Within the field of natural compound chemistry, there exists a category of molecules that science recognizes not because they are widespread or exhibit obvious effects, but because their very presence challenges the logic of systemic thinking. One such molecule is delta-9-tetrahydrocannabinolic acid-C4 (THCA-C4)-a compound detected only in trace amounts, yet whose existence alone raises a series of questions for which there are currently no definitive answers. Its appearance in the chemotype of Cannabis sativa, its rarity, variability, and structural similarity to major cannabinoids suggest a possible biological and biochemical role, although none of these have yet been experimentally confirmed. THCA-C4 is not simply a derivative of the primary tetrahydrocannabinolic acid; it is a homolog with a modified side alkyl chain, which may serve as a marker of deeper metabolic processes that remain invisible to most analytical systems.
In the traditional description of cannabinoids, the main focus has been on the so-called major components-THCA-A, CBDA, CBGA, and their decarboxylated forms. However, scientific interest is increasingly shifting toward microcomponents, which under normal conditions do not exhibit pronounced activity but may fulfill regulatory, signaling, or even inhibitory functions within more complex chemo-ecosystems. Such are the cannabinoids with unusual side chain lengths, including homologs with butyl (C4), propyl (C3), or even heptyl (C7) substitutions. Among them, THCA-C4 stands out as having the highest level of structural similarity to THCA-A, yet is modified in a way that may confer fundamentally different pharmacokinetic parameters, receptor affinity, and metabolic fate.
This compound was not identified in the early isotopic studies of Cannabis, nor was it recorded in the classical spectra of standard chemotypes. However, with the advent of highly sensitive mass spectrometers and improved extraction conditions, researchers began detecting minor peaks that did not match any known standards. Further identification revealed that these signals originated from homologs of the main acidic cannabinoids, including THCA-C4. Its exact concentration in dried material typically does not exceed 0.01-0.05% of the total cannabinoid fraction, making it virtually inaccessible for direct biological evaluation without specialized synthesis or large-scale bioextraction. Nevertheless, the presence of such a structure cannot be considered accidental. In biochemistry, coincidences rarely go unnoticed.
THCA-C4 is unique in that it belongs to a group of substances that result from natural chemogenetic variability. In a sense, it is a byproduct of the action of enzymes that typically participate in the synthesis of THCA-A, but under altered conditions or substrate availability can catalyze the formation of related compounds. For example, if a cell contains not the standard geranyl pyrophosphate, but an alternative precursor with a modified hydrocarbon chain (for instance, four instead of five carbon atoms), the end product of the synthase will also differ-resulting in the formation of a C4 homolog. In laboratory conditions, this process is difficult to reproduce, but at the level of cellular biosynthesis, it is entirely plausible. And it is precisely these micro-pathways that lead to THCA-C4 which may offer insight into the plasticity of cannabinoid biosynthesis as a whole.
Another aspect that lends THCA-C4 particular value lies in its potential as a chemomarker. In systems where microcomponents act as signal indicators of an organism’s internal chemical logic, even a trace amount of a specific homolog can indicate a state of metabolic activity, stress, mutation, or a change in environmental conditions. Some researchers are already considering THCA-C4 as a potential biomarker for atypical or deliberately modified Cannabis chemotypes resulting from selective breeding, biotechnological engineering, or adaptation to environmental pressure. In this context, THCA-C4 may be of interest not only to pharmacologists but also to botanists, bioinformaticians, and agronomists.
However, the scientific challenge is not limited to identifying or classifying this molecule. The more important question is its functional significance. Is it a random product of metabolic “noise”-or does it serve a specific, albeit non-obvious, function? In nature, it is rare to find substances with no biological purpose. Even transient or degradative molecules often act as intermediates in complex signaling cascades. In the case of THCA-C4, we are dealing with a substance that, based on its structure, is fully capable of binding to cannabinoid receptors, potentially altering their conformation, yet at the same time lacks confirmed active effects on humans or animal models. This is a paradoxical situation in which scientific logic encounters a lack of instrumental evidence.
Research is further complicated by the fact that THCA-C4 is extremely unstable under typical laboratory conditions. Some observations suggest that it undergoes partial degradation or transformation already during extraction from plant material. Depending on the type of solvent, temperature, pH, and even the duration of the procedure, this molecule may either disappear without a trace or convert into other forms. This means that most researchers may have already worked with THCA-C4-without even realizing it. Therefore, the task is not only to search for it, but also to reconstruct its lost traces in already collected analytical datasets. In this context, there is a clear need for new types of standards for high-precision identification of small cannabinoid homologs, among which THCA-C4 is merely the tip of a possible submerged iceberg.
Beyond analytical issues, there is also an epistemological dimension to this molecule. THCA-C4 is not only an object of study but also a challenge to our understanding of the boundaries of knowledge in natural chemistry. In the traditional scientific paradigm, substances are classified based on efficacy, quantitative presence, or direct utility. But recent decades have shown that scientific value often lies beyond utilitarian categories. Rare compounds may hold tremendous potential for creating new approaches to understanding biochemistry, genetic regulation, or molecular evolution. In this sense, THCA-C4 acts as an intellectual focal point-a catalyst for rethinking models of cannabinoid synthesis, enzyme interactions, cellular microenvironments, and principles of extraction.
What is THCA-C4
Delta-9-tetrahydrocannabinolic acid-C4 (THCA-C4) belongs to the group of acidic cannabinoids-natural phenolic compounds produced by the Cannabis sativa plant. A distinguishing feature of THCA-C4 is its side alkyl chain, which consists of four carbon atoms (butyl chain), whereas the more common THCA-A contains a five-carbon (pentyl) chain. This seemingly minor modification of the chain significantly affects the molecule’s physicochemical properties, as well as its biochemical behavior and potential biological activity.
THCA-C4 is the primary acidic form of the cannabinoid, meaning it naturally occurs in the plant as an acid, with a carboxylic group (-COOH) attached to the molecule. This acidic form is synthesized directly within the veins and cells of Cannabis sativa through specific enzymes that catalyze the conversion of precursors into their respective cannabinoid acids. In contrast to its decarboxylated form (which results from heating or time)-delta-9-tetrahydrocannabinol (Δ9-THC), which possesses psychoactive properties-THCA-C4 is considered inactive in the classical sense of the term, which is typical for acidic forms.
However, despite its structural backbone being similar to other cannabinoids, THCA-C4 is markedly distinct in both chemical structure and mode of origin. First, the butyl side chain increases the molecule’s hydrophobicity compared to the pentyl chain, potentially affecting the compound’s ability to pass through biological membranes or interact with protein receptors. Second, this modification alters the molecule’s conformation, particularly the spatial orientation of functional groups, which is crucial for molecular recognition.
It is also worth noting that THCA-C4 is one of a number of less common cannabinoids that comprise what is known as the “minor fraction” of cannabinoids in the plant. Although it is present in extremely small quantities, its study is important for understanding the diversity of the cannabinoid profile, which is shaped by genetic variability, climatic and agronomic conditions, and the plant’s inherent biochemical flexibility. The presence of such homologs points to the deep internal complexity of cannabinoid metabolism and provides insight into alternative synthesis pathways.
Despite its rarity, THCA-C4 is of considerable scientific importance because cannabinoid homologs with altered side-chain lengths often exhibit unique properties not replicated by the primary cannabinoids. Such molecules can influence the spectrum of biological effects, modulate receptor activity, or even reveal new mechanisms of interaction at the cellular level. Thus, studying THCA-C4 helps broaden our understanding of the cannabinoid system’s complexity, which is key not only in pharmacology but also in the molecular biology of plants.
From a classification standpoint, THCA-C4 belongs to a type of cannabinoids referred to as “butyl cannabinoids.” These form a homologous series with cannabinoids that have side alkyl chains of various lengths, ranging from propyl (C3) to heptyl (C7). This series is not arbitrary: it reflects the principle of enzymatic plasticity, where the key enzymes in cannabinoid synthesis accept different substrates and, depending on their availability, produce the corresponding products. This means that THCA-C4 is a product of a metabolic pathway that, while not dominant, plays an important role in the structural diversity of cannabinoids.
The phenomenon of THCA-C4’s presence is also tied to researchers’ efforts to discover natural and synthetic cannabinoids with improved or modified pharmacological properties. The length and structure of the side chain often determine the molecule’s affinity for cannabinoid receptors CB1 and CB2, which are the key mediators of cannabinoids’ biological effects. Therefore, despite not being thoroughly studied yet, THCA-C4 holds potential for further pharmacological research as a candidate with unique characteristics.
At the same time, THCA-C4 is not just a scientific object of interest but also a biochemical indicator. Its detection in specific Cannabis chemotypes may signal the presence of unique metabolic profiles, reflecting the plant’s genetics or the influence of external factors on biosynthetic pathways. This makes THCA-C4 a potential biomarker for classification and breeding, which is especially relevant in light of current trends toward personalized therapy and the creation of engineered chemotypes with desired properties.
In addition, it is important to emphasize that THCA-C4, like other acidic cannabinoids, in its natural state does not have psychoactive effects, which is critical for understanding its potential therapeutic value. Studying such inactive acidic forms allows researchers to separate the psychoactive impact of cannabinoids from their anti-inflammatory, neuroprotective, analgesic, or immunomodulatory properties, revealing new horizons in pharmacology.
Structural Features of THCA-C4
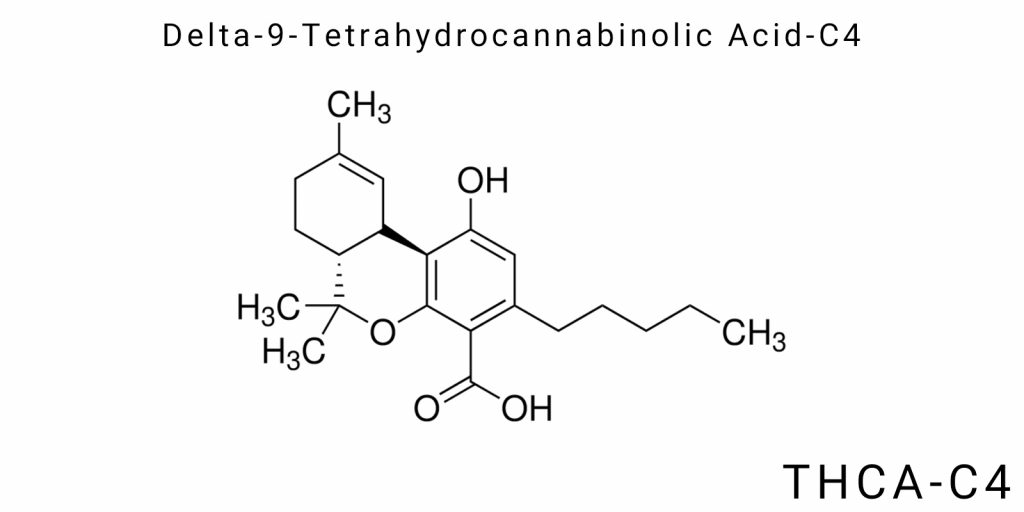
The chemical structure of Delta-9-tetrahydrocannabinolic acid-C4 (THCA-C4) reflects its classification as a cannabinoid, which is a complex tetrahydrofuran derivative featuring a phenolic core, a carboxyl group, and a side alkyl chain. The uniqueness of THCA-C4 lies in the distinctive configuration of its molecule, which determines the specificity of its chemical reactivity, physicochemical properties, and biological activity.
At the core of the molecule is the tetrahydrocannabinol scaffold, which includes a benzocyclohexane structure to which a phenolic hydroxyl group and a side alkyl chain are attached. A distinguishing characteristic of THCA-C4 is the presence of a butyl (C4) alkyl chain located at position 3 of the benzocyclohexane ring, which is critically important for its molecular geometry. On the molecular level, this chain represents a straight four-carbon sequence ending in a methyl group.
This structural feature has a significant impact on the conformational behavior of the molecule. The alkyl chain in THCA-C4 exhibits greater flexibility compared to the more common pentyl chain found in THCA-A, which partially alters the spatial arrangement of the entire molecular framework. Specifically, the butyl chain allows a different degree of rotational freedom around bonds, resulting in variations in the stability of different conformational isomers. This flexibility affects the molecule’s ability to adapt to various biological targets, modulating its pharmacological profile.
