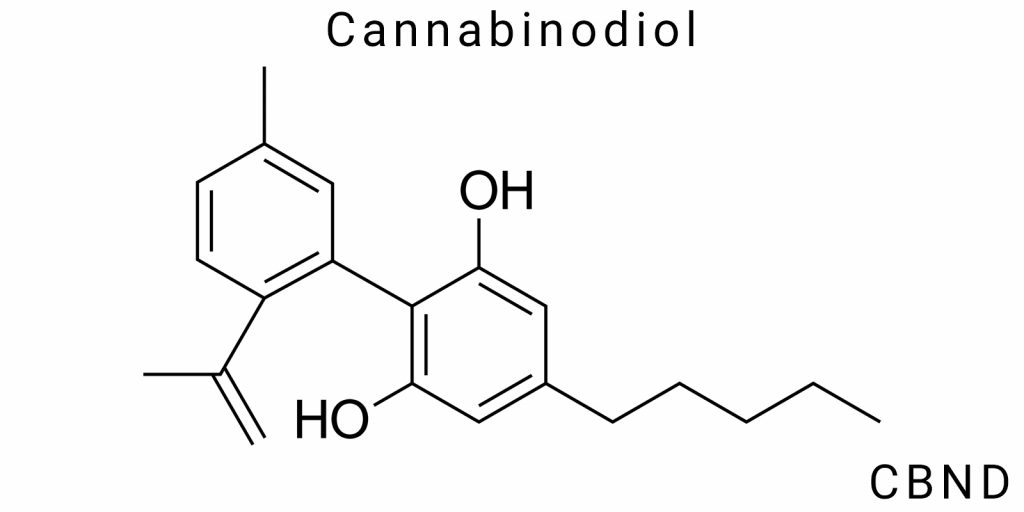
Cannabinodiol (CBND) is one of many cannabinoids found in the Cannabis sativa plant. However, despite its presence in cannabis extracts and chemical profiles, CBND remains relatively underexplored and seldom studied. This fact hides an intriguing potential for investigating its chemical properties, mechanisms of action, and possibilities for medical applications. In this section, we will attempt to delve deeper into the nature of CBND, its physicochemical properties, origin, methods of extraction, and possible uses in medical practice.
The importance of studying CBND is underscored by the fact that it is part of a larger group of cannabinoids that are actively researched due to their potentially beneficial properties. Cannabinoids found in cannabis interact with the body’s endocannabinoid system, making their study essential for understanding various physiological and psychoactive processes. Specifically, many cannabinoids, such as tetrahydrocannabinol (THC) and cannabidiol (CBD), have gained popularity due to their medical and therapeutic effects, including in pain management, inflammation, epilepsy, and mental health disorders. However, cannabinoids like CBND remain on the periphery of these studies and have not yet received sufficient attention from the scientific community.
To understand the significance of CBND, it is necessary to start by examining its chemical structure. The difference between CBND and other cannabinoids lies in its unique molecular structure, which determines its distinct physicochemical properties and interactions with biological systems. It is known that CBND is an isomer of cannabidiol (CBD), but its molecular characteristics differ significantly due to the presence of additional functional groups or changes in the configuration of the molecule during its oxidation process. This distinction imparts certain unique properties to CBND, which may be valuable for future pharmacological and biochemical research.
One of the key aspects of cannabinoid research is their origin. Typically, most cannabinoids are produced in the Cannabis sativa plant as part of its metabolic process. It is known that cannabinoids in cannabis are formed through complex biosynthetic pathways involving numerous chemical reactions, such as oxidation, decarboxylation, and other transformations. CBND is the result of the transformation of CBD under thermal or oxidative degradation conditions. This process can occur at high temperatures or through prolonged exposure to light, which leads to changes in the molecular structure of CBD and the formation of CBND. Because of this, CBND is typically found in older or improperly stored cannabis extracts.
Interest in CBND is also growing due to its potential medical applications. Although there are no large-scale clinical studies confirming its effectiveness at this time, researchers highlight the prospects of using CBND in areas such as neuroprotection, inflammation reduction, and even anti-stress therapy. Since cannabinoids interact with the endocannabinoid system, they may play a role in regulating various physiological processes such as pain sensitivity, appetite, mood, and others. Understanding the role of CBND in this system may open up new approaches to the treatment of various diseases, including chronic pain, neurodegenerative diseases, and anxiety disorders.
It is also important to note that the existing methods of obtaining CBND are crucial for understanding its potential applications. Different extraction methods for cannabinoids from plant material, such as using organic solvents or supercritical CO₂, can influence the chemical composition of the extracts and, consequently, the CBND content. Considering that CBND forms as a result of the degradation of other cannabinoids, its quantity in extracts may vary depending on processing methods and the storage conditions of the plant material.
