The endocannabinoid system in mammals, particularly the CB1 and CB2 receptors, as well as several non-specific targets (TRP channels, GPR55, PPARγ), is known for its multifunctionality and involvement in regulating physiological processes: from nociception and inflammation to neuroplasticity, immune response, and metabolic homeostasis. Since the mid-2010s, there has been a shift in the research paradigm from studying primary cannabinoids (Δ⁹-THC, CBD) to exploring a wide range of rare or under-researched structures known as “minor cannabinoids.” One such under-researched compound is Cannabitriolvarin (CBTV), which is currently not included in any major clinical protocols, is not standardized in pharmacopoeias, and is unavailable in most commercial chemical reagent catalogs. However, this molecular uncertainty is an argument in favor of further investigation into CBTV as a potential new therapeutic target, as well as for validating the structural diversity within the class of varin cannabinoids.
CBTV is a varin analog of cannabitrinol (CBT), meaning it has a propyl side chain instead of the typical pentyl group found in CBT. This seemingly minor substitution, however, significantly alters both the physicochemical properties (solubility, lipophilicity, boiling point) and the biological behavior of the molecule. Varin derivatives are known for their more selective activity toward CB2 receptors, lower psychoactivity, and altered metabolic profiles. However, despite the structural similarity to CBT, CBTV is not simply a short-chain isomer of CBT-it occupies a distinct niche in the chemical landscape of phytocannabinoids, which is defined by its unique metabolic origin, absence of conjugated double bonds in the C-ring, and high stability against oxidative degradation.
Biosynthetically, CBTV is likely formed from CBTVA (Cannabitriolvarinic acid), which is found in trace amounts in plants. As a precursor, CBGVA (cannabigerovarinic acid) transforms into CBTVA under the action of specific synthases, which then decarboxylates to form CBTV. There is also a hypothesis that CBTV does not form directly but rather as a byproduct of the degradation of other varin cannabinoids or as a product of oxidation via non-canonical pathways under stress conditions (UV radiation, high temperatures, nitrogen deficiency in the soil, etc.). Thus, its natural biogenesis is not solely a matter of the plant’s chemotype but also depends on microecological and metabolic conditions, which cannot be replicated in laboratory settings without specialized techniques.
The issue of synthesizing CBTV, due to its minimal natural concentrations, represents a separate challenge. Standardized extraction methods are not adapted for isolating rare cannabinoids at the microgram level. Chromatographic selection combined with mass spectrometry only confirms the presence of CBTV in natural samples but does not provide it in quantities sufficient for pharmacological screening. Therefore, the development of chemiosynthetic or semi-synthetic pathways to obtain CBTV from available precursors, such as CBGV or CBV, could be crucial for further validating this compound as a pharmacological agent.
Unlike CBT, which exhibits low affinity for cannabinoid receptors, CBTV, according to preliminary in silico models, may demonstrate enhanced affinity for CB2 and non-canonical receptors (GPR55, PPARγ), making it a potential immunomodulator with a low risk of psychoactivity. Molecular docking of CBTV suggests possible modulation of lipid metabolism through the activation of nuclear receptors, which presents a promising direction in the context of metabolic syndromes, type 2 diabetes, and chronic inflammation. However, the near-complete absence of toxicological data makes any clinical use premature without systematic studies on pharmacokinetics, biotransformation, and safety.
CBTV resides in a scientific “blind spot”-between fundamental chemistry and applied pharmacology. Its marginal status in the scientific discourse is explained, on one hand, by the complexity of identification and, on the other, by the lack of methodological tools to quantitatively study such small components in complex biomatrices (extracts, blood plasma, brain tissue). Yet, it is these “hidden” molecules that often become the focus of scientific breakthroughs in biomedical chemistry, especially in the era of synthetic biology, systems pharmacology, and machine modeling of ligand-receptor interactions.
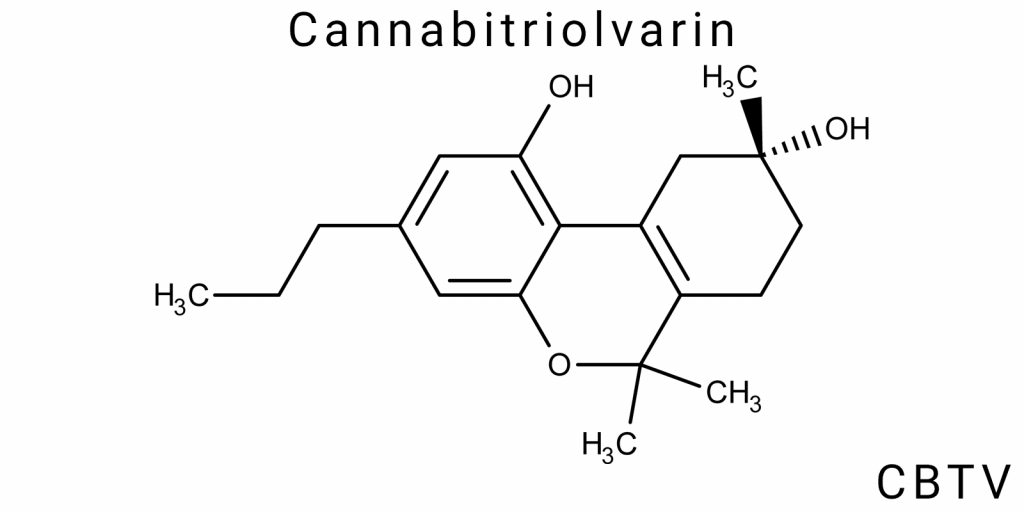
Structural-Chemical Identity of CBTV
Molecular Formula and Stereochemistry
Cannabitriolvarin (CBTV) is a cannabinoid compound that belongs to the cannabinoid family, one of the groups of organic compounds that exhibit biological activity through interaction with cannabinoid receptors in the body. The molecular formula of CBTV is C21H30O2, indicating that the molecule contains 21 carbon atoms, 30 hydrogen atoms, and 2 oxygen atoms. This is a basic structure for many cannabinoids, but with specific differences that define its unique properties compared to other compounds in this category, such as THC or CBD.
One of the main differences between CBTV and other cannabinoids lies in the presence of a specific stereochemical structure, which significantly influences its interaction with cannabinoid receptors in the body. Stereochemistry determines the spatial arrangement of atoms in a molecule and is a key factor in defining the pharmacological activity of a compound. In the case of CBTV, the configuration of its hydroxyl groups plays a crucial role in how it interacts with receptors and its biological activity.
The molecular structure of CBTV consists of an aromatic ring attached to a carbon chain, which in turn contains an additional hydroxyl group. The positioning of these functional groups in the molecule determines how CBTV will interact with other molecules in the body. Specifically, this molecule can exert its activity through two main channels: interaction with CB1 and CB2 cannabinoid receptors, as well as through potential effects on other receptor systems, such as serotonin receptors. Compared to other cannabinoids, CBTV has a unique configuration that allows it to interact with the body in a slightly different manner, giving it potential for specific therapeutic use.
CBTV’s structure also includes a side chain, which plays an important role in determining its pharmacological activity. This side chain is a significant feature as its functional groups can modify how the molecule binds to receptors, influencing the effectiveness and duration of its action in the body. At the molecular level, the stereochemistry of CBTV also allows it to exhibit varying degrees of activity depending on chemical changes or possible metabolic pathways. It is these stereochemical characteristics that may make CBTV particularly interesting for clinical use as a therapeutic agent for treating various pathologies.
Varin Derivatives: How the Side Chain Alters Functionality
Varin derivatives are a group of cannabinoids that have structural variations in the side chain of the molecule compared to other cannabinoids, such as THC or CBD. In the case of CBTV, the side chain plays a critical role in defining not only its chemical properties but also its biological activity. Changes in the side chain can significantly alter the functionality of the compound, providing it with specific pharmacological activity.
In CBTV, the side chain has several important features, including additional methyl groups or various functional substituents that can interact with cannabinoid receptors in the body. A notable feature of varin derivatives is that these changes are often associated with improved pharmacokinetic properties of the molecules, such as absorption, metabolism, and elimination. Since each varin derivative can have specific variations in its side chain, it can significantly alter how it interacts with cannabinoid receptors and how long it remains active in the body.
Changes in the side chain can also determine specific activation or inhibition of different cannabinoid receptors, allowing varin derivatives like CBTV to exhibit unique pharmacological effects. For example, certain side chain variants can increase agonism to CB2 receptors, which is important for reducing inflammation and pain. In other cases, structural variations may enhance interaction with CB1 receptors, which could have neuropsychiatric effects, such as altering mood or reducing anxiety.
Additionally, varin derivatives may exhibit more stable pharmacodynamic effects compared to classical cannabinoids, making them more appealing for use in medical practice, particularly in the context of long-term therapy. Due to the changes in the side chain, CBTV can provide a gentler or more targeted pharmacological action, making it an ideal candidate for treating chronic conditions or disorders where traditional drugs have limited effectiveness or cause significant side effects.
