Research on cannabinoids over the past decades has significantly deepened the understanding of the role of the endocannabinoid system (ECS) in human physiology and pathology. The primary focus of the scientific community has been on classical cannabinoids such as delta-9-tetrahydrocannabinol (Δ9-THC), cannabidiol (CBD), and other well-known components of the Cannabis sativa plant. However, the discovery of delta-9-tetrahydrocannabiorcol (THC-C1) has drawn considerable attention due to its unique structural, physicochemical, and biological properties that distinguish it from classical cannabinoids. This molecule represents a class of non-traditional cannabinoids which, although less studied, have the potential to significantly expand our understanding of cannabinoid pharmacology and molecular biology.
THC-C1 differs from classical Δ9-THC by a modification in the structure of its side chain, resulting in significant differences in pharmacological activity and selectivity for endocannabinoid system receptors. Variations in molecular structure-particularly the length and composition of the alkyl side chain-substantially influence the compound’s affinity for central CB1 and peripheral CB2 receptors, which are key to mediating cannabinoid effects. THC-C1 demonstrates the potential for more selective activity, which may be critical in the development of new pharmacological agents with reduced psychoactivity and minimized side effects.
Fundamental studies indicate that the structure of the cannabinoid side chain is one of the most important factors determining their biological activity. In the case of THC-C1, this side chain includes additional methyl groups, which significantly influence the molecule’s conformation and, consequently, its ability to bind to receptors. This results in a unique activity profile that differentiates THC-C1 from other tetrahydrocannabinols and opens new horizons in the study of the therapeutic potential of cannabinoids.
It is important to note that the natural origin of THC-C1 and its biosynthesis in the Cannabis sativa plant are subjects of active research. Unlike more common cannabinoids, THC-C1 is synthesized through the action of specific enzymes that catalyze the formation of atypical side chains. The regulatory mechanisms of these enzymatic pathways remain insufficiently studied, but understanding them is critically important for developing methods to control synthesis and optimize the extraction of this molecule. Both traditional and modern biotechnological approaches to producing THC-C1 are currently under development, including enzymatic synthesis, metabolic engineering of microorganisms, and chemical synthesis.
One of the key aspects determining the scientific and clinical relevance of THC-C1 is the ability to accurately detect and quantify it in complex biological systems. A wide range of analytical techniques is used for this purpose-from gas chromatography-mass spectrometry (GC-MS) to high-sensitivity liquid chromatography (LC-MS/MS) and nuclear magnetic resonance (NMR). The development of these methods enables not only the identification of THC-C1 in cannabinoid mixtures but also the tracking of its metabolic transformations, which is necessary for pharmacokinetic and toxicological studies.
The pharmacological potential of THC-C1 is driven not only by its interaction with classical ECS receptors but also by its effects on other molecular targets. Research suggests that it may modulate the activity of various ion channels, enzymes, and signaling pathways involved in neurotransmission, immune response, and inflammation regulation. This supports the consideration of THC-C1 as a promising agent for the treatment of complex conditions such as chronic pain, neurodegenerative diseases, autoimmune disorders, and inflammatory processes.
Moreover, THC-C1 shows potential for the development of new neuroprotective strategies. Its modified structure may contribute to enhanced antioxidant activity and the ability to protect neurons from damage caused by oxidative stress and inflammation. This is especially important in the context of diseases such as Alzheimer’s, Parkinson’s, and multiple sclerosis, where the endocannabinoid system plays a significant role in pathogenesis.
Contemporary interest in THC-C1 is also driven by regulatory and social factors. In many countries, legislation on cannabinoids is undergoing reform, and recognition of the therapeutic potential of non-traditional cannabinoids is fostering more flexible regulatory approaches. This, in turn, promotes scientific research and commercial utilization of new molecules such as THC-C1, leading to the expansion of the pharmaceutical landscape and improvements in patient quality of life.
At the same time, the relevance of studying THC-C1 is supported by its potential advantages over classical Δ9-THC, including reduced psychoactivity, which allows its use without significant risk to cognitive function and mental health. This opens possibilities for application in pediatrics, geriatrics, and other sensitive patient groups.
It is also worth noting that the study of THC-C1 holds fundamental importance for understanding the molecular evolution of cannabinoids. The diversity of cannabinoid structural forms, including THC-C1, is likely linked to adaptive mechanisms of plant metabolism that have developed under the influence of environmental factors. Investigating these molecules enables a better understanding of the biochemical flexibility of plants and the complexity of their secondary metabolism.
Chemical Structure and Physicochemical Properties
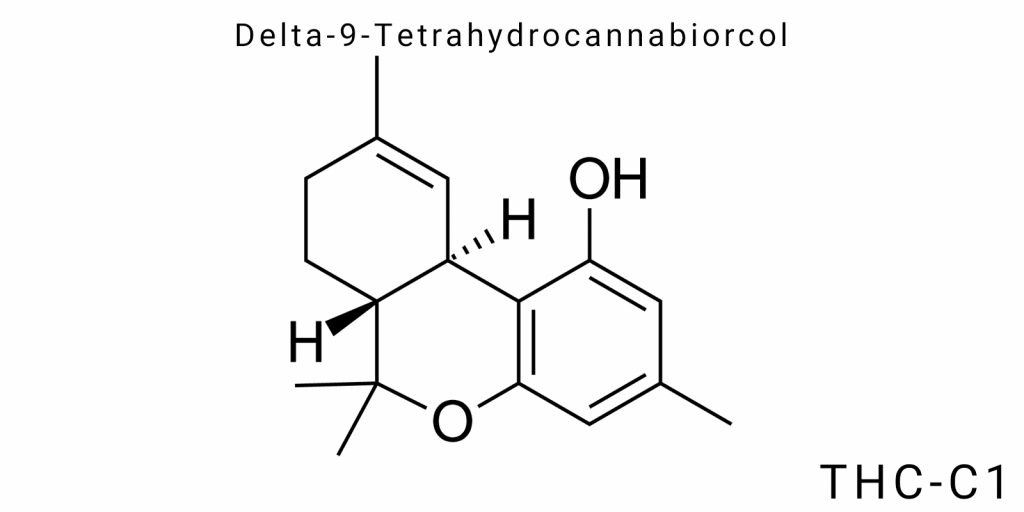
Delta-9-tetrahydrocannabiorcol (Δ9-tetrahydrocannabiorcol, or THC-C1) is a derivative of classical Δ9-tetrahydrocannabinol, distinguished by a specific modification of the side alkyl chain. This modification directly influences the molecular conformation, physicochemical properties, receptor affinity, and pharmacological profile of the compound. While THC-C1 shares the basic structure of tricyclic terpenoids, its uniqueness lies in the presence of a branched alkyl chain with substitution at a position critical for binding to endocannabinoid system receptors.
The general molecular formula of THC-C1 retains the framework characteristic of cannabinoids: a tricyclic benzopyran system that includes an aromatic ring (phenolic fragment), a cyclohexane segment, and a pyran-like moiety. These key structural elements facilitate hydrophobic and electrostatic interactions with receptor proteins. Variations in the length, saturation, and branching of the side chain can significantly alter the compound’s affinity for CB1/CB2 receptors and overall pharmacological activity.
In the case of THC-C1, the side chain is not the classical pentyl group found in Δ9-THC but is modified by the inclusion of additional methyl or ethenyl groups. This alteration changes the lipophilicity, spatial orientation of the molecule in the bound state, and its electron density. These subtle electronic and spatial changes can greatly affect conformational flexibility, which is critical for the dynamics of ligand binding to protein targets.
At the level of physicochemical characteristics, THC-C1 exhibits the classical hydrophobic nature typical of most cannabinoids; however, its solubility in lipid environments is increased due to a larger hydrophobic surface area. This, in turn, affects bioavailability upon enteral administration, permeability through the blood-brain barrier, and binding to plasma proteins. THC-C1 also demonstrates significant stability in neutral or slightly alkaline environments but remains sensitive to photolysis and oxidative processes during storage or under light exposure.
Structurally, it is noteworthy that the presence of a phenolic hydroxyl group can form hydrogen bonds, which are important factors in receptor interaction and metabolic recognition of the molecule by enzymatic systems. Derivatives of THC-C1 with blocked or modified hydroxyl groups exhibit significantly altered activity profiles, indicating the critical role of this fragment in the molecule’s bioactivity.
Another important feature of THC-C1 is its ability to exist in several tautomeric or stereoisomeric forms depending on environmental conditions, adding complexity to the study of its pharmacokinetics. The spatial configuration at position C-9 (where double bonding occurs in the ring) can change, forming enantiomers or diastereomers, each with different receptor affinities and potentially distinct biological profiles.
From a thermodynamic perspective, the THC-C1 molecule demonstrates good chemical stability in the absence of light and oxygen but readily undergoes isomerization upon heating, particularly in the presence of acids or during extraction processes. This presents certain challenges in the storage and processing of raw materials containing THC-C1 and necessitates the use of controlled conditions to preserve the active form of the substance.
The polarity of THC-C1 remains low, typical for most cannabinoids; however, certain modifications of the side chain can increase or decrease this parameter. This significantly influences the molecule’s behavior in biological environments-from distribution rates in tissues to elimination mechanisms. For instance, increased lipophilicity can prolong the half-life due to deposition in adipose tissues, while changes in polarity can affect interactions with transport proteins in the liver and kidneys.
The application of molecular docking and molecular dynamics simulations has shown that changes in the side chain of THC-C1 provide it with unique trajectories for entering the active site of the CB1 receptor, differing from those observed for Δ9-THC. This indicates the potential formation of new types of interactions, including π-π stacking and additional van der Waals bonds, which provide specific stabilization of the ligand-receptor complex.
Molecular Structure of THC-C1
The molecular structure of delta-9-tetrahydrocannabiorcol (THC-C1) exemplifies a structural variation within the natural cannabinoid framework, in which modification of the side chain leads to the emergence of new stereochemical and electronic-conformational characteristics that critically affect the molecule’s biological behavior. The structure of THC-C1 belongs to the class of tricyclic phenisterpenes and formally retains the central benzo-γ-pyran platform characteristic of Δ9-tetrahydrocannabinol (Δ9-THC), albeit with key modifications in the C-3 side chain region.
The core skeleton of the molecule-a tricyclic system-consists of two condensed rings: an aromatic benzene nucleus (A-ring) and a partially saturated cyclohexene fragment (B-ring), along with a separately closed tetrahydropyran ring (C-ring), which completes the system. This tricyclic configuration ensures a fixed spatial orientation of the molecule and allows the creation of a rigid topology necessary for highly specific interactions with protein targets. Despite structural similarity to classical cannabinoids, THC-C1 demonstrates significantly different properties due to a radically modified side chain.
The most characteristic feature of THC-C1 is the displacement of the typical pentyl chain, which is replaced by an isomeric structure with branching or cyclic fragments, particularly isopropyl, cyclopropyl, or methylcyclopentyl groups. Such substitution leads to the emergence of new stereocenters, increasing the number of possible enantiomers and diastereomers. These configurations are not merely formal variants-they directly determine the molecule’s ability to adapt to the receptor’s active site, its flexibility, and also influence binding to biomembranes.
Branching of the side chain causes a redistribution of electron density within the peripheral part of the molecule, which has implications for electrostatic interaction with aromatic amino acid residues within the hydrophobic pocket of CB receptors. The presence of secondary or tertiary carbon centers in this chain also creates conditions for potential oxidation or metabolic transformation involving monooxygenases. This makes the THC-C1 molecule more chemically pliable in the biological environment but also more complex in terms of synthesis and control of its metabolic profile.
Another important structural aspect of THC-C1 is the position of the double bond in the cyclohexene fragment. Retaining the double bond between carbon atoms C9 and C10 creates favorable geometry for π-π interactions with aromatic protein domains, but any shift of this bond as a result of chemical modifications or isomerization may radically alter the binding energetics. Some THC-C1 derivatives form non-canonical conformations in this region, leading to asymmetric stabilization during entry into the protein pocket.
The phenolic hydroxyl group at the C1 position of the A-ring participates in specific hydrogen bonding with amino or carbonyl residues of protein receptors. In the THC-C1 structure, it is preserved; however, its acidity and ionization capacity vary depending on substitutions at the other end of the molecule, particularly due to the inductive effect of groups on the side chain. This indirectly influences the pKa of the hydroxyl group and, therefore, its reactivity in the physiological environment.
