Over the past several decades, the attention of scientists, medical professionals, and pharmacologists has increasingly focused on a deeper understanding of the complex regulatory systems that maintain balance in the body. One such key system, which has only recently begun to be widely studied, is the endocannabinoid system. Despite its name, it is not solely related to cannabis but is a universal biological network that functions within the body independently of external cannabinoid influence. Its discovery opened a new era in biomedicine, pharmacology, and the understanding of homeostasis.
Before summarizing, it is important to clarify once again: what exactly is the endocannabinoid system? It is a complex of receptors, endogenous ligands (that is, natural compounds produced by the body itself), and enzymes that together regulate a wide range of physiological processes. Key components of the system include cannabinoid receptors type 1 (CB1) and type 2 (CB2), as well as endogenous cannabinoids-specifically anandamide and 2-AG-and the enzymes responsible for their synthesis and breakdown.
At the time of the endocannabinoid system’s discovery in the 1990s, the scientific community was impressed by its all-encompassing influence on vital processes such as mood, memory, appetite, pain, inflammation, immune response, sleep regulation, motor function, and more. This indicates that the endocannabinoid system does not act in isolation but interacts with many other systems-the nervous, immune, and endocrine systems.
Current scientific research shows that dysfunction or deficiency of the endocannabinoid system can play an important role in the development of chronic diseases. These may include irritable bowel syndrome, migraines, fibromyalgia, depression, PTSD, anxiety disorders, as well as some autoimmune diseases. The well-known scientific term-clinical endocannabinoid deficiency-describes a condition where natural cannabinoids are produced in insufficient amounts, disrupting the body’s ability to self-regulate.
A distinct role in researching this system is played by its interaction with phytocannabinoids-that is, compounds from the cannabis plant that can affect the human endocannabinoid system. The most well-known of these are tetrahydrocannabinol (THC) and cannabidiol (CBD), which have become the focus of numerous clinical trials. They act through CB1 and CB2 receptors, which are widely distributed in the brain, central nervous system, immune cells, and internal organs. Thus, cannabis is not an “external” agent in the classical sense-its components fit into an already existing biochemical system in the body.
However, the answer to the question “what is the endocannabinoid system” is not limited to just listing receptors and molecules. It is a holistic concept-about how the body maintains stability amid changing external and internal environments. The ECS acts like a thermostat, “sensing” deviations from the norm and activating corresponding mechanisms to restore balance. This principle, known as homeostasis, is central to modern physiology.
Considering all the above, it can be stated that the endocannabinoid system holds extraordinary importance for overall health. It acts as an invisible regulator that ensures adaptability, stability, and recovery after stress, injury, or infection. And although today we are only beginning to truly understand its role, there is already talk of the enormous potential for its therapeutic use.
For example, in neuropsychiatry, there is consideration of modulating the ECS to treat depression, anxiety disorders, and PTSD. In oncology, for pain relief and improving patient quality of life. In neurology, to reduce seizures in epilepsy. Interestingly, some countries have already officially allowed medical use of cannabinoids under specific clinical indications.
However, it is important to understand that active intervention in the endocannabinoid system is an area that requires precise dosing, control, and deep understanding. Unsanctioned or excessive use of phytocannabinoids can lead to unwanted effects, including cognitive impairments, dependency, or endogenous imbalance.
History of the Discovery of the Endocannabinoid System
Early Discoveries of Cannabinoids
The history of cannabinoid discovery begins long before understanding their effects on the body. For thousands of years, the Cannabis sativa plant was used for medicinal and ritual purposes in China, India, and the Middle East. However, it wasn’t until the 19th century that the first attempts were made to isolate the active components of cannabis using chemical methods. Despite this, the real breakthrough came in the 20th century with the advent of chromatography and spectroscopy technologies, which allowed scientists to accurately determine the chemical structure of the compounds present in the plant.
The first successful isolation of an active cannabis component was carried out by British chemist Robert C. Adams in the 1940s, but it was Israeli scientist Raphael Mechoulam and his team who made the key discoveries in the 1960s. In 1963, they first described the structure of cannabidiol (CBD), one of the main non-psychoactive components of cannabis. CBD does not produce mind-altering effects but has significant anti-inflammatory, neuroprotective, and anxiolytic properties.
In 1964, Mechoulam was the first to identify and fully characterize tetrahydrocannabinol (THC)-the psychoactive compound responsible for most of the effects commonly associated with marijuana use. This discovery was historic because it provided the first scientific explanation for cannabis’s psychoactive properties. THC was recognized as the primary exogenous cannabinoid-that is, an external compound affecting the body’s biochemistry.
Researchers paid particular attention to the fact that THC acts very specifically, as if interacting with a particular molecular “target” in the body. At that time, however, the “target” receptor to which THC bound was still unknown. This prompted a wave of further research since if such an effect exists, there must be specific structures in the body capable of responding to cannabinoids.
It’s important to note that cannabinoid research took place under regulatory restrictions. After the 1970s, cannabis was banned in many countries, significantly limiting scientific experiments. Despite this, scientists from several countries continued searching for mechanisms of action of these compounds in the body. It became clear that THC was not the only active substance and that cannabis contains over 100 different cannabinoids, each with a unique pharmacological profile.
Discovery of CB1 and CB2 Receptors
A key stage in studying the mechanisms of cannabinoid action was reached when researchers identified what exactly these compounds interact with in cells. By the 1980s, it was hypothesized that THC must bind to specific receptors, but their existence remained speculative. The breakthrough came in 1988 when scientists at Washington University in St. Louis, USA, used radioactively labeled THC to investigate its binding in the rat brain. They discovered the presence of highly specific binding sites-evidence of a new type of receptor.
In 1990, a research team led by Lisa Matsuda at the U.S. National Institute of Mental Health cloned the first cannabinoid receptor-CB1. This receptor was found to be widely distributed in the brain, especially in areas such as the hippocampus (memory), cerebellum (coordination), and basal ganglia (motor activity). CB1 proved to be the main target for the psychoactive effects of THC. It belongs to a large group of receptors known as G-protein-coupled receptors, which are membrane structures that activate intracellular signals upon ligand binding.
The second receptor type-CB2-was discovered in 1993. Its expression was found mainly in immune system cells, the spleen, tonsils, and other peripheral tissues. Although CB2 is not involved in psychoactive effects, it plays a critical role in regulating inflammation, immune response, and cellular homeostasis. Unlike CB1, this receptor is almost absent in the brain, although some studies have confirmed its presence in microglia-cells responsible for immune protection of nervous tissue.
When the Endocannabinoid System Was Discovered
The concept of the endocannabinoid system did not emerge all at once. It was formed through gradual discoveries: identification of exogenous cannabinoids, discovery of corresponding CB1 and CB2 receptors, and isolation of endogenous compounds that activate these receptors. Specifically, the key date for the discovery of the endocannabinoid system is considered 1992. That year, Raphael Mechoulam’s group identified and described the first natural ligand for the CB1 receptor-anandamide (N-arachidonoylethanolamide). This molecule was found to be remarkably similar to THC in pharmacological properties but is produced by nervous tissue itself.
The second endocannabinoid compound-2-arachidonoylglycerol (2-AG)-was discovered in 1995. Both substances can activate CB1 and CB2 receptors, but at different concentrations and contexts. With this discovery, it became clear that the body has its own internal cannabinoid system functioning independently of plant-based compounds. This led to the formation of the concept of the “endocannabinoid system” as an integrated regulatory network.
To understand what the endocannabinoid system is and what its role is, one must go beyond chemical formulas. It is a feedback regulation system: it activates only when physiological stress, overload, or homeostatic disturbance occurs. Its action is short-lived and localized-anandamide and 2-AG are rapidly synthesized “on demand” and equally quickly broken down without being stored. This mechanism allows the body to adapt to conditions, regulate neuronal excitability, modulate pain, appetite, sleep, and even emotional responses.
The system also interacts with other signaling systems-dopaminergic, serotonergic, glutamatergic-which confirms its central place in neurophysiology. Additionally, CB1 and CB2 receptors have been found in almost every tissue of the body, allowing the endocannabinoid system to coordinate responses both at the cellular level and throughout the entire organism.
Structure and Components of the Endocannabinoid System
The endocannabinoid system is a complex signaling network that performs regulatory functions in many physiological processes throughout the body. It operates at the cellular level and is responsible for maintaining homeostasis-that is, the internal balance between excitation and inhibition, between activity and recovery. The uniqueness of this system lies in the fact that it activates only when the body moves out of balance, responding to physical, chemical, or emotional stimuli. The structure of the endocannabinoid system includes three main groups of elements: receptors, endogenous cannabinoids (endocannabinoids), and enzymes responsible for the synthesis and breakdown of these molecules.
At the core of this system are two primary receptors-CB1 and CB2. They are located on the surface of cells and are proteins that activate intracellular signaling cascades after interacting with their respective ligands. CB1 receptors are most actively expressed in the central nervous system, particularly in areas responsible for memory, coordination, emotions, and pain perception. In contrast, CB2 receptors are predominantly localized in immune system cells, where they regulate inflammatory responses, phagocytosis, and cytokine release. There are also less-studied receptors that may participate in endocannabinoid activity, such as GPR55 or TRPV1, but CB1 and CB2 remain the primary targets.
The next key component is the endocannabinoids themselves-signaling molecules produced by the body’s cells as needed. The most studied among them are anandamide (AEA) and 2-arachidonoylglycerol (2-AG). Both substances are synthesized from lipid precursors in cell membranes and act briefly, activating receptors in close proximity to their site of synthesis. Anandamide, named after the Sanskrit word “ananda” (bliss), is associated with regulating mood, anxiety, appetite, and sleep. 2-AG performs similar functions but is found in higher concentrations in the brain and exerts a stronger effect on CB2 receptors.
Equally important are the enzymes that control endocannabinoid levels in tissues. They are responsible for the rapid breakdown of signals once the desired effect is achieved. The main enzyme that degrades anandamide is FAAH (fatty acid amide hydrolase), while for 2-AG it is MAGL (monoacylglycerol lipase). These enzymes regulate the duration and intensity of the signal, ensuring that endocannabinoid activity remains controlled and localized.
The overall architecture of the endocannabinoid system provides it with unique capabilities. Unlike most neurotransmitter systems, this system operates by a feedback signaling mechanism: endocannabinoids are synthesized in the postsynaptic cell and act on presynaptic receptors, suppressing the release of neurotransmitters. This mechanism allows for rapid and effective regulation of excessive neuronal activity, which is especially important under conditions of stress, pain, or pathological excitation.
Beyond the central nervous system, the endocannabinoid system encompasses numerous peripheral organs: the gastrointestinal tract, liver, heart, skin, reproductive system, and immune organs. It participates in numerous physiological processes such as glucose metabolism, lipid metabolism, regulation of appetite, energy balance, immune response, pain, inflammation, and reproduction. Due to its versatility and adaptive regulatory capabilities, this system is considered a potential target for therapy across a wide range of diseases-from epilepsy and depression to autoimmune disorders and cancer.
Describing the structure of the endocannabinoid system is impossible without considering its high level of integration with other signaling networks. Endocannabinoid receptors directly or indirectly interact with the dopamine, serotonin, and opioid systems. This explains their role in modulating pleasure, pain, anxiety, motivation, and sleep. The system itself functions as a subtle biological moderator that maintains neurochemical balance without excessive intervention-until physiological demand arises.
