Within the scope of research on natural compounds that modulate the endocannabinoid system, cannabinoids remain some of the most intriguing subjects from the perspectives of both fundamental chemistry and pharmacology. Over one hundred phytocannabinoids have been identified in species of the Cannabis genus, most of which exist in their acidic forms-as secondary metabolites synthesized in the glandular trichomes of the plants. Among these, the most extensively studied are Δ⁹-tetrahydrocannabinolic acid (Δ⁹-THCA), cannabidiolic acid (CBDA), and cannabigerolic acid (CBGA). Their active forms-Δ⁹-THC, CBD, and CBG, respectively-are formed through decarboxylation triggered by heat or time. However, with growing interest in the structural diversity of cannabinoids in the scientific community, attention has increasingly turned toward less studied isomers and derivatives, including Δ⁸-tetrahydrocannabinolic acid (Δ⁸-THCA).
Δ⁸-THCA should not be confused with Δ⁸-THC, the psychoactive isomer of Δ⁹-THC, which has recently become the focus of both media attention and regulatory scrutiny. Although Δ⁸-THC can be found in trace amounts in Cannabis sativa L. plants, it is typically a product of artificial isomerization from Δ⁹-THC. Accordingly, Δ⁸-THCA, which is its acidic precursor, is currently considered predominantly a laboratory-derived compound, although its theoretical natural origin has not been fully ruled out. Chemically, Δ⁸-THCA is a structural isomer of Δ⁹-THCA, in which the double bond in the cyclohexene ring has shifted from the Δ⁹ to the Δ⁸ position. This change is not trivial given its potential impact on the biological activity, metabolism, and pharmacokinetics of the compound. Nevertheless, research on Δ⁸-THCA remains in its early stages, and a comprehensive review of its properties, methods of synthesis, and scientific rationale is currently absent from the professional literature.
A key question arising in the study of Δ⁸-THCA is its place in the chemotype of cannabis plants. In the known biosynthetic pathways of cannabinoids, CBGA serves as the precursor for THCA, CBDA, and CBCA, depending on the enzymatic activity of the respective synthases. There is no reliable evidence for the existence of a specific Δ⁸-THCA synthase. This raises doubts about the natural formation pathway of Δ⁸-THCA in hemp plants grown under agricultural or wild conditions. Instead, isomerization of Δ⁹-THCA to Δ⁸-THCA under laboratory conditions is possible through acid-catalyzed mechanisms similar to those used for converting Δ⁹-THC to Δ⁸-THC. The stability of Δ⁸-THCA under various conditions, including before and after decarboxylation, is of particular interest given the potential pharmacological differences between the isomers.
Despite its structural similarity to Δ⁹-THCA, Δ⁸-THCA may exhibit a different interaction profile with cannabinoid receptors (CB1/CB2), especially considering previous observations regarding the less pronounced psychoactivity of Δ⁸-THC compared to Δ⁹-THC. At the same time, like all acidic forms of cannabinoids, Δ⁸-THCA likely has a distinct mechanism of action that is not related to direct CB1 activation. Early animal studies indicate potential anti-inflammatory, antiemetic, and neuroprotective activities of acidic cannabinoids, including THCA. If similar effects apply to Δ⁸-THCA, this compound could be of interest as a foundation for developing new pharmaceuticals that are not associated with psychoactive effects.
It is also important to consider scientific and technical limitations related to studying Δ⁸-THCA. First, the analytical isolation of this compound from extracts is a challenging task due to its structural similarity to Δ⁹-THCA and its tendency to decarboxylate even under slight heat exposure. Second, the lack of certified analytical standards significantly complicates qualitative and quantitative analysis of this compound using chromatography or mass spectrometry. Consequently, most available data on Δ⁸-THCA are based on indirect methods or synthetic models, which creates an additional challenge for its systematic investigation.
Another aspect deserving special attention is the regulatory and legal status of Δ⁸-THCA. In most countries, cannabinoid legislation focuses on psychoactive forms (THC) and their quantitative content in finished products. Acidic forms are often excluded from regulatory categorization or classified depending on their ability to convert into active compounds upon heating. This creates a so-called “gray area” in regulation, which on one hand allows manipulation of the compound’s legality, and on the other hand complicates its integration into pharmaceutical research according to proper clinical practices.
Cannabinoid System of Cannabis: Biochemical Context
The cannabinoid system of cannabis (Cannabis sativa L.) is a complex metabolic and regulatory network that has evolved under the influence of specific biotic and abiotic factors. At the molecular level, it is represented by multiple enzymatic pathways involved in the synthesis, transformation, transport, and accumulation of cannabinoids-compounds that play a fundamental role in the physiology of the plant itself, as well as exhibiting potent pharmacological activity in mammals, including humans. However, it is important to emphasize that the cannabinoid system of Cannabis sativa is not an isolated biosynthetic pathway of secondary metabolites, but rather an integrated part of the plant’s overall metabolome, dynamically interacting with central metabolism, hormonal regulation, and stress responses.
The biosynthetic processes responsible for cannabinoid formation involve a close interaction of two main metabolic sources: the acetate-malonate pathway, which provides the formation of olivetolic acid, and the isoprenoid pathway, which leads to geranyl pyrophosphate (GPP). The fusion of these two molecules, catalyzed by a specific enzyme-GPP-olivetolate transferase-results in the formation of cannabigerolic acid (CBGA), which is the key biochemical hub from which all major cannabinoid structures derive. Since CBGA serves as the common precursor for THCA, CBDA, CBCA, and other derivatives, the regulation of its synthesis and subsequent routing into one of the catabolic branches determines the chemotype of the plant.
This biochemical ensemble is localized within highly specialized structures-glandular trichomes, primarily of the capitate type-which provide physical separation of synthesized compounds from the internal tissues. This isolation has evolutionary value, as it allows the plant to accumulate potentially cytotoxic compounds without harming itself. Additionally, the trichomes ensure effective concentration of metabolites, their protection from degradation, as well as modulated secretion into the surrounding environment. Trichome biogenesis is a genetically coordinated process but is significantly modulated by external factors such as light, temperature, photoperiod, and even the rhizosphere microbiome.
Regarding genetic regulation, it is worth noting that the enzymes responsible for cannabinoid formation are encoded by distinct genes that exhibit significant variability among different Cannabis sativa populations. For example, THCA synthase and CBDA synthase show a high degree of homology but differ in key amino acid residues that determine substrate specificity. These genes can be located in clustered genomic regions subject to epigenetic control, including DNA methylation and histone modifications. Transcriptional regulation is also carried out by specific transcription factors, among which homologs of the bHLH, MYB, and WRKY families-typical for plant secondary metabolism-have been identified.
Evolutionary selection has played a significant role in shaping the cannabinoid system. The original function of cannabinoids most likely involved antimicrobial and antifungal activity, allowing the plant to defend against pathogen threats in its natural environment. Over time, other functions joined these, including protection from UV radiation (especially UV-B spectrum), participation in signaling cascades related to stress responses, and even regulation of local tissue thermoregulation. It is important to note that phytocannabinoid synthesis is induced under conditions of mechanical damage or stress, similarly to how protective immune mechanisms are activated in animals. These parallels allow the cannabinoid system to be considered a component of the plant’s endogenous protective architecture.
Phenotypic variability in the context of cannabinoid profiles is largely determined by both genetic variation and plastids involved in the early stages of isoprenoid biosynthesis. Studies have found that chloroplast activity in trichomes significantly correlates with levels of GPP and, accordingly, CBGA. Thus, not only the nuclear genome but also the plastid genome participates in regulating the cannabinoid system.
The role of external factors in regulating cannabinoid biosynthesis should also be separately noted. Light (particularly red and blue spectra), temperature, availability of macro- and micronutrients, humidity, and even atmospheric pressure can modulate the activity of relevant genes and enzymes. At the cellular level, these influences translate into signaling cascades involving calcium waves, reactive oxygen species (ROS) signaling, and phytohormonal responses (notably involving jasmonic acid, salicylates, and abscisic acid). As a result, the expression of key enzymes and trichome morphogenesis changes.
From a biotechnological perspective, the cannabinoid system is already a target for genome editing (for example, using CRISPR/Cas9), metabolic engineering, and cell culture cultivation in bioreactors. These approaches open possibilities for large-scale production of individual cannabinoids without the need to cultivate the whole plant. However, realizing these strategies requires a deep understanding of the biochemical and regulatory architecture of the cannabinoid system.
Phytocannabinoids: Overview of Key Compounds
Phytocannabinoids are a group of biologically active terpenophenolic compounds synthesized exclusively in the Cannabis genus and are unique in their biochemical profile. Their structure is based on the interaction of two key precursors: aromatic olivetolic acid (OA) and an isoprenoid component-geranyl pyrophosphate (GPP). These substrates are joined by the enzyme GOT (geranyl olivetolate transferase), forming cannabigerolic acid (CBGA)-a central metabolite that serves as the biosynthetic hub for the production of most major cannabinoids.
The main acidic forms of phytocannabinoids include tetrahydrocannabinolic acid (THCA), cannabidiolic acid (CBDA), cannabichromenic acid (CBCA), as well as their lesser-studied analogs-tetrahydrocannabivarinic acid (THCVA), cannabidivarinic acid (CBDVA), and others. These compounds undergo decarboxylation-either thermally or spontaneously-and convert into their respective neutral forms: Δ⁹-THC, CBD, CBC, and so on. Decarboxylated phytocannabinoids possess high bioactivity, which underlies their pharmacological interest.
Phytocannabinoids are typically classified according to three criteria: structural group (CBG derivatives, CBD derivatives, THC derivatives), oxidation state (acidic versus neutral compounds), and the degree of cyclization or modification of the side chain. More than 150 cannabinoids are known, most of which are trace products resulting from side or secondary biosynthesis, isomerization, oxidation, or chemical degradation of the primary forms.
