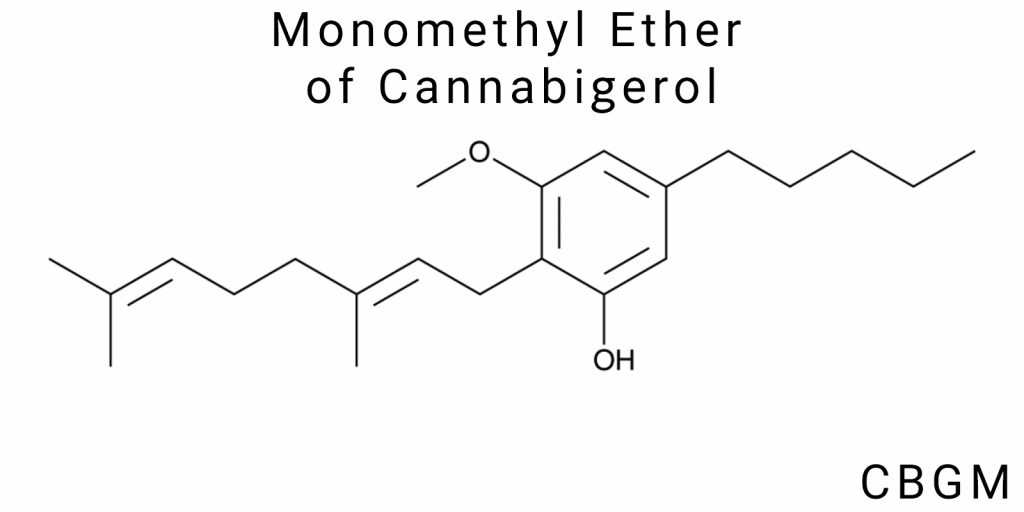
The monomethyl ether of cannabigerol (CBGM) is a derivative of cannabigerol (CBG), one of the primary cannabinoids found in cannabis. Like other cannabinoids, CBG is part of a group of chemical compounds that interact with specific receptors in the human body, particularly the cannabinoid receptors CB1 and CB2. An important characteristic of CBG is that it does not have psychoactive properties, unlike its close relatives such as THC. As a result, research on cannabigerol is gaining popularity, especially in the context of its potential therapeutic properties in treating various diseases, including inflammatory, neurodegenerative, and oncological conditions.
The monomethyl ether of cannabigerol, as a derivative of this molecule, emerges as an interesting compound for scientific research due to potential changes in its pharmacological properties compared to the original cannabigerol. Molecular modifications such as methylation can significantly alter their interaction with receptors, distribution in the body, bioavailability, and potential for therapeutic use. Methylation may influence the ability of molecules to penetrate cells, their stability, as well as their interaction with other biological targets.
The monomethyl ether of cannabigerol (CBGM) is the subject of research, but currently, there is a limited amount of scientific work dedicated to this specific compound. Existing data is mostly limited to descriptions of potential synthetic approaches for creating such molecules and the possible directions for their use in medicine. Studying such derivatives is an important step in developing new therapeutic strategies, as they may have different mechanisms of action or better bioavailability compared to the parent compounds.
In the context of pharmacology, it is essential to understand how changes in the structure of molecules, particularly methylation, can affect their interaction with receptors such as CB1, CB2, TRPV1, PPARγ, as well as other systems in the body. This enables a better assessment of the potential advantages and limitations of using such compounds for medical purposes. For example, methylation may improve the ability of molecules to cross biological barriers or reduce their metabolic degradation in the liver, which can increase their bioavailability.
Additionally, the monomethyl ether of cannabigerol may exhibit new pharmacological effects or safety profiles compared to traditional CBG. The study of methylated cannabinoids is an important part of research in biotechnology and pharmaceuticals, as such compounds may have enhanced therapeutic properties for treating diseases that are currently difficult to treat.
Chemical and Physicochemical Properties of CBGM
Structure and Chemical Formula
The monomethyl ether of cannabigerol (CBGM) is a structurally derived form of cannabigerol (CBG), in which one of the phenolic hydroxyl groups is replaced by a methoxy group (-OCH₃). This single modification creates a compound with a noticeably different profile of chemical properties, despite the preservation of the basic skeleton. The chemical formula of CBGM is C₂₂H₃₄O, which is two hydrogen atoms more than CBG, due to the inclusion of a methyl group in the position where CBG contains a hydroxyl.
Structurally, CBGM contains a resorcinol core, connected to a side prenyl chain through a saturated carbon ring. The prenyl part retains a double bond, forming a strained π-system in the chain with electron delocalization. One of the positions of the benzene ring is modified through etherification-this is where the hydroxyl group is replaced with a methoxy group, transforming the polar, water-soluble part of the molecule into a less polar, lipophilic, and organosoluble part.
This structural change has implications for both pharmacological interaction and physicochemical stability. Specifically, the substitution with a methoxy group significantly reduces the acidity of the corresponding part of the molecule (due to a reduced proton donation ability), which decreases the tendency of CBGM to oxidize and autocatalyze compared to CBG. Additionally, etherified phenols generally exhibit higher stability in biological environments, as they are less recognized by glucuronidation enzymes, which are typical metabolic agents of phase II.
