The cannabinoid system, although a relatively new subject of targeted pharmacological analysis, has already demonstrated exceptional flexibility in the context of the structure-activity relationship (SAR) of its ligands. Among natural and synthetic cannabinoids, delta-9-tetrahydrocannabinol (Δ9-THC) remains the most well-known due to its psychoactive properties and high affinity for CB1 receptors. However, limitations in its clinical use-caused by its psychotropic effects, unpredictable pharmacokinetics, and ethical/legal barriers-have driven the scientific community to search for modified derivatives with alternative or selective biological profiles. It is in this context that trihydroxy-delta-9-tetrahydrocannabinol (triOH-THC) attracts attention as a potentially important component in the chemical evolution of cannabinoids.
TriOH-THC is a poorly studied oxygenated metabolite of Δ9-THC formed as a result of hydroxylation of the cannabinoid core. Its presence was first reported in the scientific literature within the metabolic profile arising from the intensive transformation of Δ9-THC in the liver. Most studies have focused only on 11-hydroxy-Δ9-THC (11-OH-THC)-the primary active metabolite with enhanced psychoactivity. Meanwhile, triOH-THC, which contains three hydroxyl groups in different positions of the terpene skeleton, remains on the periphery of pharmacological interest despite its potential bioactivity and chemical uniqueness.
Mechanistically, hydroxylation of Δ9-THC mainly occurs through enzymes of the cytochrome P450 family (particularly CYP2C9 and CYP3A4), which perform regio- and stereoselective oxidation. In the classical metabolic scenario, the first step is the conversion of Δ9-THC to 11-OH-THC, followed by transformation to 11-nor-9-carboxy-THC (THC-COOH), which is pharmacologically inactive. However, under certain conditions or in the presence of oxidase inducers, additional hydroxylated derivatives-including triOH-THC-may form. Likely pathways include hydroxylation at positions 8α, 8β, 10α, or the 1′-methyl group-resulting in a trifunctional compound with increased polarity and altered receptor binding.
From a chemical engineering perspective, triOH-THC opens a new vector in structural modification of cannabinoids. Trihydroxylated analogs typically exhibit reduced psychoactive potential but show modified receptor selectivity, enhanced binding to CB2, or the ability to interact with non-canonical targets such as PPARγ, TRPV1, or GPR55. This potentially makes triOH-THC a promising candidate for treating inflammation, neurodegeneration, or pain without eliciting the psychotropic effects typical of Δ9-THC.
It is important to note that the presence of three hydroxyl groups significantly influences the physicochemical properties of the compound. TriOH-THC has reduced lipophilicity compared to the parent molecule, which may alter its ability to cross the blood-brain barrier but simultaneously increase its bioavailability upon parenteral administration or its effects on peripheral tissues. Furthermore, these hydroxyl groups create potential for additional hydrogen bonding, which is important in protein-receptor interactions and opens prospects for creating selective ligands or drug prototypes.
Despite this, the availability of triOH-THC in pure form remains a significant experimental challenge. Synthetic production requires highly precise regioselective hydroxylation in the presence of the terpene backbone, which is chemically unstable toward oxidation. Research groups propose the use of biocatalysts or in vitro enzymatic synthesis, which offer more controlled productivity but require complex expression of the corresponding enzymes and optimization of environmental conditions. An alternative route is modifying natural Δ9-THC from cannabis plants in the presence of activated microbial cultures or enzymes, followed by chromatographic purification of the product.
Currently, trihydroxylated forms of Δ9-THC are not commercially available and are not included in pharmacopoeial references. Their presence in the body is considered transient and limited, further complicating their study. The lack of analytical standards for triOH-THC leads to its underestimation in pharmacokinetic studies, especially when examining plasma metabolites in cannabis users. This raises questions about the potential role of triOH-THC as a hidden or “invisible” participant in cannabinoid pharmacodynamics.
In some experimental toxicology and metabolomics studies, fragments identical to hydroxylated Δ9-THC derivatives corresponding to the molecular mass of triOH-THC have been detected. This indicates its involvement in biotransformation, although further data remain incomplete. Some indirect evidence suggests triOH-THC may play a role in forming secondary effects after chronic cannabis use, possibly acting as an antagonist or modulator of other metabolites’ effects.
In the focus of modern cannabinoid chemistry, trihydroxylated derivatives represent a fundamentally new class of molecular structures with potentially selective pharmacological characteristics. Unlike fully synthetic cannabinoids, which often demonstrate excessive activity or toxicity, triOH-THC is a product of natural or semi-natural biosynthesis and can be used as a template for further ligand optimization. It is expected that in the future, triOH-THC or its analogs will become subjects of research aimed at developing non-psychoactive anti-inflammatory, analgesic, or neuroprotective agents.
Chemical Identity of triOH-THC
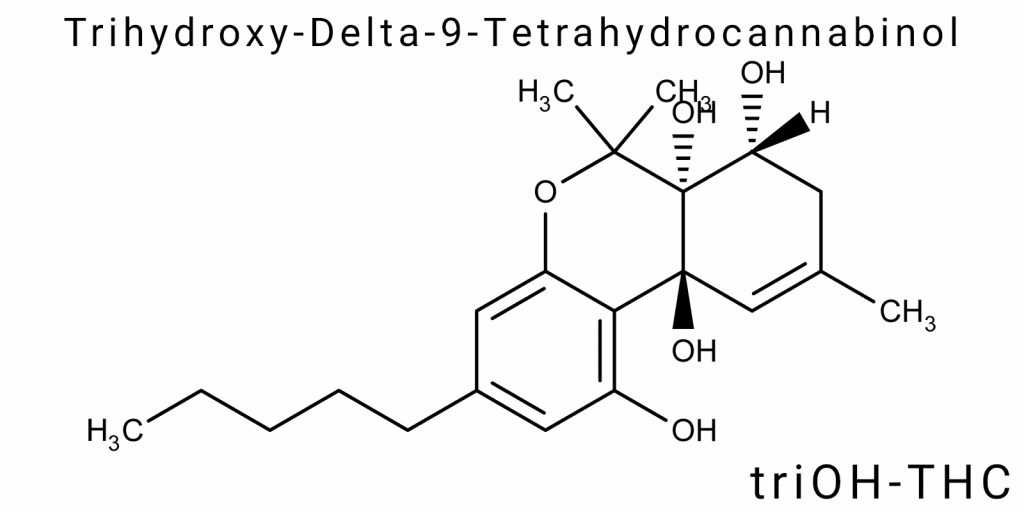
Trihydroxy-delta-9-tetrahydrocannabinol (triOH-THC) is a derivative of Δ9-tetrahydrocannabinol featuring three hydroxyl groups introduced into the molecule as secondary or tertiary alcohols. This modification significantly alters both the electronic structure of the molecule and its reactivity, creating a new class of polar cannabinoids that differ from both classical phytocannabinoids (Δ9-THC, Δ8-THC) and oxidized metabolites (11-OH-THC, THC-COOH). Unlike other Δ9-THC metabolites, triOH-THC is not monofunctional but represents an example of a polyfunctionalized molecule with potentially altered bioactivity, specific electronic density topology, and a changed pattern of interaction with protein targets.
The core cannabinoid structure of Δ9-THC consists of three key fragments: a trisubstituted benzene ring (resorcinol moiety), a terpene cycle (cis-hexahydro-6H-dibenz[b,d]pyran), and a side alkyl chain at position 3. The hydroxyl group at position 1 on the benzene ring is a common feature of all classical cannabinoids and provides the possibility of hydrogen bonding with CB1/CB2 receptors. In the case of triOH-THC, two additional hydroxyl groups are introduced, which can be localized in several positions: most frequently at positions 8 (on the terpene ring), 11 (on the methylene group near the double bond), or on the side chain. Depending on the position of hydroxylation, several structural isomers of triOH-THC are possible, each with distinct spatial configuration, degrees of conformational flexibility, and electronic density in key receptor interaction sites.
The most likely candidates for hydroxylation positions include:
- 11 – the classical pathway of Δ9-THC hydroxylation in the liver mediated by CYP2C9/CYP3A4.
- 8β or 8α – common sites of cannabinoid metabolic transformation in humans and other species.
- 10α – a less stable but documented hydroxylation site in several in vitro experiments.
- 1′-methyl group on the side chain – particularly involving microsomal enzymes.
Thus, triOH-THC is not a single molecule but a structurally heterogeneous group of derivatives sharing the same formula but exhibiting different properties. For example, 1-hydroxy-8α,11-dihydroxy-THC and 1-hydroxy-8β,11-dihydroxy-THC are stereoisomers that may differ in receptor affinity by orders of magnitude. Their isolation and characterization require methods such as chiral chromatography or nuclear magnetic resonance with 2D-COSY and NOESY techniques, which have so far been scarcely applied to triOH-THC.