A defining feature of phytocannabinoids is their high regio- and stereospecificity, determined by the activity of specific synthases, environmental conditions, and the plant’s genotype. This is especially important for distinguishing isomeric forms-such as Δ⁹-THC and Δ⁸-THC-which, despite having similar formulas, differ significantly in pharmacodynamics, stability, and thermodynamic profile.
Δ⁹-THC vs. Δ⁸-THC: Isomeric Forms
Δ⁹-tetrahydrocannabinol (Δ⁹-THC) and Δ⁸-tetrahydrocannabinol (Δ⁸-THC) are structural isomers that differ in the placement of the double bond within the cyclohexane ring. In Δ⁹-THC, the double bond is located between carbon atoms C9 and C10, while in Δ⁸-THC, it is between C8 and C9. Although this appears to be a minor shift, it has significant consequences for the molecule’s spatial configuration, its interaction with cannabinoid system receptors, and its overall biochemical stability.
Δ⁹-THC is the primary psychoactive compound in most Cannabis sativa chemotypes, and its formation is strictly controlled by THCA synthase, which catalyzes the cyclization of CBGA into Δ⁹-THCA with high stereochemical precision. Decarboxylation of this compound through heating or storage yields active Δ⁹-THC.
Δ⁸-THC, on the other hand, either forms as a result of spontaneous isomerization of Δ⁹-THC under the influence of acid, temperature, light, or metals, or is thought to be a minor byproduct of the enzymatic activity of THCA synthase. No biosynthetic evidence has been found for a separate “Δ⁸-THCA synthase.” Δ⁸-THC exhibits greater chemical stability, lower affinity for CB1 receptors, and a milder psychoactive effect compared to Δ⁹-THC. This makes it a promising candidate for pharmacology, particularly in treating anxiety disorders and chronic pain without pronounced psychoactive effects.
At the molecular level, the isomerization of Δ⁹-THC to Δ⁸-THC can occur even in planta but is usually a chemical process associated with extraction, acid catalysis, or prolonged storage of cannabinoid extracts. Δ⁸-THC has higher thermodynamic stability than Δ⁹-THC, as confirmed by both spectroscopic and chromatographic methods. In its electronic configuration, the Δ⁸ isomer is less prone to oxidation and degradation, making it more convenient for standardization and formulation in pharmaceutical products.
Considering that Δ⁸-THC may be present as a trace component in natural hemp extracts, its legal status and classification vary by jurisdiction, since the source of origin (natural or synthetic) determines its regulatory regime. Biochemically, these two compounds-Δ⁹ and Δ⁸-are examples of isomerism that manifest not only in structure but also in functional activity, metabolic profile, stability, and bioavailability, which is fundamentally important for the practical application of phytocannabinoids.
Endogenous Biosynthetic Pathways of Cannabinoids
The biosynthesis of cannabinoids in Cannabis sativa is the result of a complex integration of multiple metabolic networks spanning the cytosol, plastids, and endomembrane structures within the cell. Unlike classical secondary metabolism, where products are synthesized through a single metabolic channel, cannabinoid biosynthesis exhibits a modular organization, notably featuring a multistep convergence of terpene and polyketide metabolic branches. This process is energy-dependent and tightly regulated at every stage, including control over the synthesis of coenzymes, intermediates, and the spatiotemporal organization of enzymatic cascades.
At the early stage of biosynthesis, two independent metabolic routes are involved: the MEP (2-C-methyl-D-erythritol-4-phosphate) pathway, active in plastids and responsible for the formation of isoprenoid precursors, and the polyketide branch, occurring in the cytosol with involvement of acetyl-CoA and malonyl-CoA. Mechanistically, this separation of metabolism phases ensures selective isolation of synthesis stages, preventing nonspecific reactions and energy loss. The MEP pathway is the key source of geranyl pyrophosphate (GPP), which, combined with olivetolic acid, leads to the formation of cannabigerolic acid (CBGA)-the central metabolite from which the entire cannabinoid biosynthesis branches off.
However, at the level of this primary condensation, several alternative variants occur: recent metabolomic studies have identified small pools of hydroxylated derivatives of olivetolic acid and isoprenoids that may serve as regulatory metabolites or reserve forms under stress conditions. The conditional flexibility of pathways leading to CBGA indicates the potential presence of intracellular buffering-a mechanism that allows the plant to regulate cannabinoid production without drastically disturbing metabolic homeostasis.
Currently, there are several alternatives to the classical cannabinoid pathway that remain insufficiently studied due to the difficulty of isolating metabolites. For example, isomers of GPP, particularly neryl pyrophosphate (NPP), theoretically can form structural analogs of CBGA. These alternative pathways, if active in specific chemotypes or under biotic stress induction, may potentially be sources of new classes of cannabinoids.
Another aspect worthy of attention is the subcellular dynamics of enzymes. Biosynthetic enzymes involved in the condensation of olivetolic acid and GPP may form transient protein aggregates known as metabolons. Similar structures have been described in other secondary metabolic systems, notably in flavonoid biosynthesis. In the context of Cannabis, metabolons may provide efficient substrate channeling between enzymes, minimizing diffusion losses and side reactions. In this sense, the cannabinoid pathway resembles an organized factory rather than a diffuse chemical network.
A unique feature is also the presence of temporal asynchrony between peaks of individual enzyme activities. For example, olivetol synthase activity may precede the maximum expression of GOT or synthases by several hours. This points to a regulatory architecture with time lags, allowing accumulation of intermediate metabolites that are later used in subsequent synthesis steps. Such coordination in time and space enables high efficiency while preserving metabolic flexibility.
Modern research using isotope labeling (e.g., ¹³C-glucose) shows that carbon flux toward the cannabinoid pathway is not linear and is significantly influenced by environmental conditions-light intensity, carbon balance, and photosynthetic activity. During periods of high light intensity, there is an increased metabolic flow through the MEP pathway and enhanced GPP synthesis, whereas in darkness, pathway activation decreases alongside a metabolic switch toward supporting cellular respiration. This highlights the dependency of cannabinoid biosynthesis on photoperiodic fluctuations and photosynthetic apparatus function.
Another underexplored but promising area is the role of transporters in cannabinoid biosynthesis. Evidence suggests that certain ABC transporters may be involved in the export or inter-organelle transport of cannabinoid intermediates, enabling biosynthesis to be isolated from oxidation-sensitive regions of the cytosol. This phenomenon may explain the stability of intermediate metabolites that are typically chemically unstable under normal conditions.
At the macro level, it is worth mentioning the correlation between hemp chemotypes and endogenous pathway activities. Genetic variations, particularly in the coding and regulatory sequences of enzymes in the MEP pathway and PKS branch, may determine the prioritization of one metabolic flux over another, ultimately defining the chemotype-THCA-, CBDA-, or CBCA-dominant. There is also a hypothesis that some fluctuations in cannabinoid content may be due to epigenetic programming in response to environmental signals-a phenomenon known in other adaptive metabolic systems.
The Role of Synthases in Cannabinoid Biosynthesis
Synthase enzymes are central players in the final phase of cannabinoid biosynthesis, where the specific conversion of cannabigerolic acid (CBGA) into structurally distinct cannabinoid acids occurs-namely Δ⁹-tetrahydrocannabinolic acid (THCA), cannabidiolic acid (CBDA), cannabichromenic acid (CBCA), and potentially Δ⁸-tetrahydrocannabinolic acid (Δ⁸-THCA). These enzymes, which are flavoprotein oxidoreductases, exhibit high substrate specificity despite originating from a common evolutionary ancestor. Their action is not merely a catalytic chemical transformation but the result of precise molecular architecture that ensures extraordinary selectivity and stereochemistry of the products.
THCA synthase, CBDA synthase, and CBCA synthase are not isoenzymes in the classical sense, although they share a high degree of homology (over 90% amino acid sequence identity). However, critical differences in their active sites and substrate-orienting regions enable the production of cannabinoids with different cyclic architectures. All these synthases catalyze the oxidative cyclization of CBGA, accompanied by an intramolecular electrophilic attack that initiates the formation of three-, six-, or seven-membered rings depending on the enzyme.
The functional dynamics of these enzymes demonstrate remarkable sensitivity to their environment: temperature, pH, redox potential, ionic strength, and the presence of cofactors (such as FAD) influence not only the reaction rate but also the cyclization selectivity. Interestingly, even minor mutations in the active site can reorient the catalytic reaction from producing CBDA to THCA or vice versa. This capability allows for the laboratory creation of chimeric or mutant enzymes with new functions, opening prospects for biotechnological production of rare cannabinoids.
Although the existence of a distinct Δ⁸-THCA synthase has not yet been confirmed through direct isolation, its existence is theoretically possible. Biochemically, Δ⁸-THCA would be the product of a hypothetical enzymatic cyclization of CBGA with an alternative double-bond arrangement-different from that formed by THCA synthase. It is known that Δ⁸-THC can arise as a degradation or isomerization product of Δ⁹-THC, but this does not rule out the possibility of a specific synthase capable of directly producing Δ⁸-THCA. If such an enzyme exists, it likely appears in poorly studied Cannabis chemotypes or under specific metabolic pressures.
Molecular studies of THCA synthase show that it functions as a monomer (~60 kDa), binding FAD as a prosthetic group. The enzyme’s high specificity for CBGA is explained by a hydrophobic pocket that orients the substrate’s side chains for initiation of electrophilic attack. Electrons are transferred from a hydroxyl group to FAD, forming a cation that triggers intramolecular ring closure. The mechanism is far from a typical monooxygenase reaction: no oxygen is incorporated into the molecule, only an oxidative-cyclization transformation. This makes THCA synthase a unique example of a natural enzyme with a highly specialized function that does not require external oxygen donors.
