Cannabifuran (CBF) is a chemical compound belonging to the class of synthetic cannabinoids, which mimic the biochemical activity of natural cannabinoids isolated from plants of the Cannabis genus. Although natural cannabinoids have been extensively studied-such as tetrahydrocannabinol (THC) and cannabidiol (CBD)-Cannabifuran is characterized by unique structural features that define its specific pharmacological activity profile. The study of the CBF molecule is gaining importance both in the context of chemical synthesis and biomedical research, given its potential in various fields including pharmacology, toxicology, and biotechnology.
The molecular structure of Cannabifuran is distinguished by the presence of a furan ring fused with the core cannabinoid skeleton, which provides high lipophilicity and specific interaction properties with the receptors of the endocannabinoid system. This feature governs the fine regulation of the molecule’s binding to CB1 and CB2 receptors, which are central components of neuromodulatory and immune processes in the body. Differences in receptor affinity and activation of secondary signaling cascades place CBF among substances with a potentially broad spectrum of biological effects.
Considering its chemical structure, the synthesis of Cannabifuran represents a complex process that requires precise control of reaction conditions and the selection of appropriate precursors and catalysts. Scientific attention to methods of obtaining CBF is driven not only by the desire to produce a pure and stable product but also by the need to explore structural variability in search of optimal pharmacological properties. Modern synthetic methods include classical organic reactions as well as innovative approaches employing transition metal catalysts and “green” chemical technologies that reduce environmental impact and improve product yield.
Natural sources of Cannabifuran remain insufficiently studied, but there is evidence of potential biosynthetic activity in certain strains of Cannabis sativa, where enzymatic systems contribute to the formation of derivatives containing a furan ring. Beyond traditional plant-based pathways, biotechnological methods of synthesis using recombinant microorganisms are being investigated, opening new prospects for large-scale production of CBF with specified characteristics. Analysis of metabolic pathways and identification of enzymes catalyzing the formation of furan structures are key for the development of efficient biosynthetic platforms.
The pharmacological profile of Cannabifuran includes studying its interaction with receptors of the endocannabinoid system, which determines its potential to influence a wide range of biological processes, including neuromodulation, immune regulation, and metabolic activity. The mechanisms of CBF action are investigated using experimental in vitro and in vivo models, allowing determination of its effects on cellular signaling cascades as well as its pharmacokinetic profile, which characterizes the rate and pathways of metabolism, bioavailability, and half-life.
From a toxicological perspective, Cannabifuran is of interest as a synthetic cannabinoid for which safe use limits, potential side effects, and mechanisms of toxic action need to be established. Standardized methods for studying acute and chronic toxicity, as well as assessments of carcinogenicity and mutagenicity, are essential components in defining the safety profile of CBF. These data are critical not only for pharmaceutical development but also for understanding health impacts in cases of accidental or intentional misuse.
Analytical methods for studying Cannabifuran include spectroscopic techniques (NMR, IR spectroscopy, UV spectroscopy), chromatographic methods (HPLC, GC-MS), and mass spectrometry, which provide comprehensive characterization of the chemical structure, purity, and stability of samples. Quality control of the product requires validation of analytical methods that meet stringent criteria for accuracy, reproducibility, and specificity. The storage and stabilization of Cannabifuran are also studied to determine optimal conditions that prevent degradation and preserve biological activity.
Chemical Structure and Physicochemical Properties
Cannabifuran (CBF) belongs to the group of synthetic cannabinoids characterized by a unique molecular structure that determines their physicochemical properties and biological activity. This compound is distinguished by the presence of a furan ring fused with the cannabinoid skeleton, which significantly influences its lipophilicity, stability, and reactivity. The structural configuration of CBF defines its ability to specifically interact with molecular targets, particularly receptors of the endocannabinoid system, making it the subject of active research in biochemistry and pharmacology.
The core chemical structure of Cannabifuran consists of a five-membered furan ring saturated with one oxygen atom, creating specific electronic conditions within the molecule. The attachment of the furan fragment to the cannabinoid core forms a compound with increased electron density in certain regions of the molecule, which affects its physical and chemical parameters such as solubility in various media, melting point, and resistance to oxidation. These characteristics are fundamental to understanding the behavior of CBF in biological systems and during synthesis.
The physicochemical properties of Cannabifuran are closely related to its molecular geometry and electronic structure. Lipophilicity, a critical parameter for the molecule’s ability to penetrate cellular membranes, is ensured by the presence of nonpolar hydrophobic groups in its structure. This property determines CBF’s capacity for passive diffusion in lipid environments and, accordingly, influences its bioavailability and pharmacokinetic characteristics.
The stability of Cannabifuran is determined by both its internal structure and external environmental conditions. The molecule exhibits moderate thermal stability but is subject to photolysis and oxidation in the presence of oxygen and light, which is important for storage and transportation. Chemical stability also depends on the pH of the environment: hydrolytic or condensation reactions can occur in acidic or alkaline solutions, altering the primary structure and, consequently, the biological activity.
Cannabifuran’s interaction with various solvents shows variable solubility due to the specificity of its electronic structure and molecular polarity. CBF dissolves best in nonpolar and weakly polar organic solvents such as chloroform, dichloromethane, and lipid environments. Its water solubility is low, which is typical for most cannabinoids and limits the compound’s use in aqueous biological systems without additional carriers or emulsifiers.
The chemical structure of Cannabifuran also determines its ability for conformational flexibility, which is important for interaction with biological receptors. Variations in the spatial arrangement of functional groups provide adaptability of the molecule to different receptor types, explaining the diversity of biological effects recorded in experimental studies. Conformational variability also influences pharmacodynamic properties, particularly affinity to receptor sites.
Additionally, Cannabifuran exhibits characteristic spectroscopic properties, reflected in distinct absorption bands in the UV-visible spectrum, as well as specific signals in NMR spectra. These physicochemical characteristics serve as the basis for identification and purity control of CBF, which is crucial in synthesis processes and further applications. The use of spectroscopy allows obtaining detailed information about the electronic structure and conformation of the molecule.
Physicochemical properties of Cannabifuran also define its behavior in complex systems such as biological membranes or pharmaceutical formulations. Interactions with proteins, lipids, and other biomolecules depend on electrostatic and van der Waals forces, which are critical for forming stable complexes. This affects pharmacological activity and the distribution of the molecule in living organisms.
Molecular Formula and Structure
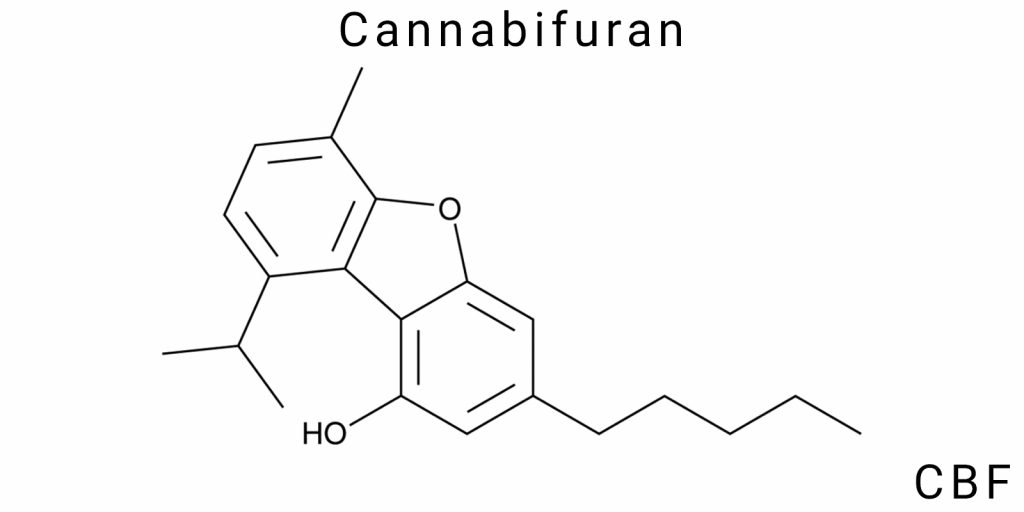
Cannabifuran (CBF) has a clearly defined molecular formula reflecting the quantitative composition of atoms in the molecule and serving as a basis for its spatial and electronic structure. The formula of CBF is C21H26O2, indicating the presence of 21 carbon atoms, 26 hydrogen atoms, and 2 oxygen atoms. This formula shows that the molecule contains two oxygen-containing functional elements, structurally presented as a furan ring, which is an invariant component of the molecule and determinative for its chemical specificity.
The structure of Cannabifuran consists of fused rings, where the base is a tricyclic aromatic cannabinoid core, to which a five-membered oxygen-containing furan ring is attached. A feature of the molecule is the spatial arrangement of these rings, creating a specific three-dimensional conformation with certain bond angles and spatial constraints. The conformation is defined both by the rigidity of the furan ring and the mobility of the side chains, collectively influencing physicochemical parameters and interaction with biological targets.
The cyclic system of the molecule is formed by an aromatic benzene ring, to which additional cyclic fragments and aliphatic side chains are attached. These chains include saturated carbon fragments of varying lengths, serving as modulators of lipophilicity and chemical stability. The structural complexity of the CBF molecule allows it to be classified as a polycondensed aromatic ether with a complex architecture, where electron density is localized in specific segments, critical for reactivity.
Chemical bonds in the molecule have specific length and angle parameters that determine electron distribution and geometric properties. In particular, the oxygen in the furan ring forms σ- and π-bonds, providing partial conjugation with the aromatic system. This creates zones of increased electron density that enhance the electrophilicity of molecular fragments and influence mechanisms of chemical interaction.
The spatial orientation of functional groups determines the potential for forming intramolecular hydrogen bonds, which stabilize the conformation and minimize the energy potential. Molecular modeling analysis shows that aliphatic side groups are arranged to avoid steric hindrance, increasing the thermodynamic stability of the structure.
Cannabifuran is an example of a molecule with a heterocyclic ring containing oxygen, which increases the polarity of local regions, creating dipole moments. These moments are important for interactions with biological receptors as well as for solubility in polar and nonpolar solvents. The molecular dipole moment is significant for predicting pharmacokinetic characteristics and transport across cellular membranes.
The specificity of Cannabifuran’s molecular structure lies in the optimal balance between rigid cyclic systems and flexible side chains, providing the molecule with the ability to adapt to various spatial conditions in receptor zones. This property is essential for selective action and high affinity to certain cannabinoid receptor subtypes.
The electronic structure of the molecule is characterized by the distribution of π-electrons in aromatic and furan rings, creating potential for participation in electrophilic and nucleophilic reactions. Such distribution defines chemical activity, including susceptibility to oxidative processes and the formation of stable radical intermediate compounds.
