In cannabinoid chemistry, the systematic study of structural variants within the phytocannabinoid series has opened the door to investigating a number of previously undescribed or scarcely studied acidic forms derived from the main cannabinoids. One such compound is Delta-9-tetrahydrocannabiorcolic acid (THCA-C1) – a metabolite with a short alkyl side chain, which chemically is an analog of the classical THCA-A but contains a one-carbon (methyl) side group instead of the pentyl radical. This seemingly minor structural difference radically changes the compound’s pharmacokinetics, bioavailability, receptor affinity, and therapeutic potential.
The cannabinoid landscape has long since expanded beyond the familiar Δ⁹-tetrahydrocannabinol (THC), cannabidiol (CBD), and their acidic forms. Within this field, there emerged a need to rethink the cannabis chemotype paradigm by taking into account the presence of short-chain homologs – C1, C3, C4 cannabinoids – which traditionally remained outside the spectrum of focus due to technological limitations in analytical methods and their low concentration in raw materials. However, modern chromatographic and mass spectrometric techniques now allow for the identification of even trace concentrations of such derivatives, opening new horizons in the pharmacological study of these lesser-known structures.
THCA-C1, as a representative of the short-chain orcolic acids, is a potentially biologically active form of phytocannabinoid that remains in a stable acidic state until decarboxylation occurs. This means its pharmacological action fundamentally differs from Δ⁹-THC-C1 (the decarboxylated form), particularly in terms of psychoactivity, transport across the blood-brain barrier, and interactions with cannabinoid receptors. The presence of a methyl group instead of a pentyl chain alters the molecule’s lipophilicity, its hepatic metabolism, as well as its bioaccumulative properties, which necessitates separate pharmacokinetic modeling and clinical testing.
From a chemical standpoint, THCA-C1 is the product of enzymatic oxidation of the precursor cannabiorcolic acid (CBCA-C1) or is formed through the specific action of THCA synthase on the short-chain form of cannabigerolic acid (CBGA-C1). Although this synthase is generally associated with the production of THCA-A, some isoforms of the enzyme in certain cannabis phenotypes have the capacity to catalyze the formation of THCA-C1 given the appropriate substrate set. This allows THCA-C1 to be regarded as a natural component rather than a byproduct or artifact.
The presence of THCA-C1 has been confirmed in some high-altitude or extremophile-adapted cannabis strains with a high degree of genetic diversification. In these samples, the predominance of short-chain phytocannabinoids is often recorded, suggesting the possibility of an evolutionary mechanism by which the plant forms an alternative cannabinoid profile to adapt to environmental pressure, ultraviolet radiation, or biotic stress. Thus, THCA-C1 is not only a chemical variation but also a potential adaptive response of the phytosystem.
In the pharmacological context, short-chain derivatives attract attention due to their potential to reduce psychoactive burden while maintaining therapeutic activity. Initial in vitro studies indicate that THCA-C1 exhibits significant antiproliferative, anti-inflammatory, and antioxidant potential. Unlike THCA-A, which demonstrates limited bioavailability when administered orally, THCA-C1 potentially may achieve more effective cell membrane penetration due to its lower molecular weight and higher water solubility. However, these hypotheses still require confirmation under in vivo conditions.
Another aspect driving scientific interest in THCA-C1 is its potential to serve as a basis for creating new synthetic analogs. Considering the pharmacophore profile of cannabinoid acids, replacing the long alkyl side chain with a short one opens the possibility of developing highly affine yet less psychoactive molecules for use in pharmacotherapy. For example, derivatives based on THCA-C1 could be created with additional hydroxyl or amino groups to modulate interactions with PPARγ or TRPV1 receptors, which are promising targets in the treatment of neuroinflammatory and metabolic disorders.
From an applied perspective, identifying and standardizing the THCA-C1 content in raw materials is an important step toward building more precise phytochemical profiles of cannabis. This will allow for strain selection not only by classical THC/CBD markers but also by the presence or ratio of lesser-known components such as THCA-C1. Achieving this requires the introduction of new analytical standards, specific reference materials, and calibration protocols. Without this, the inclusion of THCA-C1 in legal pharmacopeias or medical formularies will remain challenging.
Chemical Structure and Classification
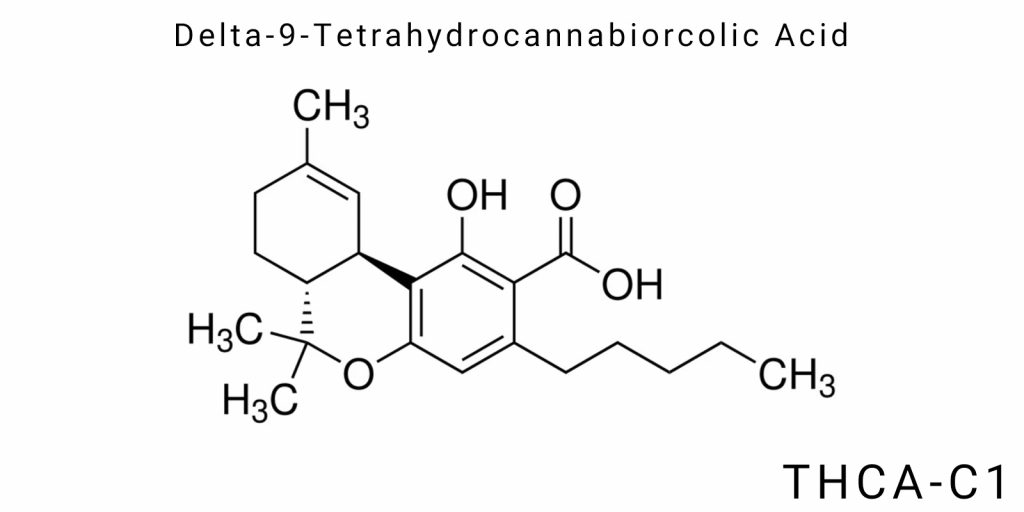
Delta-9-tetrahydrocannabiorcolic acid (THCA-C1) belongs to the class of natural acidic cannabinoids characterized by the presence of a carboxyl functional group, a phenolic core, and a variable alkyl side chain. Structurally, THCA-C1 is a shortened side-chain homolog of THCA-A, with the key difference being the substitution of the pentyl group by a methyl group. Chemically, this places it within the class of short-chain orcolic acids.
Formally, THCA-C1 is derived from cannabigerolic acid with a C1 side chain (CBGA-C1), which, in the presence of a specific form of THCA synthase, is enzymatically converted to the target compound. The main molecular framework consists of a tricyclic system, including an aromatic ring (the phenolic core), a cyclohexane ring with a double bond at the Δ⁹ position, and a pyran-like ring with an attached carboxyl group. This structure exhibits the typical configuration characteristic of classical cannabinoids, but its molecular weight is reduced due to the shortened alkyl chain, which in turn alters its physicochemical properties, including lipophilicity, acidity, and solubility.
THCA-C1 naturally exists in an inactive acidic form, which implies the presence of a carboxyl group at the 2′ position (on the pyran-like ring) that can potentially be cleaved during thermodecarboxylation, resulting in the psychoactive form-delta-9-tetrahydrocannabiorcol (Δ⁹-THC-C1). This chemical dynamic enables reversible conversion within the biochemical metabolism, a feature typical of acidic phytocannabinoids.
From a classification standpoint, THCA-C1 is grouped among cannabinoids with a short (C1) alkyl side chain. This subtype is part of a broader cannabinoid classification system based on the length of the side alkyl radical, which includes C1 (methyl), C3 (propyl), C4 (butyl), C5 (pentyl, the most typical), and C7 (heptyl) forms. This division has a clear biochemical basis: the length of the side chain affects affinity for cannabinoid receptors CB1 and CB2, bioavailability, metabolism, blood-brain barrier penetration, and consequently defines psychoactive and therapeutic properties.
As a short-chain analog, THCA-C1 shows significantly lower affinity for CB1 receptors compared to classical THCA-A, which is explained by the insufficient lipophilicity of the methyl group to optimally interact with the receptor’s lipophilic pocket. This suggests reduced psychoactivity even after decarboxylation. However, interaction with CB2 receptors or other molecular targets (such as TRPV1, PPARγ, GPR55) may be preserved or even modified toward a specific effect, forming the basis for potential therapeutic interest.
Regarding chemical reactivity, THCA-C1 demonstrates high stability at room temperature but undergoes rapid decarboxylation at temperatures above 100°C. This thermal lability necessitates specialized extraction and storage conditions if the goal is to isolate the acidic form. Consequently, analytical and preparative chemistry of THCA-C1 requires the use of mild temperature regimes, including cold ethanol or supercritical CO₂ extraction under low-temperature pressure conditions.
The molecular formula of THCA-C1 is C₂₀H₂₈O₄. Compared to THCA-A (C₂₂H₃₀O₄), this structure contains two fewer carbon atoms, which significantly impacts its chromatographic characteristics. In liquid chromatography with UV/Vis or MS detection, this cannabinoid exhibits a marked decrease in retention time due to lower polarity and smaller molecular size. Standardization and identification of THCA-C1 require the development of a separate analytical standard, as the compound does not produce a significant signal within the typical phytocannabinoid mass spectral panels.
In terms of nomenclature, the IUPAC name of the compound-2-carboxy-2,3-dihydro-6,6,9-trimethyl-3-pentyl-6H-dibenzo[b,d]pyran-1-ol-requires correction to reflect the actual methyl side group substitution. The correct nomenclature should account for replacing the pentyl group with a methyl group at the 3-position of the side chain, resulting in the name 2-carboxy-2,3-dihydro-6,6,9-trimethyl-3-methyl-6H-dibenzo[b,d]pyran-1-ol.
From a biosynthetic perspective, THCA-C1 may form in specific phenotypes of Cannabis sativa L., particularly those carrying mutations in the gene encoding THCA synthase. Alternatively, synthesis is possible through modified in vitro expression in recombinant systems based on Saccharomyces cerevisiae or Escherichia coli, opening prospects for biotechnological production of this cannabinoid without plant biomass. There is also potential for chemoselective synthesis of THCA-C1 based on cannabiorcol via localized oxidation and cyclization, which is relevant for laboratory standardization.
Within the classification framework, THCA-C1 is regarded as a subtype of acidic phytocannabinoids with a short-chain structure within the orcolin group. These substances include both natural and semi-synthetic representatives that retain the fundamental cannabinoid architecture but vary in length and hydrophobicity of the side radical. This forms the basis not only for classification by functional groups but also for the spectrum of biological activity, which will be detailed in subsequent sections.
Molecular Structure of THCA-C1
Delta-9-tetrahydrocannabiorcolic acid (THCA-C1) is a derivative of the tricyclic terpenophenolic scaffold characteristic of all cannabinoids but differs in key structural parameters that directly influence its physicochemical and pharmacological behavior. The central chemical framework of THCA-C1 is a derivative of cannabiorcolic acid with a methyl substituent at the third position of the side chain, distinguishing it from the classical THCA-A, which has a pentyl group.
The molecular formula of THCA-C1 is C₂₀H₂₈O₄. This formula includes 20 carbon atoms, 28 hydrogen atoms, and 4 oxygen atoms. The molecule contains three clearly differentiated functional parts: (1) an aromatic ring with a phenolic hydroxyl group; (2) a hydrogenated cyclohexane ring with a double bond at the Δ⁹ position; (3) a tetrahydropyran portion with an attached carboxyl group, which gives the molecule its acidic character.
The core is a condensed system morphologically classified as 6H-dibenz[b,d]pyran. At position 1, there is a hydroxyl group involved in an intramolecular hydrogen bond that stabilizes the structure in solution. The double bond at the Δ⁹ position between carbons 9 and 10 in the cyclohexane ring has a Z-configuration and is key for the potential psychoactivity of the THC-C1 derivative after decarboxylation.
The presence of the acid is explained by the attachment of the carboxyl group to the β-position of the tetrahydropyran ring, forming a β-keto acid moiety that destabilizes upon heating and releases CO₂. This reactive capability underlies the thermal conversion of THCA-C1 into Δ⁹-THC-C1. The substituent configuration at position 3 – a methyl group replacing the typical pentyl radical – reduces the flexibility and volume of the molecule’s hydrophobic region, significantly affecting its interaction with hydrophobic receptor domains.
The three-dimensional conformation of the molecule indicates the presence of several chiral centers. The most significant is at the C-6a atom (at the junction between the aromatic core and the cyclohexane ring), which defines the stereochemistry of the entire molecular backbone. Determination of absolute configuration (usually R or S) is performed via circular dichroism spectroscopy or X-ray crystallography.
The electron density distribution within the molecule shows electron localization in the phenolic core, making it an electron-donating fragment. Simultaneously, the carboxyl group induces electron deficiency at the β-position of the tetrahydropyran ring, increasing its reactivity in oxidation processes. This chemical anisotropy (distribution of electrophilic and nucleophilic sites) enables THCA-C1 to undergo selective chemical modifications – for example, esterification, amidation, or selective hydrogenation.
The thermodynamic stability of the compound is associated with several factors: intramolecular stabilization of the hydroxyl group by hydrogen bonds; resonance stabilization of the aromatic ring; and spatial conformation that minimizes steric interactions between side substituents. The enthalpy of decarboxylation, according to quantum chemical calculations, is approximately 78-82 kJ/mol, which aligns with empirical values for other acidic cannabinoids.
The pharmacophore model of THCA-C1 is based on three key binding points: (1) the phenolic hydroxyl group as a hydrogen bond donor; (2) the alkyl substituent as a hydrophobic anchor; (3) the carboxyl group as an acidic functionality capable of ionization at physiological pH. The relative acidity (pKa) of THCA-C1 is estimated between 4.6 and 4.8, allowing it to exist predominantly in an anionic form in neutral environments, thereby reducing its penetration through the blood-brain barrier compared to neutral analogs.
Spectroscopically, THCA-C1 shows a characteristic UV absorption maximum at approximately 275 nm, caused by π→π* transitions within the aromatic system. The IR spectrum includes intense bands around 1700 cm⁻¹ (carbonyl group), 1250-1300 cm⁻¹ (phenol C-O deformation), and a broad band between 3200-3500 cm⁻¹ corresponding to -OH group vibrations. In the ¹H NMR spectrum, aromatic protons resonate between δ 6.2-6.8 ppm, and the methyl substituent appears as a singlet near δ 1.2 ppm. In the ¹³C NMR spectrum, the carboxyl carbon resonates near δ ~175 ppm.
