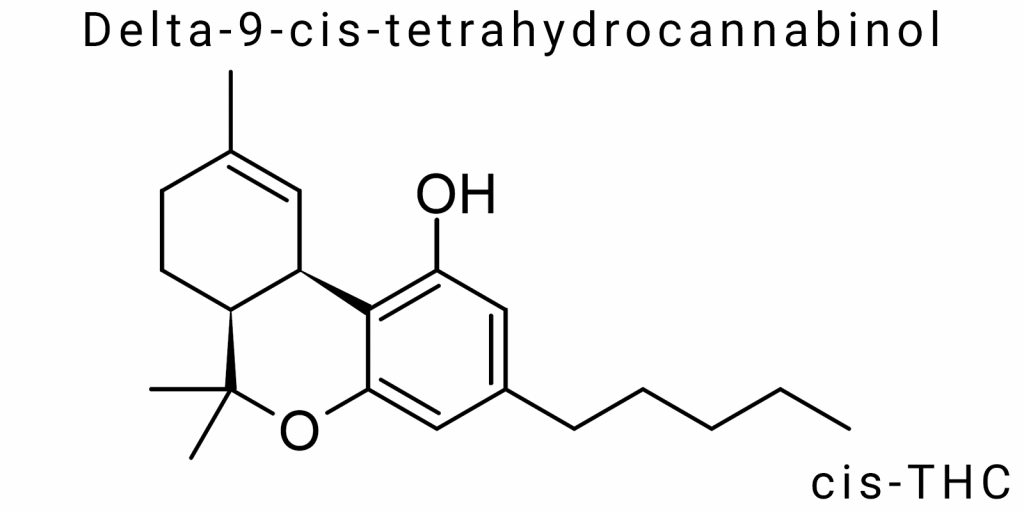
Δ⁹-cis-tetrahydrocannabinol (Δ⁹-cis-THC) is a rare but chemically and pharmacologically significant stereoisomer of one of the main psychoactive components of cannabis-Δ⁹-tetrahydrocannabinol. In the system of natural phytocannabinoids produced by Cannabis sativa L., stereoisomerism plays a crucial role in the biological activity of the molecules. Δ⁹-THC has at least four stereoisomers arising from the configuration at the chiral centers in the tetrahydrocannabinol scaffold. Among them, the most well-known is the trans isomer with the configuration (−)-(6aR,10aR)-Δ⁹-THC, while the cis form with the configuration (6aS,10aR) remains the subject of only limited research. Studying this isomer is a necessary step in a systematic analysis of structure-activity relationships among cannabinoids.
Phytocannabinoids are not classically biosynthesized secondary metabolites in terms of high enzymatic stereoselectivity. Current research demonstrates that Δ⁹-THC synthases in Cannabis sativa can produce mixtures of diastereomers, particularly in industrial (hemp) cultivars dominated by CBDA or CBGA. This means that Δ⁹-cis-THC is formed not only as a result of artificial isomerization in the laboratory but can also be present in trace amounts in the plant. Its presence in natural material is detected using highly sensitive methods-liquid chromatography combined with mass spectrometry (LC-MS), as well as nuclear magnetic resonance (NMR).
From a chemical point of view, Δ⁹-cis-THC is a cyclic triterpenoid compound, a derivative of meroterpenoic acid, featuring a characteristic tricyclic system. Its molecular formula is C₂₁H₃₀O₂. The most important structural elements include a phenolic ring, a terpene chain, and a characteristic double bond between C⁹ and C¹⁰. In the case of the cis isomer, the spatial arrangement of this double bond and substituents around the chiral centers creates a unique configuration that alters electron density, molecular volume, and the geometry of its interaction with biological targets.
Since Δ⁹-cis-THC is a stereoisomer, its laboratory synthesis is usually associated with chemical methods that do not provide high enantioselectivity without specific catalysis. Two common approaches include: (1) direct condensation of olivetol precursors with terpene aldehydes, such as citral, or with terpene epoxides-specifically (+)-trans-2-carene oxide-in the presence of acid catalysts (e.g., BF₃·Et₂O); (2) acid-catalyzed isomerization of more stable isomers, such as cannabichromene (CBC), which is accompanied by a redistribution of the double bond configuration and chiral centers. Both approaches yield mixtures of diastereomers from which Δ⁹-cis-THC must be purified by chromatography. The synthetic yield is typically moderate, and stereoselectivity requires additional purification or crystallization steps.
The biological activity of Δ⁹-cis-THC is evaluated based on its ability to bind to cannabinoid receptors of the first (CB1) and second (CB2) types. Unlike the trans isomer, which is a full agonist of CB1 with high affinity, Δ⁹-cis-THC has significantly lower affinity for this receptor, indicating weak or partial agonist activity. This reduces its psychoactivity while increasing its interest as a potential cannabinoid with a therapeutic profile that lacks central side effects. In vivo studies on mouse models show that Δ⁹-cis-THC can still induce the typical cannabinoid triad of effects (hypothermia, analgesia, reduced locomotor activity), although with less intensity.
Currently, the pharmacokinetic profiles of Δ⁹-cis-THC remain largely unexplored. It is expected that its lipophilicity, bioavailability, and metabolic pathways in the liver involving cytochrome P450 enzymes will be similar to those of other cannabinoids. However, stereochemistry can significantly affect the rate of metabolic breakdown and the formation of active metabolites, particularly 11-hydroxy-Δ⁹-THC-the main psychoactive metabolite. It is likely that Δ⁹-cis-THC is metabolized more slowly or produces less active derivatives, which could be advantageous from a toxicokinetic perspective.
At present, Δ⁹-cis-THC is not controlled as a separate substance in most jurisdictions, including the UN lists. As a result, it can be studied more freely within fundamental science or pharmacological research. However, due to its structural similarity to Δ⁹-trans-THC, regulatory authorities may interpret it as an analog, which raises questions about the legality of its circulation depending on the jurisdiction. In the context of medical cannabis, this isomer is not a standard component, adding further uncertainty to the development of products based on it.
Studying Δ⁹-cis-THC not only allows a better understanding of the nuances of cannabinoid pharmacology but also may contribute to the creation of new therapeutic agents. Its potential as a partial agonist of CB1/CB2 receptors may be relevant for conditions requiring moderate modulation of the endocannabinoid system-specifically in cases of chronic pain, spasticity, neuroinflammation, or anxiety disorders. In combination with other cannabinoids or as a standalone substance, Δ⁹-cis-THC opens opportunities for developing more controlled, targeted pharmaceutical formulations without strong psychoactive effects.
Chemical Structure and Stereochemistry of Δ⁹-cis-THC
Δ⁹-cis-tetrahydrocannabinol (Δ⁹-cis-THC) is one of the stereoisomers of Δ⁹-tetrahydrocannabinol, the primary psychoactive component of cannabis. Its uniqueness lies in the specific spatial arrangement of atoms around the chiral centers and the double bond, forming the so-called “cis” configuration. This stereoisomer differs from the naturally active trans isomer (−)-(6aR,10aR)-Δ⁹-THC, which predominates in most chemotypes of Cannabis sativa L.
Chemically, Δ⁹-cis-THC is a tricyclic terpene phenol, a derivative of cannabigerolic acid (CBGA), which is a common biosynthetic precursor for many phytocannabinoids. The molecular formula of Δ⁹-THC remains constant for all its stereoisomers: C₂₁H₃₀O₂, with a molecular weight of 314.46 g/mol. The difference lies in the spatial arrangement of atoms in the molecule, particularly around the chiral centers at positions C6a and C10a of the tetrahydrocannabinol skeleton.
In Δ⁹-cis-THC, these chiral centers have the configuration (6aS,10aR), whereas in the active trans isomer, they are (6aR,10aR). This affects the geometry of the ring and the position of the methyl and pentyl groups, which determines the molecule’s ability to bind to cannabinoid receptors. The structural formula includes one phenolic hydroxyl group (at position 1), an aliphatic pentyl side chain (at position 3), a double bond between carbon atoms C9 and C10, and a terpene fragment that is closed into the tetrahydrocannabinol ring.
Stereoisomers, and particularly the diastereomers of Δ⁹-THC, differ not only spatially but also in physicochemical properties such as melting point, optical rotation, solubility, and chromatographic mobility. In the context of Δ⁹-cis-THC, these parameters allow for its separation from other forms using chiral HPLC (high-performance liquid chromatography) or thin-layer chromatography with specialized solvents.
From a synthetic standpoint, Δ⁹-cis-THC can be obtained through several schemes. The first approach involves acid-catalyzed condensation of olivetol with citral, producing a mixture of Δ⁹-THC isomers, among which the cis isomer is present. Alternatively, the reaction of olivetol with (+)-trans-2-carene oxide in the presence of BF₃·Et₂O can lead to the preferential formation of Δ⁹-cis-THC. It can also be obtained via isomerization of cannabichromene (CBC), which cyclizes into Δ⁹ isomers in the presence of boron trifluoride etherate. All these methods require subsequent chromatographic purification because the products are usually mixtures of trans/cis diastereomers.
Besides chemical synthesis, the cis form can be found in trace amounts in natural cannabis, especially in strains with high CBD content or in so-called “non-psychoactive” chemotypes. However, in medical strains with high Δ⁹-trans-THC content, such as Bedrocan, the level of the cis isomer usually does not exceed 0.1%. Its detection is possible through a combination of LC-MS/MS and two-dimensional nuclear magnetic resonance correlation spectroscopy (2D-NMR COSY, HSQC, HMBC), which allow differentiation of stereoisomers based on chemical shifts and spin-spin couplings.
From the perspective of interaction with biological systems, the spatial arrangement of the side alkyl group as well as the orientation of the phenolic ring are crucial for binding to CB1 and CB2 receptors. Δ⁹-cis-THC demonstrates reduced binding to the CB1 receptor compared to the trans isomer, which can be explained by the displacement of key groups from the optimal orientation within the binding site. This likely accounts for the decreased psychoactive potential and possible partial agonism.
The thermodynamic stability of the cis isomer is lower than that of the trans form, which is manifested in its tendency to undergo further isomerization upon heating, light exposure, or acidic conditions. This is important for the development of stable pharmaceutical formulations: Δ⁹-cis-THC requires careful optimization of storage conditions, including temperature control, absence of UV radiation, and an inert atmosphere.
The presence of chiral centers makes Δ⁹-cis-THC an optically active compound. It is usually described as (−)-(6aS,10aR)-Δ⁹-THC, but enantiomers (+(6aR,10aS) form) may also exist depending on synthesis conditions. Their biological activity will differ, as receptor affinity is enantioselective. This is another argument in favor of applying chiral chromatography when analyzing synthesized products.
Structural Formula of Δ⁹-cis-Tetrahydrocannabinol
The molecule of Δ⁹-cis-tetrahydrocannabinol (Δ⁹-cis-THC) represents a highly organized organic structure belonging to the class of tricyclic cannabinoids. Its basic chemical formula – C₂₁H₃₀O₂ – remains constant for all stereoisomers of Δ⁹-THC; however, the spatial arrangement of functional groups and the configuration of the ring systems differ, which accounts for the unique physicochemical and biological properties of the cis form specifically. This section focuses solely on the aspects directly related to the formal structural organization of Δ⁹-cis-THC, excluding stereochemistry or receptor activity, which will be discussed in detail elsewhere.