The intense charge distribution across the molecule determines its potential for interaction with cationic and anionic environments, which is practically important for the synthesis of derivatives with modified properties, as well as for pharmacodynamic studies. Intramolecular forces influence molecular configuration and stabilize it in certain electronic states.
Besides the main molecule, the structure may include isomers with variations in the arrangement of side chains or the configuration of the furan ring. Isomerism can affect pharmacological activity, stability, and pharmacokinetics, highlighting the need for detailed study of CBF stereochemistry.
Modern methods of molecular modeling and X-ray crystallography allow obtaining precise three-dimensional structures of Cannabifuran, facilitating understanding of its interaction with biomolecular targets at the atomic level. These studies are critical for the development of synthetic analogs with improved pharmacological characteristics.
Physicochemical Characteristics (Solubility, Stability, Spectroscopy)
The physicochemical characteristics of Cannabifuran (CBF) are key to determining its behavior in various environments, as well as for controlling its quality and efficacy in research and applied processes. Solubility, stability, and spectroscopic properties of CBF characterize its interaction with the external environment, which is fundamental for analyzing its bioavailability and chemical identification.
The solubility of Cannabifuran is determined by its molecular structure and polarity, directly affecting the molecule’s ability to disperse in different solvents. CBF exhibits low water solubility due to its high lipophilicity, which is associated with the presence of a significant portion of nonpolar carbon chains and aromatic fragments in its structure. However, its solubility in nonpolar and weakly polar organic solvents such as hexane, toluene, chloroform, and dichloromethane is high, reflecting the molecule’s tendency to dissolve in environments with similar physicochemical properties. This characteristic is critical for preparing solutions for chromatographic analysis and for developing pharmaceutical formulations.
CBF’s solubility in water is on the order of lower fractions of milligrams per milliliter, which substantially limits its use in aqueous environments without the application of additional adjuvants or carriers, such as cyclodextrins or microemulsions. This physicochemical feature necessitates the use of specific technologies to improve bioavailability and form stable pharmaceutical preparations.
The stability of Cannabifuran is determined by its sensitivity to environmental conditions, specifically exposure to light, oxygen, temperature fluctuations, and pH. CBF’s photolability is associated with the ability of the furan ring to degrade under ultraviolet radiation, leading to the breaking of π-bonds and the formation of free radicals. This phenomenon requires careful control of storage conditions, especially avoiding direct sunlight and using protective packaging with UV filters.
The oxidative stability of the molecule reflects its reactive capacity toward atmospheric oxygen. The presence of an electron-rich furan fragment creates zones of heightened chemical activity where the molecule may transition into reactive intermediate states with the formation of peroxides and other oxidative products. Such processes alter the primary structure of CBF, reduce its efficacy, and can lead to the formation of toxic metabolites, highlighting the need for antioxidant stabilizers during long-term storage.
The thermostability of Cannabifuran is limited, manifesting as gradual decomposition at elevated temperatures above 120°C. Decomposition is accompanied by the destruction of the aromatic core and opening of the furan ring, resulting in the loss of molecular integrity and alteration of pharmacological activity. This feature influences the choice of synthesis, storage, and processing conditions for CBF, particularly in pharmaceutical manufacturing and research.
The spectroscopic characteristics of Cannabifuran include UV-Visible, infrared (IR), nuclear magnetic resonance (NMR), and mass spectrometry, which are integral to its identification and structural analysis.
UV-Visible spectroscopy of CBF demonstrates characteristic absorption bands in the 250-320 nm range, associated with π→π* transitions in the aromatic system and n→π* transitions of oxygen lone pair electrons in the furan ring. The intensity and position of these peaks depend on the solvent and temperature, enabling the use of this method for quantitative analysis and concentration determination in solutions.
Infrared spectroscopy reveals typical bands corresponding to the molecule’s functional groups. The absence of a broad band in the 3200-3600 cm⁻¹ region confirms the lack of free hydroxyl groups, while distinct bands in the 1000-1300 cm⁻¹ region correspond to the C-O-C bonds of the furan ring. Peaks in the 1500-1600 cm⁻¹ range reflect aromatic C=C bonds, confirming the preservation of the conjugated system. These spectral features are critical for purity control and identification of CBF.
NMR spectroscopy, particularly proton (¹H) and carbon (¹³C) NMR, provides detailed information about the chemical environment of atoms within the molecule. Proton chemical shifts in the aromatic region are found between 6.5 and 7.5 ppm, while aliphatic groups show signals in the lower 0.8-2.5 ppm range. Specific shifts related to atoms within the furan ring help confirm conformation and identify possible isomers. Carbon NMR shows signals corresponding to different carbon types, including aromatic, aliphatic, and heterocyclic carbons.
Mass spectrometry of CBF reveals a molecular ion peak corresponding to the molecular weight of 310 g/mol, with a characteristic set of fragments reflecting cleavage pathways within the molecule. Fragmentation allows differentiation of structures containing the furan ring and aliphatic chains, which is important for confirming the molecular structure and controlling the synthesis product.
Chemical Reactivity
The chemical reactivity of Cannabifuran (CBF) is determined by its structural features-the presence of a furan ring, an aromatic core, and side aliphatic chains-that interact with various reagents through specific mechanisms. These mechanisms define the range of possible reactions in which CBF can participate, as well as determine its chemical stability, transformations, and potential pathways for molecular modification.
The primary reactivity of the molecule is driven by the electron-rich nature of the furan ring. This heterocyclic ring containing oxygen exhibits high reactivity in electrophilic substitution reactions, nucleophilic ring-opening, and redox processes. Opening of the furan ring can occur under the influence of strong acids or bases, leading to cleavage of C-O bonds and the formation of reactive aldehydes or ketones. Such reactions are fundamental for producing derivatives with altered functionality.
Within the aromatic core of CBF, classical electrophilic aromatic substitution reactions are observed, which can proceed with the formation of various derivatives by replacing hydrogen with nucleophilic or electrophilic groups. Importantly, the presence of the furan ring and side aliphatic groups significantly modulates the electron density in the aromatic system, altering the reactivity of substitution sites. This interaction influences the selectivity of the reaction, enabling the synthesis of targeted functionalized compounds.
The interaction of Cannabifuran with oxidizing agents represents a distinct class of reactions where the molecule undergoes transformations involving electron transfer. Oxidation of the furan ring in the presence of peroxides or strong oxidizers leads to the formation of lactones, carboxylic acids, or other oxygenated products that alter the physicochemical properties and biological activity. Oxidative processes may be catalyzed by metal ions or proceed under light exposure, which is associated with the photochemical lability of CBF.
Reduction reactions of Cannabifuran primarily target the transformation of oxygen-containing functional groups. Upon treatment with reducing agents such as metal hydrides (NaBH4, LiAlH4), the furan ring may be reduced, forming hydrogenated cyclic derivatives or hydroxylated analogs. These reactions are important for synthesizing analogs with modified pharmacological properties.
The nucleophilic reactivity of CBF is manifested in its ability to interact with electrophilic centers due to the donor properties of the oxygen in the furan ring. Specifically, CBF can form adducts with active halogens or isotopic agents, opening possibilities for creating labeled derivatives in radiochemical studies. Nucleophilic substitution is also used in synthetic schemes modifying the side chains to increase selectivity.
Cannabifuran undergoes condensation and cyclization reactions resulting from interactions between functional groups within the molecule or with additional reagents. These reactions facilitate the formation of new rings or macrocycles, significantly expanding the chemical space of CBF derivatives. An important aspect is the choice of reaction conditions (pH, temperature, catalysts), which determine the kinetic and thermodynamic parameters of the process.
Polymerization and polymer-binding reactions involving CBF, although less studied, hold potential for creating new materials with specific properties. Mechanisms include radical and ionic initiations, where the furan ring may serve as an active center for bonding with other monomers or polymers. This opens prospects for developing functional polymers with biological activity.
Cannabifuran is capable of forming complexes with metal ions, defining its coordination chemistry. Coordination bonds are predominantly formed through the oxygen in the furan ring, granting the molecule the ability to interact with transition metal cations. These properties are exploited for catalyst development and the study of organometallic systems with specific reactivity.
The chemical reactivity of CBF is also influenced by its propensity for radical processes, particularly the formation of free radicals under ultraviolet light or chemical initiators. Radical mechanisms participate in decomposition, polymerization, and functional group transformations, which is important for understanding the stability and chemical behavior of the molecule under extreme conditions.
An important aspect includes reactions with isocyanates and isocyanurate compounds, which can interact with amino or hydroxyl groups present in CBF derivatives, enabling the synthesis of polymers or conjugates for biomedical applications.
Methods of Synthesizing Cannabifuran
The synthesis of Cannabifuran (CBF) involves a complex approach based on the sequential execution of chemical reactions that allow the formation of its unique structure with high selectivity and product purity. The primary synthesis methods include various strategies for constructing the furan ring, forming the aromatic system, and integrating aliphatic side chains into the molecule. Each synthesis step requires precise optimization of conditions, selection of reagents, and process control methods to achieve the desired outcome.
Structurally, the synthesis of Cannabifuran can be divided into three key stages: formation of the heterocyclic core, substitution of the aromatic ring, and condensation with aliphatic fragments. These stages require the use of different types of reactions such as cyclization, electrophilic substitution, alkylation, and specific redox processes, which together ensure the gradual buildup of the molecule.
The first stage, formation of the furan ring, is carried out both through classical cyclization reactions using diols, aldehydes, and ketones, as well as through more modern approaches, for example, using catalytic systems that promote selective heterocycle formation. Precise control of reaction conditions such as temperature, pH, and duration determines the yield and stability of the heterocyclic core.
The second stage, modification of the aromatic system, involves electrophilic and nucleophilic substitution reactions implemented using specific reagents capable of interacting with designated positions on the ring. The choice of method depends on the kinetic parameters and selectivity of the reaction, which are critical for obtaining structurally homogeneous products.
The third stage of synthesis is related to attaching aliphatic side chains, carried out via alkylation, acylation, or through the formation of complex esters and amides. This stage also requires optimization of reagents and conditions to avoid side reactions and ensure maximum yield of the final product.
Successful synthesis requires consideration of the reactivity of intermediate compounds, their stability, and potential unwanted reactions such as decomposition or polymerization. Therefore, various reaction environment control techniques are often applied, including inert atmospheres, cooling or heating with temperature control, and the use of specific solvents.
In addition to traditional methods, modern synthesis technologies include the use of microwave heating, ultrasonic treatment, and automated reagent dosing systems, which increase process efficiency and reduce the formation of side products.
Furthermore, to enhance yield and selectivity, diverse approaches to controlling the stereochemistry of molecule formation are employed, an important factor in the synthesis of biologically active compounds. Stereospecific synthesis is often realized through the use of chiral catalysts or chiral intermediates, ensuring the targeted production of isomers.
