Despite the growing interest in cannabinoids within pharmacology and neurobiology, cannabinol (CBN) has long remained in the shadow of more extensively studied compounds such as Δ9-tetrahydrocannabinol (THC) and cannabidiol (CBD). Its status as a secondary metabolite, primarily formed through the auto-oxidation of Δ9-THC, often led to its mistaken identification as an “inactive” or even “degraded” product. This approach overlooks the significant biological specificity of CBN, as well as its unique physicochemical and pharmacokinetic properties, which distinguish it both from primary phytocannabinoids and synthetic cannabinoid analogs.
CBN is not just a byproduct of oxygen acting on THC. It is a distinct cannabinoid with full pharmacological activity that cannot be reduced to merely its suppressed effect on CB1 receptors. Its partial agonistic action on CB2 receptors, as well as its affinity for non-mainstream cannabinoid and TRP receptors (particularly TRPA1), indicates the potential of this compound in exploring peripheral anti-inflammatory pathways, nociception modulation, and neuroinflammation. Moreover, unlike THC, CBN demonstrates a significantly lower psychoactive profile, which opens up possibilities for therapeutic applications without the restrictions associated with THC’s psychotropic effects.
Historically, CBN was the first cannabinoid to be isolated in its pure form and characterized using classical organic chemistry methods: its structure was established in the 1930s, before the identification of THC. The systematic chemical study of cannabis began with CBN, though it was later marginalized due to its low concentration in fresh plant material. It is important to note that the isolation of CBN was often an indirect indicator of the quality or “freshness” of cannabis raw material, which could mislead expectations regarding its pharmacological potential.
Another undervalued feature of CBN is its unique stability under conditions where most cannabinoids lose their activity or structure. For example, CBN exhibits greater thermal stability compared to THC, making it a promising candidate for formulations requiring sterilization or for use in long-term storage conditions. Its chemical inertia also allows the investigation of specific interactions with membrane proteins without the side effects on receptor dynamics that are characteristic of less stable molecules.
Unlike other cannabinoids, CBN is not directly produced by the plant via enzymatic biosynthesis from cannabigerolic acid (CBGA)-its formation is entirely dependent on post-biosynthetic processes, including photochemical and redox reactions. This places CBN in a distinct conceptual category of “secondary” or “decadent” cannabinoids, whose quantitative presence in a sample serves as an indicator of age, storage methods, and the degree of oxidation of primary components. This approach opens the possibility of using CBN as a chemical marker for the degradation or photodegradation of the cannabinoid system.
Considering contemporary approaches to polypharmacology and the modular analysis of the entourage effect, CBN is beginning to attract attention as a potentially co-active compound capable of influencing the pharmacodynamics of other components in the phytocannabinoid spectrum. Research suggests that CBN may alter the transcriptional activity of microglia or influence the expression of pro-inflammatory cytokines, thereby modulating the activity of CBD in peripheral environments. Under such conditions, CBN goes beyond the role of a “secondary cannabinoid” and becomes a functional component of the phytochemical composition.
Equally important is the fact that CBN is part of the group of cannabinoids that are more commonly found in processed or stored cannabis, which has led to its active study in the context of product safety, secondary processing of raw material, and long-term exposure to the body. Such research allows CBN to be considered not only as a biologically active compound but also as an indicator of the technological process-specifically in the standardization of pharmaceutical cannabis-based products.
Biogenesis and Chemical Origin of CBN
Cannabinol (CBN) is not synthesized in the Cannabis sativa plant through the classic enzymatic pathways typical of major phytocannabinoids, but rather arises as a result of post-biogenetic transformations. Its formation is a direct consequence of the instability of Δ9-tetrahydrocannabinol (Δ9-THC), particularly its susceptibility to oxidative processes. This fundamentally distinguishes CBN from primary biosynthetic cannabinoids such as cannabigerol (CBG), cannabidiol (CBD), or Δ9-THC itself, the formation of which is based on enzymatic conversion of a common precursor, cannabigerolic acid (CBGA), through the action of specific synthases.
CBN predominantly forms via a non-catalytic auto-oxidation of Δ9-THC in the presence of atmospheric oxygen and light. This process does not require enzymes or specific cofactors, allowing it to occur in already harvested, dried, or stored plant material. From an organic chemistry perspective, the transformation of Δ9-THC into CBN involves dehydrogenation and aromatization of part of the molecule: specifically, the cyclohexane ring structure of Δ9-THC loses two pairs of hydrogen atoms, leading to the formation of an aromatic benzene ring in the CBN structure. This aromatic ring is a key distinction-it stabilizes the molecule, reducing its reactivity and susceptibility to further degradation processes.
While cannabinol was historically identified as a product of time and environmental exposure to cannabis raw material, current data demonstrates that its formation processes can be controlled and intentionally induced under laboratory conditions. For instance, by modulating light, humidity, temperature, and oxygen access, the rate of conversion of Δ9-THC to CBN can be regulated with a relatively high degree of selectivity. There are also known methods of catalytic oxidative aromatization using agents like chloranil or manganese complexes, which speed up the conversion process without the simultaneous degradation of other structures. In the realm of chemical-technology approaches, such reactions can even be scaled up to semi-industrial levels, raising the question of the technological feasibility of CBN as a standalone product.
An interesting fact is that, unlike most phytocannabinoids, CBN has its own chemical history independent of enzymatic origin. In the 1940s, the structure of CBN was determined using classical organic chemistry methods, including nitration, bromination, and coupling reactions, without involving knowledge of its biogenetic role. This means that CBN was identified in the chemical realm before THC-i.e., before the discovery of the cannabinoid biosynthetic system in plants. It was later proven that the natural presence of CBN in cannabis is a derivative of storage conditions rather than a result of direct action by cannabinoid synthases.
It is also worth noting the chemotaxonomic significance of CBN in pharmacognostic research. Its accumulation in cannabis samples allows for the reconstruction of the temporal dynamics of chemical transformations in biomass, particularly under the influence of ultraviolet radiation or prolonged oxidative stress. This makes it a marker of chemical aging in phytocomplexes and an important indicator of the degradation of other phytocannabinoids. In this context, the role of CBN could be compared to that of terpene oxides in the biomonitoring of essential oils.
Special attention should be given to its chiral properties. Although CBN is an achiral molecule due to the presence of a symmetrical aromatic ring, it forms from a chiral precursor-Δ9-THC-which has multiple chiral centers. During the transformation, the chiral information is lost, which could serve as an additional marker of chemical degradation: loss of chirality = loss of biosynthetic identity.
Finally, it should be mentioned that the structure of CBN is related to the class of diphenolic triterpenoids, yet, due to its aromatic core, it tends more toward the phenanthrene group, which is characteristic of many central nervous system receptor ligands. This structural shift from a cannabinoid-type framework to an aromatic contour defines the unique receptor activity of CBN, distinct from classic cannabinoids, and deserves deeper exploration from a pharmacophore perspective.
