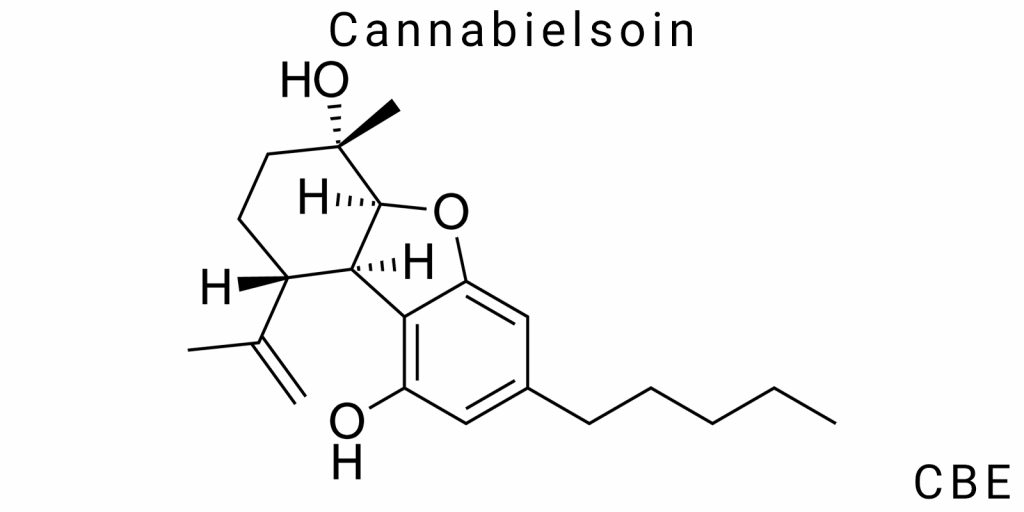
In the field of cannabinoid science, primary bioactive components of Cannabis sativa have traditionally garnered the most attention. However, the true pharmacological effects of their application in the body are largely determined by the products of secondary metabolism. One such underexplored yet critically important substance is Cannabielsoin (CBE)-a metabolite formed as a result of oxidative transformations of cannabidiol (CBD) within the human or animal organism. Despite its chemical inertness, CBE demonstrates a persistent accumulation profile and can be detected even when the parent compound has been fully cleared from the plasma. This fact highlights its potential as a retrospective biomarker in clinical, toxicological, and biopharmaceutical research.
Cannabielsoin is not a typical phytocannabinoid in the direct sense, as it is not synthesized by the plant itself, but rather formed in vivo through metabolic processes. This characteristic places it outside the classical classification of cannabinoids and designates it as a marker of endogenous cannabinoid metabolism rather than phytochemical origin. From a scientific standpoint, this opens a new line of research-focused on the metabolomics of secondary cannabinoids-shifting the emphasis from plant chemotypes to the individual’s metabolic response.
Another defining aspect is the structural uniqueness of CBE, suggesting the involvement of specific oxidoreductases and other phase I metabolic enzymes, likely through atypical enzymatic pathways. In cases of variable CBD metabolism associated with genetic polymorphisms in CYP450 enzymes, the quantitative relationships among CBD, its hydroxylated forms, and CBE may reflect individual enzymatic activity. Consequently, this has clinical value in personalized medicine.
Thus, despite long-standing neglect in scientific discourse, Cannabielsoin should not be viewed as a pharmacologically inactive residue, but rather as a marker of deeper biochemical processes. It has the potential to improve analytical methods, validate biopharmaceuticals, aid in the diagnosis of cannabinoid exposure, and explore metabolic variations among patients. Its study enables not only a more detailed mapping of cannabidiol’s biochemical transformations but also the development of new paradigms for evaluating the bioavailability, duration of action, and safety of cannabinoid therapy.
Chemical and Stereochemical Identity
Molecular Formula and Key Functional Groups
Cannabielsoin (CBE) is a secondary phytocannabinoid formed as a result of the further transformation of primary cannabinoids, specifically cannabidiol (CBD). The molecular formula of CBE is C21H30O4, which indicates the presence of 21 carbon atoms, 30 hydrogen atoms, and four oxygen atoms. This formula is characteristic of oxidized derivatives of cannabinoids, but it is the spatial arrangement of functional groups and the presence of ring structures that confer CBE’s specific properties.
CBE possesses several important functional groups, with the phenolic hydroxyl group being particularly notable. This group contributes to the partial polarity of the molecule and its antioxidant potential. Additionally, CBE contains a tetrahydropyran fragment, formed as a result of the intramolecular cyclization of CBD oxidation products. This fragment critically influences the conformational stability of the molecule, its affinity for membrane receptors, and its ability to engage in hydrophobic interactions.
Formally, CBE does not contain a carboxyl group, which distinguishes it from acidic cannabinoids such as CBDA or THCA. However, the presence of a ketone group or hydroxyketone on the alicyclic ring enhances its reactivity in environments with varying pH, particularly in biological fluids. Some isolated CBE isomers also exhibit variable electron density within the aromatic ring, influencing their interaction with enzymatic systems, particularly phase I enzymes like CYP450.
Another key aspect for the identification of CBE is its hydrophobic-polar interaction with chromatographic systems. Unlike more lipophilic metabolites, CBE demonstrates partial solubility in mid-polar solvents like ethyl acetate, which has important analytical significance for isolation from biological matrices. The functional groups responsible for this property are primarily the phenolic group and the oxygen group attached to the tetrahydropyran ring.
