Over the past few decades, cannabinoids, biologically active compounds naturally found in Cannabis sativa L., have become the focus of extensive scientific research. The primary attention has been directed toward Δ⁹-tetrahydrocannabinol (THC) and cannabidiol (CBD), which have well-characterized pharmacological profiles. However, the plant also contains other, less common cannabinoids that demonstrate unique biological properties. One such compound is cannabigerol (CBG) – a non-psychoactive molecule that serves as a metabolic precursor to THC, CBD, and CBC in the biosynthetic cascade of cannabinoids.
CBG is synthesized as a precursor acid, cannabigerolic acid (CBGA), which undergoes cyclization into other major cannabinoids with the help of enzymes. As a result, in mature plants, the concentration of free CBG typically does not exceed 1%, historically complicating its study. However, modern biotechnological methods now allow for the production of CBG in sufficient quantities, opening new opportunities to explore its anti-inflammatory, neuroprotective, antibacterial, and potentially anti-cancer effects.
Currently, interest in CBG is driven not only by its unique pharmacological profile but also by its low psychoactivity, making it a promising candidate for clinical applications in areas where traditional cannabinoids are limited.
Origin and Chemical Characteristics of Cannabigerol (CBG)
Cannabigerol (CBG) is one of the primary cannabinoids found in Cannabis sativa plants. It is a molecule that plays a central role in the complex biochemical pathway of cannabis metabolism, acting as a precursor to most other cannabinoids, such as Δ⁹-THC and CBD. A key feature of CBG is its ability to act as a matrix for subsequent transformations that occur within the plant. This makes CBG essential for understanding how cannabinoids, which possess significant therapeutic properties, are formed in the plant.
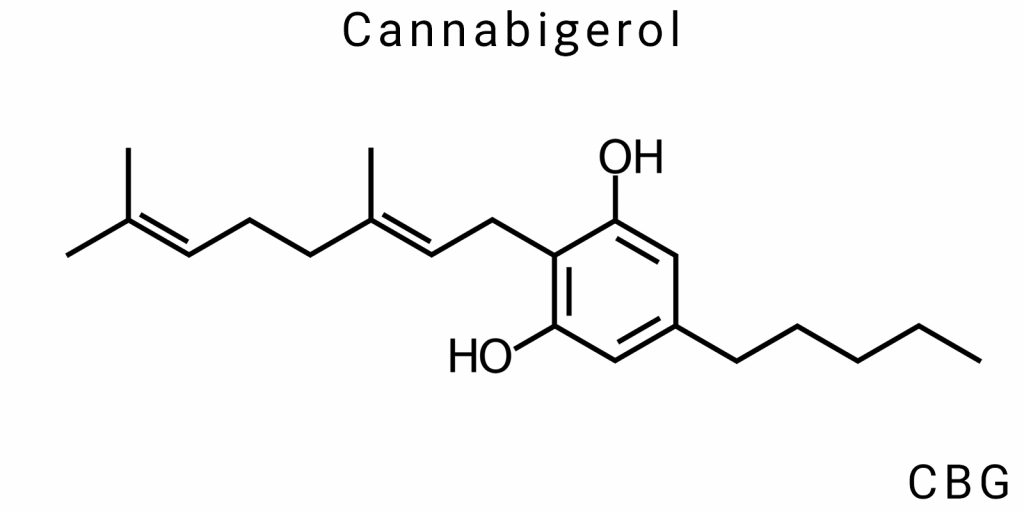
Regarding its chemical structure, CBG consists of a molecule with 21 carbon atoms, 30 hydrogen atoms, and one oxygen atom, placing it in the class of phytocannabinoids. These compounds typically feature a phenolic ring and a hydrophobic alkyl chain. A distinctive feature of CBG is the presence of a hydroxyl group (-OH) at a specific position in the molecule, which makes it more polar compared to other cannabinoids such as THC or CBD. This is significant for its biological activity and interaction with various receptors in the body.
One of the important characteristics of CBG is its biological activity at the molecular level, allowing it to have the potential for treating a wide range of conditions, from neurodegenerative disorders to inflammatory processes. However, its concentration in cannabis plants is typically very low, which limits its mass application. To increase the concentration of CBG in plants, specialized selection and genetic engineering methods are employed.