Phytocannabinoid Landscape: The Role of CBN Among Other Compounds
The phytocannabinoid profile of Cannabis sativa includes over 120 identified compounds, which are classified based on their structural similarity to derivatives of cannabigerol (CBG), cannabidiol (CBD), tetrahydrocannabinol (THC), cannabichromene (CBC), as well as their respective carboxylated forms. In this context, CBN does not belong to any of the main synthetic pathways since it does not originate from CBGA through a direct enzymatic route, as is characteristic of the aforementioned classes. Instead, it forms secondarily through post-synthetic modifications, meaning its presence in a chemotype is not a predictive marker for the genotype of the plant.
From a chemotaxonomic perspective, CBN occupies a niche as a degradative cannabinoid. Its appearance is not a result of enzymatic activity but rather a consequence of external conditions, which distinguishes it from structurally related molecules like Δ8-THC or cannabicyclol. Its molecular weight and polarity distribution influence its pharmacokinetics: reduced lipophilicity compared to THC contributes to a modified ability to cross the blood-brain barrier. As a result, CBN can be considered a boundary compound-between phytocannabinoids that are active in the central nervous system and those that primarily act peripherally.
CBN is also interesting in the context of the “second-wave” cannabinoid spectrum-a group of compounds that form during the aging process or through the technological processing of cannabis material. Along with compounds like cannabivarin (CBV) or dehydrocannabinoids, it helps to reconstruct the chemical evolution of a sample. This approach is particularly valuable in pharmacognostic and toxicological analysis.
Oxidation of Δ9-THC: The Mechanism of Cannabinol Formation
Auto-oxidation Involving Oxygen
The mechanism of conversion from Δ9-THC to CBN is based on radical auto-oxidation. It begins with the abstraction of a hydrogen atom from the aliphatic chain of Δ9-THC, primarily at the C-11 position, resulting in a stabilized allylic radical. This intermediate product reacts with molecular oxygen (O₂), forming a peroxide radical, which undergoes further intramolecular rearrangements to form an aromatic quinone-like core. A key step in this process is the establishment of a π-system via oxidative dehydration.
The final product, CBN, is thermodynamically stable due to the fully conjugated aromatic system. It is important to note that this pathway does not require catalysts and occurs even at room temperature, as confirmed by isotope-marked studies. At the same time, the process is highly sensitive to the presence of water, pH conditions, and ultraviolet (UV) radiation levels, which can induce alternative phototransformation reactions.
Light Sensitivity and Thermolability of THC
Δ9-THC is a molecule with significant photo- and thermolability due to the presence of double bonds and chiral centers in the cyclohexene fragment. Exposure to light, particularly in the 280–320 nm range, promotes the cleavage of π-bonds, the formation of radical intermediates, and, ultimately, the restructuring into a stable aromatic product.
Thermal degradation of Δ9-THC at temperatures above 70°C triggers rearrangements, also leading to the formation of CBN. This pathway is particularly activated in conditions of limited oxygen access, where intramolecular dehydration mechanisms dominate.
Thermokinetic studies show that CBN forms from Δ9-THC with a conversion efficiency of up to 90% after 72 hours at 100°C in an air atmosphere. In contrast, with increased moisture content, the formation of other degradation products, such as quinones or free aldehydes, predominates.
Alternative Synthesis Pathways
Chemical Synthesis of CBN: History and Modern Approaches
The first laboratory syntheses of CBN were conducted in the 1940s through the aromatization of structurally similar precursors. For example, the reaction of cannabiols with dichlorodimethylquinone or other aromatizing agents allowed for the formation of CBN with moderate yield. Later, electrophilic substitution methods with subsequent dehydration were employed to form the aromatic core.
Modern approaches are based on controlled oxidation reactions of Δ9-THC in the presence of catalysts like MnO₂, CrO₃, PCC (pyridinium chlorochromate), as well as newer organocatalysts such as TEMPO. Catalytic aromatization allows for precise stoichiometric regulation, minimizing the formation of byproducts. An important direction is also the selective use of photocatalysis: introducing photosensitive electron carriers allows for the initiation of dehydrogenation processes with high efficiency and without the need for harsh thermal conditions.
In the field of synthetic organic chemistry, there is growing interest in C-H functionalization as a method for the direct conversion of Δ9-THC analogs into CBN without the need to first isolate THC. This approach could be useful for large-scale chemical production of CBN from semi-synthetic sources.
Biosynthetic Models: Using Microbial Strains
Unlike traditional cannabinoid biosynthesis via plant-derived enzymes (THCAS, CBDAS, CBCAS), CBN lacks a specific enzyme that catalyzes its formation. However, modern biotechnology allows the creation of microbial strains (usually Saccharomyces cerevisiae or Escherichia coli) capable of producing Δ9-THC, which is then subjected to induced oxidation to CBN in situ or ex vivo conditions.
Research groups focus on creating strains capable of performing controlled auto-oxidation or expressing recombinant peroxidases that catalyze aromatization. Experiments involving the expression of bacterial hydroxylases combined with oxidative agents show promising results for targeted CBN synthesis without isolating Δ9-THC as an intermediate product.
Another area of interest is the use of genetically modified Aspergillus niger cultures, which demonstrate the ability to aromatize terpenoids through endogenous oxidases. Under certain cultivation conditions, such fungi could be used as biocatalysts to form CBN from cannabinoid precursors.
Unlike traditional chemical methods, biosynthetic models allow the production of CBN with a high degree of purity without the need for harsh conditions or toxic reagents. These models have advantages in the context of “green chemistry” and pharmacological standardization, making them appealing for future pharmaceutical applications.
Methods of Preparation and Isolation
The processes of obtaining and isolating cannabinol (CBN) from natural or synthetic sources involve a complex set of technologically sophisticated procedures that require precise regulation of conditions, high chemical specificity, and sensitivity to external factors. Historically, CBN was the first cannabinoid to be isolated, and this circumstance led to the early development of methodologies for its extraction, which were later optimized according to the standards of modern chemical technology, analytics, and pharmaceuticals. At the same time, obtaining pure CBN still presents a significant challenge in both laboratory and industrial settings due to its secondary nature, low concentration in fresh plant material, and structural similarity to related cannabinoids.
The primary raw material for obtaining CBN is most commonly represented by dried flowers or extracts with a high content of Δ9-tetrahydrocannabinol (THC), as CBN forms as a result of its oxidation. However, using such matrices is associated with numerous analytical and procedural difficulties. Firstly, the significant presence of lipophilic substances in the natural extract complicates the specific isolation of CBN without also extracting other components. Secondly, CBN often exists in extracts as unstable intermediate products or in a bound state, which requires prior activation or transformation of the matrix.