The molecular architecture of Δ⁹-cis-THC includes three condensed rings: a partially saturated cyclohexene ring (B), a benzene phenolic core (A), and a tetrahydropyran ring (C), which together form the cannabinoid tricyclic skeleton. The starting point for analyzing the structural formula is the cannabigerol core, which during biogenesis or chemical synthesis converts into Δ⁹ isomers, including the cis form. For accurate description, the atom numbering system approved by IUPAC is used: beginning from the phenolic ring, atoms are numbered from 1 to 10a, allowing precise localization of functional groups in the context of reactivity and analytical identification.
The phenolic ring (A) contains a hydroxyl group at position C1, which participates in hydrogen bonding and can be substituted or modified in synthetic derivatives. Parallel to this, at position C3, an attached pentyl alkyl side chain (−C₅H₁₁) serves as a key determinant of the molecule’s lipophilicity. This side chain not only influences the solubility of Δ⁹-cis-THC in lipid environments but also defines its affinity for transmembrane domains of receptors. Nevertheless, within the structural formula, it is considered an unchanging aliphatic fragment without reactive centers.
The central cyclohexene ring (B) contains a double bond between carbon atoms C9 and C10, which is a characteristic feature of the Δ⁹ isomers. The placement of this double bond in the plane of the ring creates conditions for the existence of cis- and trans-diastereomerism. In the case of Δ⁹-cis-THC, two substituents (groups at positions 6a and 10a) are located on the same side relative to the plane of the double bond. This positioning alters the ring conformation and affects the electron density around the double bond, which can be detected by NMR and IR spectroscopy methods.
Ring C is the tetrahydropyran fragment, formed by an intramolecular cyclic connection between the terpene chain and the phenolic ring. In the structural formula, this is a six-membered heterocyclic ring containing an oxygen atom at position C1′, which lacks double bonds but can adopt flexible conformations. This fragment is key for establishing the stable tricyclic structure of the molecule. Its orientation is rigidly fixed due to the cis-configuration on the adjacent cyclohexene ring, determining the overall volume and topology of the molecule.
A critical feature of Δ⁹-cis-THC is that the Δ⁹ double bond (between C9 and C10) imposes planarity on part of the molecule, which fixes the orientation of bulky substituents. In the cis form, both methyl substituents (at 6a and 10a) are positioned on the same side relative to the plane of the ring, introducing spatial asymmetry. This configuration is not the most energetically favorable – which is why the trans isomer is more stable – yet under specific synthesis or biosynthesis conditions, it may form as either a minor component or the target product.
From the standpoint of electronic structure, the molecule contains one phenolic hydroxyl group exhibiting acidic properties (pKa ≈ 10.6), as well as a double bond that determines spectroscopic properties (characteristic UV absorption in the 210-280 nm range). The dielectric influence of the pentyl group on the electron density of the aromatic ring also plays a role. The arrangement of all these groups creates a unique chromatographic profile for Δ⁹-cis-THC, which can be readily separated from other isomers using HPLC, TLC, or GC with selective detection.
To construct the full structural formula, it is important to consider the tetrahydrocannabinol skeleton, which contains six chiral centers; however, only two of these (6a and 10a) are configurationally defining. The others may exist in dynamic equilibrium due to rotational flexibility. In the structural formula, they are conventionally fixed in positions that ensure the cis configuration, though the real molecule may exhibit some mobility in solution.
The presence of three ring systems in Δ⁹-cis-THC creates a complex conjugated orbital system, rendering the molecule spectroscopically active across several ranges. This allows the use of instrumental methods such as 2D-NMR, DEPT, COSY, and HSQC for detailed structural verification. For complete characterization of the structural formula of Δ⁹-cis-THC, X-ray diffraction (XRD) analysis is often used when the compound can be crystallized, or high-resolution mass spectrometry (HRMS) with fragmentation analysis.
Another aspect of the structural formula of Δ⁹-cis-THC is the presence of potentially reactive centers available for chemical modification: the phenolic hydroxyl can be esterified or etherified, the pentyl chain can be elongated or substituted, and the double bond can be hydrogenated. These transformations allow the creation of a series of structurally related analogs, but the primary structure of Δ⁹-cis-THC remains unique.
Stereochemical Configuration of Δ⁹-cis-Tetrahydrocannabinol
The stereochemical configuration of Δ⁹-cis-tetrahydrocannabinol (Δ⁹-cis-THC) is a key parameter that determines its physicochemical properties, reactivity, stability, and molecular recognition specificity. Unlike many low-molecular-weight compounds, where stereochemistry is limited to individual chiral centers, in the case of Δ⁹-cis-THC it encompasses an interdependent system involving both stereogenic centers and the geometric arrangement of substituents relative to the plane of the double bond. Studying this configuration is essential for a precise understanding of its formation mechanisms, potential bioactivity, and conditions for selective synthesis.
In the Δ⁹-cis-THC molecule, several chiral centers are present, but for characterizing the cis-isomerism, the key atoms are those at positions 6a and 10a. Their absolute configuration is determined by the Cahn-Ingold-Prelog (CIP) system and is designated as 6aS,10aR for the most commonly described enantiomer of Δ⁹-cis-THC. A distinctive feature of the cis-isomer is the orientation of the substituents at these positions on the same side of the plane formed by the cyclohexene ring. This arrangement sharply contrasts with the more thermodynamically stable trans-configuration, where the substituents are oriented on opposite sides of the plane. This orientation defines the nature of conformational locking and alters the molecule’s topology in three-dimensional space.
The double bond at the Δ⁹ position (between atoms C9 and C10) serves as a crucial element in the formation of diastereomers. The planar π-bond system rigidly fixes the positions of the atoms and excludes free rotation, which stabilizes the spatial orientation of two adjacent fragments-the cyclohexene ring and the tetrahydropyran ring. In the “cis” configuration, the methyl group at position 10a and the hydrogen at 6a are located on the same side relative to the plane, causing a change in the angle between rings A-B and B-C. Consequently, the molecule adopts a more compact, folded conformation, as opposed to the extended trans-orientation. This difference in architecture significantly affects the molecule’s energy profile and its interactions with the surrounding environment.
Beyond the configuration at the double bond, it is also important to consider the local stereochemistry within the tetrahydropyran ring, particularly at positions C1′, C4′, and C6′. Although these atoms are not classically chiral in all cases, due to the ring’s flexibility, they can form semi-rigid pseudo-chiral configurations that may play a role in molecular recognition mechanisms. Notably, in Δ⁹-cis-THC, the tetrahydropyran fragment is oriented such that the oxygen atom included in the ring approaches the plane of the cyclohexene, reducing the steric accessibility of this part of the molecule and enhancing its conformational rigidity.
The stereochemical behavior of Δ⁹-cis-THC is the subject of intensive analysis by two-dimensional NMR spectroscopy methods (COSY, NOESY, ROESY), where through-space correlations enable determination of atomic proximities regardless of their covalent connectivity. For example, based on NOE effects, the cis-orientation between the hydrogens at positions 6a and 10a can be accurately identified. The reduced NOE intensity compared to the trans-isomer further verifies the degree of ring system twisting and confirms the existence of the folded conformation. Such stereochemical organization influences not only spectroscopic characteristics but also the kinetics of reactions with external reagents.
Studies of the configurational stability of Δ⁹-cis-THC reveal that this form is less stable compared to the trans-isomer, which is supported both by density functional theory (DFT) calculations and empirical data on the thermodynamics of isomerization. In non-aqueous media and in the presence of protonic acids, a transition from the cis- to the trans-form is possible via an electrophilic activation mechanism of the double bond, accompanied by configuration inversion. Therefore, during the synthesis of Δ⁹-cis-THC, controlling temperature and reaction conditions is critically important to prevent racemization or isomerization.
Although enantiomeric purity of Δ⁹-cis-THC is not decisive for the existence of the cis-orientation, it is significant in the context of biological recognition. In nature, the most commonly encountered enantiomer is the (-)-enantiomer, corresponding to the 6aS,10aR configuration. However, synthetic methods may produce mixtures of enantiomers that require separation by chiral chromatography. Determination of absolute configuration is typically performed by comparing experimental optical rotations with theoretical data calculated by time-dependent density functional theory (TD-DFT) or by X-ray crystallographic analysis of chiral derivatives.
The integral stereochemistry of Δ⁹-cis-THC affects molecular dynamics parameters: the formation of intramolecular hydrogen bonds, chain flexibility, and the possibility of induced phytoselective interactions. For example, compared to the trans-isomer, the cis-form has a lower dipole moment, which decreases its solubility in polar environments and alters its distribution within membranes. This also changes the likelihood of intercalation into the lipid bilayer, impacting pharmacokinetic parameters. However, within this section, the focus remains solely on the stereochemical nature rather than functional consequences.
Additionally, it should be considered that cis-isomers, unlike trans-forms, do not always form crystalline phases with well-defined spatial packing. This complicates the use of X-ray crystallography but facilitates spectroscopic identification. The conformational mobility of the cis-form allows for more pronounced IR and NMR spectra, especially under variations in temperature or pH. This makes it possible to construct conformational maps of the molecule that describe changes in its structure depending on external conditions.
Methods of Synthesizing Δ⁹-cis-THC
The synthesis of Δ⁹-cis-tetrahydrocannabinol (Δ⁹-cis-THC) presents a particularly challenging task in modern organic chemistry due to the necessity for high stereoselectivity, the limited thermodynamic stability of the cis-isomer, and the chemical sensitivity of the functional groups involved. This cannabinoid features an isomeric difference in the configuration of the double bond, which critically impacts the biological properties of the molecule. Synthetic methods for the target molecule are focused on achieving precise control over the spatial arrangement of bonds in the central part of the tricyclic system. A successful strategy must combine efficient carbon skeleton construction with exact management of configurational integrity at the later stages.
The classical approach to cannabinoid synthesis is based on the coupling of olivetol (5-pentylresorcinol) with a terpenoid structure, most often a derivative of citral or α-pinene, followed by cyclization to form the cannabinoid framework. For Δ⁹-cis-THC, this route undergoes significant modifications. The initial steps are designed to prevent the formation of the thermodynamically more favored trans-isomer. A critical factor is the choice of protecting groups for the phenolic fragments and the conditions for aldehyde activation to minimize side reactions and uncontrolled isomerization.
To ensure stereospecific formation of the Δ⁹-cis-isomer, acid-catalyzed cyclization reactions are employed under carefully selected temperature, solvent, and reagent concentration conditions. For example, the use of boron trifluoride ether complexes directs the ring formation via a carbocation intermediate followed by induced nucleophilic attack, which promotes cis-orientation of substituents in the six-membered ring. Despite some efficiency, the reaction mixture typically contains a mixture of isomers, so a subsequent separation stage is mandatory.
