Delta-8-tetrahydrocannabinol is not just a variant of an already known molecule. It is an artifact of the chemical plasticity of cannabinoids and simultaneously an example of how a slight structural deviation can lead to a completely different pharmacological profile, a different regulatory trajectory, and ultimately a different discourse surrounding the substance. The standard lineup of psychoactive compounds derived from cannabis has long needed reconsideration-not only due to the emergence of new semi-synthetic molecules but because the cannabinoid system-biochemically, regulatorily, commercially-cannot be reduced to just one or two key molecules. Delta-8 is exactly a symptom of this complexity.
The existence of this compound does not fit within the unified paradigm of “natural origin,” nor does it align with the canonical scheme of psychoactivity. Its appearance was not the result of spontaneous biosynthesis in significant concentrations, nor did it come about from selective breeding or agricultural modifications. Its growing presence is the result of chemical recoding of existing cannabinoids, particularly cannabidiol, under specific laboratory conditions. This is a transformation that happens not in the plant but in the reaction flask. In other words, we are not dealing with a cannabinoid in the classical sense, but rather the result of technological intervention within the cannabinoid class.
The fact that Delta-8-THC exists in the modern pharmacochemical landscape is evidence that classificatory boundaries in the field of natural compounds no longer function autonomously. There is no clarity-neither in biogenesis, nor in mechanisms of action, nor in legal status. Delta-8 does not have a stable position in chemical taxonomy: it is called either an isomer, a metabolic derivative, or a byproduct. It is a molecule that behaves like a conceptual trap: it’s convenient to call it safer, but it is difficult to pinpoint exactly what in its pharmacology makes it so. It is at once closer to a laboratory construct than a phytocannabinoid-and yet it is not quite a classic synthetic. It cannot be stripped of its plant origin, but it cannot honestly be called “natural.”
Particular interest lies in the way this compound entered the pharmacological field. Instead of a direct path-from phytocomponent to clinical trials-it followed a curved trajectory, where its first widespread use occurred not as a result of an evidence base but through a legal vacuum and chemical convenience. It emerged at a moment when the market was seeking a “legal alternative,” and chemists offered a technology for the rapid conversion of CBD into a psychoactive form. This is the unique situation: a substance affecting the central nervous system began circulating in the consumer space before its pharmacokinetic profiles or toxicological safety data appeared.
In other words, this is a molecule that formally belongs to the cannabinoid class but in fact is a heterogeneous phenomenon: simultaneously a chemical substance, a legal anomaly, and an object of risky use. All of this requires a more complex perspective than what is usually applied to substances of natural origin. It is not a pharmaceutical drug, but it cannot be reduced to the status of “legal doping.” It does not have a stable concentration in market products but does produce objective pharmacological effects. That is why considering it “just another isomer” is methodologically incorrect.
The questions arising in connection with Delta-8-THC are interdisciplinary. They are not only questions of chemistry, although synthesis plays a key role here. They also involve neurobiology-since mechanisms of action on CB₁ receptors only partially overlap with Δ⁹-THC. These are questions of clinical pharmacology-because the subjective effects change not only in intensity but also in quality. And finally, it is an ethical and epistemological question: how is knowledge about substances formed in the modern world when experiential use precedes laboratory verification?
Equally important is the question of source. Unlike classic cannabinoids, which are extracted from the plant as extracts, Delta-8 is almost always the result of isomerization-a chemical reaction converting CBD into a new structure under the influence of acid and heat. Accordingly, whenever Delta-8 is discussed, it implies not natural presence but an engineered realization of the molecule’s potential, which occurs only in trace amounts in nature. Therefore, the responsibility for its safety lies not with nature but with technology.
Chemical and Physicochemical Characteristics of Delta-8-THC
Structural Formula and Isomerism of Delta-8-THC
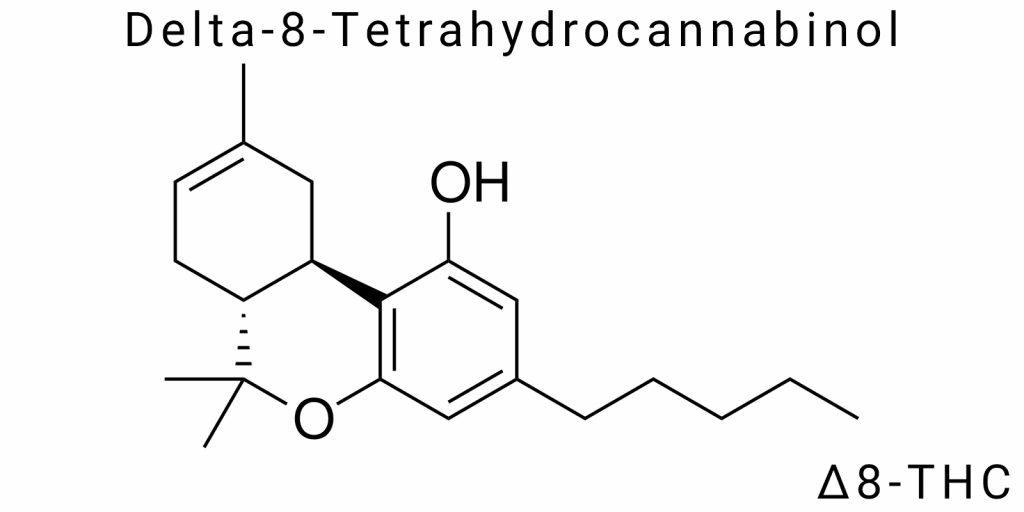
The delta-8-tetrahydrocannabinol molecule is distinguished by a unique structure that combines a tricyclic carbon framework with several chemically active centers. Formally, it can be described as a derivative of the benzopyran structure with a pentyl side chain, which significantly influences its lipid solubility and, consequently, its pharmacokinetics. A key feature of this molecule is the presence of a double bond located between the eighth and ninth carbon atoms in the central cyclohexene ring. This particular position of the double bond defines its classification as a delta-8 isomer and differentiates it from delta-9-THC, where the same bond is shifted one carbon forward-between the ninth and tenth carbons.
Despite the minimal change in the position of the double bond, the overall electronic configuration of the molecule changes enough to modify its physicochemical and biochemical properties. Within the framework of stereoelectronic analysis, it has been established that the shift of the π-bond affects the distribution of electron density throughout the molecule, decreasing the reactivity of certain functional groups and increasing structural stability according to thermodynamic criteria. This effect is especially important for a molecule that must function in varying biological environments-from the acidic pH of the stomach to the neutral pH of blood or the enzymatic activity of the liver.
Delta-8-THC is a chiral compound, meaning it has spatial asymmetry. The two most well-known chiral centers correspond to the compound’s IUPAC designation as 6aR,10aR. The natural form found in plants always has the (−)-configuration, which determines its interaction with protein structures, particularly cannabinoid CB1 receptors. However, under synthetic manufacturing conditions, the formation of racemic mixtures is possible, which in turn can affect both pharmacodynamics and potential toxicity. The spatial configuration of the molecule, especially regarding the flexibility of its pentyl side chain, also influences its conformational fit within the receptor binding pocket, partly determining the strength and duration of the agonist response.
Equally interesting is the geometry of the ring system itself. The molecule’s tricyclic framework is not completely rigid: the cyclohexene ring is capable of transitions between different conformational forms, the most stable being the chair conformation. This conformation minimizes steric interactions between atoms while preserving the molecule’s active site in a position favorable for receptor binding. Such conformational self-adjustment is an important property that makes cannabinoids, including delta-8, unique among lipophilic signaling molecules.
Although delta-8 is often mentioned as an isomer of delta-9, it is important to emphasize that isomerism here is not just a formal concept but a structural difference with consequences for thermal stability, reactivity, and even the metabolic transformation pathway. For instance, Δ8-THC shows higher chemical stability than Δ9-THC under acidic conditions, as confirmed by in vitro studies. This is due to its reduced tendency to undergo autoconversion-the process in which delta-9 spontaneously isomerizes to delta-8 or even degrades to cannabinol when exposed to oxygen or light. Unlike its isomer, Δ8-THC maintains chemical integrity across a broad temperature range and in the presence of acids, making it more attractive for pharmacological use in environments with elevated acidity, such as the gastrointestinal tract.
Special attention should be given to isomeric variants that arise during synthesis or biotransformation. This involves not only delta-9 and delta-10 isomers but also intramolecular modifications occurring under the influence of cationic agents, acids, or enzymes. All these isomers can have similar mass and even similar ultraviolet spectra, but they differ significantly in spatial orientation and activity toward cannabinoid receptors. That is why modern analytical techniques require not only chromatographic but also spectroscopic confirmation of configuration, particularly using NMR spectroscopy, where it is critical to clearly identify the chemical shifts of each hydrogen atom near the double bond.
Differences Between Δ8-THC and Δ9-THC
Despite their structural similarity, delta-8 and delta-9 tetrahydrocannabinol exhibit differences that are systemic in nature-they encompass mechanisms of interaction with biological systems as well as distinct features of synthesis, metabolism, pharmacology, and even molecular transport. The shift of the double bond within the cyclic fragment is only a formal starting point, behind which lies a whole chain of conformational and receptor-related consequences that cannot be reduced to a superficial “isomeric difference.” Understanding these differences requires a multidimensional analysis that includes electronic topography, bioavailability, specificity of enzymatic oxidation, effects on neuromediator signaling pathways, and even tendencies for the formation of side products within the metabolic cycle.
One of the most notable distinctions between the two molecules is their interaction with CB1 receptors. Although both cannabinoids are partial agonists of this receptor, the degree of affinity and the intensity of the intracellular signaling they initiate are not identical. Δ9-THC demonstrates a higher affinity for CB1, which is confirmed in vitro by a lower dissociation constant (Kd) and a more complete agonist response. In contrast, Δ8-THC exhibits reduced receptor activity, which is not only due to differences in configuration but rather microscopic shifts in the orientation of functional groups when the molecule is fixed within the receptor’s binding pocket. The reduced signaling efficiency may partially explain the lower psychoactive effect of delta-8, which empirical estimates place at around 50-75% of an equivalent dose of delta-9.
Equally important is the pharmacokinetic behavior of both isomers. After oral or inhalation administration, Δ9-THC undergoes active metabolism in the liver with the formation of 11-hydroxy-Δ9-THC-a metabolite that has higher psychoactivity than the parent cannabinoid. Δ8-THC is also transformed into its 11-hydroxy analog, but the metabolism process is slower, and the affinity of the resulting metabolite for CB1 receptors is lower. This difference has direct consequences for subjective perception of the effect, as Δ8-THC not only has a slower onset of action but also produces a more prolonged yet milder effect with a less pronounced peak activity.
Additionally, there are fundamental differences in molecular stability. Δ8-THC exhibits increased thermal stability, chemical inertness toward autoconversion, and better preservation of its structure when exposed to light and oxygen. This makes it more attractive from the standpoint of storage, formulation, and use in therapeutic products that require a long shelf life without loss of bioactivity. Δ9-THC, in contrast, shows a tendency to degrade into cannabinol, especially when stored improperly or exposed to oxygen, which limits the stability of pharmaceutical formulations based on it.
It is also worth noting differences in biotransport. Δ9-THC has a higher affinity for plasma proteins, particularly albumin, leading to faster distribution throughout the body and a tendency to accumulate in fat deposits. Δ8-THC exhibits a narrower protein binding profile, which reduces the risk of accumulation with prolonged use, although it prolongs the elimination half-life due to slower clearance. This, in turn, is significant for chronic pharmacotherapy where controlling plasma levels without excessive accumulation-which may potentiate side effects-is important.
In the context of enzymatic transformations, both isomers are primarily metabolized by cytochrome P450 enzymes, predominantly the CYP2C9, CYP3A4, and CYP2C19 isoforms. However, Δ8-THC shows a lower capacity to inhibit the enzymatic activity of these systems, reducing the risk of drug interactions. Δ9-THC, on the other hand, can inhibit or competitively suppress CYP2C9, which is important for patients taking medications with narrow therapeutic windows. This feature makes Δ8-THC promising in multimedication regimens where maintaining a stable enzymatic profile is critical.
Another aspect that differentiates the two cannabinoids is their profile of neuromediator effects. Δ9-THC activates not only cannabinoid receptors but also indirectly influences dopamine, serotonin, and GABA systems. Its effect on the limbic system is intense, which underlies both its psychoactivity and the frequency of psychogenic side effects. Preliminary data suggest that Δ8-THC demonstrates limited cross-activation of neuromediator pathways, notably not causing significant dopamine release in the mesolimbic pathway, which partly explains its milder effect and lower likelihood of anxiety, paranoia, or panic attacks.