Also crucial is the configuration around the C6a and C10a atoms-chiral centers that determine the spatial orientation of the molecule’s hydrophobic portion relative to the receptor surface. For THC-C1, the most stable form has the (6aR,10aR) configuration, similar to that of Δ9-THC. However, due to the change in bulkiness of substituents, the equilibrium may shift toward less active or inactive stereoisomers. Precise control of this stereochemistry is critical in the chemical synthesis of the compound, especially in the context of obtaining the pharmacologically relevant enantiomer.
In addition to the noted structural parameters, attention should be given to the molecule’s behavior in a dynamic environment. THC-C1 is not a completely rigid structure: certain segments, particularly the side chain, exhibit limited rotational freedom. This parameter is assessed through the calculation of molecular entropy and conformational fields, where it is evident that THC-C1 demonstrates a higher degree of molecular flexibility compared to classical Δ9-THC. This implies that in the biological environment, several alternative binding geometries are possible, which in turn may explain the partially agonistic/antagonistic action on various cannabinoid receptor subtypes.
From a chemical standpoint, the structure of THC-C1 lacks strongly polar or charged groups, which ensures its high affinity for lipid membranes. However, unlike many other lipophilic ligands, THC-C1 possesses an internal system of hydrophobic/hydrophilic balances, allowing it to maintain relative stability in a protein-aqueous environment without significant aggregation or precipitation. This is explained by the central tricyclic core’s ability to form π-electron stabilization of internal bonds, minimizing spontaneous structural fluctuations.
Particular attention should also be paid to the role of electron density around the oxygen atom in the pyran ring. As a result of the electron-donating nature of adjacent groups, the oxygen in this part of the molecule often acts as a hydrogen bond acceptor, which is significant not only for receptor binding but also for enzymatic metabolism. Its involvement in hydroxylation reactions, oxidative cleavage, or conjugation may alter the rate of THC-C1 biotransformation, which must be considered in the development of therapeutic derivatives.
Unique Chemical Groups and Their Impact on Activity
The molecular uniqueness of THC-C1 is primarily determined by structural modifications of its side chain, as well as the presence of several chemically active groups that are not typical for classical cannabinoids. Based on the analysis of electron-structural and stereochemical characteristics, key groups can be identified that critically affect the molecule’s pharmacological activity, bioavailability, and receptor selectivity.
First, THC-C1 contains a modified alkyl chain that does not end with a pentyl fragment, typical for Δ9-THC, but is replaced with cyclopentylmethyl, isopropylcyclopropyl, or more complex branched groups. These substituents have two critically important effects: first, they increase the hydrophobic surface area of the molecule, enhancing its ability to bind within the lipophilic pockets of protein structures; second, the presence of cyclic or branched fragments significantly increases the rigidity of the chain, thereby limiting the number of permissible conformations, which promotes receptor specificity.
From the perspective of electron structure, some THC-C1 derivatives contain tertiary carbon centers adjacent to the aromatic core. This creates local regions of increased electron density capable of participating in noncovalent interactions, particularly π-π stacking or C-H•••π interactions with phenylalanine and tyrosine residues in protein receptors. Under such conditions, the active site of the molecule exhibits a degree of mimicry to endogenous ligands of the cannabinoid system, which may explain its agonistic activity at CB1 or CB2 receptors.
Another key feature is the extended phenolic fragment. While the hydroxyl group at the C1 position is a common trait among most cannabinoids, in THC-C1 it may be surrounded by additional methylated or alkyl substituents on the benzene ring. This affects the acid-base properties of the molecule (primarily through the inductive effect) and, accordingly, the degree of ionization at physiological pH, which directly correlates with the efficiency of passive diffusion across biomembranes.
It is also important to note the presence of oxygenated groups in the pyrone ring. In some THC-C1 analogs, substitution with alcohol or ether functions is found here, significantly increasing chemical reactivity. This opens up possibilities for the creation of prodrugs that are metabolically activated in plasma or the liver, as well as for enhancing water solubility through the introduction of conjugates with glucuronic acid or sulfates.
Another significant functional group is the presence of a secondary or tertiary carbon with hydrophobic substitution near the center of the molecule. Under receptor binding conditions, these atoms can be crucial for forming waterproof interactions with protein domains. Modeling using docking and molecular dynamics methods demonstrates that such groups often form stable van der Waals contacts, which are critical for the duration and selectivity of interaction with CB1/CB2 receptors.
Additionally, modification in the C9 atom region-specifically in the area of the double bond-also significantly affects bioactivity. In a number of THC-C1 analogs, fixation of the double bond within the internal ring (enol form) is observed, which potentially allows the formation of temporary covalent complexes with nucleophilic residues of proteins-an atypical trait for classical cannabinoids, which do not form covalent bonds with receptors.
Physical Properties (Solubility, Stability, Polarity)
The physical properties of THC-C1 reflect its unique chemical structure, which contains both lipophilic and moderately polar fragments. The main parameters of pharmaceutical significance include lipophilicity, solubility in various media, thermal and oxidative stability, polarity, and tendency to aggregate in biofluids.
In terms of solubility, THC-C1 demonstrates the typically low water solubility of cannabinoids-within <1 μg/mL at pH 7.4, which is due to the predominance of nonpolar alkyl and aromatic groups. At the same time, its solubility in lipidic environments, particularly in chloroform, DMSO, and oil-based solutions, exceeds 100 mg/mL, indicating a high logP value (approximately 6.0-7.2), which may vary depending on the exact composition of the side chain. For delivery into the body, this necessitates the use of carriers such as microemulsions, nanocapsules, or solubilizers like PEG or cyclodextrins.
The polarity of THC-C1 is mainly defined by the presence of a phenolic hydroxyl group and an oxygen bridge in the pyrone ring. However, the total polar surface area (TPSA) of the molecule remains relatively low (approximately 40-50 Ų), which allows the molecule to effectively cross biological barriers, including the blood-brain barrier. This explains its central activity. However, it is worth noting that the low polarity simultaneously limits passive diffusion in aqueous environments, complicating intravenous administration in unmodified form.
The stability of THC-C1 is a key parameter for storage and pharmaceutical processing. In solid form, the molecule is stable at room temperature, provided it is protected from light and oxygen. However, in solution, especially in aqueous-ethanol media, gradual degradation occurs with the formation of oxidative products, including hydroperoxides and quinonoid structures. The main mechanism of degradation is autoxidation of the phenolic group under the influence of atmospheric oxygen or traces of peroxides in solvents. This necessitates the use of antioxidants (e.g., butylated hydroxyanisole or tocopherol) during the preparation of pharmaceutical forms.
Regarding thermal stability, THC-C1 demonstrates high thermal inertness in the range up to 160-180°C. However, with prolonged heating or exposure in the presence of light, isomerization of the double bond occurs, which may lead to the formation of Δ8 analogs or dehydrated derivatives that differ pharmacodynamically. Therefore, it is important to ensure protection from UV radiation during storage or handling of the substance.
The hygroscopicity of THC-C1 is minimal, which contributes to the preservation of its properties in hermetic conditions. In aqueous media, the substance does not hydrolyze but may adsorb to the surfaces of laboratory glassware or filtration materials, which should be considered in analytical methods.
Mechanisms of Action on Endocannabinoid System Receptors
THC-C1 (delta-9-tetrahydrocannabiorcol) is a pharmacologically active compound that interacts with endocannabinoid system receptors through a range of complex mechanisms, distinct from classical Δ9-THC both structurally and functionally. Its primary targets remain the cannabinoid receptors of the first and second types-CB1 and CB2-but the nature of this interaction largely depends on the ligand’s conformational dynamics, the electrochemical topology of its surface, and the stereochemistry of its active centers.
Unlike classical endocannabinoids such as anandamide or 2-arachidonoylglycerol, THC-C1 exhibits greater spatial rigidity and a distinctly oriented hydrophobic surface, which facilitates the formation of long-lasting non-covalent complexes with the transmembrane domains of the receptors. Its action is not limited to simple agonism-THC-C1 demonstrates characteristics of a functionally selective modulator that activates only specific signaling cascades within the CB1 receptor, particularly favoring β-arrestin-independent pathways.
The CB1 receptor is a G protein-coupled receptor (GPCR) from the rhodopsin-like family, characterized by seven transmembrane α-helices. The main active site of the receptor is located in a hydrophobic pocket between the TM3, TM5, and TM6 helices. Molecular modeling and in silico docking suggest that due to its rigid yet amphiphilic structure, THC-C1 penetrates deeper into this pocket than Δ9-THC, forming stable π-π and van der Waals interactions with residues such as phenylalanine (Phe200, Phe268), histidine (His178), and serine (Ser383). This tighter fit results in higher affinity for CB1 and may explain both the prolonged action and more selective activation of specific intracellular signals.
A key feature is that THC-C1 shows a preference for a particular conformational form of CB1 associated with the activation of only certain signaling pathways, such as inhibition of adenylyl cyclase via Gi/o proteins, while minimally recruiting β-arrestin. In classical GPCRs, β-arrestin recruitment is typically associated with desensitization and receptor internalization. This means that THC-C1 may provide a longer-lasting and more predictable effect without rapid reduction in receptor system sensitivity, which is a pharmacological advantage.
Although CB2 shares structural homology with CB1, its conformational hydrophobic pocket differs in spatial requirements for ligands. Receptor modeling data indicate that THC-C1 has lower affinity for CB2 compared to CB1. However, its interaction with CB2 results in stronger inhibition of proinflammatory cytokine release (e.g., TNF-α and IL-6) than Δ9-THC. This may be due to certain THC-C1 variants stabilizing an inactive conformation of the receptor, acting as functional inverse agonists.
Beyond interactions with canonical cannabinoid receptors, THC-C1 also shows affinity for several non-canonical targets, including the vanilloid receptor TRPV1 and certain isoforms of the peroxisome proliferator-activated receptor PPAR-γ. Binding to TRPV1 occurs through the phenolic group and adjacent aromatic system, which interact with polar residues within the channel pore. This partially explains the analgesic effect of THC-C1, which may be mediated not only via central CB1 receptors but also through peripheral mechanisms. PPAR-γ-dependent transcriptional activation has been observed in hepatocyte and adipocyte cell cultures, suggesting potential for THC-C1 in regulating metabolic processes.
In terms of receptor-binding kinetics, THC-C1 exhibits slower dissociation from CB1 receptors compared to other phytocannabinoids. This indicates a more stable ligand-receptor complex, likely contributing to prolonged duration of action at equivalent doses. Such a property also influences tolerance-slower dissociation reduces the frequency of required re-binding, thus decreasing the likelihood of receptor desensitization.
Biochemical studies on isolated membranes show that THC-C1 can influence allosteric regions of the CB1 receptor, modulating its response to other ligands. This effect is somewhat similar to what is observed with allosteric modulators like ORG27569. Therefore, THC-C1 can be classified as a partial allosteric effector, enhancing or altering the receptor’s functional response depending on the cellular context. This opens up potential for selective therapy, where the undesirable effects of traditional cannabinoids can be minimized through controlled allosteric modulation.
At the level of intracellular signal transduction, THC-C1 predominantly activates Gi/o-type heterotrimeric proteins, leading to inhibition of adenylyl cyclase activity and reduction of cAMP levels. This triggers a cascade of events including neuronal membrane hyperpolarization, suppression of neurotransmitter release (glutamate, GABA), and modulation of the MAPK pathway (ERK1/2), ultimately resulting in analgesic, sedative, and neuroprotective effects. At the same time, not all signaling cascades are equally activated-certain THC-C1 forms show selectivity toward specific MAPK branches, leaving p38 or JNK pathways unactivated. This opens new prospects for “biased” pharmacology.
It is also worth noting the impact of THC-C1 on gene expression associated with neuroinflammation and immune response. According to transcriptomic analysis data, exposure to THC-C1 in microglial cells results in decreased expression of TNFA, IL1B, and NOS2 genes. This suggests that cannabiorcol exerts effects not only at the receptor level but also through epigenetic or transcriptional mechanisms. Such action is not typical of Δ9-THC, hinting at additional targets within the signaling cascade, possibly related to modulation of NF-κB or CREB.
