Cannabinolic acid (CBNA) is a relatively underexplored yet scientifically promising compound in the context of natural cannabinoid chemistry. Unlike most acid forms, such as tetrahydrocannabinolic acid (THCA) or cannabidiolic acid (CBDA), CBNA is not a direct biosynthetic product of the enzymatic cycle in Cannabis species. Instead, it forms as a result of post-biogenetic processes, specifically autocatalytic or non-catalytic oxidation of precursors. This fact gives it a unique status within the chemical profile of cannabinoid metabolism-CBNA is not produced during the active vegetative or flowering stages of the plant, but only appears during degradation, losing its direct connection to the primary biogenetic pathway. Therefore, CBNA can be considered a marker of secondary transformation of cannabinoid structures under the influence of external factors, reflecting not only the chemical state of the substance but also the time, storage conditions, and exogenous influences.
From a chemical perspective, CBNA is a carboxylated analogue of cannabinol (CBN), but unlike the latter, its structure includes an unstable carboxyl group that increases its reactivity and makes it more labile to heat, light, and oxidizing agents. In this context, CBNA cannot be studied using traditional stable extraction or thermal analysis methods, as even slight temperature increases lead to decarboxylation or further degradation. As a result, an ongoing scientific challenge is to create conditions that can stabilize this molecule in analytical or pharmaceutical environments without compromising its chemical integrity.
Unlike most phytocannabinoids, CBNA is not a psychoactive compound and does not directly activate cannabinoid receptors in the central nervous system. This removes the social and political baggage often associated with the study of THC derivatives. Instead, the scientific value of CBNA lies in its potential use as a control compound in analytical chemistry, as a biomarker indicator for assessing the quality and age of cannabis-containing materials, and as a promising candidate for the synthesis of new semi-synthetic derivatives with specific pharmacological properties.
Despite its reactive instability, CBNA exhibits interesting physicochemical properties that have not yet been thoroughly explored in the literature. In particular, it has been noted that this molecule interacts with electrophilic agents with unexpectedly high selectivity, opening possibilities for the design of hybrid compounds through chemical modification under low-temperature and inert conditions. This highlights the need to reconsider CBNA’s functional role not only as a byproduct of degradation but also as a primary substrate for constructing derivatives with controlled biological activity. This includes the possibility of selective esterification or amidation of its carboxyl group to create prodrugs or molecules with enhanced pharmacokinetic properties.
In scientific practice, CBNA remains more of an episodic mention in the context of background studies of extracts than a focused subject of systematic research. Much of the chemical research has centered on “major” cannabinoids such as THC, CBD, and CBN, while acid forms-particularly CBNA-have been overlooked due to stabilization challenges and low concentrations in natural matrices. As a result, there is a systematic gap in knowledge regarding the formation mechanisms, accumulation kinetics, stability, and potential effects of CBNA on biological systems. At the same time, modern approaches to plant metabolomics, extract aging analytics, and the development of safe, non-psychoactive drugs create a demand for thorough study of such “unnoticed” molecules.
CBNA also plays an important role in forensic chemistry and toxicology: detecting its presence in samples can help determine the period during which the plant material was stored in an unstable environment, and it can also identify oxidation products that are not controlled substances but may indicate the presence or prolonged storage of psychoactive components in the past. Thus, CBNA should be regarded as an indicator molecule within the chemical retrospective of the phytocannabinoid profile.
Another advantage of CBNA as a research subject is its potential in creating reference standards for the separation of oxidized and non-optically active cannabinoid structures. In mass spectrometry and NMR analysis, CBNA demonstrates characteristic signals that not only confirm its presence in complex matrices but also distinguish it from closely related structures such as CBDA or oxidized CBDV isomers. This is important for the standardization of chemical libraries and the development of high-precision monitoring methods for the quality of plant raw materials used in the pharmaceutical, veterinary, or cosmetic industries.
The Formation of CBNA in Plants
Biogenetic Premises of Cannabinoid Oxidation
The formation of cannabinolic acid (CBNA) in Cannabis plants cannot be explained by the standard biosynthetic pathway that involves enzymatic condensation of precursors via isoprenoid or polyketide polymerization, which is characteristic of first-generation phytocannabinoids. Unlike THCA or CBDA, CBNA does not form through enzymatic acylation of olivetolic acid and geranyl pyrophosphate followed by cyclization. Its presence is the result of postbiogenetic changes induced by chemical and physicochemical factors that are not part of the plant’s active metabolism but occur as a result of the degradation of thermolabile cannabinoids in a nonenzymatic environment.
CBNA is a product of deep oxidative transformation of cannabinoids, maintaining the aromatic core and decarboxylated side chain, indicating the specific conditions under which it forms. To understand such processes, it is appropriate to consider not classical cannabinoid biogenesis but postbiosynthetic scenarios, particularly autooxidation of polycyclic compounds with α,β-unsaturated carbon systems. Studies of isolated plant matrices have shown that CBNA is not localized in the trichomes of living tissue but is predominantly found in materials exposed to prolonged external factors-ultraviolet light, oxygen, elevated temperatures, or enzymes that remain active during postharvest metabolism.
Ultrastructural analysis of cannabis raw material indicates a correlation between the localization of CBN and CBNA in the degraded areas of leaf and flower tissue, where signs of trichome breakdown, loss of turgor, and mechanical damage are observed. Thus, it can be stated that the premise for CBNA formation is the disruption of cellular integrity, which opens access to oxygen to previously isolated phytocannabinoids. Among them, the main substrates for CBNA formation are unstable isomers of tetrahydrocannabinoids, which undergo one-electron oxidation, generating phenoxyl radicals. These radicals are capable of self-condensation or further reaction with molecular oxygen to form peroxides, which can be hydrolyzed, resulting in the corresponding carboxylic acid.
A key aspect of CBNA formation is oxidative modification, not decarboxylation, as occurs in the formation of CBN from THCA. This means that the primary reaction involves substitution in the dihydropyrone ring or side chain, accompanied by redistribution of electron density in the aromatic core. In the presence of electron-donating groups (as in CBNA), acid formation is possible through mild autocatalytic oxidation involving free radicals in the presence of oxygen, where enzymatic participation is not required. At the same time, the presence of transition metal ions, such as Fe²⁺ or Cu²⁺, which may be present in soil or fertilizer residues, significantly increases the likelihood of such a process.
