Cannabiglendol is one of the rare and little-studied natural compounds of the Cannabis genus, belonging to the class of cannabinoids. This chemical substance is of particular interest to researchers because its unique molecular structure and physicochemical properties distinguish it from more common cannabinoids such as tetrahydrocannabinol (THC) or cannabidiol (CBD). Due to its low concentration in natural extracts, cannabiglendol remains one of the least studied compounds; however, it potentially could play an important role in the pharmacology and biochemistry of cannabinoids.
The biosynthetic pathways for the formation of cannabiglendol in the Cannabis plant are associated with enzymatic transformations of cannabinoid precursors, particularly cannabigerolic acid (CBGA) and other acid-forming compounds. Cannabiglendol is formed as a result of complex chemical transformations, including decarboxylation and isomerization, making it structurally and functionally distinct from other cannabinoids. Although detailed biosynthesis mechanisms have not yet been fully revealed, available data suggest that this cannabinoid arises as a byproduct in the plant’s metabolic chains.
Considering that cannabiglendol is present in plants in very small amounts, its extraction for experimental research is a technically challenging task. Therefore, various methods are applied to obtain cannabiglendol: from isolation from plant material to semi-synthetic and fully synthetic chemical synthesis pathways. Biotechnological approaches are also beginning to develop, involving the use of genetically modified microorganisms to produce this compound. Developing efficient and scalable synthesis methods is key to ensuring sufficient quantities of the substance for further pharmacological and toxicological studies.
The pharmacological properties of cannabiglendol remain insufficiently studied at present. However, it is anticipated that it may interact with various receptor systems in the body, particularly the cannabinoid receptors CB1 and CB2, as well as with other molecular targets that regulate inflammatory processes, neuroprotection, and immune responses. There are individual in vitro studies indicating the potential anti-inflammatory and neuroprotective properties of this compound, but systematic in vivo studies and clinical trials in humans are currently lacking.
Chemical Nature of Cannabiglendol
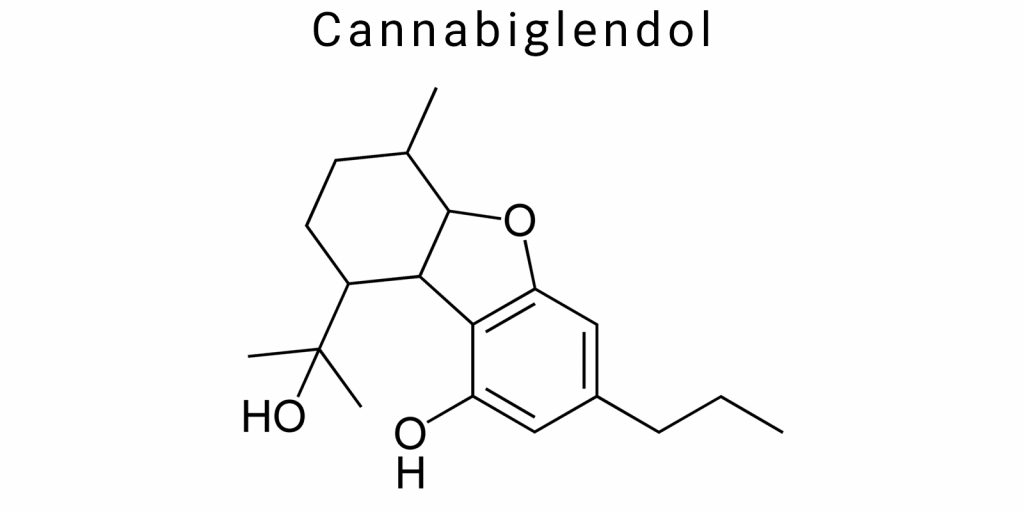
Cannabiglendol is a representative of the class of phyto-cannabinoids – natural organic compounds produced in plants of the Cannabis genus. This compound is characterized by unique chemical properties that distinguish it from other cannabinoids and determine the specificity of its biological activity. The chemical nature of cannabiglendol is closely related to its molecular structure, which forms the basic foundation for interaction with biological targets and also determines physicochemical properties affecting solubility, stability, and metabolism of the substance.
Chemically, cannabiglendol is a derivative of terpenophenol containing a phenolic group and an incomplete cyclic structure typical of many cannabinoids. The main molecule includes an aliphatic side chain and a phenolic core that interact to form the characteristic three-dimensional structure. The absence of an acidic functional group, unlike cannabinoid acids, gives cannabiglendol neutral chemical properties. This structure influences its ability to pass through cell membranes and interact with hydrophobic regions of receptors.
The chemical formula of cannabiglendol corresponds to the classic cannabinoid formula, including carbon, hydrogen, and oxygen, but has characteristic differences in the placement of double bonds and functional groups. Cannabiglendol is characterized by the presence of several saturated and unsaturated areas that determine its reactivity. A special role in the chemical nature of this compound is played by its ability to undergo electrophilic and nucleophilic reactions, allowing molecular modification for synthetic purposes.
Cannabiglendol exhibits weak acidity due to the phenolic hydroxyl group, which affects its solubility in water and organic solvents. Its solubility is relatively low in water, but it dissolves well in nonpolar and slightly polar environments, reflecting the hydrophobic nature of the molecule. These properties determine the conditions for storage and transportation of cannabiglendol, as well as its pharmacokinetics.
The thermostability of cannabiglendol is an important factor influencing its potential use in medical and pharmaceutical products. It is known that cannabinoids can undergo thermal degradation, including decarboxylation and isomerization processes. In the case of cannabiglendol, thermal stability is relatively high, allowing it to retain its chemical properties at moderate temperatures, though at elevated temperatures, side products may form.
In the chemical nature of cannabiglendol, the ability to form stereoisomers should also be noted due to the presence of chiral centers in the molecule. This affects its biological activity, as different isomers may vary in their ability to interact with receptors or enzymes. Such features of chemical nature are key in developing synthesis methods and studying the pharmacology of cannabiglendol.
An important component of its chemical nature is also the interaction of cannabiglendol with other components of the plant extract, including terpenes and other cannabinoids. Interactions between molecules can promote the formation of stable complexes that affect the bioavailability and effectiveness of the compound in biological systems.
Like other cannabinoids, cannabiglendol is capable of redox reactions, which determine its behavior in living organisms and the environment. Oxidative metabolites may form both through degradation processes under the influence of light and oxygen and as a result of enzymatic metabolism, which is an important aspect of toxicological evaluation.
Molecular Structure and Physicochemical Properties
The molecular structure of cannabiglendol defines its unique physicochemical characteristics, which distinguish this compound from other cannabinoids. The molecule of cannabiglendol is a terpenophenolic compound with the formula C₂₁H₃₂O₂. It consists of a monocyclic terpene skeleton containing three isoprene units connected in a “2-isoprene units + phenolic group” arrangement. What sets cannabiglendol apart is the specific placement of double bonds and functional groups that provide the molecule with a distinctive geometry.
A key feature of the molecular structure is the presence of a phenolic hydroxyl group positioned to provide a high level of electron density on the aromatic ring. This characteristic significantly influences the acid-base properties of the molecule, as well as its ability to form hydrogen bonds with polar solvents and biomolecules. The differing position of the hydroxyl group in cannabiglendol compared to other cannabinoids determines its unique reactivity.
The molecule includes an aliphatic side chain consisting of a linear carbon chain capable of conformational flexibility. This flexibility affects cannabiglendol’s ability to adapt to various molecular targets, particularly receptors, by altering the conformation of the side chain. The variability in spatial orientation allows the compound to optimize hydrophobic interactions deep within receptor binding sites.
The physicochemical properties of cannabiglendol include melting and boiling points, solubility, optical characteristics, and electrochemical activity. The melting point falls within a range corresponding to an amorphous state with a tendency to form crystalline structures, which exhibit specific polymorphic properties. This polymorphism is related to the possibility of different molecular packings in the solid state, significantly impacting the bioavailability and stability of cannabiglendol.
Cannabiglendol’s solubility displays a polar-apolar character: it is practically insoluble in water but exhibits high solubility in organic solvents such as methanol, ethyl ether, chloroform, and hexane. This is due to the large hydrophobic portion of the molecule, as well as the polar phenolic group, which facilitates dissolution in mediums of intermediate polarity. Excellent solubility in organic solvents is important for extraction and purification processes of cannabiglendol.
The optical properties of the molecule are determined by its chirality and the presence of conformational isomers. Cannabiglendol exhibits optical activity measurable by polarimetry and circular dichroism methods. These optical characteristics are important for determining the absolute configuration of the molecule, which is relevant for its interaction with biological receptors and enzymes.
