Among TRP channels, TRPV1, TRPV2, TRPA1, and TRPM8 play particularly important roles in processes related to both pain and thermoregulation. CBDV shows the ability to modulate these channels, opening new possibilities for its application in the therapy of a range of conditions.
TRPV1 (Transient Receptor Potential Vanilloid 1) is probably the most well-known among TRP channels and is involved in the perception of heat and pain. It is activated by elevated temperature or chemical irritants, particularly molecules such as capsaicin. Activation of this channel promotes the release of neuropeptides, such as substance P, which induce inflammation and painful sensations. CBDV may exert an inhibitory effect on TRPV1, reducing pain sensations and lowering the level of inflammation. This is particularly important for the treatment of chronic pain, which is often associated with neuropathy and inflammatory diseases.
CBDV Interaction with TRPV1: Inhibition of Inflammatory Processes
TRPV1 (Transient Receptor Potential Vanilloid 1) is a crucial target for many therapeutic approaches concerning inflammation and pain. This channel can be activated not only by temperature but also by chemical agents such as capsaicin. Upon activation, these irritants contribute to the release of molecules that trigger pain and inflammatory mechanisms, such as substance P. This process increases the permeability of cell membranes to calcium, resulting in elevated intracellular calcium levels and, consequently, the activation of various inflammatory signaling pathways.
CBDV interacts with TRPV1 by inhibiting its excessive activation. Its ability to modulate TRPV1 activity allows for the reduction of substance P activation and other inflammatory molecules, thereby decreasing pain intensity and limiting the progression of inflammatory processes. This is crucial for treating chronic pain, which is often a result of conditions such as arthritis, fibromyalgia, and nervous system injuries. Studies have shown that TRPV1 activation levels can be significantly elevated in chronic pain, worsening patient outcomes. CBDV’s interaction with this channel may help reduce pain sensitivity and, thus, decrease the need for analgesic medications, which often have serious side effects.
Studying the effects of CBDV on TRPV1 is a vital step toward developing new therapeutic strategies for treating pain, particularly in the context of chronic and inflammatory disorders where conventional treatment methods often fail to produce satisfactory results.
Effect on TRPV2: Modulation of Cellular Proliferation and Regeneration
TRPV2 (Transient Receptor Potential Vanilloid 2) plays a significant role in the regulation of cellular functions, including cell proliferation, ion transport, and mechanical sensitivity. The connection between TRPV2 and cellular activity is critical for the proper functioning of the body, especially in the context of tissue regeneration and recovery after injuries.
CBDV is capable of interacting with TRPV2, modifying its activity and thereby regulating calcium influx into cells. Activation of TRPV2 can lead to elevated calcium flows, which often result in negative cellular outcomes such as oxidative stress or even apoptosis. CBDV’s interaction with TRPV2 helps to mitigate these effects, maintaining cellular stability, which is vital for preventing the development of inflammatory and degenerative diseases.
The impact of CBDV on TRPV2 is particularly important in the context of neurodegenerative diseases. In conditions such as Alzheimer’s disease, Parkinson’s disease, and other forms of neurodegeneration, brain cells are damaged due to disruptions in calcium homeostasis. CBDV may help stabilize these processes, thus reducing cellular damage and promoting neuronal recovery.
Moreover, CBDV may have therapeutic potential in oncological diseases. The ability of TRPV2 to activate anti-apoptotic mechanisms can facilitate cell growth and tumor development; thus, regulating this process through CBDV could become an important strategy in fighting cancer. Modulation of TRPV2 activity allows for the inhibition of anti-apoptotic pathways, which can help prevent the progression of tumor cells and reduce the risk of metastasis.
Overall, CBDV’s interaction with TRPV2 opens new avenues for the treatment of various diseases, particularly neurodegenerative and oncological conditions, where alterations in cellular activity play a critical role in disease development.
Interaction with TRPM8: Cooling Effect
TRPM8 (Transient Receptor Potential Melastatin 8) is a receptor that plays a key role in the perception of cold stimuli and the sensation of cooling. This channel is activated by menthol compounds and other cooling agents such as eucalyptus or xylitol. The activation of TRPM8 not only influences temperature perception but also regulates processes associated with pain, particularly in cases of inflammation and neuropathy. The interaction of cannabidivarin (CBDV) with TRPM8 expands the potential for treating conditions associated with impaired temperature sensitivity, such as thermoregulatory dysfunctions or chronic pain, particularly in patients with neuropathy.