Key Elements: Receptors, Endocannabinoids, Enzymes
The endocannabinoid system consists of three key structural components: the CB1 and CB2 receptors, endocannabinoid ligands (anandamide and 2-AG), and enzymes that regulate their biosynthesis and degradation. These elements interact in real time, providing dynamic modulation of neuronal and immune activity depending on the physiological context. Although they function as a unified whole, each has unique molecular properties, mechanisms of action, and functional characteristics.
CB1 receptors are membrane-bound G protein-coupled receptors encoded by the CNR1 gene, which is located on chromosome 6 (6q14-q15 in humans). Their molecular weight is approximately 53 kDa, and they contain seven transmembrane domains. High concentrations of CB1 are found in the neocortex, hippocampus, basal ganglia, cerebellum, and other structures of the central nervous system. At the cellular level, they are primarily localized on presynaptic terminals, where they regulate the release of neurotransmitters such as glutamate, GABA, dopamine, and acetylcholine. Activation of CB1 inhibits adenylyl cyclase, reduces calcium channel activity, and stimulates the opening of potassium channels, leading to membrane hyperpolarization and suppression of neurotransmitter release.
CB2 receptors, encoded by the CNR2 gene (located on chromosome 1p36), share a similar overall structural plan with CB1 but differ significantly in their expression patterns. They are present in immune cells (macrophages, T lymphocytes, neutrophils, dendritic cells), the spleen, bone marrow, and in smaller amounts in the CNS, predominantly in microglia. Stimulation of CB2 does not directly affect neurotransmitter transmission but modulates cytokine release, chemotaxis, and cellular proliferation. Their activation environment is primarily associated with immune response, inflammation, and modulation of tissue damage.
At the level of endogenous ligands, anandamide (AEA) and 2-arachidonoylglycerol (2-AG) play the leading roles. Both belong to the class of fatty acid derivatives but differ in chemical structure, synthesis pathways, and pharmacological profiles. Anandamide is synthesized from N-arachidonoyl phosphatidylethanolamine (NAPE) through the action of NAPE-specific phospholipase D. Its biological activity is limited by a short half-life lasting only a few seconds due to rapid enzymatic degradation. Anandamide is a partial agonist of CB1 and has low affinity for CB2, but it can also interact with TRPV1 receptors, which are responsible for temperature sensitivity and nociception.
2-AG is produced from diacylglycerols (DAG) by the action of DAG lipase α/β. Unlike anandamide, it is a full agonist of both CB1 and CB2 receptors, exhibiting a broader range of effects. Its concentration in the brain exceeds that of AEA by 100 to 1,000 times, highlighting its dominant role in fine-tuning synaptic transmission. In addition, 2-AG participates in the regulation of vascular tone, neuroinflammation, and influences cellular adhesion through integrins and other adhesion molecules.
The enzymatic system controls endocannabinoid levels in tissues, determining their bioavailability and duration of action. The primary enzyme responsible for anandamide degradation is fatty acid amide hydrolase (FAAH)-a serine hydrolase localized in the cytoplasm. It breaks down AEA into arachidonic acid and ethanolamine. Mutations in the FAAH gene may be associated with increased pain threshold, reduced anxiety, and decreased intensity of negative affective responses. Inhibitors of FAAH are the basis for pharmacological agents with potential analgesic, anxiolytic, and antidepressant effects.
For 2-AG, the main catabolic enzyme is monoacylglycerol lipase (MAGL), which hydrolyzes 2-AG into glycerol and arachidonic acid. MAGL is expressed in both neurons and astrocytes and has high activity in the brain’s gray matter. Its inhibition raises 2-AG concentrations, which can produce analgesic effects but may also lead to the development of tolerance and desensitization of CB1 receptors. Besides MAGL, enzymes ABHD6 and ABHD12 also participate in 2-AG metabolism, playing auxiliary but important roles in tissues with low MAGL activity.
It is also important to mention several regulatory proteins that do not directly participate in the hydrolysis or synthesis of endocannabinoids but influence their transport and localization. One of these is FABP (fatty acid-binding proteins), which is involved in transporting anandamide to FAAH within the cell. Inhibition of this protein may reduce AEA degradation without affecting FAAH itself as an enzyme, representing a promising direction for pharmacological modulation.
A distinctive feature of the endocannabinoid system’s structure is that its elements are not maintained in a stable active state. Endocannabinoid synthesis is triggered “on demand,” and receptors may undergo rapid desensitization or internalization upon excessive stimulation. This gives the system high plasticity, distinguishing it from most classical neurotransmitter or hormonal systems. All these factors make it extremely sensitive to changes in internal and external environments, ensuring an effective and rapid response under fluctuating physiological balance conditions.
Types of Receptors and Their Localization in the Body
Within the structure of endocannabinoid regulation, CB1 and CB2 receptors play a central role as the primary connection points between signaling molecules and cellular responses. Although they share common activation mechanisms through G-protein-coupled cascades, their distribution throughout the human body demonstrates clear functional specialization. CB1 receptors dominate the central and peripheral nervous systems, while CB2 receptors are concentrated in immune and hematopoietic structures. This distribution ensures precise modulation of physiological processes, including nociception, motor coordination, emotional reactivity, inflammatory mechanisms, energy metabolism, and other aspects of homeostatic control.
CB1 receptors are found at extremely high density in the brain, especially in areas responsible for cognitive integration, memory, motivation, and motor control. The highest concentrations are recorded in the hippocampus (regulation of short-term memory), basal ganglia (motor activity), neocortex (higher cognitive functions), and cerebellum (movement coordination and balance). In the hypothalamus, CB1 receptors modulate appetite and energy metabolism, while in the amygdala they participate in regulating emotional reactivity, particularly anxiety and fear. Activation of these receptors in brainstem nuclei affects pain sensitivity, thermoregulation, and autonomic function.
In the spinal cord, CB1 receptors are localized in the dorsal horns, where they inhibit the transmission of pain signals from the periphery to the central nervous system. Their activation in these areas reduces the release of glutamate and substance P, weakening the intensity of the nociceptive response. At the level of the retina, CB1 receptors modulate the electrophysiological activity of ganglion cells, influencing light adaptation.
Outside the nervous system, CB1 receptors are present in peripheral neurons of the autonomic nervous system, including the vagus nerve, sympathetic ganglia, and enteric neurons. Their activation leads to changes in intestinal motility, enzyme secretion, and blood flow in internal organs. In adipose tissue, these receptors participate in regulating lipogenesis; in the liver, they influence gluconeogenesis; and in the pancreas, they affect insulin secretion.
CB2 receptors are predominantly concentrated in immune cells found in peripheral blood and tissues. The highest expression is detected in B and T lymphocytes, macrophages, neutrophils, dendritic cells, and microglia. In the spleen, tonsils, bone marrow, and lymph nodes, CB2 receptors act as inhibitors of inflammatory responses: reducing the production of proinflammatory cytokines, suppressing the proliferation of activated immune cells, and decreasing chemotaxis. In brain microglia, activation of CB2 suppresses neuroinflammation by lowering the production of IL-1β, TNF-α, and nitric oxide. In astrocytes, their action is manifested through local homeostasis control and protection of neurons from the toxic effects of inflammatory mediators.
CB2 receptors are also expressed in the tissues of peripheral organs, including the intestinal epithelium, skin, lungs, and bones. In the gastrointestinal tract, they control the balance between tolerance to microbiota and immune response. In the skin, CB2 receptors are involved in wound healing, regeneration, and inhibition of pathological cell proliferation, such as in psoriasis or dermatitis. In bone tissue, CB2 receptors are activated in osteoblasts and osteoclasts, modulating the balance between bone formation and resorption. In the lungs, they play a role in inflammatory lesions and asthmatic reactions.
A separate category includes non-classical cannabinoid receptors that do not belong to CB1 or CB2 but respond to endocannabinoids or cannabinoid agonists. These include GPR55, GPR18, TRPV1, and PPARs. The GPR55 receptor is expressed in bone cells, adrenal glands, and certain brain areas, including the brainstem. Its activation stimulates calcium signaling, cell proliferation, and may be associated with pain and inflammation. TRPV1 (the vanilloid receptor), which is also targeted by anandamide, is localized in sensory neurons and participates in thermoregulation and pain perception. PPAR-α and PPAR-γ, nuclear receptors activated by endocannabinoid metabolites, regulate the expression of genes related to lipid metabolism and inflammation.
In the cardiovascular system, CB1 and CB2 receptors perform opposing functions. CB1 receptors promote vasodilation by inhibiting norepinephrine release from sympathetic neurons, thus lowering blood pressure. CB2 receptors, in contrast, protect the endothelium from oxidative stress and reduce monocyte adhesion to the vascular wall. In the myocardium, both receptor types influence metabolism, apoptosis, and ischemic resistance.
In the reproductive system, CB1 receptors are found in the hypothalamic-pituitary-gonadal axis, regulating the secretion of gonadotropins, ovulation, and spermatogenesis. CB2 receptors are expressed in Leydig cells in the testes, where they modulate testosterone synthesis. In the female reproductive system, CB2 receptors localize in the endometrium, placenta, and ovarian follicles, regulating embryo implantation and pregnancy maintenance.
Expression of both receptor types varies depending on age, sex, hormonal status, pathology, and even circadian rhythm. Their activity is modulated not only by endogenous ligands but also by numerous external factors-diet, stress, physical activity, medication, and toxins. It has been established that certain diseases are accompanied by altered expression of CB1 or CB2: for example, obesity is associated with CB1 overexpression in the hypothalamus, while autoimmune diseases show increased CB2 activity in immune cells.
Through Which Receptors Cannabis Interacts with the Endocannabinoid System of Our Body
The mechanism of cannabis action on the human body is determined by its ability to directly or indirectly interact with receptors of the endocannabinoid system. The primary role in this process is played by cannabinoid receptors CB1 and CB2, however, the influence of cannabinoids is not limited to just these two structures. Bioactive components of cannabis, particularly Δ9-tetrahydrocannabinol (THC) and cannabidiol (CBD), have varying affinities for a number of molecular targets, which provides a broad spectrum of their pharmacological activity.
The main psychoactive component of cannabis – THC – acts as a potent partial agonist of CB1 receptors. Its high affinity for these receptors, which are predominantly located in the brain, explains the characteristic psychoactive effect. By binding to CB1, THC initiates a cascade of intracellular signaling that suppresses the release of neurotransmitters, including glutamate, GABA, acetylcholine, dopamine, and norepinephrine. This results in changes in cognitive perception, short-term memory impairment, increased emotional lability, altered motor activity, and modified sensory processing.
At the same time, THC activates CB2 receptors, although with less efficacy. This interaction influences the immune response by reducing the synthesis of pro-inflammatory cytokines and modulating the proliferation of immune cells. Clinically, this manifests as a potential anti-inflammatory and immunoregulatory effect, especially in the context of autoimmune or inflammatory diseases.
Cannabidiol (CBD), although it does not have high affinity for CB1 and CB2, modulates their activity indirectly. Specifically, CBD acts as a negative allosteric modulator of CB1, which decreases the binding efficiency of THC to this receptor. This explains why products with high CBD content can reduce the psychoactive effects of THC. Additionally, CBD increases the endogenous concentration of anandamide by inhibiting its breakdown by the enzyme FAAH, thereby enhancing the tone of the endocannabinoid system through endogenous mechanisms.