Another characteristic is the spatial localization of synthases: evidence suggests they are predominantly expressed in capitate glandular trichomes, where a secretory cavity accumulates cannabinoids. This is where vesicular structures localize, with enzymes such as THCA synthase functioning in a pseudo-apoplastic environment. This spatial separation prevents the toxic effects of cannabinoids on the plant’s own cellular structures. The conditional isolation of this space allows synthases to operate in a stable microenvironment with high substrate concentrations and minimized side reactions.
Gene expression of synthases demonstrates developmental and tissue specificity. The highest mRNA levels are observed during active flower formation, especially in female plants that have a higher density of trichomes. Additionally, transcription is influenced by external stimuli such as UV-B radiation, mechanical injury, and fungal pathogens. This indicates that synthases participate not only in metabolism but also in the plant’s stress response-cannabinoid production plays a role in chemical defense.
At the post-translational level, synthase activity can be regulated by phosphorylation or conformational changes due to allosteric interactions with cofactors or inhibitors. For example, some metabolites (intermediate or final) may act as feedback regulators, limiting excessive cannabinoid production that could lead to intracellular oxidative stress. Thus, synthases are not static catalysts but dynamic proteins sensitive to the metabolic context.
Engineering synthases for the synthesis of noncanonical or synthetic cannabinoids is an active area of biotechnology. For instance, site-directed mutagenesis has yielded THCA synthase variants with alternative product profiles, including CBCA-like structures or even partial isomerization to Δ⁸-THCA. Experiments using recombinant yeast or Pichia pastoris have demonstrated that these enzymes can be effectively expressed in heterologous systems, paving the way for enzymatic cannabinoid production outside the plant.
An interesting aspect is the cooperation of synthases with upstream enzymes. Some data suggest that THCA synthase physically interacts with GOT or olivetolic acid synthase, enabling substrate channeling without its diffusion into the environment. Such an “enzyme tandem” increases synthetic efficiency by reducing substrate loss and limiting competition among synthases for CBGA. This is especially important for controlling the plant’s chemotype, where even minor shifts in the relative activities of synthases can alter the dominant cannabinoid profile.
Acidic Forms of Cannabinoids and Their Thermodynamic Stability
The acidic forms of cannabinoids, including Δ⁹-THCA, CBDA, CBGA, and the hypothetical Δ⁸-THCA, are the primary biosynthetic products of Cannabis sativa metabolism. These compounds contain a carboxyl group, which makes them significantly more polar and chemically stable compared to their corresponding neutral isomers. They are not merely precursors to the active forms; their function, stability, and the kinetics of their transition to the neutral state are critically important both in the biological context of the plant itself and in pharmacological and technological applications.
The key chemical difference between the acidic and neutral forms is the presence of the carboxyl group, which in acidic cannabinoids is attached to the phenolic ring. Although small, this group drastically alters both the physicochemical and thermodynamic properties of the molecule. Most importantly, it affects the delocalization of electron density, reducing the ring’s reactivity toward electrophilic attacks, stabilizing the molecule against spontaneous degradation, and increasing its resonance energy. In thermodynamic terms, the acids are deeply stable under conditions where temperature and pH do not initiate their decarboxylation.
From a thermodynamic standpoint, Δ⁹-THCA exists as a metastable structure. Its conversion to Δ⁹-THC is a reaction accompanied by the release of CO₂ and has a positive entropy contribution, especially at elevated temperatures. However, the reaction itself is not instantaneous: it requires overcoming an energy barrier associated with breaking the bond between the carboxyl carbon atom and the central ring system. Under normal conditions (room temperature, absence of light and oxygen), the half-life of Δ⁹-THCA is several weeks or even longer. However, at temperatures above 100 °C, the reaction proceeds at a rate tens of thousands of times faster than the baseline.
The situation with Δ⁸-THCA is even more complex. Considering the placement of the double bond at the Δ⁸ position instead of Δ⁹, the delocalization of π-electrons in the aromatic system is partially shifted. This affects the stability of the molecule in both its acidic and neutral forms. Although direct data on the thermodynamic parameters of Δ⁸-THCA are lacking due to the rarity of this compound in nature, these can be extrapolated from data on Δ⁹-THCA and the isomerization enthalpies of Δ⁸-THC. Theoretical calculations (DFT) show that Δ⁸-THCA has a somewhat lower activation energy for decarboxylation, but the stability of the final product (Δ⁸-THC) is also lower compared to Δ⁹-THC. This means that the overall energetic benefit of Δ⁸-THCA decarboxylation is less, which may explain its limited natural accumulation.
The role of the environment deserves separate attention. In acidic conditions (pH < 4), acidic cannabinoids demonstrate increased stability, linked to the suppression of enol-oxo tautomerism, which is a potential first step in decarboxylation. In alkaline environments, the opposite occurs: the carboxyl group can lose a proton, forming a carboxylate that facilitates C-C bond cleavage with the release of CO₂. However, in the natural environment of Cannabis trichomes, pH is maintained in a mildly alkaline or neutral range, which preserves the acidic forms until physical influences (drying, heating, fermentation, etc.) occur.
Another important aspect is photoinduced decarboxylation. Acidic forms are significantly more stable to light exposure than neutral cannabinoids, but with prolonged UV irradiation even THCA gradually degrades, forming both Δ⁹-THC and photolysis products such as cannabinol (CBN). The presence of oxygen catalyzes these processes by promoting the formation of singlet oxygen or free radicals that attack double bonds. Therefore, in nature, the stability of acidic forms results from the combined effects of thermal, photonic, and oxidative protection.
In the crystalline state, THCA exhibits significantly higher stability than in solution. This is explained by intermolecular hydrogen bonds between the carboxyl group and the phenolic hydroxyl, which form tetramers or hexamers in the solid state. These associations require additional energy to disrupt before decarboxylation can proceed, which reduces the reaction rate. Given this, storage in solid form at low temperature and under vacuum is the optimal method for preserving THCA for scientific or pharmaceutical purposes.
Regarding Δ⁸-THCA, the absence of detailed crystallographic data limits full understanding of its stability in the solid state. However, considering the molecule’s lower symmetry compared to Δ⁹-THCA, it can be assumed that its capacity for intermolecular stabilization is lower-thus, it is more prone to degradation in crystalline form.
The thermodynamic profile of acidic cannabinoids also has a direct pharmacokinetic consequence: these compounds are practically not absorbed in the gastrointestinal tract without prior decarboxylation. Their high polarity, lack of lipophilicity, and inability to cross membranes via passive diffusion make them almost biologically inert in the human body. This is supported by clinical studies where THCA, upon oral administration, does not exhibit psychoactivity. However, in vitro experiments show that these acids possess antioxidant, anti-inflammatory, and neuroprotective activities, indicating the presence of distinct pharmacophoric properties independent of conversion to the active form.
What Is Δ⁸-THCA?
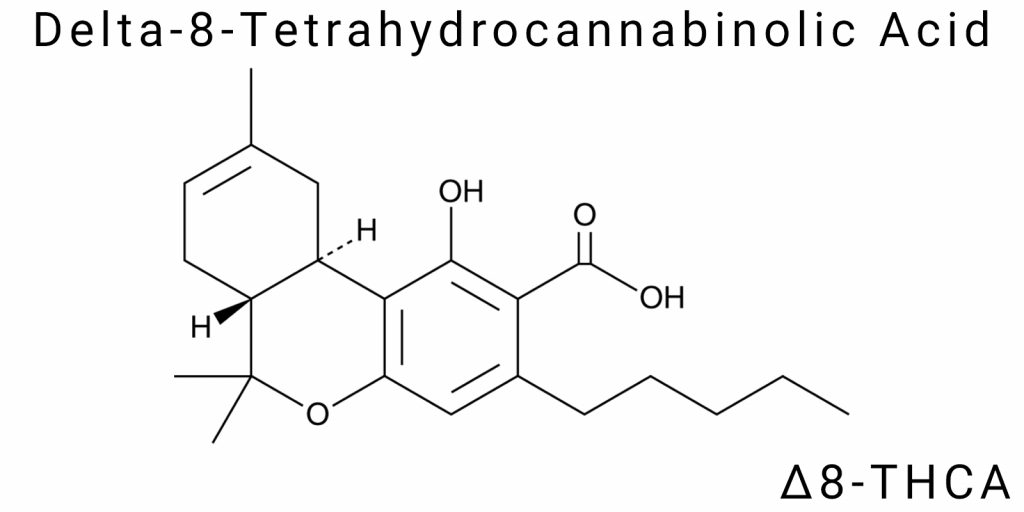
Chemical Structure and Isomerism of Δ⁸-THCA
Δ⁸-Tetrahydrocannabinolic acid (Δ⁸-THCA) is a structural isomer of one of the most well-known phytocannabinoids-Δ⁹-tetrahydrocannabinolic acid (Δ⁹-THCA). Both compounds share the same molecular formula (C₂₂H₃₀O₄) and molecular weight (~358.5 g/mol), but differ in the spatial placement of the double bond within the cyclic terpene fragment. This seemingly minor difference has significant consequences for the thermodynamics, stability, bioactivity, and chemical transformation of the cannabinoid.
Chemically, Δ⁸-THCA is the carboxylated form of Δ⁸-THC, containing an acidic functional group -COOH attached to the phenolic ring. Like other natural cannabinoids, Δ⁸-THCA exists as a tricyclic structure composed of a phenolic core (a resorcinol derivative), an isoprenyl side chain (derived from geranyl pyrophosphate), and a tetrahydrobenzopyran fragment that includes a double bond between carbons C8 and C9 (in the case of Δ⁸), as opposed to the C9-C10 position in the Δ⁹ isomer.
