The molecule Δ9-tetrahydrocannabinol (Δ9-THC) is one of the most well-known, yet simultaneously one of the most controversial and least accurately understood compounds ever discovered in nature. Its chemical stability, biological activity, and pharmacological polyvalence have long elevated this compound beyond a simple natural alkaloid or phytochemical component. Δ9-THC has become the subject of research in neuroscience, molecular biology, pharmacology, natural product chemistry, and also the epicenter of legal, ethical, and social debates. This status is determined not only by the molecule’s ability to affect the human central nervous system but also by its unique mechanism of action, which involves an endogenous signaling system that was relatively recently discovered in the human body-the endocannabinoid system.
Historically, the study of Δ9-THC began with the isolation of the active cannabis component in 1964 by Israeli chemists Raphael Mechoulam and Yechiel Gaoni. However, the use of cannabis as a psychoactive substance and phytotherapeutic agent dates back thousands of years. Archaeological evidence confirms the cultivation of Cannabis sativa as early as the 4th millennium BCE in what is now China. Over time, only the sociocultural narratives changed: from a sacred plant and meditation tool to a criminalized object in the 20th century. Yet the THC molecule itself-a stable, biochemically active, lipophilic structure-remained unchanged, and only human knowledge about its properties gradually expanded.
From a biochemical perspective, Δ9-THC is a tertiary phenol of the cannabinoid class, synthesized in trichomes-microscopic glands on the surface of female Cannabis sativa flowers. In its natural state, cannabis does not contain psychoactive Δ9-THC but rather its acidic precursor-tetrahydrocannabinolic acid (THCA), which undergoes decarboxylation through heat exposure or prolonged storage, converting into the active form. This transition unlocks the understanding of the psychoactive mechanism, which unfolds through Δ9-THC’s interaction with cannabinoid receptors of the first (CB1) and second (CB2) types. This interaction causes effects that researchers classify as changes in affect, cognition, motor coordination, pain perception, appetite, and time perception.
Modern science does not reduce THC solely to a psychoactive agent. On the contrary, with the development of neuropharmacology, biomedical technologies, and molecular diagnostics, a growing range of applications for this molecule in the medical context is emerging. THC’s analgesic, antiemetic, anxiolytic, and appetite-stimulating properties are studied as potential alternatives or supplements to traditional pharmacotherapy, especially in oncology, palliative care, psychiatry, and gerontology. Promising results from clinical studies exist in the treatment of chronic pain, post-traumatic stress disorder (PTSD), cachexia, and glaucoma. All this points to the significant biomedical potential of THC, which has yet to be fully realized, but exclusively within the bounds of rigorous scientific methodology.
At the same time, the chemical industry is developing new methods for synthesizing, extracting, and purifying THC, allowing the production of standardized, pharmacologically predictable preparations. Instead of traditional use of dried plant material, modern laboratories work with pure isolates, distillates, microemulsions, and even nanoforms. Thanks to these technologies, precise dosing, modification of bioavailability, and targeted delivery of the active substance to specific tissues are possible. For example, supercritical CO₂ extraction allows the production of a pure Δ9-THC fraction without residual organic solvents while preserving the terpene profile and the chemical structure’s integrity. Such developments are critical for the pharmaceutical sector, where bioavailability and drug stability are key parameters.
At the same time, the scientific complexity of studying THC cannot be ignored due to its high pharmacodynamic variability. It is known that individual differences in CB1 receptor expression, metabolic enzyme activity (notably CYP2C9 and FAAH), and even gut microbiome significantly influence the subjective and objective effects of the molecule. This creates a need for personalized research and the application of pharmacogenetic approaches in the future.
Currently, the scientific paradigm concerning THC is undergoing a phase of transformation: from a stigmatized substance to an object of systematic interdisciplinary analysis. It is important not to reduce the discussion to a binary dichotomy of “benefit or harm.” Δ9-THC is not simply a “psychoactive substance” or a “medicine”: it is a biologically active molecule that interacts with the complex signaling network of the endocannabinoid system, whose consequences can be both therapeutic and pathogenic depending on dosage, frequency, age, genotype, comorbidities, and many other variables. This multidirectional nature of effects makes simplified interpretations of the molecule impossible.
Moreover, THC is a phenomenon that has not only biochemical dimensions but also technological, social, legal, and philosophical ones. On one hand, it is a chemical compound that can be synthesized in a laboratory or extracted from plant material; on the other hand, it is a substance endowed with a complex historical and regulatory context. In some countries, Δ9-THC is an approved pharmaceutical agent, while in others it is an illegal psychoactive substance. However, no legal status changes the objective chemical nature of the molecule-its pharmacological properties, receptor interactions, or tissue kinetics.
In scientific understanding, it is important not to make any a priori judgments-neither positive nor negative-without strict evidence. That is why modern research on THC focuses not on the moral or political legitimacy of its use but on a precise description of its molecular action, potential risks, and benefits. This approach gradually allows THC to transition from an object of taboo to a full-fledged element of the pharmacopeia and a subject of deep interdisciplinary research.
Chemical Nature of Δ9-Tetrahydrocannabinol
Δ9-Tetrahydrocannabinol (Δ9-THC) belongs to the class of natural lipophilic terpene-phenols unique to the Cannabis genus. Its chemical nature exemplifies a complex bioorganic architecture demonstrating a high level of evolutionary adaptation for interaction with mammalian biological targets. Despite its natural origin as a plant metabolite, Δ9-THC combines features of structural flexibility, stereospecificity, functional multipotency, and pronounced chemical reactivity. It is not merely a pharmacologically active compound – it is a biochemically adapted signaling modulator capable of altering neurobiological patterns through precise and specific interactions with cannabinoid receptors, membrane lipids, ion channels, and numerous enzymatic systems.
The chemical nature of Δ9-THC reflects three fundamental characteristics: a multicomponent cyclic structure, significant hydrophobicity, and the presence of a chiral center. The tricyclic system with an aromatic core and partially saturated rings creates a molecule that is both structurally stable and conformationally dynamic. This enables reversible adaptation when binding to protein structures, particularly hydrophobic pockets of receptors. The core molecular framework of Δ9-THC contains a benzene ring fragment enabling π-π interactions, as well as an isoprenoid-derived fragment – an aliphatic pentyl side chain involved in hydrophobic bonding with protein residues. Such chains play a key role in selectivity and affinity to cannabinoid receptors, as even minimal modifications in this area lead to radical changes in pharmacological profile.
A unique feature of Δ9-THC is its ability to exist as a clearly defined stereoisomer. The natural form is the (-)-trans isomer, possessing a single asymmetric center at the carbon atom in position C9. This means the molecule can exist in two enantiomeric forms, but only one enantiomer shows biological activity at CB1 and CB2 receptors. Chirality in this context is not a secondary characteristic – it is a critical factor determining pharmacological specificity. Studies using chirally pure synthetic analogs have demonstrated that changing the configuration at the C9 position results in more than a 30-fold decrease in receptor affinity, even when all other structural elements remain unchanged.
Δ9-THC is chemically an amphiphilic compound but with markedly predominant lipophilic properties. Its partition coefficient logP exceeds 6, making it one of the most hydrophobic compounds among natural plant-derived products with pharmacological activity. This property results in several important biochemical consequences: firstly, rapid accumulation in neuronal membrane structures and adipose tissue; secondly, slowed elimination from the body; and thirdly, the ability to cross-interact with other lipophilic agents, including pharmaceuticals. It is precisely because of Δ9-THC’s exceptional lipophilicity that it exhibits a tendency for nonlinear pharmacokinetics, especially with chronic use when tissue depots become saturated.
Another important aspect of Δ9-THC’s chemical nature is its relative instability in environments rich in oxygen, light, and heat. Oxidative degradation of the molecule – mainly under ultraviolet radiation – leads to the formation of cannabinol (CBN), which has significantly lower receptor affinity and virtually no psychoactive effect. It is important to note that this process is not instantaneous but accumulates gradually, which is why aged cannabis samples have altered chemical profiles. For this reason, chemical-analytical identification and quantitative determination of THC must take into account the degree of degradation and isomerization occurring during sample storage or processing.
Δ9-THC also exhibits affinity for interaction with metal ions, particularly Mg²⁺ and Zn²⁺, which may be relevant in the context of enzymatic transformation or bioaccumulation. In vitro studies show that Δ9-THC can be a substrate for cytochrome P450 systems, especially the CYP2C9 and CYP3A4 isoenzymes, which carry out oxidative metabolism of the molecule in the liver. The main metabolites are 11-hydroxy-THC (pharmacologically active) and 11-nor-9-carboxy-THC (inactive but metabolically stable). This means that the chemical nature of Δ9-THC is not limited to its structure at the moment of absorption but includes the full spectrum of its transformations in the biological environment.
Another lesser-known aspect of Δ9-THC’s chemical nature is its ability to aggregate in lipid environments. Studies using atomic force microscopy and fluorescence spectroscopy have shown that under certain concentration conditions, THC can form nanovesicles or lipid-like domains capable of modulating the rheological properties of membranes. This opens a new direction for studying membrane-active properties of cannabinoids, which was previously almost unexplored.
From a chemical perspective, Δ9-THC is also a compound with high potential reactivity. Alongside the aforementioned degradation reactions, the molecule can undergo conjugation with glutathione, glucuronic acid, or sulfate groups – processes critical for its detoxification and elimination. These reactions convert the hydrophobic molecule into more water-soluble derivatives that can be transported by blood plasma or excreted through the kidneys. This points to a complex, multistep interaction of Δ9-THC’s chemical nature with the body’s detoxification systems.
The chemical nature of Δ9-THC also defines its interactions with other phytocannabinoids. Studies of synergy and antagonism with cannabidiol (CBD), cannabigerol (CBG), and cannabichromene (CBC) indicate that the presence of THC in a mixture with other cannabinoids alters its biological activity. Part of this is explained by changes in physicochemical properties – for example, CBD can modify THC’s solubility in aqueous environments, thermal stability, or metabolic pathway.
