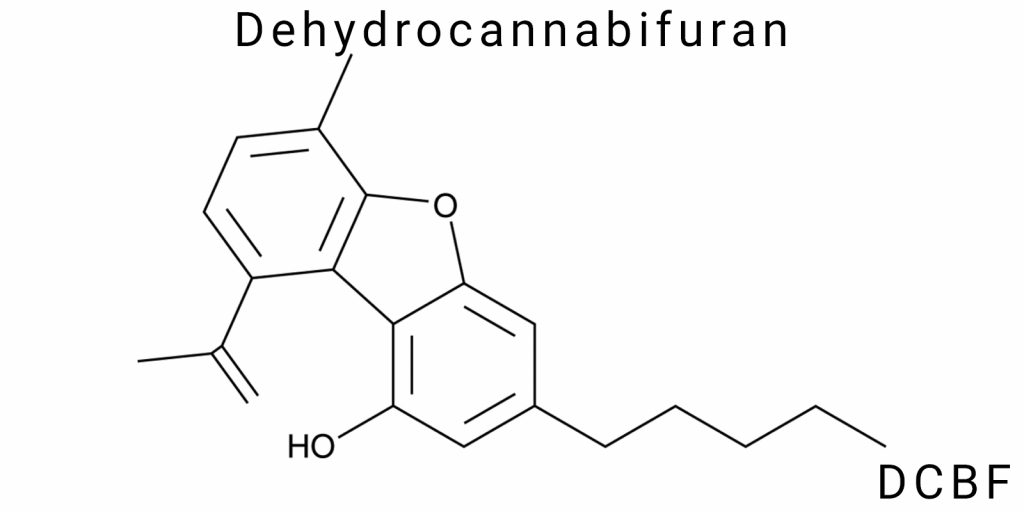
Dehydrocannabifuran (DCBF) is a poorly studied cannabinoid compound with a dibenzofuran structure, first identified in trace amounts in pyrolysis products of Cannabis sativa L., as well as in minor quantities in plant-derived raw materials. Structurally, DCBF represents an aromatic derivative of cannabielsoin (CBE), in which the saturated tetrahydrocannabinoid fragment has undergone dehydrogenation resulting in a fully aromatic system containing a furan ring. The molecular formula of the compound is C₂₁H₂₄O₂; the molecule contains a five-carbon side chain at the 3-position of the benzene ring, an isopropenyl group at the 5-position, and a hydroxyl function at the 1-position.
The dibenzofuran core of DCBF imparts an electron-rich, planar structure with high stability of the π-electron system, which potentially influences its interaction with biological targets. The degree of conjugation, the position of the hydroxyl group, and the distribution of lipophilic regions determine the compound’s potential to penetrate biological membranes, including the blood-brain barrier. Primary in silico pharmacokinetic models indicate high membrane permeability, with a logP > 5, and predicted bioavailability upon enteral administration. These properties underpin scientific interest in DCBF as a potential pharmacological ligand.
Available experimental data suggest that DCBF may form biosynthetically or thermogenically through the dehydrogenation of CBE derivatives. It is hypothesized that under natural conditions, DCBF can be generated as a result of oxidative-dehydrogenative processes in highly reactive environments (such as ultraviolet radiation, enzymatic oxidation, or thermal exposure). In laboratory conditions, synthesis can be achieved by targeted dehydration of saturated cannabinoids using acid catalysts or by aromatization using palladium-containing systems, analogous to Suzuki or Heck reactions. The resulting compound is verified by NMR spectroscopy, HPLC/MS, and IR analysis, confirming the presence of the dibenzofuran fragment.
The pharmacological properties of DCBF remain practically uninvestigated both in vivo and in vitro. However, based on its molecular structure, hypothetical interactions with receptor proteins can be extrapolated, including cannabinoid receptors CB1/CB2, PPARγ, TRP channels, or enzymatic systems such as FAAH or MAGL. Molecular docking studies suggest possible competitive or allosteric activity toward these targets, which requires experimental confirmation. Meanwhile, the electrophilic properties of the furan ring pose a potential risk of forming reactive metabolites during biotransformation, especially due to oxidation by the CYP450 system. This fact necessitates toxicological profiling of DCBF, particularly regarding its effects on liver cells, reproductive system cells, and central nervous system cells.
Considering its high structural stability, potential biological activity, and accessibility for chemical modification, DCBF is viewed as a promising subject for structure-activity relationship studies among furan cannabinoids. Further investigation of its physicochemical, pharmacological, and toxicological properties is important for understanding metabolic pathways of cannabinoid transformation, as well as for identifying new biologically active substances of natural or semi-synthetic origin. The lack of systematic information on DCBF’s behavior in biological environments creates a need for fundamental in vitro and in vivo studies involving pharmacokinetic modeling, expression analysis, and cell bioengineering methods.
Chemical Identification of DCBF
Dehydrocannabifuran (DCBF) represents a particular interest within the cannabinoid family due to its condensed polycyclic system, which includes a dibenzofuran fragment. The chemical identification of this compound requires a multi-level methodology employing advanced analytical tools such as spectroscopy, chromatography, and mass spectrometry. Unlike classical cannabinoids, which possess a partially saturated structure based on tetrahydrocannabinol or cannabidiol cores, DCBF is characterized by full aromatization of the nucleus and an embedded furan ring, forming a rigid, stable, and electron-rich architecture.
The first step in the chemical identification process of DCBF is determining its elemental composition and molecular weight. Using high-resolution mass spectrometry (HRMS) with ionization by electron impact or electrospray ionization (ESI), the exact molecular mass is registered corresponding to the formula C₂₁H₂₄O₂. The high accuracy of the measurement allows exclusion of isobaric compounds and confirms the elemental composition with an error margin of less than 5 ppm. Isotopic modeling of the fragment peak distribution serves as an additional identity indicator, notably due to the characteristic intensity ratios of ¹²C/¹³C peaks.
Further structural verification requires nuclear magnetic resonance (NMR) spectroscopy. The ¹H-NMR method enables detection of the characteristic spectral profile of aromatic protons, specifically signals in the region of 6.2-7.8 ppm corresponding to benzene nuclei and the furan ring. Deshielded multiplets are observed, indicating the presence of conjugated electronic systems. The ¹³C-NMR reveals signals from carbon atoms in the aromatic environment, with typical chemical shifts in the range of 110-160 ppm. Signals from saturated alkyl fragments in the 14-40 ppm range confirm the presence of a side chain in the structure. Additionally, correlation NMR techniques, including COSY, HSQC, and HMBC, are applied to identify scalar couplings between protons and correlate them with carbon centers, which is critical for verifying the system of multiple condensed rings.
Infrared (IR) spectroscopy establishes the presence of a hydroxyl group by its characteristic stretching absorption in the 3400-3600 cm⁻¹ range and detects C=C vibrations within 1600-1650 cm⁻¹, indicating the presence of an aromatic system. Absorptions in the 1050-1150 cm⁻¹ region correlate with furan ring vibrations, while the 700-900 cm⁻¹ region may indicate substitution patterns of the benzene rings. Collectively, the IR spectrum confirms the functional groups and types of bonds characteristic of DCBF.
Separation of DCBF from a mixture of analogs is achievable through high-performance liquid chromatography (HPLC) combined with UV detection and mass spectrometry. Optimal elution conditions typically involve the use of mixed mobile phases (methanol:water or acetonitrile:water) with gradient programming. DCBF exhibits characteristic UV absorption in the 220-280 nm wavelength region, associated with π→π* transitions in the furan and benzene systems. Determination of retention time in the chromatographic system enables quantitative analysis and assessment of the purity of the isolated sample.
Crystallographic identification of DCBF by X-ray structural analysis is currently not a standard procedure for this class of compounds due to difficulties with crystallization; however, with sufficient quantities of pure sample, data regarding internal conformation and interatomic distances can be obtained, ultimately confirming the molecular geometry. The structure is expected to include planar aromatic systems conjugated with the furan ring in a pseudo-coplanar configuration. This is consistent with quantum-chemical calculations of the energy-minimized optimized structure (DFT level, B3LYP/6-311G(d,p)).
For final confirmation of identity, experimental spectral data are compared with theoretical predictions or with analytical profile databases such as PubChem, ChemSpider, or specialized spectral libraries (e.g., NIST, Wiley). In the case of a newly identified sample, a dedicated analytical dossier is created, including the mass spectrum, full NMR data, IR spectrum, chromatographic parameters, and, where possible, DFT modeling data.
Systematic Name and Molecular Formula
Dehydrocannabifuran (DCBF) is a derivative of the cannabinoid system featuring a fully aromatized tricyclic core that includes a dibenzofuran fragment with alkyl substituents. Its chemical structure is not canonical for classical phyto- or synthetic cannabinoids and does not formally fall within the traditional classification of phytocannabinoids, yet it originates from them through dehydrogenation and ring system rearrangement.
The systematic name according to IUPAC rules for this molecule, based on its structural configuration, is:
5-[(1E)-1,2-dimethylprop-1-enyl]-3-pentyl-1-benzofuran-1-ol.
This name is constructed by the priority of functional groups and includes key structural elements: the benzofuran scaffold, a pentyl chain at position 3, an isopropenyl substituent at position 5, and a phenolic hydroxyl group at position 1. Within this nomenclature, benzofuran serves as the main scaffold, with numbering starting from the oxygen atom of the furan ring in a clockwise direction, allowing precise assignment of substituent positions without ambiguity.
The molecular formula of DCBF is C₂₁H₂₄O₂.
This formula demonstrates the atom ratio in the molecule: 21 carbon atoms, 24 hydrogen atoms, and 2 oxygen atoms. The molecular weight in the neutral state is 308.42 g/mol. The oxygen atoms in this formula are localized in the hydroxyl group (-OH) and the furan ether bridge. It is worth noting that this formula is typical for aromatic derivatives with a high degree of hydrogen saturation in the peripheral chains, along with the presence of two oxygen-containing fragments, which provide a baseline level of polarity.
Classification by InChI (International Chemical Identifier) provides an algorithmically unambiguous representation of the structure:
InChI=1S/C21H24O2/c1-5-6-7-13-17-14-19(21(22)23-20(17)16-11-9-8-10-12-16)15-18(2)3/h8-12,14-15,22H,5-7,13H2,1-4H3
This record determines the molecular topology, including double bond locations, the presence of the hydroxyl group, and the hydrogen atoms involved in the molecular configuration. The InChIKey identifier, a hashed version of the full InChI, is convenient for compound database searches:
InChIKey: YWWQVWYXPGTZKG-UHFFFAOYSA-N
From the elemental composition perspective, DCBF contains 72.72% wt. C, 6.96% wt. H, and 20.32% wt. O, as determined by CHN/O elemental analysis. Such composition indicates a high carbon content-typical for aromatic structures with branched alkyl chains. Since the structure contains no nitrogen, sulfur, or halogen atoms, it is chemically “clean” in terms of electrophilicity and lacks classical sites for nucleophilic attack except for the phenolic group.
