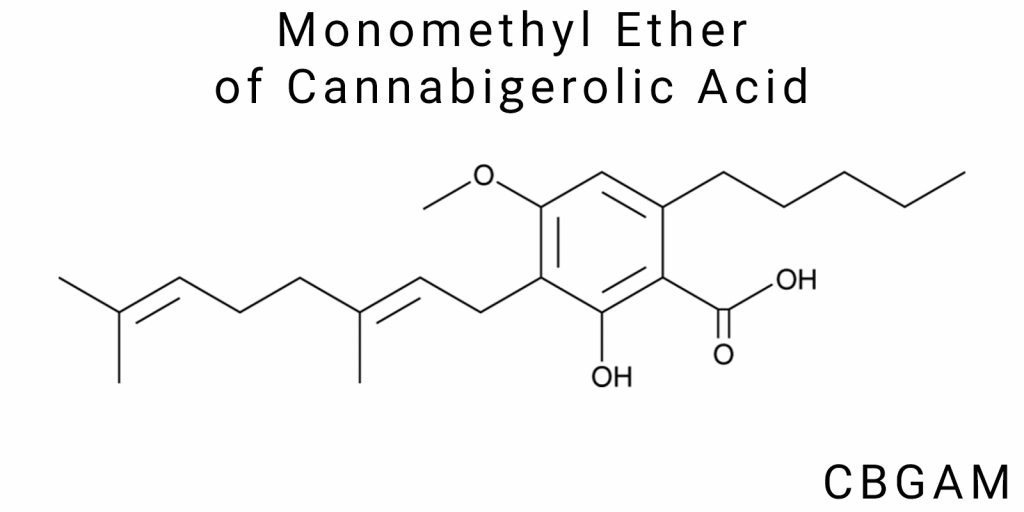
Monomethyl ether of cannabigerolic acid (CBGAM) is a novel and largely unexplored member of the class of acidic cannabinoids, arising through the specific methylation of cannabigerolic acid (CBGA). Despite its relatedness to well-characterized cannabis compounds such as Δ⁹-tetrahydrocannabinolic acid (THCA) or cannabidiolic acid (CBDA), CBGAM remains outside the scope of systematic pharmacological research. Its emergence in the scientific discourse is a relatively recent phenomenon, arising from deeper exploration of the cannabinoid profile, particularly in specialized Cannabis sativa strains developed using directed phytogenetic methods and through laboratory experiments involving methylation.
From a scientific perspective, the appearance of derivatives such as CBGAM represents a logical step in the development of research into metabolic pathways in cannabinoid biosynthesis. The chemical structure of CBGAM, containing both a carboxylic acid group and a methylated phenolic functionality, suggests potentially unique biophysical properties that may modify the interaction of the molecule with cellular membranes, receptor complexes, and transport proteins. This, in turn, provides grounds for hypothesizing distinct pharmacokinetic and pharmacodynamic properties compared to unmethylated forms. Of particular interest is the possibility of stereoselective effects related to the spatial arrangement of the methyl group, which may significantly influence affinity for receptors or enzymes.
In contrast to major cannabinoids, CBGAM has yet to be included in regulatory classification or large-scale studies on safety, toxicology, and bioavailability. This results in the absence of clinical standards or pharmacopoeial norms for this compound, leaving significant room for research initiative. On the other hand, this uncertainty presents a unique opportunity for discovery in pharmaceutical chemistry, especially in light of the molecule’s potential activity in the context of new pharmacological targets that are not limited exclusively to the cannabinoid system.
It is important to emphasize that CBGAM is not a product of simple storage or decarboxylation of traditional cannabis. Its presence may be attributed to specific metabolic environmental conditions, the influence of enzymatic pathways, or even artificial biochemical modifications carried out under laboratory conditions. This allows CBGAM to be considered not merely as a derivative molecule but as a potentially independent class of bioactive compounds capable of forming a separate pharmacological niche with unique therapeutic properties.
Chemical Nature of CBGAM
Unique Structural Characteristics of the Molecule
Monomethyl ether of cannabigerolic acid (CBGAM) belongs to the class of ethers formed through the methylation of phenolic hydroxyl groups of cannabigerolic acid (CBGA). This modification, while seemingly minor at first glance, leads to significant changes in the chemical properties of the compound. The primary feature of CBGAM is the presence of a methoxy group (–OCH₃), which replaces one of the hydroxyl groups in the aromatic ring. This radically affects the reactivity of the molecule, its affinity for specific biological targets, and its ability to interact with lipophilic environments. A distinctive feature of the CBGAM structure is the retention of the carboxyl group –COOH, which gives it its acidic nature, unlike the neutral metabolites of the cannabinoid series.
The three-dimensional spatially oriented structure of the molecule demonstrates a noticeable asymmetry between the hydrophilic and lipophilic domains, determining its specific behavior in the cellular microenvironment. It is known that methylation can reduce the number of potential hydrogen bonds, which alters the conformational possibilities of the molecule in biological conditions. Tests of isolated CBGAM samples show increased resistance to autoxidation at room temperature compared to CBGA, indicating improved chemical stability of the derivative form.