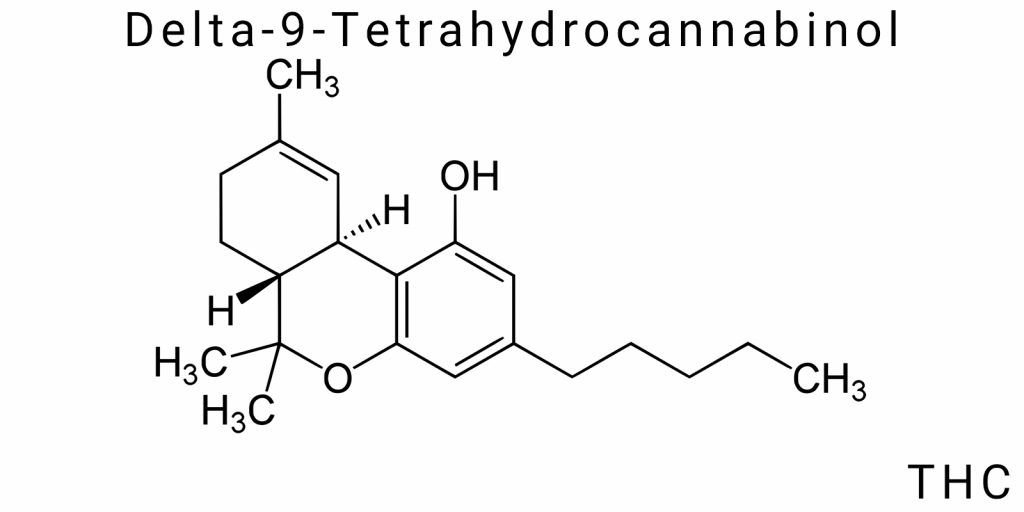
Structural Formula and Key Properties
Δ9-Tetrahydrocannabinol (Δ9-THC) is a high-molecular-weight compound with a branched structural organization, based on a tricyclic system formed by the fusion of an aromatic ring, a partially saturated cyclopentane, and a tetrahydropyran ring. This architecture is atypical among natural metabolites because it combines features of phenolic, terpenoid, and polyketide origins, indicating a complex biosynthetic evolution and deep functional specialization. The structure of Δ9-THC can be represented by an expanded constitutional formula clearly showing the localization of key functional groups, including the phenolic hydroxyl, tertiary alcohol, and methylene segments of the pentyl side chain attached to the aromatic ring at the C3 position.
The phenolic hydroxyl group at the C1 position plays a crucial role in acid-base equilibria, hydrogen bonding, and potential metabolic oxidation. Meanwhile, the tertiary hydroxyl group located in the alicyclic ring is less reactive but serves as a key site for phase II metabolism, particularly conjugation with glucuronic acid. The opposite side of the molecule is formed by the pentyl side chain-a flexible, open alkyl fragment that interacts with the hydrophobic pockets of cannabinoid receptors. Its length and branching are critical parameters: variations as small as a single methylene unit radically alter affinity for CB1 and CB2 receptors, as confirmed by numerous Structure-Activity Relationship (SAR) studies.
Another fundamental structural element is the double bond at the Δ9 position (between carbon atoms C9 and C10). This fragment defines the compound’s classification as a Δ9-isomer, as opposed to the less common Δ8- or Δ10-forms, which have altered pharmacological properties. The position of this π-bonded system affects the planarity of part of the molecule and its capacity for electron delocalization, which in turn influences affinity for membrane receptors. The molecule’s three-dimensional configuration is semi-rigid: while some bonds in the aliphatic regions allow free rotation, the presence of cyclic fragments significantly restricts conformational flexibility. This creates a unique interaction profile with biological targets, distinguishing Δ9-THC from simpler, more flexible lipophilic molecules.
Thermodynamic parameters of THC, such as melting point (~66°C), boiling point (157°C at 0.05 mm Hg), and low water solubility (<0.003 g/L), reflect its amphiphilic yet distinctly lipophilic nature. Δ9-THC’s ability to sublimate under reduced pressure and its instability under prolonged heating or UV irradiation are linked to the delicate balance between conjugated and saturated regions of the molecule. This balance also defines its spectral properties: a UV absorption maximum around 270-280 nm, along with specific vibrational modes in the IR spectrum reflecting phenolic, aliphatic, and ether vibrations.
In solution, Δ9-THC can form specific intermolecular associates, especially in nonpolar environments where hydrophobic aggregation leads to the formation of microclusters. This effect is nontrivial-it alters solubility, diffusion, and even pharmacokinetic parameters upon transdermal or inhalational administration. It is also important to note that the conformational stability of the molecule increases in lipid surroundings, which partly explains its tropism for lipid domains in biomembranes and high affinity for neuronal receptors embedded in the phospholipid bilayer.
Molecular formula: C₂₁H₃₀O₂
The molecular formula of Δ9-THC – C₂₁H₃₀O₂ – reflects the exact quantitative composition of elements forming this complex organic compound. This formula demonstrates the presence of 21 carbon atoms, 30 hydrogen atoms, and 2 oxygen atoms, corresponding to a relative molecular mass of about 314.45 g/mol. It is important not to reduce it to a mere numerical configuration, since each of these atoms participates in defining the molecule’s functional and physicochemical properties. For example, out of the 21 carbon atoms, at least 15 contribute to the formation of cyclic structures or conjugated systems critical for activity at CB1/CB2 receptors.
The presence of two oxygen atoms in the molecule is not simply functional groups but strategically located atoms forming the phenolic hydroxyl (-OH) and the tertiary alcohol. Both centers have electron-donating character, influence local electron density within the molecule, and serve as potential sites for chemical modification or biotransformation. Isotopic composition should also be considered-the presence of stable isotopes like ¹³C or ²H (deuterium) can be used for labeling the molecule in metabolomic studies or pharmacokinetic analysis via mass spectrometry.
C₂₁H₃₀O₂ represents a formula combining terpene and phenolic nature: 15 out of 21 carbon atoms originate from isoprene units, evidencing the terpene backbone of the molecule, while the other 6 are derivatives of the phenolic core and pentyl side chain. This hybrid character determines the unique behavior of Δ9-THC in biological systems, including its ability to cross-interact with other secondary metabolites that share a similar elemental formula but differ in spatial structure.
The molecular formula also dictates the potential for ionization, although Δ9-THC practically does not ionize at physiological pH. This is due to the absence of strongly acidic or basic groups, which lowers the capacity for electrostatic interactions but enhances membrane permeability through lipids. Moreover, the limited number of oxygen atoms reduces molecular polarity, directly affecting the partition coefficient between organic and aqueous phases.
The formula C₂₁H₃₀O₂ also imposes constraints on the range of possible reactions in which THC can participate. For instance, halogenation, etherification, acylation, or oxidation reactions can occur only at certain molecular regions. This is critically important for the development of semi-synthetic THC derivatives with modified bioavailability or receptor specificity. Thus, the basic molecular formula forms the foundation for structural modification and chemical redesign in the pharmaceutical context.
Structure, Chirality, Lipophilicity
Δ9-THC is a typical example of a chiral bioactive compound with pronounced lipophilic characteristics and structural complexity that define its biochemical behavior. The chiral center is located at the C9 position, where the carbon atom has four different substituents, creating the possibility of two enantiomers. The naturally occurring isomer is the (-)-trans form, which possesses affinity for the CB1 and CB2 receptors, whereas other enantiomers, although they can be synthesized artificially, lack significant biological activity.
The presence of chirality in Δ9-THC critically influences its pharmacodynamics because the CB1 receptor has a chiral binding pocket sensitive to the ligand’s configuration. This means that only the correct spatial orientation enables full intermolecular interaction. The location of the chiral center also determines the specificity of the molecule’s binding within the lipid layer of biomembranes, as only one enantiomer can adopt a thermodynamically stable conformation when embedded in the phospholipid bilayer.
The lipophilicity of Δ9-THC is pronounced and multifaceted. A high logP value (greater than 6) indicates the dominance of nonpolar regions in the molecule, which facilitates passive diffusion across membranes. This is especially important for penetration through the blood-brain barrier, underlying the central effects of THC. However, this lipophilicity causes retention in the body, accumulation in adipose tissue, and a long half-life. Additionally, it creates challenges for water-soluble pharmaceutical formulations, stimulating the search for lipid carriers or nanotransport systems for THC delivery.
The interrelationship between structure, chirality, and lipophilicity forms the foundation of Δ9-THC molecular pharmacology. It is precisely this triad that allows the molecule to function as a natural bioactive agent capable of specific, flexible, and adaptive binding with a wide range of cellular targets.
Biosynthesis of THC in Cannabis sativa
The biosynthesis of Δ9-Tetrahydrocannabinol (THC) in the Cannabis sativa plant is a complex multi-step process involving numerous enzymatic reactions within specialized cellular structures. The main biochemical pathway for THC formation is the cannabinoid pathway, which begins with the synthesis of precursors from general cellular metabolic networks and culminates in the formation of the cannabinoid acid-tetrahydrocannabinolic acid (THCA)-which subsequently converts to psychoactive Δ9-THC upon heating. The corresponding enzymes are primarily localized in the plant’s glandular oil-producing trichomes, where characteristic crystalline structures responsible for cannabinoid accumulation and concentration form.
The primary biochemical route starts with the condensation of geranyl pyrophosphate (GPP) and olivetolic acid, catalyzed by the enzyme cannabinoid synthase. The result of this reaction is the formation of cannabinoid acids, notably the THCA precursor. THCA then serves as a matrix for the formation of active THC, which is one of the main psychoactive components. Importantly, cannabinoid biosynthesis is a tightly regulated process influenced by genetic factors, environmental conditions, and the plant’s developmental stage.
THCA production mainly occurs in specialized epidermal cells of the trichomes-microscopic glandular structures that form a protective barrier while simultaneously accumulating active metabolites. The trichomes’ ability to synthesize and store cannabinoids determines the concentration of Δ9-THC in the final product, and their morphology and density are key selection criteria for strains with increased psychoactive content.
The decarboxylation process, i.e., the chemical conversion of THCA to Δ9-THC, occurs under the influence of heat or prolonged storage. This reaction involves the loss of a carboxyl group (CO₂), altering the chemical structure and activating the molecule’s ability to bind cannabinoid receptors in the human body. Incomplete or disrupted decarboxylation can significantly reduce the pharmacological activity of the final product. Therefore, optimizing this process is a crucial step in the production of medical and recreational Cannabis sativa products.
Cannabinoid Biosynthesis Pathway
The cannabinoid biosynthesis pathway is one of the most extensively studied metabolic routes in plants, defining the formation of a unique group of tetrahydrocannabinol compounds. It begins with the condensation reaction of the isoprenoid unit geranyl pyrophosphate with the phenolic precursor olivetolic acid, producing cannabigerolic acid (CBGA), which is a key intermediate. CBGA, in turn, serves as a common precursor for various cannabinoids, including THCA, CBDA (cannabidiolic acid), and CBCA (cannabichromenic acid).
The enzymatic conversion of CBGA to THCA occurs under the action of the enzyme THCA synthase, which is a highly selective oxidoreductase catalyzing the cyclization and oxidation of the molecule. Studying the structure and mechanism of this enzyme helps to understand the molecular specificity of psychoactive cannabinoid formation. Genetic variations in the enzyme, expression of relevant genes, as well as external factors such as temperature and light conditions, significantly influence the final yield of THCA.
In the context of metabolic regulation, the cannabinoid pathway interacts with other metabolic branches such as the terpene and phenolic pathways, which supply substrates and define the plant’s chemical profile. This integration ensures not only the synthesis of THC but also the formation of a complex of accompanying secondary metabolites that modulate the pharmacological activity of the final product.