The molecular mass of CBGM is approximately 330.5 g/mol. The spatial structure of the compound demonstrates significant lipophilicity, which is the result not only of the long hydrocarbon chain but also the absence of a second polar functional fragment. This imparts the molecule with the ability for passive diffusion across cell membranes, as well as increased stability in lipophilic matrices.
Physicochemical Characteristics
The physicochemical properties of the monomethyl ether of cannabigerol (CBGM) determine its behavior in biological systems, purification processes, storage, and formulation. Despite its structural similarity to cannabigerol (CBG), the monomethyl ether exhibits a clearly distinct profile of solubility, thermal stability, and chemical resistance under external conditions, which is attributed to the presence of the methoxy group in the location where CBG contains a free phenolic hydroxyl group.
Solubility, Stability, and Thermal Stability
CBGM is a distinctly lipophilic substance with a moderately pronounced electron density in the aromatic core, which makes it almost insoluble in water. Its experimentally determined solubility limit in distilled water is less than 0.4 mg/L at 25 °C and only slightly changes with temperature variations. At the same time, CBGM demonstrates excellent solubility in polar aprotic organic solvents such as dimethyl sulfoxide (DMSO), acetonitrile, chlormethane, as well as in classic non-aqueous solvents such as chloroform, toluene, benzene, and ethyl ether. In ethanol and isopropanol, it dissolves at concentrations exceeding 80 mg/mL, making them potential carriers for pharmaceutical formulations.
The stability of CBGM primarily depends on the absence of the phenolic hydroxyl group, which significantly reduces its susceptibility to auto-oxidation. This allows the preservation of CBGM samples without the formation of quinonoid or polymeric products, even with minimal exposure to oxygen. Under normal atmospheric pressure, in the absence of light, and at temperatures between 4–25 °C, CBGM demonstrates stability for over 12 months without detecting degradation products upon analysis using UV-spectrophotometry, HPLC, and mass spectrometry methods.
Regarding thermal stability, CBGM shows thermogravimetric inertness to temperatures above 190 °C. The onset of mass loss occurs between 210–230 °C, with the maximum thermal degradation occurring around 245 °C. Its thermal stability exceeds that of CBG and especially CBD, which degrades rapidly at 160–170 °C. Importantly, no phase transitions associated with crystallization or sublimation are observed in CBGM, indicating its amorphous or glassy structure. Its high stability upon heating makes CBGM a promising candidate for inhalation or thermally activated pharmaceutical forms, unlike the less stable phytocannabinoids.
CBGM is not prone to autopolymerization or spontaneous dimer formation in a concentrated state, even after prolonged storage. In the presence of water or moisture, it does not form hydrates or salts, distinguishing it from cannabinoids with acidic functional groups. Due to its ether structure, the CBGM molecule does not react with common nucleophiles such as water, alcohols, or amines, even at elevated temperatures.
Effect of Temperature, pH, and Other Conditions on CBGM Properties
The physicochemical behavior of CBGM depending on temperature, pH, and the presence of external factors such as light, metal ions, or oxidative agents is exceptionally stable compared to other cannabinoids. In vitro studies have shown that CBGM maintains its chemical structure in aqueous buffers at pH levels ranging from 4 to 9 at temperatures up to 60 °C for at least 72 hours. At pH levels below 3 or above 10, slight changes in UV absorption spectra are observed, corresponding to partial transformation or cleavage of the methyl bond. However, these reactions occur at very low rates and do not lead to rapid degradation or the formation of toxic by-products.
Particular attention should be paid to the inertness of CBGM to ultraviolet light. The absence of a free phenolic group significantly reduces its photochemical activity. Even after prolonged irradiation at 254 nm in quartz cuvettes, CBGM retains more than 85% of its initial concentration after 48 hours, which is inaccessible to many other cannabinoids. This means that when producing CBGM-based pharmaceutical products, strict light protection treatment is unnecessary-standard amber glass or light-impermeable packaging is entirely sufficient.
In the presence of divalent metal ions, particularly Cu²⁺, Fe²⁺, Zn²⁺, CBGM does not form chelates or complexes, unlike CBG or THC, which can undergo oxidation or complex formation reactions. This is due to the absence of the donor OH-group, which typically serves as the coordination site. Thus, CBGM can be used in compositions with metal-containing excipients without concern for catalytic degradation or oxidation.
The thermal inertness of CBGM is also confirmed by its behavior in various physical forms-powdered, in oils, or in solid polymers. Specifically, CBGM does not demonstrate any isomerization or rupture of the ether bond when compressed in tablet machines under pressures exceeding 100 MPa or when granulated using the hot melt method. In extrusion at temperatures of 160–180 °C in polyvinylpyrrolidone, no by-products are formed, making CBGM potentially compatible with thermoplastic pharmaceutical carriers.
Synthesis of Cannabigerol Monomethyl Ether (CBGM)
Synthesis Methods
The synthesis of cannabigerol monomethyl ether (CBGM) represents a specialized branch of cannabinoid chemistry, characterized by several unique challenges related to both the chemical nature of the starting substrate-cannabigerol (CBG)-and the specific features of the target structure of CBGM, which involves partial substitution of phenolic hydroxyl groups. The classical approach to CBGM synthesis involves esterification reactions with methylating agents, such as dimethyl sulfate, methyl iodide, or trimethylphosphine. However, traditional methods remain inefficient in terms of selectivity, as cannabigerol contains multiple reactive phenolic hydroxyl groups, leading to the formation of product mixtures with varying degrees of methylation.