Changes in the degree of hydroxylation sharply affect the compound’s acid-base properties. Additional hydroxyl groups lower the pKa of the benzene hydroxyl group due to an electron-withdrawing effect, which may alter the molecule’s ionization under physiological conditions. This is critical for predicting passive diffusion across cell membranes. While Δ9-THC shows a high partition coefficient (logP ≈ 6.4), triOH-THC has a significantly lower logP (preliminary modeling suggests values in the range of 2.8-3.5), indicating reduced lipophilicity and potentially altered pharmacokinetics.
The chemical stability of triOH-THC is also considerably lower. Trihydroxylated cannabinoid derivatives are more prone to autocatalytic oxidation and degradation, especially upon exposure to oxygen, light, or metal ions. This limits their long-term storage and complicates working with analytical samples. Under normal storage in solvents such as methanol or ethanol, triOH-THC may undergo etherification or dehydration, further complicating precise identification by mass spectrometry.
Another key aspect is the polarity of triOH-THC in the context of water solubility. Δ9-THC is practically insoluble in water (≈2.8 µg/mL), which significantly complicates its pharmaceutical formulation. In the case of triOH-THC, hydrophilicity increases, although the hydrocarbon residue remains substantial. This provides a basis for possible inclusion in formulations such as microemulsions, liposomes, or polymeric nanoparticles-technologies that allow optimization of pharmacological delivery.
Regarding analytical identification of triOH-THC, none of the existing standard methods (high-performance liquid chromatography with UV detection, LC-MS/MS, gas chromatography) are sufficiently validated for this class of compounds. Moreover, toxicological analyses of blood or urine typically lack analytical standards for triOH-THC, leading to its failure to be recognized. Even with high-resolution mass spectrometry (HR-MS), triOH-THC molecules may remain undetected due to instability during ionization or signal overlap with other metabolites.
Molecular Structure
The molecular structure of trihydroxy-delta-9-tetrahydrocannabinol (triOH-THC) is an example of a functionally complex derivative of classical Δ⁹-tetrahydrocannabinol, modified by the addition of three hydroxyl groups whose placement significantly alters the geometric, electronic, and interatomic characteristics of the molecule. Importantly, unlike simple metabolites or semi-synthetic derivatives, triOH-THC does not have a fixed configuration – it exists as an ensemble of isomers, each potentially possessing a unique molecular architecture. This complexity complicates its crystallographic mapping and modeling, requiring a comprehensive quantum-mechanical approach.
The core scaffold of triOH-THC is based on a tricyclic structure – tetrahydro-6H-dibenz[b,d]pyran – to which three hydroxyl functions are attached, typically at positions C1 (on the aromatic ring), C8 (on the saturated ring), and C11 (in the allylic position). Introduction of hydroxyl groups at saturated positions leads to a transition of carbon atoms from sp³ to partially sp²/planar hybridization states, locally disrupting the symmetry of the tetrahydrocannabinoid scaffold.
From a geometric perspective, the hydroxylation at position C11, where a primary allylic alcohol usually forms, is key: a new chiral center emerges that is subject to tautomeric instability and can convert into a carbonyl form through oxidation. The hydroxyl at C8 (especially in the β-configuration) creates a sterically strained region where deformation occurs in the C6-C7-C8-C9 ring, leading to a reduction in the internal dihedral angle and potential formation of intramolecular hydrogen bonds between the OH groups and the oxygen of the aromatic ring.
The side alkyl chain attached at position C3 plays a critical role in forming the lipophilic core of the molecule. In classical Δ⁹-THC, this is a butyl or pentyl chain, but in conversion to triOH-THC, partial oxidation or hydroxylation may occur at the ω or ω-1 positions, transforming the flexible lipophilic part into a partially polar moiety. This limits rotation around C-C bonds and reduces the molecule’s ability to adapt its conformation when interacting with proteins. This localized decrease in conformational entropy leads to a more defined fixation of the molecule within receptor cavities, potentially generating new points for hydrogen bonding or dipole-dipole interactions.
Evaluation of the molecular topology using DFT methods (B3LYP/6-31G** theory level) shows that electron density significantly increases at the hydroxylated sites, especially when all three OH groups are oriented spatially in the same molecular hemisphere. This creates a localized hydrophilic pole that drastically alters the molecule’s dipole moment (μ) – from approximately 1.7-2.0 D for Δ⁹-THC to over 4.5-5.1 D for triOH-THC, depending on conformation. Such asymmetry of the electrostatic field can result in a completely different receptor affinity profile – favoring interactions not with the hydrophobic regions of CB1/CB2, but with hydrophilic glycan structures of transport or plasma proteins.
In terms of three-dimensional structure, it is important to note that introducing three hydroxyl groups increases the Van der Waals volume of the molecule by at least 8-11%. On one hand, this enhances the density of intermolecular contacts during ligand-receptor interaction, but on the other hand, it reduces membrane permeability due to the increased molecular volume (≈590-610 ų versus ≈510 ų in Δ⁹-THC). Structural calculations via electrostatic potential surface analysis (MEP) reveal strong electronic overload in the aromatic ring region with the hydroxyl at position C1, particularly when an intramolecular cyclic hydrogen bond forms with the OH at C8.
Importantly, triOH-THC exhibits significant conformational restriction between the terpene and benzene rings. Whereas Δ⁹-THC allows almost free rotation between these fragments, the additional OH groups establish local hydrogen bonding networks that stabilize one or two primary conformations. This so-called “ring fixation” effect reduces the entropy of the ligand’s free state and influences binding to protein sites by decreasing the energetic cost of fixation during complex formation.
It is also worth noting that, as a result of triple hydroxylation, triOH-THC has the potential to form chelate complexes with divalent and trivalent cations such as Mg²⁺, Zn²⁺, or Fe³⁺. This property is absent in regular Δ⁹-THC and creates conditions for a completely different type of molecular interaction – in particular, selective accumulation in tissues with high metal ion concentrations or effects on metalloenzymes, for example, phosphatases or oxidoreductases.
Additionally, hydroxyl groups of triOH-THC may undergo phosphorylation in cellular environments, converting the molecule into a triphosphate ether – potentially capable of participating in signaling pathways or interacting with membrane kinases. This mechanism of pharmacological modulation for cannabinoids has not yet been described, but the presence of three OH groups creates a realistic basis for analogy with phenolic catecholamines such as norepinephrine.
From the perspective of covalent chemistry, the arrangement of the three hydroxyl groups in triOH-THC opens new reactive pathways: selective acylation, formation of esters, sulfates, or carbonates. In particular, the presence of two neighboring OH groups (at positions 1 and 8) may lead to the formation of cyclic six- or seven-membered esters, which are reactive intermediates – with clear spectral signatures in ¹H NMR (δ 4.2-5.1 ppm) and characteristic IR signals (νC-O 1250-1280 cm⁻¹). Such modified structures may possess entirely different pharmacodynamic properties, including prolonged half-life or altered metabolic fate.
Stereochemistry and Isomers
The stereochemistry of tri-hydroxy-delta-9-tetrahydrocannabinol (triOH-THC) is a critical aspect for understanding its biochemical behavior, as the three hydroxyl substitutions create multiple chiral centers, resulting in the theoretical existence of several dozen stereoisomers. The key variations arise not only from the presence of classical sp³ chiral carbon atoms but also from axial chirality associated with restricted rotation around the inter-ring σ-bonds, caused by the formation of hydrogen bonds between the OH groups.
The main scaffold of triOH-THC consists of a tricyclic framework with several possible chiral centers. In most natural and synthetic variants of Δ⁹-THC, the chiral centers are localized at positions C6a, C10a, and C8. By introducing hydroxyl groups at positions C1, C8, and C11, at least two additional chiral centers are added-especially when hydroxylating saturated positions-expanding the number of enantiomers and diastereomers to more than 16 theoretical forms even without accounting for tautomerism.
The hydroxyl at position C8 can be oriented in either the β- or α-configuration, spatially changing the angle between rings B and C. In the β-form, a local “chair-boat” conformation forms, in which C8-OH is held in a pseudoaxial position, promoting the formation of a stable intramolecular hydrogen bond with the oxygen of C1-OH. In the case of α-orientation, the hydroxyl shifts to an equatorial position, weakening these intergroup interactions but favoring contacts with water molecules or ions in the surrounding environment.
Chirality at position C11 arises only when a hydroxyl group is present. If a secondary or primary alcohol forms here, an asymmetric center is created, which can exist in two enantiomorphic forms. In vivo, dynamic interconversion between these forms is possible unless fixation occurs within an enzymatic or protein complex. Upon oxidation to an aldehyde or ketone, chirality is lost, but a planar sp²-hybridized center emerges, forming a π-system with electron delocalization in the side chain-this is important for interaction with aromatic residues of target proteins.
