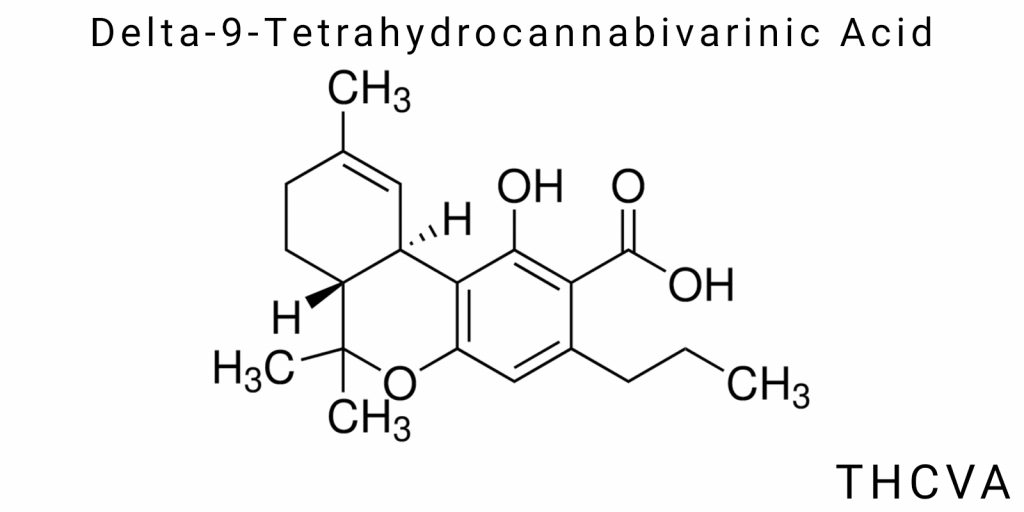
Delta-9-Tetrahydrocannabivarinic Acid (THCVA) is the acidic form of a lesser-known varin cannabinoid found in Cannabis sativa L. in extremely low concentrations, predominantly in plants of the so-called chemotype IV. Despite its structural similarity to delta-9-tetrahydrocannabinolic acid (THCA), THCVA is a derivative of divarinic acid rather than olivetolic acid and features a shortened side chain composed of three carbon atoms. This chemical distinction significantly alters its biological behavior and pharmacological profile, which has sparked growing interest among cannabinoid researchers.
Unlike more extensively studied cannabis compounds such as Δ9-THC or CBD, THCVA remains in the shadows not only because of its low natural concentration but also due to the difficulty of isolating it in a stable acidic form. The cannabinoid rapidly undergoes decarboxylation to THCV under the influence of heat or time, complicating quantitative determination and functional studies of the acidic form itself. This also partially explains why THCVA is almost entirely absent in chromatographic profiles of processed or stored extracts and why it is not included in the pharmacopoeial or regulatory standards of most countries.
At the molecular level, THCVA is formed through the varin biosynthetic cascade, which begins with the interaction of geranyl pyrophosphate with divarinic acid, catalyzed by a specific tetrahydrocannabivarinic acid synthase (THCVAS). This pathway is independent of the primary olivetolic acid route responsible for synthesizing the more common cannabinoids. However, the presence of varin derivatives such as THCVA, CBVA, or CBGVA in cannabis biomass indicates a broader spectrum of chemotypic variability than previously thought and holds potential for use as a selection or biochemical marker in research and raw material standardization.
Interest in THCVA is also driven by its hypothetical pharmacological activity. It is known that the decarboxylated form-THCV-exhibits antagonism at CB1 receptors and possesses anorexigenic, anticonvulsant, and anti-inflammatory properties. However, the pharmacodynamics of the acidic form remain largely unexplored. Data regarding its affinity for cannabinoid system receptors, stability under physiological conditions, or bioavailability lack experimental confirmation but are critical for developing new non-psychoactive drug prototypes.
Current scientific attention to acidic cannabinoid forms, particularly CBDA, CBGA, and THCA, stimulates interest in THCVA as a potentially active component that may have been misinterpreted or underestimated in previous studies due to technical limitations of identification methods. Along with advances in precision chromatography, mass spectrometry, and nuclear magnetic resonance spectroscopy, new opportunities are emerging for the isolation, identification, and pharmacological analysis of even such unstable and low-concentration molecules as THCVA.
Chemical Characterization and Biogenesis
Delta-9-tetrahydrocannabivarinic acid (THCVA) belongs to the class of varin cannabinoids-secondary metabolites characteristic of the Cannabis genus-formed via polyketide biosynthesis followed by terpenization and cyclization. Although THCVA is the acidic form that decarboxylates to the active THCV, the acidic variant represents the initial compound form in raw plant biomass and is the chemically stable product of enzymatic metabolism. From the standpoint of cannabinoid chemomics, THCVA is treated as a non-psychoactive precursor; however, its structural relatedness to classical Δ⁹-tetrahydrocannabinolic acid (THCA) suggests similar-but not identical-biological properties.
Chemically, THCVA is classified as a varin acid with a three-carbon side chain at the 5-position of the phenolic nucleus, which distinguishes it from the five-carbon olivetolic acid backbone underlying most cannabinoids. In the biosynthetic context, this difference is fundamentally important because it affects the molecule’s spatial configuration, hydrophobicity, interactions with cannabinoid receptors, and stability under different physicochemical conditions. Thus, THCVA is not merely an isomer of THCA-it represents a separate molecular category with its own unique biogenetic pathways and physicochemical characteristics.
Structurally, THCVA is a tricyclic terpene-phenol derivative with a carboxyl group that easily undergoes decarboxylation under heat or time to the neutral molecule THCV. From an analytical perspective, researchers must distinguish cannabinoid acids from their decarboxylated forms, considering that acids predominate in untreated plant matrices. This complicates the analytical identification of THCVA, especially in thermally processed, aged, or extracted materials where a significant portion of the acid has already converted to THCV.
According to current understanding of cannabis metabolism, cannabinoid synthesis occurs through the interaction of the terpene precursor geranyl pyrophosphate (GPP) with a polyketide precursor. In the case of THCVA, this polyketide is divarinic acid (the varin equivalent of olivetolic acid), which is formed via polyketide synthase activity and specific cyclase enzymes. Following condensation of GPP with divarinic acid, cannabigerovarinic acid (CBGVA) is formed-a central intermediate metabolite of the varin cannabinoid biosynthesis branch. This precursor is subsequently metabolized by specific synthases, including tetrahydrocannabivarinic acid synthase (THCVAS), which catalyzes the cyclization of CBGVA into THCVA.
The biosynthetic specificity of THCVA is also reflected in the distribution of Cannabis sativa chemotypes. It has been found that THCVA predominantly occurs in plants of chemotype IV-those that accumulate varin acids (THCVA, CBDVA, CBGVA) as their primary cannabinoids-while classical THCA or CBDA predominate in chemotypes I-III. This distribution is determined by genetic variants of the respective synthases-THCVAS, CBDVAS, and CBGA synthase-each exhibiting high substrate selectivity for CBGVA rather than CBG (the five-carbon cannabigerolic acid). Therefore, the presence of THCVA in the plant is not only a result of enzymatic activity but also a marker of the genetic configuration of biosynthetic enzymes.
Thanks to these chemotypic distinctions, THCVA may be used as a selection marker in genetic breeding programs aimed at developing cultivars with low Δ⁹-THC content or enriched varin profiles. For example, cultivars with high THCVA content could be utilized in pharmacology as sources of uncommon cannabinoids with minimal psychoactivity, which is fundamentally important when developing safe therapeutic agents for vulnerable populations such as children or individuals with psychiatric disorders.
Special attention should be paid to the physicochemical stability of THCVA. Like all cannabinoid acids, it is unstable during storage and prone to autooxidation, isomerization, and decarboxylation. This limits its use in pharmaceutical formulations without appropriate stabilization protection or controlled environmental conditions. On the other hand, the presence of the carboxyl group in the molecule makes it more polar compared to THCV, enabling targeted chromatographic separation using reversed-phase or ion-exchange chromatography.
From a chemical standpoint, THCVA can also serve as a model structure for studying the effects of shortened side chains on cannabinoid activity. Such studies hold promise for structure-activity relationship modeling and the creation of synthetic analogs with optimized receptor profiles. Pharmaceutical companies are already showing interest in non-psychoactive varin derivatives, particularly due to their potential antagonism of CB1 receptors, opening possibilities for therapy of obesity, type 2 diabetes, epilepsy, or neuroinflammatory diseases.