Attention should also be paid to molecular interactions with ion channels and TRP receptors. Although both isomers can modulate the activity of the vanilloid receptor TRPV1, Δ8-THC produces a less pronounced effect. This has potential significance in the context of pain, hyperalgesia, and neuroinflammation, as TRPV1 is linked to the regulation of pain transmission at the spinal dorsal horn level. Theoretically, Δ9-THC may provide more potent analgesia but simultaneously carries a higher risk of dependence or receptor desensitization.
Molecular Stability and Behavior in Biological Environments
Studying the stability of delta-8-tetrahydrocannabinol (Δ8-THC) at the molecular level, as well as its behavior in various biological environments, is critically important for understanding the pharmacodynamic and pharmacokinetic properties of this cannabinoid. The molecule’s ability to maintain its structure and functionality under complex biochemical conditions determines its effectiveness, safety, and therapeutic potential. In this section, we will explore in detail the mechanisms of chemical stability of Δ8-THC and its dynamics in conditions that simulate intracellular and extracellular environments.
One of the fundamental aspects of stability is Δ8-THC’s susceptibility to isomerization and oxidation. Research has shown that the position of the double bond in the molecule, characteristic of Δ8-THC, creates specific electronic conditions that make this molecule less reactive toward free radical attacks compared to other cannabinoids. This reduced reactivity directly affects the molecule’s resilience to factors such as light, oxygen, temperature, and pH balance. Specifically, Δ8-THC demonstrates greater resistance to photodegradation in the ultraviolet range, which is attributed to a more stable molecular conformation, decreased electron mobility in the double bond region, and a lower tendency to form photolabile products.
Particular attention should be given to the molecule’s behavior in aqueous environments, since most pharmacological processes occur under hydrophilic conditions, even though the molecule itself is hydrophobic. Δ8-THC shows low water solubility due to the significant hydrophobicity of the tetrahydrocannabinol backbone, yet it is capable of forming microcomplexes with proteins and lipids present in biological systems. Interaction with plasma proteins, such as albumin, occurs through non-covalent hydrophobic bonds that stabilize the molecule in the bloodstream while simultaneously reducing its availability to metabolic enzymes. This phenomenon not only prolongs the half-life of Δ8-THC but also limits the rate of its breakdown in liver cells, positively influencing its pharmacokinetic profile.
Regarding pH conditions, it should be noted that Δ8-THC is highly stable at neutral and mildly alkaline pH levels but begins to show susceptibility to hydrolytic processes in acidic environments, including the opening of cyclic structures and formation of derivatives that do not retain cannabinoid activity. These reactions occur in the gastrointestinal tract during oral administration and can affect bioavailability and conversion of the molecule into less active forms. This highlights the necessity of developing pharmaceutical formulations capable of protecting Δ8-THC from degradation in acidic environments, for example, through encapsulation or the use of enteric coatings.
Another important characteristic is Δ8-THC’s behavior within cellular membrane structures. The molecule’s lipophilicity allows for effective incorporation into the phospholipid bilayer of membranes, where it can modulate the physicochemical properties of the membranes by influencing their fluidity and permeability. This integration into the membrane also facilitates the local concentration of the molecule near membrane-bound receptors, particularly CB1 and CB2, optimizing receptor activation. Furthermore, the molecule’s stability in lipid environments is higher than in aqueous ones due to reduced exposure to oxidants and enzymes, making membrane regions an additional protective zone for Δ8-THC.
Moreover, recent studies focus on the reactions with oxidative enzymes involved in cannabinoid metabolism. Δ8-THC shows resistance to non-catalytic oxidation, but hepatic enzymatic systems, especially cytochrome P450, are capable of converting it into various hydroxy- and carboxy-derivatives. The rate and direction of these transformations largely depend on the isoform composition of enzymes, the patient’s health status, and the presence of other medications. Metabolites formed from Δ8-THC have different bioactivity profiles, which is important to consider when predicting clinical effects.
A particular interest lies in studying Δ8-THC stability under conditions of immune-related oxidation, characteristic of inflammatory processes. In the presence of reactive oxygen species (ROS), the molecule demonstrates moderate resistance, indicating potential for use in chronic inflammatory disease therapy without rapid degradation of the active agent. This distinguishes Δ8-THC from some other cannabinoids that are quickly inactivated by ROS.
Stability is also important in the context of long-term storage and manufacturing conditions. During extraction, purification, and pharmaceutical formulation, Δ8-THC exhibits high chemical inertness even at elevated temperatures, allowing its activity to be preserved without the need for complex cooling conditions. This significantly facilitates technological processes and reduces the cost of the final product while maintaining its pharmacological properties.
The behavior of Δ8-THC in intracellular environments is crucial for its pharmacological action. The molecule can penetrate organelle membranes, including mitochondria, opening new avenues for studying its effects on cellular metabolism, energy production, and signaling cascades. This permeability is related to its moderate polarity and structural flexibility. These properties may explain some of the unique pharmacodynamic effects of Δ8-THC, including its role in modulating oxidative stress and apoptosis.
Natural Origin and Sources of Delta-8-THC
Biogenesis in Cannabis Plants
The study of the biogenesis of delta-8-tetrahydrocannabinol (Δ8-THC) in plants of the Cannabis genus is an important component of understanding the chemical evolution of cannabinoids and the specifics of their synthesis. Unlike the more extensively studied Δ9-THC, the biochemical pathways leading to the formation of Δ8-THC are less well understood, which is due to its low natural content in most strains and the complexity of analytically distinguishing isomers. However, the fundamental principles of cannabinoid biosynthesis can shed light on how Δ8-THC is formed.
The primary precursor compound for cannabinoid synthesis is cannabigerolic acid (CBGA), which is produced in the secretory gland cells, or trichomes, of the flowers and leaves of Cannabis plants. This precursor undergoes condensation with an oligoprenoid molecule through an iterative mechanism, leading to the appearance of acidic forms of cannabinoids: tetrahydrocannabinolic acid (THCA), cannabidiolic acid (CBDA), and cannabichromenic acid (CBCA). THCA is the main substrate for the formation of Δ9-THC and, under certain conditions, also for Δ8-THC.
The formation of Δ8-THC begins with THCA, which undergoes enzymatic or non-enzymatic transformation resulting in a shift of the position of the double bond within the cyclic structure of the molecule. The enzymatic mechanism of Δ8-THC formation is not currently as deeply studied as that of Δ9-THC; however, some research suggests the possible existence of isomerases or reductases that may catalyze this reaction under limited cellular conditions. This process is complex and depends on specific cellular environmental factors, including the level of redox potential, pH, and availability of cofactors.
In addition to enzymatic pathways, Δ8-THC formation in the plant may occur through spontaneous isomerization of THCA under physical or chemical influences that alter the molecule’s stability. Specifically, exposure to heat, light, or pH changes can promote the shift of the double bond position from the Δ9 to the Δ8 configuration. However, unlike the more common laboratory-induced isomerization, in natural conditions this process is limited and occurs locally in certain plant tissues or under stress factors.
It is also important to consider the role of subcellular localization in biosynthesis. It is known that the enzymes responsible for cannabinoid formation are localized in specific membrane complexes in the cytoplasm and endoplasmic reticulum. The spatial organization of these enzymatic complexes determines the local environment for synthesis and modification of molecules, which may be key to the formation of less common isomers such as Δ8-THC. The formation of specific enzyme isoforms in different plant genotypes also explains variations in the ratio of Δ8-THC to other cannabinoids.
At the genetic level, research shows that the expression of genes encoding cannabinoid acid synthases is regulated by complex signals associated with plant development, light regimes, and external stressors. These factors influence not only the quantity of synthesized molecules but also the activity of enzymes that carry out isomerization and modification of cannabinoids. Therefore, the phenotypic presence of Δ8-THC is linked to a multidimensional interaction of genetic and environmental factors.
Overall, the biogenesis of Δ8-THC in Cannabis is a product of both enzymatic activity and the chemical instability of the THCA molecule, which leads to the formation of isomers with a shifted double bond. The specifics of enzymatic pathways remain a subject of active scientific discussion, and the potential role of the plant tissue microecology in the formation of these isomers opens new horizons for fundamental research.
Concentrations in Natural Conditions: Assessment of Content
Studying the quantitative content of delta-8-tetrahydrocannabinol (Δ8-THC) in natural Cannabis samples is a complex task that requires highly precise analytical methods and a detailed understanding of the factors influencing its concentration. Despite significant advances in analytical technologies, available data on natural concentrations of Δ8-THC remain limited, and reported values often vary depending on the biological material, growing geography, harvesting conditions, and analytical techniques used.
Δ8-THC is traditionally considered a minor isomer within the cannabinoid profile of the plant, compared to more dominant cannabinoids such as Δ9-THC and cannabidiol (CBD). However, with the advent of more accurate chromatographic and spectroscopic methods-particularly liquid chromatography coupled with tandem mass spectrometry (LC-MS/MS) and gas chromatography-mass spectrometry (GC-MS)-it has become possible to identify and quantify Δ8-THC at low concentrations. It is important to emphasize that the lack of standardized protocols for extraction and analysis complicates the comparison of results between different studies.
Assessment of Δ8-THC concentration in natural Cannabis specimens shows significant variability. Typically, its content does not exceed 1% of the total dry weight, making it a secondary component in the cannabinoid spectrum. However, in some specific genotypes, as well as in samples subjected to certain agricultural or post-harvest treatments, Δ8-THC content can reach 3-5%. Such elevated concentrations are usually observed in products where partial isomerization of Δ9-THC has occurred or in aged plant materials where natural degradation processes alter the cannabinoid profile.
Quantitative evaluation of Δ8-THC in leaves, flowers, and stems of Cannabis indicates that the highest concentration is localized in the floral parts, especially in the trichomes-specialized glandular structures that produce and accumulate cannabinoids. Δ8-THC content in leaves is significantly lower, and in stems it is practically absent or detected only in trace amounts. This distribution characteristic is linked to the localization of cannabinoid-synthesizing enzymes and the concentration of precursors necessary for Δ8-THC formation.
It is also important to consider the impact of the plant’s maturity stage on Δ8-THC concentration. During the flowering and ripening phases, active cannabinoid synthesis occurs, alongside degradation processes that can increase or decrease Δ8-THC levels in the tissues. In particular, the enzymatic decarboxylation of THCA to the active form Δ9-THC can be accompanied by partial isomerization producing Δ8-THC. In older, dried samples, storage may stimulate further conversion of Δ9-THC to Δ8-THC through oxidative and photochemical processes.
Regional differences also play a significant role in determining Δ8-THC content. Depending on climatic conditions, soil types, water availability, and solar radiation, Cannabis plants may modulate cannabinoid metabolism, which is reflected in the proportions and concentrations of individual compounds. For example, plants grown in subtropical and tropical zones may have more stable cannabinoid profiles with elevated levels of stable isomers, whereas in temperate climates, greater variability is observed, particularly in Δ8-THC levels.
Analytical challenges in determining Δ8-THC arise from its chemical similarity to Δ9-THC, which complicates differentiation of these isomers by standard analytical methods. Accurate identification and quantification require the use of high-performance chromatographic columns with optimized separation conditions, as well as high-resolution mass spectrometric detectors. This precision is essential because errors in determination can lead to distortions of the pharmacological profile and, consequently, clinical conclusions.
Another aspect is the instability of Δ8-THC in samples stored for prolonged periods or exposed to unfavorable conditions such as light, temperature fluctuations, and oxygen. This can cause not only a decrease in concentration but also the formation of derivative compounds with different pharmacological properties. Therefore, a standardized approach to sample collection, storage, and preparation for analysis is critical to avoid artifacts and preserve the representativeness of the data.
Additionally, in natural conditions, Δ8-THC concentrations may vary depending on the plant’s age and genetic characteristics. Genetic variability dictates different levels of expression of enzymes that synthesize or modify cannabinoids. This feature explains the wide diversity of cannabinoid profiles in Cannabis plants even within a single region and creates prerequisites for selecting strains with elevated Δ8-THC content for scientific or medical purposes.
It is worth noting separately that in wild Cannabis populations, Δ8-THC is almost absent or found only in trace concentrations, indicating that its natural accumulation is either a result of the plant’s adaptation to specific ecological stresses or a metabolic byproduct arising from oxidative or photochemical degradation of other cannabinoids.
The Influence of Environmental Factors on the Formation of Delta-8-THC
The formation and accumulation of delta-8-tetrahydrocannabinol (Δ8-THC) in plants of the Cannabis genus result from a complex interaction of genetic, biochemical, and environmental factors. Among external influences, environmental factors play a decisive role in regulating the metabolic pathways that control the synthesis, transformation, and stability of this compound. Studying ecological modulating factors is fundamentally important for understanding the biochemical flexibility of the plant and its adaptive mechanisms, as well as for optimizing cultivation to achieve a targeted chemical profile.