For scaling up the Cannabifuran synthesis process, methods that ensure reproducibility and stability of reaction conditions are applied, as well as techniques that simplify product purification. These include the use of reactors with automatic parameter control, modular synthesis systems, and flow technologies that contribute to more precise regulation of reaction kinetics and thermodynamics.
An important component of the synthesis methods is intermediate product monitoring using analytical techniques such as thin-layer chromatography, spectroscopy, and mass spectrometry. These methods allow assessment of intermediate purity and determination of the optimal timing for transition to the next synthesis stage.
Chemical Synthesis: Description of Key Reactions and Reagents
The chemical synthesis of Cannabifuran is based on a series of reactions that provide the formation of the molecule’s complex structure, which includes the furan ring, aromatic core, and specific aliphatic substituents. The synthesis is founded on the stepwise organization of molecular fragments through reactions characterized by high selectivity and chemical specificity.
The first key step is the formation of the heterocyclic furan core. Typically, this involves cyclization reactions of aldehydes or ketones with 1,4-diols or hydroxyaldehydes in the presence of acid catalysts. The reaction proceeds through intermediate stages forming oxocarbonyl compounds, which undergo intramolecular electrophilic attack, leading to cyclization. Both mineral acids (such as sulfuric or phosphoric acid) and organic acid catalysts are used, allowing regulation of the reaction rate and stability of intermediates.
The next important step is alkylation of the aromatic core. This process is performed through Friedel-Crafts reactions, where the aromatic ring undergoes electrophilic substitution by alkyl groups. The use of alkyl halides in the presence of acid catalysts such as AlCl3 facilitates the formation of carbocation intermediates that attach to specific positions on the aromatic ring. Control of reaction conditions, particularly temperature and molar ratios of reagents, is crucial to avoid polyalkylation and side products.
Oxidation reactions represent another critical point in the synthesis. Cannabifuran undergoes selective oxidation to introduce oxygenated functional groups into side chains or the core. Medium-strength oxidizers such as chromates or peroxides, as well as metal-based catalysts (e.g., rhodium or palladium), are often employed to promote high reaction selectivity. Oxidation allows the formation of aldehydes, carboxylic acids, or lactone structures that are key for subsequent reactions or final molecule properties.
Nucleophilic substitution reactions are also widely used in the synthesis, enabling the introduction of amino groups or other nucleophilic functional groups into the molecule. The use of activated halogenated derivatives combined with amines, alcohols, or thiols creates opportunities for obtaining diverse derivatives important for regulating the pharmacological activity of CBF. Choice of solvent and temperature regime are key factors influencing reactivity and selectivity.
Condensation reactions play a significant role in constructing complex Cannabifuran structures. Interactions of aldehydes or ketones with amines result in the formation of imines or Schiff bases, providing stable functional groups within the molecule. These reactions are often catalyzed by acids or bases, altering kinetic parameters and allowing selection of specific isomers.
Additionally, reduction reactions are important for transforming carbonyl groups into hydroxyl or alkyl groups. The use of reducing agents such as borohydrides or aluminum hydrides facilitates selective reduction without breaking heterocyclic structures. These reactions are necessary for obtaining final molecular forms with desired physicochemical properties.
To form specific C-C and C-O bonds, cross-coupling reactions such as Suzuki, Heck, or Sonogashira are applied, involving catalysis by palladium complexes. These methods enable the construction of complex structures by attaching aromatic or aliphatic fragments, ensuring high chemical selectivity and scalability of the synthesis.
Protection and deprotection reactions of functional groups are employed to prevent unwanted side reactions. Protective groups are introduced as ethers, acetals, or silyl ethers, which are stable under reaction conditions and easily removed at final stages. The choice of protective group depends on the specifics of the reaction environment and compatibility with other functional groups in the molecule.
Photochemical reactions are also significantly applied, where the Cannabifuran molecule is activated by ultraviolet or visible light. This allows initiation of radical processes, opening additional pathways for molecular modification, including introduction of new functional groups or conformational changes.
Use of Catalysts in Synthesis
Catalysts play a fundamental role in the chemical synthesis of Cannabifuran, enhancing reaction rates, product selectivity, and lowering the energy barriers for chemical transformations. In the synthesis conditions, which involve complex multistep reactions, catalysts act as key agents that enable difficult or thermodynamically unfavorable reactions to proceed. Depending on the type of reaction and target functional groups, various classes of catalysts are employed, among which the main ones are acidic, basic, organometallic, and biocatalysts.
Acidic catalysts hold a leading position in the synthesis of Cannabifuran, especially in cyclization and electrophilic substitution reactions. Solid acidic catalysts based on zeolites or sulfonated polymers provide high activity and regenerability, which is critical for industrial-scale synthesis. Their acidic centers activate carbonyl groups, increasing their electrophilicity and facilitating intramolecular cyclization. The use of such catalysts allows control of selectivity due to the size and electronic effects of the porous zeolite structure, which limits access to active sites by certain molecular conformations.
Basic catalysts are applied in reactions requiring the initiation of nucleophilic attack or stabilization of reaction intermediates. Basic metal oxides, such as magnesium oxide or calcium oxide, activate hydroxyl and amino functions, promoting the formation of esters or amides. The use of basic catalysts is particularly important in alkylation and nucleophilic substitution steps, where reagent conversion is regulated and side product formation is minimized.
Organometallic catalysts form the basis of modern synthesis of complex organic compounds, including Cannabifuran. Palladium and rhodium complexes are widely used in cross-coupling reactions such as Suzuki, Heck, and Sonogashira reactions. These catalysts activate halogenated functional groups, enabling the formation of carbon-carbon bonds at relatively low temperatures and with high regioselectivity. A characteristic feature is the catalysts’ ability to undergo cyclic oxidation state changes, allowing repeated reuse during catalysis. Ligand-dependent systems are of particular interest, enabling fine-tuning of the catalyst’s electronic and steric properties, influencing reaction kinetics and stereochemistry.
Iridium and platinum catalysts are used in certain oxidation and hydrogenation reactions. Specifically, they provide selective hydrogenation of carbonyl groups without damaging the heterocyclic system, which is important for producing stable final products. Using such catalysts requires control of reaction parameters, particularly pressure and temperature, to avoid excessive reduction or degradation.
Biocatalysts, especially oxidative enzymes and hydrolytic enzymes, are employed in Cannabifuran synthesis to introduce stereospecific functional groups. Enzymatic catalysts enable reactions under mild conditions, in aqueous media, with high regio- and stereochemical selectivity. The use of enzymes such as oxidases, esterases, and lipases allows for the formation of chiral centers, which is critical in synthesizing biologically active compounds.
Heterogeneous catalysts are applied to facilitate purification and regeneration processes and to enhance the environmental safety of the synthesis. Their advantages include the possibility of multiple reuse and the absence of complex separation requirements. Nanocluster catalysts, particularly those based on gold and silver, demonstrate high activity in oxidation and hydrogenation reactions, offering new prospects for optimizing Cannabifuran synthesis.
Kinetic control of reactions is achieved not only by selecting the type of catalyst but also by adjusting its concentration, form of delivery (e.g., nanoparticles, complexes, or immobilized on supports), and synergistic effects when using mixed catalytic systems. Interaction between different catalysts can accelerate reactions or alter selectivity, enabling adaptation of synthesis to specific requirements.
Regulation of catalytic activity also occurs through modification of ligand structure and their electronic and steric properties. Ligands with donor or acceptor capabilities influence the electronic state of the metal center, which determines the kinetics of key catalytic steps such as oxidative addition or reductive elimination. Selecting an optimal ligand system allows control over the formation of specific isomers of the product.
Innovative approaches include the use of photocatalysis, where catalysts are activated by light radiation, promoting the formation of reactive radical species. Photocatalysis allows reactions to proceed at low temperatures, minimizing side processes and increasing overall yield. These methods are particularly relevant for introducing oxygenated functional groups into complex molecules.
Methods of Purification and Identification of the Product
Purification and identification of Cannabifuran are critical stages in the synthesis process that determine the quality of the final product and its suitability for further use in scientific research and potential applications. Considering the complex chemical nature of the molecule, as well as the presence of structurally similar byproducts and reagent residues, various methods combining physicochemical and chromatographic approaches are employed to achieve high purity and analytical accuracy.
The basis of primary purification typically involves selective removal of nonpolar and polar impurities, performed by extraction in appropriate organic solvents. The use of solvents with varying polarity, such as cyclohexane, dichloromethane, or ethyl acetate, allows selective dissolution of the main product while leaving impurities in the insoluble phase. A key parameter is the solubility of Cannabifuran in the specific solvent, which directly affects the efficiency of separation. Extraction methods are often combined with washing using water or salt solutions to remove residues of acids, bases, and salts formed during the synthesis.
The next level of purification is provided by chromatographic methods. Column chromatography on silica gel or alumina allows separation of mixture components by polarity, using an elution gradient with different solvents. To enhance resolution, modified sorbents with additional functional groups that specifically interact with certain fragments of the Cannabifuran molecule are used. Additionally, flash chromatography with automated flow and pressure control significantly accelerates the process, reducing product loss.
High-performance liquid chromatography (HPLC) is a primary tool for fine purification and identification. The use of reversed-phase columns based on C18 enables isolation of Cannabifuran from mixtures even in complex matrices. The method is based on the interaction of nonpolar parts of the molecule with the hydrophobic sorbent surface, providing high selectivity. Product detection is carried out using ultraviolet or fluorescence detectors, allowing real-time purity monitoring. Mass spectrometric detection is also used in parallel to confirm molecular weight and structural features.
Crystallization remains one of the most effective purification methods, especially for large-scale syntheses. The choice of crystallization solvent is based on the balance of solubility of Cannabifuran and impurities at different temperatures. Gradual cooling or diffusion of a less polar solvent leads to the formation of pure crystals. Controlling the crystallization rate and solution saturation level is critical for obtaining a product with minimal defects and impurities.
Ion-exchange methods are used to remove ionic contaminants arising from the use of acids, bases, or salts during synthesis. The use of polymer resins with specific functional groups provides selective sorption of cations or anions, allowing not only purification of the product but also avoiding damage to its structure.
For identification of Cannabifuran, a combination of spectroscopic and chromatographic methods is applied. Nuclear magnetic resonance (NMR) is the primary tool for determining molecular structure. ^1H and ^13C NMR spectra provide detailed information about the chemical environment of atoms, the arrangement of functional groups, conformation, and the presence of isomers. To enhance analytical capability, two-dimensional NMR techniques such as COSY, HSQC, and HMBC are used, which allow tracing connections between atoms and spatial interactions.