According to HOMO-LUMO electron density analysis, the gap between the highest occupied and lowest unoccupied molecular orbitals indicates moderate reactivity. This allows THCA-C1 to participate in nucleophilic addition reactions while maintaining sufficient stability under physiological conditions. Quantum chemical calculations also show that the methyl group contributes less to the molecular dipole moment than the pentyl group, altering its solubility in lipid environments.
Regarding physical state, THCA-C1 at room temperature is a viscous, resinous substance, hygroscopic, with a tendency to crystallize below -10°C. Its solubility in water is practically zero, while partial solubility is observed in nonpolar solvents such as hexane, chloroform, and diethyl ether. In alcohols (ethanol, isopropanol) and dimethyl sulfoxide (DMSO), it exhibits good solubility, which guides the choice of solvents for preparative chemistry.
In the context of synthetic reproduction, the molecular structure of THCA-C1 allows for modular synthesis from three main fragments: the phenolic core (resorcinol), the terpene bicyclic component (geraniol or its derivatives), and the methyl acetate side chain. Condensation via Fries or Friedel-Crafts reactions, sequential cyclization, and regioselective attachment of the acidic functional group enable in vitro production of THCA-C1 with controlled stereochemistry.
Structural Features: Branching, Phenolic Core, Acidic Group
The THCA-C1 molecule belongs to the group of orcolinic acids characterized by a canonical cannabinoid tricyclic structure that includes an aromatic (phenolic) core, a partially saturated cyclohexane ring with a double bond at the Δ⁹ position, and a tetrahydropyran fragment bearing a carboxyl functional group. Within this architecture, three key structural regions stand out: (1) the branching system on the alkyl side chain; (2) the phenolic core with a hydroxyl group; and (3) the acidic functional group attached to the oxygenated ring.
Branching on the Alkyl Side Chain
A crucial structural element that distinguishes THCA-C1 from classical C5-type cannabinoids is the presence of a methyl group as a substituent in the side chain instead of the typical pentyl group. This shorter alkyl branch is localized on the aromatic ring at the C-3 position and creates fundamentally different spatial and electronic characteristics. Due to its shorter length and reduced flexibility, the methyl group does not actively participate in hydrophobic interactions with the CB1 and CB2 receptor domains, significantly lowering THCA-C1’s affinity for these receptors compared to Δ⁹-THCA (C5).
At the same time, this structural minimization of the lipophilic region leads to reduced molecular polarizability, decreases the overall surface area of solubility in lipid environments, improves chemical stability under oxidative conditions, and alters the molecule’s behavior in chromatographic separation. The methyl branch is planar relative to the core and, according to quantum chemical calculations, does not induce significant conformational changes in the molecule’s central skeleton.
Moreover, the absence of a long alkyl tail reduces steric hindrance around the aromatic core, allowing easier access for nucleophiles or oxidants to the phenolic hydroxyl. This significantly facilitates chemical modification of the molecule, particularly for synthesizing ethers or sulfates in pharmaceutical design.
Phenolic Core
The phenolic portion of the THCA-C1 structure forms the basis of the molecule’s electronic and donor activity. The aromatic ring includes one hydroxyl group at the C-1 position, which serves as a classical hydrogen bond donor. This fragment shows high reactivity toward electrophilic substitution and plays a key role in oxidation and conjugation processes. The phenolic hydroxyl is also the primary target for metabolic transformation in the human liver involving UDP-glucuronosyltransferases.
In spectroscopic analysis, the phenolic group determines the molecule’s UV activity, providing a characteristic absorption peak at approximately 275 nm. This feature is used for detecting THCA-C1 in chromatographic systems with diode-array detection. In IR spectra, the phenol -OH group gives a broad absorption band around 3400-3450 cm⁻¹, typical of medium-strength hydrogen bonds.
The aromatic ring of THCA-C1 is of the resorcinol type (a 1,3-dihydroxybenzene derivative), but the second hydroxyl group is substituted by an alkyl (methyl) group. This creates an asymmetric electronic distribution where the phenolic group acts as a hydrogen bond acceptor, and the alkyl substituent induces a weak +I effect, stabilizing the electron density of the core. This balanced electronic configuration contributes to the phenol’s resistance to oxidation under physiological conditions, reducing the risk of auto-oxidation.
The phenolic fragment also functions as an anchor site for binding with protein sites. Molecular docking shows that, in the case of THCA-C1, the primary hydrogen bonds are formed via the phenolic -OH with amino acid residues such as tyrosine and serine in receptor proteins.
Acidic Group
The carboxyl group in THCA-C1 is localized on the tetrahydropyran ring at the C-11 position (C-2′ in classical nomenclature relative to the side ring) and is the main functional group that determines the molecule’s acidic character. This fragment is responsible for its instability toward thermal decarboxylation and acts as a critical regulator of its bioactivity.
When heated to temperatures between 100 and 130°C, decarboxylation occurs, forming Δ⁹-THC-C1 – a neutral, potentially psychoactive form. This process is accompanied by the release of a CO₂ molecule, and the shift of electron density at the β-position activates conjugation of the π-system in the molecule’s center. Consequently, the acidic form of THCA-C1 is stable only at low temperatures or under anaerobic conditions.
The acid group has a pKa range of 4.6-4.8, allowing it to exist in an ionized form at physiological blood pH (~7.4). This limits the molecule’s ability to cross hydrophobic barriers such as the blood-brain barrier but ensures its capacity to circulate in plasma in a bound form (e.g., with albumin). The ionized state also influences behavior in chromatography-in reversed-phase high-performance liquid chromatography (RP-HPLC) conditions, the acidic form elutes with a shorter retention time than neutralized derivatives.
The carboxyl group participates in phase II metabolism conjugation reactions, notably glucuronidation and amidation. Its chemical reactivity allows selective formation of derivatives-for example, THCA-C1 glucuronides-that play a role in pharmacokinetics and excretion.
Position within the Cannabinoid System
Delta-9-tetrahydrocannabiorcolic acid (THCA-C1) constitutes a distinct analytical category within the cannabinoid system because it does not conform to the typical biosynthetic, pharmacological, or classificatory criteria of classical phytocannabinoids. Its presence is not the result of a dominant biosynthetic pathway in the plant but rather a peripheral phenomenon within the polyketide metabolism, indicating the existence of alternative enzymatic routes in the Cannabis sativa metabolome. Chemically and metabolically, this compound belongs to a poorly studied yet conceptually significant branch-the cannabiorcolic acids-with an exceptionally short side chain, which radically alters its interactions within the endocannabinoid and peripheral signaling systems.
Phytocannabinoids, in the context of modern chemical classification, are usually grouped based on the length of the alkyl side chain at position 5 of the aromatic ring, where the five-carbon (C5) pentyl substituent is considered the reference standard. This structure forms the core of the primary bioactive molecules, including Δ⁹-THCA, CBDA, and CBCA. In contrast, THCA-C1 contains only a methyl (C1) substituent, which sharply modifies not only the molecule’s lipophilicity but also its conformational stability and its electronic-spatial interactions with protein targets.
The place of THCA-C1 in the cannabinoid system cannot be reduced to a simple side chain shortening: this compound is not a reduced analog of canonical Δ⁹-THCA but the result of a parallel biosynthetic pathway involving alternative polyketide precursors. Therefore, its occurrence in cannabis tissues is not necessarily linked to typical THCA synthase activity but potentially involves other enzymatic domains with atypical substrate specificity. This allows THCA-C1 to be considered not as a variant of a known metabolite but as an indicator of metabolic branching within the plant’s secondary metabolism.
Structurally, THCA-C1 is not an isomer but a separate homolog, with clearly defined shifts in molecular mass, solubility coefficients, ionization, and stereochemistry of active centers. Compared to other classes of natural cannabinoids clustered around the C5 group, C1 homologs-specifically THCA-C1-exhibit a relatively isolated position in multidimensional classification models, including PCA and QSAR analyses.
From this classificatory perspective, THCA-C1 can be interpreted as a point of minimal molecular branching, which, despite its relative simplicity, preserves the fundamental architecture of the cannabinoid scaffold: a polycyclic structure, phenolic functionality, and an acidic group. Thus, it represents a conceptual boundary between fully formed cannabinoids and fragmentary products that biochemically may act as substrate analogs or competitive enzyme inhibitors.
From a systemic viewpoint, the presence of THCA-C1 signals the latent potential of cannabis metabolism to produce non-standard compounds under shifts in exogenous or endogenous parameters: changes in substrate availability, mutations in enzyme-coding genes, or epigenetic influences. Its detection in chemotypes with unstable synthase expression reflects the metabolic flexibility of the plant and supports the development of an expanded chemosystematics, where THCA-C1 serves as a marker of specific metabolic drift.
THCA-C1 does not fit into the binary dichotomy of “psychoactive vs. non-psychoactive cannabinoid.” It lies outside this classification because its biological activity is realized not via CB1/CB2 receptors but possibly through other signaling mechanisms or through general antioxidant or chelating activity. This creates the necessity to revise the pharmacophoric approach to cannabinoids-from the traditional focus on receptor affinity to a multivector analysis of biological interaction.
From the standpoint of bioorganic chemistry, THCA-C1 serves as a model system for studying the minimal functional units of cannabinoid activity. Its size, acidity, and electron density allow detailed analysis of noncovalent binding mechanisms, the role of phenolic action in hydrogen bonding, and the influence of the carboxyl group on kinetic behavior in model environments. In this sense, THCA-C1 holds potential as a template for synthesizing minimalist ligands capable of selective interaction with unpredictable biomolecular targets.
Another systemic characteristic of THCA-C1 is its isolation not only as a native metabolic unit but also as an intermediate under conditions of oxidative stress or degradation of larger cannabinoids. In such cases, its presence may signal endogenous transformation of the cannabinoid pool in the plant-a transition from saturated phytocomponents to low-molecular-weight acids capable of playing buffering, protective, or signaling roles. This opens the prospect of studying THCA-C1 as a secondary marker of the plant’s physiological state under biotic or abiotic stress conditions.
Classification by Chemical Nature: Orcoline Acids
The cannabinoid THCA-C1, by its chemical nature, belongs to a specific group of compounds forming the class of orcoline acids. This classification is based on the presence of a phenolic core with a short-chain substitution at the fifth position and a mandatory carboxyl functional group, which imparts high polarity and acidity to the molecule. Orcoline acids are not a universally recognized category in classical nomenclature; however, within the biogenetic and structural classification of cannabinoids, they represent an analytically justified subdivision distinct from the typical pentyl- or propyl-substituted phytocannabinoids.
The name “orcoline acids” derives from the historical term “cannabiorcol” – a chemical marker within the group of C1-substituted cannabinoids that possess a methyl or similarly short substituent at the 5-position of the phenolic core. This characteristic defines a homologous series in which THCA-C1 acts as the acidic form of tetrahydrocannabiorcol, the methyl analog of Δ⁹-THCA. Accordingly, orcoline acids can be defined as a class of natural cannabinoids with shortened side chains (C1 or C2) and a pronounced carboxyl group, which precludes activity through the CB1 receptor due to reduced lipophilicity and spatial mismatch.
Within the internal classification of orcoline acids, THCA-C1 is closely related to CBDA-C1 and CBCA-C1 – the acidic forms of cannabidiorcol and cannabichromorcol, respectively. All these derivatives form a distinct branched cluster that does not overlap with the C3- and C5-substituted phytocannabinoid groups in multidimensional metabolomic analyses. They are characterized by smaller molecular volumes, higher pKa values (due to the influence of electron-donating groups), and a tendency toward easier oxidation when exposed to phenol oxidase-type enzymes.
Biogenetically, orcoline acids are not produced via the standard condensation pathway of olivetolic acid with geranyl pyrophosphate, as seen in classical cannabinoids. Instead, their formation suggests involvement of orcetoic acid – a shortened polyketide precursor, itself a rare substrate in plant biochemistry. This implies that cannabiorcoline acids are byproducts of an alternative polyketide route that operates under conditions of altered substrate availability or enzymatic specificity.
This fact carries important implications for the chemotaxonomy of Cannabis sativa. The presence of orcoline acids in certain chemotypes or phenotypes indicates deviation from the canonical phytocannabinoid profile and reveals diversity in biosynthetic pathways within a single species. Consequently, THCA-C1 and its related acids can be utilized as chemotaxonomic signatures for identifying atypical or rare metabolic states of the plant, particularly under cultivation stresses, nutrient deficiencies, or genetic mutations affecting synthase enzymes.
From a structural standpoint, orcoline acids share several common features: an aromatic ring with an electron-donating phenolic group connected to a tetrahydropyran or tetrahydrocoumarin fragment, as well as an embedded carboxyl group positioned to confer acidity without loss of spatial compactness. This functional group constellation enables them to act as efficient hydrogen bond donors, ionized species under neutral conditions, and potential ligands for non-protein targets – for example, metal centers of enzymes or membrane transporters.
It is also important to highlight the distinction between orcoline acids and phenolic acids of non-cannabinoid origin. Despite containing phenol and carboxyl groups, orcoline acids possess a cannabinoid chiral backbone with two stereocenters in the tetrahydro component, significantly expanding their spatial interaction capabilities with biomolecules. This feature grants them a unique status among natural phenols: not only are they active as antioxidants, but they may also participate in metabolic pathways as modulators of enzymatic activity.
From an analytical perspective, orcoline acids exhibit characteristic spectrophotometric and chromatographic behaviors. Due to the polar carboxyl group and phenolic ring with a short alkyl substituent, they are retained significantly longer on reversed-phase columns compared to their C5 homologs. In mass spectrometry and UV detection spectra, these compounds display stable signature peaks, enabling their differentiation even in complex extracts without the need for further derivatization.
Beyond the context of Cannabis, the orcoline acid structure is valuable in chemical ecology: structurally analogous molecules have been found in other plant families where they perform protective, signaling, or antioxidant functions. This underscores the universality of this structural motif and justifies further investigation of cannabiorcoline acids as representatives of a broader phenolic secondary metabolite field.