Cannabiglendol’s electrochemical activity manifests in the oxidation and reduction processes of the phenolic group. This compound can participate in radical reactions, particularly forming stable phenolic radicals that play a role in biochemical processes, including antioxidant activity. Using cyclic voltammetry techniques, the oxidation potential of cannabiglendol can be studied, providing insight into its stability and potential involvement in redox reactions.
The thermodynamic parameters of cannabiglendol include bond energy and activation energy for conformational changes, which determine its stability and reactivity. Studies employing quantum-chemical methods have shown that the molecule has low activation energy for transitions between different conformations, providing dynamic flexibility within biological systems.
Cannabiglendol has significant potential to form complexes with metals, especially transition metal ions, due to the presence of oxygen donor atoms in the phenolic group. This property is a subject of research in the context of developing new catalysts and pharmacologically active metal complexes.
Molecular stability under various physical and chemical conditions is a crucial aspect that defines its practical applications. Cannabiglendol maintains its chemical integrity when exposed to moderate temperatures, neutral pH, and absence of oxidizing agents. At the same time, it can degrade under ultraviolet radiation and oxygen exposure, necessitating the development of protective methods for the molecule in pharmaceutical formulations.
Isomerism and Conformational Features
Isomerism in cannabiglendol is a complex and multifaceted phenomenon that has a determining influence on its chemical, physical, and biological properties. The isomeric forms of this molecule encompass both structural (constitutional) isomers and stereoisomers, including configurational and conformational variants. Analysis of isomerism allows revealing the diversity of molecular forms that may arise during synthesis, biosynthesis, as well as metabolism in living organisms.
Cannabiglendol contains several chiral centers that form the basis for the existence of different enantiomers and diastereomers. The positioning of the hydroxyl group and side chains creates asymmetry in the molecule, allowing for optically active isomers. The configuration of these centers critically affects receptor interactions, as different stereoisomers may have varying affinities and biological activities. Enantiomeric purity is an important characteristic for pharmacological studies and the development of cannabiglendol-based drugs.
Structural isomers of cannabiglendol are associated with changes in the placement of double bonds, positions of hydroxyl groups, and variations in the length and branching of aliphatic chains. Such changes lead to significant differences in chemical reactivity and physicochemical properties. Specifically, shifting the position of double bonds influences electron density in the molecule, its radical interaction capabilities, and spectral characteristics.
Conformational isomerism in cannabiglendol relates to the spatial flexibility of the molecule caused by rotation around single covalent bonds. The features of the conformational space are determined by energy barriers arising from steric interactions between atoms and functional groups, as well as electronic effects such as hyperconjugation. An important role is played by interactions between the phenolic group and aliphatic chains, which can create conformations with additional stabilization through intramolecular hydrogen bonds.
Analysis of conformational changes in the cannabiglendol molecule is conducted using quantum-chemical modeling and spectroscopic methods, including nuclear magnetic resonance (NMR) and infrared spectroscopy. Data from these methods allow identification of the most stable conformations and determination of energy barriers between them, which is important for understanding the molecule’s dynamics in solution and biological environments.
Conformational features directly influence cannabiglendol’s ability to interact with CB1 and CB2 receptors, as well as other molecular targets. The molecule’s capacity to adapt its shape allows effective contact with protein active sites, determining its pharmacological potential. Differences in conformations may cause changes in affinity and selectivity of interaction, highlighting the importance of studying this aspect of isomerism.
Special attention should be given to tautomerism-a form of isomerism involving proton transfer with a concurrent shift of double bond position. Cannabiglendol may exist in phenol-keto and enol-keto tautomeric forms, which differ in stability depending on the environment. Tautomerism affects the molecule’s acid-base properties, reactivity, and spectral characteristics.
Geometric (cis-trans) isomerism is another important isomeric form arising from restricted rotation around double bonds in the molecule. Such isomers in cannabiglendol can significantly differ in energy levels and spatial arrangement of functional groups, influencing their interaction with biological targets.
Like other cannabinoids, cannabiglendol can form intermolecular complexes through hydrogen bonds and van der Waals interactions, which depend on the specific isomeric form. These interactions may influence the formation of aggregates in solution, stability in pharmaceutical forms, and bioavailability.
Given the importance of isomerism for biological activity, the isomer ratio is critically controlled during the synthesis and extraction of cannabiglendol. Chromatographic methods such as high-performance liquid chromatography (HPLC), combined with spectroscopic techniques, allow separation, identification, and purity assessment of individual isomers.
Origin of Cannabigerol
The origin of cannabigerol is closely linked to the complex biochemical system of plants from the Cannabis genus, which serve as the primary natural source of this compound. Cannabigerol is formed as one of the numerous cannabinoids that arise during the metabolism of terpenophenolic compounds within the plant’s tissues. Its origin is determined by specific transformation pathways of precursors that integrate into the overall cannabinoid metabolic network.
Historically, cannabigerol was not immediately identified as a separate component of cannabis due to its lower concentration compared to other cannabinoids such as tetrahydrocannabinol or cannabidiol. However, with the advancement of analytical methods, particularly chromatography and spectroscopy, cannabigerol was clearly isolated and identified as a distinct chemical entity. Its origin is associated with natural biochemical transformations occurring in the plant’s exocrine glands (trichomes).
Cannabigerol is a derivative of the terpenoid class with a phenolic group formed on the basis of the terpenoid skeleton through sequential enzymatic reactions. The fundamental biochemical basis for its formation involves linear or cyclic terpenes synthesized in the plant from pyruvic acid, acetyl-CoA, and isoprenoid precursors. These terpenes subsequently undergo oxidation, cyclization, and hydroxylation, leading to the formation of the characteristic cannabigerol framework.
The process of cannabigerol formation often occurs in parallel with the synthesis of other cannabinoids since they all originate from a common precursor, cannabigerolic acid. In the plant, cannabigerolic acid serves as a central molecule that, through the action of specific enzymes, transforms into various cannabinoids, including cannabigerol. A distinctive feature of cannabigerol biosynthesis is the difference in enzymatic pathways and structural modifications that avoid the formation of typical tetrahydrocannabinol rings.
The origin of cannabigerol in nature is also accompanied by non-enzymatic reactions such as oxidation or degradation of precursor cannabinoids. These reactions may occur under the influence of external factors-light, temperature, and oxidizing agents. Specifically, cannabigerol can form as a product of oxidation of other terpenophenolic compounds, emphasizing its status as a derivative within the complex chemical chain of cannabis metabolism.
Cannabis plants exhibit a high degree of plasticity in the synthesis of secondary metabolites, which provides variability in cannabigerol content depending on the strain, growing conditions, and maturity stage. The genetic characteristics of individual plant lines determine the activity of enzymes catalyzing cannabigerol formation, which in turn reflects the quantitative content of this compound. It is important to note that environmental factors such as lighting, temperature, humidity, and nutrient regimen significantly influence the expression of enzymatic systems responsible for cannabigerol synthesis.
Additionally, cannabigerol can form as a result of chemical transformations during drying and storage of plant material. Such transformations include the degradation of other cannabinoids or their acidic forms, altering the chemical profile of the final product. This phenomenon is important for accurately determining the origin of cannabigerol in studied samples and for quality control of pharmaceutical preparations.
Cannabigerol, as a natural compound, reflects the evolutionary adaptation of Cannabis plants to their environment. Its synthesis likely holds ecological significance related to protection against ultraviolet radiation, pests, or microorganisms. This function is characteristic of many secondary metabolites, which act as biochemical barriers that enhance plant survival in extreme conditions.
Understanding the origin of cannabigerol is important not only from a fundamental perspective but also for practical applications-selecting plants with increased levels of this compound, optimizing cultivation conditions, and developing extraction technologies. Specifically, knowledge of biochemical pathways enables the development of methods for enzymatic modification or chemical synthesis of cannabigerol in laboratory settings.
Biosynthesis in Cannabis Plants
The biosynthesis of cannabigerol in Cannabis plants is a complex, multi-stage process integrated into the overall metabolic pathway for cannabinoid formation. This pathway is based on the interaction of two primary metabolic chains-terpenoid and phenolic-that converge in the synthesis of the characteristic terpenophenolic backbone of the cannabigerol molecule. The key precursor in this process is cannabigerolic acid, which acts as a matrix compound for the formation of various cannabinoids, including cannabigerol.
The initial phase of biosynthesis involves the formation of isoprenoid units, which are the building blocks of the terpenoid segment of the molecule. In Cannabis plants, this primarily occurs through the methylerythritol phosphate (MEP) pathway in plastids. Isoprenoid units isopentenyl pyrophosphate (IPP) and dimethylallyl pyrophosphate (DMAPP) are condensed by specific synthases to form geranyl pyrophosphate (GPP)-the primary terpenoid precursor. This step defines the terpene base of the future molecule.
