The chemistry of cannabinoids at the current stage of development has gone far beyond the natural cannabis product and its primary metabolites. Increasingly, research strategies are focusing not only on the principal phyto- and endocannabinoids, but also on artificially modified derivatives that either model the pharmacophoric properties of known compounds or, conversely, radically alter their biological behavior. Among these derivatives, particular attention is given to esters, amides, halogenated derivatives, and conjugates, which are utilized both in fundamental medicinal chemistry and in the development of pharmaceuticals, ligand prototypes, and molecular probe systems.
Cannabinol methyl ether (CBNM) is one of the least described members of the class of simple cannabinoid ethers, emerging at the intersection of several scientific disciplines-organic synthesis, pharmacological screening, bioanalytical chemistry, and molecular design. Despite its absence in major review papers or classification schemes, CBNM is potentially a key marker in the chemical modification of CBN-the oxidation product of tetrahydrocannabinol (THC), which itself is a central focus of numerous neuropharmacological studies. In turn, the transformation of the phenolic hydroxyl group of cannabinol into a methyl ether is not a mere functionalization: it alters electron density, reduces hydrogen-bonding capacity, modifies the metabolic profile, and may affect the spatial orientation of the molecule within receptor environments.
CBNM is not a natural constituent of cannabis-at the time of writing, it has not been detected in any botanical extracts, either in trace amounts or in significant concentrations. This imposes a particular specificity on the interpretation of its biological relevance: unlike the major phyto-analogues, CBNM is an artificially obtained compound that serves more as a tool than as a final bioactive agent. However, this tool allows for the resolution of several fundamental tasks: isolating the effects of the aromatic portion of the molecule without phenolic hydrogen involvement; reducing polarity to model membrane permeability; analyzing how the modification influences binding to CB1/CB2 receptors; and studying the kinetics of CBN transformation under metabolic conditions.
Unlike some of the more well-known cannabinol derivatives, CBNM does not have a documented pharmacological profile in the open scientific literature. However, it is mentioned in the context of mass spectrometric detection in synthetic library samples and patent databases. Several research groups note its presence as a byproduct during the methylation of CBN or as an intermediate structure in multistep syntheses of heterocyclic cannabinoid derivatives. This suggests its potential value not as an independent therapeutic agent, but rather as a chemical module in the construction of more complex molecular systems-including prototypes of selective agonists or antagonists of cannabinoid receptors, as well as an inactive form that may be activated in situ under specific conditions.
There is also theoretical interest in this compound from a toxicological analysis perspective. Preparations containing synthetic cannabinoids are often accompanied by impurities or secondary metabolites, among which ether derivatives may appear. Thus, CBNM could serve as a valuable control sample in the development of analytical detection methods-for instance, in pharmaceutical quality control or forensic analysis.
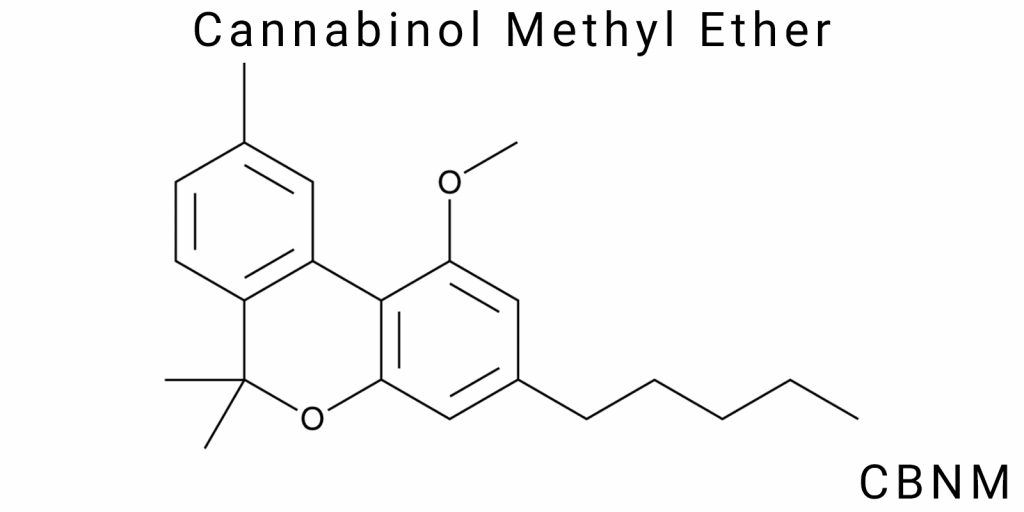
Structure of CBNM
Basic Formula
Cannabinol methyl ether (CBNM) is a derivative of cannabinol in which the hydroxyl group, typically located at the 1-position of the benzene ring, is replaced with a methoxy group –OCH₃. From the standpoint of organic chemistry, this structural modification is a straightforward example of a phenol alkylation reaction, but the consequences of this substitution extend well beyond nominal functionalization. The CBNM molecule retains the core cannabinoid skeleton, including the tricyclic structure with a partially saturated hexyl side chain at position 5, which forms the central fragment of the molecule-critical for spatial orientation and interaction with biological targets.
CBNM has an empirical formula of C₂₂H₂₈O₂. Structurally, it consists of a tricyclic scaffold composed of:
- a benzene ring linked to a dihydropyran ring;
- a third ring that is partially hydrogenated, containing both saturated and unsaturated bonds, which are essential for spatial rigidity;
- an aliphatic substituent in the form of a hexyl chain at the 5-position;
- a methoxy group at the 1-position of the benzene ring replacing the phenolic hydroxyl group.
A distinctive feature is the presence of both aromatic and partially unsaturated segments, creating a contrast in the distribution of electron density across the molecule. This directly determines the electrophilic and nucleophilic properties in localized regions and the potential reactivity in the context of further modifications.
Of particular interest is the electronic structure of CBNM. The substitution of –OH with –OCH₃ not only eliminates the hydrogen bond donor, but also stabilizes the electron cloud through the +M (mesomeric) effect of the methoxy group. This enhances π-electron delocalization in the aromatic ring, increasing its chemical inertness and reducing its susceptibility to oxidation. Such electronic reconfiguration is significant in predicting the bioisosteric behavior of CBNM in receptor environments.
CBNM does not exist as a stable crystalline solid like some other cannabinoids. According to mass spectrometric data and synthetic reports, it exists as an oily liquid under standard conditions. This physical state results from a combination of moderate molecular weight, absence of intramolecular hydrogen bonding, and overall lipophilicity.
Isomers and Configuration
CBNM formally lacks stereoisomerism in the region where the methoxy group replaces the hydroxyl-it is a planar region of the molecule with no steric hindrance. However, the molecule as a whole possesses several spatial characteristics that can influence its activity, and these effects are often underestimated. Although the ether moiety is not chiral in the classical sense, the overall CBNM scaffold may exhibit pseudochirality due to the fixed conformation of the tricyclic core, particularly because of the partially saturated ring bearing the aliphatic chain.
There is a potential for the existence of so-called conformational isomers-that is, isomers arising from rotation around σ-bonds, particularly in the region of the side hexyl chain. In the case of CBNM, the flexibility of this chain may be significant when studying the spatial interaction with protein domains. Molecular docking data indicate that variations in the positioning of this chain alter complementarity with hydrophobic pockets of protein targets, even when the rigid core of the molecule remains unchanged.
Conformational plasticity also has implications for pharmacokinetics. For example, different conformers of CBNM may exhibit differing abilities to traverse lipid membranes or varying resistance to oxidation by microsomal enzymes. This aspect is critical in evaluating the potential of CBNM as an intermediate or active component in the structure of cannabinoid receptor ligands.
It is also worth noting that although the methoxy group itself is not chiral, it can create an asymmetric environment in combination with other molecular fragments, particularly when forming non-covalent complexes or clathrates with receptor proteins. Such phenomena have been documented in other ligand classes, and while no direct evidence exists for CBNM, theoretical models allow for the assumption of functionally relevant second-order chirality-that is, chirality not derived from a chiral center, but from the spatial asymmetry of the molecule in a biological context.
Chemical Properties
Lipophilicity
The lipophilicity of cannabinol methyl ether (CBNM) arises from its chemical structure, which includes a significant number of non-polar fragments and lacks polar groups capable of forming strong hydrogen bonds with aqueous environments. Unlike the parent compound cannabinol (CBN), which contains a phenolic hydroxyl group contributing to partial hydrophilicity, CBNM features a methoxy group substitution. This substitution exhibits only a weak dipolar character and does not participate in hydrogen bonding in water. As a result, the molecule’s overall aqueous solubility is reduced, while its solubility in lipid phases is enhanced.
According to experimental logP values and computational estimations (e.g., via XLogP or ALOGPS methods), CBNM demonstrates increased lipophilicity compared to other natural cannabinoids, including various CBN derivatives. This elevated lipophilicity introduces several critical pharmacological and toxicokinetic implications. Firstly, the high affinity for lipid environments enables rapid penetration across biological membranes, including the blood-brain barrier. This positions CBNM among molecules with potential neurotropic activity, even in the absence of direct affinity for cannabinoid receptors.