Differences Between Cannabiorcol (C1) and Typical C5 Cannabinoids
The key chemical difference between C1-type cannabinoids, such as cannabiorcol (and its acidic form THCA-C1), and canonical C5 cannabinoids-such as Δ⁹-tetrahydrocannabinol (THC), cannabidiol (CBD), or cannabichromene (CBC)-lies in the length of the alkyl side chain attached to the fifth position of the phenolic ring. In the C1 class, this chain is represented by a methyl group (-CH₃), whereas in the C5 class, it is a pentyl group (-C₅H₁₁). At first glance, this structural difference may not seem drastic, but on a molecular level, it creates fundamental distinctions in the physicochemical, pharmacological, and biological properties of the two groups of compounds.
First and foremost, the length of the side chain plays a critical role in interaction with cannabinoid receptors, primarily CB1. Experimental evidence shows that C5 cannabinoids, especially Δ⁹-THC, exhibit high affinity for the CB1 receptor, which accounts for their psychoactive effects. In contrast, C1 derivatives practically do not activate this receptor. This is due both to spatial mismatch (the C1 chain is too short to fill the receptor’s lipophilic pocket) and reduced hydrophobic interactions necessary for a stable ligand-receptor complex. This means that even after decarboxylation (conversion of THCA-C1 to the active C1-THC form), the molecule remains pharmacologically inert toward CB1.
Beyond receptor affinity, the shorter alkyl chain impacts solubility and permeability through biological membranes. C1 cannabinoids are less lipophilic, significantly limiting their bioavailability in systemic circulation. As a result, they show low accumulation in adipose tissue, reduced ability to cross the blood-brain barrier, and consequently, limited systemic activity. By contrast, C5 analogs have higher tissue accumulation capacity, which provides them with prolonged effects and more pronounced activity even at low concentrations.
Pharmacokinetically, C1 cannabinoids are metabolized more rapidly, producing fewer stable intermediate metabolites. In the liver, they predominantly undergo oxidation and glucuronidation, being excreted via urine and bile within a short time frame. This rapid metabolic pathway prevents accumulation of active forms in the body, reducing the potential for chronic exposure effects. At the same time, it opens opportunities for their use in contexts where short-term localized action is needed without systemic burden.
From a structural stability standpoint, C1 derivatives are less prone to autooxidation or cyclization compared to C5 analogs. This is due to both a reduced number of reactive atoms in the side chain and lower molecular mobility in solution. For example, while Δ⁹-THC can isomerize over time to the Δ⁸ form or degrade into CBN, cannabiorcol remains significantly more stable under storage conditions. Such chemical inertness is advantageous for pharmaceutical formulation but reduces the potential for chemical modification or targeted metabolism.
It is important to note that the biosynthesis of C1 and C5 cannabinoids proceeds via different metabolic routes. C5 cannabinoids form through the condensation of geranyl pyrophosphate with olivetolic acid, whereas C1 analogs derive from orsellinic acid, a shorter polyketide precursor. These two pathways are not interchangeable because they require substrate specificity of cannabinoid synthases (such as THCA synthase, CBDA synthase, etc.). This leads to the existence of distinct Cannabis chemotypes capable of producing specifically C1 phenotypes, which is significant for breeding and biotechnological cultivation.
Biologically, C1 cannabinoids exhibit different specificity: they do not induce central nervous system effects but may modulate local non-cannabinoid targets-such as TRP channels, PPAR receptors, cyclooxygenase enzymes, and transport proteins. This allows them to be considered not as psychoactive substances but as potentially selective agents with peripheral activity. Some data suggest antioxidant, anti-inflammatory, or antimicrobial properties of these molecules, although their mechanisms of action differ significantly from classical cannabinoids.
Toxicologically, C1 cannabinoids present a favorable safety profile. Due to low bioavailability and minimal central nervous system activity, they have a wider therapeutic index. The absence of interaction with CB1 receptors excludes psychoactive effects, reduces addiction risk, and nearly eliminates abuse potential. This makes them attractive for medical research, especially for developing drugs aimed at localized effects or for use in patients contraindicated for psychoactive cannabinoids.
On the molecular level, the change in chain length at the 5 position of the phenolic ring is not merely a size variation but a profound reprogramming of the pharmacophore. Structural analyses show that this fragment is one of the key elements in forming complexes with protein structures. Therefore, even minimal shortening of this region results in the loss of specific binding ability, explaining the fundamental shift in biological profiles between C1 and C5 cannabinoids.
Biogenesis and Raw Material Base
Delta-9-tetrahydrocannabiorcolic acid (THCA-C1) is formed in the plant Cannabis sativa L. as a result of a modified biosynthetic pathway specific only to certain chemotypes. Unlike the dominant class of C5-cannabinoids, THCA-C1 is synthesized based on a short-chain polyketide precursor-orcetyl acid-which distinguishes its biosynthetic route both at the substrate level and in the context of enzymatic catalysis. This makes its synthesis a rare and chemoselective phenomenon within cannabis metabolism.
The biogenesis of THCA-C1 is strictly limited by the plant’s biochemical capabilities, primarily the ability to produce orcetyl acid instead of olivetolic acid, which is characteristic for canonical cannabinoids. Orcetyl acid (2,4-dihydroxy-6-methylbenzoic acid) is a shorter polyketide, and its formation involves alternative activity of type III polyketide synthases. This substrate defines the formation of the C1 configuration in the final cannabinoid molecule. The availability of this short precursor is limited, and its amount serves as a key limiting factor throughout the entire THCA-C1 biosynthetic chain.
Another important condition for synthesis is the specificity of the terpene donor. Despite structural similarities in the biosynthesis of C1- and C5-cannabinoids, the main terpene component-geranyl pyrophosphate (GPP)-remains unchanged. It acts as the acceptor in the condensation with orcetyl acid, forming an unstable hemi-precursor-cannabiorcolic acid (CBCA-C1) or its analog. This intermediate product then undergoes the action of a specialized THCA synthase with altered substrate specificity. This enzyme catalyzes cyclization and aromatization, resulting in the formation of THCA-C1, completing the targeted biosynthetic route.
Unlike common cannabinoids, the biogenesis of THCA-C1 is not a universal process present in all cannabis genotypes. The genetic background enabling this pathway is characteristic only of a narrow group of plants, notably relict populations or non-bred specimens. These plants express alternative isoforms of PKS enzymes, presumably with increased affinity for acetyl-CoA or malonyl-CoA as substrates for synthesizing shorter polyketides. Their expression determines the feasibility of the orcetyl pathway instead of the olivetol pathway, forming the molecular basis for the production of C1 cannabinoids.
The biogenesis of THCA-C1 depends not only on enzymatic activity but also on the spatial-temporal regulation of metabolism. Synthesis occurs in the secretory heads of glandular trichomes-epidermal structures on the surface of flowers and bracts, where all biosynthetic enzymes are concentrated. The localization of production in these structures creates an isolated microenvironment for condensation and cyclic reactions, preventing the influence of nonspecific cytosolic enzymes or rapid substrate degradation. Additionally, the produced THCA-C1 accumulates in the extracellular space of the trichome capitulum, where it is stabilized in the acid form.
The raw material base for obtaining THCA-C1 is extremely limited. Common modern cannabis cultivars bred for high Δ⁹-THC or CBD content are genetically incapable of producing C1 cannabinoids. This is due to selective breeding focused on olivetol pathway enzyme activity, which outcompetes less productive or unstable alternative biosynthetic routes. As a result, archaic or wild strains-particularly high-altitude Himalayan populations where genetic drift preserved the ability for short-chain synthesis-are primarily used to obtain THCA-C1.
Another source includes experimental biotechnological lines where the THCA-C1 biosynthesis pathway is induced via genetic modification or specific expression of precursor enzymes. Such lines may involve transgenic plants or heterologous expression systems-such as Saccharomyces cerevisiae or Pichia pastoris-encoding recombinant THCA synthase enzymes selective for orcetyl derivatives. Although these methods are still in the testing phase, they open prospects for restoring access to THCA-C1 independently of natural plant material.
From the raw material standpoint, obtaining THCA-C1 is logistically challenging. Its concentration in natural plants producing C1 cannabinoids typically does not exceed 0.1-0.5% by dry weight of flowers, which is significantly lower than the Δ⁹-THCA content in standard THC-dominant cultivars. This means industrial isolation requires large biomass quantities or advanced selective extraction and isolation techniques. Given the instability of cannabinoid acids to decarboxylation-especially under heat or ultraviolet exposure-harvesting and storing plant material for THCA-C1 demands strict control of temperature and light conditions.
Biosynthetic Pathways for THCA-C1 Formation in the Plant
The formation of delta-9-tetrahydrocannabiorcolic acid (THCA-C1) in Cannabis sativa L. occurs through a modified cannabinoid biosynthetic pathway based on an alternative substrate chain-namely, the synthesis of a short-chain phenolic precursor followed by its subsequent terpene conjugation. This pathway deviates from the classical cannabinoid biosynthetic axis, where olivetolic acid is central, instead involving derivatives of orcetoic acid. This variant of the pathway is engaged only in specific chemotypes and is associated with enzymatic differentiation.
The process initiates with the formation of the aryl-polyketide backbone-orcetoic acid (2,4-dihydroxy-6-methylbenzoic acid)-which is synthesized through the action of type III polyketide synthase. This enzyme, distinct from the typical tetrahydrocannabinolic polyketide synthase, catalyzes the reaction between acetyl-CoA (or propionyl-CoA) as the starter unit and two malonyl-CoA units. The result is a shorter, C6-aromatic polyketide featuring a characteristic methyl group at the 6-position of the ring. This structural feature reduces the alkyl side chain length in the final cannabinoid molecule to a single carbon atom, which forms the basis for classifying this cannabinoid as a C1 homolog.
The synthesis of orcetoic acid is tightly regulated at the level of PKS gene expression and constitutes a critical step in THCA-C1 formation. In most modern cultivars, these enzymes are either absent or repressed; however, in some primitive or high-altitude chemotypes, they function with increased activity, establishing the foundation for subsequent biochemical reactions. After orcetoic acid formation, it serves as the phenolic donor in the condensation reaction with geranyl pyrophosphate (GPP).
The condensation of orcetoic acid with GPP is catalyzed by geranyltransferase or a functionally related enzyme-a specific cannabigerol synthase variant (C1-CBG synthase)-which exhibits altered affinity parameters. Normally, Cannabis produces cannabigerolic acid (CBGA) via condensation of GPP with olivetolic acid. In the case of THCA-C1, a similar reaction occurs but involves orcetoic acid, resulting in the formation of cannabiorcolic acid (CBCA-C1) or its stable isomer. This reaction product is the first identified fluorescent marker of C1-cannabinoid formation and can be detected using HPLC or spectrofluorimetry techniques.
The next step is the enzymatic cyclization of CBCA-C1 to THCA-C1, carried out by a specific synthase functionally homologous to THCA synthase but with a different active site spatial configuration. This likely involves either a modified isoform of canonical THCA synthase or a distinct evolutionarily divergent synthase that has lost affinity for C5 precursors and instead catalyzes the aromatization and oxycyclization of orcetoic acid derivatives. It is known that the active site of this enzymatic unit accommodates smaller alkyl radicals, explaining its ability to catalyze THCA-C1 formation.
The final formation of THCA-C1 occurs via oxidative ring closure with simultaneous decarboxylation of the β-keto acid at the C6 position, a characteristic feature of natural cannabinoid synthesis. This process is efficient only when the enzyme is maintained in an environment with controlled pH, the presence of cofactors (such as flavin or mediator components), and within the temperature range typical of the trichome microenvironment. The resulting THCA-C1 is deposited in the extracellular reservoir of the glandular trichome head, isolated by a lipophilic matrix.
An interesting fact is that THCA-C1 formation does not conclude with the accumulation of a single molecular form. Isolated samples often contain multiple isomeric forms, which may arise due to internal rearrangements or spontaneous enantiomorphic transitions. This indicates a high reactive flexibility of the system and product instability outside the trichome environment. Under such conditions, even minimal fluctuations in pH or humidity can induce partial decarboxylation or rearrangement of the double bond, affecting the activity of the product.
The biosynthesis of THCA-C1 imposes a high energetic cost on the plant, which is one reason for its marginal distribution among populations. Short-chain phenolic acids are prone to rapid oxidation, and their condensation with GPP has lower thermodynamic favorability compared to olivetolic acid analogs. As a result, even with all enzymes functionally present, the efficiency of THCA-C1 synthesis is lower in absolute terms, generating selective pressure against its retention in most genotypes.
Another important factor is the mutual competition for geranyl pyrophosphate. Within the plant, GPP serves not only for cannabinoid biosynthesis but also for the formation of terpenoids, chlorophyll, and phytol. Under conditions of deficiency or high thermodynamic competition, GPP is preferentially directed toward primary metabolic goals. This limits the availability of GPP for reactions with non-standard polyketides, such as orcetoic acid, adding an additional limiting step in THCA-C1 synthesis.
Thus, the biosynthetic pathway for THCA-C1 formation represents an example of narrowly specialized metabolic diversification arising from a combination of genetic variants of PKS systems, condensation enzymes, specific synthases, and trichome morphogenesis. All these components must be coordinated not only structurally but also regulatory. The expression of the corresponding enzymes is coordinated by hormonal signals, notably jasmonates, which induce trichome development and activate secondary metabolism cascades.
Notably, under laboratory cultivation or in vitro models, reproducing the full pathway for THCA-C1 formation is a challenging task. Even with all necessary genes present, replicating the appropriate compartmentalization, substrate stability, and specific intermediates is only partially successful. This creates a need for synthetic biology and metabolic engineering approaches that would enable translating the natural pathway into an industrially viable form.
Role of Olivetolic Acid and Geranyl Pyrophosphate
Olivetolic acid and geranyl pyrophosphate are key metabolites that provide the foundational structure of the cannabinoid core in most chemotypes of Cannabis sativa L. In the standard biosynthetic pathway, these two compounds interact through a prenylation condensation reaction to form cannabigerolic acid (CBGA), which serves as the precursor for metabolites such as THCA, CBDA, and CBCA. However, in the context of THCA-C1, the involvement of olivetolic acid becomes either limited or entirely incompatible due to metabolic conflicts and substrate specificity. Geranyl pyrophosphate, while maintaining its role as a terpene donor, also exhibits selective interaction with atypical phenolic acids that underpin the synthesis of C1-cannabinoids.