Cannabinodiol represents an important component of the cannabinoid group that may hold significant potential for the development of new treatment methods, although further in-depth research is needed. Investigating the mechanisms of its action and properties will allow scientists to more precisely evaluate the role of CBND in human physiology and its possible clinical applications. At present, this remains primarily a theoretical interest, but the study of cannabinoids like CBND is an essential part of future scientific discoveries in pharmacology and medicine.
Chemical Nature of CBND
Cannabindiol (CBND) is one of the many cannabinoids found in Cannabis species, but it is less studied compared to other cannabinoids like cannabidiol (CBD) or tetrahydrocannabinol (THC). CBND is the product of the oxidation of cannabidiol (CBD), and its chemical properties are determined by specific structural features of the molecule. Although there is already some knowledge about its molecular structure, its role in pharmacology and human physiology remains not fully understood. This opens up new avenues for studying the potential therapeutic possibilities of CBND, as well as for a deeper analysis of its chemical nature. Therefore, studying its structure and molecular characteristics is an essential step in understanding why CBND might be beneficial in various medical fields.
Structural Formula and Molecular Characteristics
The structural formula of cannabindiol forms the basis for its identification and analysis. Like many other cannabinoids, the CBND molecule consists of a cyclic structure with aromatic properties and linear chains. It contains a benzene ring, which is a characteristic feature of many organic compounds, as well as a combination of carbon, hydrogen, and oxygen atoms. The structure of CBND is very similar to that of cannabidiol, but it contains an additional functional group – an epoxide group, which gives the molecule specific chemical properties. This epoxide may form as a result of the oxidation of cannabidiol, making CBND more stable to thermal influences and oxidative reactions compared to other cannabinoids.
The molecular weight of CBND is approximately 314.48 g/mol, which makes it similar in mass to other cannabinoids. On the other hand, the specific configuration of the CBND molecule affects its physicochemical characteristics, such as polarity, solubility in water, melting point, and its interaction with biological systems. Cannabindiol has relatively low water solubility but dissolves well in organic solvents such as ethanol, methanol, and chloroform, making it suitable for various extraction methods and chemical analysis.
Another important aspect is the conformational stability of the CBND molecule. Cannabinoid molecules can exist in various conformations, meaning different spatial orientations that determine their properties. In the case of CBND, these different conformations can significantly affect its physicochemical and biological properties. For instance, there is a specific relationship between the conformational stability of the CBND molecule and its ability to bind with receptors of the endocannabinoid system, which plays a crucial role in determining its potential pharmacological activity.
Stereochemistry and Isomers
The stereochemistry of the CBND molecule is important for its biological activity, as molecules with the same chemical formula but different spatial arrangements of atoms can differ significantly in their properties. An important aspect of the stereochemistry of CBND is the presence of stereoisomerism, which arises due to the presence of chiral centers in the molecule. Chirality means that the molecule is not superimposable on its mirror image, allowing for the existence of different stereoisomers that may have distinct physiological activity.
In the case of CBND, there are two main types of isomers: enantiomers and diastereomers. Enantiomers are pairs of molecules that are mirror images of each other but cannot be aligned by simply rotating them in space. Diastereomers, on the other hand, are isomers that are not mirror images but have different spatial orientations. From a pharmacological perspective, enantiomers can have entirely different biological effects because they may interact with receptors and other molecular structures in the body with varying strength or even in different directions. For example, one enantiomer may have a strong effect on reducing inflammation or relieving pain, while its mirror image may be inactive or even cause a reverse effect.
On the other hand, diastereomers may have similar properties but still show differences in biological activity. For CBND, there are several potential stereoisomers that may arise due to different spatial configurations of atoms in the molecule, such as changes in the positioning of hydrogen atoms or hydroxyl groups on the chain. The impact of stereochemistry on the biological activity of CBND is still insufficiently studied, but based on experience with other cannabinoids, it is likely that different stereoisomers of CBND may have different effectiveness in interacting with the receptors of the endocannabinoid system.
According to some previous studies, it is the stereochemical structure of the molecule that determines how a cannabinoid binds to CB1 and CB2 receptors, which are the main targets for cannabinoids. Changes in the configuration of atoms in CBND can influence how the molecule interacts with these receptors, thereby determining the cannabinoid’s effect on the body. As CBND is still a relatively new object of study, the presence of various stereoisomers could mean that this cannabinoid still possesses a range of unusual or unstudied effects that may be uncovered in further scientific experiments.