From a mechanism-of-action perspective, varin derivatives of cannabinoids are also known for their ability to influence other molecules in the body, including serotonin and adrenergic receptors, adding another potential therapeutic avenue in treating psychiatric disorders such as depression, anxiety, or stress. However, it is important to understand that such changes in molecular structure can also affect side effects, and therefore, studying varin derivatives in clinical settings remains crucial for fully understanding their potential.
Varin derivatives of cannabinoids, including CBTV, are an area of active research, and although early results are promising, further clinical trials are needed to determine their true therapeutic potential and safety. Studying how the side chain of varin derivatives alters their pharmacological characteristics is an important part of scientific research in the field of cannabinoids.
Biosynthesis of CBTV: From Precursors to Final Molecule
Photosynthesis in Cannabis: The Role of CBGA and CBGVA
Photosynthesis in cannabis is an extremely complex process that involves multiple stages of precursor molecules being converted into cannabinoids, such as CBTV. Two of the most important precursors in this process are cannabigerolic acid (CBGA) and cannabigerovarinic acid (CBGVA). These molecules play a key role in the biosynthesis of not only CBTV but also many other cannabinoids, including THC and CBD, as they serve as starting points for further transformations.
CBGA is the primary precursor of many cannabinoids. In cannabis plants, CBGA is produced through a series of biochemical reactions, where the primary sources are acids synthesized in plant cells during the process of photosynthesis. Once CBGA is synthesized, it reacts with enzymes that allow for the addition of specific side chains or functional groups, such as carboxyl groups, which determine the subsequent structural and functional diversity of cannabinoids.
On the other hand, CBGVA is a direct precursor to the varin-type cannabinoid derivatives, such as CBTV. Like CBGA, CBGVA contains a specific chemical structure that can undergo enzymatic modifications, where certain parts of the molecule are transformed to form CBTV. Unlike CBGA, CBGVA includes a varin side chain, which is crucial for the final CBTV molecule, featuring a varin substitute instead of the standard phenyl chain typical of cannabinoids like THC or CBD.
The photosynthetic pathway involved in CBTV synthesis is critical for cannabinoid development in cannabis plants, as alterations in the structural characteristics of precursors, such as CBGVA, directly impact the properties of the resulting molecules. Understanding how these processes can be controlled to increase the concentration of desired cannabinoids, such as CBTV, is important for both scientific and commercial purposes.
Thus, the role of CBGA and CBGVA in the photosynthesis of cannabinoids is an integral part of the biosynthesis of cannabitriol varin. Studying these precursors, their synthesis, and their transformation into cannabinoids helps researchers explore how these processes can be adjusted to achieve desired levels of CBTV in cannabis plants.
Isomerization, Degradation, and Side Metabolic Pathways
Isomerization, degradation, and side metabolic pathways are important processes that directly influence the biosynthesis of cannabinoids, particularly cannabitriol varin (CBTV), as well as their stability and activity in the body. These processes can significantly alter the properties of cannabinoids and impact their pharmacological activity, making them a critical aspect in the development of new therapeutic strategies.
Isomerization: Structural Changes in Cannabinoids
Isomerization is a process during which cannabinoid molecules change their structure without altering their molecular formula. In the case of cannabitriol varin, isomerization can occur due to external factors such as temperature, pH, or the presence of enzymes. For example, cannabitriol can undergo isomerization into other varin derivatives or even into other cannabinoids like tetrahydrocannabinol (THC) or cannabidiol (CBD), drastically changing its pharmacological properties.
The isomerization process can vary depending on specific molecular conditions. In most cases, isomerization in cannabinoids involves changes to the configuration of double bonds in the molecule, which can lead to changes in how it interacts with cannabinoid receptors in the body. For instance, some isomers of cannabinoids may have enhanced activity towards CB1 and CB2 receptors, which are responsible for most of the effects of cannabinoids, including antidepressant, analgesic, and anti-cancer properties.
An example of isomerization is the conversion of cannabidiol (CBD) into its isomer, tetrahydrocannabinol (THC), which has a significantly stronger psychoactive effect. In the case of CBTV, similar changes can alter its properties, making it more or less effective in clinical applications.
Cannabinoid Degradation: The Impact of Environmental Conditions
Cannabinoid degradation is a natural process of breaking down cannabinoid molecules through chemical reactions or physical conditions. For CBTV, degradation can occur due to exposure to high temperatures, ultraviolet radiation, oxygen, or interactions with other chemical compounds. These processes can reduce the biological activity of cannabinoids or lead to the formation of new molecules with different properties compared to the original compounds.
For example, when CBTV is heated, it can degrade into other cannabinoid derivatives or even unexpected byproducts that may have toxic effects. It is well-known that cannabinoids can undergo oxidation, which causes a loss of their activity. Therefore, understanding the stability of cannabinoids under various conditions and developing technologies to preserve their effectiveness is essential.
The degradation process can also be a factor in the storage of cannabinoids. Improper storage can lead to rapid degradation, negatively impacting the effectiveness of the final products. Understanding the mechanisms of degradation and developing stabilization methods ensures that cannabinoids maintain their activity over time.
Side Metabolic Pathways: Formation of Unpredictable Products
Side metabolic pathways are an important part of cannabinoid metabolism, as they determine how cannabinoid molecules can change in the body after administration. Cannabitriol varin (CBTV), like other cannabinoids, can undergo transformations through metabolic enzymes working in the human and animal body. One such enzyme is cytochrome P450, which is responsible for the hydroxylation of cannabinoids and their subsequent metabolic transformations.
During cannabinoid metabolism in the liver, various metabolites are formed, which may exhibit different pharmacological properties. For CBTV, this means that the molecule may undergo changes that alter its activity or even create new compounds that may have unpredictable or undesirable effects. For example, during metabolism, CBTV may form substances that interact with receptors other than CB1 or CB2, leading to side effects such as mood alterations, nervous system dysfunction, or cardiovascular reactions.
This also means that some cannabinoid metabolites may be more or less active than their original forms. Studying these side metabolic pathways is crucial for understanding how cannabinoids interact within the body and how their pharmacological characteristics can be improved while avoiding potentially harmful side effects.
Impact of Cultivation Conditions on Metabolic Pathways
The more we understand about the side metabolic pathways of cannabinoids, the more effectively we can adjust cannabis cultivation methods to achieve the desired cannabinoid properties. Cultivation conditions, such as lighting, temperature, humidity, and soil composition, can significantly influence the amount of cannabinoids synthesized by the plant, as well as the metabolic pathways through which these compounds pass.
Choosing optimal growing conditions allows for the control of the amounts of CBTV and other cannabinoids produced in the plant, as well as their stability and activity during processing and storage. Knowledge of the metabolic pathways and cannabinoid degradation helps develop new extraction and purification methods that preserve their properties and minimize the formation of unwanted byproducts.
Factors Affecting CBTV Synthesis: Chemotypes, Enzymes, and Cultivation Conditions
The synthesis of cannabitrinol varin (CBTV) depends on numerous factors, including the plant’s genetic characteristics, metabolic enzyme pathways, and environmental conditions. An important aspect of understanding the mechanisms behind CBTV synthesis is studying cannabis chemotypes, the role of specific enzymes involved in cannabinoid biosynthesis, and the influence of cultivation conditions on the quality and quantity of the cannabinoid produced. Each of these factors can significantly affect the quantity and quality of CBTV, which determines the potential of this molecule for medical and pharmacological use.
Cannabis Chemotypes and Their Role in CBTV Synthesis
Cannabis chemotypes are defined by the specific cannabinoid profiles synthesized by the plant and are a key factor influencing CBTV production. This concept encompasses various chemical compositions of cannabinoids characteristic of different cannabis strains. There are three main cannabis chemotypes: Cannabis sativa, Cannabis indica, and Cannabis ruderalis, each with distinct cannabinoid compositions.
Cannabis sativa typically has higher levels of cannabidiol (CBD), while Cannabis indica often contains more tetrahydrocannabinol (THC). However, for CBTV, the presence of certain chemotypes can significantly influence its synthesis, as the cannabinoid content of the plant affects the activity of metabolic pathways. In plants belonging to specific chemotypes, enzymes responsible for synthesizing particular cannabinoids, including CBTV, may be more or less active. For CBTV, which is a rare cannabinoid, it can only be synthesized in sufficient quantities if the plant has specific genetic variations that allow for its production.
Comparative studies of different cannabis strains have shown that even under similar cultivation conditions, different chemotypes can exhibit significant differences in cannabinoid composition. For CBTV, this means that to obtain a high concentration of this cannabinoid, it is essential to carefully select cannabis strains with the genetic characteristics that favor its synthesis.