Structural Composition and Classification
Delta-9-tetrahydrocannabivarinic acid (THCVA) is a member of a specific subgroup of cannabinoids known as the varin cannabinoids, which are characterized by a unique structural feature – a shortened side alkyl chain consisting of three carbon atoms. This structural modification distinguishes them from classical five-carbon cannabinoids, such as Δ9-tetrahydrocannabinolic acid (THCA). The structural characteristics of THCVA not only define its physicochemical properties but also significantly influence its molecular interactions with biological targets, notably cannabinoid receptors.
The chemical structure of THCVA contains three main functional components: a phenolic ring, a cyclic terpene fragment, and a carboxylic acid group. The phenolic ring consists of a benzene nucleus with a hydroxyl group, which provides the molecule with polarity and the ability to form hydrogen bonds. This fragment determines the chemical reactivity of the compound, including its susceptibility to oxidation and conjugation with other molecules. The carboxyl group confers acidic properties to THCVA, making it the dominant form in raw plant material and ensuring alkaline solubility. Upon heating or enzymatic action, decarboxylation occurs, leading to the formation of the neutral form-THCV.
The cyclic terpene portion of the molecule is a three-membered structure formed by the involvement of a pyran ring, typical for cannabinoids, which defines the three-dimensional configuration of the molecule and its ability to interact with specific receptors in the body. In the case of THCVA, the difference lies in the shortened side chain, which shifts the molecule’s conformation, resulting in both altered affinity profiles for CB1 and CB2 receptors and potential differences in its ability to cross biological barriers.
The classification of THCVA in a chemical context is based on several criteria. First, structurally, it belongs to the group of phenolic carboxylic acids-organic compounds characterized by the presence of a phenolic ring attached to a carboxyl group. This fact determines its chemical reactivity and interactions within the plant’s ecosystem as well as in biological environments. Second, in the context of cannabinoids, THCVA is classified within the varin subclass, which indicates the presence of a three-carbon alkyl side chain compared to the five-carbon chain of the main group of cannabinoids. It is important to emphasize that this classification is fundamental for determining the biological activity of the compound since the length of the alkyl chain directly affects binding to receptors and enzymatic processing.
From a molecular standpoint, THCVA represents a complex organic ester with a terpene base connected to a phenolic acid via an ether linkage. This structure provides a balance between hydrophobicity and polarity of the molecule, which is critical for its ability to penetrate cellular membranes and interact with lipophilic receptors. The structural organization of THCVA allows it to form complexes with protein targets and influence signaling pathways that regulate physiological processes.
Unlike more common cannabinoids such as THCA or CBD, varin cannabinoids, including THCVA, exhibit specific stereochemical properties. THCVA shows conformational flexibility due to its shorter alkyl chain, which affects its spatial orientation in molecular interactions. This flexibility may increase or decrease affinity to CB1 and CB2 receptors or alter its ability to activate additional receptor systems such as TRPV1 or GPR55. Studying these characteristics is crucial for developing new pharmacological agents with finely tuned selectivity.
There is also a classical division of cannabinoids based on the type of molecular core: olivetol-type (with a five-carbon side chain) and varin-type (with a three-carbon side chain). THCVA belongs to the latter group, reflecting its chemical origin from divarinic acid rather than olivetolic acid. This classification is fundamentally important not only for chemical characterization but also for pharmacodynamics, as the difference in the length of the side chain dramatically changes interactions with receptors and enzymes that modulate cannabinoid metabolism.
Another aspect of structural classification is the spatial arrangement of functional groups-the carboxyl, phenolic, and hydroxyl groups. In THCVA, the carboxyl group is positioned at carbon 1, enabling ionization at physiological pH and determining the acid-base properties of the molecule. This feature influences its solubility in aqueous and lipid environments, which has practical importance during extraction, analysis, and potential therapeutic use.
From a physicochemical perspective, THCVA is highly polar compared to neutral cannabinoids, which favors its dissolution in polar organic solvents such as methanol or acetonitrile. This property dictates the specifics of its extraction from plant material and is critical for standardizing analytical methods such as high-performance liquid chromatography (HPLC) and mass spectrometry.
A distinctive feature of THCVA is its ability to undergo rapid thermal decarboxylation with the formation of THCV, an active neutral cannabinoid. This process occurs when heated above 100 °C and determines the biological transformation of THCVA during material processing. Chemically, this transition results from the loss of CO₂ from the carboxyl group, leading to radical changes in the molecular structure and pharmacological activity.
Classification-wise, THCVA is viewed in the broader context of cannabinoids, which are subdivided based on the number of carbons in the side chain (varin-type, olivetol-type) as well as the types of functional groups (acids, neutral compounds, oxidized metabolites). Such structural distinctions are decisive for determining chemical stability, metabolic pathways, and pharmacokinetics, as well as for developing methods of analytical control.
Understanding the structural composition of THCVA enables prediction of its chemical reactivity, including the potential for forming conjugates with proteins or other macromolecules, which is important for investigating its possible toxicological profile and interactions in living systems. The structural specificity of THCVA also opens prospects for synthesizing chemical analogs with targeted modifications that may alter molecular properties to enhance therapeutic potential.
Biosynthetic Origin in Cannabis sativa
The biosynthesis of delta-9-tetrahydrocannabivarinic acid (THCVA) in Cannabis sativa results from a complex cascade of secondary metabolism pathways that combine isoprenoid and polyketide origins. Central to this process are two key molecules: geranyl pyrophosphate (GPP), which serves as the terpene precursor, and divarinic acid-a specific varin analog of olivetolic acid. Their condensation in the presence of a specific synthase enzyme leads to the formation of cannabigerovarinic acid (CBGVA), which acts as the biosynthetic precursor for the subsequent enzymatic conversion into THCVA.
Precursors: Geranyl Pyrophosphate and Divarinic Acid
Geranyl pyrophosphate is synthesized in the cytosol of Cannabis sativa cells via the mevalonate pathway (MVA) or alternatively in plastids through the methylerythritol phosphate pathway (MEP). This C10 isoprenoid diphosphate is a universal precursor for many terpenes and cannabinoids. Its electrophilic nature and activated phosphate environment make it suitable for nucleophilic attack by aromatic acids, particularly divarinic acid.
Divarinic acid (4-pentylresorcinolic acid with a three-carbon side chain) is formed through polyketide synthesis involving a specialized variation of tetraketide synthase (TKS) and olivetolic cyclase activity. Compared to olivetolic acid-the precursor of classical cannabinoids-divarinic acid is synthesized from propionyl-CoA instead of acetyl-CoA, which determines its shortened side chain. This substitution of the initial acyl donor alters the length of the final product and directs the entire metabolic flow toward the varin chemotype.
While the precise mechanism of divarinic acid synthesis remains under active investigation, experimental data using radiolabeled isotopes confirm its origin from propionic acid through malonyl-CoA-induced condensation. At the enzymatic level, cyclization of the poly-β-ketone chain occurs, forming the phenolic core-a hallmark feature of future cannabinoids.
Condensation to CBGVA: The Central Node of Varin Biosynthesis
The union of geranyl pyrophosphate and divarinic acid is catalyzed by cannabigerovarin synthase (CBGVS), which exhibits high specificity for three-carbon substrates. This enzyme facilitates the alkylation reaction to produce cannabigerovarinic acid (CBGVA)-the precursor to all varin acids. The stereochemical orientation of this reaction is critical, as it determines the subsequent specificity of enzymes involved in later biosynthetic stages.
CBGVA functions as the biochemical branching point in the biosynthetic pathway. Depending on the expression of specific oxidase synthases, it can be converted into various varin acids, including cannabidivarinic acid (CBDVA), cannabichromevarinic acid (CBCVA), or THCVA. This reflects the genetic regulation of chemotype, as the profile of expressed enzymes varies according to the genotype of C. sativa and cultivation conditions.
Specific Role of THCVA Synthase
The key enzyme responsible for converting CBGVA into THCVA is delta-9-tetrahydrocannabivarinic acid synthase (THCVAS). This enzyme belongs to the flavoprotein oxidoreductase family, utilizing FAD as a cofactor. THCVAS catalyzes the cyclization of the prenyl fragment of CBGVA through oxidative opening of the pyran ring, forming a new cyclic structure while retaining the carboxyl group. Importantly, the enzyme does not perform decarboxylation-thus, THCVA remains in its acidic form until exposed to exogenous treatments such as heating, enzymatic action, or time.