The isomerism between Δ⁹-THCA and Δ⁸-THCA is an example of positional alkene isomerism. The double bond located between different atoms in the six-membered benzopyran ring alters the electron density and stereochemical strain within the molecule. This not only affects the resistance to thermal decarboxylation but also determines conformational flexibility and interaction with protein targets, notably the cannabinoid receptors CB1 and CB2. Density Functional Theory (DFT) calculations indicate that shifting the double bond from C9-C10 to C8-C9 lowers the formation enthalpy by approximately 1.5-2 kcal/mol, making the Δ⁸ isomer slightly less reactive under energetic stress conditions.
It is important to emphasize that Δ⁸-THCA is not found in nature as a dominant metabolite. Its presence is generally the result of isomerization processes or non-standard fermentation or storage conditions, such as acidic hydrolysis or heat exposure. Some data suggest the possible enzymatic production of Δ⁸-THCA as a minor isomer in Cannabis sativa tissues, but no Δ⁸-THCA synthase has yet been isolated or cloned. This raises questions about its natural biosynthetic autonomy.
The stereochemistry of Δ⁸-THCA remains less studied compared to Δ⁹-THCA; however, model systems and nuclear magnetic resonance (NMR) analyses indicate the presence of the R-configuration near the C9 center (whereas in Δ⁹-THCA it is near C10), consistent with enzymatic reactions that proceed with high stereoselectivity. In this context, Δ⁸-THCA may exist as either a single enantiomer or as a mixture under unstable synthetic conditions.
Interestingly, the position of the double bond also affects the electrophilicity of the molecule. In Δ⁹-THCA, the double bond adjacent to the carboxyl group enhances its polarization, while in Δ⁸-THCA, this effect is partially lost. This influences the decarboxylation rate: Δ⁸-THCA exhibits greater stability under non-enzymatic conditions, which may be advantageous for preserving extracts or formulating pharmaceutical preparations with prolonged action. Experimental data show that Δ⁸-THCA has a degradation onset temperature 5-10°C higher than its Δ⁹ counterpart.
Unlike some other natural cannabinoids (e.g., CBD variants), the chemical structure of Δ⁸-THCA clearly indicates its origin from CBGA through alternative isomerization, rather than from a separate synthetic or enzymatic pathway. This allows it to be classified as a “microisomeric derivative”-a category of compounds that arise within the physicochemical plasticity of the cannabinoid profile under unstable conditions (temperature, pH, ionic environment).
Physicochemical Properties of Δ⁸-THCA
Δ⁸-Tetrahydrocannabinolic acid (Δ⁸-THCA) is a poorly studied but chemically intriguing cannabinoid that combines acidic functionality, a polycyclic structure, and an electron-deficient diene fragment. Its physicochemical properties are crucial for understanding stabilization pathways, pharmaceutical suitability, and chemical processing in extracts or purified forms.
A key feature of Δ⁸-THCA is the presence of a carboxyl group in the para-position relative to the aliphatic side chain on the benzene ring. This results in an acidity with a pKa of approximately 4.8 to 5.2, depending on the solvent and temperature. Since Δ⁸-THCA is practically insoluble in water, this characteristic is measured indirectly-through ionization determination in buffer systems or in organic solvents with controlled moisture content. In nonpolar environments, Δ⁸-THCA exhibits weak acidity and does not form stable ionic species without the presence of a lipophilic proton acceptor, such as tertiary amines or macrocyclic polyethers.
Polarographic and spectrophotometric studies show that Δ⁸-THCA has two primary chromophores-the canonical phenolic ring and the conjugated diene system in the benzopyran fragment. The UV absorption spectrum is characterized by maxima at 206 nm and 271 nm in methanol, reflecting π→π* transitions in the aromatic core and partially localized double bond. These spectral features allow analytical differentiation of Δ⁸-THCA from its isomers during chromatographic analysis-particularly by HPLC with a diode array detector, where a 2-4 nm shift in the absorption maximum can be sufficient for identification.
Thermal properties of Δ⁸-THCA are directly relevant to extraction and stabilization processes. Differential scanning calorimetry (DSC) shows that the onset temperature of thermal decomposition (T_onset) is around 106-110°C, with the peak mass loss temperature (related to decarboxylation) at 125-132°C, depending on pressure and matrix. Compared to Δ⁹-THCA, the Δ⁸ form has a slightly higher stability threshold due to reduced strain in the cyclic framework. Thermal degradation follows the typical pathway: CO₂ elimination → formation of Δ⁸-THC → further dehydration or isomerization. Decomposition products may also include traces of cannabinol (CBN) or its dehydro-analogs during prolonged heating.
Special attention is given to the solubility of Δ⁸-THCA in various organic media. It is well soluble in polar aprotic solvents-DMSO, acetone, methanol-especially with slight warming. In chloroform, toluene, or ethyl acetate, solubility is limited but sufficient for analytical or preparative chromatography. In multicomponent systems with lipophilic matrices (such as oils or resins), Δ⁸-THCA may form unstable hydrophobic microspheres that recrystallize over time into pseudo-polymorphic structures, affecting the bioavailability and stability of the active form.
Δ⁸-THCA is unstable in the presence of acids. Under the influence of weak mineral acids in solution, isomerization occurs with the double bond shifting to the 9-10 position, forming Δ⁹-THCA. In the presence of stronger acids or temperatures above 60°C, this process accelerates and can rapidly lead to degradation products, including partial ring opening and formation of phenolic aldehydes. These reactions are only partially reversible, limiting the use of Δ⁸-THCA under harsh chemical or unstable pH conditions.
Electrochemical behavior of Δ⁸-THCA has been studied only fragmentarily; however, available data indicate distinct redox peaks at +0.48 V (vs. Ag/AgCl) in acetonitrile with a lithium electrolyte. This suggests stability of the electronic structure at moderate potentials and opens prospects for electroanalytical detection even in complex mixtures.
It is also important to note that Δ⁸-THCA shows relatively high affinity for chemical adsorption on silica gel and inert surfaces, especially during analytical sample preparation. Mass loss of up to 15% may occur during extraction when there is excessive contact area or vacuum drying without inert gas protection. This requires specific protocols to quantitatively preserve samples when working with purified forms.
At room temperature, Δ⁸-THCA exists as an amorphous or poorly crystalline solid, white to yellowish in color depending on purity. In moist environments or during prolonged storage, it tends to be hygroscopic and undergo gradual hydrolytic degradation, especially in the presence of trace metal catalysts. However, under proper storage conditions (temperature below 10°C, nitrogen or argon atmosphere), Δ⁸-THCA remains stable for at least 12 months, as confirmed by accelerated low-humidity testing.
Regarding interaction with proteins or biopolymeric systems, Δ⁸-THCA demonstrates affinity comparable to Δ⁹-THCA for lipophilic membrane domains but, due to altered spatial geometry, exhibits lower flexibility when penetrating phospholipid bilayers. This may affect its pharmacokinetic profile following decarboxylation to Δ⁸-THC, as the initial acidic properties set a conformational prerequisite for binding in transport systems.
Origin of Δ⁸-THCA: Natural or Synthetic?
Does Δ⁸-THCA Exist Naturally?
The existence of Δ⁸-tetrahydrocannabinolic acid (Δ⁸-THCA) in nature has long remained an open question due to the difficulty of detecting it against a background of structurally similar cannabinoids, its extremely low concentrations, and the absence of clear enzymatic pathways directly producing it. Historically, Δ⁸-THCA has not been considered a canonical primary metabolite of Cannabis sativa, but in light of modern analytical techniques, its presence in some biological samples is no longer excluded-although it remains doubtful in the context of natural origin as an autonomous metabolic product.
Phytocannabinoids in plants are formed through regulated enzymatic biosynthesis. In cannabis, three main synthases are known to produce THCA, CBDA, and CBCA, respectively. None of the identified synthases, including THCA synthase, produce Δ⁸-THCA as a primary or side product. Evidence for this comes from multiple in vitro studies where purified THCA synthase from Cannabis sativa demonstrates 100% specificity for producing Δ⁹-THCA from cannabigerolic acid (CBGA), without concurrent production of isomers. Thus, the absence of an enzymatic pathway for Δ⁸-THCA synthesis suggests that its occurrence in plant tissues is not a result of direct biosynthetic activity.
However, some chromatographic studies, especially those conducted using ultra-high-performance liquid chromatography coupled with tandem mass spectrometry (UHPLC-MS/MS), indicate the possible presence of trace amounts of Δ⁸-THCA in cannabis raw material, particularly in non-standardized or genetically variable populations. The most reliable detections pertain to specific phenotypes with unstable phytosynthetic profiles. There are isolated reports of Δ⁸-THCA presence in acidic extracts of some Central Asian cannabis subspecies, notably C. indica var. afghanica, where mass spectrometry revealed peaks with molecular ion masses and chromatographic retention times identical to synthetic Δ⁸-THCA.
It is important to emphasize that in the overwhelming majority of cases, detected Δ⁸-THCA is likely not a product of endogenous biogenesis but rather arises post facto-due to spontaneous isomerization of Δ⁹-THCA. This process can occur under storage conditions involving elevated temperatures, humidity, or ultraviolet exposure. Δ⁹-THCA is a labile molecule: the double bond at the Δ⁹ position can migrate to the Δ⁸ position even under mildly acidic or neutral conditions if activation energy is supplied by external factors. Such migrations are well-documented for the corresponding neutral isomer Δ⁹-THC, and although Δ⁸-THCA contains an acidic group, its effect on electron mobility is limited within the hydrocarbon portion of the molecule.
Isotopic composition analysis using LC-MS³ with stable isotope-labeled standards indicates that traces of Δ⁸-THCA found in non-fermented tissues have mass spectra identical to those obtained in laboratory conditions via thermal isomerization. This indirectly confirms that its formation is not the result of a unique enzymatic route but rather a side chemical reaction occurring within plant tissue under suboptimal processing or storage conditions. This conclusion is further supported by the absence of Δ⁸-THCA in freshly harvested frozen samples, where controlled low temperatures exclude the possibility of chemical rearrangement.