Role of Oil Glands (Trichomes)
Oil glands, or trichomes, are microscopic structures on the surface of Cannabis sativa that act as bioreactors for the synthesis, accumulation, and storage of cannabinoids, including THC. They exist in three main forms: needle-like, leaf-like, and capitate trichomes, with the capitate type being primarily responsible for producing the oily resin extract.
The morphology of trichomes includes a glandular head filled with lipophilic resin rich in cannabinoids and terpenoids. These resins protect the plant from pathogens, ultraviolet radiation, and drying out. The level of trichome development directly correlates with the plant’s capacity to produce THC, making the selection and cultivation of plants with high trichome density a strategic goal for increasing the yield of psychoactive components.
Biochemical processes inside trichomes include high expression of enzymes from the cannabinoid pathway, coenzyme synthesis, and active transport activity that localizes metabolites within secretory vacuoles. Trichomes also act as a barrier, isolating toxic metabolic products from the plant’s main tissues, minimizing their impact on physiological functions.
THCA → Δ9-THC: Decarboxylation Process
The conversion of the cannabinoid acid THCA into its active form Δ9-THC is a chemical decarboxylation process characterized by the loss of a carboxyl group (-COOH) in the form of carbon dioxide. This reaction is critical for activating the psychoactive properties of the cannabinoid since THCA itself does not interact with cannabinoid receptors in the human body.
Decarboxylation occurs when THCA is heated to approximately 105-145 °C, which can happen naturally through exposure to sunlight heat, during drying of the plant material, or artificially in processing facilities. The reaction kinetics depend on temperature conditions, humidity, and treatment duration. Excessive heating can lead to further degradation of THC into cannabinol, which has reduced pharmacological potential.
At the molecular level, decarboxylation causes a change in the molecule’s electronic configuration, increasing its affinity for CB1 receptors in the brain, and consequently enhancing the psychoactive effect. Understanding the detailed mechanism of this process is crucial for optimizing extraction methods and production of medicinal products containing THC.
Production: From Plant to Active Molecule
The process of transforming cannabis from biological raw material into a highly specific active substance-Δ9-tetrahydrocannabinol-is a multi-component procedure that encompasses the entire chain: from cultivating botanical material to obtaining a pharmaceutically suitable substrate with controlled parameters of purity, stability, and bioavailability. In the context of this transformation, it is important to consider not only the technological aspect but also the biochemical, agronomic, and even regulatory factors, as these directly influence the qualitative composition of the final product.
The initial stage of the production cycle is the cultivation of Cannabis plants, which, unlike many other medicinal crops, exhibit a high degree of chemotypic variability. Even within a single species, significant differences in the profile of secondary metabolites can be observed, including the quantitative ratio of Δ9-THC, cannabidiol (CBD), cannabigerol (CBG), and other cannabinoids. This chemovariability is determined not only by genetic factors but also by cultivation conditions such as photoperiod, temperature, humidity, pH, soil composition, and intensity of ultraviolet radiation. Practice shows that even minor fluctuations in agronomic parameters can lead to changes in the cannabinoid profile, particularly Δ9-THC concentration, by tens of percent. That is why modern production approaches require full standardization of growing conditions, often using closed greenhouse systems, hydroponics, or aeroponics with computerized control of every stage.
After cultivation and harvesting of the plant biomass, a key task is stabilizing the raw material and preventing cannabinoid degradation. This involves careful drying at a temperature that does not exceed the decarboxylation threshold of THCA, which is the inactive acidic form of Δ9-THC. Drying must be sufficient to prevent microbial growth, yet not so intense as to promote terpene degradation or initiate conversion of THCA to Δ9-THC before this process is intentionally controlled at the next stage. In this context, the stability of trichomes-specialized glandular structures where cannabinoids are synthesized and accumulated-is also critical. Excessive mechanical or thermal disturbance of trichome structure leads to loss of bioactive components, reducing the yield of the target substance.
The next stage is extraction itself-the isolation of Δ9-THC from the overall matrix through selective disruption of cell walls and transfer of active compounds into a liquid or semi-solid medium. Various technologies are used at this stage, differing significantly in efficiency, specificity, safety, and environmental impact. Traditionally, mechanical methods such as sieving dry material (to obtain kief) or manual pressing (hashish) have been used. In an industrial context, these methods are considered insufficiently effective as they do not provide the necessary concentration and purification level of Δ9-THC. More advanced solvent-based methods employ polar or non-polar extractants (ethanol, butane, hexane, supercritical CO₂) followed by filtration, distillation, and fractional purification. Modern approaches also include chromatographic techniques, molecular distillation, and supercritical fluid extraction, enabling pharmaceutical-grade purity without residual toxic solvents.
The obtained extract mostly contains Δ9-THC in its acidic form-THCA-which is pharmacologically inactive. The conversion of THCA to Δ9-THC is carried out by controlled decarboxylation-a thermal process that breaks down the carboxyl group, forming the active molecule and releasing carbon dioxide. This process critically depends on temperature and heating duration: an optimal temperature is considered to be about 110-120°C for 30-60 minutes, though in industrial settings, more precise time-temperature protocols may be used, often under vacuum to prevent oxidation. As a result of this stage, the exact target form of Δ9-THC is produced, which has high bioavailability and receptor affinity.
The final stage of production involves standardization, analysis, and stabilization of the resulting substance. This includes quantitative determination of Δ9-THC by HPLC or GC-MS (high-performance liquid or gas chromatography), assessment of residual solvents, heavy metals, microbiological contaminants, and terpene profile. The product obtained at this stage can be presented as a concentrate, isolate, resin, or microemulsion-depending on its intended use (pharmaceutical, nutraceutical, or recreational).
Source: Botanical Aspects of Cannabis sativa and Cannabis indica
The Cannabaceae family includes several botanical species, the most well-known of which are Cannabis sativa and Cannabis indica. These species are the primary sources for the production of Δ9-tetrahydrocannabinol (Δ9-THC) and exhibit significant differences in morphology, chemical composition, and biological activity. First and foremost, it is important to note that the biological differences between these species are related not only to classical botanical traits but also to the differential regulation of secondary metabolite synthesis, which determines their use in medical, industrial, and recreational fields.
Cannabis sativa is historically associated with northern and temperate climate zones, characterized by taller plants with narrow leaves and less dense inflorescences. This species has a longer vegetative cycle, which can influence the final Δ9-THC content. Biochemically, sativa tends to produce a higher amount of psychoactive Δ9-THC relative to other cannabinoids, though significant variations exist depending on the genotype. From a pharmacokinetic and pharmacodynamic perspective, products derived from sativa are often characterized by a more stimulating effect, which is attributed to the cannabinoid and terpene ratios as well as interactions with central nervous system receptors.
Cannabis indica, in contrast, features a more compact stem, broader leaves, and denser inflorescences. This species is better adapted to high-altitude and subtropical climates, which is reflected in a shorter flowering cycle and more intense biosynthetic activity of secondary metabolites. A typical characteristic of indica is a generally higher CBD content relative to Δ9-THC, resulting in a more pronounced sedative effect in final products. These properties are fundamentally important for pharmacotherapy, especially in cases requiring anxiety reduction, pain relief, and sleep improvement.
The genetic and chemotypic diversity of both species determines not only physical parameters but also the productivity of Δ9-THC synthesis. Recent molecular genetic studies have shown that the regulatory mechanisms of the THCAS gene, which encodes the enzyme tetrahydrocannabinol synthase, differ between sativa and indica, supporting the concept of their separate evolution and adaptation to different ecological niches. As a result, the concentration and chemical profile of Δ9-THC can vary significantly not only between species but also among populations within a species.
Morphological Differences
The morphological features of Cannabis sativa and Cannabis indica not only define the plants’ external appearance but also affect metabolism and accumulation of active substances, particularly Δ9-THC. Sativa is characterized by tall stalks that can reach 4 to 6 meters and narrow, palmate leaves with long, thin fingers. This provides effective light capture in open spaces and during extended photoperiods. Sativa inflorescences are typically more sparse, with lower trichome density, which potentially influences the concentration of cannabinoids in the plant.
Indica, on the other hand, has a shorter, bushier form with broader leaves that are thicker and larger in size. Its inflorescences are more compact and densely covered with resinous trichomes, which ensures increased accumulation of cannabinoids and terpenes. This morphology reflects adaptation to shorter photoperiods and more extreme climatic conditions, particularly in high-altitude regions. Morphological differences also influence the ratio of leaf surface area to stem volume, which is reflected in metabolic processes and accumulation of bioactive compounds.
Additionally, differences in trichome structure between species are known as a key factor determining Δ9-THC productivity. Indica trichomes are more numerous and have a higher concentration of enzymes responsible for cannabinoid synthesis, contributing to increased active molecule concentration. Meanwhile, sativa trichomes are more sparse, explaining the lower average Δ9-THC content in the raw material. This morphological basis is important for agronomic selection of strains aimed at optimizing Δ9-THC production.
At the molecular level, morphological characteristics also determine regulation of genes involved in trichome development and cannabinoid metabolism. However, it should be noted that morphological differences do not always correlate with chemotypic diversity, since environmental factors can modify gene expression and alter the chemical profile of the plants. Thus, morphology is an important but not exclusive characteristic defining the botanical features of the Δ9-THC source.
THC Content Depending on Strain, Soil, and Agronomy
The concentration of Δ9-THC in Cannabis plants is a dynamic value largely dependent on the combination of genetic and environmental factors, as well as agronomic management methods. Significant variation in Δ9-THC productivity exists at the strain level, reflecting breeding programs aimed at enhancing therapeutic or recreational value of the plants. Some strains are characterized by extremely high Δ9-THC content exceeding 20-25%, while others focus on balance with other cannabinoids, particularly CBD.
Soil conditions play a critical role in determining Δ9-THC accumulation levels, as mineral composition, structure, aeration, and water retention capacity influence metabolic processes in the plant. For example, nitrogen deficiency may reduce vegetative growth but stimulate increased synthesis of secondary metabolites, including Δ9-THC. Conversely, excess nitrogen is often associated with decreased cannabinoid concentration. Similarly, levels of phosphorus, potassium, calcium, and micronutrients directly affect the functioning of enzymatic systems that determine biosynthetic productivity.
Agronomic practices-such as irrigation regimes, lighting, planting density, and use of growth stimulators-also determine the final Δ9-THC content. Light intensity, especially in the UV-B spectrum, stimulates cannabinoid synthesis by activating plant defense systems against ultraviolet stress. In response to these conditions, plants increase production of resinous substances, primarily Δ9-THC. Control of air and soil humidity must ensure optimal conditions for trichome growth and prevent disease development that could reduce raw material quality.
