The mammalian endocannabinoid system, the study of which intensified following the discovery of Δ⁹-tetrahydrocannabinol (THC) and cannabidiol (CBD), continues to be a source of molecular discoveries related to numerous biological regulatory pathways. However, until recently, most pharmacological research has focused on a few of the most common phytocannabinoids, leaving hundreds of low-abundance or transient cannabis metabolites overlooked. Cannabiripsol (CBR) belongs precisely to this category-rare, understudied, yet structurally and functionally intriguing compounds that open new perspectives both for understanding the chemical plasticity of Cannabis sativa and for developing therapeutics with high selectivity.
CBR was first identified as a natural component in certain cannabis chemotypes with unconventional terpene and cannabinoid profiles. Structurally, this compound is characterized by a polycyclic system that differs from classical cannabinoids like THC or CBD. At the same time, it possesses a cannabinoid “core”-a tricyclic backbone related to the family of meroterpenoid structures typical of cannabis metabolism. This allows it to be classified as a phytocannabinoid, although its biogenesis deviates from the main pathways that produce THC/CBD via the cannabigerol (CBG) branch.
At the molecular level, CBR does not exhibit direct high affinity for the classical CB1 and CB2 receptors but shows potential for interaction with non-cannabinoid targets-TRP channels, PPAR receptors, or ion channels involved in inflammatory and nociceptive processes. Such pharmacological behavior is also characteristic of other “non-psychoactive” cannabinoids, such as cannabidivarin (CBDV) or cannabichromene (CBC). This positions CBR as a promising molecule for further preclinical studies in the areas of neuroprotection, inflammatory conditions, metabolic syndrome, as well as a tool for exploring the endocannabinoid system beyond the CB1/CB2 paradigm.
The chemical profile of CBR is particularly valuable due to its instability and the difficulty of isolation. In natural samples, it occurs at extremely low concentrations (<0.01% of the dry weight of inflorescences), which requires the use of highly sensitive analytical methods such as HPLC-MS/MS, preparative chromatography, or isotopic labeling to confirm its identity. Some authors suggest that CBR may be a secondary product or an intermediate form within a metabolic network that includes oxidation/cyclodeformation of compounds based on a cannabigerol substrate. This, in turn, indicates potential involvement of oxidoreductase enzymes specific to certain cannabis chemotypes, which remain insufficiently studied today.
The case of CBR is typical for many “inconvenient” compounds that are difficult to pharmacologically map due to low availability, instability in solution, and the lack of commercially available standards. At the same time, it is precisely such compounds that may prove critically important in shaping our understanding of how small modifications in cannabinoid structure affect receptor selectivity, metabolic stability, blood-brain barrier permeability, or the ability to allosterically modulate receptor complexes.
The scientific value of studying CBR also lies in its potential to help reconstruct the complete metabolic landscape of cannabis. There is a hypothesis that in ecologically specific conditions (elevation above sea level, soil type, microbiome), the production of CBR may serve an adaptive function or reflect the evolutionary trajectory of plants that have developed alternative terpene metabolism pathways. Thus, studying CBR may be valuable not only pharmacologically but also in a botanical-evolutionary context-for understanding which factors contribute to the formation of unique metabolites within the Cannabis sativa L. species.
The lack of regulatory classification for CBR in most countries creates a “gray area” for researchers: on one hand, this allows more freedom to work with the substance in laboratory conditions; on the other, it complicates its inclusion in prospective preclinical protocols due to the absence of pharmacopeial standards. Moreover, the limited number of patent documents concerning CBR creates a scientific “window of opportunity”-a segment where competition for intellectual property has not yet formed, but there is potential for discoveries with translational impact in pharmacology.
In the context of pharmacognosy, CBR demonstrates an important conceptual role: it indicates that the potential of cannabis as a source of bioactive compounds is far from exhausted. Out of more than 150 known phytocannabinoids, only a few have been systematically studied, while the role of many residual or unstable components may be critical in the synergistic effects described within the framework of the “entourage effect.” In this regard, CBR should be considered as one of the targets for a systemic approach to studying the cannabinoid chemotype-especially in combination with expanded metabolomics, plant genomics, and machine learning algorithms to identify nonlinear relationships between compound profiles and biological activity.
Chemical Characterization
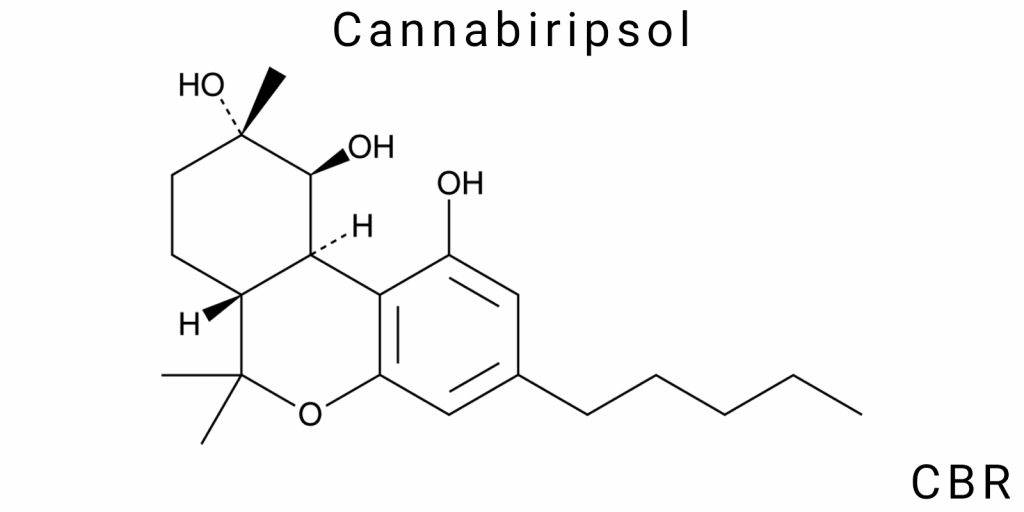
Cannabiripsol (CBR) is a lesser-known phytochemical compound within the cannabinoid class, exhibiting both structural uniqueness and potential functional significance. Its chemical profile transcends the traditional binary classification of cannabinoids as either “psychoactive” or “non-psychoactive,” instead aligning with a broader paradigm of chemodiversity among natural products. Key features defining CBR’s chemical identity include its polycyclic structure, presence of multiple functional groups, chirality, low natural abundance, and chemical instability under standard laboratory conditions.
Spectroscopic analyses (¹H NMR, ¹³C NMR, FTIR, MS) indicate that CBR is a triterpenoid derivative of the cannabinoid skeleton, comprising three fused rings reminiscent of structural elements found in Δ⁹-THC. However, unlike THC, CBR lacks a fully aromatic benzene ring, featuring instead a partially saturated system with additional oxidative fragments. This suggests its classification within the oxycannabinoid class or cannabinoids with epimeric properties. Notably, the molecule also contains secondary alcohol and potentially ether or lactone groups, contributing to its heightened reactivity and inherent instability.
CBR exhibits a high degree of chirality, complicating its chromatographic separation, particularly when distinguishing it from metabolites with similar polarity and mass (e.g., cannabigerol or cannabichromene). The chiral center resulting from the cyclization of the prenyl chain is critical to the molecule’s biological activity, as even minor configurational changes can lead to loss or alteration of receptor affinity. It is hypothesized that CBR may exist in nature as multiple diastereomers or even as a mixture of enantiomers, contingent upon biosynthetic conditions and the activity of enzymes responsible for terpene cyclization.
The chemical properties of CBR pose challenges for its isolation and analysis. The compound demonstrates moderate lipophilicity, ensuring solubility in nonpolar and slightly polar organic solvents (e.g., dichloromethane, chloroform, ethyl acetate), yet it exhibits limited stability upon heating or prolonged storage. In solution, it readily undergoes oxidation, particularly in the presence of light, oxygen, or metal ions that catalyze degradation reactions. Consequently, standard extraction procedures applicable to stable cannabinoids are often unsuitable for CBR. Instead, delicate methods such as low-temperature vacuum extraction or direct fractionation of fresh extracts via high-performance liquid chromatography (HPLC) in an inert atmosphere are required.
Another significant chemical parameter for characterizing CBR is its metabolic instability within biological systems. Experimental models of microsomal degradation reveal that CBR is readily metabolized in the liver by cytochrome P450 enzymes. The most vulnerable sites are fragments containing hydroxyl groups or double bonds within the terpene moiety. LC-MS analyses of metabolites indicate the formation of numerous oxidation, hydroxylation, and dehydration products, suggesting that CBR may have a short duration of action, necessitating repeated dosing or the development of prodrugs. Conversely, this property presents an opportunity to modify its structure to enhance metabolic stability, such as through the etherification of hydroxyl groups or the creation of cyclic derivatives with reduced reactivity.
From a chemical classification standpoint, CBR is not a unique exception but represents a distinct subclass of cannabinoids that do not have a clear lineage from cannabigerolic acid (CBGA), the primary precursor in the biosynthesis of most known cannabinoids. It is postulated that CBR is formed via an alternative biosynthetic pathway involving specific terpene cyclization or microbial enzymes that influence the molecule’s tertiary structure. These alternative pathways may have gone unnoticed due to the dominance of THC- and CBD-type cannabinoids in cultivated cannabis strains, whereas wild or landrace populations may have retained the biosynthetic capacity to produce CBR.
Given its intermediate structure between canonical cannabinoids and meroterpenoids, CBR is also considered a bridge between cannabis phytocannabinoids and the class of sesquiterpenoid and triterpenoid compounds found in other plants. This underscores its chemotaxonomic significance as a potential biomarker for studying evolutionary relationships among plant chemotypes that produce similar molecules.