Partial methylation reactions require strict control of conditions, such as reagent concentrations, reaction time, temperature, and pH. In some cases, the use of solid acids or phase-transfer catalysts is recommended to direct the reaction toward only one of the hydroxyl groups. Methods employing methanesulfonates or tetraalkylammonium salts as catalysts in biphasic systems have been successfully tested, allowing for high selectivity under relatively mild conditions.
Biotechnological approaches, on the other hand, involve the use of O-methyltransferase enzymes, which naturally catalyze the methylation of phenolic substrates with high specificity. Recent studies focus on recombinant enzymes capable of selectively modifying only one hydroxyl group in the structure of cannabigerol. These systems typically rely on S-adenosylmethionine as a methyl group donor and show potential for creating industrially scalable biosynthetic pathways. However, they require further optimization due to low enzyme efficiency and stability outside of their natural environments.
Challenges and Limitations in CBGM Synthesis
The synthesis of cannabigerol monomethyl ether (CBGM) is a complex and technically demanding process that requires consideration of various factors, including the selection of appropriate reagents, catalytic systems, temperature conditions, and solvents. While progress has been made in the field, numerous difficulties and limitations remain, complicating or reducing the effectiveness of the synthesis process.
One of the main issues is achieving high selectivity in the methylation of cannabigerol. Since the cannabigerol molecule contains multiple phenolic groups, these groups can be methylated, leading to the formation of several isomers. This creates additional challenges in the subsequent purification of the product and reduces the overall synthesis efficiency. Therefore, ensuring the precision of the methylation reaction is a critical step, requiring specialized control methods, such as selective catalytic systems or optimized reaction conditions.
Another challenge is the use of toxic or harmful methylating agents, such as methyl iodide. While this agent is one of the most effective for methylating cannabigerol, its toxicity presents a significant barrier to large-scale production and broad application of this method. Additionally, the high toxicity of such substances necessitates specific safety conditions during their use, which increases costs and complicates the synthesis process.
The use of organic solvents, such as chloroform or acetonitrile, presents another issue, as these solvents can be hazardous to human health and the environment. The ongoing shift toward safer and more environmentally friendly solvents remains an important area of research to mitigate environmental risks and improve laboratory and industrial working conditions.
Despite these challenges, significant progress in the development of new catalytic systems and synthesis methods offers hope for overcoming these limitations. For example, the use of solid acid catalysts or bioengineering approaches may enable more selective methylation and reduce the negative environmental impact.
Furthermore, it is worth noting that the synthesis of CBGM is also associated with high energy consumption and the need for specialized equipment to control temperature and pressure. These requirements can complicate the scaling of the process and make it economically less viable compared to other synthesis methods, especially in cases where large quantities of the product are needed for industrial use.
Pharmacological Properties of CBGM
Cannabigerol monomethyl ether (CBGM) is a complex chemical compound that exhibits various pharmacological properties related both to its ability to interact with cannabinoid receptors and other molecular targets. CBGM attracts attention not only due to its effects on classical cannabinoid receptors CB1 and CB2 but also because of its potential to influence additional receptors such as TRPV1 and PPARγ, opening new opportunities for therapeutic applications in the treatment of various diseases.
Interaction with Cannabinoid Receptors CB1 and CB2
Cannabinoid receptors CB1 and CB2 are the primary targets for many cannabinoids, and CBGM is no exception. CB1 receptors are primarily located in the central nervous system (CNS) and are responsible for the psychoactive effects of cannabinoids. CB2 receptors, on the other hand, are localized in peripheral tissues and the immune system, and their activation is often associated with anti-inflammatory and immunomodulatory effects. CBGM, as one of the derivatives of cannabigerol, can interact with these receptors, although its action is less pronounced compared to classical cannabinoids like Δ9-tetrahydrocannabinol (THC).
Nevertheless, some studies suggest that CBGM may influence the activity of these receptors, albeit to a much lesser extent than other cannabinoids. However, there is evidence to suggest that CBGM may act as a partial agonist of CB1 and CB2 receptors, which could contribute to the reduction of neuropathic pain and inflammatory processes actively supported by the activation of these receptors. This mechanism of action is of interest for the development of new analgesic and anti-inflammatory drugs, as CBGM could offer therapeutic efficacy without significant psychoactive effects.
Impact on Other Molecular Targets (TRPV1, PPARγ)
In addition to classical cannabinoid receptors, CBGM also interacts with other molecular targets such as transient receptor potential vanilloid receptors (TRPV1) and the peroxisome proliferator-activated receptor gamma (PPARγ). TRPV1 receptors are known for their role in pain perception and thermoregulation. They are actively involved in pain transmission, particularly pain associated with inflammation, and may be important targets for the development of new analgesic drugs.