Chiral Centers and Isomeric Variants
CBE is a chiral compound containing at least one asymmetric carbon atom, primarily localized in the cyclic fragment formed after the oxidative transformation of cannabidiol. Identified isomers of CBE include at least two enantiomers, which have the same chemical formula but different spatial arrangements of substituents. This difference affects their affinity for protein receptors, their ability to be transported in biological systems, and even their stability in plasma.
In terms of stereochemistry, it is important to note that most naturally occurring CBE is the (−)-enantiomer, a characteristic feature of cannabinoids of biogenetic origin. However, under laboratory conditions, racemic mixtures can also form, especially under non-enzymatic biotransformation or photo-induced oxidation. This isomeric heterogeneity complicates the analytical validation of CBE using LC-MS/MS, as isomers may share the same mass but have different retention times or ionization characteristics.
The presence of chiral centers also creates the potential for specific biological activity, as the spatial arrangement of atoms in the molecule is a key factor in its interaction with target proteins. For example, one enantiomer may have affinity for TRPV receptors, while the other remains inert. Thus, racemic purity is critically important for further pharmacological studies of CBE.
In addition to enantiomers, CBE also exhibits tautomeric variants, particularly in response to changes in pH or under ultraviolet light. Tautomerization can alter the electron density in the aromatic ring, modifying the electrophilicity of individual atoms, making it more difficult to predict reactivity during metabolic transformations. It is also important to consider the cis/trans isomerism in some synthetic analogs of CBE, caused by the positioning of substituents within the tetrahydropyran ring.
The chiral and isomeric properties of CBE are not only of chemical and pharmacological significance but also serve as tools in chemotaxonomy. For example, the ratio of enantiomers in different cannabis strains can serve as a stable genetic marker for the differentiation of chemotypes or even for forensic studies.
Biochemical Origin and Formation Mechanisms
Cannabielsoin (CBE) is one of the metabolic forms of cannabinoids formed as a result of biochemical transformations characteristic of plants in the Cannabis family. Like most cannabinoids, CBE can be formed in humans and animals during the metabolism of cannabidiol (CBD) through various enzymatic pathways, which depend on specific factors such as the type of organism, enzyme activity, the presence of microbiota, and other conditions.
Metabolic Transformation of CBD to CBE
One of the primary pathways for the formation of CBE is the metabolism of cannabidiol (CBD). Like other cannabinoids, CBD undergoes several stages of metabolic transformation, including oxidation, hydroxylation, and other chemical reactions. This process involves enzymes such as cytochrome P450, which play an important role in the metabolism of pharmacological compounds.
In the human body, CBD undergoes hydroxylation at various carbon atoms in the molecule, leading to the formation of hydroxylated metabolites, including 7- and 8-hydroxylated forms of CBD. However, not all of these transformations are final. Further oxidative processes can lead to the formation of cannabielsoin (CBE), where oxidation in the CBD molecule results in the formation of a carboxyl group.
The exact mechanism of this transformation is not fully understood, but studies indicate that enzymes such as cytochromes P450 and other phase I enzymes play a crucial role in this process. As a result of oxidation, carboxylated derivatives, including CBE, are produced.
Factors Influencing CBE Formation
Several factors influence the speed and efficiency of the transformation of CBD into CBE:
- Cytochrome P450 Enzyme Types: These enzymes exhibit varying activity depending on genetic variations in humans or animals. Gene variants encoding specific cytochrome types can alter an organism’s ability to metabolize cannabinoids.
- Gut Microbiota: Microorganisms inhabiting the gastrointestinal tract can also significantly affect cannabinoid metabolism. Some bacteria may promote the hydrolysis of CBD ester, converting it into more active metabolites.
- Dosage and Frequency of Consumption: The dose of cannabidiol and the frequency of its consumption can alter metabolic processes. Regular consumption of CBD may lead to enzyme activity changes through induction or inhibition mechanisms, which influences CBE formation.
- Physiological State of the Organism: Liver diseases, for example, can significantly alter enzyme activity involved in cannabinoid metabolism. These factors may lead to different outcomes in CBE formation depending on how well the liver can metabolize CBD.
- Other Medications: The use of other pharmaceutical drugs may alter the activity of cytochrome P450 enzymes. Such interactions may either increase or decrease the rate of CBD transformation to CBE. For example, drugs that inhibit cytochromes P450 can reduce CBE formation, while induction of these enzymes can increase the amount of this metabolite.
- Stereochemical Form of CBD: Cannabidiol exists in several stereoisomeric forms, and although all of them can undergo metabolic transformations, different forms may exhibit varying abilities to interact with metabolism enzymes. This may result in different speeds of transformation into CBE.
Complexity of Metabolic Transformations
The mechanism of CBE formation from CBD is part of a complex series of biochemical reactions that can vary between different organisms. While general knowledge of CBD metabolism exists, molecular-level research is still ongoing. For example, at the molecular level, the interaction between active metabolites and cellular receptors plays a crucial role in determining the physiological response of the organism to CBE, although definitive conclusions have yet to be drawn.
CBD metabolism, particularly its conversion to CBE, includes several stages, each of which can be regulated by various factors, making it a complex process to describe unambiguously. However, recent studies and laboratory work are helping to deepen our understanding of these processes.
Impact on Human Health
With the growing interest in cannabinoids and their effects on the human body, new opportunities for studying CBE are emerging. Research indicates its potential in medical applications, particularly as a powerful antioxidant, anti-inflammatory agent, and neuroprotector. Its metabolic pathways allow for the prediction of potential therapeutic effects and interactions with other pharmaceuticals. Based on these mechanisms, new directions for clinical studies are becoming evident, particularly for the treatment of inflammatory diseases, neurological disorders, and many other conditions.