Another key element of the structure is the presence of a carboxyl group (-COOH) at the 2′ position of the tetrahydrocannabinol core, which defines the acidic nature of the molecule. This functional group allows the molecule to participate in acid-base reactions and influences its solubility in polar environments. Chemically, the carboxyl group is also a central site for decarboxylation-a process that transforms the acidic form into the active neutral Δ9-THC. The influence of this group on the stability of the THCA-C4 molecule is particularly important, as it makes it more sensitive to thermal and oxidative stress.
Regarding the aromatic ring, THCA-C4 features a phenolic core with a hydroxyl group at the 1′ position, allowing for the formation of hydrogen bonds. These bonds determine intermolecular interactions in the crystalline phase and in solution, and they also affect the molecule’s affinity for protein receptors. The presence of the phenolic ring also imparts antioxidant properties to the molecule, which makes it potentially valuable in combating oxidative stress.
Structurally, THCA-C4 is a chiral molecule-it contains one or more chiral centers that confer optical activity. Chirality is fundamentally important in biochemical interactions, as isomer conformations can demonstrate different biological activities, receptor affinities, metabolic rates, and even toxicity. THCA-C4 is characterized by the presence of (−)- and (+)-enantiomers, with natural compounds typically appearing in one specific stereoisomeric form, determined by plant enzymes during biosynthesis.
A crucial part of the molecule is the tetrahydrofuran ring, which forms an additional cyclic structure that stabilizes the molecule and affects its electron distribution. This structural element is responsible for the molecule’s unique electrophysical properties, such as polarizability and the potential for donor-acceptor interactions. It also determines the specificity of the molecule’s binding to cannabinoid receptors, as the structural integrity of the tetrahydrofuran ring is essential for forming stereospecific interactions.
Considering the electronic structure of THCA-C4, it is important to highlight the influence of conjugated double bonds in the benzocyclohexane ring and the butyl side chain. The absence or presence of double bonds in the side chain significantly affects the molecule’s electron density and energy levels. In the case of THCA-C4, the absence of double bonds in the butyl chain renders it less electronegative compared to longer pentyl homologs, which in turn changes its ability to engage in electrophilic and nucleophilic reactions.
Another notable aspect is the impact of structural isomerism among cannabinoids with varying side-chain lengths on interactions with CB1 and CB2 receptors. Recent molecular modeling studies have shown that changes in chain length alter the geometry of interaction, which can either enhance or reduce receptor affinity. In the case of THCA-C4, the butyl chain leads to less hydrophobic interaction with the receptor’s lipophilic channel, distinguishing it from cannabinoids with longer chains.
The overall molecular weight of THCA-C4, taking into account the butyl chain, is lower than that of pentyl cannabinoids, which also influences its distribution in biological systems. The lower mass and specific structure result in differences in physicochemical parameters such as melting point, solubility in organic solvents, and metabolic kinetics.
Equally important is the molecule’s resistance to degradation. Due to its structural features, THCA-C4 shows notable stability at room temperature under dry conditions, but it is also sensitive to temperature fluctuations that induce decarboxylation. The chemical structure with the butyl chain accounts for some differences in degradation mechanisms compared to classical THCA-A. In particular, the rate and mechanism of side-chain breakdown during oxidation or thermal destruction have unique characteristics that are being studied to better understand the molecule’s thermal behavior.
From the standpoint of chemical reactivity, the carboxyl group also enables the formation of esters and amides, opening avenues for synthesizing derivatives with modified biological activity. The interaction characteristics of the butyl chain during these functional transformations differ significantly from analogous reactions in pentyl cannabinoids, making THCA-C4 a promising target for chemical synthesis and modification.
Finally, it is worth noting the crystalline structure of THCA-C4, which is determined by the relationship among the tetracyclic core, the carboxyl and phenolic groups, and the side chain. Crystallization of the molecule and the formation of stable aggregates in the solid state or as crystalline powders define its physical properties, such as melting point, stability, and hygroscopicity. Studying these parameters is crucial for developing methods of extraction, purification, and storage of THCA-C4.
Why THCA-C4 Is So Difficult to Detect
The detection and identification of Delta-9-tetrahydrocannabinolic acid-C4 (THCA-C4) in both natural and laboratory samples is associated with a complex set of technical, chemical, and analytical challenges that significantly complicate accurate analysis and quantification of this compound. The difficulty in detecting THCA-C4 stems from several factors related to its physicochemical properties, stability, low concentration in materials, and the limitations of current analytical techniques. A deeper understanding of these obstacles is key to developing new, more effective analytical methods as well as accurately interpreting research results.
First and foremost, one of the main challenges is the extremely low natural concentration of THCA-C4 in biological materials such as cannabis plant tissue. THCA-C4 is a structural homolog of more common cannabinoids, particularly THCA-A, but its unique butyl side chain structure occurs far less frequently, resulting in its microscopic presence. Consequently, the number of THCA-C4 molecules in a sample is often at the detection limit of many analytical instruments, necessitating the use of methods with ultra-high resolution and sensitivity. This factor complicates not only quantitative analysis but also the very fact of detection, especially in the presence of more concentrated cannabinoids.
The second important aspect relates to the chemical instability of THCA-C4 under physicochemical influences, particularly temperature and light. The molecule contains a carboxyl group, which makes it sensitive to decarboxylation-its transformation into the neutral cannabinoid Δ9-THC-C4. This process occurs during heating or prolonged storage of samples, leading to the loss of the original THCA-C4 form and alteration of the analytical profile. Since decarboxylation occurs unevenly and depends on storage conditions, this complicates data interpretation and makes it difficult to restore the initial acid concentration in the studied material. Accordingly, even properly selected methods may “miss” THCA-C4 due to its conversion into other forms.
A third reason lies in the high chemical similarity of THCA-C4 to other cannabinoids, particularly structural isomers and homologs with similar molecular masses, functional groups, and physicochemical characteristics. This similarity complicates the selection of selective chromatographic separation conditions and spectroscopic identification. For instance, in gas or liquid chromatography methods with mass spectrometric detection (GC-MS, LC-MS), multiple cannabinoids may produce overlapping peaks or isobars, making unambiguous identification difficult. The lack of unique mass spectrometric fragments that could serve as specific markers necessitates the use of complex multidimensional approaches or high-resolution chromatographic techniques.
The fourth factor is instability during sample preparation, where THCA-C4 can undergo chemical or physical changes due to solvent influence, pH, temperature, or interaction with other matrix components. Samples containing THCA-C4 often have a complex organic composition with many polar and non-polar compounds, including other cannabinoids, terpenoids, and flavonoids. The interaction of these compounds during extraction and purification can lead to degradation, column adsorption, or loss through non-selective reactions, resulting in inactivation or “masking” of THCA-C4. Therefore, optimizing sample preparation is critical for reliable detection and requires deep understanding of the molecule’s chemical behavior in different environments.
A fifth aspect is instrumental limitations. Standard analytical platforms widely used for cannabinoid research often lack sufficient selectivity or sensitivity for unambiguous THCA-C4 detection. For example, in traditional gas chromatography methods, the thermal instability of the carboxyl group means that using GC without prior derivatization may lead to molecular breakdown, loss of the acidic form, and artifact formation. On the other hand, liquid chromatography with mass spectrometry, while offering better capabilities, requires expensive and complex high-pressure systems and high-resolution mass spectrometry, as well as careful calibration, limiting its widespread use.
The sixth important factor is the lack of standardized and certified reference samples of THCA-C4 for calibration and validation of analytical methods. Due to the relatively recent discovery and study of THCA-C4, the production of pure, stable standards in sufficient quantities is technologically complex and costly. This limits laboratories’ ability to accurately quantify the compound and leads to significant variability in results depending on the methodology used and the analysts’ level of expertise.
Another scientific and technical barrier is the difficulty of separating THCA-C4 from its isomers, particularly closely related structural forms such as THCA-C3 or THCA-C5. These compounds have similar molecular weights, similar chromatographic profiles, and differ only in the length of the side chain, necessitating the use of multi-step separation methods-such as two-dimensional chromatography (2D-LC) or combinations of chromatography with nuclear magnetic resonance (NMR) spectroscopy-for accurate structural determination. Such techniques are time-consuming and require specialized equipment and deep expert knowledge, making their routine use difficult.
From a chemical kinetics perspective, THCA-C4 decarboxylation and oxidation reactions occur at different rates depending on conditions, which creates additional difficulties for standardizing analytical protocols. The absence of clearly controlled conditions and varying sample storage methods cause significant variability in THCA-C4 concentrations even from the same source. Such variability undermines the reliability of results, requiring the development of stabilization methods and the implementation of protocols to prevent degradation.
Given the above, the scientific community is actively working to improve technologies for detecting THCA-C4. This includes the development of ultra-high-resolution chromatography, multidimensional spectroscopic methods, and the integration of machine learning techniques for spectral data analysis. However, these approaches have not yet achieved widespread use due to their high cost and technological complexity.
How THCA-C4 Appears in the Plant
The appearance of THCA-C4 in the Cannabis plant is the result of a complex and multi-layered biochemical process that occurs at the level of cellular metabolic activity and genetic control. The production of this rare cannabinoid is a form of biochemical adaptation that combines specific features of the plant’s genetic apparatus, enzymatic systems, and environmental influences. To understand the mechanism behind the emergence of THCA-C4 in cannabis, one must delve into the processes of secondary metabolite metabolism, the functioning of enzymatic cascades, and the interaction between the plant’s genotype and phenotype.
Essentially, THCA-C4 is formed in specialized cells called trichomes, which are located on the surface of the plant’s flowers, leaves, and young shoots. These trichomes function as bioreactors where cannabinoids are synthesized and accumulated. THCA-C4, as a specific cannabinoid, is the product of a unique biochemical reaction that begins with basic precursors of the cannabinoid pathway, which are synthesized within the plant’s cells. It is important to understand that the emergence of THCA-C4 is preceded by a complex metabolic pathway, where a central role is played not only by enzymes but also by substrate specificity and the spatial organization of enzymatic complexes.
Despite its general similarity to other cannabinoids, the biogenesis of THCA-C4 has distinct features associated with the activation and transformation of specific precursors. The difference in the structure of the alkyl side chain-four carbon atoms (C4) instead of the more common three or five-determines the emergence of a unique enzymatic mechanism responsible for this variant of biosynthesis. Therefore, plants that produce THCA-C4 may possess characteristic variants of genes responsible for the synthesis of longer alkyl side chains.
A key factor is the localization of the biosynthesis process in specific organelles and subcellular structures, where the enzymes responsible for cannabinoid synthesis are organized into multi-enzyme complexes. This organization enables efficient control over the sequence of reactions and minimizes the loss of unstable intermediate products. Like other acidic cannabinoids, THCA-C4 is formed through specific carboxylation and cyclization processes, which are delicate and sensitive to changes in the cell’s metabolic state, oxidative stress, and precursor availability.
The natural phenomenon of THCA-C4 emergence is closely linked to the genetic variability of Cannabis plants. There are strains and lines genetically programmed to produce cannabinoids with longer side chains. This specificity is reflected in the function and regulation of key enzymes involved in the formation of these structures. Thus, THCA-C4 is not an accidental byproduct but rather the result of adaptation, potentially linked to ecological and evolutionary factors such as protection against ultraviolet radiation, pathogens, or phytophagous organisms.
The appearance of THCA-C4 in the plant is also associated with its stages of growth and development. The synthesis level of this cannabinoid is dynamic and depends on the plant’s age, health status, and external environmental conditions such as light, temperature, humidity, soil composition, and stress levels. At early stages of plant development, enzymatic systems are tuned for the active synthesis of major cannabinoids, whereas in more mature forms, structural diversity increases and the relative accumulation of rare forms, including THCA-C4, becomes more pronounced. This phenomenon indicates the existence of a complex regulatory network that controls the metabolic balance between the synthesis of primary and secondary cannabinoids.
An important component is also the interaction between cannabinoid metabolic pathways and other secondary plant metabolites, including terpenoids and flavonoids. The joint regulation and coordination of these pathways influence the availability of precursors and the cell’s energy balance, which in turn determines the intensity of THCA-C4 synthesis. Metabolic competition and feedback mechanisms may either enhance or suppress the production of this cannabinoid, creating a complex and flexible biochemical profile for the plant.
