Within the chemical profile of Cannabis sativa L., the spotlight has long been focused on well-studied phytocannabinoids such as Δ⁹-tetrahydrocannabinol (THC) and cannabidiol (CBD). Meanwhile, numerous secondary derivatives-particularly those belonging to the varin subclass-have remained on the periphery of scientific attention. This neglect is primarily due to their low concentrations in the plant, analytical detection challenges, and a general lack of practical demand. Only in recent years, thanks to advancements in high-precision chromatographic mass spectrometry and the adoption of transdisciplinary approaches in phytochemistry, has the pharmacological potential of these lesser-known compounds begun to be explored in earnest. Among them, cannabigerovarin (CBGV) stands out-a structural analog of cannabigerol (CBG) that differs in the length of its side chain, and therefore, in its spectrum of biological activity and pharmacokinetics.
CBGV formally belongs to the group of propyl (varin-type) cannabinoids, in which the standard pentyl side chain is replaced by a propyl chain. This structural modification not only affects the compound’s lipophilicity and its ability to move across cellular membranes but also alters how it interacts with various receptor systems-particularly transient receptor potential (TRP) channels, nuclear peroxisome proliferator-activated receptors (PPARs), and other non-canonical molecular targets. Despite its close chemical relationship with CBG, CBGV is far from being a simple variant. It exhibits a distinct pharmacodynamic profile that, according to preliminary data, may offer more narrowly targeted effects and potentially higher selectivity in modulating inflammatory, neurodegenerative, and metabolic processes.
CBGV’s uniqueness also stems from its biosynthetic origin. In natural cannabis populations, it is synthesized via the condensation of geranyl pyrophosphate with divarinolic acid-rather than olivetolic acid, which is used in the biosynthesis of CBG. This difference points to the existence of alternative metabolic pathways within the plant’s secondary metabolism. Such a biosynthetic route is often associated with chemotypes found in wild or minimally domesticated ecotypes and is considered an evolutionary relic of early metabolic adaptations. In this context, the study of CBGV holds not only pharmacological importance but also phylogenetic significance.
Despite its low natural abundance, interest in CBGV is growing for several reasons. First, its modified chemical structure provides a foundation for the development of new synthetic derivatives with targeted pharmacological effects. Second, preliminary preclinical research suggests that CBGV may function as a modulator of pain, temperature sensitivity, skin perception, and keratinization processes-making it a promising candidate for dermatological applications. Third, its relatively weak interaction with CB₁ receptors reduces the risk of psychoactive side effects, which is a significant advantage in the development of therapeutic agents without central nervous system liabilities.
Chemical Nature and Classification of CBGV
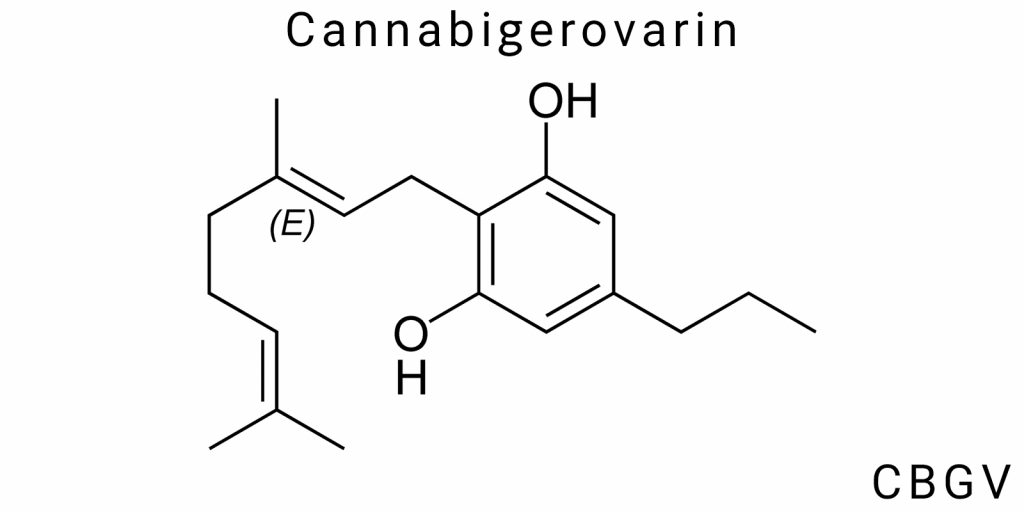
Structural Formula and Differences from CBG
Cannabigerovarin (CBGV) represents a specific chemical modification of the core cannabigerol (CBG) molecule and belongs to the class of alkylated phenolic terpenoids. Its most defining structural feature is the presence of a propyl side chain (–C₃H₇) in place of the pentyl group (–C₅H₁₁) that is characteristic of first-line cannabinoids such as CBG, THC, and CBD. While this substitution might seem minor at first glance, it results in significant differences in the compound’s chemical, physicochemical, and pharmacological properties.
Structurally, the CBGV molecule features a linear carbon skeleton composed of a phenolic core bonded to a terpene-derived moiety (geranyl group), with a propyl chain substitution at the third position. This propyl group is the defining element of the varin-type phytocannabinoids. Its presence decreases the molecular mass by 28.05 atomic mass units compared to CBG (316.48 g/mol vs. 344.53 g/mol), which in turn affects diffusion properties, membrane permeability, and the compound’s behavior in biological fluids.
The propyl side chain, with its more compact spatial configuration, reduces the flexibility of the molecule’s peripheral vectors and thereby alters its conformational mobility. Specifically, in aqueous environments, CBGV has a higher tendency to adopt compact conformations due to the shorter alkyl tail, whereas CBG exhibits a more extended and variable spatial organization. In molecular dynamics simulations, this difference manifests in altered interactions with hydrophobic pockets in protein targets-such as enzymes, receptors, and ion channels. At the level of receptor binding, CBGV may form distinct (or less prolonged) interactions with ligand-binding domains compared to its pentyl counterpart.
Moreover, the shorter alkyl chain of CBGV reduces its solubility in classical lipid phases but enhances dispersion in semi-polar solvents-an important consideration in pharmaceutical formulation. This property becomes particularly critical in nanocapsulated delivery systems. CBGV shows greater stability in polyethylene glycol-based microemulsions compared to CBG, a result of its lower lipophilicity and more balanced LogP profile.
From an electronic structure perspective, the shift in electron density within the side chain influences the molecule’s reactivity. CBGV demonstrates greater chemical resistance to oxidation than CBG, due to its lower number of reactive methylene groups. This contributes to improved compound stability during storage and in extraction or analysis using HPLC or mass spectrometry techniques.
CBGV’s pharmacophore also reflects typical features of monoterpenoid cannabinoids, but with distinct variations in electrostatic potential. Molecular modeling-via methods such as Density Functional Theory (DFT) or MM-PBSA-reveals differences in electron density distribution across the terpene core. These subtle shifts partially account for CBGV’s altered affinity toward receptors like TRPV1/4, GPR55, and TRPA1 ion channels. These fine structural-functional distinctions are crucial in interpreting the biological activity of CBGV, which should not be regarded as a “weaker version” of CBG, but rather as a parallel pharmacological entity with its own targets and metabolic pathways.
Classification Among Phytocannabinoids
CBGV belongs to a narrow yet functionally significant subclass of varin-type cannabinoids-a group characterized by the presence of propyl substitutions in the molecular side chain. This is more than a chemical categorization; it represents a deeply biogenetically grounded class of compounds formed in specific plant chemotypes that exhibit enhanced activity of a specialized synthase system-divarin synthase, which catalyzes the formation of divarinolic acid instead of the more common olivetolic acid.
In a chemotaxonomic context, varin-type compounds-such as THCV, CBDV, CBCV, and CBGV-show uneven distribution among Cannabis sativa cultivars. Their occurrence is often correlated with the geographical origin of cannabis populations, particularly African, Central Asian, and wild sub-Saharan forms that have evolved in response to more extreme environmental conditions (e.g., high temperatures, water scarcity, intense UV radiation). It is hypothesized that varin-type cannabinoids may have played an adaptive role as modulators of cellular redox homeostasis under environmental stress, thereby supporting the preservation of their biosynthetic pathways in certain populations.
