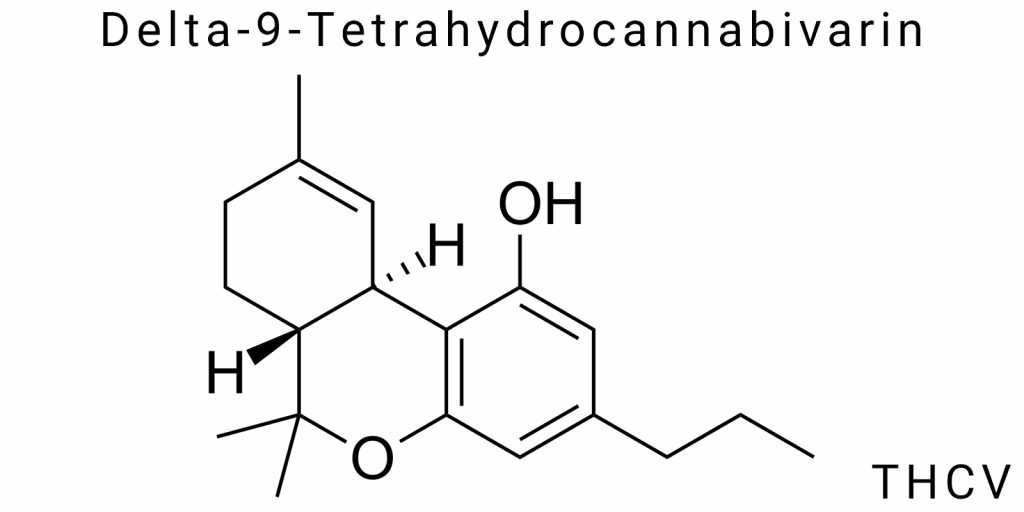
Among the numerous phytocannabinoids produced by the Cannabis sativa L. plant, Delta-9-tetrahydrocannabivarin (THCV) draws the attention of researchers as a chemically nonstandard and pharmacologically multifaceted molecule. Structurally similar to Δ9-tetrahydrocannabinol (THC), THCV differs in the length of its alkyl side chain, which significantly alters its receptor activity and metabolic profile. Despite its morphological resemblance to the primary psychoactive components of cannabis, THCV exhibits a unique ability to act as a CB1 cannabinoid receptor antagonist at low doses and as an agonist at higher concentrations, demonstrating complex biophysiological behavior.
This cannabinoid belongs to the class of varin compounds, which feature a propyl (C3) side chain instead of the pentyl (C5) chain characteristic of THC. This variation not only alters the chemical reactivity of the molecule but also affects its absorption, distribution, metabolism, and excretion. From the perspective of chemical evolution, the appearance of THCV in specific chemotypes of Cannabis sativa indicates the presence of an alternative biosynthetic pathway, involving the specific participation of divarinolic acid as a precursor. The biosynthetic isolation of this pathway through the enzymatic activity of THCVAS (tetrahydrocannabivarin synthase) opens the possibility for modular control over the plant’s secondary metabolism.
THCV exhibits multifunctional effects in biological systems, particularly in neurohumoral regulation, glucose homeostasis, and energy metabolism. In preclinical models, it has demonstrated the ability to suppress appetite, inhibit excessive fat accumulation, improve insulin sensitivity, and exhibit neuroprotective properties. At the same time, the absence of psychoactive effects at low doses provides it with a pharmacological advantage over classical cannabinoids in a therapeutic context. All of this contributes to the formation of a distinct research paradigm for THCV as a compound with potential functional selectivity.
However, current knowledge about THCV remains fragmented, mainly due to the limited number of cultivars with a high content of this compound, restricted access to standardized extraction methods, and the complexity of tracking its pharmacokinetics in vivo. Furthermore, regulatory uncertainty and a low level of commercial standardization complicate the study of THCV in a clinical context, despite its apparent potential. This article aims to collect and critically analyze the chemical, biosynthetic, pharmacological, and biotechnological aspects of THCV as a research subject within contemporary cannabinoid science. Particular attention is given to the latest approaches to analytical identification, chemotypic variability of the producing plant, purification methods, synthetic biology prospects, and regulatory factors shaping the future of this compound in medical, pharmaceutical, and agri-biotechnological practice.
Structural and Biochemical Identity of THCV
Delta-9-tetrahydrocannabivarin (THCV) belongs to a narrow class of natural cannabinoids that demonstrate molecular similarity to Δ9-tetrahydrocannabinol, but with distinct structural and biochemical divergence. Its molecular formula is C19H26O2, reflecting the presence of a carbon skeleton and the tricyclic architecture typical of cannabinoids, albeit with a modified hydrocarbon chain. THCV is formed within the tertiary metabolism of cannabis, belonging to the group of cannabivarins-structural analogs of classical cannabinoids with a shortened side chain. However, unlike most lesser-known varin compounds, THCV demonstrates functional receptor activity and synthetic reproducibility, making it a promising target molecule for both pharmacological screening and chemical identification.
Its structure is defined by the presence of three key elements: a phenolic core, an isoprenoid side system, and a variable cyclic bridge that forms a trans- or cis-configuration of the double bond within the tetrahydrocannabinol ring. The trans-configuration of the Δ9-double bond in particular ensures THCV’s specificity to cannabinoid receptors of the CB1 and CB2 types, although the degree of affinity to each varies depending on dosage and isomeric state. The THCV molecule is chiral, with one asymmetric center that forms a specific spatial orientation of functional groups necessary for receptor recognition.
Biochemically, THCV is not the product of direct decarboxylation of THC analogs but arises as a distinct metabolic entity at early stages of cannabinoid biosynthesis. Its isolation in native form is complicated by low concentration in most Cannabis sativa chemotypes and the need for precise chromatographic separation in the presence of structurally similar cannabinoids, including Δ9-THC, CBDV, and CBCV. Therefore, chemical identification of THCV requires the use of highly specific instrumental methods focused on separating isomers and alkyl chain variants.
Particular attention is warranted by the polarity characteristics of THCV, which significantly influence its solubility, bioavailability, and reactivity. Its partial hydrophobicity is due to the saturated hydrocarbon nature of its primary skeleton, while the hydroxyl group in the phenolic ring provides limited ability to form hydrogen bonds. This balances the molecule between lipophilic and amphiphilic properties, resulting in its accumulation in lipid environments (notably, cellular membranes) while enabling pharmaceutical microencapsulation and transport in aqueous systems under modification.
Unlike THC, THCV demonstrates a reduced degree of isomerization in acidic environments and under thermal processing, making it less prone to non-enzymatic conversion into CBN derivatives (cannabinols) under oxidative conditions. This stability is of significant importance for the standardization of cannabinoid extracts and long-term storage of pharmacologically active substances without loss of bioactivity. This very characteristic distinguishes THCV as a more thermodynamically stable representative of tricyclic cannabinoids, which is critical for pharmaceutical formulation.
Molecular profiling of THCV reveals distinctive spectral properties across IR, NMR, and mass spectrometry, allowing for precise recognition even in complex matrices. For example, in NMR spectra, THCV exhibits characteristic shifts of the methylene group signals in the propyl chain, which allows for clear differentiation from other cannabinoids with pentyl or butyl chains. In mass spectra, the key fragments are ions with masses of 286, 271, and 259, reflecting specific decomposition patterns under electron ionization analysis. These parameters play an essential role in developing methods for quantitative analysis of THCV in biological samples, including blood plasma, tissue extracts, or Cannabis sativa extracts.
It is also worth noting that THCV shows potentially high chemical derivatization capacity, especially through modification of the hydroxyl group or alkylation of the hydrocarbon chain. This opens prospects for creating semi-synthetic derivatives with predictable pharmacological profiles but at the same time complicates standardization in the case of natural extraction. Thus, THCV may be considered not only a biologically active molecule but also a platform for creating new cannabinoid derivatives with controlled biopharmaceutical properties.
Structural Features of the Cannabivarin Skeleton: The Varin Side Chain
The molecular uniqueness of THCV is defined by the presence of a varin side chain-a short-chain alkyl fragment that is a fundamental distinction from classical cannabinoids with a pentyl radical. The varin structure is formed by incorporating a propyl (C3) substituent instead of the traditional pentyl (C5), resulting in a chemically and physiologically distinct skeleton. This aspect is not merely a case of constitutional substitution; it radically transforms the molecule’s geometry, electron density, and spatial configuration, affecting both its pharmacodynamics and interaction with membrane receptors and enzymatic systems.
The varin side chain is formally represented by a propyl chain attached to the fifth position of the phenolic ring within the prenylated monoterpenoid skeleton. This structural feature results in a relative reduction in molecular weight, decreased lipophilicity, and a shift in the center of electron density. In a physicochemical context, this impacts molecular volume parameters, the critical microenvironment in receptor binding, and submolecular interaction properties-particularly in relation to CB1 receptors, which are highly sensitive to the length and flexibility of the side chain.
The varin radical significantly reduces van der Waals interactions with the hydrophobic pocket of the CB1 receptor, which in turn explains the antagonistic action of THCV at low doses. This also influences the so-called entropic component of binding-the reduced flexibility of the chain in varin compounds leads to smaller entropy losses during receptor complex formation. Thus, the cannabivarin skeleton optimizes the energetic profile of interaction through a more compact structure. Experimental pharmacology has established that even minimal elongation of the varin side chain to butyl or pentyl restores agonistic activity, indicating the fundamental role of the propyl radical in conformational recognition.
Stereochemically, the varin group does not introduce asymmetric centers, but it modifies the steric accessibility of other chiral centers, especially in the cyclopentane-derived portion of the molecule. This effect is critically important in the formation of so-called short-acting metabolites produced via microsomal oxidation. The shorter chain makes the molecule less prone to long-term accumulation in adipose tissue-another property that correlates with the known effects of THCV as a potential anorexigenic and antidiabetic agent.
From the standpoint of chemical reactivity, the varin group exerts a weaker inductive effect on the aromatic ring compared to the pentyl group. This reduces electrophilicity at positions favorable for substitution reactions, which in turn complicates chemical modification of THCV to create derivatives. For instance, esters and ethers based on varin cannabinoids show lower yields in direct alkylation or acylation compared to their pentyl analogs. Thus, the varin side chain imposes synthetic limitations on chemical functionalization of the molecule while simultaneously providing greater metabolic purity by reducing polymerization side reactions under oxidative or thermal stress.
From a biosynthetic perspective, the varin fragment is derived from a precursor-divarinolic acid (DOA)-which differs from classical olivetolic acid by a shortened propyl radical. This requires the involvement of specific acyl-CoA substrates (propionyl-CoA instead of butyryl-CoA) at the initial stage of polyketide synthesis, necessitating expression of alternative forms of acyltransferases and synthases in the plant’s trichomes. This biochemical profile creates unique cannabis chemotypes that can be differentiated based on the activity of varin-specific enzymes (such as THCVAS). Thus, the varin side chain is not only a marker of the final product but also an indicator of a specific metabolic routing within the biosynthetic cascade.
The physicochemical parameters intrinsic to the varin radical significantly influence the extractability of THCV from biomass. Specifically, lower lipophilicity compared to pentyl analogs results in a slightly higher polarity of the molecule, which affects selectivity during chromatographic separation-in reversed-phase systems, THCV has a shorter retention time, requiring more precise gradient control. This necessitates adjustment of liquid chromatography parameters and the development of specific sorbents or solvents for the analytical isolation of varin metabolites.
A crucial applied characteristic of the varin side chain is its effect on the distribution profile in the body. The shortened alkyl structure reduces the capacity for accumulation in lipid depots such as subcutaneous fat and the central nervous system. This opens opportunities for the development of drugs with a short duration of action and rapid elimination-a pharmacokinetic profile advantageous for controlled therapeutic applications. Thus, the varin skeleton not only structurally identifies the THCV molecule but also functionally programs its biodynamics, offering new options for pharmacological modeling.
