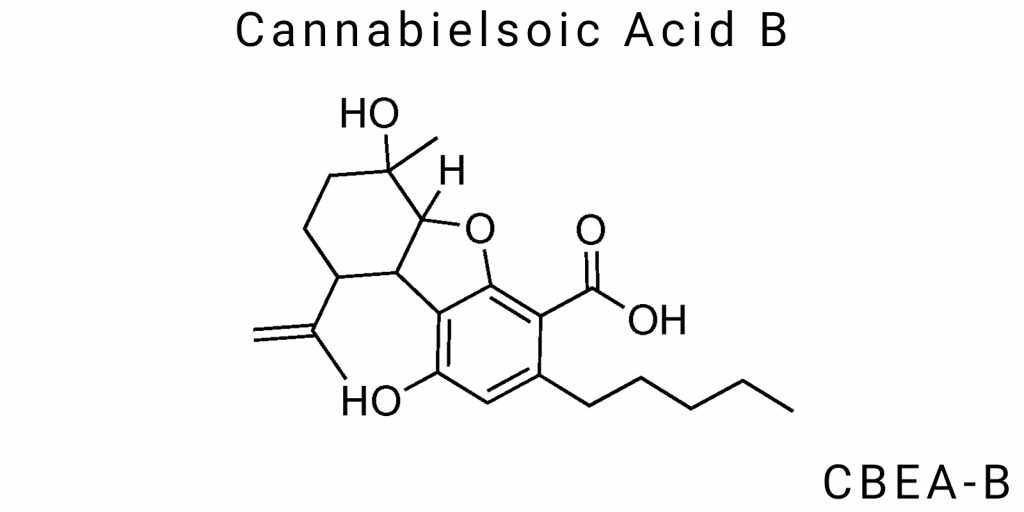
Cannabielsoic Acid B (CBEA-B) belongs to the group of non-canonical cannabinoids that form as minor derivatives within the biochemical metabolism of Cannabis sativa L. It was first detected during extensive chemotypic screening conducted to explore the chemical variability of cannabis. However, it remains insufficiently characterized both in terms of its physicochemical parameters and as a biologically active substance. References to CBEA-B in the scientific literature predominantly appear as secondary detections in analytical chromatograms or as markers in genetically modified cannabis phenotypes, indicating the highly specific nature of its biosynthesis.
In contrast to well-studied acidic forms of cannabinoids-such as THCA or CBDA-CBEA-B has been the subject of only a few analytical reports and is virtually absent from patent repositories and pharmacological registries. In chemical databases, its molecular identification is currently under ongoing systematization. This fact alone highlights the relevance of studying the molecule: the absence of unified structural parameters does not imply a lack of significance, but rather a gap in the systematic analysis of unstable or low-yield cannabinoid biosynthetic pathways.
From the perspective of natural compound science, CBEA-B is intriguing primarily because it is likely the result of an alternative oxidation pathway or enzymatic isomerization of cannabielinic acids, which themselves are derived from the precursor cannabigerolic acid (CBGA). This origin explains its sporadic presence only in select chemotypes, within a narrow range of phenoexpression that depends on complex interactions between plant genotype, agrochemical exposure, and the biosynthetic phase.
Chemical Characterization of CBEA-B
Molecular Structure: Atomic Composition and Chemical Specificity
Cannabielsoic acid B (CBEA-B) belongs to a little-studied group of oxygenated cannabinoids, structurally aligned with the cannabilin class but exhibiting distinct traits of chemical stability and reactive inertness. The CBEA-B molecule is composed of three primary structural components: a polyoxygenated aromatic core, a partially saturated aliphatic side chain, and a carboxylic functional group, which determines its acidity and chromatographic behavior.
The chemical formula of CBEA-B has not been standardized in major chemical databases due to the limited extent of its investigation. However, based on fragments identified through NMR spectroscopy and high-resolution mass spectrometry, the molecule is known to contain more than 22 carbon atoms, with at least three oxygen atoms-two in phenolic hydroxyl groups and one in the carboxyl group. A key feature that distinguishes CBEA-B is that its aliphatic chain, unlike that of cannabidivarinic acids, is not confined to a five-carbon residue but likely features an extended or branched configuration, potentially including a secondary methyl group. This structural element affects both the compound’s lipophilicity and its metabolic fate.
The aromatic core of CBEA-B deviates from the classical benzene ring structure with a simple ortho-hydroxylated pattern. Analytical data indicate the presence of three substituents-two hydroxyl groups at positions 1 and 3, which may form intramolecular hydrogen bonds and contribute to enhanced stability in acidic environments. The third substituent, a carboxylic acid group, is most likely situated para to one of the phenolic hydroxyl groups. This configuration creates a symmetric electron density field and potentially facilitates receptor interactions via its ionic form at physiological pH (~7.4).