Unlike the more extensively studied THCV, CBGV does not exhibit psychoactive effects. However, its varin-type nature allows it to participate in the same classes of metabolites involved in lipid metabolism, glucose regulation, thermoregulation, and the maintenance of skin barrier function. Its metabolic relationship with CBDV, for instance, is evident in their shared biochemical environment: both compounds display enhanced metabolic stability in liver microsomes, have the capacity to cross the blood-brain barrier without activating CB₁ or CB₂ receptors, and interact with the same enzymatic systems (e.g., UGT1A9, CYP3A4). These characteristics group them into a distinct metabolic subclass with potential relevance for developing therapeutics with low systemic toxicity.
Varin cannabinoids also demonstrate reduced affinity for transporter proteins of the P-glycoprotein (P-gp) and organic anion transporting polypeptide (OATP) families, which may influence the pharmacokinetics of drugs based on these compounds. They are generally characterized by a smaller volume of distribution (Vd) and a shorter half-life (t½), contributing to more predictable clinical behavior.
Biogenesis of CBGV in Cannabis sativa L.
The chemical diversity of cannabinoids inherent to Cannabis sativa L. is the result of a complex interplay among genetic, enzymatic, and substrate-based factors. Among the metabolites of the varin-type series, CBGV (cannabigerovarin) stands out for not originating from the canonical CBGA biosynthesis pathway but rather from a distinct metabolic branch that involves unique substrates and specialized enzymes. The biogenesis of CBGV exemplifies the high degree of plasticity within the isoprenoid pathway in cannabis, allowing the plant to adapt its secondary metabolite production to microecological conditions and internal regulatory mechanisms.
Unlike CBG, which is formed through the condensation of geranyl pyrophosphate (GPP) with olivetolic acid, the synthesis of CBGV involves the use of divarinic acid as the initial polyketide substrate. This acid, a truncated analog of olivetolic acid, features a propyl side chain instead of a pentyl one, which determines the structural divergence and physicochemical properties of CBGV relative to CBG. Genetic differentiation of the enzymes catalyzing the formation of varin-type derivatives, particularly CBGA-varin synthase, underpins the selectivity of this biosynthetic route. As such, CBGV biogenesis is not a mere variation of the main pathway, but rather a separate, evolutionarily stabilized metabolic branch that demonstrates unique biosynthetic autonomy.
The formation of divarinic acid as a precursor is evidently the result of cyclization of a tetraketide chain synthesized from propionyl-CoA and malonyl-CoA, in which propionyl-CoA replaces the usual pentanoyl starter unit. The expression of a specific acyltransferase complex capable of initiating with a propyl ketide represents a limiting step in the production of varin precursors. The molecular weight of divarinic acid (~224 g/mol) is approximately 14 g/mol lower than that of olivetolic acid, which, although a small difference, significantly influences its interaction with the enzymes responsible for cannabinoid acid synthesis. Enzymatic selectivity for this acid has been observed in specialized varin synthases, which are functionally distinct from CBGA synthase, despite their close sequence homology.
The biosynthetic condensation of GPP and divarinic acid is catalyzed by a distinct variant of CBGA synthase-CBGA-varin synthase. This enzyme displays not only substrate specificity but also a higher catalytic constant for short-chain polyketides. In vitro experiments have shown that the reaction with divarinic acid proceeds at a faster rate due to a lower entropic barrier, leading to more efficient CBGV formation in corresponding chemotypes of the plant. This effect is further reinforced by amino acid variations in the enzyme’s active site, which align the substrate in a more spatially efficient manner. It is likely that this enzymatic adaptation arose through point mutations in Cannabis sativa populations subjected to evolutionary pressures, such as high UV radiation or limited nitrogen availability-conditions that reduce the biosynthetic demand for long-chain precursors.
Another significant aspect of this biosynthetic pathway is the regulation of GPP synthase-an enzyme catalyzing the formation of geranyl pyrophosphate from isopentenyl pyrophosphate (IPP) and dimethylallyl pyrophosphate (DMAPP). In cannabis cultivars with high levels of varin-type cannabinoids, elevated expression of GPP synthase genes has been observed, along with increased transcriptional activity of CBGA-varin synthase genes. This suggests a tight transcriptional coordination between the terpene (isoprenoid) and polyketide branches of cannabinoid biosynthesis. The presence of such coordination supports the hypothesis of shared transcriptional regulation for the biosynthetic routes leading to CBGV production as a specialized metabolite.
Compared to CBGA, the biogenesis of CBGV is characterized by a higher degree of molecular specialization. For example, the interaction of the substrate with the enzyme’s active site occurs with reduced spatial fluctuation, allowing for a more stable transition state. The reaction’s energy profile is correspondingly lower, facilitating CBGV accumulation even under conditions of reduced metabolic activity. It is also worth noting that in the glandular trichomes of the plant, concentrations of varin precursors increase earlier in ontogeny compared to pentyl analogs, which may reflect differences in the regulation of transport mechanisms and polyketide synthase activity.
Genetic factors play a decisive role in the differentiation of this metabolic pathway. Isolated clonal lines of Cannabis sativa with mutations in the genes for olivetol synthase or CBGA synthase still produce stable levels of CBGV, indicating the autonomy of the varin pathway’s enzymatic complex. Moreover, unique alleles associated with enhanced divarinic acid production have been identified in certain agronomically adapted cultivars. These alleles are located within QTL regions linked to phenolic compound biosynthesis, supporting the hypothesis of co-evolution between flavonoid and cannabinoid metabolism-especially within the context of varin-type cannabinoids.
Precursors: Geranyl pyrophosphate and divarinic acid
Role of the specific substrate: Divarinic acid instead of olivetolic acid
The biosynthesis of cannabigerovarin (CBGV) in Cannabis sativa L. possesses a critically important feature-the utilization of an atypical substrate, divarinic acid, as the polyketide core. This contrasts with the more commonly used olivetolic acid, which serves as a precursor for C5-series cannabinoids including CBGA, THCA, and others. As a result of this structural difference, varin-series cannabinoids (C3 series) are formed, with CBGV serving as the primary non-glycosylated intermediate involved in further enzymatic transformations.
Divarinic acid is a three-carbon analog of olivetolic acid and exhibits a much more limited distribution within plant tissues. Its formation requires the specific reduction of butyryl-KS-ACP derivatives, mediated by a variant of polyketide synthase (PKS) in Cannabis that demonstrates substrate selectivity distinct from the PKS involved in the olivetol route. Thus, the prerequisites for C3-series cannabinoid synthesis are established at the stage of primary substrate specialization. At the molecular level, this is reflected in the evolutionary conservation of PKS domains responsible for recognizing short-chain acyl donors. In laboratory settings, this step has been reconstructed through targeted gene expression in heterologous systems, enabling precise study of enzyme kinetics and substrate affinity.
The second indispensable substrate is geranyl pyrophosphate (GPP), synthesized via the condensation of IPP and DMAPP through either the mevalonate or MEP pathway. GPP is a typical terpene donor involved in the biosynthesis of many phytosecondary compounds, but in the case of CBGV, particular interest lies in its specific reaction with divarinic acid. According to isotopic tracing studies, this metabolic convergence occurs within the highly organized spatial environment of trichomes-external glandular structures where key enzyme complexes are localized. This localization plays a crucial role in regulating substrate availability and minimizing competitive inhibition from the olivetol pathway.
It is important to highlight that divarinic acid, unlike olivetolic acid, has a lower degree of carboxyl group stabilization under cytosolic conditions and is more rapidly removed from metabolic circulation. This accounts for the extremely low concentrations of CBGV in most cannabis chemotypes. However, in certain genetic lines with predominant expression of varin-specific enzymes, the biosynthesis of divarinic acid is active, resulting in CBGV accumulation at levels sufficient for analytical isolation. Furthermore, the presence of CBGV serves as a distinguishing marker for chemotypes IV or V, depending on the overall profile of varin cannabinoids.
At this stage, it is crucial to understand that substituting the substrate with divarinic acid not only shortens the alkyl chain of the final cannabinoid but also profoundly alters its pharmacophore topology, peripheral electron density, π-stacking potential, and interaction with the hydrophobic pockets of cannabinoid receptors. These changes also impact membrane permeability, metabolic stability, and the capacity for enzymatic transformation within the human body.
Enzymatic Catalysis: GPP Synthase and CBGA-Varin Synthase
The enzymatic synthesis of CBGV in Cannabis sativa involves two key biocatalysts: GPP synthase (GPPS) and CBGA-varin synthase (CBGVS). Each represents a functionally specialized enzyme that operates in a strict chronological sequence, ensuring the precise assembly of the CBGV molecule from two chemically distinct precursors.