The THCVAS gene and its transcriptional profiles are closely linked to the plant’s chemotype. Certain C. sativa subpopulations of var. indica and var. afghanica consistently express THCVAS, leading to predominant accumulation of THCVA in trichomes. Sequence analysis of the enzyme reveals high homology to THCA synthase; however, even minor differences in the enzyme’s active site result in a radical shift in substrate specificity.
Crystallographic studies of THCVAS show that its substrate-binding pocket is more tightly organized to accommodate the smaller divarinic side chain. This structural adaptation indicates evolutionary specialization of the enzyme for varin compounds.
Regulation and Compartmentalization of the Metabolic Pathway
In Cannabis sativa cells, THCVA synthesis is localized in secretory trichomes, where enzymatic machinery concentrates within the secretory head. There, vesicles containing membrane-bound synthase complexes are formed. This compartmentalization reduces competition among different synthases and allows maintenance of high local concentrations of CBGVA for efficient THCVA biosynthesis.
On the regulatory level, light, temperature, and nutrient availability play important roles. It has been shown that short-day photoperiods stimulate THCVAS expression, whereas deficiencies in phosphorus or potassium can reduce enzymatic activity.
Phylogenetic and Environmental Sources
The biosynthesis of delta-9-tetrahydrocannabivarinic acid (THCVA) in Cannabis sativa results from the interplay between genetically determined traits of the plant and environmental stimuli that influence the expression of secondary metabolism. Unlike the more extensively studied cannabinoids with a pentyl side chain, the synthesis of varin-type acids, including THCVA, strictly depends on the presence of specific biochemical prerequisites: the formation of divarinic precursors, the activity of corresponding synthases, and tightly regulated control within the cannabinoid metabolon.
Genetic variability in Cannabis sativa provides a broad spectrum of chemotypes, each characterized by the predominance of a specific cannabinoid group. Chemotypes dominated by THCVA are less common than typical Δ⁹-THCA- or CBDA-dominant forms, yet they represent the greatest scientific interest due to their potentially distinct biological activity and chemoselectivity.
Chemotype determination is conducted through chromatographic analysis of the cannabinoid profile; however, the root causes of this chemical profile are embedded in heredity. The THCVA chemotype forms as a result of the expression of specific genes encoding THCVAS, as well as the activation of genes responsible for the biosynthesis of the precursor-divarinic acid. Accordingly, such chemotypes typically display a characteristic combination of two independent traits: (1) dominance of a short-chain acyl donor (propionyl-CoA instead of acetyl-CoA) in primary metabolism, and (2) the presence of a functional THCVA synthase allele, structurally distinct from THCA synthase.
Genetic markers associated with THCVAS expression remain incompletely mapped, but whole-genome sequencing analyses have identified specific loci on chromosome 6 (C. sativa has 10 chromosomes) that exhibit active transcription of THCVAS isoforms only under conditions of high CBGVA concentration in tissues. This highlights the importance not only of transcriptional control but also of metabolic context as a limiting factor for THCVA production.
Population analysis shows that chemotypes with high THCVA content are more frequently found among African Cannabis sativa var. indica lines and in Asian regions where indigenous varin-type forms dominate. For example, phenotypic data available for lines such as Durban Poison or Red Congolese consistently demonstrate elevated THCVA levels under appropriate cultivation practices. These lines have limited distribution due to their low overall Δ⁹-THC levels but represent a scientifically valuable source of highly specific varin-type metabolism.
Beyond genetics, environmental factors exert a significant influence on THCVA synthesis. Agroecological conditions-specifically lighting, photoperiod, temperature, humidity, and mineral nutrient availability-not only determine the overall biomass of trichomes but also the metabolic directionality of synthetic pathways. For instance, temperatures above 28°C recorded during the active flowering phase are associated with increased expression of divarinic pathway genes, whereas excessive light intensity (>1000 µmol m⁻²s⁻¹) primarily induces a THCA-dominant response.
Studies using hydroponic systems have shown that nitrogen or magnesium deficiency during the late vegetative stage shifts metabolism toward varin-type cannabinoids, likely through stress-induced alteration of primary metabolism. Meanwhile, an optimal phosphorus level (15-30 ppm) and a pH range of 5.8-6.3 in the nutrient solution correlate positively with THCVA synthase activity, as confirmed by proteomic investigations.
Particular attention has been given to the effects of altitude and ultraviolet radiation on THCVA synthesis. Mountainous regions (above 1200 meters) show higher percentages of varin-type acids, explained by the activation of protective mechanisms and an increase in flavonoid content, which presumably acts as a signal for the expression of secondary metabolism enzymes.
Another factor is soil type. Light sandy substrates, which limit root zone hydration, promote THCVA accumulation, whereas heavy clay soils with high moisture retention are associated with decreased varin fractions in the cannabinoid profile. It can be inferred that osmotic stress activates the same metabolic cascades that favor the accumulation of varin analogs.
Equally important is the biotic environment-the presence of symbiotic fungi, bacteria, and mycorrhizae. Current data indicate that symbiosis with Rhizophagus irregularis or other glomeromycetes enhances CBGVA production in trichomes, which in turn increases substrate availability for THCVA synthase. The root zone microbiome thus acts as an indirect regulator of the cannabinoid profile.
Cannabis Chemotypes with High THCVA Content
Cannabis sativa chemotypes characterized by the predominance of delta-9-tetrahydrocannabivarinic acid (THCVA) represent a distinct group within the phytochemical classification of the species. These chemotypes arise from a specific combination of genetic variants that ensure the dominance of varin-type cannabinoids in the plant’s biosynthetic profile. Typically, these phenotypes exhibit low Δ⁹-THC content and elevated levels of cannabinoids with a short (C₃) side chain.
The traditional classification of cannabis chemotypes, proposed by Small & Beckstead (1973) and refined in subsequent phytochemical studies, divides populations into three main types: I (THC-dominant), II (THC/CBD mixed), and III (CBD-dominant). However, an expanded classification includes two additional types: IV (CBG-dominant) and V (non-cannabinoid). Chemotypes with high THCVA are formally assigned to subtypes within type I or to a separate classification group VI, which is increasingly distinguished in research circles to describe varin-dominant lines.
The genetic basis of these chemotypes includes the presence of an active THCVA synthase gene (THCVAS) and a metabolic pathway favoring the condensation of geranyl pyrophosphate with divarinic acid (rather than olivetolic acid). This biosynthetic shift produces a distinct chemical signature in the cannabinoid content formed within the plant. Inheritance of an active THCVAS leads to co-production of THCVA with little or no THCA, allowing precise identification of these chemotypes even in early ontogenetic stages.
Some of the best-known high-THCVA strains originate from geographic regions where local climate and cultural selection pressures influenced breeding. African lines, notably Durban Poison, Power Plant, and Malawi Gold, exhibit a stable varin cannabinoid profile. These strains were developed in areas with high insolation, which may have favored genotypes prone to varin metabolism. Molecular identification has confirmed the presence of a conserved THCVAS allele with elevated transcription levels in these varieties.
Beyond African genetic lines, high-THCVA chemotypes have been identified among Central Asian populations (C. sativa var. afghanica) and in some hybrid strains created through modern breeding programs. For example, Doug’s Varin and Pineapple Purps lines were developed through targeted crosses of high-varin phenotypes and have THCVA levels exceeding 1.5% dry weight, significantly higher than the average for cultivated forms.
Within chemotypes, variability among phenotypes even within the same population indicates multifactorial hereditary control of THCVA biosynthesis. It is known that THCVA levels in these plants depend heavily on the developmental stage of the flowers and the induction of trichome tissue. This opens opportunities for intra-population selection based on morphological and metabolic screening.
Phylogenetic analysis employs quantitative methods to define THCVA chemotypes. High-performance liquid chromatography (HPLC) combined with mass spectrometry enables separation of cannabinoid isomers with high accuracy. Chemical profile data serve as phenomarkers for constructing dendrograms and clustering populations by metabolite similarity. This approach underpins the development of THCVA-focused genetic material banks.