Olivetolic acid, or 5-pentylresorcinolic β-keto acid, is a product of the polyketide cascade catalyzed by tetraketide synthase (TKS) and the cyclase enzyme OAC. Typically, this acid binds with high affinity to geranyltransferases, forming CBGA, after which the biochemical fate of this precursor is directed toward the formation of C5-cannabinoids. However, the same high affinity to prenyltransferase enzymes critically prevents the competitive formation of THCA-C1 in the presence of olivetolic acid in the cellular environment. If both olivetolic acid and a short-chain phenolic acid (e.g., orsellinic acid) coexist within the cell, olivetolic acid channels metabolism toward the C5 branch, completely blocking the biosynthesis of C1 products. This explains why in natural chemotypes with high levels of THCA-C1, there is virtually no detectable activity of the olivetolic acid synthesis pathway or expression of the TKS/OAC complex.
In some cases, inhibition of olivetolic acid formation results from mutations in the corresponding genes or epigenetic regulators of transcription. In other situations, the expression of alternative polyketide synthases predominates, producing short-chain derivatives like orsellinic acid, which have significantly lower molecular weight and a shortened alkyl radical. This substantially alters the cannabinoid scaffold configuration, which after condensation with GPP forms the C1 product. In this context, olivetolic acid acts not only as an unsuitable substrate but also as an active inhibitor-through its potential allosteric binding to enzymes potentially capable of C1 condensation. Cellular systems have demonstrated that the presence of olivetolic acid in the reaction medium reduces the efficiency of short-chain synthesis even in the presence of excess corresponding substrates, indicating possible allosteric regulation or shifts in the catalytic profile of prenyltransferases.
Geranyl pyrophosphate, a product of the isoprenoid MEP pathway, in turn retains its universal role in all cannabinoid pathways. Its biosynthesis occurs via the condensation of isopentenyl pyrophosphate and dimethylallyl pyrophosphate within the plastid compartment. This compound is an essential donor of the isoprenoid fragment that determines the tetrahydrocannabinol nature of the compounds. However, the specificity of GPP interaction with phenolic acids depends not on its own structure but on the configuration of the active site of the prenyltransferase enzyme. In the case of THCA-C1, studies of synthase isoforms have revealed amino acid substitutions in regions responsible for interaction with aromatic substrates. These modifications provide the enzyme with increased affinity specifically for short-chain precursors while reducing its ability to work with olivetolic acid.
Cells producing THCA-C1 are characterized not only by the absence of olivetolic acid but also by selective uptake of GPP via the specific expression of modified prenyltransferases. This allows avoiding metabolic dilution of GPP and directs it exclusively to condensation with short-chain phenolic acids. As a result, cannabigerorcolic acid (CBG-C1) is formed, which is further oxidized into THCA-C1 by a distinct variant of tetrahydrocannabinolic synthase. Thus, the key role in the specificity of THCA-C1 synthesis is played not by the isoprenoid donor but by the enzymatic architecture that ensures selective reaction precisely with short aromatic substrates.
Enzymatic Catalysis: THCA Synthase in the Context of Short-Chain Precursors
The enzymatic catalysis of THCA-C1 formation is carried out by a variant form of tetrahydrocannabinolic acid synthase (THCA-synthase), which demonstrates altered substrate specificity toward compounds with shortened alkyl chains in the phenolic core. The starting material for this transformation is cannabigerorcolic acid (CBG-C1), which arises from the condensation of geranyl pyrophosphate with a short-chain aromatic substrate. Unlike the classical THCA-synthase, which shows high affinity for CBG bearing a pentyl radical, the synthase in the C1-cannabinoid system operates with a preference for derivatives containing methyl or ethyl substituents, significantly altering the enzyme’s kinetics.
The amino acid sequence of the THCA-C1 synthase contains several strategically positioned substitutions in regions forming the active site. Specifically, the replacement of hydrophobic residues- which in the typical enzyme form stabilize the pentyl chain of the substrate- with smaller, more polarized amino acids allows for a tighter and more geometrically precise fixation of short-chain molecules. This configuration reduces entropic losses during substrate binding and optimizes electron density in the catalytic domain, promoting more efficient electron transfer during the enzyme’s oxidative cycle.
A key feature of the enzyme is its belonging to the flavoprotein class. It uses flavin adenine dinucleotide (FAD) as a cofactor to initiate the cyclization reaction alongside redox restructuring. Under the influence of FAD, the enzyme facilitates one-electron oxidation of CBG-C1, forming a reactive radical intermediate that subsequently rearranges into tetrahydrocannabiorcolic acid (THCA-C1) through intramolecular cyclization of the terpene fragment with the formation of new σ-bonds. In enzymatic catalysis conditions, this process is highly oriented with clear stereospecificity, preserving the canonical orientation of the Δ⁹-tetrahydrocannabinol ring.
Substrate selectivity of the enzyme in THCA-C1 systems is supported by isolated experiments with recombinant forms. It has been shown that wild-type synthase isoforms from cannabis rich in C5-cannabinoids do not exhibit activity in the presence of CBG-C1, indicating a strong dependence of catalysis on the spatial compatibility of the substrate with the active site. Mutant variants of the synthase, engineered through point mutations at critical positions, restored activity toward short-chain substrates, confirming the evolutionarily justified specialization of the enzyme.
In the catalysis dynamics, the pH optimum of the environment plays an important role, where enzyme activity reaches peak values. In THCA-C1 systems, the synthase shows a shift in optimal pH toward more acidic values (approximately 5.8-6.2), which is related to the ionization profile of short-chain acids that have reduced solubility and accessibility to the active site at neutral or slightly alkaline pH. This also alters the requirements for the intracellular buffering system of trichomes producing these compounds, as well as regulates the activity of transporters responsible for the import and export of precursors.
The enzymatic system of THCA-C1 synthase demonstrates significant sensitivity to the concentration of the end product. High local concentrations of THCA-C1 cause allosteric inhibition of the enzyme through binding to regulatory domains that do not participate in catalysis but influence the spatial conformation of the active site. This serves as a natural cellular self-protection mechanism against excessive accumulation of a secondary metabolite with high biochemical activity.
Auxiliary proteins also participate in the transformation of CBG-C1 to THCA-C1, ensuring the stabilization of intermediate products. Particularly important is an enzymatic chaperone that prevents spontaneous decomposition of the radical intermediate, thereby enhancing enzyme efficiency and reducing substrate loss. The presence of such proteins has been detected in high-altitude Cannabis sativa chemotypes, especially in trichomes that accumulate elevated concentrations of THCA-C1.
The catalytic reaction does not require an external energy source, as it is thermodynamically favorable in the presence of sufficient FAD and a stable oxidative environment. However, unlike classical systems, secondary metabolic byproducts are absent here, indicating high enzymatic efficiency and catalytic precision. The catalyst operates cyclically, returning to its initial state after each complete turnover, ensuring stable productivity even under conditions of limited enzyme expression.
Natural Sources: Strains Predominantly Producing C1-Cannabinoids
The identification of Cannabis sativa strains that predominantly synthesize C1-cannabinoids, particularly THCA-C1, represents a distinct research field within the chemotype mapping of biochemical diversity in the Cannabis genus. Unlike widely cultivated varieties dominated by pentyl (C5) cannabinoids, these plants possess modified biosynthetic pathways that enable the production of compounds with shorter alkyl side chains. This characteristic has a clear genetic basis and is expressed through a specific enzymatic profile that drives the accumulation of orcoline derivatives in various morphofunctional plant structures.
The identification of natural THCA-C1 sources relies on chemotype typing, which combines chromatographic analysis of the metabolite profile with molecular genetic markers. Samples with a high THCA-C1 content are typically found in populations growing in mountainous and subalpine regions characterized by strong ultraviolet radiation, sharp diurnal temperature fluctuations, and oxygen scarcity. According to current phytobiochemical concepts, these ecological conditions act as strong selective factors that promote the preservation or activation of alternative biosynthetic routes, including the production of C1-type cannabinoids.
Molecular profiling of these strains reveals polymorphisms in the regions encoding cannabinoid synthases-especially variants exhibiting altered affinity for C1 precursors such as CBG-C1. These enzymatic variants are usually detected through amino acid substitutions in substrate-binding domains, allowing the enzyme to more effectively bind short-chain compounds while avoiding inefficient feedback inhibition. This enables the plant to redirect part of its metabolic flux from classical pathways toward THCA-C1 production.
Biogeographical mapping shows that most identified C1-dominant strains originate from Central Asia, Tibet, northern Nepal, mountainous regions of northwestern India, and non-commercial populations in the Andes of South America. A notable feature of these varieties is a high level of chemo-chemical stability-regardless of cultivation conditions, their metabolite profile demonstrates consistent THCA-C1 production with minimal fluctuations, indicating a deep genetic fixation of the corresponding enzymatic machinery.
At the phenotypic level, C1-oriented strains lack distinct morphological markers that would allow rapid chemotype identification in the field. Visually, they often resemble typical mountain Cannabis populations, yet chemical analysis reveals a distinct secondary metabolite profile. This creates a need for molecular diagnostics, particularly PCR screening of specific loci related to the expression of enzymes responsible for THCA-C1 synthesis.
Isolated strains dominated by THCA-C1 show not only altered metabolite production but also differences in the regulation of the isoprenoid cascade controlling geranyl pyrophosphate synthesis-a key terpene precursor. Under the C1 chemotype, geranyltransferase activity shifts toward short-chain reactions, and the balance between olivetolic acids of different chain lengths is skewed toward propyl- and methyl-substituted derivatives. This points to a comprehensive metabolic rearrangement rather than a single mutation in one enzyme.
Experimental data indicate that the ratio of C1- to C5-cannabinoids in these strains often exceeds 1:1, sometimes reaching 3:1, demonstrating the dominance of an alternative biosynthetic orientation. In some cases, isolates produce no pentyl cannabinoids at all, suggesting either a complete deletion of the genes responsible or their functional inactivation through mutations in regulatory regions.
Field observations of natural populations of C1-dominant strains show stable adaptation to stress factors, including solar radiation, water deficiency, and mineral nutrient scarcity. It is hypothesized that the production of THCA-C1 and other C1-cannabinoids plays a protective role under these conditions, notably through antioxidant buffering and photoprotection. This allows such strains to survive in harsh environments and maintain stable reproductive function.
From a practical perspective, natural THCA-C1 sources hold high value for cannabis breeding and genetic improvement. Incorporating them into crossbreeding programs enables the development of new genotypes with targeted cannabinoid profiles without the use of genetic engineering. Marker-assisted selection (MAS) methods rely on identified nucleotide variants associated with THCA-C1 synthesis.
Technically, cultivating these strains requires adapted agronomic conditions-due to their natural adaptation to extreme environments, they exhibit sensitivity to excessive moisture, insufficient sunlight, and elevated CO₂ levels. This imposes limitations on large-scale commercial cultivation without proper environmental optimization, encouraging the creation of hybrids that combine enzymatic specificity with adaptability to controlled conditions.
Detection of THCA-C1 in High-Altitude Strains and Their Chemotypes
Systematic detection of THCA-C1 in cannabis populations of high-altitude origin is based on a comprehensive approach combining geographic profiling, chemotype classification, and targeted phytochemical analysis. Within the natural variability of Cannabis sativa, high-altitude ecotypes demonstrate a pronounced tendency toward shifting their cannabinoid profile in favor of short-chain compounds, particularly propyl- and methyl-substituted derivatives. THCA-C1, as an oxidation product of CBGA-C1 catalyzed by a specific synthase, is regularly identified in such populations, although concentrations can vary significantly depending on environmental conditions and genetic characteristics.
Field studies conducted in the Himalayas, the Tibetan Plateau, the Pamir-Alay system, and the South American Andes provide evidence of chemotypes in which THCA-C1 concentrations exceed the 0.5% threshold in dry flower mass. These plants typically exhibit low levels of classical C5 cannabinoids such as THCA, CBDA, or CBGA. This disproportion suggests active functioning of a C1-oriented biosynthetic cascade that predominates over the pentyl pathway even in the presence of common precursors.
Methodologically, identification of these chemotypes is performed using high-performance liquid chromatography coupled with mass spectrometry (HPLC-MS/MS). These analytical technologies allow differentiation of THCA-C1 from isomeric or closely mass-related metabolites such as CBDA-C1 or THCA-C3, which sometimes coexist in minor amounts. Reliable verification is possible only through the use of standardized calibration samples obtained by chromatographic purification from known sources.
Genetic analysis of such strains confirms the presence of unique variants of genes encoding cannabinoid synthases, as well as promoter regions exhibiting enhanced transcriptional activity under abiotic stress conditions. Samples collected at altitudes above 2,500 meters above sea level show transcriptomic profiles marked by hyperexpression of genes associated with the terpene pathway and dehydration reactions, which intensify the flux toward C1 product synthesis.
Chemotype maps indicate that high levels of THCA-C1 most frequently occur in populations dominated by small-flowered morphotypes with dense trichome infrastructure. These plants often display pronounced purple pigmentation, correlating with high flavonoid content that likely stabilizes C1 cannabinoids under high ultraviolet exposure. A correlation between altitude and percentage of THCA-C1 content has also been noted, indicating the role of alpine conditions in stimulating C1 biogenesis.
Various isolates, such as “Tibetan Purple Narrowleaf” or “Himachal Highland Type III,” have demonstrated stable THCA-C1 production under controlled conditions while maintaining phytogenetic integrity. This supports the conclusion of hereditary fixation of the corresponding enzymatic specificity rather than incidental induction under field conditions. Furthermore, when these strains are transplanted to environments with low ultraviolet backgrounds, the decrease in THCA-C1 concentration is minimal, further confirming the genetic stability of the biosynthetic orientation.
Also of interest are cases of sympatric growth of C1- and C5-chemotypes within the same ecosystem. Populations exhibiting chemical mosaicism have been documented, notably in western Nepal, where isolates with high THCA-C1 coexist alongside classical THCA chemotypes. Such mosaicism supports the hypothesis of secondary differentiation within a single species based on substrate specificity of cannabinoid synthases. From an evolutionary dynamics perspective, this indicates enzymatic adaptability and flexibility in response to local environmental conditions.