Moreover, it should be noted that the stereochemistry of CBND is not only important for pharmacology but also for its synthetic methods. When cannabinoids are synthesized in the laboratory, especially when creating new molecules or analogs of CBND, it is crucial to consider the stereochemical aspects, as incorrect atomic arrangements in the molecule can lead to ineffective or even toxic compounds. Therefore, ensuring the correct stereochemistry of the molecule is critical for the development of new therapeutic agents based on cannabinoids.
Biogenesis and Natural Origin of CBND
Cannabinoids, including cannabindiol (CBND), are key biologically active compounds synthesized in plants of the Cannabis genus, particularly Cannabis sativa L. The process of cannabinoid formation is part of a broader system of metabolic pathways in these plants, and each cannabinoid has its specific molecular structure, which determines its physiological and pharmacological properties.
The biosynthesis of cannabinoids begins with oleic acid, which is a component of the cell membranes in Cannabis. This acid participates in the formation of molecules that, through several stages of transformation, result in the creation of the main cannabinoids. The molecular precursors of cannabinoids include cannabigerolic acid (CBGA), from which, through enzymatic conversions, compounds such as cannabidiol (CBD), tetrahydrocannabinol (THC), and other cannabinoid derivatives are formed. However, an important aspect is not only the synthesis of these primary cannabinoids but also the formation of molecules such as CBND, which is produced through the oxidation of cannabidiol.
The Phytocannabinoid Pathway in Cannabis sativa L.
The process of cannabinoid synthesis in Cannabis sativa L. takes place in several stages, with enzymes playing a key role in catalyzing the transformation of fatty acid molecules into cannabinoids. Oleic acid, derived from lipids, is the starting precursor for the synthesis of cannabigerolic acid (CBGA), the main precursor molecule for a series of cannabinoids such as CBD and THC. Further biochemical transformations of CBGA lead to the formation of cannabidiol and tetrahydrocannabinol.
These processes occur in specialized plant cells known as trichomes, which are responsible for the production and storage of cannabinoids. Trichomes accumulate these compounds, resulting in their high concentration in various parts of the plant, particularly in the flowers and leaves. Cannabidiol (CBD) is one of the primary cannabinoids produced in these plants, and it serves as the main precursor for further transformations.
One of the key stages is the oxidation of cannabidiol (CBD) into cannabindiol (CBND). This process occurs not only through biosynthetic pathways but can also be triggered by external factors such as temperature changes, exposure to ultraviolet radiation, or plant aging.
Overview of Major Biosynthetic Pathways
The biosynthesis of cannabinoids in Cannabis can be described as a stepwise process, where each step corresponds to the formation of specific precursor molecules and enzymatic reactions that determine the final cannabinoid composition. Oleic acid, the starting molecule, is converted into cannabigerolic acid (CBGA) through a series of enzymatic transformations. This process involves the activation of fatty acids and their condensation with other molecules to form the main cannabinoid precursors.
As a result of specialized enzymes, CBGA is broken down, forming various cannabinoids, including CBD, THC, CBG, and other derivatives. After their formation, these molecules may undergo further transformations, such as oxidation or other chemical reactions. Oxidation of cannabidiol (CBD) into cannabindiol (CBND) is one such conversion pathway, which occurs under certain conditions, such as thermal or chemical processing of the plants.
The Role of CBND as a Degradation Product
Cannabindiol (CBND) is not a primary cannabinoid produced in the plant but is a result of the oxidation of cannabidiol. This process can occur in plants under the influence of various environmental factors, such as temperature, humidity, UV radiation, or aging of the plants. These changes can lead to the conversion of cannabidiol into CBND, which has its unique properties.
One of the main pathways by which CBND forms is the oxidation of CBD, which occurs due to temperature fluctuations or exposure to ultraviolet radiation. Therefore, the level of CBND in different cannabis samples may vary significantly depending on storage conditions, processing methods, or the aging of the plant. This means that controlling storage and processing conditions is a crucial factor in maintaining a stable cannabinoid profile in cannabis products, especially when a precise concentration of a specific cannabinoid is required.
Natural Occurrence: Conditions for CBND Formation
The process of CBND formation is a critical part of the biochemical processes in Cannabis sativa plants and depends on changes in the external environment. Cannabidiol (CBD), one of the primary cannabinoids in the plant, can undergo natural transformations influenced by various factors such as temperature, storage, and ultraviolet radiation. These processes not only determine the presence of CBND but also can alter its concentration and biological activity.