An important feature of chemotypes dominated by THCVA is their potential pharmacological value. However, due to limited commercial demand, these chemotypes have until recently been excluded from large agricultural companies’ breeding programs. The situation is changing now, driven by growing interest in non-intoxicating or low-intoxicating cannabinoids with potential for treating metabolic disorders, endocrine regulation, and neuroprotection. Therefore, THCVA chemotypes may become a valuable source of biomass for pharmaceutical extraction, especially under strict Δ⁹-THC regulatory conditions.
Agroecological Conditions Affecting THCVA Synthesis
The biosynthesis of delta-9-tetrahydrocannabivarinic acid (THCVA) in Cannabis sativa is a dynamic process controlled not only by genetic factors but also by agroecological environmental parameters. Expression of enzymes responsible for synthesis, such as THCVAS, and the rate of precursor accumulation (geranyl pyrophosphate and divarinic acid) are closely linked to the plant’s cultivation conditions. Key external factors most impacting THCVA levels in tissues include: light intensity, photoperiod, spectral composition of light, temperature, soil pH, humidity, microelement composition of the substrate, and biotic interactions in the rhizosphere.
Lighting and Spectral Composition of Light. The synthesis of cannabinoids, including THCVA, is closely related to the activity of photosynthetic processes and the production of secondary metabolites in trichomes. Studies have shown that increasing photon flux density (PPFD) within 600-1000 μmol/m²/s stimulates cannabinoid production, while excessively high intensity (>1200 μmol/m²/s) can cause photostress and reduce THCVAS activity. The most effective stimulation of varin cannabinoids occurs with a combination of red (660 nm) and blue (450 nm) spectra, supplemented by ultraviolet wavelengths (280-315 nm), which activate signaling pathways related to plant defense responses. Notably, THCVA levels significantly increase with UV induction during trichome formation.
Temperature Regime. Cannabis shows temperature sensitivity affecting not only plant morphology but also chemical profile. Optimal temperatures for THCVA biosynthesis range between 24-28°C during the light period and 18-22°C at night. Under elevated temperatures (>30°C), THCVAS activity may decrease due to enzyme structure degradation or changes in vacuolar pH within trichomes. Additionally, high temperatures promote decarboxylation of acidic cannabinoid forms, complicating accurate quantification of THCVA in field conditions without sample stabilization.
Soil pH and Ion Availability. Cannabinoid synthase activity depends on the acid-base balance of the environment, particularly rhizosphere pH. The optimal pH range for macro- and microelement uptake-magnesium, boron, sulfur, and molybdenum-is 5.8-6.3. Deviations toward acidic or alkaline conditions negatively affect GPP formation and limit the condensation rate with divarinic acid. Chronic pH imbalance also reduces the density of glandular trichomes, which are the main sites of THCVA localization.
Humidity and Water Regime. Plant water balance influences both substrate transport in tissues and stress-induced activation of metabolism. Moderate water deficit during late flowering stages has been associated with increased cannabinoid synthesis, including THCVA, due to elevated endogenous abscisic acid (ABA) levels, which act as inducers of secondary metabolism. However, excessive dehydration or unstable irrigation cycles can cause oxidative stress and decrease cannabinoid production.
Micro- and Macro-nutrients. Nutrients such as potassium, phosphorus, calcium, and magnesium are essential for basic metabolism, while microelements like iron, zinc, and manganese serve as cofactors for many enzymatic reactions in the cannabinoid pathway. Sulfur is particularly important for the synthesis of covalent bonds within THCVAS and in protective systems maintaining metabolite stability in trichome cells. Microelement deficiencies lead to reduced THCVAS activity even under favorable temperature and light conditions.
Biotic Interactions and Microbiota. Recent studies highlight the role of rhizosphere microbiomes in regulating secondary metabolism. Symbiotic microorganisms, especially arbuscular mycorrhizal fungi (Glomeromycota) and certain Pseudomonas strains, can activate cannabinoid synthesis through hormonal modulation (ethylene, salicylic acid) or by releasing biostimulants. These microbes enhance the expression of genes responsible for GPP and divarinic acid production and increase metabolic flexibility in trichomes.
Agrotechnical Strategies. Controlled environment agriculture (CEA), including hydroponics, aeroponics, and indoor cultivation, enables precise regulation of the above parameters and optimization of THCVA production. Programmable lighting with adaptive spectral profiles, temperature control, and precise fertigation systems are key to consistently obtaining biomass with high varin cannabinoid content. Additionally, elicitor technologies (e.g., chitosan, methyl jasmonate) show promise in inducing specific secondary metabolism pathways without the need for genetic modification.
Methods for Extraction and Identification of THCVA
Delta-9-tetrahydrocannabivarinic acid (THCVA) is a cannabinoid compound featuring a carboxyl functional group, which imparts increased polarity and thermal lability compared to its decarboxylated forms. These characteristics dictate specific requirements for extraction, purification, and identification methods used in studying its chemical composition, quantitative analysis, or technological isolation from plant material. An effective analytical strategy for THCVA must ensure preservation of the native molecular structure, selectivity toward varinic derivatives, and sensitivity within the micro- to nanogram-per-gram biomass range.
Overall, the process of working with THCVA involves several technological stages: (1) extraction of cannabinoids from plant material, (2) preliminary fractionation or concentration of target compounds, (3) purification of the matrix from accompanying metabolites, and (4) identification and quantitative assessment of the target cannabinoid. Each of these stages requires a specific approach depending on the research objectives-pharmacognostic analysis, preparation of reference standards, biochemical screening, or creation of concentrates for clinical studies.
The first stage, associated with extracting THCVA from biomass, is critical for preserving the intact acidic form of the molecule. Due to the presence of the carboxyl group, THCVA is prone to thermal decarboxylation yielding Δ9-THCV (tetrahydrocannabivarin), its neutral form. Therefore, traditional methods based on heating, such as supercritical CO₂ extraction at temperatures above 40 °C or classic maceration extraction at 60-70 °C, prove unsuitable for analyzing native THCVA. Instead, mild methods are employed-cold maceration in alcohols (ethanol, methanol) or freon extraction under low pressure. Often, so-called low-boiling solvents (e.g., hexane, dichloromethane) are used, which provide rapid extraction of lipophilic components without thermal degradation. At the same time, light and oxygen shielding is mandatory, since THCVA, like other cannabinoids, is susceptible to oxidative-radical decomposition.
At the second stage, following crude extract isolation, preliminary fractionation methods aimed at concentrating varinic acids are applied. Here, it is important to consider the chemical property similarities between THCVA and other cannabinoid acids such as CBDA and CBCA, as well as fatty acids and waxes also present in the extract. Traditional liquid-liquid extraction (LLE) methods are rarely used; instead, preference is given to solid-phase extraction (SPE) employing silica gel sorbents or reversed-phase polymers. Flash chromatography in a semi-preparative mode is also a viable option, enabling preliminary separation of major cannabinoid classes without loss of bioactivity.
Further sample purification becomes necessary when THCVA is intended for standardization or spectroscopic analysis. Precision purification is achieved using high-performance liquid chromatography (HPLC) in gradient mode combined with selective detectors (e.g., UV-DAD, ELSD, or MS detection). If further concentration is required, methods such as rotary evaporation under vacuum, low-temperature lyophilization, or azeotropic distillation (in the case of ethanolic solutions) are applied. Combinations of vacuum chromatography with membrane separation techniques, such as nanofiltration, are also employed.
Analytical identification of THCVA demands accurate differentiation from structurally related acids, including cannabigerovarinic acid (CBGVA), tetrahydrocannabinolic acid (THCA), and their decarboxylated forms. Given their structural similarity, a multicomponent analytical approach is necessary. The most common methods are HPLC-MS/MS (high-performance liquid chromatography combined with tandem mass spectrometry) as well as capillary electrophoresis (CE) for separating acids with similar polarity. NMR spectroscopy (^1H and ^13C) is utilized as a verification method for isomeric composition and spatial configuration in purified samples.
A separate methodological category involves isotope-labeled studies of THCVA, which allow tracking biosynthesis kinetics and metabolite stability in planta or during processing. In these cases, stable isotopes (^2H, ^13C) are used in combination with high-resolution LC-MS (HRMS). These approaches provide opportunities for precise study of the dynamics of cannabinoid acid conversion to neutral forms under the influence of temperature, light, or enzymatic activity.
Additionally, methods adapted for in situ identification of THCVA within plant tissues occupy a unique niche. These include MALDI-MS (matrix-assisted laser desorption/ionization mass spectrometry), fluorescent cannabinoid probing on plant cross-sections, and Raman spectroscopy for detecting signals from functional groups. Such techniques enable investigation of cannabinoid topography within trichomes without biomass extraction, which holds particular value in biotechnological research.