Particular attention should be given to position C6a, where stereochemistry plays a fundamental role in defining the spatial profile of the entire molecular framework. In classical Δ⁹-THC, the predominant configuration is 6aR,10aR. Upon hydroxylation, conformational stability changes due to an imbalance in rotational energetics and torsional strain. In triOH-THC, some diastereomers with altered C6a configurational signs may acquire greater thermodynamic stability because of hydrogen bond networks that block rotation between the rings.
It is necessary to distinguish among three types of isomerism relevant to triOH-THC: enantiomerism, diastereomerism, and conformational isomerism. Enantiomers-mirror images of each other-cannot convert into one another without breaking covalent bonds. For triOH-THC, their pharmacological effects may be radically different: one enantiomer may have affinity for the CB2 receptor, while another may interact primarily with transport proteins or exhibit no receptor activity at all. Diastereomers, on the other hand, possess different physical properties-melting point, solubility, optical rotation-and may differ in their conformational interaction profiles with membrane proteins.
A special class of stereoisomerism-axial chirality-arises in triOH-THC molecules due to partial restriction of rotation around σ-bonds, particularly between the benzene ring and the cycloalkyl core. While in Δ⁹-THC these rotations are free, the presence of hydroxyl groups at positions capable of forming inter-ring hydrogen bonds stabilizes specific torsional angles. This “freezing” of rotation creates a chiral axis that is not associated with classical asymmetric centers but nonetheless results in stable atropisomers. These forms are mutually non-interconvertible under physiological temperatures and may have distinct biological activity profiles.
Tautomeric forms also deserve mention. While not stereoisomers in the strict sense, they cause dynamic variability in electron density and dipole distribution within the molecule. Specifically, C11-OH can equilibrate with C11=O, creating a chiral center only in one form. This establishes a chemo-dynamically labile system where reactivity and the spectrum of intermolecular interactions depend on the current chemical microenvironment. In aqueous media, the hydroxyl form predominates, whereas in lipid membranes, a shift toward the enol or keto structure is observed.
Another aspect is epimerism, which involves isomers differing in configuration at only one chiral center. For triOH-THC, epimers at position C8, for example, may have opposing physicochemical properties-such as differing metabolic rates due to varying recognition by enzymes (CYP450, UGTs). From a medicinal chemistry perspective, this opens opportunities for synthesizing controlled epimeric forms with prolonged or attenuated effects.
All these types of isomerism significantly influence the pharmacokinetics, pharmacodynamics, and metabolism of triOH-THC. For example, different diastereomers may vary in their ability to cross the blood-brain barrier and show distinct excretion profiles, favoring hepatic or renal elimination pathways. It is also known that some THC stereoisomers are metabolized into the highly bioactive 11-OH form; in triOH-THC, this metabolic pathway is considerably complicated or even blocked due to the pre-existing hydroxyl group at that position.
Origin and Biosynthesis
Trihydroxy-delta-9-tetrahydrocannabinol (triOH-THC) is not a natural cannabinoid in the classical sense of the term, meaning it has not been detected in significant amounts in the native biochemistry of Cannabis sativa. However, it belongs to the class of oxidatively modified metabolites of Δ⁹-tetrahydrocannabinol (Δ⁹-THC), which are formed either through biotransformation in the human body (as tertiary metabolites) or artificially-via chemical or enzymatic hydroxylation at strategically defined positions of the molecule. For this reason, its origin is dual and deserves separate analysis within the context of modern cannabinoid chemistry.
From a biosynthetic perspective, triOH-THC is a derivative of the secondary metabolism of Δ⁹-THC, in which sequential hydroxylation of the primary cannabinoid occurs. In nature, hydroxyl modifications are typically the result of enzymatic activity of oxidoreductases or monooxygenases (notably enzymes from the CYP450 family), which perform metabolic detoxification of lipophilic xenobiotics. In the context of THC, this system is responsible for producing 11-hydroxy-THC (11-OH-THC)-the most bioactive metabolite-and its further polarized derivatives.
TriOH-THC forms as a result of further metabolic oxidation of THC or 11-OH-THC with the addition of extra hydroxyl groups. The probable sites for such hydroxylation are C1, C8, and C11, considering their accessibility to enzymatic attack and relative electrophilicity. It has been established that at high doses of THC or when the normal metabolic profile is impaired (for example, in the case of genetic variants of CYP2C9), derivatives with multiple hydroxyl groups can form, which differ significantly from the parent molecule both in pharmacokinetic properties and toxicological profile.
In the living organism, this process is phased. In Phase I metabolism, THC undergoes oxidation, hydroxylation, and dealkylation, forming intermediate metabolites. In Phase II, these metabolites conjugate with glucuronic acid or sulfates, increasing their hydrophilicity and facilitating excretion. The formation of triOH-THC belongs to a deeper stage of Phase I, where the molecule already possesses multiple functional groups, making it unstable and less active at the receptor level but potentially important as a signaling or immunomodulatory agent.
However, information about the natural occurrence of triOH-THC is limited. Its rare presence in biological fluids (such as plasma or urine) can only be detected using highly specific analytical methods-particularly liquid chromatography coupled with tandem mass spectrometry (LC-MS/MS) utilizing multiple reconstructed ion transitions-since standard screening techniques often fail to recognize this structure due to its low stability and concentration.
Despite this, triOH-THC attracts attention as a chemical artifact that may form in samples subjected to partial oxidation during storage or processing. For example, prolonged storage of THC in the presence of light, oxygen, or elevated temperature can lead to gradual transformation into hydroxylated products. Under such conditions, autocatalytic hydroxylation is possible, where radical-type reactions facilitate the formation of triOH-THC or related derivatives.
From an inorganic chemistry standpoint, the synthesis of triOH-THC is possible using controlled hydroxylation of the parent Δ⁹-THC molecule or its simple analogs. Electrophilic hydroxylation reagents such as OsO₄, KMnO₄, or RuO₄ are most commonly used in buffered systems that preserve the integrity of the aromatic system. For targeted addition of groups-such as at the C8 position-metal complex catalysts or enzyme mimetics (synthetic enzyme analogs) are employed to ensure high regioselectivity of the reaction.
In a biotechnological variant, triOH-THC synthesis is carried out using specific enzymes-particularly mutant variants of P450 BM3 or CYP3A4 with modified active sites. Such systems can provide selective hydroxylation at difficult-to-access positions by altering the local electrostatic map of the molecule. The result is controlled production of hydroxylated THC derivatives with minimal formation of side products.
The possibility of biosynthetic production of triOH-THC in model organisms is also being explored-for instance, in genetically modified strains of Saccharomyces cerevisiae or Escherichia coli expressing the full set of enzymes required for cannabinoid synthesis. These enzymes include olivetol synthase, geranyltransferase, CBGA synthase, and specific P450 oxidases. By adding additional enzymes for sequential hydroxylation, it is possible to program the production of triOH-THC in vitro without relying on natural cannabis.
There is particular interest in the formation of triOH-THC as a result of interactions with reactive metabolites of other substances. For example, it is known that certain medications (such as antiepileptic drugs or immunomodulators) affect the activity of P450 enzymes, altering the metabolic profile of THC. Under certain conditions (for example, when taking CBD together with THC), induction of additional enzymatic pathways may occur, leading to the formation of over-hydroxylated forms, including triOH-THC.
Metabolic Pathway in the Body
The metabolic fate of trihydroxy-delta-9-tetrahydrocannabinol (triOH-THC) in the body is associated with advanced stages of oxidative catabolism of Δ⁹-tetrahydrocannabinol, involving highly specific enzymatic systems focused on generating polar, functionalized metabolites with increased water solubility. These processes are not primary biotransformation reactions but arise within the context of expanded enzymatic activity of the cytochrome P450 system, predominantly in liver tissue but also in peripheral microsomal fractions.
After oral or inhalational administration, Δ⁹-THC is rapidly absorbed through the gastrointestinal mucosa or pulmonary epithelium, reaching the liver via the portal vein (in the case of enteral delivery) or directly entering systemic circulation (in the case of inhalation). In the liver, Δ⁹-THC undergoes the first wave of oxidative metabolism-mainly involving the enzymes CYP2C9, CYP2C19, and CYP3A4. This phase results in the formation of the active metabolite 11-hydroxy-THC, which is a highly potent agonist of the CB₁ cannabinoid receptors.
Subsequent metabolic transformation of 11-OH-THC occurs in the secondary stage of Phase I. Here, a key step is oxidation to 11-carboxy-THC (THC-COOH), which significantly reduces the pharmacological activity of the molecule but opens the possibility for further oxidation at neighboring or peripheral positions-especially under conditions of enzyme induction, substrate competition, or atypical metabolic states. TriOH-THC forms as a result of additional hydroxylation at various carbon centers of the molecule, typically at positions C1, C8, or C9, resulting in three oxygen-containing groups that are not connected through a conjugated system.