After obtaining the intermediate structure containing the double bond at the Δ⁹ position, maintaining the cis-configuration throughout subsequent steps is crucial. Even slight heating or exposure to acidic or basic media can cause double bond inversion or migration within the ring system. Therefore, subsequent reactions-such as reduction, etherification, or functionalization-are performed under strictly controlled conditions with minimized reaction time, temperature, and acid or base concentration.
An alternative approach involves the use of asymmetric auxiliaries that influence the spatial orientation of the molecule at early stages. For instance, employing chiral boron esters in alkylation reactions can induce cis-orientation of substituents, which later translates into the correct stereochemistry of the final product. Although these methods are less scalable, they demonstrate potential in laboratory settings for synthesizing pure Δ⁹-cis-isomer without the need for further isomerization.
A distinct category of methods includes isomerization strategies, where Δ⁹-trans-THC is deliberately synthesized first, followed by controlled isomerization to the cis-form. These methods utilize photoinduced or acid-catalyzed inversion of configuration. The use of photochemical reactors with controlled wavelength and exposure allows for selective inversion without breaking other bonds in the molecule. Organic sensitizers (e.g., benzophenone) or iridium complexes capable of efficiently transferring energy to the double bond without fragmenting the carbon framework are most commonly employed for this purpose.
Despite the promise of photochemical methods, they require thorough optimization: light intensity, wavelength, solvent polarity, and reagent concentration must be carefully chosen to avoid side photooxidative processes or polymerized byproducts. UV-induced isomerization, although potentially effective, is sensitive to oxygen impurities, so reactions are typically carried out under inert atmospheres (nitrogen or argon).
Another strategic direction is the use of microfluidic reaction environments. In such systems, precise control over reagent contact time, temperature, and flow geometry allows for high reproducibility of stereochemical outcomes. Reaction conditions can be adjusted in real time, and the product output can be directly channeled to analytical systems for purity control of the target isomer. This approach is especially valuable for process scaling or combinatorial exploration of synthesis condition variants.
Regardless of the chosen synthetic route, the final stage in Δ⁹-cis-THC synthesis involves chromatographic purification and isolation of the isomer. High-performance liquid chromatography (HPLC) with chiral stationary phases is used to achieve selective separation of even structurally similar diastereomers. Gravimetric methods employing specific complexing agents that selectively bind the cis-isomer are also applied.
Classical Methods of Synthesis
The synthesis of Δ⁹-cis-tetrahydrocannabinol (Δ⁹-cis-THC) in laboratory settings has historically developed based on methodologies aimed at constructing the tricyclic system characteristic of the tetrahydrocannabinol class. The main classical approaches involve acid-catalyzed condensation reactions between phenolic precursors, such as olivetol, and terpenoid components-specifically, derivatives of monoterpenes that provide the cyclic fragment of the molecule. In the case of synthesizing cis-isomers, a key factor is controlling the spatial orientation of substituents during cyclization, which is achieved by varying reaction conditions, choice of solvent, temperature regime, and catalyst.
One of the most well-known approaches is the reaction of olivetol (5-pentylresorcinol) with (+)-trans-2-carene oxide or with citral in the presence of Lewis acids or protic acids. In this case, the reaction conditions themselves influence the configuration of the resulting cannabinoid structure. The use of boron trifluoride etherate (BF₃·Et₂O) in ether or chlorinated solvents provides electrophilic activation of the terpenoid component, which subsequently attacks the nucleophilic center of olivetol, forming a carbocation intermediate. Depending on temperature and catalyst concentration, the formation of various isomers, including Δ⁹-cis-THC, is possible.
It is particularly important that the configuration of the cyclic monoterpene component-such as citral-determines the potential for forming the cis-configuration in the final product. When geranial-derived electrophiles like citral or microconfigurations of linalool are used, the formation of Δ⁹-cis-THC occurs with moderate selectivity. However, in most classical methods, selective control over cis- or trans-isomerization is not fully guaranteed, necessitating further chromatographic separation of the isomers.
Attention should also be paid to acid-initiated condensation of olivetol with citral in benzene solvent in the presence of hydrochloric acid (HCl), which leads to the formation of the cannabinoid carbocycle followed by cyclization. In this approach, it has been noted that variation of the molar ratio of reagents (for example, 1:1.2 or 1:2) significantly affects the yield of cis-isomers. Interestingly, in a protic acid environment, the reaction proceeds with predominant formation of Δ⁹-isomers, and at lowered temperatures (0-5 °C), a higher proportion of the cis-form is formed.
Another classical variant involves the use of sesquiterpene epoxides, particularly derivatives of caryophyllene, which undergo ring-opening in the presence of Lewis acids with formation of corresponding cations. These cations can attack olivetol, generating the tricyclic structure with cis-orientation depending on the initial epoxide configuration. Menthol derivatives are applied by analogy; in reaction with phenolic components in acidic media, they produce mixtures of Δ⁹-THC isomers containing cis-configuration impurities.
A further chemical-technical aspect of classical methods is the control of moisture in the reaction environment. For reactions producing the cis-isomer, absolute dryness of reagents and solvents is critically important, as even traces of water lead to hydration of carbocation intermediates and side formation of alcohols or ethers, thereby reducing the overall yield of cis-THC.
Classical methods also include thermally controlled cyclization of corresponding cannabinoid alcohols in acidic media. In this process, the phenolic group at position 1 reacts with the aliphatic chain or isoprenoid component under catalytic heating in the presence of polyphosphoric acid (PPA), allowing formation of tertiary centers with defined stereochemistry. Thermal parameters affect not only the reaction rate but also the cis/trans product ratio.
It is also important to note that many classical protocols, historically focused on synthesizing Δ⁹-trans-THC, produce the cis-isomer as a side product with selectivity around 5-15%. However, modern retrosynthetic interpretation allows optimization of conditions to increase the yield of the cis-form. This includes, in particular, the use of stronger electrophilic activators such as SnCl₄ or AlCl₃, and the use of ether-aromatic solvents (for example, methylene chloride or toluene) with stabilizing properties for carbocation intermediates.
Additionally, condensation conditions on solid acids like silica gel impregnated with Lewis acids have been explored. In these cases, the catalytic activity of the solid phase enables the reaction to proceed heterogeneously with minimal side product formation. It has been observed that Δ⁹-cis-THC predominantly forms at temperatures of 40-60 °C over 6-8 hours, whereas trans-isomers require higher temperatures.
Some variants of classical protocols also consider a multistep approach: first, construction of the isoprenoid side chain with controlled geometry (for example, using Z-alkenes), followed by a cyclization stage preserving cis-configuration through a stereoselective inducer. These protocols theoretically allow targeted synthesis of Δ⁹-cis-THC, although practical implementation is limited by low overall efficiency.
Isomerization from Other Cannabinoids
Isomerization of cannabinoids, particularly the conversion of neutral phytocannabinoids into isomeric forms of Δ⁹-THC, is one of the key methods for obtaining rare structures, including Δ⁹-cis-tetrahydrocannabinol. This process is based on intramolecular rearrangements caused by reactions such as rearrangement, epimerization, or double bond transposition in the presence of catalysts. The choice of starting material critically influences the efficiency of cis-isomer formation. Primary focus has been placed on the conversion of cannabichromene (CBC) and cannabidiol (CBD) using Lewis acids or mild protic acids under controlled temperature conditions.
Isomerization of cannabichromene is a potential source for producing Δ⁹-cis-THC because the structure of CBC includes precisely oriented electronic regions prone to electrophilic rearrangements. CBC is a bicyclic phytocannabinoid with an open prenyl side chain, which allows cyclization initiation in the presence of acids. Under the action of BF₃·Et₂O in a nonpolar solvent (e.g., dichloromethane), migration of the double bond occurs with formation of a carbocation at the 2-position of the prenyl fragment. Subsequent cyclization ensures formation of the tricyclic cannabinoid system. When temperature is controlled within the range of 0-10 °C, predominant generation of cis-orientation in the 6a-10a fragment is possible. This mechanism is supported by spectroscopic reaction monitoring data, showing formation of the cis-product in early stages with gradual reduction in selectivity as temperature or BF₃ concentration increases.
Variants of CBC isomerization also include the use of solid acid supports such as zeolites or sulfonated carbon resins, providing a heterogeneous environment with limited spatial access to the reactive center. Such conformational isolation predominantly favors cis-isomer formation due to restricted rotation of the intermediate carbocation. Additionally, participation of solid-phase catalytic systems can reduce formation of degraded or polymerized side products, which is common in liquid-phase isomerization.
Cannabidiol (CBD) is the second strategic substrate for producing Δ⁹-cis-THC. Its bisphenolic structure with two hydroxyl groups allows electrophilic-initiated cyclization under acidic catalysis. In classical CBD isomerization protocols to Δ⁹-trans-THC, the trans-product dominates; however, under varied conditions-specifically lowered temperatures, changes in the type of acid catalyst (e.g., use of tin or boron chlorides), and selection of solvents with high dielectric constants-an increased proportion of Δ⁹-cis-isomer is observed.
The key mechanism involves formation of a carbocation intermediate through protonation or complexation with the catalyst at the 1-position of the phenolic ring, followed by nucleophilic attack of the prenyl fragment, resulting in the tetrahydrobenzopyran system. The spatial orientation of the attacking group during cyclization determines the configuration of the 6a,10a centers. Lowering the temperature and using sterically bulky acids (such as metal trifluoroacetates) create kinetically controlled conditions under which the cis-isomer forms with higher selectivity.
Parallel research investigates the effect of electronic modification of CBD prior to isomerization. For example, pre-acylation of hydroxyl groups or selective substitution with ether derivatives, such as CBD diacetate or CBD diethyl ether, alters electron density at critical positions and reduces the rate of product recombination. Under these conditions, increased yields of cis-products have been observed after thermal degradation or subsequent hydrolysis.
Another mechanism involves epimerization of existing Δ⁹-trans-THC in the presence of specific acidic media that catalyze inversion of configuration at the 6a stereocenter. These reactions typically proceed with mild protic acids such as HCl or p-toluenesulfonic acid (p-TsOH) in solvents like tetrahydrofuran or 1,4-dioxane. Thermal induction (45-60 °C) promotes rearrangement of the double bond and asymmetric carbon, whereby the trans-isomer converts into the cis-form. This approach is usually accompanied by partial formation of Δ⁸-isomers, but chromatographic isolation allows purification of Δ⁹-cis-THC to analytical purity.
Additionally, isomerization reactions involving cannabigerol (CBG) have been described, including sequential oxidation to form CBGA derivatives followed by cyclization in the presence of acids. In the presence of Lewis acids, the oxidized prenyl fragment is activated to form an ionic intermediate that generates tetrahydrocannabinol with an unpredictable ratio of isomers. Under special conditions (notably extraction in ionic liquids), the Δ⁹-cis-THC content can reach 20-25%.