Beyond the classical cannabinoid receptors, active cannabis compounds also interact with alternative targets. One of these is GPR55 – an orphan receptor often called the “third cannabinoid receptor.” THC and some other phytocannabinoids can activate GPR55, which produces effects opposite to those of CB1/CB2, including stimulation of cell proliferation or enhancement of pain. Its significance is especially notable in oncology, as it is found in some tumor cells and potentially modulates tumor aggressiveness.
TRPV1 (transient receptor potential vanilloid type 1) is another target with which CBD interacts. This ion channel is activated by temperature, acids, and other stimuli and is involved in the formation of pain sensitivity. CBD acts as an agonist of TRPV1, explaining its potential analgesic and anti-inflammatory effect. In some cases, prolonged activation of TRPV1 by CBD leads to receptor desensitization, which may reduce hypersensitivity in chronic pain.
Moreover, CBD modulates signaling pathways through nuclear receptors – notably PPAR-γ. This transcription factor plays a key role in regulating glucose metabolism, adipose tissue function, and inflammation. Activation of PPAR-γ by CBD holds promise for the treatment of metabolic syndrome, type 2 diabetes, and certain forms of neurodegeneration.
In the central nervous system, cannabis effects through CB1 receptors are observed in various regions. In the hippocampus, synaptic plasticity is suppressed, affecting memory formation. In the basal ganglia, motor function is modulated, while in the mesolimbic dopamine pathway, motivational behavior is altered. It has been established that excessive activation of CB1 in this system contributes to changes in reward response, partly explaining the addictive potential of cannabis.
Peripherally, THC interaction with CB1 in the enteric nervous system suppresses gastrointestinal motility, clinically presenting as reduced peristalsis and appetite. Through CB2 on immune cells, cannabis can decrease secretion of IL-6, IL-1β, TNF-α, and other key inflammatory mediators, explaining its efficacy in chronic inflammatory diseases such as Crohn’s disease, rheumatoid arthritis, or multiple sclerosis.
In dermatology, cannabinoid interaction with CB1 and CB2 in the skin regulates keratinocyte proliferation, sebum secretion, and activation of local immune cells. This opens therapeutic prospects for the treatment of acne, psoriasis, and atopic dermatitis.
It is worth noting that cannabinoids can interact not only with endocannabinoid system receptors but also alter the activity of transport proteins that regulate the cellular uptake of endocannabinoids. For example, CBD can block FABPs – fatty acid-binding proteins that transport anandamide to FAAH for degradation – thereby indirectly increasing anandamide levels in tissues.
The pharmacological profile of each cannabinoid depends not only on its receptor affinity but also on metabolic transformations. For example, 11-hydroxy-THC – an active metabolite of THC formed in the liver after oral consumption – has even higher affinity for CB1, which is why psychoactive effects from cannabis edibles are generally stronger and longer-lasting than those from inhalation.
Physiological Functions of the Endocannabinoid System
The endocannabinoid system (ECS) is a universal signaling network that performs a range of regulatory functions throughout the human body. Its presence has been documented in nearly every organ and tissue, from the brain to peripheral immune cells, and its physiological activity is critical for maintaining dynamic balance within the organism. The role of the ECS is not to initiate processes but to regulate already active mechanisms at the cellular level, enabling the body to respond quickly to environmental changes, infections, injuries, or stress factors.
The foundation of the system’s regulatory action is its ability to integrate signals across different functional axes: nervous, endocrine, immune, and metabolic. Thanks to this integration, it participates in controlling a wide range of processes, including pain, inflammation, energy balance, mood, appetite, thermoregulation, motility, sleep, and memory. For example, during inflammation, the endocannabinoid system locally increases the production of endogenous cannabinoids, which, by activating CB2 receptors on immune cells, reduce the production of pro-inflammatory cytokines. Similarly, during acute pain or injury, the release of anandamide increases in synapses of the spinal cord and brain structures, which decreases the transmission of pain signals.
Special attention should be given to the system’s role in short-term and long-term neuronal changes. CB1 receptors, present in brain areas responsible for learning and memory, play a role in the formation of long-term potentiation (LTP) or long-term depression (LTD), thereby regulating neuroplasticity. This is critically important for both normal cognitive functioning and processes of adaptation to stress, new situations, or environmental changes.
In the central nervous system, the ECS acts as a “signal filter” – it does not suppress all neuronal activity but finely tunes the intensity of excitation. This helps reduce neuronal “noise” and maintain the balance between excitation and inhibition. Practically, this manifests as the body’s improved ability to tolerate sensory overloads, anxiety states, or emotional excitations.
At the metabolic level, the endocannabinoid system coordinates processes of energy storage and expenditure. CB1 receptors expressed in the hypothalamus control hunger and satiety signals, as well as regulate insulin sensitivity through peripheral mechanisms. Overactivation of the system can lead to an energy imbalance that contributes to the development of obesity, insulin resistance, and metabolic syndrome. Conversely, hypofunction of this system leads to opposite problems – hypometabolism, decreased appetite, and cachexia.
Within the immune system, the ECS functions as a control factor over the inflammatory response. CB2 receptors are actively expressed on macrophages, T cells, and microglia, where they modulate activation and cytokine profiles. This provides a “braking” mechanism after acute inflammatory reactions to prevent chronic tissue damage. Dysfunction of these mechanisms may contribute to the development of autoimmune diseases or persistent inflammation.
An important characteristic of this system is its “demand-dependent” activity – the activity of endocannabinoids is not maintained continuously but arises as needed in specific local environments. This distinguishes it from most classical neurotransmitter or hormonal systems, which have a background level of activity. Such regulation allows for precise and temporally limited activation, reducing the risk of side effects and excessive reactions.
Within the context of stress physiology, the endocannabinoid system acts as a buffering structure that suppresses hyperactivation of the hypothalamic-pituitary-adrenal (HPA) axis. Experimental models show that blocking CB1 receptors increases cortisol levels and anxiety, while their activation decreases the response to stressful stimuli. Thus, the ECS not only responds to stress but also shapes emotional stability and adaptability of the organism.
Among the many physiological processes in which this signaling network participates, a special place is held by its role in development – both prenatal and postnatal. CB1 receptors are involved in the formation of neural networks, cell migration in the embryonic brain, synaptogenesis, and maturation of glial cells. This makes the endocannabinoid system critically important in early ontogenesis, and its dysfunction may be a possible cause of neuropsychiatric disorders.
Regulation of Pain, Mood, and Appetite
The endocannabinoid system serves as a critical internal link that provides flexible regulation of key sensory and affective processes: nociception (the sensation of pain), affective behavior (mood), and metabolic motivation (appetite). These processes are interconnected both in terms of neural architecture and through shared molecular pathways, and the endocannabinoid system coordinates their function via localized and context-dependent modulation of synaptic transmission.
In the case of pain, the endocannabinoid system is involved at all levels of its processing-from peripheral nociceptors to higher integrative centers in the cerebral cortex. CB1 receptors in the dorsal roots of the spinal cord reduce the release of excitatory neurotransmitters (notably glutamate and substance P) in response to peripheral stimulation. This decreases the intensity of pain signal transmission before it even reaches higher brain structures. At the same time, cannabinoid activation in the thalamus and amygdala suppresses the emotional coloring of pain, while in frontal cortical areas it influences the cognitive evaluation of the situation.
Experimental studies have shown that increasing the concentration of endocannabinoids, particularly anandamide, is accompanied by reduced sensitivity to mechanical and thermal nociception. This has been demonstrated both pharmacologically and through genetic models, where knockout of enzymes that break down endocannabinoids (such as FAAH) results in analgesia. Similar effects have been observed in clinical studies involving patients with fibromyalgia, migraine, and irritable bowel syndrome, conditions where traditional analgesic therapy was ineffective, and modulation of endocannabinoid activity led to symptom improvement.
Regarding mood regulation, the endocannabinoid system acts as an integrative mechanism among dopamine, serotonin, norepinephrine, and GABA systems. CB1 receptors in the mesolimbic dopamine pathway (especially in the ventral tegmental area and nucleus accumbens) regulate endogenous dopamine activity, which critically influences emotional motivation and feelings of pleasure. Low levels of endocannabinoid activity disrupt the ability to experience positive emotions (anhedonia) and increase vulnerability to stress.
In the dorsal raphe nucleus of the serotonergic system, CB1 receptors modulate serotonin release, which depending on conditions can either enhance or suppress this activity. This explains why the endocannabinoid system influences both poles of affective disorders-depressive states and anxiety disorders. Neuroimaging studies reveal decreased expression of CB1 receptors in the prefrontal cortex, hippocampus, and basal ganglia clusters of patients with depression, accompanied by a reduction in tonic endocannabinoid activity.
Increasing anandamide or 2-AG in these patients leads to normalization of neuroplasticity, reduction of inflammation in the central nervous system, and restoration of stress response regulation. These effects are achieved either by direct action on CB1 receptors or by inhibiting enzymes that degrade endocannabinoids. In anxiety disorders, conversely, hyperactivity of certain regions (notably the amygdala) can be controlled by enhancing local endocannabinoid tone.
Mechanisms influencing appetite and feeding behavior operate through the hypothalamus-a central integrative area coordinating energy balance. CB1 receptors located in the arcuate nucleus regulate the activity of neurons that produce neuropeptide Y (NPY) and agouti-related peptide (AgRP)-key modulators of hunger. Activation of CB1 receptors stimulates these neurons, causing sensations of hunger even when energy stores are sufficient, explaining the “munchies” effect after cannabis consumption.
Conversely, in conditions of energy deficiency, the system also supports the drive to eat by enhancing the reward feeling from food through effects on dopaminergic pathways. This allows the body to adapt to limited resources and ensure survival. However, with chronic overconsumption of fatty, sugary, or calorie-dense foods, excessive CB1 receptor stimulation contributes to the development of obesity and metabolic syndrome.
In peripheral tissues including the liver, pancreas, intestine, and adipose tissue, the endocannabinoid system also regulates metabolic responses: it influences insulin synthesis, leptin secretion, glucose sensitivity, and lipolysis. Excessive activity of the system is associated with the development of insulin resistance, while its inhibition improves insulin sensitivity.
The human endocannabinoid system coordinates fine regulation of pain, mood, and appetite through a complex interplay of central and peripheral receptors, endogenous ligands, and signaling pathways. Its modulation provides an adaptive response to changes in internal states and the external environment and participates in correcting pathological conditions where these processes are disrupted. Because of these properties, it is a promising target for pharmacological interventions in clinical medicine.
Influence on Sleep, Immune Response, and Thermoregulation
The endocannabinoid system is involved in regulating many key physiological processes, including control of the sleep cycle, immune system functioning, and maintaining stable body temperature. Its activity manifests through context-dependent modulation of various neuronal, hormonal, and immune pathways, enabling the body to adapt to environmental changes or internal physiological states.