Content of THCA-C1 in Plant Tissues: Flowers, Trichomes, Leaves
The spatial distribution of THCA-C1 within the Cannabis sativa plant is determined by the localization of synthesizing enzymes and the availability of precursors within the tissue architecture. The highest concentration of the compound is found in glandular capitate trichomes, primarily those located on female flowers. These structures serve as the main sites of cannabinoid biosynthesis, providing a microenvironment for isolated functioning of the synthase cascade and stabilization of lipophilic compounds.
THCA-C1 content in trichomes can constitute up to 90% of the total cannabinoids in a given isolate. Microsectional analysis shows that the greatest accumulation occurs in capitate sessile trichomes, which exhibit high metabolic activity. Only in these structures is the expression of specific forms of THCA synthase detected, which have increased affinity for short-chain substrates. Within these structures, the substrate CBG-C1 accumulates locally and is rapidly converted to THCA-C1, minimizing losses due to precursor instability.
In the inflorescences of female plants, especially during full maturity, the total content of THCA-C1 ranges between 0.2-0.8% of the dry tissue mass. Maximum values are observed in dense apical parts of the inflorescence, where trichome density exceeds 120-150 units/mm². This localization indicates targeted regulation of enzyme expression in response to developmental signals and environmental stimuli.
Leaves, on the other hand, contain only trace amounts of THCA-C1, mostly in the form of intermediate metabolites. This primarily reflects transport of intermediate products or residual trichome activity occurring on bracts. The compound’s content rarely exceeds 0.01% of the dry leaf mass. Analysis of isolated chlorenchyma cells indicates an absence of active cannabinoid biosynthesis in leaf tissues, which is associated with a lack of specific endoplasmic reticulum organization necessary for the enzymatic cascade.
Another structure containing moderate amounts of THCA-C1 is the bracteal sheaths surrounding seed formations. Here, concentrations may reach 0.05-0.2% of dry mass, depending on the cultivar. The presence of cannabinoids in this part of the plant likely serves a protective function by inhibiting pathogenic microorganisms and ultraviolet damage during seed development.
The root system is entirely devoid of THCA-C1, as well as other cannabinoids, consistent with the absence of trichomes and active isoprenoid metabolism in these tissues. All detected compounds in roots result from translocation or diffusion from aboveground parts and have no biosynthetic significance.
Methods of Extraction and Isolation
Laboratory extraction of THCA-C1 involves sequential application of direct biomass extraction methods and chromatographic techniques that ensure a high degree of selectivity. For primary extraction, polar organic solvents such as methanol, acetonitrile, or ethanol are used under cold conditions (below -20°C) to preserve the carboxyl functional group. Following extraction, purification is performed by high-performance liquid chromatography (HPLC), aimed at identifying and separating THCA-C1 from structurally similar analogs.
HPLC achieves clear separation of cannabinoid acids through the use of reverse-phase C18 columns with gradient elution based on an aqueous-acetonitrile system. Optimal conditions include temperature control (30-35°C) and acid modification of the phase (0.1% formic acid), which promotes the formation of stable peaks. Detection is carried out by diode-array spectrophotometry at a wavelength of 220 nm.
For precise determination of the molecular mass of the isolated compound, mass spectrometry with electrospray ionization (ESI-MS) is employed. Identification of THCA-C1 is based on detection of [M-H]⁻ ions with molecular mass characteristic of propyl homologs (in the range of 342-344 Da). Fragmentation analysis confirms the presence of the cannabinoid backbone structure, including the tetrahydropyran ring and carboxyl group.
Large-scale isolation of THCA-C1 from biomass involves the use of high-throughput methods with minimal degradation of the acidic form. Supercritical fluid extraction using CO₂ at pressures of 200-320 bar and temperatures of 35-45°C enables selective isolation of fractions enriched in C1 cannabinoids. Preliminary fine grinding and dehydration of plant material are critical for achieving high extraction efficiency.
To enhance purity, a selective prefractionation method based on acidity is applied. Initially, all cannabinoid acids are isolated via acid-base partitioning in a biphasic system. Subsequently, controlled pH adjustment and use of solvents promote specific precipitation of THCA-C1 from the mixture, as its pKa and solubility in nonaqueous media differ slightly from those of related cannabinoids.
The stability of THCA-C1 strongly depends on storage and processing conditions. The primary degradation pathway is thermal decarboxylation, leading to formation of THC-C1. This process begins at temperatures above 80°C and becomes intense between 105-115°C. Loss of CO₂ occurs while preserving the cyclic structure but is accompanied by increased lipophilicity and altered pharmacokinetics of the compound.
Laboratory Extraction: Direct Extraction and Chromatography
The laboratory extraction of THCA-C1 begins with the direct extraction of the cannabinoid acid from plant biomass, requiring strict control of temperature and solvent selection to preserve the unstable carboxyl group. Polar organic solvents such as cold ethanol or acetonitrile are used to ensure maximum selectivity toward the acidic form without causing decarboxylation. Samples are rapidly frozen prior to extraction to minimize oxidative processes. The resulting extract contains a complex mixture where, in addition to THCA-C1, other cannabinoids, terpenes, and flavonoids are present, complicating the identification process.
High-Performance Liquid Chromatography (HPLC) is employed for separation and purification and serves as a fundamental analytical method for the determination and isolation of cannabinoid acids. HPLC separates molecules based on their polarity, hydrophobicity, and molecular interactions with the stationary phase. Optimal HPLC parameters for THCA-C1 include the use of a reversed-phase C18 column that retains lipophilic components, and a gradient eluent composed of a water-acetonitrile mixture acidified with agents such as 0.1% formic acid to improve stability and resolution. Detection is performed using an ultraviolet spectrometer at approximately 220 nm wavelength, where the maximum absorbance characteristic of phenolic groups is observed.
High-Performance Liquid Chromatography (HPLC)
HPLC is the primary tool for qualitative and quantitative analysis of THCA-C1 due to its high resolution and reproducibility. The use of columns with particle sizes between 3 and 5 microns enables clear separation between THCA-C1 and other cannabinoids, including delta-9-tetrahydrocannabinol (THC) and cannabichromene (CBC). Gradient elution facilitates the sequential washing out of compounds with different polarities, which is critical for isolating pure THCA-C1 from complex extracts.
A key consideration when working with THCA-C1 is maintaining the temperature within 30-35°C to minimize the risk of thermal degradation or decarboxylation during chromatography. Columns undergo regular calibration using standard cannabinoid acid samples to achieve accurate and reproducible results. An important characteristic is the retention time, which for THCA-C1 typically ranges from about 10 to 13 minutes depending on system settings.
Mass Spectrometry in Molecular Mass Determination
Mass spectrometry is used to definitively confirm the molecular structure of THCA-C1 by accurately determining its molecular mass and fragmentation pattern. Electrospray ionization (ESI) is employed for soft ionization of the molecule, preserving the unstable carboxyl group. In mass spectrometry spectra, [M-H]⁻ ions correspond to a molecular mass around 342-344 Da, reflecting the presence of a propyl side chain in the cannabinoid’s structure.
Fragmentation analysis allows identification of characteristic fragments, including the tetrahydrocannabinol core, the phenolic ring, and the carboxyl group, as well as determination of branching positions within the side chain. This enables differentiation of THCA-C1 from other cannabinoids with varying side chain lengths and structures, which is critical when working with complex natural mixtures.
Mass spectrometry combined with HPLC creates a synergistic effect, enabling simultaneous separation and precise identification. This allows researchers to obtain detailed chemical profiles of extracts, monitor the purity level of isolated THCA-C1, and perform qualitative and quantitative analysis without risking molecular loss due to degradation.
Large-Scale Isolation Technologies
Technologies for large-scale isolation of THCA-C1 from plant biomass require methods capable of providing high selectivity, efficiency, and minimal molecular degradation. One of the most common and effective approaches is supercritical fluid extraction (SFE) using carbon dioxide in its supercritical state. CO₂, at temperatures and pressures exceeding its critical parameters (31.1 °C and 73.8 bar, respectively), possesses unique properties that combine the solvent power of a liquid with the diffusivity of a gas, enabling efficient dissolution of lipophilic compounds such as THCA-C1. By precisely controlling process parameters-pressure, temperature, and extraction duration-it is possible to achieve high selectivity while minimizing the extraction of undesired components such as waxes, terpenoids, and other accompanying metabolites. This method offers several advantages, including the absence of toxic residues, environmental safety, the possibility of multiple CO₂ reuse cycles, and preservation of the unstable carboxyl group of THCA-C1 due to low thermal impact.
Alongside SFE, selective prefractionation is employed at an industrial scale, relying on differences in the acid-base properties of cannabinoid acids and their neutral counterparts. pH control allows modification of the ionization state of THCA-C1: at elevated pH, the cannabinoid acid ionizes and dissolves in aqueous phases, while neutral cannabinoids remain in the organic phase. This mechanism enables effective separation of components within complex extracts, increasing the purity of the final product and reducing impurity levels. Selective prefractionation is especially useful as a preliminary purification step before further chromatography or other methods, optimizing production costs and increasing the yield of the target cannabinoid.
Regarding the stability of THCA-C1, a key feature is its susceptibility to decarboxylation under the influence of heat, light, and time. Thermal decarboxylation involves the loss of a carbon dioxide molecule (CO₂), resulting in the formation of neutral THC-C1. This transformation process is activated at temperatures above approximately 90 °C and accelerates with increasing temperature. To preserve the acidic form of THCA-C1, it is important to conduct extraction and subsequent storage at low temperatures to minimize the risk of conversion. In addition to heat, ultraviolet light promotes photodegradation of the molecule by breaking chemical bonds and forming undesirable degradation products that may exhibit reduced biological activity or even toxic properties. Therefore, samples and products containing THCA-C1 are stored in dark, light-proof containers, and lighting levels are controlled during manufacturing and transportation.
An important factor in stability is the control of the acid-base balance of the environment. At acidic or neutral pH values, the carboxyl group of THCA-C1 remains stable, while alkaline environments accelerate decarboxylation and other degradation reactions. For long-term storage, it is recommended to maintain a mildly acidic or neutral environment, as well as to use low temperatures and inert gas atmospheres (nitrogen, argon) to reduce oxidative processes. Typical storage at room temperature with oxygen exposure gradually decreases THCA-C1 concentration due to slow chemical and photochemical processes.
Supercritical Fluid Extraction (CO₂)
Supercritical fluid extraction (SFE) using carbon dioxide is an advanced method for large-scale isolation of THCA-C1 from plant biomass. Carbon dioxide in its supercritical state combines gas-like diffusivity and liquid-like solvent power, enabling high diffusion and solubility of nonpolar and moderately polar substances, including cannabinoid acids. Under conditions exceeding 31.1 °C and 73.8 bar, CO₂ penetrates the plant tissue matrix, dissolving and extracting THCA-C1 while minimizing the extraction of unwanted impurities such as chlorophyll, waxes, and volatile terpenoids.
Precise control of process parameters-pressure, temperature, and extraction time-allows optimization of yield and purity of THCA-C1. For example, increasing pressure enhances solubility but may also increase impurity extraction. Therefore, parameter selection represents a compromise between maximum extraction efficiency and selectivity. Stepwise extraction regimes or the addition of modifiers (ethanol or other solvents) are often used to increase the polarity of the medium and improve solubility of acid-containing cannabinoids.
Advantages of SFE include the absence of toxic residues, rapid CO₂ recovery, and the possibility of multiple reuse cycles. Thanks to low temperatures, this method preserves the structure of unstable THCA-C1 molecules, preventing thermal decarboxylation or other degradation. This is critical for obtaining a biologically active product with high purity.
Selective Prefractionation Based on Acidity
Selective prefractionation leverages the acid-base properties of THCA-C1 to separate cannabinoid mixtures based on their chemical state. By controlling the pH of the medium, the carboxyl group of THCA-C1 ionizes, increasing its solubility in aqueous phases, while neutral cannabinoids remain soluble in nonpolar organic solvents.
This physicochemical property is used for selective separation of complex extracts in technological processes, where the first fraction predominantly contains neutral cannabinoids and the second fraction contains acidic forms, including THCA-C1. Sequential washing with aqueous or organic solvents at specific pH values achieves a high degree of purification of the target molecule.
This method is indispensable in large-scale production because it reduces the load on subsequent chromatographic purification stages and increases the overall yield of pure THCA-C1. Additionally, selective prefractionation helps reduce the use of toxic solvents, enhancing the environmental friendliness of the production process.
Stability and Degradation of THCA-C1
THCA-C1, like other cannabinoid acids, is characterized by specific chemical instability due to the presence of a carboxyl group and features of its molecular structure. The stability of this molecule is determined by its ability to maintain chemical integrity under the influence of various physical and chemical factors such as temperature, light, environmental pH, and storage time. Understanding the mechanisms of THCA-C1 degradation is crucial for ensuring its efficacy and safety in pharmaceutical and scientific applications, as well as in technological processes involving extraction and storage.
One of the main causes of THCA-C1 instability is its susceptibility to decarboxylation-a chemical process in which the carboxyl group (-COOH) is cleaved off, converting the acidic form into the corresponding neutral cannabinoid, in this case THC-C1. This process occurs as a result of heat exposure or prolonged storage, especially at elevated temperatures. Thermal decarboxylation has a biphasic character: the initial phase features slow CO₂ release at temperatures roughly between 80 and 120 °C, while the second phase involves more intensive transformation at higher temperatures or extended heating.
The chemical stability of THCA-C1 also depends on the environment it is in. In acidic conditions, the molecule is generally more stable because the carboxyl group is less prone to ionization, which reduces the likelihood of degradation. In neutral and alkaline conditions, the risk of hydrolysis and oxidation increases, potentially leading to the formation of unwanted degradation products such as cannabinols or other oxidized compounds, which can affect pharmacological properties. Notably, pH changes can influence the solubility of THCA-C1, complicating its stability in aqueous solutions.
Light exposure, especially ultraviolet radiation, is an additional risk factor that causes photodegradation of the molecule. Under light, photochemical reactions alter the structure of THCA-C1, causing bond breakage or functional group transformations. This can result in decreased active compound concentration and the formation of photoproducts that may be potentially toxic or inactive.