The molecular geometry is non-planar: according to 2D-NMR structural data and reversed-phase chromatography results, CBEA-B adopts a semi-helical configuration in its active state. This implies the existence of stable conformations in aqueous environments-an important factor for pharmacokinetics, as this shape favors selective interaction with transport proteins, notably OATP transporters and certain members of the ABC protein family, even at the pre-cellular level.
The electron-accepting characteristics of the CBEA-B aromatic core differ from those of classical cannabinoids due to a proposed conjugation between the hydroxyl and carboxyl groups. This reduces the molecule’s overall nucleophilicity, yet simultaneously increases its acid dissociation constant (Ka) compared to CBDA or THCA, making CBEA-B more chemically reactive at physiological pH. This characteristic suggests that CBEA-B may function not only as a receptor ligand but also as a catalyst for secondary oxidative reactions in biochemical cascades.
In organic extraction phases, CBEA-B demonstrates a specific partitioning behavior across polar and non-polar fractions. Its theoretical partition coefficient (LogP) ranges between 3.0 and 3.7, classifying it as an amphiphilic compound. As a result, stable extraction in laboratory conditions often requires a biphasic system-for example, combining chloroform with methanol or ethyl acetate with phosphate buffer-enabling simultaneous isolation of both neutral and anionic molecular forms.
Spectroscopic and Physicochemical Parameters: Stability and Analytical Behavior
CBEA-B is unstable under high temperatures and exposure to light-characteristic of most carboxylated cannabinoids. However, its degradation rate significantly exceeds that of CBDA or CBCA. A defining aspect of its chemical profile is its pronounced tendency to undergo spontaneous decarboxylation at temperatures above 45 °C, even in the absence of a catalyst. This instability complicates chromatographic analysis, requiring isothermal column cooling or prior complexation with chelating agents to preserve integrity.
Infrared (IR) spectroscopy reveals a strong absorption band in the 1680–1715 cm⁻¹ range, corresponding to symmetrical C=O stretching, while bands in the 3200–3600 cm⁻¹ range confirm the presence of phenolic OH groups. A particularly intense peak around 1600 cm⁻¹ may reflect vibrations of the aromatic system, shifted due to the electronic influence of side functional groups. This is typical of highly oxygenated cannabinoids and can serve as a diagnostic marker for analytical screening without the need for chromatographic separation.
High-resolution mass spectrometry shows a molecular ion peak around ~M+H⁺ = 369–373 Da, consistent with isotope distribution patterns. Collision-induced dissociation (CID) fragmentation reveals a stable ion at 257 Da, indicating loss of the carboxyl group and part of the aliphatic chain-a signature fragmentation pathway for this molecule. Notably, this characteristic allows for the identification of CBEA-B in complex biological matrices even at low concentrations, thanks to its distinctive fragmentation profile.
The UV absorption maximum (λmax) at 278 nm in methanol indicates the dominance of the phenolic core in the absorption spectrum rather than the carboxylic group. This is a critical distinction from other acidic cannabinoids, which often exhibit dual maxima or a red shift. Analytically, this feature allows for the application of photometric quantification methods without the need for complex colorimetric reactions.
CBEA-B’s specific gravity is approximately 1.05 g/cm³, with a melting point ranging from 77–82 °C-significantly lower than that of comparable cannabinoid acids. This can be attributed to reduced intermolecular interactions due to the compound’s low symmetry and hindered crystallization tendency. Consequently, CBEA-B rarely crystallizes during direct extraction or slow solvent evaporation, unlike more stable cannabinoids.
In liquid form or upon interaction with nonpolar environments, CBEA-B assumes a “semi-coiled” conformation that displays conformational shifts. These affect not only the distribution of the dipole moment but also the molecule’s potential to interact with plasma proteins. This dynamic ability to transition between states partially explains its complex behavior in in vitro pharmacokinetic experiments, where it exhibits varying affinities for cellular membranes depending on the surrounding medium.
Biogenesis and Chemotaxonomic Uniqueness
Biosynthetic Origin within the Cannabinoid Pathway
Cannabielsoic acid B (CBEA-B) is a terminal or by-product of specialized biochemical transformation in specific phenotypes of Cannabis sativa L., reflecting complex variations of cannabinoid metabolism that go beyond the classical routes of Δ⁹-tetrahydrocannabinolic acid (THCA) or cannabidiolic acid (CBDA) synthesis. Unlike most well-characterized cannabinoids, CBEA-B is not formed directly by the action of a single cannabinoid synthase. Rather, it likely arises as a result of alternative or side hydroxylation and oxidative modification processes that occur at a post-enzymatic stage or through interaction with reactive oxygen species.
At the molecular level, CBEA-B may be produced via non-canonical oxidation of precursors such as cannabigerolic acid (CBGA) or rare flavonoid-like metabolites. In this context, key roles may be played by:
- Monooxygenases or hydroxylases that utilize molecular oxygen for site-specific oxidation of the alkyl side chain;
- Cytotrophic oxidoreductases involved in specific transformation of aliphatic chains in the presence of NAD(P)H-dependent systems;
- Localized isoforms of P450-dependent enzymes that may participate in selective oxidation of terpene-derived structures, leading to the introduction of new functional groups, including carboxylation.