In sleep regulation, the primary role belongs to CB1 receptors, which are expressed in hypothalamic structures, the thalamus, brainstem, and cortex. Their activation alters sleep architecture, notably increasing the duration of slow-wave sleep (SWS) and reducing wakefulness phases. Animal model studies have shown that inhibition of FAAH or MAGL enzymes, which break down anandamide and 2-AG respectively, leads to increased concentrations of these endocannabinoids and consequently prolongs total sleep time. This mechanism operates by inhibiting the activity of arousal centers responsible for wakefulness maintenance, such as the locus coeruleus and reticular formation, as well as through indirect effects on melatonin secretion in the pineal gland.
Experiments with CB1 receptor blockers (e.g., rimonabant) demonstrate that their deactivation causes sleep fragmentation, frequent awakenings, and circadian rhythm disruption. The endocannabinoid system also regulates the rapid eye movement (REM) phase of sleep: in states of anandamide deficiency, this phase is shortened, which is associated with impaired cognitive memory consolidation. Human studies have found that anandamide levels in cerebrospinal fluid fluctuate depending on the time of day, peaking before sleep onset, indicating the system’s role in regulating daily neural activity cycles.
Regarding the immune response, the influence of the endocannabinoid system is mainly mediated through CB2 receptors, which are expressed in lymphocytes, macrophages, dendritic cells, microglia, and other immune-competent structures. Activation of these receptors suppresses the release of pro-inflammatory cytokines such as TNF-α, IL-1β, and IL-6, while simultaneously enhancing secretion of anti-inflammatory agents, including IL-10. This allows the system to act as an immunomodulator, reducing excessive immune reactions without fully suppressing protective functions.
In autoimmune disorders-such as multiple sclerosis, rheumatoid arthritis, and Crohn’s disease-endocannabinoid activity holds therapeutic potential. In vivo studies have recorded reduced infiltration of T-cells into target tissues with enhanced CB2 receptor activation. The mechanism of action involves not only inflammation reduction but also a shift in cell phenotype from pro-inflammatory (M1 macrophages) to regulatory (M2 macrophages), promoting tissue repair. Although CB1 receptors are less prominent in the immune system, they also participate in modifying neuroimmune interactions through microglia in the central nervous system.
During infections, the endocannabinoid system regulates the balance between active antibacterial defense and minimizing damage to host tissues. For example, in sepsis, CB2 receptor activation reduces mortality by lowering systemic inflammation; however, excessive suppression of immune response may increase the risk of pathogen persistence, requiring delicate regulation.
Regarding thermoregulation, the endocannabinoid system participates in maintaining temperature homeostasis via central hypothalamic structures. In the preoptic area of the anterior hypothalamus, CB1 receptors affect neurons controlling autonomic responses such as vasoconstriction, sweating, and muscle shivering. Activation of these receptors leads to a decrease in body temperature through reduced sympathetic activity and enhanced heat dissipation.
Experimental evidence confirms that anandamide causes dose-dependent body temperature reduction in animals when administered into the brain ventricles, with this effect blocked by CB1 antagonists. Meanwhile, systemic effects on temperature depend on dosage, external conditions, and baseline physiological state. In hypothermia, the endocannabinoid system helps limit heat loss, while in hyperthermia, it activates cooling mechanisms. This supports the concept of the system as an internal “thermostat” enabling rapid and dynamic responses to changes in temperature balance.
Interaction of the endocannabinoid system with other neurotransmitter systems, notably serotoninergic, histaminergic, and noradrenergic pathways, allows it to coordinate thermoregulatory responses not in isolation but within the broader homeostatic context. Clinically, these properties have potential applications in treating febrile conditions, thermoregulation disorders in neurodegenerative diseases, and after traumatic brain injury.
The endocannabinoid system and its role in sleep, immune response, and thermoregulation represent not just a structural network of receptors and ligands but a fully integrated system coordinating neuroendocrine, immune, and autonomic reactions with a high degree of adaptive precision.
Connection with Other Body Systems: Nervous and Hormonal
The endocannabinoid system is closely integrated with the body’s major regulatory systems-primarily the nervous and endocrine systems. Its role extends beyond modulating local physiological processes to coordinating intersystem interactions, which enable adaptive responses to internal and external stimuli. This functional intersection is realized through the dense presence of CB1 receptors in central nervous system structures and the expression of CB1/CB2 receptors in key regulatory nodes of the endocrine axis.
In the nervous system, endocannabinoid signals function as retrograde neurotransmitters. They are synthesized “on demand” in postsynaptic neurons and act on presynaptic CB1 receptors, temporarily reducing the release of neurotransmitters such as glutamate, GABA, acetylcholine, and norepinephrine. This creates brief phases of inhibitory transmission that allow neuronal networks to avoid hyperactivity or excessive excitation. The highest density of CB1 receptors is observed in the hippocampus, basal ganglia, amygdala, prefrontal cortex, and cerebellum-areas responsible for memory, emotions, executive functions, and movement coordination.
This mechanism plays a key role in neuroplasticity, where endocannabinoid mediators participate in long-term synaptic depression (LTD), affecting learning processes and the formation of behavioral patterns. In the mesolimbic dopamine pathway, CB1-mediated modulation regulates dopamine release from the ventral tegmental area to the nucleus accumbens, which is directly related to motivation, reward, and habits. This partly explains the involvement of the endocannabinoid system in addiction mechanisms and affective spectrum disorders.
On the hormonal side, interaction is most clearly manifested within the hypothalamic-pituitary-adrenal (HPA) axis. CB1 receptors are present in the paraventricular nucleus of the hypothalamus, where they inhibit the release of corticotropin-releasing hormone (CRH), resulting in reduced secretion of adrenocorticotropic hormone (ACTH) from the pituitary and cortisol from the adrenal glands. Thus, the endocannabinoid system acts as a natural limiter of the stress response, preventing its excessive activation.
In chronic stress states, CB1 expression decreases in hypothalamic and pituitary structures, leading to elevated circulating cortisol levels. Experimentally, increasing anandamide levels or blocking its degradation normalizes HPA axis activation. Beyond the glucocorticoid axis, endocannabinoids regulate thyroid function, gonadal activity, and pancreatic secretion by influencing the release of T3/T4, testosterone, insulin, and leptin.
Another important intersection is the interaction with centers regulating reproductive function. CB1 receptors are found in the hypothalamus and pituitary, where they affect the secretion of gonadotropin-releasing hormone (GnRH), luteinizing hormone (LH), and follicle-stimulating hormone (FSH). In women, excessive CB1 activation is associated with ovulatory cycle disruption and reduced estrogen secretion, while in men it correlates with decreased spermatogenesis. This confirms the endocannabinoid system’s role in the complex hormonal regulation of fertility.
Separately, the system’s role in peripheral hormonal mechanisms-especially in adipose tissue-should be noted. CB1 receptors influence adipogenesis, expression of leptin, resistin, and insulin sensitivity. High CB1 activity is associated with metabolic syndrome development, including obesity, insulin resistance, and hypertension. Pharmacological CB1 blockade improves glycemic control and promotes weight loss, as confirmed by multiple clinical studies before the discontinuation of rimonabant due to psychiatric side effects.
The integrative function performed by the human endocannabinoid system within the nervous and hormonal systems ensures complex feedback coordination between affective states, neuronal excitability, endocrine responses, and behavioral patterns. This multilevel interaction allows adaptation not only to short-term environmental influences but also to long-term responses that alter neuroendocrine homeostasis under conditions of stress, illness, or metabolic load.
Endocannabinoid System Deficiency
The coordinated functioning of the endocannabinoid system is critically important for maintaining physiological balance, adapting to stress, and regulating numerous internal processes. However, recent decades of scientific research have shown that its disruption can have systemic character, causing what is termed a functional or clinical deficiency. This concept is not simply about the absence of receptors or mediators but encompasses a wide range of dysfunctions affecting regulatory circuits at neural, endocrine, immune, and metabolic levels.
Deficiency may manifest as reduced activity of CB1 or CB2 receptors, decreased synthesis of key endocannabinoids-anandamide (AEA) and 2-arachidonoylglycerol (2-AG)-or excessive activity of degrading enzymes, primarily FAAH and MAGL. The imbalance between production and degradation of these signaling molecules leads to a reduced ability of the system to adapt organismal responses to pain, inflammation, stress, mood fluctuations, sleep disturbances, and metabolism. Since the endocannabinoid system encompasses both central and peripheral regulation, its decrease simultaneously causes psychoemotional and somatic symptoms.
The hypothetical clinical deficiency model was first formulated in 2001 by neurologist Ethan Russo. He proposed uniting a number of functional disorders with unclear etiology but shared pathophysiological features-these include migraine, irritable bowel syndrome (IBS), fibromyalgia, chronic fatigue, and some forms of anxiety and depressive disorders. Subsequent research confirmed decreased anandamide levels in cerebrospinal fluid of patients with chronic pain and anxiety, as well as changes in CB1 receptor expression in brain structures of patients with depressive disorders.
Mechanisms of deficiency development can be primary (genetic) or secondary-acquired due to chronic stress, neuroinflammation, infections, toxic damage, prolonged sleep deprivation, or nutritional disturbances. Hormonal factors also play a significant role, as endocannabinoid levels fluctuate depending on cyclical changes in sex hormones, cortisol, insulin, and leptin. In women, in particular, fluctuations in endocannabinoid tone during the menstrual cycle potentially explain changes in pain threshold, mood, and appetite across different phases.
Pathophysiologically, deficient system activity leads to excessive excitation of neuronal circuits, instability of hypothalamic regulators, weakened anti-inflammatory mechanisms, impaired pain signal processing, and synaptic adaptation. This, in turn, causes central nervous system hyperreactivity, sleep disturbances, reduced stress tolerance, and increased sensitivity to stimuli. Systemic reduction of endocannabinoid tone creates conditions for chronic subclinical inflammation, which is significant in the progression of metabolic syndrome, atherosclerosis, and neurodegenerative diseases.
Within immune response structure, deficiency manifests as reduced modulatory influence of CB2 receptors on macrophages, microglia, and T cells, leading to predominance of pro-inflammatory cytokines-IL-1β, TNF-α, IL-6. This promotes the development of autoimmune and allergic reactions and immune tolerance instability, especially under chronic irritant exposure.
In psychiatric contexts, insufficient endocannabinoid system function correlates with reduced dopaminergic activity, impaired serotonin production, and suppressed adaptive function of the HPA axis. This contributes to the development of depressive symptoms, anxiety, emotional lability, and sleep disorders. Under such conditions, the brain loses the ability to adequately process both emotional and sensory signals, increasing the subjective experience of discomfort and pain without an actual physiological source.
What Is Clinical Endocannabinoid Deficiency?
Clinical endocannabinoid deficiency (CECD) is a concept describing a disruption of the body’s internal regulatory system, which normally maintains physiological balance. Its essence lies not only in a quantitative reduction of active compounds but in more complex dysfunctions within the signaling pathways involving the synthesis, release, transport, binding, and degradation of endogenous cannabinoids and their associated receptors. As a result of these changes, the system loses its ability to properly regulate key functions – from pain signal transmission to emotional homeostasis.
In medical and neurobiological discourse, the concept of clinical endocannabinoid deficiency was first introduced to explain symptoms of diseases without clear etiology but sharing common features: chronic pain, sleep disturbances, hypersensitivity to sensory stimuli, reduced stress tolerance, and unstable emotional states. Early examples of such conditions included migraine, fibromyalgia, irritable bowel syndrome (IBS), and later post-viral syndromes, neuropathies of unknown origin, and certain psychiatric disorders.