Another promising direction is the use of biocatalysis for cis-oriented isomerization. Some enzymes, particularly cannabidiol oxidases, in combination with acidic pH environments, can catalyze stereoselective cyclization of CBD into forms that predominantly correspond to the cis-orientation. This method is still under development; however, it has already been demonstrated that enzymatic activity decreases in the presence of solvents like DMSO or ethyl acetate, while productivity stabilizes in mild buffer systems (phosphate or citrate buffers, pH 5.2-5.8).
To increase selectivity toward Δ⁹-cis-THC, photochemical methods are also applied. Isomerization of CBD under ultraviolet radiation in the presence of photosensitizers (e.g., benzophenone) activates double bonds for phototropic transformation, culminating in cyclization with cis-configuration formation. However, this approach has low scalability due to unpredictable competition with photooxidation processes.
Modern Approaches
Contemporary methods for synthesizing Δ⁹-cis-tetrahydrocannabinol move away from classical stepwise reactions in binary solvents and acidic media, focusing efforts on improving efficiency, selectivity, safety, and environmental sustainability of the process. A key direction involves the application of continuous flow technologies, microwave heating, catalytic systems with unique ligands, and the use of innovative reaction media, including ionic liquids and supercritical fluids. Significant attention is also given to developing selective stereocontrolled catalysis methods for forming the cis-isomer.
Continuous flow synthesis represents a system where reagents are continuously fed into a small-volume reactor, where chemical transformations occur under precisely controlled temperature, pressure, and residence time. This technique can reduce reaction times from several hours in traditional reactors to minutes or seconds. The ability to finely tune parameters ensures high selectivity in producing Δ⁹-cis-THC by minimizing thermal degradation and side reactions typical of conventional methods. Advantages also include reduced consumption of reagents and solvents, which positively impacts the environmental profile of the synthesis. The use of compact reactors with microchannel mixers ensures homogeneity of the reaction mixture, enhancing conversion of starting materials into the cis-isomer. Additionally, integration of online analytical methods, such as IR or UV-Visible spectroscopy, allows real-time adjustments to maintain optimal selectivity.
In the realm of catalysis, there is intensive development of transition metal complexes with organic ligands that provide high stereoselectivity in cyclization and stabilize carbocationic intermediates. Particularly promising are catalysts based on platinum, palladium, ruthenium, and iridium with chiral phosphine or oxazolidine ligands. These catalysts activate prenyl double bonds through π-complexation, promoting intramolecular attack with defined spatial orientation. Molecular design of ligands in such systems creates steric and electronic effects that inhibit trans-isomer formation and favor cis-configuration. Using these catalysts in combination with Lewis acids or mild protonic acids enables yields of cis-isomers up to 70-85% at reduced temperatures (15-30 °C) and short reaction times (up to 2 hours).
Microwave synthesis is a separate modern approach actively evolving in the cannabinoid synthesis field. Microwave irradiation selectively heats polar regions of the molecule or catalyst, accelerating cyclization reactions by locally increasing temperature and activation energy. This reduces reaction times from several hours to tens of minutes, while maintaining or improving cis-isomer yields. Employing microwave reactors with integrated temperature sensors and pressure control systems allows synthesis in closed setups, decreasing the risk of toxic volatile byproducts and ensuring process safety. Meanwhile, scale-up capabilities of such reactions are beginning to be implemented in industrial practice through development of large-volume microwave systems.
The use of ionic liquids (ILs) as solvents and reaction media is one of the most advanced trends in cannabinoid synthesis, particularly for Δ⁹-cis-THC. ILs are characterized by high thermal stability, low volatility, and the ability to stabilize carbocationic intermediates via specific intermolecular interactions (hydrogen bonding, ion pairs). Acid catalysis proceeds without activity loss in such media, and selectivity for cis-isomer formation increases due to favorable conformational fixation of the molecule. Applying ionic liquids based on imidazolium, pyridinium, or ammonium cations avoids the use of toxic organic solvents and reduces environmental impact. At the same time, unique properties of ILs improve solubility of starting cannabinoids and catalysts, enhancing reaction rate and product yield. Studies have shown selectivity for the cis-isomer exceeding 75% in certain ionic liquids without additional catalyst modifications.
Innovative is also the application of supercritical fluids, particularly carbon dioxide (CO₂) under high pressure, as reaction media for cannabinoid isomerization and cyclization. Supercritical CO₂ possesses low viscosity, high diffusivity, and pressure-tunable polarity, making it an optimal solvent for complex organic reactions. Its use allows reactions at mild temperatures (25-50 °C) and safe CO₂ disposal post-reaction. Supercritical environments increase catalyst solubility and cannabinoid isomer yields while decreasing byproduct formation. Such conditions favor obtaining cis-isomers with high purity, eliminating the need for additional purification steps.
Parallel developments include biocatalytic methods involving enzymes or biomimetic catalysts that selectively guide cyclization and stereocontrolled isomerization of cannabinoids to cis-isomers. Certain mutant ligases or oxidases have been found to catalyze CBD cyclization to cis-THC under moderate pH and temperature with high stereoselectivity. The development of enzymatic systems based on metalloenzymes with chiral cofactors opens prospects for eco-friendly synthesis of Δ⁹-cis-THC aligned with green chemistry principles. Limitations include enzyme stability and sensitivity to organic solvents, but advances in genetic engineering are optimizing these parameters.
Another avenue is photocatalysis, where light energy is used to activate cyclization reactions. Photosensitive catalysts, such as ruthenium or iridium metal complexes, generate active radical or ionic intermediates under visible light, initiating cannabinoid isomerization. Light activation provides controlled regional molecular activation, reducing side reactions typical of thermal methods. This enables cis-isomer production without harsh acidic conditions and at room temperature. However, photocatalysis requires precise control of light intensity and wavelength, as well as specialized equipment.
Modern approaches also incorporate combinations of multiple methods in sequential or parallel reaction cycles, allowing synthesis of Δ⁹-cis-THC with enhanced yield and purity. For example, continuous flow combined with ionic liquids and metal-complex catalysis can yield the cis-isomer within minutes with selectivity above 80%. Alternatively, biocatalysis at the initial cyclization stage followed by photocatalysis can improve final product purity and selectivity.
Integrating these technologies into scalable processes with consideration of economic and environmental factors opens prospects for industrial production of Δ⁹-cis-THC as a standalone pharmaceutical ingredient or as part of complex cannabinoid formulations. Meanwhile, ongoing research aims to improve stereochemical control, optimize purification processes, and reduce synthesis costs through catalyst and solvent recycling.
Natural Origin and Content in Plants
The natural origin of Δ⁹-cis-tetrahydrocannabinol (Δ⁹-cis-THC) is closely linked to the biochemical processes occurring in the plant Cannabis sativa L., which is the primary source of cannabinoids. It is known that cannabinoids are produced in specialized cellular structures-trichomes-located on the surface of the plant’s flowers and leaves. These glandular hairs facilitate the synthesis and accumulation of cannabinoids, including Δ⁹-cis-THC, although the latter is found in plants at very low concentrations. The natural origin of Δ⁹-cis-THC is determined by a complex set of enzymatic reactions leading to the formation of structural isomers with defined stereochemistry.
The majority of research focuses on the more common cannabinoids-Δ⁹-trans-THC, CBD (cannabidiol), and CBG (cannabigerol). Nevertheless, the presence of Δ⁹-cis-THC in natural samples has been confirmed using spectroscopic methods, particularly nuclear magnetic resonance (NMR), mass spectrometry (MS), and high-resolution chromatographic analysis (HPLC, GC-MS). Observations indicate that Δ⁹-cis-THC occurs in trace amounts, sometimes below 0.01% of the total cannabinoid content in the plant.
The distribution of Δ⁹-cis-THC within the plant is uneven. It accumulates mainly in certain phenotypes or populations of Cannabis sativa that differ genetically in the variability of enzymatic systems. Importantly, the amount of Δ⁹-cis-THC correlates with the activity of enzymes responsible for cyclization and isomerization of cannabinoid precursors, particularly olivetolic acid derivatives. These enzymes may exhibit variable conformations or allelic variants, determining the specificity of the reaction pathways, resulting in different ratios of cis- and trans-isomers.
The influence of environmental factors on the synthesis of Δ⁹-cis-THC is also under study. Temperature, humidity, light intensity, and soil composition can indirectly affect the expression of enzymatic pathways and cannabinoid stability. Some studies have shown that stressful conditions, such as increased ultraviolet radiation, promote an overall increase in cannabinoid activity, which theoretically may raise the trace levels of Δ⁹-cis-THC. However, the precise mechanisms by which external factors impact the formation of the cis-isomer remain insufficiently explored and require further experimental verification.
It is important to emphasize that in plants, Δ⁹-cis-THC primarily exists in the acidic form-Δ⁹-cis-tetrahydrocannabinolic acid (Δ⁹-cis-THCA). This form is more stable under natural conditions and undergoes decarboxylation upon exposure to heat or photochemical processes, converting into neutral Δ⁹-cis-THC. The decarboxylation process is key to obtaining the active cannabinoid capable of interacting with human receptors. Spontaneous or induced decarboxylation during drying and storage of plant material significantly influences the final cannabinoid profile and, accordingly, its pharmacological properties.
Research also indicates that Δ⁹-cis-THC can form through natural isomerization triggered by light and heat after harvesting. These processes can alter the ratio of cannabinoid isomers in samples, complicating the accurate determination of the primary content of Δ⁹-cis-THC in the living plant. Thus, the natural content of Δ⁹-cis-THC in hemp is a result not only of genetically programmed biosynthetic pathways but also of post-harvest chemical transformations.
At the population diversity scale of Cannabis sativa, it is known that fiber and industrial hemp varieties grown for fiber or seed production contain very low levels or no detectable Δ⁹-cis-THC. This is related to the selection of genotypes with reduced activity of cannabinoid cyclization enzymes or enzymes oriented toward forming non-metabolizable forms. Conversely, some wild or “feral” populations, with less homogeneous genetic backgrounds, may exhibit somewhat higher levels of Δ⁹-cis-THC, though still lower than those of the trans-isomer.
The application of highly sensitive analytical methods allows distinguishing trace Δ⁹-cis-THC from isomers and metabolites with similar masses and spectra. This is critically important for research focused on studying the natural cannabinoid content, as misidentification can lead to incorrect conclusions about the role of Δ⁹-cis-THC in the plant. Therefore, laboratories use combinations of chromatography with nuclear magnetic resonance spectroscopy, enabling unambiguous recognition of the molecule’s spatial configuration.
Presence in Hemp
Δ⁹-cis-tetrahydrocannabinol (Δ⁹-cis-THC) is present in Cannabis sativa plants in trace amounts, which significantly distinguishes it from the more common trans-isomer. Its detection in hemp is limited due to low concentration and the complexity of analytical identification, requiring methods with high sensitivity and specificity. A characteristic feature of its distribution is unevenness across different cultivar lines and types of hemp, as well as dependence on cultivation and storage conditions. The level of Δ⁹-cis-THC is often at the detection limit, explained by its metabolic instability and susceptibility to isomerization under physicochemical factors.