For these reasons, the isolation of cannabinol almost always involves a multi-step approach, including preliminary fractionation, selective isolation, concentration, and purification. Extraction is usually carried out using organic solvents that show high affinity for phenolic and aromatic structures, such as dichloromethane, ethyl acetate, or acetonitrile. Conditions are favored that do not provoke further degradation of thermolabile components. In some cases, supercritical extraction technologies, particularly supercritical CO₂ extraction with modified temperature and pressure parameters, are employed to enrich CBN fractions.
The most important step in terms of high specificity remains chromatographic separation. Traditional methods, such as flash chromatography on silica gel, demonstrate limited resolution when working with cannabinoid mixtures, so they are typically combined with more modern approaches-such as preparative HPLC, phase inversion chromatography, or centrifugal partition chromatography. Elution conditions are chosen based on the subtle differences in polarity and aromaticity between CBN and closely related structures, including dehydrogenated THC derivatives and CBD traces.
An additional level of complexity arises when CBN is obtained not from natural sources but through synthetic transformation of precursors. In such cases, there is a need for careful purification of byproducts of the reactions, such as polycyclic aromatic hydrocarbons, which may form during oxidation or thermal processes. Purification methods in these contexts include molecular distillation under vacuum, solid-phase extraction, as well as the use of microcolumn electrophoretic separation, which allows for the separation of isomers with minimal product loss.
Special attention is required when controlling the analytical purity of CBN, as even microscopic impurities of isomers or partially oxidized residues can significantly affect the results of further pharmacological or biochemical studies. Therefore, purification procedures often conclude with analytical control using nuclear magnetic resonance (NMR) spectroscopy, high-resolution mass spectrometry (HRMS), and ultra-high-performance liquid chromatography with UV-Vis or DAD detection. Only after passing these checks is the material considered suitable for further experimentation or pharmaceutical development.
Chemical Transformation of Cannabinoids in Laboratory Conditions
Under controlled chemical conditions, cannabinoids, particularly Δ9-tetrahydrocannabinol (THC), can be transformed into cannabinol (CBN) with high yield efficiency. In laboratory practice, these transformations rely on well-documented mechanisms of aromatization, dehydrogenation, and oxidative bond cleavage in the terpene portion of the molecule. The main advantage of this approach is reproducibility and the ability to control isomerization processes, which often complicate the natural degradation of THC.
Acidic and Basic Environments
The use of acid-base catalysis enables the acceleration or direction of THC conversion into CBN depending on the chosen reagent, concentration, and temperature. In an acidic environment (for example, using hydrochloric or sulfuric acid), THC undergoes dehydration, which facilitates the formation of a stable aromatic system characteristic of CBN. The application of mild protonic acids in non-polar solvents at elevated temperatures (40–80°C) is particularly effective, providing controlled degradation without excessive side polymerization or the formation of chlorine-containing artifacts.
In a basic environment, especially with the use of weak bases (such as sodium bicarbonate or organic amines), the main reaction involves initiating autocatalytic dehydrogenation, where THC loses two hydrogen atoms and forms a fully conjugated aromatic system. However, this approach has a narrower range of working conditions since unstable epoxides and peroxides, with potential cytotoxic activity, may form in alkaline environments.
Controlled Aging of THC
Modeling THC aging conditions in the laboratory allows for the simulation of the natural process of CBN formation, but with much higher efficiency and controllability. This method involves exposing a solution or semi-solid THC extract to oxygen, light, or heat under controlled conditions. For example, exposure to ultraviolet light (λ = 254 nm) in a moisture-limited atmosphere promotes the selective cleavage of π-bonds and the formation of CBN with minimal side products. Thermal aging (incubation at 70°C for 2–5 days) in dry air also shows high efficiency, especially when the process is combined with periodic stirring in the presence of micro-amounts of peroxides or ozone.
Methods of Extracting CBN from Plant Material
Since the natural content of CBN in fresh Cannabis sativa samples is usually low, effective extraction requires specialized conditions to preserve the integrity of the molecule and minimize losses. Traditional solvent methods (ethanol, hexane, chloroform) allow for obtaining an enriched fraction, but high-selectivity techniques significantly increase the yield and purity of CBN, even from materials with low initial concentrations of cannabinol.
Supercritical CO₂ Extraction with Degradation Consideration
Supercritical carbon dioxide (CO₂), combined with thermal or photochemical stimulation, is a powerful tool for selective cannabinoid extraction, particularly CBN. A key parameter is regulating pressure and temperature to activate partial degradation of THC during the extraction process-this not only enables the extraction of CBN but also enhances its concentration right at the moment of extraction. Conditions of 31°C and 150–250 bar with the addition of a modifier (5–10% ethanol) promote the enrichment of the CBN extract due to thermally stimulated formation. The advantage of this method lies in the purity of the product, absence of residual toxic solvents, and minimal thermal degradation of other compounds.
Chromatographic Separation (HPLC, Flash Chromatography)
For final isolation of CBN from the extract, high-resolution chromatographic methods are applied. High-performance liquid chromatography (HPLC) using C18 columns in gradient mode allows for the separation of cannabinoids differing in aromatic saturation and hydrophobicity. Modifiers of the mobile phase-such as acetonitrile, methanol with added water or acetic acid-affect selectivity, allowing for the isolation of CBN even in the presence of large amounts of THC acid or other isomers. For large-scale isolation, flash chromatography on silica gel with gradual increases in eluent polarity (hexane/ethyl acetate) is employed, effectively separating CBN from CBD, CBC, and cannabichromene without thermal stress.
Spectroscopic Identification
Due to the chemical similarity of cannabinoids, determining the purity and confirming the structure of CBN requires a multi-spectral approach that combines NMR, IR, and mass spectrometry. This is particularly relevant when working with synthetic analogs or fractions, where isomers may be structurally unidentified without detailed analysis of spectral features.
NMR (¹H, ¹³C), IR Spectroscopy
NMR spectra for CBN exhibit distinct signals characteristic of aromatic protons (δ 6.2–7.1 ppm) and methyl groups (δ 1.2–1.6 ppm), which differ from THC in shifts and multiplicity. In ¹³C-NMR, carbon signals from the aromatic core (δ 120–160 ppm) are clearly identified, allowing differentiation of CBN from CBD or CBC, even in the case of partial degradation. IR spectra show characteristic absorptions in the 3400 cm⁻¹ range (–OH), 1600–1625 cm⁻¹ (aromatic core), and 1260–1280 cm⁻¹, corresponding to C–O vibrations in the phenolic part of the molecule. Comparison of these spectra with library data ensures final identification.
Mass Spectrometry (MS)
CBN exhibits a characteristic molecular ion at m/z 310 in EI-MS or GC-MS spectra, corresponding to its molecular mass. The main fragments are ions at m/z 295, 279, and 264, formed by the loss of a methyl, isopropyl, or hydroxyl group. In high-resolution mass spectrometry (HRMS), ions are detected with accuracy to the fourth decimal place, enabling differentiation of CBN from its isomers and potential artifacts. Tandem mass spectrometry (MS/MS) is particularly useful, allowing the identification of intramolecular fragmentation, such as the loss of water or CO groups. This data enables the identification of even micro-impurities in analytical or pharmaceutical preparations based on CBN.
