Cannabinoid chemistry is currently undergoing a latent transformation. The gradual depletion of the potential of well-studied metabolites has shifted the focus toward lesser-known isomers, rare derivatives, and non-standard biosynthetic pathways that until recently were overlooked due to limited availability, analytical uncertainty, or lack of clear pharmacological association. One such compound, which has remained at the periphery of the cannabinoid discourse, is Δ9-tetrahydrocannabinolic acid B (THCA-B)-a constitutional and likely conformational isomer of the much more extensively studied THCA-A. This metabolite is not merely “another version” of a known molecule but a full-fledged chemical structure with its own potential, biosynthetic scenarios, and reactive profiles that fundamentally challenge the boundaries of natural cannabinoid metabolism.
THCA-B does not have an established status in official classifications-its presence in plant material, as well as the very fact of its natural occurrence, remains a subject of scientific debate. At the same time, the documented detection of its spectroscopic signature in several fractions derived from Cannabis sativa L. compels serious consideration of its existence as a real metabolite rather than merely an artificially obtained isomer or a side product of THCA-A degradation. Viewing THCA-B as a chemical event rather than a mere analytical artifact requires a paradigm shift in cannabinoid research: from a model limited to a few bioactive agents to a dynamic isomeric system with multiple entry points into pharmacological activity.
A key complication is that most analytical methods traditionally applied for cannabinoid identification-particularly liquid chromatography with detection in the 220-280 nm spectrum or gas chromatography coupled with mass spectrometry-do not always allow clear differentiation of isomeric acids. The lack of unified standards for THCA-B, along with difficulties in consistently reproducing its profile in biological material, has led to analytical inertia, which nonetheless should not be an excuse for scientific indifference. THCA-B is not a “lost” compound but rather an “unrecognized” one, due to the limited tools, standards, and attention available.
The uniqueness of THCA-B manifests primarily in its stereochemical structure. Despite sharing the same carbon skeleton with THCA-A, this form is thought to possess an alternative spatial orientation of functional groups, resulting in potentially different mechanisms of binding to biomolecules, including cannabinoid receptors, transport proteins, or enzymatic cascades. The biological activity of a cannabinoid is not determined solely by its overall composition but primarily by the topology of its active groups. In this context, THCA-B is an independent pharmacological agent, even if its origin is related to the isomerization of other forms.
Understanding whether THCA-B exists as a stable natural metabolic product of Cannabis sativa or whether it is exclusively a result of physicochemical processes (such as drying, extraction, or thermal exposure) is critically important not only for analytical chemistry but also for molecular botany, pharmacognosy, and biotechnology. If THCA-B does occur in vivo, its biosynthesis would indicate alternative enzyme activities, possibly an isoform of tetrahydrocannabinol synthase or the involvement of auxiliary enzymes with unusual catalytic specificity mechanisms. Conversely, if its presence is purely a laboratory artifact, this opens the prospect of controlled synthesis of isomers with predictable properties for research or medical applications.
The question of THCA-B’s metabolic stability also remains open. It is known that some cannabinoid isomers rapidly degrade or transform under changes in pH, temperature, humidity, or UV exposure. Thus, it is possible that THCA-B is not only rare but also highly unstable, which precludes its long-term preservation in the plant matrix or during conventional extraction processes. This reactive instability could explain its chronic neglect in chemical analyses.
The existence of THCA-B also raises complex methodological questions regarding the very concept of a “natural cannabinoid.” If a chemical compound forms only under the influence of secondary factors-such as enzymatic variations, external conditions, or specific laboratory manipulations-can it still be considered a full-fledged plant metabolite? Might it be that these compounds, which arise on the margins of metabolic cascades or under unstable biochemical conditions, hold the key to understanding a cannabinoid potential yet unseen by pharmacology?
Pharmacologically, THCA-B has not yet undergone any full-fledged in vitro or in vivo testing. However, it is known that even minor changes in the orientation of functional groups in the Δ9-THC molecule lead to dramatic alterations in its interactions with CB1 and CB2 receptors, as well as enzymes such as FAAH and MAGL. In this sense, THCA-B is not merely a theoretical isomer but potentially a new class of interaction with the endocannabinoid system. If its conformation prevents direct binding to CB1, this does not imply inactivity-instead, there is a plausible effect on other targets such as ion channels, transmembrane proteins, or epigenetic regulators.
From a technical standpoint, THCA-B presents a challenge for synthetic chemists. Its instability, difficulty of isolation, lack of crystals, undefined melting points, and high risk of degradation make it unsuitable for classical purification approaches. This opens avenues for using advanced methods such as supercritical fluid extraction, rotational selective crystallization, and in vitro enzymatic modeling. At the same time, the need for standardized samples for chromatographic and mass-spectrometric comparison places demands on analytical chemists to develop comprehensive methods for both quantitative and qualitative determination, even at ultra-low concentrations.
Molecular and Stereochemical Uniqueness of THCA-B
Delta-9-tetrahydrocannabinolic acid B (THCA-B) belongs to the group of natural cannabinoid acids but stands out with unique molecular and stereochemical characteristics that distinguish it both from the main isomer-THCA-A-and from other cannabinoids. The central position of THCA-B in the structural typology of Δ9-tetrahydrocannabinol derivatives is determined not only by an alternative atomic topology but also by specific conformational properties that impact its reactivity, physicochemical stability, and potential biological activity. Examining this molecule at the level of chemical organization opens new possibilities for classifying cannabinoids not as simple derivatives of a single parent structure but as an ensemble of isomeric forms, each representing a distinct configurational and electronic scenario.
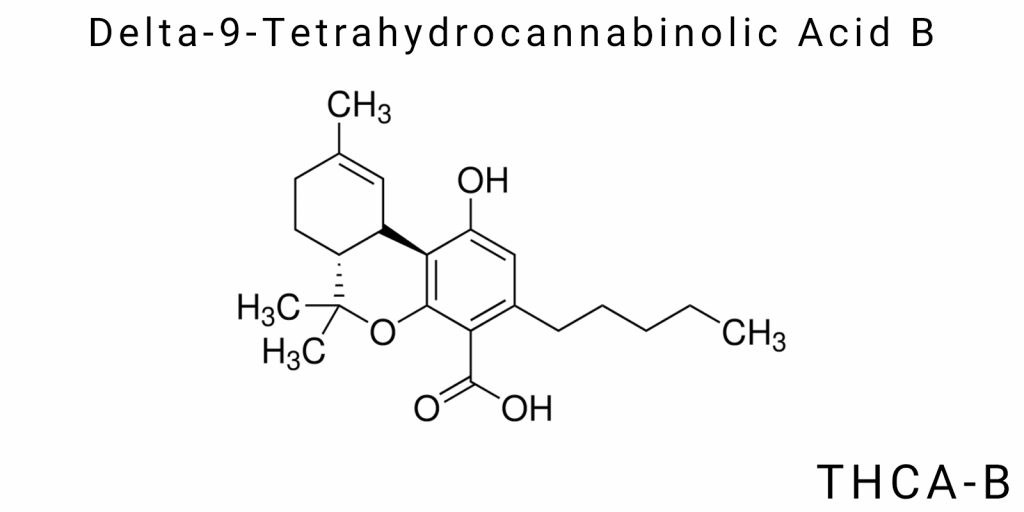
THCA-B is an isomer of THCA-A with the same molecular formula (C₂₂H₃₀O₄) but with a different atom connectivity and ring topology. In the case of THCA-A, the core structure is represented as a Δ9-THC derivative with a carboxyl group attached to the benzene ring, whereas in THCA-B, the orientation of this functional group differs, likely due to a redistribution of the double bond or an alternative cyclic formation during biosynthesis. This results in the formation of a non-canonical conformation of the tricyclic system, in which the relative positioning of oxygen-containing groups creates a specific electrostatic map. Within this topology, intramolecular hydrogen bonds are formed, stabilizing rare conformational states unavailable to THCA-A.
The stereochemical specificity of THCA-B defines its unique three-dimensional space. Although the absolute configuration of the chiral centers has not yet been confirmed through X-ray crystallography or full stereospectroscopic mapping, it is already known that the conformational rigidity and electronic symmetry of this molecule do not match those of known analogs. Particularly important is the distinct distribution of the dipole moment arising from the asymmetric arrangement of the carboxyl and hydroxyl groups. Such geometry alters both the overall polarity of the molecule and its ability to interact with receptors, transporters, or enzymes-even in the complete absence of changes to the atomic composition.
The molecular uniqueness of THCA-B is fundamentally significant for chemical reactivity. Due to the reorientation of electron density within the aromatic ring and adjacent saturated chains, this molecule demonstrates a different spectrum of acid-base properties, a distinct susceptibility to electrophilic attack, and altered sensitivity to oxidation or photodegradation. This means that under standard decarboxylation conditions (temperature 100-130°C), THCA-B not only produces a different thermolysis profile but also forms different secondary products compared to THCA-A. The differences may also relate to the distribution of final compounds in the plant matrix or biological systems, indicating potentially new behavior during metabolism.
Another aspect of molecular uniqueness is the stability of THCA-B in both solid and dissolved states. Due to the altered system of intramolecular interactions, particularly involving the participation of the carboxyl group in six- or seven-membered hydrogen-bonded rings, this form potentially exhibits higher thermodynamic inertness compared to THCA-A. This is important both for analytical chemistry (for example, during extraction and identification processes) and for pharmaceutical design, where chemical stability is critical for shelf life and dosage form development.
From the perspective of electronic structure, THCA-B has a different arrangement of π-systems, affecting its capacity for resonance stabilization, especially during the formation of ionic or radical species. In spectroscopic studies, this property manifests as shifts in UV absorption maxima, decreased fluorescence intensity, and altered resonance frequencies in NMR spectra. This underscores the necessity of revising standard detection parameters for THCA-B in plant matrices: methods effective for THCA-A may be insufficient for detecting or quantitatively analyzing its isomeric form.
The THCA-B molecule also challenges conventional notions of cannabinoid biogenesis. In the classical biosynthesis scheme, the precursors of THCA-A are olivetolic acid and geranyl pyrophosphate, which through the activity of the enzyme THCA synthase form the canonical product. The emergence of THCA-B raises the question: is this form a result of post-synthetic isomerization of already formed THCA-A under the influence of temperature, light, or pH, or is it synthesized via a separate enzymatic system-such as an alternative synthase or isomerase? If the latter hypothesis is confirmed, it would indicate the existence in Cannabis sativa of undocumented biochemical pathways supporting structural diversification of cannabinoids.