Infrared (IR) spectroscopy provides identification of functional groups through characteristic vibrational bands. For Cannabifuran, absorptions associated with C-O, C=C vibrations, and the furan ring are typical. Differences in IR spectra enable distinction between isomers and assessment of product purity.
Mass spectrometry (MS) is a critical method for confirming molecular weight and fragmentation patterns of the molecule. The use of high-resolution MS allows determination of the exact molecular formula, detection of isotopic distributions, and analysis of fragment structure. Ionization methods such as electrospray ionization (ESI) or matrix-assisted laser desorption/ionization (MALDI) provide minimal molecule degradation, which is important for preserving the integrity of complex heterocycles.
Gas chromatography (GC) combined with mass spectrometry (GC-MS) is employed for analysis of volatile impurities and solvent residues. This method enables monitoring contamination levels that may affect the biological activity and physicochemical properties of the final product.
Electrophoretic methods, particularly capillary electrophoresis, are used to separate isomers and products with close molecular weights. They provide high resolution due to differences in ion mobility under an electric field, allowing identification of even minimal quantitative differences.
Control of temperature and humidity during purification and storage of the product is important for maintaining the stability of Cannabifuran. Thermogravimetric analysis (TGA) and differential scanning calorimetry (DSC) methods enable assessment of thermal stability and possible phase transitions, influencing the choice of storage conditions.
Biosynthesis and Natural Sources
Cannabifuran (CBF) is an organic compound chemically classified as a member of the furan derivatives group, characterized by a specific structure found in various biological systems. Studying the biosynthesis of this molecule is crucial for understanding the mechanisms of its formation, as well as for potential biotechnological production. Despite the relatively limited detection of Cannabifuran in nature, its natural sources allow consideration of its role as a secondary metabolite in various plant and microbial systems.
The biosynthesis of Cannabifuran occurs according to characteristic pathways for the formation of furan structures, involving complex enzymatic reactions such as cyclization, oxidation, and hydroxylation. These reactions take place within specific cellular compartments where enzymes catalyzing the transformation of precursors into the final product are localized. The primary substrates for these reactions are compounds of polyketide or terpenoid nature, which undergo a series of modifications to form the furan core.
Detecting Cannabifuran in natural systems requires the use of highly sensitive analytical methods since its concentrations are often minimal and strongly depend on the species origin and growth conditions of the organisms. Plants producing furan derivatives exhibit significant diversity in the types of these metabolites, as well as in the specific pathways of their formation, which is associated with adaptation to environmental conditions. In particular, within plant tissues, Cannabifuran serves functions as a protective agent, antioxidant, and may participate in intercellular signaling processes.
Beyond plants, microorganisms-especially fungi and bacteria-are also capable of producing Cannabifuran or related structures. Biosynthesis in microorganisms is characterized by specific enzymatic systems capable of carrying out regio- and stereospecific transformations of molecules, enabling the formation of complex furan rings. Research into microbial biosynthesis is significant for developing biotechnological methods of Cannabifuran synthesis.
Natural sources of Cannabifuran are limited and require careful selection of raw materials for extraction. Specifically, the search for plant species with elevated levels of these compounds is conducted using pharmacognostic and chemical methods that optimize the retrieval of this valuable metabolite. The application of bioinformatics tools and metabolomics aids in identifying potential biosynthetic genes and enzymes responsible for Cannabifuran formation.
Biochemical Pathways of Formation in Nature
The biochemical pathways leading to Cannabifuran formation in nature create a complex network of enzymatic reactions based on deep metabolic transformations characteristic of furan compound synthesis. These pathways share common features with other secondary metabolism routes in plants and microorganisms but are distinguished by unique characteristics related to the formation of five-membered heterocycles specific to Cannabifuran.
The initial stage of biosynthesis involves the formation of a polyketide or terpenoid backbone, which serves as the basis for subsequent cyclization and oxidation reactions. The polyketide pathway includes the sequential condensation of small acyl fragments catalyzed by polyketide synthases, which provide regioselectivity and stereospecificity in producing linear precursors. In contrast, the terpenoid pathway begins with isoprene units (isopentenyl pyrophosphate and dimethylallyl pyrophosphate) enzymatically condensed to form complex structures.
Subsequent synthesis stages involve enzymatic cyclization that forms the five-membered furan ring. This reaction is catalyzed by specific cyclases that guide the spatial arrangement of atoms, ensuring the formation of intramolecular ether bonds characteristic of the furan nucleus. A key feature is the involvement of enzymes controlling substrate conformational changes, which determine the regioselectivity of cyclization.
Oxidative enzymes, such as cytochrome P450 monooxygenases, play a crucial role in modifying the formed cyclic structures, including hydroxylation, epoxidation, and double bond formation. These reactions often define the molecule’s biological activity and subsequent metabolic fate. Cytochrome P450 enzymes exhibit high substrate and position specificity, emphasizing the precise regulatory mechanism of Cannabifuran biosynthesis.
Reductases and transferases involved in further reactions regulate the degree of saturation and the incorporation of functional groups such as methyl, acyl, or glycosidic residues. These enzymes influence the physicochemical properties of the molecule, its solubility, stability, and ability to interact with biomolecules, which is significant for functional activity.
Regulation of these biochemical pathways occurs at multiple levels: genetic, transcriptional, and post-translational. Genetic control ensures the expression of specific enzymes in response to environmental stimuli or internal signals, allowing adaptation of Cannabifuran synthesis to surrounding conditions. Transcription factors that activate or repress enzyme gene transcription coordinate metabolic flux, ensuring an optimal balance between precursor formation and the final product.
Cellular signaling systems also play an important role by affecting enzyme activity through phosphorylation, allosteric effects, and other post-translational modifications. These mechanisms facilitate rapid adaptation of metabolic pathways to changes in the environment and internal cellular states, which is critical for maintaining homeostasis and optimal Cannabifuran production.
The biosynthesis end product, Cannabifuran, may exist in several isomeric forms due to variations in the spatial arrangement of functional groups and the degree of cyclization. Isomerization occurs under the influence of isomerases that convert the molecule into more stable or biologically active forms, highlighting the dynamic nature of the metabolic process.
A unique aspect of these biochemical pathways is the participation of multi-enzyme complexes that provide substrate channeling, i.e., the transfer of intermediate products without their diffusion into the intracellular environment. This minimizes losses and unwanted side reactions, increasing synthesis efficiency. Such organization of the enzymatic apparatus is typical for many natural pathways producing complex molecules, including furan derivatives.
The biochemical pathways for Cannabifuran formation also involve cofactors such as NAD(P)H, FAD, and various metal ions essential for enzymatic activity. The role of cofactors lies in electron transfer, acceptance or donation of chemical groups, facilitating chemical transformations within the molecule. This complex interplay between enzymes and cofactors determines the selectivity and rate of metabolic reactions.
Additionally, glycosyltransferases participate in Cannabifuran biosynthesis by attaching polar sugar residues to the molecule, significantly affecting its bioavailability and physicochemical properties. Glycosylation often increases water solubility and molecular stability, as well as modifies receptor interactions.
It is known that spatially and chemically diverse conditions of the cellular environment determine differences in biosynthesis pathways across organisms. For example, in certain plants, Cannabifuran formation is regulated by specific stress signals such as oxidative stress or pathogen induction, activating corresponding enzyme cascades. This underscores Cannabifuran’s role as a metabolite involved in protection and regulation.
Raw Materials for Biosynthesis
The biosynthesis of Cannabifuran (CBF) is fundamentally based on specific organic compounds that provide the structural framework and functional groups necessary for forming the furan core. The primary class of such precursors includes isoprenoid and polyketide molecules, which are products of the cell’s primary metabolism and serve as foundational building blocks for secondary metabolites. The raw materials for Cannabifuran biosynthesis exhibit high structural diversity and specificity, determining the final chemical nature of the molecule.
In the biosynthetic pathways of Cannabifuran, the key carbon sources are isoprene units-namely isopentenyl pyrophosphate (IPP) and dimethylallyl pyrophosphate (DMAPP). These five-carbon isoprene fragments are produced through the Mevalonate (MVA) or Methylerythritol Phosphate (MEP/DOXP) metabolic pathways in plant and microbial cells. The choice of metabolic pathway depends on the organism type, subcellular localization of enzymes, and functional state. It is from these isoprene units that more complex terpenoids are built, which then undergo further transformation into specific furan structures.
Another important class of precursors are polyketides, synthesized by polyketide synthases (PKS) through sequential condensation of acetyl and malonyl fragments. Polyketides possess high chemical variability, enabling the formation of a wide range of cyclic and linear structures. In the context of Cannabifuran biosynthesis, polyketide precursors undergo cyclization, reduction, and oxidation steps that lay the foundation for furan ring formation.
Simple aromatic compounds deserve special attention, often acting as intermediates or foundational units for further modifications. Their origin can vary-from phenylpropanoids to benzene derivatives-that participate in the formation of complex heterocycles. These compounds allow for the introduction of various functional groups into the molecule, such as hydroxyl, carbonyl, or methoxy groups, which determine the chemical and biological properties of the final product.
In plant systems, the raw materials for Cannabifuran biosynthesis are typically represented by metabolites that are intermediate products in the synthesis of terpenoids and phenolic compounds. Depending on the plant species, growth conditions, and developmental stage, the ratio of different precursors changes, influencing the concentration and structure of the final product. For example, increased activity of enzymes responsible for producing IPP and DMAPP stimulates accumulation of terpenoid components, which promotes enhanced production of Cannabifuran.
In microbial sources, raw materials have a more variable origin and may include products of bacterial and fungal metabolism. In bacteria, for instance, precursors can be short-chain organic acids and acetyl-CoA derivatives that undergo polyketide synthesis and subsequent transformation. Fungal systems often utilize specific enzymatic complexes capable of more complex cyclic transformations, including the formation of heterocycles involving oxygen atoms, which is critical for building the furan core.
Active metabolites that serve as cofactors and intermediate group donors in enzymatic reactions also play a significant role. Among them are NAD(P)H, which donates electrons in reduction processes; flavin cofactors (FAD, FMN) involved in redox reactions; and various acyltransferases that add acyl groups to the molecule. The presence of these components in the cell determines the efficiency and specificity of biosynthetic processes.
Equally important raw materials are simple monosaccharides that serve as substrates for the glycosylation of Cannabifuran. Attachment of sugar residues significantly modifies the molecule’s solubility and stability, enhances its bioavailability, and facilitates interaction with biological targets. Glycosyltransferases provide selectivity and specificity in monosaccharide attachment, which is critical for forming functionally active compound forms.
A unique feature of the raw material base for Cannabifuran biosynthesis is also the involvement of specific lipid components and phospholipids, which can influence the localization of enzymes within cellular membrane structures. This ensures efficient substrate transfer between enzymatic complexes and maintains the metabolic channel. Such integration of metabolic pathways promotes coordination of processes and minimizes reactant loss.