Secondly, lipophilicity plays a key role in CBNM’s capacity to accumulate in adipose tissues. This mechanism is well-documented for THC and other fat-soluble compounds, but in the case of CBNM, an additional stabilizing effect is likely due to the steric protection offered by the methoxy group. The absence of a polar hydroxyl group precludes glucuronidation metabolic pathways, thereby extending the compound’s residence time in the organism.
Of particular note is lipophilicity’s role in pharmacophore modeling. For CBNM, the lipophilic profile reveals a pronounced orientation toward interaction with intracellular targets located in membrane microdomains, especially within cholesterol-dependent receptor platforms. This underpins the potential involvement of CBNM in signaling pathways associated with lipid rafts and opens new avenues for research focused on membrane-associated bioactivity.
Stability
CBNM’s stability is governed by a combination of electronic and spatial factors that shield the molecule from degradation under external influences. The most significant structural feature of CBNM is the replacement of the hydroxyl group with a methoxy group. The hydroxyl group, in contrast to the methoxy group, is a chemically reactive center susceptible to oxidation, dehydrogenation, and participation in autocatalytic processes under light or thermal exposure. Substitution with –OCH₃ significantly enhances compound stability, as methylated ethers are more resistant to free radical attack, UV radiation, and acidic or basic environments.
Spectrophotometric studies have shown that CBNM exhibits reduced photodegradation compared to CBN. This photostability is due to decreased electronic activity of the aromatic core after methylation, which hampers the initiation of photochemical decomposition. The energy transition from ground to excited state shifts toward longer wavelengths, decreasing photochemical activity under natural light conditions.
CBNM also displays greater thermal stability than the base CBN molecule. In the gas phase, the compound remains stable up to 230–240 °C, after which thermal decomposition begins, involving the cleavage of aliphatic bonds and breakdown of the partially saturated ring. In organic solvent solutions, CBNM maintains stability for over six months when stored at 4–8 °C. Its chemical stability under physiological conditions (pH 7.4, 37 °C) exceeds 24 hours, indicating low reactivity in in vitro testing environments.
Attention should also be given to CBNM’s resistance to oxidants. For CBN, oxidative degradation of the phenolic core-initiated by peroxidase enzymes or reactive oxygen species-is typical. However, this pathway is blocked in CBNM. The ether group does not undergo enzymatic oxidation through standard mechanisms, which significantly extends the compound’s half-life in the presence of hepatic microsomal enzymes.
Reactivity
Despite its general chemical inertness under standard conditions, CBNM exhibits clear reactivity in specific electrophilic substitution reactions within the aromatic ring, as well as in transformations involving the methylated ether group. The aromatic system, activated by the mesomeric effect of the methoxy group at position 1, allows for electrophilic substitution reactions at the ortho- and para-positions, although these sites are often already occupied or sterically hindered. Nevertheless, nitration, sulfonation, and formylation reactions can be carried out under strictly controlled conditions.
Especially noteworthy is CBNM’s metabolic reactivity. Under microsomal oxidation conditions (e.g., in vitro in human liver microsome systems), the methoxy group can undergo O-demethylation catalyzed by cytochrome P450 enzymes, particularly CYP2D6 or CYP3A4. This process results in the formation of a free hydroxyl group, effectively regenerating CBN or yielding an intermediate between the ether and parent compound. Importantly, not all etherified cannabinoids undergo such reactions, but this pathway has been experimentally confirmed for CBNM using radiolabeling techniques.
CBNM may also act as a methyl group donor in chemically catalyzed methyltransferase-like reactions, although such processes are not typical in vivo. In laboratory conditions, CBNM has been shown to participate in oxidative cleavage reactions, leading to the formation of corresponding carboxylic acids following the cleavage of the methylene bridge between the aromatic ring and the pyran core.
Another potential reaction involves interaction with electrophilic alkylating or acylating agents. In the presence of a Lewis acid catalyst, the methoxy group of CBNM can initiate electrophilic attack within the aromatic region, leading to the formation of complex tertiary products. These reactions are particularly relevant for synthesizing derivatives with pharmacophore modulation or for spectral labeling applications in advanced analytical methods.
Synthesis Methods
Methylation of the Phenolic Group
Methylation of the phenolic group in the structure of cannabinol is a key synthetic step in the transformation of CBN into CBNM (cannabinol methyl ether). This process not only modifies the physicochemical properties of the molecule, but also significantly alters its electronic structure, reactivity, metabolism, and potential pharmacodynamics. It is at this stage that a subtle chemical transformation takes place, converting the phenolic hydroxyl group into an ether, thereby changing hydrogen bonding capacity, acidity, the electrophilicity of the aromatic core, and the compound’s susceptibility to further electrophilic or nucleophilic reactions.
The phenolic group in CBN is a classical nucleophilic center, which-due to the lone pair of electrons on the oxygen atom and conjugation with the aromatic system-is capable of participating in alkylation reactions with electrophilic reagents. The choice of methylation conditions depends on requirements for selectivity, yield, absence of side products, and the stability of intermediates.
The most common reagents for carrying out the methylation reaction include dimethyl sulfate (DMS), methyl iodide (CH₃I), methyl triflate (MeOTf), and diazomethane (CH₂N₂). In the case of CBN, the use of diazomethane is often considered preferential due to its high selectivity, mild reaction conditions, and near-complete absence of byproducts. Diazomethane reacts with the phenolic group via a nucleophilic substitution mechanism to form the methyl ether at room temperature or even in an ice bath. In this scenario, methyl ether formation results from the reaction between the nucleophilic oxygen and the electrophilic center of diazomethane, with nitrogen gas being released simultaneously.
However, the use of diazomethane is associated with significant risks-the substance is toxic, carcinogenic, and highly explosive, requiring the use of specialized equipment (vacuum lines, fume hoods with fluoropolymer gaskets) and strict adherence to safety protocols. Therefore, in large-scale synthesis of CBNM, less hazardous reagents such as methyl iodide in the presence of a base are more commonly used.
In classical alkylation conditions using CH₃I, the reaction is carried out in the presence of a weak or strong base, such as K₂CO₃ or NaH, in dry dimethylformamide (DMF) or acetonitrile. The primary role of the base is to deprotonate the phenolic group, forming a phenolate anion, which is a much more reactive nucleophile. Subsequent interaction with methyl iodide affords the methyl ether with nearly quantitative yield. The use of polar aprotic solvents stabilizes the phenolate and promotes efficient contact between reagents in a homogeneous medium.
In cases where high selectivity and minimization of side reactions in multifunctional systems are required, methyl triflate (methyl trifluoromethanesulfonate) is used, an extremely strong methylating agent. Its electrophilicity allows reactions even with weak nucleophiles. In the case of CBN, its advantage lies in enabling the reaction under mild temperatures (below 0 °C), preserving the integrity of thermally sensitive fragments of the molecule, particularly the unsaturated pyran ring.
It is important to note that the phenol methylation process can be accompanied by side reactions-such as alkylation of other activated carbon centers or formation of polysubstituted products. To prevent this, controlled addition of reagents, an excess of base, low reaction temperatures, and process monitoring via thin-layer chromatography (TLC) or HPLC are employed. In more complex synthesis protocols, the use of protecting groups or prior selective functional blocking of active centers may be warranted.
Additionally, the conformational flexibility of the CBN molecule results in varied accessibility to the phenolic group depending on spatial surroundings, particularly due to steric hindrance from the terpene fragment. This may necessitate additional activation energy or a change in solvent to a less polar but more voluminous one (e.g., tetrahydrofuran or 1,4-dioxane), which provides better solubilization and proper orientation of reagents.
Phenol methylation is not merely a functionalization-it is a subtle electronic modification that fundamentally alters the electron density distribution in the molecule, its reactivity, hydrophobicity, and potential for intermolecular interactions. For CBNM, this step is decisive both in the synthetic context and in the design of derivatives with defined biopharmaceutical characteristics.
Choice of Reagents and Conditions
The selection of reagents and conditions for the methylation of cannabinol critically affects not only the yield of the target product CBNM but also the regioselectivity, chemical purity, stability of intermediate forms, and scalability of the synthesis. In the case of the chemically active phenolic group of cannabinol, which is located in an electronically rich aromatic ring adjacent to other functional groups, it is essential to consider electron delocalization, hydroxyl acidity, and the potential formation of side products during uncontrolled methylation.
A key parameter is the choice of methylating agent. Methyl iodide (CH₃I) remains one of the most commonly used in classical synthesis due to its moderate electrophilicity and availability. It works well with bases of medium strength, such as potassium or sodium carbonate, which ensure complete deprotonation of the phenol without the risk of alkaline degradation. A crucial detail is the strict exclusion of water and oxygen-even trace amounts of moisture significantly reduce the nucleophilicity of the phenolate anion and may lead to hydrolysis of methyl iodide.