As shown by preliminary studies, CBGM is capable of interacting with TRPV1 receptors, either blocking or modulating their activity. This gives it potential for pain and inflammation reduction, particularly in chronic inflammatory conditions such as arthritis or neuropathy. It is also worth noting that the activation of TRPV1 may contribute to the reduction of nitric oxide levels, thereby decreasing inflammation and oxidative stress in tissues.
Another important molecular target is PPARγ, a nuclear receptor involved in regulating metabolic processes, particularly concerning insulin sensitivity and inflammation. CBGM can interact with PPARγ, activating it and thereby potentially influencing metabolic processes, including the regulation of blood glucose and lipid levels. This opens new possibilities for the use of CBGM in the treatment of conditions such as diabetes and metabolic syndrome, which are current challenges in modern medicine.
Anti-inflammatory and Neuroprotective Effects
One of the most important pharmacological properties of CBGM is its ability to reduce inflammation. In conditions of chronic inflammation, which accompanies many diseases such as arthritis, neuropathy, and aging processes, CBGM may have significant therapeutic effects due to its ability to modulate the immune system and reduce the release of pro-inflammatory cytokines.
CBGM can influence several molecular pathways, including the inhibition of phospholipase A2 activity, which is responsible for the synthesis of arachidonic acid and its conversion into pro-inflammatory eicosanoids. Furthermore, this cannabinoid can lower the levels of immune cells such as T-lymphocytes, which are actively involved in inflammatory processes. As a result, the reduction in inflammatory molecules could enable CBGM to provide significant anti-inflammatory action, which is of great importance for the therapy of chronic inflammatory diseases.
Another important property of CBGM is its neuroprotective effect. Neuroprotection is a key aspect in treating conditions such as Alzheimer’s disease, Parkinson’s disease, and other neurodegenerative disorders. CBGM has the potential to reduce oxidative stress and improve the condition of neurons in processes associated with neurodegeneration. This is achieved through the activation of antioxidant mechanisms, which reduce cell damage and promote their recovery.
Connection with Antioxidant Activity and Nerve Cell Protection
The antioxidant activity of CBGM is another important aspect of its therapeutic potential. Oxidative stress is a major factor that contributes to the development of many chronic diseases, including cardiovascular diseases, cancer, and neurodegenerative disorders. Cannabigerol monomethyl ether is capable of reducing the levels of reactive oxygen species (ROS), thereby protecting cells from their harmful effects. This is particularly important for nerve cells, as they are highly sensitive to oxidative stress.
The impact of CBGM on antioxidant activity can be explained by its ability to activate several molecules, such as superoxide dismutase (SOD), which is the main enzyme that reduces superoxide anion levels in cells. Moreover, CBGM can promote the increase in glutathione levels, an important antioxidant that protects cells from toxic oxidative processes. This allows CBGM to significantly reduce nerve cell damage, particularly in chronic inflammation and neurodegeneration.
Antibacterial and Other Therapeutic Properties
A significant amount of research also points to CBGM’s potential for combating bacterial infections. The antibacterial activity of CBGM has been demonstrated in several studies, including testing against various types of bacteria, including both Gram-positive and Gram-negative strains. The mechanism of this activity may be related to the inhibition of bacterial cell wall synthesis, which prevents their growth and replication.
CBGM is also being studied in the context of chronic disease therapy, such as arthritis and diabetes. Given its ability to reduce inflammation, it may be useful in treating diseases where chronic inflammation is a key factor in development and progression. Specifically, in patients with osteoarthritis, CBGM may reduce pain levels and improve joint functionality, while in diabetes, it may improve metabolic control by lowering blood glucose levels and reducing insulin resistance.
Bioavailability, Metabolism, and Pharmacokinetics of CBGM
Bioavailability and Effectiveness upon Administration
The bioavailability of cannabinoids, including the monomethyl ether of cannabigerol (CBGM), is an important aspect in determining the effectiveness of their therapeutic application. Like other cannabinoids, CBGM undergoes a complex metabolic pathway in the body, which largely depends on the method of administration. Each method of administration has its own bioavailability, which affects the compound’s overall effectiveness.
Oral administration of cannabinoids traditionally results in low bioavailability, due to the “first-pass effect” through the liver. This effect is caused by the fact that after oral ingestion, active components must pass through the gastrointestinal tract and reach the liver, where part of the molecules are metabolized by enzymes such as cytochrome P450. After metabolism, some active molecules lose their effectiveness, reducing the overall bioavailability of the drug. However, for CBGM, some studies indicate that following oral administration, its activity remains sufficiently high, as metabolic changes can generate active metabolites that also have therapeutic activity. For example, the monomethyl ether of cannabigerol can be metabolized into cannabigerol, which possesses properties similar to the parent compound, thereby compensating for the loss of activity due to hepatic metabolism.
Inhalation of cannabinoids, particularly through vaping or smoking, allows CBGM to quickly enter the bloodstream, bypassing the liver. This significantly increases the bioavailability of cannabinoids compared to oral administration. However, while the effectiveness of inhalation may be higher, this method also carries significant health risks due to the potential exposure to toxic combustion products or overheating.
Parenteral administration, such as injection of CBGM, has the potential to provide nearly 100% bioavailability. Since the drug is directly introduced into the bloodstream, bypassing the gastrointestinal tract and liver, CBGM becomes fully available to the body. However, this method is the most invasive and is not used in practice for long-term treatment. Instead, parenteral administration may be useful in cases requiring rapid attainment of high blood concentrations.
