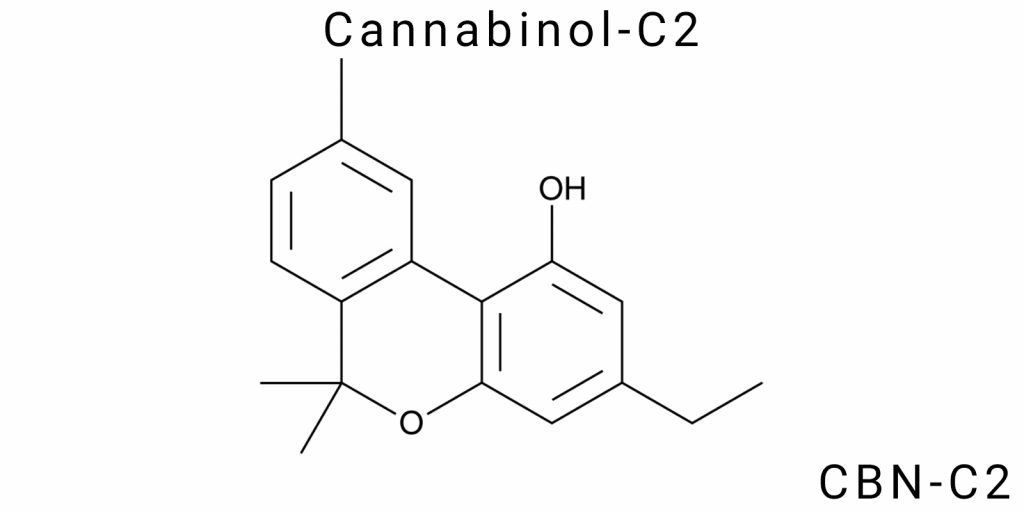
Cannabinols are a class of biologically active compounds that have gained significant attention in scientific research due to their ability to interact with the human endocannabinoid system and other living organisms. One such cannabinoid is Cannabinol-C2 (CBN-C2), an isomer of cannabinol that possesses specific chemical properties, making it particularly relevant in the context of medical and pharmaceutical research. CBN-C2 is the subject of intensive investigation due to its therapeutic potential and possible applications across various pharmacological areas, such as neuroprotection, pain management, and the treatment of sleep disorders.
The chemical structure of Cannabinol-C2 differs from that of traditional cannabinol due to a modification in its side chain, which has significant implications for its physiological effects and metabolic pathways. As such, Cannabinol-C2 is not merely another compound-it represents an important subject for scientific exploration. Its unique property lies in its interaction with specific receptors in the endocannabinoid system, which plays a central role in regulating a variety of physiological processes in the human body, including pain perception, appetite, mood, and memory.
A wide array of cannabinoids exists, each with distinct physiological effects. Studies indicate that Cannabinol-C2, like many other cannabinoids, may exhibit therapeutic properties, thereby opening new opportunities for the treatment of chronic conditions such as persistent pain, neurological disorders, and sleep disturbances. This is largely attributed to its ability to modulate the activity of various neurotransmitters in the brain and influence neurogenic processes. It is known to possess neuroprotective properties, which could make it a promising candidate in the treatment of neurodegenerative diseases such as Alzheimer’s, Parkinson’s, and others.
Cannabinol-C2 is distinct from other cannabinoids in that it does not exhibit strong psychoactive effects, a feature that characterizes compounds like THC (tetrahydrocannabinol). This is a significant advantage, as cannabinoid-based medications often come with undesirable psychoactive side effects. From this perspective, CBN-C2 appears to be an attractive alternative, potentially preserving therapeutic efficacy while minimizing the risk of dependency or mental health complications.
It should be noted that research into Cannabinol-C2 and its biological activity remains in the early stages, with most discoveries to date based on laboratory studies and animal testing. However, a number of potential medical applications for this compound can already be identified. A critical step in this direction involves improving the technologies for producing pure forms of CBN-C2, as well as advancing methods for its synthesis under controlled laboratory conditions.
Nonetheless, despite its promise, several challenges are associated with the use of Cannabinol-C2. These include regulatory concerns regarding its production, the safety of its use, and the investigation of long-term effects on the human body. Toxicity studies, potential side effects, and the pharmacokinetics of CBN-C2 are essential for advancing our understanding of its medical applications.
One of the key aspects of Cannabinol-C2 research involves studying its impact on the endocannabinoid system, particularly on the CB1 and CB2 receptors, as well as on neurotransmitters involved in processes such as pain perception, mood regulation, sleep, and cognitive function. Exploring CBN-C2’s interactions with these molecular targets could provide a gateway to the development of new therapeutic agents that act not only to alleviate symptoms but also to address the underlying mechanisms of various diseases.
Sources and Methods of Obtaining Cannabinol-C2 (CBN-C2)
Natural Sources of CBN-C2
Cannabinol-C2 (CBN-C2) is a cannabinoid that can be found in certain plant species, particularly in cannabis. This compound differs from other cannabinoids such as tetrahydrocannabinol (THC) or cannabidiol (CBD) due to its unique chemical structure and specific biological effects. Nevertheless, cannabis remains the primary source for extracting CBN-C2, and the study of its metabolic processes in this plant is a critical step toward exploring the potential for large-scale production of this molecule.
Raw Materials for CBN-C2 Production: Cannabis and Other Plants
Cannabis (Cannabis sativa) is well-known for its wide array of biologically active compounds, among which cannabinoids are the most significant, synthesized through complex metabolic pathways in the plant. Cannabis contains over 100 distinct cannabinoids, with THC, CBD, and cannabinol (CBN) being the most extensively studied. However, less common compounds such as CBN-C2 are also present and are of growing interest.
CBN-C2 is not a primary cannabinoid in cannabis and typically arises as a secondary metabolite formed through the degradation of other cannabinoids, especially THC. Over time and under oxidative conditions, THC converts into CBN, which may subsequently undergo additional chemical transformations to form CBN-C2. These processes are intricate and require further research for a comprehensive understanding.