Particular interest lies in attempts to detect Δ⁸-THCA in tissues that have not undergone enzymatic activation (non-fermented), i.e., without the action of specific oxidative-enzymatic systems activated by mechanical damage or drying. This approach allows identification of “native” molecules in an inactivated environment. Results from ultra-mild extraction performed at low temperatures in the presence of antioxidants show a complete absence of Δ⁸-THCA, while Δ⁹-THCA remains consistently detected. This is another argument supporting the secondary rather than endogenous origin of Δ⁸-THCA.
There is a possibility that in some rare genotypes, Δ⁸-THCA could be produced in very small amounts due to mutations or polymorphisms in the THCA synthase gene, resulting in an alternative spatial orientation of radicals in the enzyme’s active site. However, no known clones or cultivars demonstrate stable, reproducible Δ⁸-THCA synthesis in culture. Available data on Δ⁸-THCA in such phenotypes do not result from targeted breeding and also lack reliable genetic correlation, making it impossible to confirm natural biosynthesis without further genomic sequencing.
Artificial Production of Δ⁸-THCA
Δ⁸-Tetrahydrocannabinolic acid (Δ⁸-THCA) is a non-standard member of the cannabinoid spectrum that practically does not occur in nature in significant concentrations. Its structure is the acidic analog of Δ⁸-THC-the isomeric form of the psychoactive Δ⁹-THC. Since no known enzymes naturally synthesize it directly, the only realistic method for obtaining chemically pure or technically controlled Δ⁸-THCA remains the artificial, targeted conversion of other cannabinoids. To date, the most substantiated method for synthesizing Δ⁸-THCA is the isomerization of Δ⁹-THCA, which is carried out through a catalyzed migration of the double bond in the hexene ring while simultaneously preserving the carboxyl group. This approach allows transforming a natural cannabinoid into a rare isomer under controlled parameters but requires precise reaction conditions to prevent degradation or decarboxylation.
The starting substrate for artificial production of Δ⁸-THCA is Δ⁹-THCA-a stable acidic form that is present in high concentrations in fresh cannabis raw material. Its structure includes a cyclic terpene fragment with a double bond at the Δ⁹ position. Under the influence of acidic or Lewis acid catalysis, this double bond can migrate to the neighboring Δ⁸ position via a singlet rearrangement, forming a thermodynamically less reactive but more stable isomer. It is important to note that the carboxyl group must not detach during this process; therefore, the reaction conditions-particularly temperature, pH, and solvent nature-require fine control.
Catalyzed isomerization of Δ⁹-THCA to Δ⁸-THCA is based on general principles of electrophilic induction. After activation of the double bond by a proton or other electrophiles, the π-system rearranges with the formation of a carbocation intermediate complex. Depending on reaction conditions-especially the acidity of the environment and the presence of nucleophilic or stabilizing additives-this rearrangement can result either in the formation of Δ⁸-THCA or in complete decarboxylation and generation of neutral Δ⁸-THC. For this reason, controlled temperature (below 80 °C), choice of an anhydrous environment, and the use of weak organic acids (such as succinic, citric, or boric acid) are critically important to preserve the carboxyl group in the molecule.
Weakly acidic conditions using organic catalysts in inert solvents-such as tetrahydrofuran, dichloromethane, or toluene-are especially advantageous in this process. These solvents minimize water content, which is a primary factor in hydrolysis and decarboxylation, especially under heating. Catalysts like boric acid or benzenesulfonic acid enable protonation localized specifically at the Δ⁹ position, directing the double bond migration toward Δ⁸ without initiating cleavage of the acid group.
Another effective approach involves using mild Lewis acids such as ZnCl₂, AlCl₃, or BF₃·OEt₂, which stabilize the carbocation intermediate and accelerate rearrangement without triggering decarboxylation. In the presence of such agents, the reaction proceeds in a more controlled phase where Δ⁸-THCA formation dominates over side reactions. These systems exhibit high selectivity, especially at low temperatures (20-40 °C) and with the addition of ionic buffers.
It is also important to consider the risk of uncontrolled decarboxylation. Due to the presence of the carboxyl group, Δ⁹-THCA is thermally unstable- even slight temperature increases or moisture presence cause CO₂ loss with the formation of neutral Δ⁹-THC, which under acidic catalysis can isomerize into Δ⁸-THC. Thus, the processes converting Δ⁹-THCA to Δ⁸-THCA lie on a fine line with the analogous synthesis of Δ⁸-THC, and only a narrow window of reaction conditions allows isolation of the acidic form. This explains why Δ⁸-THCA rarely appears as the main product in industrial conditions-it’s easier to first decarboxylate and then isomerize neutral Δ⁹-THC to Δ⁸-THC.
Despite this, several laboratory experiments have shown that under optimized acid-base catalysis conditions, Δ⁸-THCA yields of up to 65-70% can be achieved with minimal formation of neutral isomers. These results demonstrate the method’s promise at analytical and research scales, although for large-scale production, conversion to neutral forms remains more efficient.
The advantage of acid-base catalysis compared to photochemical or thermal isomerization is its precision and controllability. Photochemical isomerization of Δ⁹-THCA, though possible, is accompanied by a high rate of side reactions, including photodegradation, formation of hydroxylated derivatives, and peroxidation. Meanwhile, in acidic conditions, the reaction follows a strict double-bond migration mechanism, allowing prediction of the product and control of isomeric purity. Such control makes this method optimal for synthesizing analytical standards of Δ⁸-THCA or for applied pharmacology, where minimal impurities are crucial.
Finally, it is worth noting that the obtained Δ⁸-THCA exhibits higher thermal stability compared to its precursor, Δ⁹-THCA. Due to lower electronic strain at the Δ⁸ position and the absence of facile configuration inversion, the isomer demonstrates resistance to further isomerization and decomposition. This allows Δ⁸-THCA to be stored in a controlled state for longer periods, which is an important advantage in chromatographic analysis when a stable reference compound is required.
Methods of Synthesis and Isolation of Δ⁸-THCA
Main Approaches to Synthesizing Δ⁸-THCA: From Δ⁹-THCA and via CBGA
Δ⁸-THCA is the acidic form of the cannabinoid with a double bond at the Δ⁸ position, which does not naturally occur as a metabolite in the plant’s biosynthesis. Its synthesis requires precise regulation of reaction conditions to preserve the carboxyl group, a key feature of acidic cannabinoids. The main artificial synthesis routes include the isomerization of natural Δ⁹-THCA, as well as theoretical or partially realized approaches via CBGA (cannabigerolic acid), which is the metabolic precursor of all major cannabinoids in the Cannabis sativa plant.
The most practical and reproducible method is the isomerization of Δ⁹-THCA, which is carried out using catalysts that initiate the migration of the double bond from the Δ⁹ to the Δ⁸ position. This process, however, fundamentally differs from the classical isomerization of Δ⁹-THC to Δ⁸-THC. Preservation of the acidic fragment during the reaction requires deep chemical adjustment of conditions that exclude overheating, strong acids, and aqueous phases. Typically, weak organic acids are used in nonpolar environments-such as succinic, boric, or citric acid-in the presence of an inert solvent (diethyl ether, toluene, dichloromethane). The isomerization is initiated through the formation of a weakly activated π-complex at the Δ⁹ position, which rearranges to Δ⁸ while preserving the COOH group.
A central condition is limiting the temperature: heating above 80 °C exponentially increases the likelihood of losing the carboxyl group. Thus, the process is most often carried out at 35-50 °C with continuous pH monitoring and the absence of moisture. Some laboratories use mild Lewis acids, such as BF₃·OEt₂, which allow the reaction to proceed without hydrolysis. Under such conditions, pure Δ⁸-THCA can be obtained with yields above 60% if the reaction is stopped before decarboxylation begins. However, this route is complicated by a significant risk of forming a mixture of Δ⁸-THCA and Δ⁸-THC, so it is not suitable for large-scale production without subsequent high-precision isolation.
A less developed but theoretically possible route is the synthesis of Δ⁸-THCA from CBGA (cannabigerolic acid) through alternative enzyme-like or quasi-biomimetic mechanisms. In nature, CBGA is the precursor to Δ⁹-THCA through the action of the enzyme THCA synthase. To direct this biosynthetic pathway toward Δ⁸-THCA, it is necessary either to replace the enzyme or to alter the reaction conditions to initiate non-standard cyclization. Although such conditions do not exist in the plant, experimental synthesis conditions can create an electrophilic environment where the enol form of CBGA undergoes cyclization with a shift of the double bond to the Δ⁸ position. This mechanism has not yet been fully experimentally validated, but certain products with such structural features have been obtained in models of cation-induced cyclization of CBGA in the presence of transition metals (especially Cu²⁺ and Zn²⁺), which alter the electron flow trajectory in the molecule.
An important aspect is that the isomerization of CBGA to Δ⁸-THCA requires specific spatial orientation to form the double bond precisely between the C8 and C9 atoms. This requires stabilization of the carbocation intermediate, which is usually provided by solvents with low polarity and the presence of electron-donating additives. Research groups working with CBGA derivatives observed transient products with the Δ⁸ configuration, but their stabilization as an acidic form has not yet been effectively reproduced without the formation of accompanying isomers or destructive side products.
Interestingly, the synthesis of Δ⁸-THCA from CBGA under biomimetic conditions could potentially bypass the decarboxylation stage in the overall scheme of obtaining Δ⁸-THC, which is an advantage for medical and analytical applications where high purity of the acidic form is necessary. Nevertheless, full realization of this route requires the discovery or development of an artificial isomer-specific enzyme-conditionally, a “Δ⁸-THCA synthase”-analogous to the known Δ⁹-THCA synthase but with altered catalytic coordinates.