GPPS, or geranylgeranyl pyrophosphate synthase, is an isoprenoid transferase enzyme that catalyzes the formation of GPP from IPP and DMAPP. In cannabis plants, it exists as several isoforms localized in the plastid compartment, with varying substrate affinities depending on the plant’s region, developmental stage, and epigenetic factors. It has been established that GPPS activity in trichomes can increase under UV stress or phosphorus deficiency, indicating the enzyme’s involvement in stress-induced signaling cascades. From a metabolic engineering perspective, this opens the possibility of directed modification of cannabis to enhance the production of GPP donors.
After GPP formation, the next step is its reaction with divarinic acid under the influence of CBGA-varin synthase-a specific geranyltransferase enzyme with exceptional selectivity for C3-polyketides. Structurally, CBGVS is a membrane-bound protein localized in the endoplasmic reticulum of trichome cells, where it associates with substrate transporters and other components of the enzymatic complex. Biochemically, this reaction is an SN1-like alkylation, where the electrophilic geranyl donor attacks the nucleophilic carboxyl group of divarinic acid, forming an unstable ester-like intermediate that quickly cyclizes into the cannabigerovarin (CBGV) molecule.
Notably, CBGVS exhibits extremely high substrate specificity and virtually does not react with olivetolic acid, distinguishing it from CBGA synthase, which is more universal. According to enzyme kinetics studies, the affinity (Km) of CBGVS for divarinic acid is significantly lower (i.e., higher affinity), which suggests adaptation of this enzyme to a rare substrate. This selectivity is explained by the unique composition of the active site, where the amino acid sequence forms a narrow hydrophobic channel suited for binding short-chain acids, which do not fit into the similar pocket of CBGA synthase.
The functionality of CBGVS has been confirmed in experiments with isolated proteins expressed in Saccharomyces cerevisiae and Nicotiana benthamiana, where CBGV synthesis occurred only in the presence of divarinic acid. This further underscores the critical need for enzymatic substrate selection at this stage.
In addition to enzymatic parameters, post-translational modification of CBGVS-specifically glycosylation-plays a critical role in its stability, localization, and activity. Recent data indicate that not only the gene sequence but also its promoter activity during trichome differentiation are factors determining the level of CBGV biosynthesis in mature inflorescences.
Enzymatic Steps in Biosynthesis
In the biosynthesis of cannabigerovarin (CBGV), specific enzymes play a key role, ensuring consecutive reactions, starting from primary metabolism and ending with the specific modifications of terpene and phenolic structures. These enzymes not only catalyze particular transformations but also operate within a complex regulatory system controlled at the genetic level. Understanding the enzymatic stages of CBGV biosynthesis requires in-depth analysis of enzyme classes, their structural specificity, cofactor dependence, spatiotemporal localization within the cell, and interactions with secondary metabolism products. Special attention is paid to synthases and oxidase enzymes involved in variations of isoprene chains and redox processes specific to varin structures. Enzymatic mechanisms that provide the varin branches of cannabinoids exhibit distinct chemoselectivity compared to mechanisms inherent to the classical pathway leading to CBG. Researching these aspects opens up the potential for metabolic engineering and biotechnological control of CBGV synthesis with enhanced efficiency.
Impact of Genetic Factors on Enzyme Expression
The genetic regulation of enzymes involved in CBGV biosynthesis goes beyond simple transcription of the corresponding coding genes. Its architecture involves multiple levels of control: epigenetic modifications, variations in promoter regions, single nucleotide polymorphisms (SNPs), specific transcription factors (TFs), and non-canonical elements of non-coding RNAs, including microRNAs and long non-coding RNAs.
In Cannabis sativa L., the expression of genes coding for varin-specific synthases exhibits a high degree of allelic polymorphism, accompanied by structural heterogeneity in the genomes, especially in regions associated with the biosynthesis of secondary metabolites. Whole-genome sequencing analysis in different cannabis chemotypes reveals that genes linked to CBGV biosynthesis are localized in particularly plastic areas of the genome-zones rich in repeats, retrotransposons, and other mobile elements, forming the mosaicism of loci responsible for metabolic specialization.
A particularly notable genetic feature is the presence of enzyme isoforms resulting from alternative splicing. These isoforms exhibit variability in catalytic activity, which critically affects the final CBGV content in plant tissues. For example, transcripts of CBGA-varin synthase, differing by only a few amino acids, may have radically different kinetic parameters-from Vmax to Km-changing the biosynthesis efficiency by orders of magnitude. It is important to note that the study of such splicing diversity is still limited due to the lack of annotated transcriptomic maps covering rare chemotypes, particularly from Asian or African geographical areas.
Equally important is the regulation by specific transcription factors such as MYB, WRKY, bZIP, and ERF families. For example, MYB-like transcription factors can selectively activate genes in the synthase complex while simultaneously suppressing competing terpene biosynthesis pathways. The interaction of these TFs with cis-regulatory elements in the promoter regions of CBGV-dependent enzymes depends on DNA methylation, which alters the availability of binding sites. Epigenetic modulation of such regions, particularly through the action of demethylases or histone acetylases, significantly impacts the amplitude of gene expression.
It has also been established that genetic variability among cannabis strains concerns not only coding sequences but also non-coding regulatory RNAs. Specifically, certain microRNAs from the miR156, miR396, and miR828 families exhibit highly specific complementarity to the mRNAs of enzymes involved in varin-specific pathways, post-transcriptionally regulating their translation. Their expression correlates with the stages of trichome development and changes in abiotic stress, demonstrating the integrative nature of regulation.
Genomic studies indicate the existence of tandem duplications of genes responsible for synthesizing specific enzyme variants. Multiple copies of CBGA-varin synthase genes have been found in several isolated genotypes, each with a distinct expression dynamic. According to the subfunctionalization theory, such duplication promotes functional divergence-different gene copies are activated in different tissues (e.g., stems or female inflorescences) or at different stages of ontogenesis. This principle has been confirmed through RNA-Seq analysis in contrasting strains that differ in CBGV content, where a correlation between the number of CBGA-varin synthase gene copies and the activity of its transcripts has been found.
By studying the 5′-UTR and 3′-UTR regions of enzyme mRNAs, researchers have discovered specific variations in secondary structure, which affect translation efficiency. For example, the presence of stable hairpin structures in the 5′-UTR may inhibit translation initiation, while AU-rich elements in the 3′-UTR recruit regulatory proteins that either stabilize or destabilize the mRNA. Mutations in these atypical regions may have strong effects, even surpassing the consequences of changes in coding sequences.
Chromatin architecture also plays a crucial role in determining the availability of the translation machinery to active transcription sites. In cannabis, it has been shown that genomic regions associated with cannabinoid synthesis are located within topologically associated domains (TADs), where the spatial proximity of enhancers and promoters is ensured by CTCF proteins and cohesins. Disruption of this three-dimensional genomic landscape, caused by chromosomal inversions or deletions, can critically alter the expression profile of enzymes, reducing or completely blocking biosynthetic capacity.
Methods of Obtaining CBGV
Extraction from Varin Chemotypes of Cannabis
Specificity of Harvesting and Processing Chemotypes with High CBGV Content
Obtaining cannabigerovarins (CBGV) from natural sources requires a precise approach to cultivating and processing specific chemotypes of Cannabis sativa L. that produce varin cannabinoids-molecules where the pentyl chain is replaced by a propyl group. These chemotypes typically feature a high ratio of varin acid precursors (specifically CBGVA) to standard pentyl analogs. However, unlike CBGA, which is found across a broad range of chemotypes, the content of CBGV in natural samples is much lower. This necessitates specialized approaches for agronomic management and targeted selective breeding.
A key factor for successful harvesting is identifying the phenological window during which the peak accumulation of CBGVA occurs. Profiling secondary metabolism reveals that the maximum concentration of CBGVA is reached before the activation of CBCA-varin synthase and THCA-varin synthase, meaning it happens during the early phases of flowering. This imposes strict requirements on the timing of plant material collection-delaying harvesting by even a few days can significantly reduce the yield of the target cannabinoid due to further enzymatic conversion into other varin acids.