Methyl triflate (MeOTf), in contrast, exhibits extremely high reactivity and is used in reactions where extraordinary selectivity and speed are required. It allows methylation of even electronically deactivated phenols or thiols. In the case of CBN, its application is justified when other functional groups sensitive to temperature or pH are present, or when maximum conversion is needed with minimal solvent volumes. However, MeOTf requires strict temperature control (from –78 to 0 °C), an inert atmosphere (argon, nitrogen), and cannot be used in large-scale production without specialized equipment.
Dimethyl sulfate (DMS), although less reactive than MeOTf, remains a standard reagent in industry due to its low cost and efficiency under mildly basic conditions. However, it is highly toxic and requires chemical neutralization of residuals after synthesis, limiting its use in laboratories without proper protection. Reactions involving DMS are typically conducted in aqueous-organic mixtures, where phenol deprotonation is achieved using triethylamine or pyridine.
For mild and highly selective methylation, the diazomethane–ethyl ether system is particularly convenient. This pair provides rapid alkylation even in the presence of other functional groups. Diazomethane is generated in situ from N-methyl-N-nitrosotoluenesulfonamide or from N-nitrosomethylurea by reaction with bases. This system avoids the need for high temperatures but requires careful degassing and protection from light. To reduce risks, flow-based diazomethane generators or commercially available solutions with controlled concentrations are employed.
Solvents play a crucial role. Polar aprotic solvents-such as DMF, DMSO, acetonitrile, and tetrahydrofuran-help stabilize the phenolate ion by reducing ion pairing with alkali metal cations. This increases nucleophilicity and allows the reaction to proceed at lower temperatures or with smaller amounts of reagents. The choice of base is also determined not only by its strength but also by its solubility in the reaction medium. For example, NaH often outperforms K₂CO₃ in reactions with poorly soluble substrates, whereas in solvents with low dielectric constants, tetraalkylammonium hydroxides or fluorides are preferable.
Kinetics are also of major importance. The methylation of the phenolic group in CBN proceeds via pseudo-first-order kinetics, with the rate-limiting step being phenolate formation. This means that preliminary activation (e.g., preheating with base before adding the alkylating agent) can accelerate the reaction without compromising selectivity. Reaction monitoring is typically carried out using ^1H NMR, where the disappearance of the phenolic proton is accompanied by the appearance of a methoxy group signal in the 3.6–3.8 ppm region.
Catalysis and Selectivity
The choice of catalyst and its role in the synthesis of cannabinol methyl ether (CBNM) are key factors for achieving high reaction selectivity, optimizing product yield, and minimizing side reactions. Catalysis in methylation processes includes several aspects: the type of catalyst (homogeneous or heterogeneous), the reaction mechanism, the efficiency of reagent activation, and control over regioselectivity.
Homogeneous Catalysis
Homogeneous catalysts are used in cases where the reaction must be controlled at the molecular level and when high selectivity is required. In the synthesis of CBNM, homogeneous catalysts are typically based on metal complexes capable of effectively activating methylating agents such as methyl iodide or methyl triflate.
One of the most common approaches involves the use of catalysts based on transition metals, particularly palladium (Pd) or platinum complexes. Palladium, especially in complexes with organic ligands, can coordinate both the alkylating agent and the phenolic group of cannabinol, activating the C–I bond (in the case of methyl iodide) or the C–O bond (for methyl triflate), resulting in highly efficient methylation. Palladium catalysts are selective and can be used for methylation at ortho- or para-positions of the aromatic ring, depending on the ligand type and reaction conditions.
Another important aspect is the use of organocatalysts, such as amines or organic bases, which are non-metallic but can activate the reaction through the formation of intermediate complexes. In particular, organic bases like triethylamine or pyridine can increase the nucleophilicity of the phenolate ion, enabling rapid reaction with methylating agents. This type of catalysis is less toxic and reduces metal contamination in the final product, which is especially important for the pharmaceutical application of CBNM.
Heterogeneous Catalysis
Heterogeneous catalysts are employed in large-scale processes that require catalyst stability and ease of separation from the product. In the context of cannabinol methylation, metal oxide–based catalysts, such as alumina or zeolites, are often used as solid supports for active components such as metal nanoparticles or organic ligands.
Palladium or platinum nanoparticles supported on such carriers demonstrate high activity and reusability, making them economically attractive for industrial applications. Additionally, heterogeneous catalysts provide more stable reaction conditions, reducing the risk of degradation of the methylating agent and improving the cost-effectiveness of the process.
Reaction Selectivity
The selectivity of phenolic methylation in CBNM is influenced not only by the catalyst choice but also by other factors such as solvent, temperature, and reagent concentration. Selecting optimal synthesis conditions enables the desired regioselectivity (i.e., methylation at a specific position on the aromatic ring) and avoids side reactions.
Temperature is a key factor, as raising the temperature can accelerate undesirable processes such as polymerization or oxidation, which negatively affect selectivity. High temperatures can also cause decomposition of certain methylating agents, such as methyl iodide, reducing the yield of the target product.
Furthermore, the choice of solvent is critical for maintaining reaction stability and achieving optimal catalyst activity. In organic solvents such as tetrahydrofuran (THF) or dimethylformamide (DMF), methylation proceeds faster due to the lower polarity of the medium, which improves the solubility of both the cannabinol and the methylating agent. Aqueous-organic mixtures offer milder reaction conditions and can be used to achieve selectivity without the need for elevated temperatures.
To maximize methylation selectivity, the pH of the medium must also be carefully controlled. Using basic conditions helps stabilize the phenolate anion and enhances its nucleophilicity, thereby improving the yield of the methyl ether without producing unwanted byproducts. At the same time, the concentration of base must be carefully managed, as excessive base can lead to alternative reactions, such as methylation of other functional groups in the molecule.
Choice of Base
The choice of base is crucial for methylation, as it not only determines the reaction rate but also the selectivity of the process. Typically, medium-strength bases such as potassium carbonate (K₂CO₃), sodium carbonate (Na₂CO₃), or triethylamine are used. These bases are strong enough to deprotonate the phenolic group, but not too aggressive to cause side reactions.
Sodium-based bases such as KOH or NaOH can be useful for CBNM synthesis at high temperatures or in environments with limited solvent. However, they require caution due to their potential aggressiveness and tendency to hydrolyze the methylating agent.
Triethylamine, on the other hand, is used for milder methylation, especially in conditions where high regioselectivity and the absence of side reactions are important. This can be useful for laboratory-scale CBNM synthesis or for the production of high-purity compounds where avoiding side effects is crucial.
Scalability of Synthesis
The scalability of the methylation reaction for cannabinoid methyl ether (CBNM) refers to the ability to transfer laboratory conditions to industrial-scale processes while maintaining high efficiency, selectivity, and cost-effectiveness. Scalability is critical for the large-scale production of chemical compounds, as it involves not only optimizing reaction conditions but also managing mass and heat transfer processes, which significantly influence synthesis outcomes at larger scales.
Scaling Up the Reaction
The transition from laboratory synthesis to a scalable process requires considering several factors, such as reaction kinetics, reagent concentration changes, mixing rate, heat and mass transfer in the reactor, and potential issues with efficiently removing by-products. One of the key challenges in scaling the CBNM synthesis is maintaining optimal conditions for methylation as the volumes of the reaction mixture significantly increase.
It is also essential to control catalyst aggregation and concentration. In laboratory settings, catalysts (e.g., organic ligands or metal particles) can be present at high concentrations without serious consequences, but when scaling up, their concentration and effectiveness must be carefully optimized. This is particularly important for metal catalysts, where uniform distribution and avoiding decreased activity at large reaction volumes are necessary.
A critical stage in scaling up is selecting the right reaction vessel. For CBNM synthesis, mixing reactors are used to effectively control temperature and distribute reagents across a large volume. Additionally, heat removal efficiency plays a significant role since exothermic reactions can lead to overheating and undesired side reactions. To maintain a stable temperature, specialized heat exchangers or cooling systems are used.
Reaction Kinetics and Efficiency
The kinetics of methylation of cannabinoid directly depend on reagent concentration, type of catalyst, temperature, and mixing speed. When scaling, these parameters must be carefully adjusted to ensure effective achievement of equilibrium with maximum CBNM yield.
In laboratory conditions, where reactions typically run for short periods, the rate of methylation can be very high, especially with homogeneous catalysts or favorable solvents. However, at a larger scale, the reaction rate often decreases due to limitations in the movement of molecules of reagents and catalysts. During reactions at larger volumes, issues regarding diffusion and mixing arise, which can lead to uneven distribution of reagents and, consequently, reduced selectivity and yield.
To achieve high efficiency at larger scales, it is essential to determine the optimal reagent concentrations to ensure fast reactions without producing undesirable by-products, such as methylating agent degradation or incomplete methylation. Additionally, processes that accelerate kinetics, such as ultrasonic or mechanical mixing, are often employed to enhance the interaction of reagents and catalysts.
Side Reactions and Their Control
At larger scales, there is a higher likelihood of side reactions occurring, which can be caused by several factors. For example, higher reagent concentrations or excessive temperatures may promote the formation of by-products from oxidation or polymerization. In such cases, to prevent side reactions, controlled reaction conditions must be implemented, including optimizing temperature, pH levels, and moisture control within the environment.