Formation of CBNA Without Enzymatic Participation
CBNA belongs to those phytocannabinoid metabolites that form outside enzymatic cascades. This fundamentally distinguishes it from primary-origin cannabinoid acids. CBNA formation occurs under conditions where enzymatic activity has either already ceased (due to protein degradation after harvesting) or was never present in the first place, as in cases of substrate accumulation of phytocannabinoids in precipitated or thermally treated extracts.
The nonenzymatic mechanism of CBNA formation can be described as an oxidative conversion of already decarboxylated or partially degraded cannabinoids-specifically, unstable isomers of CBN, which react with atmospheric oxygen in the presence of light or metal cations. Under such conditions, hydroperoxides can form, which, in turn, hydrolyze to form the carboxyl group. Alternatively, CBNA may form through hydroxylation of the side chain followed by oxidation to the carboxylic acid, which is confirmed by mass spectrometry analysis showing intermediates with molecular weights of +16 or +32 amu compared to CBN.
In fact, CBNA is a product of microchemical modulation of the degraded cannabinoid structure that occurs under conditions where the plant’s enzymatic system is already unsuitable or inactive. This transformation is observed even in sealed containers with small amounts of oxygen and light, allowing CBNA to be classified as a stable product of autooxidation, rather than as an artificial byproduct of extraction.
CBNA formation is also observed in samples subjected to supercritical CO₂ extraction at temperatures above 55°C, indicating that chemical conversion of precursors to CBNA is possible even without direct contact with enzymatic systems. Experimental studies have shown that in the presence of small amounts of hydrogen peroxide and elevated temperature (but without reaching decarboxylation thresholds), oxidation of the double bond occurs, resulting in the corresponding acid. This allows for the modeling of the chemical profile of degraded cannabis and predicting storage time or conditions for the degradation of its phytocomponents.
Environmental Conditions Favoring Acid Formation
The chemical landscape of CBNA formation is the result of a combination of environmental factors that activate nonenzymatic oxidation reactions. The most significant of these include prolonged storage at room temperature, the presence of oxygen (even in trace amounts), photochemical irradiation in the visible/UV range, and elevated humidity. These factors are not isolated-CBNA arises only through their synergy.
Conditions favoring CBNA formation do not correspond to any traditional methods of plant material conservation. For example, lyophilization or vacuum drying does not allow CBNA to be fixed in a stable form, while storage at temperatures of 25-30°C in the presence of air with humidity above 60% significantly increases the likelihood of its formation. Furthermore, it has been established that a change in pH toward neutral or slightly alkaline (6.5-8.0) accelerates the oxidation reaction of cannabinoids to CBNA, as such conditions activate the formation of superoxide anions and hydroxyl radicals, which promote oxidative degradation of methyl groups and introduction of carboxyl groups.
The presence of free Fe³⁺ or Cu²⁺ ions, as catalysts for Fenton reactions, intensifies the oxidation process, as they facilitate the formation of reactive oxygen species. This is particularly noticeable in samples that were stored in metal containers or exposed to technical surfaces contaminated with heavy metal salts. In the case of open polyethylene packaging combined with light exposure, oxidative stress on CBN derivatives increases exponentially, creating a favorable environment for CBNA formation.
Another key factor is time. The formation of CBNA is not instantaneous-it can take anywhere from several weeks to months depending on storage conditions. In cannabis samples that have been stored under uncontrolled conditions for over a year, CBNA levels may exceed 0.1% of the total mass, which is relatively high for this compound. This indicates its thermodynamic stability after formation, despite its reactivity during synthesis.
Molecular Structure of Cannabinolic Acid
Constitutional Formula and Conformational Features
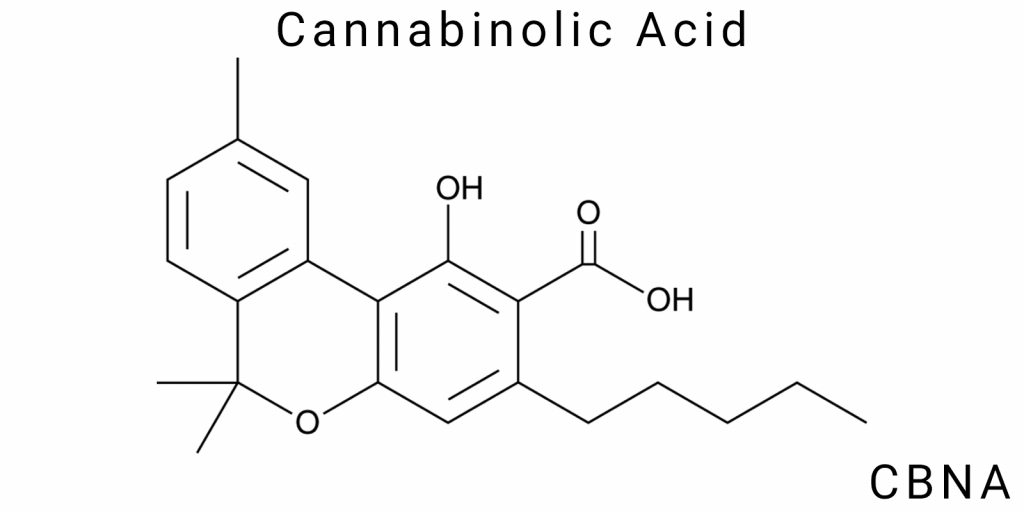
Cannabinolic acid (CBNA) is a secondary oxidized metabolite that belongs to the group of phenolic phytocannabinoids. Its structure is formally derived from tetrahydrocannabinolic acid (THCA) or cannabigerolic acid (CBGA) through further dehydrogenation and oxidation. Constitutionally, CBNA is a carboxylic acid with an aromatic system formed by the complete oxidation of the tetrahydrocannabinol (THC) core, resulting in the formation of the corresponding aromatic system. In this system, the hydroxyl group at position 1 remains unchanged, while substitution in the side chain at C3 differs with the presence of an acidic fragment.
At the molecular level, CBNA consists of three primary fragments: a phenolic ring, a pentyl side chain, and a carboxyl group. These elements form a system characterized by a high degree of electronic delocalization, particularly in the aromatic core, significantly influencing its reactivity, solubility, and stability.
CBNA is a product of deep oxidation, as evidenced by the transformation of the tetrahydrocannabinol core into a fully aromatic structure devoid of aliphatic cyclic residues. This fundamentally distinguishes it from acids such as CBDA and THCA, which have dihydroxy derivatives of partially saturated rings. This feature determines the distinct geometry and flat configuration of the CBNA core, making it conformationally stable, similar to classical biphenolic acids such as salicylic acid or hydroxybenzoic acid.