Etymology and Classification Among Cannabinoids
CBGAM stands for “cannabigerolic acid monomethyl ether,” directly indicating its origin from cannabigerolic acid and the nature of the methyl substitution. This ether represents a rare group of natural or semi-synthetic cannabinoid metabolites that contain only partial substitutions within the phenolic portion of the molecule. Such compounds are often not included in classical cannabinoid classification schemes, as most taxonomies are based on the structure of the carbon skeleton (C21, C22 types, etc.) and the degree of decarboxylation.
CBGAM occupies an intermediate position between first-order acidic cannabinoids, such as CBGA, and their neutral or fully decarboxylated derivatives. Chemically, it can be classified as a phyto-semi-synthetic ether or as a separate category of “partially modified phytoethers of cannabigerol.” This positioning is justified both by the method of its production and by the unusual presence of a single methoxy group without substitution of other active fragments in the molecule, which is not typical for natural cannabinoid profiles.
A particular challenge lies in classifying CBGAM in the pharmacological context, as in the absence of systematic clinical trials, it currently lacks a clearly established status in official pharmacopoeia reference books or regulatory systems (such as USP or EMA). However, based on its molecular structure and similarity to natural phyto-analogs, it may be classified as a small molecule in the class of non-flavonoid polyphenolic ethers, which has potential bioactive status.
Molecular Weight, Polarity, Functional Groups
CBGAM, as a monomethyl ether of cannabigerolic acid, is characterized by a complex polycondensed structure typical of acidic cannabinoid precursors. The molecular weight of CBGAM is determined as a derivative of the mass of CBGA, in which one of the hydroxyl groups is substituted by a methoxy group. For CBGA, the molecular weight is approximately 358.5 g/mol. Methylation of one of the phenolic hydroxyl groups adds 14.03 g/mol, resulting in an approximate molecular weight of CBGAM of 372.5 g/mol. This parameter influences a range of important pharmacokinetic characteristics, including solubility, ability to pass through biological membranes, and interaction with plasma proteins.
The polarity of CBGAM is lower compared to its parent compound CBGA due to the presence of the methoxy group, which is less hydrophilic than the free hydroxyl group. The reduction in the number of hydrogen bond donors leads to decreased water solubility and, simultaneously, a potential increase in permeability through lipid membranes. This property enhances the lipophilicity of the molecule, which may facilitate better absorption in tissues, especially upon enteral or transdermal administration. The polar surface area (TPSA) of CBGAM decreases by a few units, which affects its ability to cross the blood-brain barrier, determining its potential in neuropharmacology.
Structurally, CBGAM contains several functional groups that are important for its reactivity. These include the phenol residue (one hydroxyl group retained after methylation), the carboxyl group (-COOH), which is indicative of the acidic profile of the cannabinoid, an aliphatic chain with five double bonds (isoprenoid fragment), and the methoxy group (-OCH₃), which differentiates CBGAM from its parent form. The functional groups are responsible for active participation in redox, acylation, and receptor-dependent interactions. In particular, the free carboxyl group makes CBGAM a weak acid, capable of forming salts and esters, which may be useful for creating pharmaceutical derivatives with enhanced bioavailability.
CBGAM does not contain tertiary amine groups, chiral centers, or heterocyclic fragments, making it chemically more stable compared to many nitrogen-containing cannabinoids. At the same time, the conformational flexibility of the molecule is determined by the presence of aliphatic segments, which allow some changes in spatial orientation under conditions of enzymatic transformation. This creates opportunities for potential structure optimization through the synthesis of analogs with modified substitutions, without altering the fundamental skeleton of the molecule.
Biogenesis of CBGAM in Cannabis Plants
Theoretical Justification of Natural Origin
The question of the natural origin of the monomethyl ester of cannabigerolic acid (CBGM) belongs to one of the least studied, yet critically important areas of cannabinoid biochemistry. Unlike more thoroughly researched acidic forms of cannabinoids-such as THCA, CBDA, or CBGA-CBGM remained outside the scope of systematic biosynthetic studies for a long time. Its unique status lies in the fact that it combines two fundamental chemical traits: the carboxylic acid group, characteristic of primary cannabinoids in raw biomass material, and the methylated hydroxyl group, typical of specific secondary metabolites or derivatives that usually form at later stages of the biosynthetic cascade or as a result of catalytic factors.
In cannabis, as is known, biosynthetic pathways are organized as multifunctional cascades, where structural modifications of substances occur through the activity of highly specific enzymes localized in various compartments of the cell-particularly in trichomes, where the highest concentration of cannabinoids is observed. In the case of CBGAM, this is a non-trivial process that combines the functions of the classical acyltransferase cascade with the introduction of a methyl group onto the polyphenolic core. The very fact of the simultaneous presence of the carboxyl group and the etherified hydroxyl group makes this compound an exception among known acidic phytocannabinoids.
It is highly probable that methylation of CBGAA (cannabigerolic acid) within the phytosystem is an optional phenomenon, depending on ecological stress conditions, genetic variations of chemotypes, or the activity of transposons in the promoters of the responsible enzymes. In other words, the methylated ester of the acid is not the product of a classical universal biosynthetic pathway, but rather an adaptive result of the plant’s metabolic plasticity under external stress or epigenetic activation of poorly understood pathways.