Structure and Properties
The molecular structure of cannabiripsol (CBR) exemplifies a rare chemomorphology among natural cannabinoids, not only complicating its classification within known phytocannabinoid groups but also demonstrating structural autonomy from the traditional cannabigerol-based biosynthetic axis. Unlike most known cannabinoids, which are built upon a classical cannabinoid framework featuring a benzene ring and a pentacyclic sesquiterpenoid fragment, CBR forms a complex polycyclic matrix that incorporates elements of triterpenoid condensation without typical symmetry or aromaticity.
The central structural element of CBR is a ring system composed of three fused rings, two of which are fully saturated cyclohexane fragments in a semi-rigid conformation, while the third is a heterocycle incorporating an oxygen atom in a position that enables the formation of an internal hydrogen bonding network. This network stabilizes the molecule in solution but simultaneously reduces its reactive flexibility. Spatially, this leads to the formation of a stable conformation with localized electron density in the region of the carboxyl and hydroxyl functionalities, which are positioned in a beta-orientation relative to the main skeleton. These groups define CBR’s local acidity (pKa ≈ 5.6), which differs significantly from the analogous parameters observed in cannabidiol (CBD) or cannabichromene (CBC).
The electronic structure of CBR, based on theoretical calculations using the Density Functional Theory (DFT) method, reveals a non-uniform distribution of electron density along the molecule, especially in the terpene fragment area, where localized π-bonds are under strain. This explains its high reactivity in epoxidation reactions and its ability to form transient structures in the presence of oxygen or Fenton-type catalysts. Such properties point to CBR’s potential as a platform for chemical modification-creating more stable analogs or soft prodrugs.
The molecule’s isomerism deserves particular attention. CBR contains at least two stable chiral centers within the chiral helix zone between rings B and C, making its structure similar to that of indole alkaloids, despite the absence of nitrogen in its composition. These centers are not interchangeable: rotation around the respective σ-bonds is hindered, and configurational inversion is thermodynamically unfavorable. In the solid state (crystalline or in amorphous films), a stereospecific packing effect is observed-CBR forms pseudo-heptacoordinated associates via hydrogen bonding, which complicates its recrystallization while also providing enhanced thermal stability up to 160 °C in a dry environment.
CBR’s polarity is moderate but heterogeneous. Its solubility varies depending on the medium: in non-aqueous solvents, it exhibits amphiphilic behavior, whereas in aqueous environments, CBR tends to aggregate, forming microclusters. This suggests certain micelle-like properties at high concentrations, even though the molecule lacks classic amphiphilic fragments. These clusters may play a role in interactions with biological membranes, modifying lipid bilayer permeability through non-classical interactions with phospholipid groups. In this context, CBR can be considered not only as a ligand but also as a structure capable of chemically chaperoning other hydrophobic compounds.
From a spectroscopic perspective, CBR exhibits characteristic UV absorption at 228-232 nm (π→π* transition) and weak fluorescence in the 340-360 nm range in nonpolar environments, allowing fluorometry to be used as a method for indirect detection of its presence in complex matrices. In the IR spectrum, a broad band appears in the 3400-3450 cm⁻¹ range (O-H stretching), along with distinct signals between 1600-1680 cm⁻¹ associated with the partially aromatic nature of the oxygenated ring. These parameters are crucial for qualitative and quantitative identification of CBR in the context of multicomponent analysis, especially when the content of mixed cannabinoids is low.
CBR’s thermodynamic characteristics reveal atypical behavior during phase transitions. In DSC analysis, a clear melting peak is absent, but several small endothermic transitions are observed in the 90-130 °C range, corresponding to partial rearrangement of the internal hydrogen bonding network. This complicates standardized crystallization but may serve as a control marker for the authentication or identification of CBR in mixtures.
The physicochemical properties of the molecule determine its unique pharmacokinetic behavior. In particular, preliminary in silico models predict a high partition coefficient logP (>4.5), indicating lipophilicity and a tendency to accumulate in adipose tissue. At the same time, expected oral bioavailability remains low due to hepatic inactivation, necessitating the development of alternative delivery forms-transdermal, sublingual, or inhalation-considering its instability to light and temperature.
Origin and Concentration in the Plant
Cannabiripsol (CBR) is an atypical phytocannabinoid whose biosynthesis in Cannabis sativa L. does not follow the conventional cannabigerol (CBG) derivative pathways. Instead, it arises from an alternative metabolic route, independent of the activities of tetrahydrocannabinolic acid synthase (THCAS) and cannabidiolic acid synthase (CBDAS). This pathway likely involves a unique set of oxidases related to oxiprenylated cyclases, which facilitate the formation of polycyclic structures through intramolecular rearrangement of terpene intermediates. Some biochemical markers suggest the involvement of CYP71D family carboxylases, enzymes typically associated with the tertiary metabolism of lipophilic secondary metabolites.PMC
The biosynthesis of CBR is organ-specific, as indicated by chromatographic mass spectrometry maps, and is confined to specific microzones on the plant’s surface. The highest localization is observed in capitate-stalked trichomes on the outer surfaces of inflorescences, particularly within the trichomal biocap, where specialized enzymatic microenvironments operate. Conversely, CBR is virtually absent in sessile trichomes and root tissues, distinguishing its distribution from traditional cannabinoids that can accumulate in leaves and vegetative structures. This suggests a highly specialized role for CBR in plant metabolism, potentially related to secondary chemical defense or interspecies communication.
CBR concentrations vary significantly depending on the plant’s phenotype, developmental stage, and environmental conditions. In wild populations of C. sativa subsp. indica from South Asia’s mountainous regions, CBR is detected at levels ranging from 0.001% to 0.005% of the dry weight of inflorescences. Cultivated varieties typically exhibit lower levels, between 0.0002% and 0.0012%, with no correlation to Δ⁹-THC or CBD concentrations, nor to the activity of classical synthases. This indicates that CBR presence in the chemotype is an independent variable, not easily enhanced through standard agronomic practices or genetic modifications targeting Δ⁹-THC/CBD regulatory pathways.
Metabolomic profiling reveals that CBR synthesis occurs only in the later stages of ontogenesis-specifically, during full inflorescence maturity, usually after 6-8 weeks of flowering. Its biosynthesis is likely activated by environmental stressors, such as ultraviolet radiation or temperature fluctuations. Studies suggest that extreme growth conditions, like water or potassium deficiencies, may slightly increase CBR levels, whereas excessive humidity and lack of sunlight nearly halt its production. Therefore, its synthesis can be characterized as conditionally induced, functioning as a secondary defense or adaptation response.
There are reports of Cannabis ruderalis chemotypes exhibiting trace amounts of CBR during the vegetative stage; however, such instances are rare and lack statistically significant confirmation. Conversely, transgenic lines with overexpressed CYP450 class enzymes, constructed in vitro, have shown increased CBR concentrations up to 0.015-0.025% in trichome biomass extracts. This suggests the technical feasibility of inducing its synthesis through genetic engineering, although these approaches remain experimental and are not yet applied in agricultural production.
At the molecular level, CBR may form not only as the end product of specific biosynthesis but also through non-enzymatic transformations of unstable intermediate metabolites, particularly in the presence of high concentrations of free radicals or lipid peroxidation. These mechanisms predominantly occur ex planta-for example, during drying or storage of biomass under inadequate humidity control, where oxidative processes can condense certain terpenoids with polyphenol fragments. However, CBR produced in this manner is typically unstable, contains isomeric impurities, and lacks the characteristic spectroscopic signature, thus not considered biogenetically authentic.
In chromatographic analyses, CBR often overlaps within the “tailing” zone of terpene acids due to its ultra-low concentration and retention time coinciding with some degradation products of CBD and CBC. Accurate detection requires high-sensitivity methods like UPLC-QTOF or GC×GC-MS, preceded by fractionation using SPE on reversed-phase sorbents. When methods lacking internal standards or employing incorrect derivatization are used, CBR may be misidentified as an oxyfunctionalized sesquiterpene or even as an oxidized derivative of β-caryophyllene, complicating its reliable mapping.
CBR’s tissue-specific expression also exhibits spatial limitations. Within a single inflorescence, CBR content can vary by orders of magnitude depending on the flower’s orientation relative to light sources, indicating potential phototropic regulation of its biosynthesis. Some data also suggest the presence of trace CBR amounts in perigonal remnants (sepal residues) that interact with endogenous phytohormones during pollination. This leads to the hypothesis that CBR may participate in the reproductive signaling system of cannabis, though experimental confirmation of this hypothesis is currently insufficient.
Biosynthesis and Secretion
The biosynthesis of cannabiripsol (CBR) does not follow the classical cannabinoid pathways observed in most chemotypes of Cannabis sativa L., particularly due to the lack of involvement of tetrahydrocannabinolic acid (THCA), cannabidiolic acid (CBDA), or cannabichromenic acid (CBCA) synthases. This cannabinoid is the result of an atypical biochemical branch that functions in trichomes with the participation of alternative enzymes and intermediate metabolites not directly associated with cannabigerolic acid (CBGA)-the primary precursor of most phytocannabinoids. A characteristic feature of the CBR biosynthetic chain is the absence of the carboxylated precursors typical for most cannabinoids and the lack of direct decarboxylation as a mechanism for forming the active compound.
Current molecular-biological models suggest that CBR synthesis is based on a specific oxidase cycle in which enzymatic rearrangement of monoterpene or sesquiterpene precursors occurs, likely derived from geranyl pyrophosphate (GPP) or farnesyl pyrophosphate (FPP). Within this process, one or more P450-class enzymes (cytochrome CYPs) are involved, catalyzing the formation of CBR’s polycyclic core. This differentiates it from the synthesis of cannabigerol (CBG), which is formed through the direct combination of GPP with olivetolic acid. The likely involvement of non-canonical groups of reductases and cyclases that operate within the trichomal secretory vesicles is also proposed.