Biosynthesis of CBG: Transition from CBGA to CBG
The biosynthesis of CBG in cannabis plants begins with the formation of cannabigerolic acid (CBGA), which is the precursor to all major cannabinoids, such as THC, CBD, and CBC. CBGA is synthesized by the combination of two molecules: olivetolic acid and geranyl pyrophosphate (GPP), which are precursors to the cannabinoid structure. This step occurs through the action of the enzyme cannabigerolic acid synthase, which catalyzes their union.
Once CBGA is formed, it is further converted into CBG. This occurs through a decarboxylation process, which removes a carboxyl group (-COOH) from the CBGA molecule. The enzyme responsible for this transformation is cannabigerolic acid decarboxylase (CBGA-DC). The result is the neutral cannabinoid CBG, which plays an important role in pharmacology.
It is important to note that after CBG is formed, this cannabinoid can be transformed into THC or CBD depending on the action of other specific enzymes, such as Δ⁹-THC synthase or CBD synthase. This makes CBG unique in its role, as it is through CBG that the synthesis of the main cannabinoids occurs. However, CBG can also act independently of conversion into other cannabinoids, providing its own therapeutic properties.
Structural Features of CBG
The molecule of cannabigerol consists of a phenolic ring, which is a common structural unit for all cannabinoids. This ring allows the molecule to interact with cannabinoid receptors in the body, particularly with the CB1 and CB2 receptors, which are part of the endocannabinoid system. Another characteristic feature is the presence of a long carbon chain, which allows the molecule to penetrate biological membranes and interact with cells.
The CBG molecule contains a hydroxyl group (-OH), which adds polarity to the molecule. This makes CBG less hydrophobic compared to other cannabinoids such as THC, which is important for its ability to dissolve in aqueous environments and interact with the aqueous components of cells. The hydroxyl group also contributes to the stability of the molecule and can affect its interactions with other biological molecules.
Cannabigerol is not 100% stable within the plant. It may degrade or be converted into other cannabinoids under the influence of temperature, light, or certain enzymes. This is important for its potential medical use, as the conditions under which CBG is stored and produced can significantly affect its effectiveness.
Comparison with Other Phytocannabinoids
Comparing CBG with other cannabinoids such as THC and CBD is important for understanding its unique properties and therapeutic potential. CBG differs from THC in that it is a non-psychoactive cannabinoid. This means that it does not produce the characteristic “high” effect of THC, making it a potentially safer option for widespread medical use, particularly for treating chronic inflammatory conditions, neurodegenerative disorders, and some types of cancer.
While CBD is also non-psychoactive, it operates through different mechanisms compared to CBG. For example, CBD interacts with serotonin receptors, which provides calming properties, whereas CBG has a more direct effect on cannabinoid receptors CB1 and CB2, giving it stronger anti-inflammatory and antioxidant properties.
CBG also shows greater potential for treating infections, as it has proven effective against bacteria, including some antibiotic-resistant strains. This makes it a promising candidate for the treatment of infectious diseases, especially in the context of antibiotic resistance.
Methods of CBG Extraction
The extraction of cannabigerol (CBG) is a critical step in the pharmaceutical, biotechnology, and agricultural sectors, as this compound demonstrates a broad range of potential biological activities. Despite being less popular compared to tetrahydrocannabinol (THC) or cannabidiol (CBD), CBG is considered a promising therapeutic agent and an important raw material for the further chemical synthesis of cannabinoids. Its low concentration in natural sources requires the application of advanced extraction or synthesis techniques. This section will explore and analyze the main methods of obtaining CBG: natural extraction, synthetic technologies, enzymatic methods, and bioengineering approaches, including a comparative evaluation of their efficiency, product quality, costs, and environmental impact.
Natural Extraction
The most traditional yet technologically complex method for obtaining CBG is its extraction from plant material. An important factor here is the choice of strain: for CBG extraction, strains of Cannabis sativa are specifically bred with higher concentrations of cannabigerolic acid (CBGA), which is then subjected to biochemical or thermal decarboxylation to form the active CBG. This requires careful cultivation, harvesting, and storage practices.