Thermal/Oxidative Conversion of CBD
Thermal and oxidative conversions of CBD are the primary mechanisms that lead to the formation of CBND under natural conditions. When cannabis is subjected to heat treatment, particularly at temperatures above 100°C, cannabidiol may undergo a chemical reaction with oxygen, leading to its oxidation. This transformation occurs through the addition of oxygen molecules to the CBD structure, altering its chemical properties and structure. As a result of this reaction, CBND forms-the product of oxidative degradation of cannabidiol.
This process can occur not only in laboratory conditions or during special heat treatments but also during the natural aging of the plant when environmental temperature or other physical factors trigger chemical transformations within the plant. For example, storing cannabis at high temperatures over extended periods can accelerate oxidation processes, leading to increased CBND concentration. Thus, even moderate heat exposure can significantly alter the cannabinoid composition of cannabis samples.
This conversion is significant not only for the natural biosynthesis of CBND but also for the pharmacological properties of cannabis products. For instance, when producing cannabis oils or extracts, high temperatures may cause an increase in CBND levels, which alters its properties and therapeutic potential.
Impact of Storage Conditions, Temperature, and UV Radiation
In addition to thermal treatment, important factors determining the formation of CBND include the storage conditions of cannabis and the influence of the external environment, particularly temperature and ultraviolet (UV) radiation.
- Temperature: Storing cannabis at high temperatures is one of the main factors that promote the oxidation of CBD into CBND. High temperatures accelerate chemical reactions, particularly oxidation, which can lead to significant changes in cannabinoid composition. Storing cannabis above 30°C can result in the gradual formation of CBND, which may also be accompanied by other chemical changes that affect the quality and effectiveness of the product. Prolonged exposure to heat can accelerate the degradation of not only CBD but also other cannabinoids, which is an important consideration when storing cannabis products in industrial conditions.
- UV Radiation: Another important factor contributing to the formation of CBND is the exposure to ultraviolet radiation. Cannabidiol is sensitive to UV light, which can initiate photochemical reactions within the molecule. Ultraviolet radiation breaks bonds in the CBD molecule, leading to its degradation and the formation of new compounds, including CBND. These transformations are especially relevant in the production of cannabis products, such as oils or extracts, where UV light can significantly alter the final product’s composition. Since many cannabis products undergo processing under high temperatures and prolonged light exposure, this can result in substantial changes in cannabinoid concentrations, including an increase in CBND levels.
- Storage and Packaging: The storage conditions of cannabis can have a decisive impact on the formation of CBND. If cannabis is stored in conditions of high temperature and direct sunlight, this creates an environment conducive to CBD degradation. The best conditions for preserving active cannabinoids involve storing cannabis at low temperatures (no higher than 15°C) in dark, airtight containers to minimize exposure to light and heat. Under room temperature storage without adequate protection from UV radiation, the oxidation process can proceed faster.
- Humidity: Humidity is another factor that can influence the rate at which CBD is converted into CBND. High humidity levels may promote hydrolysis and other chemical reactions that affect cannabinoid molecules. Therefore, storing cannabis in controlled humidity conditions is an important aspect of preserving cannabinoid stability and preventing degradation.
Extraction and Synthesis Methods for CBND
The processes of extraction and synthesis of cannabinodiol (CBND) play a crucial role in modern scientific research and the industrial production of cannabinoids. These processes not only enable the production of high-purity CBND but also allow for the exploration of its therapeutic potential, as well as the optimization of processes across various stages-from laboratory to industrial scale.
Semi-Synthetic Methods from CBD/CBN
Semi-synthetic methods for synthesizing CBND from cannabidiol (CBD) or cannabinol (CBN) are particularly significant, as they allow for the production of CBND without needing to extract a broad range of natural compounds, which can complicate the extraction process.
One of the most studied approaches is catalytic oxidation. In this process, CBD or CBN molecules interact with oxidizing agents, resulting in the transformation of one molecule into another. The catalysts used in this process can be inorganic (metal-containing compounds) or organic compounds. The choice of catalyst depends directly on the conditions under which the oxidation occurs and the desired end result. Oxidation of CBD to CBND allows for the control of the final molecular characteristics by adjusting temperature, reaction time, and oxidizer concentration.
Solvents used to facilitate chemical reactions should be chosen to minimize impact on the cannabinoid molecule itself. For example, methanol or acetone are commonly used as solvents for oxidation reactions because they do not disrupt the molecular structure. However, the concentration of these solvents, as well as temperature and reaction time, are crucial, as uncontrolled conditions can lead to low-purity products or even the formation of by-products.