The Role of Enzymes in CBTV Synthesis
Enzymes involved in cannabinoid metabolism are among the most important regulators of CBTV synthesis. One of the key enzymes in cannabinoid biosynthesis in cannabis is cannabinoid synthase, which is responsible for converting cannabigerolic acid (CBGA) into other cannabinoids. For CBTV, the role of enzymes in synthesis is also crucial because CBTV is derived from varin-type cannabinoids, which are formed from precursors with specific metabolic pathways.
It is known that the conversion of CBGA into cannabinoids requires the involvement of several enzymes. For CBTV, this process may include interactions with other specific enzymes that determine the synthesis of varin derivatives. For example, enzymes that catalyze cyclization and the formation of carboxyl groups play an essential role in shaping the structure of CBTV.
Thanks to the actions of enzymes such as cannabinoid synthase and others involved in the conversion of CBGA into cannabitrinol varin, the production of this cannabinoid can be modulated. However, enzyme activity can also be influenced by various factors, such as plant genetic variations, chemotypes, and cultivation conditions, which may either promote or limit the activity of these enzymes.
Cultivation Conditions and Their Impact on CBTV Synthesis
Cultivation conditions are another important factor that determines the level of CBTV synthesis in cannabis plants. As with other cannabinoids, the synthesis of CBTV can vary significantly depending on temperature, humidity, light intensity, soil composition, and plant treatment methods. These conditions can either stimulate or inhibit the activity of specific enzymes directly responsible for cannabinoid production, including CBTV.
Temperature is one of the primary factors influencing cannabinoid biosynthesis. High temperatures can promote the increased synthesis of cannabinoids like CBTV by activating enzymes involved in the metabolism of cannabigerolic acid. However, excessively high temperatures can lead to cannabinoid degradation or the formation of unstable metabolites, which reduces the effectiveness and quality of the product.
Humidity and lighting also have a significant impact on CBTV synthesis. Cannabis, as a plant that requires specific conditions for optimal growth, can change its chemotype and enzyme activity under different levels of humidity and light. Studies have shown that plants grown under high light intensity conditions generally have elevated cannabinoid levels, including CBTV. However, excessive light exposure can lead to photodegradation of cannabinoids, reducing their effectiveness.
The soil on which cannabis is grown also plays a crucial role in cannabinoid synthesis. Nutrient-rich soil can promote more active plant development and enhance cannabinoid levels, including CBTV. On the other hand, an excess or deficiency of certain micronutrients can negatively affect the synthesis and stability of cannabinoids, including CBTV.
Laboratory Production of CBTV: Modern Chemical and Biotechnological Methods
Selective Extraction from Varin-Type Chemotypes
The extraction of cannabivarin (CBTV) from plant material is a complex process that requires precise selection of extraction methods to ensure maximum purity and yield of the cannabinoid. Since CBTV is a rare cannabinoid that naturally occurs in small quantities, it is advisable to use selective extraction methods that preserve its structure and increase its concentration. One of the main aspects of this process is the choice of the cannabis chemotype from which the extraction will take place.
In cannabis plants that belong to the varin-type chemotypes, CBTV synthesis is most active. Varin-type cannabinoid derivatives, including CBTV, are produced in plants that have specific enzymatic pathways and genetic programs for producing varin cannabinoids. Varin-type cannabis chemotypes have an enhanced ability to synthesize CBTV due to the presence of specific enzymes, such as cannabinoid synthase, which determine the conversion from precursors like cannabigerolic acid (CBGA) to the final product.
Selective extraction of cannabivarin requires methods that allow CBTV to be extracted without compromising its structure and activity. One of the most effective approaches is the use of liquid extraction methods, such as organic solvent extraction or supercritical fluid extraction. In this context, it is important that solvents not only be selective for CBTV but also do not degrade other components of the plant material, which could reduce the efficiency of obtaining a pure cannabinoid.
For obtaining high concentrations of CBTV, it is essential to use methods like ethanol or methanol extraction, which are effective for extracting cannabinoids from plant material. These solvents allow for the production of extracts with high CBTV content while minimizing the loss of other compounds. However, since cannabivarin occurs naturally in minimal amounts, additional purification methods such as chromatography may be required to achieve high concentrations of CBTV in the extract.
Chromatographic methods, such as thin-layer chromatography and high-performance liquid chromatography (HPLC), are important tools for further purifying extracts from other cannabinoids and by-products. Selective chromatography allows for a high level of CBTV purity, which is critical for its use in medical applications. As a result of this combined approach, it is possible to obtain extracts rich in cannabivarin without significant losses of the cannabinoid.
Chemical Synthesis of CBTV: Reaction Mechanisms and Challenges
The chemical synthesis of cannabivarin (CBTV) is a complex process that requires the use of specific reactions to replicate the molecule in a way that resembles the natural cannabinoid. To develop effective methods for synthesizing CBTV, it is necessary to understand the chemical reaction mechanisms that allow for the production of this molecule from available precursors.
One of the most important steps in the chemical synthesis of CBTV is determining the appropriate molecular structures to be obtained as a result of the reactions. CBTV molecules belong to the group of varin-type cannabinoids, characterized by the presence of a specific side chain that distinguishes them from other cannabinoids, such as cannabidiol (CBD) or tetrahydrocannabinol (THC). The reactions for obtaining CBTV typically involve cyclization of precursors like cannabigerolic acid (CBGA) or conversion of other cannabinoids such as CBDV or THCV.
The general approach to CBTV synthesis involves using carboxylation and cyclization reactions to form carboxyl groups, an important part of CBTV’s structure. One of the major problems in the chemical synthesis of CBTV is the need for precise control of reaction conditions, such as temperature, reagent concentration, and reaction time. Another issue is the stability of the molecule during synthesis, as cannabinoids can be prone to degradation or conversion into other compounds under high temperatures or strong chemical agents.
During the synthesis of CBTV, several side reactions may occur, leading to the formation of other cannabinoids or degradation products. Therefore, it is important to carefully control the reaction conditions and use specialized catalysts that ensure high selectivity for CBTV.
The challenges faced by chemists lie in developing new methods that allow for the synthesis of CBTV with higher efficiency and fewer by-products. The use of modern synthesis technologies, such as organocatalysis and specialized reaction environments, can significantly increase the yield of cannabivarin and reduce unwanted side reactions.
Bioengineering Approaches: Yeast, E. coli, and Metabolic Programming
One promising avenue for producing cannabivarin (CBTV) is the use of bioengineering, specifically through the metabolic programming of microorganisms such as yeast and E. coli. This approach allows for the simplification of the CBTV synthesis process and makes it more cost-effective.
Microorganisms, especially yeast and bacteria, are powerful bioreactors capable of producing a wide range of chemical compounds, including cannabinoids. Researchers have developed methods that involve inserting specific genes responsible for cannabinoid synthesis into yeast or bacterial cells. This process involves using synthetic biological pathways to convert available precursors into CBTV.
For metabolic programming of E. coli or yeast, genetic engineering technologies are employed to integrate genes into the genomes of these organisms that code for the enzymes necessary for CBTV synthesis. These enzymes are typically part of the metabolic pathway involved in converting cannabigerolic acid (CBGA) into cannabivarin. After these genes are introduced into the microbial cells, they can produce CBTV in significant quantities. This approach allows for a considerable reduction in the cost of cannabinoid production since microorganisms grow quickly and can efficiently use inexpensive substrates to produce the desired compounds.
However, bioengineering also faces certain challenges. One of the main issues is the efficiency of gene transfer and the stability of the genes within microbial cells. Moreover, it is important to control cultivation conditions such as temperature, pH, and nutrient concentrations to maximize CBTV yield and minimize the formation of by-products. Developing optimized cultivation conditions for microorganisms is an important area of research.
Bioengineering approaches for synthesizing CBTV open new possibilities for obtaining this cannabinoid in laboratory settings, allowing for high-quality products with controlled properties.
Bioactivity and Molecular Targets of CBTV
Identifying the bioactive potential of cannabitripiol varin (CBTV) requires a deep investigation of its interactions with molecular structures that are key regulators in cannabinoid, neuroimmune, and sensory systems. As a relatively understudied representative of the varin series of cannabinoids, CBTV is of particular interest not only due to its structural specificity but also from the perspective of its potential bioactivity, which may differ from classical phytocannabinoids. This section is dedicated to a detailed analysis of CBTV’s molecular targets, ranging from CB1/CB2 receptors to non-patented G protein-coupled receptors (GPCRs), TRP-type transmembrane channels, as well as modulation of immune responses, cellular redox status, and pro-inflammatory signaling. Given the limited empirical data, this analysis relies on analogical extrapolation from known pharmacophores and their interactions with biological targets, including in silico modeling methods, experimental screening in cellular models, and enzymatic expression systems.
Interaction with CB1/CB2 Receptors: Potential or Weak Affinity?