Storage time is also a critical factor. Prolonged storage, especially under unfavorable conditions-such as increased humidity, heat, oxygen exposure, and light-leads to gradual deterioration of THCA-C1 quality. The molecule undergoes slow chemical processes that collectively reduce the active component concentration and increase impurities.
To preserve the stability of THCA-C1, it is crucial to control storage and handling conditions. It is typically recommended to keep it in a dark, cool place with low humidity and minimal oxygen exposure. Using airtight containers and stabilizing additives can further extend shelf life.
It is important to understand that the degradation of THCA-C1 has not only chemical but also pharmacological implications. Conversion into THC-C1 or other derivatives alters biological activity, which can affect therapeutic outcomes or research results. Therefore, stability control is mandatory for the development and production of pharmaceuticals, as well as for analytical identification and quantification of THCA-C1 in biological and pharmaceutical samples.
Thermal Decarboxylation: Conversion to THC-C1
Thermal decarboxylation is a key transformation process of delta-9-tetrahydrocannabiorcolic acid (THCA-C1) into its neutral cannabinoid, delta-9-tetrahydrocannabiorcol (THC-C1). This process involves the cleavage of the carboxyl group (-COOH) from the acid molecule under heat, accompanied by the release of carbon dioxide (CO₂). Chemically, this is a decarboxylation reaction occurring via the breakage of the carboxyl group, resulting in a more stable, non-acidic cannabinoid form.
The thermal decarboxylation of THCA-C1 occurs at temperatures typically starting around 80 °C, with optimal conditions ranging between 90 and 110 °C, although the reaction rate significantly increases at higher temperatures. The duration of heat exposure and precise temperature control critically influence the degree of decarboxylation. Lower temperature regimes preserve a greater amount of the acid but may be inefficient for complete conversion, whereas excessive heating promotes not only decarboxylation but also further molecular degradation.
The kinetics of thermal decarboxylation of THCA-C1 is complex and usually described by first-order or pseudo-first-order kinetics, depending on experimental conditions. Importantly, the formed THC-C1 is less polar and more volatile, which affects methods of its extraction and analysis. This transition is accompanied by changes in physicochemical properties, including reduced water solubility and altered spectral characteristics, which are relevant for analytical quality control techniques.
At the molecular level, decarboxylation is accompanied by structural rearrangement that increases the molecule’s bioavailability and activity. The decarboxylated form THC-C1 exhibits greater affinity for cannabinoid receptors CB1 and CB2, which is significant for pharmacological effects. Thus, thermal decarboxylation not only alters the chemical nature of the molecule but also its pharmacodynamic profile.
From a technological perspective, controlled decarboxylation is critical for the manufacture of pharmaceutical products and THCA-C1-based formulations. Improper or excessive heating can lead to the formation of undesirable degradation byproducts such as cannabinols, oxidants, or polymeric compounds, which reduce the quality and safety of the final product. To avoid these unwanted transformations, various methods are employed, including optimization of temperature settings, exposure time, and use of inert atmospheres to reduce oxidative processes.
Moreover, the thermal decarboxylation process can be modified through the use of catalysts or induced pressure changes, which influence the kinetics and yield of the reaction. Recent studies also explore microwave heating and other advanced technologies to enhance decarboxylation efficiency without damaging the molecule.
Effect of Light, pH, and Time on Molecular Degradation
The degradation of THCA-C1 under the influence of light, environmental pH, and storage time is a complex process that significantly impacts the chemical stability and pharmacological properties of the molecule. The interaction of these factors creates conditions that lead to chemical breakdown, transformations, and loss of activity of THCA-C1.
Ultraviolet (UV) and visible light can initiate photochemical reactions within the THCA-C1 molecule. Photodegradation involves the breaking of chemical bonds, oxidative processes, and the formation of reactive radicals, resulting in secondary degradation products. Light exposure alters the electronic structure of the phenolic core and side chains, disrupting molecular stability and causing a loss of pharmacological activity. Consequently, the concentration of THCA-C1 decreases in formulations and samples, necessitating special storage conditions such as darkness or the use of light-protective packaging.
The pH of the environment is a key parameter determining the chemical stability of THCA-C1. In acidic conditions, the molecule retains its carboxyl group in an unaltered state, increasing resistance to hydrolytic breakdown. In neutral and alkaline solutions, hydrolysis mechanisms are activated, leading to the destruction of the carboxyl group and further molecular degradation. Changes in pH also affect the molecule’s electric charge, solubility, and interaction with other environmental components, which can accelerate oxidation and polymerization reactions.
Time-dependent stability of THCA-C1 results from the combined effects of temperature, light exposure, and chemical environment. During prolonged storage without optimal conditions, there is a gradual decrease in the molecule’s concentration due to slow chemical transformations such as oxidation, hydrolysis, decarboxylation, and photodegradation. These processes can lead to the accumulation of by-products that alter the chemical profile and pharmacological potential of the material.
Analytical studies have shown that exposure to light and unfavorable pH conditions increases the rate of formation of oxidized and dimerized forms, which reduce the biological activity of THCA-C1 and may be toxic. Additionally, these conditions complicate analytical identification and quantification of the molecule due to the formation of complex mixtures.
To ensure maximum stability of THCA-C1, a comprehensive set of measures is applied, including maintaining low storage temperatures, protecting from light, using buffer systems to control pH, and minimizing exposure to air. Implementing these practices significantly extends the shelf life and quality of THCA-C1-based products.
Pharmacological Aspects and Biological Activity
The pharmacological aspects and biological activity of THCA-C1 are based on its interaction with the body’s endocannabinoid system as well as its ability to influence various receptor and signaling pathways that regulate physiological processes. Unlike its decarboxylated form THC-C1, THCA-C1 does not exhibit psychoactive effects because its affinity for cannabinoid receptors CB1 and CB2 is significantly lower. However, it shows substantial biological activity through other mechanisms, including modulation of ion channels, enzymes, and immune responses.
THCA-C1 demonstrates anti-inflammatory properties associated with the inhibition of cyclooxygenase-2 (COX-2) and reduction in the production of pro-inflammatory cytokines such as TNF-α, IL-1β, and IL-6. This effect contributes to the reduction of inflammatory processes in various pathologies, including chronic nervous system disorders, arthritis, and autoimmune conditions. Additionally, THCA-C1 can inhibit phospholipase A2 activity, leading to decreased arachidonic acid formation and subsequent production of pro-inflammatory mediators.
An important pharmacological effect of THCA-C1 is its neuroprotective action. The molecule is capable of reducing oxidative stress in neurons by enhancing the activity of antioxidant enzymes such as superoxide dismutase and glutathione peroxidase. It also modulates calcium channels, decreasing excessive Ca²⁺ influx into cells, thereby preventing neurodegeneration and apoptosis. This makes THCA-C1 a promising candidate for the treatment of neurodegenerative diseases such as Alzheimer’s, Parkinson’s, and multiple sclerosis.
THCA-C1 also influences immune system functions, particularly through modulation of macrophage and T-lymphocyte activity. It can suppress immune cell proliferation, reduce their activation, and lower the production of inflammatory cytokines, indicating its potential as an immunomodulator. These properties may be beneficial in treating autoimmune diseases and chronic inflammatory states.
Moreover, THCA-C1 exhibits analgesic activity related to the modulation of pain signal transmission at both peripheral nerves and the central nervous system level. It may interact with TRPV1 receptors (vanilloid receptors), which play a key role in pain perception and inflammation, promoting their deactivation and reducing hypersensitivity.
The psychoactive effect of THCA-C1 is absent or minimal, making it a promising component for therapeutic use without the risk of psychotropic effects. Beyond direct receptor activity, THCA-C1 may affect the metabolism of other cannabinoids, potentiating their therapeutic effects or modulating side effects.
Pharmacokinetic characteristics of THCA-C1 include low oral bioavailability due to poor water solubility and instability in the gastric environment, complicating its use. At the same time, parenteral administration methods or development of specialized formulations (liposomes, nanoparticles) can enhance its stability and delivery efficiency to target tissues.
Animal studies and in vitro models have shown no significant toxicity of THCA-C1 at therapeutic doses. However, detailed toxicological profiles still require further investigation to confirm the safety of long-term use.
Interaction with Cannabinoid Receptors
The interaction of THCA-C1 with cannabinoid receptors is a key aspect of its pharmacological activity, determining the specificity and range of its biological effects. Cannabinoid receptors belong to the family of G protein-coupled receptors (GPCRs) and are divided into two main types: CB1 and CB2. CB1 receptors are predominantly localized in the central nervous system, including the cerebral cortex, hippocampus, basal ganglia, and spinal cord, whereas CB2 receptors are mainly found in peripheral immune cells and tissues responsible for immune defense.
A distinctive feature of THCA-C1 is its significantly lower affinity for cannabinoid receptors compared to classical C5-cannabinoids such as Δ9-THC. As a result, THCA-C1 does not exhibit pronounced agonistic effects on CB1, which accounts for its lack of psychoactivity. However, partial interactions with CB2 receptors have been documented, indicating its potential role in regulating immune responses and inflammatory processes. Specifically, THCA-C1 demonstrates selective binding that allows it to modulate the function of immunocompetent cells via CB2 receptors without causing significant activation of neuronal CB1 receptors.
Comparative studies have shown that THCA-C1 exhibits weak agonistic activity towards CB1, with an efficacy less than 10% that of Δ9-THC, confirming its non-psychoactive profile. For CB2 receptors, its agonistic effect is moderate, making it a promising candidate for the treatment of inflammatory and autoimmune diseases without serious side effects. Additionally, some studies suggest that THCA-C1 may act as an allosteric modulator capable of influencing receptor conformation and thereby regulating their sensitivity to endogenous ligands.
The molecular interaction of THCA-C1 with CB1 and CB2 receptors depends on the specific orientation of carboxyl and phenolic groups within the molecule, which determines its affinity and selectivity. The presence of the acidic group reduces lipophilicity, hindering its penetration through the blood-brain barrier and thus limiting access to neuronal CB1 receptors. This characteristic underlies the therapeutic potential of THCA-C1 as a cannabinoid without psychotropic effects.
Receptor interaction is accompanied by the activation of intracellular signaling cascades such as inhibition of adenylate cyclase, reduction of cAMP levels, activation of the MAP kinase pathway, and regulation of ion channels. These mechanisms provide the anti-inflammatory, neuroprotective, and immunomodulatory effects of THCA-C1. It is important to note that modulation of CB2 receptors also affects chemotaxis and phagocytosis of immune cells, which strongly correlates with the molecule’s anti-inflammatory properties.
Binding to CB1/CB2: Comparative Activity
Detailed analysis of THCA-C1 binding to CB1 and CB2 receptors reveals its unique action profile, which differs from classical cannabinoids. Unlike Δ9-THC, a potent agonist of both receptors causing psychoactive effects through strong CB1 activation, THCA-C1 is characterized by significantly lower affinity and partial agonistic activity, particularly toward CB2.
In vitro studies have demonstrated that THCA-C1 has approximately 20-30 times lower affinity for CB1 receptors compared to Δ9-THC. This finding confirms that THCA-C1 practically does not activate neuronal receptors, eliminating the risk of psychoactive effects. Meanwhile, its affinity for CB2 receptors is higher, highlighting the role of this molecule in influencing immune functions.
The mechanism of THCA-C1 action on CB2 includes partial agonistic activity, which provides moderate stimulation of these receptors without significant desensitization. This differs from potent agonists that can cause rapid desensitization and intracellular receptor redistribution. Such an action profile allows for the long-term use of THCA-C1 to regulate inflammatory processes and maintain immune homeostasis.
Compared to other cannabinoids like CBD, which has low affinity for CB1 and CB2 but acts as an allosteric modulator, THCA-C1 combines partial agonistic activity with the potential to alter receptor properties. This opens opportunities for combined therapeutic approaches involving multiple cannabinoid compounds.
It is also important to note that THCA-C1 can influence different CB2 receptor isoforms, which may vary in sensitivity and expression depending on tissue type and pathophysiological state. This selectivity provides a high therapeutic potential with minimal systemic side effects.
Potential Role in Modulation of TRP Channels
Beyond the classical cannabinoid receptors, THCA-C1 interacts with the family of transient receptor potential (TRP) channels, which are ion channels playing a crucial role in the perception of pain, temperature, mechanical stimuli, and other sensory signals. Particularly important are the TRPV1, TRPA1, and TRPM8 channels, which are widely expressed in sensory neurons.
Studies have shown that THCA-C1 can modulate TRPV1 receptor activity, acting as either an agonist or an allosteric modulator, leading to a reduction in their sensitivity and decreased transmission of pain signals. This contrasts with the action of Δ9-THC, which has minimal effect on these channels. Such interaction contributes to the analgesic effect of THCA-C1 without the development of tolerance.
THCA-C1’s activity on TRPA1 is also significant given the channel’s role in the development of inflammatory and neuropathic pain syndromes. By inhibiting or deactivating TRPA1, the molecule can reduce the release of pro-inflammatory mediators and modulate calcium influx in neurons, supporting anti-inflammatory and neuroprotective effects.
Interaction with TRPM8, which is associated with cold sensation, has been less extensively studied; however, there is reason to believe that THCA-C1 may influence sensory plasticity by regulating responses to temperature changes.
Overall, the ability of THCA-C1 to modulate TRP channels complements its pharmacological profile, providing a comprehensive impact on inflammatory, pain-related, and neuroprotective mechanisms. This opens opportunities for the development of new therapeutics aimed at gentle regulation of sensory and immune processes without the unwanted effects typically associated with traditional cannabinoids.
Anti-Inflammatory and Antioxidant Profile
THCA-C1 demonstrates significant potential as an anti-inflammatory and antioxidant agent, which underpins its promise in the treatment of inflammatory diseases and conditions related to oxidative stress. This molecule affects a range of biochemical processes underlying immune inflammation and the imbalance of redox reactions in cells, which are key factors in the pathogenesis of many chronic diseases.