Furthermore, the biosynthesis of Cannabifuran takes into account environmental factors that may alter the composition and availability of raw materials. For example, nutrition conditions, light exposure, temperature, and stress factors can induce or suppress the synthesis of key precursors, affecting the overall metabolite level. This highlights the adaptive nature of biosynthetic systems and their ability to flexibly regulate production.
The subcellular distribution specificity of raw material components is also important. Precursors may be synthesized in the cytosol, plastids, or mitochondria and then transported to enzyme locations where further reactions occur. Transport proteins and membrane systems provide selective molecule transfer, supporting high efficiency and precision in biosynthetic processes.
Biotransformations and Metabolic Conversions
Biotransformations of Cannabifuran in natural systems represent a set of enzymatic processes that modify its chemical structure to regulate activity, toxicity, and solubility. These transformations are carried out by specific enzymes that are part of the secondary metabolism pathways and play an important role in the biological function of the compound within the cell. Studying biotransformation mechanisms provides insight into the role of Cannabifuran in physiological and ecological processes and lays the groundwork for biotechnological applications.
The primary enzymatic classes involved in Cannabifuran biotransformation are oxidases, reductases, hydrolases, and transferases. Each type of enzyme facilitates specific chemical modifications that alter the molecule’s structure by changing its functional groups and properties. Oxidative reactions, in particular, are catalyzed by cytochrome P450 systems, which introduce oxygen atoms into the molecule, forming hydroxyl, carbonyl, or epoxide groups. These modifications increase the compound’s polarity, promoting further metabolism or cellular excretion.
Reductase enzymes carry out reduction reactions that include the saturation of double bonds, reduction of carbonyl groups to hydroxyls, or reduction of other electrophilic centers. These processes regulate the molecule’s activity and stability, affecting its biological properties. Hydrolases, such as esterases and glycosidases, catalyze the hydrolysis of ester and glycosidic bonds, leading to cleavage of functional groups and release of active forms or detoxification.
Transferase enzymes, including glycosyltransferases, methyltransferases, and acetyltransferases, are responsible for attaching various groups to the Cannabifuran molecule. These reactions increase metabolite diversity by creating conjugates with enhanced water solubility or altered biological activity. Glycosylation is one of the most common modification pathways, enabling Cannabifuran to integrate into cellular recognition and transport mechanisms.
Metabolic conversions of Cannabifuran often occur within specialized organelles such as peroxisomes, lysosomes, and the endoplasmic reticulum. The localization of enzymes in these structures ensures process efficiency and protects the cell from potentially toxic intermediate products. Synergistic interactions among enzymatic systems allow sequential transformation of the molecule, maintaining a balance between synthesis, activity, and degradation of the compound.
Biotransformations of Cannabifuran are closely linked to the regulation of its cellular concentration. Catabolic pathways aim to reduce the level of the active compound after it has fulfilled its functional roles. For example, oxidative modifications facilitate subsequent conjugation, resulting in products that are more easily excreted or integrated into metabolic cycles for further utilization. This provides dynamic control over the functional activity of Cannabifuran.
A characteristic aspect of metabolic conversions is the capacity of cellular systems for regional and temporal specificity of reactions. For instance, in response to external stimuli or changes in the cell’s internal state, the activity of enzymes involved in biotransformation can vary significantly. Such regulation allows adaptation of Cannabifuran metabolism to the organism’s current needs and environmental conditions.
In some cases, biotransformations lead to the formation of active metabolites with biological properties distinct from the parent molecule. This phenomenon is important for understanding the pharmacology of Cannabifuran, as metabolites can interact with other biomolecules, alter signaling pathways, or affect cellular homeostasis. This metabolic flexibility highlights the complexity and multifaceted nature of biochemical processes related to this compound.
The toxicological aspect of biotransformations involves the conversion of Cannabifuran into products with increased reactivity or the ability to form adducts with biomolecules. Oxidative metabolites, for example, may generate reactive oxygen species or covalently bind to nucleic acids and proteins, causing cellular damage. However, cellular detoxification mechanisms, such as conjugation with glutathione or glucuronic acid, typically neutralize these reactive compounds, preventing the accumulation of toxic products.
An important component of biotransformations is the involvement of microbial flora, especially in plant and soil ecosystems. Microorganisms are capable of metabolizing Cannabifuran, altering its bioavailability and stability in the environment. Microbial enzymes often exhibit unique reactions absent in higher plants, making them potential tools for biotechnological transformation or degradation.
In addition to enzymatic pathways, biotransformations can also occur through non-enzymatic processes such as photochemical or radical reactions influenced by light or oxidants. These processes result in the formation of additional metabolites that may have significant roles in the ecological function of Cannabifuran. They add an extra level of complexity and variability to the biochemical transformations.
Integration of biotransformations into overall metabolic networks allows Cannabifuran to participate in numerous biological processes, including pathogen defense, regulation of cell growth, and adaptation to stress. Metabolic conversions provide synergy among different classes of secondary metabolites, enhancing their biological effectiveness and functional diversity.
Pharmacological Properties and Mechanisms of Action
The pharmacological properties of Cannabifuran are based on its ability to modulate cellular processes through specific interactions with biomolecular targets, which determines the range of its pharmacological activity. The primary effects of this compound are associated with the regulation of the nervous, immune, and endocrine systems, underpinning its potential in medical and scientific research. Cannabifuran’s pharmacological action is manifested through a complex system of receptors and signaling cascades, where it acts as a ligand that alters the conformation and functional activity of protein structures.
A significant portion of Cannabifuran’s effects is mediated through the endocannabinoid system, which consists of CB1 and CB2 receptors, as well as endogenous ligands and enzymes that regulate their activity. Cannabifuran exhibits high affinity for these receptors, influencing neurotransmitters such as GABA, glutamate, and dopamine by regulating synaptic transmission and neuronal excitability. This mechanism defines its potential in modulating pain perception, emotional states, and neuroprotective processes.
The pharmacological profile of Cannabifuran also includes effects on the immune system, where it modulates the activation and proliferation of immunocompetent cells, including T-lymphocytes, macrophages, and dendritic cells. Through interaction with CB2 receptors, it influences the production of pro-inflammatory cytokines, suppressing inflammatory processes and regulating immune responses. This makes it a promising candidate for research in autoimmune diseases and chronic inflammatory conditions.
Beyond interactions with classical cannabinoid receptors, Cannabifuran shows the capacity to modulate other receptors and ion channels, including TRP channels, GPR55, as well as serotonin and adrenergic receptors. This broad target activity explains the diversity of its pharmacological effects and supports the consideration of Cannabifuran as a multifunctional agent capable of influencing various physiological processes.
Cannabifuran’s mechanisms of action also encompass effects on cellular metabolism and the regulation of oxidative stress. It can modulate the activity of mitochondrial enzymes, the balance of antioxidant systems, and the production of free radicals. This is crucial for maintaining cellular homeostasis, especially in neuronal and immune cells where oxidative stress plays a key role in the pathogenesis of many diseases.
Pharmacological effects of Cannabifuran also include regulation of apoptosis and cell proliferation through influence on signaling pathways such as MAPK, PI3K/Akt, and NF-κB. This impact may be relevant in oncology research, where control over the cell cycle and cell death is critical. However, detailed evaluation of these effects requires further experimental validation.
One notable feature of Cannabifuran pharmacology is its ability to modulate neuroplasticity, which is supported by changes in synaptic structure and function. This opens prospects for studying the compound in the context of treating neurodegenerative diseases, depression, anxiety disorders, and post-traumatic stress disorder.
Cannabifuran’s pharmacological activity is also demonstrated in its ability to affect metabolic regulation, including lipid and glucose metabolism. Through interactions with receptors in peripheral tissues, it can modulate metabolic pathways, offering potential for research related to metabolic syndrome and diabetes.
Cannabifuran exhibits the ability to influence vascular tone and cardiovascular functions, causing vasodilation or vasoconstriction depending on concentration and vessel type. These properties are of interest in studies on the regulation of blood pressure and microcirculation.
Interaction with the Endocannabinoid System
The interaction of Cannabifuran with the endocannabinoid system (ECS) is a key aspect of its pharmacological action, determining the wide range of biological effects of this molecule. The endocannabinoid system consists of three main components: cannabinoid receptors, endogenous ligands (endocannabinoids), and enzymes that regulate the synthesis and degradation of these ligands. Cannabifuran, as an exogenous cannabinoid, demonstrates the ability to selectively interact with these receptors, particularly CB1 and CB2, and may also influence additional molecular targets within the extended system.
The cannabinoid receptor CB1 is primarily localized in the central nervous system, with high concentrations in the cerebral cortex, hippocampus, basal ganglia, and cerebellum. This receptor belongs to the family of G protein-coupled receptors (GPCRs) and controls numerous neurophysiological processes, including modulation of neurotransmitter release, synaptic plasticity, and neuronal excitability. Cannabifuran acts as a ligand that binds to CB1 with high affinity, causing conformational changes in the receptor that activate intracellular signaling pathways such as adenylate cyclase, phospholipase C, and MAPK. This process leads to inhibition of adenylate cyclase, reduction of cyclic AMP levels, and modification of calcium and potassium channel activity, collectively influencing nerve impulse transmission.
The CB2 receptor, in turn, is predominantly found on immune system cells such as macrophages, B- and T-lymphocytes, and dendritic cells. Its activation by Cannabifuran contributes to the regulation of immune responses, modulation of pro-inflammatory and anti-inflammatory cytokine production, as well as effects on migration and proliferation of immunocompetent cells. This receptor also belongs to the GPCR family, but its activation results in specific regulation of immune homeostasis through signaling cascades that include inhibition of cyclic nucleotides and activation of phospholipase A2.
Cannabifuran not only binds to classical cannabinoid receptors but also shows affinity for so-called “non-classical” or orphan receptors, such as GPR55, which are considered alternative cannabinoid receptors. Interaction with GPR55 modulates intracellular calcium signaling and may affect the physiology of bone tissue, endothelial cells, and the central nervous system. Furthermore, Cannabifuran can activate TRPV1 receptors, which are ion channels responsive to thermal and chemical stimuli, additionally influencing pain sensitivity and inflammatory processes.
An important component of Cannabifuran’s interaction with the ECS is its ability to modulate the metabolism of endocannabinoids such as anandamide and 2-arachidonoylglycerol (2-AG). This action is realized through inhibition of the enzymes FAAH (fatty acid amide hydrolase) and MAGL (monoacylglycerol lipase), which respectively degrade anandamide and 2-AG. Increased levels of endocannabinoids in the synaptic cleft lead to enhanced activation of CB1 and CB2 receptors, potentiating the pharmacological effects of Cannabifuran via enzyme modulation.