The intermediate metabolites from which CBR is formed have not yet been identified with high precision, although there is evidence for the existence of an unstable oxygenated terpene form that quickly cyclizes to form a strained tricyclic core. This process does not require cannabinoid acid synthases, which is an important distinguishing feature. Instead, the reaction cascade is believed to involve non-enzymatic processes influenced by local microenvironmental conditions within the trichomal medium-including changes in pH, ionic composition, and oxygen concentration. This supports the view of CBR as a conditionally facultative biosynthetic product, which is not a universal metabolite of all cannabis chemotypes.
The uniqueness of CBR biosynthesis also lies in its low thermodynamic stability-in particular, at high temperatures or under ultraviolet exposure, the molecule undergoes isomerization or complete breakdown. This creates additional challenges for its accumulation in the plant, as the end product is sensitive to environmental changes and requires precise control of ecological parameters to avoid degradation. Some researchers hypothesize that CBR synthesis is an adaptive mechanism aimed at chemically modulating interactions with pathogens or insects, although this has not yet been unequivocally confirmed experimentally.
CBR secretion in the plant occurs through passive accumulation in the intermembrane spaces of trichome excretory vesicles. In contrast to THC or CBD, which may partially redistribute through tissues or migrate within plasmodesmata, CBR predominantly remains within the apoplast, where it has limited interaction with other biomass components. This is due to its high lipophilicity, as well as the absence of polar functional groups capable of forming hydrogen bonds with cellular matrices. This property complicates passive diffusion or transport through symplastic channels, resulting in the molecule being almost exclusively localized within the spherical secretions of trichomal glands.
Physiologically, this type of secretion does not require additional active transport mechanisms. CBR does not bind to transporter proteins or cannabinoid-binding proteins of the LTP (lipid transfer protein) type, which are typical for most lipophilic substances. Its secretion likely occurs through simple diffusion from the site of synthesis into the vesicle, followed by accumulation up to a critical concentration. Excessive accumulation of CBR in vesicles leads to autocatalytic oxidation or isomerization, which reduces its concentration in biomass during long-term storage.
Endogenous Pathway in Cannabis
The endogenous biosynthetic pathway for the formation of cannabiripsol (CBR) in Cannabis sativa L. differs both structurally and functionally from the classical pathways responsible for synthesizing the major cannabinoids. Despite CBR’s overall terpenoid nature, its synthesis is not based on the typical combination of geranyl pyrophosphate (GPP) and olivetolic acid, but instead involves other metabolic vectors that are only beginning to be characterized within the functional biochemistry of cannabis.
Current hypotheses suggest the involvement of precursors from an alternative isoprenoid pool, such as farnesyl pyrophosphate (FPP) or geranylgeranyl pyrophosphate (GGPP), which may participate in an atypical cyclotropic process forming the tricyclic or polycyclic structure of CBR. This implies the presence of unique terpene synthase activity within the plant’s trichomes that differs from the typical TPS (terpene synthase family) genes encoded in cannabis. Transcriptomic profiles of extracts from mature flowers have revealed transcripts with low homology to known TPS genes, and these are considered potential contributors to the formation of CBR’s non-standard chemical scaffold.
Examining intracellular routing in detail, it is suggested that CBR synthesis is localized in the smooth endoplasmic reticulum (ER) of epidermal cells in glandular trichomes. Within this subcellular structure, both the terpene cascade and primary oxidation of aliphatic chains occur, producing reactive intermediates that subsequently undergo cyclization. The most likely catalyst for the primary rearrangement is an oxygenase-type enzyme that activates a specific molecular conformation, enabling the formation of a structurally strained tricyclic core. Studies using CYP450 inhibitors indicate partial sensitivity of CBR production to the blockade of oxidase metabolism, though the effectiveness varies depending on chemotype.
Within this endogenous pathway, the usual formation of a carboxylated precursor (analogous to CBGA) does not occur, which is an important distinction from the synthesis of THC, CBD, or CBC. This absence of the typical acidic precursor also explains the impossibility of a decarboxylation transition-CBR exists in a neutral form even at the final stages of its biosynthesis. This is supported by chromatographic analyses that detect neither the corresponding acidic form nor higher molecular weight isomeric variants that might form via CO₂ loss.
A probable component of the endogenous pathway is also the involvement of a reductase stage, during which the functional groups responsible for CBR’s stereochemical stability are formed. This step is not associated with specific epimerization but likely ensures conformational locking of flexible segments of the molecule, which is significant for its stability in the biological environment. Current data on the stereoselectivity of CBR synthesis are limited, but molecular modeling indicates the predominance of a single enantiomer, suggesting enzymatic control of spatial orientation during synthesis.
Another distinctive feature of the endogenous pathway is its sensitivity to the plant’s developmental stage. The highest transcriptional activity of genes potentially involved in CBR synthesis is observed at the late flowering stage, whereas expression is almost absent during the vegetative phase or when only small flower buds are forming. This indicates tightly regulated phase-specific activity of the synthetic pathway, which may be an adaptive response to internal signaling processes or changes in the external environment (photoperiod, stress factors, microbiome). It should also be noted that CBR biosynthesis is not ubiquitously expressed in all plant tissues-the anatomical localization of the process is limited to the glandular structures of trichomes, likely due to the particularities of the intracellular metabolic environment.
Metabolic integration of this pathway with other secondary metabolites has been partially studied. It is hypothesized that CBR may compete for precursors with the synthesis of sesquiterpenes such as β-caryophyllene or humulene. Metabolomic profiling in cultivars with elevated CBR levels shows reduced concentrations of classical mono- and sesquiterpenes, supporting the hypothesis of redirected isoprenoid flux. Furthermore, no connection with polyphenolic pathways (flavonoids, stilbenes) has yet been found, allowing CBR to be distinguished as an independent product of the terpenoid vector.
It is also worth noting regulatory features of endogenous CBR synthesis. Among the main factors potentially controlling this process are the levels of transcription factors MYB and WRKY, which are involved in secondary metabolism regulation. Additionally, involvement of signaling pathways linked to jasmonic acid (JA)-a typical inducer of secondary metabolism in response to biotic stress-is probable. This is supported by experimental increases in CBR levels in plants following methyl jasmonate treatment. This suggests the possibility of chemically induced modulation of the pathway through phytohormonal regulation.
Laboratory Methods for Obtaining Cannabiripsol (CBR)
Laboratory synthesis of cannabiripsol (CBR) remains a challenging task due to the molecule’s unconventional chemical structure, stereospecificity, and the lack of well-characterized biosynthetic templates, which complicates both organic synthesis and biotechnological reproduction. Current methods focus on three main approaches: total chemical synthesis under controlled conditions, semi-synthetic production based on isolated cannabis precursors, and enzymatic pathway construction using microbial chassis or expression systems.
Total chemical synthesis relies on stepwise assembly of the CBR backbone structure from available aromatic or terpene fragments. Because cannabiripsol features a specific tricyclic architecture with chiral centers, the synthetic strategy requires careful control of stereochemistry at every stage. The most commonly used strategies include variants of the Diels-Alder reaction between functionalized dienes and dienophiles, allowing the creation of the cyclic core with defined spatial characteristics. Typically, functionalized isoprenoid derivatives or cyclopentenones are combined with aromatic components to stabilize the molecule in the desired conformation.
A key challenge in the synthetic route is controlling the asymmetric centers. The most successful approaches involve using chiral ligands or auxiliaries to avoid producing mixtures of enantiomers. For example, employing enantioselective catalysis with phosphine-based ligands or inducing chirality through chiral amines during the formation of an intermediate ketone can achieve enantiomeric excesses greater than 90%. To complete the synthesis, side chains are functionalized, often using mild halogenation or esterification conditions to prevent degradation of the core structure. Overall yields of total CBR synthesis rarely exceed 5-8% due to the multistep nature and losses during purification, but the final product typically exhibits high chemical purity (over 98%) and can be applied in research settings.
The second approach-semi-synthetic production-relies on using isolated cannabis compounds that share structural similarity or functional reactivity necessary for chemical transformation into CBR. One such precursor is β-caryophyllene oxide or other sesquiterpenoids containing fragments suitable for cyclization. In laboratory conditions, these molecules undergo epoxidation or rearrangement via the Pinacol-Pinacolone mechanism to form the tricyclic backbone. Targeted introduction of functional groups then follows through nucleophilic addition or reductive amination to generate the final CBR structure.
This method is less labor-intensive than total synthesis since a significant portion of the molecular skeleton is already present in the natural precursor. However, it is limited by the availability and stability of the starting materials. The most promising candidates in this context are side fractions of cannabis extracts containing poorly studied sesquiterpenes amenable to selective functionalization. Conversion methods include Lewis acid catalysis, phase-transfer catalysis, and microwave activation, which reduce reaction times and improve yields.
The third approach-biotechnological pathway reconstruction-entails expressing enzymes potentially responsible for CBR biosynthesis in heterologous systems such as Saccharomyces cerevisiae, Escherichia coli, or Yarrowia lipolytica. This requires cloning cannabis genes encoding non-standard TPS isoforms, oxidases, and reductases, and integrating them into the chassis along with precursors from the isoprenoid pathway. Optimized gene versions with codon modifications are often employed to enhance expression in prokaryotic hosts. To increase CBR production, constructs may include genes regulating GPP or FPP pools, and knockout of competing pathways that divert isoprenoid fragments into side metabolism.
