Cannabinoid chemistry has long sparked intense interest in scientific circles, bringing together researchers from organic chemistry, pharmacology, biochemistry, and medicine. Among the numerous cannabinoids isolated from the Cannabis sativa L. plant, the most attention is drawn to Delta-9-Tetrahydrocannabinol (Delta-9-THC), which is the primary psychoactive component. However, recent scientific interest has increasingly focused on the less-studied homologs and analogs of this molecule, one of which is Delta-9-Tetrahydrocannabinol-C4 (THC-C4). This homolog stands out due to structural features that determine unique physicochemical properties and potentially a different pharmacological profile compared to classic Delta-9-THC. Studying THC-C4 is a necessary step toward understanding the deeper mechanisms of cannabinoid action, as well as developing new therapeutic agents.
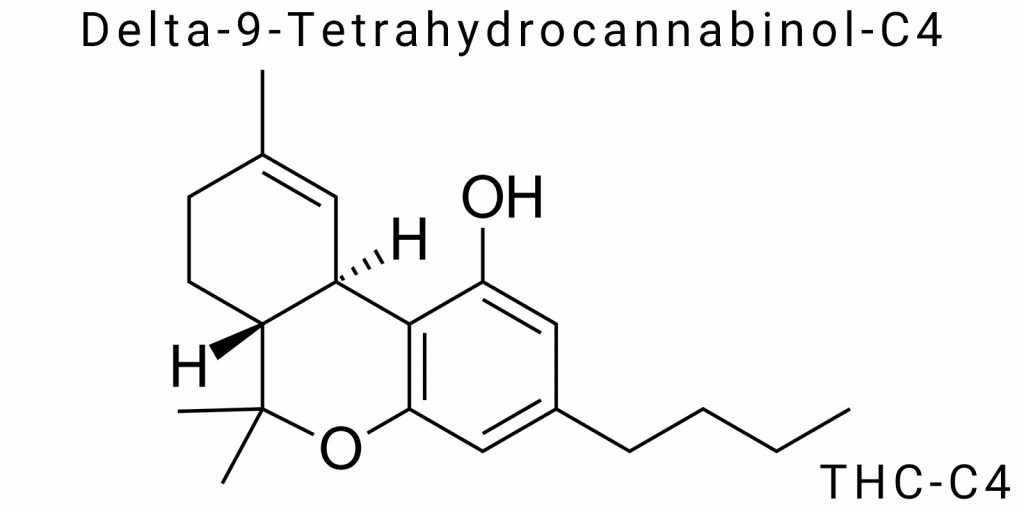
The label THC-C4 refers to a molecule belonging to the tetrahydrocannabinol class and is a structural analog of Delta-9-THC with an extended alkyl side chain containing four carbon atoms (C4). Changes in the length of the side chain in cannabinoid molecules have long been known as a critical factor influencing interaction with the endocannabinoid system receptors, and therefore the pharmacological properties of the compound. However, THC-C4 remains poorly studied in scientific literature, and its chemical and biological characterization is still not fully revealed. In recent years, thanks to advancements in synthetic methods and analytical technologies, it has become possible to obtain THC-C4 in pure form, opening new opportunities for fundamental and applied research.
It is important to note that in nature, THC-C4 is found in significantly lower concentrations than classic Delta-9-THC, which complicates its extraction from plant material. This drives the development of various synthesis methods, both chemical and biotechnological, that can provide stable production of this compound with high purity. Experimental approaches include classic organic synthesis with controlled modification of the alkyl chain, as well as enzymatic methods based on manipulating biosynthetic pathways in cannabinoid-producing cells.
From a pharmacological standpoint, the length and configuration of the alkyl side chain in the THC molecule significantly determine its ability to interact with cannabinoid receptors CB1 and CB2, which are responsible for various psychoactive and physiological effects. In the case of THC-C4, scientific data indicate a potential change in affinity for these receptors compared to Delta-9-THC, which may influence the intensity and quality of the psychoactive effects, as well as the range of therapeutic possibilities. Understanding these differences is of crucial importance for pharmacology and medicine, as it allows targeted development of new drugs with specific properties and fewer side effects.
Current research shows that THC-C4 may play a unique role in regulating the endocannabinoid system, which governs a wide range of biological functions – from pain and inflammation to emotional state and cognitive processes. This makes THC-C4 a promising candidate for further study in the context of developing medications aimed at treating various neurological, psychiatric, and chronic conditions. At the same time, the pharmacokinetics, toxicology, and safety profile of THC-C4 remain insufficiently studied, requiring systematic research using modern molecular biology, pharmacodynamics, and toxicology methods.
Beyond purely scientific aspects, interest in THC-C4 is also growing in the context of legislative and social changes related to cannabinoid regulation in many countries. Studying such less common homologs as THC-C4 helps form a more complete understanding of the spectrum of cannabinoid compounds circulating in both legal and illegal markets. This is important for creating adequate regulatory frameworks and control over cannabinoid use, especially in medical and pharmaceutical fields.
Chemical Structure and Physicochemical Properties of THC-C4
Delta-9-Tetrahydrocannabinol-C4 (THC-C4) is one of the homologs of classic Delta-9-THC, characterized by unique structural features that define its physicochemical profile and biological activity. Like other tetrahydrocannabinols, THC-C4 is a derivative of the benzopyran core to which a side alkyl chain is attached. In the case of THC-C4, this chain has a length of four carbon atoms, distinguishing it from the more common Delta-9-THC with a three-carbon (propyl) chain. This gives the molecule characteristic changes in configuration and hydrophobic properties.
The chemical structure of THC-C4 is key to its ability to interact with the receptors of the endocannabinoid system (CB1 and CB2), which mediate the physiological and psychoactive effects of cannabinoids. The change in the length of the alkyl chain affects the spatial conformation of the molecule, its solubility, as well as its ability to penetrate biological membranes, which in turn may alter affinity for receptors and its pharmacodynamic profile.
Physicochemical properties of THC-C4, such as solubility in various solvents, stability under different temperature and lighting conditions, volatility, and spectroscopic characteristics, are important for understanding its behavior in biological systems and in technological processes of production and purification. The solubility of THC-C4, particularly in nonpolar and polar environments, determines the efficiency of its extraction from plant material or synthetic mixtures and also influences pharmacokinetics – the ability for adsorption, distribution, and metabolism.
The stability of THC-C4 with respect to oxidation, photodegradation, and thermal decomposition is critical when developing pharmaceutical formulations and storing the drug. The volatility of the molecule, in turn, determines transportation and storage conditions, as well as affecting evaporation characteristics in various modes of application (inhalation, vaporization).
Spectroscopic methods such as nuclear magnetic resonance (NMR), mass spectrometry (MS), and infrared spectroscopy (IR) allow detailed characterization of the molecular structure of THC-C4, confirmation of sample purity, and study of its isomeric forms. Spectrum analysis enables identification of characteristic functional groups, configurational features, and provides insight into molecular interactions with the surrounding environment.
Studying these chemical and physicochemical parameters of THC-C4 is the foundation for further pharmacological research and for the development of synthesis and production technologies. Additionally, these properties directly influence bioavailability, efficacy, and safety of this compound’s use in medical and scientific applications. Thus, a comprehensive understanding of the chemical structure and physicochemical characteristics of THC-C4 opens new horizons in cannabinoid compound research and their potential.
Molecular Structure of THC-C4
Delta-9-Tetrahydrocannabinol-C4 (THC-C4) is a structural homolog of classic Delta-9-THC and belongs to the class of tetrahydrocannabinols, the main psychoactive components of the Cannabis sativa plant. The THC-C4 molecule consists of three main fragments: an aromatic benzopyran core, cyclic tetrahydrocannabinol, and a side alkyl chain, which in this homolog is characterized by a four-carbon length. Central to this structure is the benzopyran ring, which gives the molecule a specific three-dimensional shape and defines its electronic properties. The cyclic tetrahydrocannabinol, a saturated derivative of benzopyran, contains two main functional groups – a phenolic hydroxyl group and an oxidized oxygen atom – that provide chemical reactivity to the molecule and are critical for interaction with biological receptors. A distinctive feature of the molecule is the four-carbon (butyl) side chain attached to the benzene ring at the position known as the 3-position relative to the benzopyran core. The length and configuration of this chain directly affect the conformation of the entire molecule, determining its ability to interact with CB1 and CB2 receptors.
In the three-dimensional structure of the THC-C4 molecule, the spatial arrangement of functional groups creates a complex electronic landscape that regulates the pharmacological properties of the compound. Rotations around the bonds between the benzopyran core and the side chain form a dynamic conformer ensemble, which differs from similar configurations in classic Delta-9-THC due to the elongation of the alkyl chain. This structural feature influences the overall hydrophobicity of the molecule and its interaction with the lipid membranes of cells, determining bioavailability and mechanisms of penetration through cellular barriers.