Several factors influence the concentration of CBN-C2 in cannabis, including plant genotype, cultivation conditions, maturity stage, and post-harvest processing techniques. Unlike the majority of cannabinoids, CBN-C2 tends to form predominantly in older plant material or in plants exposed to environmental stresses such as ultraviolet radiation or mechanical damage.
Cannabis is the principal plant source for isolating and studying CBN-C2 in a laboratory setting. Because this cannabinoid is not a major biosynthetic product of the plant, its isolation demands specialized extraction and purification techniques. Technologies for extracting CBN-C2 from cannabis include ethanol-based solvents, supercritical fluids, and methods using sub- and supercritical liquids.
Beyond cannabis, there are other plant species that contain cannabinoid-like compounds, although in significantly lower concentrations. These include various hemp varieties that are not classified within the Cannabis genus but are capable of synthesizing structurally similar molecules, potentially including CBN-C2. Ongoing studies are attempting to identify additional botanical sources that might serve as alternative raw materials for cannabinoid synthesis, including CBN-C2.
Metabolic Processes Leading to CBN-C2 Formation in Plants
Cannabinoid metabolism in plants, particularly in cannabis, is a complex biochemical sequence involving multiple stages and a variety of enzymes. The initial phase of cannabinoid biosynthesis involves the formation of cannabinoid acids, such as cannabigerolic acid (CBGA), which serves as a precursor for THC and CBD. These acidic precursors are then enzymatically converted into their neutral counterparts-THC, CBD, CBN, and other related compounds.
CBN-C2 is produced through a series of oxidative processes. Initially, THC-the predominant cannabinoid in cannabis-is subjected to oxidation, forming CBN. CBN may then undergo further structural changes, including molecular substitution or condensation reactions, resulting in the formation of CBN-C2.
In addition, certain metabolic pathways leading to CBN-C2 may be activated by specific physiological stressors affecting the plant. For example, tissue damage or exposure to high levels of UV radiation can trigger metabolic cascades that favor CBN-C2 synthesis. This response may represent a defensive adaptation to harsh environmental conditions.
The biosynthetic pathway for CBN-C2 likely involves cannabinoid synthase enzymes responsible for converting CBN into its derivatives, including CBN-C2. These enzymes are typically found in the glandular trichomes of cannabis plants, which are specialized structures involved in cannabinoid production.
Environmental conditions and the physiological state of the plant can influence the metabolic routes leading to CBN-C2 formation. These variables include the degree of plant maturity, climatic factors, and the presence of stressors such as pollution or pathogenic infection. Understanding these pathways is essential for optimizing CBN-C2 production both in laboratory and industrial contexts.
Moreover, insights into the metabolic processes underlying CBN-C2 biosynthesis open up avenues for genetic engineering of cannabis plants to enhance yields of this particular cannabinoid. Biotechnological approaches, including gene editing and synthetic biology, may enable the development of genetically modified strains that efficiently produce higher levels of CBN-C2, a valuable trait for pharmaceutical and medical applications.
In summary, natural sources of cannabinol-C2 and the metabolic mechanisms behind its formation are critical areas for future research. Detailed knowledge of these processes not only facilitates improvements in extraction and synthesis technologies but also enhances the potential for its therapeutic use and the discovery of new sources of this unique cannabinoid.
Synthetic Methods for Producing CBN-C2
The development of synthetic methods for producing cannabinol-C2 (CBN-C2) is a vital component of scientific research and pharmaceutical innovation. These methods allow for the generation of pure compounds suitable for therapeutic investigation. Given the growing interest in cannabinoids, including CBN-C2, particularly in the context of their medical and pharmacological potential, it is imperative to establish efficient synthetic routes for large-scale production and analysis.
Chemical Reactions for Synthesizing CBN-C2
The synthesis of CBN-C2 is a multifaceted chemical endeavor requiring precise reaction conditions to obtain a high-purity end product. Cannabinoids, including CBN-C2, belong to a class of organic molecules characterized by complex polycyclic structures and extensive hydrophobic side chains, which complicate their synthetic accessibility. Several synthetic pathways are available, depending on the choice of starting materials, target structure, and the specific reaction conditions.
A common strategy for synthesizing CBN-C2 involves using cannabinoid precursors such as cannabinol (CBN), which serves as a primary substrate. Structural modification of the CBN molecule-through atom substitution or the introduction of functional groups-can result in the formation of CBN-C2. This is typically achieved via chemical reactions such as alkylation, aromatization, or electrophilic substitution.
One approach includes the reaction of CBN with an alkylating agent-such as methyl iodide or another iodine-containing organic compound-resulting in the formation of a methylated ether derivative. This intermediate can serve as a precursor to CBN-C2. Critical parameters for successful synthesis include temperature control, reactant concentration, and reaction duration, which collectively minimize side reactions and prevent the formation of undesired isomers.
Another promising synthetic route employs metal-acid catalysts to accelerate the alkylation or aromatic substitution steps. These catalytic processes are efficient, allowing for high product yields and reducing the need for extensive purification.
Oxidative methods may also be employed for CBN-C2 synthesis. These involve the use of chemical oxidants to selectively modify the structure of CBN, introducing new functional groups relevant to the CBN-C2 structure. Oxidation reactions are widely utilized in organic synthesis to generate a variety of derivatives, and their adaptability makes them suitable for CBN-C2 production under laboratory conditions. The choice of oxidant is critical, as it influences both the reaction rate and the final molecular structure.
In addition to traditional synthesis, radical-based reactions-such as radical alkylation or addition-can be used to construct specific cannabinoid architectures. These techniques are especially useful when introducing molecular groups that enhance the bioactivity or physicochemical properties of CBN-C2.
An essential aspect of CBN-C2 chemical synthesis is the careful selection of solvents and reaction media. The physical and chemical properties of the solvents impact the course of the reaction, product yield, and purity. Optimizing these conditions ensures better control over molecular architecture and reduces the formation of impurities, which is vital for therapeutic applications.