Chemical and Physicochemical Properties
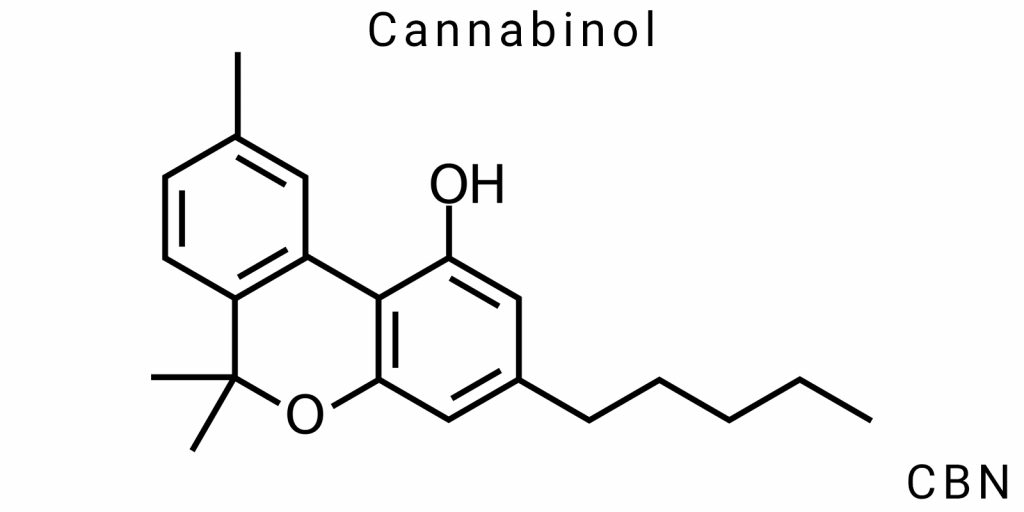
Cannabinol (CBN) belongs to the group of tricyclic phenolic terpenophenols and has a unique aromatic structure that distinguishes it from other natural cannabinoids, not only in terms of bioactivity but also in chemical behavior. Its molecular formula is C₂₁H₂₆O₂, with a precise molecular mass of 310.43 g/mol. The structure of CBN includes a conjugated aromatic system, which imparts distinct electronic properties and greater resistance to further oxidation compared to less aromatic cannabinoids.
Unlike Δ⁹-THC, the structure of CBN contains a fully aromatic ring A, which leads to increased planarity of the molecule, higher electron density in the π-bond system, and the potential for intermolecular π–π interactions. These features are crucial for crystallization, interactions with carriers, membranes, or receptors, as well as in the study of polymeric materials based on cannabinoids.
CBN typically crystallizes as colorless or light yellow needle-like crystals, with a melting point ranging from 76–77°C. Its solubility strongly depends on the polarity of the solvent: high in organic non-polar environments (hexane, chloroform, diethyl ether), limited in water, but it increases in the presence of surfactants or complexing agents. The dielectric constant of the molecule falls within ranges typical for phenolic compounds, with a logP value of approximately 6.3, indicating a high degree of hydrophobicity and preferential accumulation in lipid phases.
The optical properties of CBN are determined by its conjugated system, which results in pronounced absorption in the ultraviolet region of the spectrum (λmax ≈ 282 nm). This maximum is characteristic of π→π* transitions in benzene rings and is used for quantitative analysis of CBN through UV detection. In mass spectra and NMR characteristics, cannabinol stands out with clear structural signals, allowing it to be differentiated from other isomers, including degraded derivatives of Δ⁹-THC.
CBN is chemically less reactive to hydration, halogenation, or epoxidation at the aromatic core, which complicates its further functionalization without prior reduction of the aromatic system. However, the activity of the phenolic hydroxyl group remains, enabling reactions such as etherification, acylation, carbamate protection, or the formation of simple and mixed esters with multifunctional molecules.
Thermodynamic parameters, such as the enthalpy of fusion (~30 kJ/mol) and entropy of the transition to the liquid state, indicate a well-ordered crystalline lattice and limited sublimation capacity. At temperatures above 150°C, CBN shows signs of thermodegradation, accompanied by decarboxylation, especially in the presence of traces of cannabinolic acid or side products with incomplete aromatic rings. Its chemical stability increases in anaerobic environments or in the presence of antioxidants, allowing for predictable preservation of the substance in solid form without loss of activity for up to 12 months at temperatures up to 25°C.
The fluorescent properties of CBN are not yet fully characterized, but reports suggest weak emission when excited in the range of 290–310 nm, which opens up the potential for its application in molecular tracking systems or biosensor platforms. In terms of acid-base behavior, CBN acts as a weak acid, with a pKa of its phenolic hydroxyl group around 10.1, similar to cresol and other monophenols. This limits its ionization at physiological pH and contributes to its low water solubility.
Chemical interactions with metals, particularly with complex-forming centers (Cu²⁺, Zn²⁺), can lead to the formation of coordination complexes with potential biological functions. Research on such complexes is an emerging area of synthetic bioorganic chemistry for cannabinoids.
Molecular Structure and Stability
The molecular structure of cannabinol (CBN) is crucial in determining its chemical and physical properties, which in turn affect the stability of the compound. CBN has the molecular formula C₂₁H₂₆O₂, indicating 21 carbon atoms, 26 hydrogen atoms, and two oxygen atoms. Like other cannabinoids, CBN has a cyclic structure that includes a benzene ring. However, unlike other cannabinoids, particularly Δ⁹-THC, CBN contains a hydroxyl group (-OH), which plays a key role in defining its reactivity.
An important feature of CBN is its thermal stability. It is more thermally stable than Δ⁹-THC, which allows CBN to be more resistant to environmental conditions (high temperatures, exposure to oxygen). This means that CBN can be more stable during storage compared to Δ⁹-THC, which rapidly degrades when exposed to light and heat. However, under prolonged exposure to sunlight or elevated temperatures, CBN may degrade into other metabolites, such as cannabidiol (CBD) or cannabichromene (CBC), which also have different pharmacological effects.
From a chemical stability perspective, CBN is prone to oxidation because its structure contains an aromatic ring, which readily reacts with oxygen. Oxidation of CBN can result in changes to its pharmacological properties, such as a reduction in its analgesic effect or changes in its interaction with cannabinoid receptors.
Comparison with Δ⁹-THC and CBD
CBN, Δ⁹-THC, and CBD are three key cannabinoids found in cannabis plants, but their molecular structures and pharmacological properties differ significantly. Δ⁹-THC is the primary psychoactive compound responsible for the alteration of consciousness in cannabis users. In contrast, CBN has minimal psychoactive effects because its affinity for the CB1 receptor is much lower. This makes CBN a promising candidate for therapeutic use, where the absence of psychoactive effects is important.