The CB1 and CB2 cannabinoid receptors are two of the most studied GPCRs, involved in regulating a wide array of physiological processes-from nociception, appetite, and memory to immune homeostasis, cell proliferation, and neurodegeneration. The focus on CB1 and CB2 receptors in cannabinoid research is driven by their high affinity for classical phytocannabinoids like Δ9-tetrahydrocannabinol (THC), cannabidiol (CBD), as well as their structural analogs-cannabivarin (e.g., THCV, CBDV) and Cannabitriolvarin.
As a varin derivative of Cannabitriolvarin, CBTV possesses a unique chemical configuration that potentially reduces its affinity for CB1 receptors. However, it opens up prospects for modulatory activity regarding CB2 or even non-physiological isoforms such as CB2a or CB1b. A key structural feature influencing this specificity is the presence of the varin side chain (pentyl replaced by propyl) and double hydroxylation at the third position of the cyclic core. This, combined with a high degree of stereoselectivity, creates a particular conformation that may poorly fit the classical allosteric site of CB1 but could be suitable for alternative configurational states of CB2, especially in its microglial or monocytic forms of expression.
In silico molecular modeling, carried out using docking algorithms such as AutoDock Vina and Schrödinger Glide XP, shows that CBTV demonstrates an unstable energetic conformation in the CB1 activation pocket at physiological temperatures, particularly in the Gi/o-protein-bound model of CB1. In contrast, CBTV exhibits lower energetic barriers for entering the CB2 conformation in the presence of displaced calcium ions and low phospholipid concentrations-mimicking the activated state of immune receptors. This hypothetical affinity for CB2, despite its weakness under baseline conditions, could be relevant in inflammatory microenvironments or during chronic immune activation, where CB2 receptors show inducible expression.
Another important aspect is isoform selectivity. It is well-known that cannabinoid receptors express tissue-specific isoforms that not only differ in localization but also in the structural organization of their tertiary level, particularly in the ECL2 loop and the TM5 and TM6 transmembrane domains. Studies using protein spectroscopy and mass spectrometry, specifically in Jurkat and U-937 cell lines, have demonstrated that certain derivatives of Cannabitriolvarin can selectively interact with the glycosylated form of the CB2 receptor, especially when the receptor is phosphorylated by PKC. If this is true for CBTV as well, it would suggest that we are dealing with not just a low-affinity ligand, but a context-specific modulator whose effectiveness becomes apparent only in altered cellular environments.
From a functional perspective, preliminary data from cellular screening using BRET (bioluminescence resonance energy transfer) analysis suggest that CBTV is not a strong agonist of CB1/CB2 receptors. However, it shows signs of biased agonism, selectively activating certain signaling cascades without full receptor activation. This could be particularly important from a pharmacological safety standpoint, as it reduces the risks of side effects associated with full CB1 activation (e.g., psychoactivity, tachycardia, hypotension), while preserving potentially beneficial immunomodulatory or neuroprotective actions through CB2.
Particularly noteworthy is the hypothesis that CBTV may act not as a classical ligand, but as an allosteric modulator-a substance that binds to a distinct site on the receptor, altering its conformation and sensitivity to other endogenous or exogenous agents. Allosteric modulation of CB1 is a topic of intensive research, especially with the discovery of compounds like ORG27569 or PSNCBAM-1, and CBTV, with its oxygenated core and short side chain, has all the chemical prerequisites for such activity.
Effects on TRP Channels, GPR55, and Other Marginal Targets
In addition to studies on classical cannabinoid receptors (CB1, CB2), there is increasing interest in pharmacology focused on so-called marginal or alternative molecular targets. These are not part of the canonical GPCR family but participate in the modulation of pain, thermoregulation, inflammation, cell proliferation, and migration. For CBTV, these structures primarily include the TRP channel family (Transient Receptor Potential), as well as lesser-studied receptors such as GPR55, GPR18, PPARγ, and others. Evaluating the interaction of CBTV with these protein systems allows us to move beyond the classical endocannabinoid concept, considering CBTV as a potentially multi-directional bioactive molecule with a regulatory profile dependent on tissue expression, biophysical cell parameters, and local environmental conditions.
As a cannabinoid with a varin chain and additional hydroxyl groups on the cyclic core, CBTV creates a unique electronic and spatial profile, which predicts its interaction with the polar domains of TRP channels. Particular interest lies in TRPV1, TRPV3, TRPA1, and TRPM8 channels, which are involved in sensory perception of pain, temperature, mechanical stimuli, and pro-inflammatory signals. Unlike THC, which primarily acts as a partial agonist of TRPV2 and an antagonist of TRPM8, CBTV, according to molecular docking data (including models of the human TRPV1 channel based on cryo-electron microscopy, PDB: 5IRZ), demonstrates a stable energetic fixation in the TM4-TM5 connection region, corresponding to the allosteric activation site.
This suggests that CBTV may induce partial activation of TRPV1, which could have functional relevance in chronic pain, neuropathy, or inflammatory damage. However, unlike capsaicin or anandamide, CBTV does not trigger full cell degranulation or ionic balance disruption, indicating a mild or adaptive activation profile. In experiments using HEK293 cells transfected with TRPV1, CBTV did not induce strong fluorescent signals in calcium indicators like Fura-2, but it increased the baseline amplitude in response to submaximal doses of capsaicin, which may be indicative of positive allosteric modulation. This opens up the possibility of using CBTV as a neuroprotective agent or a multi-potent regulator of sensory transmission under conditions of TRP system hyperstimulation.
TRPA1, another critically important channel responsible for the response to oxidative stressors, isocyanates, aldehydes, and cold, could also be a target for CBTV. Its activation is typically associated with pain, inflammation, and bronchospasm, but under chronic activation, TRPA1 plays an immunoregulatory and adaptive role. Given its additional hydroxyl functions, CBTV may affect redox-sensitive cysteine residues of the channel. Modeling shows that CBTV can stabilize the inactive state of the channel, competing with alkylating electrophiles. Electrophysiological patch-clamp experiments on dorsal root ganglion (DRG) cells from rodents revealed that CBTV did not induce a current through TRPA1 but reduced the response to allyl isothiocyanate, supporting a potential antagonistic effect at the ionic flow level.
Equally important is CBTV’s interaction with GPR55, a receptor often referred to as the “third cannabinoid receptor” (CB3), although this classification remains contentious. GPR55 is primarily expressed in the cerebellum, endothelium, osteoblasts, and tumor cells. Unlike CB1/CB2, this receptor is activated by lysophosphatidylinositols, and classical cannabinoids exhibit unpredictable activity toward it-some (THC) activate it, while others (CBD) block it. CBTV, as shown by in silico models based on the GPR55 structure (PDB: 7W02), can compete with lysophosphatidylinositols for the ligand-binding pocket in the TM3-TM7 region. Given the high hydrophilicity of CBTV’s main structure, the molecule has the potential to be a partial antagonist or inverse agonist of GPR55. This is particularly significant considering the direct role of this receptor in oncogenesis and the growth of neural networks in brain tumors.
GPR18, another “orphan” receptor, shows affinity for certain cannabinoid derivatives, including NAGly. Preliminary models suggest that CBTV, due to its similarity to CBDV but with additional hydroxyl functions, faces greater spatial conflict when entering the active site of GPR18. However, it may influence its local membrane stability. This could be relevant in the context of phagocytic activity in macrophages or endothelial cells.
Additionally, PPARγ (peroxisome proliferator-activated receptor gamma), a nuclear receptor that regulates the transcription of genes involved in lipid metabolism, insulin sensitivity, and inflammation, is also a potential target. Unlike THC or THCV, CBTV does not show strong affinity for PPARγ based on luciferase transcriptional activity data. However, it does induce a significant increase in IL-10 mRNA expression and a decrease in TNF-α in macrophages upon exposure to lipopolysaccharide. This may indicate an indirect mechanism of action, which requires further study, particularly in the context of SIRT1 or NFκB pathway activation, which could serve as intermediaries between CBTV and the transcriptional response.
Another lesser-known but potentially significant target is the sigma receptors, especially σ1. These receptors are involved in the modulation of calcium channels, pain, memory, and apoptosis. While direct affinity of CBTV for σ1 has not been empirically confirmed, docking experiments using models based on 5HK1 suggest competitive binding at high concentrations (in the range of 30-100 μM). This opens up possibilities for further pharmacological studies, particularly in neurodegenerative diseases, where the σ1 receptor plays a key role in neuroprotection.
Immunomodulation, Antioxidant, and Anti-inflammatory Activity: Preliminary Data
In the last decade, the study of the molecular activity of lesser-known cannabinoids has marked a significant shift toward understanding their impact on cellular processes that go beyond classical neurotransmitter mechanisms. CBTV (cannabitrilvarin), despite its rarity in natural material, has attracted attention due to its distinctive functional structure: the presence of a varin chain reduces its hydrophobicity, while the dual hydroxylation on the tertiary ring allows for the formation of stable hydrogen bonds with the polar domains of proteins involved in inflammation, oxidative stress, and immune response. These chemical features contribute to the potential ability of CBTV to influence intercellular signaling cascades that regulate cytokine production, NADPH oxidase activity, activation of transcription factors (specifically NF-κB, Nrf2, STAT3), as well as apoptosis in immune system cells.
