Cannabinoids are a class of terpene-phenolic compounds that occur naturally, synthetically, or endogenously and interact with the endocannabinoid receptor system in vertebrates. The Cannabis sativa L. plant is a unique natural source of phytocannabinoids, among which a group of acidic forms-biosynthetic precursors to neutral (decarboxylated) cannabinoids-stands out. One of the central acidic molecules is Δ9-tetrahydrocannabinolic acid A (THCA-A), which is the direct precursor of the psychoactive Δ9-tetrahydrocannabinol (Δ9-THC). Within the biochemistry of cannabis, this compound plays a key role in the metabolic cascade and is also the subject of independent pharmacological interest due to its non-psychoactive yet bioactive nature.
THCA-A is formed at a late stage in the biosynthesis of phytocannabinoids through the enzymatic conversion of cannabigerolic acid (CBGA)-the so-called “mother” compound from which the primary acidic forms of cannabinoids are synthesized. The enzyme THCA synthase, localized in the trichomes of the plant’s flowers, catalyzes the oxidative cyclization of CBGA to produce THCA-A. This process is highly specific and genetically determined: the corresponding enzymatic pathways are activated depending on the chemotype of the plant. That is, strains with a high level of THCA synthase predominantly produce THCA-A, while others produce CBDA or CBCA depending on the presence of CBDA or CBCA synthases.
From a chemical structure standpoint, THCA-A is the carboxylated form of Δ9-THC and contains an additional carboxyl group (-COOH), which contributes to its greater hydrophilicity, reduced ability to cross the blood-brain barrier, and other pharmacokinetic properties. Importantly, upon thermal processing (heating, drying, or exposure to ultraviolet light), this carboxyl group is removed-a decarboxylation reaction occurs, resulting in the formation of Δ9-THC, a molecule that readily binds to CB1-type cannabinoid receptors and induces psychoactive effects.
Traditionally, THCA-A has been viewed primarily as an intermediate form necessary for obtaining Δ9-THC. Accordingly, many research programs and pharmaceutical strategies have focused on maximizing the efficiency of decarboxylation and stabilizing the final product-Δ9-THC. However, it was later discovered that THCA-A itself exhibits pharmacological activity through mechanisms distinct from canonical CB1/CB2 receptor binding. It can affect molecular targets outside the endocannabinoid system, including enzymes, ion channels, nuclear receptors, and signaling proteins. In particular, evidence has emerged regarding its interaction with PPARγ receptors (peroxisome proliferator-activated receptors), modulation of COX-2 expression, inhibition of pro-inflammatory cytokine production, and neuroprotective effects under conditions of hypoxia or oxidative stress.
It is important to note that under natural conditions, THCA-A does not accumulate in a stable form: it undergoes decarboxylation quickly, even with slight increases in temperature or during prolonged storage of plant material. This presents significant challenges for its laboratory study, requiring special extraction and storage conditions as well as advanced analytical control methods. Standardizing THCA-A-based preparations is a complex task even for leading pharmaceutical laboratories, as it requires taking into account time, temperature, environmental acidity, and oxygen presence at every stage-from harvesting the raw plant material to packaging the final product.
Interest in THCA-A has grown significantly over the past decade, as several independent research groups have reported its potential effects in the context of chronic pain, autoimmune diseases, neurodegenerative processes (such as Parkinson’s or Alzheimer’s disease), and chemotherapy-induced nausea. At the same time, the absence of a psychoactive profile makes this compound particularly attractive for medical use, as it does not cause euphoria, does not impair cognitive functions, and lacks abuse potential. From a regulatory perspective, this means that THCA-A may not fall under the same legal restrictions as Δ9-THC, although in many countries this distinction is not yet reflected in legislation.
THCA-A is of particular importance in the field of applied biotechnology. The study and modification of THCA synthase-the enzyme responsible for THCA-A production-allow scientists to genetically program plants to synthesize specific cannabinoids. Thus, controlling the expression of cannabinoid pathway enzymes has become one of the strategies for creating targeted cannabis strains for medical use. Experiments using yeast and other microorganisms to biosynthetically produce THCA-A in vitro have been conducted, opening the door to large-scale biotechnological production without the need to cultivate the Cannabis sativa plant itself.
It is worth emphasizing that the scientific literature on THCA-A remains limited compared to neutral forms of cannabinoids. Most data come from preclinical models or descriptive pharmacobiochemical studies. The absence of large-scale clinical trials reduces the evidence base for the medical application of this compound, necessitating the expansion of research programs, the development of standardized protocols, and a systematic assessment of its safety.
Biosynthesis and Natural Origin of THCA-A
Δ9-Tetrahydrocannabinolic acid A (THCA-A) is a key secondary metabolite of the Cannabis sativa L. plant, belonging to the class of acidic phytocannabinoids. It does not exist in the form of free Δ9-tetrahydrocannabinol (Δ9-THC) in fresh plant material; rather, it serves as its biosynthetic precursor. Unlike the final, decarboxylated form, THCA-A does not exhibit psychoactive activity, which opens up possibilities for its exploration in medical applications without the risk of inducing emotional or cognitive side effects.
The biosynthesis of THCA-A proceeds through a tightly regulated sequence of enzymatic reactions within the cannabinoid metabolic cascade. The central molecule in this process is cannabigerolic acid (CBGA), which functions as the main precursor for the synthesis of the three primary acidic cannabinoids: THCA-A, cannabidiolic acid (CBDA), and cannabichromenic acid (CBCA). CBGA itself is formed via the condensation of geranyl pyrophosphate (GPP) and olivetolic acid through the action of an enzyme of the “arylprenyltransferase” type, which is also a defining step in the cannabinoid pathway.
The subsequent fate of CBGA in the plant is determined by the activity of specific synthase enzymes. In the case of THCA-A synthesis, the key enzyme is THCA synthase, which catalyzes the oxidative cyclization reaction, transforming CBGA into THCA-A. This reaction is strictly enzyme-mediated: in the absence of THCA synthase, the conversion does not occur. Hence, the formation of THCA-A is a genetically controlled process that depends on the expression of the THCAS gene. In chemotype I plants-i.e., strains with dominant Δ9-THC synthesis-the enzymatic profile leads to the predominance of THCA-A as the main cannabinoid.
This enzymatic pathway exhibits spatial specificity: THCA-A synthesis occurs primarily in the capitate glandular trichomes located on the female reproductive organs (especially the bracts of flowers). These structures concentrate the enzymatic activity associated with cannabinoid biosynthesis. Trichomes function as micro-organelles of secondary metabolism, where, within a protected environment-specifically within their secretory vesicular space-bioactive compounds accumulate in their acidic forms. THCA-A is synthesized within such microcompartments and stored in resinous exudates secreted onto the surface of the trichomes.
The spatiotemporal regulation of THCA-A synthesis in Cannabis sativa is a dynamic process linked to the vegetative and flowering stages. Maximum production is observed during the late flowering phase when trichomes reach full morpho-functional maturity. The biosynthetic activity of THCA synthase increases significantly at this stage, correlating with a rise in THCA-A concentration in the phytomass. Importantly, during the growth phase (vegetative stage), cannabinoid concentrations are low, confirming the secondary nature of these compounds-they are not structural or energetic metabolites but serve protective and signaling functions.
The natural origin of THCA-A results from the plant’s complex bioengineering strategy aimed at chemical defense, environmental adaptation, regulation of interactions with pathogens, and mediated communication with biotic agents (such as pollinators). The biochemical synthesis of cannabinoids-including THCA-A-belongs to the class of polyketide-terpenoid hybrid pathways, which combine elements of two major metabolite classes: fatty acids and isoprenoids. This makes cannabinoids evolutionarily unique compounds with dual origins and broad functional plasticity.
Cannabinoid Biosynthetic Pathway
The cannabinoid biosynthetic pathway in Cannabis sativa represents a specific branched metabolic cascade that produces three primary acidic phytocannabinoids: Δ9-tetrahydrocannabinolic acid (THCA-A), cannabidiolic acid (CBDA), and cannabichromenic acid (CBCA). All of these compounds originate from the common precursor-cannabigerolic acid (CBGA). This pathway functions as an endogenous biosynthetic program of secondary metabolism, whose activity is coordinated by an enzymatic system governed by the transcriptional expression of specific genes. Its uniqueness lies in the combination of elements from both the polyketide and mevalonate pathways, which converge in the formation of CBGA. Specialized synthase enzymes then catalytically produce the corresponding acidic cannabinoids from CBGA.
At the onset of cannabinoid biogenesis, olivetolic acid (OA) is synthesized via the polyketide pathway through the condensation of a six-carbon unit from acetyl-CoA with successive additions of malonyl-CoA. Once formed, OA reacts with geranyl pyrophosphate (GPP)-a product of the mevalonate (or MEP, depending on tissue type) pathway-forming CBGA. This reaction is catalyzed by arylprenyltransferase (APT), which specifically recognizes olivetolic acid as an aryl substrate and transfers the geranyl group.
At this point, CBGA becomes the crucial branching node of the biosynthetic flow. Its further fate depends on which synthase enzyme is expressed in a given trichome cell. If THCA synthase predominates, the result is THCA-A synthesis; if CBDA synthase predominates, CBDA is produced; correspondingly, expression of CBCA synthase results in CBCA formation. Thus, the plant’s chemotype-that is, its cannabinoid production profile-is determined by the allelic status and activity of one or more synthase genes.
THCA synthase is a flavoprotein that catalyzes the oxidative cyclization of CBGA into THCA-A, a transformation accompanied by the formation of new cyclic structures characteristic of the Δ9-tetrahydrocannabinol core. This process is not thermodynamically spontaneous and requires high specificity and spatial orientation of the substrate within the enzyme’s active site. A notable feature of this pathway is that it is predominantly localized in the secretory cells of capitate trichomes, which are specialized for the production of resinous metabolites.
An important aspect is the synchronization of enzymatic activity with the plant’s developmental phases. In young trichomes, CBGA synthesis is dominant, while the increasing activity of THCA synthase shifts the balance toward THCA-A production. This indicates strictly regulated temporospatial control of biosynthesis, coordinated by hormonal signaling, transcriptional levels, and likely epigenetic mechanisms.
Enzymatic Formation of THCA-A from Cannabigerolic Acid (CBGA)
The enzymatic transformation of CBGA into THCA-A represents a key stage in cannabinoid biosynthesis and determines the specific cannabinoid profile in typical “THC-dominant” strains of Cannabis sativa. This process is catalyzed by the enzyme THCA synthase-a monooxygenase that carries out oxidative cyclization of the CBGA side chain. The reaction belongs to the class of aerobic enzymatic cyclizations and involves flavin adenine dinucleotide (FAD) as a cofactor.
CBGA, the substrate for the reaction, possesses an open-chain aromatic structure containing a hydroxyl group, an isoprenoid side chain, and a carboxyl functional group. In the presence of THCA synthase, CBGA undergoes oxidation to yield a reactive quinonoid intermediate, which then reorganizes into a tetrahydrocannabinolic structure through electrophilic attack on the aromatic ring. The reaction concludes with ring closure and stabilization of the electronic structure, resulting in a fully formed THCA-A molecule.
This reaction is both stereoselective and stereospecific, meaning that THCA synthase not only catalyzes the cyclization but also determines the precise configuration of the chiral centers in the final product. The absence of enzymatic activity or its replacement with CBDA synthase alters the biosynthetic trajectory, demonstrating the critical dependence of the final cannabinoid composition on this single enzyme.
Enzyme functionality is only possible under favorable physiological conditions, including the presence of oxygen, an appropriate pH level, and a temperature compatible with cellular homeostasis. Additionally, CBGA must be transported to the site of synthase localization-most commonly to the apoplastic space of trichomes-suggesting the existence of active transport systems likely involved in the intracellular trafficking of cannabinoid precursors.
The end product, THCA-A, accumulates in vesicles of the trichomal secretory system and remains stable in its acidic form until exposed to heat. Upon heating or prolonged storage, non-enzymatic decarboxylation occurs, converting THCA-A into Δ9-THC, the psychoactive form. Thus, the enzymatic formation of THCA-A is a critical stage in controlling the biological activity of phytocannabinoids and mediates the transition between the inert and active forms of the compound.
Role of the THCA Synthase Enzyme
The enzyme THCA synthase serves as the functional center for the synthesis of Δ9-tetrahydrocannabinolic acid and plays a unique role in determining the chemotype of Cannabis sativa. It is a protein belonging to the flavin-containing monooxygenase (FMO) family, with a molecular weight of approximately 60 kDa, and is encoded by the nuclear gene THCAS, which is activated in trichomal cells.