Methods of Δ9-THC Extraction
Extraction of Δ9-tetrahydrocannabinol (Δ9-THC) is a critical step in the production of both medical and recreational cannabis-based products. The technological efficiency, purity, and yield of the active molecule depend on the chosen extraction method. Historically, extraction was based on simple mechanical principles, which over time evolved into more complex chemical and physico-chemical processes that take into account the chemical nature of Δ9-THC, its lipophilicity, and thermal sensitivity. Each method has its own advantages and disadvantages, as well as specific applications depending on the final production goals, scale, and regulatory requirements.
The foundation for effective extraction is the proper selection of raw materials, degree of grinding, temperature, and duration of the process. Δ9-THC, as a fat-soluble and thermosensitive cannabinoid, requires conditions that prevent its degradation or transformation into other forms, such as Δ8-THC or cannabinol. Accordingly, optimizing extraction methods involves balancing maximum extraction efficiency with maintaining molecular stability.
Modern technological advancements involve transitioning from traditional, often low-efficiency methods, to high-tech systems that include the use of supercritical fluids, ultrasound, and membrane purification processes. At the same time, basic mechanical and chemical methods remain widely used due to their simplicity, equipment availability, and economic benefits. Understanding their mechanisms of action and limitations is important for a comprehensive approach in industrial-scale production.
Traditional (Mechanical) Methods
Traditional mechanical methods for extracting Δ9-THC rely on physically separating resinous substances and trichomes from the plant matrix without using chemical solvents. These methods were widely used long before complex technologies emerged and have persisted as basic approaches due to their ease of implementation and minimal environmental impact.
One of the most common mechanical methods is sieving or filtering frozen, ground plant material through screens of varying sizes. This process allows separation of the resin, which contains a significant concentration of Δ9-THC, in the form of so-called “kief” or dry cannabis dust – a concentrate of trichomes. Maintaining low temperatures is a crucial condition to minimize the loss of volatile components and prevent thermal degradation of Δ9-THC.
Another traditional method is pressing (cold or hot), during which mechanical pressure is applied to the plant mass to extract oils rich in Δ9-THC. Although this method produces a concentrate free of chemical contaminants, it does not ensure high purity because hydrocarbons, cellular debris, and other plant components can be co-extracted alongside active substances.
The use of ice, water, and mechanical agitation in the form of water extraction is also considered a traditional method. This technique is based on the ability of resinous trichomes to separate under the influence of cold water and agitation, allowing the production of concentrates known as “hash” or “bubble hash.” This method does not involve chemical reagents but is more labor-intensive and less scalable for industrial production.
The drawbacks of mechanical methods include a lower concentration of the final product, the presence of impurities, and significant loss of active substance due to incomplete extraction or degradation during the process. However, they remain attractive for small-scale producers and consumers who prioritize naturalness and environmental friendliness.
Chemical (Solvent-Based) Methods
Chemical extraction of Δ9-THC uses organic solvents to dissolve and extract the active molecule from plant tissue. This method dominates at the industrial scale due to its high efficiency, process control, and the ability to produce concentrates with high purity.
Among solvents, the most common are butane, ethanol, isopropanol, supercritical carbon dioxide, as well as hexane and acetone. The choice of solvent is determined by the chemical properties of Δ9-THC, particularly its lipophilicity, and the requirements for the final product. For example, butane allows rapid extraction with minimal impact on the cannabinoid structure but is associated with fire hazards and residual solvents. Ethanol is considered a safer and more environmentally friendly option but often extracts water-soluble components along with Δ9-THC, complicating further purification.
The extraction process typically consists of several stages: maceration or soaking of plant raw material with the solvent, extraction of active components, filtration, and solvent evaporation. It is crucial to strictly control temperature and contact time to avoid thermal degradation of Δ9-THC and side reactions that may alter the product profile.
Supercritical carbon dioxide as a solvent is an innovative approach that combines the physical properties of gas and liquid, allowing efficient extraction of Δ9-THC at low temperatures without residual impurities. This technology offers high selectivity, adjustable by pressure and temperature, making it especially valuable for obtaining pharmacologically pure extracts. However, capital investment and equipment complexity are barriers to its widespread use.
Chemical extraction provides better concentration of Δ9-THC compared to mechanical methods but requires careful purification of the product from solvents and impurities, which is critical for the safety of final products.
Modern Purification Technologies
After extraction, the next important step is the purification of Δ9-THC concentrates from impurities such as byproducts, solvents, chlorophyll, lipids, and waxes. Modern purification technologies are based on physico-chemical methods that allow for maximum preservation of the structure and activity of Δ9-THC while simultaneously increasing the purity and stability of the final product.
One of the main directions involves the use of chromatographic methods, including high-performance liquid chromatography (HPLC) and column chromatography, which provide selective separation of cannabinoids and impurities based on different physico-chemical properties. These methods are widely applied in the production of medical formulations where standardized concentrations of Δ9-THC are required.
Membrane technologies are rapidly developing, based on the selective permeability of molecules through semi-permeable membranes. They allow effective separation of Δ9-THC from solvents and impurities with low energy consumption and without chemical reagents. Such systems enhance the environmental friendliness of the process and simplify scaling.
Ultrasonic purification uses high-frequency sound waves to break down impurity structures and increase the solubility of target substances, accelerating and improving the quality of purification. Combined approaches using ultrasound and chromatography open new prospects for producing high-purity extracts.
Thermal methods, such as vacuum distillation and rectification, are employed to remove volatile solvents and condense Δ9-THC to obtain highly concentrated products. They require precise temperature control to prevent isomerization and degradation of the molecule.
Mechanism of Action: How Δ9-THC Interacts with the Human Body
The interaction of Δ9-tetrahydrocannabinol (Δ9-THC) with the human body is a complex biochemical process fundamental to understanding the pharmacology of this molecule. Δ9-THC is a lipophilic compound that easily penetrates cell membranes, allowing it to interact with a range of intracellular and membrane components. Its mechanism of action significantly differs from traditional pharmacological agents because it affects a specific receptor system that remained unknown before the discovery of the endocannabinoid system.
The basis of Δ9-THC pharmacodynamics is its ability to act as an agonist at cannabinoid receptors distributed throughout the nervous system and peripheral tissues. This interaction modulates neurotransmission by influencing the release of many neurotransmitters, including dopamine, gamma-aminobutyric acid (GABA), glutamate, and serotonin. Because of this, Δ9-THC can alter cognitive processes, perception, memory, as well as physiological responses in the body.
It is important to note that Δ9-THC is not a full agonist of these receptors but has partial agonist activity, which defines its unique action profile. It can trigger a complex cascade of intracellular events, including activation of G-protein-coupled receptors, leading to inhibition of adenylate cyclase, reduction of cyclic adenosine monophosphate (cAMP) levels, and influence on ion channels. This, in turn, results in modulation of neuronal electrical activity and changes in neurotransmitter balance.
Δ9-THC also interacts with receptors located outside the central nervous system, particularly in the immune system, explaining its influence on inflammatory processes and immune homeostasis. Additionally, other molecular targets are being studied, including TRPV1 receptors involved in pain signal transmission, expanding the pharmacological spectrum of Δ9-THC.
The movement of Δ9-THC within the body is characterized by rapid absorption upon inhalation and slower but more prolonged effects when taken orally. The lipophilicity of the molecule facilitates its accumulation in fat tissues and subsequent slow release, influencing pharmacokinetic parameters and duration of action. Δ9-THC metabolism occurs primarily in the liver through the cytochrome P450 system, producing active and inactive metabolites with varying effects on the body.
The result of these molecular processes is a complex range of biological effects, including psychoactive manifestations, altered sensory perception, and physiological impacts such as analgesia, antiemetic action, appetite modulation, and anti-inflammatory effects. The features of Δ9-THC’s mechanism of action determine its clinical applications and potential risks associated with long-term use.
Endocannabinoid System
The endocannabinoid system is a molecular platform that provides integrated communication between the human nervous, immune, and endocrine systems. It functions as a dynamic feedback mechanism that continuously maintains homeostasis – the state of biological balance necessary for the proper function of tissues and organs amid changing environmental conditions. The central importance of this system lies in its ability to regulate synaptic transmission, neuroinflammation, energy metabolism, as well as processes such as motivation, memory, pain, sleep, and immune response.
Unlike classical neurotransmitter systems, the endocannabinoid system primarily operates on a retrograde principle: endocannabinoids are synthesized “on demand” in postsynaptic neurons and then diffuse backward across the synaptic cleft to interact with presynaptic receptors, altering the probability of neurotransmitter release. This mechanism makes the system a unique regulatory matrix that provides fine-tuning of neuronal networks and local regulation of the cellular environment. The main endogenous ligands of the system include anandamide (AEA) and 2-arachidonoylglycerol (2-AG), both derivatives of arachidonic acid that act as transient, short-lived signaling molecules. The synthesis and degradation of these molecules are strictly controlled by enzymatic systems such as NAPE-PLD, DAGL, FAAH, and MAGL, which coordinate the appearance and disappearance of endocannabinoids at precise moments.
The regulatory potential of this system is so flexible that it can both suppress and stimulate physiological processes depending on the tissue context, cell type, homeostatic state, and presence of external stimuli. This plasticity is ensured by a wide range of effector mechanisms through which endocannabinoids influence intracellular signaling pathways, including MAP kinases, PI3K/AKT, NF-κB, and others. Thus, the ECS becomes not just a local signaling system, but a multifunctional signaling network coordinating whole-organism responses.
CB1 and CB2 Receptors: Localization and Function
CB1 and CB2 receptors are key structural components of the endocannabinoid system, executing its signaling functions through activation of G protein-coupled cascades. They differ not only structurally but also in expression patterns, ligand affinity, and functional roles. CB1 receptors predominate in the central nervous system and play a leading role in regulating synaptic transmission, while CB2 receptors are mainly found in immune system cells and have a distinct role in controlling inflammation and immune modulation.
CB1 receptors are highly expressed in regions such as the hippocampus, basal ganglia, cortex, cerebellum, and spinal cord. Their localization is responsible for controlling motor function, cognition, emotional responses, and sensory perception. At the synaptic level, CB1 receptors are located on presynaptic terminals where they inhibit the release of excitatory (glutamate) or inhibitory (GABA) neurotransmitters. Thus, CB1 mediates local feedback “braking,” reducing excessive neuronal activity. Molecularly, this occurs through activation of Gi/o proteins, which inhibit adenylate cyclase, lower cAMP levels, activate K+ channels, and block Ca2+ channels, leading to neuronal hyperpolarization.
CB2 receptors are predominantly located in cells of the peripheral immune system: macrophages, neutrophils, microglia, T-lymphocytes, dendritic cells, as well as cells in the spleen, tonsils, and bone marrow. Their function involves modulation of pro-inflammatory and anti-inflammatory cytokine production, activation of phagocytosis, control of immune cell proliferation, and apoptosis. Although CB2 receptors are rarely found in the central nervous system under normal conditions, their expression can increase during neuroinflammation or pathology, opening new therapeutic opportunities for neurodegenerative and autoimmune diseases.