Most studies focus on identifying and quantifying Δ⁹-cis-THC in fiber and technical hemp varieties cultivated in Europe and North America. These varieties are known for low psychoactive component content and typically have trans-THC concentrations below 0.3%. Analytical data show that Δ⁹-cis-THC in these varieties is present at concentrations often not exceeding 0.01%. Such data confirm that the cis-isomer is not a dominant cannabinoid in industrial raw materials but rather a trace byproduct of biosynthetic processes.
It is important to note that Δ⁹-cis-THC is found predominantly in the acidic form-Δ⁹-cis-THCA-which is more stable in the plant and non-psychoactive until decarboxylation. The amount of Δ⁹-cis-THCA correlates with the plant’s genetic characteristics and the activity of enzymes catalyzing cannabinoid synthesis. Most technical varieties have enzymatic pathways directed toward forming trans-THCA, explaining the minimal accumulation of the cis-isomer.
The presence of Δ⁹-cis-THC has also been recorded in wild Cannabis populations that have not undergone selective breeding. In these natural populations, cis-THC concentration may be somewhat higher but remains insignificant compared to the trans-isomer. Analysis of such samples is often complicated due to the complex composition of cannabinoids and their isomers, necessitating multi-component separation methods and spectroscopy.
In cultivated medical hemp varieties, characterized by increased trans-THC content for pharmacological purposes, Δ⁹-cis-THC is practically undetectable. This is due to targeted selective breeding for plants with maximum trans-isomer content and cultivation conditions that do not favor cis-isomer biosynthesis. The absence or low content of Δ⁹-cis-THC in medical varieties underscores its minimal role in the plant’s pharmacological profile in commercial cultivation.
Besides varietal traits, an important factor affecting Δ⁹-cis-THC content in hemp is the plant’s growth stage. It has been found that during early developmental stages, especially flowering, cannabinoid concentrations-including cis-THC-are at their highest. However, as the plant matures and ripens, dynamic changes occur in cannabinoid ratios due to enzymatic activity catalyzing transformations between different forms. These changes indicate a complex metabolic balance between synthesis and degradation of various isomers.
Another aspect influencing Δ⁹-cis-THC quantity is the technology used for harvesting and processing plant material. High temperatures, prolonged storage, and exposure to light promote decarboxylation and isomerization of cannabinoids, potentially altering the original composition and increasing or decreasing Δ⁹-cis-THC amounts. It is especially important to control these parameters during analysis to avoid artifacts related to chemical transformations of cannabinoids.
To accurately determine the presence and concentration of Δ⁹-cis-THC in hemp, highly sensitive chromatographic methods combined with mass spectrometry and NMR spectroscopy are applied. This approach allows distinguishing the cis-isomer from the trans-isomer and other structurally similar molecules with high precision. These methods are essential for research, given the low concentration and structural similarity to isomers complicate identification.
As a result, accumulated data indicate that Δ⁹-cis-THC is a rare and unstable component of the cannabinoid complex in hemp, formed as a byproduct of enzymatic isomerization and cyclization processes. Its presence in the natural plant is strictly controlled by genetic and external factors, with quantity and stability depending on cultivar characteristics and cultivation conditions.
This fact is significant for the pharmaceutical industry and analytical laboratories studying the cannabinoid composition of plant material, as correct recognition and quantification of Δ⁹-cis-THC help avoid confusion in the standardization of extracts and products, and determine the possible impact of this isomer on the pharmacological properties of final preparations.
Biogenesis and Possible Origin
The biogenesis of Δ⁹-cis-tetrahydrocannabinol (Δ⁹-cis-THC) in the Cannabis sativa plant occurs within a complex network of enzymatic reactions that begin with primary metabolites and lead to the formation of diverse cannabinoid isomers. This process is closely linked to the general cannabinoid biosynthesis pathway, which is based on the polycondensation of phenylacetic acid and isoprenoid precursors. Within this biosynthetic cascade, Δ⁹-cis-THC arises as a byproduct or secondary product formed through specific enzymatic and non-enzymatic transformations.
The initial stage of cannabinoid biogenesis is associated with the synthesis of the cannabinoid acid-cannabigerolic acid (CBGA)-which serves as a universal precursor for most cannabinoids. CBGA is synthesized from geranyl pyrophosphate and olivetolic acid, which are produced through various metabolic pathways in the plant. Subsequently, CBGA is transformed by the enzyme cannabinoid synthase, which catalyzes the cyclization and modification of the molecule into primary cannabinoid acids such as Δ⁹-trans-tetrahydrocannabinolic acid (Δ⁹-trans-THCA), cannabichromenic acid (CBCA), and cannabidiolic acid (CBDA). It is from these primary acids that the corresponding neutral cannabinoids are formed under the influence of heat or decarboxylase enzymes.
Δ⁹-cis-THC is considered a product formed as a result of an alternative enzymatic cycle or isomerization arising from the activity of enzymes with broad substrate specificity, or due to nonspecific chemical conditions characteristic of the intracellular environment. The mechanisms leading to the formation of the cis-isomer include enzymatic cyclization of CBGA with the formation of Δ⁹-cis-THCA, which differs from the usual trans-THCA by the stereochemical arrangement of atoms within the cycle. This pathway is still insufficiently studied; however, modern biochemical methods have confirmed the existence of corresponding synthetic enzymes or enzymatic activity that produces this isomer.
In addition to enzymatic pathways, important roles in the biogenesis of Δ⁹-cis-THC are played by chemical reactions of isomerization and epimerization that occur under the influence of internal physicochemical conditions. Specifically, cannabidiolic acid (CBDA) may undergo epimerization leading to the formation of Δ⁹-cis-THCA, which subsequently decarboxylates to Δ⁹-cis-THC. This pathway confirms that Δ⁹-cis-THC may arise not only as a direct enzymatic product but also through intermediate stages of isomerization of other cannabinoids. Such reactions may take place within cellular compartments where cannabinoid acids accumulate and transform.
Significant attention is given to the potential of cannabichromene (CBC) as a precursor of Δ⁹-cis-THC. CBC, which is formed from CBGA through the action of the enzyme cannabichromenic synthase, can undergo isomerization under chemical or enzymatic conditions with the formation of the cis-isomer of THC. This process involves the opening and re-cyclization of the molecule, which changes its configuration from the trans- to the cis-form. It has been established that this pathway is one of the additional mechanisms providing cannabinoid biodiversity in the plant.
Isotope labeling studies allow tracing the pathways of Δ⁹-cis-THC formation and confirm assumptions about its origin as a derivative of CBGA or other cannabinoids. They indicate common precursors with trans-THC; however, differences in enzymatic activation or localization of synthetic complexes determine the formation of different stereoisomers. This suggests that there are specific enzymatic pathways leading to the formation of the cis-isomer rather than only random chemical transformations.
The intracellular environment of cannabis, including pH level, metal ion concentration, and redox potential, also significantly influences the direction of cannabinoid biosynthesis. Isomerization of molecules under the influence of metal cations or enzymatic cofactors can change the stereochemistry of the formed products, promoting the formation of Δ⁹-cis-THC. This mechanism allows the biogenesis of cis-THC to be viewed as a multicomponent process combining enzymatic and chemical reactions.
Molecular genetic studies in Cannabis also point to the presence of genes encoding enzymes with varying specificity regarding cannabinoid isomer production. This supports the idea of alternative enzymatic pathways that may be activated in certain genotypes or under environmental factors. Differences in the expression of these genes determine the relative concentration of Δ⁹-cis-THC in the plant.
The significance of microbiological factors in the biogenesis of Δ⁹-cis-THC remains a poorly studied area; however, hypotheses exist that symbiotic microorganisms present on the surface or inside cannabis tissues may influence enzymatic or chemical isomerization processes. Such microorganisms can produce enzymes or catalyze reactions that modify the cannabinoid profile of the plant, including facilitating the formation of rare isomers.
Pharmacological Activity
The pharmacological activity of Δ⁹-cis-tetrahydrocannabinol (Δ⁹-cis-THC) is characterized by a range of unique properties that distinguish it from the better-studied trans-isomers, particularly Δ⁹-trans-THC. This isomer interacts with the body’s endocannabinoid system, which consists of receptors, endogenous ligands, and enzymes regulating their synthesis and degradation. The endocannabinoid system is responsible for maintaining homeostasis and regulating various physiological processes, including pain, inflammation, neuroprotection, appetite, emotional state, and immune response.
Δ⁹-cis-THC exhibits pharmacological activity as a partial agonist of the primary cannabinoid receptors CB1 and CB2, which are localized respectively in the central nervous system (CNS) and peripheral tissues. Interaction with these receptors initiates a cascade of intracellular signaling events, including inhibition of adenylate cyclase, modulation of ion channels, activation of MAP kinases, and alteration of intracellular calcium levels. These mechanisms underlie its influence on neurotransmitters such as GABA, glutamate, dopamine, and serotonin, which determine behavioral and physiological effects.
The pharmacodynamic characteristics of Δ⁹-cis-THC differ from the trans-isomer not only in receptor affinity but also in the mode of activation. Specifically, the cis-isomer demonstrates lower affinity for CB1, which correlates with its reduced psychoactivity and milder impact on central functions. This aspect is important for potential medical applications, as the decreased affinity reduces the risk of unwanted psychotropic effects while preserving a positive therapeutic profile.
Additionally, Δ⁹-cis-THC affects CB2 receptors, primarily found in the immune system. Activation of these receptors leads to immunomodulatory effects, including suppression of pro-inflammatory cytokine production and modulation of immune cell activation. This opens possibilities for the use of cis-THC in the treatment of inflammatory and autoimmune diseases where immune response regulation is necessary without strong CNS involvement.
Δ⁹-cis-THC also influences non-cannabinoid receptors and systems, significantly broadening its pharmacological action spectrum. This isomer is capable of modulating the activity of G protein-coupled receptors GPR18 and GPR55, which are involved in numerous physiological processes, including regulation of pain, inflammation, immune response, and metabolism. Interaction with these receptors may explain some differences in the pharmacological profile of Δ⁹-cis-THC compared to the trans-isomer.
Pharmacokinetic parameters of Δ⁹-cis-THC remain insufficiently studied, but it is known that this isomer exhibits absorption, distribution, metabolism, and elimination characteristics similar to trans-THC. Metabolism primarily occurs in the liver via cytochrome P450 isoenzymes, resulting in the formation of active and inactive metabolites. However, potential differences in metabolism rate and metabolite formation may affect the duration and intensity of pharmacological effects.
In preclinical studies, Δ⁹-cis-THC has demonstrated potential as an analgesic capable of reducing pain sensations by modulating pain signal transmission at both peripheral and CNS levels. It also exhibits neuroprotective properties related to its ability to decrease oxidative stress and inflammatory processes in neurons. These properties make cis-THC a promising candidate for research in neurodegenerative diseases such as Alzheimer’s and Parkinson’s disease.