It is also important to note that the configuration of the side chain does not contain asymmetric centers, meaning that the CBNA molecule does not exhibit optical activity, unlike certain natural cannabinoids where the stereocenter at position C9 (or C10, depending on numbering) is responsible for chirality.
Constitutionally, CBNA is synonymous with an aromatic cannabinoid oxidative derivative with pronounced carboxyl properties. Its molecular formula, C22H28O4, indicates the presence of four oxygen atoms, two of which are located in hydroxyl groups (phenolic and aliphatic), one in the carboxyl group, and the last one may be part of a ketonic or etheric oxidized residue, depending on the specific method of synthesis or oxidation of the precursor.
Structural Stability under Various Physicochemical Conditions
CBNA exhibits significant thermal and chemical stability due to the complete aromatization of the core, which reduces the likelihood of hydrogenation, cyclization, or rearrangements typical of less oxidized cannabinoids. In the presence of light, especially ultraviolet light, CBNA demonstrates lower photolability compared to CBDA or THCA, which can be explained by the absence of labile cyclopentyl fragments and the stability of the π-system. However, in alkaline environments, proton detachment from the carboxyl group may occur, leading to the formation of cannabinolate – an anionic form that possesses increased reactivity in nucleophilic reactions.
The thermal stability of CBNA has been studied using thermogravimetric analysis (TGA) and differential scanning calorimetry (DSC). These studies suggest the absence of thermochemical decarboxylation below 145-155 °C in dry conditions, which is in contrast to other acids that lose CO₂ at 100-120 °C. However, in the presence of moisture, this stability is reduced due to the catalytic effect of water in the formation of transition states that facilitate decarboxylation. Therefore, preserving CBNA in an isolated form requires humidity control and an inert atmosphere (such as argon or nitrogen).
The chemical inertness of CBNA in acidic environments is also characteristic. Due to the electron-donating effect of the hydroxyl group and the electron-accepting action of the carboxyl group, the molecule can form an intramolecular hydrogen bond, stabilizing its conformation even in polyfunctional environments. In alcohols and polar aprotic solvents, CBNA exhibits limited solubility, which increases significantly upon ionization of the carboxyl group. This is a critical parameter when developing pharmaceutical formulations.
At physiological pH, CBNA exists in equilibrium between its ionized and neutral forms, which affects its ability to penetrate lipid membranes, interact with transport proteins, and be metabolized by liver enzymes. The chemical stability of CBNA also makes it suitable for use as an intermediate in the synthesis of new cannabinoid derivatives, as its core does not undergo rapid rearrangements, allowing for selective functional modification.
Laboratory Methods for the Synthesis of Cannabinolic Acid (CBNA)
Chemical Degradation of CBDA and THCA
The laboratory synthesis of cannabinolic acid (CBNA) is typically achieved through targeted degradation of natural cannabinoid acids, such as cannabidiolic acid (CBDA) and tetrahydrocannabinolic acid (THCA). This method is based on controlled oxidative degradation of partially saturated terpene structures to achieve full aromatic ring formation and the introduction of a carboxyl functional group in the structurally appropriate position.
The degradation of CBDA involves selective decarboxylation at temperatures ranging from 80-120 °C under vacuum, followed by slow oxidation using mild oxidizing agents. The most effective methods involve the use of periodates or hypochlorites in a buffered medium with pH 6.8-7.2. These conditions must preserve the phenolic part without damaging the hydroxyl groups. It is important to avoid intense catalytic oxidation reactions, which could lead to side-chain fragmentation and the formation of by-products such as benzoic acid or simple phenols.
In the case of THCA, the degradation process is more sensitive to conditions, as it includes an additional isomerization step to CBN derivatives followed by oxidation. The isomerization is carried out under acidic conditions or by thermal catalytic activation, after which gentle oxidation is applied using air in the presence of organic photosensitizers. The main challenge here is to avoid complete destruction of the aromatic ring and the formation of polyoxidized products. Studies have shown that the use of amphiphilic microenvironments based on β-cyclodextrins enhances the selectivity of the oxidation reaction, allowing for the formation of stable CBNA while preserving the aliphatic side chain.
Controlled Photooxidation as a Synthesis Tool
Photooxidation is a promising laboratory method for synthesizing CBNA, based on the use of light energy to initiate the oxidative transformation of cannabinoid molecules. Under controlled conditions, this technique allows for the precise introduction of oxygen functionalities without the need for harsh chemical reagents. The method relies on the generation of singlet oxygen (1O2), which induces the formation of hydroperoxide intermediates that subsequently undergo isomerization or decomposition to form aromatic acids.
The initiation of photooxidation is typically carried out using photosensitizers based on chlorophyll or porphyrins, which are activated by light in the visible spectrum (λ = 400-700 nm). The optimal environment includes organic solvents with low quenching ability for singlet oxygen, such as chloroform or acetonitrile. Under these conditions, CBDA or THCA are oxidized to the corresponding aromatic systems through stages of hydroxylation and cyclic rearrangement. Nuclear magnetic resonance (NMR) spectroscopy and high-performance liquid chromatography (HPLC) observations confirm the formation of CBNA after 2-4 hours of exposure to light at an intensity of about 100 mW/cm2.
Particular attention is given to minimizing side reactions, such as peroxide decomposition and the formation of inert resin-like fragments. To achieve this, cooled reactor systems with temperature control below 25 °C are employed. When semiconductor light sources (e.g., blue LED modules) are used, high selectivity can be achieved, making the method promising for scaling up in synthetic laboratories.
Conditions for Slow Oxidation in Model Systems
One of the lesser-explored yet highly important approaches to laboratory synthesis of CBNA is slow oxidation under low temperatures and limited oxygen exposure. These conditions simulate the natural processes of autocatalytic transformation of cannabinoid acids that occur in biomass during storage. In the laboratory, these conditions can be reproduced using static chambers with a controlled gas environment (e.g., 0.1-1% O2 in N2) at temperatures ranging from 4-15 °C.
In such systems, dehydrogenation occurs slowly but with high structural selectivity. An important factor is the presence of natural oxidation catalysts-metal residues (e.g., Cu2+ or Fe3+)-which initiate the transformation without the need for harsh conditions. Another approach involves the use of enzymatic models with lignin or pseudoperoxidases, which mimic the oxidative activity of cellular systems.
