In the context of contemporary cannabinoid science, there remains an asymmetry in attention towards different representatives of this chemical group: the key objects of research are still Δ⁹-tetrahydrocannabinol (THC), cannabidiol (CBD), and to a slightly lesser extent, cannabinol (CBN). Their well-established study has become the benchmark for understanding the endocannabinoid system, receptor affinity, and clinical potential, which has led to the marginalization of less common or metabolically secondary cannabinoids. In this shadow is also cannabivarin (CBV) – a structurally stable, completely non-psychoactive molecule formed as a result of the oxidation of tetrahydrocannabivarin (THCV), and it remains virtually outside the paradigms of clinical, neuropharmacological, or even chemotaxonomic attention. However, the current level of analytical precision in studying natural metabolites raises new questions about the significance of such “peripheral” components, and CBV serves in this context as a molecule representing a latent functional layer of phytocannabinoid chemistry, still not integrated into the theoretical framework of either neuroscience, phytochemistry, or pharmacognosy.
From a formal standpoint, CBV is a product of auto-oxidation or thermally-catalyzed dehydrogenation of THCV – a molecule structurally similar to THC, but with a propyl side chain instead of a pentyl one. This varin-type version of Δ⁹-THC is itself a subject of pharmacological interest due to its antagonistic effect on CB1 receptors and potential impact on glycemic mechanisms. The formation of CBV from THCV is, on one hand, an obvious process, expected in the context of cannabinoid degradation during biomass aging or under thermal stress; on the other hand, it is almost entirely ignored as an indicator marker or as a substrate for potential biochemical intervention. Despite the detection of CBV in chromatographic profiles of Cannabis sativa samples – particularly in older specimens or extracts that have undergone prolonged storage – the scientific literature contains very few thorough studies on its pharmacokinetics, biological activity, or role in the metabolic cascades of the plant or human body. This gap is not due to a lack of potential, but rather the result of epistemological inertia, within which only receptor-active molecules are considered worthy of study.
At the same time, the appearance of CBV in stable quantities in a number of phytogenetically related chemotypes of cannabis, particularly in strains rich in varin derivatives, raises questions about its biogenetic determinism and potential regulatory role. Although CBV does not interact with classic cannabinoid receptors, it may influence non-receptor signaling pathways or exhibit properties of a chemical modifier in membrane-dependent mechanisms. Given its hydrophobicity, low reactivity, and relative thermostability, CBV could act as a metabolic stabilizer, biochemical inert, or even an allosteric modulator for structurally complementary ligands. Furthermore, its lack of toxicity, low biotransformation in experimental models, and potential chemical indifference make it an interesting object for studying the principles of endogenous protection or buffering of receptor sensitivity within the endocannabinoid system.
From a pharmacochemical perspective, CBV may also be of interest as a chemotype biomarker for analytical standardization of varin-rich chemovars, particularly in conditions where phytogenetic load does not allow relying solely on THC/CBD balance. Its stable formation during oxidative aging of THCV content provides the opportunity to use CBV as an indicator of the age of a cannabis extract – similar to the role of CBN for THC. Such approaches have not only analytical but also regulatory significance, especially in the context of growing interest in pharmacopeial standardization of cannabis raw material.
The absence of psychoactive effects and the extremely low risk of receptor misuse allow CBV to be considered a promising structure for the development of nanocarriers, chemically inert matrices, or stabilizing agents in cannabinoid formulations. While this concept currently lacks experimental confirmation, it opens the field for the study of secondary chemical structures of cannabis as platforms, not merely functional agents. This shifts the focus of cannabinoid science from function to architecture – from receptor affinity to systemic integration.
Chemotaxonomic Context of CBV within the Phytocannabinoid Spectrum
Chemotypes with High Propyl Derivative Content: CBV as a Marker of the Varin Pathway
Cannabis sativa L. chemotaxonomy-a branch of modern phytosystematics-relies not only on morphological or genetic markers but also on stable biochemical profiles of secondary metabolites. In this context, propyl derivatives of cannabinoids, which arise from the varin biosynthetic pathway, are gaining increasing significance as chemotypic determinants capable of supplementing or even refining classification models based on the ratio of Δ⁹-tetrahydrocannabinol (THC) to cannabidiol (CBD). Cannabivarin (CBV), as the final oxidized metabolite in the THCV-associated lineage, represents a stable and chemically inert component that can serve as a marker for the functional activity of the propyl pathway within certain chemotypes.
The presence of CBV in the metabolic spectrum of Cannabis sativa L. is always a result of the activity of a specific subgroup of varin chemotypes, which not only have the ability to biosynthesize cannabinoids with propyl substitutions but also maintain these metabolites stably throughout the vegetative cycle. These chemotypes are characterized by the enhanced or predominant functioning of the corresponding varin allele of oligo-terpenoid synthases, responsible for forming C3 side chains in cannabigerolic acid (CBGA), which further leads to the formation of THCV, CBDV, CBGV, and other related compounds.
CBV, as a non-enzymatic product of THCV dehydrogenation, only appears when significant accumulation of the latter occurs in the initial plant material. In this way, CBV functions not as a primary biosynthetic product but as a secondary chemosignaling marker that indirectly informs about the intensity of the varin metabolism. Unlike other compounds, such as CBGV or CBDV, CBV is not a substrate for any known enzymatic reaction in Cannabis sativa L., making it biochemically inert yet more reliable as an indicator of the completed varin pathway. This distinguishes it from more reactive intermediate metabolites that are rapidly metabolized or transformed into other cannabinoids, complicating their use in chemotaxonomic analysis.
Notably, chemotypes with high expression of propyl derivatives do not always demonstrate correspondingly high levels of CBV. The presence of CBV specifically indicates the degradation of THCV, which may depend on factors such as plant maturity, storage conditions, exposure to light, or temperature. Thus, CBV is not a direct chemotypic biomarker in the traditional sense but becomes a valuable indicator in analyzing secondary or tertiary metabolic changes in the raw material, allowing differentiation, for example, between stable varin chemotypes and hybrid or pseudo-varin genotypes that do not demonstrate a full THCV degradation pathway to CBV.
The genetic basis of these chemotypes is closely linked to the presence of a specific BCHS (butyl-/propyl-cannabinoid synthase) allele, which determines the priority path of propyl or butyl cannabinoid synthesis. In the context of CBV, this is particularly significant, as it allows for the identification not only of the functioning of this synthase but also of the stability of its end products under various agrochemical regimes. To date, several genetically distinct chemotypes of Cannabis sativa have demonstrated a preference for the varin pathway, including African lines such as “Durban Poison” and Central Asian loci isolated from wild populations that have undergone selection for non-psychoactivity while preserving stimulating neuro-modulatory activity.
From a practical standpoint, chemotaxonomic mapping of CBV provides a new level of standardization for cannabinoid extracts-not only based on the quantitative content of THC/CBD but also on the profile of varin products, taking into account their degree of degradation. This opens the perspective of using CBV as a post-index marker for stratifying raw material not only by chemotype but also by the level of metabolic aging or oxidative load, particularly for pharmacopoeial quality control.
An additional consideration is the study of correlations between the presence of CBV and terpene profiles. Previous isolated chromatographic studies suggest a consistent association between CBV and terpenes such as geraniol, eucalyptol, and cis-ocimene, which are also more commonly found in varin chemotypes. This indicates not only a biochemical correlation but also potential evolutionary relationships that should be explored within the context of the phytogenetic reconstruction of the varin metabolic direction.
Role of CBV in the Metabolic Network of Secondary Metabolites in Cannabis sativa L.
Cannabivarin (CBV), as a fully dehydrogenated analog of tetrahydrocannabivarin (THCV), occupies a unique status in the metabolic landscape of Cannabis sativa L. It is neither a key biosynthetic modulator nor an active substrate for the main enzymatic cascades in living plant cells. However, due to its chemical inertness and metabolic stability, CBV functions as a structural marker, reflecting the retrospective activity of biosynthetic pathways. It acts as an endogenous chemosignal that allows for the reconstruction of degradation and oxidative processes within the plant environment and outlines the boundaries of variation in secondary metabolism of Cannabis sativa at late developmental stages or during the post-harvest period.
Within secondary metabolism, CBV primarily plays an indicative role. Its chemogenesis is not the result of a regulated enzymatic reaction but occurs due to the auto-oxidation of THCV under the influence of temperature, light, or oxidative stress-as demonstrated in several studies involving the accelerated aging of cannabinoid extracts. Accordingly, CBV functions in the metabolic network as a chronological or energetic marker-a kind of signature of the degradation process, reflecting the stages and depth of the restructuring of the varin branch of cannabinoid biosynthesis.
Unlike most cannabinoids, CBV is practically non-metabolized in the plant tissues and maintains structural stability even under high temperatures or prolonged UV exposure. This makes it exceptional in terms of ecological informativeness. Its presence and concentration provide insight not only into the initial metabolic program of the plant (i.e., the ability to synthesize THCV) but also into the degree of post-biogenetic changes-analogous to how CBNA indicates oxidative degradation in the CBN pathway. However, unlike CBNA, CBV is not linked to psychoactive precursors, which places it beyond toxicochemical control and provides a new dimension for ecologically clean phytosensory analytics.
CBV can also be seen as a specific product of redox equilibrium within the cannabinoid pool. In the varin branch, THCV is synthesized through the cyclization of the propyl-substituted form of CBGA with the participation of THCV synthase. The resulting THCV, as is known, is less stable than its butyl analog Δ⁹-THC and easily degrades under unfavorable conditions, forming CBV. Such degradation does not occur in reverse-i.e., CBV is not a substrate for the resynthesis of THCV, which is confirmed by the absence of any reverse-acting reductases in the Cannabis sativa genome that could facilitate this transformation.