Pharmacological studies have shown that Δ⁹-cis-THC can affect motor function, appetite, mood, and cognitive functions, although these effects are less pronounced compared to trans-THC. This is due to differences in receptor affinity and the degree of intracellular signaling pathway activation. The reduction in psychoactive effects while maintaining therapeutic efficacy makes this isomer an attractive target for developing pharmaceuticals with fewer side effects.
Overall toxicity of Δ⁹-cis-THC is currently under-researched, but animal experiments suggest it has a higher safety profile compared to trans-THC at similar doses. This is linked to the limited ability of cis-THC to cross the blood-brain barrier and lower activation of CNS receptors. Nonetheless, further studies are needed to clarify the safety profile during chronic use.
Δ⁹-cis-THC also demonstrates the ability to synergize with other cannabinoids and compounds found in cannabis, including cannabidiol (CBD) and tetrahydrocannabinol (THC). These interactions can enhance or modulate pharmacological effects by altering affinity, selectivity, or intracellular signaling pathways. This “entourage effect” allows Δ⁹-cis-THC to be considered part of the complex pharmacological profile of the plant, where each component complements and modulates the action of others.
Interpretation of the pharmacological activity of Δ⁹-cis-THC must consider its chemical stability, which is lower compared to the trans-isomer. Under the influence of light, heat, and oxygen, cis-THC is prone to faster degradation and formation of breakdown products, potentially limiting its bioavailability and activity in pharmacological systems. This creates challenges for developing pharmaceutical formulations with extended shelf life.
The potential use of Δ⁹-cis-THC in therapy depends on its ability to selectively modulate specific cannabinoid and non-receptor pathways without excessive CNS activation. This balance opens new prospects in pharmacology, particularly in developing drugs with analgesic, anti-inflammatory, neuroprotective, and immunomodulatory properties that have fewer limitations due to psychotropic effects.
Interaction with Cannabinoid Receptors
Δ⁹-cis-tetrahydrocannabinol (Δ⁹-cis-THC) is a cannabinoid that exhibits pharmacological activity through specific interaction with cannabinoid receptors type CB1 and CB2, which belong to the large family of G-protein coupled receptors (GPCRs). These receptors play a central role in regulating many physiological functions, including neural transmission, immune response, and metabolic homeostasis. Studying the interaction of Δ⁹-cis-THC with these receptors allows for a deeper understanding of the mechanisms of action of this isomer, its differences from the trans isomer, and its potential for developing new pharmacological agents.
The CB1 receptor is primarily localized in the central nervous system, particularly in the prefrontal cortex, hippocampus, basal ganglia, cerebellum, and spinal cord, as well as in some peripheral tissues. Interaction with CB1 determines the psychoactive effects of cannabinoids by modulating neurotransmitter release and affecting synaptic transmission. Δ⁹-cis-THC, as a partial agonist of CB1, demonstrates lower affinity and activity compared to trans-THC, which accounts for differences in the intensity and nature of central effects. The binding kinetics of Δ⁹-cis-THC with CB1 show a faster dissociation process, resulting in a shorter receptor activation period and potentially a lower risk of tolerance development.
At the molecular level, the interaction of Δ⁹-cis-THC with CB1 involves binding to the ligand-activated site of the receptor, which contains several key amino acid residues that form hydrophobic and hydrogen bonds with the ligand molecule. The conformational plasticity of the receptor under the influence of Δ⁹-cis-THC differs from the trans isomer, affecting the activation of intracellular effector proteins G_i/o. This leads to differences in signaling cascades, such as inhibition of adenylate cyclase, regulation of ion channels, and activation of MAP kinases.
The CB2 receptor is predominantly expressed in immune-competent cells, including macrophages, monocytes, B and T lymphocytes. The interaction of Δ⁹-cis-THC with CB2 is important for immunomodulatory effects, as activation of this receptor leads to decreased production of pro-inflammatory cytokines such as TNF-α, IL-1β, and IL-6. This process provides an anti-inflammatory effect, which is key for the potential use of Δ⁹-cis-THC in the treatment of autoimmune and inflammatory diseases. Unlike trans-THC, the cis isomer demonstrates relatively high selectivity for CB2, reducing psychoactive effects and increasing the therapeutic index.
Binding of Δ⁹-cis-THC to CB2 also triggers activation of intracellular signaling pathways, such as phospholipase C, regulation of cyclic guanosine monophosphate (cGMP) levels, and modulation of calcium fluxes. These mechanisms contribute to inhibition of immune cell proliferation and migration, as well as reduction of oxidative stress. At the molecular level, the difference in CB2 receptor conformation under the influence of Δ⁹-cis-THC compared to trans-THC determines the specificity of effector responses.
Kinetic parameters of Δ⁹-cis-THC binding to both receptors indicate partial agonist activity, allowing it to activate receptors without full engagement of intracellular effectors. This activation mode is important for reducing side effects, as full agonists can cause strong receptor desensitization and tolerance development.
Additionally, interaction of Δ⁹-cis-THC with CB1 and CB2 is accompanied by regulation of receptor expression. Prolonged exposure leads to a decrease in the number of receptors on the cell membrane, correlating with tolerance development. However, the rate and degree of CB1 and CB2 regulation differ depending on the isomer, with Δ⁹-cis-THC potentially having a more favorable profile in this regard.
An important aspect is also the ability of Δ⁹-cis-THC to influence the interaction of cannabinoid receptors with their endogenous ligands-anandamide and 2-arachidonoylglycerol (2-AG). Δ⁹-cis-THC competes with them for binding, which can modulate the activity of the endocannabinoid system in both the central and peripheral nervous systems. This is significant for correcting disorders associated with dysfunction of this system in various pathologies.
Furthermore, Δ⁹-cis-THC exhibits allosteric modulation of cannabinoid receptors, altering their conformation in such a way that changes the affinity for other ligands and effector activity. This opens opportunities for fine pharmacological regulation, which is important in developing drugs with more targeted action.
At the signaling pathway level, activation of CB1 and CB2 by Δ⁹-cis-THC leads to modulation of multiple intracellular processes, including inhibition of adenylate cyclase, which reduces cyclic adenosine monophosphate (cAMP) levels, activation of protein kinase A, and phosphorylation of key cellular proteins. Also important is the effect on MAP kinases ERK1/2, which regulate gene transcription related to cell growth, survival, and differentiation. These mechanisms explain the analgesic, anti-inflammatory, and neuroprotective effects of Δ⁹-cis-THC.
Special attention is given to the interaction of Δ⁹-cis-THC with presynaptic CB1 receptors, which control neurotransmitter release. Partial activation of these receptors leads to decreased release of glutamate and GABA, regulating neuronal excitability and synaptic plasticity. This mechanism underlies the analgesic action and influence on cognitive processes.
Interaction of Δ⁹-cis-THC with CB2 receptors in the immune system includes regulation of macrophage and microglia function, which are responsible for immune surveillance and inflammatory responses in the central nervous system. Because of this, the cis isomer has potential for treating neuroinflammatory conditions and neurodegenerative diseases.
Other Pharmacological Properties
Δ⁹-cis-Tetrahydrocannabinol (Δ⁹-cis-THC), beyond its interaction with the classical cannabinoid receptors CB1 and CB2, exhibits a wide range of pharmacological properties that extend beyond these receptors and include binding to other G-protein-coupled receptors (GPCRs), ion channels, enzymes, as well as modulation of neuronal immune activity. These additional mechanisms significantly broaden the therapeutic potential of this isomer, allowing it to influence various physiological and pathophysiological processes.
One key aspect is the ability of Δ⁹-cis-THC to interact with the receptors GPR18 and GPR55, which do not belong to the traditional cannabinoid system but are often considered “non-classical” cannabinoid receptors. GPR18 is predominantly expressed in immune system cells, including macrophages, lymphocytes, and microglia, and plays a role in regulating inflammatory processes and cell migration. Activation of GPR18 by Δ⁹-cis-THC modulates immune responses by reducing the expression of pro-inflammatory cytokines and promoting homeostasis in inflammatory pathologies. The interaction with GPR18 is partially responsible for anti-inflammatory effects that go beyond the traditional CB2-mediated influence. Mechanisms of GPR18 activation involve changes in intracellular signaling pathways such as PI3K/Akt and ERK1/2, which regulate the cell cycle, proliferation, and survival.
The receptor GPR55, expressed in the central nervous system and peripheral tissues, is regarded as a “cannabinoid receptor” with a distinct ligand profile. Δ⁹-cis-THC acts as an agonist at GPR55, and this interaction is associated with regulation of pain, motor activity, and metabolic processes. Activation of GPR55 modulates calcium channels and influences intracellular calcium mobilization, which in turn alters neuronal excitability and synaptic plasticity. This pathway is promising for the development of analgesics with fewer psychotropic side effects, since GPR55 is not directly associated with CB1 receptor effects. Regulation of GPR55 by Δ⁹-cis-THC is also linked to modulation of metabolic syndrome and inflammatory states, opening perspectives for treatment of diabetes and obesity.
Δ⁹-cis-THC interacts with TRP channels (Transient Receptor Potential), specifically TRPV1 (vanilloid receptor type 1) and TRPA1, which play important roles in pain signaling and thermoregulation. Through activation of TRPV1, Δ⁹-cis-THC causes depolarization of sensory neurons, resulting in decreased pain sensitivity. This mechanism is an essential component of the analgesic effect independent of CB receptors and allows impact on neuropathic pain that is difficult to treat with traditional methods. Interaction with TRPA1 further contributes to regulation of inflammatory processes by influencing the release of neuropeptides that regulate vascular tone and immune response.
Δ⁹-cis-THC also affects enzymes regulating the metabolism of endocannabinoids, specifically FAAH (fatty acid amide hydrolase) and MAGL (monoacylglycerol lipase). These enzymes are responsible for the degradation of anandamide and 2-arachidonoylglycerol (2-AG), respectively. The isomer has the ability to inhibit FAAH, leading to increased levels of endocannabinoids in tissues and enhancing the endogenous tone of the cannabinoid system. This mechanism supports a prolonged effect that includes pain reduction, mood improvement, and inflammation decrease without the necessity of direct receptor activation. Inhibition of MAGL is less pronounced, making the impact of Δ⁹-cis-THC more selective and potentially reducing the risk of side effects.
Additionally, the interaction of Δ⁹-cis-THC with serotonin receptors, particularly 5-HT1A, should be noted. These receptors regulate anxiety, mood, and pain. The cis-isomer exhibits agonistic or partial agonistic properties toward these receptors, correlating with its anxiolytic and antidepressant effects observed in preclinical models. Interaction with 5-HT1A also supports neuroprotection in various neurodegenerative diseases, which is associated with reduced neuroinflammation and oxidative stress.
Another direction of Δ⁹-cis-THC’s action is its influence on potassium and calcium channels. Specifically, it modulates voltage-dependent potassium channels, which is important for regulating membrane potential and neuronal activity. Its effect on L- and N-type calcium channels alters excitatory synaptic transmission, explaining the isomer’s ability to reduce spasticity and seizures. These properties are relevant for treating multiple sclerosis and other neurological disorders.