Moreover, it is crucial to note that the formation of THCA-C4 is not an isolated process but part of a complex network of enzymatic reactions and biosynthetic cascades. This network encompasses not only the synthesis of THCA-C4 itself but also the transformation of its precursors, including specific oxidative and carboxylation reactions. The interaction between enzymes, cofactors, and substrates creates a synergistic effect that ensures the efficient synthesis of cannabinoids in optimal concentrations.
The role of epigenetic mechanisms, which may modulate the expression of genes responsible for THCA-C4 synthesis, should also not be ignored. Recent studies indicate that DNA methylation, histone modifications, and the influence of non-coding RNAs can significantly impact the metabolic profile of Cannabis, particularly by altering the balance among different cannabinoids. These mechanisms provide the plant with adaptability to environmental changes and generate variability in THCA-C4 production even among closely related genetic lines.
The combination of these factors explains why THCA-C4 appears only in certain Cannabis strains and under specific conditions. This phenomenon underscores the complexity and multifaceted nature of the metabolic processes occurring within the plant and highlights the need for comprehensive approaches to studying its biochemistry. Investigating these mechanisms is not only fundamentally important for understanding cannabinoid biology but also has practical applications in breeding plants with a defined metabolic profile, which is essential for the medical and industrial use of Cannabis.
Possible Natural Biosynthetic Pathways of THCA-C4
The biosynthesis of THCA-C4 in the Cannabis plant represents a complex and precise biochemical process that differs from classical cannabinoid formation pathways due to the unique structure of its alkyl side chain. Identifying the natural synthesis routes of this rare cannabinoid requires an analysis of key metabolic cascades, molecular substrates, and enzymatic mechanisms that ensure the specific structure of the THCA-C4 molecule.
The basis for cannabinoid formation in Cannabis involves two major metabolic pathways: the polyketide pathway (PKS) and the isoprenoid biosynthesis pathway (MEP/DOXP). These provide the respective precursors-an aromatic benzene fragment and an alkyl chain. In the classical case, THCA is formed through the condensation of ortho-coumaroyl-CoA (an aromatic component resulting from the phenylpropanoid pathway) and geranyl pyrophosphate (GPP), an isoprenoid donor synthesized via the MEP pathway. For THCA-C4, however, a key difference lies in the length and structure of the alkyl side chain-instead of the five carbon atoms found in GPP, a derivative with a shorter four-carbon chain is used. This necessitates additional enzymatic transformations or an alternative substrate pool.
The first possible biosynthetic pathway for THCA-C4 involves the participation of an alternative isoprenoid precursor-butyl pyrophosphate (BPP) or its analogs, which contain four carbon atoms in the side chain instead of the typical GPP. The difference in substrate results in a radical change in enzymatic specificity and the reaction product. Studies of isolated enzymes within the cannabinoid pathway have shown that specific teratogenases or trans-isoprenyltransferase synthases may exhibit flexibility in accepting such substrates, thereby enabling the formation of alkyl derivatives with fewer carbon atoms. This implies the existence in nature of either poorly studied enzymes or variants of known ones capable of condensing ortho-coumaroyl-CoA with butyl pyrophosphate, initiating the formation of the unique precursor to THCA-C4.
A second natural pathway is associated with the modification of basic precursors via enzymatic shortening or transformation of already existing alkyl side chains. According to this hypothesis, THCA-C4 is formed as a derivative of classical THCA through enzymatic intervention in the side chain-a process that includes oxidative hydrogenation, β-oxidation, or specific hydrolytic cleavage of terminal carbon atoms from the five-carbon chain. This transformation can be carried out by specific enzymes such as lipoxygenases, reductases, or monooxygenases localized in trichomes. This approach allows the use of already synthesized GPP as the starting substrate, and subsequent modulation of the side chain length leads to the emergence of alkyl derivatives with four carbon atoms, which condense with ortho-coumaroyl-CoA to form THCA-C4.
Enzymatic specificity in both scenarios differs from classical THCA biosynthesis. Experimental data indicate the existence of enzyme isoforms that possess substrate specificity toward alternative isoprenoid donors or modified alkyl chains. The study of such isoforms has been carried out using proteomics and genetic engineering, where mutations were identified in substrate-binding regions. These enzyme variants may result from genetic mutations or epigenetic modifications, which lead to the emergence of unique biosynthetic routes characteristic specifically of plants that produce THCA-C4.
Moreover, it is important to consider the subcellular localization of the biosynthetic complexes. The condensation of precursors and the formation of the THCA-C4 molecule likely occur in specific cytoplasmic compartments or membrane structures, where substrate and cofactor concentrations are optimal for enzymatic activity. These organelle-like complexes may have an altered enzyme composition and ensure targeted synthesis of cannabinoids with various side chains, including THCA-C4.
A significant role in this process is also played by the availability and concentration of alkyl donors. In the plant, levels of butyl pyrophosphate or similar compounds may be regulated in response to external factors-light exposure, temperature, or nutrient conditions. Variations in isoprenoid metabolism influence substrate ratios, thereby determining the intensity of THCA-C4 synthesis relative to other cannabinoids. This points to a complex and adaptive nature of biosynthetic regulation.
Special attention should also be given to the participation of cofactors and mineral components that support enzyme activity, particularly Mg²⁺, Fe²⁺, and NAD(P)H. Their quantity and availability directly correlate with the efficiency of THCA-C4 synthesis. Changes in cofactor balance may influence the preference for certain biosynthetic pathways, including the activation of alternative enzyme forms that favor THCA-C4 formation.
It is also important to consider the potential involvement of plant microbiota in the formation of specific metabolites. Certain bacteria and fungi associated with the surface and internal tissues of Cannabis may produce enzymes or intermediate compounds that stimulate or modulate THCA-C4 synthesis. This biosynthetic aspect remains understudied, but in light of current research on endophytic microbiota, it may significantly influence the quality and quantity of rare cannabinoids.
The broader metabolic context of THCA-C4 synthesis also suggests the existence of metabolons-spatially organized groups of enzymes that facilitate direct substrate transfer from one enzyme to the next, minimizing diffusion losses. Within such complexes, the sequential formation of specific cannabinoid structures occurs, and it is within these that variants leading to THCA-C4 may arise.
Participation of Enzymes and the Influence of Genotype on THCA-C4
The biosynthesis of THCA-C4, like other cannabinoids, results from the intricate interplay between the enzymatic systems of the Cannabis plant and its genetic foundation, which determines the synthetic potential and specificity of the products. The enzymatic component of this process centers around a group of enzymes that perform sequential reactions of condensation, cyclization, and modification of precursor molecules. Simultaneously, the plant’s genotype acts as a regulator of the expression levels of the corresponding genes, variations in the amino acid sequences of enzymes, and also determines the availability and efficiency of these enzymatic cascades.
A key enzyme that initiates the biosynthesis of THCA-C4 is cannabinoid synthase-a specific group of oxidases (THCA synthases) that catalyze the oxidative cyclization of the condensed product of ortho-coumaroyl-CoA with an isoprenoid donor. Considering the specificity of THCA-C4, it is hypothesized that isoforms or variants of this synthase exist with increased affinity for alternative isoprenoid substrates (particularly butylpyrophosphate or its analog), distinguishing it from the classical THCA synthase. Such enzyme variants possess modified active sites that ensure precise oriented binding with the four-carbon chain. Information about the structure of these isoforms remains limited; however, protein models based on homology with enzyme relatives demonstrate changes in key amino acids that may form a unique substrate channel.
Genetic variations that determine the emergence of such isoforms are localized in the regions of genes encoding these enzymes-particularly in regulatory and coding areas. Single nucleotide polymorphisms (SNPs) in promoter zones lead to changes in transcription levels, while changes in the coding sequence cause allelic diversity of proteins with varying activity and specificity. These variations are closely associated with different Cannabis strains, which exhibit differences in the THCA-C4 profile. Recent genomic studies, including sequencing of various strains, have identified unique alleles associated with increased THCA-C4 production, indicating the genotype’s influence on enzymatic properties and biosynthetic pathways .
In addition to cannabinoid synthase, other enzymes involved in the formation of THCA-C4 include polyketide synthase, isoprenyltransferases, and enzymes that catalyze β-oxidation and other modifications of alkyl chains. The activity of these enzymes, which directly affects the availability of specific precursors, is also regulated by genetic factors and may vary among different genotypes. Notably, plants with high THCA-C4 levels exhibit increased expression of genes encoding specific isoprenyltransferase isoforms that demonstrate higher affinity for butylpyrophosphate.
It is important to note that the enzymatic process in Cannabis does not occur in isolation but forms metabolic networks and complexes that combine several enzymes into metabolons. The plant’s genotype determines not only the quality of individual enzymes but also their ability to form these functional multi-enzyme aggregates, facilitating optimal reaction progression and minimizing intermediate substrate losses. Recent studies using mass spectrometry and co-immunoprecipitation have shown that variations in the genes of structural proteins of metabolons also correlate with the level of THCA-C4 synthesis.
Beyond the direct involvement of enzymes in biosynthesis, the genotype influences the regulation of enzyme gene expression through epigenetic mechanisms. DNA methylation, histone modifications, and microRNAs can alter the transcription levels of key enzymes, aiding the plant’s adaptation to environmental conditions and simultaneously affecting the cannabinoid synthetic profile. For instance, plants growing under stress conditions exhibit altered methylation patterns of isoprenyltransferase genes, leading to variations in THCA-C4 production.
The genetic background also determines the morphological and physiological characteristics of trichomes-specialized glandular hairs where cannabinoid accumulation and synthesis occur. The size, number, and maturity degree of trichomes correlate with the levels of enzymes and substrates necessary for THCA-C4 formation. Genetic differences affecting trichome development indirectly regulate metabolic flows and, consequently, the amount of synthesized THCA-C4.
It is worth emphasizing that identifying specific genes and enzymes responsible for THCA-C4 synthesis remains a partially unresolved task. The application of genomic, transcriptomic, and proteomic technologies, along with biochemical methods, is crucial for identifying the exact set of genetic markers and enzymatic mechanisms. These studies open prospects for genetic engineering, which can ensure increased productivity and selectivity of THCA-C4 synthesis in plants or in heterologous systems.
Stability of THCA-C4 in Plant Material
The stability of THCA-C4 in fresh and dried Cannabis plant material is a crucial aspect for understanding the preservation of the chemical integrity of this cannabinoid during storage, processing, and analysis. Scientific research focuses on the mechanisms of degradation, factors influencing decay, as well as the impact of external and internal conditions that determine the retention of the molecular structure of THCA-C4.
The physicochemical properties of THCA-C4 directly determine its stability in plant tissues. It is known that cannabinoid acids tend to undergo decarboxylation under the influence of heat, light, and oxygen, converting into their respective neutral cannabinoids. THCA-C4 is no exception; however, its unique structure with a shorter alkyl chain may affect the rate and mechanism of these transformations.
Decarboxylation of THCA-C4 occurs at temperatures significantly lower than the thermal stability of other similar cannabinoids, indicating its higher chemical reactivity. Thermal stability studies have shown that THCA-C4 begins to lose its carboxyl group at temperatures starting from 90-100 °C, which is lower compared to THCA. This means that even slight heating or prolonged storage under moderate conditions can lead to significant transformation of the cannabinoid. This process substantially affects the pharmacological properties of the plant material since the neutral form of the cannabinoid has different biological effects.
In addition to temperature effects, the stability of THCA-C4 in plant material depends on photodegradation. Exposure to ultraviolet and visible light causes photochemical reactions that lead to the breakdown of the cannabinoid ring or side chain. The chemical structure specificity of THCA-C4 with its shorter alkyl chain may influence the spectrum and rate of these reactions. Experimental studies have shown that THCA-C4 undergoes more intensive photodegradation compared to classical THCA or CBDA, which is related to the lower stability of the conjugated system and increased reactivity at the molecule’s active centers.
Chemical environmental factors in the plant material also play an important role in the stability of THCA-C4. Humidity level, pH, presence of oxidizers or reducers, as well as the concentration of metal ions can catalyze chemical breakdown or promote the formation of secondary degradation products. The influence of moisture is especially significant-elevated humidity creates conditions for hydrolytic reactions that can disrupt the integrity of the carboxyl group and trigger cannabinoid decomposition.