In clinical practice, it is observed that some patients with these diagnoses do not respond to standard treatment regimens – analgesics, anti-inflammatory drugs, antidepressants, or anxiolytics. This suggests that the primary problem may not lie in damage to terminal structures but in systemic dysregulation of the modulatory mechanism represented by the endocannabinoid system (ECS). Endocannabinoids act as retrograde messengers – synthesized “on demand” in postsynaptic neurons and feeding back to regulate presynaptic neurotransmitter release. When this feedback control is disrupted, excessive activation or suppression of neural circuits occurs, producing symptoms that don’t fit classical nosologies.
Mechanistically, clinical ECS deficiency can be caused by several biochemical deviations. For example, studies of cerebrospinal fluid in patients with chronic pain and post-traumatic stress disorder reveal lowered anandamide levels. Other data show altered CB1 receptor expression in the prefrontal cortex in depressive states. Reduced CB1 receptor density in brain regions responsible for emotion regulation and stress response leads to inadequate processing of affective information and unstable brain network interactions. Additionally, increased activity of the enzyme FAAH, which breaks down anandamide, has been linked to anxiety disorders, especially in rodent models where genetic reduction of FAAH activity reduced behavioral anxiety.
Equally important is the disruption of interactions between the ECS and other systems – serotonergic, dopaminergic, the hypothalamic-pituitary-adrenal (HPA) axis, and the immune network. Since cannabinoids influence homeostatic responses, insufficient regulatory control on their part promotes chronic low-grade inflammation, which is increasingly recognized as a key driver for a wide range of diseases – from neurodegenerative processes to metabolic disorders.
The phenomenon of clinical ECS deficiency is not simply acute or chronic reduced endocannabinoid activity. It is a dynamic dysfunction that varies depending on environment, age, hormonal status, toxin exposure, psycho-emotional factors, and even gut microbiota. Recent research increasingly considers the microbiome as a factor indirectly influencing the ECS, notably through the synthesis of fatty acids and short-chain metabolites that may modulate CB1/CB2 receptors or the activity of synthesizing and degrading enzymes.
From a clinical perspective, diagnosing this condition remains challenging. The lack of standardized biomarkers or reference levels of anandamide or 2-AG in blood or tissues prevents precise diagnosis. Assessment is mostly by exclusion, based on typical symptoms resistant to classical treatment and accompanied by signs of reduced neuroplasticity, psycho-emotional adaptation, sleep disturbances, fatigue, and chronic pain.
A key distinguishing feature of this condition, separating it from other pathologies, is its multi-organ, multisymptom presentation without morphological lesions. It is a functional disorder where instrumental methods do not reveal specific findings, yet the patient’s quality of life is significantly impaired.
In this context, endocannabinoid deficiency is viewed as a kind of “background-causal” link – not a diagnosis in the classical sense but a conceptual framework to unite a range of unclear yet clinically real conditions. This is why more researchers and clinicians recognize the value of personalized therapeutic strategies aimed at modulating ECS activity – through pharmacological, dietary, behavioral, and phytotherapeutic approaches.
Symptoms and Diseases Associated with Deficiency
Disruption of endocannabinoid tone manifests as a multivector dysfunction affecting multiple body systems simultaneously. In clinical practice, this leads to symptoms that do not fit into a single diagnostic category but form a persistent symptom complex characteristic of deficient endocannabinoid regulation.
Primarily, symptoms arise from the nociceptive system. A hallmark is chronic, widespread pain without clear localization, not accompanied by structural tissue damage. This is especially evident in fibromyalgia – a condition where the pain is musculoskeletal in nature, yet laboratory markers remain within normal limits. Patients with fibromyalgia exhibit decreased pain thresholds, hyperalgesia, and allodynia, indicating disrupted central modulation of pain signals.
Another key symptom is cyclical headaches, particularly migraines. The unique aspect of migraine involves the interaction of vascular and neuroinflammatory components. In migraine patients, impaired endocannabinoid metabolism is documented by reduced plasma levels of anandamide. This causes insufficient inhibition of trigeminal activity and excessive release of pro-inflammatory neuropeptides.
Among gastroenterological conditions, irritable bowel syndrome (IBS) is notably frequent. It is characterized by variable intestinal motility disturbances, alternating diarrhea and constipation, and chronic abdominal pain. Normally, the ECS provides fine regulation of gut motility and barrier function. Its deficiency leads to imbalance between sympathetic and parasympathetic control, manifesting as autonomic instability and enteric nervous system hyperreactivity.
An integrative manifestation of disrupted endocannabinoid activity includes the combination of anxiety, depression, and sleep disorders. Low endocannabinoid tone is associated with hyperactivity of the hypothalamic-pituitary-adrenal (HPA) axis and insufficient negative feedback of the stress response. This results in persistent internal tension, impaired emotional self-regulation, anxious thoughts, and somatic symptoms without clear organ localization.
Chronic fatigue is frequently reported – unexplained exhaustion that does not improve with rest, accompanied by cognitive impairment (commonly called “brain fog”) and physical weakness. These symptoms occur in myalgic encephalomyelitis, which some authors also link to ECS dysregulation.
From the immune system perspective, there is a tendency toward excessive inflammatory response manifested by persistent low-grade systemic inflammation. This state is not accompanied by acute fever or classic inflammatory markers but shows sustained elevation of pro-inflammatory cytokines. Over time, this condition may serve as a foundation for developing autoimmune processes or secondary immunodeficiency, depending on individual immune responses.
In reproductive health, disturbances of the menstrual cycle, endometriosis-like pain, and reduced fertility are noted. Cannabinoids participate in regulating gonadotropins and local processes in the ovaries and uterus. Their deficiency can cause hormonal imbalance and increased sensitivity to cyclic hormonal changes.
In the cardiovascular system, chronic ECS deficiency is associated with episodes of tachycardia, blood pressure instability, and a predisposition to vasospastic reactions. This results from impaired autonomic control of vascular tone. Under physiological conditions, cannabinoids regulate vasodilation and contraction, so their insufficiency disrupts hemodynamic balance.
Symptoms related to the urinary system include chronic pelvic pain, urgent urination, and detrusor instability without morphological substrate. This is typical for painful bladder syndrome, which some researchers consider a cannabinoid-sensitive pathology.
In neuropsychiatry, particular attention is given to adult attention-deficit/hyperactivity disorder (ADHD), characterized by impulsivity, reduced concentration, and emotional instability. The theoretical basis for its connection to the ECS is cannabinoids’ role in prefrontal regulation of attention and reactivity.
Among dermatological manifestations, associations between ECS deficiency and conditions such as atopic dermatitis, psoriasis, and seborrheic dermatitis have been documented. Cannabinoid receptors are found in keratinocytes, melanocytes, and hair follicles, where they regulate proliferation, inflammatory response, and sebum secretion. Their dysfunction leads to skin hyperreactivity to external irritants.
It is important to consider that the human endocannabinoid system does not function in isolation. Most symptoms arise from disrupted interactions between the ECS and other regulatory networks, especially under stress, hormonal shifts, or systemic burdens. Depletion of endocannabinoid reserves, accumulation of metabolic disturbances, sleep deficit, and chronic psycho-emotional stress lead to the consolidation of a pathological state that, without targeted intervention, shows little tendency for spontaneous resolution.
Post-infectious and post-viral syndromes deserve special attention, characterized by a combination of symptoms: decreased cognitive productivity, sleep disturbances, chronic fatigue, muscle and joint pain, and impaired thermoregulation. These conditions are thought to involve secondary depletion of endocannabinoid capacity following infections with high inflammatory burden.
Modern Approaches to Correction (Therapy, Lifestyle Changes)
Following the recognition of clinical endocannabinoid system (ECS) deficiency as a distinct functional disorder, interest in potential correction methods has increased. A comprehensive strategy includes the use of pharmacological agents, nutraceutical approaches, regulation of sleep patterns, physical activity, and modification of psycho-emotional stress. All these interventions aim not only to stimulate endocannabinoid activity but also to stabilize the metabolic background on which this system operates.
Pharmacological therapy is based on two main approaches: direct stimulation of cannabinoid receptors or inhibition of enzymes that break down endocannabinoids. The first approach uses synthetic or phytocannabinoids that mimic the action of endogenous ligands. The most studied representative is delta-9-tetrahydrocannabinol (THC), which has a high affinity for CB1 receptors. Its action temporarily compensates for the deficiency of endogenous signaling activity. However, the use of THC is limited by side effects, including psychoactivity, tolerance, and the risk of dysphoric reactions with long-term use.
An alternative is cannabidiol (CBD) – a non-psychoactive molecule that influences the ECS indirectly. It modulates the activity of TRPV1 and GPR55 receptors and alters endogenous cannabinoid concentrations by inhibiting the reuptake of anandamide. Clinical studies show CBD’s effectiveness in anxiety disorders, epilepsy, and chronic pain, indirectly indicating its ability to regulate endocannabinoid homeostasis. Using CBD in the form of standardized extracts or isolates requires precise dosing, as effectiveness depends on individual sensitivity and coexisting pathology.
The second approach involves inhibiting enzymes responsible for endocannabinoid degradation. The most well-known enzyme is FAAH, which breaks down anandamide. Pharmacological blockade of FAAH increases endogenous ligand concentration in the synaptic cleft, enhancing physiological receptor activation. Drugs in this category are still undergoing clinical trials, but preliminary results suggest their potential for treating pain, anxiety, and post-traumatic disorders.
Non-patented substances that potentially affect endocannabinoid tone include omega-3 fatty acids, magnesium, curcumin, piperine, and green tea catechins. These compounds influence enzyme activity, receptor expression, or levels of pro-inflammatory mediators, indirectly improving ECS function. For example, omega-3s are necessary for synthesizing endocannabinoids with the correct structural profile. Curcumin and piperine can reduce FAAH expression and modulate pro-inflammatory cascades that suppress endocannabinoid activity.
Physical activity is one of the most effective physiological triggers for stimulating endocannabinoids. Moderate-intensity aerobic exercise increases plasma levels of anandamide and 2-AG, which in turn improve mood, reduce pain perception, and lower anxiety levels. Regularity is important: daily or at least 3-4 sessions per week lasting 30 minutes or more provide a cumulative effect. Optimal activities include swimming, brisk walking, moderate running, dancing, or cycling. High-intensity training also stimulates the ECS, but excessive load may exhaust the system due to an amplified stress response.
Sleep regulates receptor field renewal and balances CB1 and CB2 activity. Deep sleep deficiency leads to reduced sensitivity to endogenous cannabinoids and decreased secretion. Normalizing circadian rhythms is a key component of ECS stabilization. This involves regulating bedtime and wake time, avoiding blue light exposure in the evening, refraining from electronic device use before sleep, and maintaining a consistent bedroom temperature. Low-dose melatonin or valerian extracts can be used temporarily to support transition to deeper sleep phases but should not replace proper sleep hygiene.
Psycho-emotional state critically affects ECS tone. Chronic stress, emotional exhaustion, and information overload all cause disorganization of cannabinoid signaling through hyperactivation of the cortisol axis. Conscious relaxation techniques such as deep breathing, meditation, and body-oriented practices (e.g., yoga or qigong) have demonstrated the ability to increase CB1 receptor activity and normalize interactions between the hippocampus and prefrontal cortex. Short-term interventions may yield effects after 10-15 minutes of daily practice, while systematic implementation reduces symptoms of anxiety, hyperexcitability, and somatization.