This approach is key for synthesizing CBND from CBD or CBN, as it enables the production of a highly effective product while also reducing production costs and ensuring the stability of the final result.
Chromatographic Fractionation from Natural Material
Another important method is chromatography, which effectively separates and isolates CBND from cannabis, when needed, using various chemical solvents or fractions. Chromatographic fractionation can be performed via liquid chromatography (HPLC) or gas chromatography (GC), depending on the physical properties of the cannabinoids. HPLC, for example, allows for the production of very pure CBND samples due to its high resolution and the ability to control the parameters of the process.
This method is often combined with other extraction methods to obtain the purest cannabinoids for further research or applications. As a result of this process, CBND can be isolated from plant material without compromising its biological activity.
Supercritical CO₂ Extraction
Supercritical CO₂ extraction is one of the most innovative methods for extracting cannabinoids from plant material. It offers several advantages over traditional methods, such as preserving the high purity of cannabinoid molecules, avoiding the use of chemical solvents that might leave residues in the final product, and allowing for extraction without damaging the environment. In its supercritical state, carbon dioxide exhibits both liquid and gas-like properties, allowing it to penetrate cellular membranes and extract cannabinoids without breaking down their structure.
Prospects of Biotechnological Synthesis
With the advancement of biotechnology, methods utilizing microbial chassis for cannabinoid synthesis are gaining popularity. This can be achieved through genetic engineering, enabling organisms such as yeast or bacteria to produce cannabinoids, including CBND.
Through metabolic engineering, microorganisms can be modified to synthesize cannabidiol (CBD) or cannabinol (CBN), which are then oxidized to CBND. Genetic engineering of microorganisms also allows for increased yield, reducing raw material costs and minimizing environmental impact.
Biotechnological synthesis methods hold enormous potential for large-scale CBND production without the need to cultivate cannabis plants. This allows for high production volumes while reducing the cost of the final product.
Use of Microbial Chassis
One of the most promising directions is the use of microbial chassis for biosynthetic production of CBND. Microbial chassis are microorganisms that have been modified to produce desired molecules. This allows for precise control over production conditions at the molecular level, ensuring high purity and stability of the final product.
Various types of microorganisms, such as yeast, bacteria, and molds, are successfully used for genetic modification to synthesize specific cannabinoids. The process involves transferring genes responsible for cannabinoid biosynthesis from cannabis plants to microorganisms, enabling cannabinoid synthesis without the need to cultivate plants or carry out complex extraction processes.
Microbial synthesis offers several advantages over traditional extraction methods. First, microorganisms can be cultivated in large volumes in laboratory or industrial bioreactors, reducing raw material costs and providing high productivity. Second, the biosynthetic approach allows for the production of cannabinoids without using organic solvents or toxic substances, significantly improving the ecological footprint of the process.
Genetic Engineering of Yeast or Bacteria
Genetic engineering of yeast is one of the most promising fields for biosynthesizing cannabinoids, including CBND. Using yeast allows for the creation of stable microbial systems capable of converting simple molecules, such as olive alcohol or glucose, into cannabinoids via natural biosynthetic pathways.
Furthermore, yeast-based microbial chassis can be fine-tuned for the specialized synthesis of CBND by modifying their metabolic activity and regulating fermentation processes. This enables the creation of bioreactors that can produce cannabinoids at large scales while ensuring stability and efficiency in the production process.
The process of genetically modifying yeast involves inserting genes responsible for the biosynthesis of cannabidiol (CBD), cannabinol (CBN), or other CBND precursors. This enables the microorganism to synthesize cannabinoids without the involvement of plant material, significantly simplifying the technological process and reducing costs.
Bacteria are also important tools in genetic engineering for cannabinoid production. Thanks to their ability to rapidly adapt to environmental changes, they can be used to create stable CBND synthesis systems, making them cost-effective and suitable for large-scale use.
Pharmacological Potential and Interaction with the Endocannabinoid System
Binding to CB1/CB2 Receptors
Preliminary In Silico and In Vitro Data
CB1 and CB2 receptors are the primary targets for cannabinoids in the human body. CB1 receptors are predominantly located in the central nervous system, while CB2 receptors are found in immune cells such as macrophages, microglia, and others. The study of cannabinoid interactions, including CBND, with these receptors is conducted using in silico (computer modeling) and in vitro (laboratory tests on cells or tissues) methods.
In silico research allows the creation of models that predict the binding of CBND molecules to CB1 and CB2 receptors. These data help clarify how CBND molecules may interact with these receptors, which, in turn, can predict biological effects. Using molecular modeling, potential binding sites can be identified, and the potential affinity of CBND for each receptor can be forecasted.