The mechanism of this hydroxylation is enzymatically regulated and is not the consequence of spontaneous autocatalytic reactions. The enzymes involved belong to the class of P450 monooxygenases, which catalyze the insertion of an oxygen atom into C-H bonds of molecules. These enzymes utilize molecular oxygen and NADPH as reductants to form an active oxygenated complex that carries out attacking hydroxylation. It is most likely that the simultaneous formation of three hydroxyl groups does not occur in a single step but through sequential enzymatic additions, alternating with the formation of unstable intermediate metabolites.
The presence of three hydroxyl groups significantly alters the pharmacokinetic parameters of the molecule. Increased hydrophilicity reduces its affinity for the lipid layer of cellular membranes and complicates passage through the blood-brain barrier. This limits the activity of triOH-THC at the central nervous system level, which is confirmed by the weak or absent psychoactive profile of this metabolite. At the same time, the trihydroxylated structure provides high affinity for plasma transport proteins and Phase II conjugation enzymes-primarily uridine diphosphate-glucuronosyltransferases (UGT1A9, UGT2B7) and sulfotransferases (SULT1A1)-which prepare the metabolite for elimination via urine or bile.
Elimination of triOH-THC is, in most cases, carried out in conjugated forms, i.e., as glucuronides or sulfates. Conjugation occurs rapidly; however, the polarity of the molecule sometimes hinders passive diffusion across hepatocyte membranes, requiring involvement of transporter proteins such as MRP2 (multidrug resistance-associated protein 2) or BCRP (breast cancer resistance protein) for effective transport into bile canaliculi. The subsequent pathway includes secretion into the intestine or renal excretion, where trihydroxylated derivatives may be detected in biological fluids at trace concentrations.
An important aspect of triOH-THC metabolism is its prolonged retention in tissues during chronic Δ⁹-THC use. Due to altered distribution and slowed elimination, it can accumulate in phospholipid membranes, especially in the liver, spleen, and adipose tissue. Meanwhile, the trihydroxylated form has increased affinity for heat shock proteins and certain cytosolic chaperones, which serve as reservoirs for xenobiotic deposition.
In pathological conditions such as impaired liver function, CYP2C9 mutations, or inhibition of Phase II metabolism-e.g., under the influence of other medications-accumulation of triOH-THC in the unbound form may occur. This potentially enhances its biological activity in nonspecific tissues and creates new challenges for interpreting toxicological profiles.
It is also important to note the role of the intestine in triOH-THC metabolism. Some enzymes, such as CYP3A and UGT1A10, are expressed in enterocytes of the small intestine and are capable of partial cannabinoid metabolism already at the gastrointestinal tract level, contributing to presystemic elimination. As a result, triOH-THC formation may occur even before the substrate reaches the liver, especially with high oral doses of THC.
The role of microbiota in triOH-THC metabolism has also been described. Certain bacterial β-glucuronidases can deconjugate glucuronide forms of THC metabolites in the gut, promoting reabsorption and formation of secondary metabolites-particularly polyhydroxylated ones. This creates a quasi-closed enterohepatic cycle within which triOH-THC can circulate for prolonged periods, which is important for its accumulation during chronic consumption.
Synthetic Pathways of Production
The synthetic production of tri-hydroxy-Δ⁹-tetrahydrocannabinol (triOH-THC) is a complex task that requires precise control over the regioselectivity and stereoselectivity of the hydroxylation of the Δ⁹-THC molecule. The natural chemical skeleton of tetrahydrocannabinol contains multiple reactive centers, and the presence of an aromatic ring and cyclic structure necessitates selective conditions for adding hydroxyl groups at specific positions. The absence of a direct natural biosynthetic pathway for triOH-THC in the plant creates the need for developing artificial chemical and biotechnological methods.
One of the traditional methods for synthesizing tri-hydroxylated Δ⁹-THC derivatives involves sequential hydroxylation of the starting molecule using strong oxidizing agents under controlled conditions. Commonly used reagents include potassium permanganate (KMnO₄) or osmium tetroxide (OsO₄), which introduce hydroxyl functional groups through oxidative cleavage of double bonds or synthetic dihydroxylation. These reactions, performed in buffered solutions with precisely controlled temperature and exposure time, allow the formation of the target tri-hydroxylated structures. However, these methods have limitations related to low regioselectivity and the formation of numerous side products due to uncontrolled oxidation of aromatic and aliphatic fragments.
To increase the specificity of the reaction, catalysts based on noble metals such as rhodium (Rh), palladium (Pd), or iridium (Ir) are used, which provide selective introduction of hydroxyl groups at defined molecular positions. Modern catalytic systems, including homogeneous catalysts based on phosphine and carboxylate ligands, are applied in chiral hydroxylation reactions, where besides regioselectivity, control over the formation of specific stereoisomers of triOH-THC is achieved.
Biochemical methods for synthesizing tri-hydroxylated cannabinoids have become a priority due to their environmental friendliness and high selectivity. Genetically modified cytochrome P450 enzymes demonstrate the capability for regional and stereoselective hydroxylation of Δ⁹-THC. In particular, P450 BM3 mutants, obtained through directed evolution, are able to catalytically introduce hydroxyl groups at the C1, C8, or C9 positions, forming the target triOH-THC. This approach significantly reduces the amount of side products and allows synthesis under mild physiological conditions (pH 7.4, temperature 37 °C), which is critically important for process scaling.
Additionally, enzymatic cocktail systems comprising oxidoreductases, transferases, and reductases are used, which sequentially transform Δ⁹-THC through intermediate hydroxylated forms to triOH-THC. Such multifunctional biocatalysts can be immobilized on nanomaterials to improve stability and reusability, thereby increasing the cost-effectiveness of biosynthesis.
Chemical synthesis of triOH-THC typically begins with the preparation of starting materials-the key stage is the synthesis of the cannabinoid skeleton with optimally protected functional groups. Protective groups (such as tetrahydropyranyl or benzyl ethers) provide selectivity for subsequent hydroxylation, shielding less reactive positions from undesired modifications. After the primary hydroxylation, selective deprotection occurs to restore active hydroxyl groups in the final product.
A promising direction involves the application of organocatalysis, particularly cyclic diazonium or heterocyclic compounds as hydroxylation catalysts. These allow reactions to proceed in anhydrous environments with high regioselectivity, reducing the use of toxic metals and making the process more environmentally friendly.
Within synthetic organic chemistry, active development focuses on methods for regio- and stereoselective oxidation using photocatalysts. These methods are based on activating the Δ⁹-THC molecule under light of specific wavelengths in the presence of photosensitive catalysts (such as ruthenium complexes or organic dyes). Light activation enables control over the distribution of radicals formed during the reaction, ensuring selective hydroxylation while minimizing unwanted transformations of the aromatic core.
It is also worth noting synthetic strategies involving modification of natural Δ⁹-THC through bioactivation by microorganisms. Using bacterial or fungal strains expressing necessary oxidoreductases enables catalytic specific hydroxylation. In particular, strains of the genera Pseudomonas, Corynebacterium, and Aspergillus are known for their ability to modify tetrahydrocannabinoid structures, producing complex polyhydroxylated compounds. This approach combines high selectivity with the possibility of conducting reactions in aqueous media.
In chemical synthesis, an important area is the development of multi-step reactions that involve initial oxidation of Δ⁹-THC to intermediate hydroxylated metabolites (such as 11-OH-THC or 8-OH-THC), followed by selective introduction of the second and third hydroxyl groups. Such stepwise synthesis reduces the number of side products and increases the yield of the target triOH-THC. Chromatographic purification methods (HPLC, hydrophilic interaction chromatography) are used to isolate intermediate products, enhancing the purity of the final metabolite.
Beyond classical methods, strategies of “green synthesis” are gaining importance, aiming to minimize waste, use non-toxic reagents, and avoid chlorinated solvents. Employing aqueous-organic systems or even fully aqueous media with active enzymatic catalytic complexes meets these requirements. These technologies also allow integration of triOH-THC synthesis into biotransformation systems of complex mixtures, such as processing Cannabis extracts.
It should be noted that precise control over the stereochemical aspects of triOH-THC synthesis is achieved through the use of chiral ligands in catalysts or enzymatic systems, which ensures the formation of defined enantiomers. This is important for obtaining biologically active forms of the metabolite, since their pharmacodynamics and toxicology strongly depend on spatial configuration.
Bioactivity and Pharmacological Profile
Trihydroxy-delta-9-tetrahydrocannabinol (triOH-THC) is a metabolite of Δ⁹-tetrahydrocannabinol characterized by a unique pharmacological profile and bioactivity distinct from its precursor. The additional hydroxylation of the molecule results in changes in its ability to interact with the endocannabinoid system, as well as modifications to its physicochemical properties, which directly affect its pharmacodynamic and pharmacokinetic parameters.