Expanded Formula, Isomerism, Chemical Properties
The expanded structural formula of THC-C4 reflects the precise arrangement of atoms and bonds in the molecule, which is critical for understanding its chemical and physical characteristics. In this formula, the benzopyran core is represented as a condensed aromatic and heterocyclic ring with an oxygen atom forming the pyran ring, combined with the cyclic tetrahydrocannabinol segment that contains a hydroxyl functional group on the benzene ring. At the 3-position of the benzopyran core, a butyl alkyl side chain consisting of four consecutive carbon atoms is attached, bearing saturated aliphatic bonds.
Isomerism of THC-C4 encompasses several aspects. The most important is stereoisomerism, specifically the configuration around the double bond at position 9, which determines membership in the Delta-9 isomers. Conformational isomerism is related to the possibility of rotation around single bonds, especially in the side chain and the bonds of the benzopyran core, creating different spatial forms that may differ in activity. Additionally, the presence of chiral centers in the molecular structure ensures the existence of optical isomers, which is significant for interaction with biological molecules, as different enantiomers may demonstrate different pharmacological activity.
Regarding chemical properties, THC-C4, like other tetrahydrocannabinols, is characterized by high lipophilicity due to the presence of a large hydrophobic alkyl chain and aromatic core. This provides its solubility in nonpolar organic solvents such as chloroform, methanol, and ethanol, and low solubility in water. The molecule’s ability to react to oxidation and photolysis is due to the presence of the phenolic hydroxyl group, which is an active center of chemical reactivity, especially under the influence of light and oxygen.
THC-C4 demonstrates a tendency to form stable complexes with lipids of biological membranes, facilitating its penetration into cells and interaction with intracellular receptors. The chemical activity of the molecule also manifests in its ability to form hydrogen bonds, especially through hydroxyl groups, which is important for conformational stabilization and interaction with protein receptors.
Overall, the expanded formula of THC-C4 and its isomeric properties form the foundation for detailed understanding of its chemical behavior, which is directly related to its biological activity and potential applications in pharmacology and medicine.
Physicochemical Characteristics
The physicochemical characteristics of Delta-9-Tetrahydrocannabinol-C4 (THC-C4) serve as the basis for understanding its behavior under various conditions, which directly influence its pharmacokinetic and pharmacodynamic properties. This compound, being a cannabinoid, demonstrates a complex of interrelated parameters – solubility, stability, volatility, as well as spectroscopic properties, which serve as tools for identification, quantitative analysis, and study of molecular structure. The properties of THC-C4, distinct from traditional Delta-9-THC, are shaped by structural features, particularly the length of the alkyl chain and the configuration of functional groups.
Solubility of THC-C4 is determined by the molecule’s predominant lipophilicity, caused by the presence of a large nonpolar alkyl chain and aromatic benzopyran core. Accordingly, the molecule exhibits high solubility in nonpolar organic solvents such as chloroform, benzene, ethanol, acetone, as well as in lipid matrices. At the same time, water solubility is minimal, practically negligible, which is characteristic for tetrahydrocannabinol derivatives and explained by the hydrophobic nature of the molecule. The partition coefficient (logP) for THC-C4 indicates its strong hydrophobic character, determining its ability to penetrate lipid barriers of biological membranes and complicating use in aqueous pharmaceutical solutions without emulsifiers or carriers. Compared to Delta-9-THC, increasing the length of the alkyl chain leads to a slight increase in solubility in nonpolar media, confirmed by experimental data and theoretical molecular interaction models.
Stability of THC-C4 regarding oxidation and photodegradation is an important characteristic for its pharmaceutical application and storage. The molecule contains a phenolic hydroxyl group that is sensitive to oxidative processes, leading to the formation of various oxidative products, including quinones. These reactions can cause degradation of the molecule and alter its biological activity. Under ultraviolet radiation, the THC-C4 molecule is prone to photodegradation, accompanied by cleavage of π-bonds in the aromatic core and formation of various photoisomers and decomposition products. Thermal stability is limited: at temperatures above 150-160 °C, thermal cleavage of the molecule occurs with the release of volatile fragments. To preserve the chemical integrity of THC-C4 during production and storage, it is necessary to use inert atmospheres, control temperature, and limit light exposure.
Volatility of THC-C4 is moderate, reflecting a balance between the molecular mass and its chemical structure. The molecule is capable of evaporating at elevated temperatures, which is significant for administration methods such as inhalation or vaporization. Its volatility is lower than that of low-molecular-weight compounds but sufficient to form vapor in pharmacological devices. This parameter also determines specific conditions for transportation and storage, which must exclude excessive heating and evaporation of the active substance.
Spectroscopic characteristics of THC-C4 serve as fundamental methods for analyzing the structure and purity of the compound, as well as for identifying isomers and degradation products. Nuclear magnetic resonance (NMR) provides detailed information about the position of atoms in the molecule, chemical environment of protons and carbons, and conformational features. The ^1H NMR spectra display characteristic signals of aromatic protons, methine groups of the benzopyran core, and aliphatic protons of the side chain. Chemical shifts and multiplet patterns allow differentiation of atom positions and interactions, which is critical for confirming the structure of THC-C4. The ^13C NMR spectra reflect types of carbon atoms (aromatic, saturated, aliphatic) and their relationships with functional groups.
Mass spectrometry (MS) is a key tool for determining molecular mass and the structure of molecular fragments. Characteristic is the molecular ion peak, confirming the molecular formula, as well as fragmentation spectra showing breaks in the side chain and core structure. Specific fragments allow differentiation of THC-C4 from other homologs, including Delta-9-THC, which has a shorter alkyl chain. Mass spectrometric data are also used to detect impurities and degradation products.
Infrared (IR) spectroscopy reflects the presence of functional groups such as phenolic hydroxyl, C-H aliphatic and aromatic bonds, as well as C-O bonds in the pyran ring. Characteristic peaks in the 3200-3600 cm⁻¹ range correspond to O-H groups, and peaks in the 2800-3000 cm⁻¹ range correspond to aliphatic C-H. The appearance of bands in the 1000-1300 cm⁻¹ region confirms the presence of C-O-C functionality, which is part of the benzopyran core. Comparison of IR spectra of THC-C4 with analogs allows determination of structural differences correlating with alkyl chain length and the presence of isomers.
Solubility, Stability, Volatility
The solubility of THC-C4 is characterized by pronounced lipophilicity, due to its saturated four-carbon alkyl side chain and aromatic benzopyran core. Because of this lipophilicity, the compound exhibits minimal solubility in aqueous environments, practically insoluble in water, which complicates its direct use in aqueous pharmaceutical formulations. The best solubility is observed in nonpolar and slightly polar organic solvents such as chloroform, benzene, toluene, as well as in media containing alcohols (ethanol, methanol). This property correlates with the increased length of the alkyl chain compared to classic Delta-9-THC, which promotes better solubility in lipophilic environments. Therapeutic applications of THC-C4 often require the use of emulsifiers, liposomes, or other delivery systems to enhance bioavailability in aqueous systems.
The stability of THC-C4 is a critical factor for its pharmaceutical use. The molecule is sensitive to oxidative processes, in which the phenolic hydroxyl group undergoes reactions with oxygen, forming oxidized products that lose pharmacological activity. Accordingly, storage should avoid exposure to oxygen and moisture. Photodegradation under ultraviolet radiation leads to molecular breakdown and formation of photoisomers, which may have altered pharmacological properties. Thermal stability is limited; at temperatures above 150-160 °C, thermal decomposition begins with the formation of volatile products, necessitating temperature control during production, storage, and transportation.
The volatility of THC-C4 is determined by its molecular weight and structural features. The molecule has sufficient volatility for use in vaporization and inhalation delivery methods, though its volatility is lower than that of low-molecular-weight organic compounds. Evaporation occurs at elevated temperatures, making temperature control important to minimize loss of the active substance. Volatility also affects storage conditions, requiring airtight packaging and avoidance of excessive heating.
Spectroscopic Characteristics (NMR, MS, IR)
Spectroscopic methods are fundamental for molecular analysis of THC-C4, enabling confirmation of its structure, identification of isomers, determination of purity, and detection of degradation products. Nuclear Magnetic Resonance (^1H NMR and ^13C NMR) is a basic tool for studying the spatial and electronic structure of the molecule. In ^1H NMR spectra, distinct signals of aromatic protons appear in the range of 6.0-7.0 ppm, as well as signals from methine and methylene protons of the benzopyran core and side chain in the range of 0.8-3.0 ppm. Multiplet patterns reflect interactions between neighboring protons and help determine the molecule’s configuration and conformation. ^13C NMR spectra identify different types of carbon atoms, including aromatic, aliphatic, and those involved in functional groups. These data are crucial for confirming the alkyl chain length and the position of functional groups.