The affinity of Δ9-THC for both receptor types confirms its ability to affect both neurophysiological and immune functions. However, CB1 receptors are the primary targets for psychoactive effects, whereas CB2 receptors mainly mediate peripheral immune action without pronounced changes in consciousness. This dichotomy is highly significant for pharmacology: it allows the development of selective molecules that interact only with one receptor subtype to achieve desired therapeutic effects with minimal side effects.
Agonistic Action of THC
Δ9-THC acts as a partial agonist at both cannabinoid receptors, but it shows particularly strong activity at CB1, to which it has high affinity. Its agonistic action is dose-dependent and contextually variable. At low concentrations, Δ9-THC may behave as a weak agonist or even antagonist in the presence of more potent endogenous ligands like 2-AG. However, at higher concentrations, it occupies receptors and activates a full signaling cascade that alters neurotransmission, cellular activity, and behavioral responses.
The mechanism of Δ9-THC agonism involves changing the conformational state of the CB1 receptor upon binding, triggering intracellular events through Gi/o proteins. This leads to decreased adenylate cyclase activity, reduced cAMP production, and inhibition of voltage-gated calcium channels responsible for vesicular neurotransmitter exocytosis. Thus, Δ9-THC reduces the release of excitatory mediators, shifts the balance of excitation and inhibition in neuronal networks, which in turn transforms into changes in mood, memory, attention, pain threshold, and motor coordination.
The partial agonist nature means that Δ9-THC does not activate the receptor to the full extent like full agonists (such as synthetic cannabinoids), making its effects milder but longer-lasting. This reduces toxicological risks but also limits the maximal therapeutic effect. Moreover, Δ9-THC can exhibit inverse agonist activity in the presence of high concentrations of endocannabinoids, changing receptor conformation in the opposite direction.
Its interaction with CB2 receptors is less potent but significant in the context of inflammatory and immune processes. In immune cells, Δ9-THC’s agonistic action suppresses the release of pro-inflammatory cytokines, decreases adhesion molecule expression, and modulates immune cell responsiveness to external signals. This has potential for use in treating autoimmune diseases, chronic inflammation, and even some forms of cancer.
Pharmacokinetics and Metabolism
The pharmacokinetics of Δ9-Tetrahydrocannabinol (Δ9-THC) is a complex and variable system that determines the bioavailability, distribution dynamics, metabolic transformation, and elimination of the compound from the body. All these parameters significantly depend on the route of administration, the lipophilic nature of the molecule, and individual metabolic characteristics, which result in a wide range of variations in concentrations and duration of action even with the same dosage.
After inhalation, Δ9-THC is rapidly absorbed through the alveolar membrane in the lungs, reaching peak plasma concentrations within 3 to 10 minutes. The absolute bioavailability of smoking ranges from 10 to 35%, depending on inhalation technique, inhaled volume, breath-holding duration, and combustion losses. Oral administration results in significantly lower bioavailability-between 6 and 10%-due to first-pass metabolism in the liver and slow absorption in the gastrointestinal tract. The lipophilic nature of Δ9-THC leads to active binding with dietary fats, which prolongs the time to reach Cmax (from 1 to 3 hours) and increases interindividual variability.
Once in systemic circulation, Δ9-THC is almost entirely bound to plasma proteins-up to 97%, mainly albumin-which limits its free fraction and modulates its tissue penetration rate. Due to its high lipophilicity, the molecule quickly distributes into well-perfused lipid compartments: the brain, lungs, liver, and heart. With chronic use, accumulation of Δ9-THC and its metabolites occurs in adipose tissue, from which gradual release happens over days and even weeks after cessation, explaining prolonged detection in biological samples even after a single use.
Δ9-THC metabolism primarily occurs in the liver involving cytochrome P450 enzymes, mainly the isoforms CYP2C9, CYP2C19, and CYP3A4. The main pathway is hydroxylation of the molecule to form 11-hydroxy-Δ9-THC (11-OH-THC), which is pharmacologically active and, in many respects, even more potent than the parent compound. This metabolite can rapidly cross the blood-brain barrier and cause psychoactive effects, which are especially pronounced with oral administration, where its concentration rises much more than with inhalation. Further metabolism of 11-OH-THC results in the formation of inactive 11-nor-9-carboxy-Δ9-THC (THC-COOH), which is the primary target for immunological and chromatographic detection in urine tests.
The half-life of Δ9-THC varies widely: from 1 to 2 hours with acute administration to 5 to 13 days with chronic use due to deposition in adipose tissue. THC-COOH and its conjugates are mainly excreted via feces (up to 65%) and to a lesser extent in urine (20-35%) as glucuronidated metabolites. The hydrophilicity of secondary metabolites enables renal excretion; however, a significant portion remains available for recirculation via the enterohepatic pathway, prolonging the overall elimination period.
Genetic polymorphisms of CYP2C9 play a significant role in pharmacokinetics by determining the rate of conversion of Δ9-THC to 11-OH-THC. Individuals with *2/*3 variants demonstrate slower metabolism, increasing the risk of accumulation of the active form and enhanced psychotropic effects. Additionally, concurrent use of CYP3A4 inhibitors or inducers (such as ketoconazole or rifampicin) significantly alters plasma levels of Δ9-THC, which is critical when using the compound therapeutically in patients receiving concomitant pharmacotherapy.
Special attention is given to the pharmacokinetics of other administration routes such as sublingual, transdermal, or intranasal. These routes partially bypass hepatic metabolism, allowing higher concentrations of Δ9-THC to be reached with a reduced proportion of 11-OH-THC. Consequently, the pharmacodynamic profile shifts toward a shorter but more controlled action, which is advantageous in medical use.
The pharmacokinetic properties of Δ9-THC are a key factor in determining its dose, administration frequency, expected effects, and risks. High variability, dependence on the route of administration, metabolic profile, and the patient’s fat mass complicate dose standardization. Therefore, dose individualization is a fundamentally important condition for minimizing side effects and achieving therapeutic outcomes.
Biological Effects
The biological effects of Δ9-Tetrahydrocannabinol (Δ9-THC) result from its selective interaction with receptor structures of the endocannabinoid system, which is integrated into numerous regulatory circuits of the central and peripheral nervous systems, as well as the immune, endocrine, gastrointestinal, cardiovascular, and other physiological systems. This interaction is not limited to neurotransmitter modulation but has transmembrane, transcriptional, and metabolic consequences, making Δ9-THC a unique compound with a multifunctional action profile. The biological response to Δ9-THC is not homogeneous: it varies depending on dose, route of administration, age, genetics, neurobiological status, frequency of use, and contextual environment. At the same time, it manifests in two interconnected but distinct planes: psychoactive and physiological. The first induces changes in perception, mood, and behavior, while the second modulates pain sensitivity, nausea, appetite, body temperature, vascular tone, and other parameters of autonomic function.
Psychoactive Effects (Euphoria, Perceptual Changes)
The psychoactive effects of Δ9-THC are the result of its specific impact on neurotransmitter systems, primarily dopaminergic, glutamatergic, and GABAergic. Through CB1 receptors, densely located in the mesolimbic dopamine system (especially in the ventral tegmental area and nucleus accumbens), Δ9-THC enhances dopamine transmission, causing subjectively pleasant sensations classified as euphoria. This sensation is not a simple “mood lift” but a complex cognitive-affective change involving the formation of a hedonistic appraisal of current reality, with both neuropsychological and behavioral correlates.
In addition to the dopaminergic influence, Δ9-THC modulates glutamate and GABA release in the prefrontal cortex and hippocampus. This explains typical psychoactive-state disruptions such as impaired short-term memory, altered temporal structuring of thought, spatial disorientation, and phenomena of hyperfocus on minor sensory details. Visual, auditory, and somatosensory stimuli are subjectively perceived as more intense or emotionally colored. This creates a phenomenon of altered perception, which is not a hallucination in the classical sense but rather an intensification of normal perception-a phenomenon especially characteristic of moderate doses of Δ9-THC.
Another fundamental characteristic of the psychoactive effect is the disintegration of functional brain networks. fMRI studies demonstrate decreased functional coherence in the Default Mode Network (DMN)-a network supporting introspection, self-awareness, and stream of consciousness. This may explain phenomena such as the subjective “ego dissolution,” loss of identity, and disruption of inner monologue. Simultaneously, the salience and sensory networks are activated, leading to heightened attention to sensory stimuli.
Changes in affective state are not always limited to positive valence. In some individuals, Δ9-THC induces anxiety, paranoid thoughts, heightened self-reflection, and interoceptive hypersensitivity, especially in unfamiliar environments or at high doses. This is believed to be due to individual differences in CB1 receptor sensitivity and the balance between dopaminergic and GABAergic system activity.
The psychoactive spectrum of effects is dose-dependent: low doses promote psychomotor relaxation, mood elevation, and social openness, while high doses can cause cognitive impairment, dysphoria, or even catatonia-like states. Chronic use alters receptor system sensitivity (CB1 desensitization, neuroadaptation), leading to the development of tolerance, the need for increased doses to achieve the same effect, and the phenomenon of anhedonia during withdrawal.
Physiological Effects (Analgesia, Antiemetic Effect)
The physiological action of Δ9-THC is based on the wide expression of CB1 and CB2 receptors in various organs and tissues, as well as modulation of secondary signaling pathways, including cyclic AMP systems, ion channels, and transcription factors. One of the best-studied physiological effects is its analgesic potential. Δ9-THC exerts pain-relieving effects at both peripheral and central nervous system levels. In the spinal cord, cannabinoid receptors regulate the release of substance P and glutamate at nociceptive synapses, reducing pain signal transmission. In the thalamus and cortex, they influence the integration of pain information, while in mesolimbic structures, they affect the affective perception of pain. This explains both the reduction of physical discomfort and the psychoemotional relief characteristic of cannabinoid analgesia.
The antiemetic effect of Δ9-THC is mediated by its action on the area postrema of the brainstem-the zone responsible for detecting toxins in the blood and initiating the vomiting reflex. CB1 receptors here regulate activation of serotonergic 5-HT3 receptors, inhibiting vomiting impulses. This allows the use of Δ9-THC in treating nausea and vomiting induced by chemotherapy, where classic antiemetics may be ineffective. Additionally, central activation of cannabinoid receptors stimulates hypothalamic hunger mechanisms by promoting secretion of neuropeptide Y and inhibiting pro-inflammatory cytokines, explaining the appetite-stimulating effect.
Other physiological effects include vasodilation (especially at the conjunctival level), reduction of intraocular pressure, muscle relaxation, tachycardia, transient hypotension, inhibition of gastrointestinal motility, and suppression of interleukin secretion. Importantly, cannabinoids do not cause respiratory center depression, which distinguishes them from opioids and makes them safer regarding fatal overdose.