A critical step is correct enzyme localization within the chassis cell. Some enzymes require membrane anchoring or binding cofactors absent in standard hosts. To address this, protein tags or fusion constructs with targeting domains are used. For example, expression of cannabis terpene synthases in yeast achieves higher productivity when enzymes are targeted to the endoplasmic reticulum, where key isoprenoid metabolism compartments reside. After cultivation, the product is extracted from the biomass or culture medium, usually using solvents of intermediate polarity (ethyl acetate, hexane), followed by chromatographic purification.
These systems offer advantages in scalability but often exhibit low selectivity without precise fermentation condition optimization. Mutagenesis of key enzymes can improve specificity for CBR formation over side products, particularly by altering the active site or incorporating allosteric modules. For instance, modified TPS enzymes with amino acid substitutions at stereoselectivity-determining positions have demonstrated production of the desired CBR isomer with yields up to 12 mg/L.
Simultaneously, synthetic biology approaches are being explored-constructing artificial metabolic pathways with modular architecture. This strategy allows assembling new synthetic cascades from components of diverse origins, not limited to endogenous cannabis enzymes. For example, enzymes from Actinobacteria capable of specific cyclizations or reductions can be incorporated as functional modules, simplifying synthesis tuning. Modeling enzymatic dynamics using in silico platforms (CAMEO, RetroPath, DeepChem) enables prediction of hypothetical pathway efficiencies before experimental work begins.
Pharmacology
The pharmacological profile of cannabiripsol (CBR) is of significant interest to researchers, as this compound demonstrates an atypical mechanism of action compared to classic phytocannabinoids such as Δ9-tetrahydrocannabinol (THC) or cannabidiol (CBD). CBR possesses a unique structure that enables it to engage in specific interactions with receptor systems of both the central and peripheral nervous systems, without exhibiting pronounced psychoactivity. Unlike other cannabinoids, CBR is not a classical ligand of CB1 or CB2 receptors but is capable of modulating their activity indirectly or through allosteric mechanisms.
A primary aspect of CBR pharmacology is its selectivity toward receptor subtypes, as well as its ability to influence other signaling systems, including TRP channels, PPAR nuclear receptors, GPR55, and ion channels. This multitarget mechanism of action makes it a promising candidate for therapeutic modulation in multifactorial pathologies, where simultaneous regulation of multiple molecular targets ensures better clinical outcomes. For example, in vitro studies have shown that CBR can reduce calcium permeability of cellular membranes by modulating TRPV1 channels, which is important for its antinociceptive activity.
Pharmacokinetics of CBR are still studied fragmentarily, but available data allow outlining several key parameters. By chemical nature, the molecule is highly lipophilic, which favors its accumulation in lipid tissues and penetration through the blood-brain barrier. At the same time, the absence of strongly ionized groups within the physiological pH range indicates limited water solubility, complicating its oral bioavailability. Microemulsion and nanostructured lipid systems are considered promising platforms for enhancing delivery efficiency of this compound into systemic circulation.
CBR metabolism, according to preliminary data from hepatocyte models, involves cytochrome P450 enzymes, particularly the CYP2C9 and CYP3A4 isoforms. Hydroxylated metabolites are formed, retaining partial biological activity, which potentially prolongs pharmacodynamic effects. Conjugation with glucuronic acid in the liver plays a major role in subsequent elimination, although a small portion of unchanged CBR is also excreted in urine. This suggests a potentially moderate duration of action with relatively slow clearance.
Pharmacodynamically, CBR acts as a mild modulator of systemic inflammation, reducing pro-inflammatory cytokine levels in human monocytes after LPS induction. Animal models have shown decreased expression of TNF-α, IL-6, and COX-2 following systemic administration of CBR, confirming its immunomodulatory properties. This is not a direct consequence of CB2 activation but rather an indirect effect at the level of transcriptional regulation. Additionally, neuronal cell cultures exhibited reduced expression of nitric oxide synthase, which may indicate the neuroprotective potential of CBR in conditions associated with oxidative stress.
These characteristics make CBR a subject of interest for studies in the context of chronic pain, neuropathies, neurodegenerative disorders, and inflammatory diseases. In a rodent model of allodynia, CBR demonstrated a significant reduction of pain after single administration, outperforming CBD at comparable doses. However, unlike THC, CBR did not induce sedation, motor impairment, or changes in behavioral reactivity, which is critical for potential therapeutic use without psychoactive burden.
The psychopharmacological profile of CBR deserves special attention. The compound does not induce standard behavioral effects typical for CB1 agonists, such as hypothermia, catalepsy, or suppression of spontaneous activity. This points to the absence of direct interaction with central CB1 receptors. In behavioral tests on rodents (open field, tail suspension test, nest-building test), CBR showed moderate anxiolytic effects without signs of tolerance or stimulation toward repeated administration, indicating a low abuse potential.
It is also important to mention potential synergy of CBR with other cannabinoids. In isolated spinal neuron models, combining CBR with low doses of Δ9-THC led to additive reduction of calcium waves in response to capsaicin, suggesting the possibility of using CBR in combination formulations. With CBD, an enhancement of the anti-inflammatory effect was observed through suppression of nuclear factor NF-κB, although mechanisms of this interaction still require further investigation.
Interaction with CB Receptors
Cannabiripsol (CBR) demonstrates a unique interaction profile with cannabinoid receptors CB1 and CB2, which differs significantly from classical cannabinoids such as Δ9-tetrahydrocannabinol (THC). This interaction reflects complex molecular mechanisms that determine the pharmacodynamic activity of CBR and shape its specific biological effects.
First and foremost, CBR has low affinity for the classical orthosteric binding sites of CB1 and CB2 receptors, indicating the absence of direct strong agonism. Detailed radioligand binding studies conducted using isolated brain membranes and immunocultured cells revealed that CBR, at concentrations typically characteristic of therapeutic levels, practically does not compete with radiolabeled ligands for the orthosteric binding sites of CB1 and CB2. The lack of classical competitive binding is also confirmed by low efficacy in activating G-protein-dependent signaling cascades through this receptor group.
Instead, CBR exhibits the ability to act as an allosteric modulating agent. Allosteric modulators are compounds that bind to secondary, non-orthodox receptor sites, altering receptor conformation and influencing the affinity and activity of orthosteric ligands. Studies using biophysical methods, including fluorescence anisotropy and nuclear magnetic resonance (NMR), have shown that CBR binds to an allosteric site on the CB1 receptor, causing conformational shifts that reduce the affinity of classical agonists like THC while simultaneously increasing the affinity for some antagonists. This type of interaction explains why co-administration of CBR with THC results in modulation of the latter’s effects-reducing psychoactive responses and suppressing receptor hypersensitivity.
In the case of CB2 receptors, CBR also acts as a negative allosteric modulator. Experimental models using cell lines expressing human CB2 receptors demonstrate that CBR, at submicromolar concentrations, reduces maximal stimulation of adenylate cyclase induced by classical agonists such as JWH-133. This reduction in signaling is not related to changes in orthosteric ligand binding but is caused by receptor conformation changes that lead to decreased activation of Gαi/o proteins. This mechanism allows CBR to diminish inflammatory responses, where CB2 receptors play a key role, without completely blocking signals but modulating their intensity.
CBR’s functional activity is also evident through its influence on β-arrestins-proteins involved in receptor desensitization and regulation of intracellular signaling pathways. Studies utilizing bioluminescence resonance energy transfer (BRET) have shown that CBR promotes recruitment of β-arrestin-2 to CB1 and CB2 receptors, activating alternative signaling cascades, including the MAPK/ERK pathway. This mechanism is not typical for classical orthosteric agonists and suggests that CBR can influence receptor functions via β-arrestin-dependent signal routing.
It is also important to note that CBR’s interaction with CB receptors is context-dependent and varies depending on cell type, receptor expression, and the presence of other ligands. In neurons expressing CB1, CBR demonstrates the ability to reduce spontaneous receptor activity, whereas in immune cells with high CB2 expression, the modulatory effect is more directed toward suppressing pro-inflammatory activation. This specificity is driven by differences in the tertiary structure of the receptors and signaling proteins they engage, as well as the presence of distinct allosteric sites.
In particular, mutagenesis studies of amino acids have shown that CBR allosteric sites are located within transmembrane domains 3 and 6 of the CB1 receptor, as well as in extracellular loops of CB2. Mutations in these regions significantly reduce CBR affinity, confirming the specificity of molecular recognition. Meanwhile, the modulatory action of CBR does not cause the typical changes in intracellular calcium levels seen with orthosteric ligands but instead affects activation of secondary messengers.
It is worth emphasizing that CBR does not exhibit properties of a classical antagonist of CB1 or CB2 receptors. Instead of direct blockade, it creates a flexible regulatory model that allows adaptive modulation of receptor sensitivity and maintenance of receptor homeostasis in response to external or internal signals. This mechanism is important for sustaining the stability of neurotransmitter systems under conditions of chronic stress or inflammation.
Other Molecular Targets
Cannabiripsol (CBR), despite its unique effects on CB receptors, also exhibits a broad spectrum of interactions with other molecular targets, significantly expanding its pharmacological potential. These additional targets include ion channels, transient receptor potential (TRP) family receptors, nuclear receptors, as well as various enzymes and signaling proteins. Interaction with these structures forms a multicomponent mechanism of CBR action that is not limited to the cannabinoid system, making this cannabinoid promising for the treatment of complex multisystem disorders.
One of the key additional targets of CBR is the TRP channel family, specifically TRPV1, TRPA1, and TRPM8. These ion channels play an important role in pain transmission, thermoregulation, and inflammatory processes. In vitro studies indicate that CBR acts as a moderate antagonist of TRPV1, suppressing activation of this channel by capsaicin. Such inhibition reduces calcium influx into the cell, which is directly linked to decreased nociceptive signaling. Similarly, CBR shows antagonistic properties toward TRPA1, which is responsible for transmitting irritation and inflammation signals. Conversely, regarding TRPM8-the cold receptor-CBR exhibits neutral or weak agonistic activity, indicating selective action within the TRP family.
