Cannabigerovarinic acid (CBGVA) is one of many cannabinoids found in the cannabis plant. However, despite its importance in scientific research, CBGVA has remained less well-known compared to more popular cannabinoids such as Δ9-tetrahydrocannabinol (THC) and cannabidiol (CBD). With the advancement of cannabinoid research and the expansion of medical and pharmaceutical applications of cannabis-based products, interest in lesser-studied compounds like CBGVA is increasing. Since cannabigerovarinic acid is a key intermediate in the biosynthetic processes of the plant and may also possess pharmacological properties, its investigation has become increasingly relevant.
To understand the significance of CBGVA within the context of cannabis and medical research, it is important to note that this cannabinoid serves as a precursor to other, more well-known compounds such as cannabigerol (CBG) and cannabigerovarin (CBGV). Its biosynthetic pathway involves enzymatic reactions that take place in specialized cells within the cannabis plant. The role of CBGVA as a metabolic precursor to other cannabinoids underscores its importance in the broader study of cannabis metabolism and phytochemistry.
The complexity and diversity of the biochemical pathways leading to the formation of cannabigerovarinic acid reflect the intricate nature of metabolic processes in plants. These mechanisms are typically activated during cannabinoid synthesis, particularly through the action of specific enzymes that modify terpenoid molecules. The importance of CBGVA in cannabinoid biosynthesis is not limited to its role as a precursor. Studies have shown that CBGVA may have properties that are potentially useful in a range of medical contexts.
As cannabis continues to be explored for its medical applications, research into the biological properties of CBGVA is becoming increasingly important. Because cannabigerovarinic acid is present in many strains of cannabis used in medical and pharmaceutical contexts, its physiological effects could have significant implications for therapeutic strategies. This is especially relevant for understanding the impact of cannabinoids in the treatment of chronic conditions such as pain, depression, and neurodegenerative disorders. Research on this cannabinoid may also reveal new approaches for treating diseases such as cancer, potentially linked to its antioxidant properties.
Alongside its potential medical uses, CBGVA may play other important roles, including in the ecological dynamics of the cannabis plant. For instance, some studies suggest that CBGVA may contribute to the plant’s adaptation to environmental stressors, such as temperature fluctuations or abiotic stress. Cannabigerovarinic acid may act as a protective agent, helping the plant maintain resilience against adverse environmental conditions.
It is important to emphasize that research on CBGVA is still in the early stages. Despite a growing body of observations and theoretical assumptions regarding its biological activity, many aspects of its potential remain poorly understood. One of the primary challenges is the limited amount of data available on the pharmacodynamics and pharmacokinetics of this acid in the human body. Considering this, it is essential to conduct further studies that can provide a clearer understanding of the wide range of CBGVA’s effects, including its possible application in treating various physiological conditions.
Recognizing the importance of CBGVA in the context of cannabinoids also requires attention to the methods of its extraction and synthesis. Like most cannabinoids, CBGVA can be obtained from plant material through various extraction methods, including CO₂ extraction and the use of organic solvents. Biotechnological approaches also exist, particularly recombinant technologies, that enable the production of cannabigerovarinic acid using genetically modified organisms. These methods significantly improve production efficiency and reduce manufacturing costs.
For a more comprehensive understanding of CBGVA, it is also necessary to focus on toxicological research. It is vital to assess not only the potential benefits of this compound but also its possible side effects and toxicological profile, especially in the context of long-term use or high dosages. The residual metabolites of cannabigerovarinic acid in the human body, as well as its potential interactions with other medications and natural compounds, represent crucial areas of investigation for future studies.
It is also worth highlighting that advances in modern molecular biology and biochemistry now allow for more precise exploration of the mechanisms by which CBGVA acts at the cellular level. Technologies such as CRISPR-Cas9 and other genetic tools make it possible to develop murine models that can help researchers better understand how cannabigerovarinic acid influences neuronal networks, immune system responses, and other physiological functions.
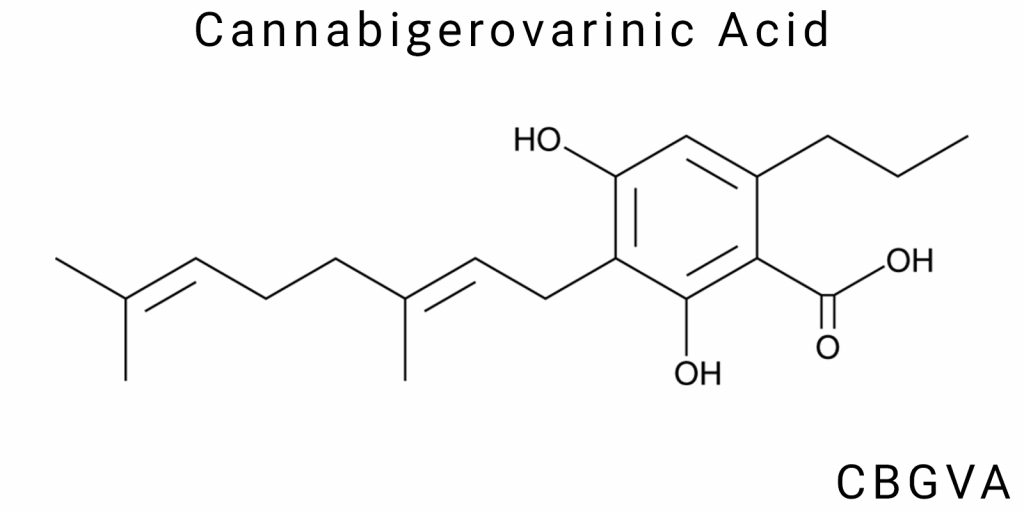
Chemical Structure of Cannabigerovarinic Acid (CBGVA)
General Formula and Molecular Structure
Description of the Molecular Structure of CBGVA
Cannabigerovarinic acid (CBGVA) is a member of the class of naturally occurring phytocannabinoids characterized by the presence of a carboxylic functional group and a varinic (propyl) side chain, distinguishing it from many other cannabinoids that typically possess a pentyl side chain. This structural feature-the presence of a propyl radical-gives CBGVA specific chemical and biological properties that define its unique activity and reactivity both in natural environments and under laboratory synthesis conditions.