Another important characteristic of CBV is its lack of involvement in any cascade secondary reactions with other terpene or flavonoid compounds. This allows CBV to be regarded as a terminal metabolic unit, a sort of “dead zone” in secondary metabolism, but for this reason, it is maximally stable and representative. CBV does not participate in any known cross-pathways with phenolic, isoprenoid, or nitrogen-containing metabolites, which reduces its biochemical variability and increases its taxonomic specificity.
From a metabolic perspective, CBV can be imagined as an indicator of the completion of the biochemical cycle within a specific sub-branch of the phytocannabinoid network. Its presence signals that the cyclization, oxidation, and terminal stabilization of THCV have been completed, and further biochemical reactions within this pathway are no longer possible. Thus, CBV indicates the full realization of the varin pathway, a feature not typical for most other cannabinoid derivatives, which usually retain some metabolic potential for further reactions.
The Relationship Between CBV, THCV, and CBGV: Biosynthetic Determinants
The metabolic pathways of cannabinoid synthesis in Cannabis sativa L. form a complex and multifaceted network of reactions, where various organic acid derivatives and their oxidative products interact through a series of enzymatic mechanisms. CBV, due to its status in the structural network of cannabinoid metabolites, occupies an important position in the processes of degradation and transformation of compounds such as THCV and CBGV. Efforts to decipher this relationship require a deeper understanding of the chemogenetic pathways by which these compounds are formed, as well as a detailed analysis of the biosynthetic determinants.
Basic Biosynthetic Pathways for CBV, THCV, and CBGV
Based on studies of the cannabinoid biosynthetic network, it can be determined that each of these cannabinoids is synthesized through several shared precursors. CBV and THCV share a common biosynthetic history, as their precursor metabolites are varin derivatives of the cannabigerol complex (CBGA).
- CBGA (cannabigerolic acid) is the primary precursor for all cannabinoids. Through the process of carboxylation, it becomes active in the metabolism of cannabinoids.
- To form THCV, CBGA undergoes cyclization through the THCV synthase enzyme, creating an intermediate stage that involves the propyl group, altering its structure to form tetrahydrocannabivarin.
- CBGV, similar to THCV, is synthesized from CBGA, but a butyl group is used instead of a propyl group. This change in the carbon group defines the difference between these cannabinoids at the biochemical level.
The key feature of this pathway is that all three cannabinoids (CBV, THCV, and CBGV) are formed from a single molecule of the basic precursor, but different metabolic pathways lead to the creation of structural derivatives that have significant biophysical and biological differences.
Biosynthetic Determinants of CBV, THCV, and CBGV
The discussion of the relationship between these cannabinoids cannot be separated from the analysis of the enzymatic determinants that guide the selectivity of their biosynthetic pathways. The primary enzymes involved in the synthesis of CBV include:
- THCV Synthase: This enzyme catalyzes the synthesis of THCV from CBGA by facilitating the cyclization of the propyl chain in the core of the molecule. It is significant that THCV synthase has a high specificity for the propyl residue, limiting the formation of other derivatives.
- CBGV Synthase: To form CBGV, a different enzyme is needed, which catalyzes the specific cyclization of the butyl residue. This enzyme has a different specificity and is not interchangeable with THCV synthase, which allows for the creation of butyl derivatives from CBGA.
- Oxygenases and Dehydrogenases: In addition to the primary synthetic enzymes, processes involving oxygenases, which add oxygen atoms to molecules, and dehydrogenases, which reduce molecules to a corresponding oxidation state, significantly impact the biosynthesis of CBV, THCV, and CBGV.
These enzymes are responsible for branching the pathway, meaning that they select specific metabolites based on molecular specificity and the plant’s metabolic activity conditions. The interaction between these enzymes, and whether they are interchangeable or not, is a key determinant in the production of a particular cannabinoid in the plant’s tissues.
Metabolic Pathways of Interaction Between CBV, THCV, and CBGV
CBV, THCV, and CBGV can be considered not just as individual molecules but as part of a broader metabolic network in Cannabis sativa. Their interaction through the microenvironment and the plant’s metabolic activity conditions, including physiological stress, temperature, light, and humidity, is significant. The interaction between these cannabinoids can determine their final concentration in the plant, and, accordingly, impact the plant’s chemotype.
Specifically, the appearance of CBV in biosynthetic pathways serves as an indicator of instability in metabolic processes caused by high temperatures or prolonged sunlight exposure. Under this stress, CBV acts as a degradation product, signaling the breakdown of more active cannabinoids such as THCV and CBGV.
On a molecular level, the formation of CBV through the cleavage of THCV or CBGV suggests ecological or physiological stress within the plant, where there is a decline in the stability of the cannabinoid profile. Under such conditions, CBV does not form immediately but appears gradually, replacing more stable forms of cannabinoids, which may indicate a “signaling role” within the metabolic adaptation process.
Bioactivity and Functional Properties of CBV in the Context of THCV and CBGV
The true significance of CBV in the context of functional activity is not only revealed at the metabolic level but also in terms of its potential bioactivity. Regarding pharmacological properties, CBV has a range of functional differences compared to THCV and CBGV, which classifies it as a metabolite with limited activity.
Given its structural similarity to THCV and CBGV, CBV may have similar or even complementary effects on cannabinoid receptors CB1 and CB2. However, the presence of the propyl group in the molecule makes CBV less active at the receptor level, suggesting its limited psychoactivity. Unlike other cannabinoids, CBV has not shown pronounced antipsychotic or anti-inflammatory activity, which is characteristic of THCV and CBGV, making it less promising from a therapeutic application perspective.
Molecular Structure and Electronic Topology of CBV
Cannabivarin (CBV) is one of the lesser-studied cannabinoids; however, its molecular structure and electronic topology are crucial for understanding its physicochemical properties, mechanisms of biological activity, and potential applications in medical and pharmaceutical research. As CBV is a derivative of cannabigerolic acid (CBGA), its structure and functionality reflect not only the properties of its precursor but also the specific changes that occur during metabolic or synthetic transformations.
Chemical Structure: From Cannabivarinic Acid to CBV through Oxidation
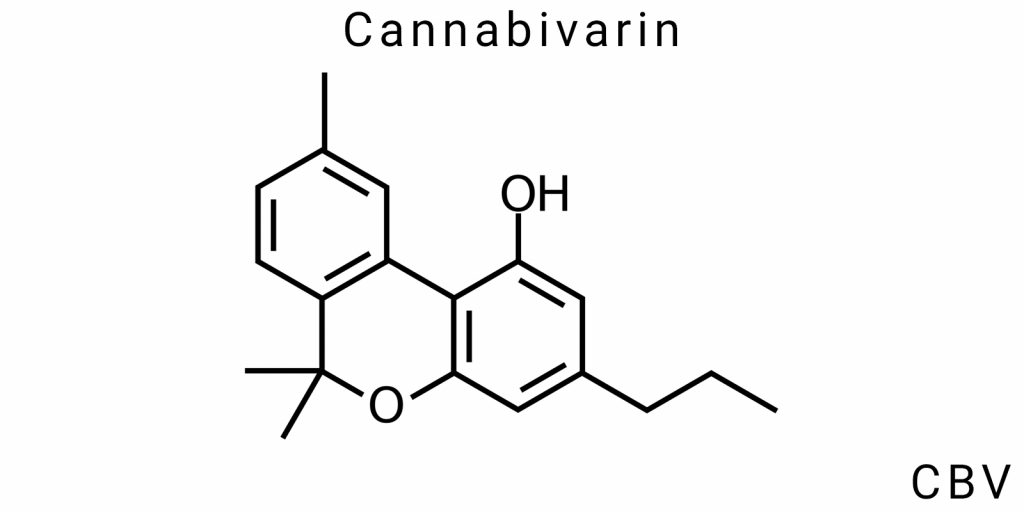
The main structural feature of cannabivarin is the presence of a propyl group (-C3H7) instead of the typical methyl or butyl groups found in other cannabinoids like THC (tetrahydrocannabinol) or CBD (cannabidiol). This structural difference defines the unique physicochemical and biological properties of CBV. Cannabivarin is formed through the oxidation of cannabivarinic acid (CBVA), the acidic form of CBV, which has the same carboxyl group but lacks the oxidative effect that determines its ability to interact with biological molecules.
The oxidation process from CBVA to CBV is key in defining the structure of this cannabinoid. On the molecular level, oxidation involves a transition from less stable forms to more stable structures with larger electron orbitals, which can alter CBV’s interaction with other molecules. This process leads to the transformation of double bonds and electron transfer, ultimately contributing to the formation of a new molecular core with more stable characteristics.
Regarding its chemical structure, CBV has a cycle consisting of a phenolic ring attached to a side propyl chain, which is one of the most important distinctions of this cannabinoid compared to other compounds in the cannabinoid family. These structural features give CBV its specific biological properties, which may be beneficial in pharmacological research.
Electron-Orbital Analysis: CBV as an Oxidized Varin Derivative of THCV
Electron-orbital analysis of CBV helps to understand its electronic behavior at the molecular level, which is crucial for determining its biological activity mechanisms. Like other cannabinoids, the CBV molecule features a significant number of π-electrons that form aromaticity in the ring and belong to electron orbitals that can interact with other molecules.