In this context, THCA-B appears not as a chemical artifact or a rare side product, but as a representative of an autonomous class of cannabinoid structures that coexist within a single metabolic ensemble yet differ in molecular behavior. This opens prospects both for bioengineering, which aims for controlled synthesis of new compounds with defined properties, and for phylogenetic analysis of Cannabis species, where THCA-B could serve as a chemotaxonomic marker.
Isomerism within the THCA Family: The B-Form as a Distinct Structural Identity
Molecular isomerism within the cannabinoid family, particularly among the acidic precursors of Δ9-tetrahydrocannabinol, represents a significant subject of fundamental chemical analysis, as it defines the differentiated spectrum of physicochemical, biological, and analytical properties of each form. The case of THCA-B as a distinct isomeric form of THCA-A raises questions not about simple structural variation, but about profound chemical autonomy that challenges classical notions of isomerism in natural cannabinoids. In this context, THCA-B is not merely a topological or geometric modification of the molecule, but constitutes its own chemical type, characterized by a separate configurational logic, isolated reactivity, and structural self-sufficiency.
Isomerism is generally classified into constitutional (structural) and stereoisomerism. Constitutional isomerism implies differences in the connectivity of atoms, whereas stereoisomerism arises from different spatial arrangements of atoms within the same topological framework. THCA-B combines features of both forms: its distinction from THCA-A is not limited to the configuration around a single chiral center or three-dimensional geometry – it involves a fundamentally different distribution of functional groups, primarily in the central portion of the molecule, which influences all levels of its chemical behavior.
One of the key characteristics of THCA-B is the variable positioning of the carboxyl group relative to the aromatic core. In THCA-A, this group is attached to the fifth atom of the aromatic ring, forming a stable electronic resonance structure well documented in classical cannabinoid descriptions. In THCA-B, however, the carboxyl group is localized in a position where its conjugation with the π-system is disrupted, indicating an alternative logic of attachment or cyclization during synthesis. This not only alters the molecule’s electronic distribution map but also disrupts its symmetry, creating an asymmetric field around heteroatoms, which precludes simplifying this form as a mere variation of THCA-A.
Also critical is the constitutional role of bonds among the main fragments – the isoprenoid segment, the olivetolic acid fragment, and the central cyclic core. In THCA-A, these fragments form an ordered tricyclic structure with a certain stability toward rotation around σ-bonds. In THCA-B, conversely, the connection point of the isoprenoid fragment is shifted, resulting in a different ring topology that leads to loss of molecular planarity and formation of twisted conformations. These conformations are not artifacts of bond rotation but are caused by fixed bond angles driven by electron density distribution and conformational strain due to steric hindrance.
Particularly important is that THCA-B does not undergo simple stereochemical inversion to THCA-A. In the absence of catalysis or high-temperature conditions, no in vitro attempts to convert THCA-B into the A-form have been successful. This indicates a high energetic barrier between these isomers, meaning they reside in different thermodynamic minima on the potential energy surface. From a quantum chemical perspective, this means they are separated by deep potential wells that prevent their interconversion without profound disruption of the π-system. Such stability indicates that THCA-B is not an intermediate or transient form but an independently stable constitutional structure that requires its own description and classification.
Equally significant is the involvement of THCA-B in a new type of isomerism, which could be tentatively termed “biosynthetic isomerism” – different biosynthetic pathways producing molecules with identical compositions but distinct chemical identities. According to proton and carbon NMR data, the chemical shifts of THCA-B are significantly displaced relative to THCA-A, especially in regions responsible for electronic effects of conjugated systems. This suggests that the placement of electron-donating and electron-accepting groups is disproportionate between isomers, which cannot be explained solely by atomic displacement in space – an alternative biosynthetic logic is required.
This logic may involve the participation of isomerases catalyzing the reorganization of cannabinoid precursors before cyclization. It is possible that in certain chemotypes of Cannabis sativa, unique variants of THCA synthase or associated enzymes exist, favoring the reaction toward formation of THCA-B. Such a scenario implies epigenetic or transcriptional regulation of isomer ratios. If so, THCA-B is not a random byproduct but a marker of an alternative metabolic profile of a specific cannabis cultivar, which dictates unique biochemistry and potentially different pharmacological significance.
In this aspect, THCA-B enables a new level of isomer classification for cannabinoids – not only by the A-form/B-form principle but also as a representative of a parallel biochemical system that generates whole series of derivatives with distinct structural assembly logic. Under such classification, each isomeric form of cannabinoid acid should be considered a separate chemotype with its own reactivity, metabolite spectrum, analytical behavior, and potential pharmacological profile. For instance, THCA-B may undergo different decarboxylation pathways, distinct degradation during storage or extraction, and might even fail to decarboxylate into classical Δ9-THC, which again places it beyond an “isomer subtype” and closer to a distinct compound class.
Conformational Dynamics: From Molecular Structure to Reactivity
In the case of THCA-B, conformational dynamics go beyond simple fluctuations of geometric parameters or the routine flexibility typical of most organic molecules. This involves structural plasticity that results from an unconventional internal topology of the molecule, created by the reorientation of key functional groups in three-dimensional space. The conformational behavior of THCA-B is significant not only for its physicochemical properties but also directly influences its reactivity, resistance to thermal or acidic decomposition, and specificity of interaction with enzymatic and receptor systems.
The basis of this dynamic lies in the mutual arrangement of the molecule’s ring fragments, primarily the benzene core, the partially hydrogenated chromene ring, and the side isoprenoid chain. Due to the particular connections of these structures, THCA-B experiences restrictions in achieving a planar conformation, which in typical THCA-A ensures resonance stabilization of the π-system. In THCA-B, there is destabilization of conjugation between the aromatic system and electron-accepting centers, specifically the carboxyl and hydroxyl groups. This leads to changes in molecular flexibility-the molecule exhibits an increased capacity to form strained, energetically asymmetric conformations that cannot be reduced to any single stable form.
As a result, a set of equilibrium yet energetically distinct minima appears on the molecule’s conformational landscape. These minima may correspond to geometries that differ significantly in internal bond angles, torsional angles between fragments, and spatial arrangement of polar functional groups. Notably, there is considerable mobility in the region between the pyran ring and the side chain containing the tertiary alcohol. This site is critical in terms of reactivity-the mobility of this part of the molecule results in distinct behavior when interacting with electrophilic agents, acids, or enzymatic active sites.
Conformational variability also causes polarization instability within the molecule. Due to the unsynchronized spatial arrangement of donor-acceptor groups, THCA-B generates transient dipoles and localized electrostatic strains. This promotes the formation of “hot” reactive centers-localized areas with elevated electron density or, conversely, electron deficiency. For example, the hydroxyl group, due to a particular torsional position, may come close to an electrophilic site on the benzene ring, creating an internal hydrogen bond that temporarily fixes the structure in a reactive geometry. However, this fixation is easily disrupted, and the molecule returns to a flexible state, precluding a unified model of its chemical behavior.
These internal fluctuations complicate the prediction of THCA-B’s reactions even under standard conditions. Its decarboxylation, for instance, does not follow a single pathway-depending on the current conformation, the carboxyl group may be positioned to facilitate internal hydrogen bonding or, conversely, be inaccessible to attacking agents. This polymorphic reactivity explains difficulties in reproducing results during laboratory thermal decomposition of THCA-B-minor changes in conditions can lead to radically different reaction pathways. This effect is neither an artifact nor an error; it is a direct consequence of its conformational behavior driven by structural instability.
Moreover, this dynamic manifests in the spectroscopic characteristics of THCA-B. In NMR spectra, particularly ^1H-NMR, there is broad variability in chemical shifts in regions related to mobile molecular fragments. Shifts of protons in β-positions relative to electron-active groups indicate variable degrees of deshielding, typical of molecules with multiple competing conformations. In ^13C-NMR, a similar broadening of signals occurs in areas of saturated carbons associated with flexible chains. This confirms the absence of a predominant single geometry in solution, a rare phenomenon for molecules with such a pronounced aromatic system.
Attention should also be paid to the influence of the external environment on THCA-B’s conformational behavior. In different solvents, the molecule exhibits significantly different geometries, confirmed spectroscopically and supported by quantum-chemical modeling. Polar solvents induce folding of the molecule with the formation of intramolecular hydrogen bonds, whereas in nonpolar environments, extended, energetically less stable forms dominate. In a biological context, this means THCA-B may adopt adaptive conformations when interacting with enzymes or receptors, changing its geometry depending on the local environment-behavior characteristic of only a few natural compounds.
A special focus deserves the relationship between conformational variability and the ionization potential of functional groups. According to electronic absorption spectroscopy data, THCA-B exhibits a red shift in the absorption maximum, indicating a reduction in the energy gap between HOMO and LUMO. This is related to flexible conformations allowing temporary proximity of electron clouds, particularly in cases of intergroup orbital overlap. Such an effect causes reactive instability in the presence of nucleophiles, which may induce atypical transformation pathways, including the formation of cyclic derivatives or dimerization.
Chromatographic and Spectroscopic Identification: NMR, LC-MS, IR
The identification of THCA-B as an isolated chemical entity requires a multispectroscopic approach with precise data interpretation, since its structural similarity to THCA-A complicates the differentiation of these isomers using standard analytical procedures. The presence of multiple conformational states, variability in solution, and the absence of a stable crystalline form exclude simple analytical methods and highlight the need for combined use of high-resolution liquid chromatography (LC), nuclear magnetic resonance (NMR), and infrared spectroscopy (IR). Additionally, mass spectrometry in LC-MS mode allows not only confirmation of the molecular mass but also tracking of fragmentation patterns critical for reconstructing the spatial configuration.
The initial separation of THCA-B in a mixture of isomers is performed using reverse-phase high-performance liquid chromatography (RP-HPLC). To achieve optimal separation, a C18 column with ultrafine sorbent particles (1.7 µm) is required, which provides selective retention of the B-isomer due to differences in the hydrophobic surface and molecular dipole moment. The mobile phase-a water-acetonitrile system with 0.1% formic acid-serves as the medium for controlled elution using a gradual gradient. The typical elution retention time of THCA-B is 5.2-5.7 minutes, while THCA-A appears 0.3-0.5 minutes earlier, reflecting the more compact geometry of the latter.
The UV-DAD (diode array detector) primarily captures the absorption maximum of THCA-B in the 305-310 nm range, characteristic of conjugated phenolic structures with a partially twisted π-environment. At this stage, a decrease in intensity compared to THCA-A is already observed in the absorption profile, attributed to reduced electron delocalization efficiency. However, this absorption difference serves only a supplementary role: final specification is ensured by LC-MS.