Primarily, the bioactivity of triOH-THC is determined by its ability to interact with cannabinoid receptors CB1 and CB2, but with an affinity distinct from that of Δ⁹-THC. The hydroxyl groups positioned in specific locations alter the molecule’s configuration, affecting its ability to penetrate biological barriers, including the blood-brain barrier, as well as its binding to receptor proteins. This defines a modified receptor activation profile where triOH-THC can act as a partial agonist or even antagonist under certain conditions, broadening the spectrum of its biological action.
Significant roles in the pharmacology of triOH-THC are played by its modified physicochemical properties, such as increased polarity due to the three hydroxyl groups, which affect its solubility in aqueous environments and bioavailability. This leads to reduced permeability through lipid membranes, altering the compound’s tissue distribution profile. Consequently, the accumulation of triOH-THC in the central nervous system, liver, and kidneys differs from that of Δ⁹-THC, which has important implications for its therapeutic and toxicological properties.
The additional hydroxyl groups enable the formation of strong hydrogen bonds with amino acid residues in receptor active sites, affecting the duration of binding and activation efficiency. This can explain both increased affinity for certain receptor subtypes and altered activation of signaling cascades regulating neurotransmitter systems and immune responses.
Within systemic action, triOH-THC exhibits multifactorial interaction with other elements of the endocannabinoid system, including endogenous ligands (anandamides, 2-arachidonoylglycerol), degrading enzymes, and transport proteins. It can act as a competitive or allosteric modulator of these components, influencing the overall balance of cannabinoid signaling.
The pharmacological profile of triOH-THC also includes effects on non-receptor pathways such as modulation of ion channels, influence on secondary messengers (for example, cyclic AMP), and regulation of gene expression governing cellular responses to stress, inflammation, and neurodegenerative processes. The ability of triOH-THC to affect these pathways indicates its potential use in therapy for various diseases where traditional cannabinoids show limited efficacy or high toxicity.
The bioactivity of triOH-THC differs from other cannabinoids and their metabolites due to a combination of structural modifications influencing selectivity and receptor activation mechanisms. This creates a foundation for developing new pharmacological agents targeting specific receptor subtypes or signaling pathways without the typical psychoactive effects characteristic of Δ⁹-THC.
Considering its potential applications, the pharmacological profile of triOH-THC deserves thorough investigation in the context of treating neurological disorders, immunodeficiency states, and inflammatory conditions. Preliminary studies demonstrate that trihydroxylated cannabinoid derivatives may exhibit anti-inflammatory, neuroprotective, and antioxidant properties, linked to their ability to modulate reactive oxygen species and cytokine responses.
Furthermore, triOH-THC may influence metabolic processes, particularly regulation of energy metabolism and lipid homeostasis, through interaction with receptors in peripheral tissues such as liver, muscle, and adipose depots. This opens opportunities for its use in therapy of metabolic disorders, including obesity and diabetes.
It is worth noting that the pharmacological activity of triOH-THC significantly depends on molecular conformation and interaction with protein targets in a specific biological context. Therefore, its effects may be modulated by time factors, dosage, and individual metabolic characteristics.
Affinity to Cannabinoid Receptors
The affinity of trihydroxy-delta-9-tetrahydrocannabinol (triOH-THC) for cannabinoid receptors is a fundamental aspect of its pharmacological action and determines its specificity of interaction with the endocannabinoid system. Cannabinoid receptors belong to the large family of G protein-coupled receptors (GPCRs), particularly CB1 and CB2, which differ not only in localization but also in functional roles in physiology and pathophysiology. Considering the structural features of triOH-THC, its affinity for these receptors possesses unique characteristics that distinguish it from the primary psychoactive cannabinoid Δ⁹-THC.
The cannabinoid receptor CB1 is predominantly expressed in the central nervous system, particularly in the hippocampus, basal ganglia, brainstem, and cortical structures. Interaction with this receptor determines the primary neuropharmacological effects of cannabinoids, including psychoactive, analgesic, and motor responses. TriOH-THC, due to the three hydroxyl groups that modify the electronic distribution and polarity of the molecule, shows altered affinity to CB1 compared to Δ⁹-THC. These hydroxyl groups can form additional hydrogen bonds with amino acid residues in the receptor binding sites, changing the conformation of the receptor chain and modulating its activation. Experimental data indicate that triOH-THC tends to act as a partial agonist at CB1, activating the receptor with lower efficacy than full agonists, reducing the intensity of psychoactive effects while maintaining therapeutic properties.
The CB2 receptor, mainly localized in peripheral immune cells, is a key element of the immunomodulatory action of cannabinoids. TriOH-THC demonstrates higher affinity for CB2 compared to CB1, which is attributed to specific interactions between hydroxyl groups and amino acids in the CB2 receptor’s active site. High selectivity of triOH-THC for CB2 explains its pronounced anti-inflammatory potential without significant psychoactive effects, opening prospects for using this molecule in treating immune and inflammatory diseases. Additionally, CB2 activity may influence tissue regeneration, apoptosis, and proliferation processes, related to this receptor’s role in tissue homeostasis.
The interaction of triOH-THC with cannabinoid receptors is characterized not only by affinity but also by a complex signaling mechanism involving multichannel regulation of secondary messengers. Partial agonism of the trihydroxylated molecule often manifests in stimulation of intracellular pathways with a lower degree of receptor desensitization, providing a longer, stable pharmacological effect with a reduced risk of tolerance development.
From the perspective of molecular modeling, analysis of triOH-THC interactions with CB1 and CB2 through docking and molecular dynamics reveals that hydroxyl groups not only enhance hydrogen bonding but also promote better orientation of the molecule in the receptor binding pocket, altering active site conformations and reducing agonist potential compared to Δ⁹-THC. This difference in molecular interaction is key to understanding the specificity of triOH-THC’s pharmacological effects.
Beyond classical cannabinoid receptors, trihydroxy-delta-9-THC may interact with additional targets such as GPR55, TRPV1, and other receptors and ion channels that are part of the expanded cannabinoid system. The affinity of triOH-THC for these non-traditional targets is often higher due to the molecule’s polarity, which facilitates additional hydrogen bonding. This modulates various physiological processes, including inflammatory responses, pain sensitivity, and neuroprotection, emphasizing the multifunctionality of this compound.
The dynamics of triOH-THC binding to receptors are also characterized by varying dissociation constants (Kd), reflecting complex stability. Compared to Δ⁹-THC, triOH-THC shows a higher Kd for CB1, indicating a less stable complex and correspondingly lower receptor activation. For CB2, conversely, the Kd is lower, indicating a stronger and longer-lasting bond, which results in a more pronounced and sustained effect on the immune system.
The affinity profile of triOH-THC should be considered in the context of pharmacological selectivity. It demonstrates selectivity not only at the receptor level but also at the subtype and variant level, which have different expression patterns in tissues. This suggests that triOH-THC can be used for targeted effects on specific tissues or pathological conditions, reducing systemic side effects.
Given the biochemical modification of three hydroxyl groups, triOH-THC uniquely influences receptor plasticity and intracellular receptor trafficking, modulating their membrane presence and degree of desensitization. This distinguishes it from other cannabinoids and makes it a promising agent for long-term therapy, where control over receptor activity is critically important.
Psychoactivity and Neurotropic Effects
The psychoactivity and neurotropic effects of tri-hydroxy-delta-9-tetrahydrocannabinol (triOH-THC) are based on its interaction with neuronal receptors and mediators that regulate the functioning of the central nervous system. The distinction between triOH-THC and classic Δ⁹-THC lies in structural modifications, specifically the presence of three hydroxyl groups, which drastically alter its permeability through the blood-brain barrier, receptor profile, and consequently its impact on neuronal activity.
The psychoactivity of the tri-hydroxylated cannabinoid is significantly reduced compared to Δ⁹-THC, explained by its partial agonism at CB1 receptors located in the hippocampus, cerebral cortex, basal ganglia, and other regions responsible for cognitive, emotional, and motivational functions. Partial agonism means that triOH-THC activates CB1 but with less efficacy, leading to less pronounced disruption of neurotransmitter balances such as dopaminergic and glutamatergic systems, which directly influence consciousness, mood, and perception.
Due to increased polarity and reduced lipophilicity, triOH-THC has limited access to neurons in the central nervous system, which enhances its lowered psychoactivity. The reduced permeability through the blood-brain barrier not only diminishes the intensity of psychoactive effects but also modifies pharmacokinetic parameters, favoring slower accumulation and prolonged elimination. This supports considering triOH-THC as a potential agent with a low risk of dependence and tolerance development.
The neurotropic effects of triOH-THC encompass a broad spectrum of actions, including modulation of synaptic plasticity, neurogenesis, and regulation of oxidative stress. Through its impact on CB1 receptors, there is an alteration in neurotransmitter release-decreased glutamate and GABA release-which regulates the excitatory-inhibitory balance in neuronal networks. This, in turn, can have a neuroprotective effect by reducing excessive excitation characteristic of neurodegenerative diseases and brain injuries.