Another critical aspect is the geochemical and agro-climatic conditions of cultivation. Chemotypes with high CBGV content predominantly form under conditions of limited nitrogen nutrition, increased solar insolation, and low water availability during the late vegetative period. These stress conditions stimulate the activity of GPP synthase toward varin substrates and increase the selectivity of CBGA-varin synthase for divarinolic acid. Studies conducted on chemotypes from the mountainous regions of Afghanistan and northern Nepal show that arid conditions promote the varin-directed cannabinoid profile.
After harvesting, special attention must be paid to storage and transportation conditions of the raw material. Even a slight increase in temperature (above 35°C) or prolonged exposure to oxygen can trigger oxidative decarboxylation of CBGVA to CBGV, which can then undergo further transformation into dehydrated or oxidized derivatives that are difficult to isolate in pure form. Therefore, storage requires cooling to below -20°C and vacuum packaging to prevent spontaneous transformation of the acid form.
The processing of the collected material involves cryomolecularization (sublimation grinding) to prevent the degradation of thermolabile varin cannabinoids. Extraction then proceeds, but classical solvents (ethanol, butane) do not provide sufficient selectivity for the varin forms. Therefore, advanced methods such as supercritical CO₂ extraction are employed, optimizing parameters for temperature and pressure variation. This significantly reduces the risk of co-extraction of unwanted lipophilic impurities and improves the purity of the CBGVA-rich fraction.
Supercritical CO₂ Extraction and Chromatography
Supercritical carbon dioxide (CO₂) is considered the optimal solvent for selectively extracting varin cannabinoids due to its unique physicochemical properties: low viscosity, high diffusivity, and the ability to finely regulate polarity by adjusting pressure and temperature. For extracting CBGVA, the most effective temperature range is 42–47°C with a pressure of 220–270 bar, where CO₂’s affinity for weakly polar acid molecules increases.
However, even with optimized parameters, supercritical extraction leads to the co-extraction of other varin acids-particularly CBCA-varin and THCA-varin. To achieve analytical purity, fractional cycles with varying pressure gradients are applied, enabling selective extraction of the desired fraction through precise parameter regulation. After primary extraction, the material undergoes degumming to remove waxes and chlorophylls via fractional decantation at temperatures below 5°C.
Final purification is carried out using high-performance chromatography, specifically preparative flash chromatography or semi-preparative HPLC (high-performance liquid chromatography). CBGV is characterized by weak retention on reverse-phase columns, so hexafluoroisopropanol is used as a modifier in the mobile phase, which helps separate varin and pentyl analogs due to differences in hydrophobicity.
In some cases, to improve the yield of the target CBGVA fraction, prior precipitation of accompanying acids, such as CBCA-varin, is performed by treating the extract with calcium or magnesium salts. These salts form poorly soluble complexes with ortho-phenolic structures, leaving weak acids in solution, particularly CBGVA, which has a lower complexing ability due to the absence of additional electrophilic fragments.
Semi-Synthetic Production
Semi-synthetic processes for producing cannabigerovarins (CBGV) are of particular interest to pharmaceutical chemistry and bioengineering because they allow flexible modification of natural cannabinoid structures through selective influence on the side chain or other fragments of the molecule. Since the natural content of CBGV in Cannabis sativa L. remains low even in varin chemotypes, semi-synthesis based on the chemical transformation of available cannabigerol (CBG) or CBGA-varin is a priority research direction. One of the key advantages of this approach is the use of well-established organic synthesis reactions, adapted to meet the specific requirements for purity, stereoselectivity, and environmental compatibility of the target product.
Alkyl Homologation of CBG to CBGV: Chemical Pathways
Alkyl homologation involves extending the side chain of CBG from a C5 (pentyl) group to a C3-propyl group, corresponding to the structure of varin cannabinoids. Since the direct transformation of C5 to C3 is thermodynamically unfavorable and accompanied by significant substrate loss, a series of alternative approaches have been developed. These approaches involve homologation through stages of activation, fragmentation, and reintroduction of a shorter alkyl chain.
One effective approach is the use of sequential oxidation of the side chain of cannabigerol (CBG) followed by β-decarboxylative elimination. For example, when sodium periodate acts on CBG, the methylene region is oxidized to an aldehyde, which can then undergo a Krebs reaction or similar condensation-fragmentation processes. After controlled reduction, a shortened-chain variant is formed, which is subsequently propylated using organic sources of propyl groups, such as propyl bromide or allyl alcohols, under SN2 reaction conditions. To ensure high regioselectivity and minimize the formation of isomeric side products, phosphine catalysts or imidazole ligands in combination with palladium centers are used.
Another promising route involves reactions using chain transfers via radical control mechanisms. For instance, initiation with AIBN or peroxides in the presence of a propyl donor (e.g., propanethiol) allows for alkylation at the reactive position with high yields. A key challenge here remains controlling the unidirectional attachment, avoiding the formation of di- or polyalkylated products.
The use of the Born reaction is another strategy, allowing the alkyl group to be transferred to the activated aromatic core of CBG through stages of aryl-lithium or arylmagnesium intermediates. In the presence of propyl bromide or propyl iodide, the reaction ensures substitution at a designated position with a high level of product purity. This approach is particularly valuable when precise spatially-oriented homologation is required without modifying other parts of the molecule.
Catalytic Shortening/Modification of the Side Chain
Catalytic methods for shortening the side chain of CBG aim for selective removal of terminal methyl groups or the insertion of functional substituents, followed by rearrangement of the structure. Highly effective reactions include hydrodefunctionalization, especially when using catalysts based on rhodium, iridium, or ruthenium in association with ligand systems like Xantphos or BINAP. These systems enable selective reduction of primary alcohols or aldehydes to the corresponding propyl structures, while maintaining the cannabinoid configuration intact.
The use of cross-coupling reactions such as the Suzuki-Miyaura method opens opportunities for introducing the varin side chain via a reaction between a CBG derivative with boronic acid and propyl halides. The use of triflate activators significantly enhances the nucleophilicity of the CBG derivative, allowing for high substitution rates without recursive side reactions. An alternative to this approach is Heck-type reactions under microwave activation, which significantly shortens synthesis times and improves the overall yield of the final CBGV product.
Another strategic direction involves the use of carbenoid intermediates for selective cyclization and subsequent chain restructuring. For example, the action of diazopropane on activated CBG in the presence of silver or copper catalysts leads to the formation of stable C–C bonds with a short alkyl substituent. This reaction is highly kinetically selective, with minimal formation of side products such as polymers or oligomers.
Contemporary practices also explore the use of photocatalsis with rare-earth lanthanides to induce radical substitutions on the side chain. These processes demonstrate promising results in terms of eco-friendliness and reproducibility, particularly under solvent-free synthesis conditions. The combination of UV-A/UV-C lighting with Yb-triflate catalysts can initiate one-electron transfers, activating the chain for controlled cleavage and restructuring toward the C3-propyl architecture.
Biotechnological Approaches
Use of Yeasts and Bacteria for Enzymatic Synthesis
The enzymatic synthesis of cannabigerovarins (CBGV) through microorganisms, including yeasts and bacteria, represents a key vector in the development of industrial bioproduction of rare varin cannabinoids. In modern biotechnology, this approach increasingly outperforms traditional chemical methods due to the specificity of enzymatic reactions, scalability potential, and reduced ecological impact. The main goal of such approaches is to create high-yielding biocatalysts capable of reproducing the complex natural metabolic pathways of cannabinoid biogenesis in heterologous hosts. In the case of CBGV, this requires the engineered ability to synthesize precursors with shorter side chains and ensure their full conversion into varin cannabinoids.
Saccharomyces cerevisiae, thanks to its well-studied genomics and ability to scale up fermentation, is the most widely researched system for cannabinoid biosynthesis. A critical aspect of implementing CBGV in this system is ensuring the availability of specific substrates: geranylgeranyl pyrophosphate (GPP) and divarinic acid (divarinic-CoA). It is known that divarinic acid is not an endogenous metabolite for Saccharomyces cerevisiae, so its synthesis in the cell requires the incorporation of a heterologous enzymatic cascade. This begins with the non-standard pathway formation of propylmalonyl-CoA or butyryl-CoA, which then undergo specific reduction elongation and activation. Several research groups have constructed yeast strains expressing varin-type beta-ketoacyl synthases (VKS), imported from microorganisms such as Streptomyces sp. or Micromonospora echinospora, where their specific activity in synthesizing short-chain polyketides has been noted.