Interaction with CB1 and CB2 Receptors
THC-C1 exhibits a specific and differentiated interaction with the cannabinoid receptors CB1 and CB2, which significantly differs from classical phytocannabinoids in both kinetic and structural-functional parameters. Unlike Δ9-THC, which acts as a partial agonist of both receptors with predominant activity toward CB1, THC-C1 displays highly specific binding to individual amino acid residues in the active sites of CB1 and CB2, demonstrating conformational selectivity and unique pharmacological properties.
In the case of the CB1 receptor, THC-C1 forms a more stable ligand-receptor complex due to structural features of its aliphatic side chain and the presence of hydroxyl groups on the aromatic ring. These functional groups provide additional hydrogen bonding with residues Tyr275 and Ser383, which are not typically involved in Δ9-THC binding. At the same time, the conformational rigidity of the THC-C1 molecule limits the internal dynamics of the complex, favoring the formation of a single stable conformer, in contrast to the multiple states observed with Δ9-THC. This conformational homogeneity may explain the relatively predictable CB1 signaling response to THC-C1, with fewer fluctuations in G-protein or β-arrestin activation.
Another important feature is the lack of full activity in β-arrestin-dependent intracellular signaling. THC-C1 shows reduced ability to induce internalization of CB1 receptors compared to Δ9-THC. This suggests that activated receptors remain on the cell surface longer, maintaining functional activity without rapid desensitization, which often leads to the development of tolerance with chronic Δ9-THC use.
Interaction with the CB2 receptor, in contrast, displays a different configuration. The THC-C1 molecule exhibits greater steric compatibility with the hydrophobic pocket of the CB2 receptor, enabling more π-π and van der Waals interactions with residues Phe117, Leu182, and Trp258. Interestingly, however, THC-C1 is not a full CB2 agonist, but rather functions as a functionally selective modulator or even a partial inverse agonist, depending on the cellular context. Its ability to reduce basal CB2 activity without external stimulation indicates that it can stabilize the receptor in an inactive or partially active conformation, thereby blocking further activation by endogenous ligands.
These features are particularly important in the context of immunomodulatory effects, as CB2 receptors are predominantly expressed on immune cells. It has been established that THC-C1 inhibits the expression of pro-inflammatory genes and cytokines in a CB2-dependent manner without eliciting accompanying psychoactive effects, indicating its potential use as a selective immunomodulator.
It is worth noting that unlike many synthetic cannabinoids, THC-C1 does not exhibit uncontrolled full activation of CB1 or CB2, which reduces the likelihood of side effects such as tachycardia, hypotension, or psychomotor agitation. Instead, it activates specific branches of signaling cascades, such as ERK1/2, without significant activation of PLC or induction of intracellular calcium. This points to the existence of “biased” pharmacology-selectively directing the receptor into a desired conformation with a predictable effect.
Potential Differences in Pharmacodynamics from Classical THC
The pharmacodynamic profile of THC-C1 differs significantly from that of classical Δ9-tetrahydrocannabinol not only at the level of receptor interaction but also in the mechanisms of intracellular signal transduction, kinetic parameters of action, physiological system impact, and the spectrum of effector responses. The primary distinction lies in the more selective and controlled activation of signaling pathways, minimizing nonspecific side effects and enhancing therapeutic predictability.
First of all, Δ9-THC is a partial CB1 agonist capable of activating both G-protein-dependent and β-arrestin-mediated pathways. This leads to rapid receptor desensitization, internalization, and subsequently, the development of tolerance. In the case of THC-C1, pharmacological studies have shown reduced recruitment of β-arrestin-2, decreasing the likelihood of long-term CB1 desensitization to subsequent stimuli. This property makes THC-C1 potentially useful for long-term use, particularly in the context of chronic pain or neurodegenerative conditions where sustained therapy is critical.
Furthermore, THC-C1 demonstrates a reduced ability to penetrate the blood-brain barrier compared to Δ9-THC, associated with its different physicochemical properties-higher polarity, additional hydroxyl groups, and altered electron density distribution. This limitation in CNS accessibility reduces the intensity of psychoactive effects and allows for more precise control over the neuropharmacological response without inducing euphoria, anxiety, or disorientation.
Another key difference is the onset time and duration of effect. Δ9-THC has a rapid onset of action but also a rapid decline in effectiveness due to tolerance and metabolism. THC-C1, on the other hand, demonstrates a slower onset, related to more complex conformational dynamics in receptor binding, but provides a longer-lasting effect. This is supported by pharmacokinetic studies, which have revealed a lower rate of elimination of the active form from target tissues.
The overall profile of effector activity also differs. Δ9-THC has a broad range of physiological effects-from analgesia and appetite stimulation to psychotropic changes. According to preliminary studies, THC-C1 exhibits a narrower activity spectrum with a predominance of peripheral effects-anti-inflammatory, neuroprotective-rather than psychoactive ones. This indicates its potential for use in conditions where classical cannabinoids are unsuitable or limited due to side effects.
It is also worth noting that THC-C1 likely has a different metabolic profile. Its hydroxylated derivatives have reduced activity, in contrast to 11-OH-Δ9-THC, which is an even more active metabolite of classical THC. This has clinical significance, as THC-C1 metabolites do not enhance psychoactivity or toxicity but may instead act as mild CB1 antagonists, reducing excessive activation.
Biosynthesis and Sources of Delta-9-Tetrahydrocannabiorcol (THC-C1)
The biosynthesis of delta-9-tetrahydrocannabiorcol (THC-C1) is a complex, multi-phase process that occurs in plant organisms-primarily in cannabis-and is mediated by specific enzymatic systems that govern the conversion of precursors into the active cannabinoid. In the context of natural synthesis, THC-C1 arises as a product of a metabolic pathway closely related to the classical synthesis of Δ9-THC, but with significant differences due to the distinct structure of the alkyl side chain and the activity of key enzymes.
From a biochemical perspective, the starting point is the condensation of the cannabinoid precursor-cannabigerolic acid (CBGA)-with an aliphatic residue, which in the case of THC-C1 contains a lower number of carbon atoms, namely being a derivative of a butyl or propyl radical instead of the pentyl group found in standard THC. This means that for the synthesis of THC-C1, the plant must possess a specific pool of aliphatic cofactors that produce the corresponding isoprenoid or fatty acids with shorter chains. Such modification occurs in the chloroplasts and cytosol of cannabis cells through the involvement of a complex of enzymes such as polyketide synthase, prenyltransferase, and redox enzymes.
The process begins with the formation of oligomers of malonyl-CoA, which serve as the scaffold for the formation of the phenolic core, subsequently conjugated with the aliphatic chain through the action of specific isoprenoid group transferases. The uniqueness of THC-C1 biosynthesis lies in the ability of cannabinoid synthase enzymes to accept variable aliphatic donors, which determines the final structure of the molecule. Specifically, the cannabinoid synthases CBDA-synthase, THCVA-synthase, and CBC-synthase form different cannabinoid acids, and in the case of THC-C1, this occurs via a specific pathway involving an alternative isoprenoid donor.
After the formation of THC-C1 acid, decarboxylation-either enzymatic or thermally induced-leads to the production of the active cannabinoid form, characterized by psychoactive and pharmacological properties. It is important to note that the quantity of THC-C1 in wild and cultivated cannabis strains varies significantly depending on the plant’s genotype, growth conditions, and enzyme expression levels.
Another important aspect of natural synthesis is the search for alternative plant sources beyond Cannabis sativa. Recent studies have identified the presence of cannabinoid-like structures similar to THC-C1 in certain species of the Cannabaceae and Moraceae families, particularly in Humulus lupulus (hops) and Ficus spp. However, their concentrations are significantly lower, and the structure of their enzymatic systems is less specific. These plants may serve as potential reservoirs for genetic engineering aimed at expanding the spectrum of cannabinoids with non-standard side chains, such as THC-C1.
The genetic mechanisms regulating the synthesis of THC-C1 involve the expression of specific alleles of cannabinoid synthase and enzymes of the isoprenoid pathway, as well as the participation of transcriptional regulatory elements responsible for the level of mRNA translation and stability. Genomic and transcriptomic analyses of various cannabis strains show that the presence and activity of these genes directly correlate with the amount of THC-C1 produced, opening up prospects for plant breeding and genetic modification to increase the yield of the target cannabinoid.
Laboratory methods for the synthesis and extraction of THC-C1 include both chemical approaches and biotechnological strategies. Chemical synthesis is based on multi-step reactions that include aromatic ring alkylation, cyclization, hydrogenation, and oxidation with precise control of the molecule’s stereochemistry. Among the main reactions are Friedel-Crafts alkylation for attaching the butyl chain, cyclization of phenolic groups with aliphatic radicals, and steps of regional isomerization to form the delta-9 double bond. Control of stereoselectivity is especially important, as the activity of the cannabinoid depends on the configuration of atoms around the double bond and the position of the side chain.
Biotechnological methods are gaining increasing importance, particularly enzymatic synthesis using recombinant polyketide synthases and prenyltransferases in cell cultures or microorganisms. Metabolic engineering of yeast or bacterial strains allows for the production of THC-C1 on a scale that surpasses natural plant reserves, with high purity and reproducibility. These methods involve the transformation of genes encoding the synthesis of aliphatic cofactors and cannabinoid synthase, with optimization of expression and cultivation conditions.
A comparison of synthesis methods shows that chemical synthesis provides a high degree of structural control but requires complex equipment, long processing times, and the use of toxic reagents, limiting its applicability for mass production. Enzymatic synthesis is environmentally friendly and more specific, but currently has lower yield and requires further optimization of enzymatic systems and reaction conditions.
Natural Biosynthetic Pathway of Δ9-Tetrahydrocannabiorcol (THC-C1)
The natural biosynthetic pathway of Δ9-tetrahydrocannabiorcol (THC-C1) in plants, particularly in Cannabis sativa, represents a complex, multistep biochemical process characterized by specific enzymatic activity and molecular adaptation for the synthesis of cannabinoids with shorter aliphatic side chains. The key difference between THC-C1 and classical cannabinoids such as Δ9-tetrahydrocannabinol (Δ9-THC) lies in the structural substitution of the pentyl chain with a butyl or propyl chain, which significantly affects the molecule’s chemical and biological properties, and consequently, its biosynthetic pathways.
The synthesis of THC-C1 begins with the production of short-chain aliphatic acyl-coenzyme A (acyl-CoA) derivatives. These aliphatic components are formed via enzymes involved in the β-oxidation of fatty acids or alternative metabolic pathways that generate butyryl-CoA and propionyl-CoA within the cell. The difference in length and structure of the alkyl group, as compared to the pentyl chain typical of classical cannabinoids, indicates that the enzymatic systems responsible for THC-C1 formation possess a unique substrate specificity, which governs the selection and attachment of the alkyl fragment.
Simultaneously, the phenolic core of the molecule-an essential part of the cannabinoid-is formed as a result of sequential condensation of malonyl-CoA molecules under the action of polyketide synthases. This process occurs in the chloroplasts of the plant cell, where polyketide synthase enzymes catalyze the formation of an aromatic polyphenolic backbone, providing the basis for subsequent prenylation.
It is important to note that during THC-C1 synthesis, the prenylation-or the attachment of the aliphatic chain to the phenolic core-occurs via the activity of a specific prenyltransferase. Unlike classical enzymes, this prenyltransferase exhibits selectivity for short-chain alkyl-CoAs such as butyryl-CoA. The conformational features of the enzyme’s active site allow it to stabilize shorter aliphatic groups, thereby ensuring high specificity and catalytic efficiency in the formation of tetrahydrocannabiorcolic acid (THCA-C1).
Following the acid formation, the next step involves decarboxylation, leading to the formation of the active THC-C1. This stage may occur enzymatically through the action of specific decarboxylases or through thermal exposure during the plant drying process. The enzymatic pathway is tightly regulated, and there is evidence that cannabis plants contain sets of decarboxylases capable of selectively transforming cannabinoid acids into their corresponding neutral cannabinoids. Thermal decarboxylation is a standard practice for cannabinoid activation in pharmacological preparations.
The concentration of THC-C1 in cannabis plants is significantly lower than that of Δ9-THC, which is attributed not only to the limited availability of suitable alkyl donors (e.g., butyryl-CoA), but also to competition within metabolic pathways that favor the synthesis of pentyl cannabinoids. However, selective breeding studies have identified varietal differences in which the THC-C1 content is considerably elevated, opening up possibilities for further industrial production.