In parallel, the synthesis of the phenolic portion occurs via phenylpropanoid pathways that start with the amino acid phenylalanine. Under the action of enzymes such as phenylalanine ammonia-lyase (PAL) and other specific enzymes, aromatic compounds like oligomeric phenols are formed. These compounds are further transformed into 1-oxy-2-phenylpropanone, which acts as a precursor for the reaction with the terpenoid segment.
Cannabigerolic acid is formed by the reaction between the terpenoid precursor GPP and the phenolic precursor oligomeric phenol. This reaction is catalyzed by a specific enzyme-cannabigerol synthase (CBGAS)-which controls the condensation and formation of cannabigerolic acid. This is the central stage of cannabinoid biosynthesis that determines the subsequent fate of the molecule in various biochemical transformations.
For the formation of cannabigerol from cannabigerolic acid, enzymatic modifications that alter the molecular structure without disrupting the primary terpenophenolic backbone play the main role. Oxidoreductases, hydroxylases, and lyases are significant in this process, facilitating selective oxidation, hydroxylation, and cyclization of specific molecular fragments.
In particular, enzymes catalyzing cyclization of the terpenoid chain promote the formation of an open aliphatic structure characteristic of cannabigerol, in contrast to the formation of cyclic structures seen in other cannabinoids. This process provides the unique chemical structure of cannabigerol and determines its specific physicochemical and biological properties.
Hydroxylation of the cannabigerol molecule occurs at defined positions, forming hydroxyl groups responsible for the molecule’s polarity and its ability to interact with aqueous environments and receptors. These reactions are catalyzed by cytochrome P450 enzymes, which selectively modify molecules, especially at positions influencing biological activity.
Besides enzymatic transformations, non-enzymatic reactions such as spontaneous oxidation and radical processes can occur during biosynthesis, influenced by environmental conditions including light and temperature. These reactions can complement the enzymatic pathway, creating additional molecular variants that enrich the chemical profile of the plant.
An important aspect of biosynthesis is the spatial and temporal regulation of enzymes in various plant tissues. The highest enzymatic activity is observed in trichomes-glandular hairs on the surface of leaves and flowers. Here, cannabinoids including cannabigerol accumulate and transform, providing localized synthesis and protection of the plant from external factors.
The expression level of genes encoding biosynthetic enzymes is strictly regulated by both genetic and epigenetic mechanisms. Environmental factors such as light, temperature, and water availability influence the regulation of these genes, modulating cannabigerol and other cannabinoid synthesis in response to stress or adaptive conditions.
Molecular studies of cannabigerol biosynthesis have identified multiple isoforms of enzymes that may have subtle differences in catalytic activity and specificity, further expanding the molecular diversity of cannabigerol. These isoforms provide flexibility in metabolic pathways and can be potential targets for genetic engineering and biotechnological methods to increase cannabigerol productivity.
Application of modern methods-metabolomics, proteomics, and transcriptomics-enables integration of data about biosynthetic pathways into a systemic understanding of cannabigerol metabolism in plants. These approaches facilitate the discovery of new enzymes, regulatory factors, and metabolic nodes shaping the plant’s adaptive strategies.
Natural Sources and Quantitative Indicators in Plants
Cannabigerol is a secondary metabolite found in plants of the Cannabis genus; however, its concentration varies significantly depending on the botanical species, cultivar, growing conditions, and the plant’s developmental stage. The natural sources of cannabigerol are limited specifically to Cannabis plants, which belong to the Cannabaceae family, although the potential presence of similar compounds in related species requires further investigation. The primary accumulation of cannabigerol occurs in specific tissues, particularly in glandular trichomes located on the leaves, inflorescences, and sometimes on the stems.
The diversity of Cannabis plant cultivars substantially influences the level of cannabigerol content. It is known that indica, sativa, and hybrid lines demonstrate different cannabinoid profiles, in which cannabigerol is present both in trace amounts and in moderate concentrations. This fact indicates a genetic determination of cannabigerol synthesis, reflecting variability in enzymatic activity and regulatory mechanisms among different genotypes.
Quantitative measurements of cannabigerol are performed using highly precise analytical techniques, including gas chromatography with mass spectrometric detection (GC-MS), high-performance liquid chromatography (HPLC), and nuclear magnetic resonance (NMR). These analytical methods provide not only identification but also accurate quantification of cannabigerol in various samples of plant material.
Quantitative indicators of cannabigerol in plants vary widely, which is associated with genotype factors as well as the influence of agronomic and environmental parameters. In traditional cultivars, the cannabigerol content usually does not exceed 0.1-0.3% of dry weight, although in specialized breeding lines, this concentration can reach 0.5% or higher. It is important to emphasize that cannabigerol in plants is often present in the form of acidic precursors, which can convert into the decarboxylated active form during extraction and storage.
Variability in the quantitative indicators of cannabigerol also depends on the plant’s developmental stage. The highest accumulation level is observed during the flowering phase, when biosynthetic enzyme activity is maximal, ensuring the greatest production of cannabinoids. During the growth and leaf formation phases, the concentration of cannabigerol is lower, indicating a close link between the synthesis of this compound and the reproductive cycle.
Environmental factors such as temperature, light regime, humidity, and plant nutrition significantly affect the concentration of cannabigerol. Specifically, increased insolation and moderate stress caused by water deficiency can stimulate biosynthetic enzyme activity, leading to increased cannabigerol content. Similarly, optimal concentrations of macro- and micronutrients in the soil ensure proper metabolism, contributing to more stable cannabigerol accumulation.
Particular attention is given to studying the impact of agronomic methods-the type of cultivation (open field or greenhouse conditions), fertilizer application methods, and growth stimulator treatments-all of which can significantly alter cannabigerol concentration. Cultivation technologies that optimize plant growth conditions and minimize stress can enhance the accumulation of this compound, which is important for the pharmaceutical industry.
Cannabigerol concentrates predominantly in trichomes, which function as bioreactors for cannabinoid biosynthesis and accumulation. Morphological characteristics of these glandular structures, including their quantity, size, and density on the plant surface, directly correlate with the level of cannabigerol content. Determining the morphometric parameters of trichomes is an important tool for assessing the plant’s potential to produce cannabigerol.
Additionally, it is worth noting that the natural content of cannabigerol can vary substantially depending on post-harvest processing technologies-drying, storage, and extraction. Elevated temperature, oxygen availability, and storage duration affect cannabigerol stability, which may lead to its partial degradation or conversion into other cannabinoids. Controlling these parameters is critical for preserving the chemical profile of the material.
Methods of Cannabigerol Production
The production of cannabigerol is the subject of complex research since its natural extraction from Cannabis plants is limited by low concentration and extraction complexity. Therefore, the development of effective methods for obtaining cannabigerol becomes important to ensure stable supplies of this compound for further research and potential pharmaceutical applications. The main approaches to producing cannabigerol include extraction from plant raw material, semi-synthetic methods, total chemical synthesis, and biotechnological technologies. Each of these methods has advantages and limitations related to yield, purity of the final product, environmental friendliness, and cost-effectiveness.
Extraction of cannabigerol from natural sources-Cannabis plants-remains a fundamental but not always sufficiently efficient method. Considering that the cannabigerol content in raw material is usually low, the amount of product obtained is limited, and the process requires significant time, solvents, and specialized equipment. Therefore, the search for alternative synthesis methods becomes critically necessary.
Semi-synthetic methods allow increasing cannabigerol yield by converting more readily available cannabinoid precursors obtained from plant raw materials. These methods are based on chemical modifications of natural compounds, which, due to the presence of characteristic functional groups, can be transformed into cannabigerol with high selectivity. The use of semi-synthetic technologies reduces the need for large-scale plant cultivation, provides better control over chemical purity and structural homogeneity of the product.
Total chemical synthesis of cannabigerol is the most complex route given the molecular structure and the presence of stereochemical centers. However, the development of organic synthesis methods makes it possible to reproduce the structure of cannabigerol from simple chemical reagents, opening opportunities for large-scale production independent of raw material sources. Advantages of this approach include control over isomeric composition and the possibility of variations in the molecular structure, which is important for creating derivatives with improved properties. The disadvantages are the complexity of reaction schemes and the use of expensive catalysts and reagents.
Biotechnological approaches using microorganisms such as genetically modified bacteria or yeast, as well as cell cultures of Cannabis plants, open prospects for environmentally friendly, highly productive, and scalable production of cannabigerol. Methods include the expression of biosynthetic enzymes of cannabigerol in host systems, controlled fermentation, and optimization of metabolic pathways. This direction requires a deep understanding of cannabinoid biochemistry and modern bioengineering technologies.
The choice of method for producing cannabigerol is determined by the required product quantity, purity requirements, economic feasibility, and application possibilities. For fundamental research, semi-synthetic or extraction methods are often sufficient, while industrial production is aimed at total synthesis or biotechnological solutions. Further development of cannabigerol production technologies will contribute to expanding its use in scientific, medical, and industrial fields, providing new opportunities for pharmacological research and therapy.