One of the most efficient methods for natural CBG extraction is supercritical fluid extraction using carbon dioxide (CO₂). This method ensures exceptional selectivity for nonpolar molecules such as cannabinoids and allows CBG to be extracted without residual organic solvents. Supercritical CO₂, in a state between liquid and gas, penetrates the plant matrix, dissolving the target components. The result is a high-purity extract suitable for further purification and fractionation. However, this method requires expensive equipment, including high-pressure extractors that operate at temperatures between 35–45°C and pressures up to 350 bar.
Another method involves extraction using organic solvents, such as ethanol, hexane, or isopropyl alcohol. Ethanol, as a solvent, has high polarity, allowing effective extraction of cannabinoids, but it also causes the extraction of undesirable components such as chlorophyll. To minimize contaminants, low-temperature extraction followed by winterization is used, which allows for the precipitation of waxes, lipids, and other high-molecular-weight substances. The next step-distillation or chromatography-ensures a high degree of CBG purity. However, these procedures significantly affect the cost of the final product.
Several studies have described the use of fractional distillation under vacuum conditions, which preserves heat-sensitive cannabinoids and increases CBG concentration without losing activity. This approach, however, is rarely applied on an industrial scale due to the complexity of scaling up and the need for precise temperature control. Additional methods, such as ultrasonic extraction, are mainly used in laboratory settings and can reduce the temperature of the process, though their effectiveness with respect to CBG remains a topic of debate within the scientific community.
Biotechnological and Synthetic Approaches
In light of the scaling challenges associated with natural extraction, synthetic and bioengineering approaches are gaining increasing attention. A key strategy involves the biosynthesis of CBG using microorganisms capable of producing cannabinoids through engineered metabolic pathways. The most commonly used organisms for this process are yeast (Saccharomyces cerevisiae) and Escherichia coli, genetically modified to synthesize cannabinoid precursors-geranylgeranyl pyrophosphate (GPP) and olivetolic acid-which are then enzymatically converted into CBGA and subsequently converted into CBG through thermal or enzymatic decarboxylation.
Innovative research in this field has demonstrated the ability to produce up to 100 mg/L of CBG in fermentation cultures, suggesting the feasibility of scaling these processes. For example, publications in Nature Biotechnology and ACS Synthetic Biology describe how modular synthetic cascades have optimized productivity and increased the stability of microorganisms to levels sufficient for industrial-scale implementation. The primary advantage of this approach lies in its independence from the cultivation conditions of plants, seasonality, and phytosanitary risks. Furthermore, microbial biorefineries significantly reduce the environmental footprint due to closed-loop production cycles.
Ex vivo enzymatic methods also show significant potential. This technology involves using purified enzymes, such as cannabigerol synthase, in reactions with isolated precursors (olivetolic acid and geranylgeranyl pyrophosphate). Enzymatic reactions are carried out in buffered environments with controlled pH and temperature parameters, ensuring high selectivity and yield. One of the challenges here is the stability and cost of the enzymes; however, synthetic biology and proteomics are actively working on producing more stable and cheaper catalytic proteins.
Regarding complete chemical synthesis, this is based on the alkylation of olivetolic acid with geranyl or its derivatives in the presence of acidic or alkaline catalysts, followed by cyclization and stabilization of the resulting structure. The problem with this approach is its low chemical selectivity and the presence of significant side products. Additionally, synthetic CBG may have different stereochemistry compared to the natural molecule, which could potentially affect its bioactivity.
Comparative Evaluation of Technologies
When selecting a method for obtaining CBG, it is crucial to consider the end goal of the compound’s use. If the aim is pharmaceutical application, key factors include purity, reproducibility, and adherence to GMP standards. In such cases, biotechnological and enzymatic approaches have the advantage due to the ability to control the process at the molecular level and reduce the risk of contamination with foreign substances.
For industrial purposes, particularly in the cosmetic or food industries, extraction from plant material remains economically viable, especially when using specialized strains with high CBGA content. On the other hand, chemical synthesis methods can be used for obtaining research samples or in cases where access to raw materials is limited due to regulatory or logistical barriers.
Pharmacological Properties of Cannabigerol (CBG)
Interaction with Receptors: CB1, CB2, TRPV1, PPARγ, and Other Molecular Targets
CBG, as a non-psychoactive phytocannabinoid, demonstrates a wide range of pharmacological properties due to its ability to interact with numerous molecular targets in the human and animal body. The most studied of these are the cannabinoid receptors type 1 (CB1) and type 2 (CB2), the transient receptor potential vanilloid type 1 (TRPV1), and the peroxisome proliferator-activated receptor γ (PPARγ).