CBDV has the ability to regulate TRPM8 activity, helping to relieve pain arising from pathological temperature sensations. For instance, patients with neuropathy often experience sensations of “icy” or “burning” pain, which can be alleviated through the activation of the TRPM8 channel. Thus, CBDV can create a cooling effect that provides therapeutic benefits for treating conditions where pain is linked to abnormal temperature sensations. Research indicates that TRPM8 activation can not only reduce unpleasant sensations but also lower inflammation levels in tissues, as this activation influences the reduction of the release of pro-inflammatory molecules, such as cytokines.
This effect is particularly important for patients suffering from chronic pain, including pain following injuries or chronic illnesses such as osteoarthritis, fibromyalgia, or thermoregulatory disorders. The interaction of CBDV with TRPM8 opens new possibilities for developing more effective analgesic therapies for patients who have difficulty managing pain with traditional painkillers. These therapies may be less aggressive than opioids or nonsteroidal anti-inflammatory drugs, while still providing consistent pain and inflammation relief.
Due to its ability to reduce temperature in pathological sites, CBDV may also be used to alleviate symptoms such as itching or discomfort arising from dermatological conditions or neuropathic disorders. This creates new opportunities for treating pain associated with temperature sensitivity disorders, such as Raynaud’s syndrome or post-surgical conditions involving altered thermoregulation.
Mechanisms of Action on Inflammation and Pain
CBDV actively influences several types of TRP channels, including TRPV1, TRPV2, TRPA1, and TRPM8, enabling it to flexibly modulate various aspects of inflammation and pain. In the case of the TRPV1 channel, which is a major receptor for pain perception, CBDV helps reduce pain sensitivity by inhibiting the excessive activation of this channel, which is especially important in chronic inflammatory diseases such as arthritis. By inhibiting TRPV1, CBDV helps reduce the release of pro-inflammatory neuropeptides, such as substance P, which actively contribute to the development of pain.
Research also shows that CBDV can modulate TRPV2 activity, which is critical for treating neurodegenerative diseases such as Parkinson’s or Alzheimer’s disease. The activity of this channel can lead to excessive calcium accumulation in cells, resulting in cell damage and death. CBDV helps to prevent this by reducing calcium influx, which is crucial for preventing cellular damage that leads to chronic inflammatory processes and neurodegeneration.
Regarding TRPA1, its activation promotes the development of inflammation, particularly as a result of chemical irritants such as acrolein, which can cause pain and swelling following injuries or in chronic conditions. CBDV, by inhibiting TRPA1, helps reduce pain caused by chemical irritants and facilitates the treatment of inflammatory states such as arthritis, migraines, and various forms of neuropathy.
All these mechanisms of CBDV interaction with TRP channels form the basis for developing new therapeutic approaches in the treatment of various forms of pain and inflammation. Modulating the activity of these channels allows for the treatment not only of pain but also of inflammatory processes arising from injuries, chronic illnesses, or even some autoimmune disorders. Systematic application of CBDV could lead to improved quality of life for patients by allowing for more precise and effective regulation of pain sensations associated with diverse pathogenic processes.
Clinical Studies: Focus on Rare Diseases
Clinical Studies of CBDV in Epilepsy
Epilepsy is one of the most common neurological disorders, characterized by recurrent seizures resulting from abnormal electrical activity in the brain. For many patients with epilepsy, standard antiepileptic drugs do not always provide the desired effect, especially in treating rare or drug-resistant forms of epilepsy such as Dravet syndrome or Lennox-Gastaut syndrome. In these cases, cannabinoids, particularly cannabidivarin (CBDV), are attracting increasing attention due to their potential therapeutic properties.
CBDV has demonstrated promising results in clinical studies, particularly in treating forms of epilepsy that are resistant to conventional therapies. One of the most significant studies is a clinical trial conducted on patients with Dravet syndrome. This study showed that CBDV can reduce seizure frequency in patients for whom traditional medications are ineffective. Among the mechanisms through which CBDV may be effective is its influence on glutamate receptors and ion channels, contributing to the stabilization of neuronal activity and decreasing the propensity for seizures.
An important aspect is CBDV’s ability to reduce neuronal hyperactivity, which is a major cause of epileptic seizures. Studies have shown that CBDV can help regulate neuroplasticity and reduce the excessive excitability of neurons. This makes CBDV a promising agent for the treatment of both typical and rare forms of epilepsy that are resistant to standard therapeutic methods.
Thanks to its properties, CBDV is also a valuable tool in treating pediatric patients. Children suffering from forms of epilepsy that do not respond to conventional treatments can greatly benefit from the use of CBDV, as it has fewer side effects compared to traditional antiepileptic drugs such as phenobarbital or phenytoin. One of the key aspects is the ability to control symptoms without the need for aggressive pharmacological therapy, preserving the quality of life for children.