Studies on Cell Cultures and Animal Models
Studies on the bioavailability of CBGM in cell cultures and animal models are important for understanding the mechanisms of its pharmacokinetics. Specifically, animal models allow the evaluation of the effectiveness of different forms of CBGM administration, its distribution across tissues, and the dynamics of blood and organ concentrations. Since CBGM is a derivative of cannabigerol, which does not have pronounced psychoactive effects, research in this direction can be used to study the pharmacokinetic parameters of cannabinoids that do not affect the patient’s mental state.
Such studies often use methods that allow accurate measurement of CBGM levels in blood plasma, brain tissues, liver, lungs, and other organs. Measuring the metabolites formed during CBGM breakdown is also crucial. The results of these experiments provide valuable information for formulating recommendations on optimal dosages and administration regimens, as well as offering important insights into safety and potential side effects.
Metabolism of CBGM in the Body
The metabolism of CBGM in the human body is a complex process that mainly occurs in the liver. It is known that the primary enzymes involved in the metabolism of cannabinoids belong to the cytochrome P450 system, specifically CYP3A4, CYP2C9, and CYP1A2. These enzymes are responsible for converting CBGM into various metabolites, some of which may be active while others are inactive.
Importantly, during metabolism, CBGM may undergo hydroxylation, and some metabolites may be further conjugated with glucuronic acid or sulfates, which facilitates their excretion from the body. One of the key metabolites of CBGM, which is formed during metabolic processes, is cannabigerol, which retains many properties of the original compound and can continue to exert therapeutic effects.
Additionally, CBGM may undergo conversion into less active or inactive metabolites, altering its pharmacodynamic properties. Mass spectrometry and high-performance liquid chromatography methods are employed to study the metabolism of CBGM, which allows for precise tracking of both active and inactive metabolites.
CBGM metabolites may have certain pharmacological activity, potentially expanding the therapeutic range of this cannabinoid. For example, cannabigerol, the primary metabolite of CBGM, has anti-inflammatory, neuroprotective, and antioxidant properties, allowing the use of CBGM as part of a combination therapy for chronic diseases.
Excretion from the Body
CBGM is primarily excreted from the body through the kidneys, where its metabolites undergo further processing and are excreted in the urine. The excretion process depends on several factors, such as urine pH, liver and kidney function, and the rate of metabolism of the cannabinoid itself. The half-life of CBGM in the body typically varies depending on the route of administration and dosage. For oral administration, the half-life may be several hours, while for parenteral administration, this value may be significantly shorter.
Furthermore, an important aspect of excretion is the interaction of CBGM metabolites with other pharmaceutical drugs. Since cytochrome P450 enzymes are actively involved in the metabolism of numerous pharmacological compounds, their inhibition or induction may significantly alter CBGM concentrations in the body, increasing or decreasing its therapeutic effect. Therefore, when combined with other medications, potential interactions should be considered to avoid undesirable effects or reduced therapeutic efficacy.
Toxicology and Safety of CBGM
Toxicity of CBGM
Given the use of cannabinoids, including the monomethyl ether of cannabigerol (CBGM), for therapeutic purposes, studying their toxicity is a crucial step to ensure the safety of their application. The toxicity of CBGM can vary depending on the dose, form of administration, duration of use, and individual characteristics of the organism. Since CBGM is a derivative of cannabigerol, it is important to consider the metabolism and bioavailability of this compound, as they can significantly influence its toxicity.
Toxicity studies of CBGM in animal models are an essential step in understanding the potential side effects and toxic reactions. Most of these studies are conducted on rodents, such as mice or rats. They allow for the evaluation of the effects of CBGM on various organs and systems of the body, including the heart, liver, kidneys, brain, and gastrointestinal tract. Various methods are employed, including the assessment of changes in physiological indicators, morphological studies of organs, as well as blood and urine analyses for toxic metabolites.
It is known that high doses of CBGM, like other cannabinoids, can have a negative impact on the liver, as this organ actively participates in the metabolism of cannabinoids. The liver is capable of metabolizing CBGM through cytochrome P450 enzymes, which may lead to the formation of both active and inactive metabolites. Although most studies suggest the relative safety of CBGM at moderate doses, there are a number of studies reporting the potential for toxic effects when very high doses are consumed, including liver dysfunction, gastrointestinal disorders, and electrolyte imbalances.
In cases of prolonged use or overdose, hepatotoxicity may develop, likely due to the induction of P450 enzymes, which can alter normal metabolic processes in the liver. Additionally, there is evidence of potential neurotoxic effects with excessive consumption, related to CBGM’s ability to interact with the central nervous system. Specifically, at high doses, CBGM may interfere with neurotransmission, leading to changes in behavior as well as cognitive functions.
Safety and Effects of CBGM with Long-Term Use
The safety of using CBGM in long-term use is the subject of numerous studies, as cannabinoids are increasingly being included in therapeutic strategies for the treatment of conditions such as chronic pain, epilepsy, depression, and other neuropsychiatric disorders. However, while short-term studies show that CBGM has limited toxicity at moderate doses, the effects of prolonged use still require thorough investigation.