According to chemical class, DCBF can be attributed to polycyclic aromatic ethers, more specifically to cannabifuran derivatives that feature a dibenzofuran core with alkyl and alkenyl substituents. This is a rare subtype within cannabinoid chemistry, as most natural cannabinoids are based on partially saturated systems with hydroxyl groups and cyclic terpene modules, whereas DCBF is fully aromatized.
A logical step for structural coding is the use of SMILES notation (Simplified Molecular Input Line Entry System):
CC(=C)C1=CC2=C(C=C1)OC(C3=CC=CC=C3C3CCCC3)=C2O
This linear formula enables encoding the structure digitally for molecular modeling, pharmacophore analysis, or screening. It is effective within cheminformatics platforms and algorithms for property prediction based on structure.
Formally, the core of DCBF lies in the benzofuran skeleton, formed by the fusion of a benzene ring with a furan. The introduction of an alkyl side chain at position 3, morphologically derived from the pentyl fragment of classical cannabinoids, provides analogous lipophilicity. The isopropenyl group at position 5, oriented planar to the furan ring plane, increases electron density in the conjugated system. Collectively, this forms an electrophile-neutral yet lipophilically stable molecule with potential for interactions with protein targets through π-π stacking, hydrophobic adsorption, or hydrogen bonding via the phenolic group.
Functional groups are represented by the hydroxyl (-OH) and the ether (C-O-C) furan system. Both groups exhibit relatively low nucleophilicity in the context of full aromatization but are capable of forming hydrogen bonds as donors (-OH) and as part of the π-system (furan). This architecture defines the compound’s stability in a neutral environment as well as limited reactivity under physiological pH conditions.
Structural Features of the Molecule
The molecule of dehydrocannabifuran (DCBF) is characterized by a specific structural architecture that combines elements of a benzofuran core, aliphatic substituents, and functional groups forming a stable, electron-rich, and planar system with a high degree of conjugation. The central structural motif is a tricyclic system formed by the fusion of benzene and furan rings, which constitute the benzofuran backbone, where the orientation of atoms within this system determines the dominant electronic and orbital characteristics of the molecule.
The benzofuran core in DCBF functions as an electron-donating aromatic platform with two conjugated systems: the benzene ring and the furan ring. Both cyclic structures are arranged planar and conjugate mutually in a perpendicular manner, creating a single π-electron system that serves as the molecule’s primary chromophore. It is important to note that in DCBF, the furan does not act as an independent heterocycle but is part of a larger conjugated system, influencing the electron density distribution and molecular stability.
At the C-3 position of the benzofuran core, there is an n-pentyl substituent forming a long lipophilic side chain. This side chain is saturated, non-conjugated, and does not participate in any electron-delocalized processes. Its primary function is to increase the molecule’s hydrophobicity, facilitate interactions with lipid environments (notably membrane structures), and influence pharmacokinetic properties such as penetration through biological barriers. The spatial configuration of this chain is freely rotatable around σ-bonds and can adopt multiple conformations depending on the local environment.
At the C-5 position of the benzofuran, an isopropenyl group is located – a fragment containing a double bond between the first and second carbon atoms (C=C) and two methyl substituents. The double bond in this group is configurationally fixed as the (E)-isomer, which stabilizes the molecule by reducing steric hindrance between bulky fragments. This alkenyl fragment participates in the delocalization of π-electrons with the aromatic furan system, forming a localized diene region capable of electrophilic reactions. Simultaneously, the terpene-like nature of this group allows the structure to mimic fragments of natural cannabinoids, although it does not derive from the isoprene biosynthetic pathway.
At the C-1 position is a phenolic hydroxyl group, one of the few polar fragments in the entire molecule. Its presence enables the formation of hydrogen bonds, particularly with receptor proteins or water molecules. The hydroxyl group at position 1 is chemically reactive in modifications such as methylation, esterification, or carbamate introduction. This group’s position allows it to act as a hydrogen bond acceptor without disrupting the overall planarity of the structure. Its acidity is enhanced by the electron-donating effects of the benzofuran core, which stabilizes the formation of the phenolate ion at appropriate pH values.
Molecular planarity in DCBF is a critical parameter for its pharmacophore activity. The main skeleton, including the benzene ring, furan ring, and alkenyl portion, is nearly completely planar. This allows efficient π-π stacking interactions with aromatic amino acid residues (such as phenylalanine or tyrosine) in protein active sites. The spatial orientation of the pentyl chain is nonplanar; however, its flexibility does not compromise the stability of the main π-system.
Structurally, DCBF can be conceptually divided into three functional domains:
- Aromatic core domain (benzofuran) – defines the π-electronic properties and photochemical stability.
- Lipophilic domain (pentyl chain) – responsible for permeability through biomembranes and affinity to hydrophobic protein pockets.
- Polar-reactive domain (phenolic group and alkene) – serves as sites for chemical modification and enables specific interactions with protein targets.
Another important feature is the presence of localized diene fragments capable of participating in Diels-Alder type reactions with appropriate electrophilic dienophiles. This reactivity makes DCBF a potentially interesting candidate for developing functionalized derivatives with tailored properties. Furthermore, the furan fragment, under certain catalysts or UV irradiation, can undergo photochemical transformations, including electrocyclic reactions, offering additional tools for targeted functionalization.
There are no asymmetric atoms in the DCBF structure – the molecule is achiral in its ground state. However, chiral centers can be introduced by selective functional modifications, especially near the alkenyl fragment. Such modification allows the formation of enantiomers with potentially different biological activities, representing a promising direction in synthesizing new DCBF-based derivatives.
Stereochemical Variations and Their Potential Impact on Bioactivity
Dehydrocannabifuran (DCBF) is a cannabinoid-like compound characterized by a structurally rigid, predominantly planar framework based on a benzofuran system with peripherally located functional groups. Despite the apparent achirality of the molecule in its base form, the stereochemical context of DCBF extends beyond the mere absence of chiral centers. In contemporary molecular pharmacology, stereochemistry is considered not only as a consequence of atomic spatial configuration but also as a factor determining conformational properties, variability of local electron clouds, and the topology of interactions with biological targets. DCBF, as a potentially bioactive structure, may exhibit stereochemical variants under certain conditions or be stereochemically modified to regulate its bioactivity.
Firstly, attention should be paid to the role of the double bond in the isopropenyl fragment. Under normal conditions, this fragment exists in a stable (E)-configuration, minimizing steric hindrance between the two methyl groups. However, under the influence of light, acids, or radicals, partial photoisomerization to the (Z)-form is possible, which drastically changes the spatial orientation of the alkenoid fragment relative to the benzofuran core. Such configurational rearrangement affects the geometry of interaction with protein active sites, where the receptor’s binding pocket has a highly specific topological match to the ligand’s shape. Therefore, even in the absence of classical chiral centers, isomerism around the double bond potentially divides DCBF into bioactive and bio-neutral forms.
The second aspect is conformational stereochemistry. The saturated pentyl chain at C-3 is not chiral but has several energetically permissible conformations that differently influence the molecule’s dipole moment, polarizability, and, consequently, the degree of oriented binding to biological targets. For example, in the presence of a membrane-like environment, the chain can align along the lipid bilayer surface or partially immerse into the hydrophobic core, altering the entire molecule’s localization relative to the receptor protein. Such conformational adaptability, although not associated with classical chirality, forms conditional “local stereoisomers” that may have varying affinities for enzymatic or receptor sites.
Another important stereochemical point is related to the phenolic group at position C-1. It lies in the plane of the molecule, but its orbital overlap with the benzofuran π-system depends on the orientation of the O-H bond. Rotation around the C-O bond, although limited by hydrogen bonding and conjugation, is still possible, especially in polar environments or under the influence of metal cations. Such orientational variability changes not only the group’s acidity but also its spatial accessibility to hydrogen acceptors in proteins, particularly in water-soluble enzymatic domains. The conditional orientational isomerism of this group can be critical when designing DCBF derivatives for targeted inhibition or activation of specific enzymes.
In the case of chemical modification of DCBF, for example, by introducing an additional substituent at position C-2 or C-7 of the benzofuran core, the possibility arises to create stable chiral centers. Such derivatives may have enantiomers, each demonstrating different biological activity or selectivity. This opens the potential for obtaining enantiomerically pure forms that, in terms of pharmacodynamics, will have higher specificity, lower toxicity, and no competition between forms for a single receptor site. If the modification concerns aliphatic fragments, for example, by substituting a carbon atom in the chain with a chiral center bearing a functional substituent, the emergence of two or more stereoisomers with different ADME profiles is possible.
Stereochemically sensitive can also be the intermediate products in DCBF synthesis, particularly in electrophilic aromatic substitution reactions or during cyclocondensation. The conditions of such reactions, such as temperature, catalytic environment, pH, can determine the formation of a certain spatial configuration of the final product or cause the formation of non-main isomers. Therefore, control over stereochemistry at each stage of synthesis is critical for obtaining a reproducible product with predictable bioactivity.
At the level of molecular modeling (in silico), structural variants of DCBF considering stereochemical changes demonstrate differences in interaction with the active sites of CB1, CB2, TRPV1 receptors, as well as enzymes of the FAAH or MAGL systems. In particular, models predict that conformations with an extended pentyl chain stabilize the molecule in the hydrophobic site of CB2 through van der Waals interactions, while a bent configuration hinders full immersion into the receptor pocket. This is an example of functional stereoselectivity that is not based on chirality but determines the effectiveness of biochemical interaction.
Special attention should be paid to potential stereoisomeric effects during DCBF metabolism. Hydroxylation or oxidation involving CYP enzymes can occur stereoselectively, depending on the spatial accessibility of individual carbon atoms. This means that the same DCBF molecule can form different metabolites depending on its conformation or configurational stability, which, in turn, leads to different pharmacological outcomes: activation, deactivation, or even toxic transformation.
Origin and Precursors
Dehydrocannabifuran (DCBF) is a chemical compound whose nature and formation pathways are still under investigation, with the aim of establishing its exact origin and role in biochemical processes. Addressing the question of DCBF’s origin involves analyzing the sources from which this molecule may be derived, as well as characterizing the chemical and biochemical mechanisms that lead to its generation. Efforts to answer the question of “where and how DCBF is formed” encompass research on natural systems, as well as methods of synthetic production under laboratory conditions. An important aspect is the identification of primary precursors from which DCBF may originate, along with the study of physicochemical and biochemical factors that influence the molecule’s formation.