Clinical studies also highlight that CBDV has limited toxicity even with prolonged use. This makes it a promising candidate for long-term therapy for epilepsy, particularly in combination with other antiepileptic agents.
CBDV in Autism Spectrum Disorders (ASD)
Autism spectrum disorders (ASD) are a group of neurological conditions characterized by impairments in social interaction, communication difficulties, and restricted or repetitive patterns of behavior. Treatment of ASD is complex and often involves a combination of medication and therapeutic interventions, such as behavioral therapy. However, research shows that cannabidivarin (CBDV) holds great potential in alleviating symptoms associated with ASD and could become an important tool in the management of this condition.
Animal studies have shown that CBDV can alter the function of neural networks, leading to improved social behavior and a reduction in symptoms such as stereotypic movements and behavioral disorders commonly observed in patients with autism. Based on these findings, a number of clinical trials in humans have been initiated to explore the use of CBDV in children with ASD.
One of the primary mechanisms through which CBDV may ease symptoms of ASD is its ability to affect neuroplasticity and synaptic connections. This cannabinoid has the potential to regulate neurotransmission in the brain, which can reduce aggressive behavior and enhance social interaction capabilities in patients with autism. Studies have indicated that CBDV can lower anxiety levels and improve cognitive function in patients, both of which are crucial aspects of autism management.
It is known that individuals with ASD often present with comorbidities such as epilepsy or hyperactivity, for which CBDV may also be an effective treatment. Research confirms that CBDV exerts a significant therapeutic effect in reducing seizure frequency in autistic patients suffering from this comorbidity.
Importantly, CBDV does not produce significant side effects and lacks psychoactive properties, making it safe for use in children and appropriate for long-term treatment. Thanks to its high safety profile and ability to alleviate symptoms such as stereotypic behavior, anxiety, aggressive actions, and even seizures, CBDV could become an essential component of comprehensive ASD treatment, especially when traditional methods fail to yield the desired results.
Ongoing clinical studies, particularly those aimed at determining optimal dosing and long-term effects, will further clarify CBDV’s potential applications in autism spectrum disorders. However, it is already clear that CBDV has the potential to become a vital tool in autism therapy, significantly improving the quality of life for individuals with ASD and their families.
Regulatory Prospects for CBDV
Potential International Regulatory Status of CBDV
Progress in understanding the pharmacological properties of cannabidivarin (CBDV) and its promising potential in treating several serious diseases, such as epilepsy and autism spectrum disorders, is driving increased efforts to establish the regulatory status of this compound in various countries. However, the legal status of CBDV remains a subject of significant debate and varies depending on the jurisdiction.
In the United States, cannabinoid regulation is undergoing evolution. In 2018, the Food and Drug Administration (FDA) approved the use of the drug Epidiolex (which contains CBD) for the treatment of certain forms of epilepsy, including Dravet syndrome and Lennox-Gastaut syndrome. This milestone marked the first step toward creating a legal framework for cannabinoids. However, CBDV has not yet received the same regulatory status. Nevertheless, several companies have already completed clinical trials based on CBDV for the treatment of specific diseases, potentially paving the way for future approvals.
In the European Union, the situation is similar. At the EU level, cannabinoids such as CBD are regulated in the context of products that do not contain psychoactive substances. The status of CBDV is currently under evaluation. Although some countries, such as the United Kingdom and Germany, have already permitted the use of cannabinoids for medical purposes, regulatory authorities must further study the pharmacological properties of CBDV and its medical indications.
One of the major challenges related to the regulation of CBDV stems from the fact that cannabidivarin originates from the same plant source as THC and CBD-cannabis plants. Therefore, for many countries, regulating CBDV faces legal obstacles related to legislative restrictions on cannabis plants. Nevertheless, positive results from clinical trials and increasing support from scientific and medical communities could drive changes in regulatory approaches.
Challenges and the Future of CBDV’s Regulatory Status
Regulatory agencies such as the FDA and the European Medicines Agency (EMA) face numerous challenges in defining clear guidelines for cannabinoids, particularly CBDV. One of the primary difficulties is establishing standard and rigorous criteria for evaluating the safety and efficacy of CBDV in global medical practice. Cannabidivarin must be thoroughly studied regarding potential side effects, especially during long-term use.
Understanding how CBDV interacts with other medications, as well as its long-term efficacy in treating chronic diseases, is equally critical. Should these questions be resolved positively, it could lay the foundation for the widespread use of CBDV in medical practice.