In vitro experimental data shows that CBND has the ability to bind to CB2 receptors but with lower affinity for CB1 receptors. This suggests that CBND may be less psychoactive compared to other cannabinoids like THC, which has a high affinity for CB1 receptors. Therefore, its use may be promising in the treatment of inflammatory and immune diseases since the activation of CB2 receptors does not induce psychoactive effects.
Neuroprotective and Anti-inflammatory Properties
Preclinical Research Data
Preclinical studies on animals and cell cultures indicate that CBND exhibits strong neuroprotective and anti-inflammatory properties. One of the mechanisms of its action is the reduction of oxidative stress and neuroinflammation, key factors in the development of many neurodegenerative diseases. In models of Parkinson’s disease and Alzheimer’s disease, CBND demonstrates the ability to lower the activity of inflammatory cytokines such as TNF-α and IL-6, as well as reduce the number of free radicals in nerve cells.
Moreover, research suggests that CBND may aid in the recovery of neuronal connections and improve cognitive function in animals, which holds great potential for treating conditions such as dementia and stroke.
Hypotheses Regarding Mechanisms of Action
The mechanisms of CBND’s neuroprotective effects may involve several pathways. Firstly, CBND may activate CB2 receptors, leading to reduced inflammation in nervous tissue. Secondly, it could inhibit the neurotoxic effect of glutamate, which is a significant factor in neurodegeneration. Additionally, CBND may stimulate neurogenesis and improve synaptic plasticity through the activation of BDNF (brain-derived neurotrophic factor), which supports the growth of new neurons and their connections.
The anti-inflammatory effect of CBND may be attributed to its ability to reduce the production of pro-inflammatory cytokines such as IL-1β and TNF-α, which are important markers of chronic inflammation. As a result, CBND holds potential for use in treating autoimmune diseases such as rheumatoid arthritis or Crohn’s disease.
Safety, Toxicokinetics, and Metabolism
Lack of Clinical Research Data
While preliminary studies in animals have demonstrated high bioavailability and low toxicity of CBND, there is still a lack of large clinical studies confirming its safety and efficacy in humans. To date, there is limited clinical experience with long-term therapeutic use of CBND.
Some clinical studies focus on assessing the safety of cannabinoids in general (including CBND), but these studies are insufficient to draw final conclusions regarding safe dosages and duration of use. It should also be noted that CBND may interact with other medications via CYP450 enzymes, which are involved in the metabolism of many drugs.
Metabolic Pathways and Elimination
Like most cannabinoids, CBND is metabolized in the liver by CYP450 enzymes. This process involves several stages, including hydroxylation and glucuronidation, which make the molecule more water-soluble and facilitate its elimination from the body through the kidneys. The metabolic pathways of CBND may also include conversion into other cannabinoids, such as CBN (cannabinol), through oxidation.
The elimination of CBND from the body may vary depending on individual metabolic characteristics such as CYP450 enzyme activity, liver and kidney health, and potential interactions with other medications. It is important to note that studies have shown that with prolonged use, its metabolites may accumulate in organs such as the liver, which may require further investigation to determine safe dosing and duration of use.
Medical Goals and Prospects of CBND Use
Cannabidiorol (CBND) has garnered attention from scientists and healthcare professionals due to its potential as a therapeutic compound, useful for treating a range of diseases. Its properties, which do not include significant psychoactivity, open new possibilities for the safe use of cannabinoids in medicine. Research at the molecular and cellular levels suggests that CBND may be useful in treating various diseases, including neurodegenerative, autoimmune, inflammatory disorders, and some mental health conditions. Since CBND can bind to CB2 receptors, the exploration of its therapeutic potential is becoming increasingly important.
Neuroprotection and Treatment of Neurodegenerative Diseases
One of the most promising uses of CBND is neuroprotection, meaning the protection of nerve cells from damage that could lead to neurodegeneration. This is crucial for treating conditions such as Alzheimer’s disease, Parkinson’s disease, and other forms of dementia.
CBND’s neuroprotective action is attributed to its ability to reduce oxidative stress and lower inflammation levels in the brain, both of which are key factors in the development of these diseases. The anti-inflammatory activity of CBND may be linked to its ability to inhibit the activity of pro-inflammatory cytokines and molecules, such as TNF-α (tumor necrosis factor) and IL-6 (interleukin-6), which play an active role in the development of neuroinflammation.
According to some animal studies, CBND may also help reduce the accumulation of toxic proteins like beta-amyloid in Alzheimer’s disease, which helps preserve cognitive functions and reduce dementia symptoms.