First of all, it should be noted that cannabinoids, including Δ8-THC, are synthesized in specialized glandular trichomes, which are sensitive to changes in the external environment. The impact of climatic conditions is expressed through modification of enzyme activity, regulation of genes responsible for cannabinoid biosynthesis, and changes in the overall metabolic state of cells. For example, temperature fluctuations can significantly affect the expression levels of enzymes that catalyze the conversion of THCA into its various isomers, including Δ8-THC.
An immediate response to increased temperature involves an acceleration of cannabinoid isomerization. Heat promotes the migration of the double bond in the tetrahydrocannabinol molecule from the 9th to the 8th position, leading to an increase in Δ8-THC concentration. In laboratory conditions, heating cannabinoid extracts is a standard method for synthesizing Δ8-THC from Δ9-THC, but this phenomenon in plant tissues is less studied, though it highlights the importance of temperature control during cultivation and storage. Excessively high temperatures can, on one hand, increase Δ8-THC but simultaneously cause degradation of other cannabinoids, which affects the overall chemical profile.
Solar radiation, particularly ultraviolet (UV) light, also has a complex effect on cannabinoid synthesis. Light induces the expression of genes regulating enzymatic biosynthetic processes; however, excessive sunlight exposure leads to free radical formation and oxidative stress, which may cause chemical transformations of already synthesized cannabinoids, including isomerization of Δ9-THC into Δ8-THC. It is important to understand that this interaction depends on the duration, intensity of light exposure, and spectral composition, making the effects difficult to predict.
Air and soil humidity also regulate plant metabolic pathways. Under drought conditions, activation of antioxidant defense systems occurs, influencing oxidative processes in cells and reducing the likelihood of spontaneous Δ9-THC isomerization. At the same time, excessive moisture can promote bacterial or fungal activity, altering metabolism and potentially increasing cannabinoid degradation. This indicates a delicate balance between hydration and stress for optimal Δ8-THC formation.
Soil chemical composition and nutrient availability act as long-term regulators of metabolism. In particular, deficiency or excess of certain micronutrients, such as iron, magnesium, or zinc, can modify the activity of enzymes involved in cannabinoid biosynthesis. It is known that copper, as a cofactor of some oxidative enzymes, influences the stability of phenolic compounds, which potentially regulates oxidative processes associated with cannabinoid isomerization.
Among other environmental factors, the influence of mechanical stress, air pollution, and plant microbiota should be noted. Mechanical damage to tissues triggers signaling cascades that activate protective metabolic pathways, which can increase or modulate the synthesis of secondary metabolites, including Δ8-THC. Pollutants, especially volatile organic compounds and heavy metals, can affect the transcription of enzymatic genes, while the root system microbiome regulates metabolism, directly or indirectly influencing cannabinoid metabolism.
The photoperiod, or lighting duration, is a critical factor for regulating cannabinoid synthesis. The light regime determines the plant’s developmental stage and accordingly alters the expression profile of key enzymes. Changes in day length stimulate the plant’s transition to the flowering phase, which is accompanied by increased cannabinoid synthesis, including potential shifts in the proportions between Δ9-THC and Δ8-THC.
In addition to the direct impact of environmental factors on synthesis, the duration of storage of plant material in natural or semi-natural conditions is also significant. During aging or natural drying, chemical transformations may occur, including the isomerization of Δ9-THC into Δ8-THC under the influence of oxidation or photochemical reactions. This indicates that the concentration of Δ8-THC in the final product results not only from initial synthesis but also from post-biosynthetic processes occurring over time.
Methods of Synthesis and Production
Isomerization of Cannabidiol (CBD): The Primary Pathway
The production of delta-8-tetrahydrocannabinol (Δ8-THC) through the isomerization of cannabidiol (CBD) is a complex chemical process based on the transformation of the molecular structure while retaining the main functional groups, but with a radical shift in the position of the double bond within the cyclic system. This transformational pathway not only forms the foundation for the industrial manufacture of Δ8-THC but also opens prospects for developing new methods to obtain structural analogs of tetrahydrocannabinol with targeted pharmacological properties.
The isomerization of CBD into Δ8-THC occurs in the presence of acidic catalysts, which facilitate electrophilic substitution within the molecule. The chemical nature of CBD as an open-chain cannabinoid containing both phenolic and cyclic structures allows, under certain conditions, the initiation of intramolecular cyclization resulting in the formation of a tetrahydrocannabinol structure with the double bond at the eighth position. This reaction involves a sequence of intermediate stages characterized by conformational changes of the molecule and the formation of stable carbocations, which are critically important for the selectivity and yield of the final product.
A key aspect is the control of the acidity of the environment. Excessively strong acids may lead to side reactions such as degradation of starting materials or formation of unwanted side isomers. Conversely, insufficient acidity reduces the rate and efficiency of isomerization. Understanding the precise acidity balance is crucial for optimizing the technological process and minimizing impurity formation.
Reaction time and temperature also critically influence the nature of the transformations. At lower temperatures, isomerization proceeds slowly but with higher selectivity, whereas increased temperatures accelerate the process but raise the risk of side product formation. Industrial conditions are often determined by a compromise between reaction speed and product purity.
The choice of solvent in which the reaction is conducted is also significant. Solvent polarity affects the stabilization of intermediate carbocations and the intensity of electrophilic reactions. Most commonly, organic solvents with moderate polarity are used, providing a balance between solubility of the starting material and stability of the catalysts.
The mechanism of isomerization begins with activation of the phenolic hydroxyl group of CBD through protonation in the acidic medium, which promotes the formation of a carbocation intermediate. Subsequent electrophilic attack on the internal double bond initiates cyclization, forming a new six-membered ring characteristic of tetrahydrocannabinols. The location of the double bond at the eighth position determines the unique physicochemical and pharmacological properties of Δ8-THC.
An additional complexity is the potential formation of Δ9-THC as a side product. Despite their structural similarity, these two isomers have different biological activities, necessitating precise regulation of technological parameters to achieve predominant synthesis of Δ8-THC. Chromatographic control methods and spectroscopic analysis are essential for monitoring the selectivity of the reaction.
Moreover, the acid-catalyzed isomerization of CBD is a classical example of a thermodynamically controlled transformation. The equilibrium between isomers is regulated not only by initial reaction parameters but also by subsequent storage and processing conditions of the product. It is known that Δ8-THC is a more thermally stable isomer compared to Δ9-THC, which affects the final quality and shelf life of the obtained preparations.
On an industrial scale, mineral acids such as sulfuric or phosphoric acid or organic acids of appropriate strength are used to initiate the isomerization reaction. It is necessary to consider not only the catalyst but also the possibility of neutralizing residual acid after the reaction, which is important for the safety and purity of the final product.
Current research focuses on increasing the selectivity and environmental friendliness of the CBD to Δ8-THC isomerization process. Catalysts based on acids with controlled activity, mild reaction conditions, and the use of green solvents are being explored. These directions are important for reducing the formation of harmful impurities and improving the efficiency of the technology.
Equally important is understanding the kinetics of the isomerization reaction. Modeling and experimental data show that the reaction proceeds through several stages with the formation of intermediate compounds of varying stability. Depending on process parameters, different reaction pathways may dominate, leading to variability in yield and purity of Δ8-THC.
Chemical Reaction in Acidic Medium
The process of isomerization of cannabidiol (CBD) to delta-8-tetrahydrocannabinol (Δ8-THC) in an acidic medium is a classic example of intramolecular cyclization initiated by a protonated catalyst, under the influence of electrophilic centers. This mechanism is based on the activation of hydroxyl groups of CBD, the formation of carbocation intermediate states, and the migration of the double bond within the tertiary ring. The corresponding chemical pathway allows the transformation of the linear CBD structure into the cyclic configuration of Δ8-THC without the need for radical reconstruction of the molecular backbone.
Mechanistically, the reaction starts with the activation of the phenolic hydroxyl group of CBD. In the presence of an acidic catalyst (for example, p-toluenesulfonic acid or hydrochloric acid in an organic medium), protonation occurs, which makes the hydroxyl group electron-deficient and increases the electrophilicity of the adjacent carbon atom. This, in turn, promotes the elimination of water or reorganization of electron density toward carbocation formation.
Following this, the most critical stage begins-the formation of a stabilized tertiary cannabinoid carbocation, which has the ability to attack the internal alkene group in the side chain of CBD. As a result of this electrophilic cyclization, a six-membered ring is formed-a characteristic feature of THC-type cannabinoid structures. The placement of the double bond between carbons C-8 and C-9 (i.e., Δ8) occurs under thermodynamic control, which is determined by the stability of molecular conformations and electronic delocalization.
One of the key components of this process is the control of cyclization stereochemistry. Under acidic catalysis, isomerization predominantly results in the formation of the Δ8 isomer; however, variations in temperature, acidity, or reaction time can lead to competing formation of Δ9-THC, Δ10-THC, and even less studied non-canonical structures such as Δ6a,10a-THC. Achieving predominant synthesis of the Δ8 isomer requires fine regulation of reaction parameters.
Solvents play a decisive role in stabilizing transition states. For example, chloroform, dichloromethane, toluene, and other nonpolar media reduce the likelihood of side reactions, including polymerization of intermediate cations or oxidation. At the same time, solvents with high donor capacity can stabilize electrophilic centers but cause undesirable shifts in equilibrium toward hydration or formation of inactive side products.
Temperature control is another crucial factor. The reaction is most often carried out in the range of 50-90 °C, depending on the chosen acid and solvent. At lower temperatures, the reaction proceeds more slowly but with fewer side isomers, whereas increasing temperature accelerates cyclization while raising the risk of forming Δ9 and Δ10 isomers. In industrial settings, the balance between selectivity and speed is often achieved by introducing modified acids or buffer systems that stabilize protonated forms of CBD without causing proton overload in the medium.
Additionally, changing the type of acid alters the isomerization mechanism. For example, when using Lewis acids such as AlCl₃ or BF₃•Et₂O, a different form of molecular activation occurs through complexation with electron pairs of CBD. Such reactions may have higher selectivity for the Δ8 isomer but require careful control of moisture levels because even trace amounts of water can alter the reaction type or initiate hydrolysis of intermediate structures.
Another challenge related to isomerization in acidic media is controlling the final pH and complete removal of acid residues. Even microscopic amounts of residual acid in the product can lead to further degradation of Δ8-THC or spontaneous re-isomerization during storage. This is especially critical in medical or research applications where product stability determines dosage accuracy.
Besides the primary transformation of CBD to Δ8-THC, under certain conditions competitive reactions may occur, producing ether side products or oxygenated derivatives, such as 11-hydroxy-Δ8-THC or acidic forms. Avoiding these reactions requires minimizing oxygen presence and controlling moisture in the reaction environment.
Finally, post-reaction purification of the product from residual acids, side isomers, and organic impurities is the stage that determines the practical value of the entire synthesis. Most often, fractional chromatography or recrystallization in an inert environment is used. In laboratory conditions, column chromatography on silica gel or flash chromatography with polarity gradients is popular.
Conditions, Reagents, and Process Control
The isomerization process of cannabidiol (CBD) to delta-8-tetrahydrocannabinol (Δ8-THC) is extremely sensitive to reaction conditions. Despite its apparent simplicity-it might seem to involve only the migration of a double bond and cyclization-this synthesis requires utmost precision in selecting every element: from the nature of the catalyst to the vapor pressure, reagent purity, and even the material of the reaction vessel walls. Any deviation from the optimal parameters can not only reduce the yield of Δ8-THC but also lead to the formation of a whole spectrum of unwanted by-products, including both inactive and toxicologically hazardous compounds.
We should begin with the acids, which are the key catalysts of the process. Most commonly used are Brønsted acids such as p-toluenesulfonic acid, sulfuric acid, orthophosphoric acid, as well as hydrohalic acids (notably HCl). Their application is based on the ability to activate the hydroxyl group of CBD, initiating electrophilic cyclization. At the same time, the choice of a specific acid influences not only the reaction rate but also the regioselectivity of the THC isomer formation-Δ8, Δ9, or Δ10. For example, the use of a strong inorganic acid like H₂SO₄ is often accompanied by partial hydrolysis or excessive isomerization, whereas organic acids (such as trifluoroacetic acid or p-toluenesulfonic acid) provide a more delicate course of the process.