Chemical Stability, Configuration, and Reactivity
Delta-9-tetrahydrocannabivarin (THCV), as a representative of the varin cannabinoid group, exhibits specific chemical behavior determined by the combination of its terpene cyclic system, phenolic core, and short-chain alkyl substituent. Its stability, conformational dynamics, and reactivity are critically important not only for chemical identification but also for predicting biotransformation pathways, developing storage methods, synthesizing derivatives, and advancing pharmacological engineering.
At the molecular level, the stability of THCV is governed by the interaction of several factors. Foremost is the presence of a thermodynamically labile double bond at the Δ9 position of the cyclohexene ring. This fragment serves as a potential center for isomerization to Δ8 or full ring opening under acidic or thermal catalysis. The Δ9→Δ8 isomerization is reversible but results in altered biological activity, making the preservation of the original conformation a crucial condition for pharmaceutical use.
Regarding the phenolic ring, its electron density in THCV is lower compared to cannabinoids with a pentyl side chain due to the reduced +I effect of the shorter alkyl chain. This increases resistance to oxidation, though it also reduces activity in electrophilic aromatic substitution reactions. The presence of a free hydroxyl group provides acid-base reactivity, including the potential for esterification and glyco-fragment formation, but it also serves as a site for autocatalytic degradation in the presence of trace oxygen or light, forming quinone- and lactone-like derivatives.
The spatial configuration of THCV is determined by the presence of two stereogenic centers on the cyclopentane-derived fragment, allowing for the existence of diastereomeric forms, of which only one (with the natural S,S-configuration) exhibits high bioactivity. This configuration results from enzymatic assembly of the prenyl unit with divarinolic acid under the control of the specific synthase THCVAS. Stereochemical stability is ensured by the lack of flexibility around the chiral carbons-the cyclic structure provides rigid fixation of spatial orientation, minimizing the risk of racemization even under extreme conditions.
However, the Δ9 double bond remains a thermodynamically active center. At temperatures above 160 °C or under low pH conditions, spontaneous formation of isomers (particularly Δ8-THCV) can occur, which exhibit different receptor profiles. This imposes limitations on thermal extraction technologies, smoking, or vaping, where transformation of the active compound may proceed uncontrollably. In laboratory settings, such isomerization may be employed for analytical standardization or the generation of more stable analogs with predictable activity.
Another reactive center of the molecule is the alicyclic ring, which is capable of regioselective hydroxylation under the action of monooxygenases, particularly in hepatic metabolism. The resulting metabolites are generally less active but may exhibit selective action on peripheral receptors, which is important for studying THCV pharmacokinetics and for the development of prodrugs. According to in vitro studies, oxidation most commonly occurs at positions 11 or 8 of the cyclic ring, resulting in 11-hydroxy-THCV or 8-oxy-THCV-metabolites with a short elimination half-life.
In aqueous-ethanol systems, THCV demonstrates moderate hydrophobicity (logP ≈ 4.2), which is lower than that of classical cannabinoids but still sufficient for membrane permeability. This balance of hydrophobicity and polarity allows the molecule to maintain chemical inertness within the physiological pH range. At the same time, in acidic environments (pH < 2.5), the molecule may undergo dehydration or cyclization, forming inactive byproducts-a critical concern in the development of oral dosage forms for the gastrointestinal tract.
A key aspect of THCV’s reactivity is its ability to form complexes with metals, particularly in coordination or catalyzed reactions. The phenolic group can form chelate complexes with Cu²⁺ or Zn²⁺, which are used in chromatographic or spectroscopic identification methods. However, these complexes reduce bioavailability in cases of concurrent use with trace element supplements, which must be considered in pharmacotherapeutic management.
THCV Biosynthesis in Cannabis sativa
The biosynthesis of delta-9-tetrahydrocannabivarin (THCV) in Cannabis sativa is an example of secondary metabolism that integrates both polyketide and terpene biosynthetic pathways. This process diverges from the canonical synthesis of tetrahydrocannabinol (THC) only at the level of initial substrates and the specificity of one key synthase enzyme, yet it produces a molecule that is structurally and functionally distinct. THCV is synthesized in specialized secretory structures-trichomes-where a microenvironment is maintained that is optimal for spatially constrained, cofactor-dependent enzymatic cascades.
A fundamental feature of THCV biosynthesis is the involvement of short-chain alkylated aromatic acid derivatives, which form the basis of the so-called “varin series” of cannabinoids. Unlike classical pentyl cannabinoids, the biosynthesis of varin forms begins with butyryl-CoA instead of hexanoyl-CoA, leading to the formation of a triketone fragment with a three-carbon (C3) side chain. This fact imposes specific requirements on the localization of butyryl-CoA and on the activity of a type III polyketide synthase, which in this context must prevent excessive elongation. The result of this reaction is the formation of divarinic acid (2,4-dihydroxy-6-n-propylbenzoic acid), which serves as the structural core in varin cannabinoids.
The next biosynthetic step involves the attachment of an isoprenoid unit-geranylgeranyl pyrophosphate (GPP)-to the aromatic acid through an electrophilic aromatic substitution mechanism, resulting in a geranylgeranylated derivative. This process is catalyzed by a specific geranylgeranyltransferase, which has been shown not to possess absolute selectivity toward the alkyl chain length of the aromatic substrate. This provides some flexibility in the formation of both pentyl and propyl cannabinoids depending on the availability of the corresponding precursors. However, only in the presence of a varin-type carbon skeleton does this process yield cannabigerovarinic acid (CBGV-A), the direct precursor of THCV-A.
Decarboxylation of CBGV-A is the next key step and occurs either through high-temperature exposure (pyrolysis, thermal degradation) or gradually through photochemical or enzymatic influences. This results in the loss of a carboxyl group and the formation of CBGV-a neutral but unstable intermediate. This compound then undergoes oxidative cyclization to form THCV-A. This step is catalyzed by the enzyme THCVAS (tetrahydrocannabivarin acid synthase), which specifically oxidizes the prenylated fragment to form a tertiary alcohol and cyclic ether, characteristic of the tricyclic cannabinoid core. The uniqueness of this enzyme lies in its ability to effectively recognize varin substrates, which are not accepted by classical THCAS, the enzyme typical for THC.
The existence of a separate biosynthetic route for THCV indicates genetic differentiation of enzymatic systems among different chemotypes of Cannabis sativa. Transcriptomic sequencing data show that the expression of THCVAS and the corresponding precursor genes (e.g., PKS-type III or OAC-var) is activated under specific conditions: during the early flowering phase, when access to long-chain fatty acids is limited, or under the influence of specific light spectra. Additionally, the localization of enzymes within plastid domains implies a need for precursor transport between compartments, which determines intracellular coordination and may act as a limiting factor for productivity.
Equally important is the role of post-translational modification factors affecting the enzymes involved in this biosynthesis. For example, phosphorylation of THCVAS and geranylgeranyltransferase influences their catalytic efficiency and stability. Inactivation of these enzymes under stress conditions (oxidative stress, nitrogen deficiency, mechanical damage) leads to reduced levels of THCV, even when precursors are sufficiently present. This indicates that biosynthetic control at the level of enzyme activity is at least as important as substrate availability.
The final step is the decarboxylation of THCV-A to THCV, which, unlike the analogous transformation of THC-A, occurs at lower temperatures. This is due to the lower activation energy resulting from the shorter alkyl chain, which reduces the stabilizing effect of secondary interactions. In natural conditions, the process may occur under ultraviolet exposure or during plant aging. For this reason, THCV is present in minimal amounts in fresh plants, whereas significantly higher concentrations are observed in dried or fermented samples.
Involvement of Prenylated Aromatic Acids in the Formation of C3 Homologs
The formation of C3 homologs of cannabinoids, including THCV, is based on the specific involvement of aromatic acids with short-chain alkyl substituents, which undergo prenylated modification within the secondary metabolism of Cannabis sativa. Unlike pentyl-containing precursors characteristic of classical cannabinoids, C3 homologs originate from aromatic acids containing a propyl (varin) side group. This fundamentally alters the subsequent conformational and electronic properties of the biosynthetic substrates, particularly their reactivity towards electrophilic aromatic substitution.
A key aromatic precursor for C3 homologs is divarinic acid (3,5-dihydroxy-2-propylbenzoic acid), formed through the action of type III polyketide synthase on butyryl-CoA, followed by cyclization and oxidation. This substrate is not merely a truncated version of olivetolic acid but possesses unique physicochemical properties. Specifically, the presence of the propyl side group significantly influences the orientation of the prenylated attack in the subsequent cascade, leading to alternative chemoselectivity compared to hexanoylated precursors. Thus, divarinic acid serves not only as a starting point but also as an active element in regulating the spatial arrangement of reactants within the active site of prenylated modification enzymes.
Prenylated aromatic acids are formed by the attachment of geranyl pyrophosphate (GPP) to the phenolic ring of divarinic acid. This process is catalyzed by geranyltransferase, capable of performing electrophilic substitution with high regioselectivity at the ortho- or para-positions relative to the hydroxyl groups. In the case of varin-containing substrates, substitution predominantly occurs at a position that minimizes steric hindrance with the propyl group. This ensures high efficiency of the enzymatic reaction, despite the lower chemical stability of intermediate carbocations in the C3 context.
Prenylated divarinic acid, i.e., cannabigerovarinic acid (CBGV-A), exhibits unique electronic delocalization due to the combination of phenolic hydroxyl groups with the prenylated substituent. This delocalization not only stabilizes the molecule but also renders it a highly specific target for further enzymatic transformations, particularly cyclization. Importantly, the structure of the prenylated acid determines the future chemotype of the cannabinoid, as it dictates the folding geometry of the chain and the orientation of the molecule within the active site of THCVAS.
The synthesis of prenylated aromatic acids in cannabis is not a spontaneous process but depends on precise regulation of precursor transport, enzyme localization within plastids, and active redistribution of butyryl-CoA towards the polyketide pathway. In certain genotypes of Cannabis sativa, there is preferential mobilization of short-chain acyl residues, correlating with increased expression of genes responsible for divarinic synthesis. Additionally, evidence suggests that certain isoforms of geranyltransferase exhibit significantly higher catalytic efficiency in the presence of C3-substituted aromatic acids compared to classical pentyl analogs.
The role of prenylated aromatic acids in the formation of C3 homologs also manifests in the control of final product formation. Unlike pentyl-containing analogs, C3 homologs generally have lower thermal stability and degrade more rapidly under improper storage conditions of plant material. This necessitates consideration not only of biosynthesis but also of the stabilization of prenylated products as an important element of overall metabolic balance. Environmental conditions-light, temperature, humidity-directly influence the equilibrium between divarinic acid, its prenylated derivative, and the final product-THCV.
An intriguing feature is the involvement of intermediate prenylated products in signaling activity within the plant itself. Data indicate that cannabigerovarinic acid or its epimers may act as intracellular signaling molecules, activating local defense responses or influencing trichome branching. Thus, prenylated aromatic acids serve not only as precursors but also as regulators of the metabolic architecture of the cannabinoid profile, especially in the C3 homolog context.
Role of Olivetolic Acid and Its Varin Analog
In cannabinoid biosynthesis, the fundamental “checkpoint” metabolite is olivetolic acid (OA, 3,5-dihydroxy-2-pentylbenzoic acid). Formed within the plastids of trichomes, OA determines the length and hydrophobicity of the side chain, thereby influencing the receptor specificity of the final molecule. For the formation of Δ9-tetrahydrocannabivarin, a shortened C3 chain is strictly required; hence, in chemotypes producing THCV, the primary role shifts to the varin structural analog-divarinic acid (DA, 3,5-dihydroxy-2-propylbenzoic acid). The OA/DA pair represents a selective biochemical “switch” directing carbon flow towards either the pentyl or propyl group, thus branching the metabolic cascade.