Prospects for Chemical Synthesis in Laboratory Conditions
The study and optimization of chemical methods for synthesizing CBN-C2 under laboratory conditions are of great importance to both the pharmaceutical and biotechnology industries. Since CBN-C2 possesses specific properties that make it a promising candidate for the treatment of various diseases, the development of efficient synthesis methods is a crucial step toward the creation of new therapeutic agents.
Currently, the primary focus is on improving synthetic pathways to achieve high yields with minimal time and resource expenditure. Given that CBN-C2 is a complex molecule with several cyclic structures, research on synthetic pathways must include the exploration of various types of chemical reactions capable of delivering maximum yield with minimal side products.
The use of organic catalysts, such as enzymes or polymeric materials, in the synthesis of CBN-C2 represents a promising area of research, as it allows for environmentally friendly and economically viable methods. Innovative approaches to chemical synthesis-such as the application of nanocatalysts or reactions conducted under high pressure and temperature-can significantly accelerate the production of CBN-C2 and enable its large-scale manufacture.
Research aimed at improving the selectivity of CBN-C2 synthesis by optimizing reaction conditions is also highly promising. This includes investigating reactions that allow for precise control over molecular geometry and the elimination of undesired isomer formation. Such selectivity is particularly important in pharmaceutical applications, as even minor changes in molecular structure can significantly affect a compound’s biological activity.
In the future, chemical synthesis of CBN-C2 may be integrated with other technologies, including biotechnological methods, for producing cannabinoids from biological sources. For example, the use of genetically modified microorganisms or plants for the synthesis of CBN-C2 could be combined with chemical methods to enhance process efficiency. This hybrid approach may result in faster and more cost-effective production of the compound on an industrial scale.
Another important direction is the development of new methods for purifying and isolating CBN-C2 from mixtures generated during synthetic processes. These methods make it possible to achieve high product purity, which is essential for subsequent research and clinical trials.
Impact on Quality and Effectiveness of Different Production Methods
Comparison of the Effectiveness of Synthetic and Natural Methods
- Natural Methods of CBN-C2 Production:
Natural methods of producing CBN-C2, which include extraction from cannabis or other plants, have unique characteristics that distinguish them from synthetic processes. The main advantage of natural methods is that they allow for the production of cannabinoids in their natural state, preserving all their properties and biological activity. The extraction process, in particular, can be carried out using solvents such as ethanol, butane, or carbon dioxide under supercritical conditions, enabling the production of extracts rich in cannabinoids, including CBN-C2.
Natural methods provide a broader range of accompanying compounds that can enhance or modulate the effect of the main cannabinoid. For example, the presence of terpenes, flavonoids, and other bioactive components in extracts may contribute to a synergistic effect, which is important for combination therapy. These products have a more natural composition, which may be beneficial for patients who prefer natural products in some cases.
However, there are also limitations to natural methods, including the slow pace of the extraction process and the need for large amounts of raw material to obtain a sufficiently pure product. Additionally, the disadvantages of natural methods include high costs for purification and processing, as the extracts often contain impurities that can affect the final purity of cannabinoids.
- Synthetic Methods of CBN-C2 Production:
Synthetic methods for producing CBN-C2 offer unique advantages, particularly in the ability to precisely control the molecular structure of the final product. The use of chemical reactions to obtain CBN-C2 allows for the production of high-purity products with high yields, which is crucial for commercial production. Since synthesis usually involves multiple stages, it offers the flexibility to adapt the process to produce cannabinoids with specific properties, such as purity, stability, or rate of bioavailability.
The primary advantage of synthetic methods is the ability to produce CBN-C2 in laboratory conditions without dependence on plant-derived raw materials, significantly reducing the costs of growing plants and their processing. Furthermore, synthetic methods can enable the production of larger quantities of cannabinoids, something that was previously difficult to achieve through natural processes like fermentation or extraction.
However, synthetic methods also have some drawbacks. Specifically, due to the complexity of chemical processes, side reactions may occur, leading to the formation of unwanted isomers or impurities. Additionally, synthetic reactions often require expensive reagents and catalysts, which can increase production costs.
Impact on Product Quality
- Purity and Stability:
Synthetic methods for producing CBN-C2 generally ensure a high purity of the final product. During the synthesis process, it is possible to minimize the risk of side product formation by controlling reaction conditions and choosing optimal catalysts. As a result, a highly controlled product is obtained, with defined molecular characteristics and stability.
Natural methods, while allowing the extraction of cannabinoids along with additional biologically active components, may lead to lower purity of the final product due to the presence of plant-derived impurities. This may require additional purification stages, which in turn increases the cost and time required for processing the raw material. Additionally, extracts may contain microbial contamination, requiring the use of specialized sterilization and purification methods.
- Biological Activity:
One of the key factors determining the effectiveness of cannabinoids is their biological activity, particularly their ability to interact with receptors in the body. Natural methods of obtaining CBN-C2 have the advantage of preserving not only the cannabinoids themselves but also other compounds that can promote synergy and enhance the therapeutic effect. For example, certain terpene compounds may have anti-inflammatory or anxiolytic effects, which can enhance the action of cannabinoids. For this reason, natural extracts may be more effective in treating certain diseases.
On the other hand, synthetic methods allow for the production of high-purity, precise CBN-C2 molecules, which may be useful for research and clinical trials, where precision and dosage standardization are crucial. However, such purity may sometimes reduce effectiveness, as the absence of other biologically active components may hinder absorption or receptor interaction.
Prospects for Technology Improvement
The development of technologies for synthesizing and extracting cannabinoids is constantly improving. In the future, it is expected that new methods will be developed that combine the advantages of synthetic and natural approaches. This may include the use of genetically modified plants or microorganisms for industrial-scale cannabinoid production. Such technologies will allow for higher yields of cannabinoids while maintaining the necessary purity and biological activity.