Mass spectrometry provides information on the molecular weight of THC-C4 and characteristic fragments resulting from molecular cleavage under ionizing radiation. The molecular ion peak corresponds to the molecular mass C23H34O2 (m/z 346), characteristic of this compound. Fragmentation typically occurs in the side chain and benzopyran core regions, allowing differentiation of THC-C4 from other cannabinoids based on unique fragment patterns. Mass spectrometry is used both for identification and for purity control of samples.
Infrared spectroscopy (IR) reveals the presence of functional groups, notably phenolic O-H groups, reflected by a broad absorption band in the 3200-3600 cm⁻¹ range, as well as C-H bonds appearing as peaks in the 2800-3000 cm⁻¹ region. Peaks in the 1000-1300 cm⁻¹ range correspond to C-O-C bonds characteristic of the benzopyran core. IR spectra of THC-C4 also indicate specific vibrations of the aromatic ring, which help distinguish its isomers and determine purity levels.
Together, the spectroscopic characteristics of THC-C4 not only confirm its structure and differences from other homologs but also allow study of chemical changes dynamics under various experimental conditions, which is critically important for scientific research and practical applications.
Methods of Synthesis and Production of THC-C4
The processes for obtaining Delta-9-Tetrahydrocannabinol-C4 (THC-C4) involve various approaches, including natural sources, chemical synthesis, and biotechnological methods. Each of these methods has its features, advantages, and limitations, which determine the choice of technology for industrial or laboratory-scale production. Considering the complexity of THC-C4’s molecular structure as well as its pharmacological significance, synthesis processes are aimed at achieving high purity, stability, and reproducibility of the final product.
The natural origin of THC-C4 is closely linked to the biosynthetic pathways of cannabinoids in plants of the Cannabis genus. The uniqueness of this compound’s structure is determined by enzymatic processes and genetic regulation within plant tissues, which define the qualitative and quantitative composition of the final cannabinoids. The search for and selection of plants with increased THC-C4 content, as well as optimization of cultivation conditions, are important factors for natural extraction of this compound.
Chemical synthesis methods for THC-C4 include classical organic reactions that allow obtaining the compound with high selectivity and isomer control. These methods are based on multi-step processes using specific reagents, catalysts, and reaction conditions. Additionally, modern approaches incorporate metal-based catalysts, enzymatic systems, and molecular modifications to facilitate synthesis or improve properties of the final product. Chemical synthesis opens possibilities for large-scale production of THC-C4 with controlled quality and structural purity.
Biotechnological methods are gaining increasing popularity due to the potential use of genetically modified microorganisms and enzymes for biosynthesis of complex molecules like THC-C4. The introduction of genes responsible for cannabinoid biosynthesis into bacteria, yeast, or other cellular systems allows production of target compounds under controlled conditions, which are more stable and environmentally friendly than traditional chemical methods. Enzymatic processes contribute to selective transformation of intermediate products into the final cannabinoid with minimal formation of byproducts.
Natural Origin: Plant Sources
Delta-9-Tetrahydrocannabinol-C4 (THC-C4) is a natural cannabinoid formed in the tissues of Cannabis plants. Its presence is caused by unique variations in biosynthetic pathways that lead to the formation of a butyl side chain, which differs from the classic pentyl side chain found in Delta-9-THC. Primarily, THC-C4 concentrates in the trichomes of cannabis flowers, where enzymatic activity is directed toward transforming precursors into specific cannabinoid compounds. The uniqueness of these plant sources lies in their genetic and metabolic heterogeneity, which determines the variability of THC-C4 content. This variability reflects not only natural differences between species and strains but also the influence of agro-climatic factors that regulate the expression of biosynthetic enzymes.
Potential of Cannabis and Hemp for THC-C4 Production
Cannabis and industrial hemp are the main natural sources of THC-C4; however, their potential for producing this compound largely depends on the selection and genetic makeup of specific lines. Traditionally, hemp has been considered a source of food and industrial materials with minimal psychoactive cannabinoid content, whereas cannabis is cultivated for pharmacological use and often has elevated levels of Delta-9-THC. The discovery and identification of strains with high THC-C4 content open new opportunities for industrial production. Unlike classic Delta-9-THC, THC-C4 is characterized by a butyl side chain, influencing its physicochemical and pharmacological properties, potentially providing unique effects and reducing undesired side effects.
Cultivating specialized cannabis strains dominated by THC-C4 requires a systematic approach, including optimization of agricultural techniques, environmental control, and the use of selection methods such as genomic screening. The application of modern biotechnologies allows rapid identification of genotypes with the desired cannabinoid profile and the development of lines with increased THC-C4 synthesis. This strategy can be effective on both laboratory and industrial scales, considering the compound’s potential commercial and medical significance.
Additionally, hemp as an agricultural crop offers advantages such as high pest resistance, relative tolerance to soil and climatic conditions, and rapid growth, making it an efficient bioreactor for accumulating specific cannabinoids. These factors significantly reduce production costs and increase the environmental sustainability of THC-C4 manufacturing processes.
Genetic and Biochemical Prerequisites for THC-C4 Formation
The genetic basis for THC-C4 synthesis is determined by polymorphisms in genes encoding enzymes responsible for forming the molecule’s side chain. Central to this is the gene encoding tetrahydrocannabinol synthase (THCAS), which catalyzes the cyclization of cannabinoid precursors into tetrahydrocannabinol. It has been found that variants of this gene determine the side chain type: pentyl (classic THC) or butyl (THC-C4). Chain elongation occurs via enzymes that regulate the length and chemical modifications of the alkyl group.
These genetic variations are not isolated but exist in complex interactions with regulatory elements that influence gene expression levels under different environmental conditions. For example, changes in temperature, lighting, or nutrition can modulate enzyme activity, leading to variations in the amount of synthesized THC-C4.
Biochemically, the formation of THC-C4 begins with enzymatic synthesis of prenyl pyrophosphate and olivetolic acid, which condense into tetrahydrocannabinol precursors. Subsequent enzymatic reactions, including oxidation, reduction, and cyclization, form the final THC-C4 molecule with its characteristic butyl side chain. The specificity of these processes lies in the high selectivity of enzymes toward the length and structure of the side chain, ensuring synthesis of this homolog among other cannabinoids.
Chemical Synthesis Methods
Chemical synthesis of Delta-9-Tetrahydrocannabinol-C4 (THC-C4) involves a complex approach based on organic reactions to create a molecule with a specific butyl side chain. These methods include multistep processes where the chromane ring is constructed, the tetrahydrocannabinol structure is formed, and the length and saturation of the alkyl chain are controlled. The development of chemical synthesis for THC-C4 is driven by the need to obtain a compound with high purity and defined stereochemistry, which is critical for pharmacological use.
Classical Organic Synthesis of THC-C4
Classical organic synthesis of THC-C4 is based on the principles of building the tetrahydrocannabinol structure through condensation and cyclization reactions, with the key step being the introduction of a butyl side chain instead of the traditional pentyl chain. Typically, starting materials are aromatic phenols or derivatives of olivetolic acid, which undergo Friedel-Crafts or similar alkylation reactions to introduce the butyl substituent. This is followed by formation of the chromane part of the molecule through cyclization involving multifunctional reagents. Precision in reaction conditions is critical to control stereocenter configuration and minimize byproduct formation.
These syntheses generally consist of several stages: alkylation of the aromatic core, subsequent attachment of functional groups forming the cyclic system, and a final stage that ensures the formation of the tetrahydrocannabinol structure. The challenge lies in the stability of intermediates and reaction selectivity, especially when working with the butyl side chain, which requires careful choice of catalysts and solvents. Despite being traditional, this approach remains relevant due to its scalability and ability to produce a product with high stereochemical purity.
Catalytic and Enzymatic Approaches
Advancements in catalytic methods for THC-C4 synthesis aim to increase selectivity and reduce harsh reaction conditions. Transition metal catalysts such as palladium, platinum, or rhodium are used for alkylation and cyclization with stereochemical control. These catalysts activate functional groups, lower the energy barriers of reactions, and allow selection of the desired isomer. Particularly effective are methods based on asymmetric catalysis, where chiral ligands provide a single stereoisomer product, important for the pharmaceutical activity of THC-C4.
Enzymatic approaches use specific biocatalysts-enzymes that mimic natural biochemical pathways of cannabinoid synthesis. These methods enable reactions under mild conditions (low temperatures, neutral pH) with high selectivity. Enzymes such as oxygenases or transferases can specifically modify side chains, providing precise control over the length and saturation of alkyl groups. Enzymatic methods also avoid toxic byproducts commonly generated in classical organic syntheses.