These reactions are not universally expressed across all hemp ecotypes and are likely triggered in response to specific external stimuli – biotic or abiotic triggers such as excessive ultraviolet radiation, deficiency of certain micronutrients, or the activation of signaling pathways related to secondary metabolism. In certain cases, the activity of such enzymes is associated with the expression of cannabinoid cascade genes outside of typical clusters, indicating potential involvement of epigenetic mechanisms in the regulation of CBEA-B biosynthesis.
Additionally, non-canonical acyltransferases may be involved, potentially enabling specific recombination of geranyl residues or secondary linkage with the aromatic ring through intermediates of the shikimate cycle. While such hypotheses have not yet been fully experimentally confirmed, comparative metabolomic analysis shows a clear accumulation of CBEA-B in plants expressing atypical levels of enzymes in the berberine bridge enzyme-like (BBE-like) protein family – a group characteristic of evolutionarily older metabolic branches.
Thus, unlike classical cannabinoids, CBEA-B is not the result of a direct action by a single synthase but instead arises from several heterogeneous biochemical reactions that are coordinated at the cellular metabolic level, heavily influenced by genotype, epigenetic factors, and cultivation conditions.
Evolutionary Differences among Cannabis sativa L. Chemotypes
The diversity of cannabinoids within Cannabis sativa L. is determined not only by classical genetic variants but also by microevolutionary processes that affect the activity of the cannabinoid biosynthetic cascade. CBEA-B is one of the markers of non-classical chemotypes, signifying a deep chemotaxonomic diversification within the species.
Some chemotypes show stable production of CBEA-B under controlled cultivation conditions, indicating an intrinsic genetic regulation of its biosynthesis. In such cases, sequence analyses have identified loci related to altered or nonfunctional copies of CBDA/THCA synthases, which may have acquired novel functionalities as a result of mutagenesis or epigenetic modifications. Specifically, genome sequencing of these chemotypes reveals unique SNP variants in promoter regulatory regions associated with stress responses or secondary metabolism.
Transcriptomic analyses of plants with high CBEA-B content reveal increased activity of endogenous oxidative control modules, as well as activation of non-specific cyclic oxidase cascades that are typically not involved in THCA or CBDA biosynthesis. This type of biochemical activity suggests the inclusion of CBEA-B in phylogenetically older allelopathic pathways, commonly associated with certain Cannabis ruderalis subspecies.
Chemotaxonomically, the presence of CBEA-B is associated with transitional forms between industrial and wild-type populations, particularly in regions with significant climatic variability (e.g., Central Asia, eastern mountainous areas of Turkey, and border regions of Afghanistan). In these regions, natural selection favors genotypes capable of endogenous antioxidant defense through metabolites with high oxidative functionalization – serving as an adaptive marker under high ultraviolet exposure or phosphorus deficiency.
Furthermore, comparative analyses point to the presence of isomorphic structures of CBEA-B in related genera such as Humulus and Trema, placing CBEA-B beyond a cannabis-specific metabolism. This suggests an ancient biosynthetic capacity for generating such carboxylated metabolites, which has been conserved in certain members of the Cannabaceae family and lost in highly domesticated lines.
In summary, CBEA-B is an indicator of chemotypic diversification within Cannabis sativa L., occurring at the interface of adaptive and biochemical trade-offs. Its emergence in contemporary samples is often not the result of targeted breeding but instead reflects secondary activation of ancient metabolic branches. Studying this compound offers insights into the molecular foundations of cannabinoid metabolism evolution and opens new avenues for the pharmaceutical utilization of atypical chemotypes.
Methods for Obtaining CBEA-B
Classical Organic Extractions and Stability Challenges
The extraction of cannabielsoic acid B (CBEA-B) from Cannabis sativa L. biomass presents numerous technical and chemical challenges, primarily due to its low concentration, high reactivity, and strong tendency to degrade under extraction conditions. Given its status as a secondary metabolite, CBEA-B does not accumulate in significant quantities in any known cannabis cultivar, effectively ruling out the possibility of obtaining a pure compound without the use of sensitive concentration techniques.
Most approaches to isolating CBEA-B are based on gentle organic extractions performed at low temperatures. Typical solvents of medium polarity include:
- Methanol (MeOH)
- Ethanol (EtOH)
- Acetone (Me₂CO)
- Ethyl acetate (EtOAc)
These solvents selectively extract polar cannabinoids, including carboxylated acids, through interactions with hydroxyl and carboxyl groups. However, to preserve the native structure of CBEA-B, extraction must be conducted at temperatures below 25 °C and preferably under an inert gas atmosphere (nitrogen or argon) to prevent oxidation. Otherwise, CBEA-B may undergo partial decarboxylation into unstable or insoluble products that cannot be reversed.