Special attention is deserved by Lewis acids-BF₃•Et₂O, AlCl₃, ZnCl₂, TiCl₄-that activate CBD by forming complexes with the oxygen electron pairs. They act not through protonation but by shifting electron density in critical regions of the molecule, allowing fine-tuning of the reaction profile. Under absolutely dry conditions and strict temperature control, these catalysts demonstrate the highest selectivity toward the Δ8 isomer. However, the necessity to avoid even traces of moisture when using Lewis acids (since they hydrolyze with heat release and formation of hazardous by-products) makes these reactions more challenging to scale up.
The reaction medium is also critically important. Typically, aprotic organic solvents with low polarity are used: chloroform, dichloromethane, toluene, benzene, or hexane. The choice depends on target parameters-solubility of reagents, stability of intermediate carbocations, heat conductivity of the medium. For example, dichloromethane dissolves both CBD and Δ8-THC well, stabilizes intermediate ions, and has a relatively low boiling point, which simplifies control of reaction temperature and subsequent solvent removal. At the same time, it is volatile and toxic, requiring sealed conditions and the use of fume hoods.
Temperature is another extremely important parameter. The typical range is from 50 to 90 °C. Lower temperatures slow the reaction, reducing the risk of excessive isomerization or product degradation but extending synthesis time to several hours. Higher temperatures, especially above 90 °C, sharply increase the likelihood of side isomer formation, including Δ9-THC, Δ10-THC, as well as peroxidized or polymerized products. When using vacuum or an inert atmosphere (nitrogen, argon), this threshold can be slightly raised but only if the system is completely sealed.
The reaction time usually ranges from 30 minutes to 4 hours depending on acidity, solvent, and temperature. Extending the reaction beyond the optimal window promotes further transformation of Δ8-THC into other products-particularly the Δ10 isomer or oxidized derivatives. The presence of molecular oxygen in the reaction medium promotes conversion of Δ8-THC into unstable peroxides or quinones, so an important step is degassing the solvent before synthesis and conducting the reaction under an inert gas atmosphere.
pH control is another strategic point on which purity and selectivity depend. An overly acidic environment (pH < 1) can be too aggressive for the CBD molecule, causing fragmentation or polymerization of products. Therefore, many protocols use buffer systems or weaker acids that maintain stable reactivity without causing excessive aggressiveness.
Regarding process control, it is based on the use of modern analytical methods. During synthesis, monitoring is performed by sampling the reaction mixture with subsequent analysis by thin-layer chromatography (TLC), high-performance liquid chromatography (HPLC), or gas chromatography coupled with mass spectrometry (GC-MS). The defining criterion for reaction completion is the appearance of the characteristic Δ8-THC signal with the disappearance of the initial CBD. Intermediate products-such as partially cyclized structures-are also identified, allowing evaluation of catalyst efficiency and adjustment of reaction parameters in real time.
After the reaction is complete, it is important to immediately quench it, usually by neutralizing residual acid, lowering temperature, and adding an organic phase that promotes extraction of Δ8-THC. This is followed by multiple washings to remove traces of acid, neutralizing agents, and by-products. Residual acidity can cause slow degradation of Δ8-THC over several days-both at room temperature and under cooling-so purification must be thorough and prompt.
Purification of the final product is also multistep. The most effective method is flash chromatography using a polarity gradient (for example, from hexane to acetone). For laboratory analysis and standardization, nuclear magnetic resonance (¹H-NMR and ¹³C-NMR) is often used, which allows confirmation of the double bond location specifically between C-8 and C-9.
Equipment materials are no less important. Acid-sensitive metals (such as aluminum or copper alloys) can catalyze unwanted side reactions or even corrode, leading to product contamination and changes in the chemical profile. For this reason, glass, PTFE (Teflon), or inert reactors are used for the reaction.
Alternative Approaches to Synthesis
Within the chemistry of cannabinoids, the concept of “alternative approaches to the synthesis of Δ⁸-tetrahydrocannabinol (Δ⁸-THC)” refers to going beyond the classical acid-catalyzed isomerization of cannabidiol (CBD). Although the latter remains a technically accessible and cost-effective method, it is accompanied by a range of technological and regulatory challenges – from impurity formation to limited control over the final isomer profile. Alternative approaches have emerged as a response to these limitations, and their development is largely driven by increasing demands for product purity, reproducibility of synthesis, and pharmaceutical compliance.
In this context, “alternative” refers to methods that do not rely on harsh acid catalysis, minimize thermal and photochemical degradation, allow control at the submolecular reactivity level, and apply new concepts of controlled isomerization, including biotransformations, electrochemistry, mechanochemistry, or selective oxidation. This section discusses these approaches as a distinct scientific field with unique logic and practical prospects.
One direction transforming the traditional approach to Δ⁸-THC synthesis is retrosynthetic planning using machine learning algorithms. Platforms such as IBM RXN for Chemistry, ASKCOS, or Chematica can model tens of thousands of synthetic route options to a target molecule, taking into account reaction selectivity, reagent availability, and potential toxicity of intermediates. For Δ⁸-THC, these systems have generated alternative synthetic routes that do not rely on CBD isomerization but include, for example, assembly of the tricyclic system via cyclization of prenylated phenols or through Diels-Alder reactions between functionalized dienes and dienophiles. Such alternative routes are generally not economically competitive for industrial production but are extremely important for expanding the chemical space of Δ⁸-THC scientifically. In particular, they allow modification of the molecular scaffold, introducing strategic changes in the aromatic core or alkyl side chain, preventing side formation of Δ⁹- or Δ⁷-isomers.
The electrochemical approach to Δ⁸-THC synthesis, although still underexplored, shows promise as a tool for selectivity control. The method involves applying an electrode potential to generate activated molecular forms capable of intramolecular cyclization. In the case of CBD, anodic oxidation of hydroxyl groups or neighboring carbon centers leads to the formation of electrophilic intermediates that rearrange into the Δ⁸-isomer in the presence of mild nucleophiles (e.g., chlorides or carbonates). The advantage of electrochemistry is the complete absence of acid or metal catalysts and exceptional scalability via modular electrochemical reactors. However, significant challenges remain: high sensitivity of the product to overpotential, the need for aqueous-organic buffered media, and pH control near the electrode surface, which significantly affects isomerization selectivity.
Among more innovative alternatives are bioorganic catalytic systems – molecules that mimic enzymatic properties but are not protein structures. For example, molecular containers based on cavitands or cyclodextrins can create a microenvironment that facilitates CBD isomerization to Δ⁸-THC. The idea is that CBD, when included inside a hydrophobic cavity, undergoes intramolecular rearrangement due to a spatial “folding” effect analogous to a protein’s catalytic pocket. These structures can be modified with functional groups acting as mild acids or bases, providing specific control over the reaction mechanism. Prototype experiments showed that cationic variants of β-cyclodextrins can induce the formation of Δ⁸-THC with purity above 95%, without generating Δ⁹- or Δ⁶a,10a-isomers. At the same time, the challenge of this strategy lies in the lengthy removal of the container phase and the poor stability of these structures under industrial conditions.
Photocatalysis also attracts scientific interest, where the isomerization process is guided not simply by UV light but through specific photosensitizers. For example, using Ruthenium(II) complexes or iridium photocatalysts in the presence of CBD in a nonpolar medium enables an energy transfer mechanism that activates CBD in a conformation favoring the formation of the Δ⁸-isomer. Importantly, this light excitation occurs in the visible range (420-480 nm), not harsh UV, greatly reducing the risk of photooxidation or product degradation. These technologies approach the frontier of modern photochemistry, where molecular activation occurs in a phase microenvironment similar to biological conditions. Their main drawbacks are the need for expensive noble metal complexes and oxygen-free controlled environments, but from a scientific potential perspective, they represent a fundamentally new class of reactions – photo-directed isomerization under reagent-free catalysis.
A distinct, still largely unexplored direction of alternative approaches is mechanochemical activation. This involves initiating a chemical reaction through physical influence: friction, pressure, or impact. Experiments with cannabidiol in the solid state have shown that under moderate mechanical stress (for example, in ball mills), partial isomerization of CBD is possible without acids or solvents. The phenomenon’s essence is that mechanical energy disrupts the π-system of the double bond and creates localized reactive centers. Despite being experimental, mechanochemistry opens the potential for fully dry synthesis of Δ⁸-THC without liquid waste formation, energy-intensive heating, or chemical impurities. However, the method’s efficiency is currently low, selectivity poorly controlled, and the mechanism insufficiently characterized.
Semisynthetic Methods
The concept of semisynthetic methods in the context of producing Δ⁸-tetrahydrocannabinol (Δ⁸-THC) encompasses a wide range of approaches that combine the biogenetic properties of natural cannabinoids with targeted chemical transformations. Unlike classical synthetic routes, where the structure is assembled de novo from simple molecular building blocks, here the starting substrates are already prepared natural phytocannabinoids-primarily cannabidiol (CBD), less commonly cannabigerol (CBG) or cannabichromene (CBC). Importantly, this class of methods not only ensures high selectivity for synthesizing the Δ⁸-isomer but also opens prospects for creating structurally related derivatives with specific pharmacological properties, since the structure of the initial natural compounds is partially preserved.
From a chemical logic perspective, the semisynthetic approach is ideal for cases where the target molecule is complex but its fragments already exist in nature. In the case of Δ⁸-THC, cannabidiol serves as such a fragment, with the preserved aliphatic side chain, cyclohexene skeleton, and the correct spatial arrangement of functional groups. The primary challenge of the synthesis in this case is inducing control over the intramolecular reaction that converts the open structure of CBD into the tricyclic system with a double bond between the eighth and ninth carbon atoms. However, unlike classical acid-catalyzed isomerization, non-standard conditions are employed here to avoid side products and promote higher purity of Δ⁸-THC.
One direction of semisynthetic approaches involves the use of mild catalytic conditions employing enzyme-mimicking organocatalysts. Specifically, it has been demonstrated that some secondary amines in the presence of non-aqueous media can activate CBD by forming an ion pair with the hydroxyl group, directing electron density in such a way that controlled cyclization to Δ⁸-THC occurs. This mechanism approaches biomimetic processes-it imitates enzymatic transformations, though without the participation of protein enzymes. The advantages of this approach include low temperature (around 30-40 °C), short reaction times (up to 1 hour), minimal side products, and importantly-the preservation of the chemical integrity of the aliphatic segments of the molecule, which often undergo hydrolysis under acidic catalysis.
Another example is the use of phase-transfer catalysis-a methodology where organic reagents and catalysts interact in a multiphase environment (for example, water/organic solvent), with the transfer of the active ion catalyst mediated by a specific agent (quaternary ammonium salts, such as tetrabutylammonium bromide). This allows regulation of each phase’s environment independently, keeping CBD in a favorable organic phase while the catalyst remains in its active ionic form at the reaction interface. This approach enables precise control of local pH activity without affecting the entire volume, reducing the risk of forming unstable isomers and destructive side reactions.
Yet another variation is the use of photochemical direction of the reaction. In this case, isomerization does not require traditional acids; instead, irradiation with a narrow UV spectrum, mainly in the 280-320 nm range, is used. In the presence of certain sensitizers (e.g., benzophenone or acetophenone), UV light activates the π-electron system of CBD, inducing the formation of an electrophilic center within the molecule and initiating intramolecular rearrangement. Conditions must be strictly controlled: excessive light flux can convert Δ⁸-THC into photolabile products-specifically, into Δ⁶a,10a-isomer or even quinonoid structures that lose psychoactivity. The advantage of the method lies in its environmental cleanliness-absence of chemical reagents, low temperature, mild conditions-but drawbacks include the complexity of scaling and the need for specialized equipment.
Particular attention is given to cationic polymer systems that function as heterogeneous catalysts. These include polymers with immobilized acidic or basic groups that are insoluble in the medium but catalyze the reaction on their surface. For example, sulfonated polymers like Amberlyst-15 or Nafion-H can serve as solid acid supports. Their use offers advantages: no need for additional acid removal, no side reactions with Δ⁸-THC, and importantly, catalyst reuse after cleaning. However, their efficiency is often lower than that of liquid catalysts, requiring longer reaction cycles (up to 6 hours).
A number of scientific studies show that electrochemical modification of CBD can also be used to selectively direct isomerization. In this approach, CBD is exposed to a controlled electrical potential in the presence of an inert electrolyte. This allows local formation of a cationic center that initiates the targeted rearrangement of the structure into Δ⁸-THC. The method is highly selective, as it provides gradual and precise generation of electrophilic conditions without adding strong acids. However, the complexity of electrochemical reactors and limitations in scaling make this technology mostly research-oriented.
Another innovative direction within semisynthetic methods is the use of microwave irradiation. Unlike photochemistry, this method uses electromagnetic radiation in the 2.45 GHz range, providing rapid and uniform heating of the reaction mixture. Under such conditions, isomerization occurs faster and at a lower overall temperature, minimizing hot spots that could cause thermal degradation. Importantly, this method works well with heterogeneous catalysts, making it especially attractive for selective isomerization without excessive acidity or oxidation.