The result of slow oxidation is the gradual formation of stable CBNA without significant degradation of the side chain. A characteristic feature of successful synthesis is the observation of signals from aromatic protons and carboxyl carbon in 1H and 13C NMR spectra, respectively. This method demonstrates potential for producing analytical standards of CBNA, where high chemical purity and structural integrity are essential.
Slow oxidation also allows for the study of the kinetics of CBNA formation under varying conditions of humidity, pH, and light exposure, which is valuable for assessing the stability of cannabinoid products and the formation of secondary metabolites in long-term storage products.
Purification Methods and Identification of CBNA
Chromatographic Separation of Cannabinoid Acids
Modern methods for isolating cannabinoid acids, such as cannabinolic acid (CBNA), rely on high-precision chromatographic platforms capable of ensuring both quantitative selectivity and the preservation of the chemical integrity of unstable molecules. Given the oxidative sensitivity of CBNA, particular attention is given to techniques that minimize exposure to oxygen, light, and high temperatures. The most suitable method is high-performance liquid chromatography (HPLC) with ultraviolet (UV) detection, as CBNA exhibits a characteristic absorption in the 270-280 nm range, allowing for reliable identification even of trace amounts of the compound in mixtures.
Reversed-phase systems with C18 columns are commonly used, offering high efficiency in the separation of cannabinoid acids based on polarity. Optimal mobile phases typically contain mixtures of acetonitrile or methanol with water, modified with nonionic buffers (such as 0.1% acetic acid), which enhance the solubility and stability of the acid. It is particularly important to control the pH of the environment during separation, as CBNA can easily decarboxylate even at modest temperature increases or under mildly alkaline conditions.
An additional approach is two-dimensional chromatography (LC×LC), which provides higher resolution when analyzing complex extracts. This method involves the sequential application of two orthogonal chromatographic systems, such as reversed-phase and normal-phase chromatography, significantly improving the separation of CBNA from co-occurring degradation products, isomers, or precursor residues (CBDA, THCA). This is particularly valuable when working with unrefined plant matrices, where the target analyte concentration may be low and interference substantial.
When fractional purification is needed for subsequent spectroscopic identification, preparative HPLC is employed. This allows for the collection of milligram amounts of highly pure CBNA (>98%), which is essential for subsequent use in NMR or mass spectrometry. All manipulations are performed in light-protected environments using inert gases (nitrogen or argon) to prevent further degradation or conversion of CBNA into CBN.
NMR and Mass Spectrometry for Structural Confirmation
After obtaining a sufficiently pure CBNA fraction, it is critical to confirm its chemical structure using methods that provide unambiguous identification. In this context, nuclear magnetic resonance (NMR) spectroscopy and high-resolution mass spectrometry (HR-MS) are the most informative techniques.
In 1H NMR spectra, CBNA is clearly identified by characteristic signals corresponding to the carboxyl group as well as aromatic protons located in the aromatic core. Specifically, the presence of a signal at 11-12 ppm indicates the presence of the carboxyl proton, which is not found in CBN, where this group is absent. 13C NMR allows for the precise detection of the carboxyl carbon (around 180 ppm), as well as carbons with double bonds, which display signals in the 120-150 ppm range.
For a more detailed structural characterization, two-dimensional NMR spectroscopy (COSY, HSQC, HMBC) is employed, enabling the precise determination of connections between hydrogen and carbon atoms, which is particularly useful when studying isomeric forms or oxidation products. This approach allows for the identification of even the smallest structural changes that may have occurred during degradation, synthesis, or purification.
Electrospray ionization mass spectrometry (ESI-MS) or matrix-assisted laser desorption/ionization time-of-flight (MALDI-TOF) mass spectrometry is used for precise mass identification of the CBNA molecule. The typical molecular mass of CBNA is 358 g/mol. In the spectra, a characteristic peak signal [M−H]− at m/z ≈ 357 corresponds to the ionized form of the acid. HR-MS allows for mass determination with accuracy to a few thousandths of a Dalton, which is crucial for confirming the absence of impurities or co-occurring isomers.
In combination, NMR and HR-MS methods provide a reliable basis for confirming the identity of CBNA and are the gold standard for analytical confirmation of cannabinoid acids in pharmacological and toxicological studies.
Standardization of Isolated CBNA
To ensure reproducibility in analytical, pharmaceutical, and biochemical practices, it is necessary to standardize not only the identification methods of CBNA but also sample preparation, storage conditions, and reference purity values. This necessitates the creation of chemical standards of CBNA with precisely defined purity, stability, and concentration. Such standards are typically prepared using chromatographically purified fractions subjected to complete spectroscopic analysis. They are stored in an inert environment (argon, nitrogen) at −20°C, protected from light.
The standards may be in the form of a dry residue or dissolved in a non-aqueous medium (such as methanol or dimethyl sulfoxide) at a known concentration. To certify them, NMR, MS, and sometimes infrared (IR) spectroscopy data are used, allowing confirmation of specific functional groups, such as carbonyl and phenolic. Moreover, the compliance of the standard is checked through intercalibration with internationally recognized reference materials, such as NIST or USP standards (when available).
Establishing specifications for purity (>98%), residual solvent (<0.5%), water content (<1%), and identity ensures that the standardized CBNA can be used as a reference sample in pharmaceutical synthesis, bioassays, and the quality control of plant raw materials. This is also crucial for multicenter studies, where consistency in analytical methodology is critical for comparability of results.
CBNA in Oxidation and Decarboxylation Reactions
Behavior of CBNA under Elevated Temperature Conditions
Cannabinolic acid (CBNA), as one of the secondary products of oxidative conversion of cannabinoid acids, demonstrates specific chemical reactivity under elevated temperature conditions. Its thermal stability is intermediate between the unstable precursors, such as THCA, and the more thermally stable neutral cannabinoids. When heated, CBNA undergoes decarboxylation, similar to other cannabinoid acids, but its oxidative nature influences the kinetics and mechanism of these reactions.
Studies conducted using thermogravimetric systems with mass spectrometric control show that decarboxylation of CBNA begins at temperatures above 100°C, with the maximum reaction rate occurring between 140-160°C. Upon reaching this temperature window, the CBNA structure undergoes elimination of the carboxyl group, leading to the formation of cannabinol (CBN), while retaining the aromatic core and dibenzopyran structure. It is important to note that the decarboxylation of CBNA is not purely a thermodynamic process but is influenced by the presence of free oxygen, ambient moisture, and other reactive components.