Semi-Synthetic Methods
Semi-synthetic methods for the production of cannabigerol are based on the chemical transformation of natural cannabinoid precursors isolated from plants of the Cannabis genus, followed by obtaining the target compound through selective modification reactions. This approach combines the biological origin of the substrate with chemical manipulation, allowing for reduced synthesis complexity and increased product yield while lowering production costs compared to total synthesis.
The use of semi-synthetic methods is driven by the low concentration of cannabigerol in natural extracts, which limits their use in pure form. Meanwhile, Cannabis plants contain other cannabinoids, such as cannabigerol (CBG), which serve as effective precursors for conversion into cannabigerol. The main challenge lies in conducting chemical reactions with high selectivity to avoid side transformations and molecular degradation.
Typically, semi-synthetic synthesis of cannabigerol begins with the isolation and purification of the precursor, for example, cannabigerol, from plant raw materials. This process includes the use of chromatography methods (column, liquid, or gas) that provide a high degree of substrate purity necessary for subsequent chemical transformations. The use of specific solvents and controlled extraction conditions helps preserve molecular integrity and prevent its degradation.
One of the key stages in semi-synthetic synthesis is the selective hydroxylation of cannabigerol, which ensures the formation of characteristic hydroxyl groups in defined positions of the molecule for cannabigerol. The use of specific oxidizers or enzymatic catalysts enables this reaction with high regio- and stereoselectivity. An important feature is the need to avoid excessive oxidation or the formation of by-products, which requires precise control of temperature, pH, and reagent concentration.
The next stage is the modification of the molecule’s side chains, which increases the stability of cannabigerol as well as improves its physicochemical properties. For example, conducting etherification or acetylation of hydroxyl groups provides protection for reactive centers, necessary for further transformations or to enhance the biological stability of the product. These reactions are typically performed in organic solvents using catalysts that minimize side reactions.
Optimizing the conditions for each stage of the semi-synthetic synthesis is critically important for achieving a high yield of cannabigerol with minimal losses. Studies show that a temperature range of 20-40 °C, along with the use of buffer systems to maintain neutral or mildly acidic environments, helps preserve active functional groups and prevents hydrolysis or molecular degradation.
Semi-synthetic methods also include the use of specific catalysts – both homogeneous and heterogeneous. For example, the application of transition metal-based catalysts (platinum, palladium, rhodium) in small concentrations allows control of hydrogenation or hydroxylation reactions with high selectivity. Additionally, supporting catalysts on carriers with a high specific surface area contributes to increased activity and stability of catalytic systems.
Advancements in semi-synthetic methods incorporate biocatalysis – the use of enzymes or microorganisms capable of selectively modifying the cannabinoid structure without harsh chemical reagents. Such biocatalysts provide mild reaction conditions, high regioselectivity, and reduced formation of by-products. However, their commercial use is limited by the need for specialized equipment and control of enzymatic activity.
The semi-synthetic production process of cannabigerol, despite its advantages, has certain challenges, including the necessity of multi-step purification of the final product. This is due to the formation of structurally similar by-products during reactions, which can be difficult to separate from the target cannabigerol. Combined chromatographic separation methods are used for this, including thin-layer, liquid, and ion-exchange chromatography.
Semi-synthetic methods allow for a high degree of control over the quality of cannabigerol, which is especially important for pharmaceutical applications where maximum purity and the absence of toxic impurities are required. The use of natural precursors minimizes the number of synthetic reagents and reduces the environmental impact of production.
Semi-synthetic technologies provide scalability of the process, adapting from laboratory conditions to industrial volumes with relatively low costs. The flexibility of the process allows modification of reaction conditions according to specific production needs, making this method one of the priorities for commercial cannabigerol production.
Total Chemical Synthesis
Total chemical synthesis of cannabigerol is the process of creating the target molecule from simple chemical reagents without using natural precursors. This approach requires careful planning of the synthetic strategy, as the cannabigerol molecule has a complex structure with multiple functional groups and stereogenic centers. Successful total synthesis ensures full control over the molecule’s configuration, enabling the production of a product with high purity and isomeric specificity, which is especially important for subsequent pharmacological applications.
The primary challenge in total chemical synthesis of cannabigerol is constructing its tricyclic structure, consisting of a phenolic core, a pyrrole or chromene system, and an aliphatic side chain. The complexity also lies in forming hydroxyl functions in defined positions and maintaining stereochemical purity, as the molecule contains several chiral centers that determine its biological activity.
The synthesis process begins with the selection of appropriate starting reagents – simple aromatic or aliphatic compounds capable of building the main structural elements of cannabigerol. Commonly used are phenol derivatives, isoprene fragments, and alkyl halide compounds for side chain formation. The synthetic strategy focuses on stepwise assembly of the molecular skeleton through sequential condensation, cyclization, and functionalization reactions.
One of the key steps is the formation of the chromene or pyrrole system through cyclic condensations. Reactions such as Friedel-Crafts or Pictet-Spengler alkylations are used, allowing introduction of the aliphatic side chain onto the aromatic core. These reactions require acid catalysts (e.g., AlCl3, BF3) and strict control of reaction conditions to avoid polymerization or by-product formation.
In parallel with cyclic system formation, selective hydroxylation of certain carbon atoms takes place, necessary for forming hydroxyl groups in cannabigerol. Hydroxylation reactions are often carried out using oxidants such as peroxides or chromates under stereospecific control. More modern methods include platinum-group metal catalysis, allowing precise regulation of hydroxyl position and configuration.
An important component of total chemical synthesis is controlling product isomerism. To ensure stereoselectivity, chiral catalysts, auxiliary agents, or reactions in specialized environments are employed to promote formation of the desired molecular configuration. Asymmetric synthesis methods, such as catalytic hydrogenation or epoxidation, are commonly used to obtain products with high optical purity.
Isolation and purification of intermediates and final products are vital parts of total synthesis. Various chromatographic separation methods, extraction, and crystallization techniques are applied. The purity of cannabigerol after total synthesis must meet strict standards, especially for pharmaceutical use, as impurities can alter the pharmacological profile of the compound.
To optimize reaction pathways, computer modeling of reaction kinetics and molecular dynamics is applied, which helps predict the most efficient synthesis conditions and prevent by-product formation. This approach significantly reduces experimental time and development costs.
An important aspect is the economic feasibility of total chemical synthesis. Although this method allows obtaining cannabigerol independently from natural resources, it often requires expensive reagents, complex catalysts, and high energy consumption. Therefore, industrial application requires further process optimization, including the implementation of reusable catalysts and reducing side reactions.
Current research combines total chemical synthesis of cannabigerol with automated organic synthesis methods, enabling parallel execution of multiple reactions with varying conditions and reagents. This facilitates rapid identification of optimal synthesis routes and allows creation of new cannabigerol derivatives with improved properties.
Total synthesis enables molecular structure modification by substituting functional groups or introducing new chemical elements at specific positions, opening opportunities for developing drugs with specific activity. Such modifications are inaccessible or difficult with semi-synthetic methods due to limitations associated with natural precursors.
Biotechnological Approaches
Biotechnological approaches to the production of cannabiglendol represent an innovative direction based on the use of biological systems for the synthesis of this complex cannabinoid molecule. Unlike traditional chemical methods, biotechnology employs living cells or their enzymatic systems, which provides high reaction selectivity, mild process conditions, and potentially greater environmental safety. The application of biotechnology not only significantly simplifies the synthesis of cannabiglendol but also opens new opportunities for genetic engineering and scalable production.
At the core of biotechnological methods is the use of microorganisms-bacteria, yeasts, fungi, or plant cells-that can express enzymes catalyzing the conversion of natural or synthetic precursors into cannabiglendol. Among the most commonly used organisms are Escherichia coli, Saccharomyces cerevisiae, and Aspergillus sp., as they have well-studied genetic mechanisms, high growth rates, and adaptability to laboratory and industrial conditions.
The first step in biotechnological synthesis is the identification and cloning of genes encoding enzymes capable of carrying out key reactions in the biosynthesis of cannabiglendol. These genes can be isolated from natural Cannabis plants or artificially synthesized with sequence optimization to enhance expression in the host. Particularly important are genes encoding oxidoreductases, hydroxylases, isomerases, and synthases responsible for the formation of the tricyclic structure and functional groups of the molecule.
The introduction of these genes into the genome of microorganisms or plant cells is carried out using vector systems (plasmids, viral vectors) that ensure stable and high expression of the target proteins. To increase production efficiency, genetic constructs are supplemented with strong promoters, signals for transport proteins, and metabolic regulation factors.