Combining catalytic and enzymatic approaches opens possibilities for multistep synthetic routes where catalysts are applied in early stages to form the basic structure, and enzymatic steps are used for fine molecular modifications. This improves yield and purity of the final product while reducing energy and material costs.
Structural Modifications for Obtaining THC-C4
Modification of the chemical structure of THC-C4 involves targeted changes to the side chains, the positions of double bonds, and functional groups, which influence the pharmacological and physicochemical properties of the molecule. These changes can be made either during synthesis or through post-synthetic transformations.
Changing the length of the alkyl chain-for example, replacing the pentyl chain with a butyl chain-is achieved by using appropriate alkylating agents or through selective catalytic hydrogenation/dehydrogenation. Adjustments to the position of double bonds or the addition of functional groups (hydroxyl, carbonyl, amino groups) occur via oxidative or reductive reactions, which can modify the biological activity of the compound, its solubility, and stability.
Isomerization reactions are also employed to alter the molecular configuration, producing various stereoisomers of THC-C4, each with a unique pharmacological profile. Using different catalysts and reaction conditions allows for the selective synthesis of a specific isomer, which is critically important for developing drugs with targeted effects.
Another approach includes introducing heteroatoms or cyclic substituents into the THC-C4 molecule, which can change its bioavailability, metabolic stability, and affinity for cannabinoid receptors. These structural modifications hold promise for the development of new THC-C4-based pharmaceuticals with improved pharmacological properties.
Biotechnological Methods
Biotechnological methods for synthesizing Delta-9-Tetrahydrocannabinol-C4 (THC-C4) rely on using living biological systems or their components for the efficient and selective production of the target compound. Unlike traditional chemical methods, biotechnology offers precise control over the molecular structure, reduces the formation of byproducts, and lowers energy consumption. Two main approaches are key in this context: the use of genetically modified microorganisms and enzymatic processes, often applied separately or in combination to optimize THC-C4 production.
Genetically Modified Microorganisms
Genetically modified microorganisms (GMMs) are among the most promising tools for the biosynthesis of THC-C4. The basic principle involves introducing genes encoding enzymes necessary for cannabinoid biosynthesis-specifically, enzymes responsible for forming the unique butyl side chain of THC-C4-into microbial cells. Using recombinant DNA technology, bacteria or yeast strains are engineered to produce precursors like prenyl pyrophosphate and oleic acid, which are then converted enzymatically into THC-C4.
Creating efficient GMMs requires not only cloning the relevant genes but also fine-tuning metabolic pathways to ensure optimal substrate flux and maximize productivity. It is essential to eliminate or suppress competing metabolic pathways that divert precursors or energy, which is critical for increasing THC-C4 yield. A distinctive feature for THC-C4 is the necessity of producing the specific butyl side chain, differing from the more common pentyl chain, so genetic constructs are accordingly modified.
An important advantage of GMMs is scalability in large bioreactors with precise control over environmental parameters such as temperature, pH, and aeration, allowing for consistent product batches with high purity. Moreover, modern approaches to enzyme evolution and gene expression optimization enhance catalytic activity and enzyme stability, significantly improving THC-C4 production efficiency.
Enzymatic Processes
Enzymatic processes involve the use of isolated enzymes that catalyze key reactions in the biosynthesis of THC-C4. These processes allow reactions to occur under mild physicochemical conditions, reducing the risk of product degradation and byproduct formation, while enhancing reaction selectivity. The main enzyme in this process is tetrahydrocannabinol synthase (THCAS), which catalyzes the cyclization and formation of the cannabinoid skeletal structure.
Immobilizing enzymes on solid supports increases their stability, permits repeated use of catalysts, and optimizes reaction conditions, thereby reducing production costs. Enzymatic systems provide high substrate specificity, which is especially important for forming the butyl side chain in THC-C4, as even slight changes in substrate structure can significantly affect the biological activity of the final product.
Optimizing enzymatic synthesis involves carefully selecting temperature, pH, substrate, and cofactor concentrations to increase product yield and shorten reaction time. Additionally, enzymatic steps can be combined with chemical or catalytic methods, expanding opportunities for molecular modification and improving THC-C4 characteristics.
Enzymatic methods are a key element in the biotechnological production of THC-C4, ensuring high selectivity, stability, and environmental friendliness of the process. They enable the development of more complex synthetic pathways that precisely shape structural and stereochemical features of the molecule, which determine its pharmacological efficacy and safety.
Integration of Genetically Modified Microorganisms and Enzymatic Systems
The integration of genetically modified microorganisms and enzymatic systems creates a powerful toolkit for industrial production of THC-C4 with high purity, controlled structure, and reproducibility. This opens prospects for scaling up and commercializing biotechnological cannabinoid-based products.
Pharmacological and Biological Aspects of THC-C4
Delta-9-Tetrahydrocannabinol-C4 (THC-C4) is one of the less studied cannabinoid isomers, differing structurally and biologically from the classic Delta-9-THC. Its pharmacological profile is shaped by its interaction with the body’s endocannabinoid system, which plays a key role in regulating many physiological processes, including pain, appetite, memory, emotions, and immune response. Research into the pharmacodynamics of THC-C4 aims to determine its affinity for cannabinoid receptors, specificity of interaction, as well as its effects on various signaling pathways that define the overall impact of the compound.
The biological activity of THC-C4 encompasses both central and peripheral mechanisms of action. Due to structural differences from the standard THC with a pentyl side chain, THC-C4 exhibits somewhat modified affinity for CB1 and CB2 receptors, which directly influences the spectrum of its psychoactive and physiological effects. Studying these properties allows for the prediction of potential therapeutic applications, as well as the assessment of safety and toxicological profiles of the substance.
Pharmacokinetic features of THC-C4, such as absorption, distribution, metabolism, and excretion, are crucial for understanding the onset and duration of its effects. Metabolic characteristics may lead to the formation of active or inactive metabolites that affect clinical outcomes. Despite limited data, it is assumed that THC-C4 may display both traditional cannabinoid properties and unique characteristics that warrant detailed investigation within the context of modern pharmacology.
Mechanism of Action on the Endocannabinoid System
The mechanism of action of Delta-9-Tetrahydrocannabinol-C4 (THC-C4) on the endocannabinoid system is based on its ability to interact with the primary receptors of this system-CB1 and CB2-which are G protein-coupled receptors (GPCRs) involved in regulating numerous physiological processes. Similar to classic Delta-9-THC, THC-C4 acts as an agonist; however, the specificity and affinity of its receptor interactions differ due to the altered side chain, which affects conformational interactions within the receptor binding sites.
Cannabinoid receptors CB1 are predominantly localized in the central nervous system (CNS), especially in brain regions associated with pain regulation, mood, motor control, and cognitive functions. CB2 receptors are primarily found in the peripheral immune system, playing a role in modulating immune responses and inflammatory processes. THC-C4, crossing the blood-brain barrier, interacts with CB1 receptors by modulating neurotransmitter activity and also affects peripheral CB2 receptors, altering immune reactions.
The biochemical mechanism involves THC-C4 binding to the receptor, causing conformational changes in the GPCR structure, activating intracellular signaling cascades, including inhibition of adenylyl cyclase, which leads to decreased cAMP levels and modulation of ion channels. As a result, the release of neurotransmitters such as GABA, glutamate, dopamine, and serotonin is altered, influencing synaptic transmission and eliciting corresponding psychoactive and physiological effects.
Differences between THC-C4 and standard THC lie in the butyl side chain, which forms unique hydrophobic contacts with the receptor, potentially altering affinity and binding selectivity. These structural nuances determine varied levels of signal activation, which may enhance or diminish certain effects related to CB1 or CB2 activation. Additionally, THC-C4 may interact with non-canonical targets, including TRP channels and other receptors, broadening its spectrum of biological activity.
The functional activity of THC-C4 within the endocannabinoid system also depends on its ability to induce receptor desensitization and intracellular receptor redistribution, which are important for the duration of action and the development of tolerance. Especially significant is its influence on the reuptake of endocannabinoids such as anandamide through competitive or allosteric interactions, further modulating system tone.
Metabolic transformation of THC-C4 in the liver produces metabolites that may vary in their activity toward CB1 and CB2 receptors, impacting the overall pharmacological profile. Some metabolites can act as partial agonists or antagonists, adding complexity to the regulation of the endocannabinoid system during THC-C4 use.
Interaction with CB1 and CB2 Receptors
The interaction of THC-C4 with CB1 and CB2 receptors defines the primary pharmacological profile of the compound and is the subject of intensive research. CB1 receptors, which belong to the family of G protein-coupled receptors, are primarily located in neurons of the central nervous system, especially in areas associated with memory, motor control, pain, and mood. THC-C4, as a lipophilic molecule, passes through cell membranes and binds to the hydrophobic pocket of the receptor, inducing conformational changes that trigger a cascade of intracellular signals.