Additionally, biological factors such as the activity of enzymes preserved in fresh material can affect the stability of THCA-C4. Enzymes, including oxidoreductases, lyases, and hydrolases, can catalyze molecular transformations during storage if the material has not been properly processed or dried. This biocatalytic degradation is intensified by temperature, which promotes the activation of enzymatic systems.
The breakdown of THCA-C4 is accompanied by the formation of various degradation products, which may include neutral cannabinoids, oxidative metabolites, and polymers. These products have different biological activities and often reduce the quality and efficacy of the plant material, which is a critical consideration for pharmaceutical use and analytical standardization.
It is important to note that the stability of THCA-C4 in plant material significantly depends on storage methods. Optimal conditions include a dark, dry, cool environment with low oxygen concentration. Using airtight containers filled with inert gases (such as nitrogen or argon) significantly improves cannabinoid preservation. Long-term storage studies show that under such conditions, THCA-C4 can maintain stability for several months to a year or more.
Scientists also study the impact of various plant material processing technologies on the stability of THCA-C4. Among these are gentle drying methods, freezing, vacuum drying, and lyophilization. Lyophilization, as results show, provides the best preservation of THCA-C4’s structure and concentration, as it reduces the risk of oxidation and photodegradation while inhibiting enzymatic activity. At the same time, aggressive thermal drying or grinding methods increase the risk of rapid degradation.
Despite some progress in studying the stability of THCA-C4, many aspects remain insufficiently explored. In particular, comprehensive data on the effects of long-term storage in different types of plant material (dried flowers, leaves, extracts) and under varying combinations of environmental factors are lacking. This complicates the development of standardized processing and storage protocols, which is critically important for medical applications and scientific research.
Artificial Synthesis of THCA-C4
Artificial synthesis of THCA-C4 is a complex, multi-step process aimed at replicating natural biosynthetic mechanisms or developing alternative pathways for the synthesis of this compound with a defined structure and functionality. Compared to classical THCA, the synthesis of THCA-C4 has specific features determined by differences in chemical structure-particularly, a shorter alkyl side chain. This factor imposes both challenges and opportunities for organic and biochemical synthetic chemistry.
The primary goal of artificial synthesis is the production of THCA-C4 in quantities sufficient for scientific research, pharmaceutical development, and potential commercial applications. The natural content of THCA-C4 in plant material is limited and highly dependent on genetic, environmental, and agricultural factors, which complicates its mass extraction by traditional methods. Therefore, a synthesized alternative allows not only for product standardization but also for avoiding issues associated with the unpredictability of the plant biosynthetic system.
Artificial synthesis of THCA-C4 can be based on several strategies, divided into fully chemical, biotechnological, and combined approaches. Fully chemical methods involve multi-step organic synthesis starting from standard building blocks-such as alkyl halides, phenols, isoprene fragments-with gradual assembly of the molecule via catalyzed reactions. A distinctive feature of this approach is the necessity to ensure high selectivity and product purity, as the cannabinoid structure involves chiral centers and sensitive functional groups. The absence of natural enzymes requires replacing biological catalysts with synthetic or metal catalysts capable of performing oxidation, cyclization, and other specific reactions.
At the same time, biotechnological methods aim to replicate natural metabolic pathways using microorganisms such as bacteria or yeast expressing the relevant Cannabis enzymes or engineered enzymes with modified properties. This approach allows the production of THCA-C4 in aqueous media, using milder conditions and potentially enhanced selectivity, as well as the possibility for scale-up through bioreactors. The main challenges in this direction are optimizing enzyme expression, precursor availability within the cell, and the stability of the final product.
Significant attention is also paid to combined strategies that integrate chemical and enzymatic synthesis elements. For example, key intermediate compounds can be produced chemically, and final steps catalyzed by specific enzymes, minimizing side products and facilitating the isolation of the target cannabinoid. Such methods demonstrate potential to improve efficiency and environmental safety of production.
It is also important to emphasize the role of chemical modeling and computer-aided design in developing synthetic routes for THCA-C4. The application of quantum chemistry and molecular dynamics simulations allows prediction of reaction energy barriers, catalyst selection, and optimal reaction conditions. This significantly reduces experimental time and resources required to develop working synthetic protocols.
Another aspect of artificial synthesis is the development of effective purification and stabilization methods for synthesized THCA-C4. Since cannabinoids tend to degrade, it is necessary to implement modern chromatography, crystallization, and other analytical techniques to ensure high purity and stability of the final product. Development of stabilizing additives and storage conditions is also part of a comprehensive approach to artificial synthesis.
Given the potential pharmacological significance of THCA-C4, artificial synthesis also includes the development of scalable technologies compliant with GMP (Good Manufacturing Practice) standards. This requires process standardization, quality control, and implementation of traceability systems at all production stages. Such requirements determine not only synthesis efficiency but also its economic viability and safety.
The current state of scientific research shows that despite the availability of basic methods for artificial synthesis of THCA-C4, this field remains under active development. It is necessary to overcome a number of technical and biochemical challenges, including increasing reaction selectivity, product yield, and creating stable enzymatic systems. In this context, an interdisciplinary approach combining organic chemistry, biotechnology, genetics, and analytical methods plays a key role.
Laboratory Reproduction of THCA-C4 Homologs
Laboratory reproduction of THCA-C4 homologs is one of the key tools in modern cannabinoid chemistry, allowing an expanded understanding of the structure-function relationships between chemical structure and biological activity of these compounds. Homologs, in the context of THCA-C4, are molecules that differ in the length or saturation of the side alkyl chain but retain the canonical tetrahydrocannabinol backbone system with a carboxyl group at the C-2 position of the benzoyl ring. It is precisely the synthesis of such variations under laboratory conditions that provides new data on the impact of minor modifications on stability, reactivity, receptor selectivity, and metabolic fate of cannabinoids.
The main challenge lies in the fact that the structure of THCA-C4 is not simply a shortened version of THCA-A-the change in side chain length alters both physicochemical parameters and the behavior of the molecule under reaction conditions. Therefore, laboratory reproduction of such homologs requires careful design of synthetic routes that enable highly selective formation of critical bonds within the molecule, particularly preserving the configuration of the five-membered tetrahydropyran ring, which easily isomerizes or degrades under mild conditions.
The first step in creating homologs is selecting alkylated precursors-typically butylbenzenes or butylphenols-that undergo directed functionalization. For THCA-C4, the introduction of a normal C4 side chain (butyl) is required, preferably in the primary configuration (n-butyl), as branching or cyclic structure of the chain affects affinity to CB1 and CB2 receptors. Introduction of such chains into the aromatic core is often achieved through Friedel-Crafts reactions using alkyl halides in the presence of Lewis acids; however, in cannabinoid structures, these reactions exhibit low regioselectivity. Therefore, a more effective strategy proved to be functionalization of an already alkylated phenol via oxidation or carbonylation with prior control of substitution position.
After constructing the aromatic core with the necessary side group, formation of the central polyketide structure-the chain from which a cyclic fragment will subsequently form-takes place. This involves condensation techniques with hexanoyl or butanoyl thioesters through Claisen-type reactions or by using acetoacetic esters in the presence of strong bases. Control over the regiochemistry of these reactions is critically important because incorrect positioning of the keto group leads to side isomers that prevent formation of the desired cycle in the final stages.
Formation of the tetrahydrocannabinol system from the oligoketide derivative usually requires acid catalysis to induce intra- or intermolecular cyclization. The nature of the solvent, temperature, concentration, and presence of additional nucleophilic/electrophilic centers all play significant roles. Since cannabinoids easily degrade in the presence of water or at temperatures above 60°C, synthesis is preferably carried out in reduced-pressure apparatuses using anhydrous conditions and limited light exposure. Achieving high diastereoselectivity during cyclization is key because failure at this stage leads to mixing the target homolog with inactive or unstable isomers.
After synthesizing the main backbone skeleton, oxidation to the carboxylic acid-a critical functional group in THCA homologs responsible for both solubility and bioactivity-is carried out. In laboratory conditions, this is achieved through selective oxidation of the secondary alcohol, typically using mild oxidizers such as PCC or TEMPO/NaOCl systems. Such oxidation requires fine control since excessive reactivity may cause decarboxylation, nullifying the synthetic outcome. After achieving the target acid, the homolog is isolated by chromatography (HPLC or flash chromatography), which allows separation of isomers, reagent residues, and side products.
It is also worth emphasizing the role of isotopic labeling in laboratory reproduction of THCA-C4 homologs. Use of ^13C- or ^2H-labeled precursors enables tracing reaction pathways at various stages and verifying structural rearrangements. This is especially important for studying stereochemistry and establishing cyclization mechanisms. Such strategies are already used in creating intracellular tracers as well as in pharmacokinetic studies of THCA analogs.
A separate point of attention is the phenomenon of creating homolog libraries. Using the same synthetic backbone, researchers modify only the side chain (from C1 to C8), which allows establishing correlations between chain length and pharmacological activity. Within such a library, THCA-C4 occupies a central niche as a representative of short-chain homologs with interesting biopharmaceutical properties. Laboratory reproduction in this case serves not only to isolate a single molecule but also as a tool for systematic characterization of the compound class.
Finally, it should be noted that even after synthesis, a significant part of research shifts into analytics-confirmation of homolog structure and function. Spectroscopic methods such as ^1H and ^13C NMR, IR spectroscopy, high-resolution mass spectrometry (HRMS), chromatographic mass spectrometry, and X-ray crystallography are used for this purpose. Without this verification, it is impossible to assert that the created sample is indeed a THCA-C4 homolog and not some close but nonfunctional analog.
Prospects for Chemical and Enzymatic Synthesis of THCA-C4
The prospects for chemical and enzymatic synthesis of THCA-C4, as a promising homolog of cannabinoid acid, unfold along two fundamentally different but complementary directions: strategic organic modeling and biocatalysis using specialized enzymes, notably synthetases and oxidoreductases, which carry out complex chemoselective transformations under mild conditions. Although these approaches differ significantly in their principles, they share the common goal of obtaining THCA-C4 in a pure form, with controlled stereochemistry and reproducible physicochemical properties suitable for further study of bioactivity and interaction with the cannabinoid system.
In the case of chemical synthesis, the central challenge remains the construction of the polyketide chain capable of stereoselective cyclization to form the tetrahydroxypyran ring characteristic of cannabinoids. Unlike the synthesis of longer-chain homologs, the THCA-C4 structure is more sensitive to rearrangements and degradation, especially during the introduction of the butyl fragment in the early stages. One promising direction is the use of phase-separated systems, where different segments of the molecule are formed in separate phases and convergently joined through activated intermediates such as enolates, acyl chlorides, or malonate esters.
Specifically, a modular synthesis strategy has proven promising, utilizing unified scaffolds with predetermined reactivity, to which the butyl residue is attached at a designated step. This approach helps avoid multistep branching and minimizes the risk of unwanted isomer formation. Experiments with butylated phenols have shown that using mild electrophiles, such as butyryl thioesters in the presence of Lewis bases (like ZnCl₂), provides better control over the site of attachment and prevents uncontrolled aryl migration. This opens prospects for scaling chemical synthesis with minimal losses during purification.
Another vector of development in the chemical approach is the use of photochemical catalysis reactions. Thanks to the application of visible light and next-generation sensitizers (such as iridium complexes), it has become possible to generate radical intermediates with high specificity. For example, attempts to initiate radical cyclization of butylated polyketones with simultaneous formation of a cannabinoid-like core have shown promise in controlling regio- and diastereoselectivity, which previously was a weak point in the chemical synthesis of such homologs. In this context, novel photocatalytic systems not only improve reaction yields but also allow transformations at room temperature, which is critical for unstable THCA homologs.
Another interesting direction is chemoselective oxidation of partially assembled cannabinoid fragments to form the terminal carboxylic acid. Instead of traditional oxidizers like chromates or permanganates, organocatalytic systems based on TEMPO or flavin derivatives are considered, which can selectively oxidize aliphatic alcohols to acids without damaging the aromatic system. Combining such catalytic systems with microfluidic reactors allows fine control of reaction time, temperature, and pH, which is key to avoiding side reactions, including decarboxylation.