To ensure the biosynthesis of cannabiglendol, multigene systems are typically developed, where enzymes catalyzing sequential reactions are expressed simultaneously. This allows the formation of a metabolic pathway in the host and minimizes the accumulation of intermediate products. The use of systems with branched regulation of expression and a specific balance of enzymes increases the yield of the target product.
One of the key directions is the optimization of cellular metabolic fluxes to provide a sufficient amount of precursors (such as isoprene units) and cofactors (NAD(P)H, ATP) necessary for biosynthetic reactions. Methods of metabolic engineering are applied for this purpose: enhancing precursor synthesis pathways, reducing the activity of competing metabolic branches, and introducing additional pathways for cofactor regeneration.
An important component is also the control of stability and product yield in the living system. Bioreactors are used to maintain optimal physicochemical parameters (temperature, pH, aeration), which ensures maximum productivity of microorganisms or cells. Culture growth parameters are adjusted to minimize stress responses that can affect enzyme expression and synthesis productivity.
The application of enzymatic systems in the form of isolated proteins is a separate direction of biotechnology. In this case, cannabiglendol enzymes are synthesized in heterologous hosts, purified, and used in reaction mixtures to catalyze specific transformations. This method allows precise control of reaction conditions, combining enzymes in cascade systems, and avoiding side reactions characteristic of living cells.
For scaling up production, fermentation technologies with high cell density are applied, as well as bioengineering systems with gene expression regulation depending on the culture growth stage. This enables adaptation of the production cycle to industrial-scale requirements and ensures product quality stability.
In the context of biotechnological approaches, a significant advantage is the ability to create new strains and lines with improved productivity and adapted to various types of raw materials. Genome editing, particularly CRISPR/Cas systems, is actively implemented for targeted metabolism improvement and increased cannabiglendol yield.
An important factor is also the environmental friendliness of the process. Biotechnological methods allow avoiding the use of toxic chemical reagents, reducing energy consumption and waste formation, making them attractive for sustainable production. The use of biocatalysis and mild reaction conditions reduces the risk of product degradation and promotes better preservation of its pharmacological properties.
However, there are challenges associated with biotechnological synthesis. These include the complexity of scaling processes, the need for long cycles of strain and reaction condition optimization, as well as high requirements for the purity and stability of enzymes or cell cultures. Intensive research in bioinformatics, systems biology, and bioreactor engineering is conducted to overcome these obstacles.
Integration of biotechnological methods with other approaches, including chemical synthesis and semi-synthetic technologies, opens prospects for hybrid production lines. Such combined systems allow merging the advantages of each method, increasing overall efficiency, and reducing the cost of the final product.
Pharmacological Properties
Cannabiglendol is one of the less studied cannabinoids that attracts significant attention in pharmacology due to its unique properties. Although research on cannabiglendol is still in the early stages, a basic understanding of its pharmacological profile has been formed, which differs from classical cannabinoids in its spectrum of action and mechanisms of interaction with the body. The pharmacological properties of cannabiglendol are determined by its ability to influence various cellular and molecular targets involved in the regulation of pain, inflammation, neuroprotection, and immune response.
The main pharmacological characteristics of cannabiglendol are related to its ability to modulate signaling pathways that regulate the homeostasis of the body, especially in the central and peripheral nervous systems. Unlike better-known cannabinoids such as tetrahydrocannabinol (THC) or cannabidiol (CBD), cannabiglendol demonstrates the absence of pronounced psychoactivity, making it a promising agent for therapeutic use without the risk of undesirable psychotropic effects. At the same time, cannabiglendol exhibits a number of pharmacological actions that may be useful in treating various pathological conditions.
From the perspective of molecular properties, cannabiglendol has the ability to affect receptors of the endocannabinoid system, but also interacts with a number of other receptors such as TRP channels, PPARs (peroxisome proliferator-activated receptors), and other proteins regulating cellular metabolism and inflammatory processes. This makes it a multifunctional agent with potential applications in pharmacotherapy.
Pharmacokinetics of cannabiglendol are still insufficiently studied, but it is known that it has high lipophilicity, which ensures its effective penetration through the blood-brain barrier. This is important for realizing neuroprotective and analgesic effects. Cannabiglendol metabolism occurs mainly in the liver involving the cytochrome P450 system, which determines potential interactions with other drugs.
Clinical and preclinical studies indicate the ability of cannabiglendol to reduce the intensity of pain sensations, especially in cases of chronic and neuropathic pain, as well as to have anti-inflammatory effects by inhibiting the release of pro-inflammatory cytokines. In addition, cannabiglendol demonstrates potential in regulating immune responses, which may be important for treating autoimmune and inflammatory diseases.
An important property of cannabiglendol is its neuroprotective potential associated with the ability to reduce oxidative stress and inflammation in neurons. This opens prospects for its use in neurology, particularly for the therapy of degenerative diseases of the central nervous system.
Although evidence of the pharmacological efficacy of cannabiglendol is still mainly based on experimental models, the obtained data provide a foundation for further research and possible therapeutic applications. Due to its properties, cannabiglendol may complement or replace traditional cannabinoid drugs, especially where the absence of psychoactivity and a low risk of side effects are required.
Mechanisms of Interaction with Biological Targets
The mechanisms by which cannabiglendol interacts with biological targets form the foundation for understanding its pharmacological effects. Defining these mechanisms is a complex task due to the intricate molecular structure of cannabiglendol and its multifaceted activity at the cellular level. Unlike classical cannabinoids, cannabiglendol exhibits a unique selectivity profile toward receptor systems, allowing it to demonstrate distinct pharmacological properties. This section provides a detailed examination of the primary biological targets of cannabiglendol, the types of interactions, as well as the molecular pathways that are either activated or inhibited upon its binding.
The most important biological targets of cannabiglendol are the receptors of the endocannabinoid system, including CB1 and CB2 receptors. However, compared to classical cannabinoids, cannabiglendol shows relatively low affinity binding to these receptors, especially CB1, which explains the lack of pronounced psychoactivity. Nonetheless, cannabiglendol can act as a modulator of these receptors, potentially influencing their conformational state and associated signaling cascades. This activity may be either partial agonistic or antagonistic, depending on the molecule’s concentration and tissue type.
In addition to CB1 and CB2 receptors, cannabiglendol exhibits significant affinity for TRP channels, particularly TRPV1, TRPV2, and TRPA1. These receptors are ion channels that respond to various physicochemical stimuli and participate in pain signal transmission, inflammation regulation, and thermoregulation. Cannabiglendol’s interaction with TRP channels modulates the permeability of cellular membranes to calcium and sodium ions, leading to changes in intracellular signaling pathways. This mechanism underlies the anti-inflammatory and analgesic effects of cannabiglendol.
Concurrently, cannabiglendol interacts with nuclear receptors, primarily PPARγ (peroxisome proliferator-activated receptor gamma), which regulate gene expression related to lipid metabolism, glucose homeostasis, and anti-inflammatory responses. Interaction with PPARγ provides transcriptional regulation that reduces the production of pro-inflammatory cytokines and reactive oxygen species, while supporting cellular antioxidant status. Thus, cannabiglendol acts as a ligand activating PPARγ, making it a promising agent for treating chronic inflammation and metabolic disorders.
Another important aspect is cannabiglendol’s interaction with enzymes regulating endocannabinoid metabolism, specifically FAAH (fatty acid amide hydrolase) and MAGL (monoacylglycerol lipase). These enzymes are responsible for breaking down the primary endocannabinoids, anandamide and 2-arachidonoylglycerol, respectively. Cannabiglendol may act as a mild FAAH inhibitor, leading to increased levels of endocannabinoids in synaptic spaces and, consequently, enhancing their physiological effects. This mechanism provides indirect potentiation of the endocannabinoid system without direct agonistic action on CB receptors.
Cannabiglendol’s interaction with membrane transport proteins also plays a role in its pharmacological activity. Specifically, cannabiglendol affects transport systems that regulate levels of neurotransmitters such as glutamate and gamma-aminobutyric acid (GABA). This modulation of synaptic transmission is relevant for neuroprotection as well as regulation of neuronal excitability.
Beyond classical receptors and enzymes, cannabiglendol demonstrates the capacity for allosteric modulation of protein complexes involved in cellular signaling cascades. In particular, in silico studies and molecular docking suggest possible interactions with protein kinases that regulate the cell cycle and apoptosis, as well as transcription factors controlling responses to oxidative stress. Such interactions may partly explain the neuroprotective properties of cannabiglendol, as well as its effects on cellular proliferation and survival.
Given the multitarget nature of cannabiglendol, its cellular-level effects are realized through complex interplay among several signaling pathways. For instance, simultaneous activation of TRP channels and PPARγ supports both acute anti-inflammatory responses and long-term gene regulation that maintains tissue homeostasis. Meanwhile, modulation of FAAH and effects on transport proteins help stabilize neurotransmitter systems and reduce hyperactivity in neural pathways.