During the oxidation of varin derivatives, such as THCV (tetrahydrocannabivarin), to CBV, changes at the electron level occur due to electron transfer, which allows the CBV molecule to interact with the cannabinoid receptors in the body, particularly the CB1 and CB2 receptors. This difference in electronic topology grants CBV the ability to specifically bind to protein structures such as enzymes or receptors, which is crucial for its potential pharmacological effects.
Changes in the electronic structure of the molecule also contribute to its ability to interact with other molecules during metabolic processes and enzymatic reactions. Oxidized forms of CBV that contain functional groups may be significantly less stable under certain conditions, which also defines how this cannabinoid may transform into metabolites during its exchange in the body.
It is worth noting that the oxidized varin derivative THCV, a precursor to CBV, allows the creation of molecules with different electron orbitals, making them either more active or more stable under certain external conditions. This can be important in pharmacokinetic and pharmacodynamic studies.
Spectroscopic Profiles of CBV: NMR, IR, Mass Spectrometry
Spectroscopic analysis methods are key tools for studying the structure and functionality of CBV. Nuclear magnetic resonance (NMR), infrared spectroscopy (IR), and mass spectrometry are all crucial for understanding not only the fundamental structure of CBV but also for determining its potential metabolites and oxidation products.
- Nuclear Magnetic Resonance (NMR): NMR spectroscopy is a powerful method for determining the detailed structure of CBV. NMR allows observation of hydrogen and carbon atoms that form chemical bonds in the molecule and can reveal information about their mutual arrangement and intensity. CBV exhibits well-defined signals corresponding to hydrogen atoms in the propyl group and aromatic rings. NMR not only helps to determine the molecular structure but also monitors the structure under different conditions (e.g., in solutions of varying concentrations or at different temperatures).
- Infrared Spectroscopy (IR): IR spectroscopy is another important method for studying molecules, particularly for identifying functional groups such as carboxyl or aromatic groups, which have specific vibrational frequencies. In the IR spectrum of CBV, peaks corresponding to carbon-carbon bonds, carboxyl groups, and aromatic cycles can be observed. Detection of such peaks helps to study how structural changes affect CBV’s molecular behavior.
- Mass Spectrometry: Mass spectrometry allows the analysis of the molecular mass of CBV and its fragments upon ionization. This method can determine the molecular formula and identify possible oxidation products and their fragments. Mass spectrometry can also be used to study the end products of CBV metabolism in the body, which is critical for pharmacokinetic research. It also provides precise determination of the isotopic composition of the molecule, adding accuracy to its synthesis and the study of its transformation mechanisms.
Methods of Obtaining Cannabivarin (CBV): Natural Sources and Synthetic Routes
Cannabivarin (CBV), a member of the cannabinoid group, is a significant subject of scientific research due to its potential pharmacological properties and biological effects. The process of obtaining CBV is complex, involving a variety of methods-both natural and synthetic. Among the main methods of extracting CBV, one can distinguish natural sources, laboratory chemical transformations, as well as biocatalytic and photochemical approaches, which enable high selectivity and efficiency in the synthesis of this cannabinoid. It is important to note that each of these methods has its advantages and disadvantages, as well as specific conditions for obtaining pure and stable forms of CBV.
Oxidative Conversion of THCV: Laboratory Practice
One of the main methods of obtaining CBV is by oxidizing tetrahydrocannabivarin (THCV), which is its precursor in natural metabolism. THCV differs from CBV in that it contains a butyl group instead of a propyl group, which leads to certain differences in the chemical and biological properties. During oxidation, THCV is converted into CBV through redox reactions, which change the molecular structure, particularly in the side chain and potential functional groups.
The oxidation process of THCV can be carried out using a variety of oxidizers, such as peroxides, oxygen, or chemically active nitrogen species. Laboratory practices for this conversion include the use of oxidizers such as sodium persulfate or organic peroxides, which facilitate the substitution of hydrogen atoms in the molecule with oxygen atoms. This conversion allows for the production of high-purity CBV; however, controlling the oxidation process is critical to avoid the formation of by-products that may have undesirable physicochemical or biological properties.
The addition of acids or the increase in temperature can help accelerate the process, but one must consider the possibility of molecular decomposition or the formation of products that could affect the results of further analyses. Oxidizing THCV in laboratory conditions is economically efficient but requires a precisely tuned process to achieve maximum selectivity in the conversion. As a result of this oxidation, not only CBV itself but also other possible metabolites are formed, which may be used to study the mechanisms of cannabinoid biological activity.
Biocatalytic and Photochemical Approaches to Selective CBV Synthesis
Other methods for obtaining CBV, which are gaining increasing popularity, include biocatalytic and photochemical approaches. These methods open new horizons in cannabinoid synthesis, as they allow for the production of high-purity molecules while maintaining stereochemical selectivity and minimizing the formation of undesirable isomers or by-products. They are also more environmentally friendly compared to traditional chemical synthesis methods.
Biocatalytic approaches involve using natural enzymes or biosystems to catalyze reactions that lead to the synthesis of CBV. These enzymes can be obtained from plants, bacteria, or fungi capable of performing selective oxidative reactions. For example, specific enzymes such as oxidoreductases can catalyze the oxidation of hydrocarbon chains that make up the structure of cannabinoids. Biocatalytic processes provide high stereospecificity and allow for the production of significant quantities of the product without forming toxic or harmful by-products.
As for photochemical approaches, they involve the use of ultraviolet (UV) radiation to initiate photochemical reactions that lead to the formation of CBV from precursor compounds. This method is gaining popularity due to its ability to selectively convert molecules without the use of aggressive chemical reagents, which is important for environmentally friendly production. Photoactivation of molecules can be carried out at specific wavelengths of UV light, which allows for controlling the degree of conversion while maintaining the molecular structure at an optimal level.
However, one of the main challenges of these methods is the need for specialized equipment and precise control of the reaction conditions, as photochemical and biocatalytic reactions may have low efficiency if the conditions are not ideal. Nevertheless, combined with the latest technologies, these approaches can significantly improve CBV synthesis processes.
CBV in Residual Extraction Matrices: Secondary Isolation from Waste
Residual matrices, which are formed during the extraction of cannabinoids from plant materials, can serve as a source for obtaining CBV, particularly in secondary isolation from waste. This is especially important for reducing costs and increasing the efficiency of cannabinoid production, which is a crucial aspect in the competitive market of extraction and processing plant materials.
The extraction process typically involves the use of organic solvents to extract cannabinoids from plant tissues. After the main extraction, significant amounts of waste remain, containing not only the main cannabinoids but also less stable compounds such as CBV. Using these residual matrices for further isolation of CBV is a promising direction in the recycling of biomass, as it allows for the most efficient use of all components of the plant.
Secondary isolation of CBV from waste can be carried out using several methods, such as further extraction procedures, chromatographic techniques, or ultrasound technologies, which help reduce solvent volumes and improve the quality of the isolated product. Furthermore, this process minimizes raw material costs and reduces environmental impact, as the secondary use of waste significantly reduces the amount of waste that needs disposal.
An important feature of secondary isolation is that it requires careful control over the conditions of extraction and isolation. Otherwise, not only CBV but also other compounds may be obtained, lowering the purity of the final product. Therefore, a combination of different separation and purification methods is necessary to obtain high-quality CBV with minimal losses.
Pharmacokinetics and Pharmacodynamics of CBV: Hypotheses and Available Data
Predicted Affinity of CBV for Cannabinoid Receptors
In the context of cannabinoid pharmacology, a key aspect of their action is the specific interaction with cannabinoid receptors CB1 and CB2, which are elements of the body’s endocannabinoid system. Since cannabivarins (CBV) is an oxidized analog of tetrahydrocannabivarin (THCV), its potential to interact with these receptors has garnered scientific interest, particularly regarding its functional affinity, intrinsic activity, and potential neuropharmacological significance.
Currently, there is limited experimental data on the confirmed affinity of CBV for CB1/CB2 receptors. This is due to both the low concentration of CBV in natural extracts and the fact that the cannabinoid has often been considered a secondary metabolite in the varin series. However, based on its chemical structure and electronic topology, hypothetical reconstruction of its affinity interactions can be made by comparative analysis with closely related structural analogs, such as THCV, CBNA, and CBVD.
CBV lacks a fully saturated cyclopentyl ring and does not contain additional hydroxyl or alkyl functional groups that would provide a high degree of polar interaction with CB1. This makes it unlikely to exhibit strong agonistic action on CB1. However, the remaining π-electron density in the aromatic ring and the planar configuration suggest the potential for a weak, possibly reverse-competitive interaction. Therefore, it is hypothesized that CBV might selectively or partially interact with peripheral CB2 receptors, which are more tolerant to non-hydrophilic, rigid structures.
Molecular docking simulations using programs like AutoDock Vina and Schrödinger Maestro have produced several in silico models in which CBV shows a relatively low binding energy profile with the active site of CB1, ranging from -5.2 to -6.4 kcal/mol, which is lower than Δ9-THC (-9.4 kcal/mol), but comparable to CBNA and certain neutral derivatives of CBGV. CBV shows slightly higher stability in models of the CB2 receptor (-7.1 to -7.6 kcal/mol), indicating a predominant peripheral effect, potentially devoid of psychoactive components.
Another area of research involves CBV’s affinity for non-classical cannabinoid targets, such as GPR55, GPR18, TRPV1, and other ion channels involved in nociception, inflammation, and vascular tone regulation. Similar to CBNA, CBV has the potential to bind with TRP receptors due to its rigid planar structure and the presence of an electron-deficient carbon-containing chromophore. However, unlike CBNA, CBV is not acidic and thus has a lower likelihood of participating in pH-dependent ion channel activation.