CBD, on the other hand, is another cannabinoid with no psychoactive activity, but it has significant therapeutic potential. CBD possesses potent anti-inflammatory and anxiolytic properties and also exhibits antioxidant activity. Compared to CBN, CBD may have a broader range of applications, particularly in treating anxiety disorders, epilepsy, and chronic pain.
Although CBN is not a psychoactive cannabinoid, it holds considerable potential for treating a range of conditions, including insomnia, chronic pain, and inflammatory processes. One of its key properties is its ability to promote sleep, which makes it potentially useful for individuals suffering from insomnia.
Lipophilicity, Solubility, and Thermal Stability
The lipophilicity of CBN is an important characteristic as it defines its ability to dissolve in fat-soluble solvents. CBN is highly lipophilic, making it more accessible for extraction using organic solvents such as ethanol or hexane. This property allows for the production of highly concentrated cannabinoid extracts, which can be used for therapeutic purposes.
Since CBN is fat-soluble, it has limited solubility in water, which can affect its bioavailability when administered orally. To improve the bioavailability of CBN, specialized delivery methods, such as liposomal systems, are often used. These systems help the cannabinoid overcome water-soluble barriers and enter the bloodstream. Additionally, the lipophilic properties of CBN enable its use in the production of various products, such as oils, tinctures, and creams.
Regarding thermal stability, CBN can withstand temperatures up to 150°C without significant degradation. However, when exposed to temperatures above 150°C for extended periods, such as during decarboxylation processes or in the production of extracts, some of the CBN may convert into other cannabinoids or metabolites. This must be taken into account when developing processing technologies for plant extracts to preserve the maximum amount of active components.
Interaction with Acidic Environments (Gastrointestinal Tract)
Oral administration of CBN involves complex processes of absorption and metabolism in the gastrointestinal tract. A critical factor is that, like other cannabinoids, CBN has limited solubility in water, which makes its absorption in the stomach more difficult. The acidic environment of the stomach can influence the molecular structure of CBN, particularly its ability to form hydrogen bonds with other molecules.
The acidity of the stomach may lead to partial cleavage or transformation of CBN, which in turn could alter its biological activity. For this reason, most cannabinoids, including CBN, have low bioavailability when taken orally. To improve the absorption of cannabinoids, specialized formulations are often used to stabilize them prior to absorption, such as controlled-release capsules or liposomal systems.
Since CBN is not particularly stable in acidic environments, its pharmacokinetics when taken orally requires careful study. This is important for developing effective delivery methods that maximize its therapeutic effects.
Pharmacological Profile of CBN
Cannabinol (CBN) is one of the cannabinoids that, while not producing a pronounced psychoactive effect, has a range of important pharmacological properties, making it an interesting subject for scientific research and clinical applications. The interaction of CBN with cannabinoid receptors CB1 and CB2 is the primary reason for its pharmacological activity. At the same time, its ability to produce less pronounced psychoactivity compared to Δ⁹-THC makes it a promising candidate for medical use, where minimal impact on the patient’s consciousness and psyche is required.
CBN has a weaker affinity for CB1 receptors compared to THC, which means it does not induce strong psychotropic effects such as changes in consciousness or euphoria. However, it is capable of interacting with CB2 receptors, which are primarily located in peripheral tissues, such as immune cells, giving CBN potent anti-inflammatory and analgesic properties. This allows CBN to be used in the treatment of chronic inflammatory diseases, such as arthritis, skin conditions, or even certain autoimmune disorders. Furthermore, CBN has moderate anxiolytic activity, which makes it useful in the treatment of stress and anxiety disorders. Its ability to reduce anxiety levels could be particularly helpful for patients with emotional or psychological disturbances, without the strong drug dependence that can occur with traditional antidepressants or sedatives.
One of the most studied properties of CBN is its ability to improve sleep quality, although this effect is less pronounced than that of Δ⁹-THC. CBN has mild sedative properties, which may be beneficial for patients suffering from insomnia. Cannabinol can help with falling asleep without causing the intense intoxication typically associated with THC. However, research in this area is still ongoing, and more precise data regarding its effect on sleep phases will require further clinical trials.
The anti-inflammatory properties of CBN are another important aspect of its pharmacological profile. As a cannabinoid, it can reduce the production of inflammatory cytokines, such as interleukins, which may help reduce inflammation in the body. This is particularly important in the treatment of conditions such as chronic pain, arthritis, psoriasis, or other dermatological disorders, as well as for supporting nervous system health in neurodegenerative diseases like Alzheimer’s or Parkinson’s disease. CBN’s ability to modulate the immune response also makes it a promising agent for treating autoimmune diseases and chronic inflammatory processes.
Another important feature of CBN is its antioxidant properties. Due to its ability to neutralize free radicals, it can help reduce oxidative stress in the body, which is a key cause of many chronic diseases such as cardiovascular diseases, cancer, and neurodegenerative disorders. This makes CBN potentially useful as a preventive and therapeutic agent for these conditions, although further studies are needed to confirm this property.
CBN also shows promise in the treatment of infections, as its antibacterial and antiviral properties may be helpful in fighting certain bacteria and viruses. Some studies indicate that cannabinol may be effective in treating respiratory infections or skin infections, making it useful in developing new therapeutic strategies, especially in cases where traditional drugs are ineffective or have significant side effects.
A unique aspect of CBN’s pharmacological profile is its interaction with other cannabinoids, enhancing or altering their effects. For example, in combination with THC, it may reduce the intensity of some side effects, such as anxiety or the psychoactive impact. This synergy between cannabinoids is one of the important areas for future research, particularly in the context of developing multi-component therapeutic agents that provide a balance between efficacy and safety.
Cannabinol also demonstrates minimal side effects compared to stronger cannabinoids like Δ⁹-THC, making it safer for therapeutic use. However, like all cannabinoids, it may cause some side effects, including drowsiness, dry mouth, or mild changes in cognitive function, but these effects are usually minor and subside once the use of the substance is stopped.
The interaction of CBN with other medications is an important aspect that requires further study. Since it can affect the metabolism of other drugs by inhibiting certain liver enzymes, this could lead to changes in their concentration in the body. This must be taken into consideration when using CBN in patients who are taking other medications, particularly for the treatment of chronic conditions.
Affinity for Cannabinoid Receptors (CB1/CB2)
Cannabinol (CBN) has specific properties that allow it to interact with cannabinoid receptors CB1 and CB2, which defines its pharmacological activity and potential clinical applications. A key characteristic of CBN is that it is a partial agonist for CB2 receptors, with relatively weak activity toward CB1 receptors. These differences in receptor interaction indicate significant variations in the effects of the cannabinoid on the central and peripheral nervous systems, as well as its potential in treating various pathological conditions.