The enzymatic approach, in turn, offers completely different advantages. The central element here is cannabinoid synthases – enzymes that catalyze the cyclization of oligoketide precursors into tetrahydrocannabinolic acids. The classic model is THCA synthase from Cannabis sativa; however, its substrate specificity is mainly oriented toward the tetrahydrocannabinol precursor with a pentyl side chain. For transferring enzymatic synthesis to THCA-C4, either modification of the natural enzyme (through site-specific mutagenesis) or the search for alternative enzymes in less studied species such as Cannabis ruderalis, Humulus lupulus, or even symbiotic fungi is required.
One of the most promising directions here is the use of protein engineering methods to reconstruct the active site of THCA synthase. Mutations in substrate interaction zones allow adaptation of the enzyme to short-chain analogs like butyl CBGA (cannabigerolic acid precursor). Research in this area has already shown that substitutions of amino acids at positions 191, 314, and 442 in THCA synthase lead to a notable increase in conversion of the C4 precursor to the corresponding acid product without loss of selectivity. Such an enzyme can be expressed in recombinant systems, notably Saccharomyces cerevisiae or Pichia pastoris, enabling sufficient yields for research needs.
Another biocatalytic tool is the use of enzyme cascades – for example, employing a system where the first enzyme forms the butylated oligoketide, and the second (a modified THCA synthase-like enzyme) catalyzes cyclization and oxidation. In such systems, it is important to ensure compartmentalization – spatial or temporal separation of stages – to avoid degradation of intermediate products. This is especially critical for C4 homologs, which exhibit instability toward enzymatic hydrolysis or auto-oxidation in solutions.
Biosynthetic platforms based on genetically modified microorganisms open a new level of synthesis control. In this context, the introduction of genes encoding polyketide synthases, olefin reductases, and specific cannabinoid synthases into strains of E. coli or Corynebacterium glutamicum is promising, where they function as a modular system. Computational modeling of these pathways (metabolic engineering) allows highly accurate prediction of metabolic bottlenecks and optimization of expression at the transcriptional or translational level. This is particularly important when working with nonstandard substrates like butylated GPP (geranyl geranyl pyrophosphate), which serves as a key precursor in the synthesis of short-chain cannabinoids.
The application of enzymes in post-synthetic processing should also not be overlooked. Oxidases, decarboxylases, and hydroxylases can be used to modify partially assembled THCA-C4 or to convert mixtures of isomers into purer forms. Successful isolation of such enzymes from rare soil bacteria or the cannabis microbiome gives grounds to hope for the creation of a complete enzymatic line without the need for harsh chemical reagents.
Methods for Detection and Structural Confirmation of THCA-C4
Identification and structural confirmation of THCA-C4 as a distinct cannabinoid homolog require the application of high-precision, sensitive, and multidisciplinary analytical methods capable not only of determining molecular weight and elemental composition but also reliably verifying the positions of functional groups, bond geometry, and stereochemical parameters. Unlike the detection of major phytocannabinoids, identification of rare and lesser-known homologs such as THCA-C4 faces several challenges: low natural abundance, significant structural similarity to other cannabinoid acids, high sensitivity to degradation, and the potential presence of isomers. Therefore, relying on a single analytical method is insufficient; a comprehensive approach combining chromatography, mass spectrometry, nuclear magnetic resonance spectroscopy, and crystallography is necessary.
The initial stage in detecting THCA-C4 usually involves high-performance liquid chromatography (HPLC), which enables separation of the analyte from the plant extract matrix or synthetic product. However, classical reversed-phase HPLC often does not provide sufficient resolution between THCA-C4 and its close analogs such as THCA-A or THCA-B. Thus, gradient chromatography with mobile phases based on ammonium formate or acetate at strictly controlled pH is used, along with stationary phases composed of enriched diol or fluorophilic materials. This improves separation based on differences in dipole moment characteristic specifically for the C4 homolog.
To increase HPLC specificity, it is combined with tandem mass spectrometry (LC-MS/MS), which allows detailed investigation of molecular fragmentation. THCA-C4 displays specific fragmentation patterns, notably the characteristic loss of the carboxyl group (-CO₂), forming ions with masses of 314 and 299 Da, correlating with the butyl side chain. For exact mass determination, high-resolution mass spectrometry (HRMS) is employed on instruments such as Orbitrap or time-of-flight (TOF) analyzers, achieving resolution above 100,000 and accuracy below 1 ppm. This enables confident differentiation of THCA-C4 from isomeric compounds or degraded forms with similar molecular weights.
Isotopic modeling analysis holds particular value. Since THCA-C4 has a distinct isotopic signature related to its ^13C content, it can be distinguished from synthetic or contaminated analogs. Isotope ratio mass spectrometry detects deviations in natural isotope distribution, which is especially useful when analyzing extracts of questionable origin or enzymatically synthesized samples.
For final structural confirmation, nuclear magnetic resonance spectroscopy (^1H-NMR, ^13C-NMR) is applied, which reconstructs molecular topology based on chemical shifts and spin-spin coupling interactions. An important aspect is analyzing protons in the 5.8-6.5 ppm range, indicative of the olefinic system of the cannabinoid core, as well as signals at 3.2-3.8 ppm corresponding to oxyfunctionalized positions, particularly the hydroxyl group at C-11. Significantly, the butyl substituent generates characteristic signals in the high-field region (0.85-1.2 ppm), distinctly different from pentyl analogs, where signal overlap with aliphatic residues occurs.
Additionally, 2D-NMR spectroscopy (COSY, HSQC, HMBC) is conducted to establish correlations between individual atoms within the molecule. The HMBC technique is particularly useful for detecting long-range interactions between carbon and proton nuclei in different molecular regions, critical for confirming the position of the carboxyl group at C-1. For the butyl fragment, determining conformational mobility is key-this parameter is studied via NOESY analysis, which tracks spatial interactions between atoms within approximately 5 Å.
Supplementing classical spectroscopy, Fourier-transform infrared spectroscopy (FTIR) is utilized. It reveals characteristic peaks in the 1680-1720 cm⁻¹ region corresponding to carboxyl group vibrations and peaks in the 3200-3500 cm⁻¹ range related to hydroxyl stretches. In THCA-C4, these peaks show slight shifts compared to THCA-A, due to the influence of the shorter aliphatic chain on electron density. Though subtle, these deviations are consistent and diagnostically important.
An indispensable tool for structural verification is X-ray crystallography (XRD), which constructs a three-dimensional molecular model based on X-ray diffraction of the compound’s crystalline form. Crystallizing the unstable THCA-C4 is challenging; however, co-crystallization methods with auxiliary molecules (such as triphenylmethane or chiral organic matrices) have, in some cases, yielded sufficiently stable crystals. The resulting crystal structure not only confirms the absolute configuration of chiral centers but also precisely measures bond lengths and valence angles-crucial for differentiating isomeric forms or confirming unique geometry.
Modern techniques also include electronic spectroscopy analyzing ultraviolet and visible light (UV-Vis). While less specific, these methods are useful for preliminary identification and purity control. THCA-C4 exhibits characteristic absorption peaks in the 275-285 nm range, associated with π→π* transitions in the conjugated system, which shift depending on aliphatic chain length.
Recently, hybrid methods such as LC-NMR-MS have gained attention, enabling simultaneous recording of chromatograms, mass spectra, and NMR data inline without the need for prior purification. This is critically important for unstable cannabinoids that may lose functionality during prolonged sample preparation. Coupled with chemometric analysis (e.g., PCA or OPLS-DA), these methods allow multidimensional discrimination of structurally similar cannabinoids.
Looking ahead, emerging technologies like single-cell spectral analysis (Raman spectroscopy, MALDI-TOF-IMS) are being explored, allowing localization of THCA-C4 within specific plant tissue cells or enzymatic biofilms. This enables not only identification but also mapping of the homolog’s distribution in biological environments, opening new frontiers in studying its biosynthesis, distribution, and functionality.
Behavior of THCA-C4 in the Organism
The behavior of THCA-C4 in the human or animal body has not yet been systematically studied; however, based on its chemical structure, homology with other cannabinoid acids, and data from analogs, a reasoned hypothetical model of its pharmacokinetics and bioavailability can be constructed. The main stages of the molecule’s metabolic fate in the body include absorption, transmembrane transport, tissue distribution, hepatic transformation, potential bioactivation or degradation, and elimination. All these phases depend on the physicochemical properties of THCA-C4, particularly its acidity, hydrophobicity, molecular size, degree of ionization in biological environments, and ability to interact with carrier proteins and enzymes.
Since THCA-C4 is a polar acid with an intact carboxyl group, its passive penetration through cellular membranes-which have a lipophilic character-is limited. In its non-ionized form, it has somewhat higher permeability, but at physiological pH (~7.4), the ionized form predominates, which poorly diffuses through the phospholipid bilayer. This implies low bioavailability upon oral administration without additional transport mechanisms. One such mechanism may involve carrier proteins of the OATP (organic anion transporting polypeptides) or MCT (monocarboxylate transporters) classes, which are expressed on the apical membranes of intestinal cells and can uptake carboxylated compounds.
An important characteristic is THCA-C4’s ability to bind plasma proteins, particularly albumin. Due to the presence of a hydrophobic cannabinoid moiety and an acidic fragment, it simultaneously forms hydrogen bonds and hydrophobic interactions, providing high affinity to transport proteins. This bound form is pharmacologically inactive but creates a reservoir in the blood from which the molecule is gradually released. This can lead to delayed plasma peak concentration, complicating the prediction of pharmacodynamic effects.
The distribution of THCA-C4 in tissues, by analogy with other cannabinoid acids, likely occurs unevenly. Predominant accumulation is observed in highly perfused organs-liver, kidneys, lungs-as well as in tissues with high lipid content, such as the brain. However, due to the molecule’s polarity and low permeability through the blood-brain barrier, limited entry into the CNS in its unchanged form is expected. This partly explains the lack of psychoactive effects in raw cannabis extracts with a high content of the acidic form.
In the liver, THCA-C4 may undergo phase I biotransformation, particularly oxidation and hydroxylation mediated by cytochrome P450 enzymes. The presence of a butyl side chain, unlike the pentyl chain in THCA-A, creates a different stereochemical profile that may alter affinity for specific CYP isoforms, such as CYP2C9, CYP3A4, or CYP2D6. The products of such transformations could theoretically include hydroxylated metabolites that acquire new properties-either increased water solubility or, conversely, latent bioactivity. However, the lack of in vivo studies complicates the assessment of the significance of these metabolites.
Phase II biotransformation-conjugation with glucuronic acid or sulfation-likely occurs rapidly, since the carboxyl group is a classical target for glucuronosyltransferases. This accelerates elimination via the kidneys, although hepatic excretion as biliary conjugates is also possible. These processes typically reduce bioactivity and systemic circulation time, but there is a theoretical possibility that certain conjugates may be reversibly hydrolyzed by gut microbiota with subsequent reabsorption-thus enabling enterohepatic recirculation.
Considering the structural features of THCA-C4, special attention should be paid to its potential involvement in interactions with endocrine regulatory systems and cellular signaling. Although cannabinoid receptors are primary targets, it is not excluded that THCA-C4 may influence other molecular pathways-specifically, inhibiting cyclooxygenase enzymes or affecting ion channels. Additionally, its acidic nature suggests the molecule might act as a lysosomotropic agent, accumulating in acidic cellular compartments-potentially altering intracellular signaling.
The pharmacokinetic profile of THCA-C4 likely depends on the route of administration. Enteral administration is expected to result in low bioavailability due to first-pass metabolism, whereas sublingual or transdermal routes may partially bypass hepatic metabolism. In the case of parenteral administration (currently not practiced for cannabinoids), the molecule would immediately enter systemic circulation; however, instability in aqueous solutions and the risk of precipitation limit this strategy.
Another hypothesis requiring verification is the involvement of THCA-C4 in microbiome-mediated mechanisms. It is known that many phenolic compounds, including cannabinoid derivatives, are metabolized by gut bacteria, forming secondary metabolites. Given the chemical reactivity of THCA-C4, microbial decarboxylation or even enzymatic modification within the microbiota is possible. Such modified molecules may possess new properties, including effects on the immune system or cholesterol metabolism, thus expanding the potential physiological roles of THCA-C4 beyond classical cannabinoid activity.