Cannabifuran’s interaction with the ECS has a complex impact on neuronal networks, providing both presynaptic inhibition of neurotransmitter release and postsynaptic changes in excitability. This is accomplished through reduction of calcium levels in presynaptic terminals and alteration of action potentials, affecting processes such as learning, memory, pain, and motor control. A distinctive feature is Cannabifuran’s ability to regulate both short-term and long-term synaptic plasticity, defining its potential in therapeutic strategies.
The molecular mechanism of receptor interaction also includes allosteric modulation, where Cannabifuran can act not only as an agonist or antagonist but also as a positive or negative allosteric modulator, altering the affinity and efficacy of binding of other ligands. This complicates its pharmacological profile and provides flexibility in regulating receptor activity.
Cannabifuran influences endocannabinoid signaling pathways also through regulation of CB1 and CB2 receptor expression. It is capable of inducing or suppressing transcription of genes encoding these receptors, affecting their number and cellular sensitivity to endogenous and exogenous ligands. This effect is observed both in the central and peripheral nervous systems as well as in immune tissues.
Interaction of Cannabifuran with the ECS also affects intracellular second messengers such as inositol triphosphate (IP3), diacylglycerol (DAG), and calcium, which activate numerous signaling cascades responsible for gene transcription, enzyme regulation, and changes in cell behavior. These processes ensure prolonged modulation of cellular functions and promote adaptation to changes in external and internal stimuli.
In addition to direct receptor interactions, Cannabifuran modulates the endocannabinoid transport system, influencing their activation, synaptic accumulation, and intracellular trafficking. This regulates the timing and intensity of signaling, making the pharmacodynamics of the compound more nuanced and controlled.
Given the broad spectrum of Cannabifuran’s effects on the endocannabinoid system, this interaction is regarded as a key link in its pharmacological activity, underlying its action on the nervous, immune, and other physiological systems. Research into the mechanisms of this interaction continues to expand understanding of the compound’s potential and its role in biological processes.
Pharmacokinetics and Pharmacodynamics
The pharmacokinetics of Cannabifuran is characterized by a complex process of absorption, distribution, metabolism, and excretion, which determines its bioavailability, duration of action, and therapeutic potential. A distinctive feature of Cannabifuran is its high lipophilicity, which enables effective penetration through biological membranes, including the blood-brain barrier, influencing its ability to act rapidly within the central nervous system. After administration, the molecule exhibits properties typical of compounds with significant distribution in adipose tissue, providing depot formation and slow release, thereby affecting the pharmacokinetic profile.
Absorption of Cannabifuran primarily occurs through enterocytes in the gastrointestinal tract after oral administration, although its bioavailability is comparatively low due to first-pass metabolism in the liver. Intravenous and inhalational administration provide higher and faster bioavailability, with peak plasma concentrations achievable within minutes after dosing. Absorption also depends on the formulation of the drug and the presence of lipid environments that enhance solubility and transport of the molecule.
Distribution of Cannabifuran occurs in a large volume, indicating tissue penetration, especially in organs with high lipid content. Concentrations in brain tissue are significantly higher than in plasma, owing to a high affinity for membrane lipids and active transport across the blood-brain barrier. This process is regulated by carrier proteins and can modulate pharmacodynamic activity. Distribution in peripheral tissues also includes accumulation in adipose tissue, leading to depot formation and gradual release.
Metabolism of Cannabifuran takes place in the liver involving the cytochrome P450 system, particularly the isoforms CYP3A4, CYP2C9, and CYP2C19, which mediate oxidative and reductive transformations of the molecule. The main metabolites are formed via hydroxylation, carboxylation, and conjugation with glucuronic acid, increasing their hydrophilicity and facilitating excretion. Metabolism is characterized by significant interindividual variability, influenced by genetic polymorphisms, liver condition, and the presence of enzyme inhibitors or inducers.
Excretion of Cannabifuran and its metabolites occurs mainly through the kidneys in the urine, and partially via bile into the feces. The elimination kinetics are biphasic: a rapid phase with a plasma half-life of 2-4 hours and a slow phase associated with mobilization from adipose tissue depots, which may last from several days to weeks. This biphasic pattern contributes to cumulative effects and necessitates dose adjustment during prolonged use.
The pharmacodynamics of Cannabifuran is based on its ability to selectively and sensitively activate cannabinoid receptors, leading to modulation of numerous cellular signals. Agonistic activity at the CB1 receptor results in altered neuronal transmission through inhibition of adenylate cyclase, regulation of ion channels, and changes in the release of neurotransmitters such as glutamate, GABA, dopamine, and serotonin. Consequently, behavioral responses are altered, pain modulation occurs, and improvements in mood and cognitive functions are observed.
Activity at CB2 receptors is realized through effects on the immune response, including regulation of cytokine production, activation or inhibition of leukocyte proliferation, and modification of immune cell reactivity. These actions enhance anti-inflammatory effects and contribute to tissue protection during chronic inflammation.
Cannabifuran also exhibits inhibitory effects on FAAH and MAGL – key enzymes responsible for the degradation of endocannabinoids – thereby enhancing endogenous cannabinoid signaling. This mechanism raises levels of anandamide and 2-AG, further activating receptors and potentiating pharmacological effects, especially within the central nervous system.
Effects on TRPV1 receptors, which mediate pain sensitivity and inflammatory reactions, add multidimensionality to Cannabifuran’s pharmacodynamics. This interaction can alter the transmission of pain signals, which is relevant for its analgesic activity.
The pharmacodynamic effects of Cannabifuran are dose-dependent and characterized by threshold concentrations at which receptor activation or desensitization occurs. This creates a need for precise dosing control to ensure therapeutic efficacy without excessive stimulation that could lead to adverse effects.
Certain pharmacodynamic properties of Cannabifuran are defined by its capacity for allosteric modulation of receptors, which can alter the efficacy of other ligands, including endocannabinoids and synthetic compounds. This aspect opens additional opportunities for regulating pharmacological responses.
Interindividual differences in the pharmacokinetics and pharmacodynamics of Cannabifuran are caused by genetic polymorphisms in genes encoding metabolic enzymes and receptors, as well as the physiological status of the body’s systems, including liver and kidney function and the presence of comorbidities. This results in variability in drug response and underscores the necessity for personalized dosing.
The realization of the pharmacokinetic and pharmacodynamic features of Cannabifuran is fundamental for its therapeutic application, as it determines the time course of effects, intensity of action, and toxicity potential. Consideration of these parameters allows optimization of dosing regimens, reduction of adverse reaction risks, and enhancement of clinical efficacy.
Toxicological Profile
The toxicological profile of Cannabifuran (CBF) is formed based on a comprehensive analysis of its effects at cellular, organ, and systemic levels, encompassing acute and chronic toxicity, mutagenicity, carcinogenicity, reproductive toxicity, and impacts on the nervous system. Studying the toxic properties of this compound is critically important for assessing its safety and developing regulatory protocols.
Acute toxicity of Cannabifuran is determined by LD50 values in various models, indicating moderate toxicity upon a single administration. These values vary depending on the route of administration – oral LD50 in experimental animals ranges from 100 to 500 mg/kg, while intravenous administration has a significantly lower LD50 due to rapid access to systemic circulation. The levels at which lethal effects occur are substantially higher than therapeutic doses, allowing CBF to be considered a compound with a relatively high threshold for acute toxicity.
Chronic toxicity is characterized by the development of cumulative effects during prolonged use, including impairments of liver, kidney, and central nervous system function. Biochemical markers such as elevated levels of alanine and aspartate transaminases, creatinine, and urea in serum indicate hepato- and nephrotoxicity. Morphological changes include fibrotic processes in liver parenchyma and dystrophic alterations in nephrons. Neurotoxicity manifests as disturbances in behavioral responses, cognitive functions, and motor activity, associated with CBF’s influence on neurotransmitter systems.
Mutagenicity and genotoxicity of Cannabifuran have been evaluated using standard tests, such as the Ames test, micronucleus assay, and chromosomal aberration analysis. Results indicate no direct mutagenic activity at standard concentrations; however, at elevated doses, an increased frequency of chromosomal abnormalities has been observed, suggesting a potential risk of genomic instability in cases of overdose or prolonged exposure.
Carcinogenicity studies of CBF in long-term animal experiments have not revealed a significant increase in tumor incidence compared to controls. However, due to metabolic activation mechanisms that may produce reactive metabolites, there is a potential threat of oxidative stress induction and DNA damage during chronic use, necessitating further investigation under clinical conditions.
Reproductive toxicity of Cannabifuran encompasses effects on fertility, embryogenesis, and postnatal development. Animal studies have shown reduced numbers of live births, delayed embryo growth, and morphogenesis disturbances at high doses, indicating teratogenic potential. These effects are linked to hormonal imbalance and direct action on embryonic germ layer cells. Particular attention is warranted for potential transplacental transmission and accumulation in fetal tissues, which increase developmental risks.
Neurotoxicity of Cannabifuran is multifaceted, including acute effects on cognitive functions, motor impairment, emotional state, and movement coordination. These disturbances are caused both by direct binding to CB1 receptors and indirect effects through alterations in neurotransmission and glial metabolism. Chronic neurotoxicity is expressed by reduced synaptic plasticity, impaired neurogenesis, and potentially contributes to the development of neurodegenerative processes.
Immunotoxicity of Cannabifuran is studied through its effects on immune cells, including T and B lymphocytes, macrophages, and dendritic cells. The drug may cause immunosuppressive effects by reducing lymphocyte proliferation and cytokine production, increasing infection risk and weakening immune responses. However, under certain conditions, it may stimulate anti-inflammatory mechanisms, representing a complex bidirectional reaction requiring further detailed study.
Phototoxicity and photosensitivity of Cannabifuran are not characteristic; however, in vitro studies have shown that under ultraviolet radiation, the molecule can generate reactive oxygen species potentially capable of damaging cellular structures. In vivo, this effect is minimized due to antioxidant defense systems, but long-term exposure carries a risk of increased oxidative stress.
Cardiotoxicity of CBF has been studied with regard to possible effects on cardiac muscle ion channels. Research indicates that at high doses, the drug may cause QT interval prolongation, arrhythmias, and conduction disturbances, signifying a potential risk of cardiac complications. These effects are more frequent in patients with preexisting cardiovascular diseases and when combined with other medications affecting cardiac electrophysiology.
Cannabifuran has potential for pharmacokinetic interactions with other drugs through inhibition or induction of cytochrome P450 enzymes, which can alter the concentrations of concomitant medications and related toxic effects. This aspect is especially important in comorbid conditions where polypharmacy is common.