Unlike THC, CBG has a relatively low affinity for CB1 receptors, which explains its lack of psychoactive effects. However, it acts as a partial agonist of both CB1 and CB2, demonstrating modulatory effects that may be significant in regulating neurotransmission, especially in cases of neurodegenerative disorders. Experimental models have shown that CBG inhibits the reuptake of anandamide-an endogenous cannabinoid-thereby enhancing the tone of the endocannabinoid system.
TRPV1, a calcium-dependent ion channel also known as the capsaicin receptor, is modulated by CBG with an activation effect. This opens potential pathways for analgesic activity, reduction of neuropathic pain, and regulation of inflammatory responses. The impact on PPARγ-a transcription factor involved in the regulation of glucose metabolism, inflammation, and cellular differentiation-is also promising. CBG activates this receptor, which may explain its anti-diabetic and neuroprotective potential.
CBG also demonstrates the ability to inhibit the activity of the 5-alpha reductase enzyme, which is relevant in the treatment of benign prostatic hyperplasia and androgenetic alopecia. Other targets include adrenergic, serotonergic, and prostaglandin receptors, although these interactions remain the subject of active research.
Anti-inflammatory, Neuroprotective, and Antibacterial Effects
CBG exhibits pronounced anti-inflammatory properties, as confirmed both in vitro and in vivo. In experiments using macrophage and microglia cell cultures, CBG reduces the expression of pro-inflammatory cytokines-interleukin-6 (IL-6), tumor necrosis factor-alpha (TNF-α), and interleukin-1β (IL-1β). Similar effects were observed in animal models of colitis, suggesting CBG’s potential in treating inflammatory bowel diseases.
Neuroprotective properties of CBG have been studied in the context of Huntington’s disease, Parkinson’s disease, and Alzheimer’s disease. In neurodegenerative models, it has been shown that CBG reduces oxidative stress, inhibits apoptosis, and promotes neuronal survival, likely through its influence on PPARγ and inhibition of microglial activation. Interestingly, CBG demonstrated effectiveness in reducing motor function loss and inflammation in the cortex of mice with a modeled Huntington’s disease.
Antibacterial properties of CBG are gaining increasing attention, especially in the context of antibiotic-resistant infections. CBG effectively inhibits the growth of methicillin-resistant Staphylococcus aureus (MRSA) and disrupts biofilms-structures that protect bacteria from the effects of antibiotics. The mechanism is believed to involve disruption of the bacterial cell membrane function.
Potential Applications in Oncology and Gastroenterology
CBG demonstrates significant potential as an adjunct or adjuvant in the treatment of certain types of cancer. Its cytotoxic effect on cancer cells, particularly colorectal, breast, and prostate cancer cells, has been confirmed in several preclinical studies. A study published in Carcinogenesis (2014) showed that CBG inhibits the proliferation of human colorectal cells, induces apoptosis, and reduces angiogenesis in tumors in mice.
A key mechanism of CBG’s anticancer action is the inhibition of signaling pathways related to cell growth, such as ERK/MAPK and PI3K/AKT, as well as modulation of the expression of genes involved in regulating the cell cycle. Additionally, CBG reduces the level of VEGF (vascular endothelial growth factor), which limits tumor blood supply.
In the field of gastroenterology, particular interest is being given to CBG’s potential in the treatment of inflammatory bowel diseases (IBD), including Crohn’s disease and ulcerative colitis. CBG reduces neutrophil infiltration into the intestinal mucosa, normalizes epithelial barrier function, and reduces oxidative stress. In animal models, recovery of gut morphology was observed after administering CBG at doses of 5-10 mg/kg.
CBG could potentially be used in the treatment of functional gastrointestinal disorders, such as irritable bowel syndrome (IBS), due to its spasmolytic, anti-inflammatory, and analgesic properties. However, additional clinical studies are needed to confirm the effectiveness and safety of these applications in the human population.
Pharmacokinetics and Safety of Cannabigerol (CBG)
Bioavailability, Metabolism, and Elimination of Cannabigerol
Cannabigerol (CBG), despite its low affinity for the primary cannabinoid receptors (CB1 and CB2), holds significant therapeutic potential due to its ability to interact with other receptors and signaling pathways in the body. To understand CBG’s potential, an important aspect is studying its pharmacokinetics, which includes bioavailability, metabolism, and elimination.