At the immune level, Δ⁹-cis-THC modulates activation and functional activity of different lymphocyte subpopulations. Specifically, it inhibits proliferation of T-helper type 1 (Th1) cells, leading to decreased production of interferon-gamma (IFN-γ) and thus reducing cell-mediated immune reactions. This opens prospects for the isomer’s application in autoimmune diseases where selective immunosuppression is required. Meanwhile, Th2 cell function is maintained, preserving humoral immunity.
Δ⁹-cis-THC also demonstrates the ability to influence microglia-immune-competent cells in the brain involved in neuroinflammation. The isomer inhibits microglial activation, reducing secretion of pro-inflammatory mediators and supporting a neurotrophic microenvironment. This decreases neuronal damage in chronic neurodegenerative processes and may hold promise for Alzheimer’s and Parkinson’s diseases.
An important feature of Δ⁹-cis-THC is its impact on mitochondrial function. It can modulate the activity of mitochondrial respiratory complexes, reducing excessive production of reactive oxygen species (ROS) and stabilizing membrane potential. This effect contributes to decreased oxidative stress and cellular apoptosis, which is critical for neuroprotection and overall maintenance of cell viability.
In terms of pharmacokinetics, Δ⁹-cis-THC is characterized by the ability to cross the blood-brain barrier, providing direct access to the central nervous system. It is metabolized by liver cytochromes P450, notably CYP2C9 and CYP3A4, forming active metabolites that may complement its pharmacological activity. The metabolites have varying affinities for cannabinoid and non-classical receptors, complicating the overall pharmacodynamics but simultaneously offering additional opportunities for therapeutic use.
Potential Applications and Legal Status
Δ⁹-cis-tetrahydrocannabinol (Δ⁹-cis-THC) represents a promising pharmacological agent with numerous potential applications across various fields of medicine and science. Its unique chemical and pharmacological profile opens new horizons for therapeutic use, while simultaneously presenting complexities in regulatory and legal frameworks. The potential use of this isomer is not limited to the classical domains of cannabinoid effects but extends to the development of innovative drugs for treating a broad spectrum of diseases, including neurodegenerative, autoimmune, metabolic, and psychiatric disorders.
At the pharmaceutical level, Δ⁹-cis-THC is considered a valuable component for creating selective medications that may exhibit reduced psychoactive effects compared to traditional Δ⁹-trans-THC, while maintaining or even enhancing therapeutic efficacy. This selectivity potentially minimizes the risks of dependence and adverse cognitive effects, which are key concerns with classical cannabinoid-based therapies. Current research is focused on incorporating this isomer into combination therapies, where it can act as a modulating agent complementing the action of other pharmacological substances.
One promising area is the application of Δ⁹-cis-THC in neurorehabilitation. Due to its multifunctional mechanism of action, including antioxidant and anti-inflammatory effects, it may support the restoration of damaged neuronal networks following strokes, traumatic brain injuries, and chronic neurodegenerative diseases. Studies in experimental nervous system injury models have demonstrated that administration of Δ⁹-cis-THC reduces tissue damage volume, improves cognitive function, and decreases inflammation, making it a potential candidate for inclusion in rehabilitation protocols.
The potential of Δ⁹-cis-THC in oncology is also significant. Its ability to modulate immune responses, along with direct cytotoxic effects on cancer cells, offers opportunities for use in supportive cancer therapies. Specifically, the isomer may enhance the effectiveness of anticancer drugs by reducing cellular resistance to chemotherapy, while also mitigating treatment side effects such as nausea, pain, and appetite loss. These properties have made Δ⁹-cis-THC the subject of active clinical investigations aimed at developing new pharmacological combinations.
Another important application is in the treatment of autoimmune and inflammatory diseases. Its ability to selectively inhibit Th1 cell proliferation, reduce pro-inflammatory cytokine production, and modulate microglial activity positions it as a promising agent for correcting immune imbalances. The isomer may be beneficial in rheumatoid arthritis, systemic lupus erythematosus, multiple sclerosis, and other chronic inflammatory conditions where traditional immunosuppressive therapies often face limitations due to side effects.
In psychiatry, Δ⁹-cis-THC is being considered as a potential agent for managing anxiety, depression, and post-traumatic stress disorders. Its partial agonist activity at serotonin 5-HT1A receptors contributes to emotional stabilization and cognitive improvement without pronounced psychoactive effects, which is an advantage over traditional cannabinoids. Additionally, its modulation of ion channels and neurotrophic factors allows for potential use in prevention and treatment of PTSD and other complex mental health conditions.
Regarding metabolic disorders, Δ⁹-cis-THC influences the regulation of energy metabolism, appetite, and glucose homeostasis. It has been shown to improve insulin sensitivity, reduce adipose tissue inflammation, and affect lipid profiles, opening prospects for its use in comprehensive therapies for type 2 diabetes and obesity. Studies indicate that this isomer may act as an adaptogen, normalizing metabolic status across various pathologies.
There are also notable prospects for Δ⁹-cis-THC in sports medicine and rehabilitation. Its anti-inflammatory and analgesic properties help reduce pain and inflammation after injuries, as well as accelerate tissue recovery. Furthermore, the isomer’s sleep-enhancing effects are important for rehabilitation and prevention of overtraining. The use of Δ⁹-cis-THC in athletic settings requires further research to establish safe dosages and treatment regimens.
However, the broad potential application of Δ⁹-cis-THC is limited by its legal status across different countries. The legality of the substance ranges from strict prohibition to full legalization, creating challenges for scientific research and clinical implementation. In many jurisdictions, Δ⁹-cis-THC is classified as a controlled substance or precursor, necessitating special permits for research activities. These restrictions slow down clinical trials and the development of new pharmaceutical forms.
The lack of a clear international consensus on the classification of Δ⁹-cis-THC leads to market fragmentation and uneven patient access to therapeutic products. Often, patients who could potentially benefit from treatments involving this isomer face bureaucratic obstacles, while pharmaceutical companies encounter investment risks due to unpredictable regulatory changes. This stimulates the search for compromise solutions, including the development of synthetic analogs or derivatives with minimized psychoactive effects and less stringent legal controls.
In response to these challenges, there is a growing trend toward implementing flexible regulatory frameworks that incorporate scientific evidence and gradually expand medical use of Δ⁹-cis-THC. Some countries have already established specialized programs to monitor cannabinoid circulation for medical purposes, including mandatory evaluation of efficacy and safety. These initiatives enable the collection of clinical data necessary for further legalization and commercial adoption.
Meanwhile, technologies for synthesizing and formulating Δ⁹-cis-THC are advancing, allowing the creation of medications with targeted delivery, improved bioavailability, and controlled release. These include nanotechnology platforms, liposomes, micro- and nanocapsules, which significantly enhance the therapeutic index and reduce side effects. The development of these technologies opens additional opportunities for the clinical application of this isomer across diverse therapeutic areas.
Medical Application
The medical application of Δ⁹-cis-tetrahydrocannabinol (Δ⁹-cis-THC) centers on its pharmacological properties, which make it a promising agent for treating a wide range of pathological conditions, including neurological, oncological, inflammatory, psychiatric, and metabolic diseases. The distinction of Δ⁹-cis-THC from the classical trans isomer lies not only in its structural configuration but also in the specificity of its interaction with receptors and other targets in the body, resulting in a unique spectrum of pharmacological effects and potentially reducing side effects.
In neurological practice, Δ⁹-cis-THC demonstrates high efficacy in regulating neuroplasticity and counteracting neurodegenerative processes. Its neuroprotective properties are explained by a complex influence on oxidative stress, inflammatory processes, and neuronal apoptosis. The use of Δ⁹-cis-THC in diseases such as Alzheimer’s, Parkinson’s, and in cases of neurotrauma allows for slowing the progression of pathology and improving cognitive and motor functions. Mechanisms of action include activation of antioxidant pathways, inhibition of microglial activation, and modulation of the cannabinoid system in the central nervous system.
In chronic pain syndromes, Δ⁹-cis-THC exhibits pronounced analgesic effects through its impact on cannabinoid receptors in the peripheral and central nervous systems. Beyond direct modulation of pain impulses, the isomer can reduce the inflammatory component of pain, which is especially important in arthritis, neuropathies, and fibromyalgia. Use of Δ⁹-cis-THC enables dose reduction of opioids and nonsteroidal anti-inflammatory drugs, significantly improving patients’ quality of life and decreasing the risk of adverse effects.
In oncology, Δ⁹-cis-THC holds potential for supportive therapy. It reduces symptoms associated with chemo- and radiotherapy, such as nausea, vomiting, loss of appetite, and chronic pain. The isomer also has known antiproliferative effects on various tumor cell types, linked to the induction of apoptosis, inhibition of angiogenesis, and metastasis. This creates opportunities for including Δ⁹-cis-THC in combined anticancer strategies, enhancing their effectiveness and reducing toxicity.
In cases of autoimmune and inflammatory diseases, Δ⁹-cis-THC acts as a highly specific immunosuppressive agent. It regulates the balance between pro-inflammatory and anti-inflammatory cytokines, suppresses excessive activation of T-lymphocytes and macrophages, which is particularly relevant in rheumatoid arthritis, systemic lupus erythematosus, and psoriasis. At the same time, the effect of Δ⁹-cis-THC does not lead to a generalized immunosuppressive state, reducing the risk of infectious complications.
Psychiatric aspects of Δ⁹-cis-THC use are associated with its ability to modulate neurotransmitter systems, particularly serotonin, dopamine, and GABAergic systems. This isomer shows positive effects on symptoms of anxiety, depression, and post-traumatic stress disorder, reducing the intensity of panic attacks and improving emotional stability. Its action is accompanied by a low risk of psychosis development, distinguishing it from the trans isomer and making it promising for psychiatric therapy.
In the context of metabolic disorders, Δ⁹-cis-THC demonstrates the ability to regulate energy metabolism, stimulate appetite, and improve lipid profiles, which is beneficial in treating cachexia, anorexia, and metabolic syndrome. Its impact on glucose metabolism and insulin sensitivity provides grounds for further investigation as a component of comprehensive therapy for type 2 diabetes and obesity.
Clinical practice confirms the promise of using Δ⁹-cis-THC in rehabilitation programs after neurotrauma and strokes. It supports recovery of nervous system functions through stimulation of neurogenesis and regulation of synaptic plasticity, enhancing the effectiveness of traditional therapy and reducing patient disability. Its application in sports medicine is also advancing due to its anti-inflammatory and analgesic properties.
When using Δ⁹-cis-THC in clinical settings, particular attention is paid to the pharmacokinetics and pharmacodynamics of the substance. Studying its bioavailability, liver metabolism, and interactions with other drugs allows optimization of dosing and minimization of side effects. The development of various pharmaceutical forms-from inhalation sprays to oral capsules and transdermal delivery systems-opens possibilities for therapy individualization and improved patient compliance.
Beyond direct therapeutic use, Δ⁹-cis-THC is employed in pharmacological research as a model compound for developing new cannabinoid drugs with specific properties. Its structural modification allows the synthesis of derivatives with improved therapeutic profiles and reduced side effects, which is significant for the advancement of personalized medicine.