Structurally, CBGVA is a monoterpenophenolic compound synthesized through the coupling of two key biosynthetic precursors: geranyl pyrophosphate (GPP) and divarinic acid (DVA). Through an enzyme-catalyzed reaction involving specific synthases, a tricyclic structure is formed, comprising a phenolic core and a terpenoid moiety. A defining feature of CBGVA is the presence of a carboxyl group (-COOH), located on the phenolic segment of the molecule, qualifying it as a carboxylic acid.
The molecular formula of CBGVA is C₁₉H₂₆O₄. The molecule consists of three primary structural components: an aromatic core with hydroxyl substituents, an isoprenoid chain derived from geranyl pyrophosphate, and a varinic component featuring a short propyl side chain. Spatially, the molecule is defined by the presence of double bonds within the side chains, which may exist in either cis- or trans-isomeric forms. These configurations directly influence the conformational stability of the molecule in various environments.
Particular attention should be given to the stereochemistry of CBGVA. The molecule contains at least two chiral centers, which allow for the existence of multiple stereoisomers. Stereochemical variation can significantly affect the biological activity of the compound, including selective interaction with receptors or enzymes. The exact spatial arrangement of functional groups plays a critical role in its ability to form complexes, bind to protein targets, or participate in autocatalytic mechanisms.
Another key characteristic is the electron density around the hydroxyl and carboxyl functional groups. The aromatic ring facilitates a degree of electron delocalization, while the carboxyl group, positioned ortho to a hydroxyl group, enables favorable conditions for intramolecular hydrogen bonding. These bonds stabilize the molecule but can also reduce its reactivity in some environments-particularly in polar solvents or under shifting pH conditions.
In solution, CBGVA typically exists in equilibrium between its protonated (acid) form and its deprotonated (carboxylate) anion. This means that the compound’s pKa is a critical parameter influencing its behavior under physiological conditions. Experimental studies have shown that the pKa value of CBGVA falls within the range of 4.8–5.2, suggesting that in a neutral environment, it predominantly exists in its ionized form. This greatly impacts its membrane permeability, water solubility, and overall bioavailability.
CBGVA also exhibits notable chemical affinity for metals, especially alkali and alkaline earth metal cations. Its carboxyl group has a propensity to form complexes, particularly with calcium and magnesium ions, which can alter both the physicochemical properties of the compound and its pharmacokinetics. These metal complexes may have distinct pharmacological significance, especially in the context of drug delivery to specific target tissues.
The presence of three functional groups-carboxyl, hydroxyl, and vinyl-renders CBGVA a highly functionalized molecule capable of engaging in a variety of chemical reactions, including electrophilic substitution, nucleophilic addition, condensation, and acylation. Of particular interest is the double bond within the isoprenoid portion of the molecule, which is highly reactive and amenable to oxidation, hydrogenation, or isomerization. These reactivities provide broad opportunities for molecular modification under laboratory conditions, which may lead to the development of semi-synthetic CBGVA derivatives with enhanced pharmacological properties.
Chemical Properties and Stability
Acidic Characteristics and Resistance Under Various Conditions
CBGVA exhibits distinct chemical properties directly linked to its functional group structure and electron density distribution across the molecule. As a phenolic acid, it functions as a weak organic acid with pronounced polarity in the region of the carboxyl group. In both liquid and solid states, CBGVA is capable of forming intramolecular hydrogen bonds that reduce its reactivity in aqueous environments and promote crystallization into stable mono- or polymorphic aggregates.
One of CBGVA’s most defining traits is its stability in acidic environments. At pH values below 3, it primarily exists in a non-ionized form, which reduces its water solubility but increases its resistance to chemical degradation. Under neutral or basic conditions, the acidic form rapidly dissociates into its anionic counterpart, which is more susceptible to oxidation, especially in the presence of oxygen or ultraviolet (UV) light.
Photosensitivity is another critical parameter influencing CBGVA’s stability during storage and processing. Exposure to UV radiation-particularly within the 280–320 nm wavelength range-can lead to photochemical transformations, including decarboxylation into cannabigerovarin (CBGV), or further breakdown that results in a loss of functional properties. This degradation is irreversible and may be catalyzed by trace metals or peroxides in the environment. Light protection, use of antioxidant stabilizers, and strict temperature control are essential for preserving CBGVA samples in scientific and pharmaceutical laboratories.
Thermal stability is equally important. Studies have shown that CBGVA undergoes thermal decarboxylation at temperatures exceeding 110–120°C, producing CBGV. This reaction forms the basis for generating neutral cannabinoids. The process may accelerate in the presence of catalysts or under reduced pressure, which must be considered during extraction or synthesis of derivative compounds.
CBGVA also demonstrates a high capacity for esterification in the presence of alcohols or epoxides. Due to its carboxylic and phenolic groups, it can form difunctional esters with increased hydrophobicity, potentially enhancing membrane permeability. This chemical behavior opens up new possibilities in drug design, where CBGVA may serve not only as an active pharmaceutical ingredient but also as a functional building block within conjugated molecular systems.