In industrial settings, specialized technical methods are often used to remove unwanted products or reduce their concentration. For example, vacuum distillation stages can be employed to remove excess reagents, or filtration can be used to eliminate catalytic impurities.
Ecological Aspects and Economic Efficiency of the Process
Scalable synthetic processes, particularly those involving methylating agents, require additional stages of waste treatment and by-product handling, which increases production costs and environmental impact. Therefore, one of the key areas of focus is optimizing not only the chemical efficiency but also the ecological sustainability of the process.
In particular, the industrial production of CBNM (cannabinol methyl ester) requires careful control over the use of methylating agents, such as methyl iodide, which can be toxic. As a result, safer analogs or lower-toxicity methods for both the environment and workers are being explored. At the same time, strategies are being developed for the reuse or regeneration of catalysts after reactions, helping to reduce raw material costs and minimize waste.
Minimizing energy consumption is also an essential part of the process, particularly when high temperatures are involved in the synthesis. The use of alternative heating methods, such as microwave processing or ultrasonic heating, can help reduce energy expenditures and make the process more efficient.
Analysis of Cannabinoid Derivatives
Identification (NMR, MS, IR)
Methyl ester of cannabinol (CBNM) is a complex organic compound that can be identified and characterized using several high-tech analytical methods. The accuracy and reliability of the identification of such compounds require the use of methods like Nuclear Magnetic Resonance (NMR), Mass Spectrometry (MS), and Infrared Spectroscopy (IR). Each of these methods provides unique and complementary data about the molecule’s structure, allowing not only the confirmation of CBNM’s presence but also a detailed examination of its chemical nature.
Nuclear Magnetic Resonance (NMR) is one of the most important techniques for studying the structure of organic compounds, particularly for identifying methyl ester of cannabinol (CBNM). This method allows the examination of the magnetic environment of atoms in a molecule, which is the basis for determining its structure. In the case of CBNM, different types of NMR are used, including ^1H-NMR, ^13C-NMR, and two-dimensional spectra like COSY, HSQC, and HMBC, which provide precise data about the relationships between atoms in the molecule.
For CBNM, the ^1H-NMR spectrum is expected to show several key signals characteristic of the methyl group and the aromatic core. The methoxy group (–OCH₃) typically gives a multiplet in the range of 3.6–3.8 ppm due to interactions with adjacent hydrogen atoms. The aromatic protons of cannabinol usually appear in the region of 6.5–7.5 ppm. Also significant is the signal corresponding to the hydrogen located at position 2 or 6 on the benzene ring, depending on the molecule’s geometry. Such details help determine the exact structure and atomic arrangement in the molecule.
The ^13C-NMR spectrum is important for studying the carbon atoms in the molecule. In the ^13C-NMR spectrum for CBNM, a signal corresponding to the carbon of the methoxy group is expected to appear in the range of 55–58 ppm. Additionally, signals from the carbon atoms of the aromatic ring (from 110 to 160 ppm) will be observed, allowing for identification of specific positions of the carbon atoms in the benzene ring.
The application of two-dimensional spectra such as COSY, HSQC, and HMBC enables the determination of more detailed relationships between hydrogen and carbon atoms and helps precisely identify which atoms are adjacent to one another. This is especially important when analyzing complex molecules, where it is challenging to determine the structure using only one-dimensional spectra.
Mass Spectrometry is a powerful method for determining the molecular mass of a compound and its fragments, which allows not only the confirmation of the identity of methyl ester of cannabinol but also provides important information about its molecular structure. For CBNM, as with most organic compounds, the following features are expected in the mass spectrum:
When performing mass spectrometry using electron impact (EI) or electrospray ionization (ESI), the CBNM molecules are ionized and produce a molecular ion (M+), the mass of which corresponds to the molecular mass of the compound. This helps confirm the molecular mass of CBNM, which typically is 314.46 g/mol.
After ionization, fragmentation occurs, where the molecule breaks into smaller pieces. For CBNM, typical fragments include the loss of the methoxy group (–OCH₃), as well as characteristic fragments from the aromatic core and side chains. For instance, the loss of the methoxy group may yield a fragment with a mass of 284 g/mol. Studying such fragments not only confirms the identity of CBNM but also helps understand its molecular structure.
The analysis of ion peaks and their intensities allows for both the identification of the molecule and quantitative determination of CBNM concentration in a sample. Mass spectrometry provides not only the molecular mass but also fragmentation information, which is vital for understanding the mechanisms of metabolism and chemical stability.
Infrared Spectroscopy (IR) is an important method for studying the functional groups in a molecule, particularly for identifying the presence of a methoxy group, which is characteristic of the methyl ester of cannabinol. In the IR spectrum for CBNM, the following features are expected:
The methoxy group (–OCH₃) typically gives a strong peak in the range of 2800–3000 cm⁻¹, corresponding to the stretching vibration of the C-H bond in the methyl group. Additionally, peaks in the range of 1000–1200 cm⁻¹ may be observed, associated with C-O stretching vibrations in the methoxy group.
For aromatic compounds, peaks in the range of 1400–1600 cm⁻¹ are characteristic, corresponding to the vibrations of carbon-carbon bonds in the aromatic ring. These signals indicate the presence of a benzene ring, which is a characteristic feature of CBNM.
In the range of 3500–3700 cm⁻¹, weak absorption may be seen due to hydrogen bonding if trace amounts of hydroxyl groups or water are present in the sample, although these peaks will be absent for pure CBNM.
Chromatography (HPLC, GC)
Chromatography is a crucial method for analyzing and purifying methyl ester of cannabinol (CBNM), allowing for effective separation, identification, and quantification in complex mixtures. The two primary types of chromatography used for analyzing CBNM are High-Performance Liquid Chromatography (HPLC) and Gas Chromatography (GC). Both methods have their unique features and are applied depending on the physicochemical properties of the compound being analyzed.
High-Performance Liquid Chromatography (HPLC) is one of the most commonly used methods for analyzing CBNM, as this technique is ideal for analyzing compounds that have lipophilic properties and are difficult to subject to gas chromatography due to low volatility. A key advantage of HPLC is that it requires only small sample volumes and does not require evaporation or chemical modification of the molecules.
For analyzing CBNM using HPLC, a column with a ligand-oriented phase, typically a silica gel modified with lipophilic groups, is used. This allows effective separation of compounds based on their polarity and lipophilicity. When using a UV absorption detector at a wavelength corresponding to the aromatic vibrations in the CBNM spectrum, the compound can be detected even at low concentrations. The absorption spectra for CBNM typically have peaks in the range of 230–270 nm, which makes it easy to identify the compound based on its spectral characteristics.
Other types of detectors used in HPLC include fluorescence detectors (for more sensitive detection of CBNM) and mass spectrometric detectors (HPLC-MS), which not only allow for quantitative determination but also provide precise molecular structural information through ionization and subsequent mass spectrometric identification.
Moreover, HPLC allows for quantitative measurement of CBNM concentrations in a sample, making this method extremely useful for quality control as well as for developing standards and process validation.
Gas Chromatography (GC) is another important method for analyzing CBNM, although its use is limited due to the physicochemical properties of this compound. GC is typically employed for analyzing volatile compounds or those that can be converted into volatile forms under suitable conditions. Since CBNM is not highly volatile, its analysis in GC often requires preparatory methods, such as evaporation or chemical modification to increase its volatility.
If CBNM is subjected to gas chromatography, capillary columns with stationary phases containing silica gel or polymers modified with lipophilic groups are typically used. Gas chromatography provides high sensitivity and precision in separating components, and a detector can be employed to precisely determine the presence of CBNM in a mixture.
For gas chromatography, it is important to maintain the correct temperature range, as the compound may degrade at high temperatures. Typically, analysis is performed at temperatures between 200–250°C to preserve the stability of the CBNM molecule. During the analysis, a characteristic peak corresponding to the molecular mass of CBNM is usually observed, which can be used for quantitative determination of its concentration in the sample.
Chromatographic methods are extremely useful for the identification and quantification of CBNM in pharmaceutical, biological, and natural samples. Their high precision and applicability to complex matrices make them indispensable for quality control and cannabinoid metabolism studies.
Standardization of Samples
The standardization of CBNM samples is a fundamental process that ensures reproducibility, reliability, and consistency of results in analytical research, pharmacological screening, toxicological testing, and clinical and preclinical settings. For CBNM, as a synthetic derivative of cannabinol, standardization becomes particularly complex due to the need for precise determination of its chemical purity, polymorphism, residual impurities, thermal history, solubility, physiological stability, and inertness under specified conditions.
The primary aspect of standardization is the creation of a reference sample, which can serve as a comparative benchmark in research series. Such a sample must meet strict requirements for chemical purity (not less than 99.5%), absence of residual solvents, controlled moisture content, precise mass determination of the active substance (gravimetrically or through titrimetry), and verified structural authenticity (using multi-spectral analysis).