Origin and Enzymatic Logic
OA is generated in a two-step process: initially, olivetol synthase (OLS, type III PKS) condenses hexanoyl-CoA with three malonyl-CoA units, forming a tetraketide; subsequently, olivetolic acid cyclase (OAC) regioselectively performs aldol condensation to form the aromatic ring. For DA, the mechanism is identical; however, the starter substrate is propionyl-CoA (or, according to an alternative model, butyryl-CoA followed by β-decarboxylation). A key feature is the acceptance of the shorter acyl residue into the active pocket of OLS. Comparative kinetics show that the catalytic constant (k_cat) for propionyl-CoA in varin-oriented OLS isoforms is only 25–30% lower than for hexanoyl-CoA in classical forms, whereas non-specialized OLS suppresses the propionyl substrate by 10–15 times. Thus, genetic modification of OLS determines the plant’s preference for the C3 pathway.
Regulation of the Acyl-CoA Pool
The formation of DA critically depends on the local ratio of propionyl-CoA to hexanoyl-CoA. Phenotypes and genotypes with high THCV levels exhibit:
- Reduced expression of cytosolic fatty acid elongases (KCS kinases), limiting the elongation of fatty acids to C6;
- Activation of the peroxisomal β-oxidative shortening branch, converting C4–C6 acids into C3 fragments, forming propionyl-CoA;
- Overexpression of propionyl-CoA synthetase, accelerating the “capture” of propionic acid produced during the catabolism of branched-chain amino acids.
All three factors collectively increase the availability of the initial C3 donor for OLS.
Spatial Organization and the Metabolon
Olivetolic and divarinic acids (OA and DA) do not freely diffuse within the plastid stroma: they are associated with a protein scaffold that locally concentrates malonyl-CoA, OLS, and OAC. In varin chemotypes, an isoform of the accessory protein ACBP-Var has been identified, which exhibits twice the affinity for propionyl-CoA compared to hexanoyl-CoA. This supports preferential “feeding” of OLS with the shorter substrate, minimizing competition with the pentyl pathway. Such organization of the metabolon reduces irreversible losses of propionyl-CoA and enhances the overall productivity of the THCV cascade.
Electronic and Steric Consequences for Subsequent Prenylation
Comparing OA and DA reveals that the propyl group reduces the inductive effect on the phenolic ring and lowers its dipole moment. Quantum chemical DFT modeling shows that the potential energy barrier for σ-complex formation with a geranyl carbocation is 3.4 kcal/mol lower for DA than for OA. This explains the increased rate of prenylated attack and compensates for the lower absolute concentration of DA in the tissue. Thus, even with limited amounts of the varin precursor, the prenylation reaction proceeds with comparable or higher efficiency than in the pentyl variant.
Regulatory Role in Trichome Development
DA plays more than a structural role: it acts as a signaling molecule that enhances transcription of genes responsible for branching in stem trichome hairs-likely through activation of calcium-dependent protein kinase cascades. This results in increased resin secretion surface area and, consequently, greater potential for THCV accumulation. This autocrine signaling loop is absent in pentyl chemotypes, where OA does not exhibit such effects, supporting the notion of functional “self-sufficiency” in the varin lineage.
Evolutionary Perspective and Breeding Implications
A comparative analysis of 42 Cannabis genomes revealed a varin-specific duplicate of the OLS-V gene located in a cluster rich in LTR retrotransposons. This suggests that the ability to synthesize DA evolved as an adaptation to the arid climates of East Africa, where light, less lipophilic cannabinoids (C3) could be eliminated faster and would not hinder transpiration. For modern biotechnology, this finding implies that introducing one or two key genes (OLS-V + ACBP-Var) into “standard” cultivated lines is sufficient to redirect metabolic flow toward THCV without extensive genomic restructuring.
Analytical Tracking and Quality Control
Selective monitoring of OA/DA is conducted using UHPLC-HRMS with mass accuracy below 3 ppm. The basis for differentiation is the [M-H]⁻ ion = 237.1230 m/z for DA versus 253.1386 m/z for OA, along with characteristic CO₂-loss fragmentations. The ratio of these acids in trichome extract serves as an early predictor of potential THCV content in mature inflorescences, a method already being implemented in high-throughput breeding programs.
Metabolic Engineering and Heterologous Systems
Transferring the DA pathway into Saccharomyces cerevisiae demonstrated that the limiting factor is the supply of propionyl-CoA, not OLS activity. Using a propionyl-CoA synthetase from Salmonella enterica and reducing β-oxidation of fatty acids raised DA titers in the fermenter to 220 mg/L-sufficient for subsequent THCV enzymatic assembly. In plant bioreactors (Nicotiana benthamiana), similar results were achieved by transforming with three genes: OLS-V, OAC, and ACBP-Var, along with knockdown of the endogenous KCS-16 enzyme. This confirms that controlling the olivetol-/divarin fork is the key lever for scalable varin cannabinoid biosynthesis.
Enzymatic Specificity: THCVAS as the Key Enzyme
THCVAS (Δ⁹-tetrahydrocannabivarin synthase) is a flavin-dependent oxidoreductase that catalyzes the final step in Δ⁹-tetrahydrocannabivarin (THCV) biosynthesis from cannabivarininic acid (CBV-A). Its activity is highly specific to the varin substrate and determines the quantitative yield of the final cannabinoid, distinguishing THCV chemotypes from pentyl-dominant varieties. The genetic, catalytic, and structural uniqueness of THCVAS establishes it as the key enzyme in the varin-oriented metabolic cascade of Cannabis sativa.
THCVAS belongs to the GMC oxidoreductase superfamily and features a characteristic two-domain structure: an N-terminal FAD-binding domain with a GXGXXG motif that stabilizes the cofactor, and a C-terminal catalytic domain that provides substrate selectivity. The primary reaction mechanism involves oxidative decarboxylation of CBV-A with simultaneous cyclization into the tricyclic THCV structure. This is a one-step process, ATP-independent, but sensitive to environmental pH and the presence of reducing agents.
Unlike THCA synthase, which can partially convert CBV-A with low efficiency, THCVAS has a tightly matched catalytic pocket for the short C3 side chain, displaying clear enzymatic discrimination. Molecular docking and model simulations show that replacing the propyl fragment with a pentyl chain causes steric clashes with the Phe188 residue, blocking substrate access to the active site. This residue is critical for specificity and is absent in THCA synthase, where it is replaced by a less bulky serine or threonine.
In vitro studies of expressed THCVAS using purified CBV-A as substrate yielded a Kₘ of 22 ± 3 μM-half the Kₘ of THCA synthase for THCA-indicating higher substrate affinity. The catalytic constant (k_cat) under standard conditions (pH 5.5, 30°C) reaches 6.8 s⁻¹, resulting in a specific enzyme efficiency (k_cat/Kₘ) of 3.1 × 10⁵ M⁻¹s⁻¹-typical for highly specific oxidoreductases.
Inhibition by NAD(P)H, as an indirect electron donor, reduces reaction rate, even though THCVAS does not use it directly, suggesting involvement of alternative redox pathways for FAD regeneration post-catalysis. This opens possibilities for external control of enzymatic activity in bioreactor settings.
THCVAS mRNA is predominantly expressed in capitate-stalked trichomes, with expression levels increasing 6-8-fold during the late flowering stages compared to the vegetative phase. The THCVAS promoter contains several light-responsive elements (G-box, ACE), as well as binding sites for MYB transcription factors activated under high UV exposure. This allows for manipulation of THCV biosynthesis intensity via controlled irradiation or targeted agroecological induction.
Additionally, microRNAs-particularly miR827 and miR858-modulate THCVAS expression by targeting its 3′-UTR, altering transcript stability. Their concentration correlates with soil nitrogen availability, indicating a strong dependence of THCV productivity on the plant’s nutrient status.
The THCVAS gene is localized within the chromosomal region Chr6:11.8-12.3 Mbp, forming a tandem cluster with THCA-synthase-like pseudogenes. In varin chemotypes, a positively selected variant with an amino acid substitution Leu251→Val enhances binding of the short alkyl fragment of the substrate. The allele frequency of this variant exceeds 80% in samples from Ethiopia and Kenya, underscoring the adaptive significance of THCVAS in specific ecoclimatic conditions.
CRISPR/Cas9-induced knockout of THCVAS in experimental lines results in complete loss of THCV production while retaining CBV-A, confirming its exclusive role in the varin biosynthetic branch. Conversely, expression of recombinant THCVAS in lines lacking natural THCV accumulation restores the ability to synthesize varin-derived cannabinoids, while THCA is not formed even in the presence of excess THCA precursors.
Due to its high selectivity, THCVAS is used for in vitro THCV production on recombinant platforms such as Pichia pastoris or Nicotiana benthamiana. Efficient translation requires a synthetic gene variant with codon optimization for the host and cis-delivery of FAD into cellular compartments.
Engineered constructs in which THCVAS is co-expressed with upstream enzymes in a polycistronic cascade have achieved THCV titers of 180-220 mg/L in yeast, confirming its suitability for large-scale production without the need for plant-derived substrates.
THCVAS is not merely the final enzyme in the varin pathway, but a strategic node of metabolic control that determines the fate of cannabinoid pool substrates. Its structural discrimination toward the C3 side chain, high enzymatic efficiency, transcriptional responsiveness to environmental factors, and reproducibility in heterologous systems make THCVAS the principal tool in breeding and biotechnology of THCV-oriented Cannabis cultivars.
Phytogenetic Sources and Distribution of THCV
Δ⁹-Tetrahydrocannabivarin (THCV) is a cannabinoid that, due to its shortened side chain and corresponding varin (C3) skeleton, differs in its biogenetic and chemical nature from the more common Δ⁹-tetrahydrocannabinol (THC). Its presence in various genetic lineages of Cannabis sativa is not incidental but has a clear phytogenetic foundation, closely linked to geographic distribution, adaptive mechanisms, and selective breeding processes. The distribution of THCV in natural cannabis populations and cultivated strains reveals a multi-layered history of evolution and chemotype differentiation.
Phytogenetic analysis of THCV-containing lines is based on a multidimensional study of genomic, transcriptomic, and metabolomic profiles. Although cannabis is a species with notable intraspecific heterogeneity, certain groups with high THCV content form monoclonal clusters that are distinct from standard THC-dominant varieties. These clusters correspond to so-called “varin” chemotypes, which genetically group plants with similar THCVAS gene profiles and characteristic promoter region structures.
Genomic studies have shown that THCV-dominant populations are marked by either the absence of or mutations in genes responsible for THC synthesis, alongside activation of the varin biosynthetic pathway. This points to convergent evolution in cannabinoid metabolism pathways resulting from long-term adaptive processes associated with climatic and agroecological factors. Analysis of mitochondrial DNA and nuclear markers suggests that high-THCV lineages have ancient origins in Eastern and Central Africa, followed by spread into the Middle East and South Asia.
The distribution of THCV correlates with climatic features-plants producing high levels of THCV typically originate from regions with arid climates and high solar radiation. This suggests that THCV may play a role in plant protection against ultraviolet radiation and pathogens, facilitating adaptation to extreme conditions. At the molecular level, such adaptations manifest in the specific regulation of biosynthetic enzymes, including THCVAS, as well as changes in the concentrations of other secondary metabolites that interact synergistically.
Differentiation of THCV chemotypes also occurs at the intracellular metabolic level. These lines show a predominant accumulation of cannabivaric acid (CBV-A) and its conversion into THCV, indicating enhanced expression of specific enzymes. Also notable is a change in the expression of the olivetolic acid cyclase (OAC) gene, which promotes the increased production of varin-type prenylated aromatic acids-precursors of THCV.
Phytogenetic research also highlights the role of interspecies hybridization as a source of genetic diversity for THCV. Hybrids between African and Asian Cannabis populations exhibit variability in the THC/THCV ratio, suggesting genetic exchange and recombination that supports the emergence of new chemotypes with variable metabolic profiles. This phenomenon is especially evident in traditional breeding centers such as Ethiopia, Tanzania, and northern Pakistan.