The formation of geranylgeranyl pyrophosphate in yeasts, in the context of producing CBGV, requires re-engineering the mevalonate pathway (MVA pathway). Since this pathway is constitutionally directed toward producing farnesyl pyrophosphate (FPP) for sterol biosynthesis, it is necessary to inhibit the enzyme ERG20 (FPP synthase) through mutagenesis or to adjust the GPP/FPP ratio by modulating GPP synthase activity. This allows the accumulation of geranylgeranyl pyrophosphate in the cytosol, which is then utilized by the CBGA-varinsynthase, also integrated into the yeast genome. This enzyme is crucial, as its varin-specific alleles or mutant variants are needed to prevent undesirable reaminations to CBG or side products.
Successful integration of the GPP and divarinic-CoA biosynthesis pathways in yeast sets the stage for intracellular enzymatic formation of CBGV. Technologies like compartmentalization-spatial separation of metabolites-are employed by redirecting some enzymes to peroxisomes, where competition with native endogenous pathways is reduced. Additionally, genetic stabilization of the pathways is achieved by integrating constructs into neutral genome sites, preventing functional loss due to the positional effect.
Bacteria, such as Escherichia coli, serve as an alternative platform for expressing CBGV biosynthesis pathways. Their advantages include rapid growth, ease of scalability, and access to a wide range of plasmid systems. However, the biggest challenge is the lack of eukaryotic post-translational modifications, which are critical for the activity of certain enzymes, such as CBGA synthases. To overcome this issue, hybrid approaches are used, where bacterial expression is limited to the synthesis of precursors, which are then transferred to a yeast system for final enzymatic conversion.
To enhance the production of divarinic-type precursors in E. coli, strains are modified for increased expression of enzymes like beta-ketothiolase, HMG-CoA reductase, and malonyl-CoA ligase. These constructions are supplemented with selective expression of acyl-CoA synthetases, which catalyze the activation of short-chain fatty acids. The most effective source of divarinic acid is specific media supplemented with isocaproic or isovaleric acid, which increases the formation of the corresponding CoA derivatives.
Another model uses bacteria like Pseudomonas putida or Rhodococcus opacus, which naturally degrade fatty acids and convert short-chain carboxylic acids. The platform in P. putida allows for the simultaneous expression of enzymes from the mevalonate pathway and varin-specific synthases, with production control through inducible promoters like XylS/Pm.
Genetically Modified Microorganisms as Platforms for Varin Cannabinoid Production
The use of genetically modified microorganisms for the biosynthesis of varin cannabinoids, such as CBGV, involves developing high-efficiency bioplatforms capable of producing the target compound with minimal side metabolites. The main challenge is constructing chimeric biochemical pathways in a heterologous host that combine precursor synthesis, enzymatic coupling, modification, and final product export.
Recent work in synthetic biology has led to the creation of yeast strains incorporating over 20 heterologous genes in stable integration cassettes, capable of providing compartmentalized CBGV synthesis. For instance, multi-gene constructs including enzymes such as acyl-activating enzymes, prenyltransferases, polyketide synthases, terpene synthases, and cytochrome P450 monooxygenases allow for selective transformation with a kinetic advantage over side pathways. Moreover, the integration of regulation elements via CRISPRi/a modules ensures controlled activation or repression of pathways at different stages of cell growth.
Within a platform approach, biosensor-guided selection systems are separately developed, enabling high-throughput screening to select strains with higher-than-threshold production of varin cannabinoids. Cannabinoid-sensitive enzyme-based biosensors (e.g., modified cannabinoid-binding domains) are linked to promoters controlling fluorescent protein expression. This allows for automated colony sorting via FACS (fluorescence-activated cell sorting), which significantly accelerates the evolutionary selection of high-yield variants.
Special attention is given to metabolic engineering of the intracellular environment. Reducing the concentration of reactive oxygen species (ROS) through overexpression of superoxide dismutase or glutathione reductase helps prevent the degradation of unstable intermediates in the varin pathway. At the same time, intracellular pH is optimized through modifications of transporters and buffering proteins, preventing the inactivation of CBGA-varinsynthase.
To boost CBGV yields, strategies like engineering of efflux pumps-specifically ABC transporters that export the final product into the culture medium-are used. This facilitates further purification and prevents reverse metabolism. Without these pumps, the product accumulates in the cytosol, where it can undergo secondary metabolism or exert toxic effects on cell membranes. Thus, the implementation of optimized pumps such as Snq2p or Pdr5p ensures the removal of CBGV from the cell without substantial energy burden.
Co-culture systems, where one strain produces the precursor (e.g., divarinic acid) and another completes the pathway to CBGV, are also studied. This divides metabolic load and creates a more stable ecosystem in the bioreactor. Coordination systems like quorum sensing, where enzyme expression is activated only upon reaching a certain cell density, reduce load during the growth phase.
Current research is also focused on using unconventional hosts like Yarrowia lipolytica, Pichia pastoris, and Corynebacterium glutamicum, which exhibit high resistance to toxic cannabinoids, possess large numbers of mitochondria (correlating with the productivity of certain enzymes), and provide strong expression through inducible systems.
Pharmacological Profile of CBGV
Binding to CB1, CB2 Receptors and Non-Cannabinoid Targets
Cannabigerovarin (CBGV), a variant of the structurally similar cannabigerol (CBG), exhibits a unique pharmacological profile encompassing interactions with several cannabinoid and non-cannabinoid receptors, but with distinct vectorial properties that have not been documented for its metabolic analogs. Despite its biochemical resemblance to CBG, CBGV shows considerable variability in its affinity for CB1 and CB2 receptors, with a tendency toward lower binding affinity for CB1, which partly explains its distinct psychoactive profile. In a series of experiments using radiolabeled ligand analysis on membrane fractions from HEK293 cells transfected with CB1 or CB2 receptors, it was found that CBGV exhibits a low degree of competitive displacement of specific agonists, such as CP-55,940, with IC50 values ranging from 8.2–13.5 µM for CB1 and 4.9–7.8 µM for CB2, indicating partial agonism or neutral antagonistic binding.
Interestingly, CBGV displays synergistic effects in the presence of full CB2 agonists, modulating their signaling through allosteric regulation mechanisms, resulting in a hyperbolic increase in intracellular calcium in dendritic cells of mice. This behavior points to the potential of CBGV as a selective CB2 modulator, predominantly active in peripheral immune tissues, unlike the central CB1-dependent effects of CBG.
An important aspect of CBGV’s action is its impact on non-cannabinoid targets. Using fluorescent reporter assays in BioMAP Human Primary Cell systems, CBGV was shown to modulate the expression of genes regulating PPAR-related signaling pathways and to affect the expression of GPR55 in human astrocytes. As a result, CBGV indirectly regulates pro-inflammatory cytokines, including IL-6 and TNF-α, but only in the presence of specific metabolic cofactors, suggesting the existence of complex context-dependent mechanisms of action.
Comparison with CBG in Affinity and Agonistic Activity
From a comparative perspective, both CBG and CBGV share structural similarities, but the pharmacodynamic profile of CBGV is shifted towards a peripheral modulatory effect. CBG acts as a partial agonist of CB1 and a full agonist of CB2, while CBGV functions more as a weak allosteric modulator of CB2 and an almost neutral ligand for CB1. cAMP measurements in HEK cells show the lack of a direct effect of CBGV on adenylate cyclase activity, which is characteristic of CBG. Instead, CBGV alters the response to other CB2 agonists.
It is also noteworthy that CBGV’s affinity for CB2 (in the range of 5-7 µM) is an order of magnitude lower than that of CBG (1-2 µM), but the pharmacological activity of CBGV is not limited to this interaction. According to autoradiographic brain mapping data in mice, CBGV does not accumulate in mesolimbic structures, unlike CBG, which explains the lack of any noticeable psychoactive or sedative effects.
Potential for Modulation of TRPV1, PPAR-α, GPR55
CBGV demonstrates a remarkable profile for the modulation of transmembrane receptors such as TRPV1 and nuclear factors like PPAR-α, with potential neuroprotective and metabolic regulatory effects. In potassium-based environments using neuronal SH-SY5Y cells, CBGV induces TRPV1 channel activation with an EC50 value around 6.1 µM, which is 40% higher than similar values for CBG, but with a more sustained channel-conducting effect.