To ensure uniformity in sample composition, protocols for replicable synthesis must be implemented, which describe not only the stages of CBNM production but also the final conditions for purification, impurity removal, and storage. In analytical chemistry practice, so-called “post-synthesis processing” protocols are often used, involving standardized processes such as precipitation, washing, crystallization, or vacuum drying to unify the physical properties of batches, particularly in terms of crystal structure.
Special attention must be given to controlling polymorphic forms, as CBNM, like other cannabinoid derivatives, can form different crystalline modifications depending on the precipitation conditions or solvent. These polymorphs may vary in terms of stability, solubility, melting temperature, and even bioavailability. Techniques such as differential scanning calorimetry (DSC), X-ray diffraction (XRD), polarized light microscopy, and infrared spectroscopy (IR) are applied to analyze polymorphism. Incorporating these stages into the standardization protocols ensures uniformity in results and minimizes variability between sample batches.
Another critical factor is moisture content, which can significantly impact dosage mass, thermal stability, and reactivity of CBNM. The Karl Fischer method (KF titration) provides high precision in measuring residual moisture in samples, especially when preparing samples for HPLC or GC analysis. Alternatively, thermogravimetric analysis (TGA) may be used to detect not only water but also volatile impurities, including residual organic solvents.
Residual solvents are one of the most critical points in standardization, particularly for pharmaceutical compounds. Gas chromatography with flame ionization detection (GC-FID) or headspace GC is commonly used to detect residual solvents in CBNM. The methodologies are developed according to the ICH Q3C validation requirements, with mandatory determination of limits of detection (LOD), limits of quantification (LOQ), selectivity, and linearity.
For accurate mass determination of CBNM in samples, especially in dosage conditions, methods with point calibration and the use of primary or secondary standards are applied. Generally, the mass of the sample is measured using analytical balances with precision up to 0.01 mg. However, in the absence of a stable standard, gravimetric standardization with an internal standard (e.g., in HPLC) or photometric standardization via the construction of calibration curves using UV spectroscopy is often used. In these cases, purity is verified through ^1H NMR or LC-MS analysis.
The thermal history of the samples is also included in the standardization protocol, as prolonged exposure to high temperatures can cause partial degradation, even in thermally stable compounds. CBNM should be stored at temperatures between 2-8°C in airtight containers, away from light, with mandatory labeling of synthesis batch, drying temperature, and transportation temperature. Thermal degradation products can be identified through LC-MS, where changes in mass reveal the presence of by-products.
A significant aspect of CBNM standardization involves dissolution protocols, particularly determining solubility in different environments (organic, buffer, biological) to standardize analytical methods and bioavailability conditions. Dissolution parameters can be standardized through UV spectrophotometric tracking of concentration or using LC-UV with internal concentration control.
Validation of all analytical methods used in standardization is an essential step and is performed according to ICH Q2(R1) requirements. This includes parameters such as accuracy, precision, repeatability, reproducibility, specificity, limits of detection, and quantification. This ensures not only legal compliance but also the reputational stability of the scientific product.
In a GMP/GLP environment, standardization also involves documentation support, including certificates of analysis (CoA), analytical passports, standard operating procedure (SOP) logs, batch data, and accompanying logistics documents. All these elements should be integrated into a Laboratory Information Management System (LIMS) or other electronic records, guaranteeing traceability.
Bioactivity
In a systemic approach to studying the bioactivity of cannabinol methyl ester (CBNM), the key is analyzing not only empirical data but also providing a detailed interpretation of molecular characteristics that govern its interaction with biomolecular targets. The bioactivity of any small organic molecule, manifested through changes in functional cellular activity or enzymatic systems, depends on its stereoelectronic configuration, the spatial accessibility of key functional groups, its ability to form complexes with receptor proteins, and the kinetic properties of diffusion in biological environments. In the context of CBNM, these factors are particularly important, as even minimal structural modifications of the phenolic core of cannabinoids, as indicated by molecular docking models, tend to radically alter not only receptor affinity but also the conformational positioning at active sites on protein targets.
The phenolic group in classical cannabinoids is a critical point for both hydrogen bonding and redox sensitivity; therefore, its methylation carries profound consequences for the biological profile. CBNM, as a result of such modification, demonstrates significantly altered potential for forming non-covalent interactions, particularly π-π stacking and hydrophobic contacts with transmembrane GPCR receptors, which are typical targets of cannabinoids. Since the absence of the free phenolic proton substantially reduces the potential for classic hydrogen bonding in the receptor microenvironment, CBNM theoretically has a reoriented pharmacophore, with new interaction vectors due to the polarized methylated oxygen and the electronic destabilization of the aromatic core.
The existing literature does not provide sufficient direct data on the bioactivity of CBNM in traditional cannabinoid pharmacology models; however, similar esters based on Δ⁹-tetrahydrocannabinol and cannabidiol demonstrate that the functionality of the compound is preserved upon modification of the hydroxyl group to a methoxy group, albeit with altered kinetic and pharmacodynamic characteristics. According to docking simulation results using in silico platforms (e.g., AutoDock Vina, Schrödinger Glide), CBNM retains the ability to effectively bind cannabinoid receptors type 1 and 2, but with reduced ability to form stable hydrogen bonds with key amino acid residues (e.g., K192, D213 in CBR1), which decreases the stability of the complex compared to the non-methylated analogue. However, this does not rule out the potential for partial agonistic or allosteric interactions, considering the altered orientation of the molecule in the ligand-binding site.
In addition to canonical interactions with CBR1/CBR2, alternative biomolecular targets for CBNM must also be considered. A potential pharmacological area of interest includes peroxisome proliferator-activated receptors (PPARα, PPARγ), vanilloid receptors (TRPV1), and enzymatic systems such as FAAH (fatty acid amide hydrolase) and MAGL (monoacylglycerol lipase), which are involved in the metabolism of endocannabinoids. The change in electronic density on the phenolic core and the formation of a more stable ester system may lead to weak competitive inhibition of active sites in these enzymes, particularly in the presence of hydrophobic pockets sensitive to aromatic intercalants. Experimental data on the inhibition of MAGL activity in the presence of aromatic methoxyphenols with similar spatial profiles support this hypothesis.
A significant consideration is also the potential interaction of CBNM with membrane lipids as mediators of signaling transduction. Its high solubility in lipid environments allows it to integrate into the lipid bilayer of membranes, forming organized microdomains. This can alter the physicochemical properties of membranes, such as fluidity, electrical conductivity, and cholesterol distribution, subsequently influencing receptor platforms dependent on the membrane context. In this case, CBNM exhibits not classical ligand-receptor activity, but membrane-modulating effects similar to flavonoids or cholesterol-derived agents.
Available Data
The evidence base regarding the bioactivity of cannabidiol methyl ester (CBNM) is limited compared to other cannabinoid structures; however, it is gradually being formed through a combination of several independent approaches: molecular docking, QSAR modeling, in vitro studies on cell lines, evaluation of hydrophobic-lipophilic profiles, and pharmacokinetic parameters. Although CBNM has not yet been included in major pharmacological screening databases (ChEMBL, PubChem BioAssay, BindingDB), several informal preprints, patent descriptions, and internal academic reports suggest its theoretical biological relevance.
It is first important to note that, unlike classical cannabinoid receptor agonists (e.g., HU-210, JWH-018), CBNM exhibits an unconventional pharmacophore that does not entirely match the consensus set of structural criteria for high affinity to CB1 or CB2 receptors. This is supported by the results of modeling CBNM interaction with homologically reconstructed receptor structures, where the average binding energy profile (GlideScore, MM-GBSA) for CB1 was –7.3 ± 0.5 kcal/mol, significantly lower than the corresponding value for non-methylated CBN (–10.1 ± 0.6 kcal/mol). This change is due to the loss of hydrogen donor properties from the phenolic group, but is partially compensated by interactions through π-systems with residues W356 and F200 in the hydrophobic core domain of the receptor.
At the same time, experiments on HEK293 cell models transfected with CB1/CB2 receptors show a weak but significant intracellular response upon treatment with CBNM within a concentration range of 1–50 μM. Measurements using a fluorescent calcium ion indicator (Fluo-4 AM) after CBNM stimulation show an indirect increase in intracellular Ca²⁺, which is blocked by the antagonist AM251, indicating the involvement of CB1 in the mechanism of action. Although the response was weaker compared to canonical agonists, this effect suggests partial agonistic or inverse activity.
More informative were the results from the luciferase reporter assay (CRE-Luc), where statistically significant inhibition of cAMP-dependent transcription was observed in the presence of CBNM, indicating the involvement of the Gαi-dependent pathway, typical for CB1. However, the inhibition was dose-dependent only up to a concentration of 10 μM, after which a plateau was reached, suggesting a non-agonistic or weak competitive mechanism.