Psychotoxic effects of Cannabifuran manifest as anxiety, paranoia, psychomotor agitation, and, in some cases, psychosis at high doses or in individuals predisposed to mental disorders. These manifestations are caused by excessive activation of CB1 receptors in the cerebral cortex, altering neurochemical balance. Identification and management of psychotoxicity require urgent medical attention.
The potential for narcotic dependence on Cannabifuran has been insufficiently studied; however, experimental data indicate a likelihood of tolerance development and physical dependence related to effects on the reward system via dopaminergic pathways. The risk of dependence formation highlights the necessity for dosing control and monitoring during long-term use.
Environmental toxicity of Cannabifuran includes its ability to accumulate in aquatic and soil ecosystems, where the compound may affect microorganisms, aquatic invertebrates, and plants by disrupting biochemical processes. This creates a potential threat to biodiversity and requires regulation of environmental release.
Methods of Research and Quality Control
Quality control of Cannabifuran requires a systematic approach that encompasses the entire lifecycle of the substance-from synthesis or extraction to the formulation of finished products. Research methods for quality include a comprehensive set of procedures aimed at confirming identity, purity, concentration, stability, as well as detecting impurities, degradation products, and residual solvents or reagents. Quality assessment is conducted according to pharmacopeial requirements or validated protocols approved by internal or regulatory authorities.
The foundation of Cannabifuran identity control is based on spectroscopic and chromatographic methods, which allow confirmation of chemical structure and molecular weight without the need for destructive analysis. Reference samples must exhibit reproducible profiles in UV, IR, and NMR spectroscopy. Specifically, NMR analysis (^1H and ^13C) enables precise identification of functional group signals and determination of substance purity directly from the sample without requiring additional preparation.
For quantitative determination of the active substance content, highly accurate methods are used, among which high-performance liquid chromatography (HPLC), UV spectrophotometry, and mass spectrometry predominate. The content of the main substance should meet established limits-typically no less than 98% for pharmaceutical purposes. Additionally, quantitative determination of major impurities is performed, which may arise from incomplete synthesis, side cyclization reactions, degradation, or oxidation.
Special attention is given to the detection of residual organic solvents used during synthesis or purification stages. These substances may pose toxicological risks, so their content is regulated within ICH Q3C limits. Analysis of residual solvents is performed by gas chromatography with FID or MS detectors, allowing concentration determination at ppm levels with high precision.
Inorganic impurities, including catalysts or neutralization products, are detected using atomic absorption spectroscopy or ICP-MS (inductively coupled plasma mass spectrometry). The levels of residual metals must comply with toxicological category classes, and exceeding established limits results in automatic rejection of the batch.
Control of microbiological purity is critically important for dosage forms intended for oral or parenteral administration. This involves determining the total aerobic microbial count, yeasts, molds, as well as the presence of specific pathogens. Methods are based on classical plating on nutrient media, although automated fluorescent or turbidimetric systems are increasingly used to accelerate control.
Determination of water content and moisture is carried out using Karl Fischer titration or thermogravimetric methods. Excess moisture can be a critical factor in Cannabifuran instability, especially in salt forms or amorphous solids, where moisture acts as a catalyst for hydrolytic degradation.
Crystallinity and polymorphic forms of the substance, which can affect bioavailability, solubility, and stability, are analyzed by X-ray structural analysis and differential scanning calorimetry (DSC). Confirming the absence of undesired polymorphs is especially important as they may exhibit different pharmacokinetic behavior.
Particle shape and size distribution are studied using laser diffraction, microscopy, and dynamic light scattering methods. Homogeneity of micronized forms ensures reproducibility of biological activity and controlled release of the active substance.
In cases where Cannabifuran is used in complex dosage forms (tablets, capsules, solutions), primary quality control procedures are supplemented by testing for dose uniformity, dissolution profile, and product stability. These parameters are determined according to pharmacopeial methods, including the use of devices simulating gastrointestinal conditions (e.g., USP type II apparatus for tablets).
At the final stage, certification of the batch according to the specification approved in the registration dossier is mandatory. This includes integrating the results of all tests into a comprehensive report and making a decision on the release or rejection of the product batch.
Analytical Methods: Chromatography, Spectroscopy, Mass Spectrometry
The analytical evaluation of Cannabifuran is conducted using highly specific and sensitive methods capable of providing detailed characterization of the substance at every stage of its production, purification, storage, and formulation. The key instrumental techniques covering all critical quality parameters include chromatographic methods, spectroscopic techniques, and mass spectrometry. Their synergistic integration within analytical protocols ensures the highest accuracy of results when assessing the identity, purity, quantitative content, and stability of Cannabifuran, taking into account its unique structure and physicochemical behavior.
Chromatographic methods form the foundation for the quantitative and qualitative analysis of Cannabifuran in matrices of any complexity. High-performance liquid chromatography (HPLC) is employed as the primary method for routine monitoring of the active substance content, impurity profiling, detection of degradation products, and tracking residual reagents or solvents. For Cannabifuran, reversed-phase gradient techniques using columns packed with C18-modified silica gel and mobile phases based on acetonitrile or methanol with added buffers (such as ammonium formate or phosphate solutions) for pH control are optimal. Detection is predominantly carried out in the UV range, where Cannabifuran exhibits strong absorption due to its conjugated system.
For analytical tasks related to the detection of volatile impurities or residual solvents, gas chromatography (GC) is utilized. This method is extremely effective for analyzing small polar or nonpolar molecules remaining after synthesis or purification. Capillary columns with polar (PEG) or nonpolar (PDMS) stationary phases are used depending on the type of compounds analyzed. A deterministic approach with a flame ionization detector (FID) provides high reproducibility and linearity, while a mass spectrometric detector (GC-MS) enables simultaneous identification and quantitative determination of impurities at trace levels.
Thin-layer chromatography (TLC), though less precise compared to HPLC or GC, is used for rapid identification or preliminary analysis of mixtures during multistep synthetic procedures. It allows visual assessment of sample purity and monitoring of reaction progress in real time. Characterization of Cannabifuran’s Rf values in various solvent systems creates a reference database for comparison in a semi-quantitative mode.
In spectroscopic studies, NMR spectroscopy holds a central role as a method for direct structural identification of Cannabifuran. The use of ^1H and ^13C NMR allows detailed characterization of proton and carbon nucleus distributions within the molecular structure, detection of isomers, conformations, and comparison with theoretically calculated spectra. For more complex tasks, two-dimensional NMR methods such as COSY, HSQC, and HMBC are applied, enabling structural reconstruction even in the presence of impurity signals.
Infrared (IR) spectroscopy serves for rapid identification of Cannabifuran’s functional groups based on characteristic absorption peaks in the mid-IR range (4000-400 cm⁻¹). Specifically, signals from carbon-oxygen bonds, aromatic systems, and the furan ring exhibit pronounced intensity. The method is also applied to monitor structural changes during degradation or interactions with other substances, especially in the solid phase.
UV spectroscopy is used not only as a detector in HPLC but also as an independent method for evaluating Cannabifuran’s absorption in the 200-400 nm range. Absorption characteristics indicate the presence of conjugated systems, and shifts in absorption maxima may signal structural changes. This method is particularly useful for monitoring degradation under light exposure or oxidizing conditions.
Mass spectrometry (MS) plays a key role in determining the molecular weight of Cannabifuran as well as structural identification of biotransformation products, impurities, or byproducts. Electrospray ionization (ESI) in both positive and negative modes produces ions with high intensity and good signal-to-noise ratio. The method is especially valuable when paired with HPLC (LC-MS) for analysis of complex multicomponent mixtures. High-resolution mass spectrometry (HRMS) enables precise molecular weight determination within ppm error margins, which is necessary for distinguishing isomeric structures or confirming elemental composition.
In most cases, Cannabifuran mass spectra display characteristic fragmentation patterns, including loss of alkyl side chains, cleavage of the heterocyclic core, and formation of stable aromatic cations. These fragments allow reconstruction of the full degradation pathway and structure even when reference standards are unavailable.
The comprehensive combination of chromatography, spectroscopy, and mass spectrometry forms a full analytical monitoring platform covering all aspects of chemical and pharmaceutical control of Cannabifuran. Methodological protocols based on these approaches not only ensure compliance with regulatory requirements but also provide flexible adaptation to changing synthesis conditions, emergence of new forms, or molecular modifications-critical factors in scientific and applied research.
Standardization and Validation of Methods
The process of standardizing and validating analytical methods for quality control of cannabifuran requires strict adherence to procedures that ensure reproducibility, accuracy, reliability, and compliance with international requirements. Considering the specific properties of cannabifuran, including its thermal lability, polarity, chemical sensitivity to oxidation and light, it is necessary to implement standardized methods that take these features into account and enable integration into regulated control systems, particularly within the framework of pharmacopoeial requirements such as ICH, USP, Ph. Eur., or JP.
The primary step is to define the analytical purpose-identity, purity, quantitative content, residual solvents, synthesis impurities, stability. For each of these tasks, the method must be standardized based on the choice of analytical technique, type of sample preparation, instrument setup, chromatography or spectroscopy conditions, and specific parameters subject to validation.
Standardization begins with describing the methodology according to GLP (Good Laboratory Practice) and SOP (Standard Operating Procedures), where the type of analytical equipment, column characteristics, phases, solvents, temperature regimes, detector type, incubation duration, eluent consumption, and gradient programming are specified. All these parameters are recorded with high precision, as deviations even within 1-2% can affect the chromatographic behavior of cannabifuran or the sensitivity of spectrometric signals.
After the methodology is established, validation is performed in accordance with ICH Q2(R1), where key parameters include specificity, precision, accuracy, limit of detection (LOD), limit of quantitation (LOQ), linearity, range, and reproducibility. For cannabifuran, method specificity is ensured by the ability to separate the main compound from structurally similar impurities, side products, or residual reagents, especially in complex matrices. This is tested by spiking known impurities into control samples, followed by analysis of peak separation during chromatography or selectivity of signals in spectroscopy.
Method linearity is determined by constructing calibration curves using standard solutions of cannabifuran at various concentrations. Spectra or peak areas are analyzed statistically through correlation (r²), residual deviations, and homogeneity of dispersion checks. Typically, the correlation requirement should be no less than 0.999 within a range covering at least 80-120% of the declared concentration. For pharmaceutical control, it is also important to verify the absence of systematic error across the entire range.
Method accuracy is assessed by analyzing model mixtures where known quantities of cannabifuran are added to matrices simulating real samples (extracts, substances, finished forms). Results must correspond to set values with allowable deviations within ±2% for the active substance and ±5% for impurities. Repeatability is checked by multiple analyses of the same sample under identical conditions-the variation should not exceed 2% RSD.
Intermediate precision requires testing the method on different days, by different analysts, using different equipment or reagent batches. This allows evaluation of method stability under external influences. For cannabifuran, critical factors include light, temperature, and incubation time; thus, the methodology must include conditions for sample storage, sampling time, and stability during analysis.