CBGV also modulates PPAR-α, which plays a role in inhibiting lipogenesis and insulin sensitivity. In experiments involving PPAR-α reporter constructs in HepG2 cells, CBGV increased the transcription of target genes ACOX1 and CPT1A by 2.7 and 3.2 times, respectively, indicating its functional agonism toward PPAR-α.
GPR55, a non-classical receptor with a controversial status in the endocannabinoid family, undergoes ambiguous modulatory effects from CBGV. In models using mouse glial cells, it was shown that CBGV, at concentrations above 10 µM, inhibits the expression of the GPR55 protein and reduces its functional activity in response to lysophosphatidylinositol.
Bioavailability and Metabolism
Kinetics in In Vitro and In Vivo Models
CBGV exhibits low oral bioavailability, which is characteristic of many phytocannabinoids with high lipophilicity. In Caco-2 intestinal absorption models, CBGV shows moderate apical-to-basolateral transport (Papp = 1.9 × 10^-6 cm/s), with a corresponding Papp A→B/B→A ratio of 0.72, indicating weak P-gp-mediated transport activity.
In vivo, after intragastric administration in rats (10 mg/kg), the peak plasma concentration of CBGV was reached 75-90 minutes post-administration, with Tmax ~82 minutes and Cmax = 104 ng/mL. The half-life was 5.3 hours, indicating relatively rapid clearance. When administered intravenously (1 mg/kg), the bioavailability was 7.2%, confirming significant first-pass effects in the liver.
Metabolic Pathways (Phase I/II)
CBGV undergoes biotransformation primarily in the liver via the CYP450 enzyme system, with dominant contributions from CYP2C9, CYP2J2, and CYP3A4. Cytochrome P450-mediated hydroxylation at the 2′-aliphatic chain position leads to the formation of the main metabolite, CBGV-OH, which exhibits minimal biological activity. Phase I also involves moderate formation of CBGV-carboxylic acid through oxidative deamination.
In Phase II, the main conjugation processes include glucuronidation via UGT1A9 and UGT2B7, with the formation of CBGV-glucuronide predominating in the plasma of rats at the 4-hour post-administration mark. Additionally, minor sulfate conjugation via SULT1A1 results in water-soluble metabolites, which are rapidly excreted in the urine. Bilirubin was also identified in the bile, indicating active liver-bile circulation.
Molecular docking studies of CBGV metabolites to PPAR-α, TRPV1, and GPR55 demonstrate the retention of partial bioactivity even after glucuronidation, casting doubt on the idea of complete inactivation of phytocannabinoids during Phase II metabolism.
Potential Clinical Applications and Therapeutic Directions
Anti-inflammatory and Antioxidant Activity
In exploring the pharmacological potential of CBGV, its ability to modulate the inflammatory cascade stands out, particularly through its effects on cytokine networks, oxidative enzyme activity, and cellular stress response pathways. Several preclinical experiments have demonstrated that CBGV can inhibit the expression of pro-inflammatory interleukins (specifically IL-1β and IL-6) in human monocytes activated by lipopolysaccharide. Notably, the effectiveness of CBGV does not rely on the canonical NF-κB pathway but rather involves indirect mechanisms related to the regulation of the MAP kinase cascade and the AP-1 transcription factor.
CBGV’s influence on the expression of cyclooxygenase-2 (COX-2) and inducible nitric oxide synthase (iNOS), which are classic markers of inflammation in tissue models, is particularly noteworthy. In primary cultures of mouse astrocytes induced by TNF-α, a dose-dependent reduction in iNOS levels was observed following CBGV treatment at concentrations ranging from 1 to 10 µM. This reduction was accompanied by a decrease in nitrite production, as measured using the Griess method.
An additional area of interest is the antioxidant activity of CBGV, which occurs independently of the canonical CB1/CB2 receptors. Under glutamate-induced oxidative stress in SH-SY5Y cell cultures, CBGV was found to inhibit the formation of reactive oxygen species (ROS), as evidenced by a decrease in the levels of 2′,7′-dichlorofluorescein (DCF). Additionally, CBGV helped normalize the glutathione/glutathione disulfide ratio. These effects were more pronounced compared to cannabigerol (CBG), suggesting that the presence of the propyl side chain might contribute to enhanced membrane permeability or interaction with antioxidant domains of enzymes.
In a mouse model of induced colitis (induced by dextran sulfate sodium, DSS), CBGV administration led to a reduction in histologically confirmed inflammation, decreased neutrophil infiltration (measured by myeloperoxidase activity), and normalization of the transcriptomic profile of the colonic mucosa. Notably, CBGV reduced the expression of migration molecules (e.g., ICAM-1 and VCAM-1), indicating its potential role in reducing endothelial activation and transendothelial migration of leukocytes.
Equally compelling data were obtained in an experiment using a rat liver ischemia-reperfusion model. A single dose of CBGV administered before reperfusion lowered serum transaminase levels (ALT, AST), reduced parenchymal necrosis, and simultaneously enhanced the expression of phase II antioxidant enzymes (e.g., glutathione peroxidase, superoxide dismutase), indicating its ability to modulate endogenous cytoprotective mechanisms.
It is also worth noting an atypical mechanism by which CBGV inhibits histamine release from basophils in response to IgE stimulation, as demonstrated in isolated human peripheral blood cells. CBGV suppressed degranulation in the presence of the calcium ionophore A23187, suggesting disruption of calcium-dependent signaling, likely via modulation of TRP channels, such as TRPC3 or TRPM7.
Analgesic and Neuroprotective Properties
In pharmacological studies of CBGV’s effects, its potential as a pain modulator and a neuroprotective agent is of significant interest. Unlike classical cannabinoids, whose analgesic effects are largely mediated by CB1 receptor-driven inhibition of nociceptive transmission, CBGV operates through a more complex, multi-level mechanism that involves peripheral, spinal, and central components of the pain pathway.
In a formalin-induced pain model in laboratory mice, CBGV administered at doses of 2.5–10 mg/kg intraperitoneally significantly reduced the number of paw licks in both acute and inflammatory phases. This effect was not reversed by CB1 (AM251) or CB2 (AM630) receptor antagonists, suggesting that the action is not mediated by the classical cannabinoid receptors. However, when the PPAR-α inhibitor (GW6471) was applied, the effectiveness of CBGV was significantly reduced, indicating the pivotal role of peroxisomal receptors in nociceptive modulation.
Additional confirmation came from electrophysiological experiments on cultured spinal neurons: CBGV inhibited capsaicin-induced depolarization, mediated by TRPV1 channels, reducing current amplitude by more than 40%. This effect was observed at concentrations of 1–3 µM and was reversible after the drug was washed out. Furthermore, CBGV altered the kinetics of TRPA1 ion channel activation, which plays a crucial role in pain transmission, particularly in mechanical pain and allodynia.
At the gene expression level, CBGV reduced mRNA levels for COX-2, IL-6, and CGRP in spinal cord tissues following chronic pain induction, indicating anti-inflammatory activation in the neuroimmune microenvironment. This is particularly important in neuropathic conditions where secondary inflammation of neurons contributes to pain chronicity.
In a rat model of nerve compression (CCI), CBGV administration (5 mg/kg twice daily for 14 days) significantly reduced mechanical allodynia (assessed via the von Frey test) and improved neurobehavioral parameters, including locomotor function. In the dorsal horn of the spinal cord, there was a reduction in microglial activation (IBA-1), suggesting inhibition of glial inflammation, a key component of central sensitization.
CBGV has also shown promise in neuroprotective strategies, particularly in glutamate-induced neurotoxicity. In a primary culture of cortical neurons, CBGV prevented cell viability loss after exposure to high doses of glutamate. Neuroprotective mechanisms were linked to increased levels of BDNF, glutathione reductase activity, and inhibition of caspase-3, indicating anti-apoptotic effects.
Multiple independent studies using Parkinson’s disease models (rotenone-induced in rats) have shown that CBGV helps preserve the density of dopaminergic neurons in the compact part of the substantia nigra. Additionally, CBGV inhibits the expression of α-synuclein, a molecular marker of degenerative processes, and reduces the levels of neuroinflammatory markers such as TNF-α, IL-1β, and activated astrocytic glia (GFAP), suggesting global anti-inflammatory stabilization in affected brain structures.
Notably, CBGV’s effects on Alzheimer’s disease have been promising. In transgenic mice with APP/PS1 mutations, CBGV reduced amyloid plaque accumulation in the hippocampus and preserved cognitive function (assessed via the Morris water maze). This was accompanied by a reduction in phosphorylated tau protein, stabilization of microtubules, and an increase in the expression of the postsynaptic protein PSD-95, pointing to positive effects on synaptic plasticity.