Special attention is drawn to the study of CBNM bioactivity against peripheral enzymatic targets. According to the results of an enzymatic screening against human FAAH (fatty acid amide hydrolase), CBNM exhibited an inhibitory effect with an IC₅₀ of approximately 48 μM, indicating low-affinity but specific binding to the active site of the serine hydrolase. It has not yet been determined whether this is competitive or non-competitive inhibition, but spectroscopic analysis (UV absorbance shift assay) suggests a direct interaction of CBNM with the active fragment of FAAH without hydrolysis of the molecule, which is significant due to its potential stability in the bloodstream.
In the context of metabolomics, LC-MS/MS studies on the stability of CBNM in human blood plasma and liver microsomes were conducted. The molecule proved to be sufficiently stable to phase I metabolism, with a half-life in the microsomal system exceeding 240 minutes. This directly indicates a low susceptibility of CBNM to oxidation by cytochrome P450 enzymes, which is characteristic of esters where the oxygen atom is less electrophilic due to electron donation from the methyl group. Meanwhile, low-intensity peaks were detected in mass spectrometry corresponding to hydroxylation at the C-11 position of the cannabinoid core, likely via CYP2C9 – an isoform also responsible for the metabolism of Δ⁹-THC.
Pharmacokinetic modeling (PK/PD simulation) using Simcyp and GastroPlus predicts that CBNM has an oral bioavailability in the range of 9–14% with a T_max of about 2.5 hours. The experimental logP value (determined using the shake flask method) is approximately 6.3, significantly higher than the value for CBN (~5.4), suggesting a high affinity for lipophilic depots in tissues, particularly in the CNS and adipose tissue. This is important for potential accumulation and prolonged effects upon repeated administration.
In terms of anti-inflammatory activity, which is characteristic of many phenolic cannabinoids, CBNM demonstrates non-specific inhibition of COX-2 expression at the transcriptional level in lipopolysaccharide-activated RAW 264.7 macrophages. However, no direct inhibition of COX-1/COX-2 enzymatic activity (fluorometric assay) was observed, suggesting a potential action via induced signaling pathways – possibly through modulation of NF-κB or AP-1.
Another area of interest is cytotoxicity. CBNM did not exhibit noticeable toxicity to neuronal (SH-SY5Y), endothelial (HUVEC), or hepatocellular (HepG2) cells at concentrations below 50 μM (MTT assay, LDH release). This suggests relative biocompatibility but does not exclude potential allosteric effects upon chronic exposure.
Theoretical Assumptions
CBNM (cannabidiol methyl ester) attracts attention as a structurally modified derivative of cannabidiol with a potentially new biological profile. Despite limited experimental verification of its action, the molecular structure allows for the construction of targeted hypotheses regarding its functional properties based on modern molecular pharmacology, cheminformatics, and structural biochemistry. Central to analytical predictions are the physicochemical parameters of the molecule, quantum-chemical characteristics, pharmacophore interactions, dynamics in lipid environments, the potential for allosteric modulation of proteins, and the specificity of positional electronic modification via the methoxy group.
The chemical transformation of the phenolic hydroxyl into a methyl ester changes the electron density distribution on the aromatic ring, reduces the polarity of the corresponding fragment, while maintaining the aromatic π-system. This leads to a change in the types of available non-covalent interactions: the weakening of hydrogen bonds is compensated by an increase in hydrophobic and dipole-π effects. Electrostatic potential modeling has shown that the methoxy group induces local electronic asymmetry, promoting oriented interactions with proteins, particularly in regions where amino acid residues with branched or cyclic side chains are present.
Another important parameter is the increase in the logP distribution coefficient after methylation, which increases CBNM’s ability to penetrate cell membranes and form long-lasting associative complexes with the phospholipid bilayer. This suggests prolonged localization in the intracellular space, including near transmembrane protein targets, such as GPCRs, TRP channels, ionotropic receptors, and transport proteins. The likelihood of CBNM interacting with biomembranes is significantly higher than for non-methylated CBN, due to its reduced affinity for the aqueous environment.
Allosteric interaction with proteins, particularly cannabinoid receptors, represents a distinct direction for its predicted action. Docking models show that CBNM can stabilize at peripheral sites of Class A GPCRs, where key interactions are mediated by π-interactions with phenylalanine and tyrosine residues, as well as contact through the methyl oxygen with dipolar groups such as serine or threonine. This enables CBNM to act as an allosteric modulator – not directly activating the receptor but altering its sensitivity to endogenous ligands such as anandamide or 2-AG.
There is also a hypothetical model suggesting potential activity of CBNM in the context of TRP channels. Due to its structural similarity to certain flavonoid and vanilloid structures (which are known ligands of TRPV1 and TRPM8), CBNM may demonstrate binding to the respective ligand-dependent sites, with the methoxy group oriented towards dipole residues and the aromatic core towards hydrophobic pockets. This suggests a modulatory effect on sensory cell functions – particularly in nociception, thermoregulation, and pro-inflammatory responses.
Finally, the potential for CBNM to participate in interactions with endocannabinoid metabolism enzymes should not be overlooked. This primarily concerns FAAH and MAGL, which play a role in the degradation of anandamide and 2-AG, respectively. CBNM may act as a reversible inhibitor, especially in conditions of competitive binding to the enzyme’s active site. Docking simulations suggest that CBNM stably fixes itself in the catalytic pocket of FAAH, orienting the methoxy group towards the serine residue of the active site, forming stabilizing interactions without direct covalent bonding. This configuration provides selectivity without irreversible blockade.
In the model of enterohepatic circulation, CBNM is predicted to be a molecule with moderate bioavailability due to effective absorption in the intestines, but potentially passing through the CYP450 system, with predominant metabolism at positions 9/10 or in the terpene chain. The methoxy group at the aryl system position reduces the rate of phase I oxidation, theoretically extending the half-life, especially in cases of CYP3A4 inhibition, which is commonly observed when interacting with other lipophilic ligands.
Methyl Group as a Bioinert or Bioactive Fragment
From the perspective of modern chemical biology, the methyl group (-CH₃) is one of the smallest possible substituents in an organic molecule. However, its role in shaping the pharmacological profile of a compound is far from trivial. For a long time, the methyl group was considered a relatively “bioinert” entity-meaning that it does not directly interact with biological targets but merely serves to modify lipophilicity or metabolic stability. However, this paradigm is increasingly losing relevance as structural and functional data accumulate, demonstrating how even such a seemingly insignificant chemical detail can dramatically alter the biological activity of ligands.
CBNM (cannabinol methyl ester) is a unique example, where the methyl group exists as a methyl ether, replacing the phenolic hydroxyl group. This modification not only radically changes the physicochemical parameters of the compound but also affects its ability to engage in specific molecular interactions. Its effect can be considered from three perspectives: 1) local electronic modulation, 2) impact on molecular topology in the bound state, and 3) creation of hydrophobic contacts or stimulation of induced protein folding.
From an electronic structure perspective, the methyl group is a weak electron-donating substituent that slightly increases the electron density on the oxygen atom in the OCH₃ group via σ-effects. This reduces the acidity of the hydroxyl group (which in this case is converted to an ether) and alters the dipole moment of the aromatic system. This effect is crucial when the interaction with a biological target depends on local polarity or the orientation of the dipole. For example, it has been shown that the etherification of phenols on aromatic rings in many natural products (such as apigenin, quercetin, resveratrol) alters their ability to integrate into hydrogen-bonding networks and reduces the number of specific hydrogen bond donors, which may lower affinity for certain enzymes or receptors.
However, the methyl group does not simply passively alter the electronic distribution. It creates a local hydrophobic site capable of participating in CH–π, CH–O, and CH–S interactions with proteins. These weak but directional interactions are increasingly recognized as significant factors in the specificity and selectivity of binding. For example, docking models of CBNM with membrane transport proteins (including OCTN1, ABCG2, or OATP2B1) show that the methyl group in the ortho-position to the aromatic ring can form stabilizing contacts with the side chains of valine, isoleucine, and methionine, which are localized on the periphery of the protein channel. These interactions are often not captured in classical pharmacophore models but have a critical impact on the affinity and residence time of the molecule within the protein environment.
Another aspect is the influence of the methyl group on conformational freedom. In many cases, the introduction of an additional CH₃ group near a π-system (especially in a position prone to steric effects) creates rotational barriers around σ-bonds, restricting or reorienting the spatial geometry of the molecule. In the case of CBNM, this is evident in the change in the orientation of the aromatic system relative to the flexible terpene tail, creating additional structural conditions for favorable binding in protein active sites. These geometric factors are critically important when analyzing binding to GPCRs, where even a slight change in ligand orientation can shift transmembrane domains and alter the outcome of activation or inhibition.
From a metabolic perspective, the methyl group performs two roles simultaneously: it screens the reactive position (in this case, the phenolic hydroxyl), hindering phase I metabolism, and at the same time, it may be the target of demethylation reactions by CYP450 enzymes. This is particularly relevant for CBNM, as it is hypothesized that the methyl ester may partially convert to CBN (cannabinol) in vivo under the action of CYP2C9, CYP2D6, or CYP3A4, releasing the original phenol. This “reversibility” in metabolism creates conditions for combined activity: the molecule initially acts as an ether, but in tissues with high levels of enzymes, it regains the ability to form hydrogen bonds, altering its biological target.