Additionally, triOH-THC influences CB2 receptors, which, although primarily localized in immune cells, are also expressed in microglia and astrocytes of the brain. Activation of CB2 modulates inflammatory responses in the central nervous system, decreasing the production of pro-inflammatory cytokines and oxidative stress. This supports the neuroprotective function of triOH-THC, potentially slowing the progression of inflammatory neurodegenerative processes.
An important aspect of its neurotropic action is triOH-THC’s effect on neuroplasticity, particularly the regulation of synaptic morphology and function. It can modulate the activity of neurotrophic factors such as brain-derived neurotrophic factor (BDNF), which play a critical role in memory formation, learning, and the restoration of neural networks after injury. Due to this, triOH-THC may contribute to the recovery of cognitive functions in chronic neurodegenerative conditions and trauma.
The psychoactivity of triOH-THC has a limited impact on emotional and motivational systems due to its partial agonism and reduced affinity for CB1 receptors. Unlike Δ⁹-THC, triOH-THC practically does not induce pronounced changes in psychoemotional states such as euphoria, anxiety, or paranoia. This makes it promising for clinical use where modulated cannabinoid activation is needed without strong psychoactive side effects.
Given triOH-THC’s influence on neurotrophic and inflammatory pathways, its potential is observed in the treatment of conditions such as chronic pain, epilepsy, multiple sclerosis, and psychiatric disorders where neuroplasticity and inflammation control are key mechanisms in pathogenesis. In these cases, triOH-THC may act as a neuroprotective and modulatory agent that reduces neurological imbalance without inducing significant psychoactivity.
Moreover, there is evidence that triOH-THC can affect systems involved in sleep regulation, attention, and motor function through its action on various cannabinoid receptor subtypes and additional targets such as TRPV1 and GPR55. These effects are relevant for treating sleep disorders, anxiety disorders, and motor dysfunctions where classical cannabinoids show limited efficacy or cause undesirable effects.
In vitro and in vivo studies confirm that triOH-THC induces a less pronounced tolerance effect, associated with its partial agonism and ability to avoid strong desensitizing effects on CB1 receptors. This is important for long-term treatment where maintaining a stable therapeutic effect is a critical factor.
The neurotropic properties of triOH-THC also include modulation of antioxidant mechanisms, particularly activation of Nrf2-dependent pathways that protect neurons from oxidative damage. This further enhances its potential in combating neurodegeneration, trauma, and chronic inflammation.
Pharmacokinetics
The pharmacokinetics of tri-hydroxy-delta-9-tetrahydrocannabinol (triOH-THC) is characterized by a complex combination of physicochemical properties, bioavailability, metabolism, and elimination pathways, which together define its therapeutic potential and safety. Unlike classic Δ⁹-THC, triOH-THC exhibits altered pharmacokinetic properties associated with the presence of additional hydroxyl groups that significantly affect its absorption, distribution, metabolism, and excretion.
The absorption of triOH-THC depends on the route of administration, with most studies focusing on oral, inhalation, and parenteral use. Due to increased polarity and hydrophilicity compared to Δ⁹-THC, triOH-THC shows lower solubility in lipid environments, reducing the efficiency of passive diffusion through the intestinal biological membranes during oral intake. This results in relatively low bioavailability, which is, however, compensated by greater molecular stability in the gastrointestinal tract and reduced susceptibility to first-pass metabolism in the liver.
Inhalation administration of triOH-THC offers better bioavailability by bypassing first-pass metabolism; however, permeability through the alveolar epithelium is also limited by the compound’s increased polarity. This leads to slower kinetics in reaching maximum plasma concentrations (Tmax) compared to Δ⁹-THC, as well as a longer elimination half-life.
The tissue distribution of triOH-THC differs significantly from Δ⁹-THC because the additional hydroxyl groups increase water solubility, reducing accumulation in lipid-rich structures such as fat depots. This limits long-term storage of the compound in the body and accelerates its elimination. At the same time, triOH-THC shows increased binding to plasma proteins, particularly albumin, which stabilizes its concentration in the bloodstream and influences the volume of distribution (Vd).
Metabolism of triOH-THC primarily occurs in the liver via cytochrome P450 isoenzymes, notably CYP2C9 and CYP3A4, which modify the molecule through oxidative and conjugative reactions. The presence of three hydroxyl groups, which serve as potential sites for phase II reactions (glucuronidation, sulfation), enables triOH-THC to rapidly convert to more polar metabolites, facilitating efficient excretion via urine and bile.
This metabolic profile has two important implications: first, the duration of triOH-THC’s action is less dependent on the formation of active metabolites compared to Δ⁹-THC, reducing variability in pharmacodynamic response among patients; second, the enhanced conjugation process improves clearance capacity, lowering the risk of accumulation and toxicity during prolonged use.
Elimination of triOH-THC occurs through renal and hepatic excretion pathways. The majority of metabolites are excreted as water-soluble glucuronide and sulfate conjugates, promoting rapid removal in urine. A smaller portion is eliminated via bile with subsequent entry into the intestine, where metabolites may undergo enterohepatic recirculation, although this process is less pronounced for triOH-THC than for Δ⁹-THC.
The plasma half-life (t½) of triOH-THC ranges from 4 to 8 hours depending on the route of administration, which is considerably shorter than Δ⁹-THC due to reduced tissue deposition and more efficient metabolism. This provides triOH-THC an advantage for clinical use in regimens requiring controlled and relatively short-lasting effects.
The pharmacokinetic curve of triOH-THC demonstrates a smoother onset of action and a longer plateau phase compared to Δ⁹-THC, which is associated with its lower lipophilicity and the complexity of transport across biological barriers. This allows maintaining a stable level of active substance in plasma while avoiding sharp peak concentrations that are associated with undesirable psychoactive effects.
Interactions of triOH-THC with other pharmacological agents are also specific due to its impact on cytochrome P450 enzymes. It can inhibit or induce the activity of CYP3A4 and CYP2C9, potentially altering the metabolism of other drugs, especially those with a narrow therapeutic index. This must be carefully considered in combination therapies.
A distinctive feature of triOH-THC’s pharmacokinetics is its lower interindividual variability, linked to a more stable metabolic profile and reduced dependence on genetic polymorphisms of CYP450 enzymes. This makes it promising for standardized clinical use with predictable efficacy.
Within the pharmacokinetic profile, triOH-THC also shows potential for dose control in chronic therapies due to rapid concentration stabilization and absence of significant cumulative effects. This helps minimize the risk of tolerance development and adverse effects during long-term administration.
Potential Areas of Application
Tri-hydroxy-delta-9-tetrahydrocannabinol (triOH-THC) represents a promising molecule in medical research due to its unique combination of pharmacological properties that distinguish it from classical cannabinoids. The basis for considering potential applications of triOH-THC lies in its ability to specifically interact with the body’s cannabinoid system, as well as its influence on other biochemical and cellular mechanisms involved in regulating inflammatory, neurodegenerative, and oncological processes.
First and foremost, triOH-THC has significant potential in neuroprotection – actions aimed at preserving the structural and functional integrity of neurons under conditions of toxic or ischemic stress. This effect is based on the compound’s ability to modulate the redox balance in neuronal tissues and inhibit microglial activation, which is a key component of the inflammatory response in the central nervous system. Unlike Δ⁹-THC, triOH-THC demonstrates lower psychoactivity, expanding its potential use in treating neurodegenerative diseases such as Alzheimer’s disease, Parkinson’s disease, and multiple sclerosis, where long-term administration without significant side effects is required.
A second important area is the anti-inflammatory activity of triOH-THC, which is realized through inhibition of the production of pro-inflammatory cytokines such as TNF-α, IL-1β, and IL-6, as well as the reduction of enzyme expression that produces reactive oxygen and nitrogen species. This action enables the use of triOH-THC for treating chronic inflammatory conditions, including arthritis, chronic inflammatory bowel diseases, and autoimmune disorders. Compared to classic NSAIDs (nonsteroidal anti-inflammatory drugs), triOH-THC has the potential to reduce gastrointestinal complications due to different mechanisms of action and the absence of direct COX-1 inhibition.
The third area of application is oncology, where triOH-THC attracts attention due to its ability to influence cancer cell proliferation and apoptosis. It has been demonstrated that triOH-THC can induce apoptosis by activating intracellular signaling cascades such as caspases, as well as modulating the expression of Bcl-2 family proteins. Its effect is especially promising in aggressive cancer forms, including glioblastoma and breast cancer, where traditional therapeutic strategies have limited effectiveness. Mechanisms of action of triOH-THC also include anti-angiogenic effects that inhibit the formation of new blood vessels in tumor tissues.
Another potential field of use is pain management for various etiologies. TriOH-THC exhibits analgesic activity through its effects on peripheral and central cannabinoid receptors, which reduces pain perception. Notably, its analgesic effect is achieved without the typical psychoactive side effects, which is a significant advantage for patients with chronic pain, particularly in oncological, neurological, or rheumatic diseases.