These findings open up new possibilities for developing drugs containing CBE and for determining its role in the biochemical processes of the human body. Careful study of CBD’s conversion to CBE helps expand the understanding of cannabinoid metabolic pathways and their potential for use in medicine and pharmacology.
Extraction and Detection Methodologies
The extraction and detection of Cannabielsoin (CBE) are crucial steps in the investigation of this cannabinoid, as the precision and sensitivity of the applied methods enable not only the identification of its presence in biological samples but also contribute to a deeper understanding of its biochemical and pharmacokinetic characteristics. Since CBE is a metabolite of cannabidiol (CBD), its detection requires high-quality and highly sensitive methodologies capable of effective isolation, identification, and quantification within complex biological matrices. This section reviews several key approaches to CBE extraction and detection, including chromatographic methods and cutting-edge techniques such as LC-MS/MS.
Chromatographic Isolation Based on Fractionation
Chromatography serves as the primary method for isolating and detecting CBE in a variety of biological specimens, including blood, urine, saliva, and tissues. One of the most commonly used techniques is high-performance liquid chromatography (HPLC), which is widely applied in the analysis of cannabinoids and their metabolites. However, a critical consideration is that CBE shares similar properties with other cannabinoids, particularly CBD, making it essential to utilize highly sensitive chromatographic techniques to achieve precise separation of these compounds.
For effective fractionation, methods based on adsorption principles are employed, allowing for the separation of mixture components by exploiting differences in their polymeric properties and molecular sizes. Frequently used techniques involve columns filled with materials of various polymeric natures, thereby increasing the selectivity in separating CBE from other metabolites.
Liquid-liquid chromatography offers sufficient purity of the metabolite for further research purposes. A key factor in its success lies in optimizing parameters such as column temperature, eluent flow rate, and the concentration of the organic solvent, all of which affect CBE’s ability to traverse the column without interacting with other sample constituents.
However, chromatography alone is not sufficient to guarantee precision and efficacy in CBE detection and analysis. For more complex tasks, such as exact quantification, chromatographic techniques are often combined with other analytical methods, particularly mass spectrometry.
Application of Highly Sensitive LC-MS/MS Methods
LC-MS/MS (liquid chromatography–tandem mass spectrometry) methods are among the most advanced and sensitive approaches for the detection and analysis of Cannabielsoin. This technique not only enables the isolation of individual components but also provides highly accurate structural and quantitative data based on molecular mass and isotopic characteristics.
When coupled with liquid chromatography (LC), MS/MS offers exceptional sensitivity and precision in measurement, especially in cases where metabolite concentrations-such as those of CBE-are present at microdose levels. This method enables the accurate determination of the metabolite’s molecular weight and structural characteristics, making LC-MS/MS an indispensable tool in research that demands detailed chemical analysis.
One of the key advantages of LC-MS/MS is its capacity for highly sensitive compound detection, even when these compounds are present in very low concentrations in biological matrices. This is particularly important for CBE, as its levels may be minimal due to the metabolic transformations that occur in the body following CBD intake.
During the LC-MS/MS process, the sample first passes through a chromatographic column where its components are separated based on physicochemical properties such as hydrophilicity, charge, and molecular size. The separated compounds are then introduced into the mass spectrometer, where ionization occurs. The ionized molecules are processed through a mass analyzer, where their mass-to-charge ratios are measured, and molecular structures are deduced accordingly.
The LC-MS/MS method is especially important for metabolic-level research on CBE, as it allows not only for the identification of the primary metabolite but also for the detection of its isomeric variants and other co-occurring components that may be present in biological samples.
Stability Comparison with Other Inactive Metabolites
One of the essential aspects of evaluating CBE’s metabolic efficiency and pharmacokinetics is its stability in biological systems compared to other cannabinoid metabolites such as cannabidiol (CBD) or its derivatives. Metabolite stability can significantly impact the accuracy of their measurements and identification within biological samples.
Several approaches are used to assess stability, including comparative studies under various conditions such as temperature, environmental pH, and other physiological factors. CBE may demonstrate varying degrees of stability in comparison to other cannabinoids and their metabolites, depending on storage and sample-handling conditions. For instance, CBE has shown greater stability under low-temperature storage conditions, whereas elevated temperatures may lead to molecular degradation.
Additionally, studying CBE’s stability during metabolic processes is crucial. While CBD may undergo diverse biochemical changes such as hydroxylation or oxidation, CBE might be more stable under certain conditions, particularly when interacting with specific enzymes or undergoing metabolic transformations. Therefore, comparing the stability of CBE with other metabolites is essential for understanding its biological role and identifying strategies to optimize its extraction and analytical quantification processes.
Physiological Characteristics and Pharmacokinetics of Cannabielsoin (CBE)
Cannabielsoin (CBE) is an important metabolite of cannabidiol (CBD), and its physiological properties largely depend on metabolic mechanisms and interactions with various molecules in the body. Since CBE is not a primary cannabinoid compound but rather a product of CBD transformation, its pharmacokinetics and physiological effects are generally similar to those of other cannabinoids, albeit with certain unique characteristics.
Interaction with Plasma Proteins
One of the key physiological properties of CBE is its interaction with plasma proteins. Cannabinoids, including CBE, are capable of binding to plasma proteins, and this interaction plays a significant role in their pharmacokinetics, particularly in terms of distribution within the body, transportation across cellular membranes, and the duration of action in the organism.