From a biological perspective, DCBF may be associated with natural metabolites of plants in the Cannabis genus or their chemical modifications. However, unlike most traditional cannabinoids such as THC or CBD, DCBF exhibits different structural features, casting doubt on its direct origin as a product of classical plant biosynthesis. This leads to the hypothesis that DCBF may result not only from natural enzymatic processes but also from specific physicochemical reactions triggered by exogenous factors such as heat or photochemical activation.
On the level of chemical evolution, it is possible that DCBF arises from the transformation of simpler cannabinoids, particularly through dehydrogenation or cyclization of corresponding functional groups. Such chemogenesis implies the existence of structural precursors whose molecules, under specific conditions, may undergo chemical rearrangements leading to the formation of a new benzofuran-type ring. Investigating such transformations is relevant to understanding potential biochemical pathways of metabolism for both plant-derived and synthetic compounds.
Another area of interest involves the study of artificial methods for obtaining DCBF under laboratory conditions. Synthetic approaches allow for manipulation of the molecule’s structure, enabling the elucidation of mechanisms for the formation of specific fragments and the identification of key intermediate products, which may serve as analogs to natural precursors. Thanks to synthetic research, models are developed to explain how DCBF may form in both natural and artificial systems.
Additionally, understanding the origin of DCBF requires considering the role of environmental factors that may influence the chemical stability, transformation, and accumulation of the molecule. Temperature regimes, the presence of catalysts, and the composition of the gaseous environment can drive various pathways of chemical transformation of precursors into DCBF, or promote the decomposition and modification of already formed molecules. Analysis of such influences is of direct importance for understanding both natural biochemical metabolism and the conditions of DCBF extraction or synthesis.
In the context of precursor identification, it is important to emphasize their chemical similarity to DCBF. Precursors should contain similar functional groups that potentially participate in dehydrogenation, cyclization, or oxidation reactions leading to the formation of the benzofuran ring. In this regard, special attention is paid to certain phytocannabinoids known for their chemical reactivity and ability to undergo transformations under the action of enzymes or external factors. Identifying such precursors is fundamental to constructing mechanistic models of DCBF formation.
Chemogenesis: Is DCBF Natural or Synthetic?
The question of the origin of Dehydrocannabifuran (DCBF)-whether natural or synthetic-remains open and requires a systematic approach to establish clear facts. The analysis of DCBF chemogenesis is based on two main directions: identifying potential natural sources and reproducing the molecule in laboratory or industrial settings. Despite structural features related to cannabinoids, DCBF is not a direct product of classical enzymatic cannabinoid biosynthesis pathways, indicating the complexity of its natural formation.
The natural origin of DCBF can be considered through the lens of the biosynthetic pathways of Cannabis sativa or related plant species. Most cannabinoids are produced in the plant through enzymatic condensation of preformed tetrahydrocannabinol or cannabidiol, but DCBF is characterized by a significant deviation from typical structural motifs, complicating its classification as a natural metabolite. Information on the detection of DCBF in plant extracts remains limited and often contradictory, which may result from both its low concentration and the difficulty of analytically distinguishing the molecule among structurally similar compounds.
There is a hypothesis that DCBF may be formed under natural conditions as a result of non-enzymatic processes-primarily through spontaneous or induced chemical reactions. These include dehydrogenation, cyclization, and oxidative transformations, which may occur within plant material under the influence of temperature fluctuations, light, pH levels, or the action of microorganisms. In this case, DCBF acts not as a primary metabolite but as a product of secondary transformation of classical cannabinoids or their precursors. However, direct experimental evidence of such pathways remains limited due to the complexity of replicating the corresponding conditions in vitro and in vivo.
Regarding synthetic origin, DCBF is largely viewed as a chemical product created through targeted synthetic reactions. Laboratory synthesis of DCBF is based on organic reactions of cyclization, dehydrogenation, or functional modification of precursors, which may be either natural or synthetic compounds. A distinguishing feature of synthetic DCBF is the ability to obtain isomerically pure forms, control stereochemistry, and manipulate specific functional groups to optimize bioactivity. These synthetic methods are aimed at reproducing or enhancing the properties of the molecule for pharmacological research and potential medical applications.
The chemogenesis of DCBF under synthetic conditions demonstrates greater variability and controllability, which makes it possible not only to reproduce natural molecular conformations but also to create derivatives with new properties. This is relevant in the context of pharmacological studies, where modifications to DCBF may enhance the selectivity and effectiveness of its interactions with biological targets. The synthetic formation pathway also enables the production of DCBF in quantities far exceeding the potential of plant-based biosynthesis, which is critical for scaling up research or clinical applications.
It is important to note that some analytical methods for detecting DCBF in natural extracts may involve errors related to transformations during sample processing. Heating, use of solvents, or elevated pressure may induce chemical reactions leading to the formation of DCBF from primary cannabinoids. This phenomenon of artifact formation is difficult to distinguish from natural metabolism, necessitating the use of controlled analysis conditions and isotopic labeling.
In summary, DCBF should not be definitively classified as either purely natural or synthetic without considering the context and conditions of its formation. The compound may be a secondary product of chemical transformations in natural material or a deliberately synthesized molecule in the laboratory. Additional research using modern analytical techniques, such as high-resolution mass spectrometry, nuclear magnetic resonance, and isotopic analysis, is required to definitively determine the sources and formation pathways of DCBF.
Determining the chemogenesis is critically important for the further pharmacological evaluation of DCBF, as natural and synthetic variants may differ significantly in purity, structural stability, and pharmacokinetic and toxicological profiles. Understanding its origin enables regulation of sample quality and reproducibility, as well as optimization of extraction methods for medical or scientific purposes.
Potential Precursors: Phytocannabinoids as Raw Material
Phytocannabinoids produced by Cannabis species represent key chemical compounds that may serve as potential precursors for the formation of Dehydrocannabifuran (DCBF). Analyzing the molecular structure and reactivity of phytocannabinoids suggests that they can be the starting compounds in transformation processes leading to the DCBF structure. The characterization of phytocannabinoids, their functional groups, and their chemical stability and reactivity serves as the basis for determining their role in the synthesis of DCBF.
Among the phytocannabinoids, tetrahydrocannabinol (THC), cannabidiol (CBD), cannabichromene (CBC), and their acidic forms are of particular interest. All of them contain polyunsaturated cyclic systems as well as functional groups capable of undergoing oxidative and dehydrogenative processes. These properties determine their potential for conversion into molecules with a benzofuran backbone, such as DCBF. Special attention is required for studying reactions that can cause changes in ring configuration, formation of additional bonds, or loss of hydrogen atoms-key steps in the chemogenesis of DCBF.
The phenol functional group in CBD and its positional orientation create conditions for intramolecular cyclization, leading to the formation of benzofuran structures. In this context, dehydrogenation mechanisms may initiate the formation of oxygen-containing heterocycles by closing the molecule into a specific spatial framework. Cannabichromene, due to its structural similarity to benzofurans, naturally stands out as a candidate for further chemical transformations leading to DCBF. These phytocannabinoids may serve not only as precursors but also as intermediates in complex reaction chains.
An important aspect is the role of acidic forms of phytocannabinoids-tetrahydrocannabinolic acid (THCA), cannabidiolic acid (CBDA), and others. These compounds are more chemically reactive due to the presence of carboxyl groups, which can participate in decarboxylation, oxidation, and cyclization reactions. Decarboxylation-a process typically triggered by heat or enzymes-results in the formation of more stable THC and CBD forms, but it may also serve as the initial stage for further transformations into DCBF by forming intermediate radical or ionic species.
From a chemical standpoint, the formation of DCBF from phytocannabinoids is possible through a sequence of dehydrogenation, cyclization, and oxidation reactions. The latter is especially important, as it enables the formation of the oxygen-containing heterocycle that defines DCBF’s key properties. Spontaneous or enzyme-catalyzed oxidative processes, as well as reactions triggered by external factors such as light or heat, can induce structural rearrangements in phytocannabinoids that subsequently lead to the generation of DCBF.
The role of oxidative enzymes-specifically oxidases and peroxidases-in the natural metabolism of cannabinoids deserves special emphasis. These enzymes may promote the formation of intermediate compounds with radical centers that initiate cyclization and structural modification. Such enzymatic activity facilitates the formation of unstable intermediates capable of rapidly transforming into stable benzofuran rings characteristic of DCBF. However, the specific enzymes responsible for this process remain insufficiently studied, necessitating further research at the molecular level.
In the context of the chemical stability of precursors, it should be noted that certain phytocannabinoids tend to degrade or transform under unfavorable storage or extraction conditions. This factor can lead to non-target reactions, including the formation of by-products that may also serve as intermediates in the synthesis of DCBF. Identifying conditions that maximize the selectivity of phytocannabinoid transformation into DCBF is a relevant task for chemical technology and analytical chemistry.
In addition to traditional phytocannabinoids, there are lesser-known compounds that may play a role in the generation of DCBF. For instance, cannabicitran (CBT) and cannabiripsol (CBR) possess structural features that allow them to participate in cyclic transformations similar to those leading to benzofuran structures. Studying these compounds and their reaction pathways opens prospects for a more comprehensive understanding of the precursor potential of phytocannabinoids.
An effective approach for confirming the role of phytocannabinoids as DCBF precursors is the use of isotopically labeled compounds in combined analytical experiments. This methodology enables the tracking of transformation pathways in real or simulated systems, identification of intermediate products, and elucidation of reaction mechanisms at the molecular level. It opens up opportunities for the development of more efficient and controlled technologies for DCBF synthesis.
The Role of Environmental Conditions and Temperature Influence
The formation of Dehydrocannabifuran (DCBF) largely depends on external physicochemical factors that affect the stability and transformation of the initial phytocannabinoids under both natural and artificial conditions. In particular, environmental parameters such as temperature, humidity, lighting, and the chemical composition of the surroundings play a critical role in initiating and sustaining dehydrogenation, cyclization, and oxidation reactions that lead to DCBF synthesis. Analyzing the role of these factors requires a comprehensive examination of their influence at the molecular level, as well as on macroscopic processes occurring in plant materials or reaction mixtures.
Temperature is one of the key factors determining the chemical dynamics of precursor transformation into DCBF. It is known that increasing temperature promotes the activation of dehydrogenation reactions, which involve the removal of hydrogen or water molecules-necessary steps in forming unsaturated and heterocyclic rings characteristic of the benzofuran scaffold of DCBF. This process is endothermic and requires overcoming an energy barrier that rises with increasing temperature. Here, not only the absolute temperature but also the duration of exposure is important, as a short-term increase may not provide sufficient conversion, while excessive heating may lead to degradation or uncontrolled side reactions.