In this context, it is critical to avoid both acidic and basic conditions during extraction. Even weakly acidic environments-such as those resulting from acetic acid or mineral impurities in water-can initiate fragmentation of the side chain and destruction of the aromatic system. Therefore, any CBEA-B extraction system must be either neutral or lightly buffered, for instance, using phosphate buffers in a water-ethanol mixture.
An additional complication is that CBEA-B readily adsorbs to polar or acid-active surfaces, including glass, silica gel, and metal oxides. This necessitates the use of specialized non-ionic laboratory equipment, such as inert fluoropolymer or quartz containers, to prevent losses due to adsorption or catalyzed degradation.
A distinct challenge is posed by extracting CBEA-B from matrices rich in lipids, chlorophylls, and phenolic impurities. In such cases, liquid-liquid extraction is employed, where CBEA-B is transferred into a polar phase after removing lipophilic components with hexane or cyclohexane. This type of fractionated extraction allows for the concentration of CBEA-B to levels suitable for further analytical purification.
Since this cannabinoid is thermolabile, subcritical CO₂ extraction methods without modifiers are not used. Even at low temperatures (35–45 °C), carbon dioxide can promote either carboxylation or decarboxylation depending on the pH of the medium. An alternative is supercritical extraction under modified conditions, such as the addition of oxidation inhibitors (e.g., α-tocopherol or BHT) and pH control using amine compounds.
For maximum extraction efficiency, research teams employ combined approaches, wherein initial extraction with organic solvents is followed by solubilization in water-ethanol microemulsions stabilized with phospholipids or polyethylene glycols. This approach minimizes losses during subsequent purification and allows stabilization of CBEA-B for long-term storage.
Chromatographic Separation and Analytical Purification
Following primary extraction, there is a need for fine separation of a complex mixture of cannabinoids, terpenoids, flavonoids, and other impurities. Considering the physicochemical properties of CBEA-B-its polarity, ionizable carboxyl group, and ability to form intramolecular hydrogen bonds-the most effective techniques are high-performance chromatographic methods, particularly:
- RP-HPLC (reversed-phase high-performance liquid chromatography)
- Gel permeation chromatography (GPC) for preliminary fractionation
- Ion exchange chromatography for analytical purposes
RP-HPLC using C18 or C8 columns enables efficient separation of extracts even from highly complex matrices. For CBEA-B, optimal mobile phases are based on acetonitrile/water with the addition of 0.1% formic acid. This stabilizes its protonated form and improves resolution by reducing secondary interactions with the stationary phase. It is important to avoid methanol as a mobile phase due to its potential to form esters with carboxyl groups under thermal conditions.
After chromatographic separation, analytical purification includes the following stages:
- Lyophilization (freeze-drying) of fractions
- Desolvation under vacuum at temperatures below 20 °C
- Purity assessment using UV detection at 210–220 nm, specific for carboxylated aromatic systems
Further confirmation of CBEA-B purity is conducted via LC-MS/MS with electrospray ionization, allowing detection of even trace amounts of structurally similar cannabinoid impurities. Identification is based on molecular ion mass and specific fragmentation profiles. If residual isomers are present (e.g., CBEA-A or unstable dehydro forms), additional purification is performed through preparative capillary electrophoresis using a boric acid-based buffer.
The purified substance must be stored at –80 °C in a sealed, inert environment-preferably under nitrogen or argon-with the addition of antioxidants to prevent oxidation. It is also worth noting that even a slight increase in storage temperature to –20 °C over several weeks may cause degradation or polymerization of CBEA-B, making its stability one of the main obstacles to scalable production.
Pharmacological Properties of Cannabielsoic Acid B (CBEA-B)
Probable Interaction with Cellular Receptors
Cannabielsoic Acid B (CBEA-B), as a little-studied cannabinoid, is currently the focus of growing scientific interest due to its potential interactions with cannabinoid receptors and other molecular targets in the human body. As a carboxylated cannabinoid belonging to the cannabilin derivative series, its chemical structure shares features with more well-known cannabinoids such as Δ9-tetrahydrocannabinol (Δ9-THC) and cannabidiol (CBD). However, its specific pharmacological effects and mechanisms of action remain poorly understood, and more detailed research is needed to uncover the exact pathways of its interaction with cellular receptors.
Cannabinoid Receptors CB1 and CB2
Cannabinoids exert their effects mainly through two principal receptors: CB1 and CB2, which belong to the G-protein-coupled receptor (GPCR) family. CB1 receptors are predominantly localized in the central nervous system (CNS), where they regulate neurotransmitter systems involved in pain sensation, mood, memory, and motor function. In contrast, CB2 receptors are primarily found in the immune system, including in microglial cells and macrophages, where they play a role in modulating inflammatory processes.