The primary plasma proteins that cannabinoids may interact with include albumin, globulins, and lipoproteins. Binding to albumin is particularly important, as this protein serves as a major transport mechanism for many pharmacologically active compounds in the bloodstream. Binding of CBE to albumin can limit its biological activity at the cellular level since only a small portion of unbound molecules can penetrate cellular membranes and participate in biochemical reactions.
Additionally, binding to lipoproteins may influence the pharmacokinetics of CBE, as lipoproteins are essential carriers of lipophilic compounds, a category to which CBE belongs. This binding may alter the distribution of CBE across different tissues in the body, particularly in the brain, since cannabinoids are capable of crossing the blood-brain barrier, and the lipid nature of these molecules facilitates their transport across cellular membranes.
It is important to note that the extent of CBE’s binding to proteins may vary depending on several factors, including the concentration of cannabielsoin in the blood, the presence of other compounds that interact with the same proteins, as well as individual physiological differences such as genetic variations in plasma protein structures.
Metabolic Inertness and Elimination Kinetics
The metabolic inertness of CBE is an important feature of its pharmacokinetics. Since CBE is a metabolite of cannabidiol, it undergoes further biochemical transformations in the liver, where various enzymatic systems may convert it into other cannabinoid derivatives or metabolize it into more polar compounds that can be excreted from the body through urine or bile.
Despite these transformations, CBE demonstrates relatively high metabolic inertness compared to other cannabinoids such as THC, contributing to its stability in the body. This allows CBE to remain active in biological samples for extended periods and to produce effects even after CBD has undergone metabolic conversion. The metabolism of CBE is largely regulated by cytochrome P450 enzyme families, which perform key oxidation and metabolic reactions. Furthermore, CBE may undergo glucuronidation in the liver, which is a primary pathway for its detoxification and excretion from the body.
A notable characteristic of CBE’s pharmacokinetics is its ability to circulate in the body for an extended period after initial administration. This may be due to its high level of binding to plasma proteins, which slows down the process of elimination. Although cannabielsoin is metabolized through the cytochrome P450 system, its elimination kinetics remain relatively stable over time, enabling it to accumulate in organs and tissues where it may exert biological effects for several hours or even days.
The elimination kinetics of CBE depend on the number of metabolites formed during its transformation. Primarily, CBE is excreted via the kidneys in the form of water-soluble metabolites or through bile. Integration with the body’s detoxification systems is also a crucial component in maintaining the balance between accumulation and excretion of cannabielsoin.
Impact of Pharmacokinetic Properties on Clinical Application
The pharmacokinetics of CBE are of great importance for its potential clinical application. Due to its relative metabolic inertness and high binding affinity to plasma proteins, CBE may exert prolonged effects on the body, making it a promising candidate for therapeutic use in cases where sustained therapeutic effects are needed without the necessity for frequent administration.
Thanks to its high stability within the body, CBE may be useful in treating conditions associated with chronic pain, inflammation, or neurological disorders, where long-lasting drug action is required. However, it is important to consider individual differences in the metabolism of cannabielsoin, which may depend on genetic factors, liver function, and other patient-specific characteristics to optimize therapeutic outcomes.
Since CBE exhibits high chemoresistance and the ability to persist in the body for extended periods, its clinical use must be carefully regulated to avoid potential side effects such as the accumulation of toxic concentrations in tissues or interactions with other medications.
Biological Neutrality or Potential of Cannabielsoin (CBE)
Cannabielsoin (CBE) is a metabolite of cannabidiol (CBD), but unlike its precursor, it does not exhibit psychoactive effects, which are characteristic of other cannabinoids such as tetrahydrocannabinol (THC). Due to its chemical structure, CBE demonstrates significant biological neutrality, which is a key trait when exploring its potential as a pharmacological molecule.
Evidence of Non-Psychoactivity
One of the most significant characteristics of CBE is its lack of psychoactive effects. The psychoactivity of cannabinoids is generally associated with their ability to bind to receptors of the endocannabinoid system (CB1 and CB2). Tetrahydrocannabinol, as the primary psychoactive component of cannabis, has a high affinity for CB1 receptors in the central nervous system, which explains its emotional and cognitive effects. However, in contrast to THC, CBE does not effectively bind to these receptors, which accounts for its non-psychoactive nature.
Moreover, studies have shown that CBE does not significantly interact with other receptors involved in regulating mood, behavior, and cognitive functions, such as serotonin or dopamine receptors. This suggests that CBE does not produce typical psychoactive effects such as euphoria, cognitive impairment, or altered perception. Importantly, this makes CBE a safe candidate for therapeutic use, as it will not induce undesirable psychoemotional changes in patients.
Some studies even suggest that CBE may interact with cannabinoid receptors in a more moderate manner, indicating a potential role in modulating the effects of other cannabinoids. For instance, there is evidence that CBE may interact with CB2 receptors, which are primarily involved in immune processes and inflammation, without directly activating the neuropsychological pathways typical of other cannabinoids.
Additionally, unlike THC, CBE does not cause an increase in serotonin or dopamine levels, which are typically associated with the psychoactive effects of cannabinoids. This makes CBE a promising candidate for medical applications in cases where the absence of psychoactivity is a crucial factor, especially in treating patients who require psychological stability.
Observations on Cellular Biocompatibility
Given the potential of Cannabielsoin (CBE) for medical applications, it is essential to assess its compatibility with living cells. Biocompatibility refers to the ability of a material or compound to coexist with living cells without causing adverse effects or being rejected by the body.