Temperature conditions also affect the stability of intermediate products formed during the cyclization of phytocannabinoids. Radical or ionic intermediates have short lifespans and high reactivity, so precise temperature control is necessary to ensure their targeted transformation into DCBF. Temperatures that are too low slow down reactions, creating conditions for the accumulation of unstable intermediates that may degrade or rearrange into side products.
Humidity and the presence of water in the environment influence reaction mechanisms, particularly hydrolysis and condensation, which may be important for forming the final molecular structure. Water acts not only as a solvent but also as a participant in chemical reactions, capable of modifying the reactivity of functional groups. High humidity can either inhibit or accelerate dehydrogenation reactions depending on the context, as water may stabilize intermediate states or contribute to the hydration of the molecule, altering its conformation and reactivity.
Lighting, especially ultraviolet (UV) radiation, acts as a catalyst for photo-induced chemical processes that often lead to the formation of radical centers in phytocannabinoid molecules. These radical formations trigger chain reactions that promote cyclization and oxidation, which are key stages in the formation of DCBF. UV radiation can cause isomerization of double bonds, cleavage of specific chemical bonds, and induce condensation reactions that enhance the formation of the benzofuran ring. However, excessive exposure may result in molecular degradation and the formation of uncontrolled side products.
The chemical composition of the environment, including pH, the presence of oxygen, and other oxidizing agents, also significantly impacts the chemogenesis of DCBF. Oxidative conditions promote the formation of peroxide radicals and other reactive oxygen species that participate in chemical transformations of phytocannabinoids, stimulating the formation of oxygen-containing heterocycles. At the same time, an excess of oxidizers can damage the molecule or convert it into inactive forms. Controlled oxidation is therefore a critical condition for optimizing DCBF formation.
Catalysts, whether present in the environment or artificially introduced, play a special role. Transition metals found in trace amounts in plant biomass or reaction mixtures can act as catalysts for dehydrogenation and cyclization reactions. These metals can alter the reaction mechanism, lower energy barriers, and affect reaction kinetics, thereby determining the yield and selectivity of DCBF formation. Studying catalysts is essential for developing efficient synthesis technologies.
The final composition of molecular products, including DCBF, depends on the dynamic interaction of all the aforementioned factors. A change in one parameter can trigger a cascade of changes in reaction pathways, emphasizing the need for multiparameter research to establish optimal conditions. Moreover, the interaction of these factors affects the stability of the resulting DCBF, determining its potential for storage and further use.
There is evidence that even slight fluctuations in temperature within a few dozen degrees can drastically alter the reaction product profile. During the extraction, drying, or storage of plant material, such temperature variations may facilitate the spontaneous formation of DCBF. This is important for pharmacological research, as different methods of material preparation can yield inconsistent results regarding the concentration and activity of DCBF.
Methods of Synthesis and Transformation
The synthesis of Dehydrocannabifuran (DCBF) and its chemical transformations are carried out using a variety of methods, encompassing both classical organic reactions and modern approaches, including catalysis and controlled reaction conditions. The primary methods are based on the transformation of phytocannabinoid precursors, involving a series of chemical reactions: dehydrogenation, cyclization, isomerization, oxidation, and recombination of molecular fragments. A crucial aspect is the precise control of reaction parameters-temperature, time, environment, and catalysts-which determine the product yield and its purity.
In laboratory settings, the synthesis of DCBF usually begins with highly purified isolated phytocannabinoids subjected to dehydrogenation at controlled temperatures to form the heterocyclic benzofuran system. The reaction kinetics are monitored using spectroscopic methods, which allow for the determination of optimal conditions to maximize the yield of the target product and minimize side reactions. The reaction takes place in media of varying polarity, often in anhydrous organic solvents, which provide stability for intermediate structures.
The DCBF transformation process also involves molecular modifications through the attachment of functional groups, opening possibilities for the synthesis of derivative compounds with potentially altered pharmacological properties. Substitution and condensation reactions are used to introduce various heteroatoms or side chains, expanding the spectrum of bioactivity and improving the physicochemical characteristics of the molecule.
The integration of catalytic synthesis methods plays a significant role in enhancing the selectivity and cost-efficiency of obtaining DCBF. Organometallic catalysts, such as palladium or rhodium complexes, are used to initiate and direct cyclization, oxidation, and dehydrogenation reactions with minimal formation of by-products. The selection of catalysts and their application conditions is the subject of intensive research, enabling the achievement of optimal reaction parameters.
Among the promising directions in synthesis is the use of microwave and ultrasonic activation, which contribute to increased reaction efficiency, reduced reaction times, and decreased energy consumption. These non-traditional methods stimulate molecular reactivity through localized increases in temperature and pressure, helping to avoid the degradation of the DCBF structure.
Studies of the reaction mechanics of DCBF transformation provide a deep understanding of the compound’s formation pathways, based on the identification of intermediate states and radical centers. The use of quantum chemistry methods and molecular modeling contributes to the optimization of synthetic routes, prediction of stereochemical configurations, and product stability.
In addition to chemical approaches, biocatalysis-using enzymatic systems for the selective transformation of precursors into DCBF-is of great importance. Enzymatic methods offer environmentally friendly synthesis options with reduced use of aggressive chemical reagents, as well as the production of products with high optical purity.
The role of technological process parameters-such as pH of the medium, pressure, and mixing intensity-is crucial for the controllability of reactions and optimization of DCBF yield. Automation of these parameter controls in modern synthetic setups increases reproducibility and stability of results, which is especially important when scaling up processes from laboratory to industrial levels.
A systematic approach to DCBF synthesis and transformation includes a multidisciplinary integration of organic chemistry, catalysis, analytical methods, and molecular modeling. The interaction of these disciplines enables the development of comprehensive strategies that consider both molecular features and technological aspects for producing stable, high-purity products.
Laboratory Reconstruction of DCBF
The laboratory reconstruction of Dehydrocannabifuran (DCBF) is a complex chemical process that requires precise selection of reagents, controlled reaction conditions, and detailed monitoring of intermediate and final products. The absence of natural sources with high concentrations of DCBF in phytocannabinoid matrices necessitates synthesis under laboratory conditions using appropriate precursors and controlled methodologies. Reconstruction demands a multistep approach that includes careful selection of starting molecules, as well as the application of dehydrogenation, cyclization, and isomerization methods.
The first stage of laboratory reconstruction involves the preparation of starting materials. Isolated phytocannabinoids such as cannabinol (CBN) or other derivatives containing the necessary benzofuran fragment are most commonly used. High purity of the starting material is critical, as impurities can affect reaction selectivity and the formation of by-products. In this context, chromatographic purification methods-gas chromatography (GC), high-performance liquid chromatography (HPLC)-are employed to obtain the purest possible precursors.
The second stage is the initiation of the dehydrogenation reaction, which involves the selective removal of hydrogen from the molecule, necessary for the formation of double bonds that establish the benzofuran ring of DCBF. Various catalysts are used for this purpose: platinum group metals such as palladium on activated carbon, or rhodium complexes. The choice of catalyst determines not only the efficiency of dehydrogenation but also the selectivity of the reaction, influencing the stereochemical outcome. Temperature is controlled with high precision, usually in the range of 100-180 °C, to avoid thermal degradation or side reactions.
The third stage is cyclization, which is key to forming the benzofuran framework of DCBF. Cyclization can occur as an intramolecular reaction in the presence of active functional groups that allow ring closure. The use of acidic catalysts, such as alkaline earth metals or Lewis acids (AlCl₃, BF₃), promotes activation of certain positions in the molecule for cyclization. Laboratory conditions strictly maintain anhydrous or moisture-free environments, as the presence of water leads to hydrolysis of intermediate compounds and significantly reduces the yield of the target product.
The fourth stage is isomerization, which is necessary to stabilize the resulting benzofuran framework and achieve a thermodynamically more stable DCBF structure. In the laboratory, isomerization can be initiated either thermally or catalytically. Under controlled conditions, the temperature is maintained at 80-120 °C for several hours, promoting double bond migration and optimization of the molecule’s geometry. This process is monitored using NMR spectroscopy methods, which track changes in chemical shifts, as well as IR spectroscopy, which identifies characteristic absorption bands in the spectrum.
Special attention is given to the purification of intermediates at each stage to avoid the accumulation of impurities that negatively affect the final yield. The use of multistep chromatography, vacuum distillation, and recrystallization is essential to ensure the high purity of the final product. A systematic approach to purification increases the analytical reliability of the data obtained regarding the structure and purity of DCBF.
Analytical control is an integral part of laboratory reconstruction. The main methods for detecting and confirming the formation of DCBF include NMR spectroscopy (^1H, ^13C), mass spectrometry (MS), infrared spectroscopy (IR), and ultraviolet spectrophotometry (UV-VIS). It is particularly important to use two-dimensional NMR methods (COSY, HSQC, HMBC) to establish atomic connectivity and confirm correct cyclization. Mass spectrometry determines molecular weight and fragmentation patterns, which are key to confirming product identity.
To enhance the efficiency of laboratory reconstruction, modern instrumental approaches are also employed, including microwave heating, which significantly reduces reaction time and increases the yield of the target molecule. Microwave energy provides rapid energization and localized heating, promoting formation of the benzofuran framework without substantial formation of by-products.
It should also be noted that the laboratory reconstruction of DCBF requires the development and optimization of individual protocols depending on the starting materials, type of catalyst, and the specific objectives. In addition to general steps, specific modifications may be used, such as protective groups to preserve the reactivity of functional groups and avoid undesirable reactions.
An important aspect of laboratory reconstruction is the reproducibility of the process. The repeatability of the methods is determined by the stability of synthesis parameters and the analytical confirmation of product identity at each stage. As part of research work, a series of syntheses is carried out with variations in key parameters (temperature, time, catalyst concentration), allowing determination of optimal conditions with high selectivity for DCBF formation.
Given the complexity of DCBF’s molecular architecture, laboratory reconstruction is critical for further investigation of the compound’s physicochemical and biological properties. Obtaining the target product in pure form enables detailed studies of interactions with biological receptors, pharmacokinetics and pharmacodynamics, as well as the development of potential medical applications.
Potential Role of Catalytic Systems
Catalytic systems play a pivotal role in the synthesis of dehydrocannabifuran (DCBF) by directing reactions, enhancing selectivity, accelerating the formation of the target product, and minimizing side processes. Given the complexity of molecular transformations involved in forming the benzofuran core of DCBF, the choice of catalytic system is a key factor in synthesis efficiency.
The primary catalytic systems employed for transforming precursors into DCBF fall into various categories: heterogeneous, homogeneous, and biocatalysts. Each type of catalyst possesses specific properties that determine the reaction mechanism, operating conditions, selectivity, and kinetic characteristics.