The biochemical activity of THCA synthase lies in catalyzing an intramolecular reaction in which CBGA undergoes oxidation to form a phenolic semiquinone. This is followed by cyclization with the formation of a pyrone ring, which constructs the canonical structure of THCA-A. The reaction proceeds under mild conditions, without the need for coenzymes, but requires the presence of oxygen and flavin. The enzyme’s spatial structure includes an active site pocket where the substrate is fixed via hydrophobic and hydrogen-bond interactions. This specificity for CBGA ensures the absence of side products and maximized efficiency.
The THCAS gene is polymorphic, with allelic variants capable of producing inactive or partially active proteins. This explains phenotypic diversity among strains: cannabis plants with low THCA synthase activity predominantly synthesize CBGA or CBDA, whereas THC-dominant genotypes contain a fully functional enzyme. Expression of THCAS is strictly tissue-specific, being found almost exclusively in capitate glandular trichomes.
The enzyme displays a high degree of conservation in its active site but contains variable regions that can influence catalytic efficiency. This presents a potential for bioengineering redesign of the enzyme to generate novel cannabinoids or increase THCA-A yield in recombinant systems (e.g., in yeast or heterologous plants). THCA synthase itself is becoming a target of biotechnological research aimed at standardized industrial production of THCA-A outside of Cannabis sativa.
Spatiotemporal Regulation of Synthesis in Cannabis sativa
The biosynthesis of THCA-A in Cannabis sativa does not occur uniformly throughout the plant, but rather exhibits pronounced spatial and developmental specialization. The highest concentration of THCA-A accumulates in glandular trichomes of female flowers, particularly on the bracts, whereas in vegetative tissues (leaves, stems) its level is low or absent. This localization is due to the differentiation of trichomal structures as organelles of secondary metabolism, which provide an isolated environment for enzymatic activity.
Spatial regulation of synthesis involves not only tissue-specific but also subcellular organization: enzymes, substrates, and synthesis products are isolated within the apoplast of trichome secretory cells, preventing unwanted reactions in the cytosol. Secretory vesicles in which THCA-A accumulates possess a multilayered lipid membrane that restricts product diffusion and protects against degradation.
Temporal regulation is equally important. The peak of synthesis occurs at the end of the flowering phase, when trichomes reach full development and the transcriptional activity of THCAS increases in response to hormonal signals-particularly the influence of jasmonates, abscisic acid, and possibly light stimuli. In parallel, CBGA levels also rise, indicating coordinated synthesis of precursors and THCA synthase activity.
Structural Characteristics of the Molecule
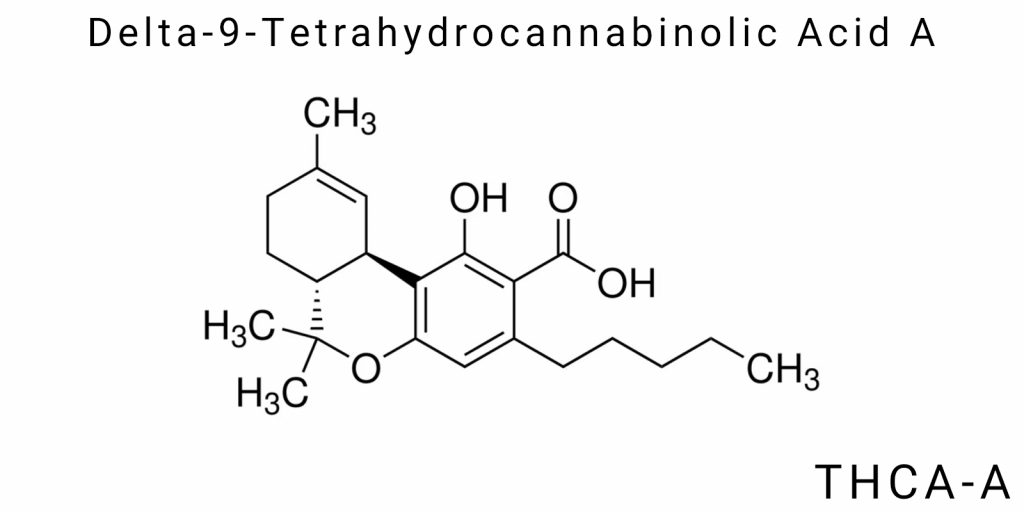
The chemical and structural organization of Δ⁹-tetrahydrocannabinolic acid A (THCA-A) is a central factor determining its biological functionality, physicochemical properties, and its distinction from its decarboxylated analog Δ⁹-tetrahydrocannabinol (Δ⁹-THC). As the precursor of the psychoactive form Δ⁹-THC, THCA-A does not exhibit psychoactive effects in its natural state; however, its molecular architecture confers a number of unique properties that define its functional role in plant biochemistry and its potential pharmacological applications. A deep understanding of the chemical nature of this molecule involves analysis of its composition, spatial configuration, and functional groups, as well as their influence on interaction with biological targets. It is critically important to distinguish THCA-A from related cannabinoids, particularly in terms of stability, polarity, and receptor affinity.
Chemical Formula and Molecular Mass
The THCA-A molecule has the molecular formula C₂₂H₃₀O₄, indicating the presence of twenty-two carbon atoms, thirty hydrogen atoms, and four oxygen atoms. Its molecular mass is approximately 358.48 g/mol. This mass is greater than that of Δ⁹-THC due to the presence of a carboxyl group (-COOH), which is the key distinguishing element. The carboxyl group renders the molecule more polar and water-soluble compared to the neutral Δ⁹-THC. Furthermore, the carboxyl group increases the acidity of the molecule, although it remains a weak organic acid. Biochemically, this means that THCA-A can engage in hydrogen bonding with proteins, lipids, or other molecules, imparting it with specific pharmacokinetic properties distinct from its psychoactive analog. From the perspective of organic chemistry, the chemical formula also reflects the presence of an aromatic ring, an alkyl chain, and an isoprenoid fragment structurally derived from cannabigerolic acid-the parent precursor for most phytocannabinoids.
The aromatic ring, characteristic of phenolic compounds, forms the molecular core and participates in numerous oxidation and hydroxylation reactions. The presence of a hydroxyl group in the ortho-position relative to the carboxyl group enhances the electron-donating properties of this part of the molecule, which may be important for metal chelation in soil or interaction with enzymatic systems. The presence of a pentyl side chain, a typical structural feature of most Δ⁹-cannabinoids, contributes to hydrophobicity and influences membrane permeability, particularly across lipid bilayers. Thus, the chemical formula of THCA-A is not merely a quantitative descriptor; it reflects structural-functional principles embedded in the molecular architecture that define its bioactivity, solubility, degradation, and metabolic pathway.
Stereochemistry and Configuration
THCA-A possesses a well-defined three-dimensional structure, which arises due to the presence of several chiral centers within the molecule. The primary chiral centers are located in the cyclohexane ring region, where conformation significantly influences the compound’s overall bioactivity. The predominant configuration of these centers is (6aR,10aR), corresponding to the natural isomer synthesized in the plant through the action of THCA synthase. The conformational stability of the molecule is maintained by intramolecular hydrogen bonds arising from interactions between hydroxyl and carboxyl groups. These interactions, in turn, affect the melting point, solubility in water and organic solvents, and chemical reactivity.
The spatial configuration of THCA-A determines its ability to selectively interact with biomolecules, including proteins, lipids, and nucleic acids, via specific spatially complementary recognition. This specificity is critically important in exploring potential therapeutic mechanisms of action, as even minor changes in stereochemistry can lead to significant alterations in biological activity-or loss of activity altogether. It is worth noting that stereoisomers not found in nature but which can be synthesized artificially typically do not exhibit the same bioactivity as the natural form. This again underscores the evolutionary adaptation of the plant’s enzymatic systems to produce only one spatial variant.
The spatial arrangement of atoms also determines the molecule’s propensity for autocatalytic decarboxylation. The presence of a carboxyl group in the α-position relative to the aromatic ring increases the likelihood of thermal instability. This factor is important in the context of cannabis extract or product preparation, as even slight heating can initiate decarboxylation and conversion to Δ⁹-THC. Thus, the stereochemistry of THCA-A not only defines its chemical inertness in terms of psychoactivity but also programs the thermodynamic fate of the molecule during external processing.
Differences from the Neutral Form Δ⁹-THC
The key distinction between THCA-A and Δ⁹-THC lies in the presence of a carboxyl group in THCA-A, which is absent in Δ⁹-THC due to the process of decarboxylation. This structural difference is decisive not only in terms of chemical composition but also in relation to pharmacodynamic and pharmacokinetic properties. THCA-A does not effectively interact with cannabinoid receptors CB1, which are the primary targets of psychoactive Δ⁹-THC. The reason for this inefficacy lies precisely in the molecule’s spatial and electronic configuration: the carboxyl group interferes with tight binding to the receptor’s active site due to steric hindrance and electrostatic mismatch.
Unlike Δ⁹-THC, which is lipophilic and readily crosses the blood-brain barrier, THCA-A is more polar and therefore characterized by weaker penetration into the central nervous system. This explains its lack of psychoactive effect while simultaneously opening avenues for therapeutic use not related to psychotropic influence. Additionally, THCA-A exhibits more stable pharmacokinetic profiles in the gastrointestinal tract, where the acid form shows greater resistance to degradation-unlike Δ⁹-THC, which is readily metabolized in the liver to 11-hydroxy-THC, a metabolite with enhanced psychoactivity.
From a thermal perspective, THCA-A is a thermolabile compound that undergoes decarboxylation upon heating to form Δ⁹-THC. This transition occurs within the temperature range of 105-120°C, which is critical during drying, smoking, or thermal extraction. In contrast, Δ⁹-THC is a more thermally stable molecule with broader applicability in pharmaceutical formulations. However, it is precisely the capacity of THCA-A for controlled transformation into Δ⁹-THC that allows regulation of psychoactive compound levels in cannabis products, which is a key factor in pharmaceutical formulation.
Technologies for Extracting THCA-A: Applications Ranging from Medical to Fundamental Science
Δ⁹-Tetrahydrocannabinolic acid A (THCA-A), despite being a precursor to the psychoactive Δ⁹-THC, is increasingly recognized as a distinct molecule with a unique spectrum of properties. Unlike other phytocannabinoids, its status as an unstable, thermolabile acidic component necessitates specific conditions for extraction, storage, and application. These characteristics have spurred the development of specialized technologies for the extraction, purification, and identification of THCA-A. However, even more significant is the question of its practical applications. Currently, THCA-A is considered a promising candidate in various fields-from applied medicine to pharmacognosy, agronomy, and molecular biochemistry.
Initially, THCA-A was primarily viewed as a transitional form to Δ⁹-THC and was not the subject of targeted research as a bioactive substance. However, advancements in methods for isolating unstable molecules without decarboxylation, particularly through the development of cryogenic extraction and precision chromatography, have paved the way for experimental and clinical studies of THCA-A in its primary acidic form. This isolated form has revealed a range of properties that distinguish it from neutralized cannabinoids. Most research has focused on its anti-inflammatory, neuroprotective, antiproliferative, and immunomodulatory potential.
In medical studies, THCA-A demonstrates selective activity toward key targets in the inflammatory cascade, such as COX-1, TNF-α, IL-6, as well as transcription factors like NF-κB. Notably, it does not stimulate psychoactive pathways, making it suitable for long-term use in patients with chronic diseases without the risk of psychogenic side effects. Particularly promising are studies on neurodegenerative conditions-such as Alzheimer’s disease, Parkinson’s disease, and multiple sclerosis. Here, THCA-A exhibits the ability to stabilize microglia and regulate glutamate neurotransmission, as confirmed by in vitro results and animal model studies .
Furthermore, THCA-A exhibits cytostatic activity in several oncological models, including colorectal cancer, breast cancer, and glioblastoma. Its mechanism of action is based on inhibiting the cell cycle at the G1/S phase and modulating the expression of apoptosis-related genes, such as BAX, BCL-2, and caspases. Researchers note THCA-A’s ability to influence signaling pathways like PI3K/Akt and MAPK, providing selective toxicity to cancer cells while preserving the viability of normal cells. Unlike Δ⁹-THC, which has psychoactive limitations in dosing, THCA-A allows for an expanded therapeutic window and the use of higher doses without adverse neurobehavioral effects .