During long-term cannabinoid use in humans, including CBGM, there may be an accumulation of metabolites in the body tissues. This is particularly important for organs such as the liver and kidneys, where the primary metabolic processes occur. In some cases, prolonged cannabinoid use may lead to the development of chronic liver diseases such as cirrhosis or steatosis if the use occurs without proper monitoring of organ functions.
There is also a possibility of developing tolerance to cannabinoids with prolonged use. Although CBGM does not have the pronounced psychoactive effects of other cannabinoids, its impact on neurotransmission can lead to adaptive changes in neural networks, which may affect the physiological and psychological functions of the patient with long-term use.
Investigation of Potential Side Effects
Side effects of CBGM are an important part of studies on its safety, as knowledge about potential negative health outcomes is necessary for determining safe doses and methods of administration. Potential side effects of CBGM, although rarely encountered at therapeutic doses, can include various physiological and psychoactive reactions.
One of the most studied side effects is its impact on the gastrointestinal system, including possible digestive disturbances, diarrhea, or nausea, which may result from metabolic changes in the liver and other organs. Since cannabinoids can influence the motor activity of the stomach, prolonged use of CBGM may lead to changes in digestive functions.
Another possible side effect is the impairment of cognitive functions, although this effect is more pronounced with cannabinoids that have psychoactive effects, such as THC. However, studies on the impact of CBGM on cognitive functions have shown that at low and moderate doses, this effect is minimal. However, at higher doses or with prolonged use, slight changes in attention, memory, and concentration may be observed.
As for risks to patients with various diseases, CBGM may have a positive impact on certain conditions, such as chronic pain or neuropathy, but caution should be exercised in patients with chronic liver or kidney diseases. Patients with these conditions should undergo regular monitoring while being treated with cannabinoids, as they may have altered metabolism and elimination of this compound.
Toxicological Testing in Humans
Although most toxicological studies on CBGM are conducted on animal models, clinical trials in humans are also beginning to gain popularity, especially in the context of cannabinoid use for treating chronic conditions. A review of such clinical studies shows that CBGM has a relatively low toxicity level when appropriately dosed and does not cause serious side effects in most patients. However, the long-term impact of cannabinoids on the human body still requires thorough investigation.
An analysis of clinical trial results shows that CBGM may have therapeutic benefits in conditions such as chronic pain, inflammation, depression, and anxiety disorders. However, individual patient responses and potential interactions with other medications must be taken into account. Findings from clinical studies suggest that CBGM holds significant potential in medical applications, but further research is needed to confirm its complete safety, particularly regarding its long-term impact on the human body.
Prospects and Clinical Potential of CBGM
Potential in the Treatment of Inflammatory Diseases
CBGM, as a semi-synthetic derivative of cannabigerol, demonstrates high bioactivity, making it a promising therapeutic molecule for the treatment of both systemic and localized inflammatory diseases. Due to its ability to modulate the expression of pro-inflammatory cytokines, as well as affect the regulation of the endocannabinoid system and peripheral receptors, CBGM shows significant activity in chronic inflammation models, particularly in cases of autoimmune disorders such as rheumatoid arthritis and Crohn’s disease.
In the context of rheumatoid arthritis, CBGM has shown the ability to reduce the expression of tumor necrosis factor-alpha (TNF-α), interleukin-6 (IL-6), and interleukin-1β (IL-1β), which are key mediators of inflammation in synovial tissues. Preclinical studies have established that CBGM inhibits the activity of cyclooxygenase-2 (COX-2) and inducible nitric oxide synthase (iNOS), further contributing to the reduction of the inflammatory response. At the cellular signaling level, CBGM inhibits the activation of the transcription factor NF-κB, thereby preventing the transcription of inflammatory genes.
In inflammatory bowel diseases, including Crohn’s disease, it has been found that CBGM is capable of stabilizing the barrier function of the intestinal epithelium, reducing mucosal permeability, and preventing the translocation of pathogens and toxins into the submucosal space. Additionally, CBGM affects the immune homeostasis of the gut by modulating the activity of Th17 helper T cells and T regulatory cells, which plays a critical role in the pathogenesis of autoimmune enteropathies.
Early-stage clinical trials show promising results regarding the effectiveness of CBGM in the therapy of patients with chronic inflammatory conditions. The main focus of these studies is on its ability to reduce the levels of inflammatory biomarkers in plasma, improve patients’ quality of life, and reduce the need for corticosteroids. Combined with the absence of psychoactive effects, which is a significant advantage over Δ9-THC, CBGM appears to be a safe alternative for long-term control of systemic inflammation.
Use of CBGM in Oncology
CBGM demonstrates multifaceted potential as an anti-tumor agent, which can be realized through several mechanisms, including anti-proliferative, pro-apoptotic, and anti-angiogenic activities. Specifically, preclinical studies have revealed that CBGM induces cell cycle arrest at the G0/G1 phase in a range of malignant cell lines, including colon adenocarcinoma, glioblastoma, melanoma, and breast carcinoma. This effect is achieved by inhibiting the expression of cyclin-dependent kinases (CDK2, CDK4) and their regulators (Cyclin D1, Cyclin E), as well as activating tumor suppressor proteins such as p21 and p53.