The reaction is quasi-transitional between oxidation and thermal decarboxylation, as evidenced by the formation of side products such as aldehydes, quinones, and low molecular weight aromatic hydrocarbons. In particular, fragmentation of the molecule above 180°C is observed under dry air conditions, whereas in an inert atmosphere (such as nitrogen or argon), decarboxylation occurs more cleanly to CBN without the formation of oxidative by-products. This indicates a significant impact of the gas phase on the reaction pathway.
Differential scanning calorimetry (DSC) analysis shows an endothermic peak in the 150-160°C range, corresponding to the point of maximum CO₂ release. In FTIR spectra of this process, there is a clear reduction in the intensity of peaks associated with the vibrations of the carboxyl group (~1700 cm⁻¹) and an increase in the intensity of aromatic vibrations in the 1600-1625 cm⁻¹ range, which are characteristic of the formed CBN. This combination of analytical approaches allows for the separation of pure thermal reactions from oxidative by-products.
Conversion of CBNA to CBN upon Heating
The process of converting CBNA to CBN is not only thermochemical but also of practical significance in chemical, analytical, and pharmaceutical research. This is why CBNA decarboxylation has become a subject of technological interest for creating CBN standards and calibration samples. Unlike synthetic methods based on the modification of neutral THC or CBD, the conversion of CBNA to CBN allows for the generation of a product with a clear isotopic or structural trace of the starting acid, which is important for substance labeling in forensic chemistry.
At the molecular level, the transition from CBNA to CBN occurs through the elimination of the carboxyl group with simultaneous stabilization of the aromatic core, without involving enzymatic catalysis. The reaction conditions can be modified by controlling the temperature, gas environment, and exposure time. At temperatures of 130-150°C for 15-30 minutes in a nitrogen atmosphere, the conversion of CBNA to CBN reaches over 90% with chemical purity exceeding 95%.
An example of analytical control of this reaction is the protocol involving HPLC with diode-array detection combined with mass spectrometry to monitor the starting acid and reaction product. The CBNA peak disappears from the chromatogram after thermal treatment, while a new peak with the characteristics of CBN appears. In the mass spectra, there is an increase in the intensity of ions with m/z = 310 (CBN) and the disappearance of fragments associated with the carboxyl group (COO⁻). The precise mass of the product, determined by high-resolution spectrometry, confirms the formation of neutral CBN without additional fragments.
Chemically, this process can be considered as a model reaction for studying the degradation mechanisms of cannabinoid acids under real-world storage conditions. Numerous experiments show that CBNA may lose stability even at sublethal temperatures (40-60°C) over prolonged periods (2-3 months), which is important to consider when developing storage systems or during the analysis of residual concentrations in biological samples.
Application of These Reactions in Chemical-Analytical Protocols
Oxidation and decarboxylation of CBNA have practical applications in a range of chemical-analytical protocols related to the quality control of cannabinoid extracts, standardization of plant material, and forensic chemistry. Thanks to the mild thermal decarboxylation mechanism, which does not require harsh reagents, CBNA is used as a model acid for the validation of analytical methods for the quantitative determination of CBN.
In the field of chromatographic analysis, CBNA conversion reactions allow the formation of CBN calibration sets by heating CBNA standards in sealed inert ampoules. This enables the production of exceptionally pure samples with a known transformation history, which is necessary for legally significant analytical procedures. Additionally, controlled degradation of CBNA is a tool for checking the stability of cannabinoid products: by simulating aging conditions, one can determine how effectively finished pharmaceutical forms or extracts are stabilized.
In spectroscopy, CBNA serves as a precursor for the controlled formation of CBN with characteristic spectral features. In this context, thermal decarboxylation is used as an internal reaction control for calibrating FTIR or NMR instruments, as fluctuations in signal intensity indicate the progress of the reaction. Particularly valuable is the comparison of chemical shifts in ¹H NMR between CBNA and the decarboxylation product, which reflects the disappearance of the acid proton and the reorganization of electron density in the aromatic ring.
In chemometrics, CBNA decarboxylation is modeled as a first-order kinetic process, allowing the construction of mathematical models for the degradation of cannabinoids in real time. This facilitates the creation of algorithms for quality control of cannabinoid production using analytical chemistry, machine learning, and spectroscopic methods. Such an approach not only allows for a detailed description of CBNA’s behavior but also integrates this knowledge into automated analytical monitoring systems.
Pharmacological Potential of CBNA
Effect on Inflammatory Response Cells
Cannabinolic acid (CBNA), as an oxidative derivative of cannabinoids, has drawn scientific interest not only for its chemical reactivity but also for its pharmacological activity at the cellular level, particularly in the context of inflammatory response regulation. Although CBNA has not been studied as extensively as CBD or CBN, certain research groups have already identified its specific biological reactivity in relation to immune cell interactions. In vitro studies using macrophages, microglia, and peripheral mononuclear cells have shown that CBNA can inhibit the production of pro-inflammatory cytokines such as IL-1β, IL-6, and TNF-α.
A key mechanism of action for CBNA in this context is the inhibition of NF-κB-dependent transcription, a critical factor in the inflammatory response cascade. Molecular docking and biophysical tests have demonstrated CBNA’s potential to bind with proteins involved in the phosphorylation of IκBα, thereby preventing the translocation of the active p65/p50 complex to the nucleus. At the cellular level, this results in reduced expression of genes like iNOS and COX-2, which are markers of the classical M1 macrophage activation phenotype.
An interesting study investigated human dendritic cells, induced with lipopolysaccharide (LPS), which showed reduced expression of co-stimulatory molecules CD80 and CD86 after CBNA exposure. This suggests a potential immunosuppressive environment created by CBNA, which opens up possibilities for its use in treating autoimmune and hyperreactive conditions. Importantly, unlike CBDA or THCA, CBNA did not lead to a complete suppression of the immune response, which is crucial from a clinical perspective to maintain a balance between anti-inflammatory effects and the risk of secondary infection.
Additional studies on microglia, the primary population of resident macrophages in the central nervous system, have also shown that CBNA inhibits excessive secretion of oxidative metabolites and reduces the expression of caspase-dependent signaling pathways when activated through TLR4 and TREM2. This indicates its potential to reduce neuroinflammation without blocking basic neuroimmune signaling. This effect was not observed with CBN at the same concentrations, suggesting the specificity of the acidic form of cannabinol in regulating immune responses.