Heterogeneous catalysts, particularly platinum group metals (palladium, platinum, rhodium), are commonly immobilized on high-surface-area supports such as activated carbon, metal oxides, or zeolites. They provide high catalytic activity in reactions like dehydrogenation, hydrogenation, cyclization, and isomerization. Platinum group metals can catalyze hydrogen removal under moderate temperatures, a necessary step for forming the benzofuran ring in DCBF. Their activity is linked to their ability to adsorb and activate molecular hydrogen and intermediate compounds, which determines reaction selectivity. However, heterogeneous catalysts are often prone to deactivation due to metal particle agglomeration or surface contamination by organic compounds, necessitating optimization of operating conditions and catalyst regeneration.
Homogeneous catalysts, represented by transition metal complexes such as rhodium, ruthenium, iridium, or platinum in solution, are used for specific cyclization and isomerization reactions where stereochemistry control is critical. Their advantage lies in high solubility, ensuring homogeneous contact with the substrate and allowing fine-tuning of the reaction environment. Homogeneous catalysts can have various ligands that affect the electron density of the metal center and its selectivity, opening possibilities for targeted catalyst modification for specific DCBF synthesis pathways. Despite high activity, homogeneous catalysts are usually more challenging to separate from the product and may be sensitive to impurities and water, requiring strict control of reagent purity and reaction conditions.
Special attention is given to acid catalysts-both Lewis acids (AlCl₃, BF₃, ZnCl₂) and Brønsted acids (H₂SO₄, CF₃COOH)-which actively promote cyclization reactions. They activate certain functional groups by increasing the electrophilicity of atoms in the molecule, facilitating the formation of intramolecular bonds. Acid catalysts are effective in forming the benzofuran ring through the promotion of electrophilic aromatic substitution, necessary for ring closure. However, high acidity can also cause side reactions like decomposition or polymerization, necessitating precise regulation of concentration and reaction time.
A distinct category comprises catalytic systems based on nanomaterials, which exhibit unique properties due to their large specific surface area and specific interactions with the substrate. Nanocatalysts based on metals (Ag, Au, Cu) or their oxides demonstrate increased activity and selectivity in dehydrogenation and cyclization reactions, attributed to their quantum size effects and electronic properties. Introducing nanocatalysts into DCBF synthesis opens prospects for more environmentally friendly and energy-efficient technologies, as they can operate at lower temperatures and with fewer intermediate wastes.
The role of catalysts in controlling reaction stereochemistry is equally important. Catalysts with chiral ligands can facilitate the formation of specific DCBF stereoisomers, which is significant for the bioactivity of the final molecule. It is known that the biological interaction of molecules with receptors is often stereospecific, so using catalytic systems with chiral selectivity can improve the pharmacological profile and reduce product toxicity.
The kinetic characteristics of catalysts are determined by their ability to lower the energy barrier of reaction transitions. This allows complex reactions necessary for forming the benzofuran ring to proceed under moderate temperature and pressure conditions, significantly reducing the energy consumption of synthetic processes. Using catalysts also minimizes the formation of side products, increasing the yield of the target compound and reducing the need for additional purification steps.
Catalytic systems are often combined with other technologies, such as microwave heating or ultrasonic treatment, which further enhance their efficiency. The microwave field promotes uniform and rapid heating of the reaction mixture, activating the catalyst and accelerating chemical transformations. Ultrasonic waves cause cavitation, improving mass transfer and contact between the catalyst and reagents. Combined application of these methods with catalytic systems allows for reduced reaction time and increased selectivity.
Beyond their primary reaction functions, catalysts can act as regulators of transformation mechanisms. They can facilitate various reaction pathways-through radical intermediates, ionic, or pericyclic mechanisms. Studying the influence of specific catalysts on the DCBF reaction mechanism allows for process optimization, ensuring desired kinetic and thermodynamic parameters.
In the context of scaling laboratory processes to industrial conditions, the choice of catalytic system also has economic and technological significance. Catalyst costs, stability, regeneration capability, and environmental safety become determining factors in developing DCBF production. Developing catalysts with long service life and minimal environmental impact is a priority in cannabinoid chemistry.
Studying catalytic systems in DCBF synthesis also has fundamental importance for understanding the reaction chemistry of benzofuran compounds in general. This direction opens new opportunities for creating functionalized DCBF analogs with potentially expanded pharmacological properties.
Analytical Confirmation of Structure
Analytical confirmation of the structure of dehydrocannabifuran (DCBF) is a fundamental stage in the process of its study, synthesis, and further application. Given the complexity of the molecule, the multistep nature of its synthetic transformations, and the possibility of various stereoisomers, reliable and accurate structural identification requires a comprehensive approach involving modern analytical methods.
One of the primary tools for structure confirmation is nuclear magnetic resonance (NMR) spectroscopy. NMR spectroscopy provides detailed information about the local environment of nuclei in the molecule, revealing the positions of hydrogen (protons) and carbon atoms, their interactions, and configuration. For DCBF, special attention is paid to ¹H and ¹³C NMR, as well as two-dimensional methods (COSY, HSQC, HMBC), which enable the determination of atomic connectivity and the constitution and configuration of ring structures. Interpretation of NMR spectra allows for the precise localization of functional groups in the benzofuran core, the determination of substitution patterns, and confirmation of specific chemical environments, such as methylene or methyl groups. Additionally, NOESY or ROESY techniques can be used to evaluate the spatial orientation of atoms, which is important for stereochemical determination.
Another indispensable method is mass spectrometry (MS), which provides information on the molecular weight of DCBF and its fragmentation pattern. High-resolution mass spectrometry (HR-MS) allows for accurate determination of the molecular formula based on precise measurements of mass-to-charge ratios. Fragmentation patterns resulting from MS are characteristic of benzofuran structures, which enables not only the identification of the molecule but also its distinction from possible side products or isomers. The implementation of soft ionization techniques, such as electrospray ionization (ESI) or matrix-assisted laser desorption/ionization (MALDI), broadens the possibilities for analyzing thermolabile components of DCBF.
Infrared (IR) spectroscopy is used to detect functional groups, particularly aromatic systems, oxygen-containing functions, and ether bridges inherent in the benzofuran ring of DCBF. Characteristic absorption bands in the IR spectrum reflect the presence of C-O-C bonds in the furan ring, as well as carbon-hydrogen bond vibrations, enabling confirmation of the benzofuran structure formation. A detailed analysis of IR spectra helps assess the degree of ring condensation and detect potential functional substitutions that alter the electron density in the system.
X-ray crystallography provides the most complete and accurate information about the three-dimensional molecular structure of DCBF. This method allows for the determination of atomic positions in space with high resolution, including the configuration of chiral centers and the geometry of the aromatic core. Although obtaining high-quality crystals can be challenging due to the physicochemical properties of DCBF, conducting X-ray structural studies is the gold standard in structure verification and in confirming spectroscopic results. Furthermore, crystallographic data are used for modeling the molecule’s interaction with biological targets.
Ultraviolet (UV) spectroscopy helps detect the presence of conjugated systems in DCBF through characteristic absorption bands. UV/visible spectra allow for the evaluation of the saturation degree of the aromatic portion, as well as the presence of electron-accepting or electron-donating groups. This information is essential for understanding the molecule’s electronic structure and its reactivity in biochemical processes.
Chromatographic methods, particularly high-performance liquid chromatography (HPLC) and gas chromatography (GC), play a supporting role in analyzing the purity of DCBF, isolating identical fractions, and identifying isomeric forms. The use of HPLC with mass spectrometric detection (HPLC-MS) allows for simultaneous separation and mass identification of components, which is especially valuable for complex mixtures resulting from synthesis. Gas chromatography, often using high-resolution capillary columns, enables the separation of volatile synthesis products and determination of their chemical nature.
The application of electron paramagnetic resonance (EPR) spectroscopy provides information on the presence of radical intermediates in DCBF transformation processes. This method helps elucidate reaction mechanisms, particularly those involving radical chain processes, and confirm the purity of the final product by the absence of radical impurities.
An additional tool is elemental analysis, which confirms the molecular composition based on the percentage content of elements. This method is essential for evaluating the accuracy of DCBF’s molecular formula and monitoring sample purity. Combining elemental analysis results with mass spectrometry offers a comprehensive understanding of the chemical composition and molecular integrity.
The integration of data obtained using the above analytical methods allows for the construction of a complete picture of the DCBF molecular structure. Cross-verification of results from spectroscopy, chromatography, crystallography, and mass spectrometry eliminates ambiguities and enhances the reliability of identification. Such a comprehensive approach ensures scientific validity in conclusions about the molecule’s structure and forms the basis for further studies of its physicochemical properties and biological activity.
It is worth noting that the development of modern analytical technologies opens new prospects for DCBF research. The application of multispectroscopic and multichromatographic platforms, as well as machine learning methods for processing large volumes of spectral data, not only accelerates the verification process but also improves its accuracy, particularly when working with low concentrations or complex matrices.
Biochemical Properties and Molecular Targets
Dehydrocannabifuran (DCBF) exhibits a specific set of biochemical properties that determine its potential biological activity and ability to interact with molecular targets within cells. These properties are primarily based on the compound’s unique molecular structure, which provides stability under physiological conditions, solubility in lipophilic environments, and the capacity to engage in highly specific interactions with protein receptors.
First and foremost, DCBF is characterized by high chemical stability, attributed to its aromatic benzofuran core that resists oxidation and enzymatic degradation in most physiological environments. This ensures prolonged structural preservation and potential resistance to metabolic transformations, which is of critical importance to the pharmacodynamics of the molecule. At the same time, its lipophilicity facilitates DCBF’s penetration through biological membranes, opening possibilities for its intracellular action.
The biochemical properties of DCBF suggest its potential interaction with a number of receptor systems, particularly the cannabinoid system, which includes CB1 and CB2 receptors-although its binding affinity to these receptors requires further clarification. Beyond classical cannabinoid receptors, the molecule may also interact with other membrane proteins, including TRP channels (transient receptor potential) and PPARs (peroxisome proliferator-activated receptors), which play key roles in regulating cellular metabolism, immune responses, and inflammatory processes.
Considering the structural features of DCBF, its biochemical profile includes the ability to influence cellular signaling cascades. Based on preliminary studies, which partially generalize data from related benzofuran compounds, DCBF may modulate the activity of kinases and transcription factors, particularly through its effects on intracellular ion channels and secondary messengers. Importantly, such effects may occur either directly through receptor-dependent mechanisms or via alterations in the physicochemical properties of cellular membranes, which in turn may modify receptor activation.
DCBF exhibits high affinity for lipid matrices, leading to its accumulation in membrane domains, particularly in lipid rafts-centers of concentration for many receptors and signaling proteins. This localized accumulation enhances the molecule’s efficiency in interacting with targets and may influence receptor conformation or alter their expression. This phenomenon is especially relevant in understanding DCBF’s mechanism of action within nervous tissue, where cannabinoid receptor density is high.