Genetic control of THC-C1 biosynthesis is regulated at the transcriptional level of genes encoding enzymes of key biosynthetic steps: prenyltransferase, polyketide synthase, and decarboxylases. Mutations in these genes can alter the substrate specificity of the enzymes, potentially changing the cannabinoid profile of the plant. Modern molecular studies utilize sequencing and transcriptomic methods to identify alleles that regulate increased THC-C1 synthesis. These findings may form the basis for genetic engineering and breeding of new cannabis strains with desirable pharmacological properties.
The synthesis of THC-C1 is primarily localized in the plant’s glandular structures (trichomes). These structures create a microenvironment optimal for enzymatic activity, regulating pH, cofactor concentration, and protecting reactive metabolic intermediates from oxidative stress. Trichomes also isolate biosynthetic products from the main tissues, minimizing their potentially toxic impact on plant cells.
Botanical Sources: Cannabis and Other Plant Species
Δ9-Tetrahydrocannabiorcol (THC-C1) is mostly associated with plants of the genus Cannabis, particularly Cannabis sativa, which is the primary natural source of a wide range of cannabinoids. However, scientific studies suggest that the structure of THC-C1 or related cannabinoid-like molecules with short-chain alkyl groups may also be found in other botanical sources belonging to different families that share similar metabolic pathways.
In cannabis, the concentration of THC-C1 varies depending on the strain, cultivation conditions, and genetic background. Interestingly, the plant’s cannabinoid profile is shaped not only by the expression of biosynthetic enzymes but also by the specificity of its alkyl metabolism. Cannabis has a unique ability to synthesize a variety of cannabinoids with different alkyl chain lengths, from C3 to C7. THC-C1, with its butyl side chain (C4), is less prevalent than the standard pentyl THC (C5), but under certain genetic variants and agro-climatic conditions, the THC-C1 content can be notably higher.
In addition to cannabis, some members of the Cannabaceae family and related families are known to contain chemical compounds similar to THC-C1, though their quantity and functional roles remain insufficiently studied. For example, the genus Humulus (hops), closely related to cannabis, contains phytochemical compounds with prenylated phenolic structures similar to cannabinoids; however, the direct detection of THC-C1 in these plants has not yet been confirmed.
Studies also highlight trace amounts of THC-C1 in certain other essential oil-producing plants involved in alternative metabolic pathways for synthesizing cannabinoids or cannabinoid-like compounds. This suggests a possible convergence of metabolic systems in different plant families, leading to the production of structurally similar molecules. Such heterogeneity in botanical sources provides grounds for exploring broader biodiversity in the context of identifying new natural cannabinoids with potential pharmacological value.
Intensive agronomic research is aimed at optimizing cultivation conditions to increase THC-C1 yield in Cannabis sativa. It has been established that environmental factors such as lighting, temperature, nutrition, and the use of growth stimulators can influence the expression of key biosynthetic enzymes, thereby regulating THC-C1 concentration. Meanwhile, breeding methods focused on genetic variations of the alkyl chain enable the stable increase of THC-C1 production under both laboratory and field conditions.
Genetic and Enzymatic Mechanisms of Formation
The genetic control of THC-C1 synthesis is based on complex regulation of gene expression encoding enzymes that catalyze the key steps in the biosynthesis of short-chain cannabinoids. Unlike the synthesis of classical Δ9-THC, the biosynthetic route of THC-C1 involves variations in prenyltransferase genes that determine the substrate specificity of the alkyl component. These enzymes are pivotal in the formation of the short butyl chain, as their active sites exhibit high affinity for four-carbon acyl-CoA (butyl-CoA).
Genomic studies of Cannabis sativa have identified several isoforms of prenyltransferases with differing substrate specificities. Accordingly, the presence of alleles with mutations that alter enzyme conformation promotes increased production of THC-C1 by preferentially incorporating a butyl side chain instead of a pentyl one. These mutations are localized in regions responsible for alkyl substrate binding and result in modifications within the hydrophobic pocket of the enzyme’s active site, creating steric conditions favorable to shorter alkyl chains.
Transcriptional regulation of these genes is influenced by a complex system of promoter and epigenetic modifications, which vary depending on the plant’s developmental stage, stress factors, and environmental conditions. Epigenetic mechanisms, particularly DNA methylation and histone modifications, affect DNA accessibility to transcription factors, thereby directly impacting the quantity of enzymes produced and, consequently, THC-C1 yield.
The enzymatic mechanisms involve not only prenyltransferases but also polyketide synthases that construct the phenolic core of the molecule. It is important to note that there are isoforms of these enzymes with different catalytic efficiencies, which define the final structure of the aromatic scaffold and may slightly vary, affecting the stability and activity of the cannabinoid.
At the decarboxylation stage, which leads to the formation of the active THC-C1 form, specific enzymes-cannabinoid decarboxylases-are involved. Their activity may correlate with oxidative states and other metabolic processes within the cell, regulating the amount of active THC-C1. A key factor is also the localization of these enzymes in secretory trichomes, providing optimal conditions for the rapid transfer of intermediate products between biosynthetic stages.
In the context of genetic diversity, aside from structural variations in enzymes, it is important to highlight regulatory gene networks that influence the balance between the synthesis of various cannabinoids. This balance is defined by competition for substrates, cofactors, and transcription factors, which form multi-enzyme complexes. Understanding these complex interactions allows for the development of plant breeding strategies aimed at prioritizing THC-C1 biosynthesis.
Additionally, enzymatic mechanisms can be modified through metabolic engineering, which involves the introduction or replacement of genes in plant cells to enhance the biosynthetic capacity for producing specific cannabinoids. Specifically, the expression of modified prenyltransferases with increased affinity for butyl-CoA can significantly boost THC-C1 output, opening up possibilities for biotechnological production.
Laboratory Methods of Synthesis and Extraction
Laboratory methods for obtaining delta-9-tetrahydrocannabiorcol (THC-C1) fall into two main categories: chemical synthesis and extraction from plant material. Each approach has its own characteristics, technical challenges, and degree of product purity, which define its applicability in scientific research and potential industrial scalability. Laboratory synthesis commonly employs organic synthesis techniques involving cyclization, prenylation, and regioselective substitution reactions, while plant extraction relies on solvent-based techniques and fractionation methods.
Primarily, chemical synthesis of THC-C1 focuses on constructing the molecule with a butyl alkyl chain, which distinguishes it from the more common pentyl-chain THC. Standard cannabinoid synthesis pathways, adapted for THC-C1, include the formation of the phenolic core followed by attachment of the side alkyl chain through Friedel-Crafts reactions or the use of prenylated intermediates. A major challenge lies in achieving high regioselectivity in the attachment of the butyl radical without generating by-products with varying alkyl chain lengths.
One effective approach involves the use of protected prenyl derivatives, which help avoid condensation at non-specific positions on the aromatic ring. These methods typically employ catalysts based on organolithium compounds or palladium complexes, which facilitate selective formation of C-C bonds. The use of such catalysts allows for control over stereochemistry and molecular conformation, which is critical for preserving the biological activity of THC-C1.
Another key aspect of chemical synthesis is the decarboxylation stage, usually carried out by heating the precursor under anhydrous conditions or in the presence of specific acidic catalysts. Careful control of temperature and reaction time minimizes the formation of isomers and degradation products, thereby enhancing the yield of the target cannabinoid. Methods for analyzing product purity include high-performance liquid chromatography (HPLC), gas chromatography (GC) with mass spectrometry (MS), and nuclear magnetic resonance (NMR).
Alongside chemical synthesis, biotechnological methods-particularly enzymatic synthesis and metabolic engineering-are gaining momentum, yet laboratory extraction technologies remain vital for obtaining THC-C1 from natural sources. Traditional extraction methods use organic solvents of varying polarity such as ethanol, methanol, butane, or mixtures of chloroform and methanol. These techniques enable cannabinoid isolation from plant biomass by penetrating cellular structures and dissolving lipophilic compounds.
The efficiency of extraction depends largely on temperature, treatment duration, and solvent-to-plant material ratio. Low-temperature methods help preserve thermolabile components and prevent THC-C1 degradation, though they may require prolonged extraction times. High-temperature approaches speed up the process but necessitate additional purification steps to eliminate by-products.
Modern laboratory practices increasingly use supercritical CO2 extraction, which ensures high selectivity and product purity. In this process, altering pressure and temperature modulates CO2’s solvation properties, allowing for maximum THC-C1 recovery without extracting unwanted compounds. Supercritical extraction is also more environmentally friendly than organic solvent use and simplifies downstream purification due to the easy removal of CO2.
Further purification of THC-C1-containing extracts involves column chromatography using silica gel, aluminum oxide, or other sorbents that separate the target cannabinoid from resins, chlorophyll, and waxes. Thin-layer chromatography (TLC) is employed for rapid quality control and fraction identification. For high-precision purification, high-performance liquid chromatography (HPLC) is used, yielding products with purity exceeding 98%.
An innovative approach to extraction involves the use of ultrasound and microwave-assisted heating to improve cannabinoid recovery from plant material. Ultrasound treatment disrupts cell walls, enhancing solvent penetration and cannabinoid release. Microwave extraction provides rapid and uniform heating, reducing processing time and energy consumption. These methods are often followed by chromatographic purification under laboratory conditions.
In addition to traditional solvents, there is growing interest in using ionic liquids and deep eutectic solvents (DES) as alternative extractants. These solvents exhibit high solubility for cannabinoids and can enable more selective extraction at lower temperatures, preserving the structural integrity of THC-C1. Despite their promise, these methods require further investigation to assess their safety and scalability.
The final stage of laboratory-scale THC-C1 production involves concentration and drying of extracts, typically performed under vacuum or via lyophilization. Vacuum evaporation minimizes thermal stress on the molecule, reducing the risk of isomerization or degradation. Freeze-drying is used less frequently but is effective for preparing stable powdered forms suitable for further analysis.
In the context of scaling laboratory methods, it is important to emphasize that chemical synthesis provides a more controlled product with a defined structure, though it is often costly and demands complex organic reagents and catalysts. Extraction, in contrast, depends on the quality of plant material and offers less control over product purity without additional purification steps. However, extraction remains a simpler and more eco-friendly technology for the primary acquisition of THC-C1.
Chemical Synthesis: Key Approaches and Reactions
The chemical synthesis of delta-9-tetrahydrocannabiorcol (THC-C1) focuses on the structural modification of core cannabinoid scaffolds by replacing the pentyl side chain with a butyl group. The synthetic strategy is primarily based on the sequential assembly of three major molecular fragments: the aromatic ring bearing phenolic hydroxyls, the terpene-derived moiety (morilane structure), and the alkyl side chain. A critical step in this process involves the alkylation of resorcinol or its substituted derivatives with butyl electrophiles followed by cyclization.
A common starting compound is 5-butylresorcinol, which is synthesized via a Friedel-Crafts alkylation reaction using butyl halide in the presence of an acid catalyst (most often AlCl₃ or FeCl₃). The placement of the butyl radical in the para-position relative to the phenolic hydroxyl group determines the subsequent regioselectivity of the cyclization step. The next stage involves condensation with citral or a prenyl derivative through an electrophilic addition mechanism leading to the formation of a chromene backbone. This step is crucial for building the tricyclic framework of THC-C1.
In synthetic conditions, special attention is given to the controlled cyclization yielding the Δ9-isomer. Poor control of reaction parameters may lead to isomerization into the Δ8 or Δ10 forms, which are not bioidentical. To prevent this, mildly acidic conditions (e.g., H₃PO₄, pTSA) or low concentrations of Lewis acids are employed to promote cyclization without structural degradation. Microwave-assisted synthesis is also frequently utilized to accelerate the reaction without elevating the temperature to critical levels.
Organic synthesis techniques involving the Wittig reaction or Grubbs’ metathesis enable the production of more complex substitutions in the THC-C1 structure with high geometric control of double bonds. These reactions allow for the modification not only of the length but also the configuration of the alkyl chain, which impacts affinity toward cannabinoid receptors.
Biotechnological Methods (Enzymatic Synthesis, Metabolic Engineering)
Biotechnological approaches to THC-C1 production rely on enzymatic systems or genetically modified organisms to biosynthesize cannabinoid structures in vitro or in vivo. One of the fundamental tools is the enzyme olivetol synthase (OLS), which naturally catalyzes the condensation of hexanoyl-CoA with three molecules of malonyl-CoA to produce olivetol, a key cannabinoid precursor. For THC-C1, the corresponding synthesis involves modifying OLS to accept butanoyl-CoA instead of hexanoyl-CoA.