- Interaction with CB2 Receptors
CB2 receptors are primarily located in peripheral tissues, particularly in immune cells such as macrophages, lymphocytes, and neutrophils. They are also found in organs like the liver, kidneys, and skin, as well as in the nervous system, mostly in its peripheral parts. CBN’s affinity for CB2 receptors is relatively high, enabling it to effectively modulate various immune and inflammatory processes. This allows CBN to exert its anti-inflammatory and analgesic effects. The interaction with CB2 also contributes to CBN’s antioxidant activity, helping reduce oxidative stress and improving cellular regeneration in conditions of chronic inflammatory diseases.
In the context of therapeutic use, this is significant because activation of CB2 receptors does not induce the pronounced psychoactive effects typical of CB1 receptors. Therefore, CBN can be useful in treating inflammatory diseases without negatively affecting the patient’s mental state. This is particularly important when treating chronic pain, autoimmune diseases such as arthritis, psoriasis, or neurodegenerative disorders like Alzheimer’s disease, where inflammation plays a key role in disease progression.
- Interaction with CB1 Receptors
Although CBN has relatively low activity toward CB1 receptors, its ability to interact with these receptors still plays an important role in its pharmacological profile. CB1 receptors are concentrated in the central nervous system, particularly in the brain and spinal cord, where they regulate a variety of functions such as pain perception, memory, appetite, emotions, and motor coordination. Since CBN has a weaker affinity for CB1 receptors compared to Δ⁹-THC, its impact on the central nervous system is much less pronounced.
Thus, CBN does not produce strong psychoactive effects, which makes it safer for use in therapeutic settings where it is important to avoid any unwanted changes in consciousness or behavior. However, its ability to interact with CB1 receptors may explain some of its sedative effects, which could be beneficial in treating insomnia or anxiety disorders. Additionally, CBN may help mitigate some of the side effects associated with high doses of THC, such as anxiety or psychosis, due to its modulatory effect on CB1 receptors.
- Partial Agonist Mechanism
Cannabinol is a partial agonist for both CB1 and CB2 receptors, but its activity at these receptors differs significantly. Partial agonism means that CBN, when interacting with cannabinoid receptors, induces a suboptimal effect compared to a full agonist like THC. This allows CBN to modulate receptor activity without excessive activation, thereby reducing the risk of developing psychoactive or negative effects that are typical of stronger cannabinoids.
Such partial agonistic action can be beneficial in clinical use, where a controlled and balanced influence on cannabinoid receptors is necessary to achieve therapeutic effects without strong side effects. For example, its anti-inflammatory effects may be achieved through CB2 receptor activation while minimizing unwanted psychoactivity through weaker interaction with CB1 receptors.
- Prospects for Therapeutic Use
The weak activity of CBN on CB1 receptors, along with its significant affinity for CB2 receptors, makes it an attractive candidate for treating inflammatory and autoimmune diseases without posing a risk to the mental well-being of patients. Additionally, CBN may become part of combination therapies with other cannabinoids or pharmacological agents to achieve more effective results without the risk of dependency or psychosis. Therefore, studying the affinity and activity of CBN on cannabinoid receptors CB1 and CB2 holds considerable potential for developing new therapeutic strategies, particularly for patients with chronic inflammatory diseases, pain, neurodegeneration, and sleep disorders.
Neuropharmacological Effects
Cannabinol (CBN) possesses specific neuropharmacological properties that make it the subject of numerous studies aimed at exploring its potential therapeutic applications. However, there is some debate in the scientific community about whether CBN truly causes a pronounced sedative effect, as well as the extent of its neuroprotective properties.
Sedation: Myth or Justified Effect?
Sedation is one of the effects often highlighted when studying cannabinoids. While at first glance, CBN’s impact on the central nervous system may seem clear-cut, its sedative nature is not as obvious as that of other cannabinoids, particularly Δ9-THC. CBN does influence the state of the patient through its partial activation of CB1 receptors, but its effect on these receptors is much weaker compared to Δ9-THC, which is why its sedative effect is often described as mild and less pronounced.
The weak activity of CBN on CB1 receptors is a crucial aspect because this type of receptor is associated with the psychoactive and sedative properties of cannabinoids. Instead of heavily activating these receptors, CBN likely acts as a partial agonist, which can reduce stress and anxiety without significantly altering emotional states or cognitive functions. Scientific studies suggest that CBN may possess moderate calming properties, which could be useful in the treatment of anxiety disorders or insomnia. However, it is unlikely to induce a powerful sedative effect like THC.
At the same time, some research suggests that higher doses of CBN may cause a more pronounced sedative effect. However, these findings require further investigation to better understand the mechanisms behind its action on the central nervous system. Therefore, while sedation is part of its neuropharmacological profile, CBN’s effect should not be considered its primary or dominant trait, and it should be seen as having a moderate impact.
Potential Neuroprotective Properties
One of the main areas of research regarding CBN is its neuroprotective properties. Neuroprotection refers to the ability of a substance to reduce neuronal damage and protect neural tissue, preserving normal nervous system function. CBN has demonstrated potential in reducing oxidative stress, a key factor in the development of neurodegenerative diseases like Alzheimer’s and Parkinson’s.
The neuroprotective mechanism of CBN is believed to involve its antioxidant properties, which allow it to effectively neutralize free radicals and reduce oxidative stress that can lead to neuronal damage. As a partial agonist of CB2 receptors, CBN may also have an anti-inflammatory effect, reducing inflammation in the nervous system. Inflammation plays a key role in the progression of many neurodegenerative diseases, so CBN’s ability to modulate immune cell activity could help slow the progression of these conditions.
CBN’s neuroprotective effects may also result from its impact on cellular stress responses. Research has shown that CBN can promote the recovery of neuronal function after damage caused by toxins or infections, which gives it potential in the treatment of brain or spinal cord injuries. Its effects on neuroplasticity may be especially useful in treating disorders associated with the loss or damage of neurons.
CBN’s neuroprotection could also be linked to its ability to reduce neurotoxic compounds in neurons. It may help mitigate the risk of apoptosis (programmed cell death), which is important for recovery after neurodegeneration. Additionally, its ability to activate antioxidant pathways, such as the glutathione system, may further reduce damage caused by oxidative stress.
Impact on Immune Response and Inflammation
Cannabinol (CBN) is one of the cannabinoids that significantly influences the immune system. Its ability to modulate the immune response makes it an interesting subject of research for potential therapeutic applications in chronic inflammatory diseases, autoimmune disorders, and other conditions related to immune system dysfunction.
CBN has a particular ability to activate CB2 receptors, which are primarily involved in immune cells. This mechanism is important because the activation of CB2 receptors typically leads to a reduction in inflammatory processes. As a result, CBN is capable of decreasing the activity of immune cells, such as T-lymphocytes and macrophages, which play key roles in the development of inflammation. Therefore, CBN may have anti-inflammatory properties, which could potentially be beneficial in treating conditions like arthritis, inflammatory bowel diseases, rheumatoid arthritis, and even some skin diseases associated with chronic inflammation.