Diet plays a fundamental role in maintaining endocannabinoid balance. Excessive intake of omega-6 fatty acids combined with omega-3 deficiency promotes formation of inactive or even antagonistic metabolites that displace endocannabinoids from receptors. Foods high in trans fats, monosodium glutamate, preservatives, or simple sugars suppress neurotransmitter plasticity and lipid metabolism. Recommended dietary adjustments include increasing plant-based foods rich in polyphenols, fatty marine fish, flaxseed, and nuts, while reducing consumption of industrially processed products. Special attention should be paid to gut microbiota, as short-chain fatty acids produced during fiber fermentation can modulate cannabinoid signaling through enteroendocrine cells.
Experimental approaches include the use of adaptogens such as rhodiola and ashwagandha, as well as selective stimulation of certain receptor types using next-generation agonists. Clinical pharmacology is investigating combinations of low doses of phytocannabinoids with modulators of serotonin, opioid, or dopamine receptors-a multitarget approach with potential for individualized therapy.
Integrating these approaches requires individualized selection and dynamic monitoring. It is important not only to increase endocannabinoid activity but also to create conditions for its stable stabilization without exhaustion. The level of effectiveness depends on the combination of methods, their mutual support, and the organism’s ability to adapt to new regulatory conditions. A systemic approach combining pharmacology, lifestyle, and behavioral interventions is the optimal path to restoring functional balance in endocannabinoid deficiency.
Endocannabinoid System and Cannabis
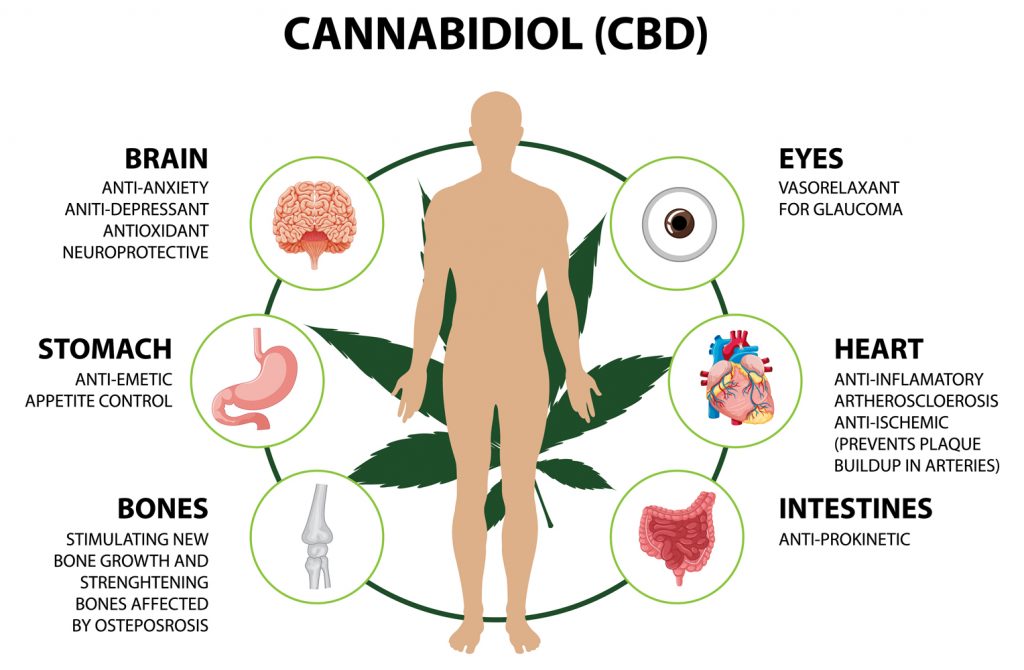
Over the past several decades, cannabis has transformed from a criminalized substance into a central focus of pharmacology, neuroscience, and clinical medicine. Modern science is increasingly uncovering the complex and multifaceted interactions of the biologically active compounds of this plant with the human body’s endocannabinoid system. The study of the mechanisms of action of phytocannabinoids, particularly Δ9-tetrahydrocannabinol (THC) and cannabidiol (CBD), has helped define key aspects of endocannabinoid regulation that were previously beyond the scope of medical knowledge.
Cannabis affects the central and peripheral nervous systems through interactions with two main receptor subtypes-CB1 and CB2-which are part of the endocannabinoid complex. CB1 receptors are primarily located in the brain, especially in the hippocampus, basal ganglia, cerebellum, and prefrontal cortex, whereas CB2 receptors are concentrated in immune system cells, the spleen, and peripheral tissues. These receptors were initially discovered through efforts to understand how cannabis interacts with the body’s endocannabinoid system, highlighting the close historical and functional link between human endogenous biochemistry and plant-derived ligands.
A key discovery was the realization that the endocannabinoid system is not designed for processing exogenous cannabinoids but has its own endogenous ligands-anandamide and 2-AG-that regulate intracellular signaling. Cannabis only mimics or modulates these processes, interfering with the delicate balance of inhibitory and excitatory signals. In this context, it is important not to oversimplify the role of phytocannabinoids as a “replacement” for endocannabinoids: the effects of cannabis are variable, dose-dependent, receptor-specific, and highly contextual-depending on the individual’s physiological, psychoemotional, and metabolic state.
Due to its lipophilicity, THC readily crosses the blood-brain barrier and binds to CB1 receptors, leading to changes in neurotransmitter release, including dopamine, glutamate, GABA, and serotonin. This explains its wide range of effects-from euphoria and sensory hypersensitivity to altered time perception, motor coordination, and appetite. CBD, on the other hand, has low affinity for cannabinoid receptors but influences the system through allosteric modulation, inhibition of anandamide reuptake, and binding to several non-cannabinoid targets such as TRPV1, PPARγ, and 5-HT1A. This interaction demonstrates the complex network of cannabis effects, involving not only the endocannabinoid system but also numerous adjacent signaling pathways.
Based on these mechanisms, the idea has emerged that controlled use of phytocannabinoids can serve as a therapeutic tool to compensate for or modulate endocannabinoid system function. Specifically, in cases of ECS hypofunction-such as clinical anandamide deficiency-phytocannabinoids may temporarily restore receptor activity and stabilize neurophysiological homeostasis. However, this raises the issue of risks: prolonged stimulation of CB1 receptors can lead to desensitization, decreased expression, and functional exhaustion of the system. This presents a challenge for clinical cannabis therapy: how to achieve correction without disrupting long-term receptor balance.
Another important aspect is that cannabis effects depend not only on THC or CBD but on the so-called “entourage effect”-the combined interaction of all bioactive plant components, including terpenes, flavonoids, and oxidative status indicators. For example, terpenes, which give the strain its aroma, can modulate cell membrane permeability and ligand sensitivity. This enhances or softens the effects of the main cannabinoids, creating a complex dynamic picture of action.
Regional differences in cannabis genetics, cultivation methods, and extraction technologies also influence the pharmacological profile. Medical extracts may contain varying ratios of THC and CBD, determining their clinical applications-for example, in epilepsy, anxiety, chronic pain, or eating disorders. Meanwhile, recreational products often have elevated THC content, increasing the risk of psychiatric complications without clinical monitoring.
From a scientific perspective, cannabis is a tool for modeling, testing, and refining our understanding of internal human regulatory mechanisms. The ECS is not an isolated system but an integrative network intertwined with dopaminergic, serotonergic, glutamatergic, and endogenous opioid signaling. Studying cannabis effects helps identify previously unseen regulatory nodes, intersections between physiology and subjective experience. In this sense, the endocannabinoid system and cannabis are not only subjects of applied medicine but keys to interdisciplinary understanding of holistic neurophysiology.
Equally important is the ethical and social dimension of the issue. The spread of cannabis as legalized or medical products raises debates about the limits of self-regulation, the role of government in controlling psychoactive substances, and the level of scientific literacy among users. The mere presence of a product on the market does not guarantee its safety or efficacy for a specific clinical case. Therapy safety requires diagnostic evaluation, medical supervision, and personalized selection of form and dose.
Interaction of Phytocannabinoids with the ECS
Phytocannabinoids are a group of biologically active plant-derived compounds that exhibit affinity for the receptors of the endocannabinoid system, mimicking or modulatory altering the action of endogenous cannabinoids. More than 100 such substances have been identified in the cannabis plant, but the most studied are Δ9-tetrahydrocannabinol (THC), cannabidiol (CBD), cannabigerol (CBG), cannabichromene (CBC), and cannabinol (CBN). Their interaction with receptor structures in the body is not uniform-each phytocannabinoid has a unique pharmacodynamic profile determined by the type of receptor binding, allosteric potential, influence on enzymatic degradation, and transport of endogenous ligands.
The primary receptor with which phytocannabinoids actively interact is CB1-a central component of neuronal regulation found in glutamatergic, GABAergic, dopaminergic, and serotonergic brain structures. Δ9-THC acts as a partial agonist of CB1, activating it in a dose-dependent manner. This causes inhibition of neurotransmitter release through a retrograde inhibition mechanism, realized by affecting calcium channels and intracellular signaling via Gi/o proteins. This mechanism allows modulation of synaptic plasticity underlying memory, affective reactivity, and motivational control. However, THC does not activate CB1 to its maximal potential, unlike some synthetic cannabinoids-this feature explains its comparatively lower toxicity but greater variability of response.
Unlike THC, CBD does not have direct agonistic activity on CB1 or CB2. Its activity is mediated indirectly: through allosteric inhibition of CB1, inhibition of FAAH (the enzyme that degrades anandamide), and binding to TRPV1, PPARγ, and 5-HT1A receptors. Such a multifaceted intervention allows CBD to modulatory influence systems involved in anxiety regulation, nociception, inflammation, and oxidative stress. Interestingly, CBD can reduce the psychoactive effects of THC when consumed simultaneously, acting as its functional antagonist. This is due to changing CB1 availability for THC and influencing anandamide transport, which competes with phytocannabinoids for receptor binding.
Other compounds such as CBG and CBC demonstrate selective activity toward CB2 receptors or interact with TRP channels without directly engaging classical cannabinoid pathways. CBG, in particular, acts as a weak CB1 agonist and a moderate 5-HT1A antagonist, as well as inhibiting norepinephrine reuptake. These properties give it potential as an anti-inflammatory, neuroprotective, and even antidepressant molecule. CBC, in turn, exhibits anti-edematous and antinociceptive effects through activation of TRPA1, TRPV3, and CB2. All these interactions have a complex time-dependent profile, involving not only direct binding but also transcriptional changes in receptor expression.
Particular attention should be paid to the mechanism of phytocannabinoid interaction with CB2 receptors. These receptors, initially considered peripheral, are now identified in microglia, astrocytes, and brainstem neurons. CB2 mediation is associated with decreased pro-inflammatory cytokines, inhibition of cell migration, and stimulation of apoptosis in immune cells. Phytocannabinoids such as CBG and CBC, as well as certain THC derivatives, activate CB2 by stabilizing the receptor conformation into an anti-inflammatory state, opening prospects for their use in treating chronic inflammatory and neurodegenerative conditions without psychoactive effects.