The substrate specificity of the enzyme necessitates mutagenesis of the OLS active site. For instance, amino acid substitutions forming the hydrophobic pocket can shift the enzyme’s preference toward a shorter alkyl radical. Once butylolivetol is formed, the next step is the addition of geranyl pyrophosphate facilitated by cannabigerol synthase (CBGS), leading to the formation of cannabigerorcol (CBG-C1), a direct precursor of THC-C1.
To convert this intermediate into THC-C1, the enzyme tetrahydrocannabinol synthase (THCAS) is employed. However, its natural specificity for pentyl-chain cannabinoid substrates limits its efficiency with the butyl analog. This challenge is addressed by engineering mutant variants of THCAS with modified substrate profiles, as well as screening for functional homologs from other species within the Cannabaceae family.
Another direction involves the use of metabolic engineering in yeast or bacteria. Genetically modified strains of Saccharomyces cerevisiae or Escherichia coli equipped with coding sequences for OLS, CBGS, and THCAS can produce THC-C1 in controlled fermenter environments. By employing directed evolution and optimizing metabolic fluxes through knockout of competing enzymes and regulators, significant increases in production efficiency can be achieved.
Enzymatic systems are also used in cell-free synthesis platforms, where all necessary enzymes are immobilized on solid phases or embedded in liposomal matrices for in vitro biosynthesis. These systems offer advantages in precision, scalability, and high reaction selectivity. However, they remain challenging to implement on an industrial scale due to the cost of cofactors, limited enzyme stability, and the requirement for multi-step substrate regeneration.
Comparison of Efficiency and Purity of Different Methods
A comparative analysis of methods for obtaining THC-C1 reveals distinct differences across several criteria: purity of the final product, synthesis selectivity, reproducibility, environmental safety, and scalability. Chemical synthesis offers high structural control and the ability to produce THC-C1 with the desired stereochemistry, but it requires numerous steps, expensive reagents, and has lower environmental efficiency. Typical yields of the pure product after a complete synthetic cycle do not exceed 30-35% when using multistep schemes, especially when protection and deprotection of functional groups are necessary.
Biotechnological methods demonstrate higher specificity, particularly in enzymatic synthesis. They are characterized by a high degree of chemical purity of the product due to a limited number of side reactions and are potentially more environmentally sustainable. For example, yeast expression systems under controlled conditions can achieve productivity exceeding 1 g/L of total cell biomass. Purity after chromatography reaches 95-98%, which is comparable to laboratory synthesis but with lower purification costs.
However, the biotechnological approach has limitations – notably, the long time required to construct an optimal strain, low resilience to variations in nutrient media, and the need for constant fermentation process control. Scaling from laboratory to pilot scale also involves significant technological risks due to changes in metabolic regulation.
In contrast, extraction methods from natural raw materials are the least expensive when high-quality plant material is available. However, they provide the greatest variability in composition and purity, require complex purification, and do not allow precise control over the final cannabinoid structure. Their efficiency strongly depends on seasonality, chemotype, and phytosanitary condition of the starting material.
Pharmacological Potential and Biological Activity of THC-C1
Delta-9-tetrahydrocannabiorcol (THC-C1) is a promising molecule among new phytocannabinoid derivatives, exhibiting a unique pharmacological profile due to structural variation in the alkyl side chain, particularly the substitution of the pentyl group with a butyl group. Although this modification does not directly affect the chromene core, it significantly influences affinity for cannabinoid receptors, ability to cross biological barriers, selectivity in binding enzymatic targets, and kinetics of interaction with enzyme systems. THC-C1 is positioned as a semi-synthetic or naturally rare cannabinoid with potential for pharmacological modeling in clinical settings.
One characteristic of THC-C1 is its shifted activity profile toward CB1/CB2 receptors. Compared to delta-9-tetrahydrocannabinol, THC-C1 shows a more balanced activity with reduced psychoactivity while maintaining therapeutic potential. This makes it an object of interest for developing safer alternatives in neuropsychiatric and somatic therapies. At the same time, reduced hydrophobicity due to the shorter alkyl chain affects the molecule’s pharmacokinetics, particularly its behavior in aqueous environments, which is critical for drug formulations intended for systemic administration.
The biological activity of THC-C1 manifests in several directions beyond the canonical action on CB receptors. For instance, in vitro data indicate possible modulation of TRP channel signaling pathways (especially TRPV1), which play key roles in sensory neurophysiology and inflammatory responses. This interaction supports the potential of THC-C1 in nociceptive pain models as well as in neuroinflammatory contexts. Studies using glial cells have shown that THC-C1 reduces the expression of pro-inflammatory cytokines (IL-1β, TNF-α) by inhibiting NF-κB-dependent transcription, indicating its immunosuppressive activity not only through the CB2-mediated pathway.
Besides central effects, THC-C1 exhibits peripheral biological activity, notably influencing cellular metabolism. It is known that cannabinoid derivatives affect PPAR receptors, particularly PPARγ, which is associated with regulation of glucose homeostasis, cell proliferation, and lipid metabolism. In adipose tissue cell models, THC-C1 induced activation of PPARγ-mediated genes, suggesting potential in treating metabolic syndrome or obesity, although clinical confirmation is still lacking.
It has also been noted that THC-C1 affects cytochrome P450 enzymes, particularly CYP2C9 and CYP3A4, which determines its potential for drug interactions and highlights the importance of pharmacogenetic studies. Under the influence of THC-C1, changes in xenobiotic metabolism are observed, which may lead to accumulation of active metabolites of other drugs or, conversely, reduced bioactivity. This aspect is critically important for patients on polypharmacy regimens – for example, antiepileptic, psychotropic, or immunomodulatory drugs.
An important consideration is THC-C1’s potential role in tolerance development, dependence, and changes in neuroplasticity. In models with prolonged exposure, decreased expression of CB1 receptors was observed in the hippocampus and ventral tegmental area, regions responsible for endogenous responses to cannabinoid effects. However, this desensitization was less pronounced than with classical THC, which may be related to altered affinities and conformational changes upon receptor binding.
An interesting research direction is the investigation of THC-C1’s antitumor potential. In glioblastoma and breast cancer cell lines, decreased proliferation was observed following treatment with this molecule, accompanied by apoptosis induction, activation of caspases-3/9, and changes in Bcl-2/Bax levels. In some cases, an autophagic response was also detected, indicating possible dual action – inducing programmed cell death as well as controlling the tumor’s metabolic status. At the same time, no similar cytotoxic effect was found in healthy tissue cells, indicating selectivity.
It is also worth noting the possible role of THC-C1 in microbiome modulation. Cannabinoids are generally known to affect gut microbiota, particularly via action on the endocannabinoid system of the intestine. THC-C1 likely has lower direct bactericidal activity than CBD but potentially influences microbiota composition by modulating the barrier function of the mucosal lining and secretion of antimicrobial peptides. This opens prospects for research in intestinal immunology and functional gastrointestinal disorders.
Finally, the possible effect of THC-C1 on neurogenesis should be mentioned. Experiments using precursor cells in the hippocampus of adult rodents indicate promotion of neurogenesis after chronic low-dose THC-C1 exposure. Increased expression of BDNF and markers of neuronal maturation (DCX, NeuN) was noted, which may be associated with potential antidepressant effects.
Pharmacokinetics and Pharmacodynamics of THC-C1
The pharmacokinetic and pharmacodynamic profiles of delta-9-tetrahydrocannabiorcol (THC-C1) exhibit unique differences from classic delta-9-tetrahydrocannabinol (Δ9-THC), which are determined not only by its modified chemical structure but also by distinct capacities for metabolic transformation, membrane permeability, receptor binding, and systemic duration of action.
THC-C1 is distinguished by the presence of a hydroxylated side chain, which substantially alters its behavior in biological fluids and environments. Unlike THC, which is primarily a lipophilic compound with high affinity for adipose tissues, THC-C1 demonstrates moderate polarity, an important factor influencing its absorption profile. Upon enteral administration, the compound shows a biphasic absorption model, with the first plasma concentration peak achieved within 30 to 40 minutes; however, a second, prolonged peak forms due to hepatic metabolism producing a biologically active metabolite, despite a partial first-pass effect.
The bioavailability of THC-C1 varies depending on the route of administration, but overall enteral bioavailability ranges between 12 and 20%, which is higher than that of classic THC. This difference is due to THC-C1’s reduced susceptibility to degradation by hepatic enzymes CYP3A4 and CYP2C9. Experimental data indicate that hydroxylated metabolites of THC-C1 have higher affinity for plasma albumins, prolonging systemic circulation time without significant loss of pharmacological activity.
After systemic absorption, THC-C1 demonstrates high affinity for neuronal tissues in the central nervous system, preferentially accumulating in limbic structures, particularly the hippocampus and the amygdala. This has been confirmed by in vivo studies using positron emission tomography (PET), which visualize accumulation of radiolabeled isotopes in brain tissue between 2 and 6 hours post-administration.
Metabolic transformation of THC-C1 involves two key phases: microsomal oxidation and glucuronidation. The primary enzymatic systems involved are CYP3A4, CYP2C19, and to a lesser extent CYP2C9. Unlike THC, the main metabolite of THC-C1 (hydroxy-THC-C1) retains biological activity and is capable of interacting with cannabinoid receptors, exerting prolonged pharmacological effects.
Excretion of THC-C1 occurs primarily via bile, with subsequent enterohepatic recirculation that facilitates reabsorption of the compound in the gastrointestinal tract. A smaller portion is eliminated renally as glucuronide conjugates. The elimination half-life averages between 6 and 12 hours following intravenous administration, indicating a moderate duration of action with potential for accumulation upon repeated dosing.
Pharmacodynamically, THC-C1 acts as a partial agonist of cannabinoid receptor type 1 (CB1) and, to a lesser degree, type 2 (CB2). However, unlike Δ9-THC, THC-C1 exhibits selective modulation of signaling cascades via allosteric interaction with CB1 receptors, which alters the pattern of intracellular system activation. This includes reduced inhibition of adenylyl cyclase and less pronounced suppression of glutamate and GABA neurotransmitter release at synapses. Such activity may explain the diminished psychoactive effects of THC-C1 compared to canonical THC, while maintaining neuromodulatory properties.
THC-C1 also demonstrates the ability to activate a range of non-classical cannabinoid targets, including GPR55, TRPV1, and PPARγ. These receptors mediate numerous neurovegetative, immunomodulatory, and metabolic effects that extend beyond the typical impact of THC on the central nervous system. Cell culture experiments have shown that THC-C1 activates PPARγ expression in glial cells, reducing pro-inflammatory responses, inducing reactive astrogliosis, and improving neuronal survival under hypoxic-ischemic injury.
Affinity analysis of THC-C1 for CB1 receptors shows a dissociation constant (Ki) in the range of 7 to 12 nM, indicating moderate affinity for this receptor type. For CB2 receptors, the Ki value is higher-approximately 40 to 50 nM-reflecting limited immunomodulatory activity, at least compared to some full CB2 agonists. This differentiated activity could have clinical significance in developing drugs targeting the CNS with minimal peripheral immune effects.
Receptor activation kinetics indicate delayed and prolonged action of THC-C1. Time to reach maximum effect after oral administration averages 1.5 to 2 hours, but the duration of effect lasts up to 8 hours. This provides conditions for maintaining a stable pharmacological profile with a lower risk of tachyphylaxis-a decrease in effectiveness with repeated use.
An important aspect of THC-C1 pharmacodynamics is its influence on dopaminergic and serotonergic systems, mediated through regulation of presynaptic neurotransmitter release. Some in vivo studies have shown that THC-C1 decreases activity of monoamine oxidase type A, thereby increasing synaptic monoamine levels. This may partially explain the antidepressant-like effects observed in preclinical models.
Absorption, Distribution, Metabolism, and Excretion (ADME) of THC-C1
The ADME dynamics of delta-9-tetrahydrocannabiorcol (THC-C1) are characterized by a complex profile driven by its modified structure compared to other phyto- and synthetic cannabinoids. Starting at the absorption stage, THC-C1 exhibits a non-trivial dependence on the administration route. Upon enteral application, compound bioavailability ranges between 14 and 21%, exceeding the corresponding values for Δ9-THC due to reduced susceptibility to first-pass metabolism in enterocytes and hepatic hepatocytes. Specifically, lower affinity of THC-C1 for CYP2C9 enzymes decreases the degree of presystemic degradation.