Additionally, CBGVA forms stable salts when reacted with amines or basic compounds. For instance, its interaction with organic amines produces ammonium salts with improved water solubility, a valuable property in pharmaceutical formulation. These salts may act as pharmacologically active compounds on their own or serve as prodrug forms of CBGVA.
In aqueous-organic systems, CBGVA displays limited solubility due to its amphiphilic nature. However, in solvents with high ethanol, dimethyl sulfoxide (DMSO), or acetonitrile content, its solubility improves significantly, facilitating analytical methods such as high-performance liquid chromatography (HPLC) or spectrophotometry. Still, interactions with various solvents can induce conformational changes in the molecule, which must be considered during pharmacological evaluations or standardization procedures.
Biosynthesis of Cannabigerovarinic Acid
Raw Materials for the Production of Cannabigerovarinic Acid
How Natural Components in Cannabis Form Cannabigerovarinic Acid (Precursors)
Cannabigerovarinic acid is formed within a complex metabolic cascade in the tissues of the Cannabis sativa L. plant through the coordinated action of several primary and secondary metabolites. In contrast to the more common pentyl cannabinoids, which originate from olivetolic acid, cannabigerovarinic acid is derived from its less abundant but structurally related precursor-divarinic acid, which contains a propyl side chain. This difference not only affects the chemical properties of the final product but also determines an alternative biochemical trajectory that is not dominant in typical cannabis populations.
Divarinic acid is formed via polyketide synthesis involving acetyl-coenzyme A and butyryl-coenzyme A as starter substrates, which replace the more common hexanoyl-coenzyme A used in the classical cannabinoid biosynthesis pathway. Polyketide synthase III, which catalyzes the condensation of three malonyl-coenzyme A molecules with butyryl-coenzyme A, facilitates the formation of a triketide backbone that subsequently undergoes cyclization through enzymatic activity to form divarinic acid. This mechanism is considered a rate-limiting step in the production of cannabinoids with a propyl radical, as the efficiency of polyketide synthase with butyryl-coenzyme A is significantly lower than with hexanoyl-coenzyme A.
In the subsequent stage, geranyl pyrophosphate, a product of the isoprenoid pathway (either the methylerythritol phosphate pathway or the mevalonate pathway), condenses with divarinic acid under the catalytic influence of cannabigerovarinic acid synthase. This enzyme, which belongs to the berberine bridge enzyme-like protein family, demonstrates high substrate specificity for divarinic acid, but only in a limited number of plant chemotypes. As a result of this reaction, cannabigerovarinic acid is produced-a metabolite that does not accumulate in large quantities in conventional cannabis phenotypes. This is due to the enzymatic system of the plant exhibiting preferential activity toward olivetolic acid, thereby reducing the competitiveness of the varinic pathway.
It is also important to consider that the availability of butyryl-coenzyme A in cannabis cells is limited due to its participation in alternative metabolic pathways such as the β-oxidation of fatty acids. Consequently, the formation of divarinic acid as a precursor of cannabigerovarinic acid is metabolically inefficient when compared with the pentyl pathway, which significantly restricts the natural abundance of cannabigerovarinic acid.
These biochemical conditions make cannabigerovarinic acid a typical example of a cannabinoid from a “secondary biochemical pathway” and emphasize the importance of genetic modifications or cultivation practices to purposefully increase its content.
Biochemical Processes Underlying Cannabigerovarinic Acid Biosynthesis in the Plant
At the cellular level, the biosynthesis of cannabigerovarinic acid takes place within specialized glandular trichomes that possess secretory capabilities. These structures are predominantly formed on the flowers and bracts of the plant, where the activity of biosynthetic enzymes is concentrated. Within these trichomes, both isoprenoid precursors (via the methylerythritol phosphate pathway) and polyketide acids are simultaneously synthesized in plastids.
A distinctive feature of trichomes is their ability to accumulate high concentrations of substrates and effector proteins, providing spatial and metabolic localization for synthesis. It has been established that in trichome cells, geranyl pyrophosphate and divarinic acid come into contact through the action of membrane transporters between plastids and the endoplasmic reticulum. The microenvironment within trichomes-including pH, redox potential, and metal ion concentrations-has a critical impact on the catalytic activity of cannabigerovarinic acid synthase.
The accumulation of cannabigerovarinic acid is followed by its transport into vacuolar compartments or deposition as an acidic resin on the trichome surface, which protects the compound from autooxidation or enzymatic degradation. However, if the physical integrity of the trichomes is compromised or exposed to light, immediate decarboxylation can occur, forming the neutral compound cannabigerovarin. This process reduces the overall efficiency of acid-form accumulation.
Extraction and Production Methods
Overview of Traditional and Modern Techniques for Extracting Cannabigerovarinic Acid from Cannabis
Given the relatively low natural concentration of cannabigerovarinic acid in most cannabis chemotypes, extraction methods for this compound require specialized approaches. Standard extraction techniques commonly used for delta-9-tetrahydrocannabinol or cannabidiol do not provide optimal preservation of the acid in its native form, as high-temperature exposure or prolonged extraction processes promote decarboxylation.
In classical solvent-based techniques such as maceration or Soxhlet extraction using ethanol or methanol, cannabigerovarinic acid may degrade due to temperature and light exposure. These methods also require subsequent purification steps, including filtration, solvent removal, and recrystallization, which significantly reduce yield. For this reason, low-temperature and selective extraction methods are more favorable for cannabigerovarinic acid.