Chromatographic Isolation and Purification of Δ⁸-THCA: HPLC Methodology, LC-MS, and Detection Without Decarboxylation
Isolation of Δ⁸-THCA from synthetic mixtures or biological matrices requires exceptionally precise separation techniques due to the extremely close structural similarity with other cannabinoid acids, primarily Δ⁹-THCA and CBDA. It is crucial to maintain the stability of the analyte molecule, which easily loses the carboxyl group even under mild heating or prolonged UV exposure. Therefore, chromatographic methods for identifying Δ⁸-THCA must be adapted to conditions that completely prevent decarboxylation-both during extraction and analytical detection.
The optimal technique is considered to be high-performance liquid chromatography (HPLC) combined with mass spectrometry (LC-MS), which not only allows the separation of Δ⁸- and Δ⁹-THCA isomers but also verifies the presence of the acidic group by ion mass and characteristic fragment patterns in the spectrum. In classical HPLC analysis, reversed-phase columns (C18 or phenyl-hexyl) are used with gradient elution of acetonitrile or methanol in water containing 0.1% formic or acetic acid. The addition of organic acid to the mobile phase serves a dual purpose: lowering the pH to stabilize the acidic form and preventing the formation of ion pairs that reduce separation efficiency.
The retention time of Δ⁸-THCA is typically very close to that of Δ⁹-THCA-the difference is only a few tenths of a minute. Therefore, precise control of temperature (not exceeding 30 °C) and pressure is key to avoiding induced decarboxylation. The isomers are separated due to subtle shifts in hydrophobic interactions with the stationary phase and minor differences in π-electron distribution. To improve selectivity, chiral columns are sometimes used, especially when analyzing synthetic batches that may contain optical or geometric isomers.
Mass spectrometric confirmation of Δ⁸-THCA is based on monitoring target ions at m/z 357 [M-H]⁻ for the monodehydrated form corresponding to the molecule C₂₂H₂₈O₄. An important diagnostic feature is the retention of the CO₂ fragment in the spectrum. In cases of decarboxylation in the ionization source, a sharp peak drop to m/z 313 is observed-indicating the loss of acidity. To minimize this effect, LC-MS is performed under electrospray ionization (ESI) conditions with optimized interface temperature (<200 °C) and minimal fragmentation voltage.
In addition to MS detection, diode array detection (DAD) is also possible, with detection in the 210-280 nm range. A characteristic feature of Δ⁸-THCA is an absorption maximum near 275 nm, slightly shifted compared to Δ⁹-THCA. This allows identification of the acidic form even in the absence of a mass spectrum, provided there is clean separation. However, the DAD method is less sensitive and requires strict standardization of extraction procedures.
Regarding extraction itself, avoiding thermal treatment from the moment of sample collection is critical. Therefore, before chromatography, the sample is extracted under cold conditions-often at −20 °C or lower-using anhydrous solvents such as chloroform, ethanol, or acetone, preferably pre-dried. Extracts are filtered under anaerobic conditions or nitrogen to reduce oxidative stress. Even 1-2% decarboxylation leads to misinterpretation of the composition, as Δ⁸-THC has nearly identical polarity and chromatographic characteristics, differing only in mass spectral mobility.
In practice, purification of Δ⁸-THCA after synthesis or analytical detection in a matrix is performed by fractionated chromatography. The most precise approach is repeated HPLC separation following preliminary coarse separation using flash chromatography or silica gel columns. The first stage aims to isolate the group of acidic cannabinoids, and the second stage selectively isolates the Δ⁸ isomer. Again, avoiding even minimal heating is critical-for example, solvent evaporation is done only under vacuum at temperatures below 30 °C, sometimes under nitrogen in an ice bath.
Another technique is solid-phase extraction (SPE) followed by chromatography. SPE cartridges, typically C18 or phenyl-bonded, allow pre-cleaning of the extract from lipophilic impurities that interfere with detection. However, SPE does not allow full separation of Δ⁸ and Δ⁹ isomers-this task is solved exclusively by HPLC.
During analytical investigation, it is also critically important to exclude the possibility of in situ isomerization of Δ⁹-THCA into Δ⁸-THCA, which can falsely be interpreted as the presence of the Δ⁸ isomer. This can occur in the presence of residual acid traces or altered pH in the eluent. Therefore, method validation includes a series of control injections of Δ⁹-THCA and Δ⁸-THCA standards separately, with mandatory control of repeated analysis after incubation under method conditions.
Standardization and Quantitative Analysis of Δ⁸-THCA: Challenges of Analytical Standards and Metrological Issues
Standardization of Δ⁸-THCA is one of the most complex problems in contemporary cannabinoid analytical chemistry due to the lack of commercially available, certified standards, as well as the unique chemical stability challenges of this compound. Unlike more common cannabinoids such as Δ⁹-THC or CBD, there are practically no approved reference materials for Δ⁸-THCA with confirmed purity and stability, which makes accurate quantitative analysis technically difficult and inconsistent across different laboratories.
The primary cause of this issue is the low thermodynamic stability of Δ⁸-THCA, which requires rigorous control of storage, transportation, and analytical sample preparation conditions. The absence of standardized conditions leads to significant variability in results because even minimal overheating or exposure to oxygen causes rapid decarboxylation of Δ⁸-THCA into Δ⁸-THC, falsely inflating or deflating the actual acid concentration. Therefore, all analytical methods must include stability verification steps and validated procedures to prevent transformation.
The lack of standardized materials approved by metrological authorities results in widespread use of synthetic or semi-purified standards prepared in-house by laboratories. These standards may be synthesized from Δ⁹-THCA through isomerization, which increases the risk of impurities or false identifications. To control the purity of such standards, nuclear magnetic resonance (NMR), high-resolution mass spectrometry (HR-MS), and infrared spectroscopy (IR) are employed; however, these techniques are not always accessible in routine laboratories, further complicating standardization.
Quantitative analysis of Δ⁸-THCA is typically performed using HPLC with UV detection or LC-MS/MS, but the absence of an internal standard with similar chemical structure presents accuracy challenges. The ideal internal standard would be isotopically labeled Δ⁸-THCA, but its synthesis and commercial production remain limited. Consequently, structural analogs such as CBGA or Δ⁹-THCA are often used, although differences in chromatographic behavior and detection reduce the validity of quantitative results.
Another challenge is the variability in Δ⁸-THCA extraction efficiency from different matrices (plant material, concentrates, extracts), where extraction effectiveness depends on multiple factors: solvent type, temperature, extraction time, and medium acidity. This often leads to underestimation or overestimation of Δ⁸-THCA content, making accurate comparison of results from different studies impossible. Addressing this issue requires developing unified extraction protocols and routine use of control samples.
Metrological difficulties for Δ⁸-THCA also include defining detection limits and quantitative analysis, especially in complex biological matrices where background impurities and isomeric cannabinoids interfere with precise identification. To reduce these interferences, high-performance chromatographic systems with multidimensional separation (2D-HPLC), as well as enhanced high-resolution mass spectrometry detectors (HRMS) and fragmentation methods (MS/MS), are applied. However, these techniques are expensive and require highly skilled analysts.
Additionally, regulatory documents concerning cannabis product control do not currently include Δ⁸-THCA as a separate parameter, resulting in practically no regulatory-level standardization for this cannabinoid. This creates challenges for the pharmaceutical industry and dietary supplement manufacturers, who lack clear requirements for Δ⁸-THCA content, consequently hindering the scientifically grounded application of this compound.
Pharmacological Potential of Δ⁸-THCA
The pharmacological potential of Δ⁸-THCA remains one of the most promising yet insufficiently studied areas in cannabinoid research. This molecule, the acidic form of Δ⁸-tetrahydrocannabinol, exhibits unique properties that distinguish it from the more extensively studied neutral cannabinoids such as Δ⁸-THC and Δ⁹-THC. The majority of current scientific work focuses on the decarboxylated forms, which display pronounced psychoactivity and act directly on cannabinoid receptors CB1 and CB2. In contrast, acidic forms, including Δ⁸-THCA, have historically been considered biologically inactive precursors, losing pharmacological activity before decarboxylation.
However, this concept is shifting due to recent discoveries. Δ⁸-THCA is not only an intermediate compound in the biosynthesis of psychoactive cannabinoids but also possesses its own spectrum of pharmacological effects that could be useful in therapeutic applications. Its potential across various biological processes is linked to specific molecular mechanisms that are not yet fully understood but are important for immunomodulation, neuroprotection, and regulation of physiological functions.
A distinctive feature of Δ⁸-THCA is that it retains the acidic functional group, which influences its ability to penetrate biological membranes and thus affects its pharmacokinetics. This means the molecule behaves differently from its neutral analogs, impacting its bioavailability, tissue distribution, and metabolism. Additionally, it demonstrates increased chemical stability under certain conditions, opening prospects for use as a pharmaceutical agent with prolonged action.
Current data suggest that Δ⁸-THCA may interact with various biological targets beyond cannabinoid receptors. This includes potential effects on TRP channels, ion channels, and other receptor systems involved in pain signal transmission, inflammatory responses, and mood regulation. Such a multifunctional profile makes Δ⁸-THCA a promising candidate for further study as a treatment for a wide range of conditions, including chronic pain, inflammation, neurological disorders, and mood disorders.
At the same time, a significant challenge in the development of Δ⁸-THCA pharmacology is the lack of systematic studies, especially those evaluating the molecule’s safety and toxicity. Contemporary scientific publications predominantly contain in vitro data and short-term animal experiments, complicating translation into clinical practice. Furthermore, analytical methodologies for accurate identification and quantification of Δ⁸-THCA in biological samples require improvement, which is essential for standardizing research and pharmaceutical development.