Moreover, CBN is capable of reducing levels of pro-inflammatory cytokines such as TNF-α, interleukin-6, and other molecules that promote immune cell activation and intensify the inflammatory process. This indicates that CBN could act as an inhibitor of inflammatory mechanisms, reducing chronic inflammation and its associated symptoms. However, to confirm these effects, further clinical studies are needed, as current data is limited to animal studies and laboratory tests.
Metabolism and Bioavailability
The metabolism of CBN is a complex process involving several stages of transformation in the body, including metabolism in the liver and the involvement of various enzymes. The primary mechanisms of CBN metabolism include oxidation, conjugation with glucuronic acid, and sulfation. Cannabinoids, including CBN, are actively metabolized in the liver via the CYP450 enzyme system. These enzymes play an essential role in metabolizing almost all cannabinoids by oxidizing molecules into active metabolites that may have varying pharmacological effects.
CBN is primarily metabolized through the enzymes CYP2C9 and CYP3A4, although other enzymes may also be involved. This is important for the clinical application of CBN because interactions with other drugs metabolized by these same enzymes can lead to changes in drug efficacy and toxicity. For example, taking CBN together with other medications that inhibit CYP2C9 or CYP3A4 may increase CBN levels in the body, which could heighten its effects or lead to side effects. Conversely, stimulating these enzymes could accelerate the metabolism of CBN and reduce its effectiveness.
As for bioavailability, cannabinoids generally have low bioavailability when taken orally due to the “first-pass” effect in the liver, where a significant portion of the active compound is metabolized before entering the systemic circulation. The bioavailability of CBN when taken orally may be even lower due to its poor water solubility and high molecular weight, which limits its ability to cross cell membranes. This makes using CBN in oil-based or lipophilic formulations a more effective method for delivery into the body.
Moreover, the bioavailability of CBN can vary depending on the route of administration. For example, when inhaled (via smoking or vaporization), the bioavailability is significantly higher because CBN enters the bloodstream directly through the lungs, bypassing the first-pass effect in the liver. This results in a faster and more potent effect, making inhalation one of the most efficient methods of administering cannabinoids, including CBN. However, inhalation also carries risks related to lung and respiratory health, which requires caution when using this method.
A comparison between oral and inhalation administration of CBN shows significant differences in their bioavailability and pharmacokinetics. Oral administration typically has a slower onset of action, as active metabolites must first pass through the liver before entering the bloodstream. Inhalation, on the other hand, provides a quicker onset of action and higher bioavailability, but the effect may be shorter-lived, as the cannabinoid is quickly metabolized.
Potential Applications
Preclinical Studies
Preclinical studies on cannabinoids, including cannabinol (CBN), are conducted on various animal models to evaluate the possible therapeutic effects and safety of this cannabinoid before transitioning to clinical trials on humans. Mice are commonly used in studies on CBN due to their genetic similarity to humans and well-established testing methods, making them crucial for exploring the pharmacological properties and mechanisms of action of cannabinoids.
One of the primary areas of research is assessing the impact of CBN on neuropharmacological effects, including its potential for sedation and appetite changes. Several studies have shown that CBN can induce a sedative effect, which, while less pronounced compared to Δ9-THC, becomes noticeable at certain doses. Animals treated with CBN displayed reduced activity, suggesting that this cannabinoid could have potential applications for conditions related to sleep disturbances or heightened anxiety.
Furthermore, CBN has been investigated for its influence on appetite. While some cannabinoids, particularly Δ9-THC, have a strong appetite-stimulating effect, data on CBN is more limited. However, it has been observed that CBN can modulate food intake behavior in animals, although this effect is not as pronounced as with other cannabinoids.
The anti-inflammatory properties of CBN represent another area actively being explored in preclinical studies. Numerous studies have demonstrated that CBN can reduce levels of pro-inflammatory cytokines and molecules such as TNF-α and interleukin-6, suggesting its potential for treating inflammatory diseases like arthritis, intestinal diseases, and skin conditions associated with chronic inflammation.
Clinical Prospects
The transition from preclinical studies to clinical trials is a significant step in the development of new pharmaceuticals, and CBN is no exception. However, several barriers exist in translational medicine that complicate the application of CBN in clinical practice. One such barrier is the insufficient research on the safety and efficacy of CBN in humans. Since most studies on cannabinol are limited to animal models, many aspects of the cannabinoid’s pharmacokinetics and pharmacodynamics, such as optimal doses, routes of administration, and potential side effects, remain inadequately explored.
Regulatory oversight is another substantial barrier to the clinical use of CBN. In many countries, cannabinoids are heavily controlled, and their use is restricted to specific conditions. This issue is particularly pertinent to the regulation of narcotic substances and their pharmaceutical forms, where legal restrictions regarding their use for medical purposes exist. Moreover, the regulatory framework can vary significantly from country to country, making it challenging to obtain permits for conducting clinical trials or to sell cannabinoids as medicines.
Another issue is the lack of clinical trials examining the effects of CBN on humans. Available data primarily come from animal studies or anecdotal observations. This means that large, randomized clinical trials are needed to confirm the efficacy and safety of this cannabinoid for treating specific conditions or symptoms. Only after obtaining evidence of its efficacy and safety, as well as determining optimal doses and methods of administration, can CBN become available for widespread clinical use.
The Role of CBN in the Polypharmacology of Cannabinoids
The polypharmacology of cannabinoids, particularly the “entourage effect,” is an essential aspect that requires further investigation in the context of CBN’s application. The entourage effect suggests that the interaction of various cannabinoids, terpenes, and other compounds in cannabis can enhance or modulate the therapeutic effects of these substances, providing more effective treatment. In the case of CBN, this effect may be observed when combined with other cannabinoids like Δ9-THC, CBD, or even other phytochemicals found in cannabis.
For example, some studies indicate that combining CBN with other cannabinoids can enhance its anti-inflammatory or neuroprotective activity while reducing potentially negative effects associated with Δ9-THC, such as paranoid reactions or anxiety. This suggests that using cannabinoid combinations for treatment could be more effective than using single molecules.
Moreover, studying the synergy between different cannabinoids is crucial for optimizing therapeutic regimens. The role of CBN in such combinations can vary depending on the therapeutic goal: it may act as the primary active compound or as an adjunct to enhance the effects of other cannabinoids.
Ethical, environmental and legal aspects of cannabinoid production
CBN as a Degradation Product: Waste or Valuable Resource?
Cannabinol (CBN) is traditionally recognized as a degradation product of Δ9-tetrahydrocannabinol (Δ9-THC), the primary psychoactive compound in cannabis. Its formation through oxidation and aging of THC, influenced by exposure to oxygen and light, presents new opportunities to explore CBN’s potential therapeutic properties. However, the debate persists: is CBN merely a byproduct with limited applications, or can it be considered a valuable resource?