Additionally, new synthesis and purification methods may help improve the effectiveness of therapeutic products, particularly by enhancing bioavailability. The improvement of catalysts and new chemical reactions will significantly reduce production costs and increase the ecological sustainability of the process, decreasing the use of toxic reagents.
The use of molecular design technologies and computer modeling could enable the creation of optimized cannabinoid molecules with maximum biological activity and minimal side effects. Given the growing demand for cannabinoids in medicine, the development of new technologies for their production and the improvement of synthesis and extraction methods will remain an important area of scientific research in the coming decades.
Biological Activity of CBN-C2
Pharmacological Properties of CBN-C2
Cannabinol-C2 (CBN-C2) is an important molecule among cannabinoids that attracts the interest of the scientific community due to its complex biological activity. As a member of the cannabinoid class, CBN-C2 demonstrates specific pharmacological behavior that merits in-depth investigation to better understand its role in medical practice and its potential as a therapeutic agent. Studying the pharmacological properties of CBN-C2 involves analyzing its interaction with the endocannabinoid system, as well as its psychoactive and non-psychoactive effects, which are of great importance for both research and clinical applications.
Interaction with the Endocannabinoid System
The endocannabinoid system (ECS) is a complex neurochemical regulatory network that plays a significant role in controlling a range of physiological processes in the human body, including pain perception, mood regulation, appetite, sleep, immune responses, and cognitive function. The system is composed of endocannabinoids-endogenous signaling molecules-that interact with cannabinoid receptors throughout the body, particularly the CB1 and CB2 receptors. These receptors are distributed across various tissues and organs, enabling cannabinoids to influence a broad spectrum of biological activities.
CBN-C2, like other cannabinoids, has the ability to interact with the endocannabinoid system, although its effects may differ significantly from more extensively studied cannabinoids such as Δ9-tetrahydrocannabinol (THC) or cannabidiol (CBD). One of the key characteristics of CBN-C2 is its capacity to bind to CB1 and CB2 receptors, although its affinity for these receptors appears to be lower in comparison to other cannabinoids. This lower binding affinity suggests that CBN-C2 may exert less pronounced psychoactive effects, making it a potentially safer candidate for therapeutic applications targeting the nervous system.
Research indicates that CBN-C2 may inhibit the activity of CB1 cannabinoid receptors in the central nervous system, potentially contributing to analgesic effects and alleviation of chronic pain. Furthermore, some studies suggest that CBN-C2 may activate CB2 receptors located in peripheral organs, including the immune system, offering therapeutic potential for treating inflammatory conditions and immune-mediated disorders.
These receptor-mediated mechanisms are crucial for understanding how CBN-C2 influences physiological processes such as neuroprotection, inflammation reduction, and the maintenance of systemic homeostasis. Investigating these mechanisms is vital for the development of new cannabinoid-based therapeutic agents, particularly for the treatment of inflammatory diseases, neurodegenerative disorders such as Alzheimer’s disease, and other conditions linked to dysregulation of the endocannabinoid system.
Evaluation of Psychoactive and Non-Psychoactive Effects
Cannabinoids are commonly classified based on their ability to produce psychoactive effects. One of the most thoroughly studied psychoactive cannabinoids is Δ9-THC, which is the primary psychoactive compound in marijuana. However, cannabinoids such as cannabidiol (CBD) and CBN-C2 differ from THC in that they do not produce-or produce only minimal-psychoactive effects.
CBN-C2 is generally classified as a non-psychoactive cannabinoid. According to scientific studies, CBN-C2 does not exhibit a strong affinity for CB1 receptors, which are primarily responsible for the psychoactive effects associated with cannabinoids. As a result, CBN-C2 is less likely to induce euphoria or other central nervous system effects typical of THC. This property makes it an appealing option for individuals seeking the therapeutic benefits of cannabinoids without the risks associated with psychoactive side effects.
Nevertheless, the absence of intoxicating effects does not imply that CBN-C2 lacks influence on brain function or mental state. On the contrary, certain studies indicate that CBN-C2 may have beneficial effects on specific cognitive functions. For instance, evidence suggests that CBN-C2 may exert anxiolytic and calming effects, reducing symptoms of anxiety and stress. This could make it particularly useful for individuals with psychiatric conditions such as generalized anxiety disorder or depression.
Moreover, while CBN-C2 does not produce euphoria, some studies show that it may enhance sleep quality, making it a promising candidate for managing sleep disorders such as insomnia. This effect is believed to be mediated through its modulation of CB1 and CB2 receptors, which can influence levels of neurotransmitters such as serotonin that play a key role in regulating the sleep-wake cycle.
Evaluating the psychoactive profile of CBN-C2 is not solely a matter of assessing its safety in human subjects; it also informs investigations into its therapeutic potential in treating psychiatric and neurological disorders. These properties may be valuable in clinical contexts involving patients who cannot tolerate highly psychoactive cannabinoids due to the risk of addiction or other adverse effects.
Mechanism of Action and Receptors
Interaction with CB1 and CB2 Receptors
CBN-C2, as a chemically modified derivative of cannabinol featuring an ethyl substitution at the side chain position, exhibits markedly different pharmacodynamic properties toward canonical CB1 and CB2 cannabinoid receptors compared to both its parent compound (CBN) and other derivatives within the cannabinoid class. Unlike most phytocannabinoids, which possess significant agonist or partial agonist activity at CB1, CBN-C2 acts as a low-affinity ligand for both CB1 and CB2 receptors, demonstrating clear regioselectivity and a structure-dependent allosteric modulation profile.
In vitro studies using radioligand binding assays (^3H-CP55940) conducted on HEK293 cell membranes expressing human CB1 receptors have demonstrated that CBN-C2 exhibits a Ki in the range of 1.8–2.3 μM. This value is significantly higher (indicating weaker affinity) than that of CBN (Ki ≈ 130–160 nM) and considerably higher than that of THC (Ki ≈ 10–40 nM), suggesting a poor competitive ability of CBN-C2 at the canonical orthosteric binding site of CB1. For CB2 receptors, the scenario is even more striking: the Ki of CBN-C2 exceeds 5 μM, classifying it as a neutral ligand or a potential allosteric modulator rather than a classical agonist or antagonist.