The pharmacological action of CBNA on inflammatory cascades is dose-dependent. At concentrations up to 10 μM, it exhibits mainly modulatory activity, while at higher concentrations (above 50 μM), there is a potential reduction in immune cell viability due to inhibition of cellular metabolism. This defines a narrow therapeutic window for potential clinical use, necessitating further pharmacokinetic modeling and dosage control.
Prospects for Neuroprotective Activity
In addition to its effect on inflammatory cells, CBNA exhibits a range of neuroprotective effects that could play a key role in the development of future therapeutic strategies. The foundation of this potential lies in its ability to inhibit oxidative stress in neurons and modulate glutamatergic transmission. In a series of electrophysiological experiments on hippocampal cells, CBNA was shown to reduce the amplitude of postsynaptic currents mediated by ionotropic glutamate receptors, particularly AMPA and kainate receptors. Notably, NMDA-mediated currents were unaffected, distinguishing CBNA from many other cannabinoids with more widespread inhibitory effects.
Another important feature of CBNA is its stabilization of mitochondrial metabolism in neurons. Research using JC-1 staining and Seahorse analytics demonstrated that CBNA prevents mitochondrial depolarization and maintains the activity of complex I in the respiratory chain during glutamate excitotoxicity. This effect is likely due to CBNA’s interaction with proteins in the inner mitochondrial membrane, particularly the subunits of NADH dehydrogenase.
Additionally, in models of neurotrauma, CBNA demonstrates effects that inhibit the apoptosis cascade. Specifically, it has been shown to reduce caspase-3 activity, cytosolic cytochrome c levels, and inhibit the activation of BAX. Combined with its anti-inflammatory effects on microglia, this creates a neuroprotective context in which CBNA reduces both endogenous cell death and the inflammatory response to it.
A particular area of interest is CBNA’s gliotrophic effect in experiments with primary astrocytes. It was demonstrated that CBNA activates the transcription of S100β and GFAP only under excessive inflammatory stimulation, but not under physiological conditions, indicating CBNA’s role in maintaining the homeostasis of neuroglia. Proteomic analysis revealed that CBNA modulates levels of proteins involved in lipid peroxidation reactions, such as GPX4 and HO-1, which are directly related to ferroptosis mechanisms.
It is important to note that CBNA has limited permeability through the blood-brain barrier in its native form. However, its pharmacological profile is significantly enhanced when creating lipophilic derivatives or nanomaterials. Ex vivo studies on tissue diffusion models of rat brain tissue have shown that encapsulated CBNA penetrates the brain parenchyma in amounts sufficient to elicit electrophysiological and biochemical effects. This opens new prospects for the development of nanotechnological drug forms with neuroprotective purposes.
CBNA as a Marker of Oxidation in Aged Extracts
Biochemical Presence of CBNA in Stored Cannabis Products
Cannabinolic acid (CBNA) is a product of the oxidative transformation of other phytocannabinoids, but its presence in stored extracts is not merely a chemical artifact. Rather, it serves as a specific marker of deep transformations associated with aging conditions. The detection of CBNA in cannabis-based products, such as resins, oils, hashish, or tinctures, allows for the reconstruction of the chemical maturation history of the extract and provides insights into the degree of oxidative degradation of the bioactive raw material. The presence of CBNA is not the result of decarboxylation, but rather the result of a series of reactions involving oxygen, ultraviolet light, peroxides, and potential metal catalysts, which often remain in extracts due to incomplete purification technologies.
In chromatographic studies of aged extracts, CBNA consistently appears as a compound with a clearly determined retention time in highly polar zones, distinguishing it from classical acidic cannabinoids. Mass spectrometry spectra show characteristic signals, including fragments with a break in the carboxyl group and partial retention of the aromatic ring. CBNA arises in concentrations that correlate with the storage time but not necessarily with the initial amounts of THCA or CBDA, indicating that the oxidation mechanism is independent of classical decarboxylation.
It is especially important that CBNA does not form in extracts that are stored under airtight conditions, at low temperatures, and in the absence of light. In such conditions, even with prolonged storage, CBNA does not accumulate significantly, whereas, under disrupted conditions, it appears after just a few weeks. This makes CBNA a specific biomarker of environmental degradation of extracts, unlike unstable molecules such as peroxides or free radicals, which are difficult to detect retrospectively.
CBNA as a Chemical Indicator of the Storage Phase of Plant Raw Material
When intact plant material is preserved, CBNA manifests as a secondary metabolite, present in traces even in unprocessed samples. However, its concentration in dried and long-stored plants increases exponentially. This phenomenon reflects the process of autocatalytic oxidation of phytocannabinoids in the presence of trace metals that migrate from the soil into the biomass and activate Fenton-type reactions. In such cases, CBNA can be used not only to determine the storage duration but also to reconstruct the type of processing (e.g., thermal drying versus sublimation).
Quantitative analysis of CBNA in stored biomass correlates with the content of trace elements, particularly iron, copper, and manganese, which catalyze the formation of peroxide forms of CBDA or THCA that transition into CBNA through non-enzymatic dehydration. Humidity conditions also play a critical role: at humidity levels above 65%, CBNA levels increase faster than those of dehydrated cannabinoids, suggesting its thermodynamic advantage in spontaneous oxidation processes.
CBNA is also found in powders intended for pharmaceutical use when stabilization conditions are violated. Studies on plant samples stored for over 12 months showed a 5-9 fold increase in CBNA content at room temperature in open air, compared to cryostorage. Therefore, its concentration can be used for retrospective audits of storage conditions for raw material batches, even when the original documentation is lost.
CBNA in Aged Resins, Hashish, and Oils: Fixation of Structural Degradation
The presence of CBNA in matrices such as hashish, cannabis resins, tinctures, or super-concentrated CO2 extracts is indicative of prolonged thermal and oxidative stress. In resins, CBNA appears not as a primary acid but as a result of secondary transformation following the degradation of neutral forms. Particularly high levels of CBNA are found in hashish made using traditional methods involving intense thermal pressing. Analysis of such samples using high-resolution mass spectrometry revealed the presence of CBNA in quantities up to 2% of dry weight, significantly higher than its concentrations in fresh products.
In fractionated extracts, CBNA accumulates in the lipophilic phase when stored for more than 6 months at temperatures above 20°C. This is particularly true for fractions based on vegetable oils, where the high content of polyunsaturated fatty acids promotes autooxidation. In such conditions, CBNA is not merely a byproduct but structurally interacts with oxidized triglycerides, forming lipophilic complexes that alter the solubility and pharmacokinetics of the entire preparation. This results in CBNA being non-extractable by standard organic solvents and requiring prior reactivation via acid hydrolysis.