Studies have shown that the presence of the butyl side chain in THC-C4 reduces hydrophobic interaction compared to the pentyl side chain of standard THC, which is reflected in the affinity and partial agonist activity at CB1. This reduces or alters the nature of receptor activation, resulting in variations in psychoactive effects. Specifically, THC-C4 may cause less intense or longer-lasting effects depending on concentration and experimental conditions.
Regarding CB2 receptors, which are predominantly expressed on immune cells, THC-C4 demonstrates a somewhat different interaction profile. Its affinity for CB2 is often higher than for CB1, suggesting a potentially more pronounced effect on immune processes and anti-inflammatory mechanisms. Interaction with CB2 occurs through specific hydrophobic and hydrogen bonds formed between the butyl side chain of THC-C4 and amino acid residues of the receptor.
In addition to the primary receptors, THC-C4 may affect non-classical cannabinoid targets, including TRPV1, GPR55, and certain ion channels. Interaction with these proteins may explain certain pharmacological effects that are not fully dependent on CB1 or CB2, such as analgesic, anti-inflammatory, and neuroprotective properties.
Structural studies, including X-ray crystallography and molecular modeling, indicate that the butyl side chain in THC-C4 changes the orientation of the molecule in the ligand-binding site, leading to variability in the receptor’s conformational state after binding. This dynamic can influence the selective activation of different intracellular signaling pathways, such as G protein or β-arrestin activation, which is important for the pharmacological effect and side effect profile.
The binding kinetics of THC-C4 with CB1 and CB2 are characterized by relatively rapid entry into the receptor site and moderate complex stability, resulting in short-term but repeatable receptor activation. This factor is important for understanding effects during repeated use and the potential for tolerance development.
Psychoactive and Physiological Effects
Delta-9-Tetrahydrocannabinol-C4 (THC-C4) exhibits psychoactive and physiological effects due to its impact on the central nervous system through interaction with CB1 receptors of the endocannabinoid system. Unlike classic Delta-9-THC, THC-C4 is characterized by a somewhat altered activation profile determined by structural differences in the side chain. These differences affect affinity and binding selectivity, reflected in changes in the intensity, duration, and spectrum of psychoactive effects.
The main psychoactive effects of THC-C4 include mood changes, sensory hypersensitivity, emotional lability, cognitive and motor function impairment, as well as possible euphoria or anxiety depending on dose and individual characteristics. Molecular structural differences lead to less pronounced or conversely more prolonged psychoactive effects compared to Delta-9-THC, which is supported by pharmacokinetic studies.
Physiological effects include pain modulation, appetite changes, cardiovascular reactions (notably tachycardia), as well as effects on the immune system through CB2 receptors. THC-C4 may cause hypotension and peripheral vasodilation, linked to mediated vasodilation through endothelial mechanisms. A significant feature is the potential modulation of inflammatory processes and neuroprotection, making THC-C4 an object of interest in therapeutic applications.
The level of psychoactivity depends on pharmacokinetic parameters including absorption rate, distribution, metabolism, and excretion. THC-C4, with its altered side chain, may demonstrate a distinct metabolic profile with the formation of specific active and inactive metabolites, which also influence the overall pharmacological effect and safety profile.
The physiological action of THC-C4 is characterized by an integrated effect on multiple body systems, including nervous, cardiovascular, and immune systems. Such effects may be beneficial in treating chronic pain, inflammatory diseases, mood disorders, and other conditions; however, there are risks of adverse reactions depending on dose and duration of use.
Toxicological Profile
The toxicological profile of THC-C4 is shaped by its pharmacodynamic and pharmacokinetic properties, including receptor affinity, metabolism, and systemic effects. Unlike classic Delta-9-THC, THC-C4 demonstrates potentially reduced acute toxicity due to less intense activation of CB1 receptors, which lowers the risk of acute psychotic reactions and overstimulation of the central nervous system.
Animal studies have shown that THC-C4 has a higher toxicity threshold compared to Delta-9-THC, reflected in a higher lethal dose and a reduced incidence of acute side effects. Chronic use may lead to the development of tolerance, accompanied by decreased efficacy and the need for dose escalation; however, the risk of accumulation of toxic metabolites remains low due to efficient metabolic pathways.
Side effects include possible impairments in cognitive function, motor skills, increased heart rate, as well as potential psychoemotional reactions-such as anxiety and paranoid states-which are especially pronounced at high doses or in individuals with predispositions. It is important to note the absence of significant organotoxic effects on the liver and kidneys at standard doses, as confirmed by biochemical markers and histopathological studies.
The interaction of THC-C4 with other medications requires further investigation, particularly due to the possibility of competitive inhibition of cytochrome P450 enzymes, which could affect the pharmacokinetics of concurrently administered drugs. These considerations are critical for safety assessment and the development of clinical guidelines.
Potential Therapeutic Properties
THC-C4 exhibits a broad range of potential therapeutic properties based on its ability to modulate the endocannabinoid system and related physiological processes. This cannabinoid may be a promising agent in the treatment of pain syndromes, especially chronic and neuropathic pain, due to its high efficacy in modulating pain sensitivity through CB1 and CB2 receptors.
The anti-inflammatory properties of THC-C4 are mediated by activity at CB2 receptors and modulation of the immune response, making it potentially useful in the therapy of autoimmune diseases, rheumatoid arthritis, as well as inflammatory neurodegenerative processes. Additionally, a possible neuroprotective effect in protecting neurons from oxidative stress and apoptosis is relevant for Alzheimer’s, Parkinson’s disease, and other degenerative conditions.
THC-C4 may influence mood disorders, anxiety, and depression indirectly through central modulation of neurotransmitters. Its effects on appetite and metabolism open prospects for use in treating conditions associated with weight loss, such as in patients with cancer or HIV.
Other potential applications include the treatment of glaucoma through the reduction of intraocular pressure, as well as effects on cardiovascular function that may be beneficial in certain clinical situations.
Results of Experimental and Clinical Studies
Research on THC-C4 is currently mainly represented by experimental work in vitro and in vivo in animal models, with a limited number of clinical trials in humans. Experimental data confirm the efficacy of THC-C4 in modulating the endocannabinoid system, as well as the presence of unique pharmacological properties distinct from Delta-9-THC.
In chronic pain models, THC-C4 shows significant reduction in pain sensitivity, which correlates with the activation of CB1 and CB2 receptors. Additionally, anti-inflammatory effects are observed, including decreased levels of pro-inflammatory cytokines and oxidative stress markers, enhancing its potential in treating inflammatory conditions.
Clinical studies, although limited, indicate acceptable tolerability of THC-C4 with minimal side effects at controlled doses. The absence of severe psychotic reactions in most patients provides grounds for further exploration of THC-C4 in therapeutic protocols. Studies on cognitive function effects show a lesser negative impact compared to standard THC, which is an important factor for clinical use.
Additional clinical trials are aimed at investigating the efficacy of THC-C4 in treating mood disorders, neurodegenerative diseases, and in palliative care, particularly in oncology contexts.
Potential Applications and Target Audience
Delta-9-Tetrahydrocannabinol-C4 (THC-C4) exhibits a wide range of potential applications that span both medical and industrial fields and generate significant interest within the scientific community. Its unique physicochemical and pharmacological properties provide a foundation for the development of new directions in pharmacotherapy, biotechnology, chemical research, and the creation of innovative pharmaceutical products. Given this, the target audience for the use of THC-C4 is multifaceted and includes medical professionals, pharmacists, scientists, biotechnologists, regulatory authorities, as well as industrial enterprises interested in implementing new technologies.
The potential medical use of THC-C4 is driven by its ability to influence the body’s endocannabinoid system, opening opportunities for the treatment of a broad spectrum of pathologies, including chronic pain syndromes, inflammatory diseases, neurodegenerative processes, and mental disorders. Differences in structure and pharmacodynamics from classical cannabinoids provide grounds to suggest a more selective and controlled therapeutic effect with a lower risk of side effects, making THC-C4 a promising candidate for pharmacotherapeutic innovation.
Furthermore, the industrial and scientific applications of THC-C4 focus on its use as a research chemical component for the development of new pharmacological agents, the creation of synthetic analogs, and the study of molecular mechanisms of receptor interaction. Considering the unique properties of the molecule, it could serve as a basis for the development of drugs with improved characteristics, including enhanced bioavailability, stability, and specificity of action.
Ethical and legal aspects of THC-C4 use are also important, regulating the framework of legality, control, and research opportunities. The legislative status of THC-C4 in many jurisdictions remains ambiguous, posing challenges in defining legal status, regulatory requirements, and oversight of production and distribution. These issues are especially relevant amid the global trend toward decriminalization of cannabinoids and the development of medical cannabis.