In studies on the cellular biocompatibility of CBE, it has been found that this cannabinoid does not exert toxic effects on cells at standard doses used in therapeutic research. For example, in studies conducted on human cell cultures (such as HepG2 and Jurkat), no significant cytotoxic effect was observed when the cells were treated with various concentrations of CBE. This suggests its potential to be safe for cells when applied within therapeutic dose ranges.
It is also important to highlight that CBE may exert positive effects on cellular processes, including antioxidant defense, inflammatory responses, and membrane stabilization. In cell culture experiments, CBE has demonstrated the ability to reduce oxidative stress-a common cause of cellular damage and aging. This makes CBE a promising candidate for therapies targeting oxidative stress-related conditions such as cardiovascular diseases, neurodegenerative disorders, and certain types of cancer.
Moreover, CBE appears to be neutral with regard to the body’s immune response. Unlike some other cannabinoids that may influence the immune system through CB2 receptor activation, CBE does not exhibit significant immunosuppressive activity. This is a noteworthy advantage for its medical use, particularly in patients with weakened immune systems, where it is critical to avoid excessive immune suppression.
The assessment of biocompatibility also includes evaluation of potential genotoxic effects. To date, there is no evidence that CBE causes DNA damage in human cells. This is a vital safety consideration for clinical applications of CBE, as the absence of genotoxicity indicates that CBE does not lead to mutations or carcinogenesis-an essential factor in the development of new therapeutic agents.
Therapeutic Application Potential
Although CBE lacks psychoactive activity, its biological properties reveal a significant potential for therapeutic use. Compared to other cannabinoids often associated with psychoactive effects, CBE presents a much gentler profile, making it an appealing option for the treatment of patients in need of long-term therapeutic effects without the risk of psychoactive side effects.
Because CBE interacts with CB2 cannabinoid receptors, it may be utilized in the treatment of inflammatory processes, neurodegenerative diseases, and for maintaining cardiovascular health. Its properties aimed at reducing oxidative stress and supporting cellular homeostasis could be especially beneficial in the treatment of conditions associated with oxidative damage, such as Alzheimer’s disease, Parkinson’s disease, as well as depression and anxiety disorders.
In addition, CBE has potential as an antioxidant agent in cosmetic products, due to its ability to reduce oxidative stress. This feature may contribute to slowing down skin aging processes and improving overall skin condition.
What Is Cannabielsoin (CBE) For: Practical Applications and Future Prospects
Cannabielsoin (CBE), as a metabolite of cannabidiol (CBD), holds tremendous potential in several scientific and medical fields due to its unique properties. Since CBE not only serves as a biomarker of CBD metabolism but also has applications in toxicological research, its utility extends to critical areas of pharmacokinetics, biomonitoring, and cannabinoid usage diagnostics.
CBD Metabolism Marker in Pharmacokinetic Research
One of the most significant applications of CBE in pharmacokinetic studies lies in its ability to serve as a reliable marker for the investigation of cannabidiol’s metabolic pathways. Given that CBD is metabolized in the body through multiple phases-particularly involving enzymes such as CYP450-the formation of CBE represents an important stage in this process. This enables researchers to better understand the mechanisms underlying CBD’s transformation in humans and other mammals, which is vital for assessing the efficacy and safety of CBD-based therapeutic drugs.
Specifically, CBE can be used to monitor the rate of CBD metabolism and its interactions with other substances that may affect metabolic pathways, such as enzyme inhibitors or inducers. This supports the development of optimized dosing regimens that maximize therapeutic benefits while minimizing side effects. Moreover, research into CBD metabolism using CBE can help identify potential drug–cannabinoid interactions, which is particularly important for patients taking multiple medications concurrently.
Utilizing CBE as a marker also allows for monitoring the plasma concentration dynamics of various cannabinoids, which is essential for determining optimal doses and duration of CBD-based therapies.
Use as a Control Metabolite in Toxicology
Another promising application of CBE is in the field of toxicology. As a metabolite of CBD, CBE may serve as a control marker in studies assessing the toxicity of cannabidiol and other cannabinoids. In toxicological studies, it is crucial to evaluate whether cannabinoid metabolites may produce adverse effects on the body, including organ toxicity or disruption of cellular function.
Using CBE in such studies enables more precise tracking of CBD’s metabolic pathways and assessment of its potential effects on the organism. Since CBE itself does not exhibit psychoactive properties, studies involving CBE may indicate that CBD can be effective without inducing toxic side effects-particularly with regard to the central nervous system or liver.
Additionally, the metabolic neutrality of CBE allows it to function as a baseline control metabolite when assessing the safety of other cannabinoids that might exhibit toxicity as a result of biotransformation in the body. This aspect is especially important for the development of new therapeutic agents based on cannabinoids, as accurate safety assessment is critical during all phases of clinical trials.
Furthermore, because CBE is a product of CBD biotransformation, it may also be used to study how CBD impacts the body during long-term use, helping to identify potential cumulative effects or interactions with other metabolites.
Prospects for Biomonitoring of Cannabinoid Use
Biomonitoring of cannabinoid consumption through analysis of their metabolites is a key tool for understanding the impact of cannabinoid products on the body, as well as for overseeing their use in clinical and research settings. Cannabielsoin (CBE), as a metabolite of CBD, is a potentially valuable biomarker for such monitoring studies. The presence of CBE in urine, blood, or saliva may indicate CBD consumption, enabling the use of this metabolite to track cannabinoid intake even when CBD itself is no longer detectable in primary samples.