Another key aspect is the need to standardize the production process for CBDV. Considering that this cannabinoid is found in specific strains of hemp, ensuring the purity and consistency of product composition is necessary to maintain high-quality standards. Innovative and efficient methods for extracting and testing CBDV will require significant investments in technology and standardization processes.
Moreover, many countries have fragmented legal frameworks regarding cannabinoids, which creates barriers to global access to CBDV-based products. In some countries, such as Canada and the Netherlands, legislation already allows the use of cannabinoids for medical purposes, and CBDV could potentially be added to this list in the future. In other countries, such as China and Australia, the legal status of cannabinoids remains more restrictive, and the process of adopting regulatory frameworks may take longer.
Considering all the above, it can be concluded that the prospects for the regulatory status of CBDV are very promising; however, significant legal and scientific challenges remain on the path toward its widespread use in medical practice. A deeper understanding of CBDV’s pharmacological properties, combined with the continued development of clinical research, is likely to enable this cannabinoid to secure a more defined place within the legislative frameworks of various countries in the coming years.
Conclusion
Cannabidivarin (CBDV) represents significant scientific and therapeutic value among cannabinoids due to its potential healing properties, particularly in the context of rare and complex diseases such as epilepsy and autism spectrum disorders (ASD). Its mechanism of action through interaction with transient receptor potential channels (TRP channels), specifically TRPV1, TRPV2, TRPA1, and TRPM8, enables the reduction of pain, alleviation of inflammation, and correction of thermoregulatory dysfunctions. These properties assign CBDV a special importance in the treatment of conditions such as chronic pain, inflammatory diseases, and neuropathies.
A major achievement in the development of CBDV has been the results of clinical studies demonstrating its effectiveness in treating rare and complex diseases. In particular, among patients with epilepsy, especially in pediatric cases, there has been a noted reduction in the frequency of seizure episodes, while patients with autism spectrum disorders have shown improvements in behavioral responses and social skills. The therapeutic effect of CBDV is the result of its ability to modulate the nervous system, reducing neuropeptide hyperactivity and enhancing neuronal function.
However, despite the promising results, the scientific understanding of the mechanisms of action of CBDV requires further research to ensure its safety and long-term efficacy. Specifically, additional clinical trials are necessary to assess possible side effects and optimal dosages, as well as to determine the duration of its impact on the human body.
Regarding its regulatory status, CBDV is currently under active study and evaluation in many countries. Although some nations have already begun to approve cannabinoids for medical purposes, CBDV faces legal and regulatory challenges due to its origin from the cannabis plant. Nevertheless, considering changes in legislation and the growing interest in the therapeutic properties of cannabinoids, it can be expected that CBDV will become more accessible for medical use in the future.
The prospects for the use of CBDV in treating rare diseases appear very promising, and this cannabinoid has real potential to change approaches to the treatment of a range of complex and chronic conditions. However, for its widespread application, further scientific research is required, as well as the harmonization of regulatory standards to ensure the safety and efficacy of CBDV’s use in medical practice.
In summary, CBDV could become an important component of future therapeutic practice, particularly in the treatment of diseases for which current treatment methods are insufficiently effective. However, to fully unlock its potential, a series of scientific studies must still be conducted, and existing legal barriers must be overcome.
Sources:
- National Institute on Drug Abuse (NIDA) – Cannabinoids and their impact on health, including CBDV.
https://nida.nih.gov - PubMed Central (PMC) – Scientific articles related to the pharmacology of cannabidivarin and its clinical applications, particularly in epilepsy and neuropathies.
https://pubmed.ncbi.nlm.nih.gov - World Health Organization (WHO) – Documentation and research on the medical applications of cannabinoids, including CBDV, for the treatment of various diseases.
https://www.who.int - Journal of Clinical Investigation – Clinical studies on the use of cannabidivarin in treating rare diseases such as epilepsy and autism spectrum disorders.
https://www.jci.org - European Medicines Agency (EMA) – Documentation on the regulatory status of cannabinoids in Europe, including CBDV.
https://www.ema.europa.eu - U.S. Food and Drug Administration (FDA) – Assessment of the safety and efficacy of cannabinoids for medical purposes, including cannabidivarin.
https://www.fda.gov - Cannabis and Cannabinoid Research Journal – Scientific studies and reviews examining the effects of cannabidivarin on various biological systems.
https://www.liebertpub.com/can - British Journal of Pharmacology – Research into the mechanisms of action of cannabinoids and their potential therapeutic effects.
https://bpspubs.onlinelibrary.wiley.com/journal/14765381