All these methods-organocatalysis, photochemistry, microwave activation, heterogeneous catalysis, and electrochemistry-belong to the paradigm of so-called “green synthesis,” aimed at reducing toxicity, energy consumption, side burdens, and ecological footprint. Although in industrial practice they remain less widespread than classical acid schemes, their research potential is undeniable: they enable the production of high-purity Δ⁸-THC without traces of Δ⁹-isomer, without side phenolic products, and without the need for extensive chromatographic purification.
Looking forward, these semisynthetic platforms may become the foundation for standardized Δ⁸-THC manufacturing for pharmaceutical use, where traces of unidentified side substances or thermolabile impurities are unacceptable. The combination of natural structure with controlled selective rearrangement is the key to reliability, purity, and reproducibility of Δ⁸-THC synthesis within modern cannabinoid chemistry.
Prospects for Biotechnological Production
Within the research on Δ⁸-tetrahydrocannabinol (Δ⁸-THC) synthesis, biotechnological approaches are considered potentially the most environmentally friendly, specific, and scalable. The idea behind applying biotechnology is to use living cells or their enzymatic systems to create cannabinoids with precise spatial configuration, high purity, and minimal byproducts. This opens the path toward shifting from chemical to bioengineered production of Δ⁸-THC, which is highly relevant given pharmaceutical standardization, regulatory requirements, and the goal of reducing environmental impact.
Current research in this area primarily focuses on engineering microorganisms-mainly yeasts (Saccharomyces cerevisiae) and bacteria (for example, Escherichia coli)-capable of producing cannabinoid precursors or even final compounds. By applying synthetic biology techniques, artificial metabolic cascades are implanted into cells, allowing the transformation of simple sugars such as glucose into key cannabinoids, including cannabigerolic acid (CBGA), cannabidiolic acid (CBDA), and subsequently cannabidiol (CBD), which can serve as a substrate for further bioisomerization into Δ⁸-THC.
In particular, a promising direction is the development of enzymes capable of selectively isomerizing CBD into the Δ⁸-isomer. To date, several enzymatic systems with functional activity in this area have been described, including those belonging to the class of oxidoreductases-flavin-dependent monooxygenases and enzymes with cyclase activity. An early example includes the expression of a cannabis synthase enzyme modified by point mutations, which reduce its affinity for forming Δ⁹-THC and shift the product profile toward Δ⁸-isomers. Alongside this, the use of enzymes immobilized on solid supports is being explored, ensuring their multiple uses and stability in continuous production environments.
One of the most ambitious directions is the creation of fully synthetic chromosomes in microorganisms-so-called chassis cells-programmed for comprehensive cannabinoid biosynthesis with minimal external intervention. Examples of this approach include projects by Ginkgo Bioworks, Amyris, or Demetrix, which develop yeast strains capable of producing tens of grams of cannabinoids per liter of medium. While current focus remains on CBD and Δ⁹-THC, metabolic flux optimization algorithms (flux balance analysis, dynamic pathway modulation) enable reorienting cascades toward producing Δ⁸-isomers by redesigning key enzymes and fermentation conditions.
Another option is the biotransformation of CBD into Δ⁸-THC using microorganisms or higher fungi capable of catalyzing specific rearrangements. For example, species from the genera Cunninghamella or Rhizopus demonstrate the ability to selectively oxidize and cyclize while preserving or altering the double bond geometry. Preincubating CBD with cultures of such fungi under controlled pH and temperature conditions allows for selective transformation without the use of acids or organic solvents. However, major limitations of these processes include metabolite instability, the need for prolonged incubation, and low product yield.
Additionally, the use of enzymes derived from cannabis plants is being considered by cloning the corresponding genes and heterologously expressing them in producer models. For instance, enzymes from the THCA synthase or CBCA synthase families modify active cannabinoid intermediate forms at the acidic precursor level. With direct evolution technologies, such enzymes can be adapted for targeted Δ⁸-THC synthesis, avoiding the thermal or acid decarboxylation stage, which significantly reduces energy consumption and the risk of forming side isomers.
The prospects for biotechnological production of Δ⁸-THC are especially attractive in the context of future pharmaceutical applications, where control over chemical purity, absence of residual acids, metals, or solvents is critical. Biotechnological platforms can ensure such purity through enzymatic catalysis, compartmentalization of metabolism, and expression of auxiliary proteins that stabilize the target molecule.
Pharmacological Profile of Delta-8-THC
Interaction with Cannabinoid Receptors
Δ8-tetrahydrocannabinol (Δ8-THC) is a complex molecule capable of interacting with several types of receptors, the most important of which are the cannabinoid receptors CB1 and CB2. These receptors belong to the family of G-protein-coupled receptors (GPCRs), which mediate numerous physiological processes, including regulation of neural activity, immune response, and metabolism. The interaction of Δ8-THC with these receptors exhibits unique pharmacodynamic characteristics that distinguish it from the more common Δ9-THC.
CB1 receptors are primarily localized in the central nervous system, especially in the cerebral cortex, hippocampus, basal ganglia, and cerebellum. Binding of Δ8-THC to CB1 causes an allosteric conformational change in the receptor, activating intracellular signaling cascades. Activated CB1 inhibits adenylate cyclase, leading to decreased levels of cAMP, regulates ion channel activity, and modulates the release of neurotransmitters such as glutamate, GABA, dopamine, and serotonin. This mechanism underlies the influence of Δ8-THC on neuronal excitability, memory, motor control, and pain.
Compared to Δ9-THC, Δ8-THC has a lower affinity for CB1 receptors, but its binding is sufficient to stimulate pharmacological activity, though the effects are generally milder and more sustained. This difference in affinity impacts clinical manifestations: Δ8-THC demonstrates less pronounced psychoactivity, which may be explained by a different spatial positioning of the double bond in the molecule, influencing receptor conformation and affinity.
CB2 receptors, which are primarily located in peripheral tissues including immune cells, spleen, liver, and gastrointestinal tract, also interact with Δ8-THC. Activation of CB2 triggers signaling pathways associated with immune regulation, including activation of MAP kinases (ERK, JNK, p38), leading to altered cytokine expression and reduced inflammation. Thus, Δ8-THC is capable of modulating immune homeostasis, which has potential therapeutic applications in inflammatory and autoimmune diseases.
Additionally, Δ8-THC shows interaction with other receptor systems beyond the cannabinoid system. Notably, its partial agonist effect on vanilloid receptors TRPV1 is linked to modulation of pain and inflammation. Effects on glutamatergic, dopaminergic, and serotonergic systems occur via indirect mechanisms that modulate synaptic transmission, providing a complex pharmacological profile.
Considering all these factors, the interaction of Δ8-THC with receptors reflects a delicate balance between affinity, intrinsic activity, and spatial molecular configuration, determining specific pharmacological effects distinct from other cannabinoids. Further studies on the molecular dynamics of binding and structural features of these interactions are key for developing targeted therapeutics using Δ8-THC.
Potential Therapeutic Effects of Delta-8-THC
Delta-8-tetrahydrocannabinol (Δ8-THC), although less studied than its isomer Δ9-THC, demonstrates a wide range of potential therapeutic properties that make it an interesting subject for pharmacological research. Its unique pharmacological profile, combining moderate affinity for cannabinoid receptors with milder psychoactive effects, creates a foundation for medical use. Below are three key areas of its therapeutic potential-antiemetic effect, analgesia, and influence on psychoemotional state, including anxiety, mood, and appetite.
The antiemetic effect of Δ8-THC is related to its ability to modulate CB1 receptor activity in central areas responsible for controlling the vomiting reflex, particularly in the chemoreceptor trigger zone (CTZ) and the adjacent nucleus in the brainstem. This allows reduction of nausea and vomiting caused by various factors, including chemotherapy, radiation therapy, or other toxic agents. A distinctive feature of Δ8-THC is a milder side effect profile compared to Δ9-THC, making it promising for use in patients with increased sensitivity to psychoactive effects.
The analgesic potential of Δ8-THC manifests through interaction with CB1 receptors in peripheral and central structures involved in pain signal transmission, as well as modulation of TRPV1 receptors responsible for sensing inflammation and thermal pain. Pharmacodynamic studies indicate that Δ8-THC can reduce acute and chronic pain, affecting both somatic and neuropathic pain syndromes. This property is due to its simultaneous influence on multiple neurotransmitter systems, allowing avoidance of tolerance seen with many opioid analgesics.
Regarding psychoemotional effects, Δ8-THC shows the ability to reduce anxiety and improve mood, which is associated with its regulatory action on dopaminergic and serotonergic systems. These effects differ from those of Δ9-THC by being less intense and more stable, potentially providing better tolerability during therapeutic use. There is also evidence that Δ8-THC stimulates appetite, which is important for patients with anorexia, cachexia, or other conditions related to weight loss, especially in oncology patients.
Antiemetic Effect
Delta-8-tetrahydrocannabinol (Δ8-THC) shows significant potential in suppressing nausea and vomiting, which are major challenges in clinical practice, especially among patients undergoing chemotherapy or suffering from various toxic exposures. Its antiemetic activity is associated with the ability to modulate central neural structures that control the vomiting reflex. The most important centers are the chemoreceptor trigger zone (CTZ), located in the medulla oblongata, and the adjacent nucleus, which integrate signals that provoke vomiting.
Δ8-THC affects these areas by binding to CB1 receptors localized on neurons that regulate the release of neurotransmitters such as dopamine and serotonin, which are key in inducing vomiting. As a result, there is decreased excitability of CTZ neurons and reduced activation of the vomiting center. Unlike Δ9-THC, Δ8-THC has a lower psychoactive effect, making it more acceptable for patients who require symptomatic relief without significant psychotropic impact.
In addition to its central action, Δ8-THC may also influence peripheral mechanisms involved in regulating digestion and gastrointestinal motility, further contributing to the reduction of nausea symptoms. Its pharmacokinetic properties provide a sufficiently prolonged effect, enabling use in extended-release regimens.
Given this pharmacological specificity, Δ8-THC is a promising candidate for developing antiemetic drugs, especially in the context of oncology therapy, where the toxicity of traditional agents often limits their use. However, further research is needed to establish optimal dosing, safety, and comparative efficacy with existing standard treatments.
Analgesic Potential
The analgesic properties of Delta-8-THC arise from its complex influence on neural networks that modulate pain signals in both the peripheral and central nervous systems. Its action is realized through activation of cannabinoid receptors, primarily CB1, which are found in high concentrations in the spinal cord and brain regions responsible for processing nociceptive information.
Δ8-THC’s effect on CB1 receptors leads to inhibition of neurotransmitter release that transmits pain signals, such as glutamate and substance P. This results in reduced neuron excitability and increased pain threshold, thereby decreasing the intensity of pain perception. Additionally, Δ8-THC interacts with TRPV1 receptors, which play a role in the transduction of inflammatory and thermal pain, broadening the range of its analgesic effect.
Furthermore, Δ8-THC can affect endocrine and immune mechanisms that amplify or sustain pain syndromes. For example, through activation of CB2 receptors, it reduces inflammation by modulating the production of proinflammatory cytokines, which is particularly relevant in chronic inflammatory conditions.
Compared to traditional opioid analgesics, Δ8-THC has the potential to lower the risk of dependence and adverse side effects such as respiratory depression. Its analgesic effect is milder but sufficient to reduce moderate and even severe pain in certain clinical cases.
Overall, the analgesic profile of Δ8-THC makes it a promising agent for treating various types of pain, including neuropathic, inflammatory, and somatic pain. However, additional preclinical and clinical studies are required to determine its effectiveness compared to existing analgesics, as well as its safety and optimal dosing regimens.
Anxiety, Mood, Appetite
Delta-8-tetrahydrocannabinol (Δ8-THC) demonstrates significant effects on the psychoemotional state, manifested in the regulation of anxiety, improvement of mood, and stimulation of appetite. These effects result from complex interactions with central neural systems that mediate emotional responses, motivation, and energy balance homeostasis.
The regulation of anxiety under the influence of Δ8-THC differs from the more psychoactive Δ9-THC due to its relatively moderate affinity for CB1 receptors and less pronounced psychoactivity. The molecule modulates the activity of glutamatergic and GABAergic neurons in the limbic system, particularly in the amygdala and hippocampus, which play a key role in forming anxiety responses. This balance between excitation and inhibition of neurons contributes to anxiety reduction without the paranoia or panic attacks typical of Δ9-THC. This effect is supported by experimental anxiety models where Δ8-THC demonstrates the ability to normalize behavioral responses without impairing cognitive functions.