Utilizing CBN as a valuable resource necessitates evaluating its economic viability and ethical implications. Efficient extraction methods from plant material not meeting standards for more prominent cannabinoids like Δ9-THC or cannabidiol (CBD) are under investigation. Given the growing demand for cannabinoids and their derivatives, future technologies may enable the processing of aging cannabis plants to obtain CBN, potentially reducing the environmental footprint of the cannabis industry.
Nonetheless, this approach raises environmental and ethical concerns. On one hand, cannabinoids resulting from degradation may have a lesser environmental impact compared to other synthesis methods. On the other hand, the need for substantial raw material to produce such compounds could lead to ecological issues related to cultivation and processing. Overexploitation of natural resources might compromise the sustainability of production chains.
Ethically, it’s crucial to consider the legality and responsibility in utilizing biological resources. Transitioning to large-scale use of CBN as a degradation product may introduce new ethical dilemmas concerning resource rights and control over cannabis genetic data. Producers must be wary of potential market monopolization and ensure equitable access to these resources for small and medium-sized enterprises.
Bioethics of Synthetic Cannabinoids
Synthetic cannabinoids, including synthetic CBN, are becoming integral to modern pharmacology and medicine. However, their use brings forth several ethical issues beyond safety, encompassing the moral aspects of employing advanced technologies to create molecules that mimic natural cannabis compounds. While synthetic cannabinoids allow for precise molecular control, their emergence poses risks of unauthorized use and potential health hazards due to non-compliance with quality standards or consumption in unsafe doses.
Moreover, synthetic cannabinoids often exhibit higher affinity for cannabinoid receptors compared to natural compounds, potentially leading to intensified or adverse effects such as psychotic reactions or severe cardiovascular issues. This necessitates stringent oversight of research and regulation to ensure both safety and ethical responsibility in medical and industrial applications.
Ethical considerations also arise in the context of developing new pharmaceutical products using synthetic cannabinoids. Synthetic analogs might reduce biodiversity and replace traditional treatment methods. According to bioethical principles, preserving natural resources and avoiding their substitution with synthetic alternatives without adequate justification or evidence of efficacy is paramount.
Legal Complexities Surrounding “Non-Psychoactive” Derivatives
The legal status of cannabinoids and their derivatives, such as CBN, remains one of the most intricate and contentious areas in drug policy and medicine. A critical issue is the legal definition of “non-psychoactive” cannabinoid derivatives. Although CBN lacks significant psychoactive effects, it sometimes falls under categories permitting medical use. However, certain jurisdictions impose legal restrictions on cannabinoids, regardless of their psychoactivity, complicating their production and market distribution.
Given that CBN results from Δ9-THC degradation, its legal classification becomes more complex. In some regions, all cannabinoids, even those without narcotic effects, are subject to strict control. This underscores the need for detailed legal frameworks defining which cannabinoids are permissible for medical and industrial purposes. Future legislative initiatives should aim to clarify classification criteria based on their impact on the central nervous system and establish clear boundaries for the authorized use of such compounds.
Legal ambiguities may also arise when cannabinoids, not classified as psychoactive substances, are still regulated due to their cannabis origin. These legal barriers pose challenges for producers seeking to commercialize CBN and other cannabinoids, often facing difficulties in obtaining licenses or market approvals. This highlights the necessity for harmonized legal standards at the international level to facilitate research and development of new medical and pharmaceutical products.
Conclusion
This article provides a detailed analysis of various aspects of cannabinol (CBN), starting with its chemical origins and synthetic pathways to its pharmacological properties and potential applications. It is noted that CBN, as one of the cannabinoids, is actively studied not only in terms of its therapeutic potential but also with regard to ecological and legal regulation. Its potential use in medical applications, particularly for reducing inflammation, alleviating pain, and offering neuroprotection, opens new possibilities for treating various conditions, although further research is needed to confirm its effectiveness and safety.
One of the key topics discussed in the article was the comparison of CBN with other cannabinoids, such as Δ9-THC and CBD. It was found that, although CBN has weak psychoactive activity and differs from these compounds, its biopharmacological profile presents interesting advantages, particularly regarding its interaction with cannabinoid receptors and potentially beneficial effects on the nervous and immune systems.
Equally important is the analysis of methods for obtaining CBN, which includes both biosynthetic and chemical approaches. The degradation of THC through aging and oxidation, as well as innovative extraction and chromatography methods, provide new opportunities for extracting and purifying CBN from plant material, promoting its commercialization and pharmaceutical applications.
At the same time, a number of ethical, environmental, and legal issues related to the production and use of CBN require careful regulation. Legislative restrictions on cannabinoids and cannabis-derived compounds, as well as the incomplete legal status of CBN as a “non-psychoactive” component, pose significant barriers to its widespread implementation in medical practice and commercialization.
Despite progress in understanding the biological and pharmacological properties of CBN, there remains a need for further research in preclinical and clinical trials to confirm its safety, effectiveness, and potential side effects. The lack of large-scale clinical studies leaves the real potential of this cannabinoid still insufficiently defined.
Sources:
- National Center for Biotechnology Information (NCBI) https://www.ncbi.nlm.nih.gov/
An official website that contains a wide range of scientific articles and studies, including those on cannabinoids such as CBN. - PubMed Central https://www.ncbi.nlm.nih.gov/pmc/
An open-access platform for scientific publications in the fields of medicine, biology, and chemistry. Specific articles on cannabinoids and CBN can be found using relevant keywords. - The Journal of Pharmacology and Experimental Therapeutics https://jpet.aspetjournals.org/
A well-known scientific journal that publishes research on pharmacology, including cannabinoids and their effects on CB1 and CB2 receptors. - Nature Reviews Drug Discovery https://www.nature.com/nrd/
A journal that contains reviews and articles on the pharmacological properties of cannabinoids, their biosynthesis, and their effects on various organs. - Frontiers in Pharmacology https://www.frontiersin.org/journals/pharmacology
An open-access journal featuring articles and studies on pharmacology, including research on the therapeutic effects of cannabinoids. - The Lancet Psychiatry https://www.thelancet.com/journals/lanpsy
An open-access journal publishing research on the psychiatric effects of cannabinoids and their role in treating neurological and mental disorders. - Cannabis and Cannabinoid Research https://www.liebertpub.com/can
A renowned journal publishing original research related to cannabis and cannabinoids, including CBN. - Harvard University – Department of Neurobiology https://neuro.hms.harvard.edu/
A prominent scientific institute with resources and research in neurobiology, where studies related to cannabinoids are conducted. - U.S. National Institute on Drug Abuse (NIDA) https://nida.nih.gov/
An official website that publishes materials and research on cannabinoids, their effects on the human body, and mental health. - American Chemical Society (ACS) https://www.acs.org/
An organization that publishes scientific research in chemistry, including studies on cannabinoids and the chemical properties of CBN.