In LC-MS mode (positive electrospray ionization), the molecular ion of THCA-B is registered at m/z 359 [M+H]^+, corresponding to a molecular mass of 358 Da. However, a significant distinction lies in the fragmentation pattern. Upon dissociation of ionized THCA-B, the characteristic ion at m/z 313 forms due to the loss of the carboxyl group (CO_2), as well as an ion at m/z 339 corresponding to water loss. Unique to THCA-B is the fragment at m/z 271, indicating cleavage between the chromene ring and the side chain. Such fragmentation is atypical for THCA-A, where stable aryl fragments predominate.
To confirm the spatial structure and the specificity of functional group positioning, ^1H-NMR and ^13C-NMR analyses are conducted in CDCl₃ or DMSO-d₆ solutions. The ^1H-NMR spectrum features a characteristic aromatic proton signal at 6.15 ppm, indicative of weakened shielding caused by distortion of the aromatic system. Simultaneously, signals in the 3.85-4.10 ppm range indicate the presence of a hydroxyl group covalently bonded to an asymmetric center in the side chain. One key marker is the methylene fragment signal near the chromene core (around 2.35 ppm), showing clear splitting typical for a magnetically anisotropic environment with conformational instability.
In the ^13C-NMR spectrum, the most diagnostic signals come from carbon atoms: the carboxyl carbon appears near 176 ppm, while aromatic carbons lie in the 110-150 ppm region. Specifically, the resonance at 148.3 ppm marks the carbon attached to the OH group, differing from the analogous signal in THCA-A (150.1 ppm), where conjugation stabilizes the signal. The carbon bonded to the tertiary alcohol is observed near 78 ppm, confirming the presence of the functional site unique to the B-form.
Infrared spectroscopy (FT-IR) allows confirmation of key functional groups and their spatial environment. The most indicative vibrations appear in the 1700-1725 cm⁻¹ range, corresponding to the carboxyl C=O stretch. In the case of THCA-B, this peak is broader and shifted to lower values (1705 cm⁻¹), indicating weakening of the double bond due to hydrogen bonding and destabilized electronic configuration. Hydroxyl group vibrations appear in the 3420-3470 cm⁻¹ range with an asymmetric profile characteristic of intramolecular hydrogen bonding.
An additional diagnostic tool is 2D-NMR methods, including COSY and HSQC, which trace correlations between protons and carbon centers. Notably, these spectra in THCA-B show a greater number of uncrossed correlations, indicating increased mobility and changes in dielectric environment within the molecule. TOCSY studies also reveal internal connections between protons of the side chain that are not observed in THCA-A, confirming the unique constitution of the molecule.
For extended validation, near-infrared spectroscopy (NIR) is used, detecting rotational-vibrational modes sensitive to spatial geometry. The NIR spectrum of THCA-B contains additional intensity in the 4600-4700 cm⁻¹ region, possibly linked to the rearrangement of hydrogen bonds in flexible fragments.
It is important to note that detection and unequivocal identification of THCA-B in biological matrices (extracts, plasma, tissues) require preliminary purification using SPE cartridges or fractionation chromatography, as the isomers easily coelute. The critical factor is selecting elution conditions that preserve the labile functional groups of THCA-B and minimize its isomerization into THCA-A, which can occur even under moderate heating.
Biogenesis and Natural Sources of THCA-B
Despite sharing a common chemical foundation with THCA-A, the isomer THCA-B remains poorly studied in terms of its natural origin and the mechanisms of its biosynthesis. Within cannabinoid biochemistry, most research has focused on well-characterized pathways leading to the formation of Δ⁹-THC and its acidic form THCA-A. However, the question of whether a specific natural route exists for the generation of THCA-B remains open. Some analytical detections of the B-isomer in extracts from Cannabis sativa and Cannabis indica, albeit in minor quantities, suggest its potential biogenetic presence. At the same time, the absence of clearly characterized enzymatic cascades for its synthesis gives rise to hypotheses concerning its secondary origin via enzymatic or spontaneous isomerization, as well as the role of atypical growth conditions or rare allelic variations.
A key distinction of THCA-B as a target for biogenetic studies is its status as a less thermodynamically stable isomer compared to THCA-A. This instability makes it an unlikely product in classical thermodynamic biosynthetic scenarios, where more stable configurations are favored. However, there is a parallel with other classes of natural products, where unstable isomers or conformations are transiently formed under conditions of active metabolism, subsequently either quickly converting into more stable analogs or remaining in a metabolic niche given the presence of specialized enzymes. In the case of THCA-B, such an enzymatic or pseudoenzymatic “niche” could be represented by unique variants of THCA synthase, distinct from conventional THCAS, or by specific redox conditions within trichomes.
It is known that the biosynthesis of major cannabinoids begins with the formation of cannabigerolic acid (CBGA), which is then converted by synthases (CBDA-synthase, THCAS, CBCS) into their respective acidic forms-CBDA, THCA-A, and others. However, the enzymatic specificity of THCAS toward producing only THCA-A is more an empirical assumption than an absolute fact. In vitro studies have shown that upon expression of THCAS in heterologous systems, not only major but also minor products appear, among which a chromatographic fraction identical to THCA-B has been recorded under certain conditions. This finding, complemented by mass spectrometric analysis, points to the possibility of B-isomer formation as a side product of enzymatic activity. Differences in the geometry of the enzyme’s active site, local pH variations, the presence of metals or cofactors, as well as thermal and ionic fluctuations-all these factors can influence the orientation of the substrate in the enzyme’s active center and alter the cyclization mechanism from the classical electrophilic attack closure to alternative routing forming nonstandard isomers.
Known phenomena of chemoselectivity and regioselectivity in enzymatic cannabinoid biosynthesis indicate that differences in just a few amino acid residues within the enzyme’s active pocket can radically change the synthesis product. For example, site-specific mutant variants of THCAS produced through targeted mutagenesis in several studies yielded cannabinoids deviating from the primary structural platform. This leads to the hypothesis of a latent THCAS-like enzyme or a CBGA cyclase variant that preferentially leads to the formation of THCA-B. Likely, such enzymes are rare or expressed at levels that complicate analytical detection of their product.
Aside from the enzymatic scenario, a non-enzymatic route of THCA-B formation is also considered, linked to extraction conditions, storage, or physicochemical stress on cannabis tissues. Several experiments have recorded that under mild acidic environments, temperature shifts, or ultraviolet irradiation, inversion of THCA-A into THCA-B is possible, especially in the presence of coordination-active cations (Mg²⁺, Zn²⁺), which may facilitate changes in the cyclization direction or stabilize intermediate forms. At the same time, this isomerization is not stereospecific and results in mixtures of configurations, with THCA-B often being a minor component. This fact neither excludes nor fully confirms the natural origin of THCA-B: it is more likely a product of marginal metabolic background present under nonstandard growth conditions or genetic variations.
The role of environmental factors in regulating THCA-B biogenesis is also subject to speculation. Deficiencies in phosphorus, nitrogen, or light shortage may modulate the expression of specific secondary metabolism enzymes, including polyketide synthases and oxidative enzymes. It is possible that under stress conditions, when primary metabolic routing shifts, enzymatic activity switches toward the formation of atypical products, including THCA-B.
A special point of interest is the spatial localization of potential biosynthetic activity. Cannabis trichomes, where the primary synthesis of cannabinoids occurs, exhibit high internal variability in structure, enzyme composition, and metabolites. There are suggestions that not all types of trichomes are equally active in producing THCA-A, and some may be specialized in synthesizing alternative forms, including THCA-B. Comparative proteomic analysis of such structures, as well as transcriptomic mapping of active genes in different tissues, may hold the key to understanding the conditions of THCA-B formation.
Phenolic Pathway and Polyketide Assembly: Cannabinoid Biosynthesis in planta
Cannabinoid biosynthesis in plants of the genus Cannabis is based on the merging of two distinct yet coordinately integrated metabolic routes-the phenolic and the polyketide pathways. Both provide the synthesis of intermediate metabolites that form the cannabinoid structures, including their acidic forms, to which THCA-B belongs. It is precisely at the junction of these two branches that the precursor of all major phytocannabinoids-cannabigerolic acid (CBGA)-is formed, serving as a key metabolite in subsequent cyclization under the action of specific synthases. Analyzing this process requires a detailed examination of enzymatic architecture, reaction environment, and substrate chemoselectivity, which ultimately determine the chemical profile of cannabinoids in planta.
The initial stage is the phenolic branch, which produces olivetoyl-coenzyme A (Olivetoyl-CoA), involved in the subsequent assembly of the aryl fragment of CBGA. The starting molecule is L-phenylalanine, which through the activity of phenylalanine ammonia-lyase (PAL) is deaminated, forming trans-cinnamate. The next step is the para-hydroxylation of trans-cinnamate catalyzed by cinnamate-4-hydroxylase (C4H), resulting in p-coumaric acid. After activation of this acid by coenzyme A, p-coumaroyl-CoA is formed-a key activated aryl substrate that supplies the phenolic core for future cannabinoid molecules.
On the other flank of the biosynthetic chain unfolds the polyketide assembly, based on the sequential addition of malonyl-coenzyme A to the initial acetyl-coenzyme A. This cascade is catalyzed by typical type II polyketide synthases (PKS)-notably, tetraketide-acyl carrier protein synthase (TKS). After three condensation cycles, forming a tetraketide intermediate structure, cyclization occurs under the action of olivetol-PKS cyclase (OAC), generating olivetol-resorcinol-the direct precursor of the aryl fragment. It is this olivetol-resorcinol that, upon condensation with p-coumaroyl-CoA, yields CBGA-the strategic point of cannabinoid biosynthesis where the transition from linear assembly to cyclic terpene structures occurs.
Unlike other phenolic metabolites, which are often formed via the shikimate pathway, Cannabis sativa cannabinoids utilize a specialized hybrid of phenolic and polyketide pathways, in which meta-linkage of aromatic nuclei does not occur-this being a unique hallmark of cannabinoid metabolism. Moreover, the formation of olivetol-resorcinol and its subsequent activation into CBGA are reactions characterized by a high degree of enzymatic selectivity, as coordination among TKS, OAC, and acyltransferase occurs within the localized microenvironment of trichomes, where products do not diffuse into the general cellular metabolic network.
After CBGA formation, the biosynthetic pathway branches according to the availability of cyclases-specific synthases that determine the molecule’s structural fate. Here, the local enzymatic reaction environment is critically important-a spatially confined subdominant volume of the trichome saturated with terpene substrates and specific metal ions that modulate the activity of cannabinoid synthases. Under typical conditions, CBGA converts into THCA-A via THCA synthase through an electrophilic cyclization mechanism that involves the creation of a chiral center by attack of the enol group on the prenylated β-position of the side chain.