Regarding mood, Δ8-THC influences dopaminergic pathways of the mesolimbic system, which stimulates centers of pleasure and reward. This leads to improved emotional tone, increased motivation, and reduced symptoms of depression. The unique conformation of Δ8-THC allows it to act as a partial agonist at CB1 receptors, creating a more controlled and predictable effect compared to Δ9-THC, which is significant for the treatment of affective disorders.
Appetite stimulation is another important therapeutic aspect of Δ8-THC. Activation of cannabinoid receptors in the hypothalamus enhances motivation to eat, including increased sensations of hunger and desire for high-calorie foods. This effect is beneficial for patients with conditions accompanied by weight loss and decreased appetite, such as cancer, HIV infection, chronic infections, and various somatic diseases. The difference with Δ8-THC lies in its reduced frequency of unwanted psychoactive reactions, which promotes better tolerability and improves the quality of life for patients.
Safety Aspects, Toxicology, and Regulation
The safety assessment of Delta-8-tetrahydrocannabinol (Δ8-THC) is critically important in the context of its increasingly widespread availability as a psychoactive agent sold on the market of legal or lightly regulated cannabinoids. Although its structural similarity to Δ9-THC suggests similarities in pharmacodynamics, the safety profile of Δ8-THC cannot be automatically inferred from the characteristics of its isomer. Differences in receptor affinity, metabolic pathways, molecular stability, and usage contexts make a separate analysis necessary. As of now, a significant portion of data on Δ8-THC is limited to preclinical models and non-randomized observations, warranting particular caution in interpreting results within a scientific framework.
Fundamentally, Δ8-THC is a partial agonist of the cannabinoid receptor type 1 (CB1), which is primarily expressed in the central nervous system. Its action is mediated through mechanisms involving inhibition of neurotransmitter release, including glutamate, GABA, dopamine, and acetylcholine. Given these properties, Δ8-THC can affect cognitive processes, motor coordination, emotional perception, and neurovegetative regulation. Therefore, toxicity evaluation should include parameters of general safety as well as specific neuropsychotropic effects that vary depending on dose, duration of use, and individual characteristics.
Currently, preclinical toxicology studies of Δ8-THC are limited and fragmented. Isolated experiments in animal models indicate that the LD50 (the lethal dose for 50% of the population) of Δ8-THC is significantly higher than typical therapeutic or recreational doses. However, lethality in such models is not the only relevant safety criterion. More important are data on sublethal doses affecting behavior, neuroplasticity, cardiovascular regulation, and liver and kidney function. So far, these results remain fragmentary: there are no large-scale studies covering a multisystem analysis of toxicity during chronic administration.
Particular concern arises from the context of synthetic isomerization of Δ8-THC from cannabidiol (CBD), which is often carried out under non-industrial conditions using acidic catalysts, heavy metals, or unstable solvents. The presence of residual acids, by-products of isomerization-including unidentified isomers or oxidized forms-can significantly affect the safety profile of the final product. In such cases, toxicological risk is determined not only by the active substance itself (Δ8-THC) but also by a mixture of opaque impurities remaining after synthesis. Independent chemical analyses show that samples marketed in the U.S. as “pure Delta-8” often contain a substantial proportion of other cannabinoids (e.g., Δ10-THC, Δ6a,10a-THC) and unwanted byproducts that have not undergone any toxicity testing.
Another important aspect is the absence of regulatory standards for purity or pharmaceutical compliance for products containing Δ8-THC. Most such substances do not undergo GMP (Good Manufacturing Practice) control or pharmacopeial standards. Because of this, consumers-especially those who use Δ8-THC for self-medication-are effectively placed in a situation of toxicological uncertainty. This represents a significant threat to vulnerable groups such as patients with oncological, neurological, or psychiatric conditions who seek a legal substitute for Δ9-THC and cannot verify the quality or stability of the product.
Currently, the only reliable source of toxicological validation remains in vitro experiments on cell lines and in vivo studies in animals. Several studies have found that Δ8-THC has effects on synaptic transmission similar to Δ9-THC but exhibits a lesser capacity to cause disorganized behavior or psychomotor disintegration. This opens possibilities for therapeutic use of Δ8-THC but does not eliminate the need for clear standardization, dose control, and understanding of its metabolism mechanisms.
Special attention should be given to Δ8-THC metabolites. The primary metabolite is 11-hydroxy-Δ8-THC, which can cross the blood-brain barrier and affect the central nervous system. Its pharmacokinetics are currently much less studied than those of 11-hydroxy-Δ9-THC, although preliminary data indicate potential activity that may exceed that of the parent compound. The lack of large-scale research in this area limits the ability to accurately predict the duration and intensity of effects, complicating the formulation of dosing recommendations and creating a risk of cumulative toxicity.
Preclinical and Clinical Research Data
Despite the growing popularity of Δ8-THC in certain regions, the volume and quality of empirical research regarding its safety and efficacy remain extremely limited, causing a significant imbalance between its prevalence of use and the scientific understanding of potential risks and benefits. Systematic study of the pharmacological, toxicological, and clinical properties of Δ8-THC is currently in its early stages, and most existing data are based on mid-to-late 20th century experiments or modern in vitro/in vivo tests that are often conducted with methodological limitations. In this context, reviewing available preclinical and clinical data requires a critical approach, with a clear distinction between preliminary assumptions and empirically confirmed conclusions.
The first attempts to study Δ8-THC date back to the 1970s, when, in a context of partial legal vacuum, researchers sought legal substitutes for Δ9-THC that could retain psychoactive and therapeutic properties with reduced potential for side effects. During this period, several small-scale clinical trials were conducted, including studies involving oncology patients who were administered Δ8-THC as an antiemetic agent within palliative care. One such study, published in 1995, reported a significant reduction in chemotherapy-induced vomiting in children who received oral Δ8-THC in encapsulated form. Despite positive outcomes (with effectiveness exceeding 90% in reducing nausea), limitations included a small sample size (only 8 participants), the absence of a control group, and a short observation period. No subsequent studies have confirmed or refuted these findings within randomized controlled trials.
To date, clinical studies of Δ8-THC have not even reached the extent characteristic of other rare cannabinoids. Existing studies are limited to incidental retrospective observations or informal consumer surveys. In such studies, patients usually self-report Δ8-THC use for pain relief, anxiety reduction, appetite stimulation, or sleep improvement. However, the lack of objective biomarkers, standardized dosing, and double-blind design makes these data more a source of hypotheses than definitive claims.
Overall, among clinically relevant questions currently lacking empirical answers are: the optimal therapeutic dose of Δ8-THC for specific pathologies; the side effect profile across different age and somatic groups; pharmacokinetics via various administration routes (oral, inhalational, transdermal); and interactions with other medications, particularly CNS depressants, antidepressants, or antipsychotics. The absence of quality control over substance sources makes even extrapolation of results to commercially available products impossible, as these rarely undergo proper chemical purification and testing.
Preclinical models, primarily in vivo studies on rodents, constitute the main body of data on Δ8-THC’s effects. They allow evaluation of basic neurobiological effects, addiction potential, interaction with other receptor systems, and influence on overall metabolism. In particular, in mice, Δ8-THC induces a significant reduction in overall motor tone, analgesia, hypothermia, and catalepsy-signs typical for CB1 agonist-class cannabinoids. However, the intensity of these effects is lower compared to Δ9-THC, confirming less pronounced psychoactivity, though not eliminating the potential for cognitive or affective impairments.
Also noteworthy are data on the neuroprotective effects of Δ8-THC. In several in vitro experiments, a reduction of neuron apoptosis induced by oxidative stress was observed after pre-incubation with Δ8-THC. The mechanisms of this effect are likely related to CB1 receptor activation, but additional involvement of signaling cascades such as PI3K/AKT or ERK1/2 cannot be excluded, indicating the potential of Δ8-THC in the context of neurodegenerative conditions. Nevertheless, none of these models has been confirmed in clinical scenarios, and extrapolation from cell cultures to human tissue requires a much more cautious approach.
Another important direction is pharmacokinetic research. Several preclinical studies using mass spectrometry have established that Δ8-THC has moderate bioavailability when taken orally and a high degree of lipophilicity, leading to accumulation in fatty tissue. This creates conditions for prolonged action but also increases the risk of unpredictable accumulation with long-term use, especially in patients with metabolic disorders or excessive body weight. Data on half-life periods and interactions of Δ8-THC with liver metabolism enzymes (such as CYP3A4, CYP2C9) are still lacking, although current observations suggest metabolic pathways similar to those of Δ9-THC.
Potential Risks and Side Effects
The question of safety regarding the use of Δ8-tetrahydrocannabinol (Δ8-THC) must be considered in the context of its biological activity, chemical instability, potential for metabolic biotransformation, as well as the lack of pharmaceutically standardized formulations. Unlike Δ9-THC, the toxicity profile of Δ8-THC remains insufficiently defined not only due to a lack of high-quality clinical research but also because of the dominance of uncontrolled distribution channels, particularly synthetic products derived from cannabidiol (CBD). In such an environment, forming a reliable understanding of side effects is extremely difficult, especially considering the various methods of administration, dosing, contaminants, and uneven bioavailability.
One of the key threats associated with Δ8-THC is the absence of molecular purity in most commercially available samples. As is known, Δ8-THC naturally occurs in Cannabis plants at extremely low concentrations, so most compounds considered Δ8-THC in commercial circulation are actually products of synthetic or semi-synthetic isomerization of CBD. This process, which typically takes place in acidic conditions with catalysts, generates numerous byproduct isomers, oxidized compounds, and unidentified intermediates that remain in the final product due to the lack of chromatographic purification. Such impurities may possess their own bioactivity or potential toxicity about which science knows almost nothing.
Retrospective data and reports to adverse event monitoring systems indicate a growing number of complaints associated with intoxication following Δ8-THC consumption. Among the most common symptoms are paranoid states, disorientation, psychomotor dysfunction, vestibular disturbances, tachycardia, hypotension, dry mouth, nausea, confusion, and seizure-like reactions. Some of these symptoms may be caused not only by the action of Δ8-THC as a CB1 agonist but also by the effects of byproduct substances in the unrefined mixture. For example, unidentified chlorinated or acetylated byproducts resulting from improper synthesis may exhibit neurotoxic effects or interact with liver enzymes, disrupting the metabolism of other drugs.
Another significant risk factor is inhalation forms of Δ8-THC, particularly vape cartridges. When substances containing residual acidic catalysts or polymer impurities are heated, toxic volatile products form, including acetates, aldehydes, phenols, and benzene derivatives. Similar mechanisms have been documented with acetate forms of Δ8-THC heated above 200°C. Particularly concerning is the potential formation of ketene-a potent alkylating agent with high toxicity to respiratory epithelium. In cases of mass intoxication in the United States in 2021 associated with Δ8-THC products, acetate and unrefined substances in vape products were the primary suspects.
From a neuropsychiatric perspective, Δ8-THC has the potential to cause cognitive impairments, especially in adolescents, individuals with a family history of mental disorders, or when combined with other psychoactive substances. Although its psychoactivity is somewhat lower than Δ9-THC, even moderate stimulation of CB1 receptors in the central nervous system can cause dysregulation of dopaminergic and glutamatergic pathways, particularly in the prefrontal cortex. This manifests as transient disturbances in attention, memory, sleep, and vulnerable individuals may experience panic attacks, psychotic episodes, or emotional destabilization.
Another under-researched vector of risk is the hepatotoxic potential of Δ8-THC. It is known that synthetically derived cannabinoids may exhibit direct cytotoxicity to hepatocytes at high doses or with prolonged use. Δ8-THC is likely metabolized by CYP2C9 and CYP3A4 enzymes, which implies its ability to compete with other substrates of these systems, including antihypertensive agents, anticoagulants, and antiepileptic drugs. While direct evidence of Δ8-THC hepatotoxicity is lacking, isolated cases of elevated liver enzyme levels in users indicate the need for further study in this area.
Additionally, there is the issue of cumulative effects. The lipophilic nature of Δ8-THC causes it to be stored long-term in fat deposits with slow release into systemic circulation. In the context of chronic use, this may lead to unexpected behavioral changes, prolonged psychomotor depression, or tolerance to other psychoactive substances. Although direct indicators of physiological dependence in experimental models are considered lower than for Δ9-THC, repeated use of Δ8-THC may cause psychological dependence or habituation at the level of conditioned reflexes.
It should also be noted that most modern Δ8-THC products do not undergo regulatory certification, so risks related to contamination with heavy metals, pesticides, solvents (heptane, toluene, ethanol), or microbiological agents (mold, bacteria) remain high. This makes any safety assessments of such products inaccurate until standards for impurity content, standard validation methods, and quality control are implemented.