Another promising area is gastroenterology, particularly the treatment of inflammatory bowel diseases (IBD), such as Crohn’s disease and ulcerative colitis. Preliminary clinical studies have shown that THCA-A reduces inflammation markers and promotes the restoration of the intestinal mucosa through modulation of the endocannabinoid system without involving CB1/CB2 receptors. Likely, alternative targets are involved-specifically PPARγ and TRP channels, which remain active in acidic cannabinoid derivatives. This aspect reflects the complex interplay between chemical form, receptor specificity, and physiological activity, opening avenues for non-canonical pharmacological approaches .
In the field of neurology, the anticonvulsant properties of THCA-A are being investigated. Preliminary results indicate that in epilepsy models, THCA-A reduces seizure frequency without altering baseline excitability, distinguishing it from Δ⁹-THC, which can have both anticonvulsant and proconvulsant effects depending on the dosage. Additionally, THCA-A does not induce tolerance even with prolonged use, making it an attractive alternative for patients with pharmacoresistant epilepsy.
Beyond the clinical context, THCA-A is considered a convenient marker in pharmacognostic studies of Cannabis sativa varieties. Its content allows for the classification of cannabis phenotypes, monitoring of inflorescence maturity stages, and optimization of cultivation conditions. In laboratory settings, THCA-A serves as a substrate for studying the catalytic properties of THCA synthase, important for synthetic biology and biotechnology. Specifically, methods are being developed for the genetic modification of yeast and bacteria capable of biosynthesizing THCA-A through the expression of cannabinoid enzymes. This opens the path to bioenzymatic production of cannabinoids without plant cultivation, with potential for industrial scaling.
Moreover, THCA-A is actively studied in fundamental biochemistry as a model structure for investigating interactions between small organic molecules and proteins. Its well-characterized stereochemistry, presence of polar and hydrophobic regions, and ability for autocatalytic transformation make it an ideal candidate for use in studies of enzymatic reactions, molecular docking, and proteomics. Spectroscopic and chromatographic methods developed for THCA-A analysis are now being adapted for other natural acidic cannabinoids, including CBDA and CBGA, expanding the analytical toolkit in phytochemistry.
Extraction Methods for Raw Cannabis
The extraction of THCA-A from raw cannabis is a key stage in obtaining a pure and stable product that preserves the cannabinoid’s acidic form. The extraction process requires adherence to specific physicochemical conditions, as THCA-A is a thermolabile compound, sensitive to heat, light, and oxidation. To preserve its biological activity and chemical integrity, low-temperature technologies are applied, and exposure to oxygen and ultraviolet radiation is minimized.
The plant material-raw cannabis-is prepared for extraction through careful harvesting at the peak stage of THCA-A accumulation, which usually occurs at the end of the flowering phase. The material is ground to increase the contact surface with the solvent, while temperature is strictly controlled to avoid the onset of thermal degradation. Once ground, the raw material is stored under cold conditions to prevent enzymatic transformations and chemical breakdown.
The choice of extraction method depends on the requirements of the final product and the specifics of its application. Low-temperature extraction using appropriate solvents ensures the maximum preservation of THCA-A. The use of both polar and non-polar solvents allows for variation in the spectrum of extracted compounds, from cannabinoids to terpenoids, although the process conditions strictly regulate temperature and contact time to avoid the conversion of THCA-A into Δ⁹-THC. Developed methodologies reduce the adverse effects of oxidative processes, particularly through the use of inert gases in the extraction zone and the hermetic sealing of the product.
Properly treated plant material undergoes technological cycles designed to optimize THCA-A yield. Within these cycles, conditions are tailored to minimize the presence of by-products such as chlorophyll, lipids, and other polar compounds that may affect extract quality. For this reason, preliminary purification and filtration steps are implemented to enhance extraction selectivity.
Technological approaches also consider the need to adapt the process to industrial-scale production, which requires a balance between product yield and quality. Optimizing operational parameters of extraction systems allows for maintaining a stable level of active compound with minimal loss. Another crucial factor is process safety, which involves monitoring potential solvent residues and preventing explosive conditions.
Conditions for Preserving the Acidic Form (Low Temperature, Absence of Light)
Δ⁹-Tetrahydrocannabinolic acid A (THCA-A) is a chemically unstable compound that requires specific conditions to preserve its acidic form during storage and handling. The main factors leading to THCA-A degradation are elevated temperatures, exposure to ultraviolet and visible light, and oxidative processes that can initiate decarboxylation-a chemical transformation of the acid into neutral Δ⁹-tetrahydrocannabinol (THC). Since THCA-A possesses unique pharmacological properties distinct from THC, preserving its structure is a top priority in the technologies of cannabinoid-based product manufacturing and storage.
Temperature control is the key parameter for THCA-A stability. At temperatures above 40°C, decarboxylation accelerates significantly, resulting in the loss of the acidic form and conversion into the active but neutral THC molecule. In laboratory and industrial settings, storage of extracts containing THCA-A is recommended at temperatures between -20°C and +4°C. This temperature range minimizes the kinetic pathways of degradation and prevents the activation of thermolabile reactions. Maintaining low temperatures is particularly critical during transport and long-term storage, as even short-term temperature rises can significantly reduce THCA-A concentration.
In addition to temperature, light exposure-especially ultraviolet (UV) radiation-has a significant impact, inducing photodegradation of the molecule. THCA-A contains a conjugated system of double bonds in its benzene ring, making it sensitive to photochemical reactions. Light disrupts the electronic structure and activates radical processes that contribute to molecular breakdown or conversion. Therefore, the entire process of extraction, purification, and storage must take place under minimal light exposure: opaque or amber containers are used, and the material is shielded from direct sunlight and laboratory lighting.
The oxidative environment also significantly affects THCA-A stability. Contact with atmospheric oxygen promotes the formation of peroxide radicals, which initiate polymerization or molecular degradation. To minimize this process, airtight containers are used, often filled with inert gases (argon or nitrogen), creating a passive environment that reduces oxygen activity. In some technologies, antioxidants are also applied to stabilize the extracts and prevent oxidative damage.
Another important aspect is the effect of humidity. High humidity can promote hydrolytic reactions or activate enzymatic processes that indirectly impact THCA-A stability. Therefore, storage facilities are designed to maintain optimal humidity levels (approximately 30-50%) using air dehumidifiers or climate control systems.
Ethanol, Carbon Dioxide, and Butane Extraction
Extraction of THCA-A from raw cannabis is carried out using various solvents, among which ethanol, supercritical carbon dioxide (SC-CO₂), and butane hold particular importance due to their physicochemical properties and specific interactions with plant material. Each of these methods is characterized by its own advantages and technological challenges that affect the yield and purity of the target cannabinoid.
Ethanol extraction is one of the most widespread and historically established technologies. Ethanol possesses polarity sufficient to extract a broad spectrum of phytochemical components, including THCA-A, terpenoids, and flavonoids. Its advantages include relative safety, availability, and the ability to control temperature, allowing for minimization of decarboxylation. Ethanol is cooled to low temperatures (-20 °C and below) to reduce the solubility of undesired components such as chlorophyll, which facilitates subsequent purification. After extraction, the solvent is removed via vacuum evaporation at temperatures below 40 °C, preserving the acidic form of THCA-A. A drawback of ethanol is its tendency to extract not only cannabinoids but also a significant quantity of other plant compounds, which complicates the purification process and necessitates additional filtration and chromatographic steps.
Carbon dioxide in its supercritical state (SC-CO₂) represents a more technologically complex but highly efficient method. SC-CO₂ combines properties of both gas and liquid, enabling deep penetration into plant tissues and selective dissolution of target compounds. Pressure and temperature control allows for manipulation of the solubility of THCA-A and other molecules, promoting the production of pure extracts with minimal impurities. This method is especially valuable as it leaves no toxic solvent residues, and the process occurs in the absence of oxygen, maintaining the stability of the acidic form. However, SC-CO₂ extraction requires expensive equipment and strict parameter control, limiting its use in small-scale laboratories.
Butane extraction is characterized by high solubility for nonpolar and weakly polar compounds, providing high cannabinoid yields, including THCA-A. It is conducted at low temperatures, reducing the risk of thermal degradation, and the solvent is easily removed through evaporation. This method is widely used on an industrial scale for producing concentrated products. However, butane is a flammable and toxic gas, necessitating strict adherence to safety protocols and meticulous control of residual solvents in the final product. Importantly, this method tends to extract predominantly nonpolar compounds, sometimes requiring combination with other techniques to obtain more complex extracts.
Cold Pressing and Live Resin Extraction
Cold pressing and the production of live resin are technologies designed to maximize the preservation of biologically active components in raw cannabis, including THCA-A, by minimizing thermal and oxidative stress on the material. These represent alternatives to traditional solvent-based extraction methods.
Cold pressing is based on mechanical extraction by compressing plant material at low temperatures (typically around 0-5 °C). The absence of heat prevents decarboxylation of THCA-A and the loss of volatile terpenoids, which are important for the full pharmacological profile of the product. The process is performed using specialized presses with slow compression, minimizing mechanical damage to tissues and the release of impurities. The resulting concentrate is characterized by a high concentration of THCA-A in its pure acidic form while retaining the natural spectrum of plant metabolites.
Live resin concentrates are produced from freshly harvested or minimally dried and frozen cannabis biomass. The process involves rapid freezing of the plant immediately after harvesting, locking in the chemical composition and cellular structure. The frozen material is then subjected to extraction-typically using supercritical CO₂ or butane-at low temperatures, which allows for maximum preservation of the full cannabinoid profile, including unstable THCA-A, and volatile terpenoids. This is a high-tech method that yields concentrates closely resembling the chemical composition of the living plant, providing maximum pharmacological efficacy.
Both methods-cold pressing and live resin-are used to produce products that are as close as possible to the natural composition of cannabis and exhibit high biological activity. They require strict temperature control and processing speed, as well as the use of specialized equipment that minimizes contact with oxygen and light, preserving the acidic form of THCA-A. These technologies have gained popularity in the production of pharmaceutical preparations, cosmetic products, and research materials where high quality and chemical stability are essential.
Laboratory Isolation and Purification
In laboratory practice, the isolated acquisition of Δ9-tetrahydrocannabinolic acid A (THCA-A) with a high degree of purity is a necessary prerequisite for subsequent pharmacological, biochemical, or analytical research. In contrast to industrial extraction methods oriented toward large-scale cannabinoid concentrate production, laboratory procedures involve precise targeting of the molecule of interest within the complex matrix of secondary metabolites characteristic of Cannabis sativa. This task is complicated by the high chemical instability of THCA-A, particularly under the influence of heat, light, or elevated pH, which induces rapid decarboxylation to the psychoactive form Δ9-THC. Therefore, any effective extraction and purification of THCA-A requires a combination of specific temperature management, environmental control, and the application of high-precision analytical techniques, particularly chromatographic and spectroscopic methods.
One of the main objectives of laboratory purification is the preservation of the carboxylic acid functional group within the THCA-A structure, which defines its biochemical specificity and pharmacodynamic distinction from decarboxylated forms. The structure of this acidic cannabinoid complicates classical crystallization methods, thereby favoring separation techniques based on differential resolution between molecular fractions according to physicochemical properties such as polarity, molecular weight, and sorbent affinity. Under strict environmental control, the combination of liquid chromatography and spectral monitoring enables the production of THCA-A preparations with purity exceeding 98%, which is essential for fundamental science and pharmaceutical synthesis.
Chromatographic Techniques (HPLC, TLC, Column Chromatography)
Chromatography plays a key role in the isolation of THCA-A, as it allows for the efficient separation of the target molecule from accompanying cannabinoids, terpenes, flavonoids, and chlorophylls without the need for aggressive chemical transformations that might disrupt the acidic form. One of the most precise methods in this context is high-performance liquid chromatography (HPLC), which is applied for both analytical and preparative separation. A primary advantage of HPLC is its ability to operate at room or reduced temperatures, which is critically important for avoiding THCA-A decarboxylation.
In HPLC, separation of the complex mixture occurs due to differential affinities of its components for the stationary phase (typically reversed-phase C18) and the mobile phase, which consists of a water-organic gradient. Optimal conditions for THCA-A separation include low-temperature eluent delivery, controlled pH, and detection at a wavelength of approximately 220-280 nm. By scaling flow parameters and concentration, HPLC can also be adapted to yield milligram and gram quantities of purified THCA-A.
Thin-layer chromatography (TLC), although less sensitive, remains a valuable method for preliminary screening of the presence and purity of THCA-A in samples, as well as for monitoring purification stages. It is based on the separation of substances within the capillary field of a thin sorbent layer (usually silica gel), where THCA-A appears as a characteristic spot when specific visualizing reagents are applied (e.g., vanillin-sulfuric acid reagent). However, TLC does not allow for the full identification or isolation of large quantities of material and is used only as a supplementary technique.