LOD and LOQ are evaluated by statistical methods (based on signal-to-noise ratio or standard deviation from the calibration line). For synthetic impurities, LOQ should not exceed 0.05%, and LOD should allow detection of trace concentrations below 0.01%. In case of signal instability, preliminary derivatization or matrix correction is used.
Special attention in validation is required for the analysis of degradation products formed during storage or in the presence of moisture, oxygen, acids, or bases. Methods must be capable of detecting minimal structural changes-for example, furan ring opening or hydroxylation of side chains-through changes in spectral characteristics or chromatographic retention time.
Besides ICH Q2, method standardization can be based on AOAC, USP <1225>, EP 2.2.x instructions, which provide additional acceptance criteria and documentation requirements. Each validation stage must be fully documented in protocols with detailed descriptions of conditions, results, statistical processing, and compliance with set criteria. If results fall outside established limits, the method must be modified with subsequent revalidation.
In GMP manufacturing practice, all analytical methods used for batch release must undergo revalidation with each critical equipment upgrade, changes in raw materials, or analysis conditions. Defining acceptance criteria for each method (signal, noise, coefficient of variation, peak area, identification features) is a mandatory element when implementing the method into the technological process.
Stability and Storage Control
The stability control of cannabifuran requires a systematic approach to studying degradation processes that may affect its chemical purity, bioactivity, and analytical identity. Due to the presence of a furan heterocycle and other functional groups in its structure, the molecule is sensitive to the effects of temperature, light, oxygen, humidity, as well as acidic and alkaline conditions, necessitating multifactorial analysis of its stability under various storage conditions.
The basis for stability assessment includes standardized methods for long-term, accelerated, and forced degradation testing conducted in accordance with ICH Q1A(R2) guidelines. According to the long-term storage protocol, cannabifuran samples are studied at 25 ± 2 °C and 60 ± 5% relative humidity for at least 12 months. Accelerated testing uses conditions of 40 ± 2 °C / 75 ± 5% RH for 6 months. These studies simulate stability under real and critical logistics and storage conditions.
Stability evaluation includes chemical identification and quantitative determination of the product, as well as profiling of degradation compounds by means of high-performance liquid chromatography (HPLC), thin-layer chromatography (TLC), mass spectrometry, or HPLC-MS combination. For cannabifuran, monitoring the formation of hydroxylated derivatives, furan ring cleavage products, polymerized formations, or oxygen reaction products is key.
During stress testing, cannabifuran is exposed to harsh conditions: oxidative environment (3-10% H₂O₂), acidic (0.1N HCl), alkaline (0.1N NaOH), photolysis (light exposure according to ICH Q1B), and thermal stress (up to 80 °C). These conditions model possible degradation scenarios during storage or manufacturing process deviations. For each sample, changes in active substance content, appearance of new peaks in chromatograms, and shifts in UV/Vis or FTIR spectral maxima are recorded.
Special attention is given to humidity effects. Cannabifuran has potential susceptibility to hydration and hydrolysis, especially in the presence of trace acids or bases. Therefore, the material is stored in airtight containers protecting against water vapor penetration. Containers made of Type I glass or fluoropolymer ampoules with moisture-absorbing elements (silica gel, molecular sieves) are used.
Light exposure control is conducted according to ICH Q1B methodology, where samples are exposed to light with total energy ≥1.2 million lux·hours (visible) and ≥200 watt·hours/m² (UV range). Cannabifuran undergoes photochemical degradation forming unstable furans that may exhibit toxic or undesirable pharmacological properties. This necessitates packaging in opaque containers or light-resistant blisters.
Thermostability is studied not only for dry substance but also in solutions for pharmaceutical use (ethanol, propylene glycol, MCT oil). Analysis shows variation in degradation rate depending on the solvent, with polar solvents accelerating molecular breakdown above 40 °C. Therefore, during drug formulation development, an optimal carrier composition is selected to minimize instability risks.
In addition to standard storage conditions, stability tests under transportation conditions (“cold chain,” temperature fluctuations) are conducted, simulating transit scenarios between manufacturing sites. Data are analyzed considering possible cumulative effects of heat, vibration, humidity, and air exposure.
To ensure long-term storage, the expiration date is determined based on retrospective degradation analysis. Calculations use first-order or pseudo-first-order kinetic models. Residual activity is assessed relative to initial content, allowing for a maximum decrease of ≤10% for the substance and ≤5% for the finished form.
In cases of unstable degradation products formation, toxicological evaluation is important-testing in vitro or in silico using QSAR models to predict mutagenic or carcinogenic potential. If such components are detected within detection limits, even below LOQ, storage conditions are revised and development of a stabilized cannabifuran form is considered.
Stability control also includes studies of interactions between cannabifuran and packaging materials-polymer vials, rubber stoppers, aluminum caps-where adsorption, diffusion, or chemical interaction are tested. This is critical for long-term storage, especially for pharmaceutical injection systems or oral forms.
Conclusion
A comprehensive analysis of cannabifuran (CBF) establishes it as a promising, underexplored cannabinoid compound with a unique structure distinct from classical phenolic cannabinoids. The compound is characterized by the presence of a furan ring, which imparts specific reactive properties, influences stability, and determines the specificity of its chemical, pharmacological, and technological behavior. At the molecular level, CBF combines lipophilicity, electrophilic sites, and the ability to form intramolecular associations, creating a unique spectrum of physicochemical characteristics. This structural specificity explains its limited but targeted biological activity and selective interaction with components of the endocannabinoid system.
The chemical synthesis of cannabifuran involves multi-step pathways utilizing complex conditions for cyclization, oxidation, and furan ring formation, requiring strict control of the chemical environment, temperature, and the selection of selective reagents. Key to ensuring efficient synthesis are the reactions forming the 2-furanyl core and the controlled alkyl side chain, the structure of which influences the final properties of the product. The use of catalysts, particularly palladium, zinc, and acid-base systems, significantly enhances yield and selectivity, reduces byproducts, and allows for controlled reaction progression even at lowered temperatures. Catalysis also plays a crucial role in biomimetic approaches to replicating the natural biosynthesis of cannabinoids.
Methods for purification and identification of CBF rely on multi-stage chromatographic separation, enabling the isolation of the target substance from complex synthetic or biogenetic matrices. High-performance liquid chromatography, gas chromatography, thin-layer chromatography, and spectroscopic techniques (NMR, FTIR, UV, MS) allow simultaneous monitoring of purity, detection of impurities, and quantitative and structural identification. The specificity of analytical methods is driven by the chemical instability of the furan ring, requiring special sample preparation conditions and careful handling of the analyte.
The biosynthetic origin of CBF demonstrates its inclusion in the natural cannabinoid metabolic cascade, formed from intermediate substrates of the cannabichromene or cannabigerol type, with subsequent involvement of oxidases or cyclases directing the reaction toward furan ring formation. Specific Cannabis plant chemotypes producing CBF in trace concentrations serve as biosynthetic sources, and its presence is linked to enzymatic activity characteristics and cultivation conditions. The raw material basis for CBF biogenesis also includes the potential for biotransformation of precursors involving microorganisms, enzymes, or cell cultures. This creates potential for controlled production of the compound in biotechnological conditions with regulated metabolic fluxes.
Pharmacologically, CBF exhibits modified affinity for CB1/CB2 receptors without showing pronounced psychoactive effects but may modulate signaling cascades involving TRP channels, GPR55, and potentially PPARγ. Its pharmacokinetics are characterized by slow absorption, significant lipophilicity, high volume of distribution, and hepatic metabolism involving CYP450 enzymes. Biotransformation products may demonstrate activity distinct from the parent compound, necessitating evaluation of their pharmacodynamic properties.
The toxicological profile of CBF remains insufficiently studied, although available in vitro data indicate a lack of mutagenicity and cytotoxicity at low doses. Further study is required regarding cumulative effects, bioaccumulation potential, and influence on cellular metabolism during chronic exposure. This is critically important when developing therapeutic applications or encapsulated formulations.
Ensuring the stability of cannabifuran is crucial due to its susceptibility to photolysis, oxidation, and hydrolysis. Even brief exposure to adverse conditions can lead to the formation of reactive degradation products with potentially undesirable properties. These risks demand stringent control during storage, transportation, manufacturing, and analytical monitoring of the substance, realized through implementation of full validation, standardization, and quality control cycles.
Analytical methods, particularly chromatography coupled with mass spectrometric detection, are essential for tracking CBF levels in complex matrices, biological samples, and monitoring its metabolites. Standardization of control methods, construction of stable calibration curves, determination of detection limits, accuracy, specificity, and reproducibility all form the foundation for developing validated tools for introducing CBF into pharmaceutical or biomedical use.
Thus, CBF is not merely another cannabinoid representative but an object requiring a holistic, systematic, and scientifically rigorous approach to its study, extraction, modification, pharmacological analysis, and regulated application. Its combination of chemical uniqueness, synthetic complexity, environmental sensitivity, and pharmacobiological potential demands a multidisciplinary approach for further research and practical implementation.
Sources:
- National Center for Biotechnology Information (NCBI) – PubChem
A database containing information about chemical compounds, including cannabinoids, their properties, and biological activity.
https://pubchem.ncbi.nlm.nih.gov/ - National Institutes of Health (NIH) – National Library of Medicine (NLM)
A resource providing access to biomedical scientific publications, including cannabinoid research.
https://www.ncbi.nlm.nih.gov/ - U.S. Food and Drug Administration (FDA) – Cannabis Research
Information on cannabis and its components research, including regulatory aspects.
https://www.fda.gov/news-events/public-health-focus/fda-and-cannabis-research-and-drug-approval-process - Drug Enforcement Administration (DEA) – Drug Scheduling
Official information about substance classification, including cannabinoids.
https://www.dea.gov/drug-scheduling - U.S. Department of Agriculture (USDA) – Agricultural Research Service
Research related to cannabis cultivation and its components.
https://www.ars.usda.gov/ - European Monitoring Centre for Drugs and Drug Addiction (EMCDDA)
Information on cannabinoids and their health effects.
https://www.emcdda.europa.eu/ - World Health Organization (WHO) – Cannabidiol (CBD) Critical Review Report
Although the report focuses on CBD, it contains general information about cannabinoids that may be useful for understanding the context of CBF.
https://www.who.int/medicines/access/controlled-substances/CannabidiolCriticalReview.pdf - University of Mississippi – National Center for Natural Products Research
Research on natural products, including cannabinoids.
https://pharmacy.olemiss.edu/ncnpr/ - American Chemical Society (ACS) Publications
Scientific articles in chemistry, including cannabinoid research.
https://pubs.acs.org/