Anti-inflammatory Properties and Treatment of Autoimmune Diseases
CBND can be used as a powerful anti-inflammatory agent in the treatment of autoimmune diseases such as rheumatoid arthritis, Crohn’s disease, psoriasis, and other conditions linked to chronic inflammation. Activation of CB2 receptors, to which CBND has an affinity, can reduce inflammatory processes without causing psychoactive effects, making it an attractive option for treating chronic inflammatory diseases.
Preclinical studies show that CBND can reduce the levels of inflammatory cytokines, such as IL-1β, IL-6, and decrease the activation of NF-kB (nuclear factor kappa-B), which plays a central role in the development of inflammation. This makes CBND promising for treating diseases where conventional anti-inflammatory drugs may have side effects.
Antioxidant Action and Potential for Treating Metabolic Disorders
CBND also has antioxidant activity, making it potentially useful in combating metabolic disorders associated with oxidative stress. For example, in the case of metabolic syndrome, type 2 diabetes, and obesity, CBND can help reduce free radical levels and mitigate oxidative damage to cells.
Oxidative stress and inflammation are key pathogenic factors in the development of insulin resistance and other metabolic disorders. Therefore, cannabinoids with antioxidant properties may become important components in treating these conditions. The anti-inflammatory and antioxidant properties of CBND could also be useful in treating cardiovascular diseases related to inflammation and oxidative stress.
Impact on Mental Health
CBND holds potential for treating mental health disorders such as anxiety and depression. Since CBND does not have psychoactive effects, unlike THC, its use does not lead to negative effects related to mental state disturbances, such as anxiety or paranoia.
Research indicates that CBND may interact with 5-HT1A serotonin receptors, which regulate mood and anxiety. This could help in treating depression and anxiety disorders without the undesirable psychoactive effects.
Current Research and Scientific Interest
Scientific interest in cannabidiorol (CBND) has grown over the last few years, particularly regarding its potential as a therapeutic compound in neuropharmacology, anti-inflammatory therapies, and as a marker for cannabis extract stability. Since cannabinoids are important biologically active components of plants in the Cannabaceae family, research concerning CBND is diverse, focusing not only on medical applications but also on technological aspects of cannabinoid storage and stability in products.
Current Research and Areas of Scientific Interest
CBND as a Marker for Extract Stability
One of the emerging topics is the use of CBND as a marker for the stability of cannabis extracts, particularly in the context of long-term storage. The issue of cannabinoid stability in extracts arises from their tendency to degrade due to temperature, light exposure, and oxidative and thermal influences. CBND, as a product of CBD (cannabidiol) oxidation, serves as an important indicator of these degradation processes.
Studies suggest that CBND can act as a marker for determining the shelf life and storage conditions of cannabis extracts. If a significant amount of CBND is found in an extract, this may indicate a decline in product quality, as CBD gradually transforms into CBND through thermal or oxidative reactions. This is crucial for the industrial production of cannabis oils and extracts, where it is essential to monitor the cannabinoid levels and stability over time.
Prospects in Neuropharmacology
One of the most intriguing areas of CBND research is its potential in neuropharmacology. Thanks to its neuroprotective properties, CBND is attracting attention from researchers in the context of neurodegenerative diseases such as Alzheimer’s disease, Parkinson’s disease, and other conditions associated with nervous system dysfunction. As some studies have shown, CBND can reduce neuroinflammation and oxidative stress, which are key factors in the development of these diseases.
An important aspect is its ability to lower neurotoxicity levels, which are commonly observed in neurodegenerative processes. Specifically, in animal experiments, CBND shows the potential to reduce the accumulation of toxic proteins like beta-amyloid in the brain, which is crucial for treating Alzheimer’s disease. Additionally, CBND may alleviate depression and anxiety symptoms, which is important for a comprehensive approach to treating mental health issues associated with neurodegeneration.
This potential of CBND in neuropharmacology remains a significant area of scientific interest, as it provides an opportunity to use it as a supportive therapy to ease symptoms and slow the progression of neurological diseases.
Knowledge Gaps and Need for Research Standardization
Despite significant progress in studying the properties of CBND, several knowledge gaps remain that require further research. For example, there are still no standard pharmacopoeial descriptions for cannabidiorol, which hinders its widespread use in clinical practice. The lack of clear protocols and standards for studying this compound is a significant barrier to its implementation in medical practice.
Most research in this area is based on animal studies or cell cultures, but clinical trials involving human patients are not sufficiently developed. This means that further scientific development requires more comprehensive and extensive clinical trials to evaluate the efficacy and safety of CBND for human use.