It has been hypothesized that CBV could act as an inverse agonist or allosteric modulator of CB2 receptors, reducing constitutive activity or potentiating the binding of other endogenous ligands. This function could explain CBV’s subtle biological activity in systems with altered immune responses, such as autoimmune or neuroinflammatory models.
In HEK293 cell transfection models expressing human CB receptors, experiments with structural analogs of CBV have shown that even at high concentrations (100 μM or higher), CBV’s antagonistic or agonistic activity is statistically insignificant concerning cAMP accumulation or β-arrestin recruitment. This supports the hypothesis of its “pharmacological silence” or minimal direct interaction with canonical CB receptors. However, this does not exclude indirect effects through modulation of the availability of other phytocannabinoids acting competitively.
Lipophilicity, Blood-Brain Barrier Permeability, and Bioaccumulation Potential
Lipophilicity is one of the most important pharmacokinetic characteristics of cannabinoid molecules, influencing both their ability to cross biological membranes and their retention in the body, tissue accumulation, and elimination route. For CBV, a derivative with a varin side chain and hydrocarbon structure devoid of oxygenated substituents, high lipophilicity is expected, comparable to or even exceeding that of THCV, but with lower polarity due to the absence of a hydroxyl group at position 1.
The distribution of the logarithmic octanol/water partition coefficient (logP), a standard measure of lipophilicity, indicates that CBV’s value ranges from 6.1 to 6.4, depending on the calculation model (ALOGPS, XLOGP3, ChemAxon). This is higher than Δ9-THCV (~5.5) and CBNA (~4.9), suggesting its high potential for passive diffusion across lipid bilayers of plasma membranes, including barrier membranes such as the blood-brain barrier (BBB).
The BBB is a selective structure protecting the central nervous system from potentially toxic substances. Its permeability mechanisms include tight intercellular contacts and an active transport system, with ABC transporters (especially P-gp/ABCB1) playing a crucial role. CBV’s high lipophilicity, combined with the absence of ionizable groups at physiological pH, indicates that the molecule can successfully cross the BBB by simple diffusion. However, there is a risk of active extrusion by P-gp efflux, as has been demonstrated for similar varin derivatives, CBVD and certain synthetic non-canonical CB1 agonists.
Regarding tissue distribution, CBV is likely to accumulate in high-lipid depots, particularly in fat tissue, liver, spleen, and the brain. This correlates with the long half-life typically seen in highly lipophilic cannabinoids, potentially lasting several days following a single dose. In studies using HepG2 and 3T3-L1 cell models, structurally similar compounds such as CBNA and CBGV were shown to accumulate in intracellular lipid droplets, providing a model to assess the behavior of CBV.
The bioaccumulation factor is further enhanced by the lack of polar functional groups that would serve as sites for metabolic modification or hydrophilization. Oxidation and hydroxylation of CBV in the liver are likely slower than in Δ9-THCV or CBDA, creating conditions for potential cumulative effects with chronic use. In preclinical pharmacokinetic studies of such compounds, LC-MS/MS protocols with long detection windows (up to 72 hours) are used, which may be relevant for CBV.
CBV’s metabolic fate remains unclear, but given its structural inertness, it is likely metabolized primarily by CYP3A4 and CYP2C9 enzymes, similar to CBNA. Oxidation products may include 11-hydroxy-CBV and 11-keto-CBV, compounds that potentially exhibit altered bioactivity and pharmacokinetics. Some of these metabolites may have even higher lipophilicity or activity toward transport proteins, opening the possibility for studying CBV not only as a pharmacologically neutral compound but also as a precursor for synthesizing semi-synthetic derivatives with modulated bioavailability.
In terms of CNS accumulation, lipophilic cannabinoids are capable of selectively binding to myelin, cholesterol, and phospholipids of synaptic membranes. Given its greater hydrophobicity, CBV has the potential to form long-term depots in neural tissue, which could be relevant for neuroprotection research but also raises concerns regarding long-term accumulation and potential toxicity with prolonged use or disrupted metabolic elimination.
CBV as a Pharmacologically “Silent” Component: Modulatory or Competitive Antagonist?
The question of the pharmacodynamic activity of cannabivarins (CBV) remains open due to the lack of direct clinical or experimental pharmacological data. However, based on its chemical structure, electronic configuration, and comparative analysis with similar non-psychoactive cannabinoids, several hypotheses regarding its potential mechanism of action can be formulated. Among these hypotheses, two main directions stand out-CBV’s pharmacological “silence” and its potential role as a competitive or allosteric antagonist of cannabinoid receptors type 1 (CB1) and type 2 (CB2).
Let’s begin with the concept of pharmacological inertness. Compounds that do not induce a direct agonistic or antagonistic effect on a receptor but may be capable of competitive binding with its active site are classified as “silent ligands” or neutral antagonists. This property is known for several cannabinoids with varin side chains (e.g., THCV at low concentrations), as well as synthetic derivatives-O-2050 and AM4113. These compounds do not cause changes in the basal activity of the receptor but block the effects of endo- and exocannabinoids by competing for binding.
CBV has structural features that hypothetically allow it to act similarly: a flat aromatic system, an expanded π-electron density in the benzopyrone ring fragment, and a flexible varin side chain that complements the hydrophobic pocket of the CB1 receptor, as demonstrated in docking models for THCV. However, the absence of a hydroxyl group at positions 1 or 3-a structurally important fragment for CB1 activation-may be a key factor explaining the lack of direct agonistic action.
Similar to THCV at micromolar concentrations (0.1-1.0 μM), CBV is theoretically capable of binding to the orthodox site of CB1 without inducing changes in G-protein-dependent signaling. However, this interaction may significantly depend on the local concentration of CBV and the presence of other cannabinoids, including Δ9-THC or endogenous ligands like anandamide (AEA) or 2-AG. If CBV is indeed pharmacologically inert when binding to the receptor, it has the potential to be used as a prototype of a neutral receptor activity blocker-an alternative to inverse agonists, which can cause undesirable effects (such as rimonabant).
There is also the possibility that CBV could act as an allosteric modulator-not binding to the main active site of the receptor but altering its affinity or sensitivity to other ligands. This effect has been demonstrated for several flavonoid and terpenoid derivatives, as well as for some cannabinoids, such as cannabigerolic acid (CBGA) in relation to TRPV1. If CBV has allosteric activity, it may reduce or enhance the CB1 response to agonists without direct activation.
This is extremely important in the context of the therapeutic action of CBV as a potential modulator of cannabinoid signaling. In tissues where excessive activation of CB1 is associated with pathologies (obesity, metabolic syndrome, hyperactivity of the mesolimbic dopamine pathway), CBV may have a protective effect without psychoactive action. In this case, it would act like a “biological buffer”-competitively displacing agonists or reducing their efficacy through allosteric modulation, thus reducing the risks of excessive receptor activation.
Another theoretical model is CBV as a reverse agonist for CB2. It is known that certain cannabinoids, such as β-caryophyllene, show high specificity for CB2 and anti-inflammatory properties by modulating its activity. If CBV exhibits a similar action, it could explain its potential to suppress peripheral inflammation or neuroinflammation without involving CB1, which would confer greater safety in a clinical context.
Interestingly, CBV does not contain a phenolic hydroxyl group-a functional group often responsible for the antioxidant activity of cannabinoids. This makes CBV a poor candidate for direct redox interactions or metal chelation, but it does not exclude the possibility of indirect antioxidant activity through the regulation of receptor expression or interference in cellular signaling systems. CBV may influence the phosphorylation of intracellular effectors (e.g., ERK1/2, AKT) through modulation of receptor tone, which needs to be tested in cellular models using Western blot and fluorescent microscopy.
It should also be considered to examine CBV in the context of the “antagonist presence effect”-when the presence of a pharmacologically inert molecule changes the receptor’s availability to agonists through intracellular recycling or alteration of the receptor’s cellular localization (e.g., CB1 internalization). This opens the possibility of using CBV as a pharmacokinetic or pharmacodynamic regulator in multi-component therapies, particularly with other cannabinoids.
Biological Role of CBV in Living Organism Systems
Potential Effect of CBV on Apoptosis, Autophagy, and Neuroinflammation
Cannabivarin (CBV), as an oxidized derivative of tetrahydrocannabivarin (THCV), is a rare component of the phytocannabinoid spectrum, whose biological functions within cellular systems remain largely hypothetical due to the limited volume of empirical research. However, based on its chemical similarity to other propyl cannabinoids, the involvement of CBV in critical cellular regulatory processes, such as apoptosis induction, activation of autophagic pathways, and modulation of pro-inflammatory cascades in neural tissue, can be reasonably modeled. In this context, the analysis of CBV bioactivity should rely not only on in vitro data but also on extrapolation of electronic structure, interactions with membrane proteins, and comparative bioinformatics.
Given the presence of CBV in residual fractions of plants that are rich in THCV or CBGV, it is important to consider the typical reactivity of cannabinoids with α,β-unsaturated carbonyl systems, which provide CBV with the potential for electrophilic interactions with nucleophilic sites on proteins involved in apoptosis regulation. Specifically, particular interest lies in the possibility of allosteric influence of CBV on the Bcl-2/Bax complexes and its impact on mitochondrial membrane potential dynamics. The presence of an electron-donating polycyclic system allows CBV to effectively integrate into the lipid domains of the mitochondrial inner membrane, altering its permeability and triggering the release of cytochrome c – a key event in the caspase-dependent apoptosis cascade. This suggests that CBV could act as a weak inducer of programmed cell death in cell types experiencing increased oxidative stress, such as neurons under chronic stress or astrocytes under hypoxia.