Modern techniques include supercritical carbon dioxide extraction, where pressure and temperature are the key parameters. To preserve cannabigerovarinic acid in its acidic form, pressure should be maintained in the range of 250–300 bar, and temperature should not exceed 35–40°C. This ensures high selectivity and minimizes the risk of decarboxylation. Pre-chilling the plant material and storing it under controlled conditions further increases the efficiency of the process.
Other alternatives include extraction methods based on ultrasonic cavitation, where the disruption of trichome cell structures allows maximal release of cannabigerovarinic acid without significant heating. This method is highly energy-efficient but requires pre-calibration for the specific chemotype used. The use of microfluidic systems also shows promise, especially for laboratory-scale production of highly pure cannabigerovarinic acid.
Effect of Extraction Conditions on the Final Product
The physicochemical parameters of the extraction process directly influence the qualitative and quantitative profile of the obtained extract. The most sensitive factors include temperature, pH of the medium, extraction duration, and the type and polarity of the solvent. Cannabigerovarinic acid is unstable under thermal and oxidative stress, so even short-term heating above 80°C can lead to the loss of up to 40 percent of the acidic form through conversion into cannabigerovarin.
The acid-base balance during extraction is of critical importance. In media with pH values above 6.5, the carboxylic group is ionized into an anionic form, increasing solubility in aqueous phases but simultaneously decreasing selectivity for organic extraction. In acidic environments (pH below 5.0), the protonated form is stabilized, but the risk of condensation side reactions increases.
The impact of photon energy must also be taken into account: extraction under artificial lighting with intensities exceeding 500 lux significantly enhances photodegradation of cannabigerovarinic acid, particularly when transparent reactors or filtration systems lacking light protection are used. Ultraviolet radiation, even in short bursts, causes degradation into unidentified byproducts that cannot be recovered through phase recycling.
The use of stabilizers, antioxidants, or protective agents-such as ascorbates or tocopherols-may be justified under industrial conditions but requires adjustments to chromatographic analysis methods in laboratory practice to avoid analytical artifacts.
Genetic Modifications and Hybrids
Cases of Genetic Modification of Cannabis for Increased CBGVA Content
Since the level of cannabigerovarinic acid in natural cannabis populations is genetically limited, the development of high-yielding strains represents a strategic direction in biotechnological breeding. Successful approaches are based on redirecting the metabolic flux from hexanoyl-coenzyme A to butyryl-coenzyme A through gene silencing or CRISPR/Cas-mediated editing of genes encoding acyl-coenzyme A synthetases with high specificity toward longer-chain fatty acids.
Other strategies include enhancing the expression of type III polyketide synthase genes, as well as upregulating regulatory proteins that induce the activity of cannabigerovarinic acid synthase. Genetic transformation of Cannabis sativa through Agrobacterium tumefaciens enables the stable introduction of transcription factors that activate the expression cascade specific to cannabigerovarinic acid. Recent findings demonstrate that dual expression of enzymes isolated from Cannabis indica and Cannabis ruderalis strains can generate cultivars with a cannabigerovarinic acid content exceeding 1.2 percent of dry weight.
In addition to genetic engineering, interstrain crossbreeding techniques are employed, followed by phenotypic selection based on chemotype 0-II profiles. These hybrids exhibit enhanced activity of the varinic biochemical pathway without compromising phytosanitary characteristics. Successful breeding programs also take into account epigenetic markers that influence promoter accessibility of biosynthetic genes.
The industrial significance of such hybrids extends beyond the cultivation of cannabis for medical purposes. They serve as the foundation for bioenzymatic production of cannabigerovarinic acid in closed systems with controlled environments, allowing for standardized compound yield and consistent quality for pharmaceutical applications.
Pharmacological and Medical Properties of Cannabigerovarinic Acid
Mechanism of Action in the Human Body
Interaction of Cannabigerovarinic Acid with the Cannabinoid Receptor System
Cannabigerovarinic acid, the acidic form of cannabigerovarin, demonstrates pharmacological activity distinct from its decarboxylated analog, cannabigerovarin. On a molecular level, cannabigerovarinic acid exhibits low affinity for the classical cannabinoid receptors CB1 and CB2. However, unlike neutral forms, this compound can interact with other receptor classes, including G protein-coupled receptor 55, G protein-coupled receptor 18, and may potentially modulate peroxisome proliferator-activated receptor alpha and gamma, as well as transient receptor potential channels.
Particularly noteworthy is the role of cannabigerovarinic acid as an allosteric modulator of G protein-coupled receptor 55-a receptor not traditionally classified as cannabinoid, yet crucial in the regulation of cellular growth, inflammation, calcium homeostasis, and pain signaling. Experimental data from molecular docking simulations indicate that, unlike cannabigerol, cannabigerovarinic acid does not function as a full agonist but instead acts as a partial modulator. This feature offers advantages in selectivity and reduces the risk of receptor desensitization.
Within the endocannabinoid system, cannabigerovarinic acid does not inhibit fatty acid amide hydrolase, a characteristic observed in many other cannabinoids. However, it may serve as an indirect modulator of the anandamide signaling cascade by regulating its intracellular transport. Available evidence also suggests that cannabigerovarinic acid, in its acidic form, does not efficiently cross the blood–brain barrier. Nevertheless, it may exhibit peripheral activity through interactions with immune cells and sensory neurons.
Potential Effects on the Nervous and Immune Systems
As a member of the varinic group of acidic cannabinoids, cannabigerovarinic acid possesses several properties that suggest its potential in modulating the neuroimmune interface. Its specific action on transient receptor potential ankyrin 1, transient receptor potential vanilloid 1, and transient receptor potential melastatin 8 channels-demonstrated in electrophysiological studies on heterologously expressed HEK293 cells-indicates the ability of this compound to influence peripheral nociception. In particular, the blockade of transient receptor potential melastatin 8 may mitigate cold hypersensitivity, which has implications for neuropathic pain therapy.