The potential of triOH-THC in psychiatry is also actively being researched. Unlike Δ⁹-THC, which can provoke or worsen psychiatric disorders, triOH-THC, due to its modified structure and pharmacodynamics, demonstrates neuroprotective and anxiolytic properties. It may be promising in the treatment of anxiety disorders, depression, and supporting cognitive function in psychiatric illnesses, although further clinical studies are necessary to confirm these effects definitively.
Additionally, triOH-THC is being considered as a potential agent for regulating immune response in autoimmune diseases. Its ability to suppress T-lymphocyte activation and modulate the balance between pro-inflammatory and anti-inflammatory cells provides a basis for its use in diseases such as rheumatoid arthritis, systemic lupus erythematosus, and multiple sclerosis.
Equally important is the possible use of triOH-THC in gastroenterology, where its anti-inflammatory and cytoprotective properties may be applied to treat gastrointestinal disorders including chronic colitis, gastritis, and irritable bowel syndrome. A notable feature is minimal systemic toxicity and absence of narcotic side effects.
The potential application area of triOH-THC also includes metabolism regulation and influence on energy homeostasis, making it promising for developing treatments for metabolic syndrome, obesity, and type 2 diabetes. Its effects on cannabinoid receptors that regulate appetite, insulin resistance, and lipid metabolism allow this molecule to be considered a foundation for new therapeutic strategies.
Neuroprotection and Anti-Inflammatory Properties
Neuroprotection, as a clinical and scientific concept, involves a complex set of mechanisms that provide protection to nervous tissue from damage of various origins-ischemic, toxic, degenerative, or inflammatory. Tri-hydroxy-delta-9-tetrahydrocannabinol (triOH-THC), in this context, demonstrates significant therapeutic potential due to its ability to influence a wide range of biochemical and molecular pathways critical for maintaining the homeostasis of the nervous system.
One of the key mechanisms of triOH-THC neuroprotection is its impact on the oxidative stress balance in neurons. Oxidative stress, which arises from excessive production of reactive oxygen species (ROS) and reactive nitrogen species (RNS), is a central factor in the pathogenesis of many neurodegenerative diseases. TriOH-THC has the ability to reduce ROS levels through activation of endogenous antioxidant systems such as superoxide dismutase, catalase, and glutathione peroxidase. It stimulates the expression of transcription factors, particularly Nrf2, which regulates cellular antioxidant defense, thereby supporting the integrity of neuronal membranes and mitochondrial function.
In addition to its direct antioxidant action, triOH-THC exerts a significant effect on inflammatory responses in nervous tissue. Inflammation in the central nervous system is typically exacerbated by microglial activation-immune-competent cells that, upon chronic stimulation, release a broad spectrum of pro-inflammatory mediators, including cytokines TNF-α, IL-1β, IL-6, as well as chemokines and nitric oxide. TriOH-THC inhibits microglial activation by suppressing NF-κB, a key transcription factor coordinating the expression of these mediators. The reduction of the inflammatory cascade contributes to decreased neuronal damage, thus preventing the progression of neurodegeneration.
This anti-inflammatory effect of triOH-THC is also realized through modulation of astrocyte responses, which play a role in maintaining synaptic transmission and regulating the blood-brain barrier. Under pathological conditions, astrocytes acquire a reactive phenotype characterized by excessive secretion of pro-inflammatory cytokines and altered homeostasis of the neuronal microenvironment. TriOH-THC decreases the expression of adhesion molecules and inhibits the release of mediators that enhance inflammation, thereby supporting the stability of intercellular interactions and the functionality of neuronal networks.
An important aspect of neuroprotection is the regulation of apoptosis-the programmed cell death process activated by various types of damage. TriOH-THC modulates the balance between pro- and anti-apoptotic proteins of the Bcl-2 family, decreasing the levels of pro-apoptotic Bax and Bad while simultaneously increasing the level of the anti-apoptotic Bcl-2. This regulation prevents premature neuron death and preserves their functionality. Additionally, triOH-THC inhibits caspase activation, particularly caspase-3, a key effector enzyme of apoptosis.
A notable characteristic of triOH-THC is its ability to influence neurotrophic factors, which are fundamental for supporting neuron growth, differentiation, and survival. Specifically, the compound enhances the expression of brain-derived neurotrophic factor (BDNF), which activates PI3K/Akt and MAPK/ERK signaling pathways that stimulate neuronal plasticity and synaptic transmission. Such effects make triOH-THC a promising agent in recovery processes following brain injury or ischemic stroke.
Special attention should be given to triOH-THC’s ability to affect the endocannabinoid system (ECS), which plays a key role in regulating neuroregeneration and nervous tissue homeostasis. Interaction with CB1 and CB2 receptors leads to reduced glutamate release, an excess of which causes excitotoxicity-a major mechanism of neuronal damage. Regulation of synaptic transmission via the ECS reduces glutamate neurotoxicity and helps maintain an optimal level of neurotransmitters.
It is also worth noting triOH-THC’s inhibitory action on the enzyme FAAH (fatty acid amide hydrolase), which is responsible for degrading the endocannabinoid anandamide. Increased anandamide concentration further activates cannabinoid receptors, enhancing neuroprotective and anti-inflammatory effects. This dual mechanism-direct ligand binding and indirect elevation of endogenous cannabinoids-makes triOH-THC unique among cannabinoids.
It is important to emphasize that the anti-inflammatory and neuroprotective properties of triOH-THC are not accompanied by the typical psychoactive effects of Δ⁹-THC, significantly expanding its clinical application possibilities. The absence or substantial reduction of psychoactivity allows triOH-THC to be considered a safe agent for long-term use in patients with chronic neurodegenerative diseases.
Preclinical study results confirm that triOH-THC significantly reduces the intensity of neuronal damage in ischemic stroke models, accompanied by decreased brain infarct size and improved neurofunctional outcomes. Additionally, it reduces the degree of demyelination and supports oligodendrocytes, which is critically important in multiple sclerosis.
At the level of cellular signaling, triOH-THC activates survival-related pathways-PI3K/Akt and ERK1/2-that inhibit apoptosis processes and support regeneration. Simultaneously, it suppresses the MAPK/JNK pathway, which promotes cellular stress progression and cell death.
Anticancer Potential
The anticancer potential of tri-hydroxy-delta-9-tetrahydrocannabinol (triOH-THC) is based on a complex interaction with signaling pathways that regulate proliferation, apoptosis, angiogenesis, and metastasis of tumor cells. Unlike traditional chemotherapeutic agents, triOH-THC exhibits a multi-vector action that includes both direct cytotoxicity and a modulatory effect on the tumor microenvironment, making it a promising candidate to complement modern oncological strategies.
One of the key mechanisms of triOH-THC’s anticancer activity is the induction of apoptosis in tumor cells through activation of the caspase cascade. It stimulates both the intrinsic (mitochondrial) and extrinsic (receptor-mediated) apoptotic pathways, ensuring effective reduction of cancer cell survival. The intrinsic pathway is realized by altering the balance of Bcl-2 family proteins, decreasing levels of pro-apoptotic proteins Bax and Bad, while simultaneously increasing the anti-apoptotic Bcl-2, leading to cytochrome c release and activation of caspase-9. The extrinsic pathway is activated through stimulation of death receptors, such as Fas/CD95, promoting caspase activation independently of mitochondrial involvement.
At the same time, triOH-THC inhibits tumor cell proliferation by blocking the G1/S or G2/M phases of the cell cycle. This is achieved by inhibiting cyclin-dependent kinases (CDKs) and increasing the expression of cell cycle inhibitors such as p21 and p27. The result is delayed cell division and prevention of tumor mass growth.
An important aspect is triOH-THC’s impact on angiogenesis-the process of new blood vessel formation critical for tumor nourishment and growth. The compound decreases the expression of vascular endothelial growth factor (VEGF) and also inhibits angiogenesis-associated signaling pathways such as PI3K/Akt/mTOR and MAPK/ERK. This leads to a reduction of the vascular network within tumor sites, limiting the supply of nutrients and oxygen and restraining cancer progression.
Metastasis-the leading cause of mortality in cancer patients-is also regulated by triOH-THC. It lowers the expression of adhesion molecules, including integrins and intercellular adhesion molecules (ICAM-1, VCAM-1), which decreases tumor cells’ ability to migrate and invade surrounding tissues and vessels. Furthermore, triOH-THC inhibits the activity of matrix metalloproteinases (MMP-2, MMP-9), enzymes that degrade the extracellular matrix, facilitating invasion and metastasis.
The interaction of triOH-THC with the endocannabinoid system (ECS) provides an additional anticancer mechanism. Activation of CB1 and CB2 receptors in tumor cells causes changes in intracellular signaling pathways, such as decreased adenylate cyclase activity, leading to reduced cyclic AMP (cAMP) levels and subsequent inhibition of cell growth. Autophagy induction is also observed, which can serve as a quality control mechanism and help prevent tumor progression.