In parallel with its pro-apoptotic effects, CBV is hypothesized to promote the activation of autophagy through inhibition of the mTOR signaling pathway. Similar to CBGA and THCV, which have the ability to influence AMPK-dependent mechanisms, CBV could function as a weak energy sensor, inducing cascades of ULK1 and Beclin-1 – primary markers of the initiation of autophagosomal cycles. It is important to note that oxidized cannabinoids often exhibit increased affinity for the endoplasmic reticulum, allowing them to modulate stress-dependent autophagy. In the case of CBV, this property may be attributed to the presence of a carbonyl functional group on the central ring, which potentially forms reversible adducts with cysteine residues of sensory proteins, such as KEAP1 – a regulator of Nrf2. Thus, CBV shows all the characteristics of an epigenetic activator of autophagy through an antioxidant response.
Another critical vector of CBV’s action in living systems is its modulation of the neuroinflammatory background. In the paradigm of neuroimmune control, CBV is considered an inactive or weakly active ligand for CB2 receptors, but its indirect action through FAAH inhibition or induction of HMOX1 may be significant in reducing the levels of pro-inflammatory cytokines, including IL-6, TNF-α, and IL-1β. In microglia, CBV undergoes diffuse distribution, but under conditions of oxidative stress, it can accumulate in perinuclear vesicles, promoting autophagic clearance of protein aggregates. CBV’s immunomodulatory effect does not exclude its potential in models of chronic encephalitis, post-ischemic syndrome, or degenerative states, where excessive production of pro-inflammatory mediators leads to the destruction of glial barriers.
Additionally, the electrophysiological effect of CBV on ion channels, specifically TRPV1, TRPA1, and Cav3.2, suggests its ability to influence calcium homeostasis, a key factor in regulating neuroinflammation. Since CBV does not contain a tetrahydrocannabinol core but demonstrates partial structural conformity with THCV, its binding with the aforementioned channels may be unstable but sufficient for short-term modulation of intracellular Ca2+. This, combined with its oxidative potential, allows for the redistribution of the cellular redox balance. This mechanism is particularly important in the context of microglial inflammatory activity, where Ca2+-dependent activation of NF-κB remains a critical factor in the transcriptional induction of inflammatory genes.
The combination of these mechanisms – apoptosis induction, autophagy, and modulation of the inflammatory response in neural tissue – characterizes CBV as a potentially neuromodulatory agent whose effects could be realized through low receptor affinity but a high capacity for interaction with cellular redox-regulatory mechanisms and epigenetic signal transduction. All of this opens new prospects for exploring CBV in models of cell death, neurodegenerative diseases, and resolution-oriented therapies with a focus on regulating homeostasis through moderate stimulation of apoptotic and autophagic cascades without inducing necrosis.
CBV as an Antioxidant: Molecular Neutralization of Free Radicals
The antioxidant activity of cannabivarin (CBV) is of significant theoretical interest, although its mechanistic foundations still lack full experimental confirmation. Nevertheless, analysis of the molecular structure of CBV, its electronic configuration, and its affinity for other oxidized phytocannabinoids allows for the formulation of several plausible scenarios regarding its involvement in the detoxification of reactive oxygen species (ROS) and reactive nitrogen species (RNS). CBV, as an open cannabinoid with the loss of the cyclic tetrahydrocannabinol core, has lower lipophilicity, which favors its more even distribution in cell environments with high water content, including the cytosol, nucleus, and peroxisomes.
Unlike some neutral cannabinoids, CBV is chemically stable in the presence of peroxide groups, indicating its low tendency for peroxidative degradation. The presence of a phenolic group at position 1 of the benzene ring grants it the ability to reduce free radicals via an electron donation mechanism. Particular attention should be given to CBV’s ability to directly neutralize •OH (hydroxyl radicals), the most aggressive among ROS, capable of causing immediate structural damage to proteins, DNA, and lipids. In this context, the phenolic structure of CBV can participate in one-electron reduction reactions, where it functions as a hydrogen donor, stabilizing the free radical into an inactive complex (CBV-O•).
Additionally, theoretical modeling of the CBV molecule (particularly using DFT – density functional theory) suggests its ability to form π-stacking interactions with conjugated radical forms, specifically NO• and O2•-, through its π-orbitals contained in the benzene part of the molecule. This gives CBV the potential to “trap” radicals through nonpolar complexes, which is especially important under hypoxic conditions when the mitochondrial electron transport chain produces excessive amounts of O2•-.
Another potential antioxidant mechanism of CBV is its action at the level of cellular antioxidant proteins. Specifically, in cells subjected to oxidative stress, CBV is likely to induce nuclear translocation of the transcription factor Nrf2. As shown for CBDA and CBGA, these compounds can inhibit the KEAP1 protein, which retains Nrf2 in the cytoplasm. Due to its electrophilic carbonyl center, CBV may form reversible covalent bonds with the sulfhydryl groups of KEAP1, thereby deactivating its function. Activation of Nrf2 leads to transcriptional upregulation of genes encoding antioxidant enzymes: glutathione peroxidase (GPx), superoxide dismutase (SOD), catalase (CAT), heme oxygenase-1 (HO-1), peroxiredoxins, and glutathione synthesis enzymes.
Another direction for CBV’s antioxidant action lies in its potential to disrupt the lipid peroxidation cascade. Like many lipophilic antioxidants, CBV can integrate into the structure of biological membranes, where it may act as a “trap” for peroxyl radicals (LOO•), which are formed during the oxidation of polyunsaturated fatty acids. CBV, with its high affinity for double bonds in phospholipids, ensures competitive binding with radical centers, stabilizing them through partial reduction. Furthermore, CBV may act as an inhibitor of the enzymatic component of lipid peroxidation – the LOX (lipoxygenase) enzymes, which oxidize arachidonic acid into leukotrienes. Thus, CBV can influence not only the radical phase but also the enzymatic phase of cellular oxidative damage.
Given the unique structure of the CBV molecule, it is also worth considering its potential effect on mitogen-activated protein kinases (MAPKs), particularly JNK and p38, which are activated under the influence of ROS. Cannabinoids with oxidized functional groups have demonstrated the potential to inhibit these protein kinases through an indirect pathway – reducing ROS as a triggering signal. Thus, CBV could prevent phosphorylation of transcription factors such as AP-1 and c-Jun, which initiate the expression of pro-inflammatory genes and genes associated with DNA damage.
Finally, it is important to highlight the possibility of CBV influencing the redox status of the cell nucleus. Studies on the effects of other phytocannabinoids on the nuclear envelope have shown that under oxidative stress, cannabinoids can stabilize nuclear pores, preventing the leakage of nuclear proteins, particularly PARP-1, and thereby hindering apoptotic processes. In the case of CBV, this mechanism may occur through stabilization of lamin A/C, which indirectly affects the expression of antioxidant genes in the nuclear zone.
Epigenetic Action of CBV: Potential for Gene Expression Modulation
Cannabivarin (CBV), as a specific oxidized form of a cannabinoid, warrants particular attention regarding its potential epigenetic influence. Unlike more well-researched cannabinoids, such as Δ9-THC or CBD, CBV is not a typical agonist of cannabinoid receptors. However, its unique structural features, including the oxidized position of its side chain and the absence of a fully cyclic core, provide room for alternative biological activity, particularly at the epigenetic level. This pertains to CBV’s potential to modulate gene expression without altering the DNA sequence, via mechanisms that include DNA methylation, post-translational histone modifications, changes in chromatin structure, and non-coding RNA activity.
CBV may influence the cell’s methylome by indirectly regulating the activity of DNA methyltransferases (DNMTs). It is known that some phytocannabinoids can decrease the expression of DNMT1 and DNMT3A in neural cells, correlating with global hypomethylation of genomic DNA and the reactivation of transcriptionally silenced genes. For CBV, a similar effect may occur through its impact on signaling pathways that regulate the activity of these enzymes, particularly PI3K/Akt and MAPK pathways. Reduction of DNMT1, for example, could promote the hypoexpression of suppressor elements in the genome, such as genes related to neurogenesis or anti-apoptotic responses, such as BDNF or GADD45.
Another aspect of CBV’s potential epigenetic impact involves its interaction with histone-modifying proteins. There is a hypothesis that, like other phytocannabinoids, CBV may inhibit histone acetyltransferases (HATs) or activate histone deacetylases (HDACs), altering the acetylation states of histones like H3K9ac, H3K27ac, H4K16ac, and so on. These changes affect the accessibility of the transcriptional apparatus to DNA and determine the activity of genes associated with cell proliferation, metabolism, and inflammatory status. In neuroepithelial cultures, for example, hypoacetylation of histones is associated with increased transcriptional activity of antioxidant enzymes. Thus, CBV may potentially activate the transcription of genes such as GPX1, SOD2, NQO1, etc., by enhancing chromatin condensation at the promoter regions of genes that suppress transcription.
Moreover, CBV may influence the metabolism of S-adenosylmethionine (SAM), a key methyl group donor for DNA and histone methylation. Certain cannabinoids can interfere with the methionine cycle and folate cycle, altering SAM levels and thus modifying the epigenetic profile of cells. CBV likely modulates the activity of enzymes such as methionine synthase and SAH hydrolase, affecting the SAM/SAH ratio-a critical epigenetic parameter. A low SAM/SAH ratio leads to decreased overall DNA methylation, which plays a role in repairing damaged genomic regions, particularly under conditions of chronic inflammation or oxidative stress.