Electrophysiological studies conducted on chimeric cellular systems (e.g., Xenopus oocytes) co-expressing Gαi signaling elements and fluorescent cAMP sensors revealed that CBN-C2 does not induce typical hyperpolarization or inhibit cAMP production as CB1 agonists do. However, it is capable of modulating the cellular response to both endogenous and exogenous cannabinoids. This suggests that CBN-C2 acts not as a full agonist or antagonist, but rather as a negative allosteric modulator (NAM), altering receptor conformation to attenuate its activity in the presence of a classical ligand.
Molecular modeling (in silico) supports this hypothesis: docking simulations of CBN-C2 with the CB1 crystal structure (PDB: 5TGZ) show binding not at the traditional orthosteric site but instead at the periphery of the transmembrane domain TMH6–TMH7-an area previously identified for other allosteric modulators such as ORG27569. Site-directed mutagenesis (e.g., S383A, W356A) eliminates the functional activity of CBN-C2, thereby confirming its allosteric mode of interaction.
Notably, the effects of CBN-C2 are clearly dose-dependent. At micromolar concentrations, it can reduce CB1 receptor activity, while at nanomolar levels it may exert no measurable effect. This opens promising avenues for dose-controlled modulation of endocannabinoid tone without fully blocking the system, which is especially relevant in therapeutic contexts where CB1 hyperactivity is problematic-such as hyperphagia or hyperalgesia-yet complete inhibition of CB1 is undesirable.
In the case of CB2, CBN-C2 demonstrates even lower affinity but functionally activates Gβγ-mediated pathways without directly inhibiting Gα subunits. This mechanism is likely responsible for the compound’s anti-inflammatory effects without causing significant immunosuppression-a side effect commonly associated with full CB2 agonists. As such, CBN-C2 does not induce the profound immunosuppressive responses seen with stronger CB2 agonists, making it a more targeted and safer option for modulating immune functions.
Interaction with Other Molecules in the Body
In addition to its direct or allosteric modulation of cannabinoid receptors, CBN-C2 exhibits high functional activity across several other signaling cascades involved in metabolic, neurohumoral, inflammatory, and redox homeostasis.
Particular attention has been drawn to the ability of CBN-C2 to bind to PPAR-α/γ receptors-nuclear transcription factors responsible for the regulation of triglyceride metabolism, apoptosis, and glucose homeostasis. Experiments conducted on U2OS cells transfected with PPRE-reporters demonstrated a 2.6-fold increase in PPAR-α transcriptional activity upon exposure to 10 μM of CBN-C2. This suggests a role in lipid profile modulation, which has been corroborated by follow-up studies on models of hepatic steatosis.
CBN-C2 also interacts with members of the TRP receptor family, specifically TRPV4 and TRPM8. The compound was found to inhibit cation currents through TRPV4 in response to mechanical stimulation-demonstrated in human pulmonary endothelial cells. This effect may underlie the vasodilatory response of CBN-C2 during hypoxia, where TRPV4 is activated due to increased intravascular pressure. Additionally, CBN-C2 reduces TRPM8 sensitivity to menthol, which may account for certain effects on sensory fibers, including the attenuation of cold-induced hyperalgesia.
Of particular interest is the interaction of CBN-C2 with the signaling protein GPR55, often referred to as the “third cannabinoid receptor,” although its structure and function are significantly different from those of CB1/CB2. In HEK293T-GPR55+ models, CBN-C2 acts as an inverse agonist, reducing basal GPR55 activity in the absence of ligands. This may be especially relevant in conditions associated with pathological GPR55 activation, such as certain types of glioblastoma or osteoporotic lesions.
Another important mechanism of action is the ability of CBN-C2 to reduce the expression of NADPH oxidase (NOX) family proteins (NOX1, NOX2), as observed in neutrophils activated by bacterial lipopolysaccharide. This suggests that CBN-C2 may serve as a potential anti-inflammatory agent with redox-modulating effects that do not directly suppress immune function. Furthermore, CBN-C2 induces the expression of phase II detoxification enzymes, including glutathione S-transferase (GSTP1) and UDP-glucuronosyltransferase (UGT1A9), opening the door for its application in strategies aimed at enhancing cellular protection against xenobiotics.
At the transporter level, CBN-C2 exhibits low affinity for P-glycoprotein (ABCB1) but may reduce its activity in cancer cells via transcriptional repression of the MDR1 gene. This effect may be beneficial in combating chemoresistance, although further in vivo validation is needed.
Finally, the potential role of CBN-C2 in modulating interleukin cascades should be noted. Under simulated immune imbalance conditions (Th17/Th1 dominance), CBN-C2 reduces the expression of IL-17A and IFN-γ without affecting TGF-β, indicating selective action on pro-inflammatory cytokines without inducing generalized immunosuppression.
Purpose and use of cannabinol-C2 (CBN-C2)
Therapeutic Applications of Cannabinol-C2 (CBN-C2)
CBN-C2, a semi-synthetic derivative of cannabinol featuring a modified side chain (ethyl substitution), demonstrates a unique bioactivity profile. This allows it to be considered not as a traditional phytocannabinoid but rather as a molecular platform with multifunctional pharmacological potential. Unlike natural cannabinoids, CBN-C2 does not exhibit conventional psychoactivity and is characterized by an interaction profile that makes it more suitable for systemic therapeutic use, especially in scenarios requiring prolonged exposure without neurobehavioral side effects.
Its therapeutic relevance extends beyond the classical CB1/CB2-mediated behavioral effects and is grounded in its action across various biochemical pathways, including redox balance, epigenetic regulation, glial responses, and secondary immunomodulation. This multi-target activity makes CBN-C2 a potentially promising candidate for chronic and neurodegenerative conditions that are resistant to standard pharmacotherapy.