There is also a possibility that CBGAM forms only in specific tissues, such as the glandular trichomes, which experience the highest concentration of phytochemical load and are subjected to specific oxidative pressure. This localization may explain not only the low detection levels of this cannabinoid in most samples but also its instability under standard extraction or storage conditions. It is also worth noting the time-dependent formation-most likely, CBGAM accumulates only within a narrow developmental window of the plant, for example, during the final stage of flowering, when enzymatic activity of O-methyltransferases is enhanced.
Interaction with Methyltransferases
In a biochemical context, the biosynthesis of CBGAM cannot be explained without the involvement of methyltransferases-enzymes that catalyze the transfer of a methyl group from a donor molecule (usually S-adenosylmethionine, SAM) to an electron-rich acceptor group. In cannabis, the presence of O-methyltransferases (OMT) is traditionally associated with flavonoid metabolism pathways; however, recent transcriptomic studies have identified the existence of structurally atypical transferases, the expression of which increases during the peak synthesis of acidic cannabinoids.
The assumption of OMT involvement in the methylation of cannabigerolic acid is supported by several critical aspects: first, the presence of an available hydroxyl group at the ortho- or para-position of the phenolic ring of CBGAA, which could serve as an electrophilic center for the methyl group attack; second, the absence of the need for prior decarboxylation, distinguishing this process from the metabolism characteristic of neutral methylated cannabinoid derivatives. This demonstrates the functional flexibility of the enzyme, capable of interacting with acidic substrates.
Another important element is the selectivity of methylation. The structure of cannabigerolic acid contains at least two potentially reactive hydroxyl groups, one of which is likely the target of enzymatic modification. In silico modeling data using enzyme banks have shown the possible conformational compatibility of CBGAA for the active site of OMT, similar to those found in plants of the genus Salvia and Erythroxylum. Such homologous analysis suggests a hypothetical functional cross-activity of enzymes that in different species have developed the ability to methylate compounds with a polyphenolic backbone, including cannabinoids.
Studies of enzyme activity in vitro using purified OMT from cannabis remain limited. However, initial results from cell lines transfected with the corresponding genes showed the emergence of new methylated derivatives of CBGA, which, according to mass spectrometric characteristics, correspond to possible isomers of CBGAM. This provides indirect evidence of the metabolic ability for natural methylation within the plant itself, without the need for external catalytic interventions. This biosynthetic pathway demonstrates a high degree of selectivity, likely governed not only by the substrate structure but also by the spatial organization of the cellular environment-such as the limited access to SAM in vacuoles or the apoplast.
Presence or Absence in the Natural Phytocomplex of Cannabis
The natural phytocomplex of cannabis is characterized by a tremendous variety of biologically active compounds, among which cannabinoids, terpenoid compounds, and phenolic metabolites play a particularly significant role. The monomethyl ester of cannabigerolic acid (CBGM), although detected in several studies, remains a rare component found in cannabis. This suggests that the synthesis of CBGAM under natural conditions is limited or triggered by specific metabolic conditions or enzymatic interactions.
The absence of CBGAM in large quantities in natural cannabis samples is confirmed by a number of analytical studies employing high-performance liquid chromatography (HPLC) with mass spectrometric detection. Specifically, analyses of cannabis leaf and flower samples show that mainly acidic cannabinoid precursors, such as cannabigerolic acid (CBGA), are detected, which may indicate that only early stages of cannabinoid synthesis are present in the natural environment. This points to the idea that methylation of CBGA to CBGAM may be hindered or not a primary biosynthetic route in the plant.
Studies of cannabinoid profiles using gas chromatography with mass spectrometric detection (GC-MS) also suggest that natural formation of CBGAM does not occur in large quantities. Only in certain cases, after the plant is exposed to specific environmental factors such as stress conditions (e.g., temperature change, drought, or chemical stresses), can traces of CBGAM be detected. However, even under these circumstances, the quantity of this compound remains minimal.
There is a hypothesis that the natural absence of significant levels of CBGAM in the cannabis phytocomplex may be related to the metabolic efficiency of the plant’s biosynthetic pathways. All known metabolic routes in cannabis are oriented towards the synthesis of major cannabinoids such as THC, CBD, and their acidic forms, which serve the plant as protective compounds against ultraviolet radiation and pests. It is possible that methylation of CBGA to CBGAM is a byproduct or an evolutionarily new process, not essential for cannabis in its natural environment.
However, some experimental studies confirm that the synthesis of CBGAM in cannabis can be possible under certain conditions. For example, under laboratory conditions, through genetic modifications or additional introduction of methyltransferases, the level of CBGAM can be significantly increased. Such results indicate that cannabis may have the potential to produce this compound under appropriate technical or biotechnological interventions, but this is not typical for the natural metabolism.
Analytical Methods for Determining CBGAM
Quantification Using High-Performance Liquid Chromatography (HPLC)
High-performance liquid chromatography (HPLC) is one of the primary analytical methods used for the determination and quantification of CBGAM in biological samples and cannabis extracts. Due to its high sensitivity, accuracy, and ability to separate various components in complex matrices, HPLC is the method of choice for studies requiring detailed information about cannabinoid content, including CBGAM.
The main advantage of HPLC is its ability to separate compounds with similar physicochemical properties, as well as provide rapid and precise concentration measurements in samples. A specialized column is used for CBGAM determination, which optimizes the separation processes, along with the application of appropriate detectors, such as ultraviolet (UV) detectors, which allow the detection of cannabinoids due to their specific UV absorption spectra.