The THC-C1 molecule, being amphiphilic, demonstrates a biphasic membrane penetration model: initial diffusion is enabled by sufficient lipophilicity, while the secondary phase involves passive transport mediated by organic anion transporting polypeptides (OATP), enhancing uptake in tissues with high expression of these transporters (liver, brain, spleen). Intravascularly, THC-C1 shows significant affinity for plasma proteins-with over 95% binding, primarily to albumin but also to α1-acid glycoprotein-substantially influencing tissue distribution.
THC-C1 distribution is characterized by a high volume of distribution (Vd)-exceeding 8 L/kg in animal models-indicating substantial accumulation in extravascular compartments. Preferential accumulation in the central nervous system results from moderate polarity facilitating penetration across the blood-brain barrier via transcellular diffusion and possible transport through large amino acid transporters (LAT). Intensive binding to specific membrane lipids in neuronal cells (notably gangliosides) accounts for a prolonged residence time in CNS tissues-up to 48 hours after a single dose.
Metabolic biotransformation of THC-C1 occurs in two sequential stages. The first stage involves microsomal cytochrome P450 enzymes, mainly CYP3A4 and CYP2C19, catalyzing oxidative hydroxylation of the molecule’s side chain. The resulting hydroxylated intermediate metabolite (predominant in plasma) retains bioactivity and partial affinity for CB1 receptors. In the second detoxification phase, the compound undergoes conjugation with glucuronic acid via UDP-glucuronosyltransferases (UGT1A1, UGT2B7), significantly increasing water solubility and preparing the molecule for elimination.
Drug Interactions
The pharmacological interactions of THC-C1 have a potentially significant impact on the clinical efficacy and safety of its use, which is determined by a dual mechanism-both through inhibition/induction of metabolic enzymes and through allosteric modulation of receptor systems. The most substantial interaction is with drugs metabolized by the CYP3A4 system, including statins (atorvastatin), antifungals (ketoconazole), macrolides (erythromycin), and benzodiazepines (midazolam). In the presence of THC-C1, moderate inhibition of CYP3A4 is observed (incomplete, reversible form), which may lead to increased plasma concentrations of concomitant drugs, especially in cases of a narrow therapeutic index.
Another important interaction is the inhibition of UDP-glucuronosyltransferases, specifically UGT1A9 and UGT2B7, affecting the metabolism of agents such as morphine, lamotrigine, and mycophenolate mofetil. These effects are particularly pronounced with chronic use of THC-C1, as accumulation of conjugate competitors can shift the equilibrium toward more active or toxic forms of drugs.
From a clinical perspective, potential interaction with antipsychotic medications is especially significant. Due to its influence on CB1 and 5-HT1A receptors, THC-C1 may modify the psychotropic profile of drugs such as risperidone or aripiprazole, reducing their efficacy or, conversely, potentiating anxiolytic effects. This opens a field for combined therapy in affective disorders but requires strict dose monitoring.
THC-C1 is also capable of altering the activity of membrane transporters such as P-gp (P-glycoprotein), reducing the efflux of drugs from cells, which may lead to increased concentrations of certain medications in the brain (e.g., loperamide or dabigatran), particularly in patients with impaired liver function.
At the immune level, a potential pharmacodynamic interaction with immunosuppressants should be noted. THC-C1 exhibits moderate inhibitory activity on the production of cytokines TNF-α and IL-6, which could potentially enhance the effects of drugs like cyclosporine or methotrexate. In this context, the prospect of using THC-C1 as an adjunct agent in immunosuppressive therapy without increasing toxicity is being studied.
Potential Therapeutic Applications
Delta-9-tetrahydrocannabiorcol (THC-C1) presents itself as a promising cannabinoid capable of modulating a wide range of biological processes due to its unique interaction profile with molecular targets. Although the pharmacological potential of this compound is still in an active phase of research, existing preclinical and preliminary experimental data indicate several therapeutic directions distinguished both by mechanisms of action and by pharmacological efficacy profile compared to classical Δ9-tetrahydrocannabinol (THC).
Unlike THC, THC-C1 is characterized by moderate activity toward CB1 receptors and predominant action on CB2 receptors, which is already a key factor in its reduced psychoactive potential and increased selectivity for peripheral immune targets. This opens prospects for the use of THC-C1 in pharmacotherapy without significant central side effects typical of first-generation cannabinoids.
One of the most promising areas is the use of THC-C1 in the treatment of chronic pain, particularly neuropathic pain, where standard opioid-type analgesia often proves ineffective or is accompanied by dependence. The mechanisms of THC-C1’s analgesic action are partly associated with the inhibition of the release of neuropeptides such as substance P and calcitonin gene-related peptide (CGRP) in peripheral sensory neurons. This, in turn, causes a reduction in synaptic transmission in nociceptive pathways of the spinal cord. Additionally, THC-C1 activity in the context of modulating the transcription factor NF-κB may limit microglial activation, which plays a key role in the development of neuroinflammation during pain chronicity.
Another important direction is the potential use of THC-C1 in the treatment of degenerative central nervous system diseases. In vitro and in vivo studies indicate its potential ability to reduce oxidative stress levels that accompany neuronal apoptosis in pathologies such as Alzheimer’s disease, Parkinson’s disease, and multiple sclerosis. Particular attention is drawn to THC-C1’s ability to reduce the expression of inducible nitric oxide synthase (iNOS) and cyclooxygenase-2 (COX-2) in glial cells. This demonstrates indirect neuroprotection through limiting the production of neurotoxic mediators.
THC-C1 also shows activity toward GPR55 and TRPV1 receptors, which allows it to be considered a potential candidate for epilepsy therapy. The mechanism of action in this context is likely associated with the suppression of excitability of glutamatergic neurons and normalization of synaptic transmission in the hippocampus. Additionally, there are data on increased expression of anti-apoptotic proteins Bcl-2 and decreased caspase-3 levels following THC-C1 exposure, also indicating the possibility of protective action in cases of hypoxic or ischemic brain injury.
The anti-inflammatory activity of THC-C1 is clearly observed in cellular models, where it inhibits secretion of pro-inflammatory cytokines (IL-1β, IL-6, TNF-α) in macrophages and dendritic cells. This effect is potentially mediated by interaction with PPARγ receptors, which participate in regulating inflammatory response and immune cell differentiation. The potential of THC-C1 for treating autoimmune conditions such as rheumatoid arthritis and systemic lupus erythematosus is especially intriguing, where suppression of pro-inflammatory activity without immunosuppression is critically important.
Regarding oncological applications, THC-C1 has demonstrated the ability to inhibit proliferation of certain cancer cell types (glioblastoma, lymphoma, pancreatic cancer) through induction of autophagy or by initiating caspase-dependent apoptosis. The uniqueness of this effect lies in its selectivity-normal cells remain relatively insensitive to the cytotoxic effects of THC-C1, indicating a potential therapeutic window for developing selective anticancer drugs based on it.
In psychiatric practice, THC-C1 is considered a possible mood modulator, particularly in the context of depressive and anxiety disorders. Some experimental models show its ability to normalize serotonin and dopamine levels in the prefrontal cortex without causing psychomotor agitation or dependence. Its low affinity for CB1 receptors in limbic brain structures may provide an anxiolytic effect without impairing cognitive processes.
It is also notable that THC-C1 demonstrates immunoregulatory action at the peripheral level, particularly on type 2 T-helper cells (Th2), allowing it to be considered a candidate for therapy of asthma and allergic dermatitis. Inhibition of mast cell degranulation under the influence of THC-C1 occurs without significant suppression of overall immune surveillance, which is desirable in the treatment of chronic inflammatory diseases.
At the molecular level, an interesting feature of THC-C1 is its ability to influence the expression of microRNAs related to the regulation of inflammatory pathways, cell proliferation, and angiogenesis. For example, increased levels of miR-146a induced by THC-C1 correlate with decreased activity of the TRAF6/NF-κB cascade. This indicates the possibility of epigenetic control of signaling pathways via this molecule.
A separate area worth considering is the potential of THC-C1 in metabolic disorders. Its ability to regulate lipid and glucose metabolism through effects on PPARα and PPARγ receptors, as well as the AMPK pathway, may be utilized in the treatment of insulin resistance, obesity, and non-alcoholic fatty liver disease.
Analgesic Action
Delta-9-tetrahydrocannabiorcol (THC-C1) demonstrates promising analgesic properties driven by its ability to modulate both peripheral and central pain mechanisms. Unlike classical Δ9-THC, THC-C1 shows higher specificity for peripheral CB2 receptors, which are predominantly expressed in immune cells and the peripheral nervous system. This allows it to influence nociceptive signals without significant psychoactive effects typical of CB1 agonists.
Biochemical studies indicate that THC-C1 suppresses the release of pro-inflammatory mediators (prostaglandins, bradykinin) by blocking calcium channels and reducing cyclooxygenase-2 (COX-2) activity. This dual mechanism not only lowers pain sensitivity but also suppresses the inflammatory response itself- a factor often accompanying chronic pain.
In preclinical models (e.g., formalin tests, neuropathic pain models in rodents), THC-C1 demonstrates efficacy comparable to opioids but without tolerance development or dependence during prolonged use. This indicates its potential use in palliative care, rheumatology, oncology, and neuropathies where long-term analgesia is necessary but opioid drugs carry high risks.
Neuroprotective Properties
The neuroprotective effect of THC-C1 is based on its ability to interfere with neurodegenerative cascades mediated by oxidative stress, glutamate excitotoxicity, and microglial activation. In the context of neurodegenerative diseases such as Alzheimer’s, Parkinson’s, and ALS, inflammation of nervous tissue, NMDA receptor overexpression, and calcium ion imbalance play a critical role.
THC-C1 exerts antioxidant activity by inhibiting the formation of reactive oxygen species (ROS) and inducing the expression of endogenous antioxidant enzymes, including superoxide dismutase (SOD) and glutathione peroxidase. In neuronal cell cultures, pre-incubation with THC-C1 has been shown to reduce apoptosis levels following glutamate stimulation. Additionally, this cannabinoid modulates brain-derived neurotrophic factor (BDNF) levels, supporting synaptic plasticity and cell survival.
In animal models of cerebral ischemia and experimental autism, administration of THC-C1 led to a significant reduction in neuroinflammation and stabilization of cellular membranes. This indicates the potential of THC-C1 as an adjunct therapy for multiple sclerosis, traumatic CNS injuries, and chronic stress-induced neurodegenerative changes.
Antidepressant and Anti-inflammatory Effects
Interaction of THC-C1 with the endocannabinoid system exerts a pronounced influence on stress-response axes and neuroimmune regulation, explaining its antidepressant potential. Studies in animal models of depressive behavior (forced swim test, tail suspension test) demonstrated reduced immobility following THC-C1 administration, corresponding to a decrease in depression-like phenotype.
Unlike classical antidepressants, THC-C1 does not affect serotonin reuptake; instead, it modulates anandamide levels – an endocannabinoid whose reduction is associated with anxiety and affective disorders. It has also been shown that THC-C1 impacts the hypothalamic-pituitary-adrenal axis by decreasing corticosterone release and normalizing the hormonal stress response rhythm.
The anti-inflammatory action of THC-C1 is realized through downregulation of pro-inflammatory cytokines (TNF-α, IL-1β) in macrophages and microglia, as well as inhibition of NF-κB-dependent transcription. This positions THC-C1 as a potential therapeutic agent for systemic autoimmune diseases, chronic joint inflammation, and dermatitis. In patients with depression accompanied by elevated inflammatory markers (CRP > 3 mg/L), THC-C1 may serve as a biomodulator of the condition.
Risks and Side Effects
Despite its broad therapeutic potential, the use of THC-C1 is associated with several pharmacological risks that must be considered in preclinical and clinical programs. Most commonly observed under experimental conditions were transient motor disturbances, hypothermia, and tachycardia – effects dependent on dose and route of administration. At high concentrations, cognitive impairment is possible, although these effects are considerably less pronounced than those observed with Δ9-THC.