Interestingly, cannabiglendol also affects immune cells, including macrophages and T-lymphocytes, via specific receptors and signaling molecules such as NF-κB and MAPK. This leads to decreased expression of pro-inflammatory factors, holding potential for therapeutic use in autoimmune and inflammatory diseases.
It is important to note that the spectrum of biological targets for cannabiglendol also includes glucocorticoid receptors, where it may exhibit effects similar to steroid drugs but with fewer side effects. This points to the possibility of developing new therapeutic agents based on cannabiglendol with minimal toxic effects.
Molecular interactions of cannabiglendol are often accompanied by changes in intracellular levels of secondary messengers-calcium, cAMP, diacylglycerol-as well as activation or inhibition of kinase and phosphatase enzymes. This creates a broad range of effects dependent on cell type, organismal state, and ligand concentration.
It is worth noting that research on the molecular mechanisms of cannabiglendol is actively evolving, with emerging data on potential interactions with non-cannabinoid targets such as serotonin receptors and modulatory effects on neurotransmitter metabolism. This makes cannabiglendol a promising subject for further study both in fundamental biology and in the development of new pharmacological agents.
Known Pharmacodynamic Effects
The pharmacodynamic properties of cannabigerol (CBG) are determined by its ability to influence a wide range of physiological processes through multifactorial interactions with diverse biological targets. The known effects of CBG include anti-inflammatory, neuroprotective, analgesic, antioxidant, and immunomodulatory activities, each grounded in specific mechanistic bases that define the potential of this compound in the treatment of various pathological conditions.
One of the key pharmacodynamic effects of CBG is its pronounced anti-inflammatory action, which is mediated through modulation of the cytokine profile and suppression of pro-inflammatory mediator expression. CBG inhibits the activity of critical transcription factors such as NF-κB, leading to reduced production of interleukins (IL-1β, IL-6), tumor necrosis factor-alpha (TNF-α), and other pro-inflammatory agents. This effect is especially important in the context of chronic inflammatory processes, where standard anti-inflammatory drugs are often accompanied by side effects. Furthermore, CBG actively influences PPARγ, which also contributes to the regulation of gene expression related to anti-inflammation, thereby enhancing its immunomodulatory effects.
The neuroprotective properties of CBG are based on its ability to reduce oxidative stress and modulate apoptotic processes in nerve cells. CBG demonstrates the capacity to protect neurons from damage caused by glutamate toxicity, which occurs during excessive excitotoxic activation. This action is achieved through regulation of intracellular calcium levels, activation of antioxidant systems, and modulation of signals that control cell survival. Additionally, CBG can increase the activity of superoxide dismutase and catalase enzymes, which helps lower free radical concentrations and maintain the structural integrity of neurons.
The analgesic effect of CBG is associated with its ability to modulate the activity of TRP channels (TRPV1, TRPA1), which participate in the transmission of pain signals in the peripheral and central nervous systems. Interaction with these channels results in decreased excitability of nociceptive neurons and reduced pain perception, making CBG a promising agent in the treatment of chronic pain of various etiologies, including neuropathic and inflammatory pain. Importantly, this analgesic effect is not accompanied by the typical psychoactivity characteristic of THC, due to the specific selectivity of CBG.
The antioxidant activity of CBG is based on its ability to directly neutralize free radicals and prevent lipid peroxidation in cellular membranes. This property is critical for protecting cells from oxidative damage, which is a fundamental mechanism underlying many diseases, including neurodegenerative processes, atherosclerosis, and metabolic disorders. In experimental models, CBG has demonstrated the capacity to reduce levels of oxidative stress markers such as malondialdehyde and 4-hydroxynonenal, indicating its efficacy as an antioxidant.
The immunomodulatory action of CBG manifests in the regulation of immune cell function, particularly macrophages, dendritic cells, and T lymphocytes. CBG promotes the transition of macrophages to an anti-inflammatory M2 phenotype, reduces dendritic cell activation, and inhibits T lymphocyte proliferation, collectively leading to decreased autoimmune and allergic reactivity. Importantly, this effect does not cause immunosuppression but rather supports a balanced immune response, making CBG promising in the therapy of autoimmune and inflammatory diseases.
CBG also influences lipid and glucose metabolism through activation of PPARγ, which is associated with its potential ability to regulate metabolic processes at the cellular level. Experimental studies have shown that CBG reduces insulin resistance, improves lipid profiles, and decreases fat accumulation in the liver, indicating potential applications in the treatment of metabolic syndrome and non-alcoholic fatty liver disease.
The neuropsychiatric effects of CBG, although less studied, also attract research interest. The compound can influence neurotransmission in serotonergic and dopaminergic systems, potentially providing anxiolytic and antidepressant effects without the risk of dependence or sedative side effects. In this context, CBG demonstrates the ability to normalize neurotransmitter imbalances characteristic of depressive and anxiety disorders.
Another known pharmacodynamic effect is CBG’s influence on muscle tone and vasodilation. CBG modulates smooth muscle function, reducing spasms and improving microcirculation, which may be beneficial in the treatment of spastic syndromes and vascular disorders. This effect is achieved both through direct action on smooth muscles and through regulation of vasoactive substance release.
Additionally, CBG exhibits effects on sleep regulation, related to its ability to influence neurotransmitter systems and ion channels responsible for sleep-wake cycle formation. Considering this, it may have potential as a sleep disorder treatment without the typical side effects associated with hypnotic drugs.
CBG also demonstrates effects on cellular metabolism and proliferation, which may be useful in cancer therapy. Some studies indicate that CBG can inhibit the growth of certain tumor cell types through modulation of apoptotic pathways and blockade of angiogenesis, opening new avenues for oncological drug development.
In Vitro and In Vivo Studies
Studies of cannabigerol (CBG) in in vitro and in vivo systems provide a deep understanding of its biological activity, pharmacological profile, and potential therapeutic applications. These studies encompass a broad range of experimental models, including cell lines, tissue cultures, and animal models, allowing for a systematic evaluation of CBG’s mechanisms of action, efficacy, toxicity, and pharmacokinetics.
In in vitro systems, CBG has been investigated on various cell types, including neurons, glial cells, immune cells, as well as endothelial cells and fibroblasts. Cultured neurons show reduced apoptosis under CBG treatment in models of induced oxidative stress, confirming its neuroprotective properties. At the cellular level, CBG demonstrates the ability to decrease the production of pro-inflammatory cytokines (TNF-α, IL-6, IL-1β) in macrophages and microglia following lipopolysaccharide (LPS) stimulation, indicating its anti-inflammatory activity mediated through inhibition of NF-κB and MAPK signaling pathways. Moreover, in vitro experiments with endothelial cells reveal a positive effect of CBG on endothelial function restoration, including increased nitric oxide production and reduced expression of adhesion molecules, which is important for preventing atherogenesis.
Investigation of CBG’s effects on TRP channels in cellular expression models has helped clarify its analgesic mechanisms. Specifically, studies using patch-clamp techniques demonstrated that CBG effectively modulates TRPV1 and TRPA1 activity by reducing their ion flux, thereby diminishing nociceptor activation. These studies support CBG’s potential as an analgesic without psychoactive effects.
In vitro models are also applied to study the antioxidant activity of CBG. Cell lines subjected to oxidative stress show decreased levels of lipid peroxidation and reduced free radical formation after CBG treatment, confirming its capacity for direct radical scavenging and stimulation of antioxidant enzymes.
At the immune system level, in vitro studies show that CBG modulates T-lymphocyte activation by suppressing their proliferation and secretion of pro-inflammatory cytokines while promoting the activation of anti-inflammatory macrophage subpopulations. These findings are significant for the potential use of CBG in the treatment of autoimmune and chronic inflammatory conditions.
Transitioning to in vivo models allows assessment of CBG’s pharmacodynamic and pharmacokinetic characteristics within complex biological systems. In animal models, CBG demonstrates efficacy in reducing inflammatory processes, confirmed in models of arthritis, colitis, and neuroinflammation. For example, in rats with induced rheumatoid arthritis, CBG led to decreased joint swelling, inhibition of cellular infiltration, and reduction of inflammatory mediator levels in synovial fluid.
The neuroprotective effects of CBG have been confirmed in models of ischemic stroke and traumatic brain injury. Animals treated with CBG after ischemia induction exhibited reduced necrotic areas, preservation of functional neuronal structures, and improved cognitive function. This effect correlated with decreased oxidative stress and inflammation levels in brain tissue, supporting the compound’s comprehensive protective mechanism.