Medical Use
Delta-9-Tetrahydrocannabinol-C4 (THC-C4) represents a promising cannabinoid agent whose unique pharmacological properties hold potential for treating a wide range of diseases. Medical use of THC-C4 is based on its ability to modulate the endocannabinoid system, affecting physiological processes such as pain, inflammation, neurological functions, and mental state. Since THC-C4 differs chemically from traditional Delta-9-THC, its pharmacodynamic and pharmacokinetic characteristics enable potentially more precise targeted intervention in pathological processes.
In clinical practice, THC-C4 may be used as a treatment for managing chronic pain syndromes, including neuropathic pain, as well as an anti-inflammatory agent due to its ability to influence cytokine cascades and reduce the expression of pro-inflammatory molecules. Additionally, its role in modulating synaptic transmission makes this cannabinoid promising for use in neurodegenerative diseases such as Alzheimer’s and Parkinson’s, where activation of cannabinoid receptors may protect neurons from oxidative stress and inflammation.
THC-C4 also demonstrates significant potential in psychiatry, particularly for addressing anxiety disorders, depression, and post-traumatic stress disorder. Given its capacity to influence brain neurotransmitter systems, this cannabinoid can modulate behavioral responses by regulating levels of dopamine, serotonin, and glutamate, making it a promising candidate for developing medications with a lower likelihood of psychoactive side effects.
In comprehensive therapy, THC-C4 may act as an adjunct agent that improves the quality of life for cancer patients by reducing chemotherapy-induced nausea and vomiting, as well as stimulating appetite, which is especially important for patients experiencing wasting. Additionally, its ability to control pain and improve mood supports the use of THC-C4 in palliative care.
Pharmacological properties of THC-C4 also open prospects in neurology, where the cannabinoid may reduce symptoms of epilepsy and spasticity in multiple sclerosis. The mechanism of action is related to regulating neuronal excitability and enhancing GABAergic transmission, which helps decrease the frequency and severity of seizures.
Pharmacotherapeutic Prospects
The pharmacotherapeutic prospects of THC-C4 are based on its ability to specifically and effectively interact with the endocannabinoid system, providing a potentially more controlled therapeutic effect compared to other cannabinoids. Due to its modified structure, THC-C4 may demonstrate higher selectivity for certain receptor subtypes or possess increased metabolic stability, reducing the formation of toxic or unwanted metabolites.
In pharmacotherapy, this cannabinoid is considered a promising agent for developing drugs with a narrow focus that minimizes psychoactive side effects, which often limit the clinical use of traditional Delta-9-THC. This is especially important in chronic conditions where long-term therapy requires high safety and minimal cognitive impact.
Pharmacotherapeutic prospects include drug development for neurological disorders, particularly chronic pain, spasticity, and mental disorders associated with neurotransmitter imbalances. Furthermore, THC-C4 may serve as the basis for combination drugs that include various cannabinoid agents for synergistic effects, allowing for an improved therapeutic profile.
Innovative pharmacotherapeutic approaches using THC-C4 involve the development of controlled-release formulations, which provide prolonged action and stable plasma concentrations. This is important for maintaining a consistent therapeutic effect and reducing dosing frequency, thereby improving patient compliance.
An important direction includes studying the combination of THC-C4 with other pharmacological agents, including analgesics, anti-inflammatory drugs, and psychotropic medications, which opens opportunities for multidisciplinary therapy of complex clinical conditions.
Differences in Therapeutic Potential Compared to Classical Cannabinoids
The therapeutic potential of THC-C4 has several distinctions compared to classical cannabinoids such as Delta-9-THC and CBD, which are determined by its molecular structure and pharmacodynamics. One of the key differences is the altered configuration of the side chain, which affects receptor affinity and metabolic pathways, ultimately resulting in a different profile of effects.
This leads to a more selective interaction of THC-C4 with CB1 and CB2 receptors, potentially reducing the intensity of psychoactive effects and lowering the risk of tolerance development. Such specificity also enhances its efficacy in treating inflammatory and neurodegenerative conditions where controlled receptor activation is critical.
Differences in the metabolism of THC-C4 also have therapeutic significance: reduced formation of active metabolites that may cause undesirable reactions, and increased plasma stability ensure a more predictable and safer action profile.
Unlike CBD, which predominantly lacks psychoactive properties, THC-C4 exhibits moderate psychoactivity, allowing it to combine therapeutic effects with mild emotional and anxiolytic influence. This opens new opportunities for treating mental disorders without a high risk of abuse.
The distinctions in the therapeutic potential of THC-C4 justify considering it as an intermediate link between classical psychoactive cannabinoids and non-psychoactive components, broadening the spectrum of possible clinical applications and creating conditions for developing new pharmacological strategies with an optimal balance of efficacy and safety.
Industrial and Scientific Fields
THC-C4, as a modified cannabinoid with unique structural and pharmacological characteristics, is gaining significant importance not only in the medical field but also in industrial and scientific contexts. On the industrial front, it opens new prospects for the development of high-tech products that can be integrated into pharmaceutical, cosmetic, food, and even chemical industries.
The significance of THC-C4 in scientific areas includes fundamental research into cannabinoid mechanisms of action, the development of innovative synthesis methods, as well as creating new technological platforms for testing biological activity.
Industrial applications of THC-C4 are focused on manufacturing pharmaceutical products with improved pharmacokinetic parameters, where dosage precision and component stability are critical. The advantages of THC-C4 compared to classical cannabinoids allow for the development of drugs with a clearer efficacy profile and fewer side effects, increasing competitiveness and opening new niches in the pharmaceutical market. Additionally, THC-C4 has potential uses in the production of cosmetic products, where cannabinoid components are employed as antioxidants and agents that improve skin condition.
Scientific research related to THC-C4 includes chemical analysis, receptor interaction modeling, and experimental evaluations of biological activity. An important aspect is the development of analytical and quality control methods that ensure the standardization of THC-C4-based products. Studying the structure-function characteristics of this cannabinoid contributes to a deeper understanding of the role of the endocannabinoid system in the body and paves the way for creating new therapeutic agents.
In biotechnology, THC-C4 stimulates the development of new approaches in biosynthesis, particularly the use of genetically modified microorganisms and enzymatic systems that optimize the production of this molecule. This enables the creation of scalable and environmentally friendly technologies that meet modern sustainable development requirements. Implementing these technologies into production processes improves the quality of the final product and reduces costs.
It is important to note that the development of industrial and scientific directions related to THC-C4 requires coordinated collaboration between scientists, industry professionals, and regulatory agencies to ensure high standards of quality, safety, and efficacy. Only a comprehensive approach promotes the realization of THC-C4’s potential and ensures its integration into various fields of human activity.
Use in Chemical Research and Pharmacological Development
THC-C4 holds a special place in chemical research due to its modified structure, which allows it to be considered a model compound for studying the interactions of cannabinoids with receptors and biomolecules. Its unique chemical properties open up opportunities for developing new methods of synthesis, analytics, and pharmacodynamics research.
The application of THC-C4 in pharmacological development involves a detailed analysis of its interaction with the endocannabinoid system, including binding specificity to CB1 and CB2 receptors, effects on signaling pathways, and bioavailability. Because of the possibility to modify the side chains of the THC-C4 molecule, scientists gain a tool for creating derivatives with targeted activity, expanding the spectrum of pharmacological effects.
In chemical research, THC-C4 is also used to study cannabinoid metabolism, which helps identify bioactive metabolites and determine the pathways of their formation. This is critically important for developing safe and effective drugs since metabolites often determine the duration of action and toxicity of substances.
Instrumental methods such as nuclear magnetic resonance (NMR), mass spectrometry (MS), and infrared spectroscopy (IR) are employed to study the structural features of THC-C4, providing high accuracy and reliability of the data obtained. These methods allow researchers to track chemical changes in the molecule during synthesis and metabolism, as well as monitor product quality.
An important direction is the use of THC-C4 as a reference standard for developing analytical methods that ensure standardization and quality control in the pharmaceutical industry. This includes the validation of quantitative determination methods and impurity detection, which is vital for the safety of the final pharmaceutical products.
Opportunities for Developing New Drugs
THC-C4 opens broad opportunities for creating new pharmacological drugs thanks to its structural flexibility and unique biological activity. Modifications of the chemical structure of this cannabinoid allow for purposeful alteration of its pharmacodynamic properties, making it possible to develop drugs with specific therapeutic profiles.
New medications based on THC-C4 can be developed to treat a wide range of conditions, including chronic pain, inflammatory processes, neurological disorders, and psychiatric diseases. A distinctive feature is the potential to reduce psychoactive effects while maintaining therapeutic activity, significantly expanding the clinical possibilities of these drugs.