Column chromatography is a classical preparative approach that allows for the separation of cannabinoids on a larger scale. For THCA-A purification, the column is packed with a polar sorbent (such as silica gel or aluminosilicates), while the mobile phase consists of mixtures of organic solvents, including hexane, ethyl acetate, methanol, or their gradients. Purification is conducted under controlled temperature conditions to prevent thermal degradation. Sequential fractionation of the eluates enables isolation of the target fraction with a dominant content of THCA-A, which is then subjected to analytical verification for purity and structural integrity.
Purity Analysis and Identification Using Spectroscopy (NMR, MS)
Following chromatographic isolation, the next critical step is confirming the identity and determining the purity level of THCA-A. High-precision spectroscopic methods are employed for this purpose, primarily nuclear magnetic resonance (NMR) and mass spectrometry (MS), which provide comprehensive structural analysis and enable verification of the presence or absence of impurities down to trace levels.
NMR spectroscopy is indispensable for verifying the chemical structure of THCA-A. The method is based on the interaction of atomic nuclei with a magnetic field and the detection of resonance signals characteristic of specific types of chemical environments. In the case of THCA-A, the greatest analytical interest lies in the protons of the carboxyl group, olefinic fragments, and aromatic ring, which allow for precise differentiation of the acidic form from the neutral Δ9-THC. The use of two-dimensional NMR spectroscopy (COSY, HSQC, HMBC) enables accurate mapping of all interatomic bonds, confirming the completeness of the structure and the absence of isomerizations or byproducts.
Mass spectrometry (MS) allows identification of the molecular weight and fragmentation profile of the compound. THCA-A has a characteristic molecular weight of 358.48 g/mol, and its ionization products produce characteristic peaks in the spectrum with exact fragment masses that confirm its presence even in extremely small quantities. When combined with chromatography (e.g., HPLC-MS), mass spectrometry provides a dual level of identification — both by retention time and spectral signature.
In some cases, Fourier-transform infrared spectroscopy (FTIR) is also used, which allows detection of specific functional groups, notably the carboxyl and phenolic fragments, which are key markers of THCA-A. Ultraviolet-visible spectroscopy (UV-VIS) is also employed as an auxiliary tool for concentration determination in analytical media.
The combination of chromatographic separation with multidimensional spectroscopic identification ensures a high degree of reliability in confirming the structure and purity of THCA-A, which is critically important for its further application in experimental biomedicine, toxicology, pharmacokinetics, and chemical synthesis of derivatives. These methods form a gold-standard procedure that helps avoid artifacts and false interpretations when studying cannabinoid biochemistry.
Pharmacological and Physiological Properties of THCA-A
Δ9-Tetrahydrocannabinolic acid A (THCA-A) is the primary acidic form of Δ9-tetrahydrocannabinol (THC) that accumulates in raw cannabis until thermal or photochemical decarboxylation occurs. Despite structural similarity to Δ9-THC, THCA-A exhibits a fundamentally different pharmacological profile due to the presence of the carboxyl group, which limits its penetration across the blood-brain barrier and alters its affinity for major cannabinoid receptors. For a long time, THCA-A was considered only as a precursor to psychoactive Δ9-THC; however, contemporary science increasingly recognizes this compound as an independent bioactive agent with its own spectrum of physiological activity. Its properties are increasingly studied within applied pharmacology, neuroscience, oncology, and immunomodulation.
One of the key features of THCA-A is its low psychotropic potential, which opens prospects for developing therapeutic agents without the risk of cognitive or behavioral intoxication. This significantly broadens the possibilities for its clinical application, particularly in patients with neurodegenerative diseases, oncological pathologies, or chronic inflammatory conditions requiring long-term pharmacological support without altered consciousness. In this context, THCA-A is primarily of interest as a potential modulator of enzymatic cascades, an inhibitor of pro-inflammatory signaling pathways, and as an agent with neuroprotective effects.
It is important to emphasize that THCA-A is not pharmacologically inert. Its bioactivity is not mediated through classical CB1 receptors, to which it has low affinity, but rather through indirect effects on other molecular targets, including cyclooxygenase cascade enzymes (COX-1, COX-2), ion channels, and likely signaling receptors such as PPARγ, TRPV1, and GPR55. Furthermore, there is growing evidence that THCA-A may act as an antiproliferative agent in certain tumor cell lines and also display the ability to stabilize neuronal activity in neurodegeneration models. Some studies indicate potential antiemetic activity, which could be utilized during chemotherapy support.
Another important aspect is the limited penetration of THCA-A across the blood-brain barrier, which on one hand restricts its central nervous system effects but on the other makes it attractive for targeted action in peripheral tissues, where it can directly influence immune cells, inflammatory components, and receptor networks without psychoactive effects. This opens the prospect of developing THCA-A-oriented pharmaceutical formulations aimed at systemic rather than neuropsychiatric treatment.
Additionally, THCA-A exhibits significant chemical lability, necessitating strict control of storage and administration conditions. The pharmacokinetic properties of THCA-A remain insufficiently studied; however, preliminary data suggest that its bioavailability upon oral administration is higher than that of the decarboxylated form, due to better solubility in aqueous environments. Nevertheless, metabolic stability and excretion pathways require further investigation, especially considering possible transformations in the gastrointestinal tract and liver.
Bioactivity Without Decarboxylation
THCA-A, as a natural acid formed in Cannabis sativa plants, differs from its neutral form Δ9-THC not only structurally but also in physiological and pharmacological properties. The most important aspect of THCA-A’s bioactivity is that it functions in the body without the need for prior decarboxylation-that is, without losing the carboxyl group, which significantly influences the molecule’s interactions and pharmacodynamics. This fact dramatically expands the potential uses of THCA-A as a bioactive component with unique characteristics distinct from psychoactive THC.
The neutralization of the carboxyl group during decarboxylation transforms the molecule into an active psychoactive cannabinoid capable of crossing the blood-brain barrier, actively binding to CB1 receptors in the central nervous system, and producing psychoactive effects. In contrast, THCA-A, retaining its carboxyl group, has a significantly lower affinity for cannabinoid receptors, especially CB1, which explains the absence of typical THC psychoactive effects when the non-activated form is administered.
THCA-A’s bioactivity is characterized by its ability to modulate various molecular targets in the body that are not associated with the classical endocannabinoid system receptors, including enzymatic systems, ion channels, and inflammatory signaling cascades. Thus, THCA-A shows potential as an agent affecting physiological processes outside the central nervous system, opening the door to pharmacological strategies without psychotropic burden.
Particular interest is drawn by data showing that THCA-A is a potent inhibitor of cyclooxygenase enzymes COX-1 and COX-2, which are key catalysts in the synthesis of pro-inflammatory prostaglandins. Because of this, it can act as a natural anti-inflammatory agent by regulating immune cell activity and reducing inflammatory mediators in tissues. This property makes it promising for research in autoimmune diseases, chronic pain, and neurodegenerative disorders, where inflammatory processes play a fundamental role.
At the same time, THCA-A interacts with various receptors outside the classical CB1/CB2 system, notably PPARγ-a nuclear receptor that regulates metabolic processes, cell differentiation, and immune response. Activation of PPARγ is associated with anti-inflammatory, antiproliferative, and neuroprotective effects, making this interaction pharmacologically significant. Additionally, THCA-A may influence ion channels such as TRPV1, involved in pain signal transmission and thermoregulation, potentially explaining some of its analgesic and neuromodulatory properties.
Thus, THCA-A’s bioactivity without decarboxylation lies in its ability to function as a multi-target molecular agent that modulates key physiological and pathophysiological processes without psychoactive effects. This opens prospects for its medical use as a foundational component of therapeutic regimens requiring inflammation reduction, immune regulation, neuroprotection, and pain mitigation without impairing cognitive functions.
Absence of Psychoactive Effects
The absence of psychoactive effects is one of the most important distinctions between THCA-A and Δ9-THC, making THCA-A attractive for medical use without the side effects related to intoxication. The psychoactivity of Δ9-THC is due to its high affinity for CB1 receptors, which are densely located in the central nervous system, including the brain, where they regulate neurotransmitter processes, psychomotor activity, cognitive functions, and perception. THCA-A, with its carboxyl group, is a more polar molecule, significantly reducing its ability to cross the blood-brain barrier and limiting its impact on the central nervous system.
The placement of the carboxyl group in the THCA-A molecule changes its spatial configuration, substantially affecting its interaction with CB1 receptors. This structural difference causes the molecule’s affinity for these receptors to decrease by several orders of magnitude compared to Δ9-THC, so THCA-A does not induce typical psychotropic effects such as euphoria, cognitive impairment, or sensory disorientation. Experimental models in vitro and in vivo have confirmed that THCA-A does not activate CB1 receptor signaling cascades, which accounts for its pharmacological safety in this context.
The lack of psychoactivity does not mean that THCA-A is pharmacologically inactive. On the contrary, it exhibits a broad spectrum of biological activities at the peripheral level, primarily through other molecular targets unrelated to psychoactivity. However, this factor significantly simplifies the use of THCA-A in therapy since patients can take THCA-A-based preparations without the risk of mental disorders and without limitations related to coordination and cognitive function.
Pharmacokinetic studies demonstrate that, upon systemic administration, THCA-A has limited capacity to accumulate in the central nervous system, which further guarantees the absence of prolonged psychoactive effects even with repeated use. This is critically important for the development of safe pharmaceutical forms targeting chronic inflammatory, neurodegenerative, and oncological conditions.
Overall, the absence of psychoactive effects is a defining pharmacological advantage of THCA-A, allowing it to be considered a safe biological agent for a wide range of medical applications where restrictions related to psychotropic effects are unacceptable.
Effects on Enzymatic Systems, Receptors, and Inflammatory Cascades
THCA-A exhibits a complex influence on several key enzymatic systems, receptors, and inflammatory cascades, shaping its pharmacological profile beyond the classical cannabinoid system. One of the most significant targets is the cyclooxygenase enzymes (COX-1 and COX-2), which catalyze the synthesis of prostaglandins-mediators of inflammation and pain. THCA-A acts as an inhibitor of these enzymes, leading to a reduction in the production of pro-inflammatory prostaglandins, effectively suppressing the inflammatory process at the molecular level. This mechanism overlaps with the pharmacology of nonsteroidal anti-inflammatory drugs, but THCA-A has the potential to be more selective and less toxic.
In addition to COX enzymes, THCA-A may influence the lipoxygenase system involved in arachidonic acid metabolism, as well as other enzymes linked to oxidative stress and the metabolism of reactive oxygen species. Such interactions contribute to the antioxidant effects of THCA-A, which is especially relevant for neuroprotection and prevention of degenerative tissue changes.
The receptor profile of THCA-A includes activation of nuclear receptors of the PPARγ type, which play a role in regulating immune response, glucose metabolism, and cell differentiation. Interaction with PPARγ provides anti-inflammatory and antiproliferative effects that are significant for the treatment of chronic inflammatory and oncological diseases. The uniqueness of this interaction lies in the ability to regulate immune processes without direct activation of the central nervous system.
Additionally, THCA-A can modulate ion channels of the TRP family, particularly TRPV1, which are involved in pain signal transmission and inflammatory responses. Influence on TRPV1 may contribute to analgesic effects and reduction of neuropathic pain, as supported by experimental data.
Furthermore, THCA-A has the potential to affect other G-protein-coupled receptors, including GPR55, which participate in the regulation of inflammatory processes and immune responses. The interaction with these receptors is still under investigation but may play a significant role in the overall pharmacology of THCA-A.
Research on Potential Effects
The potential pharmacological effects of THCA-A are the subject of intensive research due to the unique structure of this molecule, which allows it to interact with diverse biological targets without exhibiting the psychoactivity characteristic of Δ9-THC. Recent scientific data confirm that THCA-A may possess neuroprotective, anti-inflammatory, as well as antiemetic and antitumor properties. All of these characteristics grant THCA-A significant therapeutic potential and stimulate further preclinical and clinical studies.
The neuroprotective action of THCA-A is associated with its ability to modulate oxidative stress, inflammatory processes in nervous tissue, and to maintain the structural integrity of neurons. Studies in models of neurodegenerative diseases show that THCA-A can protect nerve cells from apoptotic stress by reducing free radical levels and activating antioxidant mechanisms. Moreover, the molecule demonstrates effects on specific signaling pathways such as MAPK and NF-kB, which regulate the expression of pro-inflammatory genes and cytokines in the brain, which is important for preventing degenerative processes.