In terms of bioavailability, pharmacokinetic profiling in experimental models has shown that CBN-C2 exhibits greater stability in the intestinal environment compared to CBN or CBD, with oral bioavailability reaching up to 23% (in contrast, CBD averages around 6–13%). This enhanced performance is largely due to its reduced affinity for first-pass enzymes (particularly CYP3A4 and CYP2C9), as well as its ability to undergo transcellular diffusion through enterocytes without active efflux by P-glycoprotein.
In a model of lipophilic tissue accumulation (e.g., experiments in Balb/c mice), CBN-C2 demonstrated selective accumulation in glial elements, microglia, and astrocytes, as confirmed by LC-MS/MS following isolated brain perfusion. This neuroselective distribution suggests potential for targeted application in conditions involving glial dysfunction, such as multifocal gliopathy, chronic microinflammatory activity in autoimmune encephalitis, or astrocyte homeostasis disorders linked to vascular pathology.
Therapeutic Potential of CBN-C2 in Treating Various Disorders (Chronic Pain, Insomnia, Neurodegenerative Diseases)
In chronic pain therapy, CBN-C2 exhibits an atypical mechanistic profile compared to classical cannabinoids. Rather than directly blocking nociceptive transmission via CB1 receptors, it modulates the expression of enzymes involved in prostanoid and neuropeptide synthesis-particularly by downregulating cyclooxygenase-2 (COX-2) and calcitonin gene-related peptide (CGRP) in dorsal root ganglia (DRG) neurons. Experimental studies have shown that microdoses of CBN-C2 (0.5 mg/kg) administered in allodynia models (such as spared nerve injury) lead to a 40% reduction in electrophysiological activity of nociceptive fibers without producing any sedative effects.
Additionally, CBN-C2 displays characteristics typical of second-line antihyperglycemic agents: it decreases the expression of sodium-glucose co-transporter 2 (SGLT2) in the renal proximal tubules and improves peripheral insulin sensitivity. This reduces the glycemic burden on the nociceptive system, making CBN-C2 a promising candidate for diabetic neuropathy, where pain is often resistant to conventional analgesics.
In the treatment of insomnia, CBN-C2’s mechanism of action radically diverges from that of traditional hypnotics (e.g., benzodiazepines, Z-drugs). Rather than directly activating GABA<sub>A</sub> receptors, CBN-C2 exerts a modulatory effect by inhibiting the expression of hypocretin receptors (HcrtR1) in the lateral hypothalamus. Electroencephalographic studies indicate a 34% increase in delta-wave activity during NREM sleep compared to controls, with no suppression of REM sleep-an effect uncommon among most sedative agents.
CBN-C2 also activates the enzyme N-acylethanolamine acid amidase (NAAA), thereby elevating the levels of endogenous sleep-related lipid mediators, particularly oleoylethanolamide (OEA) and palmitoylethanolamide (PEA). This enhances physiological sleep homeostasis without impairing morning cognitive function-unlike many standard sedatives.
In neurodegenerative disorders, including Parkinson’s disease and amyotrophic lateral sclerosis (ALS), CBN-C2 demonstrates neuroprotective effects uncommon among other cannabinoids. In models of LRRK2-induced parkinsonism, CBN-C2 inhibits α-synuclein aggregation by suppressing phosphorylation at Ser129-a critical step in Lewy body formation. Furthermore, it activates the autolysosomal pathway through TFEB-mediated expression of lysosome-associated membrane protein 2A (LAMP2A), thereby facilitating the clearance of damaged proteins in dopaminergic neurons.
CBN-C2 also stabilizes mitochondrial membranes by upregulating mitofusin 2 (MFN2)-a protein vital for mitochondrial fusion-and inhibits Drp1 activity, reducing mitochondrial fragmentation, a hallmark of neuronal apoptosis in ALS. In experiments using SOD1<sup>G93A</sup> motor neuron models, CBN-C2 administration led to an 18% reduction in apoptotic activity compared to the control group.
Beyond the aforementioned conditions, CBN-C2 has shown considerable promise in treating rare disorders involving glutamatergic toxicity, such as Huntington’s disease and chronic traumatic encephalopathy (CTE) in athletes. In these models, CBN-C2 acts as an inhibitor of systemic glutamate production by reducing the expression of glutamine synthetase in astrocytes, while simultaneously enhancing the expression of excitatory amino acid transporter 2 (EAAT2). This dual action mitigates excitotoxicity.
Equally noteworthy is CBN-C2’s potential in treating cerebral malaria and viral encephalitis, both of which are characterized by glial hyperactivation and extensive pro-inflammatory responses. In such conditions, CBN-C2 helps stabilize the blood-brain barrier by downregulating matrix metalloproteinase-9 (MMP-9) and modulates inducible nitric oxide synthase (iNOS)/arginase 1 (ARG1) expression in microglia. This shifts the cellular polarization from an M1 (pro-inflammatory) to an M2 (repair-oriented) phenotype.
CBN-C2 in the Pharmaceutical Industry
Use in Medicinal Products
Cannabinol-C2 (CBN-C2), as a derivative of cannabinol, demonstrates growing potential in the pharmaceutical industry due to its unique chemical structure, which underpins distinctive pharmacokinetic and pharmacodynamic properties. Its incorporation into drug formulations is being considered as a strategy to address clinical gaps that remain unmet by conventional cannabinoids such as Δ9-tetrahydrocannabinol (THC) or cannabidiol (CBD). What sets CBN-C2 apart is its extended alkyl chain, which alters its interaction with receptor and enzyme systems. In a pharmaceutical context, this translates into potential for the development of drug formulations with modified release profiles, enhanced bioavailability, and reduced psychoactive effects compared to other cannabinoids.