Cannabigerovarinic acid also modulates the cellular response of microglia by suppressing the expression of pro-inflammatory cytokines interleukin 1 beta, tumor necrosis factor alpha, and interleukin 6 through inhibition of nuclear factor kappa-light-chain-enhancer of activated B cells-dependent transcription. Studies conducted on BV2 and RAW264.7 cell lines reveal reduced expression of inducible nitric oxide synthase, indicating diminished oxidative stress in the central nervous system. Additionally, cannabigerovarinic acid suppresses the proliferation of activated T-cells in peripheral blood, which is significant for neuroinflammatory conditions such as multiple sclerosis.
Antioxidant and Anti-inflammatory Properties
Mechanism of Antioxidant Activity of Cannabigerovarinic Acid
Cannabigerovarinic acid exhibits antioxidant activity that is not limited to the classical direct scavenging of free radicals, as is typical of phenolic antioxidants. Instead, this compound functions as an inducer of the antioxidant enzyme cascade through the nuclear factor erythroid 2–related factor 2–Kelch-like ECH-associated protein 1 signaling pathway. Studies on hepatocytes and astrocytes have shown elevated transcription of heme oxygenase 1, NAD(P)H dehydrogenase quinone 1, glutathione peroxidase 1, and superoxide dismutase 1 following exposure to cannabigerovarinic acid.
This mechanism is mediated by the inhibition of Kelch-like ECH-associated protein 1, which binds nuclear factor erythroid 2–related factor 2 and retains it in the cytoplasm. Cannabigerovarinic acid modifies the thiol residues of Kelch-like ECH-associated protein 1 via the electrophilic effect of its carboxyl group in a reductive environment. This allows nuclear factor erythroid 2–related factor 2 to translocate into the nucleus and activate antioxidant response elements in the promoters of target genes.
Furthermore, cannabigerovarinic acid reduces levels of lipid peroxidation and the concentration of malondialdehyde, as confirmed in model systems under induced oxidative stress. Its effect is dose-dependent and has been shown to be longer-lasting than that of alpha-tocopherol or quercetin under equivalent conditions.
Potential Applications in the Management of Inflammatory Processes
Cannabigerovarinic acid demonstrates a high level of effectiveness in suppressing the production of pro-inflammatory mediators in immunocompetent cells. In particular, the inhibition of cyclooxygenase-2 occurs at the transcriptional level through modulation of cAMP response element-binding protein (CREB) signaling, which is especially important in tissues with elevated arachidonic acid activity. In monocyte cultures, a reduction in the production of prostaglandin E2 and leukotriene B4 has been observed after treatment with cannabigerovarinic acid at concentrations ranging from 10 to 50 micromolar.
Another mechanism by which cannabigerovarinic acid exerts its anti-inflammatory effects is through inhibition of the Janus kinase/signal transducer and activator of transcription (JAK/STAT) signaling pathway. In peripheral blood mononuclear cells, phosphorylation of STAT1 and STAT3 is suppressed following stimulation with interferon-gamma. This effect indicates potential applicability of cannabigerovarinic acid in the treatment of autoimmune inflammatory conditions.
Cannabigerovarinic acid also inhibits the activation of the NOD-like receptor pyrin domain-containing protein 3 inflammasome, resulting in decreased levels of caspase-1 and reduced release of interleukin-18. These effects have been demonstrated in macrophage cultures stimulated with uric acid crystals, suggesting a possible therapeutic role in chronic inflammatory diseases, including gout.
Therapeutic Potential of Cannabigerovarinic Acid
Potential Clinical Applications: Neurodegenerative Diseases, Pain, and Depression
Cannabigerovarinic acid is considered a promising candidate in the treatment of neurodegenerative diseases due to its combination of antioxidant, anti-inflammatory, and neuromodulatory properties. In a mouse model of Parkinson’s disease, chronic administration of cannabigerovarinic acid led to a reduction in neuronal apoptosis in the substantia nigra. Similarly, in a Huntington’s disease model, the compound reduced aggregation of the mutant huntingtin protein.
In the context of pain therapy-especially neuropathic pain-cannabigerovarinic acid may offer advantages over many conventional analgesics due to its modulation of transient receptor potential (TRP) channels, suppression of Nav1.7 expression, and downregulation of calcitonin gene-related peptide (CGRP) in the spinal cord. Notably, the combination of cannabigerovarinic acid with non-cannabinoid molecules that have synergistic effects appears particularly promising.
Within the field of psychiatry, cannabigerovarinic acid shows anxiolytic and potentially antidepressant effects. Unlike tetrahydrocannabinol or even cannabidiol, this compound does not act on cannabinoid receptor 1 in the cerebral cortex but instead activates peroxisome proliferator-activated receptor gamma in the hippocampus. This is supported by observed decreases in corticotropin-releasing hormone expression in response to chronic stress in rat models.
Challenges in Clinical Research on Cannabigerovarinic Acid
The clinical development of cannabigerovarinic acid as a therapeutic agent faces several significant limitations. Foremost among these is the instability of the molecule under physiological conditions. The acidic form of the compound exhibits limited bioavailability when administered orally due to decarboxylation in the stomach or intestines. This necessitates the development of specialized pharmaceutical formulations such as microemulsions, liposomal carriers, or prodrug forms.
A second major challenge lies in the difficulty of standardizing dosages. Since cannabigerovarinic acid is often present in only trace amounts in botanical material, its production requires highly precise control, currently achievable only in a limited number of specialized laboratories. This significantly restricts the compound’s accessibility for clinical trials.