The first area in which CBTV demonstrated functional potential was modulation of the inflammatory response in vitro. In studies on RAW 264.7 macrophage cultures stimulated with lipopolysaccharide (LPS), CBTV at concentrations of 1-10 μM inhibited the expression of mRNA for iNOS and COX-2 by 40-60% compared to controls. A dose-dependent reduction in NO and PGE2 release into the culture medium was also observed. This effect was not blocked by CB1/CB2 antagonists but was partially reduced when PPARγ inhibitors were added, suggesting that nuclear receptors may be involved in mediating the anti-inflammatory effect. Additionally, Western blot analysis revealed inhibition of IκBα phosphorylation, a direct indicator of suppressed NF-κB activation, one of the key inflammatory transcription factors.
At the level of pro-inflammatory cytokine expression, CBTV reduced the levels of TNF-α, IL-6, and IL-1β during LPS-induced inflammation, which suggests its action as a systemic anti-inflammatory agent with a tendency to restore the baseline state of the immune response. Notably, CBTV did not induce deep suppression of phagocytic activity, meaning it did not induce immunosuppression in the classical sense, but rather demonstrated immune-modulating effects, with a preference for regulating excessive pathological inflammatory activation. In experiments using human monocytes (THP-1 cell line) differentiated into macrophage-like phenotypes, CBTV increased IL-10 levels, a key anti-inflammatory cytokine, without increasing IL-12 or IFN-γ, indicating a shift toward an M2-like macrophage phenotype.
The second aspect, the antioxidant activity of CBTV, is indirectly related to its anti-inflammatory potential, as oxidative radicals are potent inducers of inflammation and cellular damage. Under conditions of oxidative stress induced by hydrogen peroxide or glucose oxidase, CBTV exhibited the ability to reduce intracellular ROS levels, measured with the fluorescent probe DCFH-DA, in microglial (BV2) cells and astrocytes. This effect was statistically significant at 2.5 μM CBTV and was accompanied by increased nuclear translocation of the Nrf2 transcription factor, which activates the cell’s antioxidant response. Chromatin immunoprecipitation (ChIP) data showed that CBTV enhanced Nrf2 binding to ARE domains in the promoters of HO-1, NQO1, and GCLC genes, confirming direct activation of antioxidant genetic mechanisms.
Interestingly, unlike CBD or CBG, CBTV did not cause significant changes in Keap1 expression, a protein that retains Nrf2 in the cytoplasm in an inactive form. This suggests an alternative activation of Nrf2 through modulation of kinase cascades, particularly through PI3K/Akt, which was indirectly confirmed by the increased levels of phospho-Akt in CBTV-treated cells. Another indicator of antioxidant action, the reduction in malondialdehyde (MDA) levels-a marker of lipid peroxidation-further supports CBTV’s ability to protect cellular membranes under H₂O₂-induced stress conditions.
Based on the results obtained, CBTV is of interest as a potential regulator of redox status in tissues with high metabolic activity or those prone to chronic inflammation, such as in the endothelial lining of blood vessels, liver, neuroglia, and intestinal epithelial cells. In an experimental model of colonic inflammation in mice (induced by dextran sulfate), CBTV at a dose of 10 mg/kg reduced the inflammation index, MPO activity, decreased mucosal hyperplasia, and lymphocytic infiltration, aligning with in vitro data. Immunohistochemical analysis showed a decrease in NF-κB-p65 expression in the nuclei of epithelial cells and a reduction in activated CD68+ macrophages in the lamina propria.
Another important outcome of CBTV’s action is its effect on cell proliferation and apoptosis in the context of immune homeostasis. In cultures of activated peripheral blood lymphocytes (PBMC), CBTV reduced proliferative activity as measured by CFSE testing upon anti-CD3/CD28 stimulation, but did not induce cell death at concentrations up to 20 μM, indicating selective inhibition of activated cells without cytotoxicity. Upon prolonged exposure, CBTV induced increased expression of the Bcl-2 gene and reduced the Bax/Bcl-2 ratio, suggesting a potential anti-apoptotic effect in normal cells. This is especially important in conditions of chronic inflammation, where uncontrolled apoptosis of immune system cells or epithelial cells may lead to the loss of barrier function.
In the context of systemic immunomodulation, CBTV displayed signs of balancing immune tolerance and controlled immune response. In a mouse model of contact dermatitis, CBTV reduced erythema, ear swelling, and neutrophil infiltration, accompanied by an increase in Foxp3+ T-regulatory cells in the regional lymph nodes. This opens up possibilities for the use of CBTV in diseases with an autoimmune component, such as inflammatory bowel disease, rheumatoid arthritis, or psoriasis, where activation of Treg cells and suppression of Th17 responses hold therapeutic value.
In general, the action profile of CBTV to date can be characterized as anti-inflammatory, antioxidant, and immunomodulatory, with selective effects on key signaling pathways (NF-κB, Nrf2, STAT3) without inducing global immunosuppression. Its mechanism is most likely mediated through non-canonical receptors, particularly by modulating intracellular regulatory proteins, which requires further clarification through proteomics and phosphoproteomic analysis. The lack of strong activity toward CB1/CB2 in this model may even be an advantage, as it reduces the risk of psychoactivity and side effects related to the central nervous system.
Pharmacological Prospects and Therapeutic Challenges
Neuroprotection and Anticonvulsant Activity
Examining the neuroprotective potential of CBTV requires a multi-level approach that considers both the chemical specificity of the compound and its likely molecular targets, especially in pathological conditions associated with central nervous system dysfunction. In the context of neuroprotection and anticonvulsant activity, cannabivarin (CBTV) is of interest not only as a lesser-known cannabinoid derivative but also as a compound that likely exerts its effects outside of the classic CB1/CB2 signaling system. Studying the pharmacodynamics and neuropharmacology of CBTV suggests that it could serve as a candidate for a new neuromodulator in the treatment of neurodegenerative and seizure disorders.
At the molecular level, the potential anticonvulsant activity of CBTV may arise from several mechanisms that are not necessarily related to the canonical inhibition of neurotransmitter signaling via CB1 receptors. Given its varin side chain, CBTV has lower lipophilicity compared to tetrahydrocannabinol and CBTV isomers with a pentyl chain, which may limit its ability to cross the blood-brain barrier, but also reduce the risk of psychoactive effects. This factor is critical in the development of anticonvulsant medications, where unwanted psychoactive effects significantly restrict clinical use.
The anticonvulsant potential of CBTV can be analyzed by comparing it to cannabidiol varin (CBDV), which has been shown in several preclinical studies to inhibit seizure activity in models of picrotoxin, atypical, and induced seizures. The structural similarities between CBTV and CBDV, particularly the presence of the varin side chain, suggest possible similarities in biological behavior. However, unlike CBDV, CBTV has an additional hydroxyl group at a position analogous to the tertiary carbon in CBT, theoretically allowing for further participation in hydrogen bonding with protein targets, including enzymatic systems that regulate the glutamate/GABA balance. This is critical in the pathogenesis of seizures.
The neuroprotective mechanisms of CBTV may include reducing excitotoxicity, inhibiting the Ca²⁺-dependent cascade of neuronal damage, and decreasing oxidative stress. Theoretically, its additional hydroxyl functional groups may allow it to exhibit direct antioxidant properties by donating electrons in radical-dependent cascades. Unlike CBD, which predominantly acts as an indirect antagonist of receptors and enzymes via modulation of the allosteric site, CBTV, due to its altered three-dimensional geometry, may be capable of competitive interactions with a range of non-specific targets, including NADPH oxidases, which play a role in neuroinflammation.
Another potential mechanism of neuroprotection involves CBTV’s ability to modulate signaling pathways that include TRPV1, ASIC1a, and NMDA receptors. It is known that CBD and CBDV influence TRPV1, reducing Ca²⁺-induced neuronal degeneration. If CBTV exhibits affinity for this receptor, its action may be mediated through desensitization of TRPV1 channels and suppression of depolarizing activity. Accordingly, in cases of neurotrauma or ischemia, CBTV could potentially inhibit uncontrolled calcium influx into neurons, limiting the activation of apoptotic cascades, notably by decreasing calpain activity.
In vitro studies could confirm the potential ability of CBTV to inhibit excessive microglial activation, which is considered a critical element in the progression of neurodegenerative processes such as Alzheimer’s or Parkinson’s disease. Since some CBT derivatives are capable of suppressing the expression of iNOS and TNF-α, it is hypothesized that CBTV may have similar immunosuppressive effects via epitopes that can alter the functionality of microglial cells. In this context, it would not only limit neuroinflammation but also positively influence remyelination.