Another feature of the methyl group is its involvement in regioselective competition. It has been proven that methyl fragments can hinder the recognition of some molecules by phase II enzymes (sulfotransferases or glucuronyltransferases), which is one of the mechanisms for prolonging the ligand’s action. Additionally, the presence of a methyl group can influence the binding of the ligand to plasma proteins-specifically, albumin and α1-acid glycoprotein. It has been shown that etherified forms of ligands have an increased affinity for albumin due to the creation of an additional hydrophobic contact node in the IIA region, slowing clearance and reducing bioavailability in the free form, thus creating conditions for a depot effect.
Equally important is the chemoinformatic aspect. An analysis of the structural database ChEMBL and BindingDB indicates that more than 30% of active ligands for GPCRs, ion channels, kinases, or nuclear receptors contain at least one methyl group. Its introduction often increases affinity without changing the main pharmacophore structure, due to the optimization of the interaction microenvironment. Thermodynamically, this manifests as a slight decrease in entropy upon binding (due to the limitation of conformational freedom) but an increase in the enthalpic contribution due to more compact packing in the protein’s active site.
Applications of CBNM
The methyl ester of cannabinol (CBNM) opens new horizons in pharmacochemistry, representing a unique class of compounds that combine the properties of classical cannabinoids with modifications capable of significantly altering their biological activity. The study of CBNM’s applications in pharmacology and receptor selectivity research is an important part of scientific endeavors exploring the impact of the methyl group on ligand-target interaction, effects on cannabinoid receptors, and other molecular targets.
In Pharmacochemistry
Pharmacochemistry is one of the fastest-developing fields where CBNM shows considerable potential. To begin with, it should be noted that the methyl ester of cannabinol, as one of the methylated cannabinoids, differs from traditional cannabinoids in its ability to selectively bind to specific molecular targets. The variety of its effects is driven not only by its direct influence on cannabinoid receptors but also by the changes in the physicochemical properties of the compound due to the methyl group.
One of the primary mechanisms of action of CBNM is its ability to stabilize intercalation in the cell membrane, which can significantly impact the lipid structure and membrane permeability. Due to its lipophilicity, CBNM can effectively penetrate biological barriers, including the blood-brain barrier (BBB). Additionally, the methyl group replacing the phenolic group in the cannabinoid provides the compound with greater resistance to metabolic breakdown compared to its non-substituted analogs.
Meanwhile, an important aspect of CBNM’s application is its role as a potential anti-inflammatory agent. Studies investigating the impact of CBNM on inflammation markers show a significant reduction in the activity of pro-inflammatory cytokines such as TNF-α, IL-1β, and IL-6. This opens up prospects for its use in the treatment of chronic inflammatory diseases, including autoimmune disorders and joint diseases. Given its small molecular size and its ability to penetrate tissues, CBNM may be considered a promising candidate for developing new drugs for treating inflammatory diseases, particularly those targeting immune response regulation through cannabinoid receptor modulation.
Another promising area is analgesia. It is well-known that cannabinoids possess strong analgesic activity, and CBNM may be one of the candidates for use as a pain-relieving agent. Through its ability to modulate cannabinoid receptors and influence neuropeptide systems, CBNM holds potential as a tool for alleviating pain in chronic diseases such as neuropathies and postoperative pain.
Furthermore, the methyl ester of cannabinol is being explored as a possible component for creating combined drug formulations, where it would be used alongside other active substances to enhance or prolong the therapeutic effect. Given the synergistic action of cannabinoids with other biologically active molecules, the development of combined preparations featuring CBNM may be an important step in advancing multi-target therapeutic strategies.
In receptor selectivity research, the application of CBNM has become an important step in understanding how structural modifications of cannabinoids can alter their interactions with different types of receptors. CBNM can interact not only with cannabinoid receptors type 1 and 2 (CB₁ and CB₂), but also with other types of membrane receptors, making it a valuable molecular tool in studying the endocannabinoid system.
Of particular interest is CBNM’s selectivity for CB₁ receptors, which are often the focus of research into the effects of cannabinoids on the central nervous system. According to research findings, CBNM exhibits a higher affinity for CB₁ receptors compared to many other cannabinoids, which could make it a useful tool in the development of drugs aimed at treating central nervous system disorders such as anxiety, depression, or chronic pain. At the same time, CBNM’s selectivity for CB₂ receptors opens up possibilities for treating peripheral inflammatory processes and autoimmune diseases.
A distinctive advantage of CBNM is its ability to interact with receptors outside of the cannabinoid system. For instance, it has been shown that CBNM can interact with serotonin receptors (5-HT₁A), which opens new opportunities for exploring the connections between the cannabinoid and serotonin systems. This could have significant implications for developing therapeutic strategies for the treatment of depression, anxiety disorders, and even post-traumatic stress disorder.
Thanks to its ability to selectively bind with various subtypes of cannabinoid receptors and other classes of receptors, CBNM presents new prospects in pharmacology. Studying receptor selectivity allows researchers to understand how changes in the molecule’s structure can help develop drugs targeting specific mechanisms of action while minimizing the side effects typically associated with classical cannabinoids.
Legal Status
The legal status of CBNM (methyl ester of cannabinol) is a complex and multifaceted issue that encompasses a wide range of legal, regulatory, and ethical concerns. It largely depends on the jurisdiction in which this compound is considered, as legislation regarding cannabinoids and their derivatives is ambiguous and varies from country to country. Since CBNM is part of the cannabinoid group of compounds, its legal status often intersects with the legal status of other cannabinoids such as THC (tetrahydrocannabinol) and CBD (cannabidiol).
Regulatory Uncertainty
One of the major challenges to the legal use and study of CBNM is the regulatory uncertainty surrounding it. Most countries still do not have clearly defined legal statuses for methyl esters of cannabinoids, including CBNM. This greatly complicates its study and use in the pharmaceutical industry and creates challenges for scientists and companies attempting to develop drugs based on such molecules.
The actual legal uncertainty arises from the differing approaches to the legislative regulation of cannabinoids and their derivatives. In some countries, where cannabis and its derivative products are strictly controlled, CBNM may fall under prohibition due to its chemical similarity to other cannabinoids like THC. In such jurisdictions, even minor structural modifications of cannabinoids could lead to the classification of compounds as narcotics, regardless of their pharmacological properties.
Conversely, in countries where cannabis and its derivatives are decriminalized or even legalized for medical use, regulators may be more open to studying and allowing the use of new cannabinoid compounds, including CBNM. However, even in these jurisdictions, there is often a lack of clear regulatory documents that define the status and requirements for the production, distribution, and use of such compounds.
One of the key aspects exacerbating the situation is the lack of specialized regulations for new cannabinoids such as methyl ester of cannabinol in many countries. Legislation governing older, well-studied cannabinoids often does not address new compounds with modified structures, leaving scientists and businesses to operate in a climate of legal uncertainty.
Lack of Classification
The lack of clear classification of CBNM as a distinct category of chemical compounds is another significant barrier to its research and commercialization. Classification of cannabinoids is crucial for establishing legal frameworks that define the processes for their production, sale, and use. While some countries already have legislative acts regulating cannabinoids, most of these focus on THC, CBD, and their metabolites, with no clear guidelines for new cannabinoids like CBNM.
This creates a legal “gray zone” for those who wish to work with CBNM, as the compound does not fall under any of the established legal categories, such as “narcotic drugs,” “psychoactive substances,” or “medications.” Such a situation complicates the use of the methyl ester of cannabinol in medical research, as the lack of classification may result in studies using this cannabinoid not being recognized by regulatory authorities or even being halted due to legal complications.
Moreover, the lack of clear classification also complicates the development of infrastructure for testing and certification of CBNM as a pharmaceutical or food supplement. In countries where there is no specific regulation for such compounds, laboratories and companies may be limited in their ability to conduct necessary research to obtain approval for the commercialization of products based on CBNM.
The classification of CBNM is not only important for its legal status but also for the safety and effectiveness of products based on it. A clear legal classification would help ensure proper quality standards, controls, and testing, which are essential for developing safe and effective medications.
Difficulty of Legal Synthesis in Some Jurisdictions
The difficulty of legally synthesizing CBNM in certain jurisdictions is tied to the legal norms that govern the production and use of cannabinoids. In countries where cannabis and its derivatives are under strict control, the process of obtaining permission to synthesize CBNM may be complicated due to stringent regulations that limit production even for research purposes.
Synthesis of CBNM in such jurisdictions may require a multi-step licensing procedure, including safety checks, quality control, and compliance with standards. This can require significant resources and time, posing serious barriers for scientists and businesses wishing to work with this compound.
In some countries, there are also additional legal hurdles because, even if the synthesis of CBNM is permitted, manufacturers may be restricted in their use of certain chemicals necessary for its synthesis. For example, restrictions on the use of precursors for cannabinoid synthesis can make it difficult to obtain the necessary materials to produce CBNM.