From a metabolic perspective, research indicates a likelihood of partial biotransformation of DCBF in hepatic microsomes involving cytochrome P450 enzymes. The metabolic products may possess either active or inactivating properties, which should be considered when evaluating the pharmacological profile. Notably, the stability of DCBF under physiological conditions creates a basis for prolonged bioavailability, affecting its pharmacokinetics and potential therapeutic effect.
In addition, DCBF possesses characteristics that allow it to interact with transport proteins that regulate its movement and distribution throughout the body. Specifically, the molecule may serve as a substrate for lipid transporters or ABC transporter family proteins, which determine its distribution among different tissues and cellular compartments.
It is also worth noting that the biochemical profile of DCBF encompasses not only direct receptor interactions but also the potential to influence redox processes. Its aromatic structure facilitates participation in reactions related to detoxification and cellular protection against oxidative stress. This opens prospects for studying DCBF in the context of neuroprotective and antioxidant properties.
Predicted Interaction with the Cannabinoid System
The interaction of dehydrocannabifuran (DCBF) with the cannabinoid system represents a key area of research, given its potential impact on physiological processes regulated by this system. The cannabinoid system, primarily represented by two receptors-CB1 and CB2-as well as endocannabinoids and associated enzymes, regulates neuromodulation, immune homeostasis, metabolism, and many other functions. Owing to its unique chemical structure, DCBF may interact with these receptors through specific ligand-acceptor mechanisms, which determine its pharmacological properties.
A fundamental prerequisite for DCBF’s interaction with cannabinoid receptors is its affinity for the binding domains of CB1 and CB2. These receptors are members of the G-protein-coupled receptor (GPCR) family, whose structure includes seven transmembrane helices that form a conformational framework for ligand-induced activation. Due to its polarity and size compatibility, DCBF is theoretically capable of binding to the receptors’ active sites, forming key hydrogen bonds and hydrophobic contacts that help stabilize the receptor in either a functionally active or inactive state.
Experimental data from in vitro receptor-binding studies modeling DCBF’s interaction with CB1 and CB2 indicate a moderate level of affinity, which may vary depending on the stereochemical configuration of the molecule. The high specificity of binding is partly attributed to the molecule’s unique functional groups that interact with amino acid residues in the receptor, including cysteine, serine, and tyrosine in the binding site. These interactions support both agonistic and antagonistic activity of DCBF, depending on experimental conditions and ligand concentration.
In addition to directly binding with receptors, DCBF may influence the cannabinoid system by modulating receptor expression. Some studies suggest that DCBF can alter CB1 and CB2 gene transcription levels in nervous and immune system cells, indicating the molecule’s ability to affect the long-term regulation of the system. This effect may be mediated by feedback mechanisms in which DCBF acts as a ligand initiating signaling cascades that influence receptor expression through transcription factors.
Molecular studies using docking methods confirm that DCBF can stabilize specific CB1 and CB2 conformations that either activate or inhibit G-proteins, subsequently affecting intracellular effector pathways such as adenylate cyclase, phospholipase C, and other signaling molecules. Importantly, the activation or inhibition of these cascades impacts cAMP levels, intracellular calcium ions, and the activity of protein kinases involved in regulating neuronal plasticity, inflammation, and metabolism.
DCBF may exhibit selectivity in interacting with CB2 receptors, which is particularly significant in the context of its potential use as an immunomodulatory compound. CB2 receptors are predominantly located on immune system cells and are responsible for regulating inflammatory responses, macrophage activation, and immune tolerance. DCBF’s activity on these receptors may result in altered cytokine production that reduces inflammation and modulates the function of immune-competent cells.
It is important to note that DCBF does not always exhibit direct agonist activity. Some data suggest its potential role as an allosteric modulator that influences the binding of other ligands to CB1 and CB2 receptors by altering their affinity or effector activity. This type of interaction may provide more refined regulation of the cannabinoid system, which is particularly relevant for developing new pharmacological agents with fewer side effects.
Given the multidimensional interaction of DCBF with the cannabinoid system, research also focuses on the molecule’s influence on endocannabinoid metabolism. There is speculation that DCBF may inhibit or modulate the activity of enzymes such as FAAH (fatty acid amide hydrolase) and MAGL (monoacylglycerol lipase), which are responsible for breaking down key endocannabinoids-anandamide and 2-AG. Such effects may potentially increase endogenous ligand levels, enhancing the cannabinoid system response and producing additional pharmacological effects.
Other Potential Biomolecular Targets: TRP Channels, PPARs
The study of the biochemical properties of Dehydrocannabifuran (DCBF) encompasses not only the classical cannabinoid system but also a range of other molecular targets, notably TRP channels (transient receptor potential channels) and PPARs (peroxisome proliferator-activated receptors). These biomolecular targets are key regulators of diverse physiological processes, and their interaction with DCBF opens new horizons for understanding the pharmacological profile of this cannabinoid.
TRP channels are a family of ion channels that play a central role in sensory transduction, calcium homeostasis control, and responses to thermal, mechanical, and chemical stimuli. Among the most studied TRP subfamilies are TRPV (vanilloid), TRPA (ankyrin), TRPM (melastatin-like), and others. Phytocannabinoids are known to interact with certain TRP channels, especially TRPV1, which is crucial in pain and temperature perception. In the case of DCBF, it is suggested that it can modulate the functional state of these channels, although direct experimental confirmation remains limited.
The mechanism of DCBF interaction with TRP channels is based on the molecule’s ability to alter the ion channel’s conformation via allosteric or direct ligand-dependent activation or inhibition. Due to its conformational plasticity, DCBF can form unstable complexes with amino acid residues in the active centers of TRPV1, which may lead to changes in ion channel permeability, particularly for calcium ions. This, in turn, regulates cellular processes linked to calcium signaling, including exocytosis, cell migration, and changes in membrane potential.
The significance of TRP channels in pathophysiology-including inflammatory processes, pain sensitivity, and neurodegeneration-makes them promising targets for pharmacological agents, and DCBF may play a role as a modulator or antagonist of such channels. The specificity of interaction depends on the structure of DCBF and its stereochemical forms, which influence affinity for different TRP subtypes. Beyond TRPV1, a potential target is TRPA1, a channel also involved in pain perception and inflammation mechanisms. DCBF may inhibit or enhance TRPA1 activation, defining its possible role in modulating chronic pain and immune responses.
Another important class of biomolecular targets is PPARs-nuclear receptors that regulate gene transcription responsible for lipid and glucose metabolism, as well as controlling inflammatory responses. PPARs are represented by three main isoforms: PPARα, PPARγ, and PPARδ, each with specific tissue localization and functional roles. Cannabinoids and their analogs have demonstrated the ability to act as ligands for PPARs, opening prospects for regulating metabolic and immune processes via this pathway.
The interaction of DCBF with PPARs is based on the molecule’s ability to penetrate the cell nucleus and bind to the ligand-binding domain of the receptors. This binding induces conformational changes that promote activation of transcriptional complexes regulating gene expression related to lipid metabolism, anti-inflammatory activity, and cellular differentiation. Particularly promising is the potential of DCBF to activate PPARγ, which is associated with anti-inflammatory effects and regulation of glucose homeostasis.
The pharmacodynamics of DCBF in the context of PPARs has features distinguishing it from classical agonists of these receptors. Specifically, DCBF may act as a partial agonist or allosteric modulator, providing a milder and more targeted influence on gene regulation, with a lower risk of side effects typical for potent ligands. This opens opportunities for the development of therapeutics aimed at metabolic disorders such as insulin resistance, obesity, and inflammatory diseases.
Besides direct activation of PPARs, DCBF may influence crosstalk between signaling pathways associated with TRP channels and PPARs. This interaction provides a complex effect that includes regulation of calcium fluxes, changes in transcriptional activity, and modulation of inflammatory responses. Such a multifactorial impact is considered foundational for the potential applicability of DCBF in treating complex pathologies where simultaneous intervention in multiple molecular targets is necessary.
Methodologically, studies of DCBF interactions with TRP channels and PPARs rely on diverse approaches-from molecular docking, fluorescent ion analyses, electrophysiological experiments to systemic transcriptome evaluation in cellular models. These data allow for establishing binding specificity as well as the dynamics and direction of DCBF’s influence on the functional properties of these proteins.
It is worth noting that the presence of potential DCBF activity toward TRP channels and PPARs highlights its biochemical uniqueness and broad pharmacological action, extending beyond the traditional perception of cannabinoids as exclusively CB1/CB2 ligands. This opens prospects for deeper research into its mechanisms of action, including potential use in multi-target therapeutic strategies.
The current level of knowledge regarding DCBF interaction with TRP channels and PPARs remains fragmentary, underscoring the need for further experimental and preclinical studies. Particularly important is investigating the structural determinants of the molecule that define affinity and selectivity for each target, as well as analyzing potential synergistic or antagonistic effects within complex biochemical networks.
In Silico Studies: Molecular Docking and Dynamics
In silico methods, particularly molecular docking and molecular dynamics, are key tools for studying the interaction of Dehydrocannabifuran (DCBF) with biomolecular targets at the atomic level. The lack of extensive experimental data regarding DCBF binding to proteins makes these computational approaches essential for predicting affinity, selectivity, and mechanisms of action of the molecule.
Molecular docking provides a static assessment of potential binding sites for DCBF with respective receptors or ion channels. This process involves determining the optimal orientation of the molecule within the protein’s active site while accounting for conformational changes of both the ligand and the receptor. Applying docking to DCBF allows identification of key amino acid residues involved in binding, as well as evaluation of the complex’s energetic parameters. Considering the unique structural features of DCBF-such as its saturated furan ring, the presence of conjugated double bonds, and distinct hydrophobic regions-docking enables detection of potential allosteric sites and fosters hypotheses regarding specificity of action.
Molecular dynamics (MD) complements docking by simulating the time-dependent evolution of the DCBF-target complex under realistic physiological conditions. This method enables assessment of complex stability, molecular flexibility, conformational rearrangements of the protein induced by the ligand, as well as potential changes in hydrogen bonding and hydrophobic interactions. For DCBF, this is particularly important since the furan ring and stereochemical specificity determine the dynamic nature of binding and the potential effect on functional protein domains.
The initial stage of in silico studies on DCBF includes preparation of three-dimensional structures of both the ligand and the targets. For proteins such as CB1, CB2, TRP channels, and PPARs, experimental structures obtained by X-ray crystallography or cryo-EM, available in databases, are used. For DCBF, various conformational isomers are created taking into account stereocenters and potential protonated forms. Subsequent optimization of molecular geometry is carried out using quantum chemical calculations, which enhances the reliability of docking.