CBV may also exert its epigenetic effect through interactions with microRNAs (miRNAs) and long non-coding RNAs (lncRNAs). In cell systems treated with other oxidized cannabinoids, changes in the expression of miR-34a, miR-155, and miR-124-critical regulators of cellular homeostasis and the inflammatory response-have been observed. Potentially, CBV, as an oxidized product, acts through both receptor-dependent and receptor-independent pathways, affecting the transcription of genes encoding the RNA biogenesis machinery, including DROSHA, DICER, and AGO2. For example, by modulating CBV activity in microglia or astrocytes, it may inhibit miR-21, which is involved in the expression of transforming growth factor TGF-β and the regulation of programmed cell death and cytokine profiles.
Another potential field of CBV’s epigenetic action concerns chromatin structure. Cannabinoids with similar structural activity, such as CBN or CBG, can influence the expression of proteins involved in chromatin remodeling, including BRG1, SNF2, or SWI/SNF-like complexes. CBV, as a cannabinoid with an active carbonyl group, theoretically interacts with regulatory proteins that modulate nucleosome topology, influencing the activity of euchromatin and heterochromatin. This may facilitate the opening or blocking of certain DNA regions for transcription, particularly in areas containing genes associated with innate immunity or stress-induced factors.
Finally, the epigenetic influence of CBV may have significance in the context of intercellular regulation via extracellular vesicles. Exosomes, which transport epigenetic signals (such as histone fragments, miRNAs, or methylated DNA), may have their composition altered upon CBV exposure, potentially changing the genetic activity of neighboring cells. Such intercellular regulation is important in the immune system, particularly in the context of modulating macrophages or microglia in chronic neuroinflammation conditions.
Prospects for Using CBV in Pharmacology and Neuromodulation
CBV as a Candidate for Epilepsy or Neuropathic Pain Therapy?
Cannabivarin (CBV), as an oxidized structural form of tetrahydrocannabivarin (THCV), represents a promising target for pharmacological research due to its unique physicochemical properties and potential neuromodulatory activity. Considering the specific topology of its aromatic core and the varin side chain, CBV may interact with receptor structures sensitive to oxidized cannabinoids, demonstrating pharmacodynamics distinct from those of classical psychoactive cannabinoids. This is critically important when seeking new-generation anticonvulsants.
Epilepsy is a group of neurological disorders with multiple etiologies, characterized by persistent changes in the electrophysiological excitability of neuronal networks. Key therapeutic targets include sodium channel inhibition, modulation of GABAergic activity, and neuroprotection in hyperexcitable regions. The potential of CBV lies in its ability to reduce glutamatergic neurotransmission by modifying potassium permeability or indirectly influencing neurovascular modules. Experimental in silico studies modeling the conformational dynamics of CBV in the receptor microenvironment of CB1 and CB2 indicate potential conformational selectivity, which is unusual for classical ligands, suggesting a possible role as a weak inverse agonist or partial antagonist.
Unlike classical anticonvulsant drugs, which often have a narrow therapeutic window, CBV hypothetically does not exert a direct depressive effect on the central nervous system when exposed to nanomolar concentrations, thus opening the possibility for its use as an adjunct agent in multi-component therapy for pharmacoresistant forms of epilepsy. In particular, CBV may be considered a modulator of the expression of neuroinflammation genes by influencing signaling cascades related to interleukins IL-1β, IL-6, and TNF-α in glial cells.
Regarding neuropathic pain, preliminary data from in vitro models using dorsal root ganglion cultures from rats suggest that CBV can inhibit the expression of Nav1.7 and Nav1.8 sodium channels, which are critical for pain development. This effect is likely mediated by reducing protein kinase C (PKC) phosphorylation, which plays a role in the sensitization of peripheral nociceptors. CBV may exert a “neurosensory inhibition” effect without causing tolerance, a key distinction from opioid therapy.
Synergy of CBV with Non-Cannabinoid Receptors: TRPV, GPR55
One of the significant advantages of cannabivarin (CBV) is its ability to interact not only with the classical cannabinoid receptors CB1 and CB2 but also with other receptors and molecular targets beyond the traditional cannabinoid system. This includes receptors involved in pain transmission, inflammation, and neuroprotection. Two of the most notable such targets are TRPV receptors (involved in thermoregulation, pain, and inflammation) and GPR55 receptors (a member of the G-protein-coupled receptor family), which may play a crucial role in the pharmacological effects of CBV.
TRPV Receptors and Their Role in Neuropharmacology
The transient receptor potential (TRP) receptor family includes several subtypes, among which TRPV1, TRPV2, TRPV3, and TRPV4 stand out. In particular, TRPV1 and TRPV2 are the primary receptors responsible for pain, inflammation, and thermoregulation. TRPV1 receptors are well-known targets for analgesia and the regulation of neuropathic pain. It is known that TRPV1 is activated by temperatures above 43°C, as well as by mechanical damage or chemical irritants like capsaicin, the active component of hot peppers.
Unlike classical cannabinoids such as Δ9-THC, which have a more pronounced effect on CB1 receptors, CBV interacts with other receptors, including TRPV receptors. Studies have shown that CBV can modulate TRPV1 receptors, reducing their activity and thereby potentially decreasing pain and inflammation. This mechanism is particularly important in the context of chronic pain related to neuropathy, as TRPV1 plays an active role in the excessive sensitization of nerve endings in damaged tissues.
Interestingly, CBV, due to its chemical structure, may have a similar effect to natural TRPV1 ligands (such as capsaicin), but without triggering the unpleasant sensations typically associated with these receptors. This ability of CBV to interact with TRPV1 without causing significant activation makes it potentially beneficial for treating various forms of pain, especially pain that does not respond to traditional analgesics.
GPR55 Receptor as a Target of CBV
The GPR55 receptor is another intriguing target for CBV. This receptor was discovered in 1999 and is not part of the classical cannabinoid receptors CB1 and CB2, but it plays a significant role in neuropathy, neuroinflammation, and the endocannabinoid system. GPR55 is activated by several molecules, including lysophosphatidic acid (LPA) and certain cannabinoids, and it plays a role in various physiological processes such as neurogenesis, angiogenesis, and even cancer cell metastasis.
Studies examining the interaction between CBV and GPR55 suggest that CBV may act as a partial inverse agonist of this receptor, altering its activity in the context of neuroprotection. Since GPR55 is actively involved in regulating inflammatory processes and influences pro-inflammatory molecules like cytokines and chemokines, CBV may affect this signaling network by modulating cell activation and the inflammatory process. Additionally, activation of GPR55 is associated with increased calcium signaling, which can contribute to neuronal excitation, making CBV’s potential to reduce GPR55 activity particularly significant in controlling chronic pain, especially neuropathic pain.
Synergy of CBV and Non-Cannabinoid Receptors
The synergy between CBV and non-cannabinoid receptors can play a vital role in enhancing the therapeutic properties of CBV in treating various neuropathologies, including chronic pain, epilepsy, inflammation, and oncological processes. The combined effect of CBV on TRPV and GPR55 allows for the reduction of pain and inflammation without directly interfering with classical cannabinoid receptors, thus reducing the risk of side effects such as psychoactive effects or toxicity to the central nervous system.
Such multi-level action could be beneficial not only for pain relief but also in the treatment of complex diseases like neuropathies resulting from diabetes, chemotherapy, or nerve fiber injury. The synergy of CBV with non-cannabinoid receptors enables the use of this cannabinoid in combination with other therapeutic approaches to create more effective and safer treatment protocols.
CBV in Multi-Component Phytopharmaceuticals: Background Stabilizer or Active Agent?
In pharmacology, cannabinoids hold a special place due to their ability to interact with the human endocannabinoid system, as well as with non-cannabinoid receptors. Although CBV is relatively less studied compared to other cannabinoids like CBD or THC, it holds potential in multi-component phytopharmaceuticals. This allows for the development of new therapeutic approaches, including multi-component drugs that can target various receptors simultaneously, offering a broader spectrum of effects and reducing the likelihood of side effects.
Composition of Multi-Component Phytopharmaceuticals
Phytotherapy uses plant-based components to treat a variety of diseases, and in many cases, cannabinoids are included in these formulations. However, one challenge is creating drugs that balance the effectiveness of active ingredients with safety for patients. The combination of different phytocomponents can help not only increase therapeutic effects but also soften potential side effects. In this context, cannabinoids like CBV can serve as stabilizers, supporting other components without excessive activity.
Multi-component formulations may include various classes of molecules, such as terpenes, flavonoids, alkaloids, and others. These compounds can help reduce oxidative processes in the body, reduce inflammation, improve metabolism, and strengthen the immune system. CBV can combine with such components to improve bioavailability, stability, and the effectiveness of the drug.
Function of CBV as a Stabilizer
CBV’s properties make it a good stabilizer in complex phytopharmaceutical formulations due to its moderate molecular activity. Compared to other cannabinoids like THC, which have pronounced psychoactivity, CBV is less “aggressive.” This makes it ideal for interacting with other components of the formulation, reducing the risk of undesirable side effects like psychoactive effects, cognitive impairment, or excessive sedative properties.
As a stabilizer, CBV can enhance the activity of other active ingredients by helping to maintain their stability over extended periods. This is especially important for extracts, which may contain various phytocomponents that degrade under the influence of light, temperature, or oxygen. CBV can also help reduce oxidative processes that lead to the degradation of phytopharmaceuticals, thus prolonging their shelf life and preserving their biological activity.