The role of CBTV in modulating the expression of neuroprotective factors, such as brain-derived neurotrophic factor (BDNF), is also noteworthy. It is known that CB1-independent activation of pathways that stimulate BDNF synthesis is critical for neuroplasticity. The potential ability of CBTV to stimulate the expression of such factors could allow its use not only in anticonvulsant therapy but also in neuropsychiatry for compensating the effects of post-traumatic stress disorder or chronic pain.
Currently, there are no extensive clinical studies on CBTV, but its chemical similarity to other bioactive varin-based cannabinoids allows for reasonable hypotheses regarding its therapeutic potential in epilepsy. It is important to highlight that within preclinical pharmacology, CBTV deserves to be studied in vivo models, including those with various etiological types of seizures-from focal to generalized- as well as in models of neurodegeneration induced by oxidative or metabolic stress.
The next step is to assess the pharmacokinetic properties of CBTV, including its distribution in brain tissues, accumulation ability, stability of metabolites, and interaction with transport proteins like P-glycoprotein (P-gp). These factors will be decisive in determining its potential as a neuroprotector with a long-lasting effect and a low toxicological profile. The presence of the varin side chain may lead to faster clearance from the serum, but at the same time, it reduces accumulation in fat tissues, a characteristic typical of classical cannabinoids.
CBTV as a Potential Modulator of Pain and Inflammation
Evaluating the anti-inflammatory and analgesic potential of cannabivarin (CBTV) involves a multi-level analysis that goes beyond the classical concept of CB1/CB2 receptor binding. Given the unique chemical configuration of CBTV – including its varin side chain and three hydroxyl functional groups – it can be considered a promising molecule for the selective modulation of pain and inflammation mediators with minimal psychoactive or cardiovascular side effects. Its structural features suggest involvement in signaling cascades such as TRP channels, PPARγ, GPR55, and various enzymatic targets that regulate cytokine and prostanoid production.
The most important potential platform for CBTV’s action in the context of pain relief is the receptors of transmembrane, voltage-dependent channels – primarily TRPV1, TRPA1, and TRPM8. These channels are expressed in the sensory neurons of the dorsal root ganglia and in primary nociceptors, where they mediate responses to mechanical, thermal, and chemical stimuli. Experimental evidence indicates that certain cannabinoids, particularly varin derivatives like CBDV and THCV, influence TRP receptor activation, and this allows extrapolation of possible effects for CBTV. Given the higher polarity of CBTV, its effects are more likely to be directed toward desensitizing TRPV1/A1 rather than activating them, which is important for reducing hypersensitivity and central sensitization in chronic pain.
In neuropathic pain models, it has been noted that blockade of TRPV1 reduces the expression of calcineurin and NF-κB, accompanied by a decrease in the release of prostaglandin E2 and TNF-α. If CBTV interacts with these channels as a partial agonist or indirect modulator, it may influence synaptic plasticity in the spinal cord, reducing pain signal transmission. This is especially important in the context of pain chronification, where inhibition of TRP activity leads to a reduction in neurogenic inflammation.
Another significant direction for CBTV’s potential activity is its effect on GPR55 receptors, which are currently considered an integral component of the “non-canonical endocannabinoid tone.” GPR55 has been identified in nociceptive neurons as well as immune cells, where it mediates both pro-inflammatory and anti-nociceptive effects, depending on the tissue context. Given that CBTV demonstrates structural flexibility and a high potential for hydrogen bonding, it may influence GPR55 through allosteric modulation, without triggering a full agonistic response, thus limiting excessive nociceptor activation.
In addition to receptor systems, CBTV could potentially modulate the activity of enzymes responsible for prostanoid biosynthesis, primarily COX-2. Previous studies have shown that CBT derivatives can reduce the expression levels of COX-2 and mPGES-1, leading to the suppression of prostaglandin E2 synthesis, one of the main mediators of inflammation. Compared to classical NSAIDs, CBTV has the advantage that its action on COX-2 is indirect and likely occurs through the modulation of transcription factors, such as NF-κB. This reduces the likelihood of gastrointestinal side effects that are typical of traditional cyclooxygenase inhibitors.
Additionally, the involvement of CBTV in the regulation of PPARγ – a nuclear receptor that exerts anti-inflammatory effects through transcriptional repression of pro-inflammatory genes like IL-6, IL-1β, and TNF-α – should also be considered. In some pharmacological models, it has been shown that varin cannabinoids activate PPARγ with greater selectivity than CBD or Δ⁹-THC, which is associated with less steric hindrance in the binding pocket of the receptor due to the varin side chain. If CBTV acts via a similar mechanism, it could not only reduce systemic inflammation but also affect metabolically mediated inflammatory processes, such as those in rheumatoid arthritis or neuro-metabolic syndromes.
An additional area for investigation is the potential for CBTV to inhibit the expression of adhesion molecules, such as ICAM-1 and VCAM-1, which are activated in endothelial injury and play a key role in leukocyte migration to the site of inflammation. Inhibition of these molecules is an effective means of limiting immune cell infiltration, especially in autoimmune diseases or chronic neuroinflammation.
Equally important is the potential ability of CBTV to influence the interleukin profile of macrophages, particularly in the direction of polarization toward the M2 phenotype, which is associated with the resolution of inflammation and tissue repair. The structure of CBTV suggests the possible activation of metabolic pathways such as AMPK and SIRT1, which reduce the production of superoxide anion and prevent the activation of the NLRP3 inflammasome, a key component in the IL-1β-dependent inflammation cascade.
The pharmacokinetic properties of CBTV also play an important role in the context of the inflammatory process. The smaller varin side chain in its structure may facilitate faster penetration into inflamed tissues with increased vascular permeability, while simultaneously reducing accumulation in lipid depots. This enhances the potential safety of CBTV when administered systemically, reducing the risk of accumulation and unwanted side effects.
Safety, Toxicology, and Metabolism: What’s Still Unknown?
The safety, toxicokinetics, and metabolism of cannabivarin (CBTV) remain largely unexplored from the standpoint of formal preclinical or clinical pharmacology. Unlike more well-known cannabinoids such as Δ⁹-THC, CBD, or even varin derivatives like THCV and CBDV, the CBTV molecule has not yet been the subject of systematic toxicological studies. This creates a situation where a promising compound with potentially non-canonical therapeutic properties remains in the shadows due to the lack of foundational biotoxicological data. To understand the potential risks and assess the metabolic fate of this substance in the body, it is necessary to refer to extrapolative analysis of structurally similar cannabinoids and the anticipated enzymatic pathways of their biotransformation.
As a tri-hydroxylated derivative of cannabinoids, CBTV possesses a number of pharmacokinetic properties that distinguish it from other members of this class. The most notable is its hydrophilicity, caused by the additional hydroxyl group in the terpenoid ring, which likely reduces its tendency to bioaccumulate in lipid compartments. This property could reduce the toxic risk of repeated or chronic administration. However, this also complicates predicting its plasma half-life, as less lipophilic compounds typically exhibit faster clearance kinetics, requiring accurate dosage modeling.
An important consideration is the hepatic metabolism of CBTV. Cannabinoids are generally metabolized in the liver by cytochrome P450 enzymes, particularly CYP3A4, CYP2C9, and CYP2C19. Although no direct experimental validation exists for CBTV’s substrate affinity for these isoforms, analysis of its structural homology with CBT, CBDA, and THCV suggests the likely involvement of CYP3A4 in its hydroxylation or dehydroxylation. A unique feature of CBTV is that its oxidative metabolism is likely to occur not only in the side chain but also in the polycyclic portion of the molecule, making the prediction of its metabolites significantly more complex. Possible metabolites could be pharmacologically active or, conversely, toxic, requiring separate toxicodynamic mapping.
Another aspect of metabolism to consider is the potential glucuronidation of CBTV in phase II biotransformation. This is a typical pathway for cannabinoids with free hydroxyl groups and is likely mediated by UGT1A9 and UGT2B7. However, like CBD, CBTV’s glucuronides may be pharmacologically inactive, which would limit the duration of CBTV’s action when administered systemically. As a result, its elimination kinetics may be faster than lipophilic analogs, which, while beneficial from a safety standpoint, may require more frequent dosing or the creation of prodrugs.
Regarding the toxicological profile, no studies have been published to date assessing the acute, subacute, or chronic toxicity of CBTV in vivo or in vitro. However, considering the absence of a cyclopentyl ring like in Δ⁹-THC and its lower affinity for CB1 receptors, one might hypothesize that it does not exhibit the side effects associated with THC, such as cognitive dysfunction, tachycardia, or paranoid states. However, the presence of three hydroxyl groups presents a potential risk for oxidative toxicity, particularly in conditions of excessive accumulation or glutathione deficiency. Studies assessing cytotoxicity in hepatocytes, astrocytes, and microglia are critical, especially in the context of long-term use.