Probable Interaction with Cannabinoid Receptors
Studying the potential interaction of THCA-C4 with cannabinoid receptors CB1 and CB2 is a complex task, as no research to date has characterized this molecule in terms of receptor affinity, functional activity, or structural-biological correspondence. However, based on the analysis of electron density, spatial conformation, functional groups, and the nature of the side chain, a hypothetical model of its possible interaction with these receptors can be reconstructed.
The initial aspect is the compliance of THCA-C4 with the structural requirements for cannabinoid receptor ligands. CB1 and CB2 receptors have a hydrophobic binding pocket within the transmembrane domains of G-protein coupled receptors (GPCRs). For effective interaction with these sites, the molecule must contain a certain set of functional elements: (1) an aromatic or partially saturated cyclic system for π-π or van der Waals interactions with phenylalanine/tryptophan residues; (2) a lipophilic side chain that “anchors” the molecule in the receptor pocket; (3) a hydroxyl or carboxyl group that forms hydrogen bonds with polar amino acids. THCA-C4 meets these requirements only partially.
Unlike the active form Δ⁹-THC, which has a neutral, lipophilic nature, THCA-C4 retains a polar carboxyl group, significantly altering its electrostatic profile and pKa. This means that in the neutral environment of the receptor (inside the hydrophobic pocket of the protein), THCA-C4 will predominantly exist in an ionized form. Such a form is energetically unstable in a lipophilic environment and complicates anchoring interaction with the receptor. However, the presence of a butyl side chain, although shorter than the pentyl in THCA-A, may partially compensate for this effect by stabilizing the molecule within the receptor’s hydrophobic core.
Crystallographic models of CB1 receptors show that affinity is highly sensitive to the length and spatial orientation of the side chain. In THCA-C4, it has four carbon atoms, making it less flexible and slightly less bulky than the canonical Δ⁹-THC. A shorter chain length is generally associated with reduced affinity for the CB1 receptor, while CB2 is less sensitive to this parameter. This allows the assumption that THCA-C4, if it binds to the receptor, is more likely to demonstrate greater affinity for CB2 or even selectivity determined by the spatial characteristics of the active site of this subtype.
An additional important factor is the volume and electrostatic asymmetry of the molecule. THCA-C4 has an acidic character but also includes aromatic and terpenoid fragments, creating a multipolar electronic surface map. This potentially provides several contact points with the receptor: one through the hydroxyl group at position 1′, another through the enol or phenolic group of the benzoid ring, and a third through the carboxyl radical. However, such multipoint interaction often does not correlate with agonism. On the contrary, it can stabilize the receptor in an inactive or partially activated state-that is, THCA-C4 may be a weak partial agonist or even an allosteric modulator.
There is also a possibility that THCA-C4 does not bind at the orthosteric site of the receptor at all but influences its function via an allosteric pocket. This action has been demonstrated for some endocannabinoids and synthetic analogs that, without activating the receptor themselves, modulate the efficacy or affinity of other ligands. If THCA-C4 acts as a negative or positive allosteric modulator, this may explain the lack of psychoactivity combined with mild immunomodulatory or neuroprotective effects sometimes observed for cannabinoid acids in general.
A separate issue is the ability of THCA-C4 to activate or inhibit other receptors of the cannabinoid system, particularly GPR55, GPR18, and TRP channels (notably TRPV1, TRPA1). Many studies have shown that acidic forms of cannabinoids exhibit activity at these targets independently of CB1/CB2. For example, THCA-A demonstrated the ability to inhibit TRPM8 and activate TRPA1, which underlies analgesic and anti-inflammatory effects. Considering the structural similarity, it can be assumed that THCA-C4 has a similar pharmacological profile. At the same time, the effect on GPR55, considered by some researchers as a third type of cannabinoid receptor, may be either agonistic or neutral depending on the molecule’s conformation in a specific environment.
Other potential scenarios include competitive inhibition of the active ligand. In the case of simultaneous administration of THCA-C4 with Δ⁹-THC, the THCA-C4 molecule may partially displace THC from the receptor site due to spatial competition, reducing the overall strength of CB1 activation. Such an action corresponds to a mechanism of partial antagonism or noncompetitive modulation. This phenomenon has pharmacological significance as it can be used to create cannabis product profiles with regulated psychoactivity.
On a molecular level, it is also important to consider receptor complex dynamics. CB1 and CB2 receptors have the capacity for desensitization and internalization following activation. If THCA-C4 binds to the receptor but does not induce its internal mobilization, this may stabilize the receptor on the cell surface, altering its responsiveness to other ligands. Such an effect is possible only in the case of weak or allosteric interaction.
What Changes After Decarboxylation
Decarboxylation is a fundamental transformation that alters not only the chemical structure of the THCA-C4 molecule but also its entire pharmacological, biophysical, and metabolic behavior. In the case of THCA-C4, as with other cannabinoid acids, this process involves the loss of the carboxyl group (-COOH) through thermal or enzymatic degradation, leading to the formation of a neutral cannabinoid, conditionally analogous to Δ⁹-THC but with a butyl (C4) side chain instead of a pentyl (C5) one. However, the effects of this reaction go far beyond the simple detachment of CO₂ – it triggers a cascade of conformational, electronic, receptor-related, and kinetic changes that define the final biological profile of the molecule.
Starting with the fundamentals: the structural rearrangement of the molecule after decarboxylation changes its electron density, charge symmetry, and hydrophobicity. The carboxyl group, which was responsible for the acidity of THCA-C4, takes with it polarity and negative charge. This converts the molecule from an ionized state to a neutral one, significantly altering its ability to cross biological membranes. While THCA-C4 was poorly absorbed through the gastrointestinal mucosa or the blood-brain barrier, its decarboxylated analog – neutral C4-THC – exhibits high membrane permeability due to increased lipophilicity.
The second key change is receptor activity. Cannabinoid receptors CB1 and CB2 show high sensitivity to the molecular form. In its unchanged form, THCA-C4 has low or almost zero affinity for these receptors due to the presence of the carboxyl group, which hinders effective positioning of the molecule within the receptor’s hydrophobic pocket. However, after decarboxylation, a structure is formed that is much closer to the active form of Δ⁹-THC, likely C4-THC, which demonstrates the ability to directly interact with CB1 – meaning it can induce psychoactive effects. The uniqueness lies in the butyl side chain, which unlike the pentyl, reduces affinity for the CB1 receptor but does not completely block it. This creates a scenario of moderate, dose-dependent CB1 activation, which may produce a mild or selectively modified psychoactive effect, different from classic Δ⁹-THC.
Decarboxylation also changes the metabolic fate of the molecule. In its acidic state, THCA-C4 is not a substrate for liver enzymes, particularly cytochrome P450 isoforms (CYP2C9, CYP3A4), due to its high polarity. But after conversion to neutral C4-THC, the molecule can undergo hydroxylation, demethylation, and conjugation reactions (glucuronidation, sulfation). This alters the profile of circulating metabolites in the blood, which can be active themselves or influence receptor feedback. For example, the formation of 11-hydroxy-C4-THC is possible – a potentially active metabolite with enhanced brain penetration ability.
It is also important that decarboxylation changes the conformational stability of the molecule. The carboxyl group, through intramolecular hydrogen bonding, stabilizes the THCA-C4 structure in a fixed conformation. Loss of this group makes the molecule more flexible, reducing its enthalpy of stability but increasing its ability to adapt to the receptor environment. Such plasticity facilitates more effective docking with receptors, including not only CB1/CB2 but also TRPV1, GPR55, GPR18.
After decarboxylation, the physicochemical behavior of the molecule in various environments also changes, including its solubility, boiling point, and oxidative stability. THCA-C4 is highly hygroscopic and poorly soluble in inorganic solvents. In contrast, decarboxylated C4-THC is more stable in nonpolar environments and dissolves better in ethanol, chloroform, and DMSO, opening pathways for encapsulation, transdermal transport, and pharmaceutical formulation.
From the perspective of bioaccumulation, C4-THC, unlike THCA-C4, can bind to plasma lipoproteins (especially albumin and HDL), which ensures prolonged circulation time and a larger volume of distribution. This leads to a longer-lasting effect, even at lower doses, and potentially increases systemic bioavailability. Additionally, the ability to cross the blood-brain barrier sharply increases – the neutral lipophilic molecule can effectively penetrate the central nervous system, where it exerts its activity.
Pharmacodynamically, C4-THC after decarboxylation may have selective receptor action because the butyl side chain not only lowers affinity for CB1 but also alters the functional profile of the interaction. It is likely the molecule acts as a partial CB1 agonist with incomplete intracellular signaling, resulting in milder effects – lowered blood pressure, pain reduction, mood modification without typical euphoria or psychomotor agitation. Simultaneously, CB2 activation, more often associated with immunomodulatory effects, may be more pronounced, considering the spatial features of the CB2 pocket and improved fit of the C4 chain.
Decarboxylation also changes the molecule’s interaction with enzymatic systems involved in xenobiotic recognition, notably UGT and SULT. THCA-C4, as an acidic molecule, avoids active glucuronidation, whereas C4-THC quickly undergoes conjugation with glucuronic acid, reducing its activity and accelerating excretion. This opens possibilities for pharmacokinetic regulation of action through modulation of phase II biotransformation enzymes.
The final important aspect is toxicological and immune behavior. THCA-C4 as an acid is significantly less active in provoking neuroinflammatory or behavioral responses. After decarboxylation, the resulting molecule may have a higher capacity to induce CB1-dependent side effects (anxiety, tachycardia, coordination changes), especially in sensitive populations. At the same time, due to reduced potency compared to Δ⁹-THC, these effects are less pronounced. Immune action also becomes more evident, as neutral cannabinoids more actively affect CB2 receptors on immune system cells.
Is it capable of having therapeutic effects?
The question of the potential therapeutic activity of THCA-C4 and its decarboxylated form C4-THC is not merely an attempt to extrapolate from already studied cannabinoids but rather a necessity for a systematic analysis of a unique pharmacophore characterized by variability in the side alkyl chain and a distinct metabolic fate. Unlike THCA-A or Δ⁹-THC, the butyl (C4) configuration gives this molecule not only reduced receptor affinity but also potential specificity in interacting with a range of non-canonical targets – from TRP channels to signaling pathways that modulate oxidative stress, autophagy, and immune response. This opens up a wide spectrum of pharmacologically relevant directions that may make THCA-C4 and its derivatives promising in the context of therapy for both common and rare diseases.
We should start with neuroprotective potential. Cannabinoid acids, likely including THCA-C4, are capable of modulating the expression of genes responsible for synthesizing antioxidant enzymes – catalase, superoxide dismutase, glutathione peroxidase. This activity does not necessarily require receptor interaction, as it may be realized through influence on the nuclear transcription factor NRF2. In chronic neurodegenerative conditions such as Alzheimer’s, Parkinson’s, and Huntington’s diseases, such activity is valuable due to its ability to reduce oxidative stress without accompanying psychoactivity. Additionally, THCA-C4 can inhibit caspase-dependent neuronal apoptosis mechanisms, adding another mechanism for preserving the neuronal pool.
The second promising direction is anti-inflammatory action. Here, THCA-C4 likely acts by selectively inhibiting cyclooxygenase-2 (COX-2), which plays a key role in generating prostaglandins during inflammation. Studies of analogs with C3-C5 side chains show that acidic forms can be selective COX-2 inhibitors with moderate activity but high safety. This effect may be enhanced through interaction with PPARγ receptors – nuclear regulators of pro-inflammatory gene expression. It is important to note that PPARγ activity is also linked to antiproliferative effects in some cancer models, notably colorectal cancer, which positions THCA-C4 as a potential candidate for adjuvant therapy.
Another relevant property is antiemetic activity. Although acidic cannabinoids do not exhibit classic antiemetic effects via CB1, there is evidence that THCA isomers can interact with serotonin 5-HT3 receptors. These receptors mediate chemotherapy-induced vomiting, so their blockade yields a pronounced effect even at low doses. If THCA-C4 has affinity for 5-HT3, this opens the way for its use as an antiemetic without the characteristic psychotropic side effects of Δ⁹-THC. Such a pharmacological window is especially valuable in palliative oncology therapy.