The anti-inflammatory potential of THCA-A is driven by its ability to suppress the activity of key enzymes and molecules involved in the generation and maintenance of inflammatory responses. Especially important is its effect on cyclooxygenases COX-1 and COX-2, which significantly reduces the synthesis of pro-inflammatory prostaglandins. This enables a reduction of local and systemic inflammatory processes, which is particularly relevant for treating autoimmune diseases, arthritis, and chronic inflammatory conditions. In addition, THCA-A can modulate the activity of leukocytes and macrophages, suppressing the production of pro-inflammatory cytokines, thereby creating an additional mechanism to counteract chronic inflammation.
The antiemetic and antitumor effects of THCA-A are being investigated in preclinical models and confirm its broad pharmacological activity. THCA-A shows the ability to inhibit the proliferation of cancer cells, particularly in studies on tumor cell cultures such as lymphoma, breast cancer, and colorectal cancer. These effects are linked to the induction of apoptosis, as well as the ability to inhibit key signaling pathways responsible for tumor growth and metastasis. The antiemetic potential of THCA-A manifests in its ability to reduce nausea and vomiting, which frequently occur during chemotherapy, through effects on receptors that regulate vomiting centers in the central nervous system.
Neuroprotective Effects
The neuroprotective properties of THCA-A represent one of the key areas of scientific research related to the potential therapeutic use of this cannabinoid in neurodegenerative diseases. The molecular mechanism of THCA-A action includes its ability to reduce oxidative stress, which is a fundamental factor in the development of diseases such as Alzheimer’s, Parkinson’s, and other neurodegenerative disorders.
In preclinical models, THCA-A demonstrates a significant reduction in the production of free radicals and markers of oxidative damage to lipids and proteins in brain tissues. This effect is associated with the activation of the endogenous antioxidant system, notably the increased activity of the enzymes superoxide dismutase and catalase. Through this, THCA-A decreases damage to cellular membranes, stabilizes mitochondrial functions, and promotes the preservation of neuronal viability.
Beyond its antioxidant action, THCA-A influences inflammatory processes in the brain by inhibiting the activity of microglia-key immune cells of the central nervous system that, when excessively activated, contribute to neuronal damage. It reduces the secretion of pro-inflammatory cytokines such as TNF-α and IL-1β, helping to control inflammation and prevent further neurodegenerative destruction.
THCA-A also modulates signaling pathways associated with apoptosis, including the caspase cascade and regulation of Bcl-2 family proteins, which govern neuronal survival. This action supports the protection of brain cells from programmed cell death, which is critically important in chronic diseases associated with progressive neuron loss.
The effect of THCA-A on TRPV1 ion channels in the nervous system aids in modulating pain sensitivity and regulating neural transmission, potentially complementing its neuroprotective effect by reducing neuropathic pain.
Anti-inflammatory Potential
The anti-inflammatory activity of THCA-A arises from its ability to influence key molecular links of the inflammatory process, distinguishing it from many other cannabinoids. Experimental data show that THCA-A effectively inhibits the activity of cyclooxygenase enzymes COX-1 and COX-2, leading to a decrease in the synthesis of prostaglandins-mediators that play a central role in the development of inflammation, pain, and swelling.
Inhibition of COX enzymes not only alleviates symptoms but also slows the progression of the inflammatory process at the molecular level. THCA-A exhibits a more selective action profile compared to traditional nonsteroidal anti-inflammatory drugs, potentially reducing the risk of side effects such as gastrointestinal damage.
Besides its effect on COX enzymes, THCA-A suppresses the production of pro-inflammatory cytokines such as interleukins IL-6 and IL-8, as well as tumor necrosis factor TNF-α. This occurs through modulation of transcription factors, particularly NF-kB, which regulate the genetic expression of inflammatory mediators. This allows THCA-A to effectively regulate immune responses and reduce chronic inflammation, often the basis for many chronic diseases, including autoimmune disorders.
THCA-A also demonstrates the ability to decrease macrophage activity and the infiltration of inflammatory cells into tissues, further contributing to the regulation of inflammatory processes and supporting the restoration of tissue homeostasis.
Antiemetic and Antitumor Effects (Preclinical Data)
The antiemetic effect of THCA-A is supported by studies on models of nausea and vomiting, which frequently accompany cancer therapy. This cannabinoid acts through interactions with central nervous system receptors, notably CB1, as well as potentially 5-HT3 serotonin receptors, which are important mediators in controlling the vomiting reflex. In preclinical studies, THCA-A reduces the intensity and frequency of vomiting, which can significantly improve the quality of life for patients undergoing chemotherapy.
It should be noted that THCA-A does not exhibit psychoactivity, making it a safer alternative to classical cannabinoids for managing chemotherapy symptoms.
The antitumor properties of THCA-A are among the most promising areas actively studied in preclinical experiments. In cancer cell cultures, THCA-A shows the ability to inhibit proliferation, induce apoptosis, and arrest the cell cycle. The molecular mechanisms of these effects include activation of caspases and modulation of signaling pathways such as PI3K/Akt/mTOR and MAPK, which play key roles in regulating tumor cell growth and survival.
Data also indicate that THCA-A may inhibit angiogenesis-the process of new blood vessel formation necessary for tumor growth and metastasis. This is achieved by reducing the expression of growth factors such as VEGF, which support vascular development in tumors.
Individual studies demonstrate the efficacy of THCA-A against breast cancer, lymphoma, and colorectal cancer, making it a promising agent for comprehensive oncological therapy, especially in combination with traditional treatment methods.
Decarboxylation: Conversion of THCA-A to Δ9-THC
The decarboxylation of THCA-A into Δ9-THC is a crucial chemical process that governs the transformation of cannabinoids from their inactive or weakly active acidic form into a biologically active psychoactive form. THCA-A (trans-tetrahydrocannabinolic acid A) naturally accumulates in the plant as an acid precursor. It does not exhibit noticeable psychoactive effects due to the presence of a carboxyl group, which limits its ability to interact with cannabinoid receptors, particularly CB1 receptors responsible for psychoactive effects. The decarboxylation process involves the loss of the carboxyl group as carbon dioxide (CO2), which occurs under the influence of heat, time, and other external factors. This chemical transformation is necessary to activate THCA-A and produce Δ9-THC, which significantly alters the pharmacological properties of the compound.
The decarboxylation process occurs naturally but can be controlled through preparation, storage, and processing conditions of cannabis. Heating raw cannabis plant material or concentrates promotes the cleavage of the carboxyl group, releasing CO2 and forming the active cannabinoid. The absence or insufficient heating preserves THCA-A in its acidic form, defining its unique pharmacological profile.
It is important to emphasize that decarboxylation is not instantaneous and passes through several intermediate states, where depending on temperature and time parameters, partial or complete conversion may occur. Besides heat, the rate and efficiency of decarboxylation are influenced by humidity, the presence of catalysts, and the type of material in which the reaction takes place.
From a chemical standpoint, decarboxylation is a straightforward reaction involving the breakdown of the carboxyl group, but biochemically it represents a transformation with profound implications for interaction with the endocannabinoid system receptors. After decarboxylation, Δ9-THC gains the ability to effectively bind to CB1 receptors, inducing the typical psychoactive effects underlying recreational and therapeutic cannabis use.
Understanding and controlling the decarboxylation process is critically important for the pharmaceutical industry and medical cannabis applications. It determines both the degree of activation of bioactive compounds and the pharmacodynamic profile of cannabis-based medications. Some therapeutic approaches deliberately avoid decarboxylation to preserve the specific effects of THCA-A, while others aim for maximum conversion to Δ9-THC.
Decarboxylation also impacts the stability and shelf life of cannabinoid products, as THCA-A is relatively more stable compared to Δ9-THC, which is more susceptible to oxidation and degradation during prolonged storage. Therefore, in manufacturing, storage, and transportation of cannabinoids, parameters must be carefully regulated to avoid unwanted decomposition or loss of activity.
Mechanism and Kinetics of the Reaction
Decarboxylation of THCA-A is a chemical reaction accompanied by the cleavage of a carbon dioxide (CO₂) molecule from the carboxyl group attached to the main cannabinoid backbone. This process occurs via thermal energy, which provides the molecule with the necessary activation energy to transition into the active form – Δ9-tetrahydrocannabinol (Δ9-THC). The reaction belongs to the class of thermally induced decomposition reactions, which do not require water or additional reagents but depend on temperature, exposure time, the reaction environment, and the physical state of the compound itself. In the case of THCA-A, a covalent bond between the carboxyl group (-COOH) and the rest of the molecule breaks, releasing carbon dioxide and forming a transient intermediate that rapidly stabilizes into the Δ9-THC structure.
At the molecular level, the mechanism involves formation of a transition state with electron density rearrangement, leading to CO₂ loss. This process is endergonic in the initial phase, meaning it requires energy input, but once the transition state is reached, the reaction becomes thermodynamically favorable. The kinetics of the reaction are complex because the rate depends not only on absolute temperature but also on physicochemical properties of the environment: humidity, pressure, presence of other components in the matrix, and sample morphology (grinding, density, degree of dispersion). It is important to note that in real conditions, decarboxylation is part of a broader set of degradation processes, so isolating the reaction for study requires controlled experimental conditions.
Pharmacological relevance of this reaction lies in the fact that only Δ9-THC exhibits full activity at central nervous system CB1 receptors, while THCA-A is antagonistically neutral in this context. Therefore, effective decarboxylation is a necessary condition for preparing medicinal formulations with psychoactive or central nervous system effects. Additionally, products of partial or incomplete decarboxylation may possess their own bioactivity, requiring separate investigation, as formation of intermediate compounds can influence the overall effect profile of the final product.
Temperature and Time Dependencies
The temperature dependence of THCA-A decarboxylation follows classical thermochemical reaction patterns governed by the Arrhenius equation. Increasing temperature accelerates the reaction by raising the average kinetic energy of molecules and the frequency of effective collisions. Studies show that THCA-A decarboxylation begins at temperatures above 90-100°C, with the optimal range for complete conversion between 110°C and 145°C. Within this range, the reaction proceeds with maximum efficiency without significant risk of thermal degradation of the produced Δ9-THC. Outside this range – both at too low and too high temperatures – side reactions occur, such as incomplete conversion, isomerization of Δ9-THC to Δ8-THC, or degradation to cannabinol (CBN).
The time factor is also critically important. Low-temperature decarboxylation requires longer durations, sometimes up to 60-90 minutes, whereas at higher temperatures (above 130°C), the reaction completes within 20-30 minutes. However, shortening the time at high temperatures carries the risk of oxidation products formation, especially in the presence of oxygen or moisture. In this context, maintaining an anaerobic environment and controlling humidity are critical technological parameters, particularly during preparation of extracts for medical use.
The presence of water or other volatile components can alter the boiling point of the system, affecting the local temperature of the molecule itself. Moisture in raw plant material can delay the reaction onset or cause uneven decarboxylation. Therefore, in analytical practice and industrial production, pre-drying of samples or humidity control through vacuum dryers is employed. It is also known that different matrices – essential oils, lipids, terpene profiles – have their own thermal conductivity properties, which change local reaction conditions and require temperature regime adjustments for each specific case.
The relationship between temperature, time, and stability of the final product is nonlinear. To achieve optimal results, mathematical modeling of reaction kinetics is often used. Such models predict system behavior under given parameters and allow for adaptation to specific technologies – drying, baking, extraction, or concentrate synthesis. Determining the reaction’s time constant within a specific temperature range enables precise control and minimizes side processes.
Catalysis and External Factors
Although the decarboxylation of THCA-A is traditionally considered a thermally induced reaction, certain external factors can act as catalysts or modifiers of the reaction kinetics. The most obvious among these are light, oxygen, moisture, and the presence of acid-base impurities, which can lower the activation energy or alter the reaction pathway. Specifically, exposure to ultraviolet or visible light leads to premature or uneven decarboxylation, especially in samples stored in clear containers. Light causes photochemical excitation that can initiate degradation reactions even before thermal exposure, reducing the yield of Δ9-THC and accelerating the formation of oxidized byproducts.
Oxygen, in the presence of light or high temperature, acts as a strong oxidizer that not only stimulates decarboxylation but also leads to further degradation of Δ9-THC into CBN, which is undesirable in most cases. For this reason, decarboxylation processes are typically carried out in an inert atmosphere-nitrogen or argon-or under vacuum conditions. This approach not only improves conversion efficiency but also preserves the stability of the produced Δ9-THC.