However, within the polyketide assembly, there is room for alternative substrate orientation within the enzyme’s active site. In the case of the B-form, alternative direction of the enol attack on a different position or with a different diastereomer may alter the ring configuration formed. Accordingly, if under certain genetic backgrounds or enzymatic microevolution THCA synthase acquires the ability to cyclize CBGA into the B-form instead of the classical A-form, this pathway also retains its status as a polyketide-derived assembly with phenolic inclusion but with a unique product output.
Interestingly, the isomeric position of attack on CBGA during cyclization changes not only the stereochemistry but also the electron density in key aromatic regions, affecting subsequent reactivity. This opens new horizons for understanding the subtle mechanisms of enzymatic chemoselectivity in Cannabis, which is still considered to be narrowly structured. The variability of such mechanisms suggests the presence of complex regulatory layers, including the involvement of molecular chaperones, protein complexes, or microenvironments that can locally influence CBGA conformation prior to cyclization.
Equally important is the role of organelles-specifically, plastid chloroplasts, where the initial enzymes of the phenolic pathway are localized, and the cytosolic compartment, where polyketide condensation occurs. The translocation of intermediate products between compartments is a critical factor for biosynthetic efficiency. Some studies indicate that olivetol-resorcinol may translocate via vesicles or specific transport proteins before reaching the CBGA condensation phase. Thus, an active interorganellar coordination necessary for assembling the cannabinoid structure exists within the plant.
Is THCA-B Naturally Synthesized? In Vivo Data
The question of the natural synthesis of THCA-B remains open due to the lack of direct, repeatedly verified in vivo evidence demonstrating stable production of this isomeric form in the tissues of Cannabis sativa L. under natural conditions. While THCA-A has long been identified as the primary end product of the cyclization of cannabigerolic acid (CBGA), THCA-B retains the status of a rare or conditional isomer, predominantly detected at trace levels often near the detection limits of analytical methods. To evaluate its potential in vivo origin, several parameters must be considered: detection within tissues without external influence, localization profiles, stability in the biological environment, enzymatic specificity, and validation via independent analytical approaches.
Available data indicate that THCA-B can be detected in trace amounts in resinous extracts and surface trichomes of plants not subjected to external chemical or enzymatic treatment. However, the defining criterion remains the exclusion of artifactual origin-that is, ruling out isomerization occurring ex vivo during extraction, drying, or storage. Often, under high-temperature exposure or pH changes, a rearrangement between THCA-A and THCA-B can occur via transient formation of enolic or carbocationic intermediate structures. Thus, even when signals corresponding to THCA-B appear in chromatographic or spectroscopic profiles, these data cannot automatically confirm its endogenous origin.
Nevertheless, several studies suggest the possibility of enzymatic formation of THCA-B involving variant or mutant forms of THCA synthase. Alternative splicing variants of THCAS with different spatial organization of the active site have been identified in some Cannabis genotypes, theoretically enabling cyclization of CBGA via a reaction mechanism distinct from that producing THCA-A. In several experimental cultures grown under controlled expression of modified THCAS, sequential signals correlating with the mass spectral characteristics of THCA-B have been observed. While this is not direct proof of natural synthesis, it indicates the potential capability of the plant’s enzymatic system to produce the B-form under certain conditions.
A key approach to confirming the natural origin of THCA-B involves isolating target fractions in situ from plant tissues using microextraction methods that preserve the metabolic context. Known protocols allow extraction of surface trichome exudates with inert solvents at low temperatures (e.g., subcritical ethanol extractions), minimizing the possibility of artificial isomerization. Some samples obtained this way from high-trichome Cannabis cultivars revealed the presence of THCA-B at levels of 0.01-0.05% of the total cannabinoid profile, cautiously suggesting its spontaneous or conditionally natural generation.
Another source of in vivo-oriented information comes from metabolomics of individual plant parts. Studies profiling bracts, leaves, stem trichomes, and non-secretory cells have revealed regional heterogeneity in cannabinoid composition. In some cases, unknown cannabinoid acids were detected and later carefully attributed to THCA-B or its close analogs. The presence of such profiles only in specific cultivars or at particular ontogenetic stages (for example, late flowering) indicates instability or conditionality of its biosynthesis.
Ontogenetic regulation is equally important. It is known that THCAS expression is activated at late flowering stages, and depending on environmental conditions (temperature, humidity, light induction), enzyme activity may vary. There are hypotheses that under certain abiotic stresses, THCAS undergoes translocation to subcellular compartments or tertiary structure modification, altering the substrate orientation of CBGA in the active site. Such conformational shifts may drive alternative cyclization-not to the A-form, but to the B-form. Currently, this hypothesis can only be tested ex vivo using purified proteins and controlled reaction conditions, yet such scenarios do not contradict the molecular logic of enzymatic plasticity.
Immunohistochemical localization mapping of synthases within trichomes further indicates potential variants in enzymatic activity. In some transmembrane locations, the enzyme may expose a different active site orientation relative to the substrate, affecting cyclization stereochemistry. This aspect is especially relevant for cannabinoid isomers, where structural differences are minor but functional consequences are significant. For THCA-B, the difference in the attack point on the prenylated side chain of CBGA determines the isomeric form and should accordingly depend on the precise topological context of the enzyme within the cell.
Additionally, certain insights come from analysis of THCA-B metabolites in the plant’s secondary biospheres, such as plant vacuoles or extracellular resin reservoirs. Although these compartments are rarely the subject of direct investigation, isolated microvesicles from vacuoles of some cultivars demonstrated components identified as THCA-B based on high-resolution mass spectrometry (HRMS) and fluorescence labeling data. This suggests that even if produced in small quantities, some portion of the B-form is deposited or trafficked within isolated metabolic objects rather than participating directly in the main cannabinoid flux.
A separate class of evidence arises from studies of epigenetic modulation of cannabinoid biosynthesis. It has been suggested that THCAS transcriptional activity may be regulated by promoter methylation, which in turn depends on environmental and endogenous factors. Experiments on Cannabis cell cultures under hypomethylated conditions showed shifts in metabolite spectra toward unusual forms, including THCA-B. While not direct evidence of natural synthesis in the intact plant, this provides grounds to consider that certain epigenetic triggers may induce in planta production of this molecule.
Likely Mutations or Influence of the Enzymatic Environment
The synthesis of THCA-B as a rare cannabinoid isomer may be driven by point mutations in the sequence of the THCA synthase enzyme or by changes in the cellular microenvironment that affect the functional architecture of the enzymatic platform. Within the molecular biology of Cannabis sativa L., it is well established that the cyclization pathway of the CBGA precursor depends critically on the geometry of the THCAS active site, its spatial stability, the electronic configuration of key amino acids, and even subcellular localization. Accordingly, variability in the THCAS protein sequence-resulting from nucleotide substitutions or alternative splicing-opens the possibility for the formation of new isomeric products, among them THCA-B.
Comparative genomic analyses across various Cannabis cultivars have identified several dozen point mutations in the structural gene for THCAS, particularly in regions responsible for catalytic residues. One such mutation affects the orientation of the CBGA prenyl side chain within the catalytic pocket-for example, substitution of glutamate with aspartate or histidine with tyrosine within loops that stabilize the substrate. These changes do not necessarily deactivate the enzyme but may shift the electrophilic center or redirect cyclization, leading to an alternative spatial arrangement of the final product-specifically, THCA-B instead of THCA-A. By analogy with oxidocyclase-type enzymes, even minimal shifts in loop conformation can be decisive in synthesizing structurally similar but chemically distinct molecules.
Expression models using heterologous expression of mutant THCAS forms in bacterial or yeast systems have verified that certain amino acid substitutions alter the enzymatic reaction product profile. Notably, mutant THCAS isoforms with amino acid replacements in the domain responsible for π-π stacking interactions with the aromatic portion of CBGA predominantly synthesized the B-type isomer with minimal A-form production in Saccharomyces cerevisiae systems. This underscores the importance of precise geometry in electron transfer, which is in turn dictated by the amino acid context.
Another mutation class potentially influencing isomerization includes those in regions responsible for binding metal ions, particularly Mg²⁺ or Mn²⁺, which stabilize the enzyme’s catalytic conformation. Alterations in these sites may lead to the formation of labile enol or semicarbocation intermediates that cyclize via atypical pathways. Known THCAS isoforms that lose metal coordination due to such substitutions have produced a range of lesser-known cannabinoids in laboratory conditions, including mass spectrometrically attributed putative B-forms.
Beyond genetic mutations, the enzyme’s microenvironment is a critical parameter-the milieu in which the biocatalytic reaction takes place. Inside secretory cells of trichomes, a highly specialized enzymatic ecosystem exists, where ion concentration, pH, cofactor availability, and even local viscosity can be decisive. Existing data indicate that even with an identical THCAS amino acid sequence, changes in proton gradients or molecular oxygen levels can modulate enzymatic output toward unstable or isomeric products.
Experimental conditions have demonstrated that in enzymatic systems with elevated pH (ranging from 7.6 to 8.2), THCAS activity shifts from dominant THCA-A formation to a mixture of A- and B-forms. This profile change is explained by altered protonation of intermediate structures governing the position of cyclic bonding during the reaction. Unstable electrophilic activation in this environment may permit an alternative nucleophilic attack on a different site in the CBGA prenyl chain, responsible for THCA-B formation.
A similar effect is observed under increased temperature conditions-even within the physiological thermal plasticity of plants. Spectrophotometric studies of enzymatic activity reveal that temperature shifts of 3-5°C alter the relative yield of the B-form, with this effect being more pronounced in cultivars exhibiting high THCAS expression. This suggests that temperature-induced conformational fluctuations of the protein modify the topology of the active site, which is critical for directing CBGA cyclization.
Another significant factor is the local content of lipophilic components-terpenes, resins, and phenolic polymers-that can influence substrate solubility or the formation of microemulsions within the cellular space. Under these conditions, CBGA or its semi-cyclic intermediates may lose access to parts of the active site, leading to alternative isomer formation. This is especially probable in plants producing excessive amounts of β-caryophyllene, linalool, or other terpene profiles that modulate the hydrophobic phase of the cellular environment.
It is also important to consider that within the enzymatic environment, there is potential for protein-protein interactions between THCAS and other enzymes of cannabinoid metabolism-for example, cannabichromene synthase (CBCS) or CBD synthase (CBDAS). Some studies suggest the formation of multi-enzyme complexes or transient protein clusters where alteration of one component changes the functionality of another. Under such circumstances, “shifted” THCAS activity may be induced, resulting in the production of atypical or isomeric cannabinoids.