During the quantification of CBGAM via HPLC, sample preparation is an important aspect, involving the extraction of cannabinoids from plant material, followed by the purification of the extract from matrix components that may interfere with the analysis. Organic solvents, such as methanol or acetonitrile, are typically used to extract cannabinoids from plant tissue. After extraction, the samples pass through the HPLC system, where separation of components occurs on a column, usually packed with silica gel. To determine the concentration of CBGAM, the obtained chromatographic data are compared with standards of known concentration.
This method provides highly accurate data on the content of CBGAM in samples, which is especially important for pharmaceutical research, where precise quantification of active compounds is required for further drug development.
Spectroscopic Approaches: NMR, IR Spectroscopy, MS
Other analytical methods, such as nuclear magnetic resonance (NMR), infrared spectroscopy (IR), and mass spectrometry (MS), are used for a more detailed analysis of the structure and molecular characteristics of CBGAM. Each of these methods has its advantages and is applied based on the specific requirements of the research.
Nuclear Magnetic Resonance (NMR)
NMR is a powerful tool for determining the structure of molecules at the atomic level. In the NMR process, the sample containing the cannabinoid is subjected to an external magnetic field, which induces resonance transitions in the nuclear spins of atoms within the molecule. NMR allows detailed information to be obtained about the chemical environment of atoms, their interactions, and the spatial orientation within the molecule.
For CBGAM, NMR allows for the precise identification of functional groups and atoms, including methyl groups that are added to cannabigerolic acid, forming the monomethyl ether. NMR also enables the determination of the molecule’s conformation and can reveal any changes that may occur during synthesis or sample handling. This method is important for studies where the confirmation of CBGAM’s structure and molecular integrity is required.
Infrared Spectroscopy (IR)
IR spectroscopy is used to identify molecular bonds and functional groups within a molecule. IR spectra reveal characteristic absorbances that occur when molecules interact with infrared radiation. Each functional group has its unique IR absorption spectrum, allowing the identification of groups such as methyl (–CH3) or carboxyl (–COOH) groups.
For CBGAM, IR spectroscopy helps identify the presence of methyl groups responsible for forming the monomethyl ether. This is especially useful for quickly verifying synthesized samples or monitoring methylation reactions, where confirming the presence of specific functional groups is essential.
Mass Spectrometry (MS)
Mass spectrometry is another powerful method for analyzing CBGAM, as it provides information about the mass and structure of the molecule, as well as its fragmentation during ionization. The mass spectrum provides data on the molecular mass of CBGAM and possible fragments of the molecule that arise during its breakdown. This method allows for the precise determination of the molecular mass of the compound, as well as its isotopic composition, which is useful for confirming the purity of the sample and detecting possible contaminants.
Mass spectrometry is particularly useful in combination with other methods such as chromatography. This enables precise spectra to be obtained for each individual fraction separated by HPLC, allowing for higher precision in quantification.
Problems with Specificity and Separation of Isomeric Compounds
One of the main challenges in analyzing CBGAM is the separation of isomeric compounds and its heightened sensitivity to experimental conditions. Since CBGAM is structurally similar to other cannabinoids, such as cannabigerol (CBG) and its acid forms (CBGA), the precise separation of these compounds is a complex task for analysts.
This is especially important when working with highly active substances, where even small amounts of other cannabinoids can significantly distort results. Technical problems may also arise due to cross-sensitivity of detectors, such as UV or MS, where peaks may overlap between different compounds.
Solutions to these problems include improving separation methods in HPLC by using specialized columns with high resolution, as well as employing combined techniques such as HPLC-MS or GC-MS to achieve maximum specificity when analyzing complex samples.
For effective separation of isomeric compounds, other technologies, such as elevated temperature or altering the concentration of the mobile phase, are also used to improve the separation of molecules with similar structures. However, even with these methods, achieving complete separation of these compounds remains a challenging task and requires careful adjustment of experimental conditions.
Pharmacological Potential of CBGAM
Monomethyl ether of cannabigerolic acid (CBGAM) is one of the promising compounds attracting significant attention from researchers due to its potential pharmacological properties. This compound can act on various receptors in the body, including cannabinoid receptors CB1 and CB2, as well as other molecular targets. Confirmation of CBGAM’s pharmacological potential could significantly alter the approach to treating a range of diseases requiring new therapeutic agents, particularly in oncology, neurology, and chronic inflammation.
Studying the mechanisms of action of CBGAM requires thorough investigation of its ability to interact with various receptors and other molecular targets, as well as evaluating important characteristics such as permeability across biological membranes and bioavailability. This will help clearly define the areas of application of CBGAM in clinical practice and predict possible side effects that may arise during its use.
Interaction with CB1, CB2 Receptors and Other Cannabinoid Targets
Like other cannabinoids, CBGAM interacts with cannabinoid receptors, particularly CB1 and CB2, which are the main molecular targets for cannabinoid therapy. CB1 receptors, found in the central nervous system, influence a wide range of functions, including the regulation of pain, mood, appetite, and cognitive functions. CB2 receptors are primarily located in the peripheral nervous system and immune cells, where they regulate inflammatory processes and modulate immune responses.