Moreover, to ensure the stability and accuracy of research results, it is necessary to create a standardized reference base for cannabidiorol and cannabis extracts. This will allow for greater reliability in comparing results across different research groups and ensure accurate determination of CBND concentrations in various extracts, which is essential for medical applications.
Conclusion
Cannabidiorol (CBND) is one of the key cannabinoids that has attracted significant attention from the scientific community due to its numerous pharmacological properties and potential applications in medical practice. Its formation, as a product of cannabidiol (CBD) oxidation, is an important aspect of research since CBND may serve as a marker for the stability of cannabis extracts and a critical element in studying the degradation mechanisms of cannabinoids during storage and processing.
The potential of CBND in neuropharmacology is the subject of intense research, especially in the context of neurodegenerative diseases such as Alzheimer’s disease, Parkinson’s disease, and others. Its neuroprotective, anti-inflammatory, and antioxidant properties make CBND a promising molecule for the treatment of such diseases. At the same time, research on this cannabinoid also highlights the limited availability of clinical data, which underscores the need for further exploration of its bioactivity and safety for patients.
Methods of extraction and synthesis of CBND are central to scientific research since the quality and stability of cannabinoids in pharmaceutical products directly depend on the selection of extraction methods and processing conditions for plant material. From chromatographic methods to cutting-edge approaches in biotechnological synthesis, which include microbial chassis and genetic engineering, there are growing prospects for creating new, effective methods of obtaining CBND for medical purposes.
However, significant gaps remain in existing knowledge about CBND. The absence of standard pharmacopoeial descriptions, precise protocols for its determination, and the lack of clinical trial data limit the application of this molecule in medical practice. Scientists call for the creation of a reference sample database and the standardization of research to improve results and ensure the high quality and efficacy of cannabinoids in pharmaceutical products.
Thus, cannabidiorol (CBND) is a promising cannabinoid with a wide range of pharmacological properties that could become an important part of therapy for neurodegenerative diseases and other conditions. However, to fully realize its potential, further research, the development of standards, clinical trials, and the implementation of new technologies for the extraction and synthesis of CBND are necessary.
Sources:
- PubMed (https://pubmed.ncbi.nlm.nih.gov/)
An official database for medical research and publications in the field of biomedicine. Thousands of articles related to cannabinoids, neuropharmacology, and their medical applications can be found here. - Google Scholar (https://scholar.google.com/)
A platform for searching scientific articles, monographs, dissertations, and other academic publications across a broad range of topics, including cannabinoids and pharmacological studies. - ScienceDirect (https://www.sciencedirect.com/)
One of the largest resources for accessing scientific articles, particularly in the fields of chemistry, pharmacology, and biotechnology. Articles about cannabinoids, their biogenesis, synthesis, and pharmacological properties can be found on this resource. - JSTOR (https://www.jstor.org/)
A database for accessing scientific articles across various disciplines. It is one of the oldest and most respected resources providing access to archives of scientific journals, including topics in pharmacology and biochemistry. - The National Center for Biotechnology Information (NCBI) (https://www.ncbi.nlm.nih.gov/)
A database that provides access to a large number of publications in molecular biology, pharmacology, and biotechnology. It is especially useful for searching articles on the biogenesis of cannabinoids and their interaction with the endocannabinoid system. - SpringerLink (https://link.springer.com/)
A platform for accessing scientific articles and books from numerous disciplines. Springer publishes a lot of research in chemistry, medicine, pharmacology, and biotechnology. - Wiley Online Library (https://onlinelibrary.wiley.com/)
Another important resource for finding scientific articles in the fields of chemistry, biomedicine, and pharmaceuticals. The website provides access to numerous journals that publish research on cannabinoids. - Scopus (https://www.scopus.com/)
A large database for scientific articles that allows users to find publications across a broad range of topics, including pharmaceutical research and biotechnology. Scopus provides access to high-quality, peer-reviewed articles and cited works. - The American Chemical Society (https://pubs.acs.org/)
Publications from the American Chemical Society include numerous articles dedicated to the chemistry of cannabinoids, methods of their synthesis, and their pharmacological properties. - Nature (https://www.nature.com/)
A well-known scientific journal that publishes articles across a wide range of disciplines, including biotechnology, chemistry, and medicine. Recent research on cannabinoids and cutting-edge biotechnological advancements can be found on this resource. - ClinicalTrials.gov (https://clinicaltrials.gov/)
This database contains information about clinical trials from around the world. Here, you can find details about trials involving cannabinoids, including CBND and other compounds, in the context of neuropharmacology and neurodegenerative diseases.