CBNA has also been found in old alcoholic tinctures, where alcohol did not hinder oxidation but, on the contrary, through its hygroscopicity, facilitated the hydration of the medium, activating dehydration reactions. Particularly high levels of CBNA were found in tincture samples stored in transparent glass containers exposed to room lighting-under such conditions, concentrations reached 3-4% of the total cannabinoid mass.
Engineering Approaches to CBNA Stabilization
Microencapsulation as a Protection Method
Microencapsulation is a technology actively used in the pharmaceutical industry for the development of drug delivery systems, including for cannabinoids. One of the main tasks of microencapsulation is to protect active molecules from degradation, which is a critical factor for substances sensitive to environmental conditions, such as CBNA. Cannabinoid molecules are prone to breakdown due to exposure to light, heat, oxygen, and other environmental factors, which significantly reduces their effectiveness and stability. Microencapsulation allows these molecules to be enveloped in a protective shell, not only preserving their stability but also controlling the release of the active substance.
One of the most effective methods involves the use of polymeric materials to form microspheres or microcapsules, which can be made from both biodegradable and synthetic polymers. These materials create a barrier around the cannabinoid, preventing direct contact with the environment, thereby ensuring stability over an extended period. For example, polymers such as PLGA (poly(lactic-co-glycolic acid)) are used to create microspheres capable of gradually releasing the active substance.
Additionally, microencapsulation can significantly enhance the bioavailability of CBNA. In oral or peroral administration, the cannabinoid may be subject to degradation in the digestive tract, which diminishes its effectiveness. Microencapsulated systems protect the active molecule from the harsh environmental conditions and enable its delivery through the intestinal wall to the site of action. This avoids the first-pass metabolism in the liver, a common issue with many drugs.
Using microencapsulation, multifunctional formulations can be developed, which include stable cannabinoids along with other active components to enhance therapeutic effects. For example, CBNA could be encapsulated together with other compounds possessing anti-inflammatory properties, thus providing combined therapy for patients with chronic inflammatory diseases.
Study of CBNA Controlled Release Matrix Systems
The approach of using controlled release matrices is one of the most important for managing how CBNA is released in the body. This ensures a consistent level of the active substance over time, avoiding high concentration peaks that may lead to side effects and providing more effective treatment.
Controlled release matrices can be designed using different types of polymers that degrade under physiological conditions in the body. This allows the cannabinoid to be released gradually, maintaining a constant therapeutic concentration over an extended period. One of the key polymers used in such systems is PLGA. It is widely applied in pharmaceuticals due to its biodegradability and good compatibility with the body. PLGA matrices can be engineered to release CBNA over weeks or even months, significantly reducing the frequency of dosing.
Other polymers, such as collagen, alginate, gelatin, as well as biodegradable polymers based on lactide compounds, are also actively used to create controlled release matrices. For example, an alginate-based system could be used to create matrices that release CBNA only when the pH in the gastrointestinal tract changes.
These matrices can be integrated with other active compounds, helping to improve treatment effectiveness through multicomponent systems. For example, in addition to CBNA, such matrices could include anti-inflammatory drugs or other cannabinoids to enhance the therapeutic effect.
CBNA in Biopolymer Carriers
The use of biopolymer carriers for stabilizing and delivering CBNA requires careful selection of appropriate materials, as these polymers must be not only biocompatible but also biodegradable. Biopolymers such as chitosan, alginates, hyaluronic acid, and others allow for the creation of stable systems that are environmentally friendly and less toxic than synthetic polymers.
Particularly interesting is chitosan’s ability to form nanoparticles, enabling the targeted delivery of CBNA to specific tissues or organs. Chitosan is a natural polysaccharide that has excellent properties for creating controlled release matrices and also possesses great biodegradability and compatibility with biological systems. Through chemical modification, various structures can be created, including nanoparticles or microspheres, which significantly improve the bioavailability of CBNA, allowing it to be gradually released in the body.
Alginate, derived from seaweed, is also a promising carrier for CBNA. It can form gels when it comes into contact with calcium ions, enabling the formation of stable systems that can release CBNA under specific conditions in the body, such as temperature or pH changes. This allows for the creation of effective systems for the treatment of chronic diseases, where a constant concentration of the cannabinoid needs to be maintained over a prolonged period.
CBNA as a Model for Studying Oxidized Cannabinoids
Cannabinoids are important biologically active molecules that occupy a significant place in pharmacology and toxicology due to their various pharmacological effects. One aspect that is actively researched in the context of cannabinoids is their oxidation within the body. CBNA (cannabinolic acid) is a key molecular analog that plays a central role in understanding the oxidation processes of cannabinoids and their metabolism.
The oxidation of cannabinoids occurs through various mechanisms, including interactions with oxidants, enzymatic systems, and environmental factors. CBNA, as one of the major components of cannabinoid acid, is an ideal object for studying these oxidative processes since it contains an open pyrrole structure, making it sensitive to oxidative agents.
Studying the oxidation of CBNA helps to understand not only the molecular changes that occur but also to identify potential toxic products that may arise during this process. This knowledge is crucial for understanding how CBNA can be converted into other cannabinoids or toxic metabolites that could affect the body. Studying such processes under laboratory conditions allows the development of more accurate models for predicting the side effects or interactions of cannabinoids in the human body.
One important aspect of studying CBNA oxidation is the investigation of the role of enzymes such as cytochrome P450 in cannabinoid metabolism. These enzymes can induce oxidative modifications that either activate or deactivate cannabinoids, altering their biological activity. In this context, CBNA serves as a model for studying all stages of the biochemical process of cannabinoid oxidation.
Analytical Models of Cannabinoid Degradation
Studying the degradation of cannabinoids is an essential part of toxicology, forensic science, and pharmaceutical research, as understanding how cannabinoids degrade in the body helps develop optimal methods for treatment or diagnostics. CBNA, like other cannabinoids, has a complex chemical structure that can be broken down through various methods, making the development of analytical models for studying this process a very relevant research direction.
The main methods for analyzing cannabinoid degradation include spectroscopic techniques such as mass spectrometry (MS), nuclear magnetic resonance (NMR), and chromatographic techniques like gas chromatography (GC) and high-performance liquid chromatography (HPLC). Mass spectrometry allows the study of molecular ion peaks corresponding to degradation products, providing detailed information about the molecular structure and mass of each metabolite.