Legal Status Worldwide and Research Challenges
The legal status of Δ8-tetrahydrocannabinol (Δ8-THC) today is one of the most controversial aspects in cannabinoid policy. This compound has not been formally included on the list of controlled substances in most jurisdictions for a long time, which has led to its widespread availability, especially in countries with liberalized cannabinoid regulations. However, the legal ambiguity surrounding Δ8-THC did not arise by chance – it is a direct consequence of the gap between regulatory frameworks based on the classification of natural cannabinoids and the reality dominated by semi-synthetic analogs derived from cannabidiol (CBD).
The 1961 Single Convention on Narcotic Drugs, ratified by most countries, does not mention Δ8-THC as a separate compound. A clearer definition appeared in the World Health Organization’s 2020 recommendations, when it was proposed to consider Δ8-THC as an isomer of Δ9-THC with similar psychoactive potential. However, this proposal was not made mandatory. Thus, at the global level, Δ8-THC often remains outside formal regulatory controls unless it is directly synthesized from Δ9-THC or obtained from cannabis plants containing it in concentrations higher than those allowed by national laws.
In the United States, the regulatory situation around Δ8-THC is especially illustrative. After the passage of the 2018 Farm Bill, which legalized hemp and all cannabinoid derivatives with Δ9-THC concentrations below 0.3%, a legal loophole emerged allowing mass production of Δ8-THC from legal CBD. Because Δ8-THC was not explicitly listed on the federal controlled substances list, it began to be sold in stores, online platforms, and vape distributors, marketed as a “legal alternative” to traditional Δ9-THC. However, starting in 2021, several states-including New York, Colorado, Alaska, and Utah-began implementing restrictions or full bans on its distribution, citing the lack of quality regulation, toxicological data, and uncontrolled availability to minors.
Regulatory agencies, including the U.S. Food and Drug Administration (FDA), have started raising concerns about the uncontrolled Δ8-THC market. In 2022, the FDA officially warned consumers about potential dangers, noting frequent cases of intoxication and hospitalizations related to Δ8-THC product use. However, the FDA emphasized that it does not have the authority to ban these substances without legislative classification of their legal status. As a result, a compound with psychoactive effects similar to Δ9-THC is spreading within a legal framework that only partially acknowledges its existence.
In the European Union, the legal regulation of Δ8-THC depends on national legislation. In most countries, it is not specifically listed as a controlled substance, but its legal status is interpreted as a derivative of Δ9-THC or as a synthetic cannabinoid, which automatically subjects it to prohibition. For example, in Germany and France, all psychoactive cannabinoids not included in officially approved medications (such as dronabinol) are considered prohibited. In the United Kingdom, Δ8-THC is classified as a Class B substance under the Misuse of Drugs Act, legally equated with Δ9-THC. Meanwhile, in Eastern European and Balkan countries, the situation is more heterogeneous: regulation partially relies on technical documentation related to hemp raw materials rather than specific identification of Δ8-THC as a substance.
One of the main challenges in researching Δ8-THC lies in difficulties obtaining pure substance legally. In most jurisdictions, there are no pharmaceutical-quality standards for Δ8-THC, no certified analytical methods, or limits on permissible impurities. This complicates the conduct of clinical studies, toxicokinetics, and drug interaction research. Furthermore, the pharmacological variability of semi-synthetic samples circulating on the market prevents definitive conclusions based on laboratory experience, since each batch may have its own spectrum of impurities and isomers.
Another systemic barrier to Δ8-THC research is the lack of international harmonization in defining “psychoactivity.” Many countries’ legislations do not have clear criteria to determine what is considered a psychoactive substance among cannabinoids. Accordingly, Δ8-THC may be classified as “non-psychoactive” in countries where its effect level is not comparable to Δ9-THC, or conversely, automatically categorized as a prohibited substance. This regulatory discrepancy creates obstacles not only for science but also for the pharmaceutical industry, which requires a predictable legal environment to develop and register new drugs.
It is also important to note the legislative inertia regarding novel cannabinoids. Existing regulations are often based on outdated classifications or approaches to fully synthetic compounds, without considering that Δ8-THC has a semi-natural origin, a structure close to Δ9-THC, and can be synthesized from compounds that are formally legal. This creates a regulatory vacuum where authorities lack sufficient grounds for prompt intervention, and the scientific community does not gain access to controlled, verified samples for research.
Prospects and Directions for Future Research
Biomedical Applications
Scientific interest in the biomedical potential of Δ8-tetrahydrocannabinol (Δ8-THC) has noticeably increased in recent years due to its unique pharmacodynamic characteristics, lower psychoactive profile compared to Δ9-THC, and potential therapeutic benefits in a range of pathological conditions. However, unlike the well-studied Δ9 isomer, Δ8-THC remains clinically underexplored. While most current knowledge comes from preclinical studies or early clinical protocols, new animal models and cell culture research suggest a significantly broader potential than previously anticipated.
Unlike Δ9-THC, which demonstrates high affinity for CB1 receptors in the cerebral cortex, Δ8-THC has somewhat reduced affinity for these structures but simultaneously shows activity at peripheral CB2 receptors, especially in immune system cells and the gastrointestinal tract. This pharmacological profile makes it an attractive candidate for developing drugs targeting immunomodulatory, anti-inflammatory, antiemetic, and gastroprotective effects without excessive neuropsychiatric stimulation. This involves not only general symptomatic applications but also potential roles in treating chronic and systemic diseases-such as inflammatory enteropathies, neurodegenerative syndromes, malignant tumors, and post-traumatic disorders.
Among the first documented clinical applications of Δ8-THC were antiemetic protocols in pediatric oncology patients published as early as the 1990s. Early studies indicated high effectiveness of Δ8-THC in reducing the frequency of nausea and vomiting attacks associated with chemotherapy-with minimal cognitive side effects. Although these results did not lead to widespread clinical adoption into pharmacotherapeutic standards, they laid the groundwork for further research into using Δ8-THC for persistent nausea, functional gastrointestinal disorders, and cyclic vomiting syndrome.
Another promising direction is the use of Δ8-THC for chronic non-cancer pain, particularly in patients with neuropathies, fibromyalgia, and chronic pelvic pain. Preliminary preclinical models demonstrate that Δ8-THC can mediate analgesic effects not only through cannabinoid receptors but also indirectly through desensitization of TRPV1 channels and modulation of prostaglandin expression in inflamed tissue. At the same time, there is a lower likelihood of psychiatric disturbances or dysphoria compared to Δ9-THC, opening prospects for using Δ8-THC in pain therapy for vulnerable patient populations-elderly individuals, those with anxiety-depressive backgrounds, or patients with comorbid central nervous system disorders.
Another possible clinical application is using Δ8-THC as an appetite stimulant in cachexia caused by AIDS, cancer, or neurodegenerative processes. Animal experiments have recorded increased feeding activity as well as modified expression of ghrelin and leptin-hormones involved in hunger regulation. Given Δ8-THC’s less pronounced psychoactivity compared to Δ9-THC, it may become more suitable for long-term use in patients with anorexia of various origins, including geriatric cases.
The potential of Δ8-THC in mental health is of particular interest. Despite some structural similarities with Δ9-THC, the Δ8 isomer shows noticeably lower tendencies to induce anxiety, panic attacks, or paranoia, which are characteristic of high doses of Δ9-THC in sensitive individuals. Conversely, some animal models demonstrate anxiolytic effects linked to activation of CB1 receptors in the hippocampus and prefrontal cortex. This allows consideration of Δ8-THC as a possible molecular platform for developing new anxiolytics, especially in combination with other non-psychoactive phytocannabinoids or terpene-modulating agents.
Some pilot observations also suggest potential for Δ8-THC in treating post-traumatic stress disorder (PTSD), where the combination of reducing emotional tension and suppressing intrusive memories is crucial. These effects are mediated through cannabinoid modulation of amygdalo-hormonal pathways. Although the evidence base here is still weak, neuropharmacological foundations justify conducting controlled clinical trials.
In dermatology, the possibility of using Δ8-THC as a topical immunomodulator for chronic skin conditions-including atopic dermatitis, psoriasis, and seborrheic dermatitis-is being studied. In vitro studies show that Δ8-THC can affect interleukin-6 and TNF-α production in keratinocytes as well as modulate CB2 receptor expression in the skin barrier. This points to the prospect of developing next-generation topical anti-inflammatory agents based on Δ8-THC or its structural analogs.
In oncology, Δ8-THC is primarily studied as a supportive therapy agent-to reduce chemotherapy side effects, stimulate appetite, alleviate pain, and improve quality of life. However, some preclinical experiments have also observed signs of direct antiproliferative effects of Δ8-THC on certain tumor cell lines, including gliomas, melanomas, and breast carcinomas. The presumed mechanism of action involves apoptosis induction through intracellular p38 MAPK signaling pathways and inhibition of angiogenesis. Nevertheless, these data require cautious interpretation, as most such effects were observed at concentrations unlikely to be achieved in vivo without toxic consequences.
Δ8-THC may serve as a model molecule for developing future cannabinoid drugs with narrow target specificity. Its pharmacophore structure allows relatively straightforward modification of the side chain, aromatic ring, or double bond to create compounds with selective action on CB2 receptors or modulation of other receptor systems-such as GPR55, TRPV1, or PPAR-γ. This opens opportunities not only for creating new drugs but also for fundamentally understanding the principles of cannabinoid binding selectivity.
Chemical Modification and Design of New Molecules
Delta-8-tetrahydrocannabinol (Δ8-THC), due to its stable cyclic structure, chemical similarity to Δ9-THC, and simultaneously reduced psychoactivity, serves as a convenient platform for creating new chemical-pharmaceutical agents. Its structure allows for diverse modifications aimed both at increasing receptor selectivity and improving pharmacokinetic properties or reducing metabolic instability. In this context, Δ8-THC is considered not only as a natural molecule but also as a pharmacophore-a basic skeleton that can be purposefully altered to obtain semi-automated lipophilic or amphiphilic derivatives with new biological properties.
One of the most promising directions of chemical modification of Δ8-THC is variation of the side alkyl chain at the 3-position of the benzene ring. In natural Δ8-THC, this chain is a pentyl group (C5H11), which is a key factor for hydrophobic interaction with cannabinoid receptors, especially CB1. Increasing the length of this chain to heptyl or replacing it with cyclic or aromatic structures can significantly change receptor affinity, as well as alter the binding profile with CB2 or even other molecular targets. For example, in a number of experiments, Δ8-THC derivatives with cyclohexyl or terpene residues demonstrated high selective affinity for CB2 receptors, reducing effects on the central nervous system. This opens the way for developing anti-inflammatory agents devoid of psychoactive potential.
A second important vector is modification of the double bond between the 8 and 9 positions of the C ring. In Δ9-THC, this bond is at the 9-10 position, which largely determines its affinity for CB1. Shifting or hydrogenating the double bond in Δ8-THC not only stabilizes the molecular structure but also reduces susceptibility to oxidative metabolism with formation of reactive intermediates. For instance, dehydrohexylated derivatives of Δ8-THC show better stability in the presence of cytochrome P450, making them promising for oral use or extended-release formulations. Additionally, saturation of the double bond leads to the formation of new tetrahydrocannabinol analogs that retain the ability to bind cannabinoid receptors but exhibit altered pharmacological properties.
Significant interest is also directed toward Δ8-THC derivatives with modified functional groups on the phenolic hydroxyl at position 1. This part of the molecule participates in hydrogen bonding with the CB1 receptor, and its acylation, ethylation, or carbamate formation allows manipulation of solubility, duration of action, and bioavailability. In some cases, such modifications are aimed at creating prodrugs activated by gastrointestinal or liver enzymes. This helps overcome first-pass metabolism limitations characteristic of cannabinoids and stabilizes the active substance until release in the target tissue.
A new class of molecules is also being formed based on hybridization of Δ8-THC with other bioactive fragments, for example, fragments of non-steroidal anti-inflammatory drugs, β-carbolines, or even serotonin antagonist structures. Such molecules are designed for polypharmacology-the ability to interact with multiple targets simultaneously, which is especially relevant in pain, inflammation, oncology, or mental disorders. In this context, Δ8-THC serves less as a targeted agent and more as a carrier or pharmacological module in multicomponent structures. Although these approaches remain mostly at the in silico design or preclinical model stages, available data confirm the promise of such a structural design approach.
Another direction involves conjugating Δ8-THC with peptide or polysaccharide residues to create new ligands with targeted action. This can be realized as a targeted delivery strategy-for example, for intestinal or brain lesions where a high local concentration of the cannabinoid agent is required with minimal systemic burden. Conjugation with mannose or galactose allows, in particular, targeting molecules to specific receptors of the colon epithelium or microglia. This approach holds promise not only in pharmacology but also in molecular diagnostics, where Δ8-THC can serve as a contrast agent for visualizing inflammation or glial lesions.