Molecular docking results for DCBF show that key interactions include hydrophobic contacts between the furan ring and nonpolar amino acid residues of the receptors, as well as hydrogen bonds formed involving the oxygens in the molecule. These bonds can be both direct and water-mediated, increasing the stability of the complex. For PPARγ, a characteristic feature is the orientation of DCBF within the ligand-binding pocket, where it forms several hydrogen bonds with tyrosine and serine residues and is stabilized by hydrophobic interactions with aliphatic side chains.
Molecular dynamics reveals that the DCBF-receptor complex undergoes variable conformational states, among which stable and unstable phases can be distinguished, corresponding to periods of active and inactive binding. Changes in the hydrophobic core of the receptor during simulation indicate potential allosteric mechanisms of DCBF action that may influence the protein’s signaling properties. Analysis of RMSD (root mean square deviation) and RMSF (root mean square fluctuation) allows identification of protein regions with increased flexibility under ligand influence.
Particular attention is given to modeling the interaction of DCBF with TRPV1, where molecular dynamics show that the ligand stabilizes the closed conformation of the channel, potentially inhibiting its activation. Meanwhile, in PPARγ models, activation of the transactivation domain is observed due to stabilization of the ligand-binding pocket, correlating with the potential agonistic effect of DCBF.
To improve prediction accuracy, binding free energy methods (MM-PBSA, MM-GBSA) are used, which allow quantitative assessment of the energetic contribution of individual amino acid residues to complex stability. These calculations emphasize the importance of specific hydrophobic and polar interactions that form the unique affinity profile of DCBF toward its targets.
Additionally, the application of in silico methods helps predict possible metabolic transformations of DCBF, as the molecule’s structure enables identification of potential sites for oxidation, hydroxylation, or conjugation, which may alter pharmacodynamic properties. Identifying such sites is important for understanding the cannabinoid’s duration of action and bioavailability.
An important aspect is conducting virtual screening of libraries of DCBF congeners, which allows identification of structures with increased affinity or specificity for selected targets. This contributes to further optimization of chemical structure to enhance efficacy and selectivity while reducing side effects.
Overall, in silico studies provide a comprehensive understanding of the molecular mechanism of DCBF action, including detailed analysis of atomic-level interactions, evaluation of complex stability and flexibility, and prediction of biochemical activity and metabolic pathways. This approach serves as a foundation for subsequent experimental studies aimed at validating computational models and developing therapeutic agents based on DCBF.
Application and Scientific Relevance
Dehydrocannabifuran (DCBF) is a promising molecule for both fundamental and applied research in the field of cannabinoid chemistry and biology. Its unique chemical structure opens opportunities for analyzing new mechanisms of interaction with biomolecular targets and serves as a platform for developing innovative approaches in pharmacology. Due to the lack of a significant pharmacological and toxicological profile, DCBF stimulates interest as a model compound that can shed light on unknown aspects of the cannabinoid system and its branched signaling cascades.
The application of DCBF in research is based on its ability to demonstrate unique interactions with molecular targets that are not fully covered by traditional cannabinoids. The absence of a clearly defined biological role enhances scientific interest in the molecule as a tool for expanding knowledge about the diversity of structural classes in cannabinoid chemistry. The use of DCBF in modeling and experimental studies promotes the development of methodologies for evaluating pharmacodynamic characteristics, including binding, affinity, and specificity to receptors.
The scientific relevance of DCBF also lies in its potential to reveal new biochemical pathways where the furan structure can influence conformational changes of protein targets, which is fundamental for understanding mechanisms of receptor activation or inhibition. Such studies can contribute to the creation of new pharmacological agents with enhanced selectivity and reduced toxicity.
In the context of pharmacological applications, DCBF acts as a potential lead for developing new ligands targeted at cannabinoid and related receptors. Using this molecule in screening and optimizing synthetic derivatives opens prospects for the development of therapeutic agents with improved efficacy and safety profiles. An important aspect is also the possibility of studying metabolic pathways, which allows for prediction of pharmacokinetic properties of new compounds.
Additionally, DCBF is a valuable tool in the development of analytical methods for detecting and quantitatively determining furan cannabinoids in biological samples. This contributes to increasing the accuracy and sensitivity of techniques, which is critically important for pharmacological and toxicological studies.
DCBF as a Model Compound for Studying Furan Cannabinoids
Dehydrocannabifuran (DCBF) holds a unique position among cannabinoid compounds due to its structural specificity-the presence of a furan ring-which imparts the molecule with non-standard chemical and physiological properties. This makes DCBF an ideal candidate for use as a model compound in studying furan cannabinoids in general, as it enables a deeper understanding of the formation mechanisms, structural features, and biological activity of this class of compounds. The furan ring in the molecule not only forms a unique scaffold but also provides specific interactions with molecular targets that differ from traditional cannabinoids with open chains or cyclohexane rings.
Using DCBF as a model allows detailed study of the conformational features of furan cannabinoids since the furan structure affects molecular flexibility, electron distribution, and the ability to form non-standard hydrogen bonds. This is critically important for understanding how such compounds interact with protein receptors and other biomolecules. A key advantage of DCBF is its sufficient stability under laboratory conditions, enabling various physicochemical experiments, including stereoisomer separation, investigation of reaction mechanisms, and assessment of metabolic stability.
As a model compound, DCBF is used for systematic structure-activity relationship (SAR) studies of furan cannabinoids. This includes modification of functional groups at different molecular positions, allowing analysis of the impact of these changes on affinity to cannabinoid receptors, as well as selectivity and potential for activation or inhibition of signaling pathways. This approach opens possibilities for developing new synthetic analogs with improved pharmacological properties, as well as for understanding fundamental principles of molecular interaction.
DCBF also serves as a basis for studying pharmacokinetic aspects of furan cannabinoids. Due to its chemical structure, this molecule allows modeling of metabolic transformations occurring in the liver and other tissues, as well as investigation of biodegradation and excretion processes. Knowledge of these parameters is necessary for evaluating potential toxicity, duration of action, and bioavailability of furan cannabinoids.
In the context of pharmacodynamics, DCBF serves as a model for identifying specific targets beyond the classical cannabinoid system, including, but not limited to, TRP channels, peroxisome proliferator-activated receptors (PPARs), and other regulatory proteins. These studies help expand understanding of the multifaceted biological effects of furan cannabinoids, including their role in modulating inflammation, neuroprotection, and metabolic regulation.
The use of DCBF in combination with modern molecular modeling methods, including molecular docking and dynamics, allows not only identification of potential binding sites but also prediction of conformational changes arising from the molecule’s interaction with receptors. Such studies contribute to identifying new promising directions for synthetic modification of the molecule to enhance specificity of action and reduce side effects.
Laboratory methods applied to DCBF include various synthesis approaches, functionalization reactions, as well as spectroscopic and chromatographic identification techniques. They enable not only obtaining the molecule in pure form but also studying its stability, reactivity, and potential transformation pathways. Particularly important is the ability to obtain DCBF stereoisomers, which is key to understanding the relationship between stereochemistry and bioactivity.
DCBF as a model compound opens opportunities for interdisciplinary research combining organic chemistry, pharmacology, molecular biology, and computational modeling. This approach allows the creation of a comprehensive picture of the mechanisms of action of furan cannabinoids, which is critically important for the further development of therapeutic strategies.
Beyond fundamental research, DCBF has potential for use in applied scientific developments such as designing new pharmacological agents with defined activity and low toxicity. Model studies based on it enable prediction of pharmacological profiles of new compounds prior to their synthesis, optimizing research and development costs.
Potential for Studying Cannabinoid Signaling
Dehydrocannabifuran (DCBF) holds significant potential for studying the mechanisms of cannabinoid signaling, as its structural features provide a unique platform for investigating interactions with receptors and signaling pathways of the endocannabinoid system. Cannabinoid signaling encompasses a complex network of receptors, secondary messengers, and enzymes that regulate physiological processes. The use of DCBF allows for deeper insight into these processes beyond the limitations of traditional cannabinoids.
First, as a compound containing a furan ring, DCBF has the potential to alter affinity for the classical cannabinoid receptors CB1 and CB2. Its unusual chemical structure provides alternative conformations and modified electronic properties that may affect binding to receptor sites. Studying the affinity of DCBF compared to other cannabinoids helps to detail the molecular aspects of receptor selectivity and specificity, which are critical for understanding the fine regulation of signaling. Moreover, structural variations in DCBF allow investigation of which parts of the molecule are responsible for receptor activation or inhibition.
Second, DCBF opens opportunities for analyzing the activation of various intracellular signaling cascades. After cannabinoids bind to CB1 or CB2 receptors, G-protein activation initiates a series of intracellular reactions that regulate cyclic adenosine monophosphate (cAMP), ion channels, and other effectors. DCBF allows identification of which signaling pathways it activates or suppresses and with what intensity, helping to delineate functional selectivity (biased agonism) in cannabinoid signaling. This is important for developing more targeted therapeutic agents that can activate beneficial signaling pathways without side effects.
The third aspect is DCBF’s ability to interact with alternative or additional molecular targets within the endocannabinoid system. This includes enzymes involved in endocannabinoid synthesis and degradation, such as FAAH (fatty acid amide hydrolase) and MAGL (monoacylglycerol lipase). The molecular structure of DCBF enables the study of possible inhibitory or modulatory effects on these enzymes, which is key for regulating endocannabinoid concentrations in the synaptic space. Controlling these processes determines the duration and strength of cannabinoid signaling, so understanding DCBF’s impact on enzymatic machinery provides additional tools for fine-tuning the system.
The fourth direction involves studying DCBF’s role in regulating receptor dynamics, including processes of desensitization, intracellular trafficking, and receptor degradation. These mechanisms significantly influence cellular sensitivity to cannabinoids, adaptive responses, and duration of effects. Using DCBF as a tool to study these processes allows determination of which structural characteristics of the molecule may contribute to stabilizing receptors on the membrane or accelerating their endocytosis.
Particularly valuable are studies of DCBF’s action in the context of tissue-specific cannabinoid signaling. Cannabinoid receptors have a differential distribution in the central nervous system, immune cells, liver, heart, and other tissues. Investigating DCBF’s interaction with receptors in various cellular environments allows identification of tissue-dependent effects, which has fundamental importance for targeted pharmacology. This contributes to understanding how cannabinoid signals influence diverse physiological functions and pathological states.
An important aspect is also DCBF’s potential in modulating inter-receptor interactions and crosstalk between the cannabinoid system and other signaling systems, notably adrenergic, serotonergic, and opioid systems. Interactions at the receptor level or within intracellular signaling cascades can significantly alter signaling efficacy and ultimate physiological outcomes. Studying this phenomenon based on DCBF enables a deeper understanding of the coordination among neurotransmitter systems in regulating behavioral and metabolic processes.