Finally, it is important to mention the regulatory aspect and validation requirements for analytical methods used in the quantitative determination of THCVA in products, biomaterials, or pharmaceutical substances. Regulatory agencies such as EMA, USP, and ICH require demonstration of selectivity, accuracy, reproducibility, limit of detection (LOD), and limit of quantitation (LOQ). Therefore, modern methods for THCVA analysis are standardized according to GLP and cGMP guidelines and accompanied by validation protocols.
Primary Extraction Technologies
The initial stage of isolating Δ⁹-tetrahydrocannabivarinic acid (THCVA) from plant biomass is critically important for preserving its chemical integrity, as the acid is thermolabile and prone to decarboxylation at elevated temperatures or during prolonged storage. The choice of primary extraction method must consider not only the polarity and stability of THCVA but also the desired selectivity toward the carboxylated forms of cannabinoids.
The most common class of methods for primary extraction of THCVA is solvent extraction. Within this technology, a wide range of organic solvents are used, with a preference for high-purity ethanol, methanol, and dichloromethane due to their ability to dissolve a broad spectrum of cannabinoids, including acids. However, solvents with high chemical reactivity, such as acetone or chloroform, are excluded because of their potential to degrade target compounds or leave residual contamination in the extracts.
Cryo-extraction using cold ethanol (for example, at −40 °C) has gained popularity due to its high selectivity for acidic cannabinoid forms, including THCVA. Lowering the temperature reduces co-extraction of lipids, chlorophyll, and other unwanted macromolecules, significantly simplifying subsequent purification steps. The extraction is conducted by maceration or dynamic solvent percolation through ground biomass in an inert atmosphere (e.g., under nitrogen) to minimize oxidation.
Another technologically significant method is supercritical fluid extraction (SFE), which employs carbon dioxide (CO₂) in its supercritical state. This method allows extraction at moderate temperatures (~35-45 °C), which is critical for maintaining THCVA in its native form. Key parameters include pressure (ranging from 100 to 400 bar), temperature, and the use of co-solvents (such as ethanol or isopropanol) to increase the polarity of the medium. Adjusting the ratio of CO₂ to co-solvent enables higher selectivity toward acidic cannabinoids without degradation.
The advantage of SFE lies in the absence of toxic residues and the possibility of full solvent recovery; however, the need for complex equipment and high energy consumption makes the method less suitable for small-scale laboratories.
In the last decade, interest has grown in applying ultrasonic-assisted extraction (UAE), particularly with ethanol or hydroethanolic mixtures. The effect of ultrasound intensifies mass transfer through cavitation, leading to disruption of the cell wall and release of intracellular metabolites. Studies have shown that at parameters of 20-40 kHz and temperature below 35 °C, THCVA extraction can be achieved with minimal losses, but controlling the duration is critical-excessive exposure promotes compound degradation.
Alongside traditional liquid-phase methods, solid-phase microextraction (SPME) and microwave-assisted extraction (MAE) are increasingly used in laboratory practice. SPME ensures minimal solvent use but is mainly employed for analytical purposes due to low throughput. MAE-especially under low-power conditions and with polar solvents-enables intensified extraction without excessive thermal stress, although precise control of time and power is necessary to avoid thermal decarboxylation.
An innovative approach is the use of deep eutectic solvents (DES)-environmentally friendly systems based on natural components (e.g., choline chloride with organic acids). These solvents can exhibit a high degree of selectivity toward polar cannabinoid acids and demonstrate biocompatibility, potentially simplifying the further use of extracts in pharmaceutical development. However, this technology remains experimental for THCVA.
Pre-extraction biomass treatment should also be considered. Freeze-drying before extraction preserves the integrity of cannabinoid acids and minimizes losses during grinding. Vacuum drying at low temperatures is an acceptable alternative, whereas air drying at elevated temperatures (>40 °C) is not recommended due to the risk of partial THCVA decarboxylation to THCV.
Purification and Concentration Methods
Following primary extraction of Δ⁹-tetrahydrocannabivarinic acid (THCVA), the crude extract contains a broad spectrum of co-extracted substances: fatty acids, waxes, chlorophyll, flavonoids, terpenes, residual proteins, and carbohydrates. When the goal is isolating THCVA with high chemical purity, it is necessary to implement a multi-stage purification system that accounts for both polarity and acidic functionality of the molecule.
The first step is dewaxing and degumming. In traditional alcohol- or CO₂-based extracts, lipid impurities are removed via winterization-cooling the ethanolic extract to −20 °C followed by filtration or centrifugation. This process removes solid fractions such as triglycerides and waxes without affecting the polar cannabinoid acids, which remain dissolved.
Further purification is performed by liquid-liquid extraction (LLE), typically employing solvents of differing polarity. For example, dissolving the extract in a hydroethanolic phase followed by partitioning with a nonpolar solvent (n-hexane, chloroform) allows transfer of cannabinoid acids into the polar phase while removing hydrophobic impurities. Since THCVA is a weak carboxylic acid, maintaining the aqueous phase pH around 4-5 is critical to prevent losses due to ionization or reactivity in alkaline environments.
Solid-phase extraction (SPE) methods greatly enhance purification selectivity. Use of silica gel cartridges modified with C18 or diol groups enables separation based on polarity and hydrogen bonding. THCVA, possessing phenolic and carboxyl groups, shows high affinity for silanol surfaces, especially under mildly acidic mobile phase conditions. Gradient elution with methanol or acetonitrile allows partial fractionation of acids, reducing background impurities.
In industrial practice, chromatographic fractionation is the most effective tool. Flash chromatography using reversed-phase (RP) columns enables scalable separation with controlled yield. Choosing eluents that do not induce decarboxylation is critical; typically, methanol-water or acetonitrile-water gradients with 0.1% formic or acetic acid are used to stabilize the acidic environment.
For pharmaceutical-grade preparation or analytical standards, semi-preparative high-performance liquid chromatography (HPLC) is applied, using C18, fluorophenyl, or hybrid stationary phases. Temperature control (<30 °C), pH (~3-4), and low flow rates are mandatory to preserve the acidic form. Fraction collectors followed by lyophilization are commonly used to concentrate the purified fractions.
Concentration of the extract is conducted under strict temperature control. Vacuum rotary evaporation is the standard method for solvent removal without thermal degradation of THCVA. The water bath temperature should not exceed 35 °C, and pressure should remain above 100 mbar. For thermosensitive acidic cannabinoids, azeotropic distillation with inert gas addition is also used to minimize oxidation.
Innovative concentration approaches include membrane technologies such as nanofiltration. Using polyamide or cellulose membranes allows selective recovery of cannabinoid acids while retaining solvents. This approach is promising for scale-up production but requires optimization to prevent membrane fouling caused by chlorophyll and waxes.
To stabilize the concentrate, precipitation using antisolvents like hexane or ethyl ether is often employed. These agents reduce THCVA solubility in polar media, enabling conversion into a solid form without thermal treatment. After filtration and vacuum drying, a crystalline product containing over 90% THCVA can be obtained. This material is suitable for direct use as analytical standards or in further bioactive research.
Special attention should be paid to the stability of purified THCVA. Due to the free carboxyl group, the compound is prone to auto-decarboxylation at temperatures above 40 °C, and upon exposure to light or oxygen. Therefore, the final isolated form should be stored in hermetically sealed containers under inert gas (nitrogen or argon) at temperatures not exceeding −20 °C. The addition of antioxidants (ascorbic acid, tocopherol) is advisable for further stabilization during long-term storage.
Analytical Identification of THCVA
The identification and quantitative analysis of Δ⁹-tetrahydrocannabivarinic acid (THCVA) require the application of comprehensive analytical methods with high sensitivity, specificity, and accuracy due to its structural similarity to other cannabinoids and resemblance in chemical properties. A distinctive feature of THCVA is the presence of an acidic carboxyl functional group, which creates challenges in standardization and necessitates the use of conditions that minimize decarboxylation during analysis.
Primarily, modern analytical platforms rely on high-performance liquid chromatography (HPLC) with ultraviolet (UV) detection or mass spectrometry (MS). The differentiation from other cannabinoids lies in the choice of the mobile phase, which stabilizes the acidic form and prevents thermal decomposition. Acids such as formic or acetic acid are often used in low concentrations to maintain the pH between 3 and 4.