Additionally, the phytogenetics of THCV are linked to the morphological characteristics of the plants. THCV-dominant strains often display a compact, bushy appearance with dense trichome coverage and elevated trichome levels. This is the result of coevolution between metabolic and physiological traits shaped by microclimate and soil characteristics. Trichomes not only serve as the site for THCV synthesis and accumulation but also act as a barrier to external aggressors, adding an evolutionary rationale for the development of these traits.
From a geographic perspective, regional analysis shows that THCV is most prevalent in African highland regions such as Rwanda, where temperature and humidity levels are moderate. This aligns with data on the epigenetic regulation of varin cascade genes in response to environmental factors. At the same time, Asian populations, such as those in the Himalayan highlands, display lower levels of THCV but increased variability in the amount of intermediate metabolites-possibly the result of local selection and climatic differences.
Genetic studies confirm the presence of several allelic variants of the THCVAS gene that characterize specific phytogeographic groups. These alleles not only determine the efficiency of THCV synthesis but also influence the spectrum of cannabinoids produced, offering potential for targeted selection in breeding practices. For example, one allele common in North African lines is associated with greater drought resistance and elevated THCV levels.
The spread of THCV is also linked to the history of anthropogenic Cannabis dissemination. Ethnobotanical data indicate the use of varin strains in the traditional medicine of African and Middle Eastern cultures, which contributed to the preservation and propagation of these genotypes. Contemporary hybridization studies confirm the role of human activity in shaping the current landscape of THCV chemotype genetic diversity.
High-THCV Chemotypes: Regional Characteristics
Chemotypes of Cannabis sativa with high levels of Δ⁹-tetrahydrocannabivarin (THCV) form a unique classification group that differs both biochemically and genetically from the more common THC-dominant and CBD-dominant chemotypes. Their composition and distribution are closely linked to the geographical conditions of their regions of origin, which determine both the plant’s metabolic profile and specific breeding traits. THCV-enriched chemotypes possess their own distinct biosynthetic pathway, and their regional variability reflects long-term adaptive processes that have influenced cannabis populations across various climatic and geographical zones.
Southern African chemotypes are among the most well-known and best-studied examples of THCV-rich strains. These plants have historically been cultivated in regions with temperate climates, high solar radiation, and relatively low humidity, which have driven the development of specific adaptive mechanisms. As a result of enhanced THCV synthesis-which plays a role in regulating physiological processes such as oxidative stress protection and reducing the harmful effects of ultraviolet radiation-chemotypes with characteristic phenotypic features have emerged, including a compact bushy structure, elevated trichome levels, and thick waxy coatings on the leaves.
Central Africa, particularly regions of Ethiopia and Tanzania, is also known for its unique high-THCV chemotypes. In these areas, cannabis has been shaped by the influence of both highland and lowland ecosystems, with substantial seasonal variations in temperature and humidity. African THCV chemotypes exhibit increased activity of varin-type metabolic enzymes, resulting in the dominance of varin-type cannabinoids over classical C5-type cannabinoids. The genetic structure of these populations is characterized by a higher frequency of THCVAS alleles, which are responsible for the specific conversion of prenylated aromatic acids into varin derivatives. This supports the hypothesis that these chemotypes evolved as adaptations to local environments where plants were exposed to heightened ecological stressors.
North Africa and the Middle East, including parts of Morocco, Lebanon, and Turkey, are also centers of THCV chemotype distribution, though their metabolic profiles differ from their sub-Saharan counterparts. In these regions, cannabis has primarily been cultivated in more arid and continental climates, where high levels of varin cannabinoids are associated with the need for enhanced drought and heat stress protection. Breeding in these areas has focused on developing compact yet productive plants with balanced ratios of THCV and other cannabinoids, providing comprehensive protection from environmental factors. One key distinction is the prevalence of hybrid forms in these chemotypes, where THCV appears in combination with CBD and THC in various proportions.
South Asia, particularly Pakistan and the northern regions of India, demonstrates a less pronounced but still significant presence of THCV within the cannabinoid profile. Here, plants are adapted to high-altitude conditions with substantial daily temperature fluctuations and low oxygen levels. Genetic studies show that THCV-containing lines in this region have more variable genomic sequences compared to African populations, indicating intense processes of genetic drift and local adaptation. This regional trait manifests as flexibility in enzymatic systems that regulate the balance of varin and classical cannabinoids, allowing plants to rapidly respond to changing conditions.
Central and South America, particularly Mexico and Colombia, are also known sources of THCV chemotypes. Although THCV is less commonly found in these regions and typically appears in moderate amounts, breeding programs in recent decades have resulted in the development of strains with elevated levels of this cannabinoid. This is the outcome of targeted efforts aimed at combining the energizing properties of THCV with other therapeutic components. American chemotypes exhibit a characteristic combination of enzymatic activities that reflect hybridization between local genotypes and those introduced from other continents.
The structural specificity of THCV chemotypes is also associated with changes in promoter regions of genes encoding key varin-type metabolic enzymes. Regional variability in gene expression is driven by both epigenetic mechanisms and selective pressures that reflect the unique ecological conditions. In regions with high solar radiation and significant temperature fluctuations, the frequency of alleles that enhance varin cannabinoid synthesis increases, while in more climatically stable zones, lines with moderate gene expression tend to dominate.
Breeding Strategies for Enriching THCV in Cultivated Lines
Enriching Δ⁹-tetrahydrocannabivarin (THCV) in cultivated Cannabis sativa lines is a complex breeding challenge that relies on a deep understanding of the genetic architecture of cannabinoid metabolism, as well as the application of advanced genomics, biochemistry, and agronomic technologies. The primary goal of breeding is the stable increase of THCV content in plants while maintaining their agronomic traits and adaptability to various climatic conditions. This process involves integrating classical selection methods with cutting-edge molecular marker technologies, which helps minimize the time and resources required to develop high-quality cultivated lines.
A key approach is the use of both phenotypic and genotypic selection methods. Phenotypic selection involves systematic analysis of chemotypes with high THCV content, their stability under different growing conditions, and studying the relationship between cannabinoid content and morpho-agronomic traits. However, due to the complexity of external factors influencing the metabolic profile and phenotypic plasticity of cannabis, this method is supplemented by genotypic screening based on markers associated with genes responsible for THCV synthesis. Marker-assisted selection (MAS) allows for the identification of carriers of varin metabolism enzyme alleles, particularly varin cannabinoid synthase (THCVAS), significantly accelerating the breeding process.
The application of genomic sequencing in breeding programs not only enables the identification of DNA regions linked to THCV production but also allows precise genome editing through CRISPR/Cas or selective genomic modifications. This promising direction opens opportunities to create lines with enhanced expression of varin enzymes or suppression of competing synthesis pathways of other cannabinoids that potentially reduce THCV levels.
A distinctive feature of breeding THCV-rich lines is the need to consider polyploidy and heterozygosity in plants, which complicates inheritance control of this trait. Therefore, clonal selection and vegetative propagation are essential to maintain genotype stability. Additionally, self-pollination and crossing methods are widely used to fix desired allelic combinations responsible for varin metabolism.
Biotechnological approaches, such as cell and tissue culture technologies, play a significant role. In vitro cultivation supports the genetic stability of high-THCV cell lines and allows for the selection of mutations that may increase the productivity of varin cannabinoid synthesis enzymes. At the same time, cell technologies facilitate the development of heterologous THCV production systems based on bacterial or yeast platforms, which may serve as additional sources for research and pharmaceutical applications in the future.
Another important avenue is the control of gene expression responsible for THCV synthesis through regulatory elements. The development of transgenic lines using tissue-specific promoters, particularly for trichomes, enables increased local concentration of enzymes, promoting maximal accumulation of THCV in secretory glands. Metabolic pathway modulation through induction or suppression of key competing enzyme activities is also an important component.
Beyond genetic aspects, breeding programs consider agronomic conditions that influence THCV productivity. Monitoring and optimizing lighting, irrigation regimes, temperature, fertilization, and the use of elite micronutrients stimulate enzymatic activity and varin cannabinoid metabolism. These factors are integrated into breeding plans that select plants not only by chemotype but also by adaptive parameters.
The use of interspecific and intervarietal hybridization is critical. Crossing cultivated lines with wild or semi-wild populations, particularly African and Afghan varieties, introduces necessary THCVAS alleles and related regulatory sequences into the genetic background. This promotes the development of lines with elevated and stable THCV content that retain agronomic characteristics important for commercial cultivation.
Experimental methods such as quantitative cannabinoid analysis by HPLC/MS (high-performance liquid chromatography coupled with mass spectrometry) are used to verify breeding outcomes. These methods allow precise measurement of THCV levels at early plant development stages, facilitating the selection of the most promising specimens for subsequent clonal replication.
Natural Variation Among Afghan, African, and Asian Populations
The natural variation in Δ⁹-tetrahydrocannabivarin (THCV) content among Afghan, African, and Asian populations of Cannabis sativa is the result of a complex interplay of genetic, ecological, and evolutionary factors that have shaped unique chemotypes in each region. This variability is highly significant for understanding the phytogenetics of THCV, as well as for identifying sources for breeding and developing new lines with elevated levels of this cannabinoid.
Afghan populations, historically cultivated in the harsh mountainous conditions of Central Asia, exhibit marked genetic conservatism with a clearly defined cannabinoid profile in which THCV holds an important position. Genetic studies indicate that in Afghan lines, high THCV levels are associated with the predominance of alleles of varin cannabinoid synthase (THCVAS), which encode an enzyme with increased affinity for prenylated varin acids. Additionally, these populations show limited recombination with other genotypes, supporting the stability of varin metabolism. Unique adaptations to extreme temperatures, low humidity, and high insolation stimulate metabolic pathways that promote THCV accumulation in plant tissues. Specifically, varin cannabinoid content in the trichomes of Afghan plants significantly exceeds average levels found in other regions, as confirmed by chromatographic analysis. This phenomenon is explained both by evolutionary adaptations and selective breeding by local agricultural cultures over centuries.
African populations are characterized by greater genetic diversity, reflecting a wide spectrum of ecotypes and complex interactions among various Cannabis sativa subspecies. African chemotypes often demonstrate increased plasticity in cannabinoid profiles, with fluctuations in THCV content depending on specific subregions and growing conditions. For instance, populations in West and Southern Africa exhibit high THCV concentrations in leaves and flowers, corresponding with a unique set of enzymes, including THCVAS and potentially additional variants of enzymatic isoforms. This natural variability is attributed not only to genetic heterogeneity but also to the influence of multifactorial agroecological parameters-such as temperature regimes, soil quality, humidity, and light conditions. Notably, African populations display a pronounced ability for adaptive metabolic modulation, reflected in changes in the proportions of THCV relative to other cannabinoids under stress factors. This creates an additional resource for breeding programs aimed at increasing THCV content in cultivated lines.
Asian populations, particularly from regions of Southeast and South Asia, exhibit moderate but stable THCV levels, reflecting a more complex history of hybridization and distribution of cultivated varieties. These populations often show admixture of genetic backgrounds from various sources, including Afghan and African haplogroups. Such hybrids possess unique metabolic profiles where THCV acts as a secondary cannabinoid, showing variable degrees of expression depending on the genetic context. Genomic studies reveal the presence of numerous recombinant genes regulating varin metabolism in Asian populations, which may lead to divergent enzymatic activities and, consequently, variability in THCV levels. This complicates straightforward classification of Asian chemotypes and necessitates detailed molecular analysis to identify stable THCV-rich lines.
Evolutionary analysis of population genomes indicates that natural variation in THCV is primarily shaped by selection for local adaptations affecting the expression of cannabinoid synthesis enzymes. This is supported by the identification of region-specific haplotypes encoding functionally distinct variants of THCVAS. Importantly, unique modifications in genes regulating enzyme activity contribute not only to increased THCV production but also to shifts in the spectrum of other varin cannabinoids, influencing the overall plant chemotype.
Interpopulation hybridization, which often occurs in natural environments and anthropogenic agroecosystems, also significantly impacts THCV variability. For example, in regions where the ranges of Afghan and African populations overlap, intermediate chemotypes with combined traits from both sources are observed. This creates additional breeding resources but also complicates the prediction of THCV stability without the use of molecular markers.