- Bioavailability
The bioavailability of CBG is determined by how the drug enters the systemic circulation after administration. When taken orally, CBG has relatively low bioavailability due to extensive first-pass metabolism in the liver. This effect can decrease the amount of active molecule reaching the bloodstream. It has been reported that, with oral administration, bioavailability can be as low as 6-10%, significantly limiting the effectiveness of CBG when taken orally.
However, with alternative methods of administration, such as inhalation or sublingual delivery, bioavailability increases as the substance does not undergo metabolic changes in the liver initially. This allows for more effective delivery of CBG into the body, achieving higher concentrations in the blood and ensuring a faster therapeutic effect.
- Metabolism of Cannabigerol
CBG is metabolized in the liver by the cytochrome P450 enzymes, particularly CYP3A4 and CYP2C9. These enzymes catalyze the hydroxylation of the CBG molecule, leading to the formation of active and inactive metabolites. The primary metabolite of CBG is 7-hydroxycannabigerol, which has significantly lower pharmacological activity compared to the parent molecule, although it may have some therapeutic potential, particularly in interactions with other molecular targets in the body.
Since CBG metabolism involves the cytochrome P450 system, there is potential for pharmacokinetic interactions with other drugs that utilize these same enzymes. For example, concomitant use of CBG with drugs that inhibit CYP3A4 may lead to increased concentrations of CBG in the bloodstream, which could, in turn, alter its efficacy and safety. Such interactions are important when creating therapeutic regimens for patients who are using multiple medications.
- Elimination of Cannabigerol from the Body
The elimination of CBG occurs primarily through the kidneys, as most metabolites are excreted in the urine. However, a portion of CBG and its metabolites may be eliminated via the bile, and thus through feces. This is important when developing dosing regimens and assessing the potential for CBG accumulation in the body during long-term use. As with many other cannabinoids, individuals with impaired liver or kidney function may experience alterations in the metabolism and elimination of CBG, which requires dosage adjustments.
Preliminary Toxicology Data of Cannabigerol
- Assessment of Acute Toxicity
Cannabigerol demonstrates low acute toxicity. Animal models show that even with high doses of CBG, no severe lethal outcomes are observed. Increasing the dose by hundreds of times above the therapeutic level does not lead to major disruptions in bodily functions. Acute toxicity studies have shown that even in cases of CBG overdose, no significant disturbances in the cardiovascular or nervous systems occur. However, mild side effects, such as temporary disorientation, drowsiness, and slight reductions in motor activity, may be seen.
- Chronic Toxicity
Currently, there is no significant data indicating chronic toxicity of CBG. It is important to note that in long-term studies with therapeutic doses in animals, no serious adverse effects were observed. Research results suggest that CBG does not exert toxic effects on the liver, kidneys, or heart over extended periods. However, since clinical studies in humans have been of a shorter duration, the full picture of potential long-term side effects is yet to be formed.
One of the greatest challenges is that CBG may cause changes in the functions of metabolism-related organs, such as the liver and kidneys, if dosages are significantly exceeded. This necessitates careful dosage control in clinical trials and during CBG use in medical practice.
- Effect on Organs
Cannabigerol may influence various organs, including the liver, kidneys, and cardiovascular system. However, to date, no serious toxic effects have been found with CBG use at recommended doses. Toxicity cases are limited to mild symptoms in the gastrointestinal tract, such as dyspepsia or slight appetite reduction. However, in the case of overdose, temporary reductions in kidney or liver function may occur, requiring dosage adjustments or a temporary cessation of the drug.
Tolerance to Cannabigerol with Different Routes of Administration
- Oral Administration
Oral administration of CBG provides stable effects with long-term use, but its low bioavailability makes it less effective. This method is the most convenient, as it allows for easy dosage of CBG in the form of capsules or oils and enables patients to maintain control over their dosage. However, it is essential to consider that oral administration involves first-pass metabolism in the liver, which reduces the amount of active molecule in the bloodstream.