Current Hypotheses on the Mechanism of Action
The mechanism of action of Δ⁸-THCA remains a subject of active scientific investigation, as this molecule differs both from neutral cannabinoids and from its better-studied isomeric form, Δ⁹-THCA. One key topic is the ability of Δ⁸-THCA to interact with endocannabinoid receptors CB1 and CB2 without prior decarboxylation, which traditionally was considered necessary for pharmacological activity to manifest.
Experimental evidence indicates a weak but specific agonist activity of Δ⁸-THCA on CB1 receptors. This interaction occurs through a mechanism distinct from the typical binding of neutral cannabinoids, particularly by influencing receptor conformation and modulating secondary messengers. At the same time, binding to CB2 receptors-primarily responsible for immune regulation-is less explored, but it is hypothesized that Δ⁸-THCA may indirectly affect immune cells through other receptor systems.
Beyond direct receptor interaction, a key role in Δ⁸-THCA pharmacology is its ability to modulate inflammatory processes. At the molecular level, this involves inhibition of pro-inflammatory cytokine and chemokine expression, as well as activation of cellular antioxidant systems. These effects have been confirmed in vitro in macrophage and neuronal cell studies, where Δ⁸-THCA reduces expression of TNF-α, IL-6, and COX-2. This suggests the molecule’s potential as an immune response regulator without triggering the psychoactive effects typical of Δ⁹-THC.
Another important aspect is Δ⁸-THCA’s influence on neurotransmission, particularly through modulation of TRP channels (transient receptor potential channels), which play roles in pain signal transmission and thermoregulation. Activation of TRPV1 and TRPA1 channels may explain the analgesic effect and ability to reduce nausea observed with Δ⁸-THCA use.
It is important to note that due to the acidic nature of the molecule, its pharmacokinetics differ significantly from neutral cannabinoids. Δ⁸-THCA has a limited ability to cross the blood-brain barrier, which restricts its direct effects on the central nervous system, but enhances local activity in peripheral tissues. This makes it a promising agent for treating peripheral inflammation and pain syndromes while minimizing psychoactive effects.
It must be acknowledged that most current hypotheses are based on limited experimental data, often obtained from in vitro models or receptor computational modeling. Confirmation of these mechanisms requires deeper in vivo studies, including application of modern molecular biology, pharmacodynamics, and pharmacokinetics methods. Particularly important is research on Δ⁸-THCA’s interaction with other components of the cannabinoid complex, which may influence the overall therapeutic effect.
Toxicological Profile and Safety of Δ⁸-THCA
Research on the toxicological profile of Δ⁸-THCA is currently in its early stages, largely due to limited access to pure isolated samples and a lack of systematic in vivo and in vitro experiments. The scarcity of data complicates determination of safe therapeutic doses, potential side effects, and the establishment of official clinical use guidelines.
Preliminary toxicological studies of cannabinoid acids suggest relatively low acute toxicity. Studies on structurally similar Δ⁹-THCA demonstrate high biocompatibility and low risk of acute toxicity even at doses exceeding pharmacologically active levels. However, direct studies on Δ⁸-THCA are still lacking, which hinders definitive conclusions.
At the molecular level, the acidic form of cannabinoids has potential for forming non-covalent complexes with membrane proteins, which theoretically could influence cellular receptor functions and transport systems. However, no data currently exist regarding toxicity from such interactions or disruptions to cellular homeostasis.
Special attention should be given to Δ⁸-THCA metabolism in the body. Enzymatic pathways for breakdown and transformation of this molecule may produce metabolites that under certain conditions have potentially toxic properties, such as generating reactive oxygen species or binding to DNA. Lack of detailed research in this area adds uncertainty about long-term safety.
Studies on the effects of Δ⁸-THCA on the liver, kidneys, cardiovascular system, and reproductive organs are practically nonexistent, posing a significant barrier to toxicological safety assessment. Animal toxicity models and cell cultures need to be developed to identify potential cumulative toxicity or organ-specific effects.
Considering the pharmacokinetic characteristics of Δ⁸-THCA-particularly its limited bioavailability with oral administration due to acidity and possible degradation in gastric conditions-experimental studies should focus on determining safe delivery forms that maintain molecular stability and reduce risk of adverse reactions.
Standardized methods for quantitative determination of Δ⁸-THCA in biological samples are also critically needed to correlate dosing with pharmacological and toxicological effects. The lack of precise analytical protocols currently complicates the evaluation of pharmacokinetics and toxicodynamic processes.
Given the rapid development of cannabinoid pharmacology, it is urgent to launch comprehensive toxicological studies addressing duration of action, accumulation effects, and potential mutagenic and carcinogenic properties. In vivo studies with models approximating human physiology will be especially important for defining a safety profile relevant to clinical applications.
Prospects for Research and Application
The prospects for research and application of Δ⁸-THCA emerge at the intersection of fundamental science, pharmaceutical technology, and legal regulation. As one of the less studied cannabinoids, this compound represents a unique subject for uncovering new properties of cannabinoid chemistry, which could significantly expand the understanding of molecular interactions with biological systems.
A fundamental prospect is that Δ⁸-THCA enables the study of cannabinoid isomerization mechanisms, which substantially influence pharmacokinetic and pharmacodynamic characteristics. The molecular structure of the isomer determines its stability, ability to interact with endocannabinoid system receptors, and passage through biological barriers. These features open new avenues for research, particularly the study of specific binding to CB1 and CB2 receptors prior to Δ⁸-THCA decarboxylation into active forms, potentially reshaping traditional views on cannabinoid mechanisms of action.
From a practical standpoint, Δ⁸-THCA holds potential as a basis for developing new pharmaceutical drugs. Investigating the properties of isomers opens opportunities for synthesizing and testing new derivatives with improved selectivity, increased bioavailability, or reduced toxicity. Modern technologies such as enzymatic synthesis and genetic engineering approaches can be applied to obtain highly pure forms of Δ⁸-THCA or to create modified molecules with unique biological properties.
Another important area concerns standardization, analytical control, and quantitative determination of Δ⁸-THCA in pharmaceutical and food products. The insufficient development of metrological methods and the absence of unified standards complicate quality control and create market uncertainty. Addressing these issues is key to the safe and effective use of Δ⁸-THCA in medical and commercial contexts.
At the same time, many prospects are tied to legal challenges. Legislation in many countries has yet to define clear regulatory criteria for acidic cannabinoid forms, leaving Δ⁸-THCA in legal “gray zones.” This creates difficulties for both manufacturers and consumers, affects market transparency, and requires regulatory frameworks to adapt to current scientific knowledge.
Applications of Δ⁸-THCA extend beyond traditional therapeutic areas, including potential uses in cosmetics, the food industry, and the development of biomaterials and nanotechnologies. Its unique physicochemical properties pave the way for innovative products with controlled bioavailability and targeted action.
Δ⁸-THCA as a Model for Studying Cannabinoid Isomerization
Δ⁸-THCA as a model for studying cannabinoid isomerization is extremely promising due to its unique chemical structure and physiological activity, which differ from classical cannabinoids. Isomerization in cannabinoid chemistry primarily manifests through different spatial and electronic configurations of molecules, significantly affecting their ability to interact with biological targets, bioavailability, metabolic pathways, and consequently, pharmacological effects.
Δ⁸-THCA is an isomer of Δ⁹-THCA, where the double bond is located at a different position in the ring. This seemingly minor change has profound implications for the molecule’s stability, reactivity, and pharmacokinetic properties. Studying Δ⁸-THCA allows for a deeper understanding of subtle isomerization mechanisms in the cannabinoid system, which typically remain overlooked due to analytical complexity and lack of pure isomers for comparison.
The pharmacokinetic aspect of studying Δ⁸-THCA as an isomer focuses on absorption, distribution, metabolism, and excretion characteristics. The shift of the double bond affects the molecule’s conformation, which in turn modifies interactions with enzymatic systems, particularly cytochrome P450 enzymes, which are key in cannabinoid metabolism. Comparing the pharmacokinetics of Δ⁸-THCA and Δ⁹-THCA reveals specific metabolic pathways that can be exploited for targeted molecule modification to enhance bioavailability or reduce toxicity.
Additionally, research on Δ⁸-THCA as an isomer opens opportunities for creating new cannabinoid derivatives with tailored properties. For example, by changing the double bond position or introducing specific functional groups, compounds with optimized affinity for CB1 and CB2 receptors or improved pharmacokinetics can be obtained. This approach is promising for developing drugs with selective action, minimal side effects, and enhanced efficacy.
Chemical synthesis and biotransformation methods of isomers based on Δ⁸-THCA are used to study structure-activity relationships. Modern analytical technologies such as nuclear magnetic resonance (NMR), high-resolution mass spectrometry (HRMS), and chromatography allow for identification and quantitative analysis of even minor structural changes, which is critical for understanding isomerization mechanisms and pharmacological activity.
Studying the pharmacokinetics of Δ⁸-THCA also contributes to the development of effective pharmaceutical formulations. There is particular interest in creating encapsulated, lyophilized, or nano-dispersed forms that improve cannabinoid stability and bioavailability. Understanding the specificity of isomerization allows adaptation of delivery technologies to ensure targeted release of the active substance, minimizing degradation and enhancing therapeutic effects.
In the context of pharmacodynamics, Δ⁸-THCA serves as a valuable model for analyzing the impact of structural changes on interactions with cannabinoid receptors and other protein targets. Different positioning of the double bond alters the electronic distribution and conformation of the molecule, correlating with changed affinity and activity toward CB1/CB2 receptors, TRPV1, and others. This facilitates deeper investigation into molecular details of receptor selectivity, aiding the development of more precise ligand-receptor interaction models.