It is also worth considering effects on peripheral nociception. C4 homologs after decarboxylation can partially activate CB2 receptors localized in immune cells, endothelium, and peripheral neurons. This enables suppression of nociceptive transmission without involvement of the central CB1 mechanism – meaning no sedation or psychoactive changes. This approach is promising in cases of chronic non-oncologic pain such as rheumatoid arthritis, neuropathy, and interstitial inflammations.
Another analgesic mechanism may be interaction with TRP channels, primarily TRPV1 and TRPA1. These ion channels participate in transmitting pain, heat, and inflammation signals. It is known that some neutral cannabinoids act as TRPV1 agonists, causing channel desensitization and reduction of pain signaling. If C4-THC has similar activity, it could be a potential component of topical formulations – ointments, gels, patches – for local pain relief in myalgias, arthritis, and injuries.
Equally important is the probable immunomodulatory effect. CB2 receptors, as well as signaling pathways involving GPR55 and PPARγ, regulate cytokine profiles – notably inhibiting IL-6, TNF-α, IFN-γ. This supports considering THCA-C4 as a potential agent for treating autoimmune diseases (systemic lupus erythematosus, sclerosis), post-infectious syndromes (long COVID), or chronic systemic inflammation (metabolic syndrome, non-alcoholic steatohepatitis). At the same time, reducing pro-inflammatory cytokine production without generalized immunosuppression is a significant advantage over corticosteroids.
The anticonvulsant activity is promising but underexplored. Some C4 cannabinoid homologs have the ability to inhibit glutamatergic transmission and activate adenosine A1 receptors, leading to reduced neuronal excitability. If THCA-C4 or its metabolites exert a similar effect, this opens possibilities for its use in epilepsy therapy, especially pharmacoresistant forms like Lennox-Gastaut syndrome or Dravet syndrome.
The influence of THCA-C4 on metabolic homeostasis is also intriguing, especially regarding glucose tolerance and lipid profile. Probable activation of PPARα and AMPK (a kinase regulating cellular energy balance) indicates the molecule’s potential in controlling insulin resistance, lowering triglyceride levels, and preventing fatty liver disease. This effect is important for patients with metabolic syndrome or type 2 diabetes who are contraindicated for appetite stimulants or psychoactive cannabinoids.
Separately, the potential of C4-THC in dermatological pathologies deserves attention. Due to its neutral form, the molecule penetrates the epidermis well, and local activation of CB2/TRPV1 in keratinocytes can reduce hyperproliferation, itching, and inflammation. This opens a path for using C4-THC in treating psoriasis, atopic dermatitis, and acne. Current data indicate that topical cannabinoids with shorter side chains have lower systemic bioavailability, reducing the risk of systemic effects.
The final direction is cardiovascular action. There is reason to believe that THCA-C4 and its derivatives can influence vascular tone by activating endothelial NO synthase, leading to vasodilation. This creates a hypotensive effect potentially beneficial for arterial hypertension or vascular dystonia. Additionally, activity on L-type calcium channels in cardiomyocytes may modulate heart contractility without significantly lowering heart rate, which is advantageous for patients with heart failure.
Prospects for Application
THCA-C4, as one of the rare homologs of cannabinoid acid with a four-carbon (butyl) side chain, holds potential for multifaceted use that extends far beyond the traditional cannabinoid context. Unlike canonical metabolites such as THCA-A or Δ⁹-THC, this compound opens new possibilities due to its unusual chemical configuration, specific receptor interactions, high structural stability, and unique presence in the chemomic profile of plants. Consequently, its applications may span pharmacology and medicine, as well as analytical chemistry, biotechnology, agronomy, and forensic science. It is precisely this interdisciplinary nature of THCA-C4’s potential that deserves detailed analysis from the perspective of strategic development across scientific, industrial, and clinical domains.
One of the most obvious vectors lies in fundamental and applied research within the framework of novel pharmacognosy – a discipline that studies bioactive natural components as foundations for developing next-generation medicines. THCA-C4, due to its non-canonical structure, can be used as a model compound to investigate SAR (structure-activity relationship) dependencies within cannabinoid chemistry. It allows experimental verification of which structural features of the cannabinoid core determine affinity for CB1, CB2, TRPV1, GPR55 receptors, or enzymes involved in inflammatory cascades. In turn, these data may lay the groundwork for designing synthetic analogs with higher selectivity and improved safety profiles.
A second area of practical significance is the development of targeted therapies based on so-called precision medicine. Within this approach, THCA-C4 can be considered not only as an active molecule but also as a pharmacological probe – a tool for mapping specific pathways within cells or tissues that can be activated only by particular configurations of receptors, proteins, or signaling systems. This is especially critical for rare or difficult-to-treat conditions that do not respond to standard treatment protocols – for example, atypical forms of epilepsy, inflammatory encephalopathies, mitochondrial disorders, or multisystem inflammatory syndromes.
Simultaneously, THCA-C4 is gaining importance in agrobiotechnology and modern cannabis breeding. Due to its limited natural occurrence in standard Cannabis sativa cultivars, this cannabinoid homolog serves as a marker of atypical metabolic pathways that are realized only under specific expression of unique variants of the THCA synthase enzyme. Consequently, the presence of THCA-C4 in a strain’s chemotype may indicate a deeply altered metabolic landscape of the plant, paving the way for creating artificially optimized phenotypes with a predefined cannabinoid spectrum. This possibility is particularly promising for industries requiring ultra-low Δ⁹-THC content and high levels of non-psychoactive metabolites for pharmaceutical or nutraceutical use.
From the viewpoint of analytical chemistry and chemometrics, THCA-C4 is a valuable biomarker. Its presence, concentration, and ratio relative to other homologs can indicate the specificity of the plant’s origin, cultivation conditions, genotype, or even peculiarities of post-processing – such as drying, fermentation, or ultraviolet exposure. This makes THCA-C4 a potential tool for identifying adulterations, verifying product authenticity, or confirming the source of raw material in forensic and customs examinations. Because C4-chain isomers rarely occur in high concentrations in wild-type strains, their detection may signal specialized cultivars or even deliberate genetic editing.
Logically, this leads to another promising field – standardization. Unlike common metabolites, which vary in concentration from harvest to harvest, THCA-C4 exhibits high stability during storage, minimal photodegradation, and predictable chromatographic behavior. This allows it to be used as an internal standard in qualitative and quantitative cannabinoid profiling, especially in low-content matrices (such as cosmetic products or cannabinoid films for nanotherapy).
Another application area is nanopharmacy. Due to its acidic nature and high polarity, THCA-C4 poorly penetrates lipid membranes but can be encapsulated in liposomes, nanospheres, or solid lipid particles. This enables the development of novel controlled-release systems aimed at local or prolonged delivery to inflamed tissues, tumor microenvironments, or barrier structures (such as the blood-brain barrier). Thus, THCA-C4 is viewed as a pharmacophore for carrier platforms that may provide targeted therapy with minimal side effects.
In a broader biomedical technology context, THCA-C4 may also be used as a metabolic sensor. Due to its specific metabolism and inactivation in the liver and small intestine, it can serve as an indicator of the activity of individual enzymatic systems – notably UDP-glucuronosyltransferases, cytochrome P450 isoforms, or epoxide hydrolases. This allows THCA-C4 to be applied in preclinical screening or as a functional probe in pharmacokinetic studies of other molecules, including polypharmaceutical preparations.
Another intriguing direction is the use of THCA-C4 in ecotoxicology and environmental monitoring. Its stability and specificity enable the detection of traces of cannabis raw material in soil, water, or air even in the absence of Δ⁹-THC or its metabolites. This can be important for identifying illegal cultivation, tracking smuggling routes, or monitoring contamination near processing facilities.
Finally, it should be noted that THCA-C4 holds promise as a molecular basis for patent protection of new formulations. Its rarity and chemical specificity allow the creation of a range of derivatives with unique properties that are not covered by existing patents on Δ⁹-THC or CBD. This opens the door to innovative pharmaceutical development where THCA-C4 can serve as the foundation for drugs with added legal and clinical value.
Precision Medicine: THCA-C4 as a Molecular Target
The application of cannabinoid compounds in precision medicine involves considering them not simply as broadly active pharmacological agents but as potential individualized molecular targets for treating specific patients, genotypes, or cellular pathologies. In this context, THCA-C4 (Δ9-tetrahydrocannabinolic acid C4), as one of the lesser-studied homologs of the classical THCA, acquires particular significance. Its unique structure, differences in physicochemical properties, unstable presence in biological material, and potential functional activity at the molecular level create the foundation for using THCA-C4 as a tool in personalized pharmacology.
The uniqueness of THCA-C4 here lies primarily in its altered profile of chemical recognition by biological receptor systems. Compared to classical THCA-A, which has a pentyl side alkyl group, THCA-C4 is characterized by a butyl chain. This small structural modification can fundamentally change not only the molecule’s spatial conformation but also its ability for complementary interaction with protein targets-specifically GPCR receptors, oxidoreductase enzymes, and membrane transporters. This difference enables preclinical modeling of THCA-C4’s effects as a narrowly specialized ligand within receptor mapping, which is the foundation of precision medicine.
Another important aspect is the metabolic fate of THCA-C4 under an individual patient’s enzymatic profile. In precision treatment, the determining factor is not only the action of the molecule itself but also how it is metabolized. It is known that some THCA derivatives undergo enzymatic hydrolysis, oxidation, or conjugation mediated by the CYP450, UGT, and NAT systems. If THCA-C4 is activated or detoxified in the body through the formation of unique metabolites-and this hypothesis has biochemical grounds considering the altered solubility and reactivity of the butyl side chain-this opens space for developing differentiated therapeutic models. In the future, such models could be adapted to a person’s individual pharmacokinetic profile, which constitutes the essence of the precision approach.
In the therapeutic context, THCA-C4 can be considered a molecule whose activity is defined by selective interaction with atypical or poorly studied cannabinoid receptors (e.g., GPR18, GPR55, GPR119). These targets do not have high affinity for classical Δ9-THC but may specifically respond to cannabinoid acids with a modified side chain. In this sense, THCA-C4 potentially plays a role as a selective modulator of signaling cascades involved in immunoregulation, angiogenesis, neuroprotection, or metabolic adaptation. All of these processes are key for implementing targeted therapy concepts in patients with severe or chronic diseases.
The use of THCA-C4 in oncology therapy based on biomarker selection appears especially promising. In several cell models (such as glioblastoma, melanoma, colorectal cancer), it has been shown that cellular expression of GPR55 and related proteins can be altered by exogenous cannabinoids. If THCA-C4 can modulate the expression of these receptors or inhibit their activity, there is potential to use this compound as a molecular target in tumor therapy with a known receptor profile.
It is also worth noting the possibility of using THCA-C4 in therapy for neurodegenerative conditions, specifically Alzheimer’s or Parkinson’s disease. The theoretical model suggests that the selective activity of the acidic form with a short lipophilic chain may influence signaling pathways associated with neuroinflammation, microglial activity, and glutamatergic transmission. Considering that THCA-C4 has relatively low permeability across the blood-brain barrier in its native form, its effects may be localized or limited to the peripheral nervous system, creating distinct pharmacological niches for its use where central nervous system action is undesirable.
Besides direct receptor action, THCA-C4 may also act as a modifier of the local environment-specifically affecting membrane viscosity, redox balance parameters, or the dynamics of intercellular communication via exosomes and vesicular structures. All these effects can be measurable and predictable within personalized medicine frameworks, taking into account a patient’s transcriptomic, proteomic, or metabolomic profile.
Of fundamental importance is also the technical possibility of incorporating THCA-C4 into computer docking platforms and in silico screening. Due to its well-defined molecular structure and small molecular weight, this compound is well suited for simulating ligand-receptor interactions using molecular dynamics methods, which are critically important in early stages of developing precision therapeutic solutions. Furthermore, since THCA-C4 still lacks a vast pharmacological background, it represents an interesting target for creating new drugs restricted to narrow patient categories or rare diseases where general therapy is ineffective.