There is reason to believe that CBEA-B may have moderate or weak agonistic activity toward both CB1 and CB2 receptors, owing to its molecular similarity to more pharmacologically active cannabinoids. However, it is not as potent as Δ9-THC, which has a high affinity for CB1. Preliminary research suggests that CBEA-B may act via a partial agonist mechanism at CB1 and CB2 receptors, potentially influencing cyclic AMP (cAMP) levels within cells and thereby modulating neurotransmission and inflammatory responses.
Investigating this effect for CBEA-B will require detailed molecular studies and the use of radiolabeled ligands to precisely assess its binding potential to cannabinoid receptors.
Other Receptors and Biological Targets
Beyond its potential interaction with cannabinoid receptors, CBEA-B, like other cannabinoids, may affect other molecular targets not directly linked to the endocannabinoid system. Notably, possible influences include:
- Transmembrane Ion Channels, particularly TRP (Transient Receptor Potential) channels, which play critical roles in sensory perception, including pain and temperature sensitivity.
- GABA Receptors: It is known that some cannabinoids can indirectly modulate the GABAergic system, promoting relaxation and reducing anxiety.
- Given that CBEA-B contains an aromatic ring with hydroxyl groups capable of donating hydrogen, it may impact the body’s antioxidant defense system, particularly through enzymes such as superoxide dismutase (SOD) and catalase, thereby reducing oxidative stress at the cellular level.
Research on Its Effects on Metabolic and Inflammatory Cascades
Inflammatory Cascades and Regulation of Immune Responses
CBEA-B holds significant potential for regulating inflammatory processes, which makes it a promising candidate for the treatment of chronic inflammatory diseases. Inflammatory cascades are typically activated through Toll-Like Receptors (TLRs) or Pattern Recognition Receptors (PRRs), which detect pathogenic molecules or internally damaged cellular components. Activation of these receptors triggers the release of pro-inflammatory cytokines such as TNF-α, IL-1β, and IL-6, which drive inflammation.
During such immune responses, CBEA-B may potentially alter the activity of microglia and macrophages, leading to a reduction in the secretion of pro-inflammatory cytokines. As such, there is a plausible hypothesis that CBEA-B exhibits anti-inflammatory properties, which could be leveraged in the treatment of conditions like inflammatory bowel disease, arthritis, or autoimmune disorders.
Impact on Lipid and Glucose Metabolism
Metabolic disorders, particularly those involving disturbances in glucose and lipid metabolism, are central to chronic conditions like diabetes and atherosclerosis. Given the known connections between cannabinoids and metabolic processes, it is reasonable to hypothesize that CBEA-B could influence metabolic pathways by regulating glucose and lipid levels in the bloodstream.
Some cannabinoids-such as CBD-have demonstrated the ability to lower blood glucose levels, improve insulin sensitivity, and reduce cholesterol levels. Due to its structural similarity, CBEA-B may offer comparable metabolic effects. Additionally, molecules involved in lipid storage and energy balance-such as PPAR-γ (Peroxisome Proliferator-Activated Receptor Gamma)-may be activated in the liver and adipose tissue by CBEA-B, potentially reducing fat accumulation and lowering the risk of metabolic syndrome.
Potential Influence on Neuroplasticity and Neurogenesis
Neuroplasticity-the brain’s ability to adapt to environmental changes-and neurogenesis-the generation of new neurons-are crucial for recovery from injury and for combating neurodegenerative diseases. Certain cannabinoids, particularly CBD, are known to positively influence these processes. Given the potential neurotrophic properties of CBEA-B, it is plausible that it could also modulate neuroplasticity and neurogenesis.
At the molecular level in the central nervous system, CBEA-B may interact with neurotrophic factors, particularly Brain-Derived Neurotrophic Factor (BDNF), which supports the survival of neurons during stress and inflammatory states. Such a mechanism could be therapeutically relevant for diseases such as Alzheimer’s and Parkinson’s, where neuronal degeneration plays a key role.
Molecular Targets and Intracellular Mechanisms of Cannabielsoic Acid B (CBEA-B)
Presumed Targets in TRP, PPAR, and GPR Systems
The TRP (Transient Receptor Potential) System
The TRP (Transient Receptor Potential) receptor system is a critical component of cellular signaling and plays a decisive role in regulating numerous physiological processes, such as the perception of pain, temperature, mechanical stimuli, as well as metabolic and inflammatory responses. TRP receptors are ion channels that allow specific ions (e.g., calcium, sodium, or magnesium) to enter the cell, thereby triggering numerous intracellular pathways.
TRP receptors include many subtypes, notably TRPV1, TRPA1, TRPM8, TRPC6, and others. The interaction between cannabinoids and TRP receptors is an area of active research, as cannabinoids typically possess the ability to modulate the activity of TRP receptors, influencing the physiological functions of the body.
TRPV1 is one of the most extensively studied TRP receptor subtypes, known for its responsiveness to temperature and certain chemical compounds such as capsaicin (the active component in chili peppers). Cannabinoids can bind to TRPV1 and modulate its activity, which can significantly reduce pain perception and inflammation. CBEA-B is likely to interact with TRPV1, potentially producing an analgesic effect.