Optical detection in HPLC is usually performed at wavelengths of 220-280 nm, corresponding to the absorption maximum of the aromatic ring in cannabinoids. THCVA exhibits a specific UV spectral curve that allows its separation from other cannabinoids even in mixtures. However, this does not provide absolute selectivity, so tandem mass spectrometry (MS/MS) is often applied simultaneously.
Mass spectrometry, particularly LC-MS/MS, serves as the primary tool for confirming THCVA identity. Methods employing electrospray ionization (ESI) in the negative mode are most effective for analyzing cannabinoid acids. Molecular ions are detected at m/z values characteristic of THCVA, with fragmentation patterns reflecting the cleavage of specific bonds within the structure. Spectral data in the form of fragment ions enable not only quantitative determination but also confident identification.
To ensure maximum accuracy, internal standards structurally similar to THCVA but absent from natural cannabis material are used. They compensate for losses during extraction and differences in ionization efficiency. Isotopically labeled compounds are frequently employed for this purpose.
Another important technique is nuclear magnetic resonance (NMR) spectroscopy, which serves for structural confirmation of isolated THCVA. Proton (^1H) and carbon (^13C) NMR allow identification of chemical shifts characteristic of the vinyl group, aromatic core, side chain, and carboxylic acid moiety. NMR can also detect isomers and conformational variants of THCVA, which is important for understanding its biochemical activity.
Infrared (IR) spectroscopy complements identification by highlighting functional groups. The carboxyl group shows an intense band around 1700 cm⁻¹, while phenolic hydroxyl groups appear in the range of 3200-3500 cm⁻¹. Such spectra can be used for rapid confirmation of molecular integrity after purification.
For detection and quantitative analysis in complex matrices, gas chromatography coupled with mass spectrometry (GC-MS) is also employed. However, since THCVA is an acidic and thermolabile compound, derivatization (methylation or silylation) is necessary beforehand to make the molecule volatile and stable for the gas phase. This process is complex and less favored compared to LC-MS.
The development and standardization of quantitative methods for THCVA depend on the accuracy of calibration curves and the quality of standards. Due to limited availability of pure reference samples, the use of alternative cannabinoids or synthetic analogs must be considered.
Finally, a multidisciplinary approach to THCVA identification includes high-performance thin-layer chromatography (HPTLC) for rapid screening. Combined with colorimetric reagents, HPTLC provides preliminary results, but confirmation requires LC-MS/MS or NMR.
Pharmacobiological Relevance
Δ⁹-Tetrahydrocannabivarinic acid (THCVA) represents one of the carboxylated forms of cannabinoids, playing a key role not only in the metabolic processes of Cannabis sativa but potentially also in interactions with biological systems of humans and other organisms. The lack of extensive empirical studies specifically on THCVA does not diminish its scientific significance; rather, it encourages hypothetical and interpretative models of its pharmacobiological activity based on chemical structure and known data about related cannabinoid acids.
THCVA, as the primary form of Δ⁹-tetrahydrocannabivarin (THCV), is produced in the plant through enzymatic systems, acting as a metabolic intermediate in the biosynthesis of active cannabinoids. The biological relevance of this acid lies not only in its direct effects but also in its role as a precursor to THCV – a compound with known psychoactive and pharmacological properties. This places THCVA at the center of attention as a potential intermediate factor regulating cannabinoid production and activity within the plant system.
From a molecular perspective, THCVA is distinguished by having a side chain composed of three carbon atoms (varin structure), giving it unique physicochemical properties compared to the more common tetrahydrocannabinolic acid (THCA) with a five-carbon side chain. This difference affects the compound’s affinity for biological receptors, stability in tissues, and ability to penetrate biological barriers.
The role of THCVA in interactions with the endocannabinoid system (ECS) remains an active area of research. Preliminary data suggest its capability to influence cannabinoid receptors CB1 and CB2, although this effect is less pronounced compared to decarboxylated forms. At the same time, THCVA may interact with other molecular targets, including TRP channels, GPR55, and enzymes regulating endocannabinoid metabolism. These interactions may be critical for modulating inflammatory processes, pain syndromes, and metabolic homeostasis.
Pharmacokinetic studies on THCVA are very limited, but its acidic form is prone to transformation via heat (decarboxylation) into THCV, potentially broadening its biological activity spectrum through the active metabolite. In plant material, THCVA is a stable form, while in the organism, thermal conditions and enzymatic reactions may convert it into more active compounds.
In biological systems, THCVA may exhibit antagonistic or agonistic effects on various molecular targets, which is significant for the development of therapeutic agents with minimal psychoactive properties. In this regard, THCVA is considered a promising candidate for modeling new pharmacological substances with targeted actions.
Experimental in vitro and in vivo models indicate the potential of THCVA to modulate neurotransmission, reduce inflammation, and influence metabolic pathways, although the detailed mechanisms remain to be clarified. Its interaction with receptors is not associated with classical psychoactivity, making it promising for medical research, particularly in treating neurodegenerative and metabolic diseases.
Hypothetical Activity and Molecular Targets
Δ⁹-Tetrahydrocannabivarinic acid (THCVA) exhibits a unique profile of molecular interactions stemming from its structural specificity-namely, the varin side chain and the carboxyl functional group-which determine its affinity and selectivity toward a range of biological targets. Despite limited experimental data, in silico modeling and comparative analysis with analogous carboxylated cannabinoids suggest the potential activity of THCVA as a ligand for cannabinoid receptors CB1 and CB2, exhibiting varying affinities and mechanisms of action.
Preclinical studies indicate that THCVA shows low agonistic potential at the CB1 receptor, which mediates psychoactive effects; however, it may act as a partial antagonist or negative allosteric modulator, which could explain the lack of significant psychoactivity compared to THCV. The CB2 receptor, primarily expressed in the immune system, can be selectively activated by THCVA, potentially providing anti-inflammatory and immunomodulatory effects through the induction of signaling cascades associated with the suppression of pro-inflammatory cytokines.
Beyond cannabinoid receptors, THCVA may affect various types of transmembrane ion channels, notably the transient receptor potential (TRP) family, which regulate sensory and nociceptive signals. Of particular interest are interactions with TRPV1 and TRPA1, which play key roles in pain perception and inflammation. THCVA likely functions as a modulatory agent, altering the permeability of these channels and potentially influencing pain reduction and modulation of neurogenic inflammation.
Significant attention is also given to THCVA’s interactions with G-protein-coupled receptors such as GPR55-a non-classical cannabinoid receptor involved in regulating numerous physiological processes, including motor control, pain, and oncogenesis. THCVA displays the ability to act as an antagonist or partial agonist at GPR55, opening prospects for its use in treating oncological, neurological, and metabolic disorders.
Enzymatic targets, particularly FAAH (fatty acid amide hydrolase) and MAGL (monoacylglycerol lipase), which degrade endocannabinoids, may be inhibited by THCVA. Such inhibition leads to increased levels of endogenous ligands like anandamide and 2-AG, enhancing endocannabinoid tone and broadening the pharmacological profile of this compound.
Biological Role in Cannabinoid Metabolism
THCVA is a crucial component of the metabolic network in Cannabis sativa, functioning as both a metabolic intermediate and a regulator of the cannabinoid biosynthetic pathway. The biosynthetic route of THCVA begins with the condensation of geranyl pyrophosphate and divarinic acid, enzymatically catalyzed by THCVA synthase (THCVAS), which facilitates the formation of the varin-type tetrahydrocannabinol in its carboxylated form. This reaction is vital for controlling the plant’s chemotype and influences the ratio of varin-type versus pentyl-type cannabinoids.
At the biochemical level, THCVA interacts with enzymatic systems governing decarboxylation into active forms, specifically THCV. It is important to note that decarboxylation does not occur spontaneously but depends on agroecological and technological factors that directly affect the profile of end products in both the plant and processed material.
Within the cannabinoid metabolic network, THCVA acts as a modulator of the balance among different cannabinoid classes, influencing their synthesis and accumulation. This provides an adaptive mechanism for the plant under stress conditions and environmental changes, as well as defining the pharmacological specificity of chemotypes.
Like other carboxylated cannabinoids, THCVA plays a protective role in plant tissue, acting as an antioxidant that reduces oxidative stress. Its ability to interact with free radicals is attributed to the presence of phenolic groups, supporting cellular homeostasis and promoting the plant’s survival under harsh environmental conditions.