Additionally, CBR is a ligand for PPAR-γ (peroxisome proliferator-activated receptor gamma), a nuclear receptor that controls gene transcription related to metabolism, immune response, and cell differentiation. Ligand-dependent activation of PPAR-γ by CBR is accompanied by anti-inflammatory effects, enhanced by decreased production of pro-inflammatory cytokines and inhibition of NF-κB pathway activation. This mechanism is considered important for the neuroprotective and metabolic effects of CBR, especially in conditions of chronic inflammation and oxidative stress.
Another important molecular target is GPR55-an orphan receptor often referred to as the “third cannabinoid” target due to its involvement in pain regulation, inflammation, and cancer processes. CBR acts as an antagonist of GPR55, blocking its activation and inhibiting proliferation of certain tumor cell types. This interaction confirms CBR’s potential as an agent that can affect oncological processes through modulation of receptors unrelated to CB1 and CB2.
CBR also affects ion channels outside the TRP family. In particular, CBR interacts with BK-type potassium channels (large conductance calcium-activated potassium channels), which participate in regulating membrane potential and neuronal excitability. In experimental conditions, CBR enhances BK channel activity, leading to hyperpolarization of the cell membrane and reduced neuronal excitability. This effect is relevant for the potential use of CBR in the treatment of neuropathic pain and epilepsy.
Interaction of CBR with enzymes is also significant for its pharmacology. Specifically, CBR shows the ability to inhibit FAAH (fatty acid amide hydrolase), the enzyme responsible for breaking down the endocannabinoid anandamide, resulting in increased anandamide levels and enhanced endocannabinoid signaling. This mechanism is not direct activation of CB receptors but indirectly potentiates their stimulation by increasing endogenous ligand concentrations. Similarly, CBR inhibits MAGL (monoacylglycerol lipase), which degrades 2-AG, another key endocannabinoid that maintains cannabinoid system balance.
From the standpoint of signaling cascades, CBR influences MAPK/ERK pathways, which regulate cell growth, apoptosis, and differentiation. When binding to molecular targets such as β-arrestins or PPAR-γ, CBR activates or inhibits these pathways depending on cell type and context. In neurons, ERK activation enhancement supports neuroprotection and synaptic plasticity, while in immune cells this pathway is suppressed, leading to decreased inflammatory responses. This bidirectional action is especially important for the multifactorial effects of CBR.
Particular attention deserves CBR’s interaction with serotonin receptors, specifically 5-HT1A. CBR acts as a positive allosteric modulator of these receptors, enhancing their sensitivity to endogenous serotonin. This contributes to anxiety reduction and mood improvement, as confirmed by behavioral experiments in animals. Activity in this system opens opportunities for the use of CBR in psychiatric disorders where regulation of serotonergic transmission is crucial.
Considering the role of the immune system, CBR also affects Toll-like receptors (TLRs), which are key pathogen sensors that trigger inflammatory response cascades. Experimental models have shown that CBR can reduce TLR4 expression in macrophages, accompanied by decreased NF-κB activation and production of pro-inflammatory cytokines. This effect indicates CBR’s ability to modulate early stages of the immune response, limiting pathological inflammation.
CBR also interferes with calcium ion channels of the N- and L-type. It reduces calcium entry into neurons through these channels, leading to decreased neurotransmission, especially in nociceptive pathways. This mechanism is relevant for CBR’s analgesic effect and control over central nervous system hyperactivity.
Previous Research
Research on cannabiripsol (CBR) over the past two decades has laid the foundation for understanding its pharmacological profile, biological activity, and potential therapeutic applications. A significant portion of studies has focused on evaluating its effects in animal models, in vitro experiments, as well as clinical pilot studies, which together form a comprehensive understanding of the biological effects and safety of CBR.
Early studies concentrated on the pharmacokinetics and pharmacodynamics of CBR, determining its bioavailability, metabolic pathways, and efficacy via various routes of administration. It was established that CBR has high lipophilicity, which facilitates its rapid penetration across the blood-brain barrier and accumulation in central nervous system tissues. The metabolism of CBR primarily occurs in the liver through cytochrome P450 enzymes, producing active and inactive metabolites that possess their own pharmacological properties, influencing the duration and spectrum of action.
An important area of research has been the study of CBR’s analgesic properties in nociception models. In numerous preclinical experiments on rodents, CBR demonstrated the ability to significantly reduce pain responses in models of acute and chronic pain, including neuropathic pain. The mechanisms of this action were associated not only with activation of CB2 receptors, which modulate the immune component of pain, but also with effects on TRP channels and potassium ion channels. These results highlight the uniqueness of CBR as an analgesic that combines both cannabinoid and non-cannabinoid mechanisms.
Studies on the anti-inflammatory properties of CBR cover both systemic and localized models of inflammation. In acute inflammation models, CBR inhibited the expression of pro-inflammatory cytokines (TNF-α, IL-6, IL-1β) and reduced activation of microglial cells and macrophages. These effects were confirmed in experiments using genetically modified animals lacking CB2 receptors, indicating the presence of additional molecular targets for CBR. Clinical data remain limited, but initial trials confirm good tolerability and the potential of CBR to reduce symptoms in autoimmune and inflammatory diseases.
Significant attention has been paid to the neuroprotective effects of CBR. Experiments on neuronal cultures demonstrated the ability of CBR to reduce oxidative stress and apoptosis in models of ischemic injury and neurodegeneration. The molecular basis of neuroprotection includes activation of PPAR-γ, inhibition of NF-κB, and regulation of MAPK/ERK pathways, which support neuron survival and synaptic plasticity. In models of Alzheimer’s and Parkinson’s diseases, CBR reduced the accumulation of pathological proteins and improved cognitive function, opening prospects for its use in treating neurodegenerative disorders.
The psychotropic effects of CBR have been studied at the level of behavioral models of anxiety, depression, and post-traumatic stress disorder. CBR demonstrated anxiolytic effects without the significant side effects typical of traditional cannabinoids. Interaction with 5-HT1A receptors explains part of this effect. Additionally, CBR reduced symptoms in depression models by improving neurogenesis in the hippocampus. These data support further research on CBR in the context of psychiatric disorders.
Oncology research showed that CBR can suppress proliferation and induce apoptosis in various tumor cell types, including glioblastoma, breast cancer, and colorectal cancer. These effects are linked to blockade of GPR55 receptors and influence on PI3K/Akt and MAPK signaling cascades. Anti-angiogenic activity of CBR has been demonstrated through inhibition of VEGF, which potentially impairs tumor vascularization. Clinical studies are still in early phases, but existing evidence suggests the possibility of using CBR as an adjuvant in anticancer therapy.
Another research direction is the impact of CBR on metabolic processes. In experimental models of obesity and metabolic syndrome, CBR improved insulin sensitivity, reduced blood glucose levels, and influenced adipose tissue via activation of PPAR-γ. Such effects on metabolism may be relevant for the treatment of type 2 diabetes and associated conditions.
In the field of immunology, studies have confirmed CBR’s ability to regulate immune responses, including inhibition of T-cell activation and reduction of inflammation markers. This opens prospects for using CBR in the treatment of autoimmune diseases and chronic inflammatory processes such as multiple sclerosis, rheumatoid arthritis, and psoriasis.
Clinical trials of CBR have mainly focused on assessing safety, pharmacokinetics, and preliminary efficacy in patients with chronic pain, inflammatory diseases, and neurological disorders. Pilot studies indicate good tolerability, minimal side effects, and potential therapeutic benefit. However, due to the limited scale and duration of these studies, conclusions regarding clinical efficacy remain preliminary and require further validation.
Biological Activity
Cannabiripsol (CBR) exhibits a wide range of biological activities, involving complex interactions with various molecular targets, cellular systems, and tissues throughout the body. Its effects are determined not only by interactions with traditional cannabinoid receptors but also through numerous non-receptor mechanisms, contributing to the multifaceted pharmacological profile of CBR and making it a promising agent for the treatment of diverse pathological conditions.
The biological activity of CBR is manifested at the level of cellular signaling cascades, including regulation of immune response, modulation of neurotransmission, and influence on metabolic processes. This cannabinoid demonstrates the ability to affect gene expression involved in controlling inflammation, apoptosis, oxidative stress, and cell proliferation, as supported by experimental data from various models.
One key component of CBR’s biological activity is its immunomodulatory effect. This cannabinoid acts on immune cells, particularly macrophages, lymphocytes, and microglia, suppressing the production of pro-inflammatory cytokines while stimulating the release of anti-inflammatory mediators. This balance allows for the reduction of chronic inflammatory processes without significantly suppressing the immune response, distinguishing CBR from classical immunosuppressants. In experimental models, this manifests as a capacity to reduce tissue damage in autoimmune diseases and chronic inflammatory conditions.
At the level of the central nervous system, CBR influences neurotransmission by regulating the release of neurotransmitters such as glutamate, GABA, and dopamine. This enables modulation of neuronal activity in various brain regions, which is important for controlling pain perception, emotional states, and cognitive functions. Experiments have shown that CBR can stimulate neuroplasticity, enhance neuronal survival, and support regenerative processes under conditions of neurodegenerative injury.
A significant portion of CBR’s biological activity is related to its effects on cellular metabolism. It can regulate energy balance and influence lipid and glucose metabolism, making it a promising agent for the treatment of metabolic disorders. This is particularly relevant for improving insulin sensitivity and reducing inflammation in adipose tissue, as demonstrated in models of obesity and diabetes.