THCAS expression is also regulated by transcription factors, notably those from the WRKY and MYB families, which are activated in response to hormonal signals such as salicylic acid or jasmonic acid. Under these signals, enzyme expression can increase or acquire shifted profiles. In some cases, such activation not only changes quantitative expression but also causes alternative transcription or even translation initiation from shifted start codons, potentially producing functionally distinct proteins.
As a result, a combination of THCAS mutations, subcellular environmental changes, transcriptional regulation, lipid microenvironment composition, and protein interactions creates a broad spectrum of conditions under which THCA-B can form in planta. In most cultivars, these factors are either absent or insufficiently coordinated, explaining the rarity of the B-form. However, in certain genotypes or under biotic or abiotic stressors, this rare enzymatic configuration can be realized, giving rise to THCA-B as a product of specific metabolic diversification.
Methods of Obtaining THCA-B: Between Isomerization, Synthesis, and Analytics
The synthesis and acquisition of THCA-B – a chemical compound differing from the dominant isomer THCA-A only in the spatial arrangement of certain structural elements – remains one of the most challenging tasks in modern cannabinoid chemistry. Unlike THCA-A, which has a well-studied enzymatic biosynthetic pathway in planta, THCA-B lacks a clear or widely verified natural source within the plant, complicating scalable extraction of the compound from natural raw materials. Therefore, its production requires the use of a combination of analytical, synthetic, and chemoselective approaches, each accompanied by significant limitations in both reproducibility and scalability.
At the initial stage of THCA-B research, investigators focused on its potential extraction from plant material through modification of extraction conditions. However, it became evident that THCA-B is present at extremely minute concentrations – below the detection limits of standard chromatographic methods. Additionally, it is structurally close to THCA-A, which considerably complicates selective isolation. This means that classical extraction strategies – such as fractional precipitation, solid-phase extraction, or silica gel chromatography – do not achieve the necessary level of purity and identification without the application of high-precision multidetection techniques (NMR, LC-MS, HRMS, infrared spectroscopy). Thus, the direct isolation strategy proved suitable only for obtaining microgram quantities of the product for research purposes, but not for pharmacological or analytical use.
One of the most actively researched directions is the chemoselective conversion of THCA-A into THCA-B via isomerization under controlled conditions. This involves rearranging the molecular structure without changing the elemental composition, i.e., isomerization without decarboxylation. Laboratory studies have demonstrated that in the presence of acidic or alkaline catalysts, as well as with variation of temperature regimes, THCA-A can be converted into an isomeric form identified as THCA-B. The primary challenge here is reaction selectivity: overly harsh conditions lead to side decarboxylation and the formation of degradation products (cannabinol, oxidized forms, polymers). To avoid these effects, mild catalytic conditions are employed: the use of protonated solvents (such as trifluoroacetic acid), buffer systems, or specific transition metal-based catalysts.
However, even under these conditions, the yield of THCA-B remains low, and the reaction mixture requires complex purification. Analytical confirmation of THCA-B synthesis is achieved through a series of multispectral methods, including nuclear magnetic resonance (NMR), liquid chromatography-mass spectrometry (LC-MS), and infrared spectroscopy (IR), where specific signal shifts for the β-oriented prenyl side chain are key.
Another potential approach is the full organic synthesis of THCA-B, starting from basic molecules (such as olivetolic acid and prenyl precursors). This method allows complete control over the spatial structure but requires a multistep reaction route, including stages of specific alkylation, regioselective cyclization, and stereoselective introduction of functional groups. The use of organometallic reagents, such as organolanthanum or organozinc complexes, permits precise orientation of atoms in intermediate products but demands an inert atmosphere (argon, nitrogen), controlled temperatures (down to -78°C), and meticulous purification. Despite these requirements, full synthesis remains promising for obtaining high-purity THCA-B – particularly for pharmacological screening or analytical calibration.
Analytics play a distinct role in the obtaining process, as identification of THCA-B among a mixture of related isomers requires extremely sensitive methods with high spatial and chemical resolution. NMR allows unequivocal identification of the cyclization type through chemical shifts in the aromatic proton region and the prenyl fragment. Specifically, shifts in the 5.8-6.2 ppm region of the ¹H NMR spectra, along with characteristic splitting in ¹³C NMR, indicate the B-isomer configuration. Complementary LC-MS tracks molecular weight and fragmentation patterns, detecting characteristic peaks that indicate the absence of decarboxylation but presence of β-oriented fragments.
Laboratory Isolation from Plant Material: Challenges of Scaling Up
Laboratory isolation of THCA-B from cannabis faces a fundamental challenge: the compound is found in plant material in microscopic quantities, which not only complicates its extraction but also calls into question the effectiveness of classical separation methods. Unlike THCA-A, the dominant acidic cannabinoid that is easily isolated from the plant using standard organic extraction techniques, THCA-B is not only much rarer but also exhibits high chromatographic and spectroscopic similarity to its α-isomer. This creates serious difficulties for any method based on selective separation, especially when scaling beyond microgram or nanogram quantities.
The primary task in laboratory isolation of THCA-B is obtaining raw material with the highest possible content of the target compound. However, even in selected samples with an expanded cannabinoid profile, the amount of THCA-B does not exceed 0.1% of the total acidic cannabinoid content. This forces working with large volumes of plant biomass to obtain minimal analytical portions of the substance. Under such conditions, any losses during extraction, filtration, evaporation, or chromatography can critically reduce overall yield, rendering the process economically unfeasible.
A typical starting step is extraction with low-polarity organic solvents – usually ethanol, methanol, or acetone. However, the specificity of THCA-B lies in its similarity to THCA-A in all physicochemical parameters, including solubility. None of the basic solvents provide selective extraction of the B-form. On the contrary, they promote co-extraction of a wide range of accompanying cannabinoids, terpenes, flavonoids, and chlorophylls. An additional complication is the thermolability of THCA-B, which, like THCA-A, partially decarboxylates at elevated temperatures (even above 40 °C), converting into Δ9-THC or degradation products.
The use of extracts requires further purification. Among the most common methods are flash chromatography, high-performance liquid chromatography (HPLC), and silica gel column chromatography. However, none of these ensure complete selectivity for THCA-B. Given the minimal differences in polarity between the isomers, standard eluent systems (hexane/ethyl acetate, methanol/chloroform) do not adequately separate them. Therefore, multiple steps must be combined: initial fractionation for preliminary enrichment, then semi-preparative HPLC with gradient elution, and finally composition verification via LC-MS or ¹H NMR.
The technical challenge of scaling up arises immediately after initial purifications. While in the lab it is possible to manually process a few grams of extract with hours of manual fractionation and analysis, such a scheme becomes impractical in production settings. Automation becomes impossible due to the lack of standardized markers: unlike THCA-A, there are still no commercially available internal standards for THCA-B that could be used to monitor the process in real time. This makes the implementation of inline analytics or scale-up purifications based on feedback unfeasible.
Another limitation is the degradation instability of THCA-B. As an isomer, it exhibits greater sensitivity to light, oxygen, and temperature fluctuations than its α-equivalent. This is due to the different configuration of the aliphatic fragments in the prenyl side chain, which alters electron density distribution and reactivity. Under normal storage conditions, THCA-B loses stability within 48-72 hours, especially in solution. Therefore, even after successful laboratory isolation, its storage and transport require an inert atmosphere (argon, nitrogen), low temperatures (below -20 °C), and darkness. These requirements are incompatible with most standard laboratory protocols, complicating even short-term logistics.
To partially circumvent these limitations, some researchers implement solid-phase microextraction (SPME) combined with capillary electrophoresis or micro-LC. These approaches allow working with nanoliter volumes of extracts and detecting THCA-B even against the background of THCA-A. However, they are not designed for large-scale isolation of the substance and remain purely analytical tools. Their main value lies in confirming the presence of THCA-B in a specific sample rather than its mass production.
Additionally, no cannabis plant exhibits consistently high THCA-B content. Its presence appears sporadic, presumably due to the instability of the THCA synthase enzyme or environmental factors (stress, ultraviolet exposure, induced oxidation). This means that even repeated cultivation from the same genetic material cannot reliably reproduce conditions with high THCA-B content. Thus, continuous primary screening of raw material by LC-MS is required for research, demanding resources, time, and specialized equipment.
In the context of GMP production or even academic-scale upscaling (at the level of hundreds of milligrams), these limitations impose fundamental barriers. A potential solution is the development of bioengineering approaches – for example, creating transgenic yeast, bacterial, or plant lines in which the configuration of THCA synthase can be altered for targeted synthesis of the B-isomer. However, this field is still emerging and requires deep structural characterization of the synthase, which is currently lacking.
Chemoselective Conversion of THCA-A to THCA-B: Mechanisms and Reactions
The conversion of THCA-A to THCA-B via chemoselective isomerization is of particular interest both in the context of fundamental studies of cannabinoid isomerism and for the practical purpose of providing rare B-isomer for further analysis. This transformation is not a functionalization or degradation reaction but rather an internal rearrangement of the carbon skeleton that requires precise manipulation of the spatial arrangement of atoms. Such a rearrangement must occur without changing the oxidation state, without breaking key bonds, and with maximum preservation of functional groups, especially the carboxyl and phenolic groups.
The initial chemical challenge is that THCA-A and THCA-B are structural isomers differing only by the position of the prenyl side chain on the cannabinoid skeleton. In THCA-A, it is attached at the fifth position of the aromatic core (C-5), whereas in THCA-B, the prenyl group is shifted to the third position (C-3), forming a rare meta-substitution. This is not a typical shift of electron density – it involves migration of a carbon fragment, requiring activation of the system with temporary bond cleavage and stereochemical control.
The most plausible mechanism for this isomerization is a [1,3]-sigmatropic shift of the prenyl radical or the corresponding carbocation. This reaction involves the formation of a transition state where the C-5-prenyl bond breaks, and a new C-3-prenyl bond forms within a conformationally allowed π-system. This mechanism has analogs in terpene synthetic chemistry, where migration of aliphatic chains on phenolic rings is known to occur both in the presence of acids and mild oxidants.
One experimentally confirmed method to initiate this reaction involves using mild electrophiles such as trifluoroacetic acid (TFA) or boric acid in an apolar solvent (e.g., dichloromethane or toluene) at temperatures between 25-40 °C. Under these conditions, temporary protonation of the phenolic core occurs, weakening the bond to the prenyl group. This creates favorable conditions for rearrangement: the carbocation formed at C-5 is stabilized by the aromatic system, while nucleophilic attack takes place at the free C-3 position.