Studying the interaction of CBGAM with these receptors is key to assessing its potential in the treatment of various diseases. Molecular-level research shows that CBGAM can bind to both receptor types, although its affinity for CB1 and CB2 may vary depending on the specific experimental conditions. Compared to other cannabinoids, CBGAM shows a somewhat greater affinity for CB2 receptors, which may suggest its potential for treating inflammatory and autoimmune diseases.
In addition to its interaction with cannabinoid receptors, CBGAM may interact with other receptors and targets that play a role in regulating physiological processes. These include opioid receptors, serotonin receptors, and other molecules that may affect pain, anxiety, and other psychological states. Such multifunctionality of CBGAM opens prospects for its use in the treatment of disorders related to nervous and mental functions.
Specifically, it has been found that CBGAM may have analgesic activity that is not solely dependent on CB1 receptor activation, which could be beneficial for patients with nervous system disorders or chronic pain. Since CBGAM can act on both CB1 and CB2 receptors, this provides an opportunity for the development of drugs with a broader action spectrum, less pronounced psychoactive effects, and a lower likelihood of dependence development.
Study of Transmembrane Permeability and Bioavailability
Assessing the permeability of CBGAM across biological membranes and its bioavailability are important aspects in the context of the pharmacological development of this cannabinoid. Transmembrane permeability determines the ability of CBGAM molecules to pass through cell and tissue membranes, which is critical for ensuring that the drug reaches its target in the body. High permeability across biological barriers, such as the blood-brain barrier (BBB), could allow CBGAM to be effective in treating neurological disorders, such as Alzheimer’s disease or Parkinson’s disease.
In studying bioavailability, it is also important to consider how quickly and in what quantities CBGAM is absorbed in the body after administration. Bioavailability determines what portion of the administered dose reaches systemic circulation in its active form and can act on target receptors and sites. If CBGAM has low bioavailability, this could limit its effectiveness, as a large portion of the compound may not reach the desired tissues or organs.
In the case of high bioavailability, CBGAM may lead to the development of drugs with low doses, significantly reducing the likelihood of side effects. To improve bioavailability, various delivery technologies, such as nanoparticles or liposomes, may be used, which can improve the permeability of molecules across biological barriers. This is especially important for therapies where the drug needs to be delivered to specific tissues, such as the brain, for the treatment of neurological diseases.
Bioavailability is also influenced by the route of administration. Oral administration may reduce bioavailability due to metabolic effects in the liver, while inhalation or transdermal methods may provide better absorption and faster achievement of therapeutic levels in the body. Therefore, for the effective use of CBGAM in clinical practice, it is essential to conduct thorough research to select the optimal method of administration and dosing of the drug.
Another important aspect is the possibility of using CBGAM in combination therapies. Since CBGAM interacts with different receptors, it can be used in the treatment of diseases that require a multifaceted approach targeting several mechanisms, such as pain, inflammation, or nervous regulation. This significantly expands the pharmacological potential of CBGAM and increases its effectiveness in treating complex pathologies.
Biological Activity: Prospects of CBGAM
Monomethyl ether of cannabigerolic acid (CBGAM) demonstrates significant potential in various areas of biological activity, which could be useful for the development of new therapeutic agents. Due to its ability to interact with diverse receptors and molecular targets, as well as its influence on various physiological processes, CBGAM has attracted considerable attention from researchers in areas such as anti-inflammatory effects, neuroprotection, and cytotoxicity, particularly in oncological models.
Anti-inflammatory Properties at the Cellular Level
One of the main biological functions demonstrated by CBGAM is its ability to reduce inflammation. Inflammation is an important physiological process; however, it can become pathological when its activation is uncontrolled or persists for an extended period, leading to chronic diseases such as arthritis, atherosclerosis, asthma, and many others. It is well known that cannabinoids can exhibit significant anti-inflammatory activity due to their ability to affect immune cells and reduce the levels of inflammatory mediators such as cytokines and chemokines.
Like other cannabinoids, CBGAM is capable of interacting with CB2 receptors, which are key regulators of immune responses in peripheral tissues. CB2 receptors are found on the membranes of immune cells such as macrophages, microglia, and neutrophils, where they play a crucial role in regulating inflammatory processes. Activation of these receptors can reduce the production of pro-inflammatory cytokines (e.g., TNF-α, IL-1β, IL-6) and other molecules that contribute to the development of inflammation.
Additionally, CBGAM is capable of influencing intracellular signaling pathways, particularly the activation of NF-κB-a key transcription factor responsible for initiating inflammatory processes. Increased NF-κB activity is commonly associated with chronic inflammation, and reducing this activity may lead to significant relief from the symptoms of inflammatory diseases. These effects suggest that CBGAM could be used as a component in the development of new anti-inflammatory drugs.
An important aspect of CBGAM’s anti-inflammatory action is its ability to reduce oxidative stress in cells. Oxidative stress is one of the primary causes of cellular damage in chronic inflammation. CBGAM, as a potent antioxidant, is capable of neutralizing excessive amounts of reactive oxygen species (ROS) that arise during inflammatory processes, thereby reducing tissue damage.