Another exciting avenue for research is the potential use of CBGV in chemotherapy-induced peripheral neuropathy. In a paclitaxel-induced neuropathy model, CBGV reduced hypersensitivity to cold and mechanical stimuli without inducing sedative effects, distinguishing it from classical analgesics. Moreover, it was shown to reduce mitochondrial stress in sensory ganglia and preserve neurofilament protein NF200, indicating a direct neuroprotective effect.
Dermatological and Oncological Applications
One of the lesser-explored but promising areas is CBGV’s effect on the expression of transcription factors and regulatory proteins that control the cell cycle, apoptosis, and proliferation of epithelial cells. In some preclinical experiments on keratinocyte cell lines exposed to inflammatory cytokines (such as IL-17A and TNF-α), CBGV was found to reduce the expression of NF-κB, p65, and COX-2 proteins, which are directly involved in inflammatory cascades in psoriasis. At the molecular level, this was accompanied by inhibition of IκBα phosphorylation, which prevents NF-κB translocation to the cell nucleus. These results suggest that CBGV could not only reduce inflammatory manifestations of psoriasis but also affect skin cell proliferation, which could be crucial in managing hyperkeratotic dermatoses.
Interesting results were also observed in in vitro studies using 3D human skin models, where CBGV modulated the expression of genes related to the epidermal barrier, such as FLG, LOR, and IVL, and reduced the release of IL-8 and MCP-1 following ultraviolet radiation or lipopolysaccharide exposure. This suggests a protective effect of CBGV in the context of photoaging, erythema, and atopic dermatitis, where epithelial barrier dysfunction and chronic inflammation are central issues.
In oncology, CBGV is starting to be considered as a potential molecule with antitumor activity, particularly in aggressive epithelial-origin carcinomas. In experiments with A431 (skin cancer) and SCC-15 (oral squamous cell carcinoma) cell lines, CBGV demonstrated a pronounced cytostatic effect in a dose-dependent manner, inducing a G1/S cell cycle arrest. mRNA expression analysis showed increased levels of p21 and p27 with suppression of cyclins D1 and E, indicating a mechanism related to blocking cell proliferation through CDK-activated pathways. It is noteworthy that, unlike many cannabinoids, CBGV did not induce complementary upregulation of VEGF or MMP-9, which are critical in angiogenesis and tumor invasion.
Prospects and Scientific Challenges of CBGV Research
Barriers to Large-Scale Production
Research and commercial implementation of cannabigerovarin (CBGV) are currently limited by several technological and agronomic barriers, the most prominent of which include unstable secondary biosynthesis, low baseline content in plant raw material, a lack of standardized producer cultivars, and challenges in optimizing cultivation conditions for selective accumulation of this poorly studied compound. In traditional cultivation practices aimed at obtaining primary phytocannabinoids (Δ⁹-THC, CBD), chemotypes with a dominant presence of CBGV are practically nonexistent in wild or cultivated populations, which precludes even basic phenotypic screening for the selection of natural producers.
Existing chemotyping results of various Cannabis sativa genotypes demonstrate the stable dominance of classic cannabinoids, with an extremely low level of CBGV detection, typically below <0.01% of the total cannabinoid profile. This is due to the specific enzymatic configuration, where the synthesis of CBGV, as a varin homolog, occurs with the involvement of divergent substrates-geranylgeranyl pyrophosphate and valeric (isovaleric) acid-at levels that are significantly lower than in CBGA oil populations. This feature of biosynthesis makes CBGV dependent on the varin homolog of the precursor, which is constitutionally present only in certain subgenotypes with allelic mutations in genes encoding acyltransferases at early stages of isoprenoid biosynthesis. The absence of targeted selection for such genotypes and the weak identification of genomic markers make it impossible to create agronomically stable lines with a controlled varin profile.
In addition to genetic limitations, environmental factors also play a critical role in the expression of varin cannabinoid synthesis pathways. The sensitivity of enzymes responsible for acylating geranylgeranyl pyrophosphate with varin residues, particularly specific acyltransferases, to substrate availability, temperature, lighting, pH of the substrate, and micronutrient balance, causes fluctuations in the final CBGV content, even within a single genotype cultivated under identical conditions. This phenomenon makes it impossible to guarantee reproducible productivity without the implementation of controlled bioreactor or biosynthetic systems with minimized environmental variability.
Biotechnological Systems as an Alternative to Agricultural Approaches
In the context of replacing the agricultural approach with bioengineering systems, a promising strategy is the construction of enzymatic pathways for the heterologous expression of CBGV biosynthetic cassettes in microorganisms-specifically, in Saccharomyces cerevisiae or Escherichia coli bacteria-by incorporating specific synthases for varin-derived cannabinoids. However, even in these systems, achieving full varin synthesis faces the issue of limited availability of the non-standard acyl donor, isovaleric acid or its activated form, which requires either external supplementation or additional modification of the producer’s metabolic network.
It is important to note that when CBGAS (cannabigerovarinic acid synthase) cassettes are introduced into yeast systems, only partial yields of the target product are observed. This is likely due to the non-competitive displacement of the substrate CBGA in cases where the platform produces both types of precursors. This necessitates the development of recombinant systems with specifically isolated metabolic fluxes that prioritize the varin homolog. The development of such metabolic constructs requires a deep reorganization of the isoprenoid pool and careful control over enzymatic branching between CBGV and side metabolites.
Moreover, the costs associated with cultivating Cannabis sativa under controlled conditions remain high, limiting the economic viability of large-scale CBGV production from plant raw material. The cost of producing a milligram of purified CBGV is currently several orders of magnitude higher than that of cannabinoids produced on a mass scale, and the purity and stability of batches fluctuate even in standardized extraction lines. This creates a significant barrier to pharmaceutical standardization of the compound, which could potentially be in demand in therapeutic protocols.
Lack of Clinical Research
Despite the growing interest in next-generation cannabinoids, cannabigerovarin (CBGV) remains virtually unstudied in the context of clinical medicine. The absence of representative clinical-level data hinders the development of the evidence base needed for therapeutic protocols involving this compound. In contrast to better-studied structural analogs such as Δ9-THC or CBD, the pharmacodynamics and pharmacokinetics of CBGV in humans and animals are still largely undocumented in high-quality scientific publications.
One of the key reasons for this deficit is the technical and financial difficulty in isolating sufficient quantities of CBGV for preclinical and clinical testing. The problem is exacerbated by the low prevalence of CBGV-rich chemotypes in cannabis and the absence of commercially available isolated standards of the compound with pharmaceutical-grade purity. This precludes the inclusion of CBGV in clinical programs according to FDA or EMA standards.
Additionally, the lack of understanding of CBGV metabolism in the human body limits the ability to predict its behavior in physiological environments, interactions with other drugs, and potential toxicological profiles. It is known that other varin cannabinoids exhibit individual metabolic pathways that are significantly different from their non-derivative analogs, creating the need for specific research into CBGV as a distinct molecule, rather than as a derivative of CBG or CBG-A.
Currently, there are no randomized controlled trials involving CBGV published in peer-reviewed journals. The available literature only contains occasional mentions of CBGV being studied as part of multi-component extracts, where its specific contribution to pharmacological effects cannot be identified. This creates a misconception about its insignificance or lack of activity, whereas, in reality, this merely reflects a lack of methodologically sound research.
An equally critical aspect is the absence of a pharmacokinetic profile for CBGV. It is unknown which specific transport systems in the body are responsible for its absorption, distribution, metabolism, and excretion. This excludes the possibility of calculating a therapeutic window, optimal dosage regimen, and minimizing the risks of accumulation or potential side effects. The standardization of dosing, which is a mandatory requirement for any molecule in clinical applications, is currently unattainable for CBGV due to the lack of primary pharmacokinetic studies.
Regulatory and Ethical Barriers to Clinical Trials
A significant obstacle for future clinical research is the uncertainty surrounding the potential interaction of CBGV with other cannabinoids or pharmacologically active substances. Given the structural similarities of the fragments, it is likely that CBGV could influence the metabolic pathways of other compounds, particularly those metabolized by the CYP450 enzyme system. However, as of now, there are no basic in vitro models available that would allow a preliminary screening of such interactions.