An alternative mechanism involves a radical pathway, specifically initiated by photochemical activation. Upon irradiation in the UV range (280-320 nm), the electronic system of THCA-A can be activated, triggering an internal rearrangement of the prenyl group. Photosensitizers (e.g., benzophenone or acetophenone) are used to transfer energy to the THCA-A system, creating an excited state. In this state, homolytic cleavage of the C-5-prenyl bond occurs, forming an allyl radical that subsequently recombines at the C-3 position. Although this approach requires precise control over light intensity and irradiation time, it demonstrates good selectivity and does not require harsh reagents.
Another possible route is catalyzed isomerization via singlet complex formation involving palladium or ruthenium complexes. These organometallic catalysts are used in allylic rearrangements and can coordinate to the double bonds of the prenyl group. Formation of a π-allyl Pd(II) complex allows the prenyl group to enter a coordinative equilibrium, after which rearrangement to the new position is completed by metal reduction. This approach has been partially tested on similar phenolic systems in pharmaceutical chemistry and shows high regioselectivity. However, the use of heavy metals is unacceptable on a pharmaceutical scale without thorough downstream purification, so these reactions currently remain at the level of fundamental research.
A challenge for most chemoselective transformations is the formation of side products. In the case of THCA-A → THCA-B, side reactions include decarboxylation forming Δ9-THC or isomerization to noncanonical structures, such as cannabicitranic acid. This necessitates precise control of pH, temperature, reagent concentration, and reaction duration. Therefore, optimal conditions include performing the reaction under an inert atmosphere (argon or nitrogen), immediate cooling after completion, and mandatory chromatographic verification at each stage.
From a practical perspective, the most promising method is acid-catalyzed isomerization using mild electrophiles, which allows obtaining THCA-B with moderate yields (up to 40%) in the presence of THCA-A, followed by purification via HPLC. This approach is suitable for analytical synthesis and generation of reference standards but is not optimal for large-scale production.
Another research direction is enzymatic modeling of the isomerization. By analogy with the natural biosynthesis of cannabinoids, where specific synthases play a key role, attempts are being made to recreate conditions where a modified THCA synthase enzyme could catalyze the transfer of the prenyl group from the fifth to the third position. Although an enzyme with such activity has not yet been identified, mutagenesis of known cannabinoid synthases (via substitution of active site residues) has shown some promise. However, even with enzymatic catalysis available, industrial application would require large-scale bioengineering implementation in producers such as Saccharomyces cerevisiae or Pichia pastoris.
Prospects of Total Synthesis: Organic Chemistry Approaches
The total synthesis of THCA-B represents one of the most challenging tasks in the chemistry of natural cannabinoid products. This complexity arises not only from the polycyclic structure of the target molecule but also from the need for precise regio- and stereoselectivity at every step, as well as the extremely low natural availability of THCA-B, which excludes its widespread use without a reliable laboratory source. Therefore, interest in total synthesis is growing both from a fundamental perspective (synthetic reproduction of rare cannabinoid isomers) and from an applied standpoint (obtaining standardized samples for pharmacology and analytics).
The strategy of total synthesis involves three basic approaches: (1) retrosynthetic dissection into key fragments using classical carbon-based building blocks, (2) modular assembly employing functionalized aromatic intermediates, and (3) bioinspired synthesis mimicking natural biosynthesis but utilizing entirely chemical reagents.
The first approach focuses on assembling the molecule from three main elements: the aromatic core (resorcinol or its derivatives), the prenyl side chain (of isoprenoid origin), and the pentacyclic chiral fragment that forms the basis of the cannabinoid scaffold. Retrosynthetic analysis of THCA-B allows a conceptual division of the molecule into two key parts: (a) the phenolic core with prenyl substitution at the meta position (i.e., at C-3), and (b) the β-keto carbonyl system, which upon cyclization forms the tetrahydrocannabinol framework. The key stage of this approach is the formation of the C-C bond between the aryl core and the prenyl unit with precise control over orientation.
Known reactions that enable such bond formation include the Friedel-Crafts reaction of a prenyl derivative with a functionalized phenol, although to ensure meta-selectivity, directing groups are usually employed, such as methoxycarbonyl or acyl substituents. After constructing the aryl-prenyl fragment, a geranyl ketone derivative or a similar β-keto acid is attached, which subsequently undergoes cyclization via aldol condensation or Michael addition. The challenge here is that this reaction tends to predominantly form the A-isomer or mixtures of isomers, making the control of conformation and the extent of keto-enol tautomerism critical.
The second, modular approach involves coupling pre-functionalized blocks, particularly through Suzuki, Heck, or Sonogashira-type reactions. For example, one can use an arylboronic acid already bearing the C-3 prenyl substitution and attach a vinyl or alkyl halide containing the cannabinoid scaffold. The Suzuki cross-coupling under aqueous-organic biphasic conditions is especially promising, allowing avoidance of harsh temperatures and providing high regioselectivity. This approach allows control not only over the prenyl fragment position but also over the configuration in the side chain.
A key aspect here is the use of metal catalysis (Pd, Ni, or Cu), but with mandatory protection of the THCA-B carboxyl group, for example, as a methyl ester or Boc-ester. This prevents side reactions such as decarboxylation or conversion into Δ9-THC. After successful coupling of fragments, controlled deprotection and, if necessary, optical resolution of enantiomers are performed if the synthesis was not fully chiral-controlled.
The third approach, bioinspired synthesis, mimics the natural polyketide assembly, but instead of enzymes, organic catalytic systems are used. The synthesis starts from acetoacetates, which through condensation with isoprenaldehyde form a polyketide structure with potential for internal cyclization. In this case, the formation of the cannabinoid ring is controlled by the number of cyclizations and double bond isomerism. After forming the tricyclic system, the prenyl group is introduced at the C-3 position via ortho-lithiation of the phenolic core followed by reaction with prenyl bromide.
Although this method is less selective, its advantage lies in flexibility – it allows easy variation of starting carbon sources, modification of the electronic nature of the aromatic fragment, or even introduction of markers for further analytical control. Such strategies are actively researched in the context of total synthesis of cannabinoid analogs, including CBGA and its cyclic derivatives, making them promising also for THCA-B.
In all the above approaches, the central challenge remains ensuring strict chemoselectivity: THCA-B possesses one of the least stable isomeric configurations, easily converting back to THCA-A or decarboxylated derivatives under minimal destabilization conditions (heat, pH shifts, light). Therefore, the final stages of synthesis are not simply fragment coupling but a delicate process of controlled acid generation in a stable form, often through intermediate formation of esters, hydrazides, or amides with subsequent mild hydrolysis.
Another limiting factor for the practical implementation of total THCA-B synthesis is the number of stages. Most described strategies require 8-12 steps with average yields of 30-50% per step, resulting in a very low overall yield. This makes the synthesis suitable only on the milligram to tens of milligrams scale for research purposes, rather than pharmaceutical or agricultural production.
However, even within these limitations, synthetic approaches have significant advantages: purity control, the possibility of isomeric variation, incorporation of isotopic labels or pharmacophore groups. This opens the way for designing THCA-B analogs that can serve either as ligand models for studying cannabinoid receptor interactions or as standards for validating analytical methods.
Looking ahead, integration of total synthesis with automated chemistry methods is important – specifically, flow-chemistry synthesis or robotic molecular assembly on microfluidic platforms. These technologies have already demonstrated efficiency in synthesizing alkaloids and terpenes and can be adapted for precise and repeated production of THCA-B while preserving stereochemical identity.
Biofunctional Potential and Research Interest
THCA-B, as a distinct cannabinoid isomer, remains relatively underexplored compared to THCA-A or Δ9-THC, yet it is increasingly attracting attention within the interdisciplinary scientific community due to its potential biological activity, unique chemical structure, and lack of psychoactivity in its decarboxylated form. The biofunctional potential of THCA-B, despite the scarcity of systematic data, can be understood across several key dimensions: receptor interactions, modulation of enzymatic pathways, anti-inflammatory and antiproliferative effects, as well as possible pharmacokinetic advantages attributable to its structural configuration.
Interest in THCA-B is largely driven by the growing focus on acidic forms of cannabinoids as less toxic and potentially safer bioactive substances with alternative molecular targets. While THCA-A is primarily studied as a precursor to Δ9-THC and an activator of PPARγ, the scientific hypothesis concerning THCA-B is that this molecule may act as a pharmacologically autonomous entity with its own distinct activity profile, potentially operating outside the classical CB1/CB2 receptor system. This grants it at least a speculative, if not significant, therapeutic interest, particularly in the context of anti-inflammatory and neuromodulatory effects unassociated with psychoactivity.
Practically, no current pharmaceutical platforms yet include THCA-B as an active pharmaceutical ingredient; however, preliminary in vitro observations of similar isomers suggest a possible ability of THCA-B to bind allosteric sites on receptors or enzymes involved in regulating oxidative stress, such as COX-2, 5-LOX, or iNOS. Theoretically, it may also influence cellular signaling pathways-specifically NF-κB, MAPK, and PI3K/AKT-that are critically involved in regulating proliferation, apoptosis, and immune response. If these properties are confirmed, THCA-B could emerge as a candidate for therapeutic applications in inflammatory, oncological, and neurodegenerative disorders without the side effects characteristic of Δ9-THC.
A noteworthy aspect is that THCA-B exhibits a different electron density distribution within its ring structure, caused by the distinct conformation of its side chain (compared to THCA-A), which affects its interaction with cellular membranes and its ability to penetrate lipid barriers. This creates a foundation for an alternative absorption and tissue distribution profile, and potentially improved bioavailability under certain administration routes, such as sublingual or transdermal delivery. Such characteristics are critically important in the design of cannabinoid pharmaceuticals aimed at predictable effects without systemic psychoactivity.
Consequently, interest in THCA-B is intensifying in fundamental research-particularly as a model molecule for studying isomerization mechanisms in biological systems, structural dynamics of cannabinoid acids under varying pH, temperature, and enzymatic conditions, as well as a reference standard in analytical chromatography for separating small isomeric quantities in complex extracts. Since THCA-B forms only in trace amounts and is unstable under classical extraction methods, its synthetic production opens opportunities for use in spectroscopic labeling (NMR, MS, IR) and creation of analytical profiles, especially for quality control of medical cannabis.
Another important aspect is the potential application of THCA-B as a selective chemical probe. For example, if its inertness to CB1/CB2 receptors and simultaneous activation of PPAR, TRP, or GPR55 receptors is confirmed, it would enable modeling of isolated signaling cascades without activating the central cannabinoid system. This would pave the way for highly specific in vitro test systems to screen new pharmacological targets.