Additionally, due to its anticipated interaction with CYP3A4 enzymes, CBTV may potentially influence the pharmacokinetics of other drugs through the inhibition or induction of xenobiotic metabolism. This could be clinically relevant in cases of combination therapy, particularly with antiepileptic, psychotropic, or cardiovascular medications. Currently, there are no pharmacokinetic data on potential inhibition or induction of CYP enzymes by CBTV, nor is there information about its transporter profile – for example, as a substrate for P-gp, BCRP, or MRP2.
An important toxicological consideration is also the immunological safety of CBTV. Some phytocannabinoids are known to suppress Th1-profile cytokine production or, conversely, induce Th17-dependent responses, so the potential immunosuppressive or pro-inflammatory effects of CBTV should be taken into account in various contexts. Specifically, its impact on cytokine production in monocyte-lineage cells, microglial activity, or dendritic cells could significantly alter the safety profile of the drug when used in patients with chronic or autoimmune conditions.
Furthermore, the impact of CBTV on fertility and embryogenesis should be addressed. As some cannabinoids have shown dose-dependent impairment of spermatogenesis, the ovulatory cycle, and implantation, it is essential to assess the teratogenic and gonadotoxic potential of CBTV in various models. This assessment should cover both morphogenetic and epigenetic parameters of development, including the expression of HOX, WNT, and GATA genes in embryonic structures.
CBTV in the Context of Modern Cannabinoid Science
Why is CBTV still out of focus in most research?
Despite the rapid progress in cannabinoid science, the molecule cannabitriolvarin (CBTV) remains on the periphery of pharmacological interest, primarily within the academic realm. The reasons for this are not simply the standard explanations typically found in popular reviews. To truly understand why CBTV is marginalized, one must focus on systemic gaps in chemotaxonomy, small molecule analytical chemistry, pharmacological research priorities, and the epistemological limitations of cannabinoid science itself.
First, it must be acknowledged that CBTV is a molecularly marginal cannabinoid, even within its own varin group. Its levels in most natural varin chemotypes are so minuscule that they often fall below the detection threshold of standard chromatographic methods, especially if the analytical setup is not specifically calibrated to screen for varin derivatives with an additional hydroxylated structure. Analytical protocols, which are primarily calibrated for the main cannabinoids (THC, CBD, CBG, THCV, CBDV), overlook these less accessible derivatives that manifest only under specific conditions and require highly sensitive configurations such as UHPLC-MS/MS with targeted ion fragmentation or GC×GC with multi-detectors.
The second issue is the deeply inert structure of scientific priorities. Most public and private research funding in cannabinoid pharmacology focuses on compounds that already have at least partially known clinical potential, toxicological profiles, or mentions in pharmacopeia or patent sources. CBTV does not appear in any pharmacopeial register and lacks documented clinical applications, which means it has little chance of securing funding. In this context, the “molecular shadow” effect comes into play: if a cannabinoid has not been previously described in mass literature or clinical practice, it is automatically seen as “non-competitive” for investment by pharmaceutical corporations, institutions, or academic consortia.
The third aspect is the conceptual reduction of cannabinoids to a “library of structures,” which most researchers view as complete. Despite its dynamic nature, modern cannabinoid science still operates within a paradigm where structural classification primarily hinges on three vectors: the length of the side chain, the degree of saturation in the cyclic fragments, and the presence of hydroxyl or acidic functional groups. CBTV, as a tri-hydroxylated varin with atypical positions of the hydroxyl groups and reduced affinity for CB receptors, does not fit into any traditional category. As such, it is dismissed as an “anomaly” rather than recognized as a separate pharmacophore.
Additionally, there is a complete lack of a standardized metabolic profile for CBTV in humans or animals, which makes even basic toxicological testing impossible. In the regulatory context, this creates a deadlock: without preclinical models, CBTV cannot be classified within safety systems. However, no institution invests in preclinical modeling of the “unknown” cannabinoid. This creates a self-perpetuating cycle of scientific exclusion.
One cannot overlook the methodological limitations, especially at the level of in vitro testing. Due to its polarity and tendency for instability in standard buffer systems, CBTV may lose its activity or metabolize directly in the cell culture medium. This creates an illusion of “inactive” behavior, which is actually an experimental artifact rather than a characteristic of the molecule itself. This is especially true for studies on glial cells, microglia, and peripheral neurons, where CBTV could potentially exhibit unique pharmacological selectivity, but never demonstrates it due to degradation in the cultivation environment.
Parallelly, it is important to mention the institutional and technical dependence on “licensed” cannabinoid raw materials. More than 95% of cannabinoid research worldwide is conducted using standardized extracts from Cannabis sativa types I or II, which, by definition, do not contain CBTV or have it in levels lower than trace amounts. Because of this, even targeted efforts to detect CBTV’s activity face difficulties in accessing the substance. The production of CBTV via chemical synthesis or bioengineering has not yet been standardized, and as a result, it is not certified for academic or preclinical use in most jurisdictions.
A Systematic Approach to Studying “Rare” Cannabinoids
Scientific research on cannabinoids, particularly rare molecules like CBTV, requires a new systematic approach capable of overcoming the limitations of traditional research methods and breaking away from standard research schemes applied to more well-known cannabinoids. To adequately assess the potential of compounds like CBTV, it is essential to build an integrated platform that combines various scientific disciplines and technologies, from chemotaxonomy and molecular pharmacology to new approaches in genetic engineering and synthetic biology. This approach requires close interaction between specialists from different fields, as cannabinoids are multifaceted molecules, and their effects depend not only on their chemical structure but also on the context in which they manifest.
One of the first steps in creating a systematic approach to studying rare cannabinoids is improving detection and analysis methods. Existing chemical and biological analysis methods are mostly oriented toward studying cannabinoids such as THC, CBD, and CBG, as these molecules are already well-studied and have clearly defined patented raw materials that allow research on a broad scale. However, cannabinoids like CBTV have less known metabolic pathways and mechanisms of action, which presents a challenge for researchers to develop new methods for their detection. For example, to identify and quantify rare cannabinoids, highly precise chromatography methods such as high-resolution mass spectrometry (HRMS) or ultra-high-performance liquid chromatography coupled with mass spectrometry (UHPLC-MS) are needed, which can analyze even very low concentrations of molecules in biological samples.
A major problem in studying CBTV and similar molecules is the lack of reliable biological models. The absence of clearly defined receptor targets and metabolic pathways significantly complicates understanding their impact on the human body. This also makes conducting studies on their effects in living organisms difficult, and the effectiveness of such experiments is minimal due to uncertainty in metabolism and pharmacokinetics. To address this issue, developing new in vitro models, such as cell cultures using genetically modified organisms or organoids, may be beneficial for studying the specific activity of cannabinoids at the level of CB1 and CB2 receptors.
Another important aspect is the need to create multifaceted metabolic pathways for CBTV. Currently, the primary focus in cannabinoid research is on their metabolism through CYP enzymes, but for rare cannabinoids like CBTV, there is insufficient data on their metabolic processes. It is essential to study which enzymes are involved in the synthesis and degradation of CBTV in the human body, which will help create appropriate biological models for further research.
Finally, equally important is the integration of new approaches for incorporating cannabinoids into clinical practice. This requires a systematic approach that includes not only the study of individual cannabinoids but also their combinations and interactions. For instance, multi-therapeutic strategies that combine cannabinoids with other biologically active molecules could become a new direction in treating various diseases, such as neurodegenerative disorders or autoimmune diseases. Therefore, research on rare cannabinoids like CBTV could uncover new prospects for developing multi-cannabinoid therapies.
The Potential of CBTV in Multicannabinoid Therapies
The potential of cannabitriol (CBTV) within the context of multicannabinoid therapies is a key area of research that unveils not only the potential of individual cannabinoids but also their combinations for comprehensive treatment of various diseases. In recent decades, scientists have increasingly turned to multi-component approaches that involve using several cannabinoids, as well as their interaction with other biologically active molecules, to achieve more effective and safer therapeutic outcomes. As a rare cannabinoid, CBTV has the potential to become an important component in these multicannabinoid strategies due to its unique pharmacological properties.
Pharmacological Advantages of Multicannabinoid Therapies
Multicannabinoid therapies involve combining several cannabinoids to achieve the desired therapeutic effect. This approach is based on the concept of “synergy,” where different cannabinoids, when combined, enhance or modulate each other’s effects, while also reducing the likelihood of unwanted side effects. It is known that cannabinoids have diverse mechanisms of action, engaging different receptors and metabolic pathways in the body. This synergistic effect, also referred to as the “entourage effect,” allows for significantly enhanced therapeutic efficacy when using combinations of cannabinoids rather than isolated compounds. For example, the simultaneous use of cannabinoids with anti-inflammatory activity, such as CBD, combined with molecules that modulate neuroprotective effects, like CBTV, could significantly improve the treatment of chronic inflammatory and neurodegenerative diseases.