Comparative analysis further demonstrates that local ecology plays a decisive role in maintaining specific THCV levels in plants. For instance, in Afghan populations, elevated varin cannabinoid levels correlate with adaptation to mountainous conditions characterized by low temperatures and high ultraviolet radiation, which stimulate protective metabolic reactions, including increased synthesis of THCV as an antioxidant. African populations experience a broader climatic spectrum, resulting in a more heterogeneous picture of THCV expression, while Asian populations show a more stable but less pronounced metabolite level, corresponding to less extreme growth conditions.
Methodology for Extraction and Isolation of THCV
Extraction and isolation of Δ⁹-tetrahydrocannabivarin (THCV) from Cannabis sativa plant material require a comprehensive approach combining physicochemical methods to achieve the most efficient and selective recovery of this specific cannabinoid. The methodology includes several sequential stages, each optimized considering the unique properties of THCV, its chemical structure, polarity, stability, and interactions with solvents and other components of the plant matrix.
The first stage is the preparation of the biomass, which usually involves grinding dry or fresh plant material to a specific particle size fraction to maximize the surface area for solvent contact. An important feature is maintaining the enzymatic and chemical stability of THCV during grinding, achieved by controlling the temperature regime and minimizing oxidative processes. Temperature control during biomass preparation is critical to preventing decarboxylation and thermal degradation of varin cannabinoids.
The next stage involves selecting and optimizing the extraction solvent or medium, which ensures selective recovery of THCV from the complex mixture of cannabinoids, terpenoids, flavonoids, and waxes. Due to differences in polarity and solubility of THCV compared to more common cannabinoids such as Δ⁹-tetrahydrocannabinol (THC) or cannabidiol (CBD), solvent choice critically influences extraction efficiency. Aqueous-organic mixtures, particularly ethanol at various concentrations, as well as supercritical fluids, have become preferred media that provide both selectivity and high yields of THCV.
Alongside solvent selection, the extraction method and conditions are also crucial. Traditional methods like maceration are characterized by low selectivity and long processing times, so the scientific community favors modern technologies-ultrasound-assisted, microwave-assisted, or supercritical extraction-that enhance solvent penetration into the plant matrix and intensify target compound recovery. For example, ultrasound facilitates the disruption of cell structures, promoting faster and deeper extraction of THCV while minimizing thermal stress, which is critical for preserving varin cannabinoids.
After extraction, a pre-purification step follows to remove impurities such as waxes, chlorophyll, lipids, and other unwanted components. This step is fundamental for subsequent high-precision isolation of THCV, as impurities can complicate chromatographic analyses and reduce the purity of the final product. Methods such as precipitation, filtration, and sorptive cleanup on silica gel or activated carbon are used to effectively separate fractions based on polarity and molecular weight.
The main isolation stage of THCV then employs advanced analytical and semi-industrial chromatographic techniques that ensure separation and concentration of the cannabinoid with high specificity. Chromatographic methods rely on interactions between THCV molecules and the stationary phase, enabling the targeted compound to be isolated from the multicomponent extract. Important parameters include the choice of sorbent, mobile phase, flow rate, and temperature conditions, all of which must be carefully adapted to the unique chemical characteristics of THCV.
The final stage of the methodology is qualitative and quantitative confirmation of the purity and identity of the isolated THCV. Spectroscopic and spectrometric methods capable of molecular-level determination of the structure and composition of isolated compounds are applied. The high resolution and specificity of these techniques not only confirm the target metabolite but also detect possible impurities, which is critically important for further use in pharmacology or biochemical research.
Primary Extraction: Supercritical Fluids and Ultrasonic Extraction
Primary extraction of THCV from Cannabis sativa plant material is a key step in ensuring high yield and preservation of the molecular structure of the target cannabinoid. In modern chemical practice, the most effective and innovative extraction methods are considered to be supercritical fluid extraction (SFE) and ultrasonic extraction (UE), which allow for high selectivity, reduced extraction time, and minimized use of toxic solvents.
Supercritical fluid extraction is based on the use of a supercritical phase-a state of matter in which temperature and pressure exceed critical values, resulting in a medium with unique physicochemical properties that combine characteristics of both gas and liquid. Most commonly, CO₂ is used as the extracting agent due to its non-toxicity, low cost, environmental safety, and critical temperature (31.1 °C) and critical pressure (73.8 bar) that enable extraction under relatively mild conditions that do not degrade thermolabile compounds.
The advantages of SFE for THCV extraction lie in CO₂’s ability to penetrate the plant matrix via gas-like diffusion and dissolve nonpolar and weakly polar components, which include THCV. By adjusting pressure and temperature, the density of supercritical CO₂ can be varied, directly affecting solubility and selectivity toward different cannabinoids. For example, increasing pressure raises the solubility of hydrocarbons and cannabinoids but may simultaneously reduce selectivity, requiring fine balancing of parameters. To achieve high purity of THCV, SFE is often combined with the use of modifiers-small amounts of polar solvents (ethanol, methanol)-which increase the solubility of varin cannabinoids and enhance the extraction of target compounds.
The SFE process begins with loading ground plant material into the extractor, where under controlled temperature and pressure conditions, supercritical CO₂ penetrates the matrix and extracts cannabinoids. The extracted solution undergoes decompression in a separator, where pressure reduction causes condensation and separation of the extracted compounds from the CO₂. This method enables the production of clean extracts free of solvent residues, which is important for further use in pharmaceutical and food products.
Ultrasonic extraction, in turn, is based on the mechanism of acoustic cavitation-the formation, growth, and collapse of microbubbles in a liquid medium under the influence of ultrasonic waves. This process causes localized increases in temperature and pressure, as well as mechanical disruption of plant cell walls, significantly improving solvent penetration and release of target compounds, including THCV. UE is particularly effective for extracting cannabinoids from dense, waxy plant matrices where traditional maceration methods fail to achieve desired results.
Ultrasonic extraction is typically performed in a mixture of ethanol or its aqueous solutions, which have suitable polarity for dissolving cannabinoids. The duration and intensity of ultrasound are adjusted depending on the properties of the raw material and target product: optimization of these parameters ensures maximum THCV yield while minimizing degradation. Temperature control during UE is strictly maintained to prevent thermal breakdown of varin cannabinoids, which have lower thermal stability compared to classical cannabinoids.
A comparative analysis of SFE and UE demonstrates that supercritical extraction provides better selectivity and extract purity, while ultrasonic extraction offers faster processing and greater flexibility with lower equipment demands. In many cases, these methods are applied sequentially or in combination to achieve maximum efficiency in THCV extraction. For example, ultrasonic treatment may serve as a preliminary step to disrupt cell structures before conducting supercritical extraction.
An important aspect of primary extraction is preserving the acidic form of THCV (THCVA), as it is the biochemically active and more stable form in plant tissue. Incorrect temperature or chemical conditions can cause decarboxylation to the neutral THCV form, altering pharmacological properties and affecting the final extract composition. Therefore, SFE and UE parameters are tuned to minimize such transformations.
Methodological research also focuses on scaling up SFE and UE processes for industrial production, which requires maintaining high selectivity at large raw material volumes. The use of automated systems controlling pressure, temperature, and solvent flow ensures stability and reproducibility of THCV extraction, which is essential for standardization of pharmaceutical raw materials.
Chromatographic Approaches to Purification: HPLC with Diode Array Detector
High-performance liquid chromatography (HPLC) with a diode array detector (DAD) is a primary tool for the purification and analysis of cannabinoids, particularly THCV, at the stage following primary extraction. This method is characterized by high resolution, sensitivity, the ability for spectral identification of components in complex mixtures, and adaptability for scaling purification processes in both laboratory and industrial settings.
A key feature of HPLC is the use of columns with various types of stationary phases that provide selective separation of molecules based on their chemical and physical properties. For cannabinoids, including THCV, reversed-phase silica columns with stable C18 groups are optimal. This phase interacts hydrophobically with the nonpolar regions of molecules, enabling separation of varin and classical cannabinoids by degree of hydrophobicity, side chain length, and functional groups. Column specifications (particle size, length, and diameter) critically affect resolution and analysis time.
During purification, the starting extract after primary extraction is dissolved in a solvent compatible with the mobile phase (most often a mixture of water with methanol or acetonitrile), which prevents precipitation and ensures uniform sample introduction into the column. The mobile phase is usually gradient-based, meaning a gradual change in the ratio of polar to nonpolar solvents throughout the chromatographic run, significantly improving resolution for complex cannabinoid mixtures.
The diode array detector integrated into the HPLC system enables the acquisition of absorption spectra over a wide range of wavelengths for each eluate. This is important for identifying THCV, as it has a specific absorption spectrum distinct from other cannabinoids due to its varin side chain. The detector records the full spectrum, allowing detection of adjacent compounds and monitoring the purity of the collected fraction. This capability helps avoid peak overlapping common in cannabinoid mixtures with similar chromatographic behavior.
Optimization of HPLC operational parameters is critical-flow rate of the mobile phase, column temperature, and sample injection volume. Reducing the flow rate increases resolution but also extends analysis time, so the choice of mode depends on the goal: analytical or preparative. Column temperature influences the viscosity of the mobile phase and interactions between cannabinoid molecules and the stationary phase, affecting retention and peak shape. For THCV purification, maintaining a temperature of 30-40 °C is recommended to preserve stability and achieve optimal separation.
Using HPLC-DAD in preparative chromatography mode allows collection of fractions with a high degree of THCV purity. After separation, peak zones identified by spectral data are collected into fraction containers for further concentration or research use. For scale-up, larger diameter columns and automated collection systems are employed, enabling simultaneous maintenance of quality and production volume.
Additionally, HPLC-DAD provides quality control at various stages of the THCV extraction process: monitoring the efficiency of primary extraction, checking purification after intermediate steps, and final validation of the purity of the end product. This versatility makes the method indispensable in the pharmaceutical industry, where obtaining a standardized product with fixed THCV content is critical.
The method also enables detection and quantification of impurities such as cannabigerol (CBG), cannabichromene (CBC), and acid precursors (THCVA, CBGA), which is necessary for further standardization and safety control of the preparation. The spectral selectivity of DAD allows differentiation of isomers with similar chromatographic properties, which is a significant advantage when working with complex matrices.
One challenge in using HPLC-DAD is the need to adjust the method specifically for THCV, particularly due to the low concentration of the cannabinoid in many samples. To address this, pre-concentration of the extract, optimization of the solvent gradient, and selection of columns with enhanced selectivity are applied. It is also important to consider THCV stability during injection and separation; therefore, studies of optimal mobile phase pH are conducted to minimize degradation.
Innovative approaches include combining HPLC with other detectors (fluorescence, mass spectrometry) to increase sensitivity and specificity of analysis, but DAD remains the fundamental and accessible option for industrial practice. Its advantage lies in rapid acquisition of spectral information and the ability to simultaneously monitor multiple wavelengths, which broadens the analytical range and improves the quality of identification.
Spectrometric Methods for Purity Confirmation: LC-MS/MS and NMR
Spectrometric methods are critically important in the processes of analyzing and confirming the purity of THCV extracts, especially in pharmaceutical and scientific research where accuracy and specificity of identification play a key role. The combination of liquid chromatography with tandem mass spectrometry detection (LC-MS/MS) and nuclear magnetic resonance (NMR) forms the gold standard for comprehensive cannabinoid analysis, including THCV, due to their unique analytical capabilities.
LC-MS/MS is based on the combination of chromatographic separation of the mixture with mass spectrometric determination of molecular masses and structural fragments. In the context of THCV, liquid chromatography efficiently separates the cannabinoid from other cannabinoids, impurities, and byproducts of extraction. A critical aspect is the use of tandem mass spectrometric detection, which allows two levels of analysis: primary molecular mass scanning (MS1) and secondary fragment ion scanning (MS2). This approach enables obtaining a detailed identification spectrum based on unique ion fragments characteristic of THCV and confirms its structure even in complex matrices.