Modern analytical techniques such as chromatography and mass spectrometry allow for the detection of low concentrations of CBE in biological fluids, making it a convenient tool for conducting clinical or toxicological investigations. Through biomonitoring, it becomes possible to estimate the duration and dosage of CBD intake, as well as to determine individual variations in metabolism across different patients or population groups.
This capability can be valuable not only for clinical trial research but also for monitoring cannabinoid use in patients with chronic illnesses such as cancer or epilepsy, where cannabinoids are used as part of therapeutic protocols. Tracking CBD metabolites via CBE allows healthcare professionals to accurately control the levels of active compounds in the body and adjust drug dosages accordingly.
Additionally, cannabinoid biomonitoring may be used to assess environmental toxicology and the safety of cannabinoid product consumption among the general public. In recent years, there has been a marked increase in the availability of CBD-based products on the market, including dietary supplements, cosmetics, and beverages. Since cannabinoids may have cumulative effects with long-term use, it is important to monitor whether regular consumption of such products leads to the buildup of metabolites in the body. CBE, as one of those metabolites, may assist in monitoring efforts aimed at evaluating the safety of cannabinoid consumption.
The Role of Cannabielsoin (CBE) in Pharmacogenomics and Personalized Medicine
Cannabielsoin (CBE), as a metabolite of cannabidiol (CBD), plays a significant role in the context of pharmacogenomics and personalized medicine. Due to its unique biochemical characteristics, CBE may be important not only in determining the efficacy of cannabinoid-based therapies but also in the development of individualized treatment plans for patients. Individual variations in the metabolism of CBE, influenced by genetic traits and metabolic pathways, make it a promising tool in scientific research and clinical practice.
Individual Metabolic Profiles and Formation of CBE
Individual metabolic profiles of cannabidiol (CBD), and accordingly the formation of cannabielsoin (CBE), are a critical factor when considering pharmacogenomics. CBE is formed via the biotransformation of CBD, a process highly dependent on genetic variations, particularly those involving genes encoding metabolic enzymes. Cannabidiol is metabolized through the cytochrome P450 enzyme system and by glucuronidation during phase II metabolism, resulting in variable levels of metabolites in different individuals.
Genetic variants in CYP450 genes (e.g., CYP3A4, CYP2C19, CYP2C9) can significantly affect the rate of CBD metabolism and the production of CBE. This has important implications for determining optimal cannabinoid dosages for therapeutic use. In some patients with specific genotypes, metabolism may be either faster or slower, leading to the accumulation of metabolites or reduced therapeutic efficacy.
A personalized approach to cannabinoid therapy involves evaluating genetic factors that may influence metabolic pathways, enabling the development of the most effective treatment strategies. For example, patients with heightened CYP450 enzyme activity may metabolize CBD more rapidly, necessitating dose adjustments to achieve therapeutic efficacy. Conversely, those with reduced enzyme activity may require lower doses or alternative therapeutic options.
Variability in CYP450 Enzyme Activity
Cytochrome P450 enzymes (CYP450) are the primary targets responsible for cannabinoid metabolism, such as that of CBD, and play a central role in the conversion of CBD into its active and inactive metabolites, including the formation of cannabielsoin (CBE). However, genetic variations in these enzymes can result in substantial differences in the metabolic pathways of cannabidiol, affecting both pharmacokinetics and therapeutic outcomes.
Key genes involved in CBD metabolism include CYP2C9, CYP3A4, and CYP2C19. Research has identified a wide range of genetic polymorphisms in these enzymes that can significantly impact the rate of CBD metabolism. For instance, patients with allelic variants leading to reduced CYP3A4 or CYP2C9 activity will have slower CBD metabolism, resulting in higher concentrations of metabolites such as CBE. This is particularly relevant for adjusting cannabinoid-based drug dosages.
As these enzymes are also involved in the metabolism of many other medications, variations in their activity can lead to significant drug interactions. For example, the use of CYP3A4 inhibitors can reduce the metabolism of CBD, increasing CBE concentrations in the body, which may necessitate close monitoring of cannabinoid levels during treatment.
Parallel Research Pathways
Research on cannabielsoin (CBE) is not limited to pharmacogenetic aspects alone. Parallel lines of inquiry include its potential neuroprotective properties and interactions with other CBD metabolites, opening new opportunities for personalized medicine. Owing to its ability to interact with molecular targets in the body, CBE may become an important component in treating neurological disorders such as Alzheimer’s disease and stroke.
This provides a foundation for the development of innovative therapeutic approaches that incorporate individual genetic variations in metabolism alongside the neuroprotective potential of cannabinoids. Therefore, exploring CBE and its influence on metabolic pathways, as well as its interactions with other drugs, is a critical direction for establishing more accurate and effective therapeutic strategies.
CBE in the Context of Neuroprotection
Recent studies indicate that cannabielsoin (CBE) may possess neuroprotective properties similar to other cannabinoids, particularly cannabidiol. As a metabolic product of CBD, CBE may retain some of CBD’s beneficial effects while exhibiting fewer psychoactive properties. This can be of great importance in the development of new therapeutic agents for neurological disorders.
The neuroprotective effects of CBE may be attributed to its ability to reduce oxidative stress, which plays a central role in the progression of neurodegenerative diseases such as Alzheimer’s and Parkinson’s. Oxidative stress arises from the accumulation of reactive oxygen species (ROS) within cells, leading to the damage of membranes, proteins, and DNA. Cannabidiol metabolites like CBE may help lower ROS levels, thereby protecting neurons from damage.