Legal Regulation Challenges
The legal regulation of Δ⁸-THCA is extraordinarily complex and ambiguous due to the unique status of the acidic form of cannabinoids, which generates a range of legal and regulatory challenges. Δ⁸-THCA, as the acidic form of the tetrahydrocannabinol molecule, exists in a sort of “legal gray area,” making it difficult to clearly classify as either a controlled substance or a permitted compound across different jurisdictions. This phenomenon arises from several factors related to the absence of standardized classification criteria, insufficient analytical identification, and varying legislative approaches to acidic cannabinoid forms.
The first challenge lies in the chemical nature of Δ⁸-THCA. Unlike decarboxylated forms such as Δ⁸-THC or Δ⁹-THC, the acidic forms contain a carboxyl group that fundamentally alters their pharmacological profile, bioavailability, and psychoactivity. Lawmakers often do not consider this difference, applying regulations designed for decarboxylated cannabinoids, which leads to ambiguous interpretations of Δ⁸-THCA’s legal status. Since this form is thermally unstable and converts to the active Δ⁸-THC upon heating, it technically holds potential psychoactivity but is itself less active. This raises the question of whether it should be classified as a prohibited substance.
The second issue concerns the lack of transparency in chemical labeling of products containing Δ⁸-THCA. Due to the absence of standardized analytical methods and certification, manufacturers may label Δ⁸-THCA products as “non-psychoactive” or “legal,” which misleads consumers and regulators. This creates legal conflicts, as such products may contain active Δ⁸-THC formed during acid degradation through storage or heating. The lack of clear quality control standards and quantitative analysis facilitates the emergence of products with undefined composition, intensifying regulatory concerns.
Another aspect is the international inconsistency in legal approaches. In some countries, Δ⁸-THCA is not listed as a controlled substance, while in others its status remains ambiguous due to the absence of regulatory acts explicitly addressing acidic cannabinoid forms. This creates barriers to international trade and scientific exchange, complicates the development of unified guidelines and standards, and poses serious restrictions on research and clinical applications for scientists and pharmaceutical developers.
Legal “gray zones” are often tied to the lack of clear methods and criteria for identifying Δ⁸-THCA within complex mixtures. Standard analytical methods, such as gas chromatography coupled with mass spectrometry, can cause thermal degradation of acidic forms, complicating reliable analysis. This leads to ambiguous results, which manufacturers and regulators may use to support divergent legal interpretations. Frequently, Δ⁸-THCA is viewed merely as a precursor to psychoactive Δ⁸-THC, raising additional questions about the legitimacy of its use.
An important factor is the general legislative focus on active cannabinoid forms, especially Δ⁹-THC, which is firmly prohibited in many countries. Δ⁸-THCA and other acids have not received the same level of regulatory attention, leaving their governance underdeveloped. This is sometimes used as an argument in favor of Δ⁸-THCA legalization, but it overlooks the acid’s potential to convert into psychoactive cannabinoids during heating or metabolism in the body.
The legal regulation of Δ⁸-THCA is further complicated by the rapidly evolving cannabinoid product market, where manufacturers constantly experiment with new formulations and delivery methods. The use of Δ⁸-THCA in cosmetics, dietary supplements, and pharmaceutical products often outpaces legislative initiatives, creating gaps in quality and safety oversight. The insufficiency of legal frameworks for acidic forms increases consumer risks and hinders the establishment of transparent standards.
Considering these factors, the key challenge in regulating Δ⁸-THCA is the lack of harmonized regulatory definitions that take into account the unique chemical and pharmacological properties of acidic cannabinoids. It is essential to develop specialized classification criteria based on modern analytical technologies and pharmacological research results to clearly distinguish Δ⁸-THCA from decarboxylated psychoactive forms and establish a definitive legal status.
It is important to emphasize that effective legal regulation of Δ⁸-THCA must incorporate scientific knowledge into regulatory documents, including standards for analytics, labeling recommendations, and product safety requirements. Such an approach would reduce health risks to consumers, support industry growth, and decrease legal uncertainty for manufacturers and researchers.
The issue of transparency in chemical labeling of products containing Δ⁸-THCA is equally critical. The lack of accurate information about product composition leads to violations in consumer choice and increases opportunities for fraud. Manufacturers should implement transparent labeling standards, including specific quantitative indicators of Δ⁸-THCA content as well as information on possible acid transformations during storage and use. This is particularly relevant for medical and food products, where safety and efficacy are critical parameters.
On the international stage, the need for harmonization of legal norms concerning Δ⁸-THCA is becoming increasingly evident. Different countries adopt fundamentally different approaches to cannabinoid regulation, which complicates scientific collaboration and the development of a global market. Manufacturers and researchers face difficulties transporting materials, sharing information, and implementing innovations due to disparate legal regimes. The absence of unified standards slows progress in the study and application of Δ⁸-THCA.
A vital step toward resolving these issues could be the development of international guidelines or protocols to standardize approaches to identification, classification, and regulation of acidic cannabinoid forms. This would not only enhance consumer safety but also stimulate scientific research, pharmaceutical development, and responsible manufacturing.
Conclusion:
Delta-8-tetrahydrocannabinolic acid (Δ8-THCA) is a little-studied yet highly promising molecule among the phytocannabinoids produced by the Cannabis sativa plant. Its uniqueness is determined by a special structural isomerism that distinguishes it from the more common Δ9-THCA, specifically the shift of the double bond within the tetrahydrocannabinol ring. This structural difference significantly impacts its physicochemical properties, stability, biochemical synthesis, pharmacological effects, and synthetic accessibility, together forming a unique profile for this acid.
In the plant, Δ8-THCA is formed through complex biochemical pathways involving synthesis from cannabigerolic acid (CBGA) via specific synthase enzymes that direct the metabolic branch toward the formation of different cannabinoid acids. However, under natural conditions, Δ8-THCA concentrations are extremely low, which is explained by the thermodynamic instability of the molecule and the preference for forming more stable isomers such as Δ9-THCA. The isomerization of the double bond that converts Δ9-THCA to Δ8-THCA is thermodynamically less favorable and requires specific conditions or catalysts, significantly limiting the natural accumulation of Δ8-THCA in cannabis tissues.
Technical advances allow for artificial production of Δ8-THCA via isomerization of Δ9-THCA, overcoming natural limitations. Catalyzed reactions involving acidic or basic agents, under precise control of conditions, enable targeted shifting of the double bond position in the molecule while preserving the carboxyl acid group-key for maintaining Δ8-THCA’s properties. This opens opportunities for synthesizing large batches of pure acid for further research and potential pharmaceutical applications.
Analytical methods, especially high-performance liquid chromatography (HPLC) combined with mass spectrometry (LC-MS), are critically important for reliable identification and quantitative determination of Δ8-THCA. A crucial aspect is preserving the acidic form during analysis, since even slight temperature increases or chemical conditions can cause decarboxylation and conversion to neutral forms, making accurate concentration assessment impossible and disrupting standardization. The lack of sufficient high-quality analytical standards poses a serious challenge to metrological accuracy, complicating the development of regulations and standards needed for industrial use.
Pharmacological activity of Δ8-THCA remains insufficiently studied, but scientific hypotheses suggest its ability to interact with cannabinoid receptors CB1 and CB2 even in the acidic form, before decarboxylation. This activity differs from traditional neutral cannabinoid forms and may explain mild yet significant pharmacological effects. In particular, the molecule shows potential in modulating inflammatory processes, immune response, and neurogenic regulation of nausea, making it promising for use in anti-inflammatory and symptomatic therapy, especially for patients with chronic conditions or cancer. However, the absence of comprehensive in vivo and in vitro studies, along with limited toxicological data, prevents a definitive safety profile assessment, which is a key barrier to clinical implementation.
Studying Δ8-THCA as a model of isomerism expands understanding of structure-function relationships in cannabinoid chemistry and pharmacokinetics, opening prospects for developing new, more effective, and selective cannabinoid compounds. There is particular interest in the possibility of creating Δ8-THCA derivatives with altered pharmacological properties, potentially leading to innovative pharmaceutical agents with unique action profiles and reduced side effects.
However, the legal regulation of Δ8-THCA remains ambiguous, as the acidic form often resides in a “gray area” within the legislation of many countries. Insufficient transparency in chemical labeling of products containing Δ8-THCA, combined with the absence of clear classification standards, creates risks for both manufacturers and consumers. This complicates quality control, safety of use, and the legality of distributing Δ8-THCA-based products, presenting a significant challenge for the further development of the cannabinoid industry.
Sources:
- PubMed Central (PMC) – Database of scientific articles on cannabinoids
https://www.ncbi.nlm.nih.gov/pmc/?term=delta+8+THCA - National Center for Biotechnology Information (NCBI) – Cannabinoid research
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7070560/ - Journal of Natural Products – Biosynthesis of cannabinoids
https://pubs.acs.org/doi/10.1021/acs.jnatprod.0c00547 - Frontiers in Pharmacology – Pharmacology and mechanisms of action of cannabinoids
https://www.frontiersin.org/articles/10.3389/fphar.2021.627908/full - Phytochemistry Reviews – Structural chemistry and isomerism of cannabinoids
https://link.springer.com/article/10.1007/s11101-019-09604-4 - Analytical Chemistry – Chromatographic methods for cannabinoid analysis
https://pubs.acs.org/doi/10.1021/acs.analchem.9b04910 - Cannabis and Cannabinoid Research – New approaches to synthesis and isolation of cannabinoids
https://www.liebertpub.com/doi/full/10.1089/can.2019.0053 - European Journal of Pharmacology – Toxicology and safety of cannabinoids
https://www.sciencedirect.com/science/article/pii/S0014299920302017 - Harvard Medical School – Cannabis Research Overview
https://hms.harvard.edu/news/harvard-experts-cannabis - National Institute on Drug Abuse (NIDA) – Information on cannabinoids and research
https://nida.nih.gov/publications/research-reports/marijuana/cannabinoids