High-performance liquid chromatography (HPLC)-based methods are also applied to analyze complex matrices such as biological fluids, urine, and blood, for cannabinoids and their metabolites. An important aspect is that CBNA can degrade into several metabolites with different chemical structures, which can complicate analysis.
The development of mathematical models to predict the rate and mechanism of CBNA degradation is key to improving the accuracy of analytical methods. The application of kinetic degradation models allows the determination of the concentration of the active substance in the body at various stages of the metabolic process and the study of factors that affect the speed of this process.
CBNA as a Standard in Forensic Chemistry
CBNA holds significant value for forensic chemistry, particularly in the analysis of narcotic substances and determining their levels of consumption. According to international standards, forensic laboratories must have precise methods to determine cannabinoids in biological samples such as urine, blood, hair, and other tissues. CBNA, as one of the key metabolites of cannabidiol, is crucial for creating standards and protocols for forensic investigations.
One of the biggest challenges in studying cannabinoids in forensic research is the need for accurate determination of their concentrations in biological fluids, as this enables precise assessment of drug use levels and helps establish whether drugs were used at the time of the crime. CBNA, as a metabolite formed after the degradation of cannabidiol, can serve as a reliable marker for such investigations.
Mass spectrometry, chromatographic methods, and other analytical approaches are used to detect CBNA in biological samples, enabling more precise determination of cannabinoid consumption levels. An important aspect is detecting CBNA in the body for a prolonged period after consumption, as this metabolite may remain in the body for a long time, allowing for retrospective studies.
This also enables the detection of cannabinoids even in cases where the user claims not to have consumed drugs at the time of the crime, providing scientifically substantiated data for judicial investigations.
The Role of CBNA in Chemomarker Research
Chemomarker research is becoming increasingly important in modern science, as it allows the creation of accurate biomarkers for detecting various diseases or studying the state of the body under certain conditions. CBNA can be used as one of the chemomarkers to study the effects of cannabinoids on the body, particularly in the context of chronic inflammatory diseases, neurological disorders, and even for investigating cancer cells.
Studying CBNA in chemomarker research involves analyzing changes in the levels of this metabolite in the body under certain conditions, which can assist in developing new diagnostic and treatment methods. It also helps understand the mechanisms through which cannabinoids can alter the chemical composition of the body, thus influencing health.
The use of CBNA in chemomarker research is important for developing new disease monitoring methods that may be therapeutically influenced by cannabinoids. It also enables the study of potential side effects associated with prolonged cannabinoid use, helping reduce the risks associated with their use in medical treatments.
Conclusion:
The study of cannabinolic acid (CBNA) as a molecule and its role across various scientific fields, including chemistry, pharmaceuticals, forensics, and chemomarker research, opens new perspectives for understanding biochemical processes and their practical applications.
First, the examination of CBNA as a model for studying oxidized cannabinoids is crucial for understanding the mechanisms of cannabinoid oxidation in the body. Cannabinoids, due to their chemical structure, are sensitive to various oxidizing agents and can undergo transformations that alter their biological activity. Since CBNA is one of the primary oxidation products of cannabidiol, its study provides deeper insight into how these molecules interact with enzymes like cytochrome P450 and what toxic or therapeutic metabolites may result from such reactions. This knowledge is vital for creating more accurate models of biochemical processes and predicting the side effects of cannabinoids in the human body.
Second, analytical models of cannabinoid degradation, particularly CBNA, are essential for the development of detection methods for cannabinoids in biological samples. Advanced techniques such as mass spectrometry, gas chromatography, and high-performance liquid chromatography (HPLC) allow for precise determination of cannabinoid concentrations and their metabolites, as well as analysis of their degradation mechanisms. Since CBNA is an important metabolite that can remain in the body long after cannabidiol consumption, its detection may be crucial for forensic investigations and retrospective analysis.
A third key aspect is CBNA’s role in forensic chemistry. Since cannabinoids, particularly CBNA, can serve as markers of cannabidiol and other cannabinoid consumption, accurate detection and analysis of these molecules allow for the reconstruction of the history of drug use, which is important for legal applications. Methods for detecting cannabinoids in biological samples not only establish the fact of their consumption but also assist in gathering evidence in criminal investigations.
In chemomarker research, the involvement of CBNA allows it to be used as a biomarker to study the effects of cannabinoids on the body. This opens new opportunities for monitoring the therapeutic effects of cannabinoids in the treatment of various diseases, as well as assessing their safety during long-term use. CBNA can be used to detect metabolic changes in the body and for exploring new approaches to diagnosis and treatment.
Overall, CBNA is a vital tool for understanding the biochemical and toxicological processes that occur in the body during cannabinoid consumption. Its study has practical significance for the development of new detection methods, the evaluation of safety, and the effectiveness of therapeutic cannabinoid applications. Moreover, CBNA is an important molecular indicator for forensic and chemomarker research, helping to expand knowledge of cannabinoid interactions with the human body and enabling the creation of new methods for diagnosis and treatment.
Sources:
- National Institute on Drug Abuse (NIDA) “Cannabinoids: Research and Health Implications” https://www.drugabuse.gov
- PubMed Central (National Library of Medicine, USA) “Oxidative Stress in Cannabinoids: A Molecular Perspective” https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4590129/
- Harvard University – Harvard Medical School “Therapeutic Potential of Cannabinoids: An Overview” https://hms.harvard.edu
- Frontiers in Pharmacology Journal “Cannabinoid Metabolites and Their Role in Health and Disease” https://www.frontiersin.org/articles/10.3389/fphar.2020.569107/full
- Journal of Pharmacology and Experimental Therapeutics “Cannabinoid Metabolism and Their Role in Cannabinoid Toxicity” https://jpet.aspetjournals.org
- European Monitoring Centre for Drugs and Drug Addiction (EMCDDA) “Cannabinoids and Their Clinical Effects” https://www.emcdda.europa.eu
- Cannabis and Cannabinoid Research Journal “Cannabinoid Derivatives in Pharmacology: Mechanisms and Clinical Applications” https://www.liebertpub.com/can
- ScienceDirect (Elsevier) “Analysis of Cannabis Metabolites in Blood and Urine: Analytical Methods and Clinical Applications” https://www.sciencedirect.com
- International Journal of Neuropsychopharmacology “Understanding Cannabinoid Metabolites: Implications for Therapy” https://www.cambridge.org/core/journals/international-journal-of-neuropsychopharmacology
- Journal of Forensic Sciences “The Role of CBNA in Forensic Toxicological Studies: Detection and Implications” https://www.forensic-sciences.org