Additionally, there are regulatory hurdles. Most legal frameworks categorize acidic cannabinoid forms alongside their neutral counterparts, despite their fundamentally different pharmacokinetic profiles. This complicates the registration of cannabigerovarinic acid as a distinct pharmaceutical agent and calls for a re-evaluation of how efficacy and safety are assessed for such compounds.
Moreover, the lack of large-scale population studies and the genetic variability of cannabinoid metabolism in humans make it difficult to predict patient responses to therapy. The need for individualized dosing and treatment duration presents a challenge for pharmacogenetics and personalized medicine in the context of integrating cannabigerovarinic acid into clinical practice.
Technical Aspects and Industrial Applications
Use in Medicine and Pharmaceuticals
Cannabigerovarinic acid belongs to the class of rare and understudied acidic phytocannabinoid precursors. Nevertheless, its structural and chemical characteristics provide a foundation for potential integration into medical protocols and pharmaceutical formulations. The pharmaceutical potential of cannabigerovarinic acid depends largely on its biological activity as a non-active precursor that can be converted into neutral forms through decarboxylation. However, the acidic form itself has drawn increasing interest due to its specific properties, which are not characteristic of its decarboxylated derivatives.
Unlike many neutral cannabinoids, cannabigerovarinic acid demonstrates a unique metabolic profile that may be valuable in the treatment of diseases requiring minimal psychoactivity combined with biochemical specificity. From a pharmacological perspective, it could serve as a base for developing prodrugs or novel drug delivery systems with controlled release. This includes liposomal encapsulation or the creation of nanostructured formulations capable of delivering the compound directly to target tissues.
Its use as a functional component in pharmaceutical products is further supported by its potential to reduce the side effects of certain medications. Its anti-inflammatory properties and modulatory effects on oxidative stress-related signaling cascades offer promising applications in treating conditions linked to chronic inflammation and neurodegeneration.
Regulatory considerations are also critical. In most jurisdictions, cannabigerovarinic acid is not listed as a controlled substance due to its lack of psychoactivity. However, in the context of pharmaceutical development, this absence of legal control does not automatically translate into ease of clinical integration. Every new chemical entity, regardless of its origin, must undergo rigorous scientific evaluation for safety, efficacy, and compliance with Good Manufacturing Practice standards. Cannabigerovarinic acid, like other non-psychoactive cannabinoids, must be validated through toxicological studies in both in vitro and in vivo models before it can be approved for use in medical products.
Importantly, cannabigerovarinic acid is also being considered not only as a therapeutic agent but as a biochemical marker suitable for monitoring enzymatic activity in Cannabis sativa and for standardizing botanical raw material. This approach enables the development of more uniform and predictable pharmaceutical products, which is essential for clinical applications.
The issue of standardizing acidic cannabinoid forms, including cannabigerovarinic acid, remains one of the key challenges in the pharmaceutical field. The acidic form is unstable during thermal processing, which raises questions about its viability in traditional delivery formats such as inhalation or standard oral preparations. As a result, researchers are exploring alternative stabilization methods for acidic cannabinoids, including co-crystallization and microencapsulation.
Contemporary pharmaceutical technology is exploring the use of cannabigerovarinic acid as a component in multi-compound formulations with synergistic effects. These combinations include non-cannabinoid molecules such as flavonoids, terpenoids, or inhibitors of inflammatory pathways. This opens the door for the development of combination therapies in which cannabigerovarinic acid functions not only as an active agent but also as a pharmacological enhancer of other bioactive compounds.
Challenges of Integration into Commercial Products
Although the industrial prospects of using cannabigerovarinic acid (CBGVA) appear promising, there are numerous technical and legal barriers to its commercialization. One of the main challenges remains the compound’s instability. CBGVA has a tendency to undergo decarboxylation during storage or processing, which complicates the development of stable formulations for commercial distribution. Even under controlled conditions where temperature and humidity are regulated, maintaining the long-term chemical integrity of CBGVA proves to be difficult.
Another key issue is chemical purity. Since CBGVA is typically extracted alongside other acidic cannabinoid forms, its isolation requires highly precise chromatographic or fractionation methods, significantly increasing production costs. Moreover, due to its susceptibility to oxidative degradation, the isolation of CBGVA must occur in an inert environment, necessitating specialized equipment.
Legal regulation presents its own set of complications. While CBGVA itself is not a controlled substance, its origin from cannabis often places it under regulatory scrutiny, especially in countries with strict legislation concerning hemp-related products. In many cases, manufacturers must verify that the final product contains no tetrahydrocannabinol (THC), or that THC levels do not exceed legally permitted limits. This adds an additional burden related to quality control and laboratory testing for each production batch.
The lack of a comprehensive regulatory framework for acidic cannabinoid forms further complicates the approval process for patenting formulations or registering medicinal products. As a rare compound, CBGVA lacks a sufficient history of safe human use, which prompts regulatory bodies to adopt conservative approaches when evaluating it. This significantly slows down innovation in the industrial application of acidic cannabinoids.
Marketing strategy challenges are also non-negligible. Given CBGVA’s limited public awareness and lack of clinical history, consumers-including those within niche pharmaceutical markets-have no established understanding of its properties or safety profile. As a result, companies must invest not only in manufacturing but also in educational campaigns, clinical research, and validation efforts, which require substantial resources.