Moreover, there is potential for modifying Δ8-THC toward creating non-cannabinoid analogs-compounds that do not bind classical cannabinoid receptors but still possess some characteristic effects such as anti-inflammatory or gastroprotective activity. This is especially relevant in conditions of regulatory restrictions or concerns about cannabinoid safety. Replacement of the C ring or partial structural reorganization toward bicyclic or linear systems allows for obtaining compounds with new mechanisms of action-specifically, through PPAR-α/γ, GPR55, or endocannabinoid system receptors.
The application of computer modeling methods, including docking, dynamic modeling, and molecular phylogenesis, allows prediction of which structural modifications of Δ8-THC might be most effective for binding to desired receptors. In this context, synthetic chemistry interweaves with bioinformatics and pharmacogenomics, opening possibilities for personalized development of cannabinoid drugs.
Finally, it should be emphasized that every chemical modification of Δ8-THC requires thorough toxicological analysis. Even minor structural changes can drastically impact biosafety, including hepatotoxicity, immunosuppression, or psychoactive effects. Therefore, the creation of new Δ8-THC derivatives requires an interdisciplinary approach involving chemists, pharmacologists, biophysicists, and toxicologists to ensure not only efficacy but also a predictable safety profile.
Challenges in Standardization and Quality Control
With the growing interest in Delta-8-THC as a pharmacologically active substance, a complex problem of standardization arises, encompassing all stages—from initial production to the final product. In conditions where the substance can originate from multiple synthetic or semi-synthetic sources, exhibit polymorphic chemical purity, and exist in various matrices (oils, capsules, inhalers, transdermal forms), ensuring stability, identity, and safety in each preparation requires not only the development of strict standards but also a revision of the methodological base of modern pharmacopeial control.
One of the main challenges is the ambiguity in defining the “reference” composition of the substance. In the context of Δ8-THC, the standard cannot be limited only to the mass fraction of the main isomer. Due to its synthetic origin, byproducts—regioisomers, residual acids, side cannabinoids (especially Δ10, Δ6a,10a, or dehydrohexylated derivatives)—are almost always present, which have their own bioactivity or may interact with the body’s enzymatic systems. This means that full chemical characterization must include a multicomponent analysis—covering isomeric profile, quantitative assessment of residual reagents, as well as characterization of secondary metabolites that may form in vivo or during storage.
The complexity of standardizing Δ8-THC also stems from the absence of globally accepted analytical methods to differentiate it from Δ9-THC, especially in mass laboratories and toxicology centers. The isomers have identical molecular masses, similar chromatographic properties, and are barely distinguishable by conventional spectrophotometric tests. As a result, there is a risk of both falsifications (for example, selling Δ9-THC under the guise of Δ8) and errors in legal disputes or clinical contexts. The creation of certified standards with clear boundary separation, supplemented by nuclear magnetic resonance spectroscopy (^1H- and ^13C-NMR), high-performance liquid chromatography with mass spectrometric detection (HPLC-MS/MS), and chiral gas chromatography, is not merely desirable but critically necessary.
A separate issue is the stability of Δ8-THC in various formulations. Due to its susceptibility to oxidation and isomerization back to Δ9- or Δ6a,10a-THC under slight heating or light exposure, irreversible changes in composition occur. This calls into question the actual dosed efficacy in the final product, especially during prolonged storage. This requires not only input substance control but also stability validation of each pharmaceutical form—taking into account packaging, temperature, humidity, oxygen levels, and type of solvent or carrier (e.g., MCT oil-based media, ethanol, or PEG-400). Standardization must include specific degradation profiles under accelerated storage conditions (ICH Q1A), determining decay kinetics and toxicity of degradation products.
An even more complicated problem is the assessment of residual impurities—acids (such as p-toluenesulfonic acid), solvents (hexane, acetone, diethyl ether), catalysts (boron trifluoride, phosphoric acid), or stabilizers. Because Δ8-THC synthesis is typically not conducted under pharmacopeial control and usually takes place in semi-industrial setups, impurities can be toxic upon systemic accumulation. Standardization must cover not only allowable limits but also mandatory declarations of purification methods, residual reagent analyses, and evidence of their complete removal—in accordance with ICH Q3A and Q3C requirements.
Quality control problems are further complicated by the commercial heterogeneity of products. Today, Δ8-THC is sold in the form of oils, vapes, capsules, edibles, cosmetics, and even veterinary medicines. Each form involves a different route of administration, varying absorption rates, metabolism, and elimination. This makes it impossible to create a universal standard scheme for all forms. Even with identical dosing, bioavailability may vary several-fold depending on the formulation. Therefore, quality must be controlled not only at the substance level but also at the final product level in a specific delivery form.
Another challenge is the gap between regulatory and scientific quality control aspects. In most jurisdictions, Δ8-THC lacks clear classification in pharmacopeias, and manufacturers rely on standards borrowed from Δ9-THC or CBD. This is a flawed practice since the properties of these substances—including solubility, stability, and chemical reactivity—differ significantly. Thus, independent pharmacopeial monographs must be developed that consider the specificity of Δ8-THC as a substance and active ingredient. This applies to titration methods, spectroscopy, chromatography, as well as requirements for moisture, pH environment, and microbiological purity control mechanisms—especially in products intended for inhalation or parenteral administration.
It is also important to mention the challenge of reproducibility of research. Due to the lack of unified standards for Δ8-THC, different research groups work with products of varying purity, sources, and production methods. This creates systemic difficulties in comparing results even under similar experimental conditions. Therapeutic efficacy, toxicity, pharmacokinetics—all depend on what exactly is called “Δ8-THC” in each specific case. Solving this problem is possible only through the creation of an international reference standard, approved by WHO, the European Pharmacopoeia, or USP, with detailed specifications, verification methods, stability criteria, and interlaboratory calibration validation.
Conclusions
Delta-8-tetrahydrocannabinol (Δ8-THC) represents a complex subject of study that combines chemical uniqueness, pharmacological potential, and significant technological and regulatory challenges. Scientific analysis of this molecule-from the perspective of its chemical structure, natural origin, methods of production, pharmacology, safety, and legal status-creates a comprehensive understanding of the potential and limitations underlying its further development as a pharmaceutical or bioactive agent.
The chemical nature of Δ8-THC is defined by a distinctive constitutional isomerism that determines its specific interaction with cannabinoid receptors, as well as its stability under various physicochemical conditions. Although its molecular formula matches that of Δ9-THC, the position of the double bond in the molecular chain fundamentally affects its conformation, capacity for isomerization, chemical reactivity, and biological behavior. These fundamental differences hold not only academic significance but also practical impact on pharmacokinetic and pharmacodynamic profiles, which are still being studied in laboratory and clinical settings.
The natural origin of Δ8-THC involves an extremely low content in cannabinoid plants of the Cannabis genus, making direct extraction of this substance from plant material practically ineffective for mass use. The biosynthetic pathways forming this isomer remain insufficiently studied, especially in the context of environmental factors that may correlate with the quantitative and qualitative composition of cannabinoids. In fact, its presence is regarded more as a byproduct of endogenous enzymatic processes or the result of degradation of other cannabinoids under physical and chemical influences. This creates a basis for searching for alternative production methods, as technological demands for synthetic or semi-synthetic methods remain crucial.
Technological approaches to synthesizing Δ8-THC demonstrate significant scientific and technical progress, particularly in the isomerization of cannabidiol (CBD) under acidic conditions. The use of catalysts, regulation of temperature and reaction time parameters, and application of various solvents and purification methods form the foundation for producing high-purity products with good yield. However, the presence of impurities and the complexity of controlling isomers and residual reagents maintain the relevance of quality concerns. Meanwhile, promising biotechnological methods-such as enzymatic catalysis, genetically modified microorganisms, and cell cultures-open new horizons for more environmentally safe and economically efficient production. These approaches, however, require further fundamental study of biosynthetic mechanisms and optimization of the expression of key enzymes defining the synthetic pathway to Δ8-THC.
The pharmacological profile of Δ8-THC reveals several important characteristics. Interaction with cannabinoid receptors CB1 and CB2 confirms its biological activity, though binding profiles and affinities differ somewhat from Δ9-THC, affecting clinical effects. Psychoactivity, while less pronounced compared to Δ9-THC, does not exclude its clinical potential. The most promising therapeutic properties include antiemetic action, one of the best-documented effects, as well as analgesic effects and influence on psychoemotional states, including anxiety reduction and appetite regulation. All these aspects require further research focused on mechanisms of action, effective dosages, and potential side effects.
Special attention should be given to analyzing safety, toxicology, and regulatory restrictions, which remain extremely complex due to limited preclinical and clinical data. Defining a safety profile requires expanded large-scale studies that include long-term observation of effects on various body systems, as well as investigation of potential toxicity of degradation products and impurities that may accumulate during synthesis. The legal ambiguity of Δ8-THC status in different countries and regions creates additional barriers for standardized evaluation and testing procedures, and the absence of unified pharmacopeial monographs complicates quality control, directly affecting patient and consumer safety.
Challenges related to standardization and quality control represent one of the biggest “bottlenecks” in the development of Δ8-THC as a pharmaceutical substance. The diversity of synthetic methods, heterogeneity of final products, presence of isomers and accompanying substances, variability in bioavailability across different forms of administration-all these demand the formation of a comprehensive control system that integrates analytical, technological, toxicological, and regulatory criteria. Only by fulfilling these conditions can reproducibility of research results, safety of patients and consumers, and legitimacy of Δ8-THC use in medical practice be guaranteed.
Regarding future directions, biomedical applications of Δ8-THC open broad opportunities for developing new therapeutic agents with efficacy and safety profiles distinct from classical cannabinoids. Chemical modification of the molecule, including the creation of derivatives and analogs, potentially allows improvement of pharmacokinetics, increased receptor selectivity, and minimization of side effects. At the same time, standardization and quality control remain critically important aspects for translating fundamental knowledge into clinical solutions.
Overall, Delta-8-THC is a complex, multifaceted object combining unique chemical, biological, and technological properties. Studying this molecule requires an interdisciplinary approach that integrates chemistry, biology, pharmacology, toxicology, and regulatory science. Only through the interaction of these fields is it possible to create a reliable platform for the development of Δ8-THC as a safe and effective bioactive agent with potential medical and commercial applications in the future.
Sources:
- National Center for Biotechnology Information (NCBI), PubMed Central (PMC) – a database of scientific articles in the fields of biomedicine and pharmacology:
https://www.ncbi.nlm.nih.gov/pmc/ - ScienceDirect – Elsevier’s platform with a large collection of publications in chemistry, pharmacology, and biotechnology (some content is open access):
https://www.sciencedirect.com/ - Journal of Cannabis Research – an open-access journal with current research on cannabinoids:
https://jcannabisresearch.biomedcentral.com/ - U.S. National Library of Medicine (NLM) – the official U.S. health resource containing scientific materials about cannabinoids:
https://www.nlm.nih.gov/ - Frontiers in Pharmacology – open access, section on cannabinoid pharmacology:
https://www.frontiersin.org/journals/pharmacology - ResearchGate – a scientific network with a large number of open research articles, where you can search for studies on Delta-8-THC:
https://www.researchgate.net/ - Journal of Natural Products – the official journal of the American Chemical Society focused on natural compound research:
https://pubs.acs.org/journal/jnprdf - European Monitoring Centre for Drugs and Drug Addiction (EMCDDA) – reports and scientific documents on cannabinoids and their effects:
https://www.emcdda.europa.eu/ - Harvard Health Publishing – publications and reviews on the medical use of cannabinoids from Harvard Medical School:
https://www.health.harvard.edu/ - U.S. Food and Drug Administration (FDA) – official documents and statements regarding cannabinoid regulation:
https://www.fda.gov/ - American Chemical Society (ACS) Publications – a large collection of peer-reviewed articles in chemistry and pharmacology:
https://pubs.acs.org/ - Cannabis and Cannabinoid Research (Mary Ann Liebert, Inc.) – a specialized journal with open access to selected articles:
https://www.liebertpub.com/can - The Journal of Clinical Pharmacology – open and peer-reviewed research on clinical pharmacology of cannabinoids:
https://accp1.onlinelibrary.wiley.com/journal/15524604 - World Health Organization (WHO) – official reports on the safety and pharmacology of cannabinoids:
https://www.who.int/ - National Institute on Drug Abuse (NIDA) – scientific reviews, research data, and publications on cannabinoids:
https://www.drugabuse.gov/