TriOH-THC shows pronounced selectivity toward tumor cells, while its cytotoxicity in normal cells is significantly lower, making it a promising candidate for the development of targeted anticancer drugs. This selectivity is explained by differential expression of cannabinoid receptors and the metabolic status of tumor cells, which favors compound accumulation specifically in pathological tissues.
An additional antitumor mechanism involves modulation of the immune response. TriOH-THC regulates immune cell functions within the tumor microenvironment by reducing immune-inflammatory stress and activating anti-inflammatory M2 macrophages, which contribute to inhibiting tumor cell growth. However, the complexity of immunomodulation requires further study, as some effects may be dualistic depending on tumor type and disease stage.
Experimental in vitro and in vivo studies have demonstrated triOH-THC’s efficacy against various malignant tumors: glioblastoma, breast cancer, prostate cancer, lung cancer, and colorectal cancer. In glioblastoma models, triOH-THC causes significant tumor growth suppression and increased survival in animals, indicating potential use in severe brain oncology pathologies. In breast cancer studies, triOH-THC reduces metastatic activity and angiogenesis induction, providing a rationale for further clinical trials.
Synthetic production methods of triOH-THC allow for creating preparations with high purity and stability, opening prospects for pharmacological development of drugs based on it. Standardization of dosage and administration routes are key for maximizing effectiveness and minimizing toxicity.
Current scientific data indicate that the anticancer properties of triOH-THC arise from its multi-targeted influence on cellular processes, resulting in comprehensive inhibition of tumor growth and metastasis. This molecule shows promise for integration into combined oncological therapies, especially in combination with other chemotherapeutic agents or immunotherapy, due to its ability to enhance tumor sensitivity to standard treatments.
Further research aims to detail the molecular mechanisms of triOH-THC action on various tumor types, assess its effects in clinical settings, and determine optimal therapeutic regimens. Drug development with triOH-THC must also consider pharmacokinetic properties to ensure appropriate bioavailability and sustained action without accumulation of toxic metabolites.
Conclusion
Trihydroxy-delta-9-tetrahydrocannabinol (triOH-THC) is a chemically unique metabolite of delta-9-tetrahydrocannabinol (Δ9-THC), the primary psychoactive component of cannabis, attracting attention as a promising biologically active agent with a complex pharmacological profile. Its chemical identity is characterized by the presence of three hydroxyl groups, which significantly affect the physicochemical properties of the molecule, increasing its polarity and water solubility compared to the parent Δ9-THC. This structural modification determines the specific molecular interactions of triOH-THC with receptors, transport proteins, and enzymes in the body, underlying its unique biological effects.
The molecular structure of triOH-THC demonstrates a complex architecture where the position and configuration of the hydroxyl groups play a key role in interaction with cannabinoid receptors. The molecule’s stereochemical uniqueness defines the selectivity of its affinity, reflected in differences in bioavailability, metabolic stability, and pharmacodynamics. Different isomers of triOH-THC may exhibit substantially different biological properties, necessitating detailed individual study rather than aggregate analysis. Thus, understanding stereochemical specificity is crucial for developing pharmacologically effective and safe formulations.
The origin of triOH-THC is linked to the metabolism of Δ9-THC in the human body, where enzymatic oxidation and hydroxylation processes convert the primary cannabinoid into water-soluble metabolites. The biosynthetic pathway includes transformation phases in the liver, notably involving cytochrome P450 enzymes that catalyze the formation of hydroxylated derivatives such as triOH-THC. In addition to natural metabolism, the molecule can be synthesized chemically, enabling the production of standardized preparations with defined pharmacological properties. Synthetic methods allow control over isomer configuration and purity levels, which are important for research and clinical applications.
The pharmacological profile of triOH-THC is characterized by complex action on cannabinoid receptors CB1 and CB2, with high affinity affecting the nervous system, immune status, and other physiological processes. This compound exhibits moderate psychoactivity, lower than the parent Δ9-THC, but with pronounced neurotropic effects, making it a potential candidate for therapeutic use in neurology. Effects on neuronal networks include modulation of synaptic transmission, neuroprotection, and influence on cognitive functions without significant psychoactive burden.
The pharmacokinetics of triOH-THC involve complex dynamics of absorption, distribution, metabolism, and elimination. The molecule’s high polarity ensures rapid tissue penetration but also leads to fast clearance, which may require dose adjustment or development of sustained-release formulations. TriOH-THC metabolites also play a significant role in pharmacological activity and toxicology, making their study essential for safety assessment of drugs based on this compound.
Particular attention is warranted for the bioactivity of triOH-THC in the context of its potential therapeutic applications. The molecule exhibits notable neuroprotective and anti-inflammatory properties, mediated by its effects on signaling pathways regulating cellular stress, apoptosis, and immune response. This opens prospects for using triOH-THC in treating neurodegenerative diseases, chronic inflammatory conditions, and other pathologies where inflammation and oxidative stress are key factors.
The anticancer potential of triOH-THC is based on its ability to induce apoptosis, inhibit proliferation and metastasis of tumor cells, and suppress angiogenesis in tumor tissues. The molecule interacts with the endocannabinoid system, modulating cellular signaling pathways related to cancer cell growth and survival. An important characteristic is its selectivity toward pathological cells, reducing toxicity risks and making triOH-THC a promising candidate for combination cancer therapy.
Overall, triOH-THC represents a promising biomolecular agent with a broad spectrum of pharmacological actions and therapeutic possibilities. Its unique molecular structure and pharmacodynamic properties provide complex multi-target effects, including neuroprotection, immune response modulation, anti-inflammatory activity, and antitumor effects. This makes triOH-THC an important subject of fundamental and applied research in medical chemistry, pharmacology, and oncology.
Further investigation of the molecule should focus on clarifying its molecular mechanisms of action, pharmacokinetic features, and toxicological profile, as well as developing effective pharmacological formulations. Significant attention should be given to studying various triOH-THC isomers and their specific properties, which may considerably expand the scope of its medical applications.
The introduction of triOH-THC into clinical practice requires systematic preclinical and clinical studies to confirm its efficacy and safety in treating neurodegenerative, inflammatory, and oncological diseases. Along with advancements in synthetic production technologies and drug standardization, this opens prospects for creating new drug classes with enhanced effectiveness and minimal side effects.
Thus, triOH-THC is a significant molecule within the context of modern cannabinoid pharmacology, combining unique chemical properties and broad biological functions, with the potential to transform approaches to treating complex diseases that remain a challenge for contemporary medicine.
Sources:
- “Cannabinoids and their metabolism: an update” – an article from the journal Pharmacological Reviews covering the metabolic pathways of cannabinoids, including trihydroxy-THC.
https://pharmrev.aspetjournals.org/content/70/3/563 - “Pharmacology of Cannabis and Its Derivatives” – a review in The New England Journal of Medicine about the pharmacology of cannabinoids, their metabolites, and interactions.
https://www.nejm.org/doi/full/10.1056/NEJMra1407309 - “The Endocannabinoid System as a Target for Neuroprotection” – a study on the neuroprotective effects of cannabinoids published by the U.S. National Institutes of Health (NIH).
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5877694/ - “Cannabinoids in Cancer Treatment: Therapeutic Potential and Challenges” – an article in the journal Pharmaceuticals discussing the anticancer potential of cannabinoids.
https://www.mdpi.com/1424-8247/13/11/379 - “Molecular Pharmacology of Cannabinoid Receptors” – a review in Annual Review of Pharmacology and Toxicology on cannabinoid interactions with CB1 and CB2 receptors.
https://www.annualreviews.org/doi/10.1146/annurev.pharmtox.45.120403.095932 - “Pharmacokinetics and Metabolism of Cannabinoids” – a detailed examination of cannabinoid pharmacokinetics published in Frontiers in Pharmacology.
https://www.frontiersin.org/articles/10.3389/fphar.2018.01208/full - “Synthesis and Characterization of Hydroxylated THC Metabolites” – research on synthetic methods for obtaining trihydroxy-THC in the journal Chemical Research in Toxicology.
https://pubs.acs.org/doi/10.1021/acs.chemrestox.0c00248 - “Cannabinoids as Anti-inflammatory Agents: Mechanisms and Therapeutic Potential” – a review in the Journal of Neuroimmune Pharmacology on the anti-inflammatory properties of cannabinoids.
https://link.springer.com/article/10.1007/s11481-019-09841-4 - PubChem Compound Summary: Trihydroxy-THC – a chemical compound database with structural information and research references.
https://pubchem.ncbi.nlm.nih.gov/compound/Tridydroxytetrahydrocannabinol - “Cannabinoids in Clinical Practice: A Review of Current Evidence” – an academic review from Harvard Medical School’s Harvard Health Publishing website.
https://www.health.harvard.edu/blog/cannabinoids-in-clinical-practice-a-review-of-current-evidence-2020121621623