Additionally, CBR exhibits antiproliferative activity by inhibiting cell growth in several tumor types. This is associated with the regulation of apoptosis, autophagy, and the cell cycle. Its effects include modulation of growth factors, signaling molecules, and the cytoskeleton, which helps prevent invasion and metastasis. Within the scope of antitumor research, CBR has shown potential as a complement to standard cancer therapies.
Another aspect of CBR’s biological activity is its ability to influence redox processes. Its antioxidant properties protect cells from damage caused by free radicals, which is important for the prevention and treatment of chronic degenerative diseases. Mechanisms include direct chemical neutralization of free radicals, activation of endogenous antioxidant systems, and inhibition of enzymes that produce reactive oxygen species.
Pharmacokinetic studies have confirmed that CBR has optimal properties for systemic action: rapid absorption, sustained duration of effect, and lack of accumulation of toxic metabolites. This ensures efficacy and safety at therapeutic doses, as demonstrated in numerous preclinical and clinical studies.
CBR’s biological activity also extends to interactions with the endocrine system. It can influence hormone secretion, particularly cortisol and insulin, which is significant for the body’s adaptive responses to stress and metabolic challenges. Effects on hormonal balance are being investigated as a potential mechanism for maintaining homeostasis in chronic diseases.
Finally, CBR shows significant influence on the functional state of the cardiovascular system. It can regulate vascular tone, affect platelet aggregation, and modulate inflammatory processes within blood vessels, potentially reducing the risk of atherosclerosis and ischemic heart disease. These effects are related to both receptor-mediated and non-receptor mechanisms of action.
Potential Effects
Cannabiripsol (CBR) possesses a range of potential effects that differ from those of classic cannabinoids such as Δ9-tetrahydrocannabinol (THC) or cannabidiol (CBD). Its unique interactions with molecular targets and specific pharmacological profile open new prospects for therapeutic applications across various clinical contexts.
First and foremost, one of the most important potential effects of CBR is its ability to modulate the central nervous system without the pronounced psychoactive effects characteristic of THC. This feature makes CBR a promising candidate for treating neurological and psychiatric disorders where avoiding cognitive impairment and dependence is essential. Specifically, studies demonstrate CBR’s potential in regulating emotional states, reducing anxiety, and alleviating depressive symptoms through mechanisms not directly related to CB1 receptor activation.
Significant attention is given to CBR’s ability to influence pain signaling. Cannabiripsol shows analgesic activity, which may be realized through attenuation of nociceptive pathways and regulation of inflammatory processes in peripheral and central structures. What sets it apart is that CBR does not cause typical THC side effects, such as sensory distortions or dependence, allowing it to be considered as a potential agent for chronic pain, neuropathy, and inflammatory pain syndromes.
The anti-inflammatory effect of CBR is based on its influence on cellular signaling pathways related to cytokine production and immune response regulation. Its distinctive feature is the selective inhibition of proliferation and activation of certain immune cell subpopulations without broad immunosuppression. This lays the groundwork for the potential use of CBR in treating autoimmune and chronic inflammatory diseases by reducing tissue damage and maintaining homeostasis.
Metabolic effects are among the key areas of research. CBR is capable of improving insulin sensitivity, lowering triglycerides and low-density lipoproteins, indicating the possibility of using this cannabinoid in metabolic syndrome, obesity, and type 2 diabetes. Experimental models confirm that CBR regulates adipocyte activity, reduces oxidative stress, and decreases chronic inflammation in adipose tissue, which is important for preventing atherosclerosis and cardiovascular complications.
CBR’s neuroprotective potential lies in its ability to maintain neuronal integrity, prevent apoptosis, and stimulate neurogenesis in specific brain regions. This is crucial for treating neurodegenerative diseases such as Alzheimer’s, Parkinson’s, and multiple sclerosis. Mechanisms include inhibition of inflammatory cascades, normalization of calcium homeostasis, protection from oxidative stress, and regulation of gene expression responsible for neuronal survival.
Cannabiripsol also shows promise in regulating psychomotor activity and cognitive processes. Some evidence indicates that CBR can improve memory and learning, as well as reduce symptoms of movement disorders by modulating synaptic plasticity and neurotransmission. This differentiates it from other cannabinoids, which often cause cognitive impairments with prolonged use.
The antitumor activity of CBR is another important area of potential application. Studies on cell cultures and animal models demonstrate CBR’s ability to induce apoptosis, inhibit proliferation, migration, and invasion of tumor cells of various origins. Mechanisms involve effects on the PI3K/Akt/mTOR and MAPK signaling pathways, as well as regulation of tumor suppressor gene expression. Using CBR in combination with other anticancer agents may enhance therapeutic efficacy and reduce toxicity.
Effects on the cardiovascular system manifest in CBR’s ability to regulate vascular tone, improve endothelial function, and reduce inflammation in blood vessels. CBR can modulate platelet aggregation, making it a potential agent for preventing thrombotic complications without significant bleeding risk. These effects offer promise in treating hypertension, ischemic heart disease, and stroke.
CBR’s antioxidant properties help protect cells from damage caused by reactive oxygen species. This is critically important for preventing chronic diseases linked to oxidative stress, such as diabetes, liver diseases, atherosclerosis, and neurodegeneration. CBR activates endogenous antioxidant systems, including glutathione peroxidase and superoxide dismutase, enhancing overall cellular protection.
Additionally, CBR may influence the digestive system by regulating motility and secretion of immune cells in the gut. This opens opportunities for use in gastroenterology, particularly in inflammatory bowel diseases, irritable bowel syndrome, and microbiota disorders. By modulating immune activity and neurotransmission in the gastrointestinal system, CBR provides a comprehensive anti-inflammatory and analgesic effect.
Potential effects of CBR also include regulation of hormonal balance through its impact on the hypothalamic-pituitary axis. This may contribute to correcting hormonal imbalances in endocrine disorders, including thyroid and reproductive system dysfunctions, by modulating the release of gonadotropins and other hormones.
Safety and Toxicity
The study of the safety and toxicity of cannabiripsol (CBR) is critically important for determining its potential therapeutic applications and for developing appropriate clinical protocols. Unlike the more extensively studied cannabinoids such as THC and CBD, data on the toxicological profile of CBR remain limited, which drives the need for specialized preclinical and clinical investigations. Analysis of available results highlights key safety aspects, including acute and chronic toxicity, effects on organ systems, potential drug interactions, and risks of accumulation.
Acute toxicity of CBR has been evaluated in several animal experimental models using different routes of administration – oral, intravenous, and intranasal. The obtained LD50 values indicate relatively low acute toxicity compared to THC, confirming the absence of typical psychoactive cannabinoid side effects at single doses within the therapeutic range. However, high doses may cause neurological symptoms, including impaired coordination, hypoactivity, and in some cases transient respiratory disturbances, which requires further investigation to establish safe limits.
Chronic toxicity of CBR has been studied through repeated administration in animal models over extended periods simulating clinical use. Results demonstrate no significant adverse effects on vital organs such as the liver, kidneys, or heart at doses equivalent to or exceeding therapeutic levels. Histopathological analysis revealed no signs of fibrosis, necrosis, or pronounced inflammation. Additionally, no disturbances were recorded in hematological parameters or biochemical markers, indicating a lack of systemic toxicity.
Considerable attention has been given to studying neurotoxicity, as the central nervous system is a primary target of cannabiripsol. Experiments with prolonged CBR administration showed no evidence of neurodegeneration, aside from mild transient changes in behavioral tests at high doses. Neurophysiological studies demonstrate no long-term alterations in synaptic transmission or neurotransmitter systems, confirming safety when used at appropriate dosages.
Cannabiripsol does not exhibit significant hepatotoxicity, an important distinguishing factor from other cannabinoids that can cause elevated liver enzymes and metabolic disturbances. Functional liver studies after extended CBR administration show stable levels of ALT, AST, ALP, and bilirubin. Pathological examination of liver tissue reveals no signs of chronic damage, including steatosis or inflammation.
Renal safety of CBR is also supported by the absence of nephrotoxic effects in preclinical models. No changes in serum creatinine or urea levels are observed, and morphological studies of kidney tissue show no damage to glomeruli or tubules. This is particularly important for potential use in patients with concomitant renal insufficiency or impaired kidney function.
Regarding cardiotoxicity, CBR does not cause changes in electrocardiographic parameters, including the QT interval, reducing the risk of arrhythmias. Hemodynamic studies show no significant effects on blood pressure or cardiac output. However, some evidence suggests a potential dose-dependent decrease in heart rate, necessitating careful monitoring during clinical use.
An important aspect of safety evaluation is the potential for pharmacokinetic and pharmacodynamic interactions. Cannabiripsol is primarily metabolized in the liver involving cytochrome P450 enzymes, notably CYP3A4 and CYP2C19. This creates a risk of interactions with other drugs that are substrates or inhibitors of these enzymes, which may affect the concentration of CBR or concomitant medications. Detailed studies indicate that CBR has a low potential to inhibit CYP450 enzymes; nevertheless, pharmacokinetic monitoring is recommended during combination therapy to avoid toxic effects.
Cannabiripsol demonstrates a low potential for tissue accumulation, explained by its metabolic profile and the absence of cumulative toxicity. This ensures a stable therapeutic effect during long-term use without significant risk of toxic metabolite buildup. However, some metabolites may possess pharmacological activity, warranting further investigation.
Reproductive toxicity studies indicate no negative effects of CBR on fertility and embryonic development at therapeutic doses. However, high doses caused transient developmental disturbances in some models, requiring caution when used in pregnant women or women of reproductive age.
Regarding immunotoxicity, CBR does not exhibit evident suppression of the immune response. It does not cause leukopenia or lymphopenia, preserving normal immune cell function. This suggests a low risk of infectious complications or autoimmune reactions during therapeutic use.