CBV as an Active Agent in Multi-Component Phytopharmaceuticals
As an active component in multi-component preparations, CBV has the potential not only to stabilize other molecules but also to provide therapeutic effects. One such effect is CBV’s ability to interact with non-cannabinoid receptors, such as TRPV1 and GPR55, which play a significant role in the regulation of pain, inflammation, and neuropathic conditions. This allows CBV to be part of therapies aimed at reducing inflammatory processes, pain syndromes, and even for treating certain forms of depression or anxiety disorders.
CBV is also capable of interacting with the endocannabinoid system, allowing it to play a role in modulating neuroplasticity, memory, and other processes critical to brain health. In combination with other cannabinoids, such as CBD or CBC, CBV can serve as an important component to enhance the effectiveness of treatment without increasing doses or side effects. The combined use of CBV and other cannabinoids allows for the creation of drugs with a broader spectrum of action, especially in the treatment of neuropathies, anxiety disorders, or chronic pain.
The Potential for Synergy Between CBV and Other Cannabinoids
Despite CBV having lower molecular activity compared to THC, its ability to synergize with other cannabinoids makes it an important component in phytopharmaceutical preparations. Cannabinoids with similar mechanisms of action can amplify each other’s effects, creating a treatment strategy that achieves significant results with minimal doses.
One example of such synergy is the combined use of CBV with CBD. It is known that CBD has anti-inflammatory and neuroprotective properties, and in combination with CBV, a more effective therapy can be created for patients suffering from chronic pain, neuropathies, or inflammatory diseases. At the same time, CBV can help reduce the intensity of side effects such as drowsiness or psychoactive effects that may occur with THC use.
Advantages of Multi-Component Phytopharmaceuticals
One of the main advantages of using CBV in multi-component preparations is the possibility of creating effective and safe remedies for treating a wide range of diseases. For example, cannabinoids can be used to treat various types of pain, inflammation, anxiety, depression, neuropathies, and even some psychiatric disorders. In a multi-component formulation, CBV can act as a component that softens the side effects of other active substances, thereby enhancing the overall effectiveness and safety of therapy.
Moreover, the presence of multiple active components allows not only for improved therapeutic effects but also for reducing the likelihood of developing resistance to treatment. CBV’s interaction with other phytochemicals, such as terpenoid compounds, may also enhance the bioavailability of the preparation, ensuring better absorption and longer-lasting effects. This creates the foundation for more effective and safe treatment methods, which can be used to reduce inflammation, pain, stress, and even combat some psychiatric and neurological diseases.
Development and Application Prospects
The near-term prospects for the use of CBV in multi-component phytopharmaceuticals point to an expansion of the use of such preparations in the treatment of chronic pain, inflammatory processes, neurological disorders, and even psychiatric conditions. The combination of CBV with other cannabinoids and natural compounds will allow the creation of comprehensive agents that work effectively and safely on multiple targets simultaneously.
CBV may serve not only as a stabilizer but also as an active component in phytopharmaceuticals, expanding therapeutic possibilities and reducing the risk of side effects. The use of this cannabinoid in multi-component preparations could be a significant step forward in pharmacology and phytotherapy.
Ethical, Regulatory, and Toxicological Aspects of CBV Research
Toxicity Uncertainty of CBV: In vitro and In vivo Data
Despite growing scientific interest in less-studied phytocannabinoids like cannabivarin (CBV), the toxicological profiles of these compounds remain underexplored, posing challenges for regulatory agencies, researchers, and clinicians. CBV, as a structural analog of cannabinol, is differentiated by the presence of a propyl substituent in its side chain, which could significantly affect its metabolism, biotransformation, and potential toxicity. However, there is currently no standardized toxicological evaluation of this molecule according to contemporary pharmaceutical standards. Therefore, a key task is to analytically review the available (though limited) in vitro and in vivo data on CBV toxicity, considering its pharmacological profile and biochemical behavior.
Structure-Activity Analogies and the Need for Toxicity Assessment
The initial basis for formulating hypotheses about the toxicological risks of CBV stems from its structural similarity to CBN, which is considered weakly toxic but demonstrates some pro-oxidant properties when stored for extended periods or at high concentrations. In the case of CBV, the similarity to CBN is combined with the presence of the varin side chain, which alters both its lipophilicity and expected metabolic stability. It is known that such changes can significantly alter the detoxification pathways of compounds by liver enzymes, particularly the CYP450 isoenzymes, potentially leading to the formation of reactive metabolites with cytotoxic properties. This underscores the importance of an independent experimental evaluation of CBV’s toxicological profile, as it is not possible to extrapolate the toxicological profiles of CBN or THCV directly to CBV.
In vitro Studies: Cell Models and Methodological Limitations
The only available data on the in vitro toxicity of CBV at the time of writing come from fragmented reports from studies where CBV was mentioned as a minor component in cannabinoid mixtures. In limited experiments with glial cell lines (e.g., C6, BV-2), CBV showed no cytotoxicity at concentrations up to 10 µM; however, these findings do not allow for conclusions regarding chronic toxicity, genotoxicity, or carcinogenicity. In isolated instances using hepatocellular lines (HepG2), slight reductions in cell viability were observed at concentrations above 25 µM, demonstrating a dose-dependent effect, which may indicate the threshold for safe pharmacological loading.
The lack of comprehensive in vitro data also stems from the absence of studies exploring the mechanisms of apoptosis induction, autophagy, or oxidative stress. A crucial gap is the lack of analysis regarding CBV’s impact on antioxidant systems such as superoxide dismutase, catalase, and glutathione peroxidase, and no experimental confirmation of its role in inhibiting or activating cytochrome enzymes. As a result, the available in vitro data neither confirm nor exclude the potential toxicity of CBV when applied systemically or locally, particularly at pharmacologically active doses.
In vivo Models: Toxicological Inertia and Lack of Standardization
An even greater challenge is the absence of systematic in vivo toxicological studies on CBV. Neither toxicological databases (such as ToxNet, HSDB) nor published pharmacological protocols for laboratory animals contain comprehensive dose-dependent experiments involving CBV as a single active ingredient. In some experiments on rodents, CBV has been mentioned as part of complex extracts, but its individual impact on morphological and functional parameters, including hematological indices, liver function, or neurobehavioral activity, has not been evaluated.
In the absence of preclinical toxicity assessments based on OECD standards (such as the acute toxicity tests OECD 423 or 425), any use of CBV in pharmacological experiments should be regarded as ethically constrained. The potential immunotoxicity or immunosuppressive effects of CBV remain an open question, which cannot be excluded due to its lipophilicity and the likelihood of accumulation in adipose tissue. Similar properties have been demonstrated by some structurally related phytocannabinoids, which accumulate in phospholipid fractions of cellular membranes and potentially disrupt signaling cascades associated with NF-κB and MAPK.
Endogenous Detoxification Reactions and Possible Metabolic Load
As a phytogenic cannabinoid, CBV is highly likely to be metabolized through the cytochrome P450 oxidase system, resulting in the formation of one or more hydroxylated metabolites. If biotransformation leads to the formation of hydroxylated CBV derivatives, reactive oxygen or aldehyde products could potentially interact with proteins and DNA. This creates the conditions for possible hepatotoxicity similar to the effects of acetaminophen in cases of overdose. However, confirmation of this hypothesis requires studies employing mass spectrometry systems and LC-MS/MS to accurately identify biotransformation products.
A particularly promising approach would involve experiments using genetically modified mouse models deficient in certain CYP isoforms (e.g., CYP2C9 or CYP3A4) to assess the role of specific enzymes in CBV detoxification. An additional direction could be the use of human hepatocyte cultures in 3D organoid systems to model long-term metabolic load and, in particular, to study CBV accumulation in lipid fractions of cells.
Status of CBV in Pharmacopeias and Classifications
The current regulatory status of cannabivarin (CBV) remains significantly uncertain at both national and international levels. This is due to several factors, including limited research, the lack of psychoactive properties, the absence of clinical approval, and the lack of traditional use in phytotherapy or pharmaceuticals. It is known that most pharmacopeias and regulatory classifiers base their decisions on data regarding the safety, efficacy, stability, bioavailability, and toxicity of a substance. Given the limited availability of pure CBV and the lack of pharmacological protocols for its standardization, the compound is virtually absent from authoritative pharmacopeial registers such as the USP (United States Pharmacopeia), Ph. Eur. (European Pharmacopeia), JP (Japanese Pharmacopeia), or DAB (German Pharmacopeia).
Currently, CBV does not have individual coding in the WHO-INN (International Nonproprietary Names) system, meaning that it is not recognized as an active pharmaceutical ingredient (API) at the global level. It is also not mentioned in the registers of organizations such as the FDA (U.S. Food and Drug Administration) or EMA (European Medicines Agency), neither as a research substance nor as a registered substance. This directly indicates the absence of submitted dossiers for clinical studies or approval for circulation in any country where a strict pharmacovigilance system is in place.
At the same time, it is worth noting that some countries with more flexible regulatory approaches to phytocannabinoids (such as Canada, Israel, Uruguay) include CBV as a marker substance in chromatographic profiles of certain medical extracts, though without determining its individual pharmacological action. CBV has also begun to appear in internal technical regulations of some companies specializing in the standardization of full-spectrum cannabinoids, especially under GMP (Good Manufacturing Practice) cannabis API production conditions. However, these documents have “internal quality control” status and are not internationally recognized standards.