Long-term effects include modification of neuroendocrine axes, particularly the hypothalamic-pituitary-gonadal axis, manifested as decreased luteinizing hormone and testosterone in men with chronic use. The immunomodulatory action of Δ9-THC is mediated through CB2 receptors on lymphocytes, macrophages, and microglia: there is inhibition of T-cell proliferation, reduction of TNF-α, IL-2, and interferon-gamma production, which holds therapeutic potential for autoimmune diseases.
Areas of Application: From Medical to Fundamental Science
Δ9-Tetrahydrocannabinol, as a pharmacologically active substance with a unique profile of action on the endocannabinoid system, has transcended the boundaries of purely psychoactive or recreational use and has gained the status of a molecule with high intellectual potential for medicine, biotechnology, and fundamental sciences. Its molecular action, which integrates signaling cascades of the central nervous system, immunity, endocrine regulation, and metabolism, allows Δ9-THC to be considered not only as a model compound for symptomatic treatment but also for studying the basic principles of homeostatic mechanisms in humans.
The sphere of Δ9-THC application unfolds across three interrelated dimensions: clinical, research, and technological. Clinically, it is used as an analgesic, antiemetic, muscle relaxant, appetite stimulant, and even as an immunomodulator – with each application grounded in strictly defined receptor and transduction mechanisms. This distinguishes Δ9-THC from many nonspecific phytopharmaceuticals and brings it closer to the class of precision medicines, whose efficacy correlates with receptor expression, metabolic enzyme polymorphisms, and the state of neuronal networks.
Beyond clinical use, Δ9-THC is the subject of extensive research in neuroscience, molecular biology, pharmacogenetics, and psychiatry. The cannabinoid action model allows highly precise study of mechanisms of synaptic plasticity, short-term memory formation, neurogenesis, and epigenetic changes in the adult brain. Thanks to Δ9-THC’s ability to influence intracellular signaling pathways, including MAPK, PI3K/AKT, and CREB-dependent processes, it is regarded as an instrumental tool in neurobiology at the cellular and network regulation levels.
Of particular interest are studies using Δ9-THC to model mental states such as anxiety, dissociation, depersonalization, and paranoid ideation. This enables testing hypotheses regarding the neurophysiological nature of schizophrenia, anxiety disorders, PTSD, and even neuroethological mechanisms of consciousness. In this context, cannabinoid effects do not appear as artifacts but as tools granting access to latent psychophysiological processes that cannot be initiated by other pharmacological agents with the same selectivity.
From an applied perspective, Δ9-THC has opened a new paradigm in pharmaceutical design – drugs based on it have become a testing ground for concepts such as nanodelivery targeting, CNS vector transport, optimization of lipophilic agent bioavailability, and personalized medicine considering pharmacogenetic parameters. Commercial prospects include the development of inhalation forms, transdermal systems, oral liposomal capsules, and even integration of Δ9-THC into “smart delivery” platforms based on functionalized nanoparticles capable of targeted penetration through the blood-brain barrier.
Fundamental science, in turn, uses Δ9-THC as a probe to study regulatory mechanisms of the immune system, neurovascular interaction, interoception, and neuroimaging. Cannabinoid receptors activated by this compound have been found in bone tissue, cardiac muscle, microglia, intestines, and pancreas, making it relevant for research fields such as regenerative medicine, metabolomics, microbiomics, and oncology. Additionally, Δ9-THC enables tracking the impact of psychoactive agents on brain development-both in experimental models of prenatal exposure and studies of adolescent neuroplasticity.
Medical Use
The medical application of Δ9-Tetrahydrocannabinol is based not on the side or secondary effects of psychoactivity but on the targeted pharmacological influence on the cannabinoid system, which is integrated into key mechanisms regulating pain, nausea, appetite, muscle tone, inflammation, and neuroendocrine homeostasis. The endocannabinoid system acts as a neuromodulatory overlay over classical neurotransmitter networks, and Δ9-THC, as its potent exogenous agonist, provides therapeutic intervention in pathological conditions accompanied by disruption of these regulatory axes. This justifies its inclusion in medical protocols across various clinical scenarios-primarily in chronic pain syndrome, refractory nausea, and cachexia induced by immunodeficiency states. These effects are not sporadic or empirical: they are documented in systematic reviews, double-blind randomized trials, and clinical meta-analyses that confirm both the efficacy and acceptable safety profile of Δ9-THC at therapeutic doses.
A distinctive feature of its medical use is that its effects often manifest in cases where classical pharmacological agents lose effectiveness or have unacceptable toxicity levels. This grants Δ9-THC the status of not a first-line drug but a highly specialized therapeutic option, applicable when conventional means fail to achieve the targeted outcome. Moreover, the significance lies not only in its action on individual symptoms but also in its multicomponent modulation of somatic and neuropsychiatric parameters of the patient, enabling multivector clinical improvement.
Chronic Pain Therapy
The analgesic effect of Δ9-THC in the context of chronic pain is not synonymous with the suppression of pain signals as seen with opioids; rather, it is realized through flexible modulatory intervention at multiple levels of the nociceptive process: peripheral, spinal, thalamocortical, and affective-motivational. Through CB1 receptors located on afferent neurons of the dorsal horns of the spinal cord, Δ9-THC reduces the release of glutamate and substance P, which are key mediators in transmitting the pain signal at the first synaptic stage. Within the central nervous system, its action extends to the thalamus, hippocampus, amygdala, and prefrontal cortex, where it modifies the emotional coloring of pain and its cognitive integration into the patient’s overall body schema.
In cases of neuropathic pain resistant to NSAIDs or antidepressants, Δ9-THC demonstrates the ability to reduce sensitization induced by glial cells, particularly microglia, which become activated in response to nerve tissue damage. By acting on CB2 receptors expressed by immune cells in the central nervous system, it blocks the production of pro-inflammatory cytokines (TNF-α, IL-1β, IL-6) that exacerbate neuropathic hyperalgesia. This is especially important in conditions such as multiple sclerosis, chronic radiculitis, postherpetic neuralgia, or diabetic neuropathy.
Clinical studies using standardized cannabis extracts containing Δ9-THC have shown a 30-50% reduction in pain intensity, measured by the visual analog scale, in patients with refractory chronic pain. This effect is achieved with a significantly lower risk of dependency or respiratory depression compared to opioids. Furthermore, Δ9-THC not only reduces the intensity of pain impulses but also improves sleep quality, decreases muscle rigidity, anxiety, and depressive symptoms often accompanying chronic pain and contributing to its psychosomatic component. Importantly, in dose-dependent regimens, its effects can be tailored to the individual clinical picture, and delivery formats-from inhalation to sublingual sprays-provide controlled pharmacokinetic profiles.
Given this, Δ9-THC is increasingly used as an adjunct to standard analgesic therapy and, in some cases, as monotherapy. Its efficacy is especially pronounced in patients contraindicated for NSAIDs due to gastrointestinal toxicity or who cannot tolerate opioids because of nausea, constipation, or cognitive side effects. In these cases, Δ9-THC shows a stable clinical response without the development of drug dependence or pronounced withdrawal syndrome.
Antiemetic in Chemotherapy
The antiemetic potential of Δ9-THC is based on its ability to modulate serotonergic activity in central vomiting centers, particularly in the area postrema and nucleus tractus solitarius, where 5-HT3 receptors sensitive to chemotherapeutic agents are concentrated. Δ9-THC blocks activation of these receptors by inhibiting serotonin release from enterochromaffin cells of the gut, preventing transmission of toxicity signals to the CNS. This explains the effectiveness of cannabinoids in preventing and treating nausea resistant to classical 5-HT3 antagonists.
Patients receiving platinum-based chemotherapy often develop both acute and delayed nausea that is difficult to control even with triple antiemetic therapy. Δ9-THC demonstrates efficacy in such cases both as monotherapy and in combination regimens. Its advantage lies not only in the receptor-level mechanism but also in its impact on the integral affective assessment of the condition, allowing reduction not only of somatic but also emotional discomfort associated with chemotherapy. Additionally, Δ9-THC normalizes appetite, stabilizes mood, improves sleep, and reduces asthenic symptoms secondary to intense vomiting.
The pharmacokinetic property of Δ9-THC-prolonged action upon oral administration-makes it especially useful in preventing delayed nausea occurring two to three days after chemotherapy. In patients with frequent vomiting episodes, Δ9-THC use helps avoid dehydration, electrolyte imbalance, nutritional deficiency, and disruption of treatment schedules. These advantages led to official approval of synthetic Δ9-THC analogs (dronabinol, nabilone) for oncological use as second-line antiemetics when standard therapy proves ineffective.
Critically, Δ9-THC does not block normal gastric motility, unlike some antiemetics that cause gastroparesis and worsen nutrient absorption. This is particularly important in the wasting conditions typical for patients undergoing chemotherapy. Effective doses can be minimal and not accompanied by pronounced psychoactive effects, which is crucial for elderly or weakened patients.
Appetite Stimulant in HIV
Δ9-THC has demonstrated clinical efficacy in treating HIV-associated cachexia-a condition characterized by anorexia, muscle atrophy, loss of subcutaneous fat, and overall decline in nutritional status. Its mechanism involves activation of CB1 receptors in the hypothalamus, particularly in the arcuate and paraventricular nuclei responsible for regulating hunger and satiety. Stimulation of these nuclei leads to increased expression of neuropeptide Y and agouti-related protein, which initiate feeding behavior and increase caloric intake.
Clinical trials have shown that Δ9-THC not only boosts appetite but also supports stable weight gain without muscle loss, a critical factor for survival in HIV patients. Its effect is not limited to short-term euphoric activation; with regular dosing, it normalizes feeding behavior, reduces nausea, eliminates food aversion, and decreases overall asthenia. As a result, physical condition improves, metabolic stability is restored, and the risk of secondary infections decreases.
Δ9-THC also modulates systemic inflammation via CB2 receptors, lowering pro-inflammatory cytokine levels involved in the pathogenesis of HIV-induced cachexia. This achieves a more stable nutritional effect than appetite stimulants based on steroids or progestins, which are accompanied by significant side effects. Unlike those agents, Δ9-THC does not cause hyperglycemia, fluid retention, or gynecomastia, making it suitable for long-term use.
The efficacy of Δ9-THC in this area is especially valuable given the limited arsenal of safe and effective treatments for HIV-associated anorexia. Its use promotes adherence to therapy, improves quality of life, and enhances the overall prognosis for patients with severe immunodeficiency.
Research in Neuroscience and Psychopharmacology
Intensive research on Δ9-THC in neuroscience has revealed a far more complex profile of its action than initially assumed based on its superficial characterization as a “recreational psychoactive substance.” Modern neuropharmacological approaches have uncovered the fundamental role of cannabinoid signaling in regulating cognitive processes, emotional homeostasis, synaptic remodeling, and neural recovery after stress or injury. Δ9-THC, interacting with CB1 receptors expressed in highly organized structures of the central nervous system – the hippocampus, prefrontal cortex, amygdala, and ventral striatum – modulates mechanisms of memory, learning, affective state, and neuroplasticity. This influence is not uniformly inhibitory or stimulatory, as is often inaccurately presented in simplified popular sources, but rather context-dependent: it changes depending on the phases of memory consolidation, the type of information (emotionally charged or neutral), stress conditions, chronic anxiety, or neurovisceral dysfunction.