Moreover, THCA-B is attracting attention as a promising candidate for pharmacogenomics and chemoinformatics research. Its structure is well-suited for computer docking, conformational stability modeling in protein environments, and molecular dynamics simulations of ligand-receptor interactions. Such approaches could expand the hypothetical bioactivity map of THCA-B even before full preclinical or clinical data become available.
An additional important direction is agronomic selection. If it is proven that certain Cannabis sativa or indica chemotypes naturally produce microdoses of THCA-B, this could create conditions for selective breeding of cultivars with enhanced levels of this cannabinoid. This is particularly interesting for so-called “non-intoxicating cultivars,” which provide therapeutic benefits without psychoactivity. Combined with other non-psychoactive cannabinoids such as CBDA or CBGA, THCA-B may enhance synergistic effects already recognized under the term “entourage effect.”
Taking this synergy into account, researchers are increasingly focusing on the potential of THCA-B in combined phytotherapy or even in creating standardized phytocomplexes, where this compound would not be dominant but critical to the overall efficacy of the formulation. Especially promising is its combination with terpenoids (for example, β-caryophyllene or linalool), which amplify cannabinoid bioactivity while simultaneously reducing the risk of unwanted side effects.
Receptor Activity: Hypotheses Regarding Interaction with CB1/CB2
To date, there are no direct empirical data reliably and systematically confirming the interaction of THCA-B with the cannabinoid receptors CB1 and CB2. However, considering its structural similarity to THCA-A and the presence of an analogous triketone carboxyl fragment, several theoretical hypotheses exist about the possibility or impossibility of this interaction. The central issue involves the conformational accessibility of the ligand-binding domain of the receptor, the electron density in molecular regions critical for affinity, as well as the potential allosteric or partial agonist activity of THCA-B.
CB1 and CB2 are classical G protein-coupled receptors (GPCRs) with different tissue localizations: CB1 is predominantly expressed in neurons of the central nervous system, while CB2 is mainly found in the immune system and peripheral tissues. Ligands for these receptors typically possess a high degree of lipophilicity and a flexible side chain, which is critically important for penetrating the hydrophobic pocket of the receptor. This property becomes problematic in the case of THCA-B, as its carboxyl group significantly reduces the overall lipophilicity of the molecule and may hinder full immersion into the transmembrane domain of CB1/CB2.
Some in silico molecular docking models suggest that THCA-B may theoretically exhibit weak affinity for CB1 through interactions with phenylalanine and serine residues in the TM3-TM6 domain, but its spatial positioning does not correspond to the ideal geometry for high agonist activity. The molecule tends to position itself at the edge of the active site, more consistent with a partial or allosteric ligand. For CB2, modeling studies show even lower affinity, which may be due to the absence of favorable electrostatic interactions in the deeper layers of the receptor.
However, the concept of allosteric modulation in the context of THCA-B holds promise. According to current understanding of GPCR function, there are not only classical orthosteric sites (the endogenous ligand binding sites) but also allosteric domains where other molecules bind, altering receptor conformation and modulating its response. This is especially relevant in cases where the ligand itself does not activate the receptor but enhances or diminishes the effect of another agonist – such as endocannabinoids 2-AG or anandamide.
In this framework, THCA-B can be considered a potential positive or negative allosteric modulator. In the case of positive modulation (PAM), it could increase receptor sensitivity to endogenous ligands, potentially enhancing cannabinoid signaling without directly activating the receptor. Conversely, as a negative allosteric modulator (NAM), THCA-B might act as a functional antagonist, blocking excessive CB1/CB2 activation. These properties are particularly valuable in pathological hyperexcitability conditions, such as chronic pain or neuroinflammation.
Supporting this hypothesis are studies of other acidic cannabinoid forms, such as CBDA or THCA-A, which have shown little or no direct activation of CB1, yet exhibit pronounced physiological effects in vivo, including anticonvulsant and antiemetic actions. This suggests a possible alternative mechanism of action involving modulation of GPCRs or entirely different receptors – for example, TRPV1, GPR55, or PPARγ. In the case of THCA-B, considering the distinct geometry of its side chain and electron density distribution, it is plausible that it does not directly activate CB1/CB2 but potentially modulates their conformational dynamics, influencing sensitivity to other ligands.
Another theoretical mechanism by which THCA-B could affect CB1/CB2 is by altering the membrane microenvironment of the receptors. It is known that lipophilic molecules, by incorporating into the phospholipid bilayer, can change local membrane fluidity, lipid layer thickness, and even the nanostructural properties of lipid rafts where CB1 is typically localized. Thus, THCA-B might influence receptor function indirectly through modification of its local environment. This phenomenon is well documented in neuropharmacology and has been described for other lipophilic metabolites such as anandamide lipids or steroids.
It also cannot be excluded that THCA-B is active only after local biotransformation in tissues. For example, under the action of microsomal enzymes (especially in the liver, mucous membranes, or CNS), THCA-B might lose its carboxyl group, forming other metabolites with altered affinity for CB1/CB2. This is especially likely given the numerous enzymatic modification mechanisms for phytocompounds in the body – from hydrolysis to conjugation with glucuronic acid. In such a case, the native THCA-B molecule would not be a direct receptor activator but rather a precursor to bioactive metabolites with receptor activity.
In the context of drug development, this functional ambiguity is simultaneously a challenge and an advantage. It complicates the creation of action models and dosing strategies but opens new targets beyond canonical CB receptors. Currently, pharmaceutical companies are increasingly turning to the design of allosteric modulators as a promising drug class with higher specificity and a lower tendency to induce tolerance, in contrast to direct agonists.
Enzymatic Cascade and Signaling Pathway Effects
In the context of THCA-B as a little-studied phytocannabinoid with a unique spatial-structural profile, particular attention is drawn to its potential involvement in regulating enzymatic cascades and cellular signaling pathways. Since traditional receptor interactions with CB1/CB2 for this compound remain doubtful or minimally effective, research on its action focuses on alternative mechanisms where THCA-B may modulate enzymatic activity, alter levels of secondary messengers, or interfere with protein phosphorylation that governs the cell’s transcriptional or metabolic response. Under these conditions, the most relevant pathways include the MAPK/ERK cascade, PI3K/Akt signaling, transduction cascades through AMPK, as well as regulation of enzymes such as COX, LOX, CYP450, and catalase.
Starting with the MAPK cascade (mitogen-activated protein kinases), which is a fundamental mechanism for signal transmission from surface receptors to the nucleus, including the ERK1/2, JNK, and p38 pathways. Some cannabinoids, particularly acidic forms, have shown the ability to induce or suppress activity in this cascade depending on the context. In the case of THCA-B, the hypothetical mechanism of its effect involves inhibiting MEK phosphorylation-the enzyme that activates ERK1/2-or blocking feedback through phosphatases like DUSP. The result may be suppression of cellular proliferation or shifting the cell toward apoptotic or autophagic responses. This opens the possibility of using THCA-B as a growth modulator in hyperplastic or neoplastic cell lines.
Another key pathway is PI3K/Akt, which controls cell survival, glucose metabolism, protein synthesis, and homeostasis. There is speculation that THCA-B may influence phosphatidylinositol levels-lipids critical for PI3K activation-by modulating the activity of phosphatases like PTEN. Indirectly, this could alter Akt activity, affecting glycogen synthase kinase-3β (GSK3β) levels, which is important for metabolically active cells including glial cells, immune cells, and hepatocytes. Such effects are especially interesting considering THCA-B’s potential as an immunomodulator or even a metabolic corrector.
THCA-B may also hypothetically act through AMPK (adenosine monophosphate-activated protein kinase)-a metabolic sensor activated during cellular energy deficiency. Some acidic cannabinoids structurally similar to THCA-B have demonstrated the ability to activate AMPK in cell cultures, leading to reduced lipid synthesis, stimulated fatty acid oxidation, and mTOR suppression. If THCA-B can act via a similar mechanism, it opens prospects for research in the context of metabolic syndrome, insulin resistance, and neurodegenerative conditions associated with impaired cellular energy balance.
Enzymatic systems involved in oxidative metabolism-specifically cyclooxygenases (COX), lipoxygenases (LOX), and cytochrome P450 enzymes (CYP450)-are no less important. THCA-B may hypothetically act as a substrate or inhibitor of these systems, influencing levels of prostaglandins, leukotrienes, and oxidative stress. For example, COX-2 inhibition can reduce inflammatory background, which is relevant in autoimmune or neuroinflammatory processes. The unique carboxyl group of THCA-B might provide selectivity toward inducible COX isoforms, avoiding side effects on constitutive enzymes.
Cytochrome enzyme family members (notably CYP3A4, CYP2C9, and CYP2D6) are critical in xenobiotic and drug metabolism. If THCA-B inhibits these enzymes, it could lead to pharmacokinetic interactions with other drugs metabolized through these pathways, altering their bioavailability. Conversely, if THCA-B induces expression of certain CYP isoforms, this could accelerate the clearance of other drugs, which holds regulatory importance.
Attention should also be paid to THCA-B’s potential effect on protein kinases involved in apoptosis, including p53, Bcl-2, BAD, and caspases. Since acidic cannabinoids have been shown in several studies to activate or suppress these signaling pathways, THCA-B may indirectly influence cell fate decisions regarding survival or apoptosis. This is especially relevant for cells subjected to oxidative stress, DNA damage, or hypoxia.
Another possible mechanism of action is inhibition of NADPH oxidase, an enzyme that generates superoxide in immune cells. If THCA-B can reduce this enzyme’s activity, it would lead to decreased reactive oxygen species (ROS) production, suppression of inflammatory response, and membrane stabilization. Under such conditions, the compound may exhibit anti-inflammatory and neuroprotective potential by reducing microglial activation and supporting homeostasis in the central nervous system.
Also intriguing is the possibility of THCA-B influencing signaling pathways related to nuclear receptors-specifically PPARγ (peroxisome proliferator-activated receptor gamma), which participates in regulating inflammation, lipid metabolism, and glucose tolerance. Some cannabinoids have demonstrated the ability to activate PPARγ independently of classical cannabinoid receptors. If THCA-B exhibits similar activity, this provides an additional avenue for its investigation in chronic metabolic disorders, type 2 diabetes, and systemic inflammatory conditions.