Equally important is the interaction of phytocannabinoids with enzyme and transporter systems. THC and CBD can inhibit FAAH and MAGL-enzymes responsible for the degradation of anandamide and 2-AG, respectively. This leads to elevated tonic levels of endocannabinoids, which in turn act on CB1 and CB2. Thus, phytocannabinoids not only mimic the action of endogenous molecules but also increase their bioavailability by reducing catabolism. Additionally, influence on transport proteins, such as FABP, alters cellular localization of anandamide, further modulating receptor activity.
Cannabinoids also affect nuclear receptors, particularly PPARγ, which controls gene expression related to inflammation, lipid metabolism, and oxidative stress. CBD and CBG activate PPARγ, inducing transcriptional changes that provide antioxidant protection and improve insulin sensitivity. This opens possibilities for use in therapies for metabolic syndrome, type 2 diabetes, and neurodegenerative diseases.
Phytocannabinoid interaction is not limited to receptor action. Terpenoids present in cannabis enhance or modify the pharmacokinetics of major cannabinoids by altering blood-brain barrier permeability, modulating liver P450 enzymes, and even changing receptor sensitivity to ligands. For example, β-caryophyllene acts as a selective CB2 agonist, while myrcene potentiates the sedative effect of THC. This ensemble, known as the “entourage effect,” represents a fundamental distinction of natural cannabis action from isolated cannabinoids or synthetic drugs.
The intensity, duration, and specificity of phytocannabinoid effects largely depend on dosage, administration route, bioavailability, and individual variations in receptor expression. Intra-individual variability caused by genetic polymorphisms, microbiota status, barrier permeability, and metabolic enzyme activity influences subjective responses to the same dose. This necessitates a personalized approach when therapeutically applying cannabis.
THC, CBD, and Other Active Compounds of the Plant
The chemical composition of cannabis is not limited to cannabinoids alone; however, cannabinoids are the primary bioactive substances affecting human physiology. Among more than a hundred cannabinoids, the most thoroughly studied are Δ9-tetrahydrocannabinol (THC) and cannabidiol (CBD), but important roles are also played by cannabigerol (CBG), cannabinol (CBN), cannabichromene (CBC), and a variety of minor compounds, including terpenoids and flavonoids. All these components act through different pharmacological vectors and collectively define the broad biological activity of cannabis.
THC is the psychoactive component of cannabis that exhibits high affinity for CB1 receptors, which are predominantly located in the brain. It acts as a partial agonist of this receptor, allowing it to modulate neurotransmitter activity including glutamate, GABA, dopamine, and serotonin. The THC molecule is lipophilic, easily crosses the blood-brain barrier, and has a long half-life. Its effects-euphoria, altered perception, temporary reduction in cognitive flexibility, and enhanced sensory integration-are consequences of central neuromodulation.
With prolonged use, THC triggers a series of compensatory changes in neuronal networks, including reduced sensitivity of CB1 receptors (desensitization), alterations in intracellular signaling cascades (especially through Gi/o proteins), and allosteric reorganization of membrane domains. These adaptations can alter the efficacy of endogenous cannabinoids and shift the balance between excitation and inhibition in neuronal circuits. This mechanism is significant in the development of dependence and tolerance.
CBD is the second most popular but non-psychoactive cannabinoid. It does not directly act on CB1 or CB2 receptors, but indirectly influences both by inhibiting FAAH (the enzyme that breaks down anandamide), allosterically modulating CB1, and stimulating PPARγ, TRPV1, and 5-HT1A receptors. Thanks to these pathways, CBD exhibits anxiolytic, anti-inflammatory, anticonvulsant effects, and stabilizes brain electrical activity in epilepsy. Its effects depend on dose, individual pharmacokinetics, and the presence of other cannabinoids.
CBD also modulates gene expression through interaction with nuclear receptors, affecting cellular differentiation, apoptosis, and antioxidant defense. Unlike THC, it does not cause receptor desensitization and, conversely, can restore receptor sensitivity to endogenous ligands in conditions of their deficiency.
Cannabigerol (CBG) is a precursor to THC and CBD in the biosynthetic pathway. Its concentration in mature plants is low, but it shows weak activity on CB1 and CB2 receptors, and interacts with TRPA1, TRPM8, and 5-HT1A. CBG has neuroprotective and antibacterial properties, inhibits MRSA aggregation, modulates gut motility, and shows efficacy in glaucoma by reducing intraocular pressure. Unlike THC, it is non-psychoactive and does not cause tolerance.
Cannabinol (CBN) forms through the oxidation of THC, especially in aged or poorly stored material. Its affinity for CB1 is weak but moderate for CB2. It exhibits sedative properties, anti-inflammatory effects, and may be useful in treating insomnia and neurodegenerative conditions. CBN influences cell proliferation processes, inhibits the release of pro-inflammatory cytokines, and shows selective activity in tissues under oxidative stress.
Cannabichromene (CBC) is another non-psychoactive component with anti-inflammatory, antidepressant, and neurogenic effects. It activates TRPA1, CB2, and partially PPARγ. Experimental data demonstrate its ability to stimulate neurogenesis in the hippocampus, which could be applied in depression therapy or neural recovery. In combination with other cannabinoids, CBC shows synergistic effects, especially in analgesia.
Besides cannabinoids, cannabis contains over 200 terpenes that significantly influence the plant’s biological activity. For example, β-caryophyllene is a selective CB2 agonist, myrcene affects cell membrane permeability, and linalool modulates GABA receptors. The interaction of cannabinoids with terpenes forms the phenomenon known as the “entourage effect,” where the action of the mixture exceeds that of individual compounds.
Active components also include flavonoids, which possess antioxidant, neuroprotective, and anti-inflammatory properties. For instance, cannflavin A inhibits the production of PGE2-a potent inflammation mediator-30 times more effectively than aspirin. Although flavonoids are present in low concentrations in cannabis, their contribution to the plant’s pharmacological profile is significant.
It is important to understand that each of these substances not only has its own effects but also modifies the pharmacodynamics of other components. For example, CBD can reduce the psychoactive potential of THC, whereas CBG enhances its analgesic effects. In clinical practice, this interaction becomes relevant when developing composite medications and selecting therapeutic profiles tailored to individual needs.
The pharmacokinetics of phytocannabinoids depends on the route of administration. With inhalation, THC reaches peak plasma levels within 5-10 minutes, while oral ingestion peaks in 1.5-2 hours. CBD has lower bioavailability, especially when taken orally, so higher doses are often required to achieve therapeutic effects. Metabolism occurs primarily in the liver via the cytochrome P450 system, producing active metabolites that may also have pharmacological activity.
Response to cannabinoids varies individually depending on genetic polymorphisms, metabolic status, presence of inflammation, microbiota composition, and the overall state of the endocannabinoid system and cannabis in the body. Therefore, a standardized approach to dosing or selecting cannabinoid profiles is often ineffective.
Medical Use of Cannabis and Its Impact on the ECS
In clinical practice, cannabis is regarded as a multi-component pharmacological tool that influences a broad spectrum of pathological processes. Its application is not limited to symptomatic therapy but is increasingly incorporated into models of pathogenetic intervention. Thanks to the ability of cannabinoids to regulate homeostatic mechanisms, medical cannabis is used in the treatment of chronic pain, epilepsy, anxiety disorders, chemotherapy-induced nausea, muscle spasticity in multiple sclerosis, sleep disturbances, and appetite loss in HIV/AIDS.
One of the most well-validated areas of use is neuropathic pain, which responds poorly to traditional analgesics. Cannabinoids modulate pain signal transmission at the spinal cord and thalamus levels by reducing the activity of pronociceptive neurons while also influencing the emotional perception of pain through the mesolimbic system. Combinations of THC and CBD in formulations such as Sativex have demonstrated effectiveness in managing chronic pain in patients with cancer, rheumatoid arthritis, and fibromyalgia.
In the treatment of pharmacoresistant epilepsy, particularly Lennox-Gastaut and Dravet syndromes, purified cannabidiol is successfully applied. The FDA-approved drug Epidiolex has shown a 30-50% reduction in seizure frequency in most patients. This effect is associated with CBD’s action on ion channels (especially Na+ and Ca2+), TRPV1 and 5-HT1A receptors, and modulation of synaptic plasticity in epileptogenic zones. Importantly, CBD is non-psychoactive and has a favorable safety profile even at high doses.
Muscle spasticity in multiple sclerosis is another condition where cannabinoids have demonstrated clinical benefit. Reduction of muscle tone, pain, and improvement in quality of life are linked to THC’s action via CB1 receptors in the motor cortex and spinal cord. Studies show a 40-50% reduction in spasticity among patients unresponsive to other medications when treated with combined extracts.
In oncology, cannabis is used not only for pain control but also to reduce nausea and vomiting caused by chemotherapy. Synthetic cannabinoids such as dronabinol and nabilone are approved for treating chemo-induced nausea in patients unresponsive to 5-HT3 antagonists. Additionally, cannabinoids may support weight stabilization by stimulating appetite, especially in patients with HIV/AIDS or severe cachexia.
In psychiatry, cannabinoid use is being investigated for treating post-traumatic stress disorder (PTSD), generalized anxiety, and obsessive-compulsive disorder. The primary therapeutic mechanism involves CBD’s ability to affect fear networks, neuroplasticity, and weaken pathological emotional memories. Clinical observations report reductions in anxiety levels, improved sleep, and decreased flashbacks in PTSD patients using CBD doses of 300-600 mg/day.
In neurodegenerative diseases such as Alzheimer’s, Parkinson’s, and Huntington’s, cannabinoids exhibit antioxidant, anti-inflammatory, and neuroprotective effects. These are linked to activation of CB2 receptors on microglia, reduction of neuroinflammation, inhibition of protein aggregation, and stabilization of mitochondrial function. Although no approved protocols exist yet, experimental data support further research in this area.
The immunomodulatory potential of cannabinoids also attracts attention. CB2 receptors are present on immune cells such as B lymphocytes, macrophages, neutrophils, and dendritic cells. Their activation leads to decreased production of pro-inflammatory cytokines (TNF-α, IL-1β, IL-6), inhibition of T-cell proliferation, and stimulation of regulatory phenotypes. This opens possibilities for cannabinoid use in autoimmune diseases like systemic lupus erythematosus, rheumatoid arthritis, and multiple sclerosis.
In dermatology, cannabinoids are applied for atopic dermatitis, psoriasis, and acne. Their effects are due to suppression of sebum production, reduction of keratinocyte proliferation, and modulation of local immune responses. CBD-based creams or ointments demonstrate efficacy in reducing inflammation, itching, and hyperkeratosis.
Cardiometabolic effects of cannabinoids are still being explored. There is evidence of improved insulin sensitivity, reduced C-reactive protein levels, and some decrease in body mass index with chronic CBD use. Mechanisms include modulation of PPARγ, AMPK, and effects on adipose tissue via CB1 receptors. However, excessive THC use may be associated with cardiopulmonary risks.
Cannabis is also used in palliative care. Patients with terminal stages of cancer, neurodegenerative diseases, or severe heart failure benefit from reduced pain, anxiety, nausea, and appetite disturbances. Cannabinoids improve quality of life and reduce opioid requirements, which is especially important considering addiction risks.