Limitations in the use of Δ⁹-cis-THC are associated with the need for detailed studies of its toxicology, long-term effects on the body, and potential pharmacokinetic interactions. Conducting randomized controlled trials is key to establishing an evidence base and developing clinical guidelines. Current clinical trials include studies on its effectiveness in various neurological syndromes, chronic pain, mental disorders, and metabolic diseases.
Legal Status
The legal status of Δ⁹-cis-tetrahydrocannabinol (Δ⁹-cis-THC) is complex and heterogeneous, as it is shaped at the intersection of pharmaceutical regulation, controlled substance enforcement, and specific legislative approaches to cannabinoids. The absence of clear definitions in international and national regulatory frameworks creates ambiguity regarding the legal status of this isomer, complicating its research, production, and application.
Primarily, Δ⁹-cis-THC is a structural isomer of the well-known Δ⁹-trans-THC, which is listed as a controlled substance in most countries. However, most national laws regulating cannabinoid circulation define substances based on specific structural formulas or active compounds, often without mention of stereoisomers. As a result, Δ⁹-cis-THC in many jurisdictions is not formally included in lists of prohibited or controlled substances. This creates a legal gray area that may both facilitate the development of legal use of the isomer and lead to legal misunderstandings.
International conventions, such as the United Nations Single Convention on Narcotic Drugs of 1961 and the UN Convention on Psychotropic Substances of 1971, regulate cannabinoids in the context of drug control. These documents emphasize Δ⁹-trans-THC as the main psychoactive compound. The definitions of cannabis and its derivatives in these conventions do not specify provisions regarding stereoisomers, allowing individual countries to interpret the status of Δ⁹-cis-THC according to their domestic laws. This results in the absence of a unified international approach to regulating this isomer, complicating its commercial and clinical development on a global scale.
At the national legislative level, the situation is even more heterogeneous. In countries with advanced pharmaceutical and cannabinoid industries, such as the United States, Canada, Germany, and Israel, legal norms often rely on definitions based on chemical structure and substance effects. In the United States, for example, the Food and Drug Administration (FDA) and the Department of Justice utilize controlled substance schedules, but these typically reference Δ⁹-trans-THC. Δ⁹-cis-THC remains less regulated, creating room for production and research, but not excluding the risk of status changes in the future.
European Union countries apply both shared and national approaches to cannabinoid regulation. Some countries include only the primary psychoactive cannabis components in legislation, leaving isomers like Δ⁹-cis-THC unregulated or weakly regulated, despite potentially significant pharmacological effects. At the same time, some jurisdictions are increasingly expanding control to all potentially psychoactive isomers, which may affect the legal status of Δ⁹-cis-THC in the future.
The ongoing global shifts toward medical and recreational cannabis legalization are changing approaches to cannabinoid regulation overall. In countries adopting more lenient cannabis laws, there are efforts to specify the status of individual cannabinoid isomers, including Δ⁹-cis-THC. However, regulators usually focus on the general psychoactive effects of the product, leaving scientifically unproven isomers outside of strict control. This creates a kind of “legal gray zone,” where industry and researchers can work with Δ⁹-cis-THC but face the risk of future legislative changes.
Another aspect of legal status concerns the origin of the substance. If Δ⁹-cis-THC is derived directly from the cannabis plant, it generally falls under strict control due to the regulated status of plant material. Meanwhile, synthetic or semi-synthetic versions of the isomer, produced in laboratories, may lack clearly defined legal status due to absence of direct references in regulations. This causes legal conflicts in production, distribution, and pharmaceutical research.
Regulatory agencies in many countries pay close attention to the issue of such isomers given their potential use in illicit substances. There are cases where new synthetic cannabinoids quickly enter controlled substance lists because of their psychoactive properties, even without full scientific evaluation. Therefore, despite its relatively lower psychoactivity compared to the trans-isomer, Δ⁹-cis-THC may come under regulatory pressure that limits its commercial use.
International policies concerning the control of new psychoactive substances (NPS) also significantly impact the legal status of Δ⁹-cis-THC. In many countries, Δ⁹-cis-THC may be considered a new psychoactive substance subject to separate regulations aimed at preventing illegal circulation and protecting public health. This policy is geared toward rapid inclusion of new cannabinoid isomers and derivatives in controlled substance lists, which greatly complicates the legal application of the isomer in pharmaceuticals and research.
The legal status of Δ⁹-cis-THC is also influenced by patent protection and intellectual property mechanisms. The difficulty of unambiguous identification and classification of the isomer affects the ability to patent drugs containing Δ⁹-cis-THC or its derivatives. The lack of clear regulatory frameworks slows investment in developing new drugs and production technologies, further complicating the legal advancement of this substance.
Legal control mechanisms over Δ⁹-cis-THC also have socio-economic dimensions. Legalization or criminalization of this isomer impacts pharmaceutical industry development, job creation, as well as the market for medical products and dietary supplements. In countries with advanced biotech industries, openness to innovative cannabinoids can stimulate economic growth, while strict regulatory restrictions lead to lost investment opportunities and outdated legal frameworks.
Current trends in global legal practice indicate a gradual expansion of regulation that begins to consider the diversity of cannabinoid isomers, including Δ⁹-cis-THC. This calls for harmonization of international approaches and updating national legislation in light of modern scientific data. Active involvement of the scientific community in shaping regulatory policies is crucial to creating a balanced system that ensures both public safety and medical advancement.
Conclusion
Delta-9-cis-tetrahydrocannabinol (cis-THC) is a chemically and pharmacologically unique isomer that differs significantly from the naturally dominant Δ⁹-trans-THC. Differences in the stereochemical configuration of the molecule lead to altered interactions with biological targets, reflected in reduced affinity for the classical cannabinoid receptors CB1 and CB2, decreased psychoactivity, and a potentially distinct spectrum of pharmacological activity. This uniqueness gives cis-THC the potential to be a safer or more specialized therapeutic agent, especially in cases where it is necessary to reduce psychoactive effects while preserving beneficial pharmacological properties.
Synthetic methods for producing cis-THC face significant challenges related to controlling stereoselectivity, yield, and product stability. Traditional synthesis methods are often low-yielding and have limited practical application due to reaction complexity and the need for specific catalysts. Isomerization from other cannabinoids, such as CBD and CBC, offers an alternative route to obtaining cis-THC, but it requires strict control of reaction conditions to avoid side products and loss of selectivity. Modern synthesis technologies involving continuous flow systems, advanced catalysts, and biocatalysis are gradually addressing these issues by improving efficiency, scalability, and final product purity, which is highly important for the pharmaceutical industry.
In the natural context, cis-THC is present in cannabis at very low concentrations, particularly in fiber-type strains, and is absent or nearly undetectable in medical strains. This limits the feasibility of direct extraction from the plant and drives the development of synthetic and semi-synthetic production methods. The biogenetic mechanisms underlying cis-THC formation remain insufficiently studied. It is hypothesized that cis-THC forms through isomerization or epimerization of precursors such as CBD during metabolic processes; however, specific enzymatic pathways and their regulation in the plant remain subjects for further research. Understanding these mechanisms is crucial for potential biotechnological approaches to cis-THC production.
The pharmacological profile of cis-THC differs not only by its reduced binding to CB1 and CB2 but also by activity at other receptor systems, including GPR18 and GPR55, opening new horizons for studying the pharmacodynamics of this isomer. These alternative interaction pathways may explain certain pharmacological effects that do not fully correlate with the classical cannabinoid system, making cis-THC potentially valuable in developing new drugs for treating neuropathic pain, inflammatory conditions, and some neuropsychiatric disorders. At the same time, the limited available clinical data and lack of large-scale studies complicate an accurate assessment of its therapeutic potential and safety profile.
Legal regulation of cis-THC is unstable and ambiguous. The absence of clear definitions in international treaties and national legislation creates legal gaps and zones of uncertainty, complicating commercial use and scientific research. The isomer is often not included in controlled substance lists or addressed separately, which provides potential for its use in medical and scientific applications without complex legal barriers but simultaneously creates risks for the legitimacy of such activities in different jurisdictions. Effective integration of cis-THC into pharmaceuticals requires the development of specialized regulatory frameworks that consider its unique properties, potential, and risks.
Thus, cis-THC is an important molecule in the context of modern cannabinoid chemistry and pharmacology with a broad range of potential applications arising from its chemical and pharmacodynamic uniqueness. Its prospects for medical implementation directly depend on further advancement of synthesis methods, deeper understanding of biogenetic mechanisms, detailed pharmacological research, and adaptation of the legal framework. Considering the complexity of the challenges and its potential, cis-THC occupies an intermediate position between classical cannabinoids and emerging pharmaceutical opportunities, requiring focused interdisciplinary research and regulatory initiatives for its optimal use.
Sources:
- FDA (U.S. Food and Drug Administration) – Regulation of Cannabis and Cannabis-Derived Products, Including Cannabidiol (CBD)
https://www.fda.gov/news-events/public-health-focus/fda-regulation-cannabis-and-cannabis-derived-products-including-cannabidiol-cbd - Federal Register (U.S. Federal Register) – Implementation of the Agriculture Improvement Act of 2018
https://www.federalregister.gov/documents/2020/08/21/2020-17356/implementation-of-the-agriculture-improvement-act-of-2018 - PubMed Central / NCBI (National Center for Biotechnology Information, USA) – Analysis of Potency and Safety of Δ⁹-Tetrahydrocannabinol Products Derived from Hemp
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC10369762/ - SAMHSA (Substance Abuse and Mental Health Services Administration, USA) – Tetrahydrocannabinol Isomerism: Summary of the Scientific Advisory Board Meeting of DTAB
https://www.samhsa.gov/sites/default/files/meeting/documents/thc-isomerism.pdf - PubChem / NCBI (U.S. National Library of Medicine) – Structure and Properties of Delta-9-cis-Tetrahydrocannabinol (cis-THC)
https://pubchem.ncbi.nlm.nih.gov/compound/Delta-9-cis-Tetrahydrocannabinol - NCBI / NIH (National Institutes of Health, USA) – Cannabis sativa and Cannabinoids: Chemical Composition and Biological Activity
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6770351/ - SpringerLink / Archives of Toxicology – Pharmacological Effects of Δ⁹-Tetrahydrocannabinol and Other Cannabinoids
https://link.springer.com/article/10.1007/s00204-021-03003-3 - DEA (Drug Enforcement Administration, USA) – Classification of Controlled Substances Including Cannabis Derivatives
https://www.dea.gov/drug-information/drug-scheduling - ScienceDirect / Elsevier – Cannabinoids: Natural Occurrence and Metabolism in Cannabis sativa
https://www.sciencedirect.com/science/article/pii/S0031942219304381 - U.S. Department of Justice / Office of Legal Counsel – Legal Status of Δ⁹-THC Isomers Under the Controlled Substances Act (CSA)
https://www.justice.gov/olc/opinion/legal-status-delta-9-thc-isomers-under-controlled-substances-act