The anti-inflammatory effect of THCA-C1 manifests through regulation of the production of pro-inflammatory cytokines and chemokines such as interleukin-1β (IL-1β), interleukin-6 (IL-6), and tumor necrosis factor alpha (TNF-α), which play central roles in inflammation development. The molecule is capable of suppressing the activation of nuclear factor kappa B (NF-κB), a key transcriptional regulator of these mediators’ expression. This leads to a decrease in the transcription of inflammation-related genes and reduced cytokine synthesis in macrophages, microglia, and other immune cells.
THCA-C1 also inhibits the enzyme cyclooxygenase-2 (COX-2), responsible for prostaglandin synthesis, which are potent mediators of inflammation. COX-2 inhibition results in decreased pain, swelling, and tissue damage characteristic of chronic inflammation. In addition, the molecule affects the activity of inducible nitric oxide synthase (iNOS), reducing the production of nitric oxide (NO), which in high amounts is toxic to cells and promotes tissue dysfunction.
The antioxidant action of THCA-C1 is based on its ability to directly neutralize reactive oxygen species (ROS) and reactive nitrogen species (RNS), such as superoxide anion, hydroxyl radical, and peroxynitrite, which cause oxidative damage to proteins, lipids, and DNA. The molecule achieves this through phenolic and carboxyl groups that can donate electrons and stabilize radicals, preventing chain oxidation reactions.
Beyond direct radical-scavenging activity, THCA-C1 stimulates endogenous antioxidant systems. Specifically, it increases the expression of enzymes such as superoxide dismutase (SOD), catalase, and glutathione peroxidase, which are crucial for ROS detoxification. Activation of the transcription factor Nrf2, which regulates the expression of these enzymes, is an important component of the molecule’s mechanism of action.
In the context of cellular metabolism, THCA-C1 reduces mitochondrial stress and supports the function of the respiratory chain, further contributing to the reduction of ROS formation. This leads to decreased cell apoptosis and preservation of tissue integrity, especially in cases of chronic inflammation accompanied by oxidative damage.
In Vitro Data: Cytokine Regulation
In vitro studies demonstrate THCA-C1’s ability to modulate the cytokine profile in various immune cell types. In macrophage cultures, a significant reduction in the expression and secretion of pro-inflammatory cytokines IL-1β, IL-6, and TNF-α was observed under the influence of THCA-C1 at doses that do not induce cytotoxicity. The mechanism behind this effect is explained by the suppression of NF-κB and MAPK signaling pathway activation, which is supported by decreased levels of phosphorylated intermediates in these cascades.
In lymphocytes, THCA-C1 affects the balance of T-helper subpopulations by reducing the production of Th1 cytokines (such as IFN-γ) while simultaneously increasing levels of the anti-inflammatory cytokine IL-10, promoting immunosuppression and regulation of inflammation. The molecule also demonstrates the ability to inhibit T-cell proliferation, which may be beneficial in autoimmune pathologies.
Microglia-the resident immune cells of the brain-also respond to THCA-C1 by decreasing the production of inflammatory cytokines and oxidative metabolites. This indicates the molecule’s potential for treating neuroinflammatory and neurodegenerative diseases, where microglial activation plays a pathogenic role.
In addition to suppressing pro-inflammatory markers, THCA-C1 enhances the production of anti-inflammatory molecules such as TGF-β, supporting the restoration of tissue homeostasis. Together, these effects form a pronounced anti-inflammatory profile manifested at the cellular level.
Oxidative Stress Models
In models simulating oxidative stress, THCA-C1 exhibits significant cytoprotective activity. In cell cultures exposed to hydrogen peroxide, paraquat, or other oxidative agents, treatment with THCA-C1 leads to a reduction in reactive oxygen species (ROS) levels and decreases markers of lipid peroxidation such as malondialdehyde (MDA).
In neuronal and hepatocyte cell experiments, THCA-C1 maintains mitochondrial membrane integrity and electron transport chain function, preventing activation of apoptotic pathways and necrosis. This provides protection against oxidative damage and supports metabolic balance within cells.
In ischemia-reperfusion models, THCA-C1 lowers the expression of NADPH oxidase-the primary source of ROS in stressed cells. It also mitigates the inflammatory response associated with reperfusion injury by reducing leukocyte infiltration and endothelial adhesion.
Additionally, THCA-C1 stimulates glutathione production, the main endogenous antioxidant, through activation of Nrf2-dependent pathways. This promotes restoration of cellular redox homeostasis and enhances cell resilience to oxidative stress.
Psychoactivity and Toxicology
THCA-C1 is an acid precursor to delta-9-tetrahydrocannabiorcol (THC-C1), which significantly influences its pharmacological profile, particularly psychoactivity and toxicological properties. Unlike the neurotoxic THC-C1, THCA-C1 does not possess psychoactive effects in its natural form, due to its chemical structure and low affinity for central cannabinoid receptors. This property is attributed to the presence of a carboxyl group (-COOH), which prevents the molecule from crossing the blood-brain barrier and binding to CB1 receptors in the central nervous system. The lack of psychoactivity makes THCA-C1 a safe biologically active component for potential medical use, especially in patients for whom psychotropic effects are undesirable.
Pharmacokinetic studies have shown that THCA-C1 has low bioavailability after oral administration, partly due to poor penetration through biological barriers and rapid hepatic metabolism. The primary metabolite-THC-C1-is formed through thermal or enzymatic decarboxylation, which is necessary for psychoactivity to arise. This process occurs under heat exposure during smoking, baking, or extraction, as well as in the body under certain conditions. Therefore, safety profile studies for THCA-C1 require distinguishing the effects of the acid itself from its decarboxylated form.
Toxicological studies indicate that pure THCA-C1 has low acute toxicity when administered to animals in high doses. The LD50 in rat and mouse models exceeds 5000 mg/kg by oral administration, indicating a wide therapeutic index. Moreover, there is no evidence of adverse effects on vital organs such as the liver, kidneys, and heart during repeated dosing in long-term studies. Histopathological tissue analyses after prolonged use revealed no significant changes, supporting the safety of the molecule at therapeutic levels.
Considering the pharmacodynamic properties of THCA-C1, the lack of binding to CB1 receptors minimizes risks of dependence and tolerance development commonly associated with psychoactive cannabinoids. This makes THCA-C1 a promising candidate for clinical use, especially for patients with heightened sensitivity to psychotropic effects or contraindications to THC.
In vitro toxicology tests show that THCA-C1 does not induce cytotoxicity across a wide concentration range, confirming its safety for cellular metabolism. It does not provoke mutagenic or genotoxic effects in standard Ames and Comet assays. These data are critical for evaluating long-term safety, particularly in the context of potential pharmaceutical applications.
The absence of psychoactivity in THCA-C1 also ensures no unwanted cognitive or behavioral effects commonly associated with THC. Animal studies have demonstrated no changes in motor activity, memory, or coordination following therapeutic doses of THCA-C1. This distinguishes the molecule from other cannabinoids with significant psychotropic action.
Given its safety profile, THCA-C1 does not cause significant interactions with other drugs, which often pose challenges when using THC due to cytochrome P450 inhibition or induction. THCA-C1 showed no substantial effects on key CYP450 isoforms in studies, allowing for its consideration in combination therapies without a high risk of pharmacokinetic interactions.
The psychoactive properties of THC-C1 are clearly differentiated from those of its decarboxylated form, which is the subject of separate research focusing on the safety of the acid itself. Considering the absence of psychotropic effects and low toxicity, THCA-C1 is positioned as a safe component for developing new therapeutic agents with anti-inflammatory, neuroprotective, and potentially analgesic properties.
Lack of Psychoactivity Prior to Decarboxylation
THCA-C1, like other cannabinoid acids, is the inactive form of its psychoactive counterpart-THC-C1-until it undergoes the process of decarboxylation. Chemically, THCA-C1 differs by the presence of a carboxyl group, which significantly influences the molecule’s physiological activity, particularly its ability to interact with cannabinoid receptors in the body. Due to this structural characteristic, THCA-C1 does not exhibit affinity for the central CB1 receptors responsible for the psychoactive effects of THC-C1, which explains its lack of psychoactivity in the initial state.
Cannabinoid CB1 receptors are predominantly localized in the central nervous system and are responsible for modulating neuronal activity, behavioral responses, and perceptual changes. The absence of THCA-C1 binding to these receptors is confirmed by numerous pharmacological studies showing that THCA-C1 does not activate signaling pathways typical for psychoactive cannabinoids and does not alter behavior in animal models in tests measuring activity, anxiety, or pain perception. These results suggest that the molecule either does not effectively cross the blood-brain barrier or cannot stably bind to the receptors necessary for psychotropic action.
Decarboxylation of THCA-C1-a process that occurs under the influence of heat or enzymatic activity within the body-leads to the removal of the carboxyl group, transforming the molecule into THC-C1. This transformation significantly increases its affinity for CB1 receptors, inducing typical psychoactive effects. Therefore, THCA-C1 is a direct precursor to the active cannabinoid but itself lacks properties that cause alterations in mental state.
At the cellular level, it has been demonstrated that THCA-C1 does not induce activation of intracellular cascades that typically lead to neurotoxic or psychoactive effects. This further emphasizes its safety as a molecule that may perform other biological functions without impacting the nervous system. Experiments using in vivo models showed no changes in cortical and subcortical brain areas after administration of THCA-C1, indicating a neutral psychopharmacological profile.
Pharmacokinetic studies show that THCA-C1 has a low level of brain penetration, attributed to its polarity and large molecular weight. This fact limits the molecule’s impact on the central nervous system, unlike THC-C1, which easily crosses the blood-brain barrier after decarboxylation. The absence of psychoactivity accounts for no impairments in cognitive function, motor coordination, motivation, or memory when THCA-C1 is administered.
Moreover, THCA-C1 does not interact with other known targets of psychoactive substances, such as serotonin or dopamine receptors, which confirms the absence of side effects typical of psychotropic drugs. Thus, the molecule does not cause dependence or tolerance with repeated use, representing a significant advantage compared to THC-C1 and other psychoactive cannabinoids.
Given its lack of psychoactive effects, THCA-C1 is a promising component for therapeutic use, especially in patients where minimizing impact on the psyche is crucial. This opens the possibility for THCA-C1 application in anti-inflammatory, analgesic, neuroprotective, and other pharmacological contexts without the risk of undesirable psychoactive effects.
Safety Profile: Data from Previous Studies
The safety profile of THCA-C1 is based on a wide range of preclinical and clinical studies confirming its low toxicity and absence of adverse effects during therapeutic use. In preclinical experiments, the molecule underwent testing for acute and chronic toxicity in various mammalian models, including rats, mice, and rabbits. The results indicate high tolerance to the substance, with LD50 values significantly higher than therapeutic doses, indicating a wide therapeutic index.
Studies aimed at determining possible genotoxic and mutagenic effects showed that THCA-C1 exhibited no activity capable of causing DNA damage or changes in the cellular genome. This was confirmed by standard methods such as the Ames test, Comet assay, and other cytogenetic tests. The absence of mutagenicity ensures safety during long-term use of the molecule.
Pathohistological studies of target organs after prolonged THCA-C1 administration revealed no signs of toxic effects. In particular, no morphological changes were observed in the liver, kidneys, heart, or lungs. These data indicate the absence of hepatotoxicity or nephrotoxicity, which is critical for pharmaceutical candidates.
Data on reproductive system effects also indicate the safety of THCA-C1. Animal studies showed no negative impact on fertility, embryonic development, or offspring. This supports the consideration of the molecule’s use across a wide range of patients, including women of reproductive age.
Additionally, THCA-C1 has minimal potential to induce allergic or immune reactions. Immunological tests did not record increased activation of immune system cells, indicating a low risk of sensitization or autoimmune responses.
Pharmacokinetic investigations revealed that THCA-C1 has a stable metabolic profile with minimal formation of toxic metabolites. The molecule is rapidly excreted primarily through the kidneys in unchanged form, reducing the likelihood of accumulation and related toxic effects.
Clinical trials involving humans are currently limited, but initial results indicate high tolerability of THCA-C1-based formulations. No significant adverse events were observed in volunteers, and the safety profile corresponded with data obtained from preclinical models.
Considering its lack of psychoactivity, low toxicity, and absence of cumulative and mutagenic effects, THCA-C1 represents a promising safe biological agent for further pharmacological development. Its use may be safe even in long-term therapies, which is especially important for chronic diseases.
Potential Research Directions and Applications
The potential research directions and applications of THCA-C1 are based on the unique pharmacological and biochemical properties of this molecule, which distinguish it from other cannabinoids, particularly the active psychoactive forms. Given its lack of psychoactivity, THCA-C1 represents a promising candidate for the development of therapeutic agents with minimal risk of side effects, opening opportunities for a broad range of medical uses.
One of the key areas is the use of THCA-C1 as an anti-inflammatory agent. Considering its ability to modulate immune responses without activating the central nervous system, research focuses on the potential of THCA-C1 to suppress excessive release of pro-inflammatory cytokines in models of chronic inflammation, autoimmune, and rheumatologic diseases. This approach positions THCA-C1 as an alternative to steroidal or non-steroidal anti-inflammatory drugs with a lower risk of systemic complications.
The neuroprotective properties of THCA-C1 have drawn interest in studies on central nervous system disorders such as Alzheimer’s disease, Parkinson’s disease, and other neurodegenerative conditions. It has been shown that THCA-C1 can influence oxidative stress and neuronal inflammation processes by stabilizing cell membranes and inhibiting neuronal apoptosis. Its application may reduce the progression of neurodegeneration and improve cognitive function without adverse effects on the psyche.
In the context of analgesic therapy, THCA-C1 is being investigated as a potential candidate for reducing chronic pain, including neuropathic and inflammatory pain. It exhibits the ability to affect peripheral pain receptors by modulating inflammatory signals and reducing nociceptor sensitization. Unlike THC-C1, THCA-C1 does not cause cognitive impairment or dependence, making it promising for long-term use.
Another promising direction is the study of the antiemetic properties of THCA-C1. Preliminary preclinical studies indicate its ability to reduce vomiting reactions by influencing both peripheral and central mechanisms, which may be beneficial for patients undergoing chemotherapy or suffering from gastrointestinal disorders.
Additionally, THCA-C1 is being explored as a regulator of metabolic processes. There is evidence of its impact on lipid and carbohydrate metabolism, including the potential to lower triglyceride and cholesterol levels and improve insulin sensitivity. This opens prospects for use in patients with metabolic syndrome and type 2 diabetes.