A significant advantage of CBGAM is its ability to exert anti-inflammatory effects not only through cannabinoid receptors but also via other molecular targets. This opens up opportunities for the development of new combination drugs that may act on multiple mechanisms simultaneously, providing a broader range of action.
Potential Neuroprotective Effects
Since CBGAM interacts with receptors that activate neuroprotective mechanisms, this opens up new possibilities for treating neurodegenerative diseases such as Alzheimer’s disease, Parkinson’s disease, and other central nervous system disorders. Many studies have shown that cannabinoids can have a positive impact on the central nervous system, particularly by protecting neurons from oxidative stress, inflammatory processes, and toxic substances.
By activating CB1 and CB2 receptors, CBGAM may regulate neurotransmitters and neuroprotective molecules in the brain. The influence on CB1 receptors could be beneficial for alleviating symptoms related to neuropathy and pain, as these receptors are actively involved in regulating pain signals and behavioral responses. Meanwhile, activation of CB2 receptors may help reduce inflammation in the brain, which is important for treating diseases like Alzheimer’s, where chronic inflammation is one of the key mechanisms of pathogenesis.
These effects of CBGAM could have practical significance in neuroprotection, particularly in the context of treating neurodegenerative diseases where neuronal damage is irreversible and accompanied by progressive cell loss. Since CBGAM can reduce the level of neurodegeneration, it may have significant potential as a therapeutic agent capable of slowing or even halting disease progression.
It is also worth noting that CBGAM may assist in the recovery of neuronal functions, particularly through its action on glial cells, which play a supportive role in neuronal activity and the regulation of brain homeostasis. This provides new opportunities for developing drugs that could not only offer symptomatic treatment but also slow down the development of neurodegenerative processes.
Cytotoxicity Studies in Oncological Models
One of the most promising areas of research for CBGAM is its potential cytotoxicity in the treatment of oncological diseases. Since carcinogenesis is associated with disruptions in the cell cycle, metastasis, and apoptosis, cannabinoids such as CBGAM may affect these processes, opening up new possibilities for the creation of therapeutic agents against cancer.
Studies have shown that cannabinoids can induce apoptosis (programmed cell death) in cancer cells through the activation of CB2 receptors or through other mechanisms that do not rely on classical cannabinoid receptors. Research on the effects of CBGAM in various oncological models helps to determine whether this compound can induce cytotoxic effects in specific types of cancer cells, such as colorectal, breast, lung, and other types of cancer.
CBGAM has the ability to interact with numerous molecular mechanisms, such as the inhibition of protein kinases involved in regulating the cell cycle. This influence on cellular metabolism may lead to a reduction in tumor growth or its cessation. One of the key advantages of CBGAM is its ability to affect multiple molecular pathways, enabling the creation of a multifunctional therapeutic approach for fighting cancer.
The cytotoxicity of CBGAM in oncological models has shown promising results in experimental conditions, where the compound demonstrates the ability to induce apoptosis in tumor cells without significantly damaging healthy tissues. This makes CBGAM an attractive candidate for further clinical research, which could aid in the development of new types of anticancer drugs.
Therapeutic Application and Clinical Interest of CBGAM
The monomethyl ether of cannabigerol acid (CBGAM) is known for its significant therapeutic potential, which spans several fields of medicine. Due to its ability to influence numerous molecular targets and biological processes, CBGAM has attracted the attention of researchers as a possible candidate for treating a variety of diseases. Its effectiveness in treating chronic inflammation, neurodegenerative disorders, as well as its potential in pain management, epilepsy, and diabetes, opens new avenues for its use in medical practice. Since cannabinoids, including CBGAM, can affect various receptors and molecules in the body, their therapeutic potential remains actively investigated.
Hypothetical Use in the Treatment of Chronic Inflammation
Chronic inflammation is a major cause of many widespread diseases, such as cardiovascular diseases, type 2 diabetes, arthritis, and autoimmune disorders. In such cases, traditional anti-inflammatory drugs may be ineffective or cause unwanted side effects. As a result, scientists are turning to cannabinoids as possible alternative or adjunctive therapies. CBGAM holds particular promise in this regard due to its ability to reduce inflammation through interactions with cannabinoid receptors CB1 and CB2, as well as other molecular mechanisms.
Since CBGAM actively interacts with CB2 receptors located on immune cells, its use may help reduce the overactivation of the immune system, which occurs in chronic inflammation. This effect is particularly important in cases where conventional medications fail to provide the desired results. CBGAM may influence the reduction of pro-inflammatory cytokines (IL-1β, TNF-α) and chemokines, which promote the development and maintenance of chronic inflammation. Because of these properties, CBGAM shows potential in treating diseases such as osteoarthritis, rheumatoid arthritis, chronic bronchitis, and others.
Furthermore, there is evidence that CBGAM can reduce oxidative stress levels in cells. Oxidative stress plays a crucial role in the development of inflammation, as well as in tissue damage that occurs due to prolonged inflammatory processes. Therefore, using CBGAM as an antioxidant may not only reduce inflammation but also preserve tissue integrity, slowing the progression of inflammatory diseases.
In general, the therapeutic use of CBGAM in chronic inflammation is a promising direction, particularly with the possibility of combining it with other therapeutic methods, which may enhance the effectiveness of therapy and reduce the risk of side effects associated with traditional non-steroidal anti-inflammatory drugs.