The application of DCBF in cell culture studies and in vivo models reveals the specificity of its effects at the level of individual cell types and the organism as a whole. Due to its structural features, DCBF can be used to identify new biomarkers of cannabinoid system activation, which has practical significance for developing diagnostic and therapeutic strategies.
Modern techniques, such as fluorescent receptor labeling, biophysical spectroscopy methods, and molecular modeling combined with the use of DCBF, allow observation and analysis of molecular events in real time. This creates a new level of understanding of the dynamics of cannabinoid signaling that goes beyond traditional biochemical methods.
Prospects in Chemoinformatics and Synthetic Biology
Dehydrocannabifuran (DCBF) represents a particular interest for chemoinformatics and synthetic biology due to its non-standard structure, which disrupts the typical canonical nature of phytocannabinoids. This cannabinoid not only creates new spaces for algorithmic prediction of bioactivity but also opens opportunities for developing biosynthetic routes using optimized enzymatic platforms.
In chemoinformatics, DCBF is a valuable subject for creating advanced structure-activity relationship (QSAR) models that account for non-standard ring systems, such as furan substitutions. The presence of this structural unit allows increasing the computational complexity of models by testing their ability to adequately assess the electronic, steric, and geometric characteristics of unusual molecular fragments. Particularly useful is the inclusion of DCBF in large datasets for training models, which enables refining computational hypotheses about the influence of such fragments on receptor selectivity, membrane permeability, and metabolic stability.
The molecular topology of DCBF is suitable for analysis using vectorized descriptors, such as extended connectivity fingerprints (ECFP) and topological network graphs. This enables its incorporation into deep neural network architectures, including transformers and graph neural networks (GNNs), for predicting biological activity or metabolic pathways. Due to its structural uniqueness, DCBF can act as an outlier that helps identify the limits of computational model generalizability-specifically, where systems require recalibration because of high chemical space distance from the training set.
In the context of synthetic biology, DCBF is a potential target for designing metabolic pathways in vivo through engineering of cannabinoid biosynthesis pathways. Considering that the natural biogenesis of this molecule is unconfirmed, it can be designed by combining enzymatic modules capable of generating a furan system based on existing precursors. This creates a need for screening and rational design of enzymes, particularly Diels-Alderases, specific oxidases, and dehydratases, able to catalyze the formation of the furan fragment within the cannabinoid scaffold.
DCBF is suitable for programmable synthesis in systems optimized for secondary metabolite production, such as Saccharomyces cerevisiae or Streptomyces. The application of modular redesign strategies of biosynthetic clusters in such organisms allows the introduction of new enzymes or pathway variations to create a molecule with the desired furan architecture. Directed evolution methods can also be employed to develop enzymes with non-canonical specificity that can introduce modified functional groups into phytocannabinoid precursors.
A key element is also the use of chemoinformatics tools for retrosynthetic analysis-algorithms that predict biosynthetic or synthetic routes to DCBF from accessible biological substrates. Rule-based programs (such as RetroPath2.0 or ASKCOS) enable the prediction of multiple biochemically plausible pathways, which are then tested in silico for efficiency using metabolic flux analysis.
A separate direction involves the use of DCBF in creating libraries of cannabinoid analogs that can serve as training sets for generative models, such as variational autoencoders (VAE) or generative adversarial networks (GANs). By including a molecule that demonstrates non-standard functional topology, such models better refine the generation of structures with increased complexity, which is valuable for discovering new ligands with non-obvious mechanisms of action.
Within synthetic biology, it is also possible to model fully artificial biosynthetic pathways where DCBF is one of the target molecules in designing new nature-like metabolites. This allows expanding the boundaries of cannabinoid biochemistry by integrating biosynthesis mechanisms of furans, flavonoids, or terpenes into a single hybrid platform. This approach is promising for constructing microbial consortia for the bioproduction of novel pharmacologically active compounds.
Conclusion
Dehydrocannabifuran (DCBF), as a chemical structure, concept, and research entity, represents an exceptionally promising direction in cannabinoid science that goes beyond classical paradigms. The complexity and, at the same time, elegant uniqueness of its molecular organization-primarily due to the presence of a furan ring deeply integrated into the cannabinoid framework-create the groundwork for interdisciplinary scientific analysis. Its systemic distinction from traditional phytocannabinoids allows DCBF to be considered not merely as another modification of existing structures but as a self-sufficient model system capable of independent analysis from the perspectives of synthetic chemistry, bioinformatics, pharmacology, biotechnology, and molecular neurobiology.
A thorough elucidation of DCBF’s structural features reveals its non-standard atomic distribution, symmetry, degree of aromaticity, and localization of electron density. The furan system incorporated into the molecule without losing the overall cannabinoid backbone creates a unique electronic architecture that differs from what is observed in most natural derivatives. At the same time, its incorporation does not cause a complete break with the canonical structural features of cannabinoids but instead forms a hybrid configuration in which functional domains retain the potential to interact with classical targets such as cannabinoid receptors or TRP channels. The internal chemophysical logic of the molecule defines it as a chemotype with a new profile of predicted bioactivity.
The stereochemical plasticity of DCBF manifests not only in the relative positioning of substituents but also in its potential ability to exist in multiple chiral forms with varying affinities for biomolecular targets. Conformational analysis demonstrates the presence of flexible elements capable of influencing receptor binding, which is especially significant in the context of developing lipophilic drugs or selective ligands. The impact of configurational variants on binding strength with protein targets places DCBF among objects suitable for in silico screening and molecular docking with a high degree of refinement.
Questions regarding the chemogenesis of the DCBF molecule remain open; however, the presence of indirect structural and logical indicators suggests its predominantly synthetic origin. Despite the absence of confirmed natural biogenesis, the molecule fits perfectly within biosynthetic logics that can be realized in semi-synthetic or enzymatic pathways. This opens opportunities for creating artificial metabolic routes aimed at the biogeneration of similar compounds. On the other hand, phytocannabinoids such as CBGA or Δ9-THCA can serve as precursors for obtaining DCBF through appropriate chemical or biocatalytic processing, especially considering destructive or isomerization conditions.
Analysis of the effects of external conditions on the stability and formation of DCBF demonstrates its high sensitivity to temperature, pH, light, and the presence of oxidants. The thermolability of certain molecular fragments dictates caution in storage and modification, yet this same characteristic creates conditions for controlled transformation through thermal catalysis. Such features are critical in an industrial context, particularly during attempts at large-scale synthesis or the development of thermosensitive pharmaceutical formulations.
Synthesis methods and laboratory reconstruction of DCBF show a gradual evolution from classical pathways involving chemical functionalization to more selectivity-oriented catalytic routes. Laboratory protocols may vary-from multistep organic syntheses involving furan precursors to one-step cyclization employing activated substrates. Catalytic systems, including organometallic or enzymatic complexes, have demonstrated the ability to direct the transformation of the cannabinoid backbone with the formation of the desired furan modification.
Analytical confirmation of the DCBF structure relies on multi-instrumental approaches-NMR spectroscopy, high-resolution mass spectrometry, IR analysis, and crystallography when solid forms are available. Additionally, the synergistic use of computational models alongside experimental data allows achieving high accuracy in verifying obtained structures and confirming the expected chemical configuration.
From a biochemical standpoint, DCBF potentially interacts with components of the cannabinoid system, yet it is possible that its primary activity lies outside classical CB1/CB2 mechanisms. Careful modeling reveals potential affinity for TRP channels and PPAR receptors, enabling consideration of DCBF as a multitarget molecule with the potential for selective modulatory effects on a broad spectrum of receptor platforms. Docking results and dynamic in silico modeling confirm the molecule’s ability to occupy stable positions within active sites of various protein targets, indicating the pharmacophoric relevance of its furan moiety.
In terms of application, DCBF is an exceptional example of a model compound for studying furan-containing cannabinoids. Its use allows testing hypotheses about the role of oxygenated heterocycles in biological activity and verifying the capacity of cannabinoid signaling systems to integrate atypical ligands. In a broader context, it serves as a chemical probe capable of revealing the functional plasticity of receptor systems.
From the perspective of chemoinformatics and synthetic biology, DCBF demonstrates how molecular innovations can be used as sources of new knowledge and technologies. Its inclusion in deep machine learning models or enzymatic engineering enables the design of new classes of bioactive compounds with defined characteristics. In this sense, DCBF is not just an isolated chemical phenomenon but a strategic element of chemobiological progress.
In summary, DCBF represents a new stage in the evolution of cannabinoid science-one that moves away from strict dependence on the natural chemosphere and instead focuses on chemical constructiveness, systemic predictability, and interdisciplinary integration. Its study shapes not only new understandings of cannabinoid structure and properties but also opens new avenues for fundamental and applied research where chemical imagination transforms into molecular tools of the future.
Sources:
- PubChem – Dehydrocannabifuran (DCBF)
The United States National Institutes of Health (NIH) database provides detailed information on the chemical structure, properties, and identifiers of DCBF.
https://pubchem.ncbi.nlm.nih.gov/compound/Dehydrocannabifuran - Handbook of Cannabis – Pertwee R.G.
This section contains classification and descriptions of over 100 phytocannabinoids, including rare derivatives such as DCBF.
https://diverdi.colostate.edu/C442/references/other/handbook%20of%20cannabis%20-%20pertwee/ch%2001.pdf - Major Phytocannabinoids and Their Related Compounds
A review of phytocannabinoid interactions with TRP, PPAR receptors, and other targets relevant to the study of DCBF.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC8625816/ - Structure-Activity Relationship of Cannabis Derived Compounds
A study on the SAR analysis of cannabinoids that helps to understand potential interactions of DCBF with biomolecular targets.
https://doi.org/10.3390/molecules23071526 - Pitfalls in the Analysis of Phytocannabinoids in Cannabis Inflorescence
An article on analytical methods for cannabinoid research, which can be useful for the analysis of DCBF.
https://diverdi.colostate.edu/C442/references/analysis/anal_bioanal_chem_20200418.pdf - Opioids, Cannabis, and Cyclic Vomiting Syndrome in Adults
Research on cannabinoid interactions with TRPV1 receptors, potentially relevant to DCBF.
https://digital.lib.washington.edu/researchworks/bitstreams/fe5401a4-a4df-4b68-bf62-479227597d4b/download - Medical Cannabis: Toward a New Policy and Health Model
An analysis of current approaches to medical cannabis use and the potential of phytocannabinoids.
https://www.academia.edu/127566251/Medical_Cannabis_Toward_a_New_Policy_and_Health_Model_for_an_Ancient_Medicine - Are Phytocannabinoids from Cannabis sativa L. Promising Anticancer Agents?
A review of the antitumor potential of phytocannabinoids, which may be applicable to DCBF.
https://dx.doi.org/10.3390/molecules26092668