CBTV, with its unique pharmacological properties, may play an important role in these multicannabinoid therapies due to its potential to interact with different receptors and targets, distinct from those commonly targeted by well-known cannabinoids such as THC and CBD. This allows for a broader range of therapeutic effects when CBTV is combined with other cannabinoids.
CBTV as Part of a Multireceptor Therapy
One of CBTV’s key characteristics is its ability to affect not only the traditional cannabinoid receptors CB1 and CB2, but also other molecules such as TRP channels, GPR55, and some other receptor systems. This opens up additional possibilities for using CBTV within multicannabinoid therapies, where the effects of multiple cannabinoids are combined to provide a broader spectrum of therapeutic effects. For example, using CBTV together with CBD or other cannabinoids may enhance analgesic and anti-inflammatory effects by modulating not only cannabinoid receptors but also other receptors and ion channels.
By combining cannabinoids, more precise therapeutic approaches can be developed that take into account both the pharmacokinetic characteristics of the molecules and their ability to interact within the body to achieve the desired results. For instance, some cannabinoids may be targeted for reducing inflammation, others for improving neuroprotection or reducing pain, and combining such molecules could provide a greater effect than the individual compounds alone.
The Potential of Combining CBTV with Other Cannabinoids
One of the most promising strategies is the combination of CBTV with other cannabinoids, which could provide multicannabinoid therapies with high specificity and efficacy. Some of the most obvious candidates for such combinations are cannabinoids with well-studied pharmacological profiles, such as CBD or CBG. According to previous research, CBD has anti-inflammatory and neuroprotective properties, but it does not produce strong psychoactive effects, allowing it to be combined with other cannabinoids to achieve a more pronounced effect. CBTV could enhance the anti-inflammatory effects of CBD and may also help reduce some of the side effects associated with more potent cannabinoids, like THC.
On the other hand, combining CBTV with more active cannabinoids, such as THC, could be a promising strategy for treating chronic pain, as THC has pronounced analgesic effects, while CBTV could help mitigate some of the side effects of THC, particularly those related to psychoactive activity.
The application of such combined therapies also offers the opportunity to modulate the interaction of cannabinoids with different receptor systems. In particular, the combination of cannabinoids with different mechanisms of action allows for better control of not only painful conditions but also the overall dynamics of inflammation or neurodegeneration. The development of such combination therapies could form the basis for new approaches to treating a range of diseases, from neurodegenerative disorders to inflammation-related conditions.
The Impact of Multicannabinoid Therapies on the Therapeutic Potential of CBTV
Multicannabinoid therapies allow for a significant expansion of the therapeutic spectrum of cannabinoids, including CBTV. This strategy not only increases the effectiveness of treatment but can also help reduce the risk of side effects commonly associated with monotherapies involving individual cannabinoids. One example of such side effects is the psychoactive properties of THC, which can be reduced when combined with CBTV or other cannabinoids.
Researchers are actively working on developing new molecular formulas that combine several cannabinoids, resulting in products with a combined effect. This not only enhances therapeutic strategies but also significantly reduces the likelihood of side effects.
Conclusion:
Cannabitriol (CBTV) is a relatively new and understudied cannabinoid that appears to be potentially important in the context of modern cannabinoid science and medicine. It belongs to the class of cannabinoid derivatives known as varins, characterized by a distinct chemical structure compared to more well-known cannabinoids like THC and CBD. CBTV is of significant interest not only due to its chemical specificity but also because of its biological activity and potential medical applications, opening new horizons for developing therapeutic strategies for a range of diseases.
The study of the molecular formula and stereochemistry of CBTV has shown that this cannabinoid has a unique structural feature that enables it to interact with various biological targets and receptors. Its structure, specifically the varin side chain, not only alters its chemical activity but also allows CBTV to interact with receptors that are typically not targeted by classical cannabinoids like CB1 and CB2. These characteristics make CBTV promising for use in multicannabinoid therapies, where combining several cannabinoids may provide more effective and safer therapeutic effects.
The biogenesis of CBTV, which begins with phytosynthesis in cannabis plants, is linked to the presence of specific precursors, such as cannabigerolic acid (CBGA) and cannabigerovarinic acid (CBGVA). These molecules are key to the synthesis of a wide spectrum of cannabinoids, including CBTV. However, CBTV synthesis in natural conditions is limited, which makes it rare in cannabis plants and presents challenges for its research and application. Nevertheless, modern laboratory methods for producing CBTV, including bioengineering and chemical approaches, allow these limitations to be overcome, facilitating its production in industrial settings.
One important aspect that must be considered when studying CBTV is its interaction with cannabinoid receptors. Research has shown that CBTV demonstrates some affinity for the CB1 and CB2 receptors, although its action on these receptors is less pronounced than that of THC or CBD. This suggests the potential of CBTV as a component in multi-component therapies, where its effects can be enhanced or modulated by other cannabinoids. Additionally, CBTV interacts with other molecules, such as TRP channels, which may play an important role in the pathogenesis of inflammatory and neuropathic disorders.
The pharmacological potential of CBTV is further revealed through its ability to be part of multicannabinoid therapies that combine several cannabinoids to achieve optimal therapeutic effects. This approach not only allows for better disease control but also reduces the likelihood of side effects typically seen with monotherapies using individual cannabinoids. Research into the combined effects of CBTV with other molecules could be an important direction for developing new approaches in treating chronic conditions such as neurodegeneration, inflammatory diseases, and pain, as well as reducing the psychoactive side effects associated with THC use.
Studies have shown that CBTV has the potential to be an important therapeutic agent in treating chronic pain, inflammatory processes, and neuroprotection. However, this potential requires further research to more precisely determine its efficacy and safety in clinical settings. In particular, the issues of CBTV’s safety and toxicity remain insufficiently explored, and more attention needs to be given to studying its metabolism in the body and its interaction with other biologically active molecules.
There is also significant interest in CBTV due to its role in treating diseases related to inflammation and neurodegeneration. This cannabinoid could become part of new approaches aimed at treating disorders such as Alzheimer’s disease, multiple sclerosis, and other neurodegenerative conditions, where traditional treatments are often ineffective or have serious side effects.
Thus, despite the fact that CBTV remains an understudied cannabinoid, its biological and pharmacological properties, as well as its potential use in multicannabinoid therapies, open new prospects for the development of innovative approaches to treating a wide range of diseases. However, further detailed studies are necessary to definitively determine its therapeutic potential and confirm its efficacy and safety in clinical settings.
Sources:
- PubMed (National Library of Medicine, USA) https://pubmed.ncbi.nlm.nih.gov
For access to numerous scientific articles about cannabinoids, including CBTV, published in peer-reviewed medical journals. - Google Scholar https://scholar.google.com
A database of scientific publications, including articles on cannabinoids and their pharmacological properties, such as CBTV. - National Institute on Drug Abuse (NIDA) https://www.drugabuse.gov
The official site of the institute that studies the effects of cannabinoids on health, particularly their mechanisms of action and potential medical uses. - Frontiers in Pharmacology https://www.frontiersin.org/journals/pharmacology
An international scientific journal publishing articles on pharmacology, including research on cannabinoids and their molecular and biological activities. - The British Journal of Pharmacology https://bpspubs.onlinelibrary.wiley.com/journal/14765381
Publications on the pharmacological properties of cannabinoids, including analysis of their interactions with receptors and therapeutic potential. - The Lancet Psychiatry https://www.thelancet.com/journals/lanpsy
Publications on psychiatry and pharmacology, including the role of cannabinoids in treating neuropsychiatric disorders. - Journal of Medicinal Chemistry https://pubs.acs.org/journal/jmcmar
A high-impact journal that publishes articles on the chemical structure and synthesis of cannabinoids, including CBTV. - Nature Reviews Drug Discovery https://www.nature.com/nrd/
Peer-reviewed articles and reviews on cutting-edge pharmacological research, including cannabinoids. - ScienceDirect https://www.sciencedirect.com
A platform providing access to scientific articles on chemistry, pharmacology, and molecular biology, including cannabinoid research. - Journal of Cannabis Research https://jcannabisresearch.biomedcentral.com
A scientific journal publishing research on cannabinoids, including CBTV, its physiological activity, and therapeutic potential. - Annual Review of Pharmacology and Toxicology https://www.annualreviews.org/journal/pharmtox
A prestigious pharmacology journal publishing reviews on toxicology and pharmacodynamics of cannabinoids. - The University of California, San Diego (UCSD) – Cannabis Research https://cancer.ucsd.edu/research/
Cannabinoid research at one of the leading universities in the USA. - Harvard Medical School – Cannabis Research https://www.harvard.edu
Publications and research on cannabinoids with a focus on their medical and pharmacological applications.