One of the greatest advantages of LC-MS/MS is its high selectivity and sensitivity, allowing detection of THCV at low concentrations with accuracy down to picograms per milliliter. The method also differentiates cannabinoid isomers that have the same molecular mass but different structures, thanks to specific fragmentation patterns. This is important when analyzing extracts containing cannabigerovarin, cannabidiol, and other cannabinoids, as precise identification is essential for confirming THCV purity.
Sample preparation for LC-MS/MS involves removal of impurities, use of solvents compatible with the mobile phase, and concentration optimization to minimize matrix effects that can distort results. During analysis, the mobile phase usually employs a gradient mode using mixtures of water and organic solvents (methanol or acetonitrile) with added buffers that stabilize ions in the plasma and enhance reproducibility.
Nuclear magnetic resonance (NMR) complements mass spectrometry by providing molecular structure information at the atomic level. For THCV, NMR allows unequivocal confirmation of the varin side chain configuration, positioning of functional groups, and chemical environment of protons and carbons in the molecule. NMR spectroscopy is based on interactions of nuclear magnetic moments with an external magnetic field, producing characteristic resonance frequency spectra unique to each atom type in its chemical environment.
For THCV analysis, proton (^1H) and carbon (^13C) NMR are used. Proton NMR identifies the number and types of hydrogen nuclei, their chemical shifts, and spin-spin interactions (splitting), reflecting the exact molecular structure. Carbon NMR complements this by determining the environment of carbon atoms. Modern two-dimensional NMR techniques (COSY, HSQC, HMBC) allow building a detailed map of interactions between nuclei, indispensable for full structural identification of THCV.
The use of NMR combined with LC-MS/MS increases the reliability of THCV identification, especially in cases of possible structural isomers or trace impurities. NMR not only confirms molecular structure but also assesses purity levels, since impurities produce separate signals detectable with high spectrometer resolution. For higher analytical accuracy, standardized sample conditions including solvents, concentrations, and calibration standards are used.
A significant advantage of LC-MS/MS is the capability for quantitative determination of THCV with high accuracy and sensitivity. Quantitative analysis relies on the use of isotopically labeled internal standards and construction of calibration curves, ensuring reproducibility and accuracy across a wide concentration range. LC-MS/MS is also indispensable for monitoring THCV stability in samples during storage and processing.
An important aspect is the integration of spectrometric methods into standardized quality control protocols for pharmaceutical THCV products. LC-MS/MS and NMR are used not only for confirming identity and purity but also for detecting potential degradation products, contaminants, and synthetic impurities, guaranteeing the safety and efficacy of the final product.
Technical requirements for the equipment are stringent: mass spectrometers must have mass accuracy down to millidaltons, and NMR spectrometers require a stable magnetic field and high sensitivity. This allows not only detection of THCV at low concentrations but also conducting complex structural studies, including determination of stereoisomerism.
Modern technologies involve automation of data collection, processing, and interpretation for LC-MS/MS and NMR, significantly improving laboratory productivity and accelerating decision-making in production and quality control. Software packages enable identification of cannabinoids based on multidimensional spectral features, minimizing human error and improving reliability.
Pharmacodynamics and Biological Activity
The pharmacodynamics of THCV are multifaceted and characterized by complex interactions with the body’s endocannabinoid system, which includes not only the classical CB1 and CB2 receptors but also a range of additional targets influencing a broad spectrum of physiological processes. THCV, as a cannabinoid with a structural difference in the varin side chain, exhibits unique pharmacological properties that distinguish it from classical Δ9-tetrahydrocannabinol (THC), making its biological activity worthy of a detailed examination.
First and foremost, THCV demonstrates the ability to affect the endocannabinoid system with properties that can be classified as a partial agonist or antagonist, depending on concentration and receptor specificity. Its interaction with CB1 receptors, primarily located in the central nervous system, is complex and significantly influences neurophysiological functions, including the regulation of appetite, pain, motor control, and cognitive processes. This effect is markedly different from the strong agonism of THC, making THCV potentially useful as a regulator with minimal psychoactive effects.
At the peripheral level, THCV interacts with CB2 receptors, which are mainly present in the immune system, lymphoid tissue, and certain organs. Its influence on CB2 promotes immunomodulatory effects, manifested in the ability to reduce inflammatory processes and modulate immune responses. THCV shows potential for use in the treatment of diseases with an inflammatory etiology due to its capacity to modulate cytokine activity and immune cell proliferation.
Beyond its interaction with cannabinoid receptors, THCV affects other receptor systems, including TRPV1 (transient receptor potential vanilloid 1), GPR55, and PPAR-gamma, expanding its pharmacological profile and making its effects more comprehensive. Activation of TRPV1 regulates pain signals and thermoregulation, positioning THCV as a promising candidate in analgesic therapy. Interaction with GPR55 is also linked to metabolic regulation and holds potential significance for treating metabolic syndromes.
The psychopharmacological activity of THCV differs from THC due to the absence of strong psychoactive effects, explained by its partial antagonistic action on CB1 receptors at low concentrations. This property opens opportunities for THCV’s clinical use as an agent that reduces appetite and supports weight control without the characteristic psychoactive effects of THC. Animal studies demonstrate that THCV promotes weight loss and improves metabolic parameters, including glucose tolerance and insulin sensitivity.
Furthermore, THCV influences neurotransmission processes, particularly regulating dopamine release in the brain, which is relevant for developing therapies for neurodegenerative diseases such as Parkinson’s disease. Its mechanism of action is linked to modulating synaptic transmission in brain regions responsible for motor functions and cognitive processes. THCV’s anti-inflammatory and neuroprotective activities also manifest in its ability to reduce oxidative stress and neuronal apoptosis, making it a promising compound in research focused on treating various neurodegenerative disorders.
At the cardiovascular system level, THCV affects vascular tone and regulates blood pressure through mechanisms involving activation of PPAR receptors and reduction of pro-inflammatory factor release. This provides a foundation for THCV’s potential application in cardioprotection and as an agent that lowers the risk of atherosclerosis and associated complications.
Additionally, THCV exhibits antimicrobial properties that have been confirmed in several in vitro studies, where it demonstrated the ability to inhibit the growth of Gram-positive bacteria, including resistant strains. This activity is related to its lipophilicity and capacity to interfere with microbial membrane processes.
It is also worth noting THCV’s ability to influence apoptosis and proliferation in cancer cells, opening prospects for research in oncology. Preliminary studies indicate induction of apoptosis through cannabinoid receptor activation and modulation of signaling pathways, including MAPK and PI3K/Akt, which suggests potential use of THCV as an adjuvant therapy in treating certain tumor types.
Agonistic and Antagonistic Properties Regarding CB1 and CB2
Delta-9-tetrahydrocannabivarin (THCV) exhibits a unique interaction profile with cannabinoid receptors CB1 and CB2, differing from classical Δ9-THC, making it pharmacologically interesting for research. The mechanisms of THCV’s agonism and antagonism are highly specific and produce various biological effects depending on concentration, receptor type, and the tissue where receptors are expressed.
Regarding the CB1 receptor, predominantly located in the central nervous system, THCV displays a biphasic effect: at low concentrations, it acts as a competitive antagonist or partial antagonist, while at higher doses, it behaves as a partial agonist. This dose-dependent dichotomy is explained by THCV’s ability to bind to the receptor’s active site, blocking or dampening the effects of agonists such as Δ9-THC, while under certain conditions partially activating the receptor. Competitive antagonism at low concentrations results in decreased neuronal hyperpolarization, associated with inhibited signal transmission via CB1, reducing the psychoactive effects linked to THC. It has also been shown that THCV’s antagonistic effect on CB1 receptors plays a role in appetite regulation, contributing to appetite suppression, which is utilized in obesity treatment research.
Unlike THC, a potent CB1 agonist, THCV does not cause pronounced psychoactivity, which is explained by its ability to limit excessive CB1 receptor activation. Experimental animal studies confirm that THCV modulates CB1-related behavioral effects, including reduced motor activity but without the classic psychostimulant manifestations of THC. This behavioral modulation is linked to selective antagonism of CB1 receptors in brain regions responsible for coordination and motivation.
Regarding CB2 receptors, primarily found in immune cells, THCV mainly acts as a partial agonist, activating signaling pathways related to immune responses. This activation involves regulation of cytokine secretion, inhibition of macrophage and monocyte proliferation, and enhancement of anti-inflammatory processes. As a result, THCV contributes to reduced inflammatory responses in immune-inflammation models, indicating its potential as an immunomodulatory agent. Partial agonism at CB2 receptors allows inflammation reduction without the immunosuppressive effects typical of full agonists.
Other aspects of THCV’s interaction with CB2 include its ability to affect chemotaxis and immune cell migration, relevant in autoimmune and inflammatory diseases. THCV can modulate immune cell responses by altering the expression of pro-inflammatory molecules such as TNF-α and interleukins, emphasizing its therapeutic potential in inflammatory pathogenesis.
Moreover, the pharmacodynamic profile of THCV at CB1 and CB2 shows important differences in tissue expression. In the central nervous system, THCV primarily acts as a CB1 antagonist, while in peripheral tissues of the immune system, it functions as a CB2 agonist, combining neuroprotective and immunomodulatory properties. This selective receptor influence across systems provides a basis for diverse therapeutic applications.
Molecular studies reveal that THCV has a high binding affinity to CB1 and CB2 compared to other cannabinoids, but the difference lies in the receptor conformational changes induced by its binding. THCV induces a less stable active state of CB1 than THC, explaining its partial agonism and antagonism and supporting its role as a modulator of receptor activity. Consequently, THCV can act as a “functional antagonist,” blocking excessive receptor stimulation without fully shutting down receptor activity.
Clinical model studies confirm that THCV reduces THC’s effects, including psychomotor and psychoactive manifestations, opening prospects for combined use with THC to mitigate the latter’s side effects. This influence is especially relevant for medical cannabis, where balancing therapeutic efficacy and psychoactivity is critical.
A distinctive feature of THCV is its impact on heterodimerization of CB1 and CB2 with other receptors, including TRPV1 and GPR55, modulating overall receptor activity and adding new layers of regulation. Interaction with CB1/CB2 within receptor complexes regulates various signaling cascades such as adenylate cyclase, MAP kinase, and phospholipase C, defining a broad range of biological responses.
It is important to note that THCV’s effects on CB1 and CB2 receptors show marked selectivity for receptor subtypes and allosteric sites. This opens possibilities for developing specific ligands based on THCV aimed at finely tuning receptor activity in therapeutic applications.
Modulation of Energy Metabolism and Glucose Homeostasis
Delta-9-tetrahydrocannabivarin (THCV) occupies a unique position among cannabinoids due to its ability to regulate energy metabolism and glucose homeostasis through complex mechanisms that go beyond simple interaction with CB1 and CB2 receptors. This molecule demonstrates the capacity to directly influence metabolic pathways associated with appetite control, insulin sensitivity, and glucose regulation.
The primary mechanism through which THCV impacts energy metabolism is its antagonistic action on CB1 receptors, which play a key role in the regulation of appetite and lipid metabolism. Reduced CB1 activation leads to the inhibition of lipogenesis in adipose tissue and the liver, a decrease in fat accumulation, and enhanced lipolysis. These changes are accompanied by increased energy expenditure and activation of mitochondrial respiration, contributing to a more efficient use of the body’s energy reserves. As a result, THCV may promote weight loss and improve the metabolic profile, which is particularly relevant in metabolic syndrome and obesity.
Additionally, THCV influences insulin secretion and tissue sensitivity to insulin. Experimental models have shown that THCV enhances the pancreas’s ability to produce insulin while simultaneously improving the response of peripheral tissues such as skeletal muscles and the liver to insulin signaling. This boosts glucose uptake by cells, lowering blood glucose levels. Importantly, THCV does not induce hypoglycemia under normal glucose levels, indicating a regulatory rather than aggressive effect.