Another important receptor is TRPA1, which is activated by various irritants, including chemical substances and temperature changes. The interaction of CBEA-B with TRPA1 may lead to the blocking of pain and inflammation, contributing to a therapeutic effect in chronic inflammatory conditions.
Cannabinoids are also capable of affecting other TRP receptor subtypes, which may be relevant for treating conditions such as arthritis, neuritis, and neuropathic pain by modulating various ion channels and decreasing inflammation levels.
Paracellular Nuclear Receptors – PPARs
PPARs (Peroxisome Proliferator-Activated Receptors) are a group of nuclear receptors that significantly influence lipid and carbohydrate metabolism as well as other key metabolic processes. There are three primary PPAR subtypes: PPAR-α, PPAR-β/δ, and PPAR-γ. These receptors, when bound by specific molecules, regulate the expression of genes responsible for maintaining metabolic balance, substance exchange, and reduction of inflammation.
PPAR-γ regulates critical processes associated with lipid metabolism and glucose homeostasis. Cannabinoids can activate PPAR-γ, which leads to reduced inflammation, improved insulin sensitivity, and lowered levels of cholesterol and triglycerides in the body. This makes PPAR-γ activation potentially beneficial for managing diseases like type 2 diabetes and metabolic syndrome.
In addition, PPAR-α participates in the regulation of lipid levels and fatty acid metabolism in the liver and other tissues. Modulating this activity with cannabinoids, including CBEA-B, may positively impact cardiometabolic health by reducing the risk of cardiovascular diseases such as atherosclerosis and hypertension.
Therefore, CBEA-B holds potential to act through PPAR receptors, making it a promising candidate for the development of therapeutic agents targeting inflammatory and metabolic diseases, as well as for regulating energy homeostasis in the body.
G Protein-Coupled Receptors (GPRs)
G protein-coupled receptors (GPRs) are a large family of receptors that regulate intracellular signaling through interaction with G-proteins. They are essential components in processes like neuroplasticity, inflammation, and energy metabolism regulation.
A particularly interesting receptor is GPR55, which is involved in the modulation of various physiological processes, including neuroplasticity and pain perception. According to research, GPR55 may play a significant role in the regulation of neurogenesis and synaptic plasticity, making it a promising target for treating neurodegenerative disorders.
Interaction with GPR55 can lead to changes in neural conductivity, pain reduction, and improved neuroplasticity. There is also a theoretical possibility of using CBEA-B in therapies for conditions such as chronic pain, memory disorders, or even depression through mechanisms involving GPR55.
Epigenetic Potential and Transcriptional Activity
Interaction with Epigenetic Mechanisms
Epigenetics studies changes in gene expression that do not involve alterations in the DNA sequence itself. These changes may include DNA methylation, histone acetylation, and microRNA regulation, all of which can modify gene activity in response to external or internal signals. Epigenetic mechanisms are critical for cell development and are implicated in various pathological processes such as cancer, neurodegeneration, metabolic disorders, and chronic inflammation.
The influence of cannabinoids, particularly CBEA-B, on epigenetic mechanisms is a promising area for future research. It is known that cannabinoids can affect deacetylases and methyltransferases, which are enzymes responsible for activating or repressing specific genes. This could be significant in the context of diseases such as cancer, cardiovascular conditions, neurological disorders, and autoimmune diseases.
CBEA-B may modulate the activity of epigenetic regulators such as HDACs (histone deacetylases), which control inflammatory gene expression levels in the body. This modulation may suppress or enhance the expression of genes involved in inflammation, antioxidant defense, immune response, and metabolism.
Transcriptional Activity
Cannabinoids have the capacity to influence gene transcription by activating specific nuclear receptors such as PPARs, LXRs (Liver X Receptors), and NRF2 (Nuclear Factor Erythroid 2-Related Factor 2). These receptors play pivotal roles in regulating antioxidant activity, lipid metabolism, and glucose processing, making them critical targets for treating metabolic and cardiovascular disorders.
CBEA-B likely has the potential to affect the activation of transcription factors that regulate antioxidant defenses and inflammatory processes within the body. In particular, CBEA-B may activate NRF2, which is responsible for cellular detoxification and protection from oxidative stress. This function is especially important for treating neurodegenerative diseases and cardiovascular impairments.
Biotransformation and Metabolism of Cannabielsoic Acid B (CBEA-B)
Transformation in the Body and the Role of Phase I–II Enzymes
The biotransformation of Cannabielsoic Acid B (CBEA-B) in the human body is a complex process that involves multiple stages carried out by Phase I and Phase II metabolic enzymes. These stages determine the molecule’s metabolic activity and stability, which are essential for its pharmacokinetic characteristics.