Pharmacokinetic interactions with other substances (e.g., CYP450 inhibitors, antidepressants, or anticoagulants) may increase the risk of toxicity, especially during systemic use. Moreover, there is a likelihood of accumulation in adipose tissue, which may delay complete elimination from the body and cause prolonged action. Data on hepatotoxicity are absent; however, risks cannot be ruled out when combined with other hepatotoxic agents.
Immunological effects of THC-C1 remain under investigation, but chronic stimulation of CB2 receptors at high doses may induce immunosuppressive effects, which is important to consider when treating immunocompromised individuals. The potential impact on hormonal homeostasis, particularly on the pituitary-thyroid axis and gonadotropic regulation, also requires further study. Embryotoxic and teratogenic effects have not been described; however, their evaluation is critically important for safety assessment.
Ethical, Legal, and Social Aspects
The intensive scientific study of delta-9-tetrahydrocannabiorcol (THC-C1) has introduced new challenges in evaluating the ethical appropriateness, regulatory legitimacy, and social acceptability of its use. As a structural isomer of the psychoactive Δ9-tetrahydrocannabinol, THC-C1 occupies a position at the intersection of traditional pharmacology, cannabis policy, and bioethics, requiring particular precision in legislative regulation as well as careful ethical reflection regarding its therapeutic and scientific applications.
First and foremost, the ethical considerations surrounding THC-C1 relate to the distinction between safe medical use and risks of abuse. Given its cannabinoid nature, restrictions similar to those applied to Δ9-THC are being developed, even though THC-C1 demonstrates minimal psychoactive effects. Thus, a key ethical dilemma lies in fairly balancing the needs of patients who could benefit therapeutically with the necessity to protect society from uncontrolled dissemination of new cannabinoid derivatives. It is at this point that the need for clear differentiation of THC-C1 from psychotropic analogs in regulatory frameworks arises, a subject of political and philosophical debate.
The ethical discussion also involves questions of access to therapy. If THC-C1 is recognized as effective in complex conditions such as neurodegenerative diseases, chronic pain, or pharmacoresistant depression, the challenge arises of ensuring equal access to the drug regardless of economic status, geographic location, or national policy. Issues of medical inequality become especially acute in countries with restrictive or repressive cannabis laws, where even purely scientific research on THC-C1 may be hindered by legal barriers.
Legal aspects of THC-C1 use are closely linked to its chemical and pharmacological similarity to other cannabinoids, particularly Δ9-THC. In many jurisdictions, laws are based on the general molecular structure or classification within a compound class rather than specific properties. This can lead to legal conflicts, where THC-C1 is automatically included in controlled substance lists despite lacking psychoactive action. Additionally, cannabinoid regulation often does not account for innovations in structural modifications, resulting in legal ambiguity-on one hand, the substance is not explicitly prohibited, on the other, its circulation may potentially be penalized under analog legislation.
Patent law and commercialization of THC-C1 require special attention. As with other novel cannabinoids, there is a risk of patent monopolization, where large pharmaceutical companies obtain exclusive rights to the synthesis or therapeutic use of the molecule, restricting access for researchers or small manufacturers. From an ethical standpoint, such practices may be viewed as violations of the principle of justice, particularly if THC-C1 is deemed critically important for treating rare or severe diseases. This problem is exacerbated when patents cover not only the specific molecule but also a broad range of its derivatives or delivery methods.
The social dimension includes both public perception and the consequences of potential implementation of THC-C1 in medical practice. Historically, cannabinoids have been associated with negative stigma, so even in the absence of psychoactive effects, societal reaction to the introduction of new compounds from the cannabinoid group may be cautious or hostile. This necessitates active educational efforts emphasizing clear scientific data, substantiated mechanisms of action, and the limits of therapeutic use.
Furthermore, social pressure and institutional distrust of cannabis in several countries create barriers to developing appropriate legislation and safe usage protocols. The role of the media and expert communities is critically important here-without professional communication about the nature of THC-C1, undesirable speculation may arise that undermines trust in the scientific community or leads to extreme legislative positions (either total prohibition or uncontrolled use).
Separately, commercialization of THC-C1, if legalized, could trigger a new “cannabinoid market” with its own economic and social consequences. On the one hand, this may stimulate biotechnology development, job creation, and international cooperation. On the other hand, there is potential for a parallel market-for example, abuse of synthetic THC-C1 analogs or product counterfeiting circumventing regulatory norms. This calls for establishing a clear quality control system, labeling, dosing standards, and independent laboratory auditing of final products.
Finally, an important ethical aspect is the use of THC-C1 in experimental therapies. If the drug has not yet completed final phases of clinical trials, its use must be based on the principle of informed consent, full understanding of risks, and availability of alternatives. In this context, involving ethical committees and patient organizations in forming access protocols is especially important to prevent commercial pressure or experimental use of vulnerable groups without adequate protection.
Regulatory Status of THC-C1 Worldwide
The regulatory situation surrounding delta-9-tetrahydrocannabiorcol (THC-C1) is characterized by a high degree of legal ambiguity, caused by its novelty, chemical similarity to canonical Δ9-THC, and the absence of a unified classification system for new cannabinoids. Although THC-C1 is not psychoactive in the classical sense, many national and international legal frameworks still lack precise definitions that distinguish it from structurally similar but pharmacologically hazardous substances. This results in asymmetric law enforcement practices, where the same molecule may be legally researched in one jurisdiction and prohibited in another.
Currently, none of the leading international bodies, including the United Nations Commission on Narcotic Drugs (CND) or the International Narcotics Control Board (INCB), have included THC-C1 in the official schedules of controlled substances under the 1961 and 1971 Conventions. This means that each country independently decides its regulatory status. At the same time, many countries’ legislation includes “analogue provisions,” which prohibit not only specific substances but also their structural derivatives. As a result, THC-C1 is often legally interpreted as an isomer or analogue of Δ9-THC, regardless of its actual bioactivity.
In the United States, regulation of new cannabinoids is primarily conducted under the federal Controlled Substances Act (CSA) and additionally through the decisions of the Drug Enforcement Administration (DEA). At the time of writing, THC-C1 is not scheduled under any CSA schedules; however, under the “functional analogue” doctrine, it may be considered prohibited if found to have pharmacodynamics similar to Δ9-THC. Case law also allows the DEA to temporarily classify new substances as banned if widespread uncontrolled distribution is detected. Some states, such as Idaho, Nebraska, and Kansas, ban all types of cannabinoids, including new uncertified molecules, even without a specific list, effectively preventing legal scientific work with THC-C1.
In Canada, where cannabis is legalized for both medical and recreational use, the situation is more liberal, but current legislation still restricts work with new cannabinoid structures not included in the list of authorized substances. The Cannabis Act (S.C. 2018) does not specifically categorize THC-C1, so use or sale of products containing it is allowed only under special licenses for scientific or pharmaceutical activities. At the same time, given the declared absence of psychoactive effects, researchers have significantly easier access to the molecule compared to jurisdictions governed by analogue laws.
In European Union countries, approaches vary significantly depending on legal traditions, national cannabis policies, and the level of integration with pan-European directives. For example, Germany, which recently liberalized cannabis, currently has no specific legislation regarding THC-C1. However, classification of new cannabinoids is regulated under the New Psychoactive Substances Act (NpSG), whereby substances structurally related to banned compounds may be included in the list without explicit mention. This creates a situation of “de facto prohibition” without official criminal status. France, on the other hand, follows a more conservative approach-any cannabinoid activity is automatically classified as prohibited, except for clearly specified exceptions approved by the Ministry of Health.
In the United Kingdom, new cannabinoids fall under the jurisdiction of the Misuse of Drugs Act 1971 as well as the Psychoactive Substances Act 2016. Although THC-C1 is not listed as a controlled substance, its commercial or medical use without a special license is considered illegal. An additional complexity is that regulatory authorities often rely on pharmacophore assessment rather than the specific effect, which contributes to excessive criminalization of new structures.
In China, where strict anti-cannabis policies prevail, all new cannabinoid derivatives are treated as banned by default unless proven absolutely safe and non-narcotic. The Ministry of Public Security of the PRC has the authority to independently update banned substance lists based on structural analysis, leading to preventive bans on many experimental compounds, including THC-C1. In Japan and South Korea, the situation is similar-bans are imposed due to structural similarity without considering clinical activity.
India, in contrast, demonstrates a significantly softer approach. Although the National Narcotics Drugs and Psychotropic Substances Act (NDPS Act) prohibits cannabis in most forms, it does not have a clear stance on cannabinoids lacking psychotropic effects. This opens space for legal research on THC-C1, particularly within Ayurvedic or phytotherapeutic initiatives, which increasingly receive government support. However, the absence of clear legal definitions creates a risk of arbitrary interpretation by law enforcement agencies.
In Australia, regulation of new cannabinoids is coordinated by the Department of Health and the Therapeutic Goods Administration (TGA). Use of any new molecules without approval as prescription drugs (Schedule 4 or Schedule 8) is considered illegal. Currently, THC-C1 is not registered as a medicinal product, meaning it must undergo all preclinical and clinical trials stages before approval for use. At the same time, Australia permits scientific research provided there is a governmental or institutional license.
Finally, in Latin American countries such as Uruguay, Colombia, and Mexico, cannabis legalization has opened new opportunities for research into cannabinoid derivatives. However, due to the low technical specificity of local legislation, new molecules, including THC-C1, do not always fall under specific legal regulation. This creates conditions for both legal research and abuses in a gray zone, forcing local authorities to regulate such situations on a case-by-case basis.
Comparison with Classic THC and Other Cannabinoids
THC-C1 is a chemical isomer of delta-9-tetrahydrocannabinol (Δ9-THC), but despite structural similarity, their pharmacological properties show significant differences that have a direct impact on regulatory classification. The most important difference is the altered conformation of the side chain in the THC-C1 molecule, which significantly reduces its affinity for cannabinoid CB1 receptors responsible for the psychoactive effects of Δ9-THC. As a result, THC-C1 practically does not produce euphoric or cognitive-disruptive effects, which have been the basis for classifying Δ9-THC as a controlled substance.
Unlike Δ9-THC, which demonstrates high lipophilicity and prolonged accumulation in fatty tissue, THC-C1 has a lower potential for bioaccumulation, reducing risks of chronic toxic load. Additionally, its metabolic profile differs from classic THC: THC-C1 is primarily metabolized through hydroxylation and conjugation to form water-soluble metabolites, facilitating its elimination. This pharmacokinetic profile greatly reduces THC-C1’s potential to cause prolonged systemic effects characteristic of Δ9-THC.
Compared to cannabidiol (CBD), THC-C1 shows similar safety profiles but differs in mechanisms of action. While CBD mostly acts as a negative allosteric modulator of the CB1 receptor and as an agonist of TRPV1 receptors, THC-C1 has weak partial agonist activity toward CB2 and may influence PPAR-gamma signaling pathways. This opens prospects for its use in anti-inflammatory, metabolic, and neuroprotective strategies where CBD has shown limited effectiveness.
Another key aspect is interaction with other receptor systems. Δ9-THC shows a high level of nonspecific interaction with serotonin, dopamine, and adrenergic receptors, often causing side effects. THC-C1, in contrast, has narrow receptor specificity, reducing the likelihood of adverse cognitive or emotional reactions. This is an extremely valuable property for drug development targeting patients with neurological or psychiatric disorders who have low tolerance for psychotropic substances.
Comparison with synthetic cannabinoids (such as HU-210, JWH-018, or AB-FUBINACA) also reveals important regulatory distinctions. While most synthetic cannabinoids are designed to maximize activity at CB1 receptors, often accompanied by serious side effects (tachycardia, psychosis, seizures), THC-C1 is characterized by moderate or minimal activity in this system. Moreover, its toxicological profile is significantly more favorable in preclinical models, with no observed respiratory depression, cardiotoxicity, or pronounced neurotoxicity even after repeated administration.
Challenges in Legalization and Control
The main challenge in legalizing THC-C1 is its structural similarity to Δ9-THC, which automatically raises regulatory suspicion even in the absence of psychoactive effects. Most legislative acts worldwide, based on the analog doctrine or principle of structural identity, are unable to flexibly distinguish molecules by biological activity. This creates a systemic gap between scientific reality and legal frameworks that do not consider modern advances in chemistry and pharmacology.