Acid-base catalysis is potentially possible in the presence of impurities such as mineral acids or bases that may remain in the material after extraction. Under such conditions, decarboxylation can proceed via alternative mechanisms, producing side products or even altering the molecule’s stereochemistry. It has also been established that the presence of metal ions, especially Fe³⁺, Cu²⁺, and other transition elements, can catalyze the reaction through coordination with the carboxyl group, lowering the activation barrier.
Moreover, the matrix structure itself influences heat transfer efficiency. For example, when using plant material containing lipids, terpene components, or cellulose residues, thermal conductivity is uneven. This creates temperature gradients that locally accelerate or delay the reaction. Consequently, modern decarboxylation technologies involve the use of homogenized matrices standardized for moisture content, organic impurities, and particle size.
Biochemical Consequences of Decarboxylation
Following the decarboxylation of tetrahydrocannabinolic acid A (THCA-A) into the neutral form Δ9-tetrahydrocannabinol (Δ9-THC), a significant transformation of the molecule’s pharmacological and receptor profile occurs. This process involves the loss of the carboxyl group with the formation of a new spatial electron distribution in the molecule, which drastically changes its biochemical activity. THCA-A in its natural state does not effectively bind to the CB1 receptor, accounting for its non-psychoactive nature. In contrast, Δ9-THC is a potent CB1 receptor agonist, which explains the primary psychoactivity of cannabis after heating.
Besides interacting with cannabinoid receptors, the decarboxylated form also exhibits an altered profile of interactions with other molecular targets-specifically, transmembrane ion channels and enzymatic complexes such as COX-2 and FAAH. These changes have profound biochemical and physiological consequences, affecting the spectrum of Δ9-THC activity in the body.
Receptor Profile Changes
The receptor profile of THCA-A is largely limited by its structural polarity associated with the presence of a free carboxyl group, which reduces the molecule’s ability to penetrate the hydrophobic cellular membrane. Accordingly, even if THCA-A theoretically has affinity for CB1 or CB2 receptors, its actual bioavailability in the central nervous system is limited. This contrasts sharply with Δ9-THC, which, due to decarboxylation, acquires lipophilic properties that allow it to freely cross the blood-brain barrier and directly activate CB1 receptors in the CNS. These receptors are Gi/o-coupled GPCRs highly expressed in the hippocampus, basal ganglia, cerebellum, and prefrontal cortex-structures involved in memory, motor control, emotions, and perception. Interaction of Δ9-THC with CB1 triggers an intracellular signaling cascade including decreased cAMP levels, inhibition of calcium channels, and activation of GIRK channels, resulting in neuromodulation.
Although THCA-A shows some affinity for CB2 receptors, it does not activate them to the degree observed with Δ9-THC or other cannabinoids. Some data suggest possible allosteric modulation of CB1 receptors by THCA-A; however, these effects remain weak and transient. Outside of cannabinoid receptors, THCA-A exhibits activity toward certain TRP channels, particularly TRPM8 and TRPV1, which potentially explains its mild anti-inflammatory effects. After decarboxylation, the Δ9-THC molecule gains additional activity toward a broader range of targets: PPARγ, GPR55, and inhibitors of fatty acid amide hydrolase (FAAH), providing a deeper impact on neuroendocrine and immune systems.
Onset of Psychoactivity
A key consequence of the decarboxylation of THCA-A is the transition to a state in which the molecule is capable of inducing changes in a person’s psychoemotional condition. At the core of this is the formation of Δ9-THC-a compound with high affinity for CB1 receptors in the brain. This property underlies the psychoactivity-the ability to influence cognitive, sensory, and affective processes. Activation of the CB1 receptor alters the release of neurotransmitters such as glutamate, GABA, dopamine, and serotonin, which accounts for the characteristic effects of Δ9-THC: euphoria, changes in the perception of time and space, reduction of anxiety or, conversely, its increase depending on individual neurophysiology.
Psychoactivity does not appear gradually-it results from reaching a certain pharmacological threshold concentration of Δ9-THC in the central nervous system. This threshold depends on the route of administration, the degree of decarboxylation, and the extent of first-pass metabolism. In the case of inhalation, Δ9-THC reaches the brain almost immediately, whereas with oral consumption it undergoes transformation into 11-hydroxy-THC, which has even greater psychoactive potency. THCA-A is unable to follow these pathways because it does not cross the blood-brain barrier in sufficient concentrations and does not activate CB1 receptors.
In the context of clinical pharmacology, the onset of psychoactivity is a critical parameter that determines the therapeutic window, dosage, administration regimen, and population restrictions for patients. Although decarboxylation is necessary for activating cannabinoids as neurotropic substances, it creates significant risks of side effects, especially with long-term or uncontrolled use. The psychoactivity of Δ9-THC is one of the main reasons for the development of tolerance, dependence, and short-term cognitive impairments. Conversely, THCA-A is not associated with any of these risks, making it promising for the development of non-psychoactive therapeutic agents.
At the neurophysiological level, the psychoactivity of Δ9-THC is manifested in modulation of gamma rhythms in the cerebral cortex, changes in local synchronization of neuronal activity, and disruption of the standard balance between excitatory and inhibitory signals. This explains the emergence of altered perception, creative activity, and possible transient paranoid thinking. In contrast, THCA-A does not induce any of these reactions, as confirmed by both clinical observations and fMRI data.
Current and Prospective Applications of THCA-A
Despite limited bioavailability and inertness in terms of psychoactivity, THCA-A is gaining increasing interest from medical, pharmacological, analytical, and biotechnological communities. Its value is not limited to its status as a precursor of Δ9-THC but unfolds across a range of unique functional applications where maintaining the acidic form is not only desirable but necessary. The study of THCA-A is conducted within the framework of searching for effective yet safer phytocompounds capable of performing specific functions without the effects characteristic of neutralized cannabinoids. Its potential spans fundamental science, clinical pharmacology, quality control of plant raw materials, and even biotechnology focused on genetic modification of enzymatic systems.
One of the main areas of interest in THCA-A remains its natural form, which unlike Δ9-THC does not interact with CB1 cannabinoid receptors with high affinity. This opens the possibility for targeted therapy without accompanying psychoactive effects. In this context, the role of THCA-A as a research tool grows, enabling separation of effects related to CB1 activation from others, possibly peripheral or non-cannabinoid mechanisms of action.
Alongside medical aspects, THCA-A is increasingly considered a biomarker of the biogenetic status of cannabis. Its stable presence in non-extracted plant biomass until the moment of thermal processing underlies the creation of quality control systems, strain authentication, and phytosanitary monitoring. In analytical chemistry, THCA-A serves not only as an object of quantitative determination but also as an indicator of storage, transportation, and extraction conditions of cannabis.
Biotechnological research directions also show growing interest in the enzyme THCA synthase, which catalyzes the final step in THCA-A biosynthesis. Since this enzyme has well-defined specificity for CBGA, its modification may allow regulation of the ratio of acidic cannabinoid forms in plant biomass. This opens the way to developing next-generation cannabis strains-focused on medical needs without the risk of conversion to Δ9-THC even under temperature influence.
Furthermore, THCA-A is gradually being integrated into the comprehensive concept of phytotherapeutic standardization. It is included in phytocontrol protocols, particularly in the evaluation of “live extracts” that preserve the full acidic profile. These extracts are considered promising because they retain the original chemical composition without thermal degradation, which is important when developing products for microdosing or topical therapy.
Medicine
THCA-A, as the acidic precursor of Δ9-tetrahydrocannabinol, exhibits unique properties that distinguish it from other cannabinoids and make it extremely promising for medical research and therapeutic application. Unlike its decarboxylated analog, THCA-A does not interact with cannabinoid CB1 receptors in the central nervous system in a way that would cause psychoactive effects. This immediately places it in a special category: it retains biological activity potentially beneficial for treating chronic and systemic diseases, while remaining free from negative impacts on cognitive functions or psycho-emotional states.
This profile is particularly attractive for clinical pharmacology, which increasingly focuses on finding compounds with maximal therapeutic selectivity and minimal side effects. THCA-A does not cross the blood-brain barrier in significant amounts, thereby avoiding interaction with neuronal CB1 receptors, yet it demonstrates activity through other molecular targets, including PPARγ, TRP channels, and potentially GPR55. This provides influence on inflammatory, immune, metabolic, and neurodegenerative processes without central psychoactive effects.
The issue of Δ9-THC psychoactivity during long-term treatment is fundamental in modern medical cannabinoid therapy. That is why THCA-A is considered a strategically important candidate as a molecule for safe, long-term use in therapeutic practice, where maintaining patient consciousness, mental stability, and functionality is critically important. Special attention is given to the use of THCA-A in pediatric neurology, geriatrics, and in conditions of chronic autoimmune inflammation, where minimizing psychoactive effects is a non-negotiable requirement.
Rheumatologic conditions, including rheumatoid arthritis and systemic lupus erythematosus, exhibit an immune-inflammatory nature, where key mechanisms include macrophage hyperactivation, production of pro-inflammatory cytokines, and pathological tissue remodeling. THCA-A affects these processes by modulating cyclooxygenase-2 (COX-2) activity, inhibiting production of TNF-α and IL-6, and also demonstrating antioxidant properties. These actions not only reduce the intensity of inflammation but also slow its chronification, which is especially important in the treatment of chronic connective tissue inflammatory diseases.
In addition to immunomodulatory properties, THCA-A shows high potential for neurological pharmacology. Its neuroprotective effect is mediated, in particular, through activation of the nuclear receptor PPARγ, which can suppress neuroinflammation, support mitochondrial function, and prevent oxidative stress. This opens prospects for using THCA-A in treating conditions such as Alzheimer’s disease, Parkinson’s disease, amyotrophic lateral sclerosis, and traumatic brain injuries. Importantly, this neuroprotection is not accompanied by reductions in cognitive performance, altered motivation, or disorganization of consciousness-effects characteristic of Δ9-THC.
In many neurological conditions, including epilepsy, multiple sclerosis, and chronic central-origin pain, the use of THCA-A may potentially reduce relapse frequency, symptom severity, and the need for combinations with systemic immunosuppressants or narcotic analgesics. In clinical protocols where cannabinoids are already integrated into palliative or supportive therapy, replacing Δ9-THC with THCA-A could improve treatment tolerability, especially in vulnerable populations.
Special attention should be given to the potential use of THCA-A in oncology as part of supportive care. Although its antitumor potential requires further verification, it is already known that this molecule can reduce chemotherapy-induced nausea, improve appetite, and at the same time avoid causing dependence or cognitive dulling. This is critically important for patients undergoing aggressive treatment who need to remain mentally active over extended periods.
Search for Non-Psychoactive Alternatives to Δ9-THC
The problem of Δ9-THC psychoactivity remains one of the main barriers to its full integration into pharmacotherapy. Despite its proven analgesic, anxiolytic, anti-inflammatory, and neuroprotective potential, its ability to alter cognitive functions, cause dependence, and tolerance significantly limits its use in outpatient settings, especially among vulnerable groups-children, the elderly, and patients with mental disorders. In this context, the search for non-psychoactive molecules with similar or close biological activity but without effects on the central nervous system via CB1 receptors has intensified. One of the most promising candidates in this area is THCA-A-the acidic form of Δ9-THC, which does not cross the blood-brain barrier in significant concentrations and does not bind to the CB1 receptor in a functionally relevant manner.
THCA-A does not cause euphoria, does not impair concentration, and does not induce changes in perception of space, time, or self-core signs of Δ9-THC psychoactivity. At the same time, it demonstrates a wide spectrum of biological activity, including anti-inflammatory, antioxidant, antispasmodic, and neuroprotective effects. This allows it to be considered a safe alternative for conditions where use of Δ9-THC is inappropriate due to risks of cognitive or psycho-emotional impact. The potential of THCA-A is especially valuable in the context of therapy for chronic pain, autoimmune disorders, functional neuroses, and muscle rigidity syndromes-where maintaining mental clarity without compromising analgesic or immunosuppressive efficacy is desired.
At the pharmacodynamic level, THCA-A interacts with a number of non-cannabinoid targets, among which PPARγ, certain TRP channels (especially TRPA1, TRPM8), and potentially GPR55 predominate. These targets are involved in regulating inflammation, apoptosis, oxidative stress, mitochondrial dynamics, and glial reactivity. While Δ9-THC exerts its activity mainly through CB1 and CB2 receptors, THCA-A acts outside this axis, enabling it to have a distinct spectrum of effects while maintaining baseline similarity in antinociceptive and neuroprotective potential. This creates a unique situation where the structural similarity of the two compounds does not imply identical mechanisms of action but provides a therapeutic enhancement without psychotropic side effects.