Phase I Metabolism
The initial stage of CBEA-B metabolism involves oxidative reactions, primarily catalyzed by cytochrome P450 (CYP450) enzymes. These enzymes mediate oxidation, hydroxylation, or other functional group modifications of the molecule. For CBEA-B, the key enzymes may include:
- CYP3A4 – This enzyme plays a major role in the metabolism of numerous pharmaceuticals, including cannabinoids. Oxidation mediated by CYP3A4 can lead to the formation of hydroxylated metabolites, which may exhibit different biological activity compared to the parent compound.
- CYP2C9 – Another crucial enzyme involved in cannabinoid metabolism. CYP2C9 may also contribute to the biotransformation of CBEA-B, especially in its conversion to hydroxylated products.
Oxidation of the CBEA-B molecule by CYP enzymes often leads to metabolites with reduced pharmacological or toxic effects. However, in the case of CBEA-B, some oxidative products may retain or even acquire new interactions with cellular targets, potentially offering therapeutic avenues for chronic diseases.
Phase II Metabolism
In Phase II, the oxidative metabolites formed in Phase I undergo conjugation reactions that facilitate their excretion. Major conjugation processes include glucuronidation, sulfation, and methylation, which convert hydroxylated metabolites into more polar compounds that are more easily excreted in urine or bile.
- UDP-glucuronosyltransferases (UGTs) – This class of enzymes attaches glucuronic acid to CBEA-B metabolites. This reaction reduces the compound’s lipophilicity and enhances its renal excretion. Glucuronide forms of CBEA-B may exhibit altered biological activity compared to the parent compound, which must be considered in therapeutic development.
- Sulfotransferases (SULTs) – These enzymes catalyze the addition of sulfate groups to the molecule, facilitating urinary excretion.
In the case of CBEA-B, glucuronidation and sulfation can reduce the activity of its metabolites by converting them into inactive forms, thereby lowering toxicity risks. Nonetheless, some metabolites may retain activity or even produce specific effects by interacting with receptors or cellular targets.
Comparative Stability in Plasma, Liver, and Microbiome
Stability in Plasma
Upon entry into the body, CBEA-B rapidly binds to plasma proteins, facilitating its transport to various tissues. The degree of protein binding may vary depending on the compound’s concentration and pharmacokinetic parameters. Through this binding, CBEA-B can maintain its activity for a certain duration in the bloodstream, allowing it to interact with molecular targets during different metabolic stages.
Stability in the Liver
The liver is the central organ for metabolism, where both Phase I and Phase II enzymes perform active transformations of pharmaceuticals and other xenobiotics. In the liver, CBEA-B undergoes extensive metabolism, with CYP450 enzymes playing a critical role in converting it into active or inactive metabolites. Therefore, hepatic stability of CBEA-B is a key factor determining its duration of activity and clearance rate.
Stability in the Microbiome
The human microbiome, particularly the gut microbiota, may also contribute to cannabinoid metabolism, including that of CBEA-B. Some bacterial strains are capable of metabolizing a wide range of organic compounds, including cannabinoids, via enzymatic cleavage of molecular bonds. This can lead to the degradation of CBEA-B or the formation of novel metabolites with distinct biological activities. Thus, the microbiome may significantly influence CBEA-B stability in the gastrointestinal tract and its overall effectiveness.
Detection Methods in Biological Samples
LC-MS/MS Approaches for Microdose Concentrations
One of the primary methods for detecting and quantifying CBEA-B in biological samples is liquid chromatography coupled with tandem mass spectrometry (LC-MS/MS). This technique allows for the accurate and sensitive detection of even microdose concentrations of the compound in blood, urine, or other biological fluids.
LC-MS/MS combines the molecular separation capabilities of liquid chromatography with the mass-based detection of mass spectrometry, enabling precise identification of the molecular weight and structural characteristics of CBEA-B metabolites. This approach is especially useful for analyzing small cannabinoid doses that may not be detectable using techniques like gas chromatography.
Isomer and Artifact Separation in Complex Matrices
One of the key challenges in detecting cannabinoids in biological samples is the presence of isomers and artifacts, which can complicate accurate metabolite identification. Like many cannabinoids, CBEA-B can exist in multiple isomeric forms, and precise differentiation of these isomers is essential for understanding their biological activity and metabolic pathways.
Mass spectrometry-based techniques are highly effective for distinguishing isomers and minimizing the impact of artifacts, which may arise during analysis of complex matrices such as blood serum or microbiota samples. Advanced separation methods, particularly high-resolution liquid chromatography (HPLC), are valuable in obtaining accurate results even in the presence of contamination and at low compound concentrations.
Innovative Research Directions for Cannabielsoic Acid B (CBEA-B)
CRISPR/Cas-Driven Enhancement of Biosynthesis in Culture
One of the most promising areas of research for CBEA-B lies in the application of CRISPR/Cas technology to modify the genome of organisms used in the biosynthesis of this molecule. This allows for precise regulation of cannabielsoic acid production processes in cellular cultures or microorganisms such as yeast or bacteria.