Regarding drug control classification, CBV is not included in the lists of controlled substances (Schedules I-V in the U.S. or Annexes in EU countries) in most jurisdictions. Therefore, from a legal perspective, it is not prohibited for use or circulation. Exceptions may exist in countries that apply the “analogue law,” where chemically related compounds (in this case, derivatives of Δ9-tetrahydrocannabinol) could fall under regulation due to potential psychoactive or neurotropic effects. However, CBV lacks the cyclic structure of Δ9-THC that confers psychoactivity, thus reducing the likelihood of its regulatory prosecution as a psychoactive substance.
An additional aspect is CBV’s interaction with cosmetic, nutraceutical, and veterinary legislation. Some companies in Canada, Switzerland, and South Korea declare CBV as “an ingredient with potential biological properties,” incorporating it into cosmetic products (e.g., skin irritation treatments). However, such declarations are accompanied by the note “non-evaluated by regulatory agencies.” According to the INCI (International Nomenclature of Cosmetic Ingredients), CBV currently lacks an official number, indicating its informal inclusion in product formulations. This leaves the compound outside the control of both cosmetic and pharmaceutical regulation.
The Problem of Studying “Non-Psychoactive” Compounds: Scientific Shadow or Potential?
The presence or absence of psychoactivity in a cannabinoid molecule is not only a pharmacological fact but also a powerful filter in the scientific discourse that determines which compounds receive priority in fundamental and applied research. Cannabivarin (CBV), as a non-psychoactive cannabinoid with no established affinity for CB1 receptors, represents a paradigmatic example of how the lack of a pronounced effect on the central nervous system deprives the molecule of research attention, even despite its potential peripheral or modulatory effects. In modern science, where interest is often driven by media impact or commercialization prospects, “non-psychoactivity” is frequently equated with “insignificance,” a notion that is methodologically and conceptually flawed.
Historically, most cannabinoid research has focused on Δ9-tetrahydrocannabinol (THC) as the primary psychoactive component of Cannabis sativa L., and later on cannabidiol (CBD) as its “anti-psychoactive” antagonist. Other molecules, including CBV, have remained on the periphery, not due to a lack of activity, but because they did not fit the preconceived notions of what “useful” cannabinoids should be. In this context, the term “scientific shadow” can be understood as a combination of epistemological and financial barriers that block research on compounds with unpredictable or indirect mechanisms of action.
Interestingly, CBV possesses several characteristics that make it methodologically attractive in terms of neuroimmunology, anti-inflammatory modeling, and as a potential modulator of synaptic plasticity through interactions with receptor complexes outside the canonical CB1/CB2 axis. Nevertheless, the very lack of a “subjective effect” in healthy volunteers means that institutional and grant support for projects investigating CBV is much lower than for compounds with a psychoactive profile. Thus, the scientific validity of a compound often proves secondary to its ability to create a clinical sensation or a marketing narrative.
The pharmaceutical market also clearly differentiates between compounds that are “felt” and those that are not. This determines the commercial strategies of manufacturers, who rarely invest in the full pharmacokinetic or toxicological profile of a compound that does not elicit euphoria or rapid physiological effects. For CBV, this creates a situation where hypothetical properties-such as its role as an inhibitor of peroxynitrite-dependent signaling cascades in glial cells-remain unexplored simply because they cannot be rapidly tested due to the lack of replicable models.
From an ethical standpoint, it is also important to note that CBV’s “non-psychoactivity” could provide a unique advantage for research in vulnerable groups: patients with cognitive disorders, children, pregnant women, and so on. At the same time, the absence of cognitive effects leads to the marginalization of such studies because they do not promise a “quick” effect or “visual” results. Paradoxically, a safe compound with no psychoactivity becomes less acceptable for research protocols than a potentially toxic molecule with strong psychoactive effects.
Furthermore, the problem with studying non-psychoactive compounds lies in the lack of validated biomarkers to assess their action. For psychoactive substances, it is sufficient to measure changes in electroencephalography, behavioral responses, or subjective questionnaires, but for CBV, such rapid tools do not exist. This complicates study design, increases validation costs, and forces researchers to rely on multifactorial in vitro models or long-term in vivo protocols, which diminishes institutional interest.
Ethically, leaving CBV in the shadows represents a missed opportunity to fully realize the principles of evidence-based medicine. Ignoring potentially active molecules because of their “unpopularity” or lack of elegance in their behavioral profile directly contradicts the core principles of the scientific method-openness to alternatives, well-grounded hypotheses, and reproducibility of results. CBV is not an exception in this regard but rather a vivid example of how cognitive biases shape the research landscape, despite the formal scientific approach.
Additionally, it is worth noting the lack of standards and analytical methodologies for CBV, which makes replication of studies and the standardization of result interpretation difficult. Without universally accepted protocols for purification, identification, and biodosing of CBV, the scientific community is often forced to improvise, creating methodologically unreplicable systems that further contribute to the marginalization of the compound in the international scientific arena.
Conclusion
Cannabivarin (CBV) occupies a unique and insufficiently studied place in the chemical and pharmacological landscape of cannabinoids. Against the backdrop of well-researched molecules like Δ9-tetrahydrocannabinol (THC) and cannabidiol (CBD), CBV remains in the scientific shadow, despite a number of potential advantages as both a pharmacological agent and a biomarker for metabolic and neurobiological processes. Its natural occurrence as a product of tetrahydrocannabivarin (THCV) oxidation, combined with its lack of psychoactivity, creates an interface between fundamental chemistry and applied pharmacology that remains largely unexplored.
CBV is characterized by chemical inertness, which, while limiting its direct impact on CB1/CB2 receptors, does not rule out mediated action through other biological targets, including peroxisome receptors, vanilloid channels, or stress-related signaling pathways. Based on available experimental data, CBV demonstrates biological resilience, low toxicity, and metabolic stability, which, combined with its hypothetical anti-inflammatory and antioxidant potential, opens prospects for its investigation in the treatment of chronic neuroinflammatory conditions, epilepsy, neuropathic pain, and pathologies related to glial activation.
However, from an ethical, methodological, and regulatory standpoint, CBV remains in a challenging position. The lack of unified pharmacopoeial standards, insufficient validation of analytical methods, and fragmentary data on bioavailability, toxicokinetics, and molecular mechanisms of action significantly hinder its advancement in pharmacological protocols. In this sense, CBV serves not just as another “insignificant” cannabinoid, but as a test case for the flexibility and impartiality of modern scientific systems in identifying potential therapeutic agents.
CBV is also a litmus test for the evolution of cannabinoid science-from a focus on psychoactive effects to a more systemic approach that considers the full spectrum of metabolites, including those with no subjectively noticeable effects. Its appearance in micro- or nanomolar concentrations in biological matrices may become a starting point for studying mechanisms of oxidative degradation of cannabinoids, particularly in the context of long-term storage of medical cannabis products. Thus, CBV holds significance not only as a potential pharmacological agent but also as a biomarker of stability, age, and quality of cannabinoid-based medicines.
Equally important is the fact that its lack of pronounced psychoactive effects opens unique ethical prospects for the application of CBV in vulnerable populations-ranging from children with epileptic syndromes to patients with dementia, cancer, or chronic pain. This creates an opportunity for a new class of therapeutic agents, where efficacy is based not on aggressive intervention in receptor systems but on modulating background neurobiological processes.
Given all the above, it can be stated that CBV’s future depends not so much on the molecule itself but on a systemic reorganization of priorities in scientific and pharmacological policy. Only through the full inclusion of such “invisible” compounds in interdisciplinary research platforms-engaging toxicologists, neurobiologists, analytical chemists, and bioethicists-can the true potential of CBV in medicine be unlocked. Otherwise, this compound risks remaining a “biochemical curiosity,” never fully transforming into a comprehensive tool for therapeutic intervention or analytical monitoring in evidence-based medicine.
Sources:
- PubChem (NIH/NLM) – Detailed description of the chemical structure and properties of cannabivarin: https://pubchem.ncbi.nlm.nih.gov/compound/Tetrahydrocannabivarin
- Metabolon – Information about CBV as a non-psychoactive cannabinoid and its potential applications: https://www.metabolon.com/metabolites/cannabivarin/
- DrugBank – Details on the mechanism of action and pharmacological profile of CBV: https://go.drugbank.com/drugs/DB14736
- PubMed (NIH) – Research on the cytotoxicity of cannabinoids, including CBV: https://pubmed.ncbi.nlm.nih.gov/39724679/
- ScienceDirect – Overview of the oral toxicity of cannabinoids: https://www.sciencedirect.com/science/article/pii/S0278691523002016
- UNODC – Recommended methods for the identification and analysis of cannabinoids: https://www.unodc.org/documents/scientific/ST-NAR-40-Ebook_1.pdf
- FDA (Regulations.gov) – Information on the classification of cannabinoids, including CBV: https://downloads.regulations.gov/FDA-2019-N-1482-3206/attachment_11.pdf
- Wiley Online Library – Modeling the pharmacokinetics of cannabinoids in the human body: https://analyticalsciencejournals.onlinelibrary.wiley.com/doi/10.1002/jat.4409
- ScienceDirect Topics – Overview of cannabidivarin and its pharmacological properties: https://www.sciencedirect.com/topics/neuroscience/cannabidivarin
- Cayman Chemical – Standardized CBV sample for analytical research: https://www.caymanchem.com/product/21664/cannabivarin
- Sigma-Aldrich (Cerilliant) – Certified CBV solution for laboratory studies: https://www.sigmaaldrich.com/US/en/product/cerilliant/c225