Clinical priorities also dictate the need for drugs with long-term safety. Decarboxylated Δ9-THC causes development of tolerance, withdrawal syndrome, and can impair memory, attention, provoke anxiety disorders, or trigger latent psychoses. In the case of THCA-A, these risks are absent even with long-term use, allowing the development of new pharmacotherapeutic regimens designed for months or years without loss of clinical efficacy or cognitive burden on the patient. At the same time, THCA-A does not accumulate in nervous tissue, does not disrupt dopamine homeostasis, and does not participate in CB1-dependent neuronal plasticity, ensuring its pharmacological inertness in terms of dependence formation.
Therapeutic Potential in Neurology and Rheumatology
Neurological and rheumatological diseases often share a range of pathogenic mechanisms, including chronic inflammation, glial activation, oxidative stress, as well as disturbances in cellular energy metabolism and mitochondrial homeostasis. In these conditions, traditional pharmacotherapy usually relies on polypharmacy – combining anti-inflammatory, neuromodulatory, immunosuppressive, and analgesic drugs – which increases the risk of toxic side effects. In this context, THCA-A emerges as a promising molecule capable of simultaneously targeting several key pathophysiological pathways without the need for multiple pharmacological agents.
In neurodegenerative diseases, such as Alzheimer’s disease, THCA-A demonstrates the ability to reduce glial activation and the secretion of pro-inflammatory cytokines, thereby slowing neuronal degeneration and protecting synaptic plasticity. Its mechanisms of action are associated with the induction of antioxidant enzymes, reduction of nitric oxide production, normalization of calcium homeostasis in neurons, and suppression of IL-1β and TNF-α expression in microglia. In Parkinson’s disease, an additional mechanism includes improving mitochondrial metabolism and reducing the formation of reactive oxygen species, which lowers neuronal apoptosis levels in the nigrostriatal complex.
For patients with multiple sclerosis, THCA-A may reduce the intensity of inflammatory lesions in the central nervous system without causing sedative or psychoactive effects. This makes it suitable for daytime use in outpatient settings, which is critically important for maintenance therapy. Similarly, in epilepsy-particularly pediatric epilepsy-THCA-A exhibits anticonvulsant effects through mechanisms unrelated to CB1 receptor inhibition, thus avoiding associated changes in mood, attention, or sleep.
In rheumatology, the key effects of THCA-A include lowering the production of pro-inflammatory mediators in synovial tissue, inhibiting neutrophil migration, and suppressing COX-2 enzymatic activity without the risk of gastric toxicity. This effect is especially beneficial in rheumatoid arthritis, where inflammation has a systemic nature. THCA-A can modulate the immune response without deeply suppressing cellular immunity, which distinguishes it from steroidal and nonsteroidal anti-inflammatory drugs.
Systemic connective tissue diseases, including systemic lupus erythematosus, often involve chronic activation of the interferon response and disruption of the balance between T-helper and regulatory T-cells. THCA-A influences this axis by reducing pro-inflammatory pathway activity without impairing overall immune competence, which is important to avoid secondary infections. Moreover, its antioxidant activity reduces the risk of vascular endothelial damage – a key mechanism of organ injury in systemic vasculitides.
Analytical Chemistry and Phytochemical Control
Analytical chemistry in the context of cannabinoids is a fundamental field that ensures precise identification, quantitative analysis, and standardization of complex plant material compositions. THCA-A, as the most abundant cannabinoid acid in raw cannabis, acts not just as one of many components but as a critical marker reflecting the plant’s biochemical state prior to extraction or thermal processing. Its presence and concentration serve as a foundation for quality control, strain differentiation, and determination of harvesting and storage conditions. Accurate measurement of THCA-A enables the development of standardized protocols for phytomaterial control, which is extremely important for pharmaceutical and medical industries where every gram of raw material must meet established standards.
THCA-A quantification is performed using modern chromatographic techniques, including HPLC, GC-MS, and high-resolution spectrometry. These methods provide not only analytical precision but also allow differentiation of THCA-A from decarboxylated forms such as Δ9-THC, which is critically important due to their differing pharmacological properties and regulatory status. These analytical approaches enable establishing the optimal harvest time when THCA-A content is at its peak, and conversion to psychoactive THC is minimal. Such control facilitates more efficient cultivation and storage and shifts the quality assessment from subjective judgment to objective quantitative indicators.
During the analytical process, it is critical not only to quantify THCA-A itself but also to assess its ratio relative to other cannabinoids and terpenes, which forms a unique “phytochemical fingerprint” of a particular strain. This component allows for precise classification and differentiation of cannabis strains, preventing adulteration or unauthorized expansion of product ranges. Thus, THCA-A functions as a biomarker that underpins the creation of global cannabinoid profile libraries with a high degree of reproducibility.
Innovations in analytical chemistry also focus on developing rapid and cost-effective methods for THCA-A detection in field conditions, opening new horizons for quality control in production and laboratory workflows. The emergence of portable devices based on spectroscopy and biosensors sensitive to the acidic form of THC has the potential to transform traditional phytochemical control approaches, reduce costs, and enhance decision-making speed. This is particularly relevant for legalized markets where accurate accounting of active substances is a matter of legal responsibility and consumer safety.
Integration of analytical data with bioinformatics and systems analysis opens prospects for in-depth study of THCA-A biosynthesis dynamics within specific cellular and tissue structures of the plant, which could lead to genetic improvement of strains with tailored pharmacological properties. This direction holds great potential for developing individualized phytopharmaceuticals with precise dosing and predictable efficacy.
Biomarker of Raw Cannabis
The identification of THCA-A as a biomarker of raw cannabis is based on its unique property of being the dominant cannabinoid acid in the primary plant material before any thermal processing begins. This distinguishes it from most other cannabinoids, which are primarily present in their decarboxylated forms in dried or processed products. THCA-A, as the precursor to Δ9-THC, reflects the metabolic state of the plant at the moment of harvest, making it an unequivocal marker of freshness and the degree of biochemical maturity of the material. The absence or significant reduction of THCA-A levels indicates the onset or completion of degradation and decarboxylation processes, which impact the quality and pharmacological profile of the final product.The use of THCA-A as a biomarker is critically important in the raw material base for pharmaceutical products, where strict control of composition and stability is a decisive factor. Monitoring the concentration of THCA-A allows assessment not only of harvest quality but also the effects of storage conditions, including temperature, humidity, and duration of exposure. Even minor changes in THCA-A concentration can signal processes detrimental to the integrity of the cannabinoid profile, potentially reducing product efficacy or safety.
Additionally, THCA-A enables differentiation of raw cannabis from edible or extract products that have undergone heat treatment, where acid levels are nearly absent. This biomarker-based approach significantly simplifies identity qualification procedures, preventing counterfeiting and abuse in commercial activity.
Studies of raw material freshness based on THCA-A also open possibilities for monitoring biological activity and predicting the therapeutic profile of products. High THCA-A content correlates with an optimal spectrum of activity, especially in the use of cannabis for treating inflammatory and neurodegenerative conditions, allowing therapeutic outcomes to be anticipated at the raw material stage.
In large-scale cannabis cultivation, where agronomic conditions, fertilizers, lighting, and harvest timing vary, THCA-A becomes an indispensable indicator for raw material standardization and avoiding variability in the pharmacological properties of the final product. This reduces the risk of unpredictable clinical results and increases trust in cannabis-based medications.
Quality Control and Strain Compliance
Precise determination of cannabinoid composition, primarily THCA-A, serves as the foundation for quality control, which is critically important in the industrialization of cannabis cultivation. Quality control is based not only on quantitative analysis but also on full identification of the chemical profile, which includes ratios of cannabinoids, terpenoids, and flavonoids. In this context, THCA-A is a key marker that determines whether the product matches the claimed strain and profile.
A cannabis strain is a complex of genetic and phenotypic characteristics manifested through the phytochemical profile. The presence and amount of THCA-A, combined with other components, create a distinctive “chemical passport” used for plant verification and classification. This information prevents confusion in commercial supply chains and ensures consistency between expected and actual pharmacological effects, especially in the medical field.
Implementing control standards based on THCA-A enables detection of counterfeits and falsifications that may occur in illicit markets. Determining the ratio of acid to decarboxylated forms helps identify storage and transport conditions that violate the technological chain, as well as using “chemical fingerprints” to trace product origin. This facilitates the development of traceability and quality certification systems necessary for legalized markets.
In modern production, quality control becomes a multi-level process where THCA-A is measured at several stages-from cultivation to the final product. Such standards ensure stability and predictability of effects, forming the basis for trust among healthcare professionals and patients. Without this control mechanism, it is difficult to guarantee that the pharmacological properties of products comply with declared requirements.
Biotechnologies
Modern biotechnological approaches to cannabis, particularly regarding the metabolism and synthesis of THCA-A, open new horizons for improving plant productivity, raw material quality control, and creating specialized pharmacological products. Biotechnology allows not only a deeper understanding of the molecular mechanisms of enzymatic cannabinoid synthesis but also a fundamental change in the way plants are grown and prepared, directing their metabolism toward desired pathways. In this context, the engineering of THCA synthase-the key enzyme catalyzing the transformation of precursors into the active cannabinoid acid-is of critical importance, alongside the development of genetically modified strains capable of accumulating high levels of THCA-A with minimal or no Δ9-THC formation. These directions not only increase production efficiency but also create conditions for safer, controlled, and standardized use of cannabinoids in pharmacy and medicine.
Engineering of THCA Synthase Enzymes
The enzyme THCA synthase, which catalyzes the conversion of cannabigerolic acid (CBGA) into THCA, is a central element in the cannabinoid biosynthetic pathway. Understanding its structure, catalytic mechanism, and regulation allows for targeted molecular-level interventions that alter the enzyme’s activity, specificity, or stability. Through genetic engineering techniques-particularly directed evolution, site-specific mutagenesis, and recombinant expression-it is possible to produce enzyme variants with enhanced productivity, reduced susceptibility to degradation, or altered substrate specificity.
One of the key goals is to increase the efficiency of THCA synthesis without raising the formation of byproducts or toxic intermediates. Engineering THCA synthase enables optimization of its active site for more efficient interaction with CBGA, resulting in accelerated reaction rates and higher yields of the final product. Attention is also given to stabilizing the enzyme under cultivation conditions, where environmental factors may affect its activity.
An important task is modifying the regulation of THCA synthase gene expression, which allows control over enzyme quantity in plant tissues. Using promoters with cell-type-specific activity, enzymatic activity can be localized to particular organs or developmental stages. This approach opens possibilities for finer control over THCA synthesis, reducing losses and ensuring cannabinoid composition stability.
Enzyme engineering also involves developing artificial biocatalysts that can operate in isolated systems such as bioreactors, which opens prospects for large-scale THCA-A production outside of the plant organism. This approach can significantly reduce production costs, eliminate dependence on agroclimatic factors, and increase the purity of the final product.
Additionally, research is underway to understand THCA synthase’s interactions with other enzymes in the biosynthetic pathway, potentially enabling the creation of complex enzymatic systems with synergistic activity. This opens the potential for manipulating metabolic fluxes within the plant, allowing cannabinoid profiles to be tailored to specific therapeutic objectives.
The integration of bioinformatics approaches and machine learning into enzyme engineering significantly accelerates the search for optimal variants, allowing modeling of enzyme structure and function prior to laboratory experimentation. This reduces the time and cost of developing new biocatalysts, making them accessible for commercial and scientific applications.
Cultivating Cannabis with Increased THCA-A Content and No THC
Developing cannabis strains capable of accumulating significant amounts of THCA-A while simultaneously reducing or completely eliminating psychoactive Δ9-THC is one of the most important directions in modern plant biotechnology. This allows for creating safe products with pronounced therapeutic potential, avoiding legal and clinical issues related to psychoactivity.
Genetic control of Δ9-THC synthesis occurs at the level of expression of enzymes responsible for decarboxylation and further modification of THCA. Genetic engineering techniques enable selective silencing or mutagenesis of genes encoding key enzymes in this pathway, particularly THC synthase, which transforms THCA into Δ9-THC. Meanwhile, THCA synthase remains active, ensuring accumulation of the acidic form.
Genome editing methods like CRISPR/Cas9 open unprecedented possibilities for precise and effective manipulation of individual genes regulating psychoactive cannabinoid synthesis. This allows creation of hybrids and mutants with stable THC absence even while maintaining high levels of THCA-A, representing a revolutionary step in pharmaceutical raw material production.