CRISPR/Cas9 enables the introduction of point mutations into genes encoding enzymes responsible for CBEA-B synthesis. This makes it possible to engineer artificial cells with an enhanced ability to produce CBEA-B, which is of critical importance to the pharmaceutical industry, particularly in the context of developing biopharmaceutical drugs based on this compound.
One of the key advantages of this approach is the potential for the expression of genetically modified organisms in industrial-scale settings, ensuring stable and large-scale production of CBEA-B for further use in scientific research and medical applications.
For example, using CRISPR/Cas to activate or upregulate genes encoding phase I and II metabolism enzymes in cultures may significantly improve the efficiency of CBEA-B biosynthesis and reduce the number of steps needed in its production. This approach also offers the potential to lower manufacturing costs and increase product stability, thereby making CBEA-B more accessible for future research.
Potential of CBEA-B as a Biomarker for Specific Chemotypes
Given its complex molecular structure and interactions with numerous receptors and metabolic pathways, CBEA-B holds significant promise as a biomarker for identifying specific chemotypes in organisms, including humans. Determining such chemotypes could support the development of personalized medicine, where treatment or dietary choices are based on a patient’s genetic or metabolic profile.
It is already well known that cannabinoids, including CBEA-B, can produce varying effects depending on the genetic predisposition of the individual. These include variations in CB1 and CB2 receptors, PPAR receptors, and distinct cannabinoid metabolism pathways in the body. Investigating correlations between CBEA-B levels and specific genetic or metabolic markers could serve as a foundation for the development of novel diagnostic and therapeutic tools for diseases related to disruptions in cannabinoid metabolic pathways.
CBEA-B could also serve as a marker for personalized therapy, as different individuals may respond differently to cannabinoids based on their genetic makeup. For instance, chemotypes associated with high or low cannabinoid metabolism rates could be used to determine the optimal doses of CBEA-B or other cannabinoids for therapeutic purposes.
Conclusion:
Cannabielsoic acid B (CBEA-B) represents a significant member of the cannabinoid family, garnering attention from the scientific community due to its unique molecular structure and potential in medical and pharmaceutical applications. It belongs to the oxidized derivatives of the cannabilin series and demonstrates notable biological activity through interactions with various receptors and metabolic pathways, opening new opportunities for the treatment of inflammatory, neurotropic, and metabolic disorders.
Research on CBEA-B in the context of biogenesis and molecular targets shows that this molecule can modulate the TRP, PPAR, and GPR systems, making it a critical focus for the development of therapeutic strategies. Techniques such as CRISPR/Cas allow for the genetic modification of organisms to enhance CBEA-B production, which could greatly improve its efficiency and application in medicine and biotechnology.
The metabolic pathways and in vivo stability of CBEA-B require further investigation, particularly regarding its interactions with phase I and II metabolic enzymes. This is essential for understanding its pharmacokinetic properties and its potential inclusion in medicinal products. The application of LC-MS/MS technologies provides precise detection of CBEA-B metabolites in biological samples, paving the way for novel diagnostic tools and treatment monitoring systems.
The potential of CBEA-B as a biomarker for specific chemotypes of Cannabis sativa L. also appears promising, as this could support advances in personalized medicine and improve therapeutic precision. Chemotype identification could help tailor treatment to the patient’s genetic and metabolic profile, which is critical for the successful application of cannabinoids in clinical practice.
Therefore, CBEA-B holds substantial promise across various fields of science and medicine. Continued research in this area may lead to new discoveries and applications that will drive progress in biomedical technologies and pharmaceutical sciences.
Sources:
- PubMed Central (PMC) – The U.S. National Institutes of Health (NIH) provides access to research on cannabinoids and their molecular properties, useful for understanding CBEA-B and its biochemistry: https://www.ncbi.nlm.nih.gov/pmc/
- NIH RePORTER – A platform for exploring federally funded research and publications covering cannabinoids, including CBEA-B, pharmacological properties, and biogenesis: https://reporter.nih.gov/
- Frontiers in Pharmacology – An open-access pharmacology journal that publishes research on cannabinoids, their molecular targets, and pharmacological effects relevant to CBEA-B: https://www.frontiersin.org/journals/pharmacology
- The Royal Society of Chemistry (RSC) – Publications in chemistry and biochemistry offering articles on cannabinoid structure and biochemistry, including CBEA-B: https://www.rsc.org/
- NCBI (National Center for Biotechnology Information) – A platform offering access to research in cannabinoid molecular biology and other relevant topics: https://www.ncbi.nlm.nih.gov/
- ScienceDirect (Elsevier) – Open-access articles in pharmacology and biochemistry, including studies on cannabinoid molecular targets relevant to CBEA-B: https://www.sciencedirect.com/
- ResearchGate – A scientific platform for accessing studies on cannabinoids and their pharmacological properties: https://www.researchgate.net/