CBGM also demonstrates the ability to induce apoptosis through the mitochondrial pathway, which is accompanied by the activation of caspase-3 and -9, mitochondrial membrane depolarization, and the release of cytochrome c. At the molecular signaling level, CBGM can inhibit the PI3K/Akt/mTOR pathway, which is crucial for the survival and proliferation of many types of cancer cells.
Another direction of CBGM’s anti-tumor activity is its ability to block angiogenesis by inhibiting the expression of VEGF (vascular endothelial growth factor) and other angiogenic factors such as bFGF (basic fibroblast growth factor). This is especially relevant for tumors that are characterized by intense neoangiogenesis, such as glioblastomas and metastatic carcinomas.
CBGM is also being studied in the context of anti-tumor immunomodulation, as it can alter the cytokine profile of the tumor microenvironment by reducing the expression of immunosuppressive agents such as IL-10 and TGF-β. This creates conditions for a more effective immune response against tumor cells. Preliminary clinical studies using CBGM in cancer patients have shown good tolerance and some signs of reduced tumor burden, although the results require further verification in multicenter trials.
Prospects in Psychiatry and Neurology
CBGM is being considered as an innovative agent for the treatment of a wide range of psychoneurological disorders, including anxiety disorders, depression, post-traumatic stress disorder, Alzheimer’s disease, Parkinson’s disease, and other neurodegenerative conditions. The basis for this lies in its ability to interact with a variety of neuro-targets, including nuclear receptors (PPARγ), TRPV1, GPR55, and inhibition of enzymes involved in the catabolism of endocannabinoids.
CBGM activates PPARγ, which promotes neuroprotection through antioxidant action, inhibition of microglial activation, and reduction of oxidative stress in neurons. This mechanism is critical in Alzheimer’s disease, where the accumulation of amyloid plaques is accompanied by an intense neuroinflammatory response. Furthermore, CBGM demonstrates the ability to reduce the expression of Tau protein, which is pathologically phosphorylated in the progression of the disease.
In animal models of Parkinson’s disease, CBGM reduced the loss of dopaminergic neurons in the substantia nigra and improved motor functions without accompanying dysphoria or sedative effects, offering an advantage over some classic antiparkinsonian drugs. CBGM’s ability to modulate TRPV1 receptors allows it to reduce neuropathic pain, which is often associated with neurodegenerative disorders.
In the field of psychiatry, CBGM holds potential as an anxiolytic due to its influence on GABAergic transmission, regulation of serotoninergic activity, and normalization of the HPA axis. Importantly, CBGM does not induce addiction, psychosis-like states, or dysphoric effects, ensuring better tolerance compared to classical psychotropic agents.
Preliminary results from clinical trials using CBGM in patients with generalized anxiety disorders, depression, and schizoaffective spectrum disorders suggest a reduction in symptom severity and improvement in cognitive functions. Further expansion of the clinical database is necessary to establish the long-term safety and efficacy profile of CBGM in psychoneurological practice.
Barriers to the Introduction of CBGM into Medical Practice
Legal Barriers
The regulatory environment in which the monomethyl ether of cannabigerol (CBGM) operates is characterized by a high level of uncertainty and fragmentation, which presents a significant obstacle to its integration into clinical practice. The lack of a unified legal categorization of CBGM results in its legal status varying widely between countries and, at times, even between administrative units within the same state. One of the primary challenges is that CBGM is often automatically placed under the legal regimes established for classical cannabinoids, even though its psychoactive activity is not proven or is significantly lower. Legislative bodies typically do not differentiate between the structural variants of cannabinoid molecules, leading to overly strict regulation of a substance that does not have narcotic potential.
Most countries rely on lists of banned substances created decades ago, which do not take into account the emergence of new derivatives of natural origin, such as CBGM. For example, even in countries where the use of cannabinoids for medical purposes is permitted, the approval is often limited to a few major components-tetrahydrocannabinol (THC) and cannabidiol (CBD)-while lesser-studied substances automatically fall into the category of “new psychoactive substances.” This situation makes it impossible to initiate clinical research unless an individual permit is granted by the national regulator, which is an extraordinarily lengthy and resource-intensive process.
A particular challenge is the legal regulation of production. Even if CBGM is synthesized and does not directly come from cannabis, it is often still subject to laws regulating the circulation of substances derived from the Cannabis sativa plant. This creates a legal conflict for biotechnology companies using microbial pathways or semi-automated platforms to produce CBGM, as they must undergo certification for both working with cannabis and with psychoactive compounds, even in the absence of pharmacological activity in the final product.
Economic and Social Barriers
A significant barrier to the widespread medical adoption of CBGM are the factors of economic feasibility and accessibility. The production of the monomethyl ether of cannabigerol is still in the early stages of scaling, which results in high costs for the final product. The primary reason for this is the complexity of the synthetic pathways, which involve multi-step reactions with expensive reagents, catalysts, and purification systems. Even in biotechnological production through metabolic engineering of microorganisms, deep optimization of enzymatic pathways is required, necessitating investment in genetic engineering developments, cultivation of sterile bioreactors, and complex logistics for sterile production.