- Inhalation
Inhalation of CBG via vapor or specialized devices allows for rapid achievement of high concentrations in the bloodstream, as the substance does not undergo metabolic changes in the liver initially. This method also minimizes the impact on the digestive system, making it more effective for quickly treating symptoms. However, due to the lack of long-term studies on the effects of inhaling CBG, this method requires further investigation.
- Sublingual Administration
Sublingual administration of CBG ensures rapid absorption and high concentrations in the bloodstream. By entering directly into circulation through the mucous membranes of the mouth, CBG bypasses the liver, allowing for high bioavailability and a swift effect. This method is convenient for patients who need a rapid therapeutic response but also requires precise dosing to avoid overdose.
Current Research and Clinical Potential of Cannabigerol (CBG)
Cannabigerol (CBG), as one of the primary cannabinoids, is garnering increasing attention regarding its medical potential. Due to its pharmacological properties, including anti-inflammatory, neuroprotective, antibacterial, and antidiabetic effects, CBG shows promise in treating a wide range of diseases. The growing body of scientific research provides a strong basis for further clinical trials aimed at confirming the safety and efficacy of this cannabinoid in medical practice.
Preclinical Models Results
Preclinical studies of cannabigerol have demonstrated its significant therapeutic potential across various model systems. Animal trials and cell culture experiments have revealed promising results in treating a number of conditions, including neurological, inflammatory, oncological, and bacterial diseases.
One of the key areas of research is the investigation of CBG’s effects on neurodegenerative diseases such as Huntington’s disease, Parkinson’s disease, and Alzheimer’s disease. In preclinical models, CBG has been shown to reduce oxidative stress levels, decrease microglial activation (cells responsible for inflammation in the brain), and promote neuron preservation, providing a foundation for further exploration of CBG as a potential neuroprotector.
Moreover, CBG has demonstrated encouraging results in combating infections, particularly bacterial ones. Research has shown its ability to inhibit the growth of methicillin-resistant Staphylococcus aureus (MRSA), as well as effectiveness against other pathogenic microorganisms such as Escherichia coli and Pseudomonas aeruginosa. Additionally, positive effects of CBG have been observed in combating bacterial biofilms, which is a significant achievement in the context of antibiotic resistance.
Another promising area is the use of cannabigerol for treating inflammatory bowel diseases such as Crohn’s disease and ulcerative colitis. Animal studies have shown that CBG can reduce the levels of pro-inflammatory cytokines, improve the intestinal barrier function, and decrease neutrophil infiltration into the gut mucosa. This opens up new possibilities for utilizing cannabigerol in the therapy of inflammatory gastrointestinal diseases.
Preclinical models have also demonstrated that CBG has analgesic properties, opening the door for its use in treating chronic pain, including neuropathic and inflammatory pain. Animal experiments have shown that CBG can reduce pain levels through the activation of TRPV1 receptors, which are responsible for pain and temperature perception.
One of the most intriguing research directions is the use of CBG in the fight against cancer. A number of preclinical studies have shown that CBG has the potential to inhibit the proliferation of cancer cells, particularly in colorectal, breast, and prostate cancers. The mechanism of CBG’s anti-cancer action involves blocking signaling pathways that promote tumor growth and development, such as ERK/MAPK and PI3K/AKT. This makes CBG a promising candidate for further investigation as an adjunct to standard cancer therapies.
Clinical Trials: A Brief Overview
Clinical trials of cannabigerol are still in the early stages, but preliminary data already supports its safety and efficacy in treating certain diseases. It is important to note that most clinical studies of CBG remain small-scale and are in phases 1 and 2, which means that conclusions regarding its therapeutic potential are still preliminary.
One of the first clinical studies of CBG focused on its use in patients with chronic pain, particularly neuropathic pain. The results showed that CBG application had a positive effect on reducing pain intensity and improving patients’ quality of life. However, while these results are promising, the study did not show significant differences when compared to placebo, indicating the need for further clinical research to confirm these findings.
Another clinical study examined the use of CBG in patients with inflammatory bowel disease. CBG application resulted in reduced levels of inflammation and improved symptoms in patients with Crohn’s disease. However, these results also require further validation in a larger patient population and over longer treatment periods.
Yet another area of clinical research is the potential use of CBG for treating neurodegenerative diseases such as Parkinson’s disease and Alzheimer’s disease. Several small clinical studies have indicated improvements in motor functions in Parkinson’s patients after CBG application, but its efficacy and safety still require further large-scale studies.