In terms of pharmacokinetics, formulations with improved bioavailability, prolonged action, or targeted delivery to specific tissues can be developed, enhancing treatment effectiveness and safety. For example, encapsulating THC-C4 in nanoparticles or using ligand-targeting systems can provide selective action on affected organs or cells.
Innovative approaches to drug development include combining THC-C4 with other active substances, enabling synergistic effects and reducing the dosage of each component, thereby minimizing the risk of side effects. This approach opens prospects for developing complex therapeutic systems.
Alongside classical pharmaceutical forms, work is ongoing to create cosmetic and dietary supplements based on THC-C4, which can improve quality of life due to antioxidant and anti-inflammatory properties. These products enjoy increasing demand and possess significant commercial potential.
Ethical and Legal Aspects
The ethical and legal aspects concerning THC-C4 form a critically important area for its research, production, and application. The peculiarities of its chemical structure, psychoactive properties, and potential therapeutic use create a complex interplay between scientific innovation, social norms, and legal frameworks. Central issues include patient safety, fair access to therapy, and effective regulation of the substance’s circulation, considering the risks of abuse. Given the lack of a clear global stance on regulating next-generation cannabinoids such as THC-C4, there is a need for systematic analysis of ethical dilemmas and legislative gaps.
The main challenge lies in balancing the stimulation of scientific progress with the protection of public health. The absence of clear regulatory standards contributes to inconsistencies in approaches to research and production, which may lead to the use of substandard or unsafe products. Ethical responsibility for scientists and manufacturers includes ensuring transparency in research methodologies, publication of results, and quality control of final products. Meanwhile, society demands high safety standards, especially regarding psychoactive compounds, necessitating the improvement of risk assessment methods and criteria.
Issues of access to THC-C4 for medical purposes touch on ethical principles of justice and equality. Ensuring patients with severe conditions have access to advanced therapeutic options is part of humanitarian policy, yet often conflicts with stringent legal restrictions. Additionally, ethical concerns include evaluating the long-term consequences of THC-C4 use, particularly potential side effects and risk of dependency, requiring careful monitoring and open communication with users.
An important aspect is intellectual property and patent law related to the synthesis and use of THC-C4. Proper regulation of these issues encourages investment in research and development but also raises debates about monopolization and access to therapeutic agents. Ethical standards call for compromises that promote innovation without limiting public access.
Equally significant is the cultural and social context of THC-C4 use. In different countries, perceptions of cannabinoids range from outright condemnation to broad legalization, complicating the formation of international norms and standards. Ethical reflection involves taking these cultural differences into account when developing policies and regulations to avoid social inequality and discrimination.
Thus, the ethical and legal aspects of THC-C4 constitute a multifaceted field requiring a systematic approach, interdisciplinary cooperation, and continuous revision in light of new scientific data and societal trends.
Legal Status of THC-C4
The legal status of THC-C4 remains unstable and ambiguous on a global scale due to its relative novelty and the lack of unified international standards. In most jurisdictions, legislation focuses on regulating traditional cannabinoids such as Δ9-THC and CBD, while synthetic and structurally modified analogs, including THC-C4, often remain overlooked or fall under general prohibitions.
In some countries, THC-C4 is classified as a controlled substance, prohibiting its manufacture, distribution, and use without special licenses. Legislative acts in these jurisdictions often include provisions that treat substances structurally similar to Δ9-THC as potentially dangerous, regardless of their specific pharmacological properties. This creates challenges for the development of scientific research and commercial use of THC-C4, as the licensing process is complicated and costly.
At the same time, some countries strive to adapt their regulations by introducing more differentiated approaches to the control of new cannabinoids. Legislative initiatives aim to establish classification systems that consider the pharmacological profile, abuse risks, and therapeutic potential of each substance individually. Such changes promote the formation of a more flexible and scientifically grounded regulatory environment that allows innovation while minimizing threats.
Particular attention is given to controlling the import and export of THC-C4, regulated by international agreements such as the United Nations Conventions on Narcotic Drugs. However, the specifics of THC-C4 complicate its precise classification under existing treaties, leading to discrepancies in how different countries control this substance.
At the legislative level, discussions are ongoing regarding the need to create specialized regulatory acts that would address the unique features of synthetic cannabinoids, including THC-C4. The absence of such acts threatens market fragmentation and creates obstacles for international cooperation in science and manufacturing.
Thus, the legal landscape regarding THC-C4 is characterized by a high degree of uncertainty, highlighting the need for further harmonization and improvement of regulatory mechanisms based on current scientific data and practical requirements.
Challenges in Regulation and Control
Regulating THC-C4 involves a number of significant challenges arising at the intersection of scientific, legal, and social factors. The first challenge is the lack of a uniform classification for new cannabinoids, complicating the determination of THC-C4’s status and causing inconsistencies between jurisdictions. The insufficient amount of scientific data on the safety and pharmacological properties of THC-C4 prevents the development of clear control criteria, leading to the application of preventive bans or, conversely, a lack of regulation.
The second significant challenge is the technological progress in the synthesis and modification of cannabinoids, which outpaces the speed of legislative updates. The constant emergence of new structural analogs of THC-C4 complicates market monitoring and creates the risk of uncontrolled substances with potential health hazards. This calls into question the effectiveness of existing control systems and requires the development of flexible, adaptive regulatory models.
The third challenge is maintaining a balance between encouraging scientific research and limiting the risks of abuse. Developing regulations that support innovation while ensuring public safety demands a high level of coordination among regulatory agencies, the scientific community, and manufacturers. The absence of such coordination results in market fragmentation and legal conflicts.
The fourth challenge relates to quality control and standardization of THC-C4-based products. Ensuring product compliance with safety and efficacy requirements necessitates the advancement of analytical methods, laboratory infrastructure, and transparent verification procedures. The lack of unified standards and testing methods complicates market access for quality products and increases the risk of the spread of uncertified substances.
The fifth challenge is social stigmatization and uneven perceptions of cannabinoids across different countries and cultures. This influences legislative initiatives and the level of support for scientific programs, which in turn hinders the development of the regulatory framework and creates additional barriers to integrating THC-C4 into medical practice.
Current Challenges and Research Prospects for THC-C4
Current challenges in researching THC-C4 are primarily related to the limited amount of scientific data on its pharmacodynamics, pharmacokinetics, and long-term effects. Given the relative novelty of this compound, there is a significant shortage of systematic experimental and clinical studies, which complicates the formation of clear scientific recommendations. The absence of standardized models for studying THC-C4 hinders comparison of results obtained between different laboratories and clinical centers. This slows down the determination of accurate therapeutic dosages, safety profiles, and potential interactions with other medications.
Additionally, a major challenge is identifying the metabolic pathways of THC-C4 in the human body, which is critical for assessing its toxicity and duration of action. The low amount of data on metabolic products and their pharmacological activity makes it difficult to predict clinical effects and side effects. The lack of reliable biomarkers for monitoring THC-C4 levels in the blood complicates therapeutic monitoring and safe dosing.
Research prospects are linked to the implementation of advanced analytical methods such as high-resolution mass spectrometry and nuclear magnetic resonance, which allow detailed analysis of the chemical structures and metabolites of THC-C4. The integration of “omics” technologies, including metabolomics and proteomics, opens opportunities for a deep understanding of THC-C4’s interaction with cellular systems, which will aid in identifying new therapeutic targets.
The active development of pharmacogenomics and personalized medicine is also an important direction, allowing adaptation of THC-C4 therapy based on individual genetic characteristics of patients, significantly improving the effectiveness and safety of its use. The application of in silico modeling enables prediction of THC-C4 interactions with various receptors and enzymes, accelerating the development of optimized drugs based on it.
The most promising research involves studying combinations of THC-C4 with other cannabinoids and pharmacological agents to enhance therapeutic effects or reduce side effects. Investigating synergistic or antagonistic interactions may revolutionize approaches to treating complex diseases.
Alongside this, it is important to foster interdisciplinary cooperation among chemists, pharmacologists, clinicians, and legal experts to comprehensively address issues related to THC-C4. Transparency in scientific communication and standardization of research methodologies will support rapid progress in this field.
Technical Barriers in THC-C4 Production
Technical barriers in producing THC-C4 involve the complexity of controlling chemical synthesis, biotechnological processes, and standardization of the final product. First, the low stability of intermediate compounds and high reactivity during THC-C4 synthesis complicate scaling production from laboratory to industrial levels. Chemical reactions require strictly controlled conditions of temperature, pressure, and catalysts, which increases equipment costs and energy consumption.
The second problem is ensuring product purity and selectivity. Manufacturing processes may lead to the formation of isomers or by-products that require complex and costly purification methods. Imperfect analytical quality control methods, especially at the mass production stage, create the risk of releasing low-quality or potentially harmful products to the market.