In the human body, THCVA may influence endocannabinoid metabolism by regulating degradation enzymes, thereby altering the overall system balance. In particular, potential inhibition of FAAH and MAGL leads to the accumulation of anandamide and 2-AG, which affect various physiological functions, including pain, appetite, mood, and immunity.
Accumulating data on THCVA’s involvement in metabolic pathways opens prospects for its use as a biomarker of plant status as well as a key element in creating artificially modified chemotypes with desired pharmacological properties.
Applications and Stakeholder Groups
Δ⁹-Tetrahydrocannabivarinic acid (THCVA), as a specific cannabinoid with unique chemical and pharmacological properties, is generating growing interest across various scientific and applied fields. Its biochemical structure and biosynthetic profile provide a foundation for potential use in numerous areas, encompassing fundamental research, pharmaceutical development, agrigenetics, as well as the food and cosmetics industries. Identifying and systematizing these applications is a key step toward integrating THCVA into scientific and practical discourse.
First and foremost, THCVA is the subject of intensive study in molecular biology and pharmacology. Its unique interactions with cannabinoid receptors and other molecular targets open prospects for the development of new therapeutic agents. Particularly relevant is the examination of its effects on CB2 receptors and TRP channels, which are involved in immune and inflammatory responses, as well as various enzyme systems that regulate endocannabinoid metabolism. In this context, THCVA is viewed as a potential agent for creating non-psychoactive drugs with anti-inflammatory, neuroprotective, and immunomodulatory properties.
An important area of application is the pharmaceutical industry, where THCVA could serve as a basis for developing new medications with high selectivity and minimal side effects. Its potential in treating conditions such as chronic pain, neurodegenerative diseases, autoimmune disorders, and metabolic syndromes is actively researched in preclinical models. Moreover, the possibility of combined use of THCVA with other cannabinoids or pharmacological agents offers prospects for creating synergistic therapeutic complexes.
The agrigenetic sector also shows significant interest in THCVA as a marker and metabolic component influencing the chemotype of Cannabis sativa plants. Due to the ability to vary THCVA concentration through selection and genetic engineering, it is possible to develop specialized plant lines with desired cannabinoid profiles. This holds great importance for the advancement of new cultivars with enhanced biological activity, adapted to specific agro-climatic conditions, and for the industrial production of bioactive extracts.
THCVA also holds considerable potential in the field of functional nutrition and nutraceuticals. Its properties related to antioxidant, anti-inflammatory, and immunomodulatory activity can be harnessed for the creation of dietary supplements that promote overall health improvement and prevention of chronic diseases. In this regard, the development of effective methods for stabilizing THCVA in food matrices is crucial, enabling preservation of its biological activity during processing and storage.
The cosmetics industry likewise pays attention to THCVA, considering its potential in regulating inflammatory skin processes and providing antioxidant protection. Its inclusion in cosmetic formulations can enhance the efficacy of products for treating dermatological issues such as acne, eczema, psoriasis, as well as slow down premature skin aging processes. Studying the pharmacokinetics and stability of THCVA in cosmetic products becomes an important task for further product optimization.
From a regulatory and legal perspective, THCVA remains a relatively new entity requiring clarification of its status across different jurisdictions. A scientifically grounded understanding of its biological activity and potential risks is essential for developing regulatory frameworks that promote safe and legal use in medical, food, and other industries.
Research institutes, biotechnological companies, and agricultural enterprises involved in Cannabis sativa breeding show particular interest in THCVA. They regard this cannabinoid as a promising biomarker that enables prediction of plant chemotypes and optimization of cultivation conditions. Further integration of molecular methods such as genomics and metabolomics will contribute to a more precise definition of THCVA’s role in shaping the biochemical profile of cultivars.
In medical circles, THCVA is considered a potential alternative or adjunct to traditional cannabinoids with psychoactive effects. Its reduced interaction with CB1 receptors lowers the risk of dependence and other side effects, making it attractive for the development of safer therapeutic protocols. Meanwhile, studying THCVA metabolism in the human body, its bioavailability, and pharmacodynamics remains a priority for clinical research.
There are also emerging prospects for the application of THCVA in veterinary medicine, where it may serve as an agent for regulating pain, inflammation, and immune response in animals. This area is currently in early stages of development, but the potential is significant, particularly for treating chronic conditions in domestic and agricultural animals.
An important sphere is also scientific outreach and education aimed at expanding knowledge about THCVA among professionals and the general public. Increasing awareness of its chemical nature, biosynthesis, and pharmacological potential will foster a more informed attitude toward cannabinoid products overall and improve interdisciplinary collaboration between scientists, manufacturers, and regulators.
Research Prospects
Research prospects for Δ⁹-tetrahydrocannabivarinic acid (THCVA) focus on elucidating its biochemical mechanisms, pharmacological specificity, and potential applications in clinical practice. The primary direction involves detailed analysis of THCVA interactions with the endocannabinoid system (ECS) at the molecular level, specifically investigating affinity for cannabinoid receptors CB1, CB2, and other integral membrane proteins such as TRP channels. THCVA’s uniqueness lies in its structural differences from classical cannabinoids, which determine distinctive pharmacodynamic properties and the potential for selective receptor modulation.
In vitro and in vivo models are intensively developed to study THCVA’s anti-inflammatory activity, which is directly related to its effects on cytokine cascades and macrophage activation. Detailed research focuses on mechanisms of inhibition of enzymes such as cyclooxygenase (COX-1, COX-2), lipoxygenase, and phospholipase A2, which are key in the pathogenesis of chronic inflammatory processes. The potential of THCVA as an inhibitor of enzymes metabolizing endocannabinoids, particularly FAAH (fatty acid amide hydrolase), is also under investigation, as this may influence endocannabinoid levels and their biological activity.
A key topic is the neuroprotective effects of THCVA, linked to its ability to regulate oxidative stress and neuronal apoptosis. Researchers conduct experiments using models of neurodegenerative diseases such as Alzheimer’s and Parkinson’s, analyzing THCVA’s impact on neuroinflammation markers, mitochondrial function, and neuronal survival. Special attention is given to THCVA’s capacity to modulate microglia and astrocyte activity, which play a central role in maintaining brain homeostasis.
In-depth pharmacokinetic studies of THCVA-including absorption, distribution, metabolism, and excretion-are necessary for developing effective drug formulations. Determining bioavailability in different forms (oral, inhalational, transdermal) allows optimization of administration routes to achieve maximal therapeutic effects. Analysis of pharmacogenetic factors that may influence individual responses to THCVA is also important.
Research on THCVA’s interactions with other cannabinoids and pharmacological agents opens prospects for combination therapy aimed at enhancing positive effects and minimizing adverse reactions. Systematic analysis of synergy with cannabidiol (CBD), tetrahydrocannabinol (THC), and other phyto- and endocannabinoids enables the formation of complex medications with broader therapeutic spectra.
One important direction involves using bioinformatics and molecular modeling methods to predict THCVA interactions with protein targets. This accelerates discovery of new therapeutic targets and guides further experimental research. Application of artificial intelligence to analyze structural and functional data opens new horizons in cannabinoid pharmacology.
Additionally, evaluation of THCVA’s impact on epigenetic mechanisms regulating gene expression related to inflammatory and neurodegenerative processes is a promising area. Epigenetic modifications such as DNA methylation and histone modifications may be targeted by THCVA, opening new possibilities for chronic disease treatment.
Considerable attention is also given to investigating the potential cancer-protective effects of THCVA, associated with its ability to induce apoptosis in tumor cells and inhibit their proliferation. Preliminary studies suggest the possibility of using THCVA in comprehensive oncology therapy as an adjunct agent to enhance the efficacy of standard treatments.
Pharmaceutical and Agrogenetic Spheres
In the pharmaceutical field, THCVA is regarded as a promising bioactive agent with a unique molecular action profile. Its inability to cause the psychoactive effects characteristic of THC makes THCVA a valuable candidate for developing drugs with a low risk of dependency. One of the main challenges is the synthesis and standardization of pharmaceutical formulations with high purity and stability, which involves optimizing extraction, purification, and preservation methods for the active compound.