From a technical standpoint, the incorporation of CBGVA into food or cosmetic products faces significant legalization challenges at the level of regulatory standards, such as the Generally Recognized As Safe (GRAS) classification in the United States or corresponding European Food Safety Authority (EFSA) norms in the European Union. In the absence of clear regulatory positions, manufacturers are often forced to adopt workaround strategies, which can limit functionality or increase the final cost of the product.
Scaling up the production of CBGVA also presents difficulties. Due to its low natural concentration in the plant and the high cost of synthetic or biotechnological production methods, industrial integration requires the development of high-performance bioreactors or genetically modified cannabis strains. Such initiatives involve long development cycles and carry high investment risks.
Future Use of CBGVA: Research Potential and Applications
Research Potential
Amid growing interest in underexplored phytocannabinoids, cannabigerovarinic acid (CBGVA) stands out for its exceptional scientific potential, which remains largely unexplored. The primary reason for this gap in research lies in the limited availability of the pure compound, owing to its naturally low concentration in Cannabis plants and the technical challenges associated with its isolation and stabilization. However, this deficiency simultaneously underscores the need for developing more advanced analytical and biochemical evaluation techniques, which opens considerable opportunities for innovation.
One highly relevant direction is the synthesis of CBGVA analogs with modified physicochemical properties that preserve or enhance biological activity. This approach would facilitate the study of structure–activity relationships (SAR), revealing which molecular fragments are responsible for specific biochemical effects. A deeper understanding of these relationships would not only allow for optimization of the pharmacological profile but also support the development of derivative compounds with targeted actions-broadening the therapeutic arsenal based on cannabinoid precursors.
In addition, applying multi-omics technologies-especially metabolomics and proteomics-offers a promising path to evaluate the biological effects of CBGVA at the cellular level. These technologies enable systematic assessments of metabolic changes induced by the compound, which is particularly important for substances that do not exhibit clear effects through classical receptor-mediated mechanisms. Comprehensive bioinformatic modeling could help forecast pharmacokinetic behavior, interactions with enzyme systems, and the compound’s metabolic stability.
Special focus should also be placed on optimizing biotechnological methods of CBGVA production-particularly through genetic engineering involving model microorganisms such as Saccharomyces cerevisiae or Escherichia coli for biosynthesis of cannabinoid acids with controllable productivity parameters. This strategy entails the construction of synthetic metabolic pathways incorporating enzymes involved in CBGVA biosynthesis in plants, followed by their optimization through evolutionary design. Ultimately, this would enable industrial-scale production of the compound without the need to cultivate cannabis.
Application Potential in Alternative Medicine
In light of the global trend toward integrating non-conventional medical practices within the framework of evidence-based medicine, cannabigerovarinic acid is emerging as a promising candidate for the development of innovative therapeutic platforms. Due to its unique interaction profile with the body’s regulatory systems-extending beyond the classical cannabinoid system-CBGVA is considered a key component in future approaches to personalized medicine.
A leading concept involves the use of CBGVA in complementary pharmacotherapy, where the compound functions as a modulator of endogenous defense mechanisms rather than directly blocking or activating conventional receptors. In this context, a crucial research area is its potential influence on the regulation of epigenetic mechanisms, such as histone acetylation and microRNA-mediated pathways. These mechanisms may play critical roles in chronic pain, neuroinflammation, and impaired neuroplasticity.
There is also a conceptual hypothesis regarding CBGVA’s ability to modulate signaling cascades associated with glial cells in the brain, which are key participants in neurodegenerative processes. This opens potential applications in neuroprotection strategies, particularly during the early stages of Parkinson’s disease, amyotrophic lateral sclerosis, and Huntington’s disease-conditions where traditional pharmacological interventions often show limited efficacy.
Furthermore, CBGVA could be integrated into multi-component phytotherapeutic formulations designed to harmonize the interactions among the endocrine, immune, and autonomic nervous systems. Although the “entourage effect” hypothesis still requires further validation in the context of CBGVA, it is reasonable to expect that combining this compound with other unfragmented phytocomplex components may produce synergistic effects without the need for high dosages.
Alternative medicine may also consider CBGVA as a mild regulator of metabolic processes, particularly in cases of carbohydrate metabolism disorders or oxidative stress. Its potential inclusion in nutraceutical or adaptogenic products warrants in-depth clinical evaluation. However, preliminary experimental results suggest that CBGVA can indirectly influence metabolic homeostasis-especially relevant for individuals with metabolic syndrome or prediabetic conditions.
Within the broader movement toward ecological pharmacology-which emphasizes the use of natural bioactive compounds with minimal toxicity-CBGVA holds particular promise as a chemosensitizer. Such compounds are capable of enhancing the efficacy of traditional pharmaceuticals while reducing required dosages and side effects. When integrated into personalized treatment protocols, this approach could form the basis of the next generation of cannabinoid-based medical products.
Conclusion
Cannabigerovarinic acid (CBGVA) is a relatively unexplored yet highly promising phytocannabinoid that opens new frontiers in pharmacology, biochemistry, and biotechnology. It emerges as a key intermediate metabolite in the biosynthesis of varinic cannabinoid derivatives, particularly tetrahydrocannabivarin (THCV) and cannabidivarin (CBDV). However, unlike its decarboxylated counterparts, CBGVA possesses a unique spectrum of biological activity that remains only partially characterized to date.
Studies of its chemical structure have revealed a complex interplay between aromatic and carboxylic fragments, which underlies its distinctive chemical behavior-including increased reactivity under acid-base conditions, photoinstability, and thermal lability. Despite these challenges, the molecular structure of CBGVA demonstrates specific chemoselectivity, opening the door for the development of novel derivatives via chemical modification or functionalization.