The field of personalized cannabinoid therapy is rapidly developing. Genetic variations in enzymes (CYP2C9, CYP3A4), CB1 and CB2 receptors, as well as individual endotypes of endocannabinoid system deficiency or hyperactivity, are taken into account. This approach minimizes side effects, avoids unwanted drug interactions, and ensures effective bioindividualized support.
Understanding the therapeutic potential of cannabis requires awareness of the receptors through which cannabis interacts with our body’s endocannabinoid system. These primarily include CB1, CB2, as well as various vanilloid, serotonin, adenosine, nuclear, and transient receptor channels. Thanks to this spectrum of targets, phytocannabinoids can modulate both central and peripheral mechanisms, providing a wide therapeutic amplitude.
Side Effects, Risks, and Prospects
Despite its significant therapeutic potential, cannabis use is associated with a range of risks that depend on dosage, chemical composition, duration of use, administration method, and the sensitivity of the individual patient. Studying side effects is critically important because improper dosing or self-administration of cannabis-based products can not only reduce treatment efficacy but also cause unwanted physiological or psychological reactions. Medical cannabis is not universally safe and requires clear standards for pharmacovigilance.
One of the most common side effects is cognitive impairment, particularly decreased concentration, slowed reaction times, and temporary weakening of short-term memory. These changes are most pronounced when using products containing high doses of delta-9-tetrahydrocannabinol (THC), especially in patients without tolerance to its psychoactive effects. In adolescence, regular THC use is linked to an increased risk of IQ decline, changes in the prefrontal cortex structure, and disrupted neurodevelopment.
Psychiatric complications may manifest as anxiety, panic attacks, paranoid thoughts, and sometimes THC-induced psychosis. Individuals with a genetic predisposition to schizophrenia or bipolar disorder are particularly vulnerable. Neuroimaging data show that THC affects dopaminergic and glutamatergic pathways involved in regulating emotions and cognition, explaining the potential risk of psychotic episodes in sensitive individuals.
Cardiovascular effects include tachycardia, blood pressure fluctuations, and vasodilation. In people with latent ischemic heart disease or arrhythmias, these symptoms may worsen. There are reports of myocardial infarction, stroke, and cardiac arrhythmias associated with high doses of THC, especially in patients who concurrently use tobacco or other psychoactive substances.
Respiratory effects are primarily linked to cannabis smoking rather than pharmaceutical formulations. Chronic inhalation of smoke can cause bronchitis, cough, shortness of breath, and bronchial hyperreactivity. Cannabis smoke contains polycyclic aromatic hydrocarbons similar to those in tobacco, increasing oncological risks with long-term use. Alternative delivery methods-sublingual sprays, capsules, tinctures, inhalers-minimize these effects but require precise dosing.
Motor coordination impairment, slowed reactions, and drowsiness increase the risk of accidents. Patients using cannabinoids should avoid driving or operating heavy machinery for at least several hours after use. This is especially important for inexperienced users or those combining cannabis with alcohol or sedatives.
Dependence can develop, though the risk is lower compared to opioids, nicotine, or alcohol. About 9-10% of regular cannabis users develop dependence, characterized by psychological discomfort during withdrawal, irritability, insomnia, and decreased appetite. The withdrawal syndrome is less severe than opioid or benzodiazepine dependence but can be clinically significant in sensitive patients.
The risk of drug interactions also requires attention. Cannabinoids are primarily metabolized by CYP3A4 and CYP2C9 isoenzymes, which also process many other drugs, including anticoagulants, antidepressants, and immunosuppressants. Potential inhibition or induction of these enzymes may cause toxicity or reduced efficacy of concurrent therapies.
Given individual variations in metabolism, receptor sensitivity, and cannabinoid responses, personalized prescribing models are essential. Genetic testing, pharmacokinetic and pharmacodynamic monitoring, and precise patient risk stratification enhance therapy safety. Special attention should be paid to children, pregnant women, and the elderly, where adverse effects risk increases due to altered pharmacological responses.
At the same time, the question of long-term consequences of regular cannabinoid use remains relevant. While some data indicate reduced inflammation, neuroprotection, and metabolic benefits, others point to potential CB1 receptor desensitization that may impair endocannabinoid system function under prolonged excessive load. This can provoke dysfunctions in pain regulation, mood, sleep, and immunity.
Regarding prospects for cannabinoid therapy development, selective agonists and antagonists of CB1/CB2 receptors, molecules with low psychoactive potential, and synthetic CBD analogs with improved bioavailability are anticipated. Development continues on combined regimens with other drugs-analgesics, anticonvulsants, anxiolytics-to achieve synergistic effects while minimizing cannabinoid doses.
A new direction is creating formulations targeting not only cannabinoid receptors but also other targets: TRP receptors, GPR55, adenosine, PPAR. This approach aims to expand therapeutic effects and reduce psychoactive side effects. Nanotechnology delivery platforms are being developed to provide controlled release of active compounds, precise dosing, and reduced systemic toxicity.
Clinical use prospects also depend on regulatory environments. Legalization of medical cannabis in various countries has opened access to systematic clinical trials, expanded therapeutic indications, and manufacturing standardization. However, in many regions cannabis is still classified solely as a narcotic, hindering scientific progress and patient access.
Another promising vector is integrating cannabinoid therapy into complementary medicine: combining it with nutritional support, physical therapy, and cognitive-behavioral therapy. This approach is especially relevant for chronic, multi-etiological conditions requiring multifaceted intervention with minimal side effects.
Conclusion:
The endocannabinoid system (ECS) is a complex, integrative signaling network functionally connected to most key physiological processes in the body. It consists of endogenous ligands (anandamide, 2-AG), specific receptors (primarily CB1 and CB2), transport proteins, and enzymes that regulate cannabinoid synthesis and degradation. The interaction among these components ensures fine-tuning of intercellular communication, rapid response to stressors, maintenance of homeostasis, and dynamic adaptation to internal and external changes. Its activity encompasses the central and peripheral nervous systems, immune response, metabolism, hormonal regulation, modulation of pain, sleep, thermoregulation, and mood.
The ECS does not operate as an isolated system but as a regulatory overlay influencing the functions of other body systems, including the nervous, endocrine, immune, digestive, and cardiovascular systems. Its specificity lies in the retrograde signaling mechanism, whereby endocannabinoids are synthesized “on demand” by the postsynaptic cell and suppress presynaptic neuron activity. This allows regulation of neuronal activity intensity by inhibiting excessive stimulation or excitation. CB1 receptors are densely distributed in the brain, especially in the hippocampus, basal ganglia, and cerebellum, accounting for their role in cognitive, motor, and emotional regulation. CB2 receptors are predominantly expressed in immune cells, modulating pro-inflammatory cascades, apoptosis, and cell migration.
One of the key physiological challenges is the clinical deficiency state of the ECS-a dysfunctional condition in which reduced endocannabinoid levels or impaired receptor response lead to pathological dysregulation. This deficiency may be primary (genetically determined) or secondary (acquired due to chronic stress, neuroinflammation, injury, infection, or aging). Low ECS activity is associated with a broad range of conditions-from migraine, fibromyalgia, and irritable bowel syndrome to depression, anxiety disorders, and neurodegenerative diseases. These connections are not merely correlational; current experimental evidence confirms a causal role of ECS tone disruption in the development of persistent pathology.
In this context, the role of therapeutic ECS modulation is growing. Modern approaches to correcting deficiency include pharmacological interventions-using natural and synthetic cannabinoids, FAAH/MAGL enzyme inhibitors, selective CB1/CB2 agonists-as well as non-pharmacological methods such as dietary strategies, physical activity, stress reduction practices, and sleep normalization. The ECS modulation approach must be personalized and based on validated measurements of endocannabinoid levels and receptor activity. Excessive or uncontrolled stimulation can, conversely, worsen the system’s adaptive capacity through receptor desensitization.
A special place is occupied by the interaction between the ECS and cannabis. Phytocannabinoids, primarily THC and CBD, have the potential to modulate CB receptor activity and related signaling cascades. THC acts as a partial CB1 agonist, which accounts for its psychoactive effects, while CBD has low affinity for classical cannabinoid receptors but influences allosteric modulation, anandamide reuptake, and other signaling targets such as PPARγ, TRPV1, and GPR55. The pharmacological profile of cannabis depends not only on the THC/CBD ratio but also on the content of terpenes, flavonoids, cannabigerol, and other non-canonical cannabinoids.
Medical use of cannabis shows promising results in treating pain, epilepsy, multiple sclerosis, Parkinson’s disease, autism, PTSD, and oncological palliative care. However, its implementation carries risks: cognitive impairment, dependence, psychotic episodes, cardiovascular complications, and negative impacts on neurodevelopment in adolescents. Safe use requires regulated dosing, strict quality control of products, medical monitoring, and legal regulation. Scientific research focuses on finding selective cannabinoids with minimal side effects, developing unconventional delivery forms (nanoformulations, inhalers, transdermal systems), and using the ECS as a therapeutic target for next-generation drugs.
Looking ahead, the ECS is no longer viewed solely as a pharmacological target. It is becoming a system integrated into clinical diagnostics, risk screening, prevention strategies, and a platform for personalized medicine. Its unique ability to coordinate functions across body systems, respond to changing conditions, and maintain balance between activation and inhibition processes makes it one of the key regulators of homeostasis and a target for future multisystem therapeutic strategies.
References:
- Endocannabinoid System: Overview and Clinical Implications
Source: National Center for Biotechnology Information (NCBI), PubMed Central
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3998222/ - The Endocannabinoid System and its Therapeutic Exploitation
Source: Nature Reviews Drug Discovery
https://www.nature.com/articles/nrd.2015.16 - Endocannabinoid System in Health and Disease: An Overview
Source: Frontiers in Neuroscience
https://www.frontiersin.org/articles/10.3389/fnins.2018.00025/full - Cannabinoid Receptors and Their Endogenous Ligands
Source: British Journal of Pharmacology, Wiley Online Library
https://bpspubs.onlinelibrary.wiley.com/doi/full/10.1111/bph.13118 - Clinical Endocannabinoid Deficiency: Can This Concept Explain Therapeutic Benefits of Cannabis in Migraine, Fibromyalgia, Irritable Bowel Syndrome?
Source: Neuroendocrinology Letters
https://www.nel.edu/userfiles/articlesnew/NEL320509A04.pdf - Pharmacology of Cannabinoids
Source: Pharmacological Reviews, American Society for Pharmacology and Experimental Therapeutics (ASPET)
https://pharmrev.aspetjournals.org/content/68/4/1103 - Therapeutic Potential of Cannabinoids in Neurological Disorders
Source: Journal of Neurology, Neurosurgery & Psychiatry, BMJ
https://jnnp.bmj.com/content/89/7/741 - Endocannabinoid System and Immune Regulation: A Role for Cannabinoids in Modulating Immune Response
Source: Journal of Leukocyte Biology
https://jlb.onlinelibrary.wiley.com/doi/full/10.1189/jlb.3RI0713-379R - Medical Cannabis and Cannabinoids: Evidence and Practice
Source: Royal Australian College of General Practitioners (RACGP)
https://www.racgp.org.au/clinical-resources/clinical-guidelines/key-racgp-guidelines/view-all-racgp-guidelines/medical-cannabis - Safety and Side Effects of Cannabinoids
Source: Drug Safety Journal, SpringerLink
https://link.springer.com/article/10.2165/00002018-200746090-00004