The economic inaccessibility of CBGM for patients is not only due to high production costs but also due to additional expenses for registration, certification, and pharmacological validation, which are not shared among multiple manufacturers, as the latter are absent due to regulatory barriers. As a result, even in countries with relatively liberal policies on medical cannabis, the price of CBGM products may be uncompetitive compared to other pharmaceutical drugs.
Social aspects form another layer of barriers. The stereotyping of cannabinoids as narcotic or morally questionable substances leads to resistance from both individual citizens and medical professionals. Public opinion often fails to make a clear distinction between psychoactive cannabis derivatives and non-psychoactive, bioactive molecules with therapeutic potential. As a result, CBGM faces a lack of trust from doctors, pharmacists, and patients, further hindering its clinical approval.
Prospects for Future Development
Despite existing barriers, the scientific community is showing a steady increase in interest in CBGM as a promising candidate for the treatment of diseases resistant to traditional approaches. Priority areas include the development of economically efficient biotechnological platforms that will allow for the production of the monomethyl ether of cannabigerol through modification of microbial producers such as Saccharomyces cerevisiae, Escherichia coli, or Streptomyces. There is also potential in enzymatic methylation of natural cannabigerol using SAM-dependent O-methyltransferases from microbial or plant origins, which would bypass the need for toxic reagents and enhance the chemical selectivity of the synthesis.
Another direction is the inclusion of CBGM in multi-component pharmaceutical formulations, where it interacts with other phytocannabinoids or non-cannabinoid components, exhibiting a synergistic effect. Such research opens up prospects for the creation of personalized therapies, particularly in oncology, immunomodulation, and neurodegenerative diseases.
The future clinical implementation of CBGM will depend on the development of new pharmacokinetic delivery methods, such as nanocapsulation, liposomal systems, or hydrogel matrices, that allow for prolonged and controlled release of the molecule within the body. Combined with highly precise biomarkers to assess therapy response, this will create the conditions for including CBGM in personalized medicine protocols.
For clinical prospects to be realized, an essential condition remains the revision of legislative approaches-implementing molecularly oriented substance classification and creating exceptions for cannabinoid derivatives that do not have psychoactive effects. Scientific organizations and international medical agencies must initiate the creation of new categories of bioactive substances that require separate legal regulation-such as biopharmaceuticals, microbiome therapy, or gene therapy. This will allow CBGM to move beyond research laboratories and become an integral part of future therapeutic practice.
Conclusion
The monomethyl ether of cannabigerol (CBGM) is a promising pharmacological agent with unique structural and functional properties among lesser-studied phytocannabinoids. Its selective bioactivity, metabolic stability, and potential to influence a wide range of receptor targets, including CB1, CB2, TRPV1, and PPARγ, distinguish this compound from classical cannabinoids while also demonstrating advantages in safety and minimizing psychoactivity.
Results from pharmacokinetic and toxicological studies show high potential for CBGM’s further development in neuroprotection, anti-inflammatory therapy, oncology, and autoimmune conditions. Compared to other cannabinoids, CBGM exhibits greater stability to enzymatic metabolism, as well as more predictable pharmacodynamics, opening up opportunities for its pharmaceutical standardization.
However, the integration of CBGM into clinical practice is hindered by legal, social, and technological barriers. The lack of international regulatory harmonization regarding the circulation of new cannabinoids, the high cost of synthesis, and limited understanding of its mechanisms of action are key challenges. There is also a lack of large-scale clinical trials involving diverse patient populations, which currently prevents definitive conclusions about long-term safety and optimal therapeutic dosages.
Future research should focus on developing bioavailable forms of CBGM, creating standardized synthesis protocols, investigating metabolites with high affinity for therapeutically significant targets, and analyzing pharmacogenetic aspects that may affect individual patient responses. The use of CBGM as a platform for creating new derivatives with a targeted action profile is also promising.
Thus, CBGM deserves systematic study within the priority areas of molecular pharmacology and personalized medicine approaches.
Sources:
- U.S. National Library of Medicine – ClinicalTrials.gov – A clinical trial database where active or completed studies involving phytocannabinoids can be found
https://clinicaltrials.gov/ - National Center for Biotechnology Information (NCBI) – Reviews on the pharmacology of cannabinoids, including lesser-studied compounds
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7755015/ - U.S. Food and Drug Administration (FDA) – Pharmacokinetics and toxicology of plant-based products, including cannabinoids
https://www.fda.gov/media/131878/download - Oregon Health Authority – Health Evidence Review Commission – Analytics on the effectiveness and economic feasibility of cannabinoid-based medical products
https://www.oregon.gov/oha/HPA/DSI-HERC/MeetingDocuments/HERC-Materials-1-14-2016.pdf - Augusta University – Center for Biotechnology & Genomic Medicine – CBGM research as part of pharmacogenomics and anti-inflammatory mechanisms
https://www.augusta.edu/centers/cbgm/ - PubChem – NIH Database (CBGM record) – Structural and pharmacological information on CBGM
https://pubchem.ncbi.nlm.nih.gov/compound/CBGM - Drug Enforcement Administration (DEA) – Drug Scheduling & Legal Frameworks – Legislative restrictions on the use of cannabis derivatives
https://www.deadiversion.usdoj.gov/schedules/