From a biosynthetic perspective, CBGVA is formed through a specific terpenoid condensation involving geranyl pyrophosphate (GPP) and divarinolic acid, a varinic analog of olivetolic acid. While this biochemical route shares similarities with the biosynthesis of cannabigerolic acid (CBGA), it involves specific enzymatic catalysts, particularly synthases with high regioselectivity. Experimental data suggest that the quantitative accumulation of CBGVA closely correlates with the expression levels of corresponding enzymes in the glandular trichomes of Cannabis sativa, laying a foundation for targeted genetic breeding and engineering.
Current technologies for extracting CBGVA face numerous challenges, largely due to its acidic nature and sensitivity to heat. Among the most effective methods are cryogenic extraction combined with ultrasonic stimulation, supercritical CO₂ extraction with modified pressure and temperature parameters, and microfluidic systems that preserve the integrity of the acidic form. Of particular interest are enzyme-assisted bioextraction techniques, which offer high selectivity under mild energy input conditions.
Genetic modification efforts to increase CBGVA content in cannabis are still in the early stages. Developing cultivars with intentionally suppressed THCA and CBDA biosynthesis in favor of varinic pathways may not only enhance CBGVA yield but also expand the portfolio of cannabinoids with narrowly targeted biological properties.
Pharmacologically, CBGVA has demonstrated an indirect ability to interact with CB1 and CB2 receptors, although its affinity for these targets is lower than that of its active decarboxylated forms. Nonetheless, its role in modulating the endocannabinoid system through allosteric mechanisms or by influencing enzymes responsible for the metabolism of endogenous ligands remains a subject of active investigation. Preliminary in vitro findings indicate neuromodulatory effects, suppression of microglial activation, and potential antinociceptive activity independent of psychoactive pathways.
Equally important is CBGVA’s antioxidant activity, which manifests through chemical quenching of free radicals, restoration of cellular glutathione levels, and protection of membrane structural components from lipid peroxidation. These properties position CBGVA as a candidate for the treatment of chronic inflammation, neurodegenerative diseases, and as part of multimodal anti-inflammatory strategies in systemic medicine.
Despite its significant scientific potential, the implementation of CBGVA in clinical practice is currently hindered by a number of technical and regulatory barriers. These include the instability of the native form in biological environments, the need for stabilized formulations, limited access to standardized extracts, and a fragmented preclinical evidence base-all of which slow the transition to large-scale clinical research. Additionally, in many jurisdictions, CBGVA remains legally unclassified apart from psychoactive cannabinoids, complicating regulatory and ethical research pathways.
From a technical standpoint in medicine and pharmaceuticals, CBGVA is being considered as a promising raw material for the development of novel pharmaceutical substances-particularly in the form of acid-resistant microcapsules, sustained-release nanoformulations, or as a substrate for semi-agonistic derivatives. Encapsulation strategies using liposomes, solid lipid particles, or polymer-based carriers help preserve the chemical integrity of the molecule during delivery to biological systems. In this context, standardizing industrial production methods-adaptable to GMP-level manufacturing-remains a critical task.
Looking to the future, CBGVA represents a new phase in the exploration of non-decarboxylated acidic cannabinoid forms, which have long remained on the periphery of pharmacological interest. The application of CBGVA in alternative medicine appears increasingly viable, particularly within integrative approaches to managing chronic pain, anxiety disorders, immune-mediated pathologies, and metabolic dysfunctions. Its combination with other acidic forms or natural adaptogens could lead to the development of a new class of multi-component phytotherapeutics with minimal toxicity and broad pharmacological profiles.
Sources:
- Udoh, M., et al. – One of the few experimental studies on CBGVA, demonstrating its interaction with ion channels and GPR55 receptors. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC9652735/
- Valliere, M.A., et al. – A biosynthetic platform for cannabinoid production, including CBGVA, outside of the living plant. An important source from a biotechnological perspective. https://www.nature.com/articles/s41467-019-08385-z
- Shoyama, Y., et al. – Original study on the isolation of CBGVA from Thai cannabis, important for the history of its exploration. https://diverdi.colostate.edu/C442/references/other/handbook%20of%20cannabis%20-%20pertwee/ch%2001.pdf
- National Institute of Standards and Technology (NIST). – Report on the quality of cannabinoid analytics, including new acids. https://nvlpubs.nist.gov/nistpubs/ir/2024/NIST.IR.8519.pdf
- U.S. Department of Energy, Office of Scientific and Technical Information (DOE OSTI). – Official publication on alternative biosynthesis methods for cannabinoids, including CBGVA. https://www.osti.gov/biblio/1613646
- Academia.edu. – An open-access study on the pharmacology of CBGVA and its effects on neuronal channels. Convenient access to key findings. https://www.academia.edu/123143988/The_anticonvulsant_phytocannabinoids_CBGVA_and_CBDVA_inhibit_recombinant_T_type_channels
- PubChem. – Comprehensive chemical information on CBGVA: structure, identifiers, properties, CID. https://pubchem.ncbi.nlm.nih.gov/compound/Cannabigerovarinic-acid
- European Monitoring Centre for Drugs and Drug Addiction (EMCDDA). – Official European Union agency report on the legal status and research of cannabinoids. https://www.emcdda.europa.eu/publications/topic-overviews/cannabis-legal-status-europe_en
- U.S. Food and Drug Administration (FDA). – FDA’s stance on cannabinoids in medicine. Relevant for sections on medical applications. https://www.fda.gov/news-events/public-health-focus/fda-regulation-cannabis-and-cannabis-derived-products-including-cannabidiol-cbd
- ClinicalTrials.gov. – Official clinical trials database. Keywords can be used to find both direct and related CBGVA studies. https://clinicaltrials.gov/