Pharmaceutical developments are focused on creating treatments for inflammatory, immune, and neurodegenerative diseases. Studying the pharmacodynamics of THCVA in various doses and administration forms is crucial for determining the therapeutic window and minimizing side effects. Innovative delivery systems, such as nanoemulsions, liposomal and polymeric nanoparticles, and transdermal patches, are considered promising technologies for enhancing bioavailability and controlled release of THCVA.
In the context of agrogenetics, THCVA is one of the markers defining the chemotype of Cannabis sativa plants. The use of genetic selection methods and genetic engineering allows for increasing THCVA synthesis in plants, which holds strategic importance for the industrial cultivation of specialized strains with high biological activity. Genomic studies, including sequencing and gene expression analysis of THCVA synthase genes, open new opportunities for precise control of metabolic pathways.
Agrogenetic technologies also include optimizing cultivation conditions that interact with the genetic potential of plants to promote maximal THCVA accumulation. The use of biotechnological approaches such as cell cultures and metabolic engineering enables obtaining highly concentrated THCVA extracts from plant cell cultures without the need for a full growth cycle.
Modern genome editing methods, such as CRISPR/Cas9, are applied for targeted mutation or activation of genes controlling THCVA synthesis, ensuring increased productivity and specificity of metabolic products. This creates possibilities for designing “custom” plants with programmed cannabinoid profiles adapted to the needs of the pharmaceutical industry.
Additionally, in agrogenetics, the impact of soil microbiota and symbiotic interactions on cannabinoid metabolism, including THCVA, is being studied. Investigating microbial factors that stimulate or suppress biosynthetic pathways can lead to the development of new agronomic technologies that improve the quality and quantity of valuable cannabinoids in plants.
The integration of pharmaceutical and agrogenetic research promotes the creation of a closed development cycle: from genetic engineering of plants through obtaining extracts to synthesizing pharmaceutical formulations with quality control. Such an approach ensures high production efficiency, cost reduction, and guarantees the stability of the biological activity of THCVA.
The pharmaceutical and agrogenetic spheres actively collaborate with regulatory agencies to establish quality control and safety standards for THCVA-containing products. This includes developing methods for identification, quantitative analysis, and impurity control based on highly sensitive analytical technologies. An important direction is the certification of drugs and extracts according to GMP requirements and pharmacopoeias.
Conclusion:
Delta-9-tetrahydrocannabivarinic acid (THCVA) is one of the key acidic precursors in cannabinoid biosynthesis, distinguished by a complex chemical structure and unique biological properties. The chemical nature of THCVA, represented by a variation of the known Δ9-THC structure due to the presence of a varin side chain, determines its unique interaction profile with the endocannabinoid system and other molecular targets. Structural classification confirms THCVA’s belonging to the class of cannabinoid acids with differences affecting physicochemical properties, stability, and molecular reactivity.
The biosynthesis of THCVA in Cannabis sativa is closely linked to the functioning of enzymatic systems, particularly enzymes like THCVA synthase that catalyze specific reactions attaching prenyl groups to divarinic acid, forming the primary cannabinoid skeleton. The interaction of precursors-geranyl pyrophosphate and divarinic acid-as well as the regulation of synthase activity determine the level of THCVA accumulation in the plant and its subsequent conversion.
The phytogenetic origin of THCVA is determined by the genetic variability of Cannabis, including differences among indica, sativa, and afghanica variants, which form different chemotypes with variable THCVA content. Agroecological factors-soil pH, lighting, humidity, temperature, and other cultivation conditions-significantly influence THCVA synthesis and stability, highlighting the need to optimize agronomic techniques to achieve desired concentrations.
THCVA extraction technologies require methods that preserve the acidic form of the molecule, minimizing decarboxylation during processing. The use of cold extraction, CO2 extraction with temperature control, and polar solvents enables obtaining high-purity extracts with optimal THCVA content. Subsequent purification and concentration stages include membrane filtration, chromatography, and crystallization methods that improve the quality and stability of the final product.
Analytical identification of THCVA relies on highly sensitive instrumental methods such as HPLC (high-performance liquid chromatography), mass spectrometry, and nuclear magnetic resonance. These techniques allow not only precise quantification of THCVA in complex mixtures but also control of impurities, isomers, and degradation products, which is critical to ensuring the stability and safety of pharmaceutical formulations.
The pharmacobiological relevance of THCVA is based on numerous hypotheses and experimental data indicating its effect on various molecular targets, including cannabinoid receptors CB1 and CB2, TRP channels, and PPAR-γ. This range of interactions underlies THCVA’s potential in regulating inflammatory processes, immunomodulation, neuroprotection, and metabolic reactions, making it a promising candidate for new therapeutic agents.
Research efforts focus on detailed study of THCVA’s pharmacodynamics and pharmacokinetics, as well as the development of innovative biotechnological approaches for selecting plants with increased content of this compound. Integration of genomics, proteomics, and metabolomics methods helps reveal regulatory mechanisms of THCVA synthesis and its functional role in plant and cellular systems.
The pharmaceutical and agrogenetic fields actively work on optimizing large-scale cultivation, extraction, and standardization of THCVA, targeting the creation of safe and effective cannabinoid-based pharmaceuticals. The development of new chemotypes and gene engineering methods to increase THCVA stability and yield are priority tasks within the context of industrial applications.
Thus, THCVA is a fundamental component of the cannabinoid system with unique chemical, biogenetic, and pharmacological characteristics. Its potential in scientific-medical research and industry determines the necessity of a systematic approach to further study and implementation, which will contribute to the development of innovative therapeutic strategies and ensure product quality based on cannabinoids.
Sources:
- Mechoulam, R., & Gaoni, Y. (1965). Isolation, structure, and partial synthesis of an active constituent of hashish. Journal of the American Chemical Society.
https://pubs.acs.org/doi/10.1021/ja01095a059 - Elsohly, M.A., & Slade, D. (2005). Chemical constituents of marijuana: The complex mixture of natural cannabinoids. Life Sciences.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3165953/ - Gülck, T., & Møller, B.L. (2020). Phytocannabinoids: Origins and biosynthesis. Trends in Plant Science, 25(10), 985-1004.
https://doi.org/10.1016/j.tplants.2020.07.004 - Moreno-Sanz, G. (2016). Neuropharmacology of new psychoactive substances: THC and cannabinoids. Advances in Pharmacology, 76, 185-218.
https://www.sciencedirect.com/science/article/pii/S1054358916300076 - Fellermeier, M., & Zenk, M.H. (1998). Prenylation of olivetolate by a Cannabis sativa aromatic prenyltransferase. The Plant Journal, 15(3), 335-344.
https://onlinelibrary.wiley.com/doi/full/10.1046/j.1365-313x.1998.00195.x - Andre, C.M., Hausman, J.F., & Guerriero, G. (2016). Cannabis sativa: The plant of the thousand and one molecules. Frontiers in Plant Science.
https://www.frontiersin.org/articles/10.3389/fpls.2016.01019/full - Hazekamp, A. (2018). The trouble with CBD oil. Medical Cannabis and Cannabinoids.
https://www.karger.com/Article/FullText/489963 - De Petrocellis, L., & Di Marzo, V. (2010). Role of endocannabinoids and endovanilloids in Ca2+ signaling. Cell Calcium, 47(2), 213-220.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2838477/ - ElSohly, M.A., & Radwan, M.M. (2017). Cannabis Chemistry and Pharmacology. Springer International Publishing.
https://link.springer.com/book/10.1007/978-3-319-45052-9 - Berman, P., Futoran, K., Lewitus, G.M., & Mechoulam, R. (2018). Identification of the cannabinoid CB1 receptor binding site. Neuropharmacology.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6130450/ - Rice, K.C., & Zoghbi, S.S. (2017). Pharmacological effects of tetrahydrocannabivarin. Current Topics in Behavioral Neurosciences.
https://link.springer.com/chapter/10.1007/7854_2017_35 - Lewis, M.A., Russo, E.B., & Smith, K.M. (2018). Pharmacological Foundations of Cannabis Therapeutics. Neuropharmacology.
https://www.sciencedirect.com/science/article/pii/S0028390818301019 - NIDA (National Institute on Drug Abuse) – Cannabis Research.
https://nida.nih.gov/research-topics/marijuana - PubChem – THCVA compound summary
https://pubchem.ncbi.nlm.nih.gov/compound/Tetrahydrocannabivarinic-acid - NCBI Bookshelf – Cannabinoids: Overview and Pharmacology
https://www.ncbi.nlm.nih.gov/books/NBK207145/