Use in Neurodegenerative Disorders
Neurodegenerative diseases, such as Alzheimer’s disease, Parkinson’s disease, and other disorders associated with neuronal damage, are among the biggest challenges in modern medicine. The processes underlying these diseases include oxidative stress, neuropathic inflammation, and degeneration of nerve cells, which gradually leads to the loss of cognitive functions and motor activity. CBGAM has significant neuroprotective potential due to its ability to interact with cannabinoid receptors, especially CB2, which play a key role in regulating neuroinflammation.
One of the main mechanisms by which CBGAM may influence neurodegeneration is its ability to reduce chronic inflammation in the central nervous system. In diseases like Alzheimer’s, microglia-the immune cells of the brain-become activated, and their excessive activity can lead to neuronal damage. By activating CB2 receptors, CBGAM may reduce microglial activation and lower the levels of pro-inflammatory molecules, thereby decreasing the intensity of neuroinflammation.
CBGAM also has the ability to influence neurotransmitters, which may be disrupted in neurodegenerative disorders. For example, studies have investigated the ability of cannabinoids to affect dopamine levels, a neurotransmitter that is key to motor function and is specifically impaired in Parkinson’s disease. This opens new possibilities for treating such disorders, where restoring normal neuronal activity could improve motor functions.
CBGAM’s use in neurodegenerative diseases has the additional advantage of its potential to aid in neuronal regeneration. This can be achieved by supporting glial cells, which maintain normal neuronal function and contribute to tissue repair after damage.
Thus, CBGAM holds great therapeutic potential in neurodegenerative diseases, particularly in alleviating symptoms and slowing the progression of diseases such as Alzheimer’s and Parkinson’s.
Exploring the Potential in Epilepsy, Pain, and Diabetes
CBGAM is also a promising candidate for treating conditions such as epilepsy, chronic pain, and diabetes. Epilepsy is a neurological disorder characterized by recurrent seizures caused by abnormal electrical discharges in the brain. Research has shown that cannabinoids, including CBGAM, may have anticonvulsant properties, allowing them to be used as adjunctive treatments for epilepsy. This is due to CBGAM’s ability to interact with CB1 receptors, which regulate neuronal activity and reduce the likelihood of seizures.
Regarding chronic pain, CBGAM also demonstrates significant potential in pain reduction due to its ability to affect cannabinoid receptors as well as other molecular targets. Cannabinoids are known to alleviate pain through interactions with CB1 and CB2 receptors, which can be beneficial in treating pain associated with chronic diseases or injuries.
Additionally, CBGAM may be effective in treating diabetes, particularly type 2 diabetes, where inflammation and oxidative stress play a central role in disease progression. CBGAM may reduce inflammation and improve insulin sensitivity, offering potential for diabetes therapy and better glycemic control.
Biotechnological Strategies for CBGAM Production
The development of efficient methods for the production of cannabigerol acid monomethyl ester (CBGAM) is an important step toward the commercialization of this promising cannabinoid. CBGAM has significant therapeutic potential, and considering its complexity, the search for optimal biotechnological production pathways is crucial for its implementation in medical practice and the pharmaceutical industry. Modern approaches for the production of CBGAM include the use of genetically modified microorganisms, metabolic engineering of cannabis, and enzymatic biosynthesis. Each of these methods has its characteristics, advantages, and limitations, which must be considered when selecting the most suitable strategy for large-scale production.
Use of Genetically Modified Microorganisms
One of the most promising methods for the biosynthesis of CBGAM is the use of genetically modified microorganisms, such as bacteria and yeast. Genetic modification allows the creation of microorganisms capable of producing cannabinoids, including CBGAM, without the need to cultivate cannabis plants, which is a significantly less economically efficient and resource-intensive process.
Microorganisms, especially Escherichia coli and Saccharomyces cerevisiae, are often used for cannabinoid production through genetic engineering processes because they have well-understood molecular biology and capabilities for genome manipulation. Genetically modified microorganisms can be equipped with genes that encode key enzymes for cannabinoid synthesis, such as cannabigerol (CBG) synthases, as well as methyltransferases required for the production of methylated derivatives like CBGAM.
For the production of CBGAM in such microorganisms, genes encoding cannabigerol synthase and other enzymes responsible for the methylation of cannabigerolic acid can be integrated, providing the organism with the ability to synthesize CBGAM. Additionally, this approach allows optimization of production by altering the growth conditions of microorganisms, using different carbon and nitrogen sources, regulating temperature and pH, which ensures high yields of the final product.
The advantage of using genetically modified microorganisms is the significant economic benefit and convenience in production conditions. Microorganisms can grow rapidly in laboratory and industrial settings, ensuring a high rate of CBGAM production with minimal cultivation costs. However, there are certain challenges, such as the need to create specific conditions for stable gene expression and the control of concomitant metabolic processes, which may reduce production efficiency.
Metabolic Engineering of Cannabis for the Production of Methylated Derivatives
Metabolic engineering of cannabis is another important direction in CBGAM production. Cannabis is a natural source of many cannabinoids, including cannabigerol (CBG), but its ability to produce methylated derivatives of cannabinoids, such as CBGAM, requires certain genetic modifications. Metabolic engineering of cannabis involves the introduction of specific genes or the modification of existing metabolic pathways to enable the synthesis of CBGAM.