The role of Δ9-THC in exogenous modulation of the endocannabinoid system within the psychoneurophysiological context is supported by experimental animal models, functional MRI, PET studies, electrophysiology of synaptic transmission, and transcriptional profiling of neurons. Specifically, it has been found that Δ9-THC action may lead to short-term suppression of glutamatergic transmission in the CA1 region of the hippocampus, reducing the amount of information that can be consolidated into long-term memory. At the same time, in other contexts, Δ9-THC exhibits neuroprotective properties: in experimental models of induced anxiety or post-traumatic reactions, it normalizes amygdala hyperactivity and reduces pathological formation of fear memories.
Neuroscience research confirms that cannabinoid tone is one of the key regulators over synaptic plasticity, functioning as a form of feedback control: CB1 receptors located on presynaptic terminals are activated by retrograde endocannabinoids released postsynaptically, ensuring focal synaptic inhibition without global suppression. Exogenous Δ9-THC, activating these same mechanisms, interferes with the fine dynamics of synaptic weight, sometimes enhancing it, sometimes reducing it depending on network activity. This opens possibilities not only for studying potential cognitive risks but also for controlling pathological synaptic remodeling, for example, in PTSD or neurodegenerative diseases.
Effects on Memory, Learning, and Synaptic Plasticity
Experimental data demonstrate that Δ9-THC disrupts short-term memory in healthy subjects by suppressing synaptic transmission in the hippocampus – the primary structure responsible for episodic memory consolidation. Presynaptic CB1 receptors activated by Δ9-THC block the release of glutamate and GABA, altering the excitation/inhibition balance in key neuronal microcircuits. This can lead to impaired formation of long-term potentiation (LTP) – the electrophysiological basis for learning. In young healthy subjects, this manifests as transient impairment of verbal memory or slowed cognitive processing speed.
At the same time, under conditions of pathological neuronal hyperexcitability, such as chronic stress, traumatic experiences, epilepsy, or neuroinflammation, Δ9-THC acts as a normalizing agent that reduces excessive synaptic activity and protects against synaptotoxicity. In such cases, it blocks pathological hyperpotentiation and prevents the fixation of overly affectively charged information. In animal models of PTSD, Δ9-THC prevents consolidation of pathological fear-associated memory and accelerates its extinction, opening prospects for therapy of disorders related to pathological persistence of traumatic memories.
Additionally, Δ9-THC can influence neurogenesis mechanisms. In the adult hippocampus, CB1 receptor expression on neural stem cells determines the rate of proliferation and differentiation of new neurons. Some data indicate that chronic low-dose Δ9-THC administration in aged animals leads to restoration of neuroplasticity, improvement in cognitive functions, and reduction of the brain’s inflammatory profile. This raises questions about the potential of Δ9-THC or its modified analogs in correcting cognitive decline associated with aging or mild cognitive impairment in early stages of dementia.
However, there is a downside: chronic Δ9-THC abuse during adolescence, when the prefrontal cortex and hippocampus are still in active development, is associated with long-term reduction of LTP, decreased synapse volume, and reduced expression of genes related to neuroplasticity (such as BDNF), correlating with deficits in working memory and executive functions. This demonstrates the critical importance of dosage, age, duration, and context in evaluating the cognitive impact of Δ9-THC.
Potential in PTSD and Anxiety Therapy
Post-traumatic stress disorder (PTSD) is a complex neuropsychiatric condition rooted in dysregulation of endocannabinoid control over emotional memory systems, stress response, and fear inhibition. Patients with PTSD show reduced baseline levels of endocannabinoids in blood plasma, hypoactivity of CB1 receptors, and hyperactivity of the amygdala when exposed to traumatic triggers. In this context, Δ9-THC acts as a functional modulator that compensates for the deficiency in endocannabinoid signaling, reduces the affective intensity of memories, inhibits hypothalamic-pituitary-adrenal (HPA) axis reactivity, and accelerates the extinction of pathological fear memory. This has been demonstrated in clinical pilot studies, where administration of Δ9-THC before exposure therapy sessions lowered anxiety levels, promoted the formation of new safety associations, and enhanced the effectiveness of psychotherapeutic interventions.
Beyond PTSD, clinical and neuroimaging data indicate that low doses of Δ9-THC may reduce pathological anxiety by normalizing amygdala hyperactivity and decreasing the pathological connectivity between the amygdala and prefrontal cortex. In cases of generalized anxiety disorder, social phobia, and obsessive-compulsive disorder, positive clinical outcomes have been observed with Δ9-THC use, including reduction of physiological anxiety symptoms such as tachycardia, muscle tension, and gastrointestinal disturbances. However, these effects have a narrow therapeutic window: exceeding the dose of Δ9-THC can itself induce anxiety or panic attacks, necessitating strict control and individualized dosing protocols.
Within the spectrum of anxiety disorders, Δ9-THC functions not as a sedative or suppressant but as a subtle restorer of neuroaffective balance, with the primary target being pathological excitability of neural networks that respond to minor triggers as threats. Its pharmacological action is not isolated; it complements psychotherapeutic approaches by providing a neurobiological “plasticity window” within which therapy can be more effective.
Modern research, including PET imaging, has identified a deficit in CB1 receptor density in PTSD patients, especially in the left amygdala and hippocampus. In such cases, Δ9-THC partially compensates for the loss of endocannabinoid sensitivity, explaining its efficacy in this patient group. On this basis, research is ongoing into synthetic Δ9-THC analogs with modified affinity for CB1/CB2 receptors or partial agonism, aiming to minimize psychoactive risks while preserving therapeutic profiles. This is particularly promising for future psychopharmacotherapy of anxiety disorders resistant to SSRIs or SNRIs.
Industrial and Pharmaceutical Production
Scaling up production lines for Δ9-THC raw material and its derivative pharmaceuticals requires exceptionally strict technological, pharmaceutical, and regulatory discipline. This is not simply the scale-up of botanical extraction but rather a complex, multi-step biotechnological process integrating agrogenomics, precision extraction, chromatographic purification, concentration standardization, and formulation into clinically suitable forms. At the intersection of chemistry, bioengineering, and molecular pharmacology, modern Δ9-THC production has transcended cannabis cultivation, incorporating cutting-edge developments in nanotechnology, targeted delivery vectors, pharmacokinetic programming, and molecular stabilization. Dominant paradigms include microdosing, maximizing bioavailability with minimal psychoactive load, and precise controlled action targeted to the patient’s receptor profile.
The pharmaceutical production cycle includes cultivation of genetically standardized strains with predictable cannabinoid profiles, followed by supercritical CO₂ extraction, chromatographic separation, fractional purification, decarboxylation, and final stabilization of Δ9-THC. The purification process achieves isolation not only of pure Δ9-THC but also synthesis of pharmaceutically adapted analogs – for example, dronabinol, levonantradol, or newer variants with modified affinity for CB1/CB2 receptors. GMP standards regulate allowable impurities down to micrograms per gram, transforming cannabis raw material from an agricultural product into a high-purity pharmaceutical substance. This level of control is necessary to guarantee pharmacokinetic stability, eliminate batch-to-batch variability in psychoactivity, and ensure dosing reliability in clinical use.
The formulation format also plays a significant role: isolated Δ9-THC in oil base, capsules, microemulsions, nasal sprays, or transdermal systems. Each form is developed considering disease specificity, onset speed, duration of effect, and side effect profile. Pharmaceutical technologists modulate release kinetics through matrix polymers, lipid carriers, or nanosuspensions, adapting formulations to patient-centered therapy requirements. This becomes especially critical in neuropsychiatric disorders or oncology, where the permissible plasma concentration range is extremely narrow.
Industrial transformation of Δ9-THC also demands deep legal regulation. In countries with legalized medical cannabis, licensing models, controlled protocols, electronic prescription systems, and full batch traceability – from plant to final product – are implemented. This establishes a new approach to standardization of phytocannabinoids as pharmacologically active substances that must meet all parameters of the European Pharmacopoeia or USP. At the same time, an innovative ecosystem is developing – from biobanks of strains to artificial intelligence optimizing cultivation conditions to ensure the cannabinoid chemotype necessary for subsequent pharmaceutical synthesis.
Precision Medicine
Precision medicine based on Δ9-THC is grounded in the principle of the patient’s individual pharmacogenetic profile-taking into account polymorphisms in the CB1/CB2 receptor genes, CYP2C9 and FAAH enzymes, endocannabinoid tone status, and metabolic characteristics. It has already been established that variants in the CNR1 gene can modulate sensitivity to Δ9-THC, shaping susceptibility to both therapeutic and adverse effects-such as cognitive suppression or psychotic reactions. Similarly, the CYP2C9*3 polymorphism reduces the metabolism rate of Δ9-THC, prolonging its action and increasing the risk of accumulation. Within a personalized approach, this necessitates dose adjustment, dosing schedule, and formulation selection.
Precision Δ9-THC therapy also considers the specifics of the clinical condition: for example, in patients with neuropathic pain, preference is given to fast-acting inhalation or sublingual forms with peak concentration within 5-10 minutes, whereas in oncologic cachexia syndrome, slow-release transdermal systems providing a stable level throughout the day are preferred. For patients with PTSD or anxiety disorders, microdoses with extended release are chosen, which do not induce psychoactivity but normalize amygdala tone.
Technologically, this is implemented through advanced methods of Δ9-THC microencapsulation in biodegradable polymers, allowing controlled release rates and avoiding peak concentration fluctuations. Systems with context-dependent activation (such as photosensitive or pH-sensitive nanocapsules) open the possibility of delivering Δ9-THC as “smart drugs” activated only within the specific microenvironment of the pathological process-for example, in areas of neuroinflammation or tumors.
A separate direction is algorithmic programming of pharmacotherapy-the use of artificial intelligence to adapt Δ9-THC prescription schemes based on patient biomarkers, neuroimaging profiles, psychometric data, and metabolic indicators. Such systems, in experimental conditions, have already demonstrated improved control of chronic pain, reduced anxiety, and fewer side effects due to precise dynamic dosing in real time. This creates a new dimension in Δ9-THC-based pharmacotherapy-not only as a substance but as a controllable platform.
Vector-Based Nanotechnology Developments of THC
The application of nanotechnology in developing vector delivery systems for Δ9-THC has become a key breakthrough in overcoming the pharmacokinetic barriers of this molecule. Due to its high lipophilicity and susceptibility to first-pass metabolism, Δ9-THC has low oral bioavailability-about 6-10%. Nanoformulations in the form of liposomes, nanostructured lipid carriers, polysaccharide matrices, or polymeric nanocapsules protect the molecule from enzymatic degradation, improve its solubility in aqueous environments, extend circulation time, and enable targeted delivery to specific tissues.