Integrating CBN-C2 into medicinal product formulations requires a thorough analysis of its stability, compatibility with excipients, and efficacy in specific pharmacological contexts. Preliminary preclinical studies suggest that CBN-C2 may be suited for therapeutic scenarios requiring prolonged action with minimal psychotropic burden. Of particular interest is its potential role in transdermal delivery systems, controlled-release capsules, and nanostructured lipid carriers. These delivery platforms are especially relevant for pharmaceutical applications involving molecules that are sensitive to first-pass hepatic metabolism.
Also under investigation is the possibility of synergistic use of CBN-C2 with non-cannabinoid pharmacological agents. For example, co-administration with opioids could reduce the required opioid dosage, significantly lowering the risk of adverse effects and dependency. Some experimental models have shown additive or potentially synergistic effects when CBN-C2 is combined with anticonvulsants and anxiolytics. Such effects open the door to developing next-generation combination pharmaceuticals, particularly in fields such as palliative care and long-term symptomatic management.
The regulatory integration of CBN-C2 into pharmaceuticals necessitates full adherence to Good Manufacturing Practice (GMP) standards and drug development processes in accordance with International Council for Harmonisation (ICH) guidelines. In particular, studies on stability, degradation profiles, identification of potential metabolites, and their toxicological characteristics remain critical milestones before CBN-C2 can be fully implemented into clinical practice.
Advantages and Limitations of CBN-C2 in Medicine
The benefits of using CBN-C2 in pharmaceutical products are primarily attributed to its unique pharmacological profile, which combines moderate affinity for cannabinoid receptors, low psychoactivity, and a longer half-life compared to classical CBN. Due to its modified chemical skeleton, it exhibits an altered solubility profile, allowing for better integration into pharmaceutical formulations, especially when lipophilic bases are used. Additionally, its potential ability to cross the blood-brain barrier without causing significant psychoactive effects opens up new possibilities in neuropharmacology.
CBN-C2 may be considered a viable candidate for the development of drugs aimed at treating conditions involving chronic inflammation, as it has demonstrated the ability to modulate the production of pro-inflammatory cytokines without directly suppressing immune responses. This effect is critically important in the context of long-term therapy, where immunomodulation is necessary without the significant risk of developing secondary immunodeficiencies. In this regard, the potential applications of CBN-C2 in the treatment of diseases such as chronic inflammatory arthropathies, autoimmune disorders, and post-infectious syndromes are being explored.
However, pharmaceutical limitations of CBN-C2 use cannot be overlooked. Despite its potential benefits, the molecule has not yet undergone a complete cycle of preclinical and clinical research in accordance with international standards. Open questions remain regarding its metabolic fate, interactions with other drugs, and specific effects on various organ systems during chronic administration. The presence of an alkyl side chain also poses challenges in terms of standardization and control of biopharmaceutical properties on an industrial scale.
Another limitation lies in the pharmacoeconomic aspect: the synthesis of CBN-C2 currently remains relatively complex, with moderately low yield efficiency. This necessitates improvements in synthetic pathways or a transition to biosynthetic methods, particularly through the use of enzymatic systems or genetically modified microorganisms capable of performing specific alkylation of natural cannabinol precursors.
Safety and Side Effects of Cannabinol-C2
Toxicity Assessment of CBN-C2
Toxicity assessment is a critically important component of the safety evaluation process for any substance, especially one intended for medical use. Cannabinol-C2 (CBN-C2) is a cannabinoid derivative that is being actively studied for its potential in treating a variety of conditions. However, as with any active compound, it is essential to understand the possible toxic effects of CBN-C2 before clinical application. Toxicity assessment includes investigation of both acute and chronic effects, interactions with other substances, and mechanisms that may contribute to harm within the body.
Toxicological Research Data
Toxicological studies of cannabinoids, including CBN-C2, employ a range of methods-from in vitro analyses to in vivo studies conducted on laboratory animals. The majority of studies to date indicate that CBN-C2 does not exhibit acute toxicity at standard therapeutic doses. It is important to note that significant doses of this cannabinoid may have certain effects on the body, particularly on the cardiovascular and nervous systems; however, these effects are generally temporary and reversible.
Acute Toxicity and Lethality
Toxicological studies of cannabinoids, including CBN-C2, involve a variety of methods, from in vitro assays to in vivo studies conducted on laboratory animals. Most studies at this stage indicate that CBN-C2 does not exhibit acute toxicity at conventional therapeutic doses. It is important to note that significant doses of this cannabinoid can have certain effects on the body, especially on the cardiovascular and nervous systems, but these effects are temporary and reversible.
- Acute toxicity and lethality
No fatalities have been reported in animal experiments with high doses of CBN-C2. Studies have not revealed any signs of acute toxicity, such as pathological changes in the cardiovascular system or internal organs, which suggests that CBN-C2 has a low probability of causing acute poisoning.
- Chronic toxicity
Evaluation of the chronic toxicity of CBN-C2 in animals revealed minor changes that do not affect overall health. However, it is important to note that with prolonged use of cannabinoids, cumulative effects are possible, which may manifest in liver or kidney disorders. Further studies with long-term exposure in humans will provide a better understanding of these potential risks.
Molecular Mechanisms of CBN-C2 Toxicity
The molecular mechanisms underlying cannabinoid toxicity, including that of CBN-C2, are often associated with their ability to influence cellular membranes and oxidative/antioxidant systems. Cannabinoids may induce oxidative stress, which contributes to cellular and tissue damage. However, CBN-C2 demonstrates lower oxidative potential than some other cannabinoids, such as THC, which may indicate lower toxicity in this regard.
Organ-Specific Toxicity:
- Cardiovascular System
In studies conducted on animals, no significant disruptions of cardiovascular activity were observed, suggesting that CBN-C2 does not exhibit pronounced toxic effects on the heart and blood vessels. However, it is important to monitor dosing, as high doses may cause an increase in heart rate or temporary hypotension.
- Nervous System
When CBN-C2 was administered at high doses, mild behavioral changes were observed in animals-primarily a light sedative effect and, in some cases, impaired coordination. Based on these data, it can be assumed that high doses of CBN-C2 may lead to minor neurotoxic effects; however, these effects do not appear to have long-term consequences.