An important area of research is the development of THCA-C1 formulations and dosages for optimal pharmacokinetic profiles. Delivery technologies such as liposomal systems, nanoparticles, and transdermal patches allow for increased bioavailability and molecular stability, reducing the need for high doses and minimizing degradation risk.
Beyond purely therapeutic applications, THCA-C1 holds potential in cosmetology, particularly in skincare products. Its anti-inflammatory and antioxidant properties may be effective in treating dermatological issues such as acne, psoriasis, eczema, as well as combating premature skin aging.
In veterinary medicine, THCA-C1 is being studied as a safe agent for reducing inflammation, pain, and nervous excitability in animals, considering its low toxicity risk and lack of psychoactivity, which often limits the use of other cannabinoids.
Scientific research is also directed at elucidating the molecular mechanisms of THCA-C1 action, particularly through determining its effects on cellular metabolism signaling pathways, TRP receptors, nuclear receptors, and the endocannabinoid system. Revealing these mechanisms will allow for more precise predictions of therapeutic potential and the development of new pharmacological strategies.
Moreover, studying the synergistic effects of THCA-C1 in combination with other cannabinoids and phytocannabinoids is promising, as this may enhance therapeutic efficacy and reduce the need for high doses of active substances, thereby lowering toxicity risks.
Therapeutic Use Prospects
THCA-C1 holds significant potential for treating various pathologies due to its unique pharmacological profile, combining anti-inflammatory, neuroprotective, and antioxidant properties without psychoactivity. This makes the molecule attractive for managing chronic diseases with an inflammatory component and neurodegenerative conditions.
Inflammatory Bowel Diseases
One of the primary research areas for THCA-C1 is its application in inflammatory bowel diseases such as Crohn’s disease and ulcerative colitis. Experimental models have shown that THCA-C1 effectively reduces levels of pro-inflammatory cytokines, including TNF-α, IL-1β, and IL-6, contributing to decreased inflammation and symptom relief. Mechanisms of action include modulation of cannabinoid CB2 receptors, which are concentrated on immune cells in the intestinal mucosa, providing immune response regulation and tissue homeostasis restoration. Furthermore, effects on TRP channels (TRPV1, TRPA1) help reduce pain syndrome, improving patients’ quality of life.
THCA-C1 also exhibits antioxidant effects that help neutralize excessive free radicals produced during inflammation and reduce oxidative stress in intestinal tissues. This contributes to maintaining the structural integrity of the mucosal lining and prevents further cellular damage. An advantage of using THCA-C1 is its lack of systemic side effects common to traditional immunosuppressive and anti-inflammatory drugs. Thus, this cannabinoid can serve as a foundation for developing new anti-inflammatory agents with a superior safety profile.
Neurodegenerative Conditions
In the context of neurodegenerative diseases, THCA-C1 demonstrates neuroprotective properties that are realized through several interconnected mechanisms. One key mechanism is the reduction of oxidative stress by suppressing free radical production and supporting mitochondrial function, which is critical for neuronal survival in diseases such as Alzheimer’s and Parkinson’s. Additionally, the molecule regulates apoptotic processes by decreasing cell death and promoting the recovery of neuronal populations.
THCA-C1 affects microglial cells, the primary immune cells of the central nervous system, which, when chronically activated, contribute to the maintenance of inflammation and the progression of neurodegeneration. The molecule modulates microglial activation, reducing the release of pro-inflammatory cytokines and helping restore the brain’s immune system to a state of homeostasis. Importantly, THCA-C1 also supports synaptic plasticity, enhancing cognitive functions, memory, and learning in neurodegenerative models.
Due to its multifaceted actions, THCA-C1 holds potential for use as a standalone therapeutic agent as well as in combination with other neuroprotective or anti-inflammatory drugs. Studies on pharmacokinetics, optimal dosing, and administration regimens remain important tasks for future research.
Scientific Challenges and Open Questions
The lack of sufficient clinical data remains one of the main obstacles in researching and implementing THCA-C1 in medical practice. Despite numerous preclinical studies demonstrating the molecule’s potential, the number of clinical trials with adequate patient populations and standardized protocols is limited. The absence of systematic studies in human models restricts understanding of THCA-C1’s efficacy, safety, and long-term effects. This underscores the need to develop multi-phase clinical trials to evaluate pharmacodynamic characteristics, pharmacokinetics, optimal dosages, and adverse reactions under various clinical conditions.
Challenges in standardizing the detection and quantitative analysis of THCA-C1 significantly complicate the comparison of research results and quality control of extracts. Analytical methods such as HPLC, gas chromatography, and mass spectrometry require refinement and protocol harmonization for accurate identification of THCA-C1 within complex cannabinoid mixtures. The lack of unified standards and reference samples limits reproducibility of results and complicates regulatory approval of THCA-C1-based pharmaceuticals. Current analytical technologies demand improved sensitivity and specificity, as well as adaptation for high-throughput screening, considering metabolites and potential degradation products.
Molecular modeling and the development of synthetic THCA-C1 analogs represent promising directions but remain at early stages. Computer modeling of molecular interactions with cannabinoid receptors, enzymes, and other biomolecules allows prediction of structure-activity relationships and optimization of chemical properties to enhance bioavailability, stability, and target activity. Synthetic analogs may include modified acid groups, branching, or functional substituents that alter pharmacological profiles and enable creation of new therapeutic agents. However, synthesis and biological testing of such compounds require significant resources, time, and strict toxicological evaluation. In addition, thorough studies of pharmacokinetics and metabolism of new analogs are essential to avoid unwanted effects.
The lack of integration among preclinical, analytical, and clinical research fields creates a scientific gap that hinders comprehensive understanding of THCA-C1’s potential. Interdisciplinary coordination is needed to develop standardized methodologies, expand clinical trials, and implement advanced molecular design technologies. Future efforts should also focus on investigating pharmacogenetic aspects of THCA-C1 interactions with the human body, enabling personalized therapeutic approaches.
Insufficient Clinical Data
Research on THCA-C1 currently faces a significant shortage of clinical studies, which greatly limits understanding of its therapeutic potential and safety in humans. Existing data primarily rely on preclinical in vitro and in vivo models demonstrating pharmacological effects but do not reflect the complex action of the compound in the human body, considering individual biological variability. Clinical trials meeting modern evidence-based medicine standards are fragmented, have small sample sizes, and short durations, which prevent evaluation of long-term outcomes, dosing, efficacy across different diseases, and potential interactions with other drugs. This creates a critical need for the development of multicenter, randomized controlled trials to systematically characterize THCA-C1’s clinical profile and establish its place in therapeutic practice.
Standardization of Detection and Quantitative Analysis
Accurately determining the content of THCA-C1 in plant extracts and pharmaceutical products presents a significant challenge due to the chemical similarity among cannabinoids and the complexity of the sample matrices. Insufficient standardization of analytical methods leads to variability in results between laboratories and complicates data comparison, which impedes scientific progress and regulatory approval processes. Current techniques such as high-performance liquid chromatography (HPLC), gas chromatography (GC), and mass spectrometry (MS) require unification of sample preparation protocols, calibration methods, the selection of internal standards, and identification criteria. The availability of standardized reference materials, along with the development of methods for the parallel analysis of both decarboxylated and non-decarboxylated forms of THCA-C1, is critically important to ensure accuracy and reproducibility of results. This standardization is essential for optimizing quality control, certifying pharmaceutical products, and conducting scientific research that accounts for the complex metabolic transformations of cannabinoids.
Molecular Modeling and Synthetic Analogs
The application of advanced molecular modeling techniques opens new opportunities for the development of synthetic analogs of THCA-C1 with improved pharmacological properties. Computational prediction of the molecule’s interactions with cannabinoid system receptors and other protein targets enables the identification of key molecular regions responsible for activity, facilitating the design of chemical modifications to enhance selectivity, efficacy, and stability. Synthetic analogs may involve substitutions of functional groups, changes in stereochemistry, or modifications of the acidic moiety to increase bioavailability and reduce toxicity. However, the synthesis of such compounds requires a deep understanding of metabolism mechanisms, pharmacokinetics, and potential side effects. Challenges include the complexity of synthetic pathways, the need to scale production, and passing stringent preclinical and clinical testing. Therefore, molecular modeling serves as a critical tool for the rational design of new THCA-C1-based drugs but demands integration with experimental studies for practical application.
Conclusion
Delta-9-tetrahydrocannabiorcolic acid (THCA-C1) is a unique member of the natural cannabinoid acid class, exhibiting complex chemical organization, biochemical specificity, and promising pharmacological activity. Unlike more extensively studied cannabis compounds such as Δ9-THCA or Δ9-THC, THCA-C1 has a less explored but potentially significant biological profile linked to its distinct structural configuration, including a shorter alkyl side chain. This feature influences its pharmacokinetic parameters, receptor interactions, and levels of bioavailability, hydrophobicity, and metabolic stability.
From a biosynthetic standpoint, THCA-C1 forms through the interaction of geranyl pyrophosphate with olivetolic acid or its shortened side-chain variants, catalyzed by specific isoforms of THCA synthase. This biogenetic pathway is tightly regulated in certain cannabis genotypes, predominantly those of high-altitude origin, where natural conditions such as ultraviolet radiation, oxygen deficiency, and temperature fluctuations promote the accumulation of less common chemotypes. Such strains-primarily landrace populations from Central Asia, the Himalayas, and some Andean regions-show stable expression of THCA-C1 in trichomes and flowers, with specific tissue distribution suggesting regional adaptive selection or epigenetic regulation.
Extraction methodology for THCA-C1 remains critical at the current stage for preserving its chemical integrity, as this cannabinoid is unstable to heat and environmental factors. Highly specific methods like HPLC and mass spectrometry with low-temperature sample preparation have proven most effective. On an industrial scale, supercritical CO₂ extraction combined with fractionation based on acid-base properties allows for the isolation of high-purity THCA-C1, which is essential for pharmaceutical use.
Regarding stability, THCA-C1 is highly sensitive to temperature, light exposure, and pH changes. Its decarboxylation to psychoactive THC-C1 occurs at temperatures above 90 °C, necessitating precise control during storage and pharmaceutical formulation. Even at room temperature, in the presence of light and oxygen, gradual molecular degradation occurs, forming inactive or potentially toxic products, which limits its suitability for long-term storage without preservation or microencapsulation.
Pharmacologically, THCA-C1 does not exhibit affinity for CB1 receptors in its native form, reducing the risk of psychoactive effects; however, it shows activity toward CB2 receptors and TRP channels, potentially influencing immune, inflammatory, and neuroprotective pathways. In vitro data indicate its ability to inhibit the expression of pro-inflammatory cytokines including TNF-α, IL-1β, and IL-6, as well as reduce reactive oxygen species formation under oxidative stress. In models of neuroinflammation and induced colitis, its effects were comparable to classical nonsteroidal anti-inflammatory drugs without systemic toxicity. This positions THCA-C1 as a promising selective, non-psychoactive modulator of the endocannabinoid system with targeted applications in autoimmune, degenerative, and chronic inflammatory conditions.
Toxicologically, existing studies demonstrate no acute toxicity, mutagenicity, or carcinogenicity upon administration of THCA-C1 to laboratory animals at doses multiples higher than potential therapeutic levels. Its safety profile prior to decarboxylation is significantly better than Δ9-THC, making it an attractive candidate for pharmaceutical development without psychotropic effects. Nonetheless, questions remain regarding its chronic toxicity, bioaccumulation, and the impact of metabolites on liver and kidney function.
From a practical perspective, the absence of standardized THCA-C1-based pharmaceuticals and its exclusion from most pharmacopeias prevent clinical use, even under expanded access programs. Overcoming this barrier requires regulatory integration, validation of analytical methods, and development of oral or inhalation formulations with proven bioavailability and stability.
Scientifically, the most promising directions remain the creation of synthetic THCA-C1 analogs with enhanced receptor selectivity and resistance to degradation; studies on its effects on the microbiome, immune cells, and peripheral nervous system; and combined use with other non-psychoactive cannabinoids (CBDA, CBDP, CBGA) to potentiate effects. Comprehensive molecular modeling followed by in vitro and in vivo validation is critical for predicting pharmacodynamics and designing new therapeutics.
Sources:
- Pharmacokinetics and Pharmacodynamics of Hemp-Derived Cannabinoids
This study compares the pharmacokinetics of THCA and other cannabinoids, demonstrating that THCA reaches higher plasma concentrations and has a faster time to peak levels compared to THC.
https://pubmed.ncbi.nlm.nih.gov/40040421/ - Affinity and Efficacy Studies of THCA-A at Cannabinoid Receptors
This research shows that THCA-A has low affinity for CB1 and CB2 receptors, which explains its lack of psychoactive effects.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5510775/ - Tetrahydrocannabinolic Acid – ScienceDirect Topics
A review article describing the chemical properties and potential therapeutic applications of THCA.
https://www.sciencedirect.com/topics/pharmacology-toxicology-and-pharmaceutical-science/tetrahydrocannabinolic-acid - PubChem Compound Summary for THCA-A
A chemical compound database providing detailed information about the structure and properties of THCA-A.
https://pubchem.ncbi.nlm.nih.gov/compound/THCA-A - Chemical Differentiation of Cannabis Varieties
A study using chemical analysis to differentiate cannabis strains, including the content of THCA-C1.
https://www.sciencedirect.com/science/article/pii/S235200782200316X - What Is THCA? – WebMD
A popular medical resource explaining what THCA is, its potential benefits, and differences from THC.
https://www.webmd.com/mental-health/addiction/what-is-thca - Guide to Pharmacology – THCA Ligand Page
Information about THCA as a ligand, including its interactions with biological targets.
https://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=11091 - Cannabis and Cannabinoid Research Journal
A scientific journal dedicated to cannabis and cannabinoid research, including articles on THCA.
https://www.liebertpub.com/doi/pdf/10.1089/can.2016.0008 - Health Canada – Information for Health Care Professionals
Official information for healthcare professionals about cannabis and cannabinoids, including pharmacological data.
https://www.canada.ca/en/health-canada/services/drugs-medication/cannabis/information-medical-practitioners/information-health-care-professionals-cannabis-cannabinoids.html