In biotechnological production involving genetically modified microorganisms or enzymatic systems, challenges include maintaining the stability of biological agents at scales required for commercial production. The complexity of cultivation, low enzyme productivity, and the risk of mutations and degradation of genetic constructs lead to instability in production processes.
Environmental safety and regulatory requirements also pose a significant barrier. Production of THC-C4 using toxic reagents or hazardous catalytic systems demands the implementation of environmentally safe technologies and waste disposal, complicating and increasing the cost of production. International standards such as GMP (Good Manufacturing Practice) and HACCP increase oversight and affect the final product’s price.
Moreover, integrating new technologies like microwave synthesis, enzymatic synthesis in biphasic systems, or enzyme immobilization requires substantial capital investment and scientific-technical support, representing a serious limitation for small and medium-sized enterprises.
Development and implementation of continuous quality control systems and process automation, including the use of artificial intelligence to optimize reaction parameters, can significantly overcome existing barriers. However, these solutions require systemic investments and interdisciplinary expertise.
Scientific Questions Requiring Further Study
Scientific questions concerning THC-C4 focus on uncovering its mechanisms of action, safety, and therapeutic potential. Priority areas include studying molecular targets beyond the classical CB1 and CB2 receptors, as there is evidence of possible interactions with other receptor systems (TRP channels, GPR55) that influence the compound’s pharmacology.
Detailed pharmacokinetic studies are needed, including absorption, distribution, metabolism, and excretion of THC-C4 in various models, as well as the impact of genetic polymorphisms of enzyme systems (e.g., cytochrome P450) on individual responses. These data will allow more accurate predictions of therapeutic effects and toxicity.
Equally important is researching THC-C4’s potential influence on immune processes, since cannabinoids possess immunomodulatory properties that may be beneficial or harmful in different pathologies. Revealing these aspects will enable the development of targeted therapies.
Long-term toxicological studies are also necessary to identify cumulative effects, potential for tolerance development, or dependence. Studying safe dosages and duration of use is particularly relevant for chronic conditions.
Another key direction is the development of effective THC-C4 delivery systems aimed at improving bioavailability and reducing unwanted side effects. This includes nanotechnologies, lyophilized forms, and other innovative pharmaceutical platforms.
Prospects for Integrating THC-C4 into Therapeutic Practice
The prospects for integrating THC-C4 into therapy are linked to its potentially unique pharmacological properties, which could expand the arsenal of treatment options. There is potential for using THC-C4 in managing pain syndromes, neurodegenerative diseases, and psychiatric disorders due to its specific interaction with the endocannabinoid system.
An important aspect is THC-C4’s potential to reduce side effects compared to traditional cannabinoids, allowing its use in a broader range of patients, including those with heightened sensitivity to psychoactive compounds. This opens up opportunities for combination therapy with lower doses of traditional cannabinoids.
Developing standardized treatment protocols with THC-C4 and evaluating its effectiveness across different clinical groups is a key task for its rapid integration into medical practice. This requires the implementation of multicenter clinical trials involving diverse patient populations.
The use of THC-C4 in pediatrics and geriatrics is also promising, where restrictions on other cannabinoids due to toxicity and side effects are especially relevant. Studying safety and efficacy in these age groups will help expand therapeutic options.
Integrating THC-C4 into the healthcare system demands the development of appropriate regulatory frameworks that guarantee drug quality control and patient safety. This also includes training medical personnel on the specifics of working with the new cannabinoid.
Conclusion:
Delta-9-Tetrahydrocannabinol-C4 (THC-C4) is a promising member of the cannabinoid group with unique chemical and pharmacological properties, opening new horizons in scientific research and medical practice. Analysis of existing scientific data and issues related to synthesis, production, pharmacology, and regulatory status of this compound indicates the complexity and multifaceted nature of its study. On one hand, THC-C4 demonstrates significant potential both for the development of new medications and for deeper understanding of the endocannabinoid system and its role in physiological and pathological processes. On the other hand, there are numerous scientific, technical, and legal barriers that must be overcome to ensure the safe and effective use of this cannabinoid.
Scientific research on THC-C4 is still in early stages, particularly concerning its mechanisms of interaction with CB1 and CB2 receptors, as well as potential additional targets that could influence the development of more targeted therapeutic strategies. Insufficient data on pharmacokinetics, metabolism, and long-term safety complicate the determination of precise dosing regimens and prediction of side effects. At the same time, the introduction of modern analytical methods and molecular modeling opens new opportunities for comprehensive study of THC-C4, which supports its further scientific development.
Technical aspects of THC-C4 production also remain challenging due to the complexity of controlling synthetic processes, the need to ensure high purity and product stability, and difficulties related to scaling from laboratory to industrial levels. Although biotechnological methods promise more environmentally friendly and potentially efficient approaches, challenges remain regarding the stability of biological systems and enzyme productivity. Manufacturing difficulties are compounded by strict environmental and regulatory requirements, creating additional barriers to commercialization.
From a pharmacological perspective, THC-C4 holds potential for medical practice, especially in treating chronic pain, neurodegenerative diseases, and psychiatric disorders, where traditional cannabinoids show certain limitations due to side effects. Its more selective receptor interaction may reduce the risk of psychoactive reactions, opening possibilities for use in a broader patient population. However, realizing this potential requires rigorous clinical trials, standardization of treatment protocols, and development of safety monitoring systems.
Beyond medical applications, THC-C4 has significant potential in industrial and scientific fields. Its use in pharmacological development may contribute to creating new classes of drugs with improved properties and expanding knowledge about the functioning of the endocannabinoid system. However, realizing these possibilities depends on overcoming technological barriers and implementing modern analytical and biotechnological approaches.
Legal and ethical aspects of THC-C4 remain complex and ambiguous. The lack of a unified international regulatory framework, unclear legal status, and absence of clear control standards create difficulties for scientists, manufacturers, and medical professionals alike. Developing clear regulatory bases that consider the specifics of THC-C4 is a key factor for its safe integration into medical and social practice. Regulatory challenges are also connected with ethical issues of using psychoactive substances, protecting patient rights, and public safety.
It is also important to note that the prospects for implementing THC-C4 into therapeutic practice depend on close interdisciplinary cooperation, involvement of modern technologies, openness of the scientific community, and adaptation of legislative mechanisms. Only a comprehensive approach combining fundamental research, technical development, and socio-legal solutions can ensure the successful use of THC-C4 to improve patient health and quality of life.
Sources:
- PubChem – THC-C4 (Delta-9-Tetrahydrocannabinol-C4) Profile
https://pubchem.ncbi.nlm.nih.gov/compound/Delta-9-Tetrahydrocannabinol-C4
Detailed chemical information, physicochemical properties, and structural data. - National Center for Biotechnology Information (NCBI) – Cannabinoids and Their Receptors
https://www.ncbi.nlm.nih.gov/books/NBK507798/
Review of the mechanisms of cannabinoid action on the endocannabinoid system. - Journal of Medicinal Chemistry – Synthetic approaches to cannabinoids
https://pubs.acs.org/doi/10.1021/jm00015a001
Scientific review of synthesis methods for various cannabinoids, including modified structures. - Frontiers in Pharmacology – Pharmacology and Therapeutic Potential of Cannabinoids
https://www.frontiersin.org/articles/10.3389/fphar.2018.01263/full
Current research on cannabinoid pharmacology and prospects for their therapeutic use. - Nature Reviews Drug Discovery – Cannabinoid-based medicines and their applications
https://www.nature.com/articles/nrd.2017.94
Overview of the development of cannabinoid-based pharmacotherapy. - ScienceDirect – Biotechnological production of cannabinoids
https://www.sciencedirect.com/science/article/pii/S1369702118302874
Discussion of biotechnological methods for cannabinoid production, including genetically modified organisms. - WHO – Cannabis and cannabinoids: Pharmacology and toxicology
https://www.who.int/medicines/access/controlled-substances/Cannabis_and_cannabinoids_2019.pdf
Official report on the pharmacology, toxicology, and regulatory aspects of cannabinoids. - U.S. Drug Enforcement Administration (DEA) – Controlled Substances and Legislation
https://www.dea.gov/drug-information/cannabis
Information about the legal status and regulations of cannabinoids in the United States. - European Monitoring Centre for Drugs and Drug Addiction (EMCDDA) – Legal status of cannabis and cannabinoids
https://www.emcdda.europa.eu/publications/topic-overviews/legal-status-cannabis-and-cannabinoids_en
Analysis of European legislation regarding cannabinoids. - American Journal of Botany – Genetic and biochemical pathways in Cannabis sativa
https://bsapubs.onlinelibrary.wiley.com/doi/full/10.1002/ajb2.1435
Genetics and biochemistry of cannabinoid formation in cannabis plants.