Purity or Origin Marker in Analytical Chemistry of Cannabinoids
THCA-C4, as a less common homolog of Δ9-tetrahydrocannabinolic acid, possesses all the characteristics of a marker compound that can be used in analytical chemistry to differentiate cannabis chemotypes, identify botanical origin, establish cultivation conditions, and confirm the chemical purity of isolated extracts. In analytical practice, the presence or absence of specific homologs in a given sample serves not only as a descriptor but also attains the status of a chemical “fingerprint,” by which conclusions can be drawn both about the natural origin of the substance and about the presence of adulterations, technological impurities, or violations of processing protocols.
The appearance of THCA-C4 in phytocannabinoid profiles is not accidental. This compound is synthesized in the plant via a biosynthetic pathway similar to that of THCA-A but uses butyryl-Coenzyme A instead of the pentanoyl precursor. This particular feature makes THCA-C4 a sensitive marker of biogenesis conditions, specifically enzyme activity of particular synthases (THCA synthase with alternative specificity), levels of available coenzymes, and the balance of carbon substrates in the cytosol of trichome cells. Therefore, analysis of THCA-C4 presence in a mixture allows inference about the physiological status of the plant at the time of biosynthesis.
From an analytical perspective, detection of THCA-C4 is performed using high-precision instrumental methods such as high-performance liquid chromatography (HPLC), tandem mass spectrometry (LC-MS/MS), gas chromatography with prior derivatization, and nuclear magnetic resonance spectroscopy. It is particularly noteworthy that THCA-C4, unlike its more common homolog, has a different mass, altered chromatographic polarity, and characteristic fragmentation in MS2 spectra. This makes it practically unmistakable from other cannabinoids, even in complex matrices. This is the core of its value as an analytical indicator-THCA-C4 can identify the botanical or chemotype profile of a sample even at ultra-low concentrations.
In forensic chemistry practice, where differentiation of samples by regional or cultivation origin is required, THCA-C4 can be used as a marker of the geochemical index. Some chemotypes, especially those grown under conditions with limited access to long-chain fatty acids, produce higher amounts of THCA-C4. Thus, the presence of this homolog in a phytoprofile is significant evidence of botanical source-whether outdoor, greenhouse, or indoor cultivation, which differ in substrate availability for synthesis. Moreover, combining THCA-C4 with other minor homologs (for example, THCA-C1 or THCA-C3) enables construction of a multicomponent analytical panel that identifies not only botanical origin but also specific strains or hybrids with high accuracy.
Another important aspect is the application of THCA-C4 as a purity marker during isolation of pharmaceutical-grade cannabinoids. During extraction, fractionation, or purification of active compounds from cannabis, it is important to monitor the presence of extraneous homologs that may indicate incomplete purification, cross-contamination, or temperature control violations. In this context, unexpected detection of THCA-C4 in fractions where it theoretically should not be present (for example, after supercritical CO₂ extraction with a temperature gradient) may indicate chemical transformations during extraction or falsification of raw materials.
It is also necessary to highlight THCA-C4’s role in detecting synthetic or modified samples. Since many synthetic cannabinoids currently available often lack natural homologs, their absence-including THCA-C4-can serve as a criterion for identifying chemically altered or laboratory-produced substances. In samples where THCA-C4 is completely absent despite the presence of main THCA-A or THC, it may indicate cannabinoid reengineering through deconstruction and resynthesis. Therefore, THCA-C4 can serve as a marker of naturalness and authenticity of the substance.
Within GMP control and pharmaceutical certification analysis, THCA-C4 may act as a “negative control indicator.” In purity specifications for medicinal formulations based exclusively on a single active cannabinoid, its presence may be unacceptable. At the same time, in full-spectrum extract-based phytoproducts, the presence of THCA-C4 at micro concentrations can confirm the integrity of the botanical profile. This creates a dual-purpose tool for controlling both authenticity and degree of purification.
Additionally, THCA-C4 has the potential to be used for creating digital chemotype libraries within molecular traceology frameworks. The combination of high-precision mass spectrometry and machine learning algorithms enables detection of recurring homolog distribution patterns in samples, forming unique “cannabinoid fingerprints.” THCA-C4 functions in this system as a validation point: its presence or absence records matrix stability over time and space, allowing verification of identity between product series or extract batches.
Biotechnology and Breeding: Custom Production of Cannabinoid Profiles
Modern biotechnology opens fundamentally new opportunities for breeding cannabis plants with specified cannabinoid profiles, including the precise content and ratios of rare homologs such as THCA-C4. In this context, biotechnological approaches transform traditional breeding-which was primarily based on phenotypic traits-into a high-tech science guided by molecular design. This enables the creation of original combinations of cannabinoids with tailored therapeutic properties that meet the demands of precision medicine and pharmacology.
The foundation for producing specified cannabinoid profiles is a deep understanding of the genetic and biochemical mechanisms controlling the expression of enzymes that determine cannabinoid biosynthesis pathways. Genetic modification, CRISPR/Cas9 editing, and marker-assisted selection allow manipulation of the activity of genes encoding THCA synthase, CBDA synthase, and other enzymes that produce specific homologs. Notably, variations in genotypes can influence the ratios among different side-chain lengths that form homologs-from C1 to C5 and beyond, including C4. This means that precise manipulation can regulate the amount of THCA-C4, directing the plant’s metabolism to increase or decrease its production.
Alongside genetic approaches, cell culture technologies are actively employed, enabling the cultivation of cannabis cells or tissues in vitro, maintaining the production of specific cannabinoids under controlled conditions. Cell cultures, organoids, or bioreactors with cannabis cells can be modified to boost THCA-C4 output by regulating nutrient conditions, exposure to stresses, and epigenetic factors. This approach is particularly important for industrial production as it allows for a homogeneous product without the fluctuations typical of plants grown in field conditions.
An essential component of the biotechnological strategy is the application of enzymatic synthesis. It is known that the enzymes catalyzing the final stages of cannabinoid synthesis have certain substrate specificity but also a degree of plasticity. Using mutant variants of these enzymes with increased affinity for substrates with shorter side chains-specifically butyryl-CoA-enables enhanced THCA-C4 synthesis in bioreactors. Such enzymatic synthesis, combined with genetic optimization of plants, paves the way for large-scale production of specified cannabinoid profiles necessary for developing new pharmaceutical formulations.
Hybridization of plants with different chemotypes also plays a significant role. Selecting parental lines with a high content of THCA-C4 or predisposition to its formation allows, through successive generations, the creation of stable cultivars with the desired profile. Unlike traditional breeding, modern methods enable tracking the presence of genes regulating cannabinoid metabolism in progeny using molecular markers and genotyping. This considerably accelerates the process and reduces unnecessary breeding cycles.
It is also worth noting the prospects of synthetic biology for creating artificial microorganisms capable of producing THCA-C4. Bacteria or yeasts engineered to express key genes of the cannabinoid pathway can serve as efficient bioreactors. An important task is tuning metabolic fluxes, particularly the pathways synthesizing acyl-CoAs, which serve as donor groups for cannabinoid synthase enzymes. Regulating the expression of acyltransferase enzymes in combination with optimizing enzymatic activity opens the door to large-scale and controlled synthesis of THCA-C4 outside the plant.
In the context of breeding, special attention is given to developing “green” biotechnologies-environmentally safe methods that maintain stability and reproducibility of cannabinoid profiles with minimal environmental impact. This is especially relevant for developing medical cultivars with strictly controlled active ingredient content. For example, by regulating light regimes, temperature parameters, and nutrient media in closed cultivation systems, it is possible to enhance the expression of genes responsible for THCA-C4 production without the need for chemical stimulants or aggressive genetic modification methods.
Another important aspect is integrating multi-omics data-genomics, transcriptomics, proteomics, and metabolomics-into the breeding process. Comprehensive analysis of these data allows for more accurate prediction of the effectiveness of specific genetic changes and assessment of cannabinoid profile stability. For instance, transcriptomic analysis enables identification of key regulatory genes that activate the synthesis of specific homologs, including THCA-C4, in response to external or internal stimuli. This opens possibilities for creating cultivars with programmable responses to environmental factors.
Custom production of cannabinoid profiles is also a matter of quality control and product standardization. In this regard, biotechnology provides the ability to obtain homogeneous raw material with minimal composition variability. This is especially important for the pharmaceutical industry, where dose and active ingredient profile stability are critical. Cell cultures and bioreactors with modified microorganisms allow eliminating dependence on climatic and soil conditions, which often complicate stability maintenance in field cultivation.
It is also worth highlighting the prospects of hybrid approaches that combine classical breeding methods with biotechnological ones. For example, creating hybrids where traditional methods enhance or guide biotechnological modifications can produce stable cultivars with predictable and consistent THCA-C4 profiles that meet the requirements of customers in the pharmaceutical, cosmetic, or food industries.
Conclusion
THCA-C4, as a rare homolog of tetrahydrocannabinolic acid, exhibits unique properties shaped at the levels of its biosynthesis, chemical structure, and pharmacokinetics. The complexity of its detection is caused not only by chemical instability and sensitivity to external factors but also by its low concentration in plant material, necessitating the use of highly precise analytical methods. The natural biosynthetic pathways of THCA-C4 are closely linked to the activity of specific enzymes and the genetic variability of plants, which opens opportunities for regulating its content through genomic engineering and breeding. The biochemical stability of this molecule within the plant environment determines its availability for further research and commercial use.
Artificial production of THCA-C4, both in laboratory and industrial conditions, requires the combination of chemical, enzymatic, and biotechnological methods that allow control over the product’s structure and purity. Modern analytical approaches using spectroscopy, chromatography, and mass spectrometry provide accurate detection and confirmation of the molecular structure, which forms the foundation for further pharmacological investigation.
In biological systems, THCA-C4 exhibits a specific interaction with cannabinoid receptors that differs from the action of classical THC, opening prospects for developing new therapeutic agents with improved safety profiles. Decarboxylation radically changes the pharmacodynamics by converting the acid form into psychoactive forms, which is a critical point for understanding the action and potential use of THCA-C4. At this stage of research, the molecule demonstrates significant therapeutic potential, especially in the areas of anti-inflammatory, neuroprotective, and analgesic effects, but requires deeper mechanistic elucidation.
The application prospects of THCA-C4 encompass precision medicine, where it can serve as a molecular target for individualized therapeutic strategies, as well as analytical chemistry as a marker of origin and purity for cannabinoid preparations. Biotechnological approaches to breeding and producing custom cannabinoid profiles open new horizons for the pharmaceutical and cosmetic industries, enabling the creation of standardized, highly effective products.
Thus, THCA-C4 is a complex research subject with unique chemical and biological characteristics that require an interdisciplinary approach. Further study of its metabolism, molecular interactions, and synthetic pathways will provide a basis for the development of new medical and biotechnological applications, as well as for creating innovative quality control methods for cannabinoid products.
Sources
- PubMed Central (PMC) – U.S. National Library of Medicine
https://www.ncbi.nlm.nih.gov/pmc/
A database of biomedical and pharmacological publications, including many studies on cannabinoids. - National Center for Biotechnology Information (NCBI)
https://www.ncbi.nlm.nih.gov/
An authoritative resource with scientific articles, including genetics and biochemistry of cannabinoids. - Journal of Natural Products – American Chemical Society (ACS Publications)
https://pubs.acs.org/journal/jnprdf
Publishes high-quality research on natural compounds, including cannabinoids. - Frontiers in Pharmacology
https://www.frontiersin.org/journals/pharmacology
Open access publications on the pharmacological properties and biochemistry of cannabinoids. - Cannabis and Cannabinoid Research (Mary Ann Liebert, Inc.)
https://www.liebertpub.com/can
A well-known journal dedicated to scientific research on cannabinoids. - ScienceDirect (Elsevier)
https://www.sciencedirect.com/
Although not all content is open access, many studies are available through university access, especially on the chemistry and biochemistry of cannabinoids. - Directory of Open Access Journals (DOAJ)
https://doaj.org/
A catalog of open access scientific journals across various fields, where publications on cannabinoids can be found. - ResearchGate
https://www.researchgate.net/
A platform where scientists publish their work; full-text articles on THCA-C4 and related topics can often be found. - European Journal of Medicinal Chemistry
https://www.journals.elsevier.com/european-journal-of-medicinal-chemistry
Publications on the synthesis and biological properties of cannabinoids. - American Journal of Botany
https://bsapubs.onlinelibrary.wiley.com/journal/15372197
Publications on plant biology and genetics, including hemp.