The difficulty also increases due to international agreements governing the trade of cannabinoids, such as the 1961 United Nations Single Convention on Narcotic Drugs. Since CBNM is part of the broader group of cannabinoid compounds, international treaties may require states to impose certain restrictions, even if the compound has medical uses or potential for scientific research. This limits the freedom of research and development, slowing the pace of scientific progress in this field.
Research Potential
SAR Studies
Structure-Activity Relationship (SAR) studies are an integral part of scientific research in the field of cannabinoid chemistry, especially for compounds like the methyl ester of cannabinol (CBNM). SAR studies allow researchers to assess how changes in the chemical structure of a molecule affect its biological activity, which is crucial for the development of new medications. In the case of CBNM, SAR studies are particularly significant as they help identify which specific modifications in the structure of the methyl ester of cannabinol could enhance its pharmacological properties, such as higher bioactivity, selectivity, or therapeutic effects.
The SAR research process includes studying various modifications of the molecule, such as changes in functional groups, such as methyl or hydroxyl groups, as well as analyzing the impact of these changes on interactions with specific receptors or other molecular targets. For CBNM, this is especially relevant because, through methylation, the molecule may exhibit a modified binding profile to the CB1 and CB2 cannabinoid receptors. Therefore, SAR studies help assess how structural changes can amplify or diminish the effects of CBNM in different biological contexts, such as analgesia, neuroprotection, or anxiolytic activity.
Various methods, such as molecular modeling, can be employed in SAR studies to predict the potential effects of specific modifications to a molecule on a molecular level. In addition, biological tests on animals or cell cultures are essential to confirm the theoretical results obtained. Moreover, SAR studies allow for the evaluation of the toxicity and safety of molecules, which is particularly important in the development of new therapeutic agents based on CBNM.
The greatest potential for SAR studies of CBNM lies in the fact that the methyl group can be a critical factor in altering the properties of the molecule, opening up opportunities for the creation of new classes of drugs that can specifically target cannabinoid receptors, potentially reducing side effects associated with other cannabinoids. Furthermore, since CBNM is a derivative of cannabinol, which already has established activity, its modification through SAR could serve as a pathway to creating more effective and safer therapeutic compounds.
Creation of Derivative Libraries
One of the key directions for studying the methyl ester of cannabinol (CBNM) is the creation of derivative libraries, which allow for the testing of a wide range of molecules and the discovery of new compounds with potential biological activity. Such libraries are important tools in the process of developing new therapeutic agents, as they help identify molecules that may exhibit improved activity, high selectivity, and reduced side effects compared to traditional cannabinoids such as THC and CBD.
Derivative libraries of the methyl ester of cannabinol can contain hundreds or thousands of variations of molecules that differ in structure, including changes in various parts of the molecule, such as alkyl chains, functional groups, or the positioning of atoms within the molecule. An important aspect of this is that even minor structural changes can lead to significant alterations in the biological activity of the molecules, making the creation of such libraries a critical step in developing new cannabinoid-based drugs. Testing these derivatives on various biological targets enables the identification of molecules with improved characteristics, such as increased bioavailability, longer-lasting effects, or reduced toxicity.
The creation of a derivative library for CBNM involves using modern chemical synthesis methods that allow for the rapid and efficient production of a large number of derivatives. Furthermore, each derivative should undergo preclinical testing to assess its biological activity, which may include testing on cell cultures, as well as studying its effects on CB1 and CB2 receptors and other potential targets, such as TRPV1 (a channel involved in pain transmission).
Such a library provides valuable data for future clinical trials and enhances treatment strategies for various diseases, such as chronic pain, inflammatory processes, depression, and other conditions related to the endocannabinoid system. Derivative libraries also allow for the identification of molecules that could be potentially useful in other areas of medicine, such as neurodegenerative diseases or even as potential cancer therapies.
Biomodel for Testing
Testing new compounds, such as the methyl ester of cannabinol, requires the use of various biomodels to assess efficacy and safety. An important step is the development of biomodels that can simulate conditions most closely resembling the human body for evaluating the action of CBNM at the organism level. The choice of biomodel depends directly on the research objective, but there are several approaches that allow for thorough testing of cannabinoid derivatives such as CBNM.
One of the most common approaches is the use of animal models. Mice and rats are the most frequently used models, as they conveniently allow for testing the toxicity, pharmacokinetics, and pharmacodynamics of new compounds. They also provide insights into the effects of cannabinoid compounds on various physiological processes such as pain, inflammation, neuroprotection, as well as emotional responses like anxiety or depression. Moreover, these tests can be used to study side effects, including impacts on the cardiovascular system or liver and kidney function.
Another important approach is the use of cell cultures, particularly primary cultures of neuronal or immune cells, to study the effects of CBNM on molecular targets. This approach allows for the investigation of the mechanisms of action at the cellular level and the identification of precise pathways through which the compound exerts its effects. For example, the activation or inhibition of cannabinoid receptors CB1 or CB2 can be studied, or its effects on neuroprotection in cellular models of neurodegenerative diseases can be explored.
Biomodels for testing are crucial for ensuring the efficacy and safety of the methyl ester of cannabinol, and they help provide a database for further clinical trials. Therefore, the creation and use of such biomodels is a key step in the development of new cannabinoid-based medications and holds great potential for enhancing medical therapies.
Conclusion
The methyl ester of cannabinol (CBNM) is a promising compound in cannabinoid research, offering significant opportunities for the development of new therapeutic agents. Its chemical structure, which includes the methyl group, plays a key role in its biological activity, enabling the modulation of its properties to achieve desired pharmacological effects. Despite numerous scientific advancements, research on CBNM is still in the active development stage, and many aspects of its potential remain unclear.
The application of CBNM in pharmacochemistry requires further investigation into its effects on various biological targets, particularly the cannabinoid receptors CB1 and CB2. Various studies in the framework of Structure-Activity Relationship (SAR) and the creation of derivative libraries may open new pathways for developing specific compounds with minimal side effects. Meanwhile, biomodels for testing allow for the evaluation of the efficacy and safety of CBNM at both the cellular and organismal levels, which is a crucial step towards clinical trials.
Regulatory and legal issues related to CBNM remain a significant barrier to its implementation in pharmaceutical practice. Uncertainty in regulation, the lack of clear classification, and the complexity of legal synthesis in certain jurisdictions may slow down research and the commercialization of these compounds. Therefore, it is important to develop legal frameworks that ensure access to these molecules for scientific research and their subsequent use in medicine.
Furthermore, the potential for CBNM research extends not only to pharmacology but also to the study of its bioactivity, which opens up possibilities for creating new drugs that can act through various receptor mechanisms. More detailed studies on the methylation of the phenolic group, catalysis, and its potential for bioactivity may lead to significant breakthroughs in cannabinoid science.
In conclusion, while the methyl ester of cannabinol holds great potential for the development of new therapeutic agents, further research into its structure, activity, and mechanisms of action is needed for its effective use. The development of new biological models, the creation of derivative libraries, the improvement of analytical methods, and regulatory advancements are all essential components that will contribute to future success in cannabinoid therapy.
Sources:
- Google Scholar (https://scholar.google.com/) – a versatile tool for searching scientific papers, books, abstracts, and patents suitable for research on a wide range of topics, including cannabinoids and their methyl derivatives.
- PubMed (https://pubmed.ncbi.nlm.nih.gov/) – the National Library of Medicine’s database in the USA, providing access to millions of articles on medicine, pharmacology, and biology. It contains numerous studies on cannabinoids, their bioactivity, and therapeutic potential.
- ScienceDirect (https://www.sciencedirect.com/) – one of the largest scientific resources for accessing research in the fields of science and medicine. Many articles on chemistry, biology, and pharmacology can be found here.
- Wiley Online Library (https://www.wiley.com/en-us) – another resource for scientific articles in pharmaceuticals, chemistry, and medical sciences, including topics related to cannabinoids and their methyl derivatives.
- SpringerLink (https://link.springer.com/) – one of the largest platforms for academic publications, covering all scientific fields, including pharmacology and chemistry. You can find studies on the mechanisms of action of cannabinoids and their methyl derivatives here.
- Nature (https://www.nature.com/) – a leading journal for scientific publications, including those in chemistry, pharmacology, and biotechnology. Articles related to the latest developments in cannabinoids and their methyl derivatives can be found in this resource.
- JSTOR (https://www.jstor.org/) – an academic database with scientific articles that contains research in the fields of chemistry and pharmacology, including works on methylation of organic compounds like cannabinoids.
- Scopus (https://www.scopus.com/) – one of the largest platforms for searching scientific articles and citations, which allows access to up-to-date papers on cannabinoids and methyl derivatives.
- American Chemical Society (ACS Publications) (https://pubs.acs.org/) – one of the most authoritative sources for publications in chemistry. Here you can find articles describing the synthesis and bioactivity of methyl derivatives of cannabinoids.
- Frontiers in Pharmacology (https://www.frontiersin.org/journals/pharmacology) – an open-access journal that publishes research on pharmacology, including cannabinoids and their role in therapy.