Selective breeding approaches based on phenotypic and molecular screening of plants naturally low in THC are also widely used. Combining traditional breeding with molecular markers accelerates the development of new cultivars with targeted chemical profiles.
Improving agronomic technologies such as control of lighting, temperature regimes, nutrition, and humidity also significantly influences the balance between THCA and THC. Biotechnology enables integration of these factors into comprehensive models of metabolic control, ensuring stability and reproducibility of production.
Cultivating such “non-psychoactive” strains is critically important for developing medical products suitable for patients with low tolerance for psychoactive effects or when it is necessary to avoid cognitive impairment. Moreover, this expands the legal product market, making them available in jurisdictions with strict Δ9-THC restrictions.
The ecological aspect also gains importance: strains with high THCA-A content and no THC can be grown in conditions that do not require complex controls and ensure production stability without additional costs for disposal of psychoactive byproducts.
Ethical and Regulatory Aspects
The development of THCA-A use in medical, scientific, and industrial fields faces a range of complex ethical and regulatory challenges related to the positioning of this cannabinoid within legal frameworks, consumer safety, and societal norms. The non-psychoactive nature of THCA-A creates potential for its application as a safe alternative to traditional cannabinoids; however, the lack of clear regulatory definitions in many jurisdictions generates ambiguity in its control and distribution. The ethical dimension involves balancing the interests of patients and researchers with legal restrictions shaped by historical, political, and social factors, as well as ensuring transparency, quality, and safety of THCA-A products. Defining the status of THCA-A within regulatory documents and international agreements is crucial for establishing an appropriate infrastructure to support the safe integration of this compound into pharmaceutical practice and scientific research.
The Role of THCA-A in Cannabis Regulatory Documents
Given its lack of psychoactive effects, THCA-A is not always clearly identified in legal frameworks regulating cannabinoid trafficking. This results in uncertainties regarding classification and the application of such products. Most countries’ legislation focuses regulation primarily on Δ9-THC as the main psychoactive component of cannabis, leaving THCA-A in a gray area. However, because THCA-A is a precursor to Δ9-THC, many regulations include it under the general term “cannabinoids” without detailed distinction. This approach creates difficulties for producers and users, especially in cases where products contain high levels of THCA-A with minimal or no Δ9-THC.
This generates legal uncertainty, particularly concerning the legality of transporting, storing, and using raw materials with high THCA-A content. In countries with strict control over psychoactive substances, the presence of THCA-A can lead to seizures and confiscations, even though the substance lacks psychoactive effects. This situation underscores the need for developing specific criteria to identify and regulate THCA-A, taking into account its pharmacological properties and potential.
Furthermore, regulatory documents increasingly emphasize quality control standards and labeling requirements for THCA-A products, aiming to ensure clear consumer information about the absence of psychoactivity and to foster trust in these preparations. At the same time, regulators face challenges in standardizing analytical methods for precise determination of THCA-A in raw materials and finished products, which is fundamental for legal circulation and scientific research.
Integrating new knowledge about THCA-A into the regulatory framework requires harmonization with international conventions and national laws, a lengthy and complex process but necessary to overcome existing legal conflicts and support the development of innovative cannabinoid-based medical products.
International Approaches to Classification
At the international level, the classification and regulation of cannabinoids, including THCA-A, remain subjects of multifaceted discussions and inconsistent approaches. Different countries and international organizations exhibit significant disparities in the interpretation of THCA-A’s status compared to Δ9-THC, influenced by historical, cultural, and political factors, as well as the level of scientific advancement and regulatory systems’ readiness for innovation.
Most international conventions, such as the United Nations Single Convention on Narcotic Drugs of 1961, focus on psychoactive cannabinoids, particularly Δ9-THC, without designating THCA-A as a separate substance, which creates legislative “blind spots.” This complicates the development of coordinated policies and contributes to regional divergences in control and usage approaches.
Some countries, especially those with advanced medical cannabis legalization programs, strive to differentiate THCA-A from Δ9-THC in national legislation, enabling more flexible regulation of product circulation, including setting threshold values for psychoactive substance content and implementing safety standards. This approach ensures legal access to THCA-A-based products and supports scientific research development.
Meanwhile, many countries’ regulatory agencies avoid such distinctions due to concerns about illicit use or inability to effectively control the conversion of THCA-A into Δ9-THC. This is reflected in prohibitions or strict restrictions on all cannabis products, regardless of their specific cannabinoid profiles.
International organizations, such as the World Health Organization (WHO), are gradually incorporating assessments of non-psychoactive or inactive cannabinoids into their recommendations, but this process is slow and dependent on accumulating scientific data. WHO recommendations regarding the decriminalization or relaxation of Δ9-THC regulations do not always include THCA-A, which adds further ambiguity.
Conclusion:
Delta-9-Tetrahydrocannabinolic Acid A (THCA-A) is a fundamental chemical compound in the biosynthetic pathway of cannabinoids within the Cannabis sativa plant. Its status as the precursor to the psychoactive Δ9-THC defines its key significance in cannabis chemistry, pharmacology, and industry. Analysis of the structure, chemical nature, and reaction mechanisms of THCA-A reveals the complex nature of this molecule, which is both stable at low temperatures and sensitive to thermal and temporal influences, leading to its decarboxylation and formation of Δ9-THC with properties that are fundamentally different from the original compound.
From a chemical perspective, THCA-A is characterized by a carboxyl group that determines its acidic properties and imparts unique physicochemical characteristics to the compound, which significantly influence its biological activity. The primary biochemical process governing THCA-A’s subsequent behavior is thermal decarboxylation, which not only modifies the molecular structure but drastically changes the pharmacological profile of the cannabinoid, transforming it from an inactive or weakly active form into the potent psychoactive agent Δ9-THC.
The decarboxylation mechanism requires detailed study due to its complex kinetics and dependence on external factors, including temperature, heating time, presence of catalysts, and environmental conditions. The temperature dependence of the reaction exhibits an exponential character with a sharp increase in rate as temperature rises, explaining the need for careful control of technological processes, especially in the production of medical and food cannabis-based products. Time factors and catalysts, including metallic and chemical agents, can modulate the process, accelerating or inhibiting the transformation, which is critical for ensuring stability and predictability of the final product.
Pharmacologically, THCA-A lacks the classic psychoactive properties characteristic of Δ9-THC, due to its inability to effectively bind to CB1 cannabinoid receptors in the central nervous system. Instead, it demonstrates significant potential in regulating immune responses, anti-inflammatory effects, and may modulate other biological systems without causing the typical side effects associated with Δ9-THC. This feature creates prospects for the use of THCA-A as a safe therapeutic agent, especially in fields where psychoactivity is undesirable or harmful.
An important component of THCA-A’s pharmacological profile is the change in receptor interaction after decarboxylation, leading to the emergence of psychoactivity and a significant increase in affinity for CB1 receptors. This allows THCA-A and Δ9-THC to be considered as two interconnected stages of a single biochemical process with different clinical and social implications. The onset of psychoactivity following this transformation dictates strict requirements for controlling decarboxylation processes, especially in medical and commercial sectors where clear distinction between psychoactive and non-psychoactive products is necessary.
From the analytical chemistry standpoint, THCA-A serves as an important biomarker of raw cannabis, enabling identification of raw material quality, origin, and degree of processing. Precise methods for quantifying THCA-A content form the basis for quality control and strain compliance, which directly impacts product standardization and legality. The determination of the THCA-A to Δ9-THC ratio is a key indicator of the technological status of the raw material and allows for prediction of the pharmacological effect of the final product.
In the biotechnological context, THCA-A opens new horizons for enzyme engineering, specifically of THCA synthase responsible for its biosynthesis in the plant. The possibilities for modifying the activity and specificity of this enzyme allow the development of cannabis strains with increased THCA-A content while simultaneously reducing or completely eliminating psychoactive Δ9-THC levels. Such technologies fundamentally change approaches to cultivation and production of cannabinoid products, paving the way for legalization and application without psychoactive risks.
Cultivating plants with elevated THCA-A content and no Δ9-THC is a strategically important direction that supports the development of medical cannabinoid therapy, as it enables the creation of effective medications with minimal side effects. This also has social significance by reducing the risks of abuse and dependency traditionally associated with Δ9-THC and meets regulatory requirements for safety and control.
Ethical and regulatory aspects of THCA-A use are highly relevant given its legal status and therapeutic potential. Regulatory frameworks in most countries lack clear provisions governing the circulation of THCA-A as a distinct chemical substance, creating difficulties in legalization, quality control, and scientific research. The absence of precise definitions and distinctions from Δ9-THC leads to legal ambiguities that negatively affect innovative development and access to therapeutic agents.
Managing the risks associated with THCA-A decarboxylation to Δ9-THC becomes a critical factor in developing quality and safety standards. Differentiating raw materials high in THCA-A from those high in Δ9-THC requires official recognition and incorporation into regulatory acts, which would allow classification of medical products according to their potential activity and risks. Such approaches would promote the formation of a transparent and scientifically grounded legal framework supporting innovation in pharmaceuticals and agriculture.
International regulatory practice demonstrates heterogeneous approaches to THCA-A classification, complicating the formation of a unified strategy for cannabinoid product development. The lack of harmonized international standards limits the exchange of scientific knowledge, complicates trade, and causes varying levels of therapeutic product availability across countries. Overcoming these challenges requires harmonization of regulatory frameworks that take into account the specific nature of THCA-A as a precursor to psychoactive Δ9-THC but lacking psychoactive properties itself.
Thus, THCA-A can be regarded as a key component in the future development of pharmaceutical and biotechnological fields related to cannabinoids. Its unique properties lay the foundation for safe, non-psychoactive therapeutic agents as well as improved agronomic processes for cultivating cannabis with optimized chemical profiles.
This cannabinoid opens new prospects for personalized medicine and precise control of pharmacological effects, balancing therapeutic efficacy and safety. Further research should focus on detailed investigation of biochemical mechanisms of THCA-A interactions with various biomolecules, as well as development of advanced technologies for controlling decarboxylation and stabilizing the compound in final products.
Sources:
- PubMed Central (PMC) – U.S. National Library of Medicine
An open database of scientific articles in medicine, pharmacology, and biochemistry.
https://www.ncbi.nlm.nih.gov/pmc/ - National Institute on Drug Abuse (NIDA)
An authoritative resource with research on cannabinoids, pharmacology, and health effects.
https://www.drugabuse.gov/ - Journal of Cannabis Research
An open-access journal publishing articles on cannabis topics, including pharmacology and biochemistry.
https://jcannabisresearch.biomedcentral.com/ - Frontiers in Pharmacology
A reputable open-access journal featuring articles on cannabinoid pharmacology, biochemistry, and therapy.
https://www.frontiersin.org/journals/pharmacology - ScienceDirect (Open Access Articles)
Elsevier’s platform providing many open-access articles on cannabinoid pharmacology and biochemistry.
https://www.sciencedirect.com/ - United States Food and Drug Administration (FDA)
Official documents and regulatory positions on cannabinoids, including regulatory guidelines.
https://www.fda.gov/ - European Monitoring Centre for Drugs and Drug Addiction (EMCDDA)
European center for monitoring drugs and their impact, offering analytical reports and regulations.
https://www.emcdda.europa.eu/ - World Health Organization (WHO) – Expert Committee on Drug Dependence Reports
Evaluations and recommendations on cannabinoids and their classification.
https://www.who.int/teams/health-product-policy-and-standards/controlled-substances/expert-committee-on-drug-dependence - Journal of Natural Products (American Chemical Society)
Publications on the chemical composition and biosynthesis of cannabinoids.
https://pubs.acs.org/journal/jnprdf - Cannabis and Cannabinoid Research (Mary Ann Liebert, Inc.)
A scientific journal specializing in contemporary cannabinoid research.
https://www.liebertpub.com/can - National Center for Biotechnology Information (NCBI) – Gene and Protein Databases
Information on enzymes, genetics, and biochemistry, including THCA synthase.
https://www.ncbi.nlm.nih.gov/ - Scopus (Open Access Articles)
A bibliographic database providing access to open articles in medicine and biotechnology.
https://www.scopus.com/ - Harvard Health Publishing
Review materials and publications on cannabinoids in medicine.
https://www.health.harvard.edu/ - The Journal of Biological Chemistry
Publications on enzyme biochemistry, including THCA synthase and other enzymes in the cannabinoid pathway.
https://www.jbc.org/ - National Agricultural Library (NAL) – US Department of Agriculture
Materials on agriculture, biotechnology, and plant quality control.
https://www.nal.usda.gov/