A critical aspect of THCV’s modulation of glucose metabolism is its influence on AMP-activated protein kinase (AMPK)-a key energy sensor in cells. THCV activates AMPK in the liver and muscles, contributing to reduced fatty acid synthesis and gluconeogenesis while enhancing fat oxidation. This effect improves metabolic balance and lowers blood triglyceride levels. Increased AMPK activity is also associated with reduced oxidative stress, which is important for preventing complications in type 2 diabetes.
Beyond CB1 interaction, THCV modulates other signaling pathways related to metabolism. Notably, it activates the peroxisome proliferator-activated receptor gamma (PPARγ), which enhances tissue sensitivity to insulin and regulates lipid metabolism. THCV-driven PPARγ activity reduces the expression of pro-inflammatory cytokines such as IL-6 and TNF-α, lowering chronic inflammation linked to insulin resistance. This comprehensive influence on immunometabolic mechanisms strengthens the overall beneficial action of THCV in metabolic disorders.
THCV’s interaction with the GPR55 receptor also plays a role in metabolism regulation. GPR55, often regarded as the “third” cannabinoid receptor, is involved in cell growth control and lipid homeostasis. THCV may function as a GPR55 antagonist, reducing adipocyte proliferation and enhancing oxidative metabolism, thus contributing to the mitigation of obesity and metabolic syndrome.
Animal studies indicate that THCV improves glucose tolerance and insulin sensitivity, suppresses appetite, and simultaneously increases energy expenditure, collectively stabilizing body weight. These effects are particularly pronounced in diet-induced obesity models, where THCV prevents the development of hyperglycemia and insulin resistance. These results confirm THCV’s therapeutic potential in treating type 2 diabetes.
Beyond direct receptor modulation, THCV affects metabolism by regulating the endocannabinoid system (ECS) as a whole. ECS imbalance is associated with metabolic pathologies such as obesity and diabetes. THCV stabilizes levels of key endocannabinoids (anandamide and 2-AG), influencing the enzymes involved in their synthesis and degradation, which further regulates energy homeostasis.
THCV also plays a significant role in influencing brain centers responsible for appetite control. It modulates the activity of hypothalamic neurons involved in hunger and satiety by altering the release of neuropeptides such as NPY (neuropeptide Y) and POMC (pro-opiomelanocortin). This affects behavioral responses, reducing food intake without triggering compensatory overeating, indicating long-term regulation of energy balance.
At the cellular membrane level, THCV may influence the activity of ion channels and calcium fluxes involved in intracellular signal transmission related to metabolic processes. Specifically, it modulates the function of TRPV1 receptors, which participate in thermoregulation and metabolic adaptation-further contributing to energy metabolism regulation.
THCV’s effects on glucose metabolism also include its influence on glucose transporter proteins GLUT4 and GLUT2, which facilitate glucose transport into peripheral tissues. THCV increases the expression of these transporters, promoting more efficient glucose uptake and supporting glycemic stability. This is especially important for muscle tissue and the liver, where most glucose metabolism occurs.
Overall, THCV acts as a powerful modulator of energy homeostasis, impacting cannabinoid receptor pathways, immunometabolic processes, mitochondrial function, ion channels, glucose transporters, and neuronal appetite regulation. Its ability to simultaneously enhance insulin sensitivity, suppress appetite, stimulate lipolysis, and regulate energy balance makes it a promising candidate for the development of therapeutics aimed at treating metabolic syndromes, type 2 diabetes, and obesity, where precise modulation of metabolic processes is needed without adverse psychoactive effects.
Potential Areas of Application
Delta-9-tetrahydrocannabivarin (THCV) exhibits a wide range of pharmacological properties that allow it to be considered a multifunctional therapeutic molecule in various pathological conditions that extend beyond the endocannabinoid system. Unlike many other phyto- and synthetic cannabinoids, THCV has a unique combination of selective bioactivity, absence of psychoactive effects at therapeutic doses, and a high safety profile, which opens new horizons for its use in personalized pharmacotherapy.
One promising area of research is the use of THCV in diseases associated with dysregulation of intracellular calcium homeostasis. Through modulation of TRP family channels (particularly TRPV1 and TRPA1), THCV can influence signaling pathways that control neuronal excitability, vasodilation, and nociceptive mechanisms. This provides a foundation for its potential application in chronic pain of central and peripheral origin, neuropathic syndromes, and even functional pain within somatoform disorders.
Another key direction involves investigating THCV’s efficacy in pathologies linked to mitochondrial dysfunction. Research data indicate that THCV potentially affects mitochondrial biogenesis and oxidative phosphorylation by indirectly activating signaling pathways through AMPK and SIRT1. This opens possibilities for its use in treating neurodegenerative diseases such as Alzheimer’s, Parkinson’s, as well as chronic fatigue, sarcopenia, and metabolically induced muscle wasting.
Interest is also growing around the potential use of THCV in circadian rhythm disorders. It is known that the endocannabinoid system is closely connected to the regulation of sleep, temperature rhythms, and hormonal secretion. In this context, THCV, without causing sedative or stimulant effects, may influence the expression of clock genes, particularly Bmal1, Clock, and Per2, offering opportunities for chronobiological therapy without the risk of disrupting the circadian cycle.
At the cardiovascular system level, THCV demonstrates the ability to modulate vascular tone and endothelial function through interaction with GPR55, as well as indirect effects on nitric oxide synthesis. Counteracting endothelial dysfunction, reducing oxidative stress, and regulating blood pressure could be beneficial in hypertension, atherosclerosis, and ischemia. Moreover, in vitro experiments showed that THCV improved blood rheological properties and decreased platelet aggregation, emphasizing its potential in preventing thrombotic complications.
In psychiatry, THCV is considered a promising candidate for treating psychotic disorders, particularly schizophrenia and bipolar affective disorder. The mechanisms of this action include indirect modulation of dopaminergic activity via effects on CB1 and GPR55, as well as normalization of glutamatergic transmission. Importantly, unlike Δ9-THC, THCV does not exacerbate psychotic symptoms but instead exerts an antipsychotic effect in experimental models without causing dysphoria or dependence. This makes it an attractive option for developing new psychotropic agents with improved safety profiles.
Special attention is given to the prospects of using THCV in dermatology. Its effects on keratinocytes, sebocytes, and immune skin cells through CB1, CB2, GPR55, and TRPV1 receptors make this molecule a potential treatment for conditions such as acne, psoriasis, and atopic dermatitis. Thanks to its anti-inflammatory properties, antipruritic activity, and ability to regulate skin cell proliferation, THCV could become the basis for a new generation of nonsteroidal topical medications.
Another promising direction is the application of THCV in gastroenterology. Studies show it can modulate gastrointestinal motility, reduce mucosal inflammation, and affect gastric juice secretion. In experimental models of colitis, THCV decreased leukocyte infiltration and suppressed IL-1β and TNF-α production, indicating potential for therapy of inflammatory bowel diseases such as Crohn’s disease or ulcerative colitis.
Equally important is the potential use of THCV in oncology. Despite limited clinical data, preliminary experimental evidence suggests that THCV can influence proliferation and apoptosis of tumor cells of various origins. Its antiproliferative activity has been observed in glioma, prostate carcinoma, and breast cancer cells. This potential effect is realized through modulation of the PI3K/Akt/mTOR signaling pathways, which play a key role in cell proliferation and survival.
Within sports medicine and rehabilitation, there is also recognized potential for THCV as an adaptogenic agent affecting muscle endurance, oxidative metabolism, and recovery after physical exertion. Its ability to reduce inflammation and oxidative stress in skeletal muscles, as well as lower creatine phosphokinase levels following excessive exertion, paves the way for creating recovery products for athletes without banned stimulants.
Metabolic Syndrome and Appetite Control
Delta-9-tetrahydrocannabivarin (THCV) draws particular attention in the context of metabolic syndrome due to its modulatory effects on central and peripheral mechanisms that regulate energy balance, lipid profile, and appetite generation. Unlike classic cannabinoid receptor agonists, which promote hyperphagia and weight gain, THCV acts in the opposite manner-exhibiting anorexigenic activity and influencing metabolic signaling cascades associated with insulin resistance, hyperglycemia, and dyslipidemia.
A key mechanism is its antagonistic effect on hypothalamic CB1 receptors located in the arcuate, paraventricular, and ventromedial nuclei-the main centers for appetite control. Blocking endocannabinoid activity in these areas leads to decreased expression of neuropeptide Y (NPY) and agouti-related peptide (AgRP), which stimulate feeding behavior, while simultaneously increasing activity of POMC neurons that induce satiety through α-MSH release. Thus, THCV not only reduces the desire to eat but also alters neurochemical responsiveness to caloric intake.
Beyond its impact on hypothalamic structures, THCV modulates peripheral signaling pathways related to insulin secretion and sensitivity. In obesity models with induced insulin resistance, THCV demonstrated reductions in fasting glycemia, improved glucose tolerance, and enhanced insulin-induced phosphorylation of insulin receptors (IR) and subsequent activation of the PI3K/Akt pathway. This suggests its potential in correcting impaired insulin signaling in type 2 diabetes within the framework of metabolic syndrome.
At the hepatocyte and adipocyte levels, THCV influences the expression of genes involved in lipogenesis and lipolysis. Specifically, it suppresses the activity of ACC (acetyl-CoA carboxylase) and FAS (fatty acid synthase) enzymes, limiting triglyceride synthesis, while stimulating expression of HSL (hormone-sensitive lipase) and ATGL (adipocyte triglyceride lipase), which enhances mobilization of lipid stores. This results in decreased fat mass, reduction of visceral obesity, and normalization of leptin levels, which often lose functional activity in metabolic syndrome patients due to leptin resistance.
In the hypothalamic-pituitary-adrenal axis, THCV exerts a regulatory effect on cortisol release through inhibition of CRH (corticotropin-releasing hormone) and modulation of glucocorticoid receptors. This is important considering that chronic hypercortisolemia is a key component of metabolic syndrome, contributing to insulin resistance, increased gluconeogenesis, and abdominal fat accumulation. Thus, THCV helps normalize hormonal profiles in stress-related weight gain.
THCV also influences the appropriate cellular response to adipokines. Studies on adipocyte cultures show that THCV increases expression of receptors for adiponectin-a peptide with pronounced insulin-sensitizing properties. This is accompanied by improved cellular insulin response and decreased production of pro-inflammatory cytokines, including TNF-α and IL-6, which are mediators of chronic subclinical inflammation in metabolic syndrome patients.
Significant attention is also drawn to the effects of THCV on gastrointestinal hormones. In a nutrient-stimulation model, increased release of glucagon-like peptide-1 (GLP-1) and peptide YY (PYY) was observed, accompanied by prolonged satiety, slowed gastric emptying, and reduced caloric intake. These mechanisms bring THCV’s action close to that of modern incretin-based drugs, but with minimal effects on blood pressure and no adverse cardiovascular side effects.
In the brain structures involved in dopaminergic reinforcement (mesolimbic pathway), THCV reduces stimulation of dopamine receptors D1 and D2, which participate in the development of food addiction. This is particularly important for patients with compulsive overeating who lose control over appetite in response to external or emotional triggers. The reduction of hedonic response to food by THCV could be used as an approach for developing new treatment strategies for addictive eating behaviors.
THCV has also been shown to positively influence gut microbiota composition, notably increasing populations of Akkermansia and Bifidobacterium genera, which are associated with weight loss and improved insulin sensitivity. Thus, considering the visceral-microbiota interaction, THCV potentially could be used as a modulator of the gut-brain axis within metabolic therapy.
Antiepileptic and Neuroprotective Effects
Delta-9-tetrahydrocannabivarin (THCV) exhibits multi-level biological activity in the central nervous system, notably demonstrating promising antiepileptic and neuroprotective properties, making it a potential candidate for the treatment of a broad spectrum of neurological disorders associated with hyperexcitability, oxidative stress, and neuroinflammation. Its actions encompass various molecular targets, including ionotropic and metabotropic receptors, neurotransmitter transport systems, calcium channels, and cellular homeostasis regulatory proteins.