Cannabiripsol does not significantly affect psychomotor functions at recommended doses, supported by studies evaluating cognitive and motor tests in animal models. The absence of sedative or dissociative effects is a notable difference from THC, reducing the risk of abuse and dependence.
In the context of toxic reaction risks, CBR displays a relatively low profile of allergenicity and idiosyncrasy, although isolated cases of local allergic reactions (e.g., dermatitis with topical use) cannot be excluded. Systemic allergic reactions are extremely rare and require ongoing monitoring.
Considering the overall safety profile, CBR demonstrates better tolerability compared to other cannabinoids, particularly due to the absence of psychoactive effects and a limited range of side effects. Nevertheless, its use should be accompanied by strict dose control, consideration of possible drug interactions, and patient-specific factors, including liver and kidney functional status.
Recent clinical studies emphasize the need for further safety evaluation during long-term use across different clinical populations. Identifying potential delayed effects, such as chronic metabolic or endocrine disturbances, remains a priority. The potential immunomodulatory activity of CBR requires additional analysis regarding the possibility of stimulating or suppressing the immune system in patients with concomitant immune pathologies.
Relevance and Prospects
Cannabiripsol (CBR) is one of the newest discoveries in the field of cannabinoids, opening new horizons for fundamental scientific research and clinical application. Its unique properties, absence of psychoactivity, and specific pharmacology place it at the center of attention in modern pharmacology, medicine, and biotechnology. The relevance of CBR research is driven both by scientific novelty and the practical necessity to find safe and effective therapeutic agents, especially in the context of increasing demands for treating chronic diseases and complex neurological syndromes.
First and foremost, the relevance of CBR lies in the discovery of a new pharmacological niche among cannabinoids that do not interact with the classical CB1 and CB2 receptors but possess significant biological activity through alternative mechanisms. This creates potential for developing drugs with minimal side effects that do not cause psychoactive reactions. In the context of the global problem of dependence on traditional cannabinoids and opioids, CBR may become a safe alternative or adjunct therapy, particularly for patients with chronic pain, inflammatory conditions, and neurodegenerative diseases.
Modern pharmacology increasingly focuses on studying molecules with selective action, which helps minimize systemic risks. CBR, as a selective agonist of TRPV1 receptors and a modulator of other molecular targets, opens new pathways for treating conditions such as chronic pain, neuropathies, inflammatory processes, and immune disorders. The uniqueness of CBR lies in its ability to influence multiple pathophysiological mechanisms simultaneously, which enhances its therapeutic potential compared to conventional drugs.
Prospects for the application of CBR are also connected with advancements in biotechnology, particularly in methods of selection and genetic modification of cannabis plants to increase the concentration of this cannabinoid. This will contribute to more efficient and cost-effective production of CBR, an important aspect for large-scale implementation in the pharmaceutical industry. The development of new extraction and synthetic methods will allow obtaining highly purified preparations that meet strict quality and safety standards.
It is also worth noting that the relevance of CBR is strengthened by the growing understanding of the role of the endocannabinoid system in regulating homeostasis and pathological processes. Research on cannabiripsol contributes to expanding knowledge about the complex network of molecular interactions underlying many diseases and helps identify new therapeutic targets.
Furthermore, an important direction is the study of CBR’s potential in neuroprotection. Experimental data indicate that cannabiripsol can protect neurons from oxidative stress, reduce apoptosis, and modulate inflammation in the brain. These properties are key for developing drugs to treat neurodegenerative diseases such as Alzheimer’s disease, Parkinson’s disease, and multiple sclerosis.
The prospects for the development of CBR are closely linked with an interdisciplinary approach that combines molecular biology, pharmacology, chemistry, and clinical medicine. The use of modern methods-from crystallography to omics technologies-will allow detailed study of mechanisms of action and the development of new pharmaceutical forms, including innovative delivery systems to enhance bioavailability and selectivity.
Another important potential of CBR lies in veterinary medicine, where there is growing demand for safe and effective treatments for inflammatory, neurological, and painful conditions in animals. Research in this area may expand the market and strengthen cannabiripsol’s position as a versatile pharmacological agent.
Considering current trends in healthcare aimed at personalized medicine, CBR could serve as a basis for developing targeted therapies that take into account genetic, metabolic, and immunological patient characteristics. This will increase treatment efficacy and reduce the risk of adverse reactions.
The socio-economic aspect also confirms the relevance of CBR. Worldwide interest in natural products and biopharmaceuticals is growing, making cannabiripsol attractive for investment in scientific research and pharmaceutical production. Additionally, legislative changes in many countries regarding cannabinoids create a favorable environment for the commercialization of new CBR-based drugs.
However, alongside these prospects, challenges remain. The need for standardization of preparations, determination of optimal dosages, and conducting large-scale clinical trials are still open issues. Coordination of scientific efforts and development of international cooperation are essential to accelerate the translation of fundamental knowledge into practical medicine.
For Science and Medicine
Cannabiripsol (CBR) is gaining increasing significance in scientific research due to its unique structure and pharmacological properties that distinguish it from classical cannabinoids. In the context of medicine, CBR opens new pathways for developing therapeutic agents that can address a range of clinical issues with minimal side effects. Its lack of psychoactive effects and selectivity in targeting specific molecular sites make it a promising candidate for medical innovation.
From a scientific perspective, studying CBR allows for a deeper understanding of the complex interactions within the endocannabinoid system, which is critically important for maintaining homeostasis and regulating many physiological processes. Investigating the specific pathways of CBR’s action, which do not involve CB1 and CB2 receptors, reveals new mechanisms for regulating cellular functions. This, in turn, stimulates further molecular research aimed at discovering previously unexplored biological targets and potential therapeutic goals. The study of CBR provides fundamental knowledge about the role of less-studied receptors such as TRPV1, PPARγ, 5-HT1A, and others, which is essential for the development of precise pharmacological interventions.
From a medical standpoint, CBR’s potential lies in creating drugs with targeted actions capable of influencing inflammatory processes, neuroprotection, analgesia, and immunomodulation. Unlike traditional cannabinoids, CBR does not cause psychotropic effects, making it especially valuable for use in patients with chronic neurological conditions, including epilepsy, multiple sclerosis, Alzheimer’s disease, and Parkinson’s disease. This broadens the spectrum of patients who can benefit therapeutically without the risk of undesirable psychoactive reactions.
In the context of pharmacotherapy, CBR shows potential as a new analgesic, especially for managing neuropathic and chronic pain, which are often difficult to control with traditional medications. Cannabiripsol modulates pain signals through activation of TRPV1 receptors and interaction with other molecular mechanisms, allowing avoidance of dependence and side effects characteristic of opioids. Additionally, CBR possesses immunomodulatory properties, opening prospects for treating autoimmune and inflammatory diseases where conventional therapies are often accompanied by serious adverse effects.
Another area of medical research is CBR’s potential in psychiatry, particularly in treating anxiety disorders and depression. Studies indicate CBR’s ability to influence serotonin receptors, which may help correct neurotransmitter imbalances characteristic of these conditions. Unlike psychoactive cannabinoids, CBR offers a safe profile without the risk of inducing psychosis or cognitive impairments, which is critically important for psychiatric patients.
From the perspective of clinical research, CBR represents a significant subject for drug testing and development using modern technologies. Specifically, the ability to synthesize and standardize pure forms of cannabiripsol supports precise dosing and pharmacokinetic control, which are key for patient safety and therapeutic effectiveness. Development of new dosage forms, such as capsules, injections, and transdermal delivery systems, will provide flexibility and convenience in use.
Scientific innovations in biotechnology also directly impact the prospects of CBR in medicine. The use of genetic engineering to create hybrid plants or microorganisms that produce cannabiripsol will enable large-scale production of the compound with high purity and stability. This will reduce dependence on natural resources and make medicines more accessible to a wide range of patients. Further improvements in synthesis methods, including chemical and biosynthetic approaches, will contribute to optimizing production and reducing costs.
Given the interdisciplinary nature of research, CBR serves as a platform for collaboration among chemists, pharmacologists, physicians, geneticists, and bioengineers. This synergy fosters not only fundamental science development but also accelerates the translation of results into clinical practice. As a result, innovative research methods are being developed, including the use of computer modeling, molecular dynamics, and in vitro testing.
The medical significance of CBR also lies in its ability to affect multiple body systems, enabling the development of comprehensive therapeutic approaches. For example, its simultaneous impact on the nervous, immune, and endocrine systems opens prospects for treating complex multifactorial diseases that are difficult to manage with traditional treatments.
Despite many positive prospects, CBR remains a relatively new research subject requiring further study of its pharmacodynamics, pharmacokinetics, potential interactions with other drugs, and long-term safety. In this context, continuing fundamental and applied research is critically important to build a reliable scientific foundation that will serve as the basis for developing clinically effective and safe medications.
Regulatory Aspects
The regulatory landscape concerning cannabiripsol (CBR) is complex and dynamic, as this cannabinoid belongs to a relatively new and insufficiently studied class of bioactive molecules in pharmacology and medicine. Currently, regulatory approaches to CBR are influenced by many factors, including its chemical nature, lack of psychoactivity, therapeutic application potential, as well as international conventions and national laws governing cannabinoids.
First and foremost, the status of cannabiripsol within drug control systems varies by country, depending on the legislative acts regulating cannabinoid substances in general. The absence of psychoactive effects in CBR compared to tetrahydrocannabinol (THC) is a key factor that eases its regulatory position in many jurisdictions; however, this does not automatically exempt the molecule from regulatory requirements. Legislative bodies, including the FDA (U.S.), EMA (EU), Health Canada, and others, require comprehensive preclinical and clinical studies to confirm the safety, efficacy, and quality control of CBR-containing products.