Potential Role in Regulating Oxidative Stress
One of the most promising directions in CBE research is its role in regulating oxidative stress. Oxidative stress is a major contributing factor to the progression of many chronic conditions, including neurodegenerative disorders, cardiovascular diseases, and cancer. Cannabielsoin, as a CBD metabolite, has demonstrated the capacity to interact with cellular antioxidant defense mechanisms, particularly by activating various antioxidant enzymes such as superoxide dismutase (SOD) and catalase.
This has significant implications for developing therapeutic agents that can be used to treat conditions associated with high oxidative stress levels. Utilizing CBE as a component to reduce ROS levels in the body could form the basis for new strategies in treating age-related diseases and central nervous system disorders.
Interaction with Phase II Enzymes: Glucuronidation
A crucial characteristic of CBE metabolism is its interaction with phase II metabolic enzymes, especially those involved in glucuronidation. Phase II metabolism involves reactions that attach a glucuronic acid moiety to molecules, thereby facilitating their excretion from the body. In the case of CBE, glucuronidation is the primary pathway for detoxification and elimination.
Cannabielsoin is glucuronidated by UDP-glucuronosyltransferase (UGT) enzymes, which are essential for the metabolism of many pharmaceuticals. Variations in genes encoding these enzymes may influence the rate at which CBE is metabolized and eliminated, which is important for optimizing cannabinoid dosage in medical applications. Understanding the mechanisms behind CBE glucuronidation is essential for improving the pharmacokinetics of cannabinoids and ensuring more precise control of active metabolite levels in the body.
Bioanalytical Methods for Studying Cannabielsoin (CBE) and Its Metabolites
In scientific and medical research on cannabinoids such as cannabielsoin (CBE), a critical aspect involves the application of bioanalytical methods to accurately study their pharmacokinetics, metabolism, and biological effects. Bioanalysis encompasses the use of various techniques to quantitatively determine cannabinoids in biological matrices such as blood, urine, saliva, and tissues. To achieve this, it is essential to develop reliable and sensitive methods capable of accurately detecting low concentrations of cannabielsoin in complex biological environments.
Calibration Methods for CBE in Biological Matrices
Calibrating analytical methods for detecting cannabielsoin in biological matrices is a key component of bioanalytical research. Given that CBE levels in biological samples are typically low, highly sensitive methods must be employed to precisely determine its concentrations.
One of the most commonly used techniques for measuring cannabielsoin concentrations is high-performance liquid chromatography coupled with mass spectrometry (HPLC-MS). This method allows for precise quantification of low-level metabolites in biological samples with high specificity and sensitivity. Calibration of these methods involves the use of standard solutions of cannabielsoin prepared at defined concentrations. Calibration curves are constructed based on the relationship between metabolite concentration and signal intensity obtained during analysis. A crucial part of calibration also includes determining the limit of detection (LOD) and limit of quantitation (LOQ) to ensure measurement accuracy at low concentration levels.
Other methods, such as gas chromatography-mass spectrometry (GC-MS), are also used to detect cannabielsoin, but they tend to be less sensitive than HPLC-MS, particularly when analyzing biological samples with low metabolite concentrations. Nevertheless, GC-MS can be valuable for elucidating structural characteristics of metabolites due to its capability for detailed mass spectrometric analysis.
Differentiation of Artifacts and Conversions Under Laboratory Conditions
One of the main challenges in using bioanalytical methods is differentiating between true metabolites and artifacts or conversion products that may form during storage or processing of biological samples. Cannabinoids and their metabolites are prone to chemical transformations under changing storage conditions (e.g., temperature, pH), which may lead to the formation of false or secondary products. These can distort results and complicate accurate determination of metabolite concentrations.
To minimize the risk of artifacts, it is essential to employ methods capable of detecting specific metabolite markers, particularly through the use of mass spectrometry. One approach involves selecting specific molecular fragments (ions) formed during metabolite ionization, which helps to avoid false signals from byproducts. Additionally, research involves the use of stable isotope-labeled internal standards, which allow for correction of errors caused by molecular transformations during storage or sample handling.
Differentiation between artifacts and true metabolites can also be achieved by comparing results with standard reference samples or by using a combination of analytical methods. For instance, integrating mass spectrometry with liquid chromatography enables precise distinction between cannabielsoin and potential artifacts, such as structurally distinct metabolites.
Prospects for Further Research
As cannabielsoin is a relatively novel cannabidiol-derived metabolite, more detailed research into its biological activity and metabolic pathways is needed. One promising direction involves studying its interactions with various metabolic enzymes, particularly those from the cytochrome P450 system and phase II enzymes responsible for glucuronidation. Understanding this process could lead to the development of new therapeutic strategies involving cannabinoids for the treatment of various diseases.
Additionally, there is significant interest in studying the effects of cannabielsoin on oxidative stress and neuroprotection, especially in the context of neurodegenerative diseases. These studies may contribute to the development of new pharmacological agents for combating Alzheimer’s and Parkinson’s disease.
On another front, exploring the pharmacokinetics and pharmacodynamics of cannabielsoin in different biological matrices will help determine its efficacy and safety at therapeutic doses. More precise data are needed on the various metabolic pathways depending on the patient’s genetic profile, which would enable the development of personalized treatment regimens tailored to an individual’s genetic makeup.
Use of Cannabinoid Metabolomics in Bioinformatics
With advancements in bioinformatics, modern approaches allow for the creation of models of cannabinoid metabolic pathways, including cannabielsoin, facilitating the prediction of their biological activity, pharmacokinetics, and potential clinical effects. Studying cannabinoid metabolomes using bioinformatic platforms enables the construction of integrated models of metabolic pathways that can aid in forecasting metabolic transformations and drug interactions.