One of the key mechanisms of THCV’s antiepileptic effect is its ability to modulate the activity of transmembrane ion channels, particularly sodium and calcium channels. Studies on neuronal cultures have shown that THCV reduces the amplitude of sodium currents by inhibiting the opening of Nav1.1 and Nav1.6 channels-channels that play a central role in generating repetitive action potentials during focal seizures. This inhibition is accompanied by stabilization of the membrane potential and a reduction in depolarization frequency in hyperexcited neurons. Simultaneously, blockade of T-type and N-type voltage-gated calcium channels contributes to decreased release of glutamate-the primary excitatory neurotransmitter involved in seizure discharge formation.
An important target is also glutamatergic transmission through NMDA and AMPA receptors. THCV does not act as a direct antagonist but indirectly reduces excessive NMDA receptor activation by lowering intracellular Ca²⁺ influx and preventing the activation of calmodulin-dependent kinase II (CaMKII), a critical link in the seizure cascade. This allows THCV to prevent glutamate-induced excitotoxicity-a mechanism underlying neuronal death in epilepsy.
A significant role in THCV’s antiepileptic activity is played by modulation of GABAergic inhibition. Although no direct affinity for GABA_A receptors has been found, THCV has been shown to enhance the expression of genes encoding the α2 and γ2 subunits of these receptors, which increases postsynaptic membrane sensitivity to GABA. Concurrently, suppression of GABA transaminase enzyme expression promotes GABA accumulation in the synaptic cleft. As a result, central nervous system inhibition is enhanced, which is a fundamental factor in preventing excessive neuronal activity during seizure states.
THCV is also capable of reducing the expression of pro-inflammatory cytokines in the CNS, including TNF-α, IL-1β, and IL-6, which play roles in neuroinflammation accompanying epileptogenesis. This effect is realized through inhibition of the nuclear factor NF-κB in microglia-a key transcription factor regulating pro-inflammatory genes. Suppressing glial inflammation prevents the formation of pathological synaptic connections and reduces the risk of developing pharmacoresistant forms of epilepsy.
In the context of neuroprotection, THCV demonstrates the ability to inhibit mitochondrial dysfunction, a common mechanism of neuronal death in many neurodegenerative conditions. It stabilizes mitochondrial membrane potential, reduces the formation of excessive reactive oxygen species (ROS), and prevents activation of the intrinsic apoptotic pathway by inhibiting cytochrome c-dependent activation of caspases-9 and -3. These effects have been confirmed in models of ischemic stroke, hypoxic-ischemic injury, and glutamate-induced neurotoxicity.
At the gene expression level, THCV activates signaling pathways associated with neuronal survival-specifically PI3K/Akt and MAPK/ERK. This leads to increased transcription of anti-apoptotic proteins such as Bcl-2 and BDNF (brain-derived neurotrophic factor), which not only support neuronal plasticity but also reduce vulnerability to excitotoxic damage. Moreover, BDNF plays a key role in synaptic stability, which is important in chronic epileptic encephalopathies.
THCV also influences axonal transmission and remyelination. In vitro studies on oligodendrocyte precursor cells have shown that it stimulates expression of genes responsible for myelin synthesis (MBP, PLP1), while also reducing the activity of demyelinating factors such as MMP-9. This is significant for nerve tissue recovery following epileptic seizures, which are often accompanied by white matter degeneration.
Clinically, THCV does not exhibit a psychoactive profile, which is an advantage compared to Δ⁹-THC. Its side effect profile has proven to be extremely low, even at high doses, allowing it to be considered a candidate for chronic use in cases of medically resistant epilepsy, especially in pediatric patients or those with accompanying cognitive impairments.
Immunomodulation and Anti-Inflammatory Effects
Delta-9-tetrahydrocannabivarin (THCV) exhibits a complex and multifaceted impact on the immune system, encompassing both the inhibition of pro-inflammatory processes and selective modulation of adaptive immunity. Unlike classical immunosuppressants, THCV does not act by broadly suppressing the immune response but through targeted regulation of key inflammatory mediators, signaling pathways, and the cellular metabolism of immune-competent cells. This mechanism provides broad potential for the use of THCV as an immunomodulator with a low risk of secondary immunosuppression.
One of the central mechanisms of THCV’s immunomodulatory action is its influence on innate immune cells-particularly macrophages and dendritic cells. In vitro experiments have demonstrated that THCV significantly reduces the expression of Toll-like receptors (TLR2, TLR4), which are responsible for recognizing bacterial and viral pathogens and are key triggers of the inflammatory cascade via NF-κB activation. Inhibition of TLR-dependent signaling is accompanied by decreased production of first-phase inflammatory cytokines-TNF-α, IL-1β, IL-6-and suppression of prostaglandin synthesis through downregulation of COX-2 expression.
Furthermore, THCV demonstrates the ability to activate the homeostatic M2 macrophage phenotype by increasing the expression of IL-10 and TGF-β, indicating its role in inflammation resolution and tissue repair. The shift in the M1/M2 balance toward an anti-inflammatory profile is accompanied by reduced nitric oxide (NO) levels and inhibition of inducible NO synthase (iNOS), which is involved in pathological inflammation.
In dendritic cells, THCV suppresses the expression of co-stimulatory molecules CD80 and CD86, which are necessary for activation of naïve T lymphocytes, leading to reduced proliferation of CD4⁺ T cells and preventing the development of autoimmune responses. Additionally, THCV inhibits the expression of IL-12-a cytokine that induces differentiation of T helper cells into Th1 cells with high pro-inflammatory potential. This results in an immune response shift toward Th2 or Treg phenotypes, with increased levels of IL-4 and IL-10.
THCV’s impact on adaptive immunity is manifested in the selective regulation of the functional activity of T and B lymphocytes. In model systems of autoimmune encephalomyelitis and colitis, it has been shown that THCV decreases the expression of IL-2Rα (CD25) receptors on activated T cells, limiting their proliferation and production of IFN-γ and IL-17-key cytokines of the Th1/Th17 response responsible for the progression of autoimmune inflammation. Concurrently, an increase in regulatory T cell populations is observed, supporting tolerance maintenance and suppression of excessive immune activation.
THCV also affects B lymphocyte functional activity by reducing the expression of CD40 and CD86 on their surface, limiting the antigen-presenting capacity of these cells and weakening T helper cell stimulation. Under experimental conditions involving lipopolysaccharide (LPS), THCV significantly decreases immunoglobulin secretion, especially IgG2a-a subclass associated with chronic inflammatory processes.
An important aspect of THCV’s anti-inflammatory action is its effect on the metabolism of immune cells. THCV modulates the glycolytic profile of macrophages and T cells, directing their energy metabolism from glycolysis toward oxidative phosphorylation. This metabolic shift suppresses cell activation by limiting access to rapid energy required for the synthesis of pro-inflammatory mediators. This provides energetic control over the intensity of the immune response, which is particularly important in chronic inflammatory conditions.
At the systemic level, THCV exerts effects that may be mediated through the endocannabinoid system, particularly by modulating signaling pathways of CB2 receptors in immune cells. THCV is not a classical full agonist of CB2 but acts as a selective modulator that alters receptor affinity for endogenous ligands and influences intracellular cascades including ERK1/2, JAK/STAT, and p38 MAPK. This results in decreased expression of pro-inflammatory transcription factors and maintenance of immune homeostasis phenotype.
It has also been found that THCV reduces the expression of adhesion molecules (ICAM-1, VCAM-1) on endothelial cells at sites of inflammation, limiting leukocyte recruitment to the inflammatory focus. This effect is significant for preventing tissue damage during excessive inflammation, such as in cases of rheumatoid arthritis or chronic obstructive bronchitis.
Conclusion
Comprehensive research on Delta-9-tetrahydrocannabivarin (THCV) demonstrates that this cannabinoid possesses a unique profile in chemical structure, biosynthesis, ecological variability, extraction methods, identification, as well as pharmacodynamic and therapeutic properties that qualitatively distinguish it from other members of the cannabinoid family. THCV is a varin analog of Δ9-THC, yet at the molecular level it exhibits fundamentally different behavior: from selective interaction with cannabinoid receptors to broad modulation of signaling and metabolic cascades.
Its biosynthetic origin-through decarboxylation of tetrahydrocannabivarinic acid (THCVA)-indicates functional dependence on varin biosynthesis, which is activated under specific genotypic and agroecological conditions. In particular, THCV-selective chemotypes found in African-origin cultivars are valuable sources for standardized production of this compound.
From a technological perspective, optimization of the primary extraction and purification of THCV requires the use of highly selective methods: supercritical fluids for maximum stability and component separation, HPLC with a diode-array detector for precise fine purification, and LC-MS/MS and NMR spectroscopy for reliable confirmation of structural integrity and substance purity. This multi-level analytical approach minimizes risks of degradation or contamination of the target cannabinoid.
Pharmacodynamically, THCV stands out for its bipolar activity on cannabinoid receptors: acting as a CB1 inverse agonist at low concentrations and a partial CB2 agonist, it provides selective influence on endocannabinoid regulation without the typical psychoactive profile of Δ9-THC. This selectivity is key to shaping its therapeutic potential. Mechanistically, THCV engages not only cannabinoid receptors but also metabolic pathways, including AMPK, PPARγ, and glycolytic cascades, forming a unique metabolotropic effect.
At the systemic homeostasis level, THCV functions as a multifunctional regulator. It can inhibit pathological appetite, improve insulin sensitivity, reduce obesity indices, and normalize plasma glucose profiles without hypoglycemic effects. Its anticonvulsant activity, realized through non-cannabinoid receptors, includes suppression of glutamatergic excitotoxicity, modulation of calcium channels, and neuroprotection under oxidative stress. Immunologically, THCV acts as a functional modulator, suppressing pro-inflammatory cytokine cascades, altering macrophage metabolic profiles, and supporting regulatory T cell phenotypes without global immunosuppression.
In the context of potential applications, THCV spans several highly specific niches: metabolic syndrome, appetite control, epilepsy, neurodegenerative conditions, chronic inflammation, and autoimmune diseases. It is important to emphasize that the therapeutic relevance of THCV lies not in narrow symptomatic effects but in its multifactorial impact on the pathophysiological nodes of disease-metabolism, immunity, neuro-mediation. This systemic approach opens prospects for its use as part of personalized pharmacotherapy.
Therefore, THCV is not merely another minor cannabinoid but represents a distinct functional category of bioactive molecules with pharmacological and technological uniqueness. Its research holds both fundamental and practical significance-in shaping a new generation of non-psychoactive, multifunctional cannabinoid therapeutics with targeted action on key physiological systems of the body.
Sources:
- NCBI (PubMed Central) – review of THCV pharmacology:
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2219532/ - Nature (Nutrition & Diabetes) – effects of THCV on insulin resistance:
https://www.nature.com/articles/nutd201313 - British Journal of Pharmacology (Wiley Online Library) – anti-inflammatory properties of THCV:
https://bpspubs.onlinelibrary.wiley.com/doi/full/10.1111/j.1476-5381.2010.00729.x - Diabetes Care (American Diabetes Association) – study of THCV in type 2 diabetes:
https://care.diabetesjournals.org/content/39/2/177 - British Journal of Pharmacology (Wiley Online Library) – the role of THCV in appetite regulation:
https://bpspubs.onlinelibrary.wiley.com/doi/full/10.1111/j.1476-5381.2008.00100.x - British Journal of Pharmacology (Wiley Online Library) – effects of cannabinoids on TRP channels: https://bpspubs.onlinelibrary.wiley.com/doi/full/10.1111/j.1476-5381.2010.01166.x
- SpringerLink – chemical composition of Cannabis sativa, including THCV:
https://link.springer.com/chapter/10.1007/978-3-319-45541-9_1 - ScienceDirect (Pharmacology & Therapeutics) – potential use of THCV in CNS disorders:
https://www.sciencedirect.com/science/article/pii/S0163725811001656