Several approaches to genetic modification of cannabis have been proposed to enable it to efficiently produce CBGAM. One approach involves integrating genes encoding enzymes that methylate cannabigerolic acid, as well as enzymes involved in CBG synthesis. By doing so, the cannabis metabolic network can be adjusted to produce methylated derivatives, including CBGAM, in greater quantities.
Another approach involves integrating genes that activate or enhance the activity of methyltransferases in cannabis. Methyltransferases are enzymes that transfer methyl groups to specific molecules, including cannabigerolic acid. By doing so, cannabis plants can be created that are capable of efficiently producing high-quality methylated cannabinoids, such as CBGAM. However, this process can be challenging due to the need for careful gene expression control to avoid disrupting other metabolic pathways that are also important for the overall health of the plant.
Metabolic engineering of cannabis for CBGAM production also has the potential for use in industrial production conditions. Cannabis has a high capacity for cannabinoid biosynthesis, allowing for the achievement of high levels of methylated derivatives even with relatively low cultivation costs. However, the use of cannabis as a source for the production of methylated cannabinoids requires addressing legal, regulatory, and economic issues related to the cultivation and processing of cannabis plants.
Enzymatic Biosynthesis Using SAM-Dependent Methyltransferases
Enzymatic biosynthesis is another promising method for CBGAM production. This method involves the use of enzymes, specifically SAM-dependent methyltransferases, to methylate cannabigerolic acid, resulting in the formation of CBGAM. SAM (S-adenosylmethionine) is the primary donor of methyl groups in the body, and its use as a substrate for methyltransferases enables the specific methylation of cannabigerolic acid molecules.
This approach has several important advantages. First, the enzymatic process is highly specific, allowing for the production of high-purity methylated derivatives such as CBGAM with minimal impurities. Second, enzymatic synthesis allows for the control of reaction conditions, which enables the optimization of methylated cannabinoid yields. Since enzymes generally have high catalytic activity, this method can be more efficient compared to traditional chemical synthesis methods.
For the enzymatic biosynthesis of CBGAM, it is necessary to have the appropriate methyltransferases, which can be obtained either by extraction from natural sources, such as cannabis plants, or by biotechnological production using genetically modified microorganisms. Since SAM-dependent methyltransferases have broad applications in the production of other bioactive molecules, this method can be expanded for industrial-scale CBGAM production.
Enzymatic biosynthesis offers significant advantages compared to traditional synthesis methods, as processes utilizing enzymes are more environmentally friendly and do not require toxic solvents or high temperatures, making them safer and more ecological.
Conclusion
Cannabigerol monomethyl ether (CBGAM) is one of the most promising molecules among cannabinoids due to its potential therapeutic properties and unique chemical nature. It demonstrates significant activity, particularly in areas such as anti-inflammatory effects, neuroprotection, and potential cytotoxicity in oncological models. Through the methylation of cannabigerolic acid, CBGAM exhibits specific properties that can be effectively utilized in the development of new pharmaceutical products.
The biosynthesis of CBGAM in cannabis plants, as well as its induction through genetically modified microorganisms and metabolic engineering, opens new avenues for its production. The application of enzymatic methods, particularly the use of SAM-dependent methyltransferases, significantly improves the efficiency and scalability of production. This will facilitate the industrial-scale production of CBGAM, which is crucial for its further clinical application.
Issues related to the separation of isomeric compounds and the specificity of analytical methods are important for ensuring the high purity of the product at all stages of its production. However, despite existing technical and legal barriers, the pharmaceutical potential of CBGAM continues to grow each year, especially with the advancement of biotechnology and genetic engineering approaches.
The potential use of CBGAM in treating various chronic diseases, neurodegenerative disorders, pain, epilepsy, and diabetes confirms its significant therapeutic potential. However, to fully unlock this potential, further clinical studies are needed to better understand the pharmacokinetics, safety, and efficacy of CBGAM in patient treatment.
Thus, CBGAM is a promising candidate for the development of new therapeutic agents, and its production, enhanced by the latest biotechnological strategies, will be a crucial step toward the commercialization of this molecule in the pharmaceutical industry.
Sources:
- National Institutes of Health (NIH) – PubMed Central (PMC)
Platform for searching peer-reviewed scientific articles and research. https://pubmed.ncbi.nlm.nih.gov/ - American Chemical Society (ACS Publications)
Authoritative source for chemistry and pharmaceutical articles. https://pubs.acs.org/ - Frontiers in Pharmacology
Publisher for scientific articles in pharmacology and biotechnology, including cannabinoid research. https://www.frontiersin.org/journals/pharmacology - Nature Reviews Drug Discovery
Peer-reviewed reviews on biopharmaceuticals and new drugs. https://www.nature.com/nrd/ - European Journal of Medicinal Chemistry
Reviews and research in medicinal chemistry, particularly on cannabinoids and their derivatives. https://www.journals.elsevier.com/european-journal-of-medicinal-chemistry - Cannabis and Cannabinoid Research
Journal dedicated to cannabis research and its derivatives, including CBGAM. https://www.liebertpub.com/can - The National Academies of Sciences, Engineering, and Medicine
Publisher of conclusions and research in medicine and pharmaceuticals. https://www.nap.edu/