In vivo studies on pain syndrome models, such as neuropathic pain, demonstrated significant reduction of pain behaviors after CBG administration. Experiments measuring pain sensitivity thresholds to nociceptive stimuli showed dose-dependent pain relief. Importantly, CBG did not cause typical side effects associated with opioid or cannabinoid drugs, such as sedation or cognitive impairment.
Pharmacokinetic studies in in vivo systems revealed that CBG has satisfactory bioavailability and a moderate half-life, ensuring stable maintenance of therapeutic concentrations. CBG metabolism occurs primarily via hepatic cytochrome P450 enzymes, producing metabolites with lower pharmacological activity that may contribute to modulating the parent compound’s effects. Excretion occurs mainly through bile and kidneys, which is important for dose adjustments in patients with impaired organ function.
The immunomodulatory effect of CBG was studied in autoimmune disease models, particularly experimental autoimmune encephalitis (EAE) and colitis models. Administration of CBG resulted in reduced clinical symptoms and decreased inflammatory activity in the nervous system and intestines, associated with regulation of the balance between T-helper and regulatory T cells. These results confirm CBG’s immunomodulatory potential in complex pathogenic processes.
In vivo toxicity studies indicate high tolerability of CBG at doses significantly exceeding therapeutic levels, with no acute or chronic toxicity observed under standard administration protocols. Experimental models showed no adverse effects on the cardiovascular system, liver, or kidneys, indicating a favorable safety profile.
Application and Target Groups
Cannabiglendol is attracting attention as a promising compound with multifaceted potential applications across various fields, including medicine, pharmaceuticals, science, and research interests. Its unique pharmacological profile and mechanisms of action open up possibilities for use in a range of clinical situations, particularly in diseases accompanied by inflammation, pain, neurodegeneration, as well as in the comprehensive treatment of immune and metabolic disorders. Thus, the target groups who may benefit from cannabiglendol encompass a broad spectrum of patients with both chronic and acute pathologies.
One of the key areas of application for cannabiglendol is the treatment of inflammatory diseases. Thanks to its ability to effectively inhibit pro-inflammatory signaling pathways, this compound may be used in managing rheumatic diseases such as rheumatoid arthritis, psoriatic arthritis, and other autoimmune conditions characterized by chronic tissue inflammation. For patients with such diagnoses, cannabiglendol represents an option as a potentially less toxic alternative or adjunct to classical immunosuppressive drugs, given its immunomodulatory properties.
Target groups also include patients with nervous system disorders, especially those suffering from neurodegenerative diseases, including Alzheimer’s disease, Parkinson’s disease, multiple sclerosis, and other conditions associated with oxidative stress and neuroinflammation. In these cases, cannabiglendol can be applied as a neuroprotective agent that helps reduce degenerative processes, mitigate neuronal damage, and improve cognitive functions. For patients with chronic pain syndromes, particularly of neuropathic origin, cannabiglendol offers an alternative analgesic pathway without the development of dependence typical of opioid analgesics.
Additionally, cannabiglendol has significant potential in the context of metabolic disorders. Research indicates its influence on glucose metabolism regulation, making this compound promising for patients with type 2 diabetes and metabolic syndrome. Its effects on endothelial function and vascular tone provide grounds for exploring the use of cannabiglendol in cardiovascular diseases associated with endothelial dysfunction, atherosclerosis, and hypertension.
Among the target groups, patients with mental health disorders, particularly anxiety disorders and depression, should not be overlooked. Preliminary experimental data suggest the anxiolytic and antidepressant potential of cannabiglendol, linked to its ability to affect neurotransmitter systems that regulate emotional state, opening prospects for the development of new psychopharmacological drugs.
Given the wide spectrum of pharmacological activity of cannabiglendol, its use covers both specific therapeutic areas and multidisciplinary approaches that combine effects on inflammatory, immune, neuronal, and metabolic processes. Accordingly, the target groups include not only patients with classical diseases but also subpopulations requiring comprehensive support, such as individuals with multimorbidity or chronic conditions with complex pathophysiology.
Alongside clinical applications, cannabiglendol is important to the scientific community as a tool for investigating fundamental biochemical and physiological processes. Its ability to specifically modulate various biomolecular targets makes it a valuable object for studying mechanisms of cellular signaling, immune regulation, neuronal plasticity, and metabolism. This creates additional target groups in the form of researchers, pharmacologists, biochemists, and molecular biologists.
Medical and Scientific Directions of Application
Cannabiglendol attracts the attention of the medical and scientific communities due to its unique pharmacological properties, allowing an expansion of therapeutic strategies in various fields. The medical use of this compound focuses on its ability to modulate inflammatory, immune, and neurodegenerative processes and influence the body’s metabolic functions. In the scientific field, cannabiglendol is used as a tool for studying fundamental biological processes and molecular mechanisms of diseases.
One of the most promising medical applications of cannabiglendol is the treatment of chronic inflammatory diseases. Inflammation is the primary pathogenetic mechanism of many chronic conditions, including rheumatoid arthritis, psoriasis, and chronic inflammatory bowel diseases. Cannabiglendol demonstrates the ability to selectively inhibit the synthesis of pro-inflammatory cytokines and reduce the activity of nuclear factor kappa B (NF-κB), which is a key regulator of the inflammatory cascade. This effect opens prospects for using cannabiglendol as an alternative to classical anti-inflammatory drugs, lowering the risk of side effects typical for nonsteroidal anti-inflammatory drugs (NSAIDs).
An important direction is the use of cannabiglendol in neurology, particularly in neurodegenerative diseases. Cannabiglendol exhibits neuroprotective properties, capable of reducing oxidative stress and stabilizing mitochondrial functions, which play a key role in maintaining cellular homeostasis. This is significant for diseases such as Alzheimer’s, Parkinson’s, and multiple sclerosis, where neuronal damage is caused by both immune and metabolic disturbances. Moreover, cannabiglendol supports the normalization of synaptic plasticity, improving cognitive functions and memory in animal model studies.
In the field of pain management, cannabiglendol shows potential for treating both acute and chronic pain syndromes, especially those of neuropathic origin. Studies have shown that cannabiglendol not only affects receptors associated with the endocannabinoid system but also modulates signaling pathways that reduce central and peripheral sensitization, which underlies many chronic pain conditions. Its ability to decrease inflammation further enhances its analgesic effect, making cannabiglendol a promising candidate for integration into comprehensive pain therapy.
Metabolic disorders represent a separate area of application. Cannabiglendol influences the regulation of energy metabolism and tissue sensitivity to insulin, making it a potential agent in the treatment of type 2 diabetes and obesity. Preclinical studies have found that cannabiglendol helps reduce blood glucose levels, improve lipid profiles, and decrease liver fat accumulation, indicating its possible effect on metabolic pathways involved in homeostasis regulation.
Another direction is the use of cannabiglendol in psychiatry. Research demonstrates its anxiolytic and antidepressant properties, associated with effects on serotonergic, dopaminergic, and other neurotransmitter systems. The compound can regulate neurochemical balance, reducing symptoms of anxiety and depression, making it promising for developing new pharmacological agents with a more favorable safety profile compared to existing drugs.
From a scientific standpoint, cannabiglendol is widely used to study mechanisms of cellular signaling, particularly those related to immune response, inflammation, and regulation of cellular metabolism. Its unique ability to influence various biochemical pathways allows cannabiglendol to serve as a model compound for investigating the pathogenesis of chronic diseases and developing new therapeutic approaches. Studying cannabiglendol’s interactions with receptors, enzymes, and transporter proteins contributes to a better understanding of complex processes in human cells.
Significant attention is also given to the study of cannabiglendol in the context of pharmacokinetics and pharmacodynamics. Its metabolism in the liver, tissue distribution, bioavailability, and excretion pathways are considered critical for optimizing dosing regimens and dosage forms. The development of new pharmaceutical forms with controlled release can improve treatment effectiveness and reduce the risk of adverse reactions.
It is also important to note the prospects for using cannabiglendol in combination with other pharmacological agents. A synergistic effect from simultaneous use with anti-inflammatory, neuroprotective, or analgesic drugs may significantly enhance therapeutic outcomes and reduce the dosage of each component, which is important for minimizing side effects.
In pharmaceutical research, cannabiglendol serves as a foundational compound for creating new synthetic derivatives with improved characteristics. Molecular modifications allow optimization of biological activity, target selectivity, and pharmacokinetic properties, opening opportunities for developing new classes of medications.
Scientific studies also consider cannabiglendol as a tool to explore the role of the endocannabinoid system in regulating physiological processes, including immune control, inflammatory responses, nerve conduction, and metabolism. This contributes to expanding the understanding of biological systems and creating a foundation for further therapeutic innovations.