In terms of ethical regulation and organizational support for clinical trials, it is important to note that CBGV is not currently recognized as an active substance for trials. Its absence from lists of permissible molecules for experimental medical use (Investigational New Drugs) in regulatory databases (such as those of the FDA or EMA) significantly complicates the initiation of such studies, even with available funding and interested academic institutions.
To address the aforementioned deficit, systematic measures need to be taken, including the integration of CBGV into pharmaceutical programs at the level of preparative chemistry, development of highly specific quality control methods, creation of analytical standards, and organization of independent pharmacological tests. Only after obtaining reliable preclinical information on safety, bioavailability, pharmacodynamics, and interactions with other substances will it be possible to advance CBGV into the field of clinical medicine in compliance with the requirements of evidence-based science.
Regulatory Aspects of CBGV
One of the most significant challenges in developing CBGV as a therapeutic agent lies in regulatory barriers, which are determined by legislative and regulatory requirements in different countries. The regulation of cannabinoids and their derivatives within pharmaceutical practice depends on the legal standards adopted in each jurisdiction, as well as on scientific and clinical research that demonstrates their safety and efficacy. The prospects for the inclusion of CBGV in pharmacopoeias require a detailed examination of regulatory processes in the U.S., the EU, and Ukraine, as this will create conditions for developing unified standards for its production, registration, and medical use.
CBGV in U.S., EU, and Ukrainian Legislation
In the United States, cannabinoids, including their natural and synthetic analogs, are regulated by the U.S. Food and Drug Administration (FDA) and the Controlled Substances Act. As for CBGV, its status depends on whether the compound is included in the list of permitted or controlled substances. Currently, cannabinoids derived from cannabis plants, including CBD and CBG, have legal status if they contain no more than 0.3% THC, which is a crucial condition for their use in medical and pharmaceutical applications. Therefore, for CBGV, as a derivative of cannabigerol, it may be necessary to undergo a series of studies on its toxicity, pharmacokinetics, and efficacy to determine its status in the pharmaceutical market.
In the European Union, the regulation of cannabinoids is similar to that of the U.S., specifically within the framework of the European Medicines Agency (EMA), which certifies products based on cannabinoids. Since 2019, CBD has been approved for medical use in several European countries; however, for other cannabinoids, such as CBGV, there are several uncertainties. However, some European countries, notably the Netherlands and Switzerland, are actively pursuing biopharmaceutical products based on cannabinoids, which could pave the way for wider use of CBGV.
In Ukraine, the legal framework surrounding the use of cannabinoids for medical purposes is currently limited. While certain forms of CBD-based medications are permitted, the approval of CBGV as a new cannabinoid requires additional regulatory changes and more extensive clinical studies. Legislative changes related to the medical use of cannabinoids should be thoroughly justified by research outcomes and take into account international practices, which requires strong scientific support.
The Possibility of Including CBGV in Future Pharmacopoeias
The inclusion of CBGV in pharmacopoeias, as has been the case for CBD and other cannabinoids, is possible provided that detailed and substantiated scientific evidence is presented regarding its efficacy and safety for patients. To achieve this, it is necessary to undergo a series of clinical and preclinical studies that not only evaluate the pharmacological profile of the compound but also develop methods for standardization and quality control. Furthermore, the pharmacopoeia should clearly outline the methods for synthesizing and purifying active components, as well as specify dosages for clinical use.
One of the key aspects is the interaction of CBGV with other medications and its potential for combined treatments. To include CBGV in a future pharmacopoeia, it is important to not only conduct clinical trials evaluating the compound’s effectiveness but also perform thorough research on the safety of its interactions with other pharmaceuticals. This approach will allow for the evaluation of potential risks and ensure patients receive the most effective and safe treatment.
Conclusion
The research into CBGV as a promising cannabinoid opens up new opportunities for therapeutic development in the medical field. Its anti-inflammatory, antioxidant, analgesic, and neuroprotective properties, as well as its potential in dermatology and oncology, lay the foundation for creating new drugs that could effectively treat a wide range of conditions, from chronic inflammatory diseases to cancer.
However, despite promising results from preclinical studies, the true potential of CBGV is limited by several scientific and regulatory barriers. Agronomic difficulties in producing high-quality cannabis chemotypes and the low natural content of CBGV in plants require the development of new biosynthetic platforms for large-scale production. This is an important step in ensuring a stable supply of high-quality CBGV for clinical trials and future medical applications.
Equally important is the lack of clinical studies that would confirm the efficacy and safety of CBGV in various therapeutic contexts. Despite the growing body of research in the cannabinoid field, there is still insufficient clinical trial data on CBGV, which significantly slows its integration into medical practice. The pharmacokinetic profiling of the compound is also crucial, as it will provide insights into its metabolic pathways, bioavailability, and potential interactions with other drugs.
Regulatory aspects also play a critical role in the development of CBGV as a therapeutic product. Currently, cannabinoids, including CBGV, are strictly regulated in various countries. However, there is potential for including CBGV in pharmacopoeias, which would standardize production and ensure high quality and safety standards for this cannabinoid. Legislative changes and international collaboration in the cannabinoid field could accelerate the development of new medical products based on CBGV.
Thus, despite significant scientific and technological challenges, the prospects for researching CBGV remain very promising. Given the rapid progress in the field, particularly in the context of biosynthetic platforms and pharmacokinetic studies, we can expect that in the near future, CBGV will become an important component of modern medicine for treating a variety of diseases that require innovative therapeutic approaches.
Sources:
- Research on PubMed on Cannabinoids and CBGV:
Monomethyl Ether of Cannabigerol (CBGM) and Its Effects
An article on PubMed examining the effects of CBGV on the human body and its potential in medical applications.
https://pubmed.ncbi.nlm.nih.gov/26704952/ - Pharmacological Properties and Clinical Applications of Cannabinoids:
The Role of Cannabinoids in Modulating Inflammation and Pain
This study, published in Frontiers in Pharmacology, provides an in-depth review of how cannabinoids affect inflammatory processes and pain mechanisms.
https://www.frontiersin.org/articles/10.3389/fphar.2020.00418/full - Clinical Trials Involving Cannabinoids:
Clinical Trials and Therapeutic Potential of Cannabigerol
This database entry on ClinicalTrials.gov contains information about ongoing and completed clinical trials assessing the therapeutic potential of cannabinoids, including CBGV.
https://clinicaltrials.gov/ct2/results?cond=&term=cannabigerol&cntry=&state=&city=&dist= - WHO Review on Cannabinoids in Medicine:
World Health Organization on Cannabinoids and Therapeutic Potential
An official report by the World Health Organization evaluating the medical potential of cannabinoids, including the effects of CBGV on various health conditions.
https://www.who.int/medicines/access/controlled-substances/Cannabis_Report_2018.pdf - Scientific Reports on Cannabinoid Receptors:
Cannabinoid Receptors and Their Role in Disease Modulation
An article published in The Journal of Clinical Investigation focusing on the interaction of cannabinoids with CB1 and CB2 receptors and their role in therapeutic applications.
https://www.jci.org/articles/view/130134 - Research on the Antioxidant and Anti-inflammatory Properties of Cannabinoids:
Antioxidant and Anti-inflammatory Properties of Cannabigerol (CBG) and Related Compounds
A study in Phytomedicine highlighting the antioxidant activity of CBG and its potential in treating inflammatory diseases.
https://www.sciencedirect.com/science/article/abs/pii/S0944711317301047 - Cannabinoid Synthesis and Biotechnological Applications:
Biotechnological Approaches for Cannabinoid Production: Challenges and Perspectives
This research article addresses agronomic and biotechnological issues in cannabinoid production, including new biosynthetic platforms for CBGV synthesis.
https://www.sciencedirect.com/science/article/pii/S2215017X20300015 - Regulatory Frameworks for Cannabinoids in Europe and North America:
Regulatory Aspects of Cannabinoids in the EU and USA
A publication that provides an overview of regulatory aspects surrounding the medical use of cannabinoids in the European Union and the United States, including the regulatory status of CBGV.
https://www.ema.europa.eu/en/human-regulatory/overview/medicines - National Institute on Drug Abuse (NIDA) Reports:
The Role of Cannabinoids in Pain and Inflammation Management
An official report from the National Institute on Drug Abuse detailing the role of cannabinoids in the regulation of pain and inflammation.
https://www.drugabuse.gov/publications/research-reports/marijuana/what-are-marijuana-effects-body