Meanwhile, clinical studies in oncology, investigating the effects of CBG on cancer cells, are in the early trial stages. Preliminary data suggests that CBG may be beneficial as an adjunctive treatment for certain types of cancer, such as colorectal cancer. However, as with other research areas, more clinical trials are necessary to confirm this hypothesis.
Barriers to the Implementation of CBG in Medical Practice
There are several significant barriers to the implementation of cannabigerol in medical practice, including legal, economic, and scientific challenges. Although CBG shows substantial therapeutic potential, some of these barriers could delay or complicate its widespread use.
- Legal Barriers
One of the major barriers to the introduction of CBG into medical practice is legal restrictions associated with cannabinoids in general. In many countries, cannabinoids, even in non-psychoactive forms like CBG, are still classified as controlled substances. This complicates clinical trials and limits the widespread use of CBG in medical practice.
Currently, only a few countries have approved cannabigerol as an authorized drug, significantly limiting opportunities for research and clinical application. However, with the gradual relaxation of cannabinoid legislation worldwide, the situation may change in favor of greater recognition of CBG as a therapeutic agent.
- Economic Barriers
The development and production of CBG-based pharmaceuticals require significant investments in scientific research and clinical trials. Therapeutic drugs based on cannabigerol incur high costs for development, testing, and obtaining necessary licenses for commercial production. Additionally, the production of such drugs may be subject to taxes and restrictions, increasing their cost for end consumers.
- Scientific Barriers
While CBG research has shown its potential in treating various diseases, further clinical trials are needed to better understand its mechanisms of action, optimal dosages, and treatment durations. Only after conducting large, multiple, and long-term studies will it be possible to definitively assess whether CBG can become an effective and safe treatment for a broad patient population.
Conclusion
Cannabigerol (CBG) represents one of the most promising cannabinoids, attracting significant attention in the context of modern medical research due to its broad pharmacological properties. Its potential in treating various diseases, such as neurodegenerative disorders, infectious diseases, inflammatory processes, and cancers, based on preclinical studies, appears promising. However, while preliminary results are encouraging, CBG still requires further clinical trials to confirm its effectiveness and safety in real-world conditions.
A critical issue remains the legal regulation and economic barriers that hinder the widespread implementation of CBG in medical practice. However, given the changes in cannabinoid legislation and the gradual expansion of research, there is a real possibility for greater recognition of this cannabinoid as a therapeutic agent in the near future.
Based on current research, it can be concluded that CBG holds substantial clinical potential, but for its full application in medicine, several issues must be resolved, including its legal status, economic aspects of production, and the expansion of scientific research. Therefore, for CBG to realize its full medical potential, continued scientific research, overcoming barriers to its implementation, and careful monitoring of clinical trial outcomes are necessary, which will help establish this cannabinoid as a vital component of modern therapy.
Sources:
- PubMed (NCBI) – scientific studies, peer-reviewed articles, clinical trials: https://pubmed.ncbi.nlm.nih.gov/
This is one of the largest databases containing articles and research on medical, biological, and pharmacological topics. - Google Scholar – scientific articles, peer-reviewed journals, and dissertations: https://scholar.google.com/
A platform where you can find scientific publications and research from various fields, including pharmacology and cannabinoid chemistry. - Frontiers in Pharmacology – open-access scientific journals on pharmacology: https://www.frontiersin.org/journals/pharmacology
The journal provides access to articles from various fields of pharmacology, including cannabinoids and their therapeutic properties. - Cannabis and Cannabinoid Research – a journal publishing articles and research on cannabis and cannabinoids: https://www.liebertpub.com/can
A specialized journal focusing on scientific and clinical research on cannabinoids. - National Institute on Drug Abuse (NIDA) – official resource publishing research on cannabis and its components: https://www.drugabuse.gov/
Scientific articles and studies on cannabinoids and their impact on health. - World Health Organization (WHO) – reports and reviews on cannabinoids: https://www.who.int/
The official WHO site publishes reports and scientific reviews, including on the medical use of cannabinoids. - ScienceDirect – scientific articles and reviews on the pharmacology of cannabinoids: https://www.sciencedirect.com/
A platform for accessing scientific articles and peer-reviewed journals covering various aspects of medicine and pharmacology.