In vector transport systems, Δ9-THC can be encapsulated in nanoparticles with modified surface functionalities-for example, using ligands targeting CD44 receptors (expressed during inflammation) or anti-epitopes targeting tumor cells. This ensures precise localization of the drug to pathological zones with minimal systemic exposure. In neurodegenerative diseases, where the blood-brain barrier is compromised, nanoformulated Δ9-THC shows the ability to penetrate the brain more effectively than standard lipophilic molecules.
Hybrid systems are also under development, in which Δ9-THC is combined with other bioactive components, such as anti-inflammatory peptides or FAAH inhibitors, providing synergistic effects. These compositions allow modulation not only of the strength and duration of Δ9-THC’s effects but also of their qualitative nature-for example, reducing the risk of psychoactivity while maintaining analgesia.
Vector forms also open new routes of administration-intranasaI, inhalation, transdermal, or even intracranial-which is especially relevant for treating localized CNS tumors or focal epilepsy. All these technologies are actively patented and are already partially entering clinical practice within experimental protocols or expanded access programs for severe patients.
Conclusion
A comprehensive study of Δ9-tetrahydrocannabinol (Δ9-THC) within a multidisciplinary approach-encompassing chemistry, botany, biochemistry, molecular pharmacology, neuroscience, medicine, and industrial technologies-demonstrates that this compound is an exceptional example of a bioactive natural product with a multivector mechanism of action, high biochemical potential, and pharmacological selectivity. Unlike many psychoactive plant-derived substances, Δ9-THC has not only become the subject of intense scientific interest but has also formed an entire industry governed simultaneously by rigorous scientific criteria, clinical protocols, pharmaceutical standards, and at the same time, regulatory, legal, and social constraints. All of this creates a unique case in modern science where a chemical substance isolated from a natural matrix acts as a multifunctional agent in both fundamental and applied research.
The chemical nature of Δ9-THC, manifested in the complex structural organization of the molecule, determines its extremely specific bioactivity. The classical terpene structure with an aromatic ring, an aliphatic chain, and a hydroxyl group not only provides high affinity for endocannabinoid system receptors but also affects its lipophilic properties, chirality, metabolic stability, and pharmacokinetics. The lipophilic nature allows THC to freely cross the blood-brain barrier, ensuring a rapid psychoactive effect. The molecule’s stereochemistry, particularly its chiral centers, determines affinity for specific receptor subtypes, resulting in partial agonist specificity, especially toward CB1 receptors in the central nervous system. The biosynthetic origin of Δ9-THC in the Cannabis sativa plant is another critically important link: from the synthesis of the precursor CBGA to its conversion into THCA and subsequent decarboxylation to the active molecule-all these stages are tightly controlled by enzymatic cascades and environmental conditions, including temperature, light, and the metabolic status of trichomes.
The transition from natural origin to pharmaceutical availability requires complex technological processing. Production of Δ9-THC involves careful selection of cannabis strains with high cannabinoid content, adaptation of agricultural conditions-specifically soil pH, ionic composition, light indices, humidity, and phenophases. Morphological features of C. sativa and C. indica-differences in inflorescence arrangement, leaf shape, trichome density-directly correlate with the qualitative and quantitative cannabinoid profile, as confirmed by analytics using HPLC, GC, and mass spectrometry methods. Variability in Δ9-THC content among strains can reach dozens of percent, which necessitates raw material standardization, especially in the clinical context.
Modern extraction methods are not merely technological processes of isolation but a whole engineering discipline aimed at preserving the chemical integrity of Δ9-THC, reducing impurity levels, and standardizing dosage. Traditional mechanical methods such as dry sifting and manual trichome collection have historical value but cannot compete with modern chemical and supercritical extraction systems. The use of ethanol, butane, supercritical CO₂ not only increases yield but also allows precise fractionation, removal of chlorophylls, waxes, and oxidation products. Subsequent multi-stage purification-including winterization, distillation, adsorption on silica gel or activated charcoal-ensures pharmaceutical-grade purity of the substance.
The molecular target of Δ9-THC-the endocannabinoid system-is one of the most complex and insufficiently studied regulatory clusters in the human body. CB1 receptors, located predominantly in the brain (hippocampus, basal ganglia, prefrontal cortex), regulate memory, mood, motivation, pain perception, and motor coordination. CB2 receptors, more represented in the immune system and peripheral tissues, mediate anti-inflammatory, immunomodulatory, and tissue-regenerating effects. Acting as a partial agonist of both receptor subtypes, Δ9-THC modulates their function based on conformational plasticity, allowing influence over a wide range of physiological parameters without a universal mechanism. This property is simultaneously the source of therapeutic potential and challenges in standardizing clinical use.
The pharmacokinetic profile of Δ9-THC varies depending on the administration route: inhalation provides maximum bioavailability and rapid achievement of peak plasma concentrations, whereas oral administration causes significant first-pass liver metabolism forming the active metabolite 11-OH-THC, which has increased psychoactivity. The presence of enterohepatic circulation prolongs the effect, contributes to accumulation, and thus complicates dosing. Metabolism occurs primarily via cytochrome P450 isoenzymes-CYP2C9, CYP3A4-with subsequent conjugation to glucuronides and excretion via kidneys and bile. More than 80 metabolites can be identified in biological fluids after a single dose, creating a complex picture for toxicokinetics, especially under chronic use or polypharmacy conditions.
Pharmacodynamic effects of Δ9-THC cover an exceptionally wide range. At the CNS level, changes are observed in dopamine, glutamate, GABA, and acetylcholine neurotransmission, leading to psychoactive phenomena-euphoria, short-term memory impairment, altered sensory perception, synesthesia, and changes in time perception. Peripheral effects-vasodilation, hypothermia, antiemetic action, muscle relaxation-are mediated by a complex cascade of neuroimmune mechanisms, where receptor action is combined with indirect effects such as inhibition of adenylate cyclase or modification of calcium channels. The biological action of Δ9-THC is not a homogeneous phenomenon but varies depending on dose, timing of administration, genetic factors, and the state of endocannabinoid tone.
In clinical use, Δ9-THC demonstrates efficacy in treating chronic neuropathic pain, chemotherapy-induced nausea and vomiting, and appetite stimulation in cachexia patients with HIV. However, its application requires strict standardization, particularly regarding the ratio with CBD, treatment duration, dosing, and route of administration. In neuroscience, Δ9-THC serves as an experimental tool for modeling changes in synaptic plasticity, LTP/LTD dynamics, studying neurovulnerability in PTSD, anxiety disorders, and even depression. New approaches involving nanoformulations, liposomal delivery systems, and microemulsions aim to improve bioavailability, minimize psychoactivity, and target specific tissues.
Vector developments include strategies using carriers, modified peptides, and anti-TAG antibodies aimed at integrating Δ9-THC into precision medicine systems. A promising direction is designing selective agonists/antagonists based on Δ9-THC with predefined affinity for CB1 or CB2 receptors, enabling creation of drugs with predictable therapeutic profiles. Modern industrial THC production lines are based on integrating biotechnology, organic synthesis, analytical control, and pharmaceutical engineering, forming the basis for regulatory approval in the form of GMP-certified substances.
Thus, Δ9-tetrahydrocannabinol is a multifaceted object integrating natural science, clinical, and technological dimensions. Its study has not only fundamental significance for understanding endogenous regulatory mechanisms but also utilitarian value within personalized medicine, pharmaceutical development, and bioengineering innovations. This cannabinoid embodies the transition from a stigmatized plant component to a full-fledged molecular tool capable of transforming treatment practices, understanding neurobiology, and the paradigm of pharmacotherapy as a whole.
Sources:
- Tetrahydrocannabinol (THC) – StatPearls, NCBI Bookshelf
A review of the pharmacology, clinical application, and mechanism of action of THC.
https://www.ncbi.nlm.nih.gov/books/NBK563174/ - Therapeutic Effects of Cannabis and Cannabinoids – National Academies of Sciences, Engineering, and Medicine
A systematic review of evidence regarding the therapeutic potential of cannabinoids.
https://www.ncbi.nlm.nih.gov/books/NBK425767/ - Cannabis and Cannabinoids (PDQ®) – National Cancer Institute
A government resource on the use of cannabis in oncology practice.
https://www.cancer.gov/about-cancer/treatment/cam/hp/cannabis-pdq - FDA and Cannabis: Research and Drug Approval Process – U.S. Food & Drug Administration
Information about FDA policy on scientific research and cannabis legalization.
https://www.fda.gov/news-events/public-health-focus/fda-and-cannabis-research-and-drug-approval-process - Cannabis and Brain Health – Centers for Disease Control and Prevention (CDC)
Information on the effects of THC on brain function.
https://www.cdc.gov/cannabis/health-effects/brain-health.html - Cannabis and the Brain – Harvard Medical School
Neurobiological effects of THC from the perspective of evidence-based medicine.
https://hms.harvard.edu/news-events/publications-archive/brain/cannabis-brain - UChicago Research on Demographic Effects of Cannabis – University of Chicago
Research on how THC impacts different demographic groups.
https://news.uchicago.edu/story/uchicago-research-examines-how-cannabis-impacts-distinct-demographics-differently - Industrial Hemp and Medical Cannabis Research – Georgia Southern University
Information on strains, composition, and industrial cannabis research.
https://ww2.georgiasouthern.edu/research/researchintegrity/cannabis-research/ - Medical Marijuana Research – Philadelphia College of Osteopathic Medicine (PCOM)
Overview of current medical cannabis research.
https://www.pcom.edu/research/research-areas/medical-cannabis.html - Cannabis and Working Memory – University of Colorado Anschutz Medical Campus
The largest study to date on cannabis and brain function showing effects on working memory.
https://news.cuanschutz.edu/news-stories/largest-study-ever-done-on-cannabis-and-brain-function-finds-impact-on-working-memory - Rising THC Potency and Health Risks – Yale School of Medicine
On increasing THC concentrations in modern strains and potential health risks.
https://medicine.yale.edu/news-article/not-your-grandmothers-marijuana-rising-thc-concentrations-in-cannabis-can-pose-devastating-health-risks/ - Know the Risks of Marijuana – Substance Abuse and Mental Health Services Administration (SAMHSA)
Physiological and psychological consequences of THC use.
https://www.samhsa.gov/substance-use/learn/marijuana/risks - MedlinePlus – Marijuana Overview
Medical aspects of marijuana and the Δ9-THC component.
https://medlineplus.gov/marijuana.html - Study on THC Enhancement via CBD – University of Mississippi
Research on the potential of CBD to enhance THC effects.
https://olemiss.edu/news/2025/2/cbd-study-chittiboyina/index.html - Cannabis Facts & Statistics – CDC
Current statistical data on cannabis use.
https://www.cdc.gov/cannabis/data-research/facts-stats/index.html