Significance for Fundamental Research
Cannabigerol has gained considerable significance in fundamental research due to its unique chemical structure and broad spectrum of biological activity, allowing researchers to study complex molecular mechanisms that regulate physiological and pathological processes. The distinctive biochemical specificity of cannabigerol makes it an important tool for uncovering the role of the endocannabinoid system, as well as for investigating the interactions between different signaling pathways within cells.
One of the key aspects of cannabigerol’s significance in fundamental research is its ability to selectively influence molecular targets that regulate immune responses. The use of cannabigerol enables detailed analysis of the roles of specific cytokines and transcription factors in inflammatory processes. Consequently, studying cellular responses in the presence of cannabigerol contributes to a better understanding of immunoregulation mechanisms, which form the basis for developing new therapeutic strategies for autoimmune and inflammatory diseases.
Cannabigerol also serves as an important tool for studying neurobiological processes. Research on the effects of cannabigerol on neurons and glial cells helps to reveal mechanisms of neuroprotection, synaptic plasticity, and neurogenesis. Particular attention is given to how cannabigerol regulates oxidative stress, mitochondrial metabolism, and apoptosis in nervous tissue. These studies facilitate a deeper understanding of the pathogenesis of neurodegenerative diseases such as Alzheimer’s and Parkinson’s and lay the foundation for further experiments with potential neuroprotective agents.
Cannabigerol opens opportunities for investigating the relationships between metabolism and regulation of cellular activity. It is used to analyze molecular pathways controlling the balance between anabolic and catabolic processes, as well as to study its effects on metabolic disorders, including insulin resistance and lipid metabolism disturbances. Cannabigerol allows researchers to determine the mechanisms of cellular adaptation to stress conditions and regulation of energy homeostasis, which are relevant to studying diseases such as diabetes and obesity.
An important area of fundamental research involving cannabigerol is the study of its impact on molecular and cellular mechanisms of pain. Due to its ability to modulate sensory neurons and receptors, cannabigerol serves as a model for investigating processes of central and peripheral sensitization, which underlie chronic pain. This enables the examination of interactions between ion channels, neurotransmitters, and inflammatory cytokines at the molecular level, forming the basis for developing new analgesic agents.
Cannabigerol also holds significant importance in pharmacological research, particularly in the study of pharmacokinetics and pharmacodynamics. Its use helps clarify the mechanisms of cannabinoid metabolism and the influence of enzymatic systems, including cytochrome P450, on bioavailability and molecular transformation within the body. This knowledge allows for the development of more effective drug formulations with optimized distribution profiles and duration of action.
In the field of molecular biology, cannabigerol is employed to study structural and functional aspects of receptors, including CB1, CB2, TRPV1, GPR55, and other potential targets. Research on the interactions of cannabigerol with these proteins helps reveal the nuances of receptor activation, ligand selectivity, as well as feedback and desensitization mechanisms. These data are important for developing new classes of drugs with increased selectivity and reduced risk of side effects.
Cannabigerol also serves as a valuable tool in research on epigenetic mechanisms regulating gene expression. It is known that cannabinoids can influence chromatin modifications and transcription factor activity. The use of cannabigerol in cellular models allows for analysis of how changes in the epigenetic landscape affect cell functions in inflammatory and neurodegenerative processes.
Thanks to its biological activity, cannabigerol is widely used in modeling pathological conditions at the cellular and tissue levels. This enables the creation of more relevant disease models that correspond to clinical manifestations and the evaluation of the efficacy of potential therapeutic agents under controlled laboratory conditions. Accordingly, cannabigerol acts as a catalyst for integrating biochemical, molecular, and cellular research in the search for new treatment approaches.
Another important aspect is the application of cannabigerol in interdisciplinary research combining pharmacology, biochemistry, molecular biology, neuroscience, and immunology. Such a comprehensive approach allows for obtaining a holistic picture of cannabigerol’s effects on the body and developing new therapy concepts based on a multitarget mechanism of action.
Conclusion
Cannabigerol represents a chemically unique compound belonging to the class of cannabinoids, with a distinctive structure that determines its unique physicochemical properties and biological activity. Detailed analysis of the molecular structure of cannabigerol shows that it possesses chemical groups characteristic of phenolic cannabinoids, including an aromatic ring, hydroxyl functions, and a lipophilic tail, which provide its ability to effectively interact with biological molecules and membranes. Features of molecular isomerism and conformational dynamics of cannabigerol define its binding specificity with receptors, stability, and physicochemical parameters that are crucial for pharmacokinetics and pharmacodynamics.
The origin of cannabigerol is determined by its natural biosynthesis in plants of the Cannabis genus, where cannabigerol is formed through sequential enzymatic transformations of specific precursors. The biosynthetic pathway of cannabigerol is part of a complex network of metabolic reactions regulated genetically and dependent on environmental factors that determine the concentration and distribution of cannabigerol in different parts of the plant. Its quantitative ratio varies depending on the strain, cultivation conditions, and the plant’s maturity, which is important to consider in both fundamental research and industrial production.
Various methods are used for laboratory and industrial production of cannabigerol. Semi-synthetic approaches are based on the conversion of natural cannabinoids, which allows preserving specificity and high quality of the final product. Complete chemical synthesis provides control over all stages of molecule formation but requires complex reactions and high technological costs. Biotechnological methods, including the use of microorganisms or genetically modified cells, are becoming promising for large-scale and more environmentally friendly production of cannabigerol, allowing simultaneous regulation of yield and product purity.
The pharmacological activity of cannabigerol is associated with its ability to interact with numerous biological targets, including classical cannabinoid receptors (CB1, CB2) as well as other protein structures such as TRP channels, G-proteins, and enzymes. These interactions determine a broad spectrum of pharmacodynamic effects, including immunomodulatory action, neuroprotection, analgesia, anti-inflammatory effects, and influence on metabolic processes. Mechanisms of cannabigerol action are studied in vitro and in vivo models, enabling systematic assessment of its therapeutic potential and safety.
Cannabigerol finds applications in various medical and scientific fields. In medical practice, its potential is considered for treating inflammatory, neurodegenerative, and immunological diseases. Cannabigerol acts as a promising agent for the development of new pharmaceutical drugs capable of effectively modulating pathological processes without typical side effects associated with traditional medications. In scientific research, cannabigerol is used as a model compound for studying the endocannabinoid system and mechanisms of cellular regulation.
The significance of cannabigerol for fundamental research is difficult to overestimate. Its unique structure and functions allow revealing subtle aspects of molecular ligand-receptor interactions, effects on epigenetic modifications, transcription regulation, and cellular metabolism. The use of cannabigerol contributes to a deeper understanding of the molecular basis of immunoregulation, neuroprotection, metabolic homeostasis, and pain, creating a platform for developing innovative therapeutic strategies and optimizing existing ones.
In summary, cannabigerol is an important chemical and pharmacological agent combining the complexity of natural biochemistry with medical application prospects. Its study promotes not only the advancement of fundamental scientific knowledge about the cannabinoid system and molecular biology but also opens new horizons in pharmaceutical development. Understanding its chemical nature, biosynthesis pathways, production methods, and pharmacological action forms a comprehensive approach necessary for the further effective use of cannabigerol in science and medicine.
Sources
- Atalay, S., Jarocka-Karpowicz, I., & Skrzydlewska, E. (2019). Therapeutic Properties of Cannabidiol (CBD). Pharmaceuticals (Basel).
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7023045/ - Izzo, A. A., Borrelli, F., Capasso, R., Di Marzo, V., & Mechoulam, R. (2009). Non-psychotropic plant cannabinoids: new therapeutic opportunities from an ancient herb. Trends in Pharmacological Sciences.
https://www.sciencedirect.com/science/article/abs/pii/S0165614709000369 - Citti, C., Braghiroli, D., Vandelli, M. A., & Cannazza, G. (2018). Medicinal Cannabis: Principal Constituents and Their Therapeutic Potential. Pharmaceuticals.
https://www.mdpi.com/1424-8247/11/1/17 - PubChem – Cannabigerol (CBG). National Center for Biotechnology Information (NCBI).
https://pubchem.ncbi.nlm.nih.gov/compound/Cannabigerol - University of California, San Francisco (UCSF) Cannabis Research. Overview of cannabinoids including CBG.
https://www.ucsf.edu/news/2019/03/412446/understanding-cannabis-cannabinoids-and-what-they-do - Healthline – Cannabigerol (CBG): What Is It and What Does It Do?
https://www.healthline.com/health/cannabigerol - Hammell, D. C., Zhang, L. P., Ma, F., Abshire, S. M., McIlwrath, S. L., Stinchcomb, A. L., & Westlund, K. N. (2016). Transdermal cannabidiol reduces inflammation and pain-related behaviors in a rat model of arthritis. European Journal of Pain.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4851925/ - Project CBD – Research Library. Articles and research on cannabinoids including CBG.
https://www.projectcbd.org/science/cannabinoids/cannabigerol-cbg