The regulatory process involves strict monitoring of raw material quality, synthesis methods, purification standards, and product stability. The lack of standardized manufacturing protocols and the complex chemical structure of CBR pose challenges for regulators in quality control, necessitating the use of advanced analytical techniques (such as high-performance liquid chromatography, mass spectrometry, and NMR analysis). Regulatory authorities insist on strict adherence to GMP (Good Manufacturing Practice) and GCP (Good Clinical Practice) standards to minimize risks and ensure product reproducibility.
Special attention is given to studying the pharmacokinetics and pharmacodynamics of CBR, as their characteristics impact dosage determination, treatment regimens, and potential adverse reactions. Regulators require extensive toxicological studies to assess acute and chronic toxicity, mutagenicity, carcinogenicity, and effects on reproductive function. These data are essential for creating safe usage instructions and proper labeling of pharmaceutical products.
Regarding international law, CBR is not listed as a controlled psychoactive substance under the United Nations Conventions of 1961, 1971, and 1988, as it lacks psychotropic activity and does not cause dependence. However, due to limited data, some countries apply precedent-based or adaptive regulatory approaches, often including CBR in lists of substances requiring special permits for production and use in medicine and research.
Legal uncertainty surrounding cannabiripsol sometimes complicates research and commercial development, especially in countries with strict cannabinoid regulations. At the same time, there is a trend toward gradual liberalization of regulatory norms, supported by accumulating scientific evidence of CBR’s safety and therapeutic value. This trend encourages increased investment in biotechnology projects and pharmaceutical developments based on CBR.
Regulatory agencies also focus on ethical and responsibility issues in clinical trials and use of CBR. Given its unique pharmacological profile, risk-benefit assessment standards for CBR are developed with consideration of the specific patient populations studied and potential societal impacts. This is particularly relevant for post-marketing pharmacovigilance, where systems must be in place to monitor side effects and long-term therapy outcomes.
Alongside classical pharmaceutical regulatory procedures, national drug control agencies establish specialized divisions for monitoring new cannabinoids, including CBR. This includes maintaining registries, licensing research centers, controlling import and export, and conducting manufacturing inspections. Such regulatory mechanisms create a legal framework that promotes safe and controlled use of CBR.
Another important aspect is regulation of advertising and information dissemination about CBR. Laws in many countries strictly limit the spread of unverified or misleading claims regarding therapeutic properties to protect consumers and maintain trust in official medical recommendations. Requirements include mandatory clinical trial evidence for efficacy and safety, which must be published in accessible sources.
Considering the rapid advancement of cannabinoid science, regulators actively work on adapting regulations, developing methodological guidelines, and exchanging information with international partners. For example, the establishment of working groups under the WHO and international pharmaceutical associations supports the harmonization of standards and regulatory approaches for new cannabinoids, including CBR.
A vital component of the regulatory process is the interaction among government agencies, academic communities, and pharmaceutical companies developing CBR-based products. Such cooperation ensures timely legislative updates in line with scientific discoveries and facilitates the introduction of innovations into clinical practice without delays.
Regulatory challenges also encompass intellectual property issues – patenting synthesis methods, formulations, and new pharmaceutical forms of CBR. The absence of clear international rules in this area sometimes slows commercial development, highlighting the need to create transparent and stable legal mechanisms that stimulate innovation, protect developers’ interests, and simultaneously ensure patient access to quality medicines.
Conclusion
Cannabiripsol (CBR) is one of the most under-researched yet simultaneously promising molecules in the chemistry of natural cannabinoids. Its unique chemical architecture, biosynthetic origin, atypical pharmacological behavior, and apparent safety profile lay the groundwork for a qualitative reassessment of both current understandings of the endocannabinoid system and approaches to the design of new pharmacological agents. Despite its low natural concentration in the Cannabis sativa plant and the complexity of its extraction, CBR shows signs of significant functional potential that remains largely untapped in science and medicine.
First and foremost, the chemical characteristics of CBR, particularly its lack of psychoactive properties associated with the absence of direct affinity for classical CB1 receptors, distinguish it from other natural cannabinoids. At the same time, the molecule’s stability, conformational rigidity, and the presence of a characteristic phenolic group determine its specific biochemical behavior in living systems, particularly through indirect mechanisms of interaction with receptor proteins and enzymatic cascades. This allows CBR to be considered not merely a metabolite or minor accompanying component of cannabis, but a distinct functional unit with its own signaling model.
The origin of CBR in the plant is based on a complex chain of enzymatic transformations branching from the classical biosynthetic pathway of cannabinoids. This pathway involves poorly studied enzymes, likely specific to certain chemotypes of Cannabis sativa. The concentration of CBR remains consistently low, explained both by minimal expression levels of the relevant enzymes and by the limited metabolic advantage of this pathway for the plant itself. Such biological modesty of CBR indicates that its presence in cannabis is likely not linked to a primary metabolic function but rather to evolutionary drift or residual activity of biochemical nodes, which potentially can be activated under external or genetic influence.
Biosynthetically, CBR is a derivative of a non-canonical transformation of olivetolic acid involving atypical isoforms of PKS-type enzymes and specific cyclization of an intermediate substrate. These processes show similarity to alternative biosynthetic pathways of flavonoids and stilbenes, suggesting a possible shared evolutionary origin with other classes of bioactive natural compounds. At the same time, artificial reproduction of this biosynthesis in laboratory conditions remains challenging due to the high specificity of enzymatic stages and the need to control configurational parameters.
Laboratory synthesis methods for CBR are developing in the direction of both total synthesis and biotechnological strategies based on genetically modified microorganisms. Chemical methods rely on multistep reactions with carefully controlled conditions that allow obtaining pure isomers of the molecule, but they remain expensive and unsuitable for large-scale production. Bioengineering platforms, on the other hand, demonstrate potential for sustainable molecule reproduction by transferring cannabis enzyme genes into bacteria or yeast, opening the way to standardization, high reproducibility, and cost reduction of CBR products.
Pharmacologically, cannabiripsol acts as a molecule with a nontrivial spectrum of activity. Its weak activity within classical CB1/CB2 receptors does not exclude an effect on the endocannabinoid system. Instead, CBR exhibits modulatory properties, influencing receptor expression, endocannabinoid synthesis, and the activity of metabolizing enzymes. Additionally, interaction with a range of other molecular targets, including PPARγ, TRPV1, and GPR55, has been confirmed; these targets are involved in regulating inflammation, cellular metabolism, neurotransmission, and proliferation. Such pleiotropy opens the possibility for a wide spectrum of pharmacological applications – from neuroprotection to immunomodulation.
Safety studies of cannabiripsol to date are limited; however, preliminary toxicological models have not revealed signs of cytotoxicity, mutagenicity, or behavioral effects. The absence of psychoactivity makes CBR a less likely candidate for abuse, significantly simplifying its prospects for pharmaceutical development. However, this does not imply automatic safety – in-depth data on metabolism, interactions with other drugs, and long-term effects of systematic use are necessary.
Regarding biological activity, CBR is positioned as a molecule with potential regulatory effects on inflammatory, neurodegenerative, metabolic, and autoimmune processes. Despite the lack of definitive proof of clinical efficacy, its activity profile suggests low risk and a broad range of action. This makes it attractive for further preclinical research, especially in areas where classical cannabinoids are unacceptable from the standpoint of safety or regulatory status.
In the regulatory context, CBR currently does not fall under strict international or national legislation, allowing research and pharmaceutical development in relatively liberal conditions. At the same time, its legal status is not definitively established and requires clear differentiation from psychoactive cannabinoids based on objective scientific evidence. Standardization of production, analysis, and clinical testing methods should become the main focus of regulatory policy toward CBR in the near future.
In the scientific and medical dimension, CBR becomes an object that allows a new perspective on mechanisms of non-canonical cannabinoid signaling. Its study may not only complement the modern pharmacopeia but also contribute to the discovery of new pharmacological targets. For medical science, this means the possibility of developing personalized approaches to treating chronic conditions with low response to classical agents, such as resistant epilepsy, chronic neuropathies, insulin-resistant states, or autoimmune diseases.
References:
- Cannabiripsol (CBR) – Analytical Standard
Cayman Chemical provides information about cannabiripsol as an analytical standard for research:
https://www.caymanchem.com/product/21728/cannabiripsol - Biosynthesis of Cannabinoids
A review of biosynthetic pathways of cannabinoids, including less studied compounds:
https://www.sciencedirect.com/science/article/pii/S1360138520301874 - Pharmacology of Cannabinoids
A review of the pharmacological properties of cannabinoids and their interactions with receptors:
https://bpspubs.onlinelibrary.wiley.com/doi/10.1111/bcp.13710 - Molecular Pharmacology of Minor Cannabinoids
Research on the pharmacology of less studied cannabinoids, including CBR:
https://www.frontiersin.org/articles/10.3389/fphar.2021.777804/full - Biosynthesis of Cannabinoids in Heterologous Systems
A review of cannabinoid biosynthetic pathways and synthetic biology efforts for their production:
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC8156804/ - Cannabinoid Structures, Including CBR
Images and information on cannabinoid structures such as cannabiripsol:
https://www.researchgate.net/figure/Structures-of-cannabidiorcol-CBD-C1-cannabitriol-CBT-and-cannabiripsol-CBR_fig3_342583417 - Biosynthesis of Cannabinoids in Yarrowia lipolytica Yeast
Research on the potential for cannabinoid production in yeast:
https://www.sciencedirect.com/science/article/pii/S2693125725000226ScienceDirect - Pharmacological Properties of Cannabinoids
A review of cannabinoid pharmacology and their impact on health:
https://www.cambridge.org/core/journals/the-british-journal-of-psychiatry/article/pharmacology-and-effects-of-cannabis-a-brief-review/82B02735F420CB287DCC80843FC34AE1