This is especially relevant for future research on cannabinoids in personalized medicine, as it enables consideration of individual metabolic differences and the development of the most effective therapeutic strategies for each patient. Bioinformatics can also be utilized to develop tools for identifying potential new metabolites of cannabielsoin and assessing their biological effects.
The Need for Multicenter Validation Studies
As research on cannabielsoin is still in a phase of active development, there is a pressing need to conduct multicenter validation studies to evaluate the accuracy and reliability of bioanalytical methods for detecting this metabolite. The importance of such studies lies in their ability to ensure standardization of methodologies and establish international criteria for determining cannabielsoin concentrations in biological matrices.
Validation studies should include different sample types (blood, urine, saliva, tissues) and a range of metabolite concentrations to ensure accurate measurements across a broad spectrum. These studies also facilitate comparisons of the effectiveness of different bioanalytical techniques and help determine the optimal approaches for their use in both medical and scientific applications.
Conclusion
All the aforementioned aspects of studying Cannabielsoin (CBE) highlight the importance and complexity of this cannabidiol metabolite in the context of bioanalytical, pharmacokinetic, and pharmacodynamic research. CBE, as one of the active cannabinoids, has numerous potential applications in medical practice, particularly in the areas of neuroprotection, regulation of oxidative stress, and personalized medicine. However, for the effective use of Cannabielsoin in therapeutic settings, it is crucial to thoroughly investigate its mechanisms of action, metabolism, and interactions with other drugs and biological systems.
The pharmacokinetics and metabolism of Cannabielsoin continue to be key factors in evaluating its safety and effectiveness. High-sensitivity bioanalytical methods need to be developed to accurately measure the low concentrations of this metabolite in biological samples. Modern methods, such as high-performance liquid chromatography coupled with mass spectrometry, demonstrate high precision and sensitivity, but continuous improvements in calibration processes and artifact minimization are essential for obtaining reliable results.
Differentiating artifacts and conversions in laboratory conditions is an important part of ensuring the accuracy of bioanalytical methods. This allows for the acquisition of trustworthy data, which forms the foundation for further use of Cannabielsoin in medical and clinical research.
An essential part of future studies is the bioinformatics of cannabinoid metabolites, which enables the creation of models of metabolic pathways, prediction of biological activity, and interactions between Cannabielsoin and other metabolites and drugs. This opens new possibilities for treatment individualization and the development of new therapeutic strategies for patients with different genetic and metabolic profiles.
The prospects of further research lie in conducting multi-center validation studies, which will allow for the standardization of bioanalytical methods and the determination of optimal parameters for measuring Cannabielsoin in various biological matrices. These studies will also contribute to the development of international criteria for evaluating the concentrations of Cannabielsoin and its metabolites, an important step in the medical use of cannabinoids.
The role of Cannabielsoin in personalized medicine and pharmacogenomics has great potential due to its ability to account for individual patient metabolism profiles. Variability in the activity of CYP450 enzymes involved in Cannabielsoin metabolism is an important factor that may influence the efficacy and safety of cannabinoid therapies. Understanding this process will allow for the creation of more precise and safe treatment strategies based on the genetic characteristics of each patient.
Therefore, for a complete understanding of Cannabielsoin’s potential, research must continue, particularly in the areas of pharmacogenetics, metabolism, bioanalytics, and clinical practice. The application of cutting-edge bioanalytical methods and bioinformatics platforms for analyzing cannabinoid metabolites will enable more accurate prediction of Cannabielsoin’s effects, ensuring the effective and safe use of this metabolite for medical purposes.
Sources:
- National Institutes of Health (NIH)
PubMed (https://pubmed.ncbi.nlm.nih.gov/) – PubMed is a database of peer-reviewed scientific articles in the field of medicine and biology. You can find articles related to cannabinoid metabolism, such as Cannabielsoin (CBE), pharmacokinetics, and interactions with various enzymes.
- National Institute on Drug Abuse (NIDA)
NIDA – Cannabinoids (https://www.drugabuse.gov/drug-topics/marijuana) – NIDA is part of NIH and specializes in research related to drugs and cannabinoids, their pharmacology, and biology.
- U.S. National Library of Medicine (NLM)
Toxicology Data Network (TOXNET) (https://toxnet.nlm.nih.gov/) – A database containing important information on the toxicological properties of substances, including cannabinoids, and their potential effects on the body.
- Centers for Disease Control and Prevention (CDC)
CDC – Marijuana and Public Health (https://www.cdc.gov/marijuana/index.htm) – CDC provides data and recommendations on the impact of cannabinoids on health, including research on their mechanisms of action and metabolism.
- Journal of Pharmacology and Experimental Therapeutics
J Pharmacol Exp Ther (https://jpet.aspetjournals.org/) – A scientific journal publishing research on pharmacokinetics, cannabinoid metabolism, and their pharmacological effects.
- The Journal of Clinical Investigation
J Clin Invest (https://www.jci.org/) – A renowned journal publishing high-quality research on biochemistry and pharmacology, including cannabinoids and their effects on the body.
- ScienceDirect
ScienceDirect – Cannabinoids (https://www.sciencedirect.com/topics/pharmacology-toxicology-and-pharmaceutical-science/cannabinoid) – A platform providing access to articles on pharmacology and biology. You can find studies on cannabinoids and their metabolism, including the role of CYP450 enzymes.