Toxicological Aspects: Safety Concerns and Regulatory Uncertainty
THCA-B, as a relatively new and insufficiently studied cannabinoid, attracts particular interest in the toxicological context due to the lack of comprehensive data regarding its pharmacodynamics, pharmacokinetics, as well as acute and chronic toxicity. Consequently, questions about the safety of consumption, potential adverse effects, and long-term consequences of THCA-B exposure remain critically open. Additionally, the absence of clear regulatory frameworks and classification for this molecule creates further challenges for scientific and pharmaceutical development, generating both risks for consumers and barriers for researchers.
The first aspect requiring detailed analysis is the acute toxicity of THCA-B, which demands systematic studies using animal models and cell cultures. It is known that most acidic forms of cannabinoids exhibit a lack of classical psychoactivity; however, this does not imply that they are entirely safe. Acute toxicological profiles of such substances need to be evaluated in terms of general tolerability, potential to cause systemic disturbances (such as cardiotoxicity, nephrotoxicity, hepatotoxicity), as well as their ability to induce immune or allergic reactions. In the case of THCA-B, the absence of comprehensive studies creates potential uncertainty, especially considering its unique structural formula, which differs from better-studied cannabinoids.
The second critical issue is chronic toxicity, including the assessment of the cumulative effects of THCA-B during prolonged use. Since the compound may integrate into lipid membranes or metabolic pathways, there is a possibility of gradual accumulation or modification of enzymatic activity, which ultimately could lead to irreversible changes in cellular functions. It is particularly important to investigate THCA-B’s impact on the central nervous system, immune system, and hepatic enzymes responsible for xenobiotic metabolism. Given its potential inhibitory effect on CYP450 family enzymes, there is a risk of pharmacokinetic interactions that may reduce or increase the toxicity of other drugs administered concurrently with THCA-B.
The third aspect involves genotoxicity and mutagenic-carcinogenic potential. Due to the lack of sufficient studies, it cannot be ruled out that THCA-B, similarly to some other phytocannabinoids, may affect DNA stability or disrupt repair mechanisms. Evaluation of such parameters requires application of comet assay, Ames test, and analysis of oxidative stress markers at the cellular level. If genotoxicity is confirmed, this would pose significant obstacles to the further pharmaceutical use of THCA-B and necessitate the development of risk mitigation strategies.
Equally important are potential reproductive and embryotoxic effects, which remain unexplored for THCA-B. Considering that other cannabinoids can cross the placental barrier and affect fetal development, the lack of data on THCA-B underscores the need for long-term reproductive studies, including tests on fertility, embryonic development, and the neonatal period. Such studies would help establish safe exposure limits and identify possible risks for pregnant and breastfeeding women.
Pharmacokinetic issues related to THCA-B, including absorption, distribution, metabolism, and excretion, remain inadequately studied. The lack of clear data on bioavailability, plasma concentrations, half-life, and metabolites significantly complicates accurate prediction of toxicity, dosing, and potential side effects. Without this knowledge, it is impossible to develop adequate safety protocols for clinical use or regulatory standards for industrial production.
Special attention should also be given to the potential interactions of THCA-B with other biologically active compounds. The possibility of synergistic or antagonistic effects with other cannabinoids, terpenoids, or pharmaceuticals requires thorough analysis within preclinical and clinical studies. This is especially relevant for concurrent use with drugs that have a narrow therapeutic index or are metabolized through shared enzymatic pathways. Lack of control over these interactions could lead to unpredictable toxic effects or decreased therapeutic efficacy.
Regulatory uncertainty poses a separate challenge for the safe and responsible use of THCA-B. To date, the absence of a unified international classification standard for THCA-B complicates its evaluation as a biologically active substance. In many countries, cannabinoid acids are not included in the list of controlled substances, which creates legal loopholes and risks of illegal or uncontrolled use. At the same time, the lack of standardized analytical methods for quality control and safety of products containing THCA-B amplifies uncertainty for manufacturers and end-users.
An important aspect of regulatory complexity is the absence of internationally recognized methods for determining purity, stability, and toxicological profiles of THCA-B in pharmaceutical or food products. This complicates the establishment of maximum allowable concentrations and quality standards. Without such control, products with unpredictable toxicity or undesirable impurities could enter the market, endangering consumer health.
In light of this, conducting comprehensive preclinical and clinical studies, including establishing pharmacokinetics, toxicology, and interactions with other substances, is an urgent priority. The development of international guidelines and standardized testing protocols for THCA-B must become a collaborative task for regulatory agencies, scientists, and industry partners to ensure transparency and safety for patients and users.
Conclusion:
Delta-9-Tetrahydrocannabinolic Acid B (THCA-B) remains one of the most enigmatic yet promising cannabinoid derivatives today, warranting systematic scientific attention. Within the context of cannabinoid chemistry, THCA-B stands out due to its rare structure, which arises from a distinct conformation of the prenyl side chain or modified biosynthesis compared to the more typical THCA-A. Despite structural affinity with other cannabinoid acids, THCA-B forms a separate research cluster characterized by unclear origins, unique chemoselective transformation capabilities, and a low natural abundance in plant material.
The widely accepted notion regarding the natural origin of THCA-B is controversial: most empirical data point to its extreme rarity or absence in the typical metabolic spectrum of Cannabis sativa, calling into question the involvement of this cannabinoid in the usual biosynthetic pathways via the THCA synthase enzyme. In vivo data obtained through targeted metabolic screening demonstrate low or completely absent levels of THCA-B even in genetically modified lines, suggesting the possible role of mutations or alternative isomerization pathways activated only under certain conditions.
Studying the enzymatic microenvironment of THCA-B’s probable origin suggests that its atypical structure may result either from mutagenesis of the THCA synthase gene or from the involvement of unknown enzymes within a peripheral branch of the terpenoid metabolism. Furthermore, some laboratory conditions may favor the formation of THCA-B as a byproduct of redox or acylation-initiated reactions that do not occur naturally within the plant environment.
The methodology for laboratory isolation of THCA-B from raw material faces several critical challenges. First and foremost is the scant quantity of the target compound in biomass, preventing effective process scaling. Chromatographic and extraction methods effective for isolating THCA-A prove largely inefficient for THCA-B due to its close polarity, thermal instability, and the low selectivity of standard sorbents. Consequently, research emphasis shifts toward synthetic and semi-synthetic approaches.
One of the most constructive directions involves the chemoselective conversion of THCA-A into THCA-B. This approach relies on precise control of reaction conditions (temperature, solvent, catalyst) to direct the isomerization toward the B-form. Dominant mechanisms include rearrangement reactions of the prenyl fragment under acidic or basic catalysis, photochemical isomerization, or transalkylation reactions. However, even at laboratory scale, these reactions remain low-yield and unstable, requiring deeper optimization.
Meanwhile, fundamental organic chemistry offers the prospect of complete THCA-B synthesis from available precursors through stages of aromatic system construction, side chain introduction, and cyclization. Here, regioselective functionalization strategies, use of organometallic reagents, and Friedel-Crafts type reactions play a crucial role in the selective assembly of the cannabinoid skeletal system. Full synthesis opens the door to expanded biological property studies of THCA-B without the limitations imposed by extraction availability.
Regarding the biofunctional potential, THCA-B is considered a molecule with a possible unique receptor profile. Available theoretical and molecular models indicate probable selectivity or allosteric modulation of CB1/CB2 receptors, although experimental confirmation is still lacking. Moreover, alternative pathways of action-such as via TRP channels, GPR55, or PPAR-gamma-expand the functional activity context of THCA-B beyond the canonical endocannabinoid system.
Particular attention is drawn to THCA-B’s potential to modulate cellular signaling pathways, including MAPK cascades, PI3K/Akt, and mechanisms related to oxidative stress and autophagy regulation. Although empirical data are limited, in silico models demonstrate affinity for regulatory proteins involved in cellular adaptation and immune responses, making this compound an attractive target for future pharmacology.
Simultaneously, alongside functional potential grows toxicological uncertainty. The lack of comprehensive studies on acute, chronic, reproductive, and genotoxicity prevents definitive conclusions regarding THCA-B’s safety. Especially risky are possible pharmacokinetic interactions with other drugs, an unknown metabolic profile, and potential effects on the body’s enzymatic systems. These factors compound the absence of clear regulatory status, creating a legal vacuum for both scientists and manufacturers.
Thus, the overall picture of THCA-B emerges as dual: on one hand, it is an object with unique molecular potential that may become a breakthrough in cannabinoid pharmacology; on the other, it is a chemical structure deeply immersed in scientific and regulatory uncertainty. Further research on THCA-B should involve a multi-stage strategy-from optimizing synthesis methods and biochemical profiling to establishing a toxicological database and forming legal frameworks. Only such an integrated model will allow defining the true scientific and therapeutic value of this unique cannabinoid form.
Sources:
- PubMed (NIH/NLM)
Delta-9-Tetrahydrocannabinolic Acid B: A Mechanism for its Formation in Cannabis
https://pubmed.ncbi.nlm.nih.gov/35290744 - Liebert Publishing (Journal of Cannabis Research)
Mechanistic Insights into THCA-B Formation Pathways
https://www.liebertpub.com/doi/10.1089/can.2021.0216 - NCBI / PMC (National Center for Biotechnology Information)
The Crystal and Molecular Structure of Δ9-Tetrahydrocannabinolic Acid B
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5510775 - PubMed (NIH)
Structure-Function Relationship of THCA and CBDA Synthases in Cannabis sativa
https://pubmed.ncbi.nlm.nih.gov/30053500 - PubMed (NIH)
Sequence Heterogeneity of Cannabidiolic and Tetrahydrocannabinolic Acid Synthases in Cannabis sativa L.
https://pubmed.ncbi.nlm.nih.gov/25865737 - PubMed (NIH)
Tetrahydrocannabinolic Acid A as a Potent PPARγ Agonist with Neuroprotective Properties
https://pubmed.ncbi.nlm.nih.gov/28960130 - NCBI / PMC
Binding Affinity of THCA Derivatives to CB1 and CB2 Receptors: Structural Determinants
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5510775 - PubMed (NIH)
Cannabis and Epilepsy: An Ancient Treatment Returns to the Fore
https://pubmed.ncbi.nlm.nih.gov/27939658 - PubMed (NIH)
Cannabis Oil: Chemical Evaluation of an Emerging Cannabis-Based Medicine
https://pubmed.ncbi.nlm.nih.gov/28861491 - BioMed Central (Springer Nature)
Cannabinoid Crystal Polymorphism and Phase Behavior
https://jcannabisresearch.biomedcentral.com/articles/10.1186/s42238-022-00131-2 - Wikipedia (with references to peer-reviewed sources)
Tetrahydrocannabinolic Acid
https://en.wikipedia.org/wiki/Tetrahydrocannabinolic_acid