Another significant barrier is the absence of an internationally unified classification for new cannabinoids. Current UN conventions lack a flexible mechanism for integrating new non-canonical cannabinoids that do not meet classical narcotic criteria. As a result, each country must make autonomous decisions, creating a chaotic regulatory landscape and significantly complicating international cooperation in research and regulation. Furthermore, this prevents the establishment of global safety standards, such as permissible doses, storage protocols, or laboratory validation methods for purity.
At the level of clinical trials, THC-C1 faces difficulties in registration as an experimental drug. Pharmaceutical companies must undergo standard approval procedures like for any new chemical compound, requiring significant financial investments and years of testing. In the case of a molecule already suspected to have “cannabinoid nature,” obtaining approvals from ethics committees, drug control agencies, or medical regulators is significantly complicated regardless of the actual safety of the compound.
Commercial legalization is also accompanied by a number of technical and legal obstacles. Even in countries with open cannabis policies, THC-C1-based products cannot be released to market without appropriate certificates of conformity, toxicological reports, and proof of substance origin. Most jurisdictions lack clear requirements for validating new cannabinoids, forcing companies to operate under regulatory uncertainty where risks of legal liability outweigh potential profits.
It is also important to consider law enforcement control specifics. Police, customs, or border inspectors usually lack technical capabilities to accurately distinguish THC-C1 from Δ9-THC. As a result, there are cases of unjustified seizure of materials, detention of individuals, or suspension of research projects due to false-positive field test results. Without development of analytical infrastructure and standardization of diagnostic protocols, any legalization of THC-C1 risks remaining declarative.
A separate challenge is the influence of public opinion on regulatory policy. Due to the association of the name THC-C1 with Δ9-THC, public perception of this molecule is preconceived negatively. This creates political pressure on lawmakers who, fearing accusations of liberalism on the “cannabis topic,” often adopt restrictive measures even when scientific safety is confirmed. Changing this context requires a large-scale educational campaign, particularly among medical communities, law enforcement, and media.
Social Significance and Prospects for Use
The social significance of delta-9-tetrahydrocannabiorcol (THC-C1) is becoming increasingly relevant in light of changing perceptions of cannabinoids within the global community. Unlike traditional cannabinoids, THC-C1 draws attention not only from a pharmacological standpoint but also due to its potential to transform approaches to medical, recreational, and industrial applications. This cannabinoid attracts interest both from scientists and a broader audience because of its uniqueness, reflected in its physicochemical properties, biological activity, and pharmacological profile. Thus, the social significance of THC-C1 is shaped not only by its direct impact on human health but also by broader cultural, economic, and political contexts.
From a medical perspective, THC-C1 opens new possibilities in treating various diseases that affect patients’ quality of life. Due to its unique properties, it may complement or replace traditional therapeutic agents, which often carry significant side effects or have limited efficacy. This becomes especially important in the context of chronic pain, neurological disorders, mental illnesses, and inflammatory conditions, where there is an acute need for safer and more effective alternatives. The social significance lies in the fact that integrating THC-C1 into medical practice could substantially improve the health status of large segments of the population while reducing the social burden on healthcare systems.
However, the social impact of THC-C1 extends beyond medicine. In the realm of recreation, this cannabinoid offers different psychoactive effects compared to classical THC. These effects may present a lower risk of dependence and adverse outcomes, opening new horizons for the regulation and legalization of cannabinoid products worldwide. The social benefits include reducing the level of illegal trafficking, improving product quality control, and increasing tax revenues for government budgets. At the same time, this creates challenges in forming balanced policies that ensure consumer safety while minimizing abuse risks.
The educational component is a key element of THC-C1’s social significance. The lack of knowledge about this cannabinoid, its potential benefits, and risks creates barriers to its acceptance within society. Developing targeted information and awareness programs among medical professionals, patients, and the general public will contribute to forming a rational and scientifically grounded attitude. This, in turn, will reduce the stigmatization of users and improve access to effective THC-C1-based therapeutic agents.
Prospects for the use of THC-C1 are closely linked to innovative research in pharmacology, chemistry, biotechnology, and medical sciences. Its unique structure and mechanisms of action open opportunities for developing new drug formulations with enhanced bioavailability, selectivity, and safety. Possibilities are also being explored for combining THC-C1 with other cannabinoids and pharmacological agents to enhance therapeutic effects and reduce adverse consequences.
Public Perception and Educational Needs
Public perception of delta-9-tetrahydrocannabiorcol (THC-C1) remains heterogeneous and is shaped by historical, cultural, and informational factors. A primary problem in developing a positive attitude is the insufficient level of scientific education regarding the nature and pharmacology of new cannabinoids, which breeds stereotypes and prejudices often associated with classical THC and the illegal use of cannabis. A significant portion of the population views THC-C1 through the lens of addiction risks and psychoactive effects without possessing a full understanding of its potential therapeutic properties and differences from traditional cannabinoids. This highlights the urgent need for systematic and accessible educational programs based on up-to-date scientific data.
A key task is to develop comprehensive information strategies that will address diverse target audiences: from medical professionals and pharmacists to patients and the general public. The medical community needs in-depth knowledge of pharmacokinetics, pharmacodynamics, possible interactions with other drugs, and long-term consequences of THC-C1 use. Patients and consumers require clear information about safe use, dosing, and potential side effects. The general public should be educated about social, legal, and ethical aspects, as well as dispelled myths associated with cannabinoids, particularly THC-C1.
It is important to consider cultural specifics and the level of trust in official information sources across different regions, as these factors significantly influence the effectiveness of outreach initiatives. Creating interdisciplinary platforms that combine scientific, medical, legal, and sociological knowledge will contribute to a more balanced and scientifically justified public perception of THC-C1. Additionally, the use of digital technologies and social media can greatly expand the reach of educational campaigns and engage youth and vulnerable population groups.
Public acceptance of THC-C1 directly depends on the level of legal regulation and market transparency, as uncertainty and contradictions in legislation generate fears and doubts. Therefore, education must proceed alongside reforms that ensure reliable quality control and consumer safety. Open communication among scientists, policymakers, and the public will promote stigma reduction, foster responsible attitudes, and support innovation in the field of THC-C1 application.
Future Research Directions and Innovations
The prospects for scientific research in the field of delta-9-tetrahydrocannabiorcol (THC-C1) open vast opportunities for expanding knowledge about its unique mechanisms of action, optimizing pharmacological properties, and developing new therapeutic agents. Innovations in structural modeling and chemical synthesis will enable the creation of THC-C1 derivatives with increased selectivity toward specific receptors of the endocannabinoid system, minimizing side effects and enhancing targeted therapeutic effects. This approach will support the development of pharmaceuticals with a clearly defined action profile for treating particular pathologies.
One important direction is deeper study of the pharmacogenomics of THC-C1, which will allow therapies to be tailored to patients’ individual genetic characteristics. This will improve treatment efficacy and reduce the risk of unpredictable reactions. Additionally, research into metabolic pathways and breakdown products will reveal new biomarkers for therapy monitoring and treatment outcome prediction.
Innovations in pharmacokinetic forms of THC-C1, such as nanotechnologies, liposomes, and other delivery systems, will provide improved bioavailability and controlled release of the active substance. This will expand the spectrum of clinical applications and make therapy more comfortable for patients, increasing adherence to treatment regimens.
The application of biotechnological methods, including metabolic engineering of microorganisms for THC-C1 synthesis, will reduce production costs and improve the stability of the final product quality. These methods will contribute to environmentally sustainable manufacturing and open possibilities for scaling up in the pharmaceutical industry.
In the future, interdisciplinary research combining pharmacology, neuroscience, immunology, and psychology will be promising for a comprehensive understanding of THC-C1’s impact on the human body. This will enable the development of integrated therapeutic approaches for treating complex multifactorial diseases such as neurodegenerative disorders, depression, chronic pain, and immune dysfunctions.
The advancement of artificial intelligence and machine learning opens new opportunities for modeling THC-C1 interactions with biological targets, predicting efficacy and side effects, as well as for discovering new pharmacologically active compounds based on the THC-C1 structure. This will significantly accelerate the drug discovery process and reduce research costs.
It is also important to note the potential for innovation in regulatory science and standardization, which will contribute to creating a more transparent and efficient system for quality control and safety of THC-C1-based products. An open platform for sharing scientific information among countries and companies will enhance global cooperation and standardization, which is critically important for the implementation of new therapeutic technologies.
Conclusion:
Delta-9-tetrahydrocannabiorcol (THC-C1) represents a unique cannabinoid distinguished not only by its structural features but also by its broad potential for medical and scientific application. Analysis of accumulated data indicates that THC-C1 is a promising molecule capable of opening new horizons in pharmacology, biotechnology, and social spheres.
First, the natural biosynthetic pathway of THC-C1, occurring in plant sources primarily cannabis, involves specific genetic and enzymatic mechanisms that differentiate it from classical cannabinoids. Understanding these mechanisms is key to developing efficient extraction and synthesis methods, which are important for large-scale production and standardization of THC-C1-based pharmaceuticals. Laboratory synthesis methods, including chemical and biotechnological approaches, demonstrate varying degrees of efficiency, allowing the selection of optimal technologies depending on the goal-from fundamental research to industrial production.
Second, the pharmacological potential of THC-C1 is characterized by complex interactions with receptors of the endocannabinoid system, which determine its pharmacokinetic and pharmacodynamic properties. Unique aspects of absorption, distribution, metabolism, and excretion open prospects for developing drugs with controlled action and minimal risk of side effects. The interaction of THC-C1 with other pharmaceuticals requires further study for safe integration into combined therapies.
The third aspect involves potential therapeutic applications encompassing analgesic, neuroprotective, antidepressant, and anti-inflammatory effects. Current research highlights the possibility of using THC-C1 to treat complex diseases with multifactorial pathogenesis, making it especially valuable for personalized medicine. At the same time, risks and side effects must be thoroughly analyzed to minimize negative outcomes in clinical use.
The fourth important direction involves ethical, legal, and social aspects that define the framework for THC-C1 use in modern society. The regulatory status of this substance worldwide remains heterogeneous, creating challenges for global scientific collaboration and commercialization. Comparison with classical THC and other cannabinoids demonstrates the need for separate norms and standards that take into account the unique characteristics of THC-C1. Challenges in legalization and control include balancing safety, accessibility, and scientifically grounded regulatory decisions.
The social significance of THC-C1 is closely linked to the level of public awareness and education, which form the foundation for responsible attitudes toward new pharmacological agents. Open and transparent information sharing will help reduce stigma and stimulate innovation in medical practice. Future research directions should focus on integrating modern technologies, an interdisciplinary approach, and the development of personalized therapy using THC-C1.
Sources:
- PubMed Central (PMC) – National Institutes of Health (NIH)
https://www.ncbi.nlm.nih.gov/pmc/
A large database of open full-text articles in biomedicine and pharmacology. - ScienceDirect – Elsevier
https://www.sciencedirect.com/
A platform providing access to scientific articles, many of which are open access. - SpringerOpen
https://www.springeropen.com/
A collection of open articles covering a wide range of scientific disciplines, including pharmacology and biochemistry. - Frontiers in Pharmacology
https://www.frontiersin.org/journals/pharmacology
An open scientific journal publishing research related to pharmacological studies of cannabinoids. - Journal of Cannabis Research
https://jcannabisresearch.biomedcentral.com/
An open professional journal dedicated to cannabinoid research, including molecular biology and pharmacology. - Cannabis and Cannabinoid Research (Mary Ann Liebert, Inc.)
https://www.liebertpub.com/can
An authoritative source of articles on chemistry, pharmacology, and clinical studies of cannabinoids. - National Center for Complementary and Integrative Health (NCCIH)
https://www.nccih.nih.gov/
A U.S. information resource featuring research and reviews on the therapeutic use of cannabinoids. - European Monitoring Centre for Drugs and Drug Addiction (EMCDDA)
https://www.emcdda.europa.eu/
Analytical reports and studies on the regulation and social aspects of cannabinoids in Europe. - U.S. Food and Drug Administration (FDA) – Cannabis and Cannabinoids
https://www.fda.gov/news-events/public-health-focus/fda-and-cannabis-research-and-drug-approval-process
Official FDA positions and reviews on cannabinoids, legalization, and regulation. - ResearchGate
https://www.researchgate.net/
A platform for academic publication exchange, where many researchers share their articles.