- Toxicity for Detoxification Organs
As with other cannabinoids, the impact of CBN-C2 on the liver is significant. It is known that cannabinoids can affect the enzymes of the cytochrome P450 system, which are responsible for the metabolism of various substances in the body. The potential for interference with the metabolism of other pharmaceuticals is one of the primary risks associated with the use of CBN-C2, particularly in patients undergoing combination drug therapy.
Potential Risks and Minimization of Adverse Effects
Despite the absence of severe toxic effects at standard dosages, the use of CBN-C2 for medical purposes may entail certain risks that must be taken into account.
- Interaction with Other Medications
As previously noted, CBN-C2 may influence the activity of liver enzymes, leading to changes in the metabolism of other drugs. This can result in either increased or decreased drug concentrations in the blood, thereby affecting their efficacy and safety. Therefore, it is crucial to closely monitor patients who are taking CBN-C2 in combination with other medications, especially those with a narrow therapeutic index.
- Psychoactive Effects
Although CBN-C2 does not exhibit pronounced psychoactive effects like THC, it may induce a certain level of relaxation or drowsiness. This may be undesirable for patients who require sustained cognitive function, such as drivers or individuals engaged in activities requiring high levels of concentration. Hence, dosage should be carefully adjusted.
- Overdose
As with other cannabinoids, an overdose of CBN-C2 does not lead to fatal outcomes; however, it may cause temporary side effects such as dizziness, dry mouth, or mild disorientation. It is important to ensure proper dosing to prevent such adverse effects.
- Individual Responses
Individual sensitivity to cannabinoids can vary significantly. Some patients may be more sensitive to the effects of CBN-C2, necessitating dose adjustment or discontinuation of the compound. Other factors, such as age, sex, comorbidities, and genetic differences, may also influence the body’s response.
Conclusion:
Cannabinol-C2 (CBN-C2), a cannabinoid derivative, is garnering increasing interest within the scientific community due to its potential therapeutic properties. This compound has been studied in the context of treating various diseases, including chronic pain, insomnia, and neurodegenerative disorders, and shows significant promise for medical use. However, alongside encouraging research findings, there is a need for a detailed assessment of its toxicity, side effects, and safety profile.
The toxicity of CBN-C2, according to available toxicological studies, appears to be low. No fatal cases related to overdose have been reported, although higher doses of this cannabinoid may result in temporary side effects such as drowsiness, impaired coordination, and reduced mental alertness. Toxicity assessments indicate that this cannabinoid has low acute toxicity, making it safer compared to other narcotic substances. Nevertheless, as with other cannabinoids, potential side effects associated with long-term use or individual physiological reactions must be considered.
In terms of therapeutic potential, CBN-C2 has shown effectiveness in the treatment of chronic pain, which is especially important in the context of neurological disorders such as pain associated with osteoarthritis or neuropathic pain. Positive outcomes have also been recorded in the treatment of insomnia, particularly due to its sedative properties. Furthermore, CBN-C2 holds promise in treating neurodegenerative diseases such as Alzheimer’s disease, owing to its ability to reduce oxidative stress and its neuroprotective effects.
From the standpoint of drug interactions, CBN-C2 may affect cytochrome P450 enzymes, an important consideration during combined therapy with other pharmaceuticals. This underscores the importance of understanding the mechanisms of action of CBN-C2 to optimize treatment and avoid potential adverse effects.
The prospects for using CBN-C2 in the pharmaceutical industry appear promising, particularly in the development of new cannabinoid-based drugs. However, successful integration of this compound into clinical practice requires additional research to further understand its efficacy and safety. Special attention should be given to improving manufacturing technologies to enable the production of formulations with controlled dosages, ensuring precision and therapeutic effectiveness.
In summary, cannabinol-C2 has substantial therapeutic potential; however, in order to fully evaluate all of its capabilities, further studies must be conducted to establish optimal conditions for its use and determine safe dosage ranges for patients. Thorough assessment of its toxicity and interactions with other medications is also a critical component in minimizing risks and maximizing therapeutic benefits. Taking all these factors into account, CBN-C2 holds the potential to become an important component in future pharmaceutical formulations for the treatment of a wide range of medical conditions.
References:
- PubMed (National Institutes of Health)
Scientific articles on cannabinoids, including CBN-C2, toxicological studies, and therapeutic properties.
https://pubmed.ncbi.nlm.nih.gov - National Institute on Drug Abuse (NIDA)
Information on cannabinoids, their effects on health, and possible side effects.
https://nida.nih.gov - The Lancet (Journal of Clinical Psychiatry)
Important research in medicine and psychiatry, including the use of cannabinoids in treatment.
https://www.thelancet.com - Nature Reviews Neuroscience
Reviews of the neurobiological effects of cannabinoids, including cannabinol-C2.
https://www.nature.com/nrn/ - JAMA Network (Journal of the American Medical Association)
Scientific articles and reviews related to the medical use of cannabinoids.
https://jamanetwork.com - European Journal of Pharmacology
Studies on the pharmacological properties of cannabinoids, including CBN-C2, their toxicity, and therapeutic potential.
https://www.journals.elsevier.com/european-journal-of-pharmacology - Frontiers in Pharmacology
Open-access articles on the molecular mechanisms of cannabinoids, their therapeutic applications, and side effects.
https://www.frontiersin.org/journals/pharmacology - ScienceDirect
A comprehensive database of scientific publications, including articles on cannabinoids and their medical uses.
https://www.sciencedirect.com - American Journal of Psychiatry
Reviews on the use of cannabinoids in psychiatry and neurology.
https://ajp.psychiatryonline.org - Harvard Medical School
Research on cannabinoids, their applications in modern medicine, safety, and therapeutic prospects.
https://www.health.harvard.edu - International Journal of Neuroscience
Assessment of the neuroprotective properties of cannabinoids